Photocatalytic Degradation of Sulfamethoxazole and Enrofloxacin in Water Using Electrospun Composite Photocatalytic Membrane
Abstract
1. Introduction
2. Methods and Materials
2.1. Materials and Regents
2.2. Preparation of PAN Membrane and Composite Membrane
2.3. Characteristics of PAN Membrane and Composite Membrane
2.4. Photocatalytic Degradation of SMX and ENR Using Photocatalytic Membrane
3. Results and Discussion
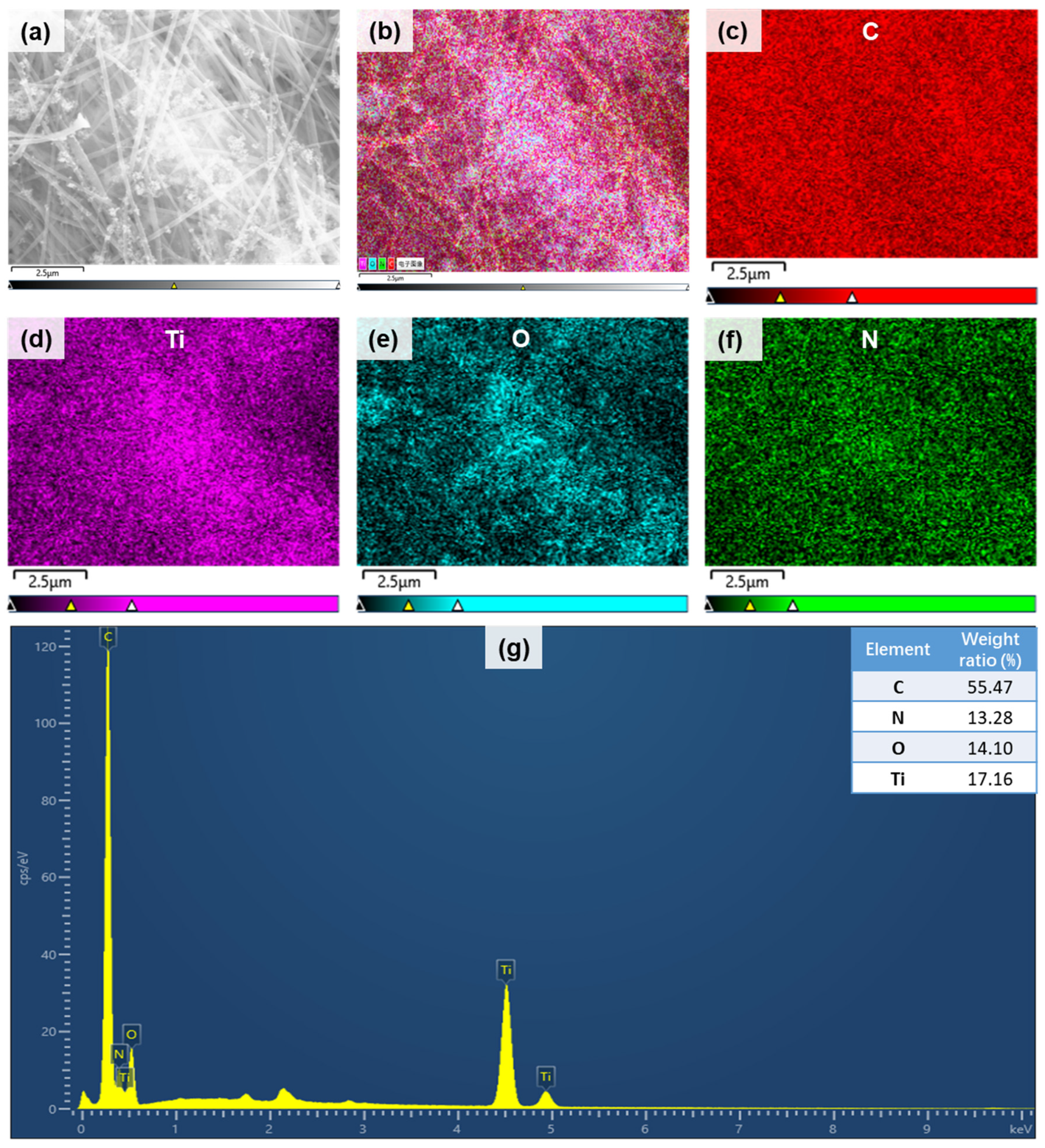
3.1. The Characteristics of PAN and Composite Membrane
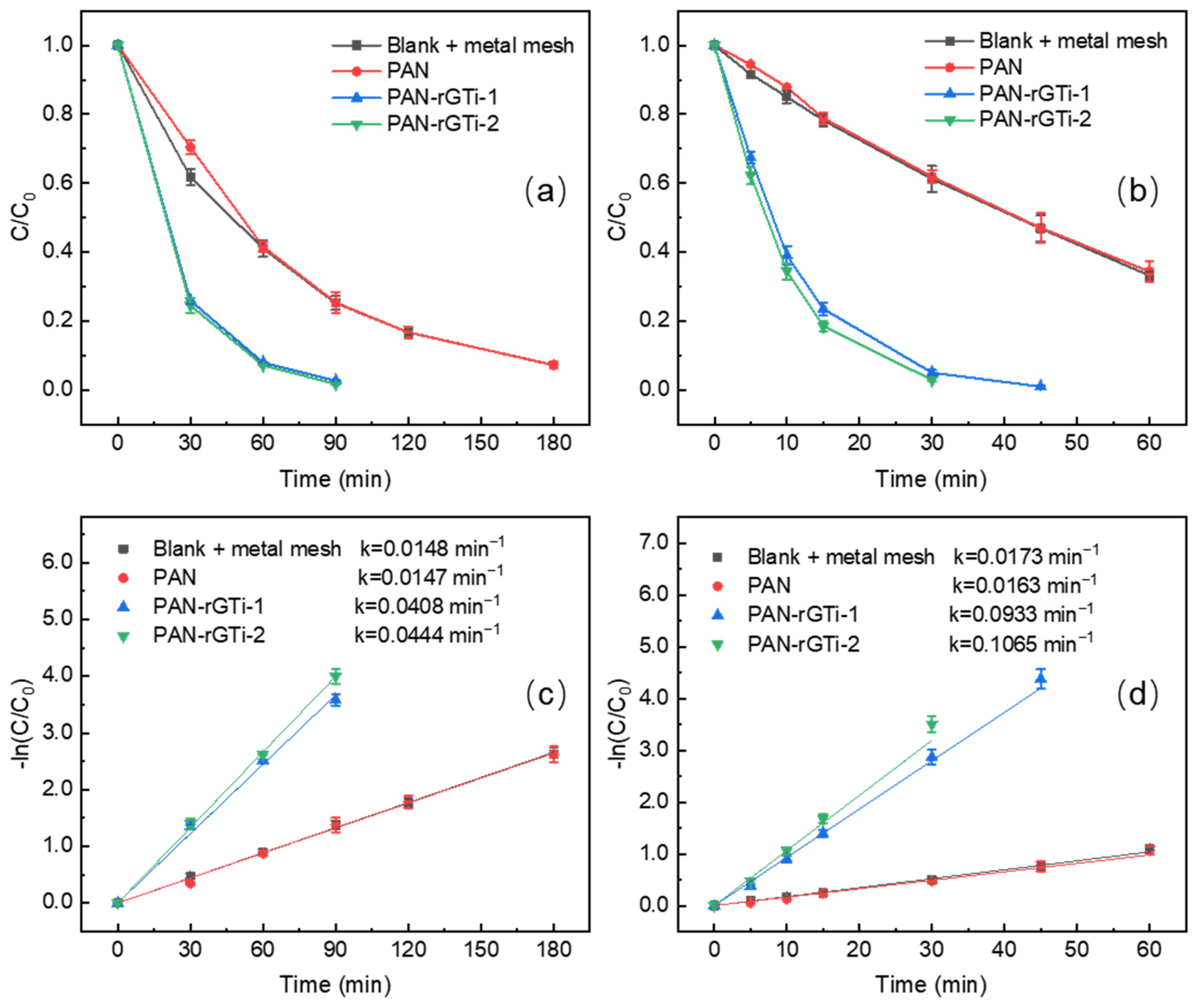
3.2. Photocatalytic Degradation of SMX and ENR
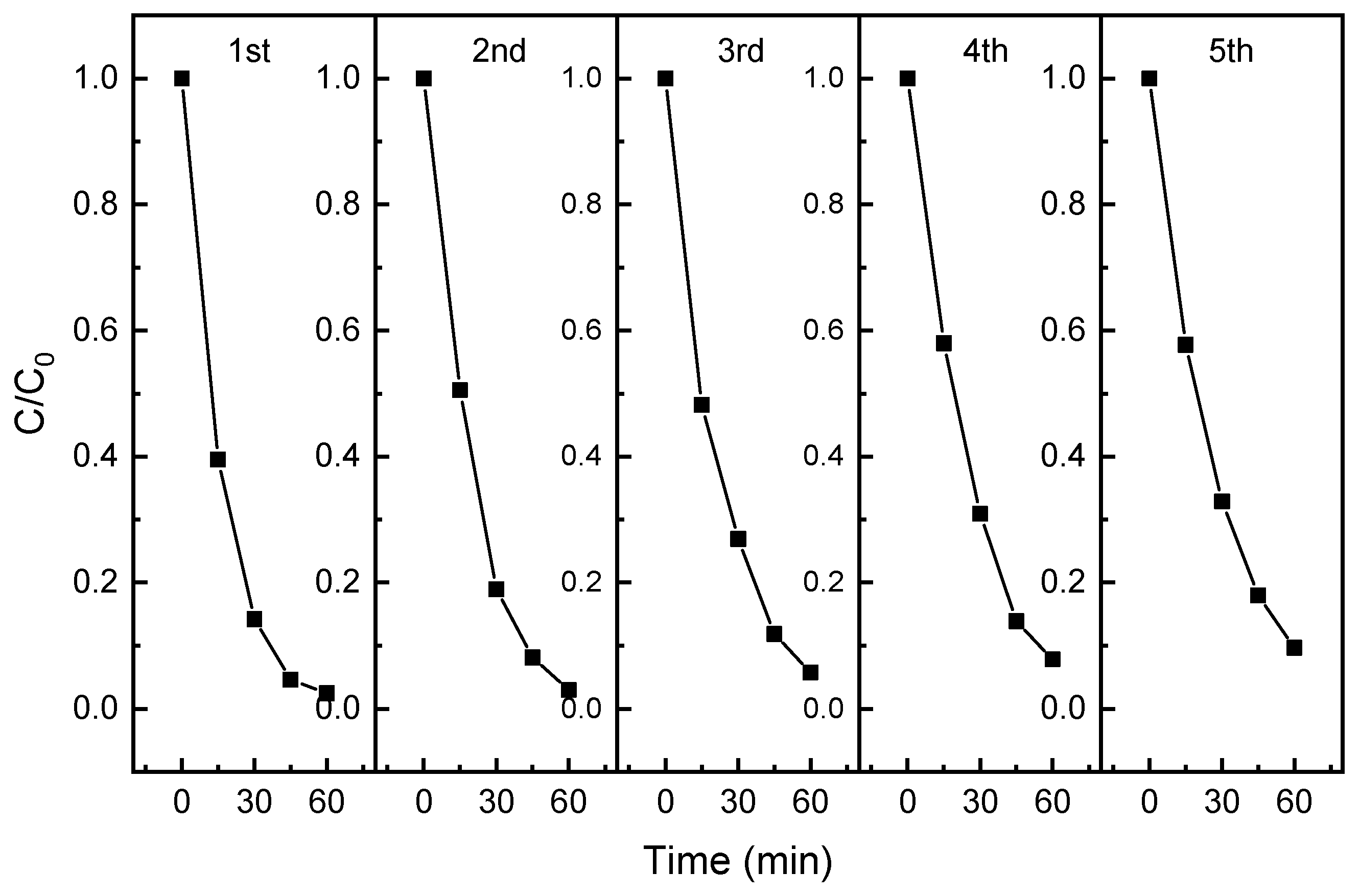
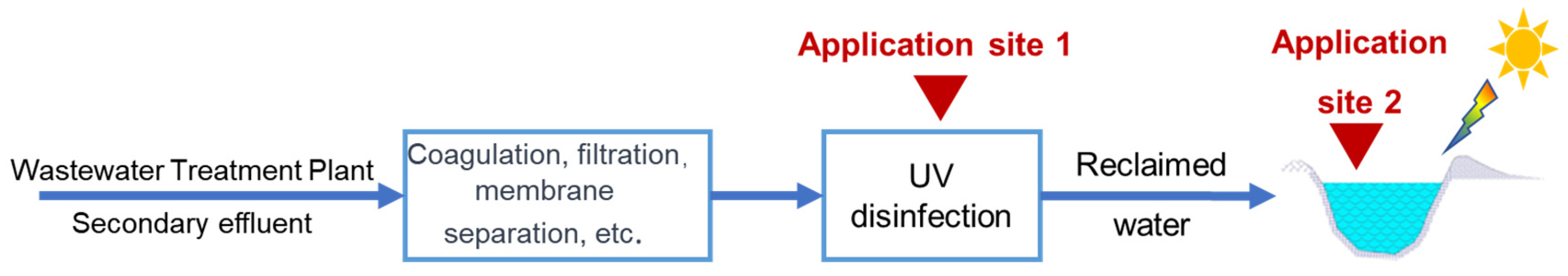
3.3. Recyclability and Application Feasibility of Photocatalytic Composite Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, X.H.; Xu, J.C.; Keller, A.A.; He, L.; Gu, Y.H.; Zheng, W.W.; Sun, D.Y.; Lu, Z.B.; Huang, J.W.; Huang, X.F.; et al. Occurrence and risk assessment of emerging contaminants in a water reclamation and ecological reuse project. Sci. Total Environ. 2020, 744, 11. [Google Scholar] [CrossRef] [PubMed]
- Sabri, N.A.; Schmitt, H.; Van der Zaan, B.; Gerritsen, H.W.; Zuidema, T.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Prevalence of antibiotics and antibiotic resistance genes in a wastewater effluent-receiving river in The Netherlands. J. Environ. Chem. Eng. 2020, 8, 102245. [Google Scholar] [CrossRef]
- Schmidt, C.; Kumar, R.; Yang, S.; Buettner, O. Microplastic particle emission from wastewater treatment plant effluents into river networks in Germany: Loads, spatial patterns of concentrations and potential toxicity. Sci. Total Environ. 2020, 737, 139544. [Google Scholar] [CrossRef] [PubMed]
- Cundy, A.B.; Rowlands, F.M.; Lu, G.; Wang, W.X. A systematic review of emerging contaminants in the Greater Bay Area (GBA), China: Current baselines, knowledge gaps, and research and management priorities. Environ. Sci. Policy 2022, 131, 196–208. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef]
- Wols, B.A.; Hofman-Caris, C.H.M. Review of photochemical reaction constants of organic micropollutants required for UV advanced oxidation processes in water. Water Res. 2012, 46, 2815–2827. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced oxidation processes: Performance, advantages, and scale-up of emerging technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef]
- Samal, K.; Bandyopadhyay, R.; Dash, R.R. Biological Treatment of Contaminants of Emerging Concern in Wastewater: A Review. J. Hazard. Toxic Radioact. Waste 2022, 26, 04022002. [Google Scholar] [CrossRef]
- Deng, F.; Shi, H.; Guo, Y.; Luo, X.; Zhou, J. Engineering paths of sustainable and green photocatalytic degradation technology for pharmaceuticals and organic contaminants of emerging concern. Curr. Opin. Green Sustain. Chem. 2021, 29, 100465. [Google Scholar] [CrossRef]
- Silvestri, S.; Fajardo, A.R.; Iglesias, B.A. Supported porphyrins for the photocatalytic degradation of organic contaminants in water: A review. Environ. Chem. Lett. 2022, 20, 731–771. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals and endocrine disrupting compounds from water by zinc oxide-based photocatalytic degradation: A review. Sustain. Cities Soc. 2016, 27, 407–418. [Google Scholar] [CrossRef]
- Feng, Y.; Rijnaarts, H.H.M.; Yntema, D.; Gong, Z.; Dionysiou, D.D.; Cao, Z.; Miao, S.; Chen, Y.; Ye, Y.; Wang, Y. Applications of anodized TiO2 nanotube arrays on the removal of aqueous contaminants of emerging concern: A review. Water Res. 2020, 186, 116327. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yu, Z.; Li, Z.; Zhao, X.; Yuan, Y. In-Situ Synthesis of TiO2@GO Nanosheets for Polymers Degradation in a Natural Environment. Polymers 2021, 13, 2158. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.-J.; Lee, S.-H.; Kwon, Y.-B.; Kim, K.; Lee, K.-H.; Kim, S.M.; Kim, Y.-K. Fabrication of sustainable and multifunctional TiO2@carbon nanotube nanocomposite fibers. Appl. Surf. Sci. 2021, 541, 148332. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Lu, Y.; Song, Z.; Wang, C.; Li, D.; Tang, X.; Zhou, X. Surface hydroxylation of TiO2/g-C3N4 photocatalyst for photo-Fenton degradation of tetracycline. Ceram. Int. 2022, 48, 1306–1313. [Google Scholar] [CrossRef]
- Jansson, I.; Garcia-Garcia, F.J.; Sanchez, B.; Suarez, S. Key factors to develop hybrid photoactive materials based on mesoporous carbon/TiO2 for removal of volatile organic compounds in air streams. Appl. Catal. A Gen. 2021, 623, 118281. [Google Scholar] [CrossRef]
- Melinte, V.; Stroea, L.; Chibac-Scutaru, A.L. Polymer Nanocomposites for Photocatalytic Applications. Catalysts 2019, 9, 986. [Google Scholar] [CrossRef]
- Epelle, E.I.; Okoye, P.U.; Roddy, S.; Gunes, B.; Okolie, J.A. Advances in the Applications of Nanomaterials for Wastewater Treatment. Environments 2022, 9, 141. [Google Scholar] [CrossRef]
- Luna, M.; Gatica, J.M.; Vidal, H.; Mosquera, M.J. One-pot synthesis of Au/N-TiO2 photocatalysts for environmental applications: Enhancement of dyes and NOx photodegradation. Powder Technol. 2019, 355, 793–807. [Google Scholar] [CrossRef]
- Chu, Y.; Fan, J.; Wang, R.; Liu, C.; Zheng, X. Preparation and immobilization of Bi2WO6/BiOI/g-C3N4 nanoparticles for the photocatalytic degradation of tetracycline and municipal waste transfer station leachate. Sep. Purif. Technol. 2022, 300, 121867. [Google Scholar] [CrossRef]
- Oulhakem, O.; Zahdi, H.; Belaiche, M.; Laalioui, S.; Naimi, Z.; Ikken, B.; Sekkat, Z.; Alaoui, K.B. One-step immobilization of tungsten oxide on microporous silica surface as a photocatalyst for water pollutant removal. Microporous Mesoporous Mater. 2022, 335, 111784. [Google Scholar] [CrossRef]
- Xue, Y.; Kamali, M.; Zhang, X.; Askari, N.; De Preter, C.; Appels, L.; Dewil, R. Immobilization of photocatalytic materials for (waste)water treatment using 3D printing technology-advances and challenges. Environ. Pollut. 2023, 316, 120549. [Google Scholar] [CrossRef] [PubMed]
- Mansurov, R.R.; Safronov, A.P.; Lakiza, N.V.; Beketov, I.V. Photocatalytic Activity of Titanium Dioxide Nanoparticles Immobilized in the Polymer Network of Polyacrylamide Hydrogel. Russ. J. Appl. Chem. 2017, 90, 1712–1721. [Google Scholar] [CrossRef]
- Hasnan, N.S.N.; Mohamed, M.A.; Anuar, N.A.; Sukur, M.F.A.; Yusoff, S.F.M.; Mokhtar, W.N.A.W.; Hir, Z.A.M.; Shohaimi, N.A.M.; Rafaie, H.A. Emerging polymeric-based material with photocatalytic functionality for sustainable technologies. J. Ind. Eng. Chem. 2022, 113, 32–71. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Xiao, G.; Su, H. Fabrication of biomaterial/TiO2 composite photocatalysts for the selective removal of trace environmental pollutants. Chin. J. Chem. Eng. 2019, 27, 1416–1428. [Google Scholar] [CrossRef]
- Zhao, Y.; Tao, C.; Xiao, G.; Su, H. Controlled synthesis and wastewater treatment of Ag2O/TiO2 modified chitosan-based photocatalytic film. RSC Adv. 2017, 7, 11211–11221. [Google Scholar] [CrossRef]
- Enesca, A.; Cazan, C. Polymer Composite-Based Materials with Photocatalytic Applications in Wastewater Organic Pollutant Removal: A Mini Review. Polymers 2022, 14, 3291. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Anae, J.; Khan, M.Z.; Sabir, S.; Yang, X.J.; Thakur, V.K.; Campo, P.; Coulon, F. Visible light-conducting polymer nanocomposites as efficient photocatalysts for the treatment of organic pollutants in wastewater. J. Environ. Manag. 2021, 295, 113362. [Google Scholar] [CrossRef]
- Zakria, H.S.; Othman, M.H.D.; Kamaludin, R.; Kadir, S.; Kurniawan, T.A.; Jilani, A. Immobilization techniques of a photocatalyst into and onto a polymer membrane for photocatalytic activity. RSC Adv. 2021, 11, 6985–7014. [Google Scholar] [CrossRef]
- Lin, Q.; Zeng, G.; Yan, G.; Luo, J.; Cheng, X.; Zhao, Z.; Li, H. Self-cleaning photocatalytic MXene composite membrane for synergistically enhanced water treatment: Oil/water separation and dyes removal. Chem. Eng. J. 2022, 427, 131668. [Google Scholar] [CrossRef]
- Zhou, X.; Shao, C.; Yang, S.; Li, X.; Guo, X.; Wang, X.; Li, X.; Liu, Y. Heterojunction of g-C3N4/BiOI Immobilized on Flexible Electrospun Polyacrylonitrile Nanofibers: Facile Preparation and Enhanced Visible Photocatalytic Activity for Floating Photocatalysis. ACS Sustain. Chem. Eng. 2018, 6, 2316–2323. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, C.; Li, X.; Han, C.; Yang, S.; Ma, J.; Li, X.; Liu, Y. Immobilization of ZnO/polyaniline heterojunction on electrospun polyacrylonitrile nanofibers and enhanced photocatalytic activity. Mater. Chem. Phys. 2018, 214, 507–515. [Google Scholar] [CrossRef]
- Sun, F.; Xie, Y.; Xu, D.; Liu, F.; Qi, H.; Ma, Q.; Yang, Y.; Yu, H.; Yu, W.; Dong, X. Electrospun self-supporting double Z-scheme tricolor-typed microfiber oriented-heterostructure photocatalyst with highly effective hydrogen evolution and organic pollutants degradation. J. Environ. Chem. Eng. 2023, 11, 109169. [Google Scholar] [CrossRef]
- Li, S.; Thiyagarajan, D.; Lee, B.-K. Efficient removal of methylene blue from aqueous solution by ZIF-8-decorated helicoidal electrospun polymer strips. Chemosphere 2023, 333, 138961. [Google Scholar] [CrossRef] [PubMed]
- Sarkodie, B.; Amesimeku, J.; Frimpong, C.; Howard, E.K.; Feng, Q.; Xu, Z. Photocatalytic degradation of dyes by novel electrospun nanofibers: A review. Chemosphere 2023, 313, 137654. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.W.; Xu, J.C.; Wang, L.Y.; Zhang, J.L.; Chu, W.H.; Liu, J.; Lu, L.J.; Cai, C.; Peng, K.M.; Huang, X.F. Electro-enhanced rapid separation of nanosized oil droplets from emulsions via the superhydrophilic micro-sized pore membrane. Sep. Purif. Technol. 2023, 314, 123453. [Google Scholar] [CrossRef]
- Diamantopoulou, A.; Sakellis, E.; Romanos, G.E.; Gardelis, S.; Ioannidis, N.; Boukos, N.; Falaras, P.; Likodimos, V. Titania photonic crystal photocatalysts functionalized by graphene oxide nanocolloids. Appl. Catal. B Environ. 2019, 240, 277–290. [Google Scholar] [CrossRef]
- Tolosana-Moranchel, A.; Casas, J.A.; Bahamonde, A.; Pascual, L.; Granone, L.I.; Schneider, J.; Dillert, R.; Bahnemann, D.W. Nature and photoreactivity of TiO2-rGO nanocomposites in aqueous suspensions under UV-A irradiation. Appl. Catal. B Environ. 2019, 241, 375–384. [Google Scholar] [CrossRef]
- Yu, L.; Tang, B. Photocatalytic Degradation of Phenolic Compounds from Wastewater Using Titanium dioxide@reduced Graphene Oxide (TiO2@rGO) Nanocomposites. Int. J. Electrochem. Sci. 2021, 16, 210915. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, W.; Li, S.; Fang, H.; Fu, S.; Xu, J.; Huang, J. Photodegradation of Sulfamethoxazole and Enrofloxacin under UV and Simulated Solar Light Irradiation. Water 2023, 15, 517. [Google Scholar] [CrossRef]
- Su, J.; Yang, G.; Cheng, C.; Huang, C.; Xu, H.; Ke, Q. Hierarchically structured TiO2/PAN nanofibrous membranes for high-efficiency air filtration and toluene degradation. J. Colloid Interface Sci. 2017, 507, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, X.; Huang, Y.; Liu, J.; Wang, D.; Feng, S.; Huang, X.; Wang, H. Electrospinning preparation of PAN/TiO2/PANI hybrid fiber membrane with highly selective adsorption and photocatalytic regeneration properties. Chem. Eng. J. 2020, 399, 125749. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, D.; Li, Y.; Qu, J.; Yu, Z.-Z. Fabrication of PAN@TiO2/Ag nanofibrous membrane with high visible light response and satisfactory recyclability for dye photocatalytic degradation. Appl. Surf. Sci. 2017, 426, 622–629. [Google Scholar] [CrossRef]
- Sun, X.-X.; Liu, G.; Li, R.; Meng, Y.; Wu, J. Polyporous PVDF/TiO2 photocatalytic composites for photocatalyst fixation, recycle, and repair. J. Am. Ceram. Soc. 2021, 104, 6290–6298. [Google Scholar] [CrossRef]
- Moreno-Andrés, J.; Rueda-Márquez, J.J.; Homola, T.; Vielma, J.; Moríñigo, M.A.; Mikola, A.; Sillanpää, M.; Acevedo-Merino, A.; Nebot, E.; Levchuk, I. A comparison of photolytic, photochemical and photocatalytic processes for disinfection of recirculation aquaculture systems (RAS) streams. Water Res. 2020, 181, 115928. [Google Scholar] [CrossRef]


| Blank + Metal Mesh | PAN Membrane | PAN-TiO2-2 | PAN-TiO2-4 | PAN-rGTi-1 | PAN-rGTi-2 | |
|---|---|---|---|---|---|---|
| Removal efficiency in adsorption stage | 2.86% | 16.82% | 37.72% | 42.61% | 47.96% | 50.85% |
| Removal efficiency in photocatalytic degradation stage | 92.7% | 92.6% | ~100% | ~100% | ~100% | ~100% |
| Total removal efficiency | 92.9% | 93.8% | ~100% | ~100% | ~100% | ~100% |
| Reaction constant in photocatalytic degradation stage (min−1) | 0.148 | 0.147 | 0.259 | 0.330 | 0.408 | 0.444 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Fang, H.; Wang, L.; Sun, D.; Zhao, G.; Xu, J. Photocatalytic Degradation of Sulfamethoxazole and Enrofloxacin in Water Using Electrospun Composite Photocatalytic Membrane. Water 2024, 16, 218. https://doi.org/10.3390/w16020218
Lin X, Fang H, Wang L, Sun D, Zhao G, Xu J. Photocatalytic Degradation of Sulfamethoxazole and Enrofloxacin in Water Using Electrospun Composite Photocatalytic Membrane. Water. 2024; 16(2):218. https://doi.org/10.3390/w16020218
Chicago/Turabian StyleLin, Xiaohu, Haifeng Fang, Libing Wang, Danyan Sun, Gang Zhao, and Jingcheng Xu. 2024. "Photocatalytic Degradation of Sulfamethoxazole and Enrofloxacin in Water Using Electrospun Composite Photocatalytic Membrane" Water 16, no. 2: 218. https://doi.org/10.3390/w16020218
APA StyleLin, X., Fang, H., Wang, L., Sun, D., Zhao, G., & Xu, J. (2024). Photocatalytic Degradation of Sulfamethoxazole and Enrofloxacin in Water Using Electrospun Composite Photocatalytic Membrane. Water, 16(2), 218. https://doi.org/10.3390/w16020218








