Abstract
Starting with the Remane diagram, various conceptual models have been proposed to show how species richness varies along a salinity gradient. However, as relatively few estuaries experience extreme hypersalinity, quantitative data are lacking to evaluate the model. We used data for 1891 samples of benthic macroinvertebrates from 12 estuaries in southwestern Australia (salinity 0–122 ppt) to determine the salinities in which 257 taxa were recorded. The pattern of richness differed from the conceptual models, with relatively few species (≤20%) recorded in freshwater and oligohaline salinities. Richness peaked at 35 ppt (seawater, 44%) before declining precipitously, with 21% and 10% of taxa recorded in hyperhaline salinities of 40 and 48 ppt, respectively. Taxa were recorded across the full salinity range, and several holohaline annelids, crustaceans, and insects were identified. Descriptive statistics and the frequency distribution of each taxon along the salinity gradient are provided. These identify stenohaline taxa and those with different extents of euryhalinity and how the occurrence of these taxa changes with salinity. The results help predict how benthic macroinvertebrate species and assemblages in estuaries in southwestern Australia and other Mediterranean climatic regions may shift due to climate change, particularly increased incidences and magnitude of hypersalinity.
1. Introduction
Being located at the interface of freshwater derived from land drainage and marine water from tidal movement, estuaries are dynamic, and their environmental conditions vary markedly over a range of temporal scales, e.g., from a tidal cycle to several years of drought [1,2]. Many definitions of estuaries include statements such as “free connection with the open sea” and “seawater is measurably diluted with fresh water”, e.g., [3,4] and thus salinity values would typically range from 0 to 35 ppt. However, subsequent definitions have been broader and recognise that some estuaries become periodically disconnected from the sea and can become hypersaline [5,6]. These systems, which represent ~3% of all estuaries, have received increasing attention in recent years [7,8] and salinities > 300 ppt have been recorded in several [9,10].
The highly variable salinities present in estuaries pose osmoregulatory challenges compared to the relatively stable environments found in freshwater and marine ecosystems [11,12]. As a result, estuaries typically harbour a unique fauna, often comprising a smaller number of species, many of which are relatively euryhaline, meaning they can tolerate a wide range of salinities [13]. In contrast, stenohaline species, which can only tolerate a narrow range of salinities (<10 ppt), are less common in these environments [14]. Sampling conducted for the UK’s National Marine Monitoring Programme recorded 784 species (14 phyla) from coastal areas and 296 species (11 phyla) from estuaries, resulting in a marked difference in faunal composition [15]. Typically, the benthic macroinvertebrate species in macrotidal estuaries (tidal range > 4 m) are annelids, crustaceans (excluding insects), and molluscs. For example, species in these three groups comprised 93.2% of the 296 species recorded in 16 UK estuaries [15]. Similarly, of the species recorded from intertidal areas from the mouth to the tidal freshwater reaches of the Scheldt Estuary (The Netherlands and Belgium), 45% were annelids, 29% were molluscs, and 23% were crustaceans [16]. However, insects are an additional group that can occur in microtidal estuaries (tidal range < 2 m) in Europe [17], South Africa [18], and Australia [19].
Numerous natural environmental variables influence the composition of the benthic macroinvertebrate fauna within estuaries. These include habitat, particularly the structural complexity provided by macrophyte beds and shellfish reefs, sediment composition (e.g., grain size and organic matter content), and the physicochemical properties of water [17,18,19]. Salinity, in particular, plays a crucial role as species have evolved physiological and life-cycle adaptations to specific salinity ranges [20]. Osmoregulation, the physiological process by which organisms maintain the balance of water and salts in their bodies, is essential for survival in estuarine environments. When animals are exposed to salinities outside their tolerance range, it can disrupt homeostasis, leading to physiological stress, reduced fitness, altered behaviour, and even mortality [21].
Elevated salinity levels can cause dehydration and ion imbalance as organisms lose water to their hypertonic environment, leading to cellular and systemic stress. Conversely, low salinity levels can result in water influx, causing cells to swell and potentially rupture, disrupting normal cellular functions. Osmoregulation can be energy-intensive, as it involves the active transport of ions across cell membranes, which demands a significant amount of energy [14]. There are two main strategies for dealing with salinity changes: osmoregulation or osmoconformation. Osmoregulators actively regulate their internal osmolarity to remain constant, regardless of the external environment. This strategy is common in many fish, mammals, and birds and involves specialised organs like kidneys, salt glands, or gills to remove excess water or ions [22]. In contrast, osmoconformers match their internal osmolarity to the osmolarity of their surrounding environment. This strategy is typical of many marine invertebrates and is less energetically demanding but limits their ability to survive in fluctuating salinity conditions. Few organisms can osmoregulate or complete their life cycles along the full salinity gradient from fresh to seawater, and many are adapted to a salinity range that is taxon-specific [23]. Consequently, biological communities are structured by the salinity gradient, e.g., [24,25].
The relationship between salinity and species richness has become a paradigm of estuarine ecology [26] and is illustrated by the Remane diagram [27]. This conceptual model, which was based on benthic macroinvertebrate fauna from the Baltic Sea, was designed to show how the number of species changes along a salinity gradient (0–35 ppt) and the salinity tolerance of those species, i.e., freshwater, brackish, and marine. Numerous authors have adapted the Remane diagram since its original publication in 1934; see [28] for a review. In particular, Hedgpeth [29], based on studies from bays and lagoons in Texas, most notably the Laguna Madre, extrapolated the salinity gradient to 310 ppt. He also developed two terms: (i) holoeuryhaline, i.e., species distributed from freshwater to extreme hypersalinity that may be absent from marine waters, and (ii) ultrahaline, i.e., marine species occurring in oligohaline to extreme hypersaline waters. Similarly, Khlebovich [30] also extended the salinity gradient to 100 ppt. The revised diagrams produced by both authors showed a decrease in the number of species after 35 ppt, albeit with a slightly different shape to the curve. Based on a review of the many modifications to the Remane diagram and examining patterns of richness for various estuarine faunas, Whitfield et al. [28] produced a conceptual model for estuarine biodiversity from freshwater to hyperhaline conditions (0–60 ppt). It shows that richness is moderately high in freshwater (0–0.5 ppt), declines to a low in the horohalinicum (5–8 ppt), and increases to a maximum at 35 ppt and then decreases to 60 ppt. These authors also termed holohaline (i.e., species found between 0 and 100+ ppt), which includes both holoeuryhaline and ultrahaline species.
Despite its wide usage in estuarine ecology literature, the Remane diagram provides a qualitative assessment of the biodiversity in different salinities [28]. It has seldom been tested, particularly in estuaries that can become hyperhaline. Therefore, the primary aim of this study is to collate data across a range of estuaries in the same climatic region that experience a salinity gradient from oligohaline to markedly hyperhaline conditions and determine how the number of species changes and compare this to the various modifications to the Remane diagram. A secondary aim is then to examine the occurrence distribution of species along the salinity gradient. The results are useful in assessing and predicting how climate change-induced changes in salinity might impact the fauna of estuaries.
2. Materials and Methods
2.1. Site Description and Sampling
Southwestern Australian estuaries are microtidal and experience a Mediterranean climate, with hot, dry summers and cool, wet winters [31]. Over 85% of rainfall occurs between May and October, and there is a gradient ranging from ~1400 mm per year in the southwestern corner to a minimum of ~400 mm north and eastwards, where it becomes more variable [32]. These conditions result in some of the estuaries becoming disconnected from the ocean for several years at a time and, if rainfall is low, evaporation can cause them to be amongst the most hyperhaline globally [33,34].
Data on the benthic macroinvertebrate fauna were collected from 12 estuaries distributed along the coast of southwestern Australia (Figure 1) and differed in their physical characteristics and environmental conditions (Table 1 and Table S1). The Swan–Canning and Peel–Harvey estuaries are large, permanently-open systems on the lower west coast. Oyster Harbour and Waychiniup Estuary are also always open to the ocean, albeit smaller and located on the south coast. The Vasse–Wonnerup (west coast) and Broke, Torbay, Taylor, Normans and Cheyne inlets and Cordinup River (all south coast) are open to the ocean for part of each year (annually-open), while Beaufort Inlet may be closed for several years at a time (normally-closed). Due to these differences in bar status, the size of their catchments and riverine flow, they experience marked variations in salinity ranging from 0 to 122 ppt (Figure A1).
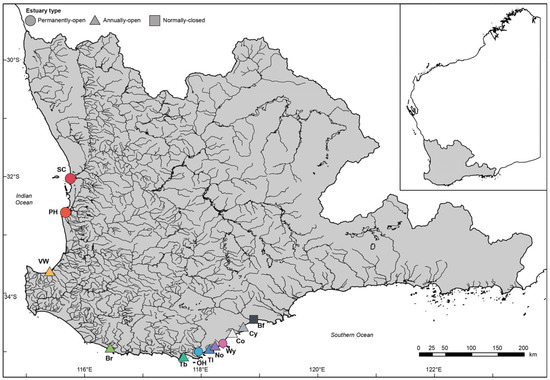
Figure 1.
Map of southwestern Australia showing the rivers in the southwest drainage division (grey shading) and the location of the 12 estuaries where samples of benthic macroinvertebrates were collected. SC, Swan–Canning Estuary; PH, Peel–Harvey Estuary; VW, Vasse–Wonnerup Estuary; Br, Broke Inlet; Tb, Torbay Inlet; OH, Oyster Harbour; Tl, Taylor Inlet; No, Normans Inlet; Wy, Waychinicup Estuary; Co, Cordinup River; Cy, Cheyne Inlet; Bf, Beaufort Inlet. Insert shows the location of the southwest drainage division within Western Australia.
Full details of the sampling protocols are provided in the relevant source publications and are summarised briefly in Table 1. Sampling was conducted along the longitudinal axis in all estuaries to incorporate the full range of salinities experienced during sampling. Moreover, the sites (12–60 per estuary) encompassed a range of sediment types and areas with and without submerged aquatic vegetation (e.g., seagrass and charophytes). Sampling was conducted between two and 15 times in each estuary to account for temporal variation. This extensive sampling yielded 1891 samples, with between 48 and 480 samples from each of the 12 estuaries.
Two broad sampling methods were employed depending on the depth of the water body. Sediment from shallow waters (0.5–1 m deep) was collected using a cylindrical corer with a diameter of 11 cm (sampling area: 96 cm2) that sampled to a depth of 15 cm. For sites where sediments are collected in waters deeper than 1 m, an Ekman grab (225 cm2) that sampled to a depth of 15 cm was employed. Peel–Harvey Estuary was the only exception where samples were collected (regardless of water depth) using a smaller corer with a 6 cm diameter (sampling area: 28 cm2). Once collected, sediment was wet sieved through a 500 µm mesh field sieve, and the residual material was transferred into a container and preserved with 4% formalin solution buffered in estuary water and/or 70% ethanol. At each site on each sampling occasion, water temperature (°C), salinity (ppt), and dissolved oxygen concentration (mgL−1) were measured at the bottom of the water column using the YSI 556 or ProDSS Multiparameter Digital Water Quality Meter (Yellow Springs Instrument, Yellow Springs, OH, USA). As the instruments were only capable of accurately reading to a maximum salinity of 70 ppt, water samples were diluted using deionised water to allow accurate measurement [9]. Although the SI unit of salinity is the practical salinity scale (PSU), this is a ratio (i.e., has no units) and its calculation was validated only for values between 2 and 42 [35]. As such, salinities in this study are provided in parts per thousand (ppt), which is also the unit of measurement provided by the water quality probes.

Table 1.
Summary of the characteristics of each of the 12 estuaries taken from [36], and details of the sampling regime and the mean and range of values recorded for a suite of water physicochemical parameters. n = number of samples; PO = permanently-open, AO = annually-open and NC = normally-closed.
Table 1.
Summary of the characteristics of each of the 12 estuaries taken from [36], and details of the sampling regime and the mean and range of values recorded for a suite of water physicochemical parameters. n = number of samples; PO = permanently-open, AO = annually-open and NC = normally-closed.
| Estuary Characteristics | Sampling Regime | Water Physicochemical Parameters | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area (km2) | Mean Catchment Rainfall (mm) | Median Annual Flow (ML) | Estuary Type | Region | Sites | Timing | n | Method | Salinity (ppt) | Temperature (°C) | DO (mgL−1) | |||||
| Mean | Range | Mean | Range | Mean | Range | Reference | ||||||||||
| Swan- Canning Estuary | 38.1 | 409 | 600,000 | PO | Upper | 5 | 11 months (2010/11) | 309 | Ekman grab (225 cm2) | 19.0 | 4.0–32.5 | 21.2 | 11.6–29.7 | 3.3 | 0.2–11.0 | [37] |
| Lower | 4 | 4 seasons (2023/24) | 80 | Corer (96 cm2) | 34.5 | 19.1–39.5 | 22.4 | 14.3–30.9 | 8.3 | 6.4–10.5 | [38] | |||||
| Lower | 6 | 1 season (2023) | 90 | Corer (96 cm2) | 35.2 | 34.8–35.6 | 25.1 | 23.5–27.6 | 8.0 | 6.1–10.6 | [39] | |||||
| Peel-Harvey Estuary | 133.6 | 662 | 810,000 | PO | All | 60 | 2 seasons (2017/18) | 120 | Corer (28 cm2) | 26.7 | 2.1–56.2 | 21.3 | 13.9–28.9 | 5.8 | 0.1–10.6 | [40] |
| Vasse- Wonnerup Estuary | 6.1 | 905 | 40,300 | AO | All | 30 | 15 seasons (2017–20) | 344 | Ekman grab (225 cm2) | 26.5 | 0.6–96.5 | 20.5 | 12.0–34.3 | 8.8 | 0.6–18.2 | [41,42] |
| Broke Inlet | 45.6 | 1331 | 162,000 | AO | All | 40 | 5 seasons (2007–08) | 480 | Corer (96 cm2) | 22.9 | 2.1–45.1 | 18.1 | 12.6–28.3 | 6.0 | 2.5–20.4 | [43] |
| Torbay Inlet | 0.9 | 943 | 75,000 | AO | All | 12 | 4 seasons (2020) | 48 | Corer (96 cm2) | 14.1 | 1.0–34.7 | 19.1 | 12.4–25.7 | 8.0 | 2.2–11.6 | [44] |
| Oyster Harbour | 17.7 | 949 | 97,200 | PO | All | 12 | 4 seasons (2020) | 48 | Corer (96 cm2) | 34.1 | 17.4–37.6 | 17.6 | 12.3–22.3 | 8.3 | 1.5–12.2 | [44] |
| Taylor Inlet | 0.5 | 800 | 1400 | AO | All | 12 | 4 seasons (2020) | 48 | Corer (96 cm2) | 20.0 | 16.1–24.2 | 17.5 | 12.5–22.5 | 9.6 | 6.1–16.3 | [44] |
| Normans Inlet | 0.2 | 810 | 1800 | AO | All | 12 | 4 seasons (2020) | 48 | Corer (96 cm2) | 4.2 | 2.4–6.2 | 16.9 | 12.5–22.0 | 8.4 | 4.3–10.4 | [44] |
| Waychinicup Estuary | 0.1 | 760 | 8000 | PO | All | 12 | 4 seasons (2020) | 48 | Corer (96 cm2) | 31.9 | 3.4–35.4 | 18.3 | 15.5–21.5 | 8.1 | 6.3–10.3 | [44] |
| Cordinup River | 0.1 | 610 | 1700 | AO | All | 12 | 4 seasons (2020) | 48 | Corer (96 cm2) | 12.6 | 7.9–19.3 | 17.6 | 14.3–23.2 | 8.4 | 4.4–13.9 | [44] |
| Cheyne Inlet | 0.2 | 610 | 1800 | AO | All | 12 | 4 seasons (2020) | 48 | Corer (96 cm2) | 42.5 | 33.0–54.3 | 18.0 | 13.4–25.7 | 7.6 | 3.7–10.7 | [44] |
| Beaufort Inlet | 6.5 | 410 | 36,000 | NC | All | 12 | 11 times (2020–23) | 132 | Corer (96 cm2) | 56.3 | 3.2–121.8 | 17.7 | 8.6–25.5 | 7.4 | 3.6–16.2 | [45] |
In the laboratory, invertebrates were removed from the residual sediment and stored in 100% ethanol. Each specimen was identified to the lowest possible taxon. Insects were identified to a higher taxonomic level than other taxa due to the differences in the resolution of identification keys for the various life stages (i.e., larva, pupa, adults). To ensure consistency in identification, the reference collections from the various studies were examined, and the taxonomy of all species was checked and updated where necessary using the World Register of Marine Species [46]. The abundance of each macroinvertebrate taxon in each replicate sample was converted into a density (individuals 100 cm−2).
2.2. Data Analysis
The density and water physicochemical data were used to create a data matrix of the salinities of the samples in which each of the 257 taxa was present. These data were then used to calculate the minimum and maximum salinities in which a taxon occurred. As not all species were recorded in each salinity between 0 and 122 ppt, the values for the range were used to generate a presence/absence data matrix of each taxon in each salinity (1 ppt resolution). Species were assigned as stenohaline or euryhaline based on the range of salinities in which they were recorded, i.e., ≤10 or ≥10 ppt, respectively. The number of taxa in each salinity was counted and used to construct a quantitative version of the Remane diagram. Published modifications of the Remane diagram made by Whitfield et al. [28], Hedgpeth [29] and Khlebovich [30] were taken from the former paper, and the data for the total number of species in each salinity (1 ppt resolution) were extracted using https://www.graphreader.com/. As the y-axis in some versions of the Remane diagram has no quantitative unit of measure, the results from those papers and those generated in the current study were converted to a percentage of the maximum to compare how the values change along the salinity gradient. A stacked area chart was produced to show the number of stenohaline and euryhaline species in each salinity. Note that this plot was restricted to the 208 taxa recorded more than once.
To visualise how the proportional richness of various phyla and classes differed along the salinity gradient (groups of 10 ppt), the percentage contribution of taxa in each salinity category was calculated. Only phyla and classes comprising at least five taxa were included.
The minimum and maximum salinity in which each annelid, mollusc, and arthropod taxon was recorded were used to create a dumbbell plot. For taxa occurring in ≥10 of the 1891 samples, the distribution of those occurrences across the salinity gradient was plotted in a ridgeline plot using kernel density estimation. Dumbbell and ridgeline plots were generated using rStudio v4.3.1; [47], utilising the ggplot, ggridges, and ggdist packages [48,49,50].
3. Results
3.1. Faunal Description
A total of 257 benthic macroinvertebrate taxa, representing 11 phyla, were recorded across 1891 samples from the 12 estuaries in southwestern Australia (Table 2). Among these taxa, 94% were arthropods (118 taxa), annelids (82 taxa), and molluscs (42 taxa) and combined, they contributed 98% of all individuals. The 118 arthropod taxa included 58 malacostracans and 47 hexapods. Malacostracans were more numerous and frequently recorded than hexapods, representing 17% of all individuals and occurring in 58% of samples (vs. 5 and 32%, respectively). Although relatively few ostracod species (7 taxa) were recorded and not abundant, they were found in 7% of samples. The vast majority of the 82 annelid taxa were polychaetes (94%). Polychaetes represented 51% of the 53% of annelid individuals present and were found in 85% of samples. Oligochaetes were also relatively frequently recorded (14%). Of the 42 mollusc taxa, 24 were gastropods and 16 were bivalves. Bivalves were more abundant (19 vs. 3% of individuals) and more common (48 vs. 21% of samples; Table 2). Among the other phyla, nematodes and nemerteans were also relatively frequently recorded (~5% of samples).

Table 2.
Richness (# taxa), percentage contribution to total abundance (%C), number of samples (n) and frequency of occurrence (%F) of taxa belonging to each phylum (boldface) and order or class.
Five taxa, namely the polychaetes Capitella spp. (which includes the Capitella capitata species complex), Simplisetia aequisetis and Pseudopolydora cf. kempi, the bivalve Arthritica semen, and amphipod Corophium minor were the most abundant taxa, representing 24 to 5% of all individuals (54% combined) and were found in 51 to 22% of samples (Table S2). Other common species included the polychaetes Scoloplos normalis, Prionospio cirrifera, Desdemona ornata, and the amphipod Grandidierella spp. (i.e., Grandidierella japonica and Grandidierella propodentata).
3.2. Relationship Between Richness and Salinity
The number of taxa increased rapidly from 13 (5% of all taxa) in freshwater to 13–75 in oligohaline, 88–106 in mesohaline and 105–124 in euhaline waters, reaching a peak of 173 taxa (66%) at a salinity of 35 ppt (Figure 2a). After this, richness declined steeply to 83 by 40 ppt, 38 by 50 ppt, 21 by 60 ppt, and 11 by 90 ppt. Six taxa were present in salinities > 100 ppt, and a single species was found in the highest salinity recorded (122 ppt). This pattern of richness along the salinity gradient differs markedly from the conceptual models by Remane and the various modifications in several regards (Figure 2b). Firstly, the other models have a relatively high richness in freshwater, which declined rapidly through the horohalinicum before increasing. In contrast, in southwestern Australia, there was a rapid increase in richness in oligohaline salinities. Secondly, although the curves for all models peaked at a salinity of 35 ppt, the decline in richness in southwestern Australia was more rapid in salinities between 40 and 90 ppt. In the current study, 65% of the taxa were classified as euryhaline (i.e., found in a salinity range ≥ 10 ppt), including 55%, 70%, and 76% of the annelid, arthropod, and mollusc taxa, respectively. Stenohaline species were generally found in salinities between 32 and 38 ppt, representing 15–33% of all taxa (Figure 2c). While some stenohaline taxa were recorded in salinities < 5 ppt, they comprised 8% of the total number of taxa. Almost a third of all taxa were recorded across a salinity range greater than 35 ppt, and for five taxa, this exceeded 100 ppt (Figure 2d).
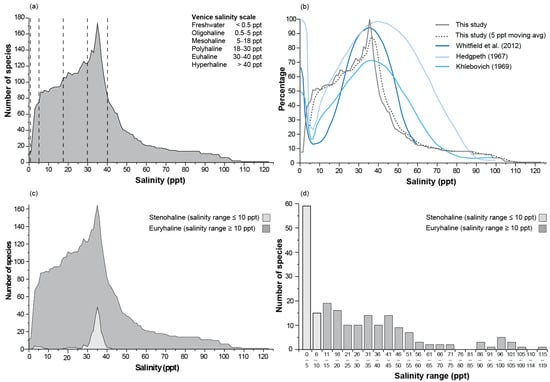
Figure 2.
(a) The number of benthic macroinvertebrate taxa in southwestern Australian estuaries recorded in each salinity increment (1 ppt), (b) relationship between the number of taxa expressed as a percentage of the maximum value and salinity found in southwestern Australia and various authors’ modifications to the Remane diagram [28,29,30], (c) the number of stenohaline and euryhaline species in each salinity increment, and (d) the number of taxa within each salinity range, defined by the difference between the minimum and maximum salinity that each taxon was recorded in. Dashed lines in (a) denote boundaries between salinity categories on the Venice salinity scale.
Among the phyla, arthropods were recorded in all salinity categories, with annelids only absent from salinities > 111 ppt and molluscs from those >100 ppt (Figure 3a). In contrast, echinoderms were restricted to euryhaline conditions (31–40 ppt). The percentage richness of arthropod, annelid, and mollusc taxa increased sequentially to a peak in euhaline conditions (31–40 ppt) before declining rapidly, with only 19, 11, and 13% of taxa recorded in salinities > 50 ppt. The pattern for these three phyla was mirrored by those for polychaetes, malacostracans, gastropods, and particularly bivalves, which were not recorded in salinities > 60 ppt (Figure 3b). However, that for hexapods was different, with the percentage richness being similar in salinities between 0 and 30 ppt (~67%) before declining more gradually and remaining > 10% until salinity exceeded 100 ppt.
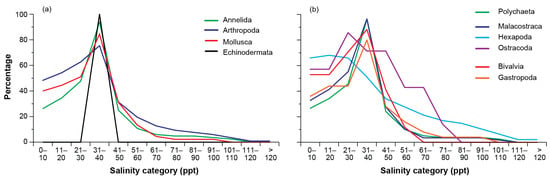
Figure 3.
Percentage number of taxa belonging to each (a) phylum and (b) class in each salinity category (10 ppt).
3.3. Distribution of Annelids
Polychaetes were absent from freshwater, but 15 taxa were recorded in oligohaline conditions and 20 from hyperhaline conditions (Figure 4). Among the relatively common taxa, Eusyllis sp., Aricidea (Acmira) lopezi, Spirorbis sp., Namanereis sp., Exogone sp., and Neanthes cricognatha occurred over a narrow range of euhaline salinities. All taxa recorded in oligohaline conditions were also found in salinities > 35 ppt. Among these, some occurred over a wide range, including D. ornata (2–43 ppt), P. cirrifera (2–51 ppt), Scoloplos normalis (1–54 ppt), S. aequisetis (1–63 ppt), and particularly Capitella spp. (0–103 ppt) and P. cf. kempi (1–107 ppt). Among the other annelids, Hirudinea spp. and the oligochaetes Enchytraeidae sp. and Naididae sp. were recorded in salinities < ~30 ppt and Sipuncula spp. in 28–45 ppt. However, Oligochaeta spp. occurred over a far broader range of 1–86 ppt (Figure 4).
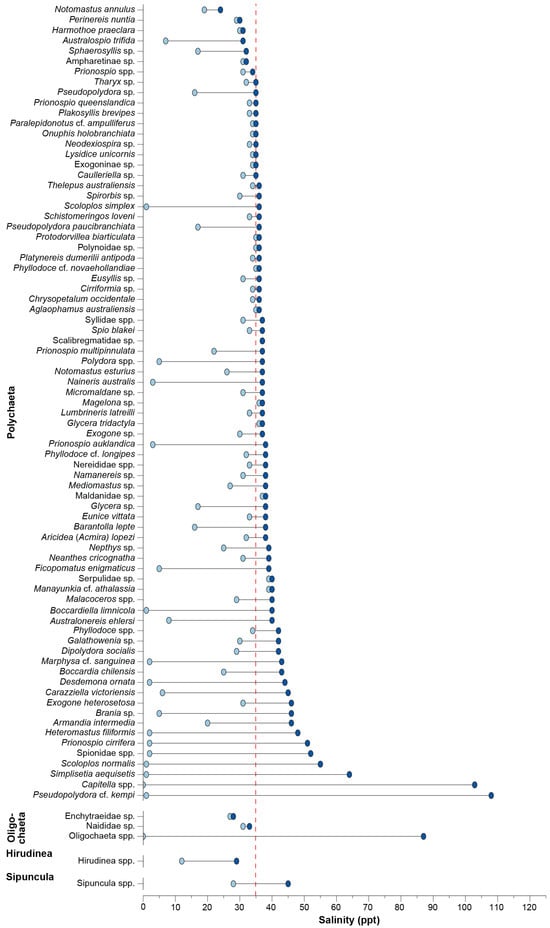
Figure 4.
Dumbbell plot of the minimum and maximum salinity in which each annelid taxon was recorded. The dashed red line denotes salinity around full-strength seawater.
Visual interpretation of the ridgeline plots provides evidence for a shift in the distribution of common annelid taxa along the salinity gradient from oligohaline to hyperhaline conditions, after which occurrences are limited. However, due to the relatively broad range of salinity values in which many of these species were recorded, this trend is based more on their median salinity and relative frequency of occurrences (Figure 5). Although none of the common polychaete species were restricted to salinities < 35 ppt, species such as Boccardiella limnicola and D. ornata had the largest proportions of their occurrences in oligohaline to polyhaline salinities (median = 16 and 19 ppt, respectively). Pseudopolydora cf. kempi, Ficopomatus enigmaticus, and P. cirrifera were most often found in polyhaline salinities (median = 22–24 ppt), albeit they were present across a marked range of salinities. Similarly, a group of taxa comprising S. normalis, S. aequisetis, Pseudopolydora sp., Capitella spp., and Armandia intermedia were most often recorded in salinities of ~30 ppt (median = 26–32 ppt). Most of the remaining taxa, e.g., Heteromastus filiformis, Spirorbis sp., Eusyllis sp., and Exogone sp., were mainly found in a salinity of ~35 ppt and all except H. filiformis (range = 3–47 ppt) were recorded in a relatively restricted range of salinities (i.e., range = ~5 ppt).
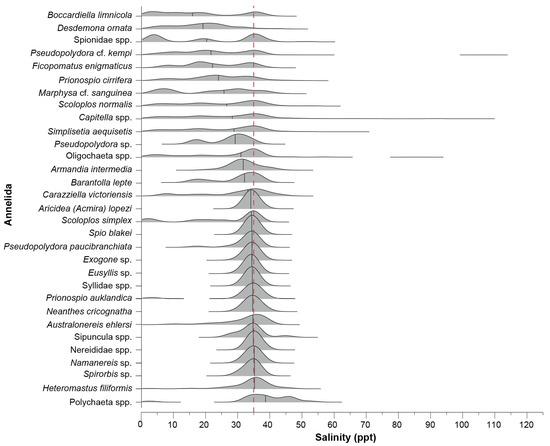
Figure 5.
Ridgeline plot of the frequency distribution of each annelid taxon recorded in ≥10 samples across the salinity gradient. The dashed red line denotes salinity around full-strength seawater and the black vertical solid lines indicate the median salinity of each taxon generated by the kernel density estimation.
3.4. Distribution of Molluscs
As with the polychaetes, no bivalves were recorded in freshwater, and almost all taxa found in oligohaline conditions occurred over a salinity range of >35 ppt (Figure 6). Of these, Arcuatula senhousia and Xenostrobus securis were recorded in salinities of up to 36 and 39 ppt, respectively, Hiatula biradiata and Fluviolanatus subtortus up to ~46 ppt, and Spisula trigonella and A. semen to 51 and 54 ppt, respectively. Other species such as Macomona deltoidalis, Venerupis galactites and Theora lubrica occurred over ranges of 13–21 ppt, albeit in salinities that were polyhaline and euhaline. The gastropods Potamopyrgus spp. and Coxiella striatula occurred over the widest range of 0–97 ppt and 3–70 ppt, respectively. Among the other relatively common species, Ascorhis occidua and Batillaria australis occurred over a slightly narrow range of 5–40 ppt and 1–47 ppt, while Tatea rufilabris occurred in a more restricted range of lower salinities (1–24 ppt) than both Tritia burchardi and Nassarius spp. (16–46 ppt and 16–37 ppt, respectively). The scaphopod Paradentalium hemileuron and polyplacophorans Leptochiton matthewsianus and Acanthochitonidae sp. were essentially restricted to salinities ~35 ppt (Figure 6).
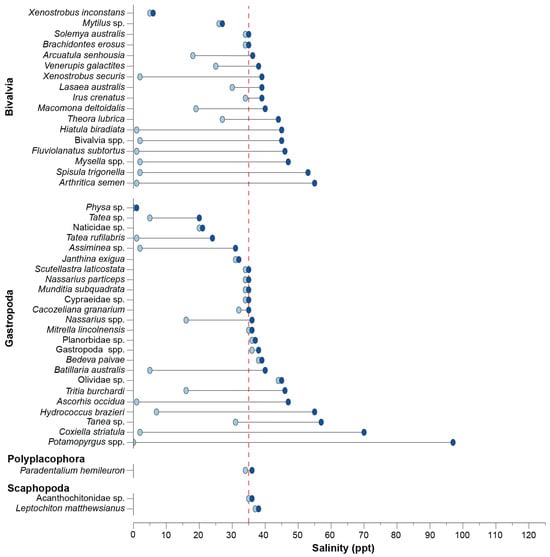
Figure 6.
Dumbbell plot of the minimum and maximum salinity in which each molluscan taxon was recorded. The dashed red line denotes salinity around full-strength seawater.
As with the annelids, there was a shift in the occurrences of species along the salinity gradient. The tateid (formerly hydrobiid) gastropod A. occidua was most frequently recorded in oligohaline salinities, while another species from this family, T. rufilabris and the bivalve F. subtortus, were mainly found in mesohaline waters (Figure 7). Arthritica semen, X. securis, Potamopyrgus spp., and S. trigonella were broadly distributed from fresh to hyperhaline waters but were most often recorded in polyhaline salinities. Other molluscs, such as Tritia burchardi, B. australis, and M. deltoidalis, had a more restricted distribution along the salinity gradient in euhaline waters.
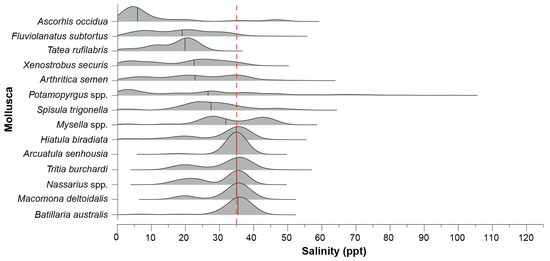
Figure 7.
Ridgeline plot of the frequency distribution of each molluscan taxon recorded in ≥10 samples across the salinity gradient. The dashed red line denotes salinity around full-strength seawater and the black vertical solid lines indicate the median salinity of each taxon generated by the kernel density estimation.
3.5. Distribution of Arthropods
Most of the malacostracan taxa tended to be either recorded over a narrow range of salinities around full-strength seawater (euhaline) or recorded over a broad range of salinities from oligohaline to hyperhaline (Figure 8). Species in the first group tended to be uncommon and included the amphipods Aora maculata, Birubius eake, and Ampithoe valida and the hymenosomatid and portunid crabs Halicarcinus ovatus and Ovalipes australiensis. Twenty four of the 58 malacostracans were found in a range of ≥20 ppt. With the exception of species such as the isopod Paracerceis sculpta, mysid Gastrosaccus sorrentoensis, amphipod Caprella scaura, and hymenosomatid Lucascinus bedfordi, most of these taxa were recorded in salinities < 5 ppt (oligohaline). Because of this, the extent of their salinity range was determined by the highest salinity in which they were recorded. For example, the isopods Syncassidina aestuaria and Cirolanidae sp., the tanaidid Tanais dulongii, and C. scaura were recorded in salinities up to 39–45 ppt. Other species, such as the caridean shrimp Palaemon australis and the amphipods Perthia sp., Barnardomelita matilda, Austrochiltonia subtenuis, and Paracorophium excavatum, were recorded in salinities up to 57 ppt. The malacostracans found in the highest salinities, i.e., 93 and 102 ppt, were the amphipods Corophium minor and Grandidierella spp., respectively.

Figure 8.
Dumbbell plot of the minimum and maximum salinity in which each malacostracan taxon was recorded. The dashed red line denotes salinity around full-strength seawater.
There was a clear sequential pattern of minimum and maximum salinities among the hexapods. A group including larval horse flies (Tabanidae sp.), mayflies (Caenidae spp.), beetles (Dytiscidae sp. 2 and Coleoptera spp.), and springtails (Collembola sp.) were restricted to freshwater and oligohaline salinities (Figure 9). Larvae of several mosquitoes (i.e., Aedes sp. and Coquillettidia spp.) and non-biting midges (chironomid; i.e., Procladius villosimanus, Paratanytarsus grimmii, Chironomus alternans, and Chironomus occidentalis) were recorded in mesohaline and polyhaline salinities (range up to 28 ppt). Almost all of the taxa recorded in hyperhaline salinities (≥40 ppt) were found over a wide range of salinities (range for a taxon of between 37 and 119 ppt). The taxa with the broadest ranges were also those recorded in the highest salinities, e.g., larvae of the water scavenger beetle (hydrophilid) Berosus spp. (97 ppt), Diptera spp. (102 ppt), biting midges Ceratopogonidae spp. (103 ppt), and the non-biting midge Tanytarsus barbitarsis (122 ppt). The latter species was also recorded over the widest range, i.e., 118 ppt (Figure 9).
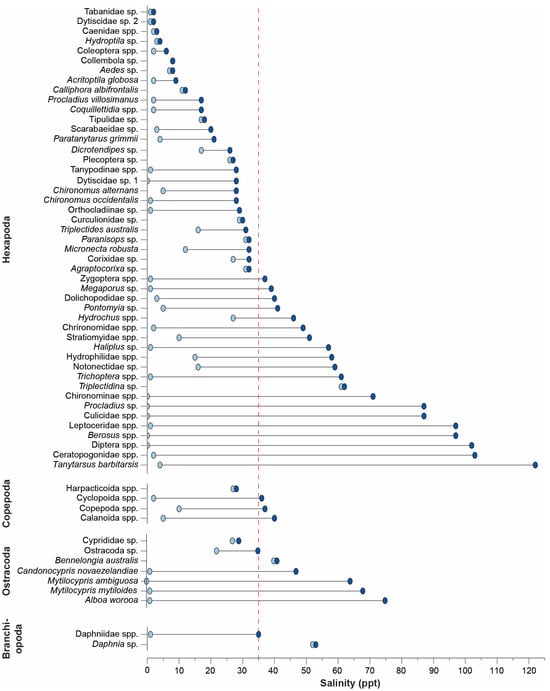
Figure 9.
Dumbbell plot of the minimum and maximum salinity in which each hexapod, copepod, ostracod, and branchipod taxon was recorded. The dashed red line denotes salinity around full-strength seawater.
All ostracod taxa recorded on ten or more occasions were found in essentially freshwater (0 or 1 ppt) to hyperhaline conditions. For example, Candonocypris novaezelandiae was recorded in salinities up to 47 ppt, Mytilocypris ambiguosa and Mytilocypris mytiloides to 64 and 67 ppt, respectively, and Alboa worooa to 74 ppt (Figure 9). Copepod and branchiopod taxa were typically found in salinities of 0 to ~35 ppt, although individuals were only identified to a relatively low taxonomic resolution.
Despite the relatively broad range of salinities in which the most common taxa were recorded, there was a sequential pattern in the peak occurrences along the salinity gradient (Figure 10). Species most common in oligohaline waters included the ostracods C. novaezelandiae and M. ambiguosa and the non-biting midges C. occidentalis and P. grimmii. A similar suite of higher taxa was frequently recorded in mesohaline salinities, e.g., ostracods (M. mytiloides and A. worooa) and non-biting midges (P. villosimanus, Chironominae spp. and Procladius sp.). In contrast to oligohaline and mesohaline salinities, the taxa most common in polyhaline and euhaline salinities were a range of malacostracans, including amphipods (e.g., Grandidierella spp., B. matilda, and C. minor), isopods (e.g., Cyathura hakea and S. aestuaria) and decapods (P. australis and L. bedfordi). Tanytarsus barbitarsis was the only very common species with a median occurrence in hyperhaline conditions (92 ppt).
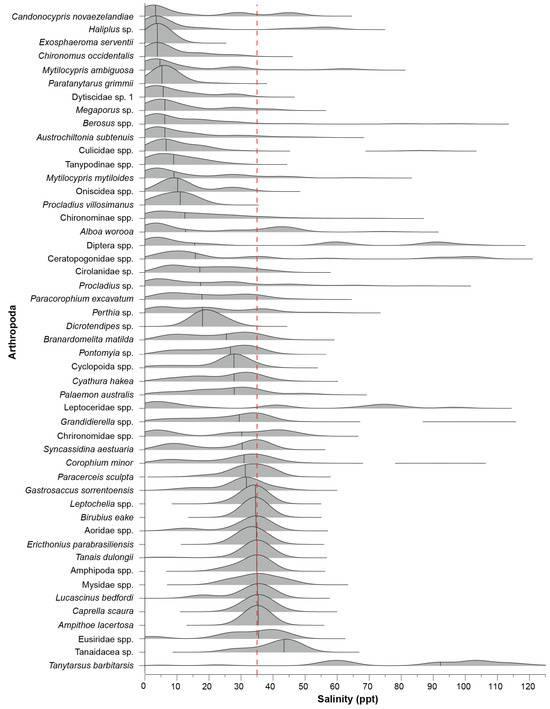
Figure 10.
Ridgeline plot of the frequency distribution of each arthropod taxon recorded in ≥10 samples across the salinity gradient. The dashed red line denotes salinity around full-strength seawater and the black vertical solid lines indicate the median salinity of each taxon generated by the kernel density estimation.
4. Discussion
4.1. Comparisons to the Remane Diagram
The primary aim of this study was to determine, using quantitative data, whether the patterns of benthic macroinvertebrate species richness along a pronounced salinity gradient of freshwater to hyperhaline conditions matched the conceptual models in the literature. The pattern of richness in southwestern Australian estuaries peaked at a salinity of 35 ppt, as in all three models; however, the richness in (i) freshwater/oligohaline and (ii) hyperhaline waters differed. The Whitfield et al. [28], Khlebovich [30], and Hedgpeth [29] models all indicate, albeit to varying relative extents, that freshwaters are relatively speciose, and richness then declines through the horohalinicum before increasing. However, in southwestern Australia, taxa richness was lowest at a salinity of 0 and increased sequentially until 35 ppt. This could reflect the environmental characteristics of the region. Rainfall is highly seasonal, and thus most rivers are intermittent or ephemeral, and some are saline due to natural geology and/or secondary salinisation [32,51]. This would prohibit the occurrence of stenohaline freshwater species, which is supported by studies on wetlands on the Swan Coastal Plain in southwestern Australia, where salinity is one of the major drivers of invertebrate composition [52]. Moreover, the temporary nature of rivers is a driver of the relatively depauperate native fish fauna (8 species), all of which are small-bodied (typically < 5 cm total length) and endemic to the region [53]. The seasonal and/or saline riverine flow, together with the microtidal regime, is not conducive to the formation of tidal freshwater zones that are present in many macrotidal estuaries and support a unique faunal community [54,55]. From an evolutionary perspective, the aridification of the Australian climate, which began in the early Cenzoic era and accelerated in the last 33 ma, has resulted in increased salinity in rivers and lakes [56]. This process could have also led to the creation of selection pressures that favoured invertebrates with the ability to cope in saline water [57]. Similar to the current study, benthic macroinvertebrate species richness was lowest in the upstream reaches of the Gamtoos Estuary in South Africa [58]. The fauna in this region of the estuary comprised taxa typically associated with fluvial habitats (e.g., chironomids and oligochaetes) and a few estuarine taxa with tolerances to very low salinities (e.g., corophiid amphipods and some polychaetes) [58]. This mirrors the 18 taxa found in salinities of ≤1 ppt in southwestern Australia, which comprised the larvae of six hexapods (including several chironomids), nematodes, oligochaetes, three polychaetes, two gastropods, two bivalves, and a single ostracod and single amphipod species (Table S2). Sixteen of these 18 taxa were also recorded in salinities > 35 ppt, with seven found in salinities > 80 ppt. Thus, very few taxa are stenohaline. This supports the work of Cronin-O’Reilly et al. [42], who found that the benthic macroinvertebrates taxa recorded in the upper reaches of the Vasse–Wonnerup were mainly saline wetland-associated that can persist in a wide range of salinities.
The decline in richness between 40 and 90 ppt in southwestern Australia was greater than depicted in all three models. Moreover, whereas the models estimated a relatively equal richness on either side of a peak at 35 ppt, the decline in southwestern Australia was greater in hyperhaline than polyhaline salinities. For example, 173 taxa were recorded in 35 ppt, but 124 taxa occurred in a salinity of 30 ppt compared to 83 taxa at 40 ppt, a difference of 41 taxa. Furthermore, this differential increased to 50 taxa at ±10 ppt and 74 taxa at ±15 ppt. The proportionally fewer taxa able to survive in higher than lower salinities could reflect the low number of estuaries and the relative frequency with which these salinities occur [34] and also the smaller area they cover, leading to reduced selection pressure to develop behavioural strategies and physiological adaptations. Moreover, in permanently-open estuaries with a mesotidal or macrotidal regime, much of the estuary may become polyhaline or mesohaline each day, depending on the tidal cycle [59]. In contrast, hyperhaline conditions only occur in a subset of estuaries and typically over prolonged periods [8,9], which would reduce the effectiveness of some behavioural responses. However, these results highlight the vulnerability of benthic macroinvertebrates to hypersalinity [60]. Several studies have shown that pronounced hypersalinity, typically >60 ppt, simplifies the fauna and truncates food webs [61,62]. Extreme salinities can also result in the absence of benthic macroinvertebrates [10,63].
A far larger proportion of the species recorded in 35 ppt and euhaline salinities were stenohaline than in other salinities. Although reproductive information, e.g., the timing and location of spawning, is not known for many of these species, they are likely marine. An example of this is provided by the echinoderms, which all occurred in a narrow range of 33–37 ppt. This trend mirrors that in the UK, where 33 echinoderm taxa, which represented 4.2% of all invertebrates, were recorded in coastal waters, but only four such taxa comprising 1.4% of invertebrates were found in nearby estuaries [15]. Echinoderms are regarded as stenohaline as they lack an excretory organ, have highly permeable body walls, and their body fluids are ionically and osmotically similar to that of the surrounding water [64,65]. Although some species can tolerate short-term exposure to hyposaline conditions for several hours to days [66], echinoderm species in southwestern Australia are restricted to the lower reaches of permanently-open systems, which remain euhaline [44,67,68]. These species have also not been recorded in hypersaline conditions [34] and have been shown to be sensitive to discharges of desalination brine [69].
4.2. Holohaline Taxa
Globally, few species are regarded as holohaline [28,70,71], however, several holohaline taxa were identified in the current study, i.e., T. barbitarsis, P. cf. kempi, Grandidierella spp. (i.e., Grandidierella japonica and Grandidierella propodentata), Capitella spp. (which includes C. capitata), Diptera spp., and Ceratopogonidae spp. Furthermore, several other taxa, i.e., Berosus spp. and Leptoceridae spp., were found in salinities of ~0 to 97 ppt and in the future could be found in salinities ≥ 100 ppt. Among these eight taxa, Capitella spp., P. cf. kempi and Grandidierella spp., could be regarded as ultrahaline species as these polychaete and amphipod taxa are all known to occur and breed in coastal marine waters [72,73,74]. The remaining taxa, all of which are hexapods, are holoeuryhaline as they are typically absent from local marine waters [75] and more often associated with wetlands in Australia, some of which become markedly hypersaline [76].
While these taxa belong to several classes and exhibit a range of physiological adaptations to cope with the huge variation in salinities (see below), they all must obtain sufficient energy to survive, the need for which increases in more saline water [77]. At the same time, the diversity and abundance of faunal communities decreases [78,79], which has been found to telescope the food chain [80]. It is not surprising that many of the aforementioned holohaline taxa are deposit feeders (e.g., P. cf. kempi and Capitella spp. [81]) or suspension feed on phytoplankton and detritus or graze on microphytobenthos (e.g., Grandidierella spp. [82] and Tanytarsini larvae [83]). Moreover, T. barbitarsis is also known to ingest its faecal pellets [84]. Such a diet is beneficial as, in microtidal estuaries, most of the fine sediments and detritus that are transported into the estuary become ‘trapped’ and, in the Lagoa de Araruama (Brazil), benthic diatoms were present in salinities of 0–180 ppt [2,85]. The exceptions to this are larval ceratopogonids that suspension feed, graze, and predate on meiofauna [86], and larval Berosus spp., which are predators [87,88]. However, chironomids, capitellids, and ostracods were abundant and co-occurring. As a result, unlike the situation with the zoobenthivorous sparid in Beaufort Inlet [62], sufficient prey were always available for the beetle larvae.
4.3. Annelids
Among the three main phyla, annelids had the lowest proportion of taxa present in both freshwater/oligohaline and hyperhaline salinities, and this was particularly true for the sipunculans. This reflects the fact that annelids are mostly osmoconformers, although some taxa can osmoregulate and/or use behavioural adaptations to limit salinity exposure [89,90]. While sipunculans can tolerate considerable water loss and gain, they lack active regulation [91]. The range of salinities in which these taxa were recorded in the current study was relatively narrow, i.e., 28–45 ppt (median = 35 ppt), which matches that in the review by Murina [92]. The low number of polychaetes found in freshwater/oligohaline salinities can be attributed to their thin, permeable body walls and being osmoconformers [93]. However, several families of polychaetes have been documented to withstand low salinities, most notably nereids, spionids, sabellids, and fabriciids, which is consistent with the current study [93]. Many of the species recorded in oligohaline and mesohaline waters in southwestern Australia are also found in similar salinities in other estuaries. For example, B. limnicola was first described from lakes in New South Wales and was found in salinities between 0 and 12 ppt in the Hawkesbury Estuary (Australia) [94]. In addition to some of these species being able to osmoregulate [89,95], they often have thicker body walls, reside in tubes and use reproductive strategies such as brooding or embryo encapsulation to reduce the mortality of the more sensitive larval stage [93].
Some of these adaptations would also be effective in hyperhaline salinities and could potentially explain the broad salinity ranges in which some of the species, e.g., P. cf. kempi, Capitella spp., and S. aequisetis, were recorded in both southwestern Australia and elsewhere. For example, Capitella has been recorded in salinities of 1–138 ppt in the Coorong Lagoon (Australia) [96], the spionid Polydora cornuta and the nereid Nereis pelagica were recorded in salinities up to 75 ppt in the Laguna Madre (USA) [29], and several nereids were among the most abundant macrofaunal species in St Lucia (South Africa) during a prolonged drought [77]. The presence of polychaetes in salinities up to ~100 ppt is greater than that recorded in the Ría Lagartos (Mexico), where no such taxa were recorded in salinities of ≥60 ppt [97], and instead similar to hypersaline waterbodies in Crimea, where such species were recorded in salinities of up to 80–100 ppt [71].
It should also be noted that C. capitata (the main representative in Capitella spp.) and P. cf. kempi are thought to be species-complexes, and the holohaline nature of these ‘species’ could be an artefact of cryptic species. Capitella capitata was originally described in Greenland, and more detailed morphological and molecular studies are needed to confirm whether the species in southwestern Australia are the same as those elsewhere and if there are sibling species e.g., [98].
4.4. Molluscs
The richness of molluscan taxa was substantially greater than annelids in oligohaline to mesohaline waters before following a similar peak at 35 ppt and subsequent decline in hyperhaline salinities. The distribution of the common species largely conformed to the categories proposed by Chalmer et al. [99] and applied by Wells and Threlfall [100], i.e., freshwater (none in this study), estuarine (e.g., A. occuidua and F. subortus), marine affinity (e.g., T. burchardi and S. trigonella), and marine (e.g., M. deltoidalis). Moreover, the presence of X. securis in oligohaline and mesohaline waters and of B. australis in euhaline salinities aligns with previous surveys in the Swan–Canning Estuary [101,102]. As with annelids, most molluscs are osmoconformers [103], but they possess a range of behavioural adaptations. For example, B. australis was recorded infrequently in salinities as low as 6 ppt. Laboratory experiments conducted at 9, 18, and 36 ppt for two weeks resulted in no mortalities, which was due to individuals having a closed operculum preventing water exchange and being inactive, thus reducing metabolic needs [104]. Similar adaptations are also used by Nassarius spp. and T. burchardi [105] and some bivalves, e.g., Brachidontes pharaoni and Cerastoderma endures, in other parts of the world [106,107].
Species such as A. semen (1–74 ppt), C. striatula (3–70 ppt), and the hydrobiids Hydrococcus brazieri (7–55 ppt) and Potamopyrgus spp. (0–97 ppt) were all recorded over a wide range of salinities. Arthritica semen and an unidentified hydrobiid species were recorded in the Coorong Lagoon in salinities of 125 and 130 ppt, respectively [61]. Moreover, A. semen in the Peel–Harvey Estuary survived for 60 h in salinities of up to 54 ppt. The survival rate exceeded 90% in late summer, suggesting that individuals acclimatised as salinities increased over the dry summer period. The occurrence of C. striatula in hyperhaline conditions reflects the fact that, despite being an osmoconformer, Coxiella is the only gastropod genus where all species are halophilic and that individuals are active in salinities of 10 to 70 ppt, with a maximum recorded range of 0.3 to 130 ppt [108]. The hydrobiids Hydrobia ulvae and Potamopyrgus jenkinsi are capable of osmoregulating over a moderate range of salinities below 35 ppt [109], while H. ulvae and Hydrobia glyca were highly active in all salinities tested (i.e., 20–80 ppt) [110]. Wells and Threlfall [111] suggested that reproductive strategies such as embryo protection and rapid growth rates help A. semen and H. brazieri cope with salinity stress.
4.5. Arthropods
Arthropods were also found across the entire salinity gradient and, of the four main phyla, had the highest percentage of number of taxa in all salinity categories except 31–40 ppt. The pattern of relative richness varied among classes; hexapods were most rich in freshwater to mesohaline salinities, malacostracans in euhaline salinities, and ostracods and hexapods in moderately and extremely hyperhaline salinities, respectively.
Malacostracans typically occurred over a more restricted range of salinities than the other classes of arthropods. However, several species of amphipod were recorded in oligohaline and hyperhaline salinities. Among these, A. subtenuis was mainly recorded in salinities below 20 ppt, but was found in 52 ppt. This species is regarded as freshwater based on its pattern of occurrence but was recorded in wetlands in the Wheatbelt region of WA in salinities between 0.2 and 77 ppt [112]. The corophiid amphipods C. minor and P. excavatum, both of which are common in southwestern Australian estuaries and likely breed there [38,113], were found in salinities of 1–92 ppt and 1–49 ppt, respectively. Although the osmoregulation ability of these species is unknown, the estuarine-breeding Corophium volutator maintains hyperosmotic haemolymph concentration until 50 ppt [114]. In the current study, Grandidierella spp. (i.e., G. japonica and G. propodentata) was the malacostracan found in the broadest range of salinities (1–102 ppt). Species in this genus are known to occur in hypersaline waters, e.g., Grandidierella lignorum in South Africa (0–56 ppt) and Grandidierella halophila in Malaysia (7–80 ppt) [115,116]. Moreover, G. propodentata was first described from a solar salt farm in Australia [117]. The ability of Grandidierella spp. to occur in a broad range of salinities could be related to the presence of salt-excreting tissues, as is present in the coxal gills of G. japonica [118].
Several ostracod species belonging to the Mytilocypridinae were found across the gradient from oligohaline through to hypersaline conditions in this study. Both M. ambiguosa and M. mytiloides are common in Australian salt lakes and have been reported in salinities of 0.5–65 ppt and 1.3–173 ppt, respectively [119]. The congeneric Mytilocypris praenuncia is an osmoregulator with mitochondria-rich cells in the inner carapace layer, which help excrete salt in hypo-osmotic regulation [119]. Furthermore, the desiccation-resistant eggs produced by mytilocyprid ostracods would enable recolonisation from drying [108], which often occurs during dry periods and resulting hyperhaline conditions in southwestern Australian estuaries [120]. Copepods and daphniids were also found in a broad range of salinities, reflecting their occurrences in freshwater and marine environments. Although species-level identifications were not conducted in the source publications, species such as the copepod Tigriopus californicus have been recorded in salinities of 2 to at least 190 ppt and temperatures up to 40 °C [121]. Moreover, Pinder et al. [112] recorded 49 freshwater and 25 halophilic copepod species in wetlands in the Wheatbelt. The same authors also found that species of Daphniopsis (which some authors argue should be subsumed into Daphnia [108]) were most frequently recorded in <50 ppt but did occur in salinities > 150 ppt. Despite being common in salt lakes, no brine shrimp, i.e., Artemia and Parartemia, were recorded in hypersaline estuaries, although Tweedley and Krispyn [120] did observe an individual in Gordon Inlet in a salinity of 179 ppt.
The majority of the hexapods recorded in southwestern Australian estuaries, particularly those that are abundant, are characteristic of saline wetlands [52]. Thus, it is unsurprising that 30 of the 37 taxa recorded at least twice were classified as euryhaline and around 42% of all hexapods were present in salinities >35 ppt. Moreover, some of the taxa found in the highest and broadest ranges of salinities, i.e., Berosus, Procladius, Tanytarsus, and the ceratopogonid Culicoides, were identified as among the most salt-tolerant genera in Australia by Chessman [76] in his Invertebrate-Based Salinity Index designed to detect the ecological impact of salinisation in lentic and lotic inland waters. Species of Berosus have been recorded in salinities of up to 150 ppt in a salt pan in the Camargue (France) [122] and Berosus munitipennis and Berosus discolor in 0.8–91 ppt and <0.1–120 ppt, respectively, in the Wheatbelt [112]. Chironomids often dominate the benthic macroinvertebrate fauna of hypersaline waters [61,123] and, among these taxa in Australia, T. barbitarsi is regarded as the most salt-tolerant, being recorded in up to 210 ppt [112]. This species is known to osmoregulate; however, the high energetic cost has consequences, i.e., that it has a relatively long generation time and only occurs in eutrophic systems, suggesting a large food demand [124].
4.6. Limitations and Future Directions
In many Mediterranean climatic regions precipitation will decrease due to climate change, which can also have a disproportionate influence on freshwater discharge [125]. Combined with localised anthropogenic impacts, e.g., water diversion and secondary salinisation, these estuaries will generally become more saline, and the spatial extent of oligohaline waters will decrease [31,102]. Increasing salinity has varying effects on estuaries depending on their morphology [31]. On one hand, in estuaries that are permanently open to the ocean and those that experience regular bar breaches, marinisation can facilitate the recruitment of marine taxa, thereby increasing species richness [38,68], albeit the intrusion of more saline water may also lead to a decline in species adapted to lower salinities [102]. In contrast, in estuaries that are normally closed (i.e., Beaufort Inlet), a lack of tidal exchange, combined with reduced freshwater inflow and increased evaporation, can lead to hypersalinity and a dramatic decrease in richness and, in extreme cases, mass mortality events [45,62]. As such, there is a need to document the in situ environmental conditions in which species are recorded. Where possible, these observations should be paired with controlled laboratory experiments to determine the impacts of salinity alone and the synergistic effects of salinity with other variables (e.g., temperature). Most of the existing information details the effect of relatively low salinities on freshwater taxa rather than the effect of hyperhaline salinities on estuarine fauna [126,127]. This information is vital in helping to understand how estuaries may change in the future. The current study does have some limitations; as there were relatively few samples collected in salinities > 50 ppt, some of the taxa in the source publications were not identified to species level, and the analyses conducted only considered the frequency of occurrence and not the abundance of each taxon. Given the predicted continued drying climate and the shifts in species along the salinity gradient, more work should be conducted in hyperhaline estuaries. Finally, the accuracy of future studies would greatly benefit from improved taxonomy to resolve the status of cosmopolitan species such as P. kempi and C. capitata [128].
5. Conclusions
The pattern of benthic macroinvertebrate richness in southwestern Australia differs from that in the various modifications of the Remane diagram. This reflects a lack of stenohaline freshwater species and the proportionately fewer taxa that can survive in hyperhaline conditions. Most taxa were either stenohaline marine or euryhaline, with some euryhaline taxa also being holohaline. Although many of the euryhaline species of annelid, mollusc, and arthropod were recorded in oligohaline to euryhaline salinities, their frequency distribution showed zonation along the salinity gradient. Thus, although they can tolerate a broad range of salinities, there is a narrower suite of preferred salinities. This research highlights that future changes in the salinity regime of estuaries will have a marked impact on the suite of benthic macroinvertebrate species present.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17111642/s1, Table S1: Summary of the characteristics of the 12 estuaries sampled in southwestern Australia. Table S2: Mean density (100 cm−2), percentage contribution (%C), number of samples (n), and frequency of occurrence (%F) of each benthic macroinvertebrate taxon and the minimum, maximum, mean and median salinity and salinity range (R) in which it was recorded in south-western Australian estuaries.
Author Contributions
Conceptualization, J.R.T. and R.L.; methodology, J.R.T., S.C.-O. and E.M.R.; software, J.R.T.; validation, R.L. and J.R.T.; formal analysis, R.L., J.R.T. and B.J.R.; investigation, R.L., E.J.S., S.C.-O., J.R.T. and S.A.F.; resources, J.R.T., S.C.-O. and E.M.R.; data curation, R.L., S.A.F., E.J.S., S.C.-O. and J.R.T.; writing—original draft preparation, R.L., J.R.T. and E.M.R.; writing—review and editing, all authors; visualization, J.R.T. and B.J.R.; supervision, J.R.T., S.C.-O. and E.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors acknowledge the Noongar people as the Traditional Custodians of the land on which this research took place and pay their respects to Elders past, present and emerging. We also thank the friends and colleagues who assisted with collecting the samples in the original studies, particularly Kurt Krispyn, Charles Maus, and Chris Hallett. Gratitude is also expressed to the organisations that funded the original studies, namely the Department of Biodiversity, Conservation and Attractions through the Swan River Trust, the Australian Research Council and its Linkage partners, the Department of Water and Environmental Regulation, and Murdoch University. Ben Roots is supported by an Australian Government Research Training Program (RTP) Scholarship.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
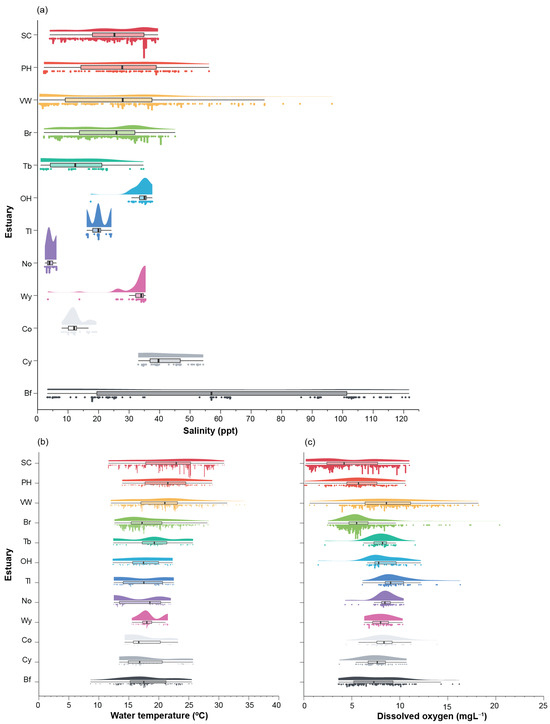
Figure A1.
Raincloud plots, i.e., the combination of a scatter plot, probability density and a box plot of the (a) salinity (ppt), (b) water temperature (°C) and (c) dissolved oxygen concentration (mgL−1) in each of the 12 estuaries in southwestern Australia. Data for each estuary is shown in a different colour; SC, Swan-Canning Estuary; PH, Peel-Harvey Estuary; VW, Vasse-Wonnerup Estuary; Br, Broke Inlet; Tb, Torbay Inlet; OH, Oyster Harbour; Tl, Taylor Inlet; No, Normans Inlet; Wy, Waychinicup Estuary; Co, Cordinup River; Cy, Cheyne Inlet; Bf, Beaufort Inlet.
References
- McLusky, D.S.; Elliott, M. The Estuarine Ecosystem: Ecology, Threats and Management, 3rd ed.; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Tweedley, J.R.; Warwick, R.M.; Potter, I.C. The contrasting ecology of temperate macrotidal and microtidal estuaries. Oceanogr. Mar. Biol. Annu. Rev. 2016, 54, 73–172. [Google Scholar] [CrossRef]
- Pritchard, D.W. What is an estuary: A physical viewpoint. Am. Assoc. Adv. Sci. 1967, 83, 3–5. [Google Scholar]
- Tagliapietra, D.; Sigovini, M.; Ghirardini, A.V. A review of terms and definitions to categorise estuaries, lagoons and associated environments. Mar. Freshw. Res. 2009, 60, 497–509. [Google Scholar] [CrossRef]
- Day, J.H. The nature, origin and classification of estuaries. In Estuarine Ecology: With Particular Reference to Southern Africa; Day, J.H., Ed.; A.A. Balkema: Cape Town, South Africa, 1981; pp. 1–6. [Google Scholar]
- Potter, I.C.; Chuwen, B.M.; Hoeksema, S.D.; Elliott, M. The concept of an estuary: A definition that incorporates systems which can become closed to the ocean and hypersaline. Estuar. Coast. Shelf Sci. 2010, 87, 497–500. [Google Scholar] [CrossRef]
- McSweeney, S.L.; Kennedy, D.M.; Rutherfurd, I.D.; Stout, J.C. Intermittently closed/open lakes and lagoons: Their global distribution and boundary conditions. Geomorphology 2017, 292, 142–152. [Google Scholar] [CrossRef]
- Largier, J.L. Recognizing low-inflow estuaries as a common estuary paradigm. Estuaries Coasts 2023, 46, 1949–1970. [Google Scholar] [CrossRef]
- Hoeksema, S.D.; Chuwen, B.M.; Tweedley, J.R.; Potter, I.C. Factors influencing marked variations in the frequency and timing of bar breaching and salinity and oxygen regimes among normally-closed estuaries. Estuar. Coast. Shelf Sci. 2018, 208, 205–218. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Krispyn, K.N. Protracted bar closure temporarily transforms an estuary into a salt lake. Pac. Conserv. Biol. 2024, 30, PC24007. [Google Scholar] [CrossRef]
- Charmantier, G.U.Y. Ontogeny of osmoregulation in crustaceans: A review. Invertebr. Reprod. Dev. 1998, 33, 177–190. [Google Scholar] [CrossRef]
- González-Ortegón, E.; Pascual, E.; Cuesta, J.A.; Drake, P. Field distribution and osmoregulatory capacity of shrimps in a temperate European estuary (SW Spain). Estuar. Coast. Shelf Sci. 2006, 67, 293–302. [Google Scholar] [CrossRef]
- Whitfield, A.; Elliott, M. Ecosystem and biotic classifications of estuaries and coasts. In Treatise on Estuarine and Coastal Science; Wolanski, E., McLusky, D.S., Eds.; Academic Press: Waltham, MA, USA, 2011; pp. 99–124. [Google Scholar]
- Willmar, P.; Stone, G.; Johnston, I. Animal water balance, osmoregulation and excretion. In Environmental Physiology of Animals, 2nd ed.; Willmar, P., Stone, G., Johnston, I., Eds.; Blackwell: Oxford, UK, 2005; pp. 76–110. [Google Scholar]
- Tweedley, J.R.; Warwick, R.M.; Potter, I.C. Can biotic indicators distinguish between natural and anthropogenic environmental stress in estuaries? J. Sea Res. 2015, 102, 10–21. [Google Scholar] [CrossRef]
- Ysebaert, T.; Meire, P.; Maes, D.; Buijs, J. The benthic macrofauna along the estuarine gradient of the Schelde Estuary. Neth. J. Aquat. Ecol. 1993, 27, 327–341. [Google Scholar] [CrossRef]
- Edgar, G.J.; Shaw, C.; Watsona, G.F.; Hammond, L.S. Comparisons of species richness, size-structure and production of benthos in vegetated and unvegetated habitats in Western Port, Victoria. J. Exp. Mar. Biol. Ecol. 1994, 176, 201–226. [Google Scholar] [CrossRef]
- Teske, P.R.; Wooldridge, T.H. What limits the distribution of subtidal macrobenthos in permanently open and temporarily open/closed South African estuaries? Salinity vs. sediment partical size. Estuar. Coast. Shelf Sci. 2003, 57, 225–238. [Google Scholar] [CrossRef]
- Edgar, G.J.; Barrett, N.S. Benthic macrofauna in Tasmanian estuaries: Scales of distribution and relationships with environmental variables. J. Exp. Mar. Biol. Ecol. 2002, 270, 1–24. [Google Scholar] [CrossRef]
- Whitfield, A.K. Estuaries—How challenging are these constantly changing aquatic environments for associated fish species? Environ. Biol. Fishes 2021, 104, 517–528. [Google Scholar] [CrossRef]
- Thabet, R.; Ayadi, H.; Koken, M.; Leignel, V. Homeostatic responses of crustaceans to salinity changes. Hydrobiologia 2017, 799, 1–20. [Google Scholar] [CrossRef]
- Marshall, W.S. Osmoregulation in estuarine and intertidal fishes. In Fish Physiology; McCormick, S.D., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 32, pp. 395–434. [Google Scholar]
- Cloern, J.E.; Jassby, A.D.; Schraga, T.S.; Nejad, E.; Martin, C. Ecosystem variability along the estuarine salinity gradient: Examples from long-term study of San Francisco Bay. Limnol. Oceanogr. 2017, 62, S272–S291. [Google Scholar] [CrossRef]
- Ysebaert, T.; Herman, P.M.J.; Meire, P.; Craeymeersch, J.; Verbeek, H.; Heip, C.H.R. Large-scale spatial patterns in estuaries: Estuarine macrobenthic communities in the Schelde estuary, NW Europe. Estuar. Coast. Shelf Sci. 2003, 57, 335–355. [Google Scholar] [CrossRef]
- Valesini, F.J.; Wildsmith, M.D.; Tweedley, J.R. Predicting estuarine faunal assemblages using enduring environmental surrogates, with applications in systematic conservation planning. Ocean. Coast. Manag. 2018, 165, 80–98. [Google Scholar] [CrossRef]
- Elliott, M.; Whitfield, A.K. Challenging paradigms in estuarine ecology and management. Estuar. Coast. Shelf Sci. 2011, 94, 306–314. [Google Scholar] [CrossRef]
- Remane, A. Die brackwasserfauna. Verhandlungen Der Dtsch. Zool. Ges. 1934, 36, 34–74. [Google Scholar]
- Whitfield, A.K.; Elliott, M.; Basset, A.; Blaber, S.J.M.; West, R.J. Paradigms in estuarine ecology—A review of the Remane diagram with a suggested revised model for estuaries. Estuar. Coast. Shelf Sci. 2012, 97, 78–90. [Google Scholar] [CrossRef]
- Hedgpeth, J.W. Ecological aspects of the Laguna Madre, a hypersaline estuary. In Estuaries; Lauff, G.H., Ed.; AAAS Publication 83, American Association for the Advancement of Science: Washington, DC, USA, 1967; pp. 408–419. [Google Scholar]
- Khlebovich, V.V. Aspects of animal evolution related to critical salinity and internal state. Mar. Biol. 1969, 2, 338–345. [Google Scholar] [CrossRef]
- Hallett, C.S.; Hobday, A.J.; Tweedley, J.R.; Thompson, P.A.; McMahon, K.; Valesini, F.J. Observed and predicted impacts of climate change on the estuaries of south-western Australia, a Mediterranean climate region. Reg. Environ. Change 2018, 18, 1357–1373. [Google Scholar] [CrossRef]
- Brearley, A. Ernest Hodgkin’s Swanland, 1st ed.; University of Western Australia Press: Crawley, UK, 2005; p. 550. [Google Scholar]
- Hodgkin, E.P.; Hesp, P. Estuaries to salt lakes: Holocene transformation of the estuarine ecosystems of south-western Australia. Mar. Freshw. Res. 1998, 49, 183–201. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Dittmann, S.R.; Whitfield, A.K.; Withers, K.; Hoeksema, S.D.; Potter, I.C. Hypersalinity: Global distribution, causes, and present and future effects on the biota of estuaries and lagoons. In Coasts and Estuaries; Wolanski, E., Day, J.W., Elliott, M., Ramachandran, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 523–546. [Google Scholar]
- Lewis, E.L. The practical salinity scale 1978 and its antecedents. IEEE J. Ocean. Eng. 1980, 5, 3–8. [Google Scholar] [CrossRef]
- Krispyn, K.N. The Fish Faunas of Estuaries in the Albany Region of South-Western Australia; Murdoch University: Perth, Australia, 2021. [Google Scholar]
- Tweedley, J.R.; Hallett, C.S.; Warwick, R.M.; Clarke, K.R.; Potter, I.C. The hypoxia that developed in a microtidal estuary following an extreme storm produced dramatic changes in the benthos. Mar. Freshw. Res. 2016, 67, 327–341. [Google Scholar] [CrossRef]
- Lim, R. Multidecadal Changes in the Benthic Macroinvertebrates Assemblages of the Swan-Canning Estuary; Murdoch University: Perth, Australia, 2025. [Google Scholar]
- Stout, E. Benthic Macroinvertebrate Communities of the Swan Estuary Marine Park; Murdoch University: Perth, Australia, 2025. [Google Scholar]
- Cronin-O’Reilly, S. Benthic Community Structure, Health and Function of a Microtidal Estuary in South-Western Australia; Murdoch University: Perth, Australia, 2021. [Google Scholar]
- Tweedley, J.R.; Cronin-O’Reilly, S.; Cottingham, A.; Beatty, S.J. Vasse-Wonnerup Integrated Monitoring Review of 2017-20: Benthic Macroinvertebrate Component; Report for the Department of Water and Environmental Regulation; Murdoch University: Perth, Australia, 2021; p. 65. [Google Scholar]
- Cronin-O’Reilly, S.; Cottingham, A.; Kalnejais, L.H.; Lynch, K.; Tweedley, J.R. Tidal exclusion barriers fragment an invertebrate community into taxonomically and functionally distinct estuarine and wetland assemblages. J. Mar. Sci. Eng. 2025, 13, 635. [Google Scholar] [CrossRef]
- Tweedley, J.R. The Relationships Between Habitat Types and Faunal Community Structure in Broke Inlet, Western Australia. Ph.D. Thesis, Murdoch University, Perth, Australia, 2011. [Google Scholar]
- Fourie, S.A. Benthic Macroinvertebrate Faunas of Microtidal Estuaries in Albany, South-Western Australia; Murdoch University: Perth, Australia, 2024. [Google Scholar]
- Cronin-O’Reilly, S.; Krispyn, K.N.; Maus, C.; Standish, R.J.; Loneragan, N.R.; Tweedley, J.R. Empirical evidence of alternative stable states in an estuary. Sci. Total Environ. 2024, 954, 176356. [Google Scholar] [CrossRef]
- WoRMS Editorial Board. World Register of Marine Species. Available online: https://www.marinespecies.org (accessed on 27 March 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; p. 260. [Google Scholar]
- Wilke, C.O. ggridges: Ridgeline Plots in “ggplot2”; R Package Version 0.5.6. 2024. Available online: https://cran.r-project.org/web/packages/ggridges/index.html (accessed on 16 October 2024).
- Kay, M. ggdist: Visualizations of distributions and uncertainty in the grammar of graphics. IEEE Trans. Vis. Comput. Graph. 2024, 30, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.L.; Brock, M.A.; Rees, G.N.; Baldwin, D.S. Effects of increasing salinity on freshwater ecosystems in Australia. Aust. J. Bot. 2003, 51, 655–665. [Google Scholar] [CrossRef]
- Davis, J.A.; Rosich, R.S.; Bradely, J.S.; Growns, J.E.; Schmidt, L.G.; Cheal, F. Wetland classification on the basis of water quality and invertebrate community data. In Wetlands of the Swan Coastal Plain; Water Authority of Western Australia and Environmental Protection Authority: Perth, Australia, 1993; Volume 6, p. 242. [Google Scholar]
- Unmack, P.J. Biogeography of Australian freshwater fishes. J. Biogeogr. 2001, 28, 1053–1089. [Google Scholar] [CrossRef]
- Barendregt, A. Tidal freshwater wetlands: The fresh dimension of the estuary. In The Wetland Book: II: Distribution, Description, and Conservation; Finlayson, C.M., Milton, G.R., Prentice, R.C., Davidson, N.C., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 155–168. [Google Scholar]
- Little, S.; Wood, P.J.; Elliott, M. Quantifying salinity-induced changes on estuarine benthic fauna: The potential implications of climate change. Estuar. Coast. Shelf Sci. 2017, 198, 610–625. [Google Scholar] [CrossRef]
- Fujioka, T.; Chappell, J. History of Australian aridity: Chronology in the evolution of arid landscapes. In Geological Society, London, Special Publications; Bishop, P., Pillans, B., Eds.; Geological Society of London: London, UK, 2010; Volume 346, pp. 121–139. [Google Scholar]
- Lawrie, A.D.A.; Chaplin, J.; Rahman, M.; Islam, M.A.; Webzell, K. Insights into the recent evolutionary history of salt lake gastropods (Coxiella) in Australia. Hydrobiologia 2025, 852, 3309–3325. [Google Scholar] [CrossRef]
- Bate, G.C.; Whitfield, A.K.; Adams, J.B.; Huizinga, P.; Wooldridge, T.H. The importance of the river-estuary interface (REI) zone in estuaries. Water SA 2002, 28, 271–280. [Google Scholar] [CrossRef]
- Valle-Levinson, A. Classification of estuarine circulation. In Treatise on Estuarine and Coastal Science; Wolanski, E., McLusky, D., Eds.; Academic Press: Waltham, MA, USA, 2011; pp. 75–86. [Google Scholar]
- Warwick, R.M.; Tweedley, J.R.; Potter, I.C. Microtidal estuaries warrant special management measures that recognise their critical vulnerability to pollution and climate change. Mar. Pollut. Bull. 2018, 135, 41–46. [Google Scholar] [CrossRef]
- Dittmann, S.; Baring, R.; Baggalley, S.; Cantin, A.; Earl, J.; Gannon, R.; Keuning, J.; Mayo, A.; Navong, N.; Nelson, M.; et al. Drought and flood effects on macrobenthic communities in the estuary of Australia’s largest river system. Estuar. Coast. Shelf Sci. 2015, 165, 36–51. [Google Scholar] [CrossRef]
- Krispyn, K.N.; Loneragan, N.R.; Whitfield, A.K.; Tweedley, J.R. Salted mullet: Protracted occurrence of Mugil cephalus under extreme hypersaline conditions. Estuar. Coast. Shelf Sci. 2021, 261, 107533. [Google Scholar] [CrossRef]
- Wooldridge, T.H.; Adams, J.B.; Fernandes, M. Biotic responses to extreme hypersalinity in an arid zone estuary, South Africa. S. Afr. J. Bot. 2016, 107, 160–169. [Google Scholar] [CrossRef]
- Diehl, W.J. Osmoregulation in echinoderms. Comp. Biochem. Physiol. Part A Physiol. 1986, 84, 199–205. [Google Scholar] [CrossRef]
- Barrett, N.J.; Harper, E.M.; Peck, L.S. The impact of acute low salinity stress on Antarctic echinoderms. Proc. R. Soc. B Biol. Sci. 2024, 291, 20241038. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.F.; Russell, M.P. The distribution and behaviour of Patiriella mortenseni and P. regularis in the extreme hyposaline conditions of the southern New Zealand fiords. J. Exp. Mar. Biol. Ecol. 2008, 355, 76–84. [Google Scholar] [CrossRef]
- Poh, B.; Tweedley, J.R.; Chaplin, J.A.; Trayler, K.M.; Crisp, J.A.; Loneragan, N.R. Influence of physico-chemical and biotic factors on the distribution of a penaeid in a temperate estuary. Estuar. Coast. Shelf Sci. 2019, 218, 70–85. [Google Scholar] [CrossRef]
- Wildsmith, M.D.; Rose, T.H.; Potter, I.C.; Warwick, R.M.; Clarke, K.R.; Valesini, F.J. Changes in the benthic macroinvertebrate fauna of a large microtidal estuary following extreme modifications aimed at reducing eutrophication. Mar. Pollut. Bull. 2009, 58, 1250–1262. [Google Scholar] [CrossRef]
- Fernández-Torquemada, Y.; González-Correa, J.M.; Sánchez-Lizaso, J.L. Echinoderms as indicators of brine discharge impacts. Desalin. Water Treat. 2013, 51, 567–573. [Google Scholar] [CrossRef]
- Seale, A.P.; Cao, K.; Chang, R.J.A.; Goodearly, T.R.; Malintha, G.H.T.; Merlo, R.S.; Peterson, T.L.; Reighard, J.R. Salinity tolerance of fishes: Experimental approaches and implications for aquaculture production. Rev. Aquac. 2024, 16, 1351–1373. [Google Scholar] [CrossRef]
- Anufriieva, E.V.; Shadrin, N.V. Diversity of fauna in Crimean hypersaline water bodies. J. Sib. Fed. Univ. Biol. 2018, 11, 294–305. [Google Scholar]
- Blake, J.A.; Woodwick, K.H. Reproduction and larval development of Pseudopolydora paucibranchiata (Okuda) and Pseudopolydora kempi (Southern) (Polychaeta: Spionidae). Biol. Bull. 1975, 149, 109–127. [Google Scholar] [CrossRef]
- Méndez, N.; Inez, L.-G.; Forbes, V.E. Variability in reproductive mode and larval development within the Capitella capitata species complex. Invertebr. Reprod. Dev. 2000, 38, 131–142. [Google Scholar] [CrossRef]
- Munari, C.; Bocchi, N.; Mistri, M. Grandidierella japonica (Amphipoda: Aoridae): A non-indigenous species in a Po delta lagoon of the northern Adriatic (Mediterranean Sea). Mar. Biodiv. Rec. 2016, 9, 12. [Google Scholar] [CrossRef]
- Wildsmith, M.D.; Potter, I.C.; Valesini, F.J.; Platell, M.E. Do the assemblages of the benthic macroinvertebrates in nearshore waters of Western Australia vary among habitat types, zones and seasons? J. Mar. Biol. Assoc. UK 2005, 85, 217–232. [Google Scholar] [CrossRef]
- Chessman, B.C. A new salinity index for the invertebrate fauna of Australian inland waters. Hydrobiologia 2023, 850, 3539–3550. [Google Scholar] [CrossRef]
- Anufriieva, E.V.; Shadrin, N.V. General patterns of salinity influence on the energy balance of aquatic animals in hypersaline environment. Biol. Bull. Rev. 2023, 13, 420–430. [Google Scholar] [CrossRef]
- Roots, B.J.; Lim, R.; Cronin-O’Reilly, S.; Fourie, S.A.; Rodgers, E.M.; Stout, E.J.; Tweedley, J.R. Hypersalinity leads to dramatic shifts in the invertebrate fauna of estuaries. Animals 2025. [Google Scholar]
- Pillay, D.; Perissinotto, R. The benthic macrofauna of the St. Lucia Estuary during the 2005 drought year. Estuar. Coast. Shelf Sci. 2008, 77, 35–46. [Google Scholar] [CrossRef]
- Deegan, B.M.; Lamontagne, S.; Aldridge, K.T.; Brookes, J.D. Trophodynamics of the Coorong: Spatial Variability in Food Web Structure Along a Hypersaline Coastal Lagoon; CSIRO: Water for a Healthy Country National Research Flagship: Canberra, Australia, 2010; p. 52. [Google Scholar]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of worms emended: An update of polychaete feeding guilds. Annu. Rev. Mar. Sci. 2015, 7, 497–520. [Google Scholar] [CrossRef]
- Aikins, S.; Kikuchi, E. Grazing pressure by amphipods on microalgae in Gamo Lagoon, Japan. Mar. Ecol. Prog. Ser. 2002, 245, 171–179. [Google Scholar] [CrossRef]
- Ingvason, H.R.; Ólafsson, J.S.; Gardarsson, A. Food selection of Tanytarsus gracilentus larvae (Diptera: Chironomidae): An analysis of instars and cohorts. Aquat. Ecol. 2004, 38, 231–237. [Google Scholar] [CrossRef]
- Paterson, C.; Walker, K. Recent history of Tanytarsus barbitarsis Freeman (Diptera: Chironomidae) in the sediments of a shallow, saline lake. Mar. Freshw. Res. 1974, 25, 315–325. [Google Scholar] [CrossRef]
- Sylvestre, F.; Beck-Eichler, B.; Duleba, W.; Debenay, J.-P. Modern benthic diatom distribution in a hypersaline coastal lagoon: The Lagoa de Araruama (R.J.), Brazil. Hydrobiologia 2001, 443, 213–231. [Google Scholar] [CrossRef]
- Hribar, L.J. Mouthpart morphology and feeding behavior of biting midge larvae (Diptera: Ceratopogonidae). In Functional Morphology of Insect Feeding; Schaefer, C.W., Leschen, R.A.B., Eds.; Thomas Say Publications in Entomology: Proceedings of the Entomological Society of America: Lanham, Maryland; Entomological Society of America: Annapolis, MD, USA, 1993; pp. 43–58. [Google Scholar]
- Rodriguez, G.; Fikáček, M.; Minoshima, Y.N.; Archangelsky, M.; Torres, P.L.M. Going underwater: Multiple origins and functional morphology of piercing-sucking feeding and tracheal system adaptations in water scavenger beetle larvae (Coleoptera: Hydrophiloidea). Zool. J. Linn. Soc. 2020, 193, 1–30. [Google Scholar] [CrossRef]
- Yee, D.A.; Kehl, S. Order Coleoptera. In Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Thorp, J.H., Rogers, D.C., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 1003–1042. [Google Scholar]
- Preston, R.L. Osmoregulation in annelids: Cells and animals. In Osmotic and Ionic Regulation; Evans, D.H., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 135–165. [Google Scholar]
- Castellano, G.C.; Lana, P.d.C.; Freire, C.A. Euryhalinity of subtropical marine and estuarine polychaetes evaluated through carbonic anhydrase activity and cell volume regulation. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Oglesby, L.C. Salinity-stress and desiccation in intertidal worms. Am. Zool. 2015, 9, 319–331. [Google Scholar] [CrossRef][Green Version]
- Murina, G.-V.V. Ecology of Sipuncula. Mar. Ecol. Prog. Ser. 1984, 17, 1–7. [Google Scholar] [CrossRef]
- Glasby, C.J.; Erséus, C.; Martin, P. Annelids in extreme aquatic environments: Diversity, adaptations and evolution. Diversity 2021, 13, 98. [Google Scholar] [CrossRef]
- Hutchings, P.A.; Murray, A. Taxonomy of polychaetes from the Hawkesbury River and the southern estuaries of New South Wales, Australia. Rec. Aust. Mus. Suppl. 1984, 3, 1–118. [Google Scholar] [CrossRef]
- Mucciolo, S.; Desiderato, A.; Leal, S.M.; Mastrodonato, M.; Lana, P.; Freire, C.A. Variability in the degree of euryhalinity of neotropical estuarine annelids. J. Exp. Mar. Biol. Ecol. 2021, 544, 151617. [Google Scholar] [CrossRef]
- Rolston, A.; Gannon, R.; Dittmann, S. Macrobenthic Invertebrates of the Coorong, Lower Lakes and Murray Mouth Ramsar Site: A Literature Review of Responses to Changing Environmental Conditions; Report to the Department for Environment and Heritage; Flinders University: Adelaide, Australia, 2010; p. 36. [Google Scholar]
- Dávila-Jiménez, Y.; Papiol, V.; Hernández-Alcántara, P.; Enriquez, C.; Sauma-Castillo, L.; Chiappa-Carrara, X. Polychaete assemblages in a tropical hypersaline coastal lagoon of the southeastern Gulf of Mexico during the rainy season. Rev. Biol. Trop. 2019, 67, 136–156. [Google Scholar] [CrossRef]
- Silva, C.F.; Seixas, V.C.; Barroso, R.; Di Domenico, M.; Amaral, A.C.Z.; Paiva, P.C. Demystifying the Capitella capitata complex (Annelida, Capitellidae) diversity by morphological and molecular data along the Brazilian coast. PLoS ONE 2017, 12, e0177760. [Google Scholar] [CrossRef]
- Chalmer, P.N.; Hodgkin, E.P.; Kendrick, G.W. Benthic faunal changes in a seasonal estuary of south-western Australia. Rec. West. Aust. Mus. 1976, 4, 383–410. [Google Scholar]
- Wells, F.E.; Threlfall, T.J. Molluscs of the Peel-Harvey Estuary with a comparision with other south-western Australian estuaries. J. Malacol. Soc. Aust. 1981, 5, 101–111. [Google Scholar]
- Wildsmith, M.D.; Valesini, F.J.; Robinson, S.F. The value of enduring environmental surrogates as predictors of estuarine benthic macroinvertebrate assemblages. Estuar. Coast. Shelf Sci. 2017, 197, 159–172. [Google Scholar] [CrossRef]
- Cottingham, A.; Bossie, A.; Valesini, F.; Maus, C.; Tweedley, J.R. Habitat compression of an estuarine mytilid following half a century of streamflow decline. Estuar. Coast. Shelf Sci. 2023, 282, 108253. [Google Scholar] [CrossRef]
- Medeiros, I.P.M.; Faria, S.C.; Souza, M.M. Osmoionic homeostasis in bivalve mollusks from different osmotic niches: Physiological patterns and evolutionary perspectives. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 240, 110582. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Wernberg, T.; Tuya, F.; Silliman, B.R. Ecological performance and possible origin of a ubiquitous but under-studied gastropod. Estuar. Coast. Shelf Sci. 2010, 87, 501–509. [Google Scholar] [CrossRef]
- Wells, F.E.; Threlfall, T.J.; Wilson, B.R. The Peel–Harvey Estuarine System Study 1976–1980, Technical Report, Biology of Molluscs; Department of Conservation and Environment: Perth, WA, Australia, 1980; p. 121.
- Sarà, G.; Romano, C.; Widdows, J.; Staff, F.J. Effect of salinity and temperature on feeding physiology and scope for growth of an invasive species (Brachidontes pharaonis—MOLLUSCA: BIVALVIA) within the Mediterranean Sea. J. Exp. Mar. Biol. Ecol. 2008, 363, 130–136. [Google Scholar] [CrossRef]
- Verdelhos, T.; Marques, J.C.; Anastácio, P. The impact of estuarine salinity changes on the bivalves Scrobicularia plana and Cerastoderma edule, illustrated by behavioral and mortality responses on a laboratory assay. Ecol. Indic. 2015, 52, 96–104. [Google Scholar] [CrossRef]
- Lawrie, A.D.A.; Chaplin, J.; Pinder, A. Biology and conservation of the unique and diverse halophilic macroinvertebrates of Australian salt lakes. Mar. Freshw. Res. 2021, 72, 1553–1576. [Google Scholar] [CrossRef]
- Todd, M.E. Osmotic balance in Hydrobia ulvae and Potamopyrgus jenkinsi (Gastropoda: Hydrobiidae). J. Exp. Biol. 1964, 41, 665–677. [Google Scholar] [CrossRef]
- Pascual, E.; Drake, P. Physiological and behavioral responses of the mud snails Hydrobia glyca and Hydrobia ulvae to extreme water temperatures and salinities: Implications for their spatial distribution within a system of temperate lagoons. Physiol. Biochem. Zool. Ecol. Evol. Approaches 2008, 81, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Wells, F.E.; Threlfall, T.J. Reproductive strategies of Hydrococcus brazeri (Tenison Woods, 1876) and Arthritica semen (Menke, 1843) in Peel Inlet Western Australia. J. Malacol. Soc. Aust. 1982, 5, 157–166. [Google Scholar]
- Pinder, A.M.; Halse, S.A.; McRae, J.M.; Shiel, R.J. Occurrence of aquatic invertebrates of the wheatbelt region of Western Australia in relation to salinity. Hydrobiologia 2005, 543, 1–24. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Warwick, R.M.; Clarke, K.R.; Potter, I.C. Family-level AMBI is valid for use in the north-eastern Atlantic but not for assessing the health of microtidal Australian estuaries. Estuar. Coast. Shelf Sci. 2014, 141, 85–96. [Google Scholar] [CrossRef]
- Dobrzycka, A.; Szaniawska, A. The effect of salinity on osmoregulation in Corophium volutator (Pallas) and Saduria entomon (Linnaeus) from the Gulf of Gdańsk. Oceanologia 1995, 37, 111–122. [Google Scholar]
- Masikane, N.F.; Newman, B.K.; Scharler, U.M. Salinity tolerance of the South African endemic amphipod Grandidierella lignorum (Amphipoda: Aoridae). Afr. J. Aquat. Sci. 2014, 39, 151–156. [Google Scholar] [CrossRef]
- Shahin, S.; Okomoda, V.T.; Ishak, S.D.; Waiho, K.; Fazhan, H.; Azra, M.N.; Azman, A.R.; Wongkamhaeng, K.; Abualreesh, M.H.; Rasdi, N.W.; et al. Lagoon amphipods as a new feed resource for aquaculture: A life history assessment of Grandidierella halophila. J. Sea Res. 2023, 192, 102360. [Google Scholar] [CrossRef]
- Moore, P.G. A new species in the genus Grandidierella Coutière (Crustacea: Amphipoda) from an Australian solar salt-works. J. Nat. Hist. 1986, 20, 1393–1399. [Google Scholar] [CrossRef]
- Kikuchi, S.; Matsumasa, M. The osmoregulatory tissue around the afferent blood vessels of the coxal gills in the estuarine amphipods, Grandidierella japonica and Melita setiflagella. Tissue Cell 1993, 25, 627–638. [Google Scholar] [CrossRef]
- Rahman, M.; Chaplin, J.; Pinder, A. The biology of giant ostracods (Crustacea, Cyprididae), a review focusing on the Mytilocypridinae from Australian inland waters. Mar. Freshw. Res. 2023, 74, 1–19. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Krispyn, K.N. Fishes and salinities of low-inflow estuaries in the Fitzgerald Biosphere. FiSHMED Fishes Mediterr. Environ. 2025, 001, 1–14. [Google Scholar]
- Terry, C.E.; Liebzeit, J.A.; Purvis, E.M.; Dowd, W.W. Interactive effects of temperature and salinity on metabolism and activity of the copepod Tigriopus californicus. J. Exp. Biol. 2024, 227, jeb248040. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.H.; Johnson, A.R. An ecological account of a Mediterranean salina: The Salin de Giraud, Camargue (S. France). Biol. Conserv. 1987, 42, 185–230. [Google Scholar] [CrossRef]
- Shadrin, N.V.; Anufriieva, E.V.; Belyakov, V.P.; Bazhora, A.I. Chironomidae larvae in hypersaline waters of the Crimea: Diversity, distribution, abundance and production. Eur. Zool. J. 2017, 84, 61–72. [Google Scholar] [CrossRef]
- Kokkinn, M. Osmoregulation, salinity tolerance and the site of ion excretion in the halobiont chironomid, Tanytarsus barbitarsis Freeman. Mar. Freshw. Res. 1986, 37, 243–250. [Google Scholar] [CrossRef]
- Cowley, P.D.; Tweedley, J.R.; Whitfield, A.K. Conservation of estuarine fishes. In Fish and Fisheries in Estuaries; Wiley: Hoboken, NJ, USA, 2022; pp. 617–683. [Google Scholar]
- Kefford, B.J.; Fields, E.J.; Clay, C.; Nugegoda, D. Salinity tolerance of riverine microinvertebrates from the southern Murray Darling Basin. Mar. Freshw. Res. 2007, 58, 1019–1031. [Google Scholar] [CrossRef]
- Kefford, B.J.; Papas, P.J.; Metzeling, L.; Nugegoda, D. Do laboratory salinity tolerances of freshwater animals correspond with their field salinity? Environ. Pollut. 2004, 129, 355–362. [Google Scholar] [CrossRef]
- Hutchings, P.; Kupriyanova, E. Cosmopolitan polychaetes—Fact or fiction? Personal and historical perspectives. Invertebr. Syst. 2018, 32, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).