Abstract
Wine lee generation, a by-product of the wine industry, implies economic challenges for producers in terms of management due to its high organic load and low pH value. Biological treatment based on controlled anaerobic digestion may emerge as a viable management alternative given its promising potential for biogas production thanks to the organic content of the substrate. However, the complex properties of wine lees may lead to microbial activity inhibition and process kinetics failure. Various solutions have already been explored, including co-digestion with other substrates, or the application of different pretreatments, to mitigate the effects of the accumulation of phenolic compounds, volatile fatty acids, antioxidants, or the acidic pH value of the medium. In this study, laboratory-scale batch reactors were established, adding iron- (magnetite) or carbon (graphite)-based microparticles to assess their impact on the kinetics of the process. The results demonstrate a significant improvement of 35% in the potential production of biomethane after four days of operation with graphite particles and 42% after five days using magnetite particles. Methane production rates, as determined by the Gompertz model, were 45.38 and 46.54 mL CH4∙gVS−1∙d−1 for the application of graphite and magnetite microparticles to the medium, respectively, compared to the value of 33.46 mL CH4∙gVS−1∙d−1 for the control trial, confirming kinetic process improvements of 36% and 39%, respectively. Evidences of the acceleration of the methanogenesis phase were detected along the essays; however, the strong inhibition mediated by the carboxylate accumulation was not avoided in any of the tested conditions.
1. Introduction
The sustainable management of agricultural by-products is both an opportunity and a challenge for the industries within the sector [1,2]. On one hand, there is the potential to enhance the value of their processes, increasing profitability by reusing these materials as substrates for bioenergy or biomaterials. On the other hand, addressing this challenge promotes the minimization of environmental impact, directly contributing to the circular economy [3,4]. The wine sector, particularly in countries like Chile, Italy, and Spain, serves as a notable example [5]. Over recent years, the significant increase in overall productivity has resulted in substantial waste and wastewater generation during wine production [6]. By using different winery types of waste, such as stems, grape pomace, and wine lees, valuable chemical substances can be extracted, bioenergy produced, and new applications developed in agriculture and the environment [7]. Concerning wine lees, these constitute a semi-solid residue that is recovered from the bottom of wooden barrels after the end of the fermentation process. They can account for up to 5% of the overall weight of the processed grapes [8]. However, many times managing these by-products is complex due to their physicochemical properties, featuring high chemical oxygen demand (COD) and low pH values. This complexity is the result of the composition of dead yeast cells, tartrates, proteins, polysaccharides, and other substances produced and settled during fermentation, often implying an economic cost for the producers that should be invested in its management [9]. In particular, the high content of digestible matter present in wine lees is expected to yield a good energy performance through their biological transformation [7].
Anaerobic digestion (AD) is an interesting option to decompose organic waste materials by the activity of microorganisms in the absence of oxygen. Consequently, organic materials like wine lees are transformed into a biogas and a digestate, providing bioenergy and fertilizers [10,11]. However, the process faces limitations because of the high concentration of complex phenolic substances, inhibitory chemicals, and antioxidants, all of which adversely affect the kinetics of the process [12]. In this contest, the optimal operational conditions during anaerobic digestion have not been clearly unraveled. Some previous studies have explored the inhibitory effects of phenolic compounds using synthetic substrates, identifying concentration limits for the process [13]. Similar investigations have been conducted regarding the accumulation of volatile fatty acids in the medium, pH variations, and increases in hydrogen sulfide (H2S) content in the biogas [14,15,16].
To address these challenges, various strategies have been suggested, including optimizing operational parameters, applying co-digestion, and implementing pretreatments or using additives. Where Da Ros et al. (2017) [17] blended winery wastewater sludge and wine lees to enhance methane production, Hungría et al. (2021) [18] assessed the anaerobic co-digestion of various lees sourced from organic crops. Other authors have explored pretreatment methods, including fungal and enzymatic approaches [19], electro-oxidation [9], and microwave or ultrasound treatments [20]. A widely used pretreatment method for wastewater AD treatment is thermal hydrolysis (liquid hot water or steam explosion, commonly), which has demonstrated a high efficiency in AD processes by improving the degradability of bio-recalcitrant organic substances [21]. However, the diverse characteristics of substrates can influence biogas production, as well as the presence of polyphenols, phytotoxic, and antimicrobial compounds, along with low pH values, complicating waste treatment and disposal. This study introduces an alternative approach to the anaerobic digestion of wine lees by the application of two diverse types of microparticles to enhance the kinetics of the process.
The addition of microparticles to anaerobic digestion processes holds the potential to improve the process by providing larger active sites for the various microorganisms involved. Additionally, microparticles may serve as crucial mediators, capable of breaking cell membranes and facilitating direct electron transfer [22,23]. Concerning the substrate, the introduction of microparticles can accelerate the hydrolysis of organic matter, thereby enhancing substrate decomposition, increasing biogas production, and reducing the lag phase [24]. A number of essays have been devoted to demonstrating the efficiency of the addition of these microparticles to the AD process, consistently yielding positive results, particularly with substrates such as sewage sludge or livestock waste, as shown in Table 1. In this study, various discontinuous anaerobic reactors were used considering wine lees as substrate, which were supplemented with iron-based (magnetite) or carbon-based (graphite) microparticles, to determine their effect on the improvement of biological methane potential (BMP). Based on their electric conductivity figures and different natures, positive outcomes are expected in accordance with the existing literature on this topic. Concurrently, monitoring encompassed other key process parameters, such as biogas quality, pH, volatile fatty acids (VFAs), and phenolic compound contents.

Table 1.
Review of the addition of metal-based and carbon-based particles to enhance the anaerobic digestion process of organic substrates, including the type of particles, biomass type, the content of particles, and the effect on the biogas production reported by each study.
2. Material and Methods
2.1. Anaerobic Inoculum and Feedstock
Wine lees, as agricultural waste from the wine process, were collected from a winery placed in Mélida de Peñafiel province (Valladolid, Spain) and were stored at 4 °C before use. Inoculum was sampled in the wastewater treatment plant (WWTP) of Soria (Spain), specifically from the anaerobic treatment digester tank. The physico-chemical characteristics of substrates in terms of total organic carbon (TOC), total nitrogen (TN), dry matter, organic matter, pH, chemical oxygen demand (COD), and phenolic compounds content are presented in Table 2.

Table 2.
Characteristics of the substrate and anaerobic sludge used in the bioreactors (TS, VS: total and volatile solids; CODt: total chemical oxygen demand; TOC: total organic carbon; TN: total nitrogen; C:N: carbon-nitrogen ratio).
2.2. Microparticles as Additives
Two types of commercial microparticles were used in the experiments. On one hand, the iron-based microparticles consisted of magnetite (iron (II–III) oxide) microparticles (<5 μm), which were supplied by Aldrich Chemistry (St. Louis, MO, USA), specifically from batch 310069-25 G. On the other hand, the carbon-based microparticles consisted of 99.8% graphite microparticles (<20 μm) supplied by Alfa Aesar (Kandel, Germany), batch W24B028, specifically. The selected concentration for the experiments was 200 mg L−1, following the recommendations of other studies [26,38,39].
2.3. Anaerobic Digestion Tests
AD of the substrates was assessed in batch conditions for 15 days with the working flow design shown in Figure 1.
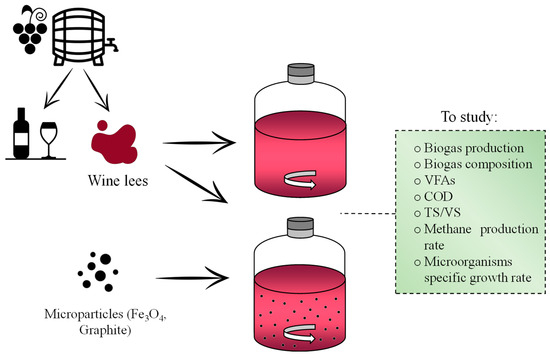
Figure 1.
Study proposal for the anaerobic digestion process using wine lees as substrate with and without the addition of different microparticles.
A BPM methodology (biochemical methanogenic potential) was applied following the protocol described by Holliger C. et al. (2016) [40] and the experimental conditions set are explained in Table 3. Serological 120 mL glass bottles were used as anaerobic reactors under a mesophilic temperature condition of 35 ± 0.5 °C that was set in an incubator (GL-Hotcold, Selecta, Barcelona, Spain). Agitation conditions were performed by an orbital stirring plate (Rotabit, Selecta, Barcelona, Spain). The ratio of inoculum to substrate was 1.5:1 g of volatile solids (VS) [41]. Furthermore, 1.5 g of CaCO3∙L−1 was introduced as a buffering agent to prevent pH fluctuations. The reactors were purged with an inert gas (N2, 99.9% purity) to displace the air from the system and establish an anoxic environment at the beginning of the essay [42].

Table 3.
BMP (biochemical methanogenic potential) experimental conditions.
The tests were performed in triplicates for each substrate condition, namely, lees standalone (Lees Control), lees with graphite microparticles (Lees Gr), and lees with magnetite microparticles (Lees Fe), as well as a blank test incorporating just inoculum to assess the endogenous production of biogas (blank test). The measurements were adjusted by subtracting the endogenous production. Daily biogas production was quantified in terms of water displacement and the volume was corrected to standard conditions. The composition of the biogas was measured using gas analysis equipment (Biogas 5000-GeoTech, Leamington Spa, UK). NaOH 0.1 M was added to adjust the pH to 7.5 at the beginning of the experiment and the microparticles were subsequently and separately added into the glass bottles.
2.4. Analytical Procedure
Total solids (TS) and volatile solids (VS) were assessed following the American Public Health Association’s standard methods for the examination of water and wastewater [43]. The calculation included the drying process of the samples at 105 °C for a period of 24 h and their posterior incineration in a muffle at 550 °C for 30 min.
Chemical oxygen demand (COD) was also measured according to the standard methods for the examination of water and wastewater [43]. Absorbance was measured using a spectrophotometer (Genesys 10uv, Thermo Spectronic, Waltham, MA, USA) at 600 nm.
Phenolic compound concentration was measured according to the Folin–Ciocalteu methodology [44]. This approach relies on the interaction between phenolic compounds and the Folin–Ciocalteu reagent under alkaline conditions, resulting in the development of a blue coloration. The absorbance of this coloration was subsequently determined at 765 nm by the spectrophotometer. Lastly, volatile fatty acids (VFAs) were determined in a 5 mL sample filtered through 0.45 μm of previous centrifugation at 5000 rpm (Centromix, Selecta, Barcelona, Spain). Subsequently, a second centrifugation was required at 13,000 rpm (MicroStar 17, VWR, Radnor, PA, USA) and filtration at 0.2 microns was carried out to subsequently run the samples in a gas chromatograph (7820A Gas chromatograph, Agilent, Santa Clara, CA, USA).
2.5. Modelling
Experimental biomethane production data were adjusted to the Gompertz mathematical model [45,46] and first-order models [47] to fit the biogas production of each test and their anaerobic digestion phases according to Equations (1) and (2):
where M (t) is the cumulative methane production (mL CH4·g−1 VS at standard pressure and temperature conditions), P is the potential methane production (mL CH4·g−1 VS), R is the maximum methane production rate (mL CH4∙g VS−1∙day−1), K is the specific growth rate of microorganisms (day−1), t represents the elapsed time (days), and λ is the lag phase (days).
The adjustment of the model to the experimental results was carried out using the least squares procedure [48]. The adjusted coefficient of determination (R2, correlation coefficient) and the root mean square error (E) were determined by applying Equation (3):
where m is the data pairs number, Y is the measured production of methane production (mL∙g−1 VS added), and d is the deviation between the predicted and the experimental methane production results.
2.6. Data Analysis
All trials were carried out in triplicates. Results were processed with Microsoft Excel (Microsoft 365, MSO version 16.0) to calculate the mean and standard deviation values of the results. A model adjustment was performed using the Solver Tool provided by MS Excel.
3. Results and Discussion
3.1. The Effect of Microparticles on the Production of Biogas
The daily CH4 production is shown in Figure 2. The control trial without particles yielded 136 mL CH4∙g SV−1 at the end of the essay; whereas, the addition of microparticles increased this value until 166 mL CH4∙g SV−1 for Lees Gr and up to 192 mL CH4∙g SV−1 in the case of Lees Fe. CH4 production in the tests was registered during the first 6 days of operation, declining from Day 5 onwards. Daily methane production has been virtually nonexistent since Day 8 of incubation. The lag phase for all three trials was minimal addressing production figures without significant differences among the essayed conditions. The detected values of methane production were in the range of previously reported studies for these kinds of substrates, which reported values between 100 and 350 mL CH4∙g SV−1 using wine lees in batch anaerobic experiments [49,50,51]. In Table 4, the biomethane production performance of wine lees as an organic substrate is displayed in comparison to other agricultural waste substrates of different origins, confirming a standard yield per unit of volatile solids and adding value to the management of this wine by-product.
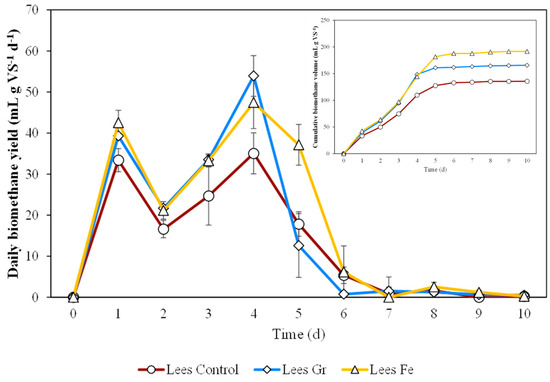
Figure 2.
Time course evolution of daily methane yield in the trials, with and without microparticles in addition to the anaerobic digestion of wine lees. On the upper-right side is experimental cumulative methane production.

Table 4.
Review of literature studies investigating the anaerobic digestion of organic substrates’ various biomasses used for biogas production.
The positive impact of the microparticles, which resulted in an increase in cumulative CH4 production of 22.1% and 41.3% in the cases of iron-based (magnetite) and carbon-based (graphite) microparticles, respectively, at the end of the essay, was clearly related to an increased methanogenic phase of the anaerobic digestion. This fact was evidenced by the rapid decrease in VFA concentration that was detected after 5 days of incubation (Figure 3). Where the control experiment presented a VFA concentration of 23 g·L−1, the concentration in essays with microparticles was considerably lower: 6.4 and 14.4 g·L−1 in Lees Gr and Lees Fe experiments, respectively. This acceleration of the carboxylate consumption was concomitantly detected with a considerably higher value of biogas production, which reached the highest values at Day Four, when 53.9 and 47.5 mL of biogas were produced in Lees Gr and Lee Fe, respectively; whereas, the production of the control experiment was considerably lower, addressing an average value of 35.1 mL per day (Figure 2). During that phase of the experiment, the biomethane difference was maximal compared to the control trial, reaching 35.2% for graphite particles on the fourth day. In the case of iron-based particles, a 42.3% positive difference was registered on the fifth day. In the case of magnetic particles, this stimulation of the methanogenic phase of the anaerobic digestion process might be related to their electronic and physical properties.
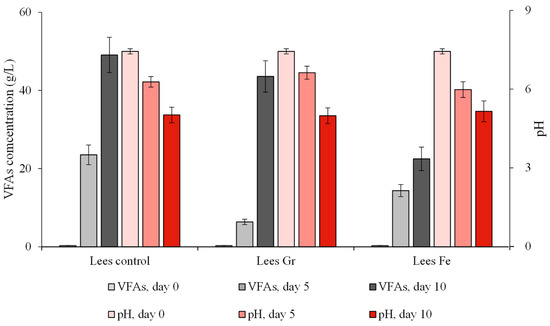
Figure 3.
Time course of VFA concentration and pH in the BMP experiments with and without microparticle addition from the anaerobic digestion of wine lees.
The mechanism of direct interspecies electron transfer (DIET), consisting of a syntrophic metabolism where free electrons move between cells by shared physical (microbe to microbe and microbe to electrode) and electrical connections (conductive pili), has previously been described in biogas production systems [59,60]. Particularly, DIET does not necessitate reduced electron carriers (redox mediators) like molecular hydrogen [61]. Instead, it relies on oxidizing bacteria that extracellularly release electrons, which are then transferred to methanogenic archaea with the assistance of electrically conductive materials, such as iron oxides included in the particles [62,63]. This mechanism allows a reduction in CO2 to CH4 through direct electron transfer between species [64]. Preliminary experiments carried out in our lab showed that the addition of a similar quantity of inert deionized sand microparticles did not enhance biogas production by improving mixing or any other mechanical effect in the anaerobic digestion process, thus confirming the DIET mechanism to be the most plausible explanation to the herein addressed biogas production improvement (data not published).
According to previous studies, the presence of magnetite particles proves beneficial in enhancing hydrogenotrophic methanogenesis. This enhancement occurs as magnetite contributes electrons through iron corrosion, leading to increased hydrogen (H2) production. The generated H2 subsequently reacts with carbon dioxide (CO2), resulting in elevated methane production. However, the results herein presented suggested also an enhancement of the acetoclastic methanogenesis, with considerably lower values of VFAs in the early steps of the anaerobic digestion when microparticles were added. In addition, Fe3O4 particles, via Fe2+, contribute to breaking down volatile solids present in the substrate by binding with the substrate. It increases the substrate’s surface area, which is particularly beneficial for improving hydrolysis, especially when dealing with substrates with a diameter smaller than 6 mm. Increases in the hydrolysis phase were not detected in the experiment herein reported, with small differences in the lag phase according to the modeling applied. The latency phase detected (λ) was virtually negligible in all experiments, registering at 0.38, 0.49, and 0.53 days for the Control, Lees Gr, and Lees Fe treatments, respectively, when the Gompertz model was applied (see Section 3.3).
The 200 mg L−1 concentration used for iron microparticles was sufficient to enhance biogas production without the negative effects of the bioprocess. An over-addition of microparticles resulted in an excessive iron accumulation that could be potentially toxic in the medium, leading to the cessation of biogas production by inhibition of the process [38]. An additional advantageous characteristic of iron-based particles is their retrievability after use, which can be achieved by methods like filtration or magnetic separation. This is particularly significant as the failure to recover and manage these microparticles can adversely impact the environment, potentially affecting both aquatic and terrestrial ecosystems, as well as human health.
Conversely, the introduction of graphite microparticles also resulted in an immediate rise in the generation of methane and in a higher conversion of VFAs. The significance of carbon-based microparticles lies in their diverse geometric forms, including graphene, and carbon nanotubes. These variations offer distinct properties, such as high conductivity, large surface area to promote chemical reactivity and thermal stability, and the promotion of microbial colonization, particularly attributable to their extensive specific surface area available [65]. Similar to iron oxide particles, carbon-based particles were proved to be valuable in anaerobic digestion processes by facilitating DIET due to their conductivity figures. Additionally, they offer the advantage of being more cost-effective than other types of particles [66].
Iron-based microparticles not only addressed a better performance than graphite microparticles but the addition of magnetite microparticles would additionally be more practical in a continuous mode anaerobic digestion process because they could be recovered from the digestate by means of magnet-assisted devices, which are conventionally used in organic waste management systems [67,68].
3.2. Potential Inhibitors along the Anaerobic Digestion Process
The abrupt reduction in CH4 production, mentioned in Section 3.1, might be linked to the increase in the content of volatile fatty acids (VFAs). Chiappero et al. [69] observed a similar inhibition of the methanogenic phase at VFA concentrations around 21–24 g VFAs∙L−1 while working with the anaerobic digestion of wine lees. In contrast, other researchers have reported lower values, ranging between 6 and 10 g VFAs∙L−1, when working with cattle dung and sewage sludge as substrates [70,71].
VFA concentration was measured on Test Days 0, 5, and 10, as illustrated in Figure 3, revealing a notable increase in VFA concentration throughout the experiment. This growth may be attributed to the slower degradation of VFAs by acetogenic and methanogenic microorganisms compared to their production by acidogenic microorganisms [72]. In the trials involving graphite microparticles, a reduced accumulation of VFAs was detected, particularly during the initial 5 days, as compared to the control test. Between Days 5 to 10, VFA accumulation was lower in the Lees Fe trials than in the Lees Gr trials, contributing to producing a higher CH4 yield compared to graphite microparticle addition essays. At the end of the experiment, all trials exhibited a VFA concentration exceeding 20 g∙L−1 by the 10th day of the processes. Although the presence of microparticles promoted a higher conversion of VFAs to methane, this positive effect was insufficient to circumvent the negative effects produced by VFA accumulation in the methanogenic stages of AD processes.
Another parameter influenced by the accumulation of VFAs is pH, which, despite initial pH adjustments and the inclusion of CaCO3 as a buffering agent, stabilizes around 5 at the conclusion of the trials. This pattern is consistent across all three treatments, with and without microparticles. Similar effects on pH were also noted by other authors [51,69].
The accumulation of phenolic compounds in the medium could be another factor influencing the inhibition of the process [73,74]. The initial measured value exceeds 0.3 g∙L−1 (Figure 4), suggesting a potential mild impact on the process according to [13]. Other studies have reported that inhibition in anaerobic digestion can occur when the concentration of phenolic compounds surpasses 0.1–0.6 g∙L−1, leading to high system instability and, consequently, reduced biogas production [9,75,76]. Figure 4 illustrates a decline in the phenolic compound content in the medium throughout the essay, which, in this instance, does not appear to be a greater adverse effect than VFA content or pH value. After 10 days of testing, the reduction in the phenolic compound content in all three treatments, with and without microparticles, reached about 65%. This reduction may also be attributed to the substantial dilution of the wine lees substrate in the medium. Although lees hold a phenolic compound content of 1.59 g∙L−1 (Table 1), it decreases to 0.3 g∙L−1 after being mixed with the WWTP sludge at the beginning of the trials. No significant effect of the microparticles’ presence was detected over the phenolic compound concentration (Figure 4).
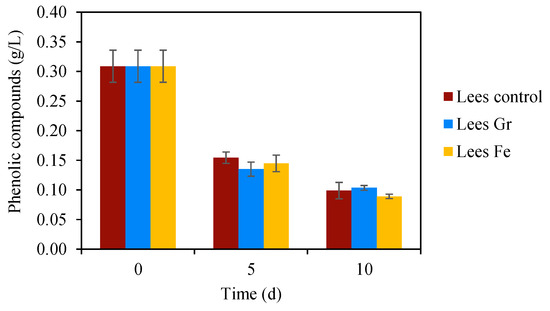
Figure 4.
Time course evolution of phenolic compound concentration in the BMP experiments, with and without microparticle application to wine lees’ anaerobic digestion.
Biogas composition obtained along the tests revealed also the presence of inhibitory phenomena. Two noteworthy outcomes were observed in Figure 5. Firstly, a decline in methane concentration was detected from Day 10 onwards, which may potentially be attributed to the inhibition of the process mediated by VFA accumulation, specifically affecting methanogenic microorganisms, which are known for their sensitivity to such changes [77,78], and the fact that the methanogenesis stage is slower than the preceding phases [79]. Secondly, the hydrogen sulfide content experienced a significant increase from the beginning of the trial, reaching values exceeding 5000 ppm in all three treatments, with or without microparticles. This accumulation has also been documented as an inhibitory effect on methanogenic microorganisms [80]. The drastic increase may be attributed to the fact that lees from winemaking residues contain substantial concentrations of sulfates formed during the winemaking process. Consequently, the rise in H2S concentration may result from the reduction in the sulfates present in the wine lees during anaerobic digestion [81]. Additionally, the biogas composition in the uninhibited stage averaged values of 57.2%, 62.8%, and 61.7% of biomethane content for the Lees Control, Lees Gr, and Lees Fe trials, respectively, corresponding to an expected energy content of 5.7 to 6.3 kW·h·m−3, which corresponds to a calorific value of 4904 to 5421 kcal∙m−3 [82].
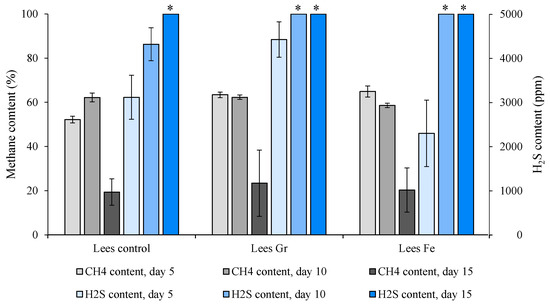
Figure 5.
Time course evolution of the biogas composition (CH4 and H2S) along BMP experiments, with and without microparticle addition, from the anaerobic digestion of wine lees. The asterisk indicates that the H2S measurement has arrived at the maximum value measured by the gas analyzer.
3.3. Modelling
The examination of the kinetics for adjusting biomethane production using the Gompertz and first-order models exhibited satisfactory fits relative to the curves derived from experimental data, as depicted in Figure 6. For the Lees Control, Lees Gr, and Lees Fe tests, R2 values for the Gompertz model reached 98.6%, 98.1%, and 98.4%, respectively; whereas, the corresponding values for the first-order model were 96.5%, 94.9%, and 93.6%, respectively.
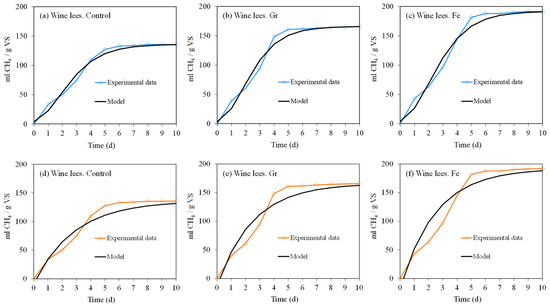
Figure 6.
Biomethane generation (mL) per quantity of volatile solids (g) for wine lees, with and without microparticle addition, in the anaerobic digestion of wine lees in a BMP experiment under mesophilic conditions. Gompertz modeling (a–c) is represented in the blue color, and first-order modeling (d–f) is represented in the orange color.
Table 5 illustrates that the latency phase (λ) was insignificant in all experiments, registering at 0.38, 0.49, and 0.53 days for the Lees Control, Lees Gr, and Lees Fe treatments, respectively, in the Gompertz model; the values were 0.17, 0.18, and 0.21 days for the first-order model, respectively, as well. This fact evidenced the absence of a limiting step in the hydrolysis. This is attributed to the highly biodegradable nature of the substrate that had been used in these trials when inhibitory conditions had not been generated in the AD process. Other studies have similarly documented the absence of a lag phase for wine lees’ AD [50,83].

Table 5.
Kinetic parameters obtained from Gompertz and first-order equation modeling from the BMP experiments of the anaerobic digestion of wine lees, adding different microparticles.
Methane production results for Rm (mL CH4∙gVS−1∙d−1) were 33.46, 45.38, and 46.54 mL CH4∙g−1 SV∙d−1 for the Lees Control, Lees Gr, and Lees Fe treatments, respectively, for the Gompertz model. Previous research documented by Arenas Sevillano et al. [9] reported a daily production rate of 27 mL CH4∙g−1 SV∙d−1; whereas, Da Ros et al. [49] found a rate of 24 mL CH4∙g−1 SV∙d−1, similar to the values observed in the control trial of this study. Once again, the positive effect of the presence of microparticles resulted in a considerably higher rate of organic matter conversion to methane. Complementarily, the production kinetics were estimated by the specific growth rate of microorganisms (K) when the first-order model was applied, resulting in 0.350, 0.398, and 0.401 d−1 for the Lees Control, Lees Gr, and Lees Fe treatments, respectively. Consequently, the addition of iron particles contributed to reporting higher values in the indices for both models.
The substantial variation in daily biomethane production rates, ranging from 35% to 40%, according to the Gompertz model for the respective two distinct particle types, suggests the potential operational benefits of integrating it into the AD process of wine lees in an anaerobic reactor. This integration would optimize, on the one hand, the biomethane production, and, on the other hand, the potential reduction in the hydraulic residence time (HRT), by accelerating the organic decomposition of the substrate. Consequently, this acceleration could lead to a smaller digester size, lower HRT, decreased investment costs for the operator, and, ultimately, a more sustainable process. However, inhibition mediated by the VFA accumulation and acidification considerably limited the bioprocess, leading to a poor biogas quality generation holding very low methane concentration, as detected in the last steps of the digestion process.
4. Conclusions
The addition of iron-based microparticles to the anaerobic digestion (AD) process of wine lees resulted in a substantial 39.1% increase in biomethane production rates; whereas, the introduction of graphite microparticles yielded a significant 35.6% increase. Nevertheless, inhibiting factors, such as an elevated volatile fatty acid (VFA) content and low pH values, were identified during the AD process and were not avoided with the presence of the particles. Additionally, the biogas content of hydrogen sulfide (H2S) exhibited a rapid increase along the anaerobic digestion process of wine lees, highlighting a potential area for further investigation and process optimization aiming to mitigate the impact of the presence of inhibitory substances in the medium on the efficiency of biomethane production from wine lees. The next steps of research development will focus on the design and development of a reactor under continuous or semi-continuous conditions aiming to verify whether the inhibition period mediated by VFAs or phenolic substances may be overcome by adapting the microorganism to the substrate. This will complementarily require the design of a magnetic system for a microparticle separation system that would enable the recovery and reuse of magnetic iron-based materials after anaerobic digestion.
Author Contributions
A.G.Á.: Conceptualization, Methodology, Investigation, Writing—Original Draft; C.R.P.: Methodology, Investigation. D.H.: Conceptualization, Methodology, Writing—Review and Editing, Supervision. Funding acquisition; A.G.: Conceptualization, Writing—Review and Editing; R.M.: Conceptualization, Supervision; I.d.G.: Writing—Review and Editing, Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Regional Government of Castilla y León and the EU-FEDER Programme (CL-EI-2021-07), Grant PID 2020-114918RB-I00; funded by MCIN/AEI/10.13039/501100011033; and funded by LIFE Programme through LIFE SMART AgroMobility (LIFE19 CCM/ES/001206).
Data Availability Statement
The data and material used in this research are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ginni, G.; Kavitha, S.; Kannah, Y.; Bhatia, S.K.; Kumar, A.; Rajkumar, M.; Gopalakrishnan, K.; Arivalagan, P.; Nguyen, T.L.C.; Rajesh, B.J.; et al. Valorization of agricultural residues: Different biorefinery routes. J. Environ. Chem. Eng. 2021, 9, 105435. [Google Scholar] [CrossRef]
- Mahari, W.A.W.; Waiho, K.; Fazhan, H.; Necibi, M.C.; Hafsa, J.; Ben Mrid, R.; Fal, S.; El Arroussi, H.; Peng, W.; Tabatabaei, M.; et al. Progress in valorisation of agriculture, aquaculture and shellfish biomass into biochemicals and biomaterials towards sustainable bioeconomy. Chemosphere 2021, 291, 133036. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Summary Progress Update 2021: SDG 15-Life on Land. 2021. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=SDG_15_-_Life_on_land (accessed on 1 June 2023).
- United Nations. Summary Progress Update 2021: SDG 13-Climate Action. 2021. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=SDG_13_-_Climate_action (accessed on 1 June 2023).
- Wei, M.; Ma, T.; Ge, Q.; Li, C.; Zhang, K.; Fang, Y.; Sun, X. Challenges and opportunities of winter vine pruning for global grape and wine industries. J. Clean. Prod. 2022, 380, 135086. [Google Scholar] [CrossRef]
- Mastoras, P.; Zkeri, E.; Panara, A.; Dasenaki, M.E.; Maragou, N.C.; Vakalis, S.; Fountoulakis, M.S.; Thomaidis, N.S.; Stasinakis, A.S. Application of a pilot-scale solar still for wine lees management: Characterization of by-products and valorization potential. J. Environ. Chem. Eng. 2023, 11, 111227. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Romero-García, J.M.; López-Linares, J.C.; Romero, I.; Castro, E. Residues from grapevine and wine production as feedstock for a biorefinery. Food Bioprod. Process. 2022, 134, 56–79. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive compounds from vine shoots, grape stalks, and wine lees: Their potential use in agro-food chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Sevillano, C.B.A.; Chiappero, M.; Gomez, X.; Fiore, S.; Martínez, E.J. Improving the anaerobic digestion of wine-industry liquid wastes: Treatment by electro-oxidation and use of biochar as an additive. Energies 2020, 13, 5971. [Google Scholar] [CrossRef]
- Álvaro, A.G.; Palomar, C.R.; Torre, R.M.; Redondo, D.H.; Crespo, I.d.G. Hybridization of anaerobic digestion with solar energy: A solution for isolated livestock farms. Energy Convers. Manag. X 2023, 20, 100488. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and future strategies for wine yeast lees valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef]
- Poirier, S.; Chapleur, O. Inhibition of anaerobic digestion by phenol and ammonia: Effect on degradation performances and microbial dynamics. Data Brief 2018, 19, 2235–2239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jay, J.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.; Arjmand, M.; Eskicioglu, C. Nanomaterial-amended anaerobic sludge digestion: Effect of pH as a game changer. Environ. Res. 2024, 240, 117463. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, D.; Zhang, K.; Ma, Y.; Liu, F.; Li, Z.; Gao, X.; Gao, W.; Du, L. Effects of initial volatile fatty acid concentrations on process characteristics, microbial communities, and metabolic pathways on solid-state anaerobic digestion. Bioresour. Technol. 2023, 369, 128461. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, C.; Cavinato, C.; Pavan, P.; Bolzonella, D. Mesophilic and thermophilic anaerobic co-digestion of winery wastewater sludge and wine lees: An integrated approach for sustainable wine production. J. Environ. Manag. 2017, 203, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Hungría, J.; Siles, J.A.; Chica, A.F.; Gil, A.; Martín, M.A. Anaerobic co-digestion of winery waste: Comparative assessment of grape marc waste and lees derived from organic crops. Environ. Technol. 2021, 42, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- Strong, P.J.; Burgess, J.E. Fungal and enzymatic remediation of a wine lees and five wine-related distillery wastewaters. Bioresour. Technol. 2008, 99, 6134–6142. [Google Scholar] [CrossRef] [PubMed]
- Romero-Díez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-Rojo, S.; Cocero, M.; Matias, A. Microwave and ultrasound pre-treatments to enhance anthocyanins extraction from different wine lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Li, Y.; Li, Q.; Li, P.; Luo, L.; Zhen, F.; Zheng, G.; Sun, Y. Low-Temperature Pretreatment of Biomass for Enhancing Biogas Production: A Review. Fermentation 2022, 8, 562. [Google Scholar] [CrossRef]
- François, M.; Lin, K.-S.; Rachmadona, N.; Khoo, K.S. Advancement of nanotechnologies in biogas production and contaminant removal: A review. Fuel 2023, 340, 127470. [Google Scholar] [CrossRef]
- Hassanein, A.; Kumar, A.N.; Lansing, S. Impact of electro-conductive nanoparticles additives on anaerobic digestion performance-A review. Bioresour. Technol. 2021, 342, 126023. [Google Scholar] [CrossRef] [PubMed]
- Dehhaghi, M.; Tabatabaei, M.; Aghbashlo, M.; Panahi, H.K.S.; Nizami, A.-S. A state-of-the-art review on the application of nanomaterials for enhancing biogas production. J. Environ. Manag. 2019, 251, 109597. [Google Scholar] [CrossRef] [PubMed]
- Suanon, F.; Sun, Q.; Mama, D.; Li, J.; Dimon, B.; Yu, C.-P. Effect of nanoscale zero-valent iron and magnetite (Fe3O4) on the fate of metals during anaerobic digestion of sludge. Water Res. 2016, 88, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Mahar, R.B.; Soomro, R.A.; Sherazi, S.T.H. Fe3O4 nanoparticles facilitated anaerobic digestion of organic fraction of municipal solid waste for enhancement of methane production. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1815–1822. [Google Scholar] [CrossRef]
- Hassanein, A.; Lansing, S.; Tikekar, R. Impact of metal nanoparticles on biogas production from poultry litter. Bioresour. Technol. 2018, 275, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; Mayumi, M.; Syo, K.; Satoshi, A.; Seiichi, Y.; Takashima, S.; Ono, H.; Ap, Y.; Yamashiro, T.; Ahmed, M.M.; et al. Potential of biogas production from manure of dairy cattle fed on natural soil supplement rich in iron under batch and semi-continuous anaerobic digestion. Bioresour. Technol. 2020, 309, 123298. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, Q.; Ye, X.; Wang, C.; Jia, Z.; Du, J.; Kong, X.; Xi, Y. Effect of different charged Fe3O4 nanoparticles on methane production for anaerobic digestion of wheat straw. J. Clean. Prod. 2021, 328, 129655. [Google Scholar] [CrossRef]
- Ünşar, E.K.; Perendeci, N.A. What kind of effects do Fe2O3 and Al2O3 nanoparticles have on anaerobic digestion, inhibition or enhancement? Chemosphere 2018, 211, 726–735. [Google Scholar] [CrossRef]
- Abdallah, M.S.; Hassaneen, F.Y.; Faisal, Y.; Mansour, M.S.; Ibrahim, A.; Abo-Elfadl, S.; Salem, H.; Allam, N.K. Effect of Ni-Ferrite and Ni-Co-Ferrite nanostructures on biogas production from anaerobic digestion. Fuel 2019, 254, 115673. [Google Scholar] [CrossRef]
- Ma, H.; Hu, Y.; Kobayashi, T.; Xu, K.-Q. The role of rice husk biochar addition in anaerobic digestion for sweet sorghum under high loading condition. Biotechnol. Rep. 2020, 27, e00515. [Google Scholar] [CrossRef]
- Lee, J.T.; Lim, E.Y.; Zhang, L.; Tsui, T.-H.; Tian, H.; Yan, M.; Lim, S.; Majid, M.b.A.; Jong, M.-C.; Zhang, J.; et al. Methanosarcina thermophila bioaugmentation and its synergy with biochar growth support particles versus polypropylene microplastics in thermophilic food waste anaerobic digestion. Bioresour. Technol. 2022, 360, 127531. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Qiao, S.; Li, X.; Zhang, M.; Zhou, J. Nano-graphene induced positive effects on methanogenesis in anaerobic digestion. Bioresour. Technol. 2017, 224, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Y.; Ji, D.; Li, X.; Zhang, J.; Zang, L. Synergetic promotion of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with graphite felt in anaerobic digestion. Bioresour. Technol. 2019, 287, 121373. [Google Scholar] [CrossRef] [PubMed]
- Muratçobanoğlu, H.; Gökçek, B.; Mert, R.A.; Zan, R.; Demirel, S. Simultaneous synergistic effects of graphite addition and co-digestion of food waste and cow manure: Biogas production and microbial community. Bioresour. Technol. 2020, 309, 123365. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Reyes, F.L.d.L.; Call, D.F. Call, Amending anaerobic bioreactors with pyrogenic carbonaceous materials: The influence of material properties on methane generation. Environ. Sci. Water Res. Technol. 2018, 4, 1794–1806. [Google Scholar] [CrossRef]
- Casals, E.; Barrena, R.; García, A.; González, E.; Delgado, L.; Busquets-Fité, M.; Font, X.; Arbiol, J.; Glatzel, P.; Kvashnina, K.; et al. Programmed Iron Oxide Nanoparticles Disintegration in Anaerobic Digesters Boosts Biogas Production. Small 2014, 10, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; de Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Álvaro, A.G.; Palomar, C.R.; Redondo, D.H.; Torre, R.M.; Crespo, I.d.G. Simultaneous production of biogas and volatile fatty acids through anaerobic digestion using cereal straw as substrate. Environ. Technol. Innov. 2023, 31, 103215. [Google Scholar] [CrossRef]
- Ghofrani-Isfahani, P.; Baniamerian, H.; Tsapekos, P.; Alvarado-Morales, M.; Kasama, T.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Effect of metal oxide based TiO2 nanoparticles on anaerobic digestion process of lignocellulosic substrate. Energy 2019, 191, 116580. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water & Wastewater, 22nd ed.; American Water Works Association: Denver, CO, USA, 2012. [Google Scholar]
- Box, J.D. Investigation of the Folin-Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res. 1983, 17, 511–525. [Google Scholar] [CrossRef]
- LAY, J.J.; Li, Y.Y.; Noike, T. Student, Effect of moisture content and chemical nature on methane fermentation characteristics of municipal solid wastes. Doboku Gakkai Ronbunshu 1996, 552, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Nopharatana, A.; Pullammanappallil, P.C.; Clarke, W.P. Kinetics and dynamic modelling of batch anaerobic digestion of municipal solid waste in a stirred reactor. Waste Manag. 2007, 27, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Shamurad, B.; Gray, N.; Petropoulos, E.; Tabraiz, S.; Membere, E.; Sallis, P. Predicting the effects of integrating mineral wastes in anaerobic digestion of OFMSW using first-order and Gompertz models from biomethane potential assays. Renew. Energy 2020, 152, 308–319. [Google Scholar] [CrossRef]
- Cano, R.; Nielfa, A.; Fdz-Polanco, M. Thermal hydrolysis integration in the anaerobic digestion process of different solid wastes: Energy and economic feasibility study. Bioresour. Technol. 2014, 168, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, C.; Cavinato, C.; Bolzonella, D.; Pavan, P. Renewable energy from thermophilic anaerobic digestion of winery residue: Preliminary evidence from batch and continuous lab-scale trials. Biomass Bioenergy 2016, 91, 150–159. [Google Scholar] [CrossRef]
- Fabbri, A.; Bonifazi, G.; Serranti, S. Micro-scale energy valorization of grape marcs in winery production plants. Waste Manag. 2014, 36, 156–165. [Google Scholar] [CrossRef]
- Jasko, J.; Skripsts, E.; Dubrovskis, V. Biogas production of winemaking waste in anaerobic fermentation process. In Proceedings of the 11th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 24–25 May 2012. [Google Scholar]
- Dinuccio, E.; Balsari, P.; Gioelli, F.; Menardo, S. Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses. Bioresour. Technol. 2010, 101, 3780–3783. [Google Scholar] [CrossRef]
- Kalia, V.; Kumar, A.; Jain, S.; Joshi, A. Biomethanation of plant materials. Bioresour. Technol. 1992, 41, 209–212. [Google Scholar] [CrossRef]
- Almeida, P.; Gando-Ferreira, L.; Quina, M. Biorefinery perspective for industrial potato peel management: Technology readiness level and economic assessment. J. Environ. Chem. Eng. 2023, 11, 110049. [Google Scholar] [CrossRef]
- Khoufi, S.; Louhichi, A.; Sayadi, S. Optimization of anaerobic co-digestion of olive mill wastewater and liquid poultry manure in batch condition and semi-continuous jet-loop reactor. Bioresour. Technol. 2015, 182, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Wall, D.M.; Herrmann, C.; Murphy, J.D. A detailed assessment of resource of biomethane from first, second and third generation substrates. Renew. Energy 2016, 87, 656–665. [Google Scholar] [CrossRef]
- Galí, A.; Benabdallah, T.; Astals, S.; Mata-Alvarez, J. Modified version of ADM1 model for agro-waste application. Bioresour. Technol. 2009, 100, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Vivekanand, V. Combined fungal and bacterial pretreatment of wheat and pearl millet straw for biogas production—A study from batch to continuous stirred tank reactors. Bioresour. Technol. 2020, 321, 124523. [Google Scholar] [CrossRef] [PubMed]
- Sravan, J.S.; Tharak, A.; Mohan, S.V. Chapter 1-Status of biogas production and biogas upgrading: A global scenario. In Emerging Technologies and Biological Systems for Biogas Upgrading; Aryal, N., Mørck Ottosen, L.D., Wegener Kofoed, M.V., Pant, D., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 3–26. [Google Scholar] [CrossRef]
- Park, J.-H.; Kang, H.-J.; Park, K.-H.; Park, H.-D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Dhar, B.R. Advances towards understanding and engineering direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 244, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Gahlot, P.; Ahmed, B.; Tiwari, S.B.; Aryal, N.; Khursheed, A.; Kazmi, A.; Tyagi, V.K. Conductive material engineered direct interspecies electron transfer (DIET) in anaerobic digestion: Mechanism and application. Environ. Technol. Innov. 2020, 20, 101056. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kang, H.-J.; Kim, Y.; Kim, N.-K.; Park, H.-D. Different contribution of exoelectrogens in methanogenesis via direct interspecies electron transfer (DIET) by the different substrate in continuous anaerobic bioreactor. Bioresour. Technol. 2022, 364, 128115. [Google Scholar] [CrossRef]
- Kassab, G.; Khater, D.; Odeh, F.; Shatanawi, K.; Halalsheh, M.; Arafah, M.; van Lier, J.B. Impact of Nanoscale Magnetite and Zero Valent Iron on the Batch-Wise Anaerobic Co-Digestion of Food Waste and Waste-Activated Sludge. Water 2020, 12, 1283. [Google Scholar] [CrossRef]
- Başar, I.A.; Eskicioglu, C.; Perendeci, N.A. Biochar and wood ash amended anaerobic digestion of hydrothermally pretreated lignocellulosic biomass for biorefinery applications. Waste Manag. 2022, 154, 350–360. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, Z.; Zhao, Z.; Quan, X.; Zhao, Z. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. J. Clean. Prod. 2017, 149, 1101–1108. [Google Scholar] [CrossRef]
- Powell, C.D.; Atkinson, A.J.; Ma, Y.; Marcos-Hernandez, M.; Villagran, D.; Westerhoff, P.; Wong, M.S. Magnetic nanoparticle recovery device (MagNERD) enables application of iron oxide nanoparticles for water treatment. J. Nanoparticle Res. 2020, 22, 48. [Google Scholar] [CrossRef]
- Nurmi, J.T.; Sarathy, V.; Tratnyek, P.G.; Baer, D.R.; Amonette, J.E.; Karkamkar, A. Recovery of iron/iron oxide nanoparticles from solution: Comparison of methods and their effects. J. Nanoparticle Res. 2010, 13, 1937–1952. [Google Scholar] [CrossRef]
- Chiappero, M.; Berruti, F.; Fiore, S. Biomethane potential of wine lees from mesophilic anaerobic digestion. Biochem. Eng. J. 2023, 196, 108954. [Google Scholar] [CrossRef]
- Yeole, T.; Gokhale, S.; Hajarnis, S.; Ranade, D. Effect of brackish water on biogas production from cattle dung and methanogens. Bioresour. Technol. 1996, 58, 323–325. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, N. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438. [Google Scholar] [CrossRef]
- Gavala, H.N.; Angelidaki, I.; Ahring, B.K. Kinetics and Modeling of Anaerobic Digestion Process. In Biomethanation; Ahring, B.K., Angelidaki, I., de Macario, E.C., Gavala, H.N., Hofman-Bang, J., Macario, A.J.L., Elferink, S.J.W.H.O., Raskin, L., Stams, A.J.M., Westermann, P., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 57–93. [Google Scholar] [CrossRef]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef]
- Jakubíková, M.; Sádecká, J.; Hroboňová, K. Determination of total phenolic content and selected phenolic compounds in sweet wines by fluorescence spectroscopy and multivariate calibration. Microchem. J. 2022, 181, 107834. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Pramanik, A.; Maji, S.K.; Haldar, S.; Mukhopadhyay, U.K.; Mukherjee, J. Utilization of vinasse for production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei. AMB Express 2012, 2, 34. [Google Scholar] [CrossRef]
- Freitas, P.V.; da Silva, D.R.; Beluomini, M.A.; da Silva, J.L.; Stradiotto, N.R. Determination of Phenolic Acids in Sugarcane Vinasse by HPLC with Pulse Amperometry. J. Anal. Methods Chem. 2018, 2018, 4869487. [Google Scholar] [CrossRef]
- Girault, R.; Bridoux, G.; Nauleau, F.; Poullain, C.; Buffet, J.; Steyer, J.-P.; Sadowski, A.G.; Béline, F. A waste characterisation procedure for ADM1 implementation based on degradation kinetics. Water Res. 2012, 46, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Pavlostathis, S.G.; Giraldo-Gomez, E. Kinetics of Anaerobic Treatment. Water Sci. Technol. 1991, 24, 35–59. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.A.; Vavilin, V.A. The IWA Anaerobic digestion model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources. In Focus on Catalysts, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2011; p. 8. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Singh, R.N.; Tripathi, A.K.; Rauniyar, S.; Saxena, P.; Thakur, P.; Sani, R.K. Chapter 11-Biochemical and molecular mechanisms of sulfate-reducing bacterial biofilms. In Understanding Microbial Biofilms; Das, S., Kungwani, N.A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 165–172. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Pandey, A. Chapter 6-Landfill Gas as an Energy Source. In Current Developments in Biotechnology and Bioengineering; Kumar, S., Kumar, R., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 93–117. [Google Scholar] [CrossRef]
- Gunaseelan, V. Biochemical methane potential of fruits and vegetable solid waste feedstocks. Biomass Bioenergy 2004, 26, 389–399. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).