Abstract
The warming trend of the Mediterranean Sea is a long-term process. It has resulted in a northwards and westwards range expansion and abundance increase of thermophilic species, both native and non-indigenous, and in a shrinking of the range of cold-affinity species. Marine heatwaves (MHWs) are relatively short-term extreme episodes that are responsible for spectacular mortality events in some species and have been extensively reported in the literature. In contrast, the species that benefit from MHWs (the ‘winners’) have been much less studied. A record-breaking MHW occurred in 2022 in the north-western Mediterranean Sea. We focus on three ‘winner’ species, the thermophilic green macroalgae Penicillus capitatus and Microdictyon umbilicatum and the endemic seagrass Posidonia oceanica. Penicillus capitatus, which is mainly present in the area as an inconspicuous turf of entangled filaments (espera stage), produced the erect paintbrush-like stage where sexual reproduction takes place. Microdictyon umbilicatum, usually uncommon, bloomed to the point of clogging fishing nets. Finally, a mass flowering of P. oceanica occurred in late August–September, followed the following year (April–May 2023) by the extensive production and dissemination of fruits and seeds. Both processes, the long-term warming trend and one-off heatwaves, both ‘losers’ and ‘winners’, shape the change in structure and functioning of Mediterranean ecosystems.
1. Introduction
1.1. Mediterranean Warming Trend and Heatwaves
Although the climate warming since the beginning of the 19th century has been partly natural (the end of the Little Ice Age—LIA), there is little doubt that greenhouse gas emissions due to human activities have amplified this warming, until they became the main warming driver in the late 20th century and early 21st century [1,2,3,4,5,6]. The rate of warming is higher in the terrestrial realm than in the global ocean, including the Mediterranean Sea [6,7]. The Mediterranean is a hot spot for climate change [8], and all simulations point to a rapid warming of the Mediterranean by the end of the 21st century (e.g., [9]). In the upper layer, the simulated warming ranges from 0 to 2 °C under scenarios RCP2.6 and RCP4.5 up to 2 °C to 4 °C under scenario RCP 8.5 (see Table 4 of [9] for details), with trends ranging from ~0.2 to ~0.6 °C/decade (e.g., [10]). The warming anomalies display heterogeneity in intensity as well as in space at the Mediterranean basin scale (see Figure 2 of [9]; Figure 1 of [10]). One of the consequences associated with global ocean warming is the increased occurrence of marine heatwaves (MHWs), both longer and more frequent in the following decades [11,12,13,14,15,16,17,18,19,20,21]; see [22] for a review. Note that there is no consensus on the definition of MHWs, nor on the way to compute them (e.g., [10,23]), primarily since the definition depends on the time span considered for the climatology, hence, on time series availability. It is also worth noting that heatwaves, terrestrial and probably marine, are not a new phenomenon: they have been reported for almost a millennium [3]. In any case, MHW occurrence, intensity and duration have significantly increased over the last 20 years, beyond as well as within the Mediterranean (e.g., [24,25,26,27,28]). MHWs impact both the upper and the deeper layers (e.g., [10,29]), with dramatic ecological and economic consequences [19,20,30,31,32,33,34,35,36,37,38].
Long-term sea water warming of the Mediterranean has three main ecological consequences. (i) The spread of thermophilic native Mediterranean species, long confined to the warmest areas (the east and the south), whose range has been expanding westwards and northwards. This is the case for some teleosts, e.g., the ornate wrasse Thalassoma pavo [39,40], the parrotfish Sparisoma cretense [41,42] and the scorpaenid Scorpaena maderensis [43]. The thermophilous painted comber Serranus scriba, absent in 1990–1993 from the Côte Bleue Marine Park west of Marseilles, is now six times more common than S. cabrilla in shallow habitats (Éric Charbonnel, unpublished data). (ii) The spread of non-indigenous thermophilic species introduced to the Mediterranean Sea via, e.g., the Suez Canal and shipping, such as the seagrass Halophila stipulacea [44,45,46] and the red alga Lophocladia trichoclados (as L. lallemandii) (kingdom Archaeplastida) [47,48]. And (iii) the shrinking of the range of cold-water species, such as some teleosts (e.g., the common sole Solea solea and the European seabass Dicentrarchus labrax [49]), the eelgrass Zostera marina Linnaeus [50,51] and the brown alga Fucus virsoides (Fucales, kingdom Stramenopiles) in the Adriatic Sea [52,53,54]. For the latter species, most authors have only attributed its decline to pollution; it is a doxa dating from the middle of the 20th century (pollution was then claimed to explain everything; see [55]); but global warming must also be considered for this cold-affinity species.
The basket star Astrospartus mediterraneus is a filter-feeder endemic to the Mediterranean, although also present in the neighbouring Atlantic Ocean (Morocco, Portugal) [56]. It was considered as rare. From 2013 to the present, an impressive outbreak occurred in Catalonia; its abundance also conspicuously increased in Liguria (Italy) [56]. No correlation was found between basket star occurrence and the sea surface temperature (SST); however, it mainly dwells at depths ranging from 50 to 80 m, a depth where water temperature is poorly known; in addition, a correlation was found with summer rainfall [56]. As a result, the basket star could be a candidate species for the status of ‘global change winner’.
Relatively short-term extreme events, such as heatwaves, rather account for local mortality within a narrow depth range of sessile invertebrates, such as gorgonians (e.g., Paramuricea clavata, Eunicella singularis), sponges (Spongia officinalis) and the precious red coral (Corallium rubrum). Recovery has proved to be very slow or absent, at least in some localities, with mortality even worsening after the end of the surface heatwave [57,58]. The heatwave of 2003 had a severe impact on the leaf epibionts of the seagrass Posidonia oceanica in Liguria (Italy): the red calcified alga Hydrolithon sp. declined by more than 60% but quickly recovered, while the bryozoan Electra posidoniae declined seven-fold and took 16 years to recover [59]. On the other hand, heatwaves can work in favour of some species. Here, we develop this last point, much less often addressed in the literature than the issue of mass mortality, focusing on three species, the ‘flowering’ of the green alga Penicillus capitatus, the bloom of the green alga Microdictyon umbilicatum and the exceptional flowering event of the seagrass Posidonia oceanica.
1.2. The Record-Breaking 2022 Marine Heatwave
The meteorological (and climatological) processes behind this extreme event are beyond the scope of this paper, so readers are referred to [60,61] and references therein.
The description of the 2022 MHW is only tentative, firstly because there is as yet insufficient literature to serve as a basis for a global survey, and secondly because the criteria for MHW detection and characterization vary according to the authors or the aims. The MHW affected the Western Basin more severely (see Figure 1 from [61]; T-MedNet website).
There is general agreement [60,61,62] that the intense warming of the surface layer started early, during May 2022, since by June SSTs (sea surface temperatures, based on satellite data) were already above normal in the western Mediterranean. Martinez et al. [10] even compute that the first 2022 severe MHW started by 15 June 2023. Then, in the NW part of the Mediterranean as well as throughout the Mediterranean, the SST has constantly been higher than the climatological baseline until spring 2023 (at least). The MHW displayed several peaks, impacting various areas. In the NW Mediterranean, the preceding average temperature/SST record (25.6 °C) dating back to 2003 (for the period 1982–2011) was broken with 26.1 °C, and maximum daily anomalies could exceed 4 °C. A main factor for the occurrence of the MHW was the negative anomaly of wind episodes [60], as mistral and tramontane wind episodes mix the upper layer and generate upwelling cells in the Gulf of Lions that prevent overheating in the surface layer (note, however, that in the stretches of coastline where they induce cascading [63], the MHW stress on benthic populations should be increased).
At the Mediterranean scale, the SST anomalies ranged between 1.5 and 2 °C during the meteorological summer, and until April 2023 all anomalies ranged between 0.5 and 1.5 °C. At the local scale, some SST anomalies higher than 2 °C persisted in the south-western part. In their Mediterranean main MHW catalogue, Martinez et al. [10] found two MHW events classified as ‘severe’ (as in 2003): from 15 June to 21 August 2022 and from 24 October to 22 November 2022. Whatever the domain considered and the criteria applied, this extreme event had a record-breaking duration.
The T-MedNet website ([64]; https://t-mednet.org/visualize-data/temperature?view=tfigure, accessed on 7 December 2023) clearly showed that, by the end of 2022, the temperature anomalies reached deeper than 40 m. This resulted in the event with the highest cumulative intensity just after the well-known 2003 event [10].
2. Material and Methods
This work is based on the opportunistic observations of the authors, spread across the entire studied area (Occitania, Provence, French Riviera and Corsica; France), who dive frequently and all year round, from very shallow to deep (30–40 m) habitats (sand, reef, seagrasses, coralligenous outcrops), in the course of various field research and monitoring programs. They have also collected testimonies from artisanal fishermen, managers of marine protected areas and owners of diving clubs.
3. Results and Discussion
3.1. The Green Alga Penicillus capitatus
The green alga Penicillus capitatus Lamarck (Ulvophyceae, Viridiplantae, kingdom Archaeplastida) occurs as two stages. The first stage is a tangled turf of filaments that carpet the substrate; it is called the espera stage as, for a long time, it was regarded as a distinct species, Espera mediterranea. The second stage, which arises from the basal filaments, is a simple stalk terminating in a capitular tuft of bright green, free, dichotomously branched filaments, looking very like a paintbrush (hereafter: paintbrush stage) [65,66,67,68,69].
As shown by Alexandre Meinesz, in P. capitatus, the sexual reproduction is holocarpic: all the cytoplasm of the plant turns into gametes, after which the plant dies. This process occurs in the paintbrushes, which in a way are the sexual organs of the plant, a kind of flower—although the term is of course inappropriate for algae [70,71].
Penicillus capitatus is a thermophilic species, occurring worldwide in tropical and warm seas: the Indian Ocean, Pacific Ocean and Atlantic Ocean [65,71]. It is present in the Mediterranean Sea, especially in its warmer areas, the eastern and southern basins. The espera stage is relatively common; it appears in the form of a turf of branched and intertwined filaments. In contrast, the paintbrush stage, relatively common although very localized in the eastern Mediterranean, is quite uncommon in the western Mediterranean; there, it is localized at a very few sites: Sainte-Maxime in eastern Provence, Villefranche-sur-Mer, Antibes and Golfe Juan (Croton Cove) in the French Riviera; Taverna, Portivechju Gulf and Sant’Amanza Gulf in Corsica; Elba Island and Secca della Meloria in Tuscany, Italy; Korbous, Hergla, Kerkennah Islands, Jerba Island, Zarzis and Ras-el-Ketef in Tunisia; and Cala Blava, south of Mallorca, Balearic Islands ([66,67,68,72,73,74,75,76,77,78]; Gérard Pergent and Thierry Thibaut, unpublished data). The occurrence of the paintbrush stage is on the increase [78].
The range of the espera stage is more extensive than that of the paintbrush stage, the occurrence of which is very sporadic, at least in the western basin of the Mediterranean. The paintbrush stage generally occurs between August and December in shallow (1–5 m) and soft-bottom areas: dead matte of the seagrass Posidonia oceanica, meadows of the seagrass Cymodocea nodosa and meadows of the green alga Caulerpa prolifera [66,68,78]. However, especially in the eastern Mediterranean basin, the paintbrush stage can also be observed in much deeper sites (Gérard Pergent, personal observations).
It seems that the formation of paintbrushes from the underlying espera turf could be uneven, occurring after hot summers, then absent for several years, at least in the north-western Mediterranean. The occurrence of the espera stage has been interpreted as a stress response induced by suboptimal environmental conditions [79]. Unfortunately, long-term monitoring of the presence, abundance or absence of paintbrushes is lacking. This is probably the case in Sant’Amanza Gulf (southern Corsica). In September 2022, paintbrush stages were observed at 14 m depth on dead matte of Posidonia oceanica at a site where they were definitely absent the previous years (2020 and 2021) (Figure 1).

Figure 1.
Paintbrush stage of Penicillus capitatus, Taverna, eastern Corsica, August 2023. Left: a voucher specimen; centimetric scale. Right: in situ aspect of the stand. Photo © Gérard Pergent.
3.2. The Green Alga Microdictyon umbilicatum
The green alga Microdictyon umbilicatum (Vellay) Zanardini (Ulvophyceae, Viridiplantae, kingdom Archaeplastida), also referred to as M. tenuius [80,81], is a thermophilic species that has been reported from all tropical and warm marine areas: the Atlantic Ocean, Pacific Ocean, Indian Ocean and Red Sea [82,83,84,85].
In the Mediterranean Sea, M. umbilicatum seems widespread, with the exception of the north-western basin, although it is usually uncommon everywhere; it thrives at various depths, from the deep infralittoral zone (up to 30–40 m) to the circalittoral zone (beyond 30–40 m depth) [69,80,81] (Figure 2). In Corsica, the only record is that of Coppejans [86] at Calvi; it was collected in September 1977 at 25 m depth. In mainland France, M. umbilicatum has only been reported from Mala Cove and Golfe Juan (French Riviera) in June 1927 [72,87], then from Port-Cros Archipelago (Eastern Provence) in October 2019 [88].
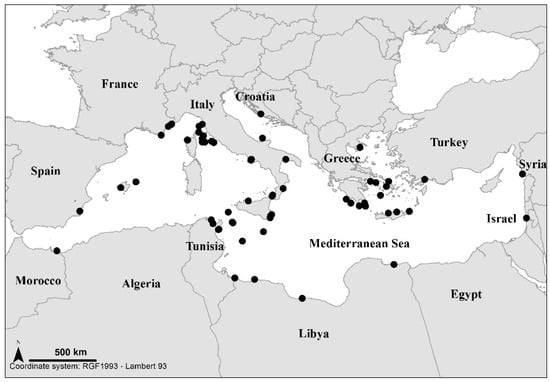
Figure 2.
Location of Microdictyon umbilicatum known records (black points) in the Mediterranean, according to the Plateforme macrophytes database (OSU Pytheas, Aix-Marseille University). Its rarity in Spain, France and Turkey, well-explored regions, is undoubtedly not an artefact. On the other hand, its absence in Algeria and Egypt perhaps constitutes an artefact.
From late summer to fall 2022, M. umbilicatum proliferated off the Provence coast. Many fishermen found it trapped in their nets and some of them, intrigued by this alga, brought it to our institute. We also directly observed M. umbilicatum in situ (Figure 3 and Figure 4). Since then, M. umbilicatum is still present, sometimes abundant, in several localities along the Provence coast, e.g., Prado Bay at Marseilles (Sandrine Ruitton, personal observations).

Figure 3.
Microdictyon umbilicatum from Prado Bay, Marseilles (France).

Figure 4.
An accumulation of Microdictyon umbilicatum within the artificial reefs of Prado Bay, Marseilles (France), 28 m depth, on 14 December 2022. Photo © Sandrine Ruitton.
3.3. An Exceptional Flowering of the Seagrass Posidonia oceanica
The seagrass Posidonia oceanica (Linnaeus) Delile (Magnoliophyta, Viridiplantae, kingdom Archaeplastida) is endemic to the Mediterranean Sea [89]. It constitutes extensive meadows from sea level down to 10–40 m depth, depending on the water transparency, throughout the Mediterranean, with the exception of Lebanon, Israel, the northernmost Adriatic, the westernmost Alboran Sea, part of the Gulf of Lions and the vicinity of the mouths of rivers, such as the Rhone river in France [90,91].
The flowering of Posidonia oceanica occurs in fall, usually between September and November; an autumnal flowering is a not-uncommon feature for land plants in the Mediterranean area. Hermaphrodite flowers are grouped in an inflorescence at the tip of a 10–30 cm long stalk (Figure 5). The fruits, resembling green olives, are ripe in spring, usually between April and July, when they drop off, float and are dispersed by currents and winds over long distances [90,92,93].

Figure 5.
An inflorescence of Posidonia oceanica. Each flower includes an ovary (with a stigma) and stamens (with an anther). Photo © Vincent Maran, courtesy of the author.
Flowering is not as rare as was believed in the mid-20th century (e.g., [94]), but its intensity is very variable, with mass flowerings generally spaced 5 to 12 years apart. According to Diaz-Almela et al. [95]), they occur every 9–11 years. Mass flowering usually concerns a wide area, such as the north-western Mediterranean basin. Mass flowering has been reported, e.g., in 1975 [92,96], in 1993–1994 [97,98,99,100,101], in 2003 [91,95,101,102,103,104,105] and in 2009 [106]. However, the synchronism of flowering sometimes does not extend to the whole of the Mediterranean: for example, in Sicily, between 1974 and 1999, the most extensive mass flowering events were those of 1997 and 1998 [107,108].
In 2003, an unusual flowering was observed in July in the Bay of Calvi (Corsica), with flowers quickly aborting, followed by a new and massive flowering in fall and then by a massive fruiting in the spring of 2004 (Gérard Pergent and Christine Pergent-Martini, unpublished data).
According to the literature, flowering may be triggered and/or enhanced by several factors, e.g., age of the orthotropic shoot, high summer temperature, peaks of annual SST, intense solar activity (with peaks every 11 years on average), the amount of carbohydrate compounds stored within rhizomes or a combination of these factors [95,100,103,104,108,109,110].
In the 1950s, the relative rarity of flowering was attributed to ‘the steady loss of adaptability of P. oceanica to the Mediterranean environment’ [94]. The assumption was somewhat naive, P. oceanica being a species that possibly resisted the partial drying up of the Mediterranean during the Messinian crises (5.7 through 5.3 Ma ago), then thirty glacial/interglacial cycles (since 2.5 Ma) and finally the succession of hot (such as the Medieval Warm Period—MWP) and cold (such as the Little Ice Age—LIA) episodes since the Last Glacial Maximum (LGM) 20 000 years ago. As to how P. oceanica possibly survived the Messinian crises, the debate remains open [111,112,113]: its presence in the Mediterranean is only formally attested by fossils after these crises [114,115]. Subsequently, the hypothesis in vogue was that flowering was the consequence of stress: sexual reproduction was seen as the last effort of the plant to survive in an environment that was becoming less suitable due, e.g., to pollution [116]. In fact, the most likely hypothesis to account for the irregularity and rarity of flowering seems to be the predator satiation strategy (PSS) (e.g., [117,118,119,120,121]). A regular production of offspring leads to the occurrence of predators consuming all of them. To be successful, be unpredictable! In the years when mass production of offspring occurs, predators are overwhelmed with potential prey (here inflorescences, fruits and seeds); they can consume only a certain amount, so that a significant number of them can escape being consumed. Obviously, for a long-lived species, such as many trees and P. oceanica (up to several millennia [122]), reproduction every year is unnecessary. Accumulating carbohydrate reserves during several years to allow an unpredictable mass production of offspring is a better strategy. As far as P. oceanica is concerned, while leaves are only moderately grazed [123], inflorescences are actively consumed by herbivores, such as the teleost Sarpa salpa and the sea urchin Paracentrotus lividus [100,110,124,125] (Figure 6). This high rate of consumption of the inflorescences is surprising, because they are better defended chemically, with higher levels of phenolic compounds, and have a lower nutritive value (proteins, nitrogen); in fact, the main factor in herbivore deterrence is the structural defences of the tissues [125]. The fruits are also consumed by sea urchins and the hermit crab Clibanarius erythropus [126]. The role of carbohydrate reserves and the reproductive cost are confirmed by the loss of rhizome elongation and production in the two years following mass flowering ([104,108]; but see [100]).

Figure 6.
Grazing of Posidonia oceanica leaves and inflorescences by the teleost salema Sarpa salpa. Reserve of Carry-le-Rouet, Côte Bleue Marine Park (west of Marseilles), 5 m depth, 1st December 2022. Photo © Bruno Belloni.
In late August–September 2022, an exceptional mass flowering of P. oceanica occurred from the Italian border to Camargue (mainland France), in the Gulf of Lions and in Corsica. We observed this flowering in situ (Figure 7 and Figure 8) and on sand beaches, where broken inflorescences were massively cast ashore (Figure 9). The inflorescence density values reached may never have been recorded previously (Table 1 and Table 2), with up to 100% of the shoots bearing an inflorescence at some sites (Figure 8). Of course, since the shoot density decreases with depth and is dependent on the health status of the meadow and micro-distribution patterns [90,127,128,129], the inflorescence density per m2 can decrease with depth, while the percentage of flowering shoots actually increases [130]. In April and May 2023, large rafts of floating fruits were observed on the surface of the sea, together with fruits and seeds stranded on beaches (Figure 10 and Figure 11).

Figure 7.
Patrick Astruch observing the flowering of Posidonia oceanica, 10 m depth, Montremian, Bagaud Island, Port-Cros Archipelago (eastern Provence), on 17 November 2022. Photo © Bruno Belloni.

Figure 8.
Mass flowering of Posidonia oceanica at Montremian, Bagaud Island, Port-Cros Archipelago (eastern Provence), on 17 November 2022. Almost all shoots bear an inflorescence. The metal frame measures 20 cm × 20 cm. Photo © Bruno Belloni.

Figure 9.
Inflorescences—in fact, young fruits—cast ashore. Tamarone beach, Macinaghju, Corsica, 12 October 2022. Photo © Charles-François Boudouresque.

Table 1.
Some historical data on inflorescence density of Posidonia oceanica.

Table 2.
Inflorescence density of Posidonia oceanica in fall 2022.

Figure 10.
Fruits of Posidonia oceanica floating at the sea surface in late April 2023, after the massive flowering of fall 2022. Sant’Amanza Gulf, southern Corsica. Photo © Bruno Belloni.

Figure 11.
Fruits and seeds of Posidonia oceanica cast ashore in May 2023. Palombaggia Beach, southern Corsica. Photo © Bruno Belloni.
Posidonia oceanica is a relatively thermotolerant species [137,138]; it tolerates temperatures as low as 9 °C and as high as 30 °C [139,140,141]. Interestingly, the populations from the warm (Cyprus) and cold (Catalonia) range limits of P. oceanica are the most resistant to extreme temperatures, whether low or high [142]. As far as 4- and 16-month-old germlings are concerned, photosynthesis is highest at 28–30 °C, the balance of photosynthesis/respiration becomes negative at 32 °C and photosynthesis ceases at 36 °C [143]. In the context of climate warming, the occurrence of sexual reproduction and the production of a number of offspring that escape predation owing to mass flowering could trigger better genetic diversity, with higher resistance to warming and other environmental changes [138]. Some modelling works predict, under the RCP8.5 scenario, the disappearance or very strong decline of P. oceanica from much of the Mediterranean [144,145], but debating the credibility of their conclusions is beyond the scope of the present article.
4. Conclusions
Global warming is a long-term trend. In the Mediterranean Sea, it has resulted in the northwards and westwards expansion of the range of native species with warm affinity, e.g., the teleosts Scorpaena maderensis, Sparisoma cretense and Thalassoma pavo [39,40,41,42,43], together with many Lessepsian species introduced into the eastern basin. It has caused a shift in ecosystem functioning in the eastern part of the Mediterranean. One of the most spectacular species resulting from this upheaval are teleosts of the genus Siganus (rabbitfish), which are formidable herbivores that have completely disrupted the habitats of photophilous rocky reefs in the eastern basin [146,147,148], although their success could be a consequence of the pre-existing thermal conditions in this eastern basin (or much of it).
Marine heatwaves, however they are defined and delimited (questions that are open to debate), are discrete extreme events. They provide additional evidence of climate warming, highlighted by changes in their intensity, frequency and duration. However, it must be noted that marine heatwaves in the Mediterranean are not a new or recent phenomenon. The first documented event dates from 1983. But it is likely that for some of the heatwaves reported for centuries on land (see, e.g., [3] in western Europe) there may have been a corresponding phenomenon in the marine environment.
Most of the literature about Mediterranean marine heatwaves reports associated mortality events (e.g., [11,19] and references therein). Extreme events always induce extreme reactions from the public, from users (divers, fishermen) and from the media, but also from scientists when only the negative impacts are emphasized and perhaps sometimes exaggerated. The impacts on sessile invertebrates are localized to particular sites and within a relatively reduced depth range. Currently, these underwater mortality events, although spectacular for divers, have a relatively limited impact over time: the resilience of some (not all) of the impacted species must be considered, and no species is threatened with rapid extinction by these heatwaves because their distribution, in particular their depth range (e.g., Paramuricea clavata), goes well beyond the impacted areas and depths [149,150,151,152]. Cases of recovery following mass mortality events of gorgonians (P. clavata) have been reported in different locations after different MHWs over time [153,154]. In addition, these species may have dispersal capacities via planktonic larvae higher than expected, and connectivity between populations is maintained, since it is rare that all of the individuals are killed [155]. Resistance to thermal stress varies between individuals within a population and between populations, e.g., in Corallium rubrum and Paramuricea clavata [156,157,158]. Larval exchange between sites hundreds of metres apart and between different depths has occurred, supporting the hypothesis that deeper subpopulations unaffected by sea surface MHWs may provide larvae for shallower ones, enabling recovery after climate-induced mortality events [144,159,160]. Of course, the frequency and intensity of these events could have more significant long-term consequences in the future, such as regional species extinctions, especially when slow-growing and long-lived species are considered; this is already the case for the sponge Spongia officinalis, regionally extinct in western Provence [38]. Even if species impacted by MHWs are generally not threatened, the consequences are mainly related to the alteration of the seascape and the related ecosystem functioning [161]. After the collapse of animal forests such as gorgonian facies, ecological functions can be strongly affected in the long term [20,58].
The record-breaking 2022 heatwave had a spectacular impact on certain species (the ‘losers’) and probably on ecosystems yet to be described and analysed.
Parallel to the mortality of certain species, marine heatwaves may favour others, the thermophilic species (the ‘winners’), that may benefit from these extreme events to develop and disperse. Here, we have chosen three case studies in the north-western Mediterranean. (i) The possible induction of ‘flowering’ (in the form of the paintbrush stage) of the green alga Penicillus capitatus. (ii) The unprecedented bloom of the green alga Microdictyon umbilicatum. (iii) The massive flowering of the seagrass Posidonia oceanica. Other possible winners could have been proposed, but they require further investigation, and our aim was not to be exhaustive.
The equilibrium of ecosystems is a shifting status influenced by environmental factors. The current warming is obviously one of the main stressors affecting the Mediterranean Sea. In this context, heatwaves constitute milestones: unlike long-term warming, the consequences of which for ecosystems are often not perceptible in the short term, heatwaves cause spectacular, clearly visible events, either mortality or, on the contrary, the proliferation or massive reproduction of certain species. Both processes, the long-term warming trend and one-off heatwaves, both losers and winners, shape the change in structure and functioning of Mediterranean ecosystems.
Author Contributions
Conceptualization, C.-F.B. and M.P.-B.; Methodology, C.-F.B., G.P., P.A. and S.A.; Investigation, B.B., B.M., C.P.-M., G.P., I.T.-L., M.M., P.A., S.A. and S.R.; Writing—Original Draft Preparation, C.-F.B., M.P.-B. and T.T.; Writing—Review and Editing, A.B., A.C., B.B., B.M., C.-F.B., C.P.-M., É.C., G.P., I.T.-L., J.-M.C., M.M., M.P.-B., P.A., R.D.d.l.G., S.A. and T.T.; Supervision, C.-F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All available data are provided in the tables.
Acknowledgments
The authors thank Vincent Maran for flower photos, Michael Paul, a native English speaker, for proofreading the text and two anonymous reviewers for valuable suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stott, P.A.; Tett, S.F.B.; Jones, G.S.; Allen, M.R.; Mitchell, J.F.B.; Jenkins, G.J. External control of 20th century temperature by natural and anthropogenic forcings. Science 2000, 290, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Chuine, I.; Yiou, P.; Viovy, N.; Seguin, B.; Daux, V.; Le Roy Ladurie, E. Grape ripening as a past climate indicator. Nature 2004, 432, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Le Roy-Ladurie, E. Histoire Humaine et Comparée du Climat: Canicules et Glaciers (XIII–XVIII siècles); Fayard Publisher: Paris, France, 2004. [Google Scholar]
- Stott, P.A.; Stone, D.A.; Allen, M.R. Human contribution for the European heatwave of 2003. Nature 2004, 432, 613. [Google Scholar] [CrossRef] [PubMed]
- Neukom, R.; Steiger, N.; Gómez-Navarro, J.J.; Wang, J.; Werner, J.P. No evidence for globally coherent warm and cold periods over the preindustrial Common Era. Nature 2019, 571, 550–554. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2022: Impacts, Adaptations and Vulnerability; Summary for Policymakers, Technical Summary and Frequently Asked Questions; Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2022. [Google Scholar]
- Lejeusne, C.; Chevaldonné, P.; Pergent-Martini, C.; Boudouresque, C.F.; Perez, T. Climate change effects on a miniature ocean: The highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 2010, 25, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, F. Climate change hot-spots. Geophys. Res. Lett. 2006, 33, L08707. [Google Scholar] [CrossRef]
- Soto-Navarro, J.; Jordá, G.; Amores, A.; Cabos, W.; Somot, S.; Sevault, F.; Macías, D.; Djurdjevic, V.; Sannino, G.; Li, L.; et al. Evolution of Mediterranean Sea Water Properties under Climate Change Scenarios in the Med-CORDEX Ensemble. Clim. Dynam. 2020, 54, 2135–2165. [Google Scholar] [CrossRef]
- Martínez, J.; Leonelli, F.E.; García-Ladona, E.; Garrabou, J.; Kersting, D.K.; Bensoussan, N.; Pisano, A. Evolution of marine heatwaves in warming seas: The Mediterranean Sea case study. Front. Mar. Sci. 2023, 10, 1193164. [Google Scholar] [CrossRef]
- Cerrano, C.; Bavestrello, G.; Bianchi, C.N.; Cattaneo-Vietti, R.; Bava, S.; Morganti, C.; Morri, C.; Picco, P.; Sara, G.; Schiaparelli, S.; et al. A Catastrophic Mass-Mortality Episode of Gorgonians and Other Organisms in the Ligurian Sea (North-Western Mediterranean), Summer 1999. Ecol. Lett. 2000, 3, 284–293. [Google Scholar] [CrossRef]
- Romano, J.C.; Bensoussan, N.; Younes, W.A.N.; Arlhac, D. Anomalie thermique dans les eaux du golfe de Marseille durant l’été 1999. Une explication partielle de la mortalité d’invertébrés fixés? Comptes Rendus L’Académie Sci.-Ser. III-Sci. Vie 2000, 323, 415–427. [Google Scholar] [CrossRef]
- Perez, T.; Garrabou, J.; Sartoretto, S.; Harmelin, J.G.; Francour, P.; Vacelet, J. Mortalité massive d’invertébrés marins: Un événement sans précédent en Méditerranée nord-occidentale. Comptes Rendus L’Académie Sci.-Ser. III-Sci. Vie 2000, 323, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Coma, R.; Linares, C.; Ribes, M.; Diaz, D.; Garrabou, J.; Ballesteros, E. Consequences of a mass mortality in populations of Eunicella singularis (Cnidaria: Octocorallia) in Menorca (NW Mediterranean). Mar. Ecol. Prog. Ser. 2006, 327, 51–60. [Google Scholar] [CrossRef]
- Garrabou, J.; Coma, R.; Bensoussan, N.; Bally, M.; Chevaldonné, P.; Cigliano, M.; Diaz, D.; Harmelin, J.G.; Gambi, M.C.; Kersting, D.K.; et al. Mass mortality in northwestern Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Glob. Chang. Biol. 2009, 15, 1090–1103. [Google Scholar] [CrossRef]
- Hobday, A.J.; Oliver, E.C.; Gupta, A.S.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Neil, J.; Holbrook, N.J.; Pippa, J.; Moore, P.J.; et al. Categorizing and naming marine heatwaves. Oceanography 2018, 31, 162–173. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Azzola, A.; Bertolino, M.; Betti, F.; Bo, M.; Cattaneo-Vietti, R.; Cocito, S.; Montefalcone, M.; Morri, C.; Oprandi, A.; et al. Consequences of the marine climate and ecosystem shift of the 1980–90s on the Ligurian Sea biodiversity (NW Mediterranean). Eur. Ecol. J. 2019, 86, 458–487. [Google Scholar] [CrossRef]
- Garrabou, J.; Ledoux, J.B.; Bensoussan, N.; Gómez-Gras, D.; Linares, C. Sliding toward the collapse of Mediterranean coastal marine rocky ecosystems. In Ecosystem Collapse and Climate Change; Canadell, J.G., Jackson, R.B., Eds.; Ecological Studies; Springer: Cham, Switzerland, 2021; pp. 291–324. [Google Scholar]
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; Bensoussan, N.; Turicchia, E.; Sini, M.; Gerovasileiou, V.; et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Glob. Chang. Biol. 2022, 28, 5708–6725. [Google Scholar] [CrossRef] [PubMed]
- Estaque, T.; Richaume, J.; Bianchimani, O.; Schull, Q.; Mérigot, B.; Bensoussan, N.; Bonhomme, P.; Vouriot, P.; Sartoretto, S.; Monfort, T.; et al. Marine heatwave on the rise: One of the strongest ever observed mass mortality event in temperate gorgonians. Glob. Chang. Biol. 2023, 29, 6159–6162. [Google Scholar] [CrossRef]
- Orenes-Salazar, V.; Navarro-Martínez, P.C.; Ruíz, J.M.; García-Charton, J.A. Recurrent marine heatwaves threaten the resilience and viability of a key Mediterranean octocoral species. Aquat. Conserv. Mar. Freshw. Ecosyst. 2023, 33, 1161–1174. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Benthuysen, J.A.; Darmaraki, S.; Donat, M.G.; Hobday, A.J.; Holbrook, N.J.; Schlegel, R.W.; Sen Gupta, A. Marine heatwaves. Ann. Rev. Mar. Sci. 2021, 13, 313–342. [Google Scholar] [CrossRef]
- Rosselló, P.; Pascual, A.; Combes, V. Assessing marine heat waves in the Mediterranean Sea: A comparison of fixed and moving baseline methods. Front. Mar. Sci. 2023, 10, 1168368. [Google Scholar] [CrossRef]
- Oliver, E.C.; Donat, M.G.; Burrows, M.T.; Moore, P.J.; Smale, D.A.; Alexander, L.V.; Benthuysen, J.A.; Feng, M.; Sen Gupta, A.; Hobday, A.J.; et al. Longer and more frequent marine heatwaves over the past century. Nat. Comm. 2018, 9, 1324. [Google Scholar] [CrossRef] [PubMed]
- Darmaraki, S.; Somot, S.; Sevault, F.; Nabat, P. Past variability of Mediterranean Sea marine heatwaves. Geophys. Res. Lett. 2019, 46, 9813–9823. [Google Scholar] [CrossRef]
- Darmaraki, S.; Somot, S.; Sevault, F.; Nabat, P.; Cabos-Narvaez, W.D.; Cavicchia, L.; Djurdjevic, V.; Li, L.; Sannino, G.; Sein, D.V. Future evolution of marine heatwaves in the Mediterranean Sea. Clim. Dynam. 2019, 53, 1371–1392. [Google Scholar] [CrossRef]
- Androulidakis, Y.S.; Krestenitis, Y.N. Sea surface temperature variability and marine heat waves over the Aegean, Ionian, and Cretan Seas from 2008–2021. J. Mar. Sci. Engin. 2022, 10, 42. [Google Scholar] [CrossRef]
- Pastor, F.; Khodayar, S. Marine heat waves: Characterizing a major climate impact in the Mediterranean. Sci. Tot. Environ. 2023, 861, 16621. [Google Scholar] [CrossRef] [PubMed]
- Juza, M.; Fernández-Mora, À.; Tintoré, J. Sub-regional marine heat waves in the Mediterranean Sea from observations: Long-term surface changes, sub-surface and coastal responses. Front. Mar. Sci. 2022, 9, 785771. [Google Scholar] [CrossRef]
- Smale, D.A.; Wernberg, T.; Vanderklift, M.A. Regional-scale variability in the response of benthic macroinvertebrate assemblages to a marine heatwave. Mar. Ecol. Prog. Ser. 2017, 568, 17–30. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Burrows, M.T.; Donat, M.G.; Sen Gupta, A.; Alexander, L.V.; Perkins-Kirkpatrick, S.E.; Benthuysen, J.A.; Hobday, A.J.; Holbrook, N.J.; Moore, P.J.; et al. Projected marine heatwaves in the 21st century and the potential for ecological impact. Front. Mar. Sci. 2019, 6, 734. [Google Scholar] [CrossRef]
- Santora, J.A.; Mantua, N.J.; Schroeder, I.D.; Field, J.C.; Hazen, E.L.; Bograd, S.J.; Sydeman, W.J.; Wells, B.K.; Calambokidis, J.; Saez, L.; et al. Habitat compression and ecosystem shifts as potential links between marine heatwave and record whale entanglements. Nat. Commun. 2020, 11, 536. [Google Scholar] [CrossRef]
- Suryan, R.M.; Arimitsu, M.L.; Coletti, H.A.; Hopcroft, R.R.; Lindeberg, M.R.; Barbeaux, S.J.; Batten, S.D.; Burt, W.J.; Bishop, M.A.; Bodkin, J.L.; et al. Ecosystem response persists after a prolonged marine heatwave. Sci. Rep. 2021, 11, 6235. [Google Scholar] [CrossRef]
- Bernal-Ibàñez, A.; Gestoso, I.; Ramalhosa, P.; Campanati, C.; Cacabelos, E. Interaction of marine heatwaves and grazing on two canopy-forming algae. J. Exp. Mar. Biol. Ecol. 2022, 556, 151795. [Google Scholar] [CrossRef]
- Smith, K.E.; Burrows, M.T.; Hobday, A.J.; King, N.G.; Moore, P.J.; Sen Gupta, A.; Thompsen, M.S.; Wernberg, T.; Smale, D.A. Biological impacts of marine heatwaves. Ann. Rev. Mar. Sci. 2023, 15, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.J.; Smith, R.O.; Micaroni, V.; Strano, F.; Balemi, C.A.; Caiger, P.E.; Shears, N.T. Marine heat waves drive bleaching and necrosis of temperate sponges. Curr. Biol. 2023, 33, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Dayan, H.; McAdam, R.; Juza, M.; Masina, S.; Speich, S. Marine heat waves in the Mediterranean Sea: An assessment from the surface to the subsurface to meet national needs. Front. Mar. Sci. 2023, 10, 1045138. [Google Scholar] [CrossRef]
- Grenier, M.; Idan, T.; Chevaldonné, P.; Perez, T. Mediterranean marine keystone species on the brink of extinction. Glob. Chang. Biol. 2023, 29, 1681–1683. [Google Scholar]
- Francour, P.; Boudouresque, C.F.; Harmelin, J.G.; Harmelin-Vivien, M.L.; Quignard, J.P. Are the Mediterranean waters becoming warmer? Information from biological indicators. Mar. Pollut. Bull. 1994, 28, 523–526. [Google Scholar] [CrossRef]
- Vacchi, M.; Morri, C.; Modena, M.; La Mesa, G.; Bianchi, C.N. Temperature changes and warm-water species in the Ligurian Sea: The case of the ornate wrasse Thalassoma pavo (Linnaeus, 1758). Archo Oceanogr. Limnol. 2001, 22, 149–154. [Google Scholar]
- Astruch, P.; Bonhomme, P.; Goujard, A.; Rouanet, E.; Boudouresque, C.F.; Harmelin, J.; Harmelin-Vivien, M. Provence and Mediterranean warming: The parrotfish Sparisoma cretense is coming. Rapp. Comm. Int. Mer Médit. 2016, 41, 362. [Google Scholar]
- Bianchi, C.N.; Caroli, F.; Guidetti, P.; Morri, C. Seawater warming at the northern reach for southern species: Gulf of Genoa, NW Mediterranean. J. Mar. Biol. Assoc. UK 2018, 98, 1–12. [Google Scholar] [CrossRef]
- Astruch, P.; Belloni, B.; Rouanet, E.; Schohn, T.; Harmelin-Vivien, M.; Harmelin, J.G.; Boudouresque, C.F. Dominance of Scorpaena maderensis among scorpaenids of littoral rocky reefs in Corsica (NW Mediterranean): Further evidence of Mediterranean Sea warming. J. Fish Biol. 2022, 100, 601–604. [Google Scholar] [CrossRef]
- Acunto, S.; Maltagliati, F.; Rindi, F.; Rossi, F.; Cinelli, F. Osservazioni su una prateria di Halophila stipulacea (Forssk.) Aschers. (Hydrocharitaceae) nel Mar Tirreno meridionale. Atti Soc. Tosc. Sci. Nat. Mem. Ser. B 1995, 102, 19–22. [Google Scholar]
- Gambi, M.C.; Barbieri, F.; Bianchi, C.N. New record of the alien seagrass Halophila stipulacea (Hydrocharitaceae) in the western Mediterranean: A further clue to changing Mediterranean Sea biogeography. Mar. Biodiv. Rec. 2009, 2, e84. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Holon, F.; Agel, N.; Descamps, P.; Deter, J.; Pavy, T.; Delaruelle, G.; Verlaque, M. Distribution of the seagrass Halophila stipulacea: A big jump to the northwestern Mediterranean Sea. Aquat. Bot. 2022, 176, 103465. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Perret-Boudouresque, M.; Ruitton, S.; Thibault, D. The invasive thermophilic red alga Lophocladia lallemandii reaches the Port-Cros National Park (northwestern Mediterranean). Sci. Rep. Port-Cros Natl. Park 2022, 36, 59–66. [Google Scholar]
- Golo, R.; Vergés, A.; Díaz-Tapia, P.; Cebrian, E. Implications of taxonomic misidentification for future invasion predictions: Evidence from one of the most harmful invasive marine algae. Mar. Pollut. Bull. 2023, 191, 114970. [Google Scholar] [CrossRef] [PubMed]
- Ben Lamine, E.; Schikele, A.; Goberville, E.; Beaugrand, G.; Allemand, D.; Raybaud, V. Expected contraction in the distribution ranges of demersal fish of high economic value in the Mediterranean and European seas. Sci. Rep. 2022, 12, 10150. [Google Scholar] [CrossRef] [PubMed]
- Boudouresque, C.F.; Bernard, G.; Pergent, G.; Shili, A.; Verlaque, M. Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: A critical review. Bot. Mar. 2009, 52, 395–418. [Google Scholar] [CrossRef]
- Pergent, P.; Bazairi, H.; Bianchi, C.N.; Boudouresque, C.F.; Buia, M.C.; Calvo, S.; Clabaut, P.; Harmelin-Vivien, M.; Mateo, M.A.; Montefalcone, M.; et al. Climate change and Mediterranean seagrass meadows: A synopsis for environmental managers. Medit. Mar. Sci. 2014, 15, 462–473. [Google Scholar] [CrossRef]
- Mačić, V. Distribution of seaweed Fucus virsoides J. Agardh in Boka Kotorska Bay (South Adriatic Sea). Ann. Ser. Hist. Nat. 2006, 16, 1–4. [Google Scholar]
- Falace, A.; Alongi, G.; Cormaci, M.; Furnari, G.; Curiel, D.; Cecere, E.; Petrocelli, A. Change in the benthic algae along the Adriatic Sea in the last three decades. Chem. Ecol. 2010, 26, 77–90. [Google Scholar] [CrossRef]
- Blanfuné, A.; Boudouresque, C.F.; Verlaque, M.; Beqiraj, S.; Kashta, L.; Nasto, I.; Ruci, S.; Thibaut, T. Response of rocky shore communities to anthropogenic pressures in Albania (Mediterranean Sea): Ecological status assessment through the CARLIT Method. Mar. Pollut. Bull. 2016, 109, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Boudouresque, C.F.; Perret-Boudouresque, M. Qualité de l’eau de mer, de l’environnement marin et de la biodiversité: Fausses pistes et vrais enjeux. In L’eau Dans Tous Ses États; Piel, G., Ed.; Éditions Émile Communication: Marseille, France, 2022; pp. 81–135. [Google Scholar]
- Canessa, M.; Betti, F.; Bo, M.; Enrichetti, F.; Toma, M.; Bavestrello, G. Possible population growth of Astrospartus mediterraneus (Risso, 1826) (Ophiuroidea, Gorgonocephalidae) in the Mediterranean Sea. Diversity 2023, 15, 122. [Google Scholar] [CrossRef]
- Gómez-Gras, D.; Linares, C.; López-Sanz, À.; Arnate, R.; Ledoux, J.B.; Bensoussan, N.; Bianchimani, O.; Marschal, C.; Torrents, O.; Zuberer, F.; et al. Population collapse of habitat-forming octocorals in Scandola Marine Protected Area: The long-term gorgonian populations affected by the 2003 extreme warm summer. In Proceedings of the Workshop on Marine Reserves (REMAR 2020), Barcelona, Spain, 1–3 July 2020; pp. 24–25. [Google Scholar]
- Gómez-Gras, D.; Linares, C.; Dornelas, M.; Madin, J.S.; Bramvilla, V.; Ledoux, J.B.; López-Sendino, P.; Bensoussan, N.; Garrabou, J. Climate change transforms the functional identity of Mediterranean coralligenous assemblages. Ecol. Lett. 2021, 24, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.; Oprandi, A.; Bianchi, C.N.; Morri, C.; Azzola, A.; Montefalcone, M. Unexpected slow recovery of seagrass leaf epiphytes after the impact of a summer heat wave and concomitant mucilage bloom. Mar. Environ. Res. 2023, 189, 106034. [Google Scholar] [CrossRef] [PubMed]
- Guinaldo, T.; Voldoire, A.; Waldman, R.; Saux Picart, S.; Roquet, H. Response of the sea surface temperature to heatwaves during the France 2022 meteorological summer. Ocean Sci. 2023, 19, 629–647. [Google Scholar] [CrossRef]
- Marullo, S.; Serva, F.; Iacono, R.; Napolitano, E.; di Sarra, A.; Meloni, D.; Monteleone, F.; Sferlazzo, D.; De Silvestri, L.; de Toma, V.; et al. Record-breaking persistence of the 2022/23 marine heatwave in the Mediterranean Sea. Environ. Res. Lett. 2023, 18, 114041. [Google Scholar] [CrossRef]
- CMCC. Available online: www.cmcc.it/marine-heat-wave-in-the-mediterranean (accessed on 25 November 2023).
- Odic, R.; Bensoussan, N.; Pinazo, C.; Taupier-Letage, I.; Rossi, V. Sporadic wind-driven upwelling/downwelling and associated cooling/warming along Northwestern Mediterranean coastlines. Cont. Shelf Res. 2022, 250, 104843. [Google Scholar] [CrossRef]
- T-Mednet Project. Marine Heatwave Tracker at 4 km in Near Real Time (Mediterranean). Available online: https://t-mednet.org/visualize-data/marine-heatwaves (accessed on 18 November 2023).
- Taylor, W.R. Marine Algae of the Eastern Tropical and Subtropical Coasts of the Americas; The University of Michigan Press: Ann Arbor, MI, USA, 1960. [Google Scholar]
- Huvé, P.; Huvé, H. À propos de Penicillus capitatus Lamarck forma mediterranea (Decaisne) comb. nov. (Caulerpale, Udotéacée). In Proceedings of the 4th International Seaweed Symposium, Biarritz, France, 18–25 September 1961; Pergamon Press: Oxford, UK, 1963; pp. 99–111. [Google Scholar]
- Meinesz, A. Sur la croissance et le développement du Penicillus capitatus Lamarck forma mediterranea (Decaisne) P. et H. Huvé (Caulerpale, Udotéacée). Comptes Rendus L’Académie Sci. 1972, 275, 667–669. [Google Scholar]
- Meinesz, A. Contribution à l’Étude des Caulerpales (Chlorophytes). Avec Une Mention Particulière aux Espèces de la Méditerranée Occidentale. Ph.D. Thesis, Nice University, Nice, France, 1980. [Google Scholar]
- Cormaci, M.; Furnari, G.; Alongi, G. Flora marina bentonica del Mediterraneo: Chlorophyta. Boll. Accad. Gioenia Sci. Nat. Catania 2014, 47, 11–436. [Google Scholar]
- Meinesz, A. Premières observations sur la reproduction du Penicillus capitatus Lamarck forma mediterranea (Decaine) P. et H. Huvé (Caulerpale, Udotéacée). Ann. Mus. Hist. Nat. Nice 1975, 3, 19–20. [Google Scholar]
- Meinesz, A. Connaissances actuelles et contribution à l’étude de la reproduction et du cycle des Udotéacées (Caulerpales, Chlorophytes). Phycologia 1980, 19, 110–138. [Google Scholar] [CrossRef]
- Olivier, G. Étude de la flore marine de la Côte d’Azur. Ann. Inst. Océanogr. 1929, 7, 54–173. [Google Scholar]
- Cinelli, F. Biologia delle secche della Meloria (Mar Tirreno). IV. Contributo alla conoscenza della vegetazione bentonica marina. Boll. Pesca Piscic. Idrobiol 1971, 26, 5–20. [Google Scholar]
- Cinelli, F.; Salghetti-Drioli, U. Observations en plongée sur les peuplements à Penicillus capitatus et sur la floraison de Posidonia oceanica de l’île d’Elbe (Méditerranée occidentale). Rapp. Comm. Int. Mer Médit. 1983, 28, 169–170. [Google Scholar]
- Ben Maiz, N.; Boudouresque, C.F.; Ouachi, F. Inventaire des algues et phanérogames marines benthiques de la Tunisie. G. Bot. Ital. 1987, 121, 259–304. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Perret-Boudouresque, M. A Checklist of the Benthic Marine Algae of Corsica; GIS Posidonie Publisher: Marseille, France, 1987. [Google Scholar]
- Boudouresque, C.F.; Ballesteros, E.; Ben Maiz, N.; Boisset, F.; Bouladier, E.; Cinelli, F.; Cirik, S.; Cormaci, M.; Jeudy de Grissac, A.; Laborel, J.; et al. Livre Rouge “Gérard Vuignier” des Végétaux, Peuplements et Paysages Marins Menacés de Méditerranée; IUCN and Programme des Nations Unies pour l’Environnement (UNEP): Athens, Greece, 1990. [Google Scholar]
- Cerrato, M.D.; Mir-Rosselló, P.M.; Ferriol, P.; Gil, L.; Montserrat-Mesquida, M.; Tejada, S.; Pinya, S.; Sureda, A. Oxidative stress response in the seaweed Padina pavonica assiociated to the invasive Halimeda incrassata and Penicillus capitatus. Water 2023, 15, 557. [Google Scholar] [CrossRef]
- Friedmann, E.I.; Roth, W.C. Development of the siphonous green alga Penicillus and the Espera state. Bot. J. Linn. Soc. 1977, 74, 189–214. [Google Scholar] [CrossRef]
- Gallardo, T.; Gomez-Garreta, A.; Ribera, M.A.; Cormaci, M.; Furnari, G.; Giaccone, G.; Boudouresque, C.F. Check-list of Mediterranean seaweeds. II. Chlorophyceae Wille s.l. Bot. Mar. 1993, 36, 399–421. [Google Scholar] [CrossRef]
- Rodríguez-Prieto, C.; Ballesteros, E.; Boisset, F.; Afonso-Carrillo, J. Guía de las Macroalgas y Fanerógamas Marinas del Mediterráneo Occidental; Ediciones Omega: Barcelona, Spain, 2013. [Google Scholar]
- Womersley, H.B.S. The Marine Benthic Flora of Southern Australia. Part I; Woolman D.J. Publishing: Adelaide, Australia, 1984. [Google Scholar]
- Silva, P.C.; Basson, P.W.; Moe, R.L. Catalogue of the Benthic Marine Algae of the Indian Ocean; University of California Press: Berkeley, CA, USA, 1996. [Google Scholar]
- Alves, A.M.; de Souza Gestinari, L.M.; Moura, C.W.d.N. Microdictyon (Chlorophyta, Anadyomenaceae) di Estado de Bahia, Brazil. Sitientibus Ser. Cienc. Biol. 2011, 11, 57–61. [Google Scholar] [CrossRef]
- Einav, R.; Guiry, M.D.; Israel, A. A revised list of seaweeds from the Red Sea (1756–2020). Isr. J. Plant Sci. 2021, 68, 175–247. [Google Scholar] [CrossRef]
- Coppejans, E. Végétation marine de la Corse (Méditerranée). III. Documents pour la flore des algues. Bot. Mar. 1979, 22, 257–266. [Google Scholar] [CrossRef]
- Hamel, G. Chlorophycées des côtes de France (fin). Rev. Algol. 1931, 6, 9–73. [Google Scholar]
- Boudouresque, C.F.; Perret-Boudouresque, M.; Blanfuné, A. Diversity of marine and brackish macrophytes in the Port-Cros National Park (Provence, France, Mediterranean Sea): Taxa and research effort over space and time. Diversity 2022, 14, 329. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Does the seagrass Posidonia really occur in Madagascar? Phycologia 2008, 47, 435–436. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Bernard, G.; Bonhomme, P.; Charbonnel, E.; Diviacco, G.; Meinesz, A.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Tunesi, L. Protection and Conservation of Posidonia oceanica Meadows; RAMOGE and RAC/SPA Publishers: Tunis, Tunisia, 2012. [Google Scholar]
- Pergent, G.; Bazairi, H.; Bianchi, C.N.; Boudouresque, C.F.; Buia, M.C.; Clabaut, P.; Harmelin-Vivien, M.; Mateo, M.A.; Montefalcone, M.; Morri, C.; et al. Mediterranean Seagrass Meadows: Resilience and Contribution to Climate Change Mitigation. A Short Summary; IUCN: Gland, Swizerland; Málaga, Spain, 2012. [Google Scholar]
- Giraud, G. Recensement des floraisons de Posidonia oceanica (L.) Delile en Méditerranée. Rapp. Comm. Int. Mer Médit. 1977, 24, 126–130. [Google Scholar]
- Pergent, G.; Ben Maiz, N.; Boudouresque, C.F.; Meinesz, A. The flowering of Posidonia oceanica over the past fifty years: A lepidochronological study. In International Workshop on Posidonia Beds; Boudouresque, C.F., Meinesz, A., Fresi, E., Gravez, V., Eds.; GIS Posidonie: Marseille, France, 1989; Volume 2, pp. 69–76. [Google Scholar]
- Molinier, R.; Picard, J. Recherches sur les Herbiers de Phanérogames Marines du Littoral Méditerranéen Français. Ann. Inst. Océanogr. 1952, 27, 157–234. [Google Scholar]
- Diaz-Almela, E.; Marbà, N.; Duarte, C.M. Consequences of Mediterranean warming events in seagrass (Posidonia oceanica) flowering records. Glob. Chang. Biol. 2007, 13, 224–235. [Google Scholar] [CrossRef]
- Giraud, G. Floraison de Posidonia oceanica à Port-Cros. Trav. Sci. Parc Natl. Port-Cros 1976, 2, 191–193. [Google Scholar]
- Piazzi, L.; Balestri, E. Observations on flowering and fruiting of Posidonia oceanica (L.) Delile transplants. Biol. Mar. Medit. 1997, 4, 429–430. (In Italian) [Google Scholar]
- Sandmeier, M.; Caye, G.; Molenaar, H. Seed enzyme polymorphism and autogamy of the seagrass Posidonia oceanica from the western Mediterranean. Bot. Mar. 1999, 42, 359–366. [Google Scholar] [CrossRef]
- Balestri, E.; Cinelli, F. Sexual reproductive success in Posidonia oceanica. Aquat. Bot. 2003, 75, 21–32. [Google Scholar] [CrossRef]
- Balestri, E.; Vallerini, F. Interannual variability in flowering of Posidonia oceanica in the north-western Mediterranean Sea, and relationships among shoot age and flowering. Bot. Mar. 2003, 46, 525–530. [Google Scholar] [CrossRef]
- Diaz Almela, E.; Marbà, N.; Álvarez, E.; Balestri, E.; Ruiz-Fernández, J.M.; Duarte, C.M. Patterns of seagrass (Posidonia oceanica) flowering in the Western Mediterranean. Mar. Biol. 2006, 148, 723–742. [Google Scholar] [CrossRef]
- Ferrari, B. Étude Synécologique de Posidonia Oceanica et de Sarpa Salpa le Long de la Côte Rocheuse des Albères (Pyrénées-Orientales, France); Influence d’une Aire Marine Protégée. Ph.D. Thesis, Université de Perpignan, Perpignan, France, 2006. [Google Scholar]
- Calvo, S.; Tomasello, A.; Di Maida, G.; Pirrotta, M.; Buia, M.C.; Cinelli, F.; Cormaci, M.; Furnari, G.; Giaccone, G.; Luzzu, F.; et al. Seagrasses along the Sicilian coasts. Chem. Ecol. 2010, 26, 249–266. [Google Scholar] [CrossRef]
- Montefalcone, M.; Giovannetti, E.; Morri, C.; Peirano, A.; Bianchi, C.N. Flowering of the seagrass Posidonia oceanica in the NW Mediterranean: Is there a link with solar activity? Medit. Mar. Sci. 2013, 14, 416–423. [Google Scholar] [CrossRef]
- Sghaier, Y.R.; Zakhama-Sraieb, R.; Charfi-Cheikhrouha, F. Patterns of shallow seagrass (Posidonia oceanica) growth and flowering along the Tunisian coast. Aquat. Bot. 2013, 104, 183–192. [Google Scholar] [CrossRef]
- Urra, J.; Mako, Á.; Marina, P.; Rueda, J.L.; García Raso, J.E. First record of Posidonia oceanica flowering at its westernmost distributional limit (Málaga, Alboran Sea). Bot. Mar. 2011, 54, 101–104. [Google Scholar] [CrossRef]
- Di Martino, V. Eccezionale fruttificazione nel Posidonieto dell’isola di Capo Passero di Portopalo (SR) (Sicilia, S.E.). Biol. Mar. Medit. 1999, 6, 383–384. [Google Scholar]
- Calvo, S.; Lovison, G.; Pirrotta, M.; Di Maida, G.; Tomasello, A.; Sciandra, M. Modelling the relationship between sexual reproduction and rhizome growth in Posidonia oceanica (L.) Delile. Mar. Ecol. 2006, 27, 361–371. [Google Scholar] [CrossRef]
- Caye, G.; Meinesz, A. Observations sur la floraison et la fructification de Posidonia oceanica dans la baie de Villefranche et en Corse du Sud. In International Workshop on Posidonia Oceanica Beds; Boudouresque, C.F., Jeudy de Grissac, A., Olivier, J., Eds.; GIS Posidonie: Marseille, France, 1984; pp. 193–201. [Google Scholar]
- Ferrari, B.; Raventos, N.; Planes, S. Assessing effects of fishing prohibition on Posidonia oceanica seagrass meadows in the Marine Natural Reserve of Cerbère-Banyuls. Aquat. Bot. 2008, 88, 295–302. [Google Scholar] [CrossRef]
- Taviani, M. The Mediterranean benthos from late Miocene up to present: Ten million years of dramatic climatic and geologic vicissitudes. Biol. Mar. Medit. 2002, 9, 445–463. [Google Scholar]
- Boudouresque, C.F. Marine biodiversity in the Mediterranean: Status of species, populations and communities. Sci. Rep. Port-Cros Natl. Park 2004, 20, 97–146. [Google Scholar]
- Bianchi, C.N.; Parravicini, V.; Montefalcone, M.; Rovere, A.; Morri, C. The challenge of managing marine biodiversity: A practical toolkit for a cartographic, territorial approach. Diversity 2012, 4, 419–452. [Google Scholar] [CrossRef]
- Moissette, P.; Koskeridou, E.; Cornée, J.J.; Guillocheau, F.; Lécuyer, C. Spectacular preservation of seagrasses and seagrass-associated communities from the Pliocene of Rhodes, Greece. Palaios 2007, 22, 200–211. [Google Scholar] [CrossRef]
- Koskeridou, E.; Thivaiou, D.; Giamali, C.; Agiadi, K.; Mantzouka, D. Seagrass associated molluscan and fish communities from the early Pleistocene of the Island of Rhodes (Greece). IOP Conf. Ser. Earth Environ. Sci. 2019, 221, 012050. [Google Scholar] [CrossRef]
- Boyer, M.; Bussotti, S.; Guidetti, P.; Matricardi, G. Notes on the flowering and fruiting of Posidonia oceanica (L.) Delile beds in the Ligurian Sea (North-Western Mediterranean). Boll. Mus. Ist. Biol. Univ. Genova 1996, 60–61, 21–29. [Google Scholar]
- Janzen, D.H. Seed predation by animals. Annu. Rev. Ecol. Syst. 1971, 2, 465–492. [Google Scholar] [CrossRef]
- Karban, R. Increased reproductive success at high densities and predator satiation for periodical cicadas. Ecology 1982, 63, 321–328. [Google Scholar] [CrossRef]
- Curran, L.M.; Leighton, M. Vertebrate responses to spatiotemporal variation in seed production of mast-fruiting Dipterocarpaceae. Ecol. Monogr. 2000, 70, 101–128. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Keesing, F. Oh the locusts sang, then they dropped dead. Science 2004, 306, 1488–1489. [Google Scholar] [CrossRef][Green Version]
- Patten, N.L.; Mitchell, J.G.; Middelboe, M.; Eyre, B.D.; Seuront, L.; Harrison, P.L.; Glud, R.N. Bacterial and viral dynamics during a mass coral spawning period on the Great Barrier Reef. Aquat. Microb. Ecol. 2008, 30, 209–220. [Google Scholar] [CrossRef]
- Migliaccio, M.; De Martino, F.; Silvestre, F.; Procaccini, G. Meadow-scale genetic structure in Posidonia oceanica. Mar. Ecol. Prog. Ser. 2005, 304, 55–65. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Paracentrotus lividus. In Sea Urchins: Biology and Ecology, 4th ed.; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 447–485. [Google Scholar]
- Piazzi, L.; Balestri, E.; Cinelli, E. Grazing of inflorescences of the seagrass Posidonia oceanica (L.) Delile. Bot. Mar. 2000, 43, 561–584. [Google Scholar] [CrossRef]
- Vergés, A.; Becerro, M.A.; Alcoverro, T.; Romero, J. Variation in multiple traits of vegetative and reproductive seagrass tissues influences plant-herbivore interactions. Oecologia 2007, 151, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lizaso, J.L. Las praderas de Fanerógamas marinas. Reproducción sexual: Floración, fructificación y germinación. Estructura genética de las praderas. In Praderas y Bosques Marinos de Andalucía; Luque, A.A., Templado, J., Eds.; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2004; pp. 67–69. [Google Scholar]
- Pergent-Martini, C. Impact d’un Rejet d’Eaux Usées Urbaines sur l’Herbier à Posidonia oceanica, Avant et Après la Mise en Service d’une Station d’Épuration. Ph.D. Thesis, University of Corsica, Corte, France, 1994. [Google Scholar]
- Pergent, G.; Pergent-Martini, C.; Boudouresque, C.F. Utilisation de l’herbier à Posidonia oceanica comme indicateur biologique de la qualité du milieu littoral en Méditerranée: État des connaissances. Mésogée 1995, 54, 3–27. [Google Scholar]
- Bacci, T.; Rende, F.S.; Scardi, M. Shoot micro-distribution patterns in the Mediterranean seagrass Posidonia oceanica. Mar. Biol. 2017, 164, 85. [Google Scholar] [CrossRef]
- André, S.; Astruch, P.; Boudouresque, C.F.; Belloni, B.; Bardinal, V.; Boada, J.; Charbonnel, É.; Chéré, É.; Cottalorda, J.M.; Estaque, T.; et al. The 2022 mass flowering of Posidonia oceanica in the French Mediterranean Sea: Is it unprecedented? Sci. Rep. Port-Cros Natl. Park 2023, 37, 65–100. [Google Scholar]
- Pergent, G.; Pergent-Martini, C. Phénologie de Posidonia oceanica (Linnaeus) Delile dans le bassin Méditerranéen. Ann. Inst. Océanogr. 1988, 64, 79–100. [Google Scholar]
- Romero, J.; Pérez, M.; Alcoverro, T. L’alguer de Posidonia oceanica de les illes Medes: Més de trenta anys d’estudi. In El Fons Marí de les Illes Medes i el Mongrí. Quatre Dècades de Recerca per a la Conservació; Hereu, B., Quintana, X., Eds.; Recerca i Territori: Volume 4; Càtedra d’Ecosistemes Litorals Mediterranis: L’Estartit, Spain, 2012; pp. 79–100. [Google Scholar]
- Dural, B. Phenological observations on Posidonia oceanica (L.) Delile meadows along the coast of Akkum (Sıgacık Bay, Aegean Sea, Turkey). J. Black Sea/Medit. Environ. 2010, 16, 133–144. [Google Scholar]
- Moreno, D.; Guirado, J. Nuevos datos sobre la floración, fructificación y germinación de fanerógamas marinas en Andalucía. Acta Bot. Malacit. 2006, 31, 51–72. [Google Scholar] [CrossRef][Green Version]
- Muñoz-Ramos, G. L’alguer de Maraeó, cinc anys d’estudi (1997–2001). L’Atzavara 2002, 10, 23–28. [Google Scholar]
- Balestri, E.; Vallerini, F.; Lardicci, C. On the unusual flowering of plagiotropic shoots in the seagrass Posidonia oceanica. Aquat. Bot. 2005, 82, 82–88. [Google Scholar] [CrossRef]
- Augier, H.; Robert, P.; Maffre, R. Étude du régime thermique annuel des eaux au niveau des peuplements de phanérogames marines de la baie de Port-Cros (îles d’Hyères, Méditerranée, France). Trav. Sci. Parc Natl. Port-Cros 1980, 6, 69–131. [Google Scholar]
- Cantasano, N. The effects of climate changes on Posidonia oceanica meadows in the Mediterranean Basin. Nat. Res. Conserv. Res. 2023, 6, 1961. [Google Scholar] [CrossRef]
- Vela, A. Fonctionnement et Production Primaire des Herbiers à Posidonia oceanica (L.) Delile en Méditerranée. Ph.D. Thesis, University of Corsica, Corte, France, 2006. [Google Scholar]
- Tomasello, A.; Di Maida, G.; Calvo, S.; Pirrota, M.; Borra, M.; Procaccini, G. Seagrass meadows at the extreme of environmental tolerance: The case of Posidonia oceanica in a semi-enclosed coastal lagoon. Mar. Ecol. 2009, 30, 288–300. [Google Scholar] [CrossRef]
- Mateo, M.A.; Renom, P.; Michener, R.H. Long-term stability in the production of a NW Mediterranean Posidonia oceanica (L.) Delile meadow. Palaeogeogr. Palaeoclim. Palaeoecol. 2010, 291, 286–296. [Google Scholar] [CrossRef]
- Bennett, S.; Alcoverro, T.; Kletou, D.; Antoniou, C.; Boada, J.; Buñuel, X.; Cucala, L.; Jorda, G.; Kleitou, P.; Roca, G.; et al. Resilience of seagrass populations to thermal stress does not reflect regional differences in ocean climate. New Phytol. 2022, 233, 1657–1666. [Google Scholar] [CrossRef]
- Rinaldi, A.; Martinez, M.; Badalamenti, F.; D’anna, G.; Mirto, S.; Marín-Guirao, L.; Procaccini, G.; Montalto, V. The ontogeny-specific thermal sensitivity of the seagrass Posidonia oceanica. Front. Mar. Sci. 2023, 10, 1183728. [Google Scholar] [CrossRef]
- Llabrés, E.; Blanco-Magadán, A.; Sales, M.; Sintes, T. Effect of global warming on Western Mediterranean seagrasses: A preliminary agent-based modelling approach. Mar. Ecol. Prog. Ser. 2023, 710, 43–56. [Google Scholar] [CrossRef]
- Beca-Carretero, P.B.; Winters, G.; Teichberg, M.; Procaccini, G.; Schneekloth, F.; Zambrano, R.H.; Chiquillo, K.; Reuter, H. Climate change and the presence of invasive species will threaten the persistence of the Mediterranean seagrass community. Sci. Total Environ. 2024, 910, 168675. [Google Scholar] [CrossRef]
- Sala, E.; Kizilkaya, Z.; Yildirim, D.; Ballesteros, E. Alien marine fishes deplete algal biomass in the Eastern Mediterranean. PLoS ONE 2011, 6, e17356. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.N.; Corsini-Foka, M.; Morri, C.; Zenetos, A. Thirty years after: Dramatic change in the coastal marine ecosystems of Kos Island (Greece), 1981–2013. Medit. Mar. Sci. 2014, 15, 482–497. [Google Scholar] [CrossRef]
- Vergés, A.; Tomas, F.; Cebrian, E.; Ballesteros, E.; Kizilkaya, Z.; Dendrinos, P.; Karamanlidis, A.A.; Spiegel, D.; Sala, E. Tropical rabbitfish and the deforestation of a warming temperate sea. J. Ecol. 2014, 102, 1518–1527. [Google Scholar] [CrossRef]
- Bramanti, L.; Manea, E.; Giordano, B.; Estaque, T.; Bianchimani, O.; Richaume, J.; Mérigot, B.; Schull, Q.; Sartoretto, S.; Garrabou, J. The deep vault: A temporary refuge for temperate gorgonian forests facing marine heat waves. Medit. Mar. Sci. 2023, 24, 601–609. [Google Scholar] [CrossRef]
- Piazzi, L.; Kaleb, S.; Ceccherelli, G.; Montefalcone, M.; Falace, A. Deep coralligenous outcrops of the Apulian continental shelf: Biodiversity and spatial variability of sediment-regulated assemblages. Cont. Shelf Res. 2019, 172, 50–56. [Google Scholar] [CrossRef]
- Sartoretto, S.; Ledoux, J.B.; Gueret, E.; Guillemain, D.; Ravel, R.; Moirand, L.; Aurelle, D. Ecological and genomic characterization of a remarkable natural heritage: A mesophotic ‘giant’ Paramuricea clavata forest. Mar. Ecol. Prog. Ser. 2023, hal-04342474. [Google Scholar] [CrossRef]
- Varzi, A.G.; Fallati, L.; Savini, A.; Bracchi, V.A.; Bazzicalupo, P.; Rosso, A.; Sanfilippo, R.; Bertolino, M.; Mazzupappa, M.; Basso, D. Geomorphology of coralligenous reefs offshore southeastern Sicily (Ionian Sea). J. Maps 2023, 19, 2161963. [Google Scholar] [CrossRef]
- Cupido, R.; Cocito, S.; Barsanti, M.; Sgorbini, S.; Peirano, A.; Santangelo, G. Unexpected long-term population dynamics in a canopy-forming gorgonian coral following mass mortality. Mar. Ecol. Prog. Ser. 2009, 394, 195–200. [Google Scholar] [CrossRef]
- Santangelo, G.; Cupido, R.; Cocito, S.; Bramanti, L.; Priori, C.; Erra, F.; Iannelli, M. Effects of increased mortality on gorgonian corals (Cnidaria, Octocorallia): Different demographic features may lead affected populations to unexpected recovery and new equilibrium points. Hydrobiologia 2015, 759, 171–187. [Google Scholar] [CrossRef]
- Aurelle, D.; Tariel, J.; Zuberer, E.; Haguenauer, A.; Ribout, C.; Masmoudi, M.; Kara, H.; Chaoui, L.; Garrabou, J.; Ledoux, J.B.; et al. Genetic insights into the recolonization processes of Mediterranean octocorals. Mar. Biol. 2020, 167, 73. [Google Scholar] [CrossRef]
- Cau, A.; Bramanti, L.; Cannas, R.; Moccia, D.; Padedda, B.; Porcu, C.; Sacco, F.; Follesa, M.C. Differential responses to thermal stress of shallow and deep dwelling colonies of Mediterranean red coral Corallium rubrum (L., 1758). Adv. Oceanogr. Limnol. 2018, 9, 13–18. [Google Scholar] [CrossRef]
- Pratlong, M.; Haguenauer, A.; Brener, K.; Litta, G.; Toulza, E.; Garrabou, J.; Bensoussan, N.; Pontarotti, P.; Aurelle, D. Separate the wheat from the chaff: Genomic scan for local adaptation in the red coral Corallium rubrum. Peer Comm. J. 2021, 1, e31. [Google Scholar] [CrossRef]
- Ramirez-Calero, S.; Bensoussan, N.; López-Sendino, P.; Gómez-Gras, D.; Montero-Serra, I.; Pagès-Escolà, M.; Medrano, A.; López-Sanz, A.; Figuerola, L.; Linares, C.; et al. Temporal variability in the response to thermal stress in the red gorgonian, P. clavata: Insights from common garden experiments. In Proceedings of the 4th Mediterranean Symposium on the Conservation of Coralligenous and Other Calcareous Bio-Concretions, Genoa, Italy, 20–21 September 2022; RAC/SPA: Tunis, Tunisia, 2022; pp. 102–107. [Google Scholar]
- Pilczynska, J.; Cocito, S.; Boavida, J.; Serrão, E.; Queiroga, H. Genetic diversity and local connectivity in the Mediterranean red gorgonian coral after mass mortality events. PLoS ONE 2016, 11, e0150590. [Google Scholar] [CrossRef] [PubMed]
- Padrón, M.; Costantini, F.; Bramanti, L.; Guizien, K.; Abbiati, M. Genetic connectivity supports recovery of gorgonian populations affected by climate change. Aquat. Cons. Mar. Freshw. Ecosyst. 2018, 28, 776–787. [Google Scholar] [CrossRef]
- Shalders, T.C.; Champion, C.; Benkendorff, K.; Davis, T.; Wernberg, T.; Morris, S.; Coleman, M.A. Changing nutritional seascapes of kelp forests. Front. Mar. Sci. 2023, 10, 1197468. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).