Abstract
This study addresses the challenge of mitigating ammonia and greenhouse gas (GHG) emissions from stored pig slurry using chemical and biological additives. The research employs dynamic chambers to evaluate the effectiveness of these additives. Chemical agents (sulfuric acid) and biological additives (DAB bacteria) containing specific microbial strains are tested (a mixture of Rhodopseudomonas palustris, Bacillus subtilis, Bacillus amyloliquefaciens, Bacillus licheniformis, Nitrosomona europea, Nictobacter winogradaskyi, and nutritional substrate). Controlled experiments simulate storage conditions and measure emissions of ammonia, methane, and carbon dioxide. Through statistical analysis of the results, this study evaluates the additives’ impact on emission reduction. Sulfuric acid demonstrated a reduction of 92% in CH4, 99% in CO2, and 99% in NH3 emissions. In contrast, the biological additives showed a lesser impact on CH4, with an 8% reduction, but more substantial reductions of 71% for CO2 and 77% for NH3.These results shed light on the feasibility of employing these additives to mitigate environmental impacts in pig slurry management and contribute to sustainable livestock practices by proposing strategies to reduce the ecological consequences of intensive animal farming.
1. Introduction
Pig farming is a significant sector of global livestock production, and pig manure is generated in substantial quantities worldwide. The quantity of pig slurry production varies across countries and is influenced by different factors such as the size of the pig population, farming practices, and the intensity of pig production [1]. Some of the top pig-producing countries include China, those in the European Union (EU), and the United States [2]; these countries have large pig populations, and as a result, they generate significant amounts of pig manure. Indeed, the high demand for pork production results in a significant generation of pig manure (PM) globally; it is estimated that approximately 1.7 billion tons of pig manure is generated annually worldwide [3]. It is important to note that pig manure production is influenced not only by the number of pigs but also by the management practices employed, including manure handling, storage, and treatment systems. Different countries and regions have varying regulations and practices in place to manage pig manure and mitigate its potential environmental impacts; in the United States, for example, the U.S. Environmental Protection Agency (EPA) regulates pig manure under the Clean Water Act. Large-scale pig farms, known as Concentrated Animal Feeding Operations (CAFOs), are required to obtain permits and implement manure management plans [4]. These plans often involve the use of manure storage structures, nutrient management practices, and strategies to minimize runoff and water pollution. The European Union also has specific regulations regarding pig manure management through the Nitrate Directive (1991/676/EEC) [5] and the Water Framework Directive (2000/60/EC) [6]. Member states are required to implement measures to control nutrient losses from agricultural activities, including pig farming, which involves nutrient management planning, restricting manure application during certain periods, and promoting the best agricultural practices to reduce environmental impacts.
These examples highlight the diversity of the approaches taken by different countries and regions to manage pig manure and mitigate its environmental impacts. Specific regulations and practices can vary based on local environmental conditions, agricultural practices, and government priorities, all aimed at promoting sustainable pig farming and minimizing environmental pollution.
This substantial amount of pig manure poses challenges in terms of its management, environmental impact, and associated issues such as greenhouse gas emissions and nutrient management [7]. Improper storage, handling, and disposal of manure can lead to nutrient runoff, contaminating water bodies and causing eutrophication. It can also release greenhouse gases such as methane (CH4) and ammonia (NH3), which contribute to climate change [8]. Treating pig manure can help mitigate greenhouse gas emissions, such as methane and nitrous oxide, and mitigate the environmental impact of ammonia [9].
The concept of a circular economy in pig slurry treatment aims to reduce environmental impact, specifically focusing on mitigating the ammonia, methane, and carbon dioxide emissions associated with pig farming. By integrating innovative technologies and the most sustainable practices, the circular economy model for pig slurry treatment offers a comprehensive and environmentally friendly solution, aligning with broader goals of sustainability and climate mitigation in agriculture [10]. In this study, two different treatments will be applied to the same type of pig manure in order to study their mitigation of ammonia, methane, and carbon dioxide emissions.
This study is situated within a context of increasing interest in reducing emissions of ammonia and greenhouse gas in the agricultural sector, especially concerning livestock manure. In addressing this challenge, there has been a trend towards exploring a wide range of treatments and techniques. These include widely used approaches such as solid–liquid separation [11], aerobic biological nitrogen removal [12], and anaerobic digestion [13,14]. Nevertheless, the application of these treatments comes with significant expenses and requires specialized expertise for an effective and optimal implementation. An alternative approach involves the incorporation of chemical or microbial additives into slurry-managed manure. This approach offers a more accessible and cost-effective solution, as it can be readily applied by farmers following provided guidelines and instructions. These additives are designed to influence specific properties of the slurry by either inhibiting or stimulating specific microbiological processes [15].
One common approach has been the use of acidification, and recent research has demonstrated the effectiveness of this technology in reducing ammonia emissions of livestock manure [16,17,18]. Another promising approach has been the use of biological additives. However, the few studies conducted in this line have reported a suboptimal efficacy of these products [19]. The varying results observed in prior experiments with additives highlight the need for a more comprehensive comprehension of the impact of specific products when implemented in real-world scenarios, and not in controlled laboratory conditions.
Recently, novel categories of biological additives have entered the market, boasting additional attributes such as the potential to facilitate nitrogen removal through denitrification, achieved with the introduction of anaerobic bacteria [20]. While this potential effect holds promise for alleviating nitrogen excess in intensive livestock areas, it is still under investigational conditions.
In a study by Wheeler [21], a total of 22 additives were tested. These additives encompassed microbial digestion products, oxidizing agents and chemicals, disinfectants, odor-masking agents, and adsorbents. The results revealed a diverse range of effects: certain additives led to a decrease in ammonia emissions, while others resulted in an increase, and some demonstrated no influence on ammonia emissions.
Another study, by Van der [22], conducted an assessment of several biological additives tailored to mitigate ammonia emissions from dairy slurry. These formulations included Agri-mest (Agriton), designed to augment the anaerobic fermentation of manure via microorganisms; Effective Microorganisms (EMs), composed of lactic acid bacteria, yeast, and smaller quantities of diverse microorganisms; and Euro Mestmix (Ecostyle), a blend of pH buffering agents, clay minerals, and undisclosed supplements designed to enhance microorganism activities. In general, no discernible reduction in ammonia emissions was observed with the application of these products [22].
The aim of the present research was to assess the impact of a commercially available biological additives, anticipated to enhance denitrification, when administered to the slurry within a commercial pig fattening farm. The use of these biological additives, particularly the DAB bacteria formula, represents a novel approach in this field of study, marking new contributions for scientific knowledge. Furthermore, this research aims to facilitate decision-making in the choice between chemical and biological agents by comparing their effectiveness when applied to the same type of slurry under identical conditions.
The outlined study has two main objectives: the first objective is to validate the use of dynamic chamber floats for measuring gaseous emissions from slurry storage. This research intends to assess the effectiveness and accuracy of using dynamic chamber floats as a measurement method for quantifying gaseous emissions (specifically NH3, CH4, and CO2) from slurry storage facilities. By testing the validity of this method, the researchers aim to establish its reliability for future emission measurements. The second objective is to characterize the dynamics of gaseous emissions during the storage of slurry. This objective seeks to investigate the patterns and variations in gaseous emissions (NH3, CH4, and CO2) during a slurry storage period with and without any chemical or biological additives. The goal is to analyze the temporal and spatial changes in gaseous emissions throughout the storage period, capturing the emissions’ dynamics. This information will help in understanding the factors influencing emission variations and guide the determination of appropriate periods and durations for estimating gaseous emission factors in pig manure storage with and without additives.
2. Materials and Methods
2.1. Experimental Design
The experiment was conducted at a white pig farm in Fuente Alamo, located in the Murcia region, a municipality in the southeastern part of Spain, from 18 April to 22 June 2023. The pilot study was designed to simulate manure conditions in a swine manure storage pool; this portable pool was purchased from Bestway®, España, Spain (ref. 84265026), and is a circle with dimensions of Ø 457 × 122 cm.
Slurry was representatively divided among the experimental pools, and thus each pool was filled with 13 m3 of slurry (1 m depth); the selected volume was determined with the objective of fulfilling the requirements of VERA protocol [23]. Two pools were subjected to manure storage with chemical and biological additives, whereas one pool was not treated and served as a control. Slurry composition and gaseous emissions were monitored during the experiment.
The experiment consisted of three phases. The initial phase lasted for the first 7 days of storage and all pools were subjected to the same management. During this period, none of the additives were applied. During this phase, the pig slurry was sampled to study differences among the pools; also, the GHG and ammonia emissions were evaluated.
The second phase lasted for 10 days, during which the slurry was acidified with sulfuric acid (H2SO4) to pH 5.5, whereas the slurry in the other pool was fed a biological additive. The last phase involved greenhouse gas flux measurements, which were performed twice per week by placing the floating dynamic chamber open on the pools and collecting gas samples at T0 = 0 min and at T30 = 30 min following chamber deployment and the recommendations of the VERA protocol [23].
2.2. Use of Additives
The biological additive used in this experiment was purchased from DAB-Biotecnología, Spain. This product is commercialized under references DAB ACF-32 and DAB ACF-AD, and contains a mixture of Rhodopseudomonas palustris, Bacillus subtilis, Bacillus amyloliquefaciens, Bacillus licheniformis, Nitrosomona europea, Nictobacter winogradaskyi, and nutritional substrates.
The bacterial complex was designed to accelerate the process of organic matter degradation (biological oxidation). Through a controlled bacterial process, we aimed to achieve an improvement in slurry management via liquefaction, reaching an effective control and reduction in total nitrogen, GHG, and ammonia. The addition of the DAB product was performed following the manufacturer’s directions, and it was distributed homogeneously on the pig manure. The dosage used was 0.4 kg of DAB biotechnology product per m3 of pig manure.
The acidification process in storage tanks can be categorized as either short-term or long-term acidification, depending on when it is conducted. Long-term acidification was chosen for this study, and this design is illustrated in Figure 1; the effectiveness of this method has been discussed in previous studies [24,25,26].
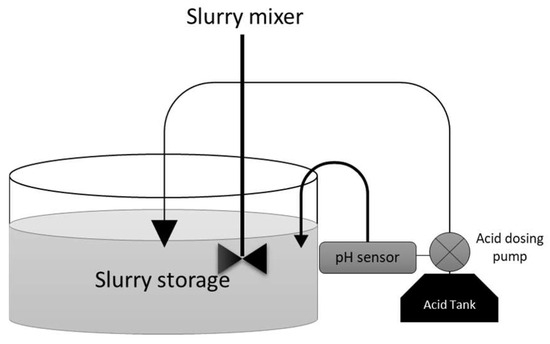
Figure 1.
Storage tank acidification.
In this investigation, long-term acidification was implemented using concentrated sulfuric acid (96% H2SO4) to gradually attain a pH of 5.5. The total volume added amounted to 32.76 L, equivalent to 12 cubic meters of the treated pig slurry [27]. This process was meticulously automated, with a specialized dispenser overseeing the precise control of pH levels. This was achieved through the integration of a pH sensor with an acid-dosing pump, ensuring consistent and accurate acid dosage throughout the acidification process.
2.3. Dynamic Chamber
A dynamic chamber is a specialized piece of equipment used in greenhouse gas emission studies to measure the release of gases, such as methane (CH4), nitrous oxide (N2O), carbon dioxide (CO2), and ammonia (NH3) from various sources, including pig slurry.
The dynamic chamber technique involves enclosing the emission source (in this case, pig slurry) within a chamber, which is made of a material that is non-sticky to the gases that are being investigated [23]; in this study, PVC was chosen [28], and it was carefully sealed to prevent gas exchange with the surrounding environment, allowing the collection and measurement of GHG emissions over a specific time.
In this study, an open dynamic chamber was used to cover a part of the liquid surface; a 0.564 m2 area of the pig slurry surface was covered with the dynamic chamber respecting the recommendation of the VERA protocol [23], which specifies the optimal size of the sampling area for measurement with a partial covering exposed to flow (at least 0.5 m2).
This chamber was designed to have an opening or an inlet for the entry of ambient air and an outlet for the exit of the chamber air. This design facilitates the continuous exchange of gases between the chamber and the surrounding environment. The airflow through the chamber has to be carefully regulated and monitored; for this reason, in this study regulation was achieved by using a controlled and adjustable pump at the input, and a suction device at the output of the chamber.
The rate of airflow into the chamber is an essential parameter for determining the dilution of emitted gases and the overall ventilation conditions. Therefore, an anemometer was installed inside of the chamber to control the airflow rate during the measurement and configurated at a speed of 0.2 m s−1, as is recommended by the VERA protocol [23].
The chamber includes strategically positioned gas sampling ports, through which air samples can be extracted. These ports are designed to ensure representative sampling of the air within the chamber. The input and output ports are connected to the analytical equipment for subsequent gas concentration analysis; in this study, GASERA ONE was used to measure the gas concentrations of interest to this research.
The sampling duration operates over a specific sampling period to capture a representative range of emissions. The duration of the sampling period can vary depending on the emission source, measurement objectives, and other factors [29]. Regarding the VERA protocol [23], the sampling duration should be at least 30 min per sampling point, which was considered during measurement.
The gaseous emission was expressed as F (flux measured within the dynamic chamber) in kg (gas), ha−1 h−1, and is calculated using the following equation [23]:
where Cout and Cin are the time-averaged gaseous concentrations of the gas (kg m−3) in the outlet and inlet air, respectively, Ai is the cross-sectional area of the inlet (m2), Vi is the measured wind speed at the tunnel inlet (m s−1), and Ab is the source surface area covered by the tunnel canopy (m2).
The environmental conditions and parameters, such as temperature, humidity, and pressure, inside the chamber were measured often to ensure the measurement conditions were accurately documented and accounted for in the emission calculations.
Overall, the dynamic chamber technique provides a means to quantify GHG emissions from pig slurry and helps in understanding the environmental impact of pig farming operations. It can also aid in the development of mitigation strategies to reduce GHG emissions in the agricultural sector.
2.4. Sampling Frequency
Our study aimed to assess seasonal variations in gas emissions by determining the total annual gas emissions from stored slurry. This assessment involved using multiple measurement periods, each lasting approximately four weeks, with a measurement frequency of twice per week, as recommended by the VERA protocol [23], conducted under different ambient temperature conditions. The specified measurement periods occurred in spring 2023 (from 18 April to 22 June 2023).
During each measurement period, the 30 min average emission rates for each gas were calculated based on air flow rates and gas concentrations obtained at intervals of 10 min. Additionally, continuous records were kept for manure management practices, slurry properties, and environmental conditions, such as slurry temperature and ambient temperature, throughout the measurement periods.
2.5. Slurry Sample Analysis
The pH and electrical conductivity were measured in situ using HANNA multiparameter equipment (ref. HI98194). Samples of stored slurry were collected at the start of each GHG and ammonia measurement. At each of the three locations in the storage tank, the slurry was collected from the surface to 20 cm depth and was well mixed before being sampled for analysis, then stored and transported at 4 °C to the laboratory. The Kjeldahl nitrogen (KN) content was measured using a modified Kjeldahl method [30]. In this method, 1 mL of pig slurry was used for digestion. Ammonium nitrogen (NH4+–N) was determined via steam distillation, followed by titration with HCl 0.1 N. Total nitrogen (TN) encompassed both organic and inorganic forms, including Kjeldahl nitrogen, nitrite, and nitrates. To determine total phosphorus (TP), acidic hydrolysis and oxidation at 120 °C was carried out, followed by photometric analysis using a molybdenum blue reagent (Macherey–Nagel GmbH & Co., KG, Nanocolor Test; ref. 985-055, Düren, Germany). Potassium (K+) was determined using an atomic absorption spectrometer (PerkinElmer AA-Analyst 800, Jyväskylä, Finland).
For the determination of total suspended solids (TSSs), the sample was filtered through a pre-weighed standard glass-fiber filter. The residue retained on the filter was then dried at 105 °C until a constant weight was obtained, using the 2440-D method (APHA–AWWA–WEF, 2012). The biochemical oxygen demand in five days (BOD5) was determined using the OXITOP WTW equipment and measured with a manometer (Darmstadt, Germany). Chemical oxygen demand (COD) was determined via photometric analysis of the chromium (III) concentration after 2 h of oxidation with potassium dichromate/sulfuric acid and silver sulfate at 148 °C (Macherey–Nagel GmbH & Co., KG, Nanocolor Test; ref. 985 028/29, Weilheim, Germany) according to German standard methods DIN 38 409-H41-1 and DIN ISO 15 705-H45 [31]
2.6. Statistical Analysis
The data underwent statistical analysis using one-way ANOVA followed by Tukey’s HSD post hoc test for pairwise comparison of means (at a significance level of p < 0.05), employing the SPSS 24.0 software package, to finally identify significant differences through comparisons of the greenhouse gas emissions and ammonia, as well as by analyzing parameters of the pig slurry for both treatments (acidification and bacterial).
3. Results
3.1. Greenhouse Gas Emissions and Ammonia
Figure 2, Figure 3 and Figure 4 present a comprehensive overview of the carbon dioxide (CO2), methane (CH4), and ammonia (NH3) emissions observed from both the raw and treated pig slurry samples over a period of five weeks. Notably, it is important to highlight that the treatment application was initiated after the initial measurement, ensuring a thorough evaluation of the effects of the additives. These figures provide valuable insights into the emission dynamics and variations in the greenhouse gases during the specified timeframe, allowing for a deeper understanding of the impact of these treatments on the slurry’s composition and environmental implications.
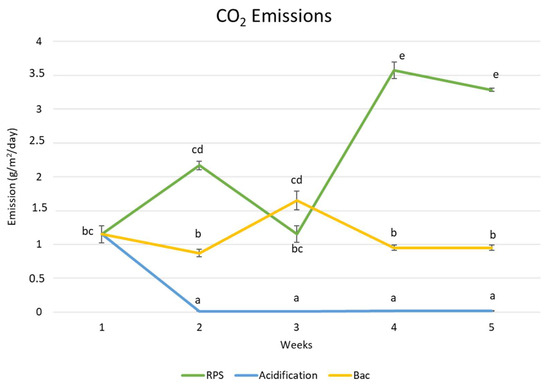
Figure 2.
Carbon dioxide (CO2) emission evolution of raw and treated pig slurry (RPS: raw pig slurry, Bac: bacteria). Different letters indicate significant differences between the sampling times and treatments (p < 0.05).
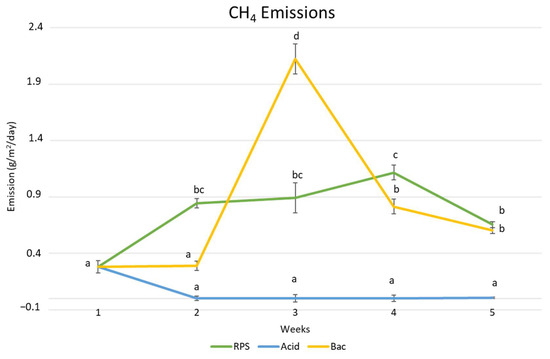
Figure 3.
Methane (CH4) emission evolution of raw and treated pig slurry (RPS: raw pig slurry, Bac: bacteria). Different letters indicate significant differences between the sampling times and treatments (p < 0.05).
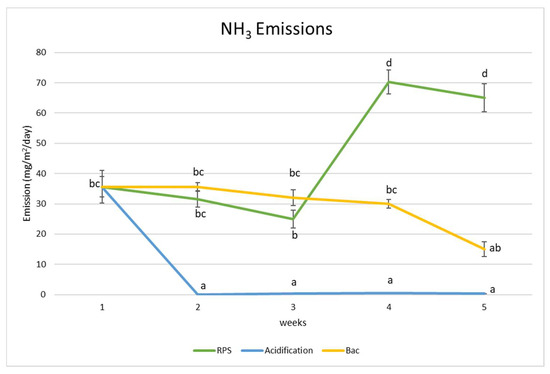
Figure 4.
Ammonia (NH3) emission evolution of raw and treated pig slurry (RPS: raw pig slurry, Bac: bacteria). Different letters indicate significant differences between the sampling times and treatments (p < 0.05).
3.2. Raw and Treated Pig Slurry Analysis
Table 1, Table 2 and Table 3 present the physicochemical characteristics, macro-nutrients, and micronutrients of pig slurry, respectively. This study’s research involved analyzing each parameter for both raw and treated pig slurry, enabling a comprehensive study of the treatment’s evolution and reaction during slurry storage. It is crucial to note that the treatment application occurred after the initial slurry sampling (first week). Significant differences (p < 0.05) in different parameters’ concentrations were found between the raw pig slurry pond and the treated slurry.

Table 1.
Means and standard deviation values of the physicochemical parameter’s evolution in raw and treated pig slurry.

Table 2.
Means and standard deviation values of the macronutrients parameter’s evolution in raw and treated pig slurry.

Table 3.
Means and standard deviation values of the micronutrients parameter’s evolution in raw and treated pig slurry.
4. Discussion
4.1. Methane (CH4) Emissions
As can be seen in Figure 3, the weekly emission of CH4 followed different trends for both treatments comparing to the control slurry; for acidification, the methane emission was significantly low (p < 0.05) compared to the control raw pig slurry (0.655 g/m2/day and 0.005 g/m2/day, respectively), while the biological additives showed no significant differences compared to the control slurry (0.602 g/m2/day).
The CH4 emissions from the PS were efficiently reduced through acidification. Methane emission was detected in the control (RPS) from the second week, with a significant increase compared to the first week, as well as during all of the study periods. According to Hjorth [32], acidification of slurry reduces methanogenesis. Shin [33] also confirmed the inhibition of methanogenesis due to the acidification of slurry, and Ottosen [18] explains how this acidification has an inhibitory effect on microbial activity and how the pH range affects it. However, other authors do not attribute this to the lower pH value but to the associated increase in free protonated fatty acids in the slurry. These have an inhibitory effect on microbial activity [34]. The optimal methane production through anaerobic digestion of pig slurry typically falls between 6.5 and 7.5 [35]. This range is considered ideal because it allows the microbial communities responsible for methane production to function most efficiently. The potential factor behind this phenomenon could involve an increase in the concentration of protonated acids, which function as inhibitory agents, according to Ottosen [18]. Only a small amount of research has explored the effects of acidification on methane emissions during the storage of slurry. Berg [36] reported that acidifying slurry was associated with a decrease in CH4 emissions during storage regardless of the specific pH target, as long as it remained below pH 5. The effects on CH4 emissions show notable variations depending on the type of acid. Several studies focusing on different pH levels have recorded reductions of more than 90% when utilizing lactic acid, in comparison to 67–87% with H2SO4, 40–65% with HCl, and 17–75% with nitric acid [25,36,37].
The inhibition of methanogenesis due to adding sulphates via sulfuric acid is demonstrated in our study, confirming reports of other studies [25,38,39]. This outcome could potentially account for the reduced CH4 emissions observed in our study’s research. Lowering the slurry pH reduces CH4 emission, but the mechanism of this inhibition is not completely understood. Other studies have demonstrated that acidification could only reduce emissions by 67% [34], while a common practice of lowering the pH to 5.5 with H2SO4 resulted in a CH4 reduction of 63–99% [17]. A reduction in CH4 was also detected in our research on acidified slurry, which is similar to previous studies [40,41]. Various acids, such as sulfuric acid [16,42,43], acetic acid, lactic acid [16,42,44], hydrochloric acid [34,42], or citric acid [16,44], have been investigated in various previous studies. However, both the cost of sulfuric acid and the amount of it required are very low compared to the other acids [43], and this was the reason why sulfuric acid was chosen for our study, since the results will be taken into account for the management of pig slurry by different farmers.
Additionally, the temperature of the slurry exerts a potent sway over CH4 emissions; elevated slurry temperatures correspond to heightened CH4 emissions from the slurry. Furthermore, the slurry temperature has a strong impact on CH4 emissions: with a higher slurry temperature, the CH4 emissions from the slurry increase [26,45]. In our study, the slurry temperature was higher in the third week (21.9 °C), which therefore resulted in an augmentation of CH4 emissions, but this was more noticeable when we used biological additives. Since the CH4 emissions can be reduced via acidification, the CH4 emissions in the acidification were very low during the research period. Hence, the effect of the slurry’s temperature was hardly noticeable.
Methane emissions after the biological additives were also reduced compared to the control raw pig slurry, as shown Figure 3. In the second week, significant differences were found with respect to the control, probably due to the fact that the bacterial formulations present in the additive reduce the anaerobic habitat present in pig slurry. This is because they promote the digestion of organic sludge, with a consequent inhibition of the anaerobic bacteria that carry out methanogenesis [46,47]. However, during the third week, there was a significant increase in CH4 emissions in the pond with biological additives compared to the control pond. The rise in temperature is directly related to the rise in CH4 emissions due to the fact that higher temperatures favor methylotrophic methanogenesis, which produces three more CH4 molecules than acetoclastic methanogenesis and two more CH4 molecules than hydrogenotrophic methanogenesis [48].
From the fourth week, there was a tendency towards reduced methane emissions in the pond with additives; once the temperature stabilized, the processes of the digestion of organic sludge and inhibition of methanogenesis due to the reduction in anaerobic bacteria began again [46,47]. Additionally, a pH below 5.5 or above 8 could reduce the gene abundance or activity of most enzymes involved in methanogenesis, such as acetate kinase, formylmethanofuran dehydrogenase, and tetrahydromethanopterin S-methyltransferase [49].
Furthermore, anaerobic oxidation combined with denitrification, carried out via methane-oxidizing bacterial strains that oxidize methane independently, uses the excess electrons from methane oxidation to reduce nitrates, effectively removing both nitrogen and methane [50,51].
4.2. Carbon Dioxide (CO2) Emissions
In livestock production, CO2 emissions mainly derive from the respiration of the animals inside the barns, and from the CO2 that is produced as a consequence of consuming fossil fuels, directly or indirectly, used for energy in the production, transportation, and processing of feed, and the maintenance of lighting, heating, or transport systems within the farm (vehicles, machinery, etc.), including changes in land use as a consequence of feed production; all of this would determine what is called a “carbon footprint” (Guía de las Mejores Técnicas Disponibles para reducir el impacto ambiental de la ganadería, 2017). The emission of CH4 and CO2 during the storage of manure depends on aeration conditions [52] and the microbial communities’ methanogenic activity, because acidic pH conditions can trigger the deceleration of methanogenic biomass growth [53], thus inhibiting the production of these gases and preventing their emission into the atmosphere.
The treatment based on slurry acidification can take place in situ where the livestock is generated, at the slurry storage unit, or right before valorization in the field. When acidification is performed in situ, it is considered long-term acidification, as the acid is added on a daily or weekly basis to the slurry channels or treatment tank. However, acidification performed in storage units can be either short- or long- term, depending on the time between the acidification and subsequent field application of the slurry [54].
According to Balnes-vidal [55], during the storing process, wastewater gas bubbles are formed in the bulk liquid containing 55 to 65% of CH4, 35 to 45% of CO2, and many other gases and volatile organic compounds, the majority of which are still unknown [56]. Therefore, as a consequence, the emission of these gases is strongly influenced by slurry agitation and could be explained by the high volatility and the release of these gases contained in gas bubbles during the storing process.
The tendency observed in this study towards CO2 emissions in the storage pond under acidification is caused by the different physicochemical properties that determine the mechanisms occurring in the release of this gas. In Figure 2, the significant differences (p < 0.05) between the acidified pig slurry and raw pig slurry for weeks 2–5. In our investigation, we demonstrated that pH values over 6 could effectively reduce CO2 emissions. Ambrose [53] affirmed that acidification is more effective, minimizing the emissions of pig slurry with a low content of DM and solids. The results of the present study show reductions of 45–71% for TSSs and 62–75% for COD, which are related to these parameters, and can influence the release of emissions.
In a previous study, Ni [56] pointed that most of the CO2 produced inside the manure could come out of the pig slurry more speedily than other gases, and a relatively small amount of CO2 could be retained in the manure “reservoir.” This phenomenon could explain the low mean values for CO2 emissions in this study, being 0.019 g/m2/day for the acidified slurry compared to 3.28 g/m2/day in the raw pig slurry in the fifth week. Thus, to clarify, the CO2 release rate is largely dependent on its production rate; after manure disturbances, the CO2 ventilation changes and reaches steady-state conditions compared with the other gases, such as NH3 or H2S. After the application of acid, if the slurry is kept under these steady-state conditions, it can exhibit a smoother reduction in CO2.
On the other hand, the pig slurry to which bacteria were added showed significant differences (p < 0.05) during weeks 2–4, but did not for week 3. However, the increase in the mean value observed during week 3 could have been influenced by the improved microbiological activities caused by the better mixing of microorganisms and substrates [56] in the storage pond. Although, the slurry treated with biological additives maintained a stable pattern compared to the raw pig slurry.
In addition, as can be seen in Figure 2 and Figure 3, the emission trend over the weeks with the biological additive for CH4 and CO2 is similar and tends to decrease from week 3, confirming that methanogenesis takes place less efficiently when the temperature is lower [48], as well as that the pH in week 3 did not favor the activity of the enzymes involved in methanogenesis [48,49].
Carbon dioxide reduction can also be influenced through denitrifying bacteria [57]. Denitrification requires an oxidizable substrate, either organic or inorganic, that acts as an energy source so denitrification can be carried out via both heterotrophic and autotrophic bacteria. In autotrophic denitrification, these bacteria are generally characterized by resorting to an inorganic source of carbon, such as CO2 [58].
4.3. Ammonia (NH3) Emissions
As can be observed in Figure 4, the weekly NH3 emission peaked on the fourth and fifth week for the raw pig slurry, while it decreased in both treatments in the end of the experiment: from 35.5 to 0.287 mg/m2/day for acidification and to 15.21 mg/m2/day for the bacterial treatment. The NH3 emission did differ significantly (p < 0.05) between the control (RPS) and the treatments during the experiment; however, the acidified treatment significantly reduced (p < 0.05) these emissions from the second week onwards and remained stable throughout the experiment. It is important to mention that the acidification was controlled via an automatic acidifier, which allowed us to maintain a stable acidic pH (6.2–5.12), as shown in Table 1. This pH was chosen according to X.R. Dai’s recommendations [59], while the bacterial treatment maintained stable with no significant differences during the four weeks. Then, a significant reduction (p < 0.05) was observed at the end of the treatment compared to the control (RPS).
The NH3 emissions for the acidification decreased through the maintenance of a low and stable pH via the addition of sulfuric acid, due to the acid causing the ammonium–ammonia (NH4+:NH3) equilibrium in the slurry to shift towards non-volatile ammonium (NH4+) [34]. The results of this study are similar to other results reported in other studies [59,60], where the acidification of pig slurry reduced NH3 emissions by 70%, sometimes reaching up to 90%, according to Eihe [61]. Ammonia (NH3) is a gas, and its volatility increases as the pH of the slurry increases (becomes more alkaline) [25,62]. When pig slurry is more alkaline, a higher concentration of free ammonia is present, and more ammonia molecules can escape into the atmosphere [34]. However, by adding acid to the slurry, the pH is lowered, and this decreases the volatility of ammonia [26,36]. Acidification effects the equilibrium of total ammoniacal nitrogen in favor of NH4+ rather than NH3, which effectively curtails the release of ammonia into the atmosphere [63]. As a result, the incorporation of acidified pig slurry as an amendment played a crucial role in decreasing the susceptibility of urea to nitrogen losses caused by ammonia volatilization. This phenomenon explains the results of this study.
Regarding biological additives, ammonia is monitored for the efficiency of the nitrification–denitrification process, in which ammonia gas is converted to nitrogen gas (N2); also, NH3 emissions depend mainly on the concentration of mineral N available in the slurry and on the pH [64]. Therefore, ammonium can be oxidized by nitrifying bacteria in an aerobic environment, and, under such conditions, the molecules produced from nitrification can be denitrified [62]. In this process of nitrification–denitrification, Nitrosomona europea consumes and accelerates the passage of ammonia to nitrates during the first stage of oxidation, since it intervenes in the nitrification process, while denitrifying heterotrophic bacteria (Nitrobacter winogradskyi) and facultative bacteria (Rhodopseudomona Palustris) lead the execution of denitrification with the consequent transformation of nitrogen into N2, an innocuous gas. This process carried out by the bacteria present in the DAB formulations enables reductions in the ammonia [65,66]. In addition, NH4+ production depends on urease activity, with urease being abundant in animal manure. Consequently, the ammonium–ammonia (NH4+:NH3) equilibrium in the slurry shifts towards the non-volatile ammonium (NH4+), which is available for the nitrification denitrification process [58].
4.4. Compositions of the Treated and Control Raw Pig Slurry
The compositions of the slurry during the treatment’s applications are presented in Table 1, Table 2 and Table 3.
As shown in Table 1, there is a noticeable contrast in electrical conductivity (EC) between the first and fifth week, with an increase from 22.9 (µS cm−1) to 27.7 (µS cm−1), which holds statistical significance (p < 0.05). Acidification of pig slurry has an influence on the electrical conductivity (EC) of the pig slurry; when sulfuric acid is introduced to the slurry, it dissociates into hydrogen ions (H+) and sulfate ions (SO4 2−), which leads to a decrease in the pH due to the release of hydrogen ions (H+), thus contributing to a higher electrical conductivity [67], as presented in Table 1. Biological additives presented no significant effects on the electrical conductivity of the slurry and it had almost the same comportment as the control tank, while the pH had a significant effect between the first and fifth week; this can be explained by the microbial degradation of organic matter in the slurry, which results in the formation of carbonate and ammonium [68] and thus leads to an increase in pH value, from 7.41 to 8.2.
The storage of pig slurry can have a significant impact on the behavior and concentration of total suspended solids (TSSs) within the slurry. TSSs refers to solid particles that are suspended in the liquid phase of the slurry. During storage, pig slurry undergoes natural settling, where heavier solid particles begin to separate from the liquid. This settling process can lead to the accumulation of TSSs at the bottom of the storage tank over time [69]. This explains the significant difference (p < 0.05) in the TSSs between the first and fifth weeks: between the acidification treatment and the control, the evolution of TSSs during the whole treatment period was from 77.7 g L−1 to 22.2 and 24.1 g L−1, respectively. The acidification treatment presented no significant effect on the removal of TSSs compared to the control tank of raw pig slurry during the study period. Adding bacteria had a small significant effect (p < 0.05) on the removal of TSSs compared to the control slurry. Microbial cultures are designed to break down organic matter in the slurry; as organic compounds are broken down, some solid particles might become solubilized or transformed into smaller particles, which leads to a decrease in TSSs, according to [70].
Correlations between the COD and TSSs vary with the amount of inorganic and organic solids present [71]. In pig slurry, TSSs and COD are often correlated because the suspended solids include organic and inorganic particles that contribute to the COD content [72]. This work confirms this phenomenon, as a lower amount of suspended solids contributed to reduced COD concentrations in the three tanks with a high significant effect (p < 0.05).
TSSs and BOD5 are also related, as the BOD5 can be associated with the organic matter present in suspended solids. Microorganisms responsible for BOD5 consumption can be attached to these solids [73,74]; therefore, an effective removal of TSSs can lead to reduced BOD5 levels because a low amount of suspended solids can decrease the microbial habitat and organic matter available for biological degradation. In this study, a significant BOD5 removal was observed in the three tanks (p < 0.05), while there was no significant effect between the control tank and the treated slurry at the end of the study period.
Acidification of pig slurry does not alter the total nitrogen content of the slurry, even though this process mainly affects the behavior and forms of the nitrogen [75]; the results of this study demonstrate that there is no correlation between acidification and total nitrogen because the TN had the same comportment in both the control and acidified slurry, with a concentration of 2.46 g L−1 and 2.55 g L−1, respectively. Nitrification is an exclusively aerobic process, wherein ammonium (NH4+) is oxidized to nitrates (NO3−), with nitrite (NO2−) as an intermediate product. Two different groups of bacteria are highly important in the nitrification process: NH4+-oxidizing bacteria and NO2−-oxidizing bacteria [76]. Nitrification is often followed by a denitrification process. The initiation time for denitrification is approximately 2 weeks [77]. However, unlike nitrification, the required retention time is less significant [78]. Furthermore, denitrification requires the presence of a sufficient amount of carbonaceous material in the fluid being treated (in this case, the pig manure).
The goal of biological denitrification is to reduce nitrogen from slurries, which was achieved through the action of heterotrophic denitrifying bacteria (Nitrobacter winogradskyi) and facultative bacteria (Rhodopseudomona Palustris), active components of the DAB formulation that convert nitrogen passing through nitrates (NO3−) during the process into molecular N2 that evaporates into the atmosphere. This explains the substantial reduction in total nitrogen observed in this study, where the initial sampling results of the total nitrogen were 3.52 g L−1 and 1.88 g L−1 at the end of the study period, showing a significant effect (p < 0.05).
In conclusion, the biological treatment on this farm has yielded the expected results, achieving a 47% reduction in total nitrogen compared to untreated slurries through the nitrification–denitrification process.
Acidification had no significant effect on various macro and micronutrients, such as K+, Mg+2, Cu+, Zn, Fe, while it had a significant effect on others, such as Mn+, Ca2+, and P.
Acidic conditions, typically associated with a lower pH, have the potential to induce the precipitation of total phosphorus within pig manure. When the pH of pig manure is lowered due to processes like acidification, chemical reactions can transpire that result in the formation of insoluble compounds containing phosphorus [79], which explains the reduction in total phosphorus in the acidified pig manure.
Acidosis decreases protein binding, resulting in increased free calcium levels [80]. As the pH decreases, calcium compounds that were previously bound or less soluble can start to dissolve, releasing calcium cations (Ca2+) into the solution, which can lead to an increase in the concentration of calcium ions [74]. The Ca2+ concentration showed a significant effect (p < 0.05) between the raw and acidified slurry, with values of 457 mg L−1 and 882 mg L−1, respectively.
Biological additives have no significant effect (p < 0.05) on Cu, Zn, Fe, and Mg2+. On one hand, they have an important significant effect on some micro and macronutrients, such as Mn and P, and on the other hand, they have a low significant effect on other parameters, namely Ca2+, K+, and Na+.
According to Rongrong Wu [75], there are some types of biological methods for removing Mn through microorganisms, including biosorption, bioaccumulation, and biological oxidation. Therdkiattikul and Katsoyiannis [81,82] confirmed that biological oxidation using microorganisms has been considered as an alternative method for manganese removal from water; this can explain the significant difference between raw pig slurry and pig slurry with added bacteria, which had concentrations of 4.48 and 2.48 μg L−1, respectively.
All species of nitrifying bacteria require a number of micronutrients, and the most important among these is their need for phosphorus for ATP (Adenosine Tri-Phosphate) production. The conversion of ATP provides energy for cellular functions, and phosphorus in pig slurry is normally available to cells in the form of phosphates (PO43−) [83,84]; these phenomena explain the significant reduction in total phosphorus seen when comparing raw pig slurry and pig slurry with biological additives, which present concentrations of 153 mg L−1 and 69.4 mg L−1, respectively.
5. Conclusions
In conclusion, this study has made a significant stride in addressing the pressing challenge of mitigating ammonia and greenhouse gas (GHG) emissions from stored pig slurry using chemical and biological additives. By employing innovative dynamic chambers, the effectiveness of these additives has been studied and evaluated.
This study demonstrates that sulfuric acid exhibits remarkable capabilities, achieving an impressive reduction of 92% in methane emissions, and a 99% reduction in both carbon dioxide and ammonia emissions. Acidification’s ability to control pH aligns with prior studies, curbing NH3 volatility by favoring NH4+ over NH3 and thus reducing emissions significantly. On the other hand, biological additives, while showing a lower impact on methane reduction (8%), demonstrate high decreases in carbon dioxide (71%) and ammonia (77%) emissions.
The application of biological additives, particularly bacteria in DAB formulations, effectively reduces total nitrogen (TN) levels and NH3 emissions. Additionally, it leads to a consistent decline in CO2 emissions and a gradual reduction in methane (CH4) emissions over time. This highlights the promising impact of biological additives in addressing emissions from stored pig slurry.
Acidification also increased electrical conductivity due to hydrogen ion release, while biological additives had no significant impact on this. Settling caused a decrease in the total suspended solids, with bacteria slightly enhancing this reduction. This settling process over time caused a significant decrease in the total suspended solids (TSSs) in both the acidified and control tanks. The addition of bacteria exhibited a small but significant effect on TSS reduction, likely due to microbial degradation of organic matter. A strong correlation was observed between the TSSs and chemical oxygen demand (COD), where lower amounts of TSSs resulted in reduced COD concentrations. Acidification had little impact on the total nitrogen (TN), while biological denitrification significantly lowered TN levels. Overall, both additives led to significant changes in certain micronutrients, such as manganese and phosphorus, attributed to microbial activities, and also changes in phosphorus precipitation and increased free calcium levels in the acidified slurry.
Overall, this study demonstrated the potential of acidification and biological treatments to modify slurry properties, manage nutrient content, and reduce environmental impact, providing valuable insights for sustainable agricultural practices.
Author Contributions
Conceptualization, O.E.b. and J.A.A.; data curation, O.E.b. and J.A.A.; formal analysis, O.E.b.; investigation, O.E.b., A.G.-V. and J.A.A.; methodology, O.E.b.; resources, M.A.T.T., A.G.-V. and Á.F.C.; software, O.E.b.; supervision, Á.F.C. and J.A.A.; validation, O.E.b., Á.F.C. and J.A.A.; visualization, O.E.b. and J.A.A.; writing—original draft, O.E.b., M.A.T.T. and A.G.-V.; writing—review and editing, O.E.b., M.A.T.T., A.G.-V. and J.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ayuntamiento Fuente Alamo, Murcia, Spain. Project reference: CUE-AYTO FUENTE ÁLAMO 2020-T.
Data Availability Statement
The data presented in this study are of a confidential nature and are part of an ongoing project. This publication is the first related to this project. Due to the privacy of the data, access to the full project data is restricted to authorized personnel only.
Acknowledgments
We express our gratitude to DAB company for their invaluable support and cooperation, which significantly contributed to the success of this research endeavor.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Whalen, J.K.; Thomas, B.W.; Sharifi, M. Novel Practices and Smart Technologies to Maximize the Nitrogen Fertilizer Value of Manure for Crop Production in Cold Humid Temperate Regions. Adv. Agron. 2019, 153, 1–85. [Google Scholar] [CrossRef]
- STATISTA. Number of Pigs Worldwide in 2023, by Leading Country (in Million Head); STATISTA: Hamburg, Germany, 2023. [Google Scholar]
- Zhang, L.; Sun, X. Addition of Seaweed and Bentonite Accelerates the Two-Stage Composting of Green Waste. Bioresour. Technol. 2017, 243, 154–162. [Google Scholar] [CrossRef] [PubMed]
- EPA United States Environmental Protection Agency. Available online: https://iaspub.epa.gov/tdb/pages/treatment/treatmentOverview.do?treatmentProcessId=1934681921 (accessed on 1 November 2023).
- European Union. Directive 91/676/EEC. Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources; European Union: Brussels, Belgium, 1991; Volume 375, pp. 1–8. [Google Scholar]
- European Union. Water Framework Directive (2000/60/EC); European Union: Brussels, Belgium, 2000. [Google Scholar]
- Varma, V.S.; Parajuli, R.; Scott, E.; Canter, T.; Lim, T.T.; Popp, J.; Thoma, G. Dairy and Swine Manure Management—Challenges and Perspectives for Sustainable Treatment Technology. Sci. Total Environ. 2021, 778, 146319. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sheng, L.; Xu, J. Clogging Mechanisms of Constructed Wetlands: A Critical Review. J. Clean. Prod. 2021, 295, 126455. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, H.; Zhu, Z.; Gerber, P.J.; Xin, H.; Smith, P.; Opio, C.; Steinfeld, H.; Chadwick, D. Mitigating Greenhouse Gas and Ammonia Emissions from Swine Manure Management: A System Analysis. Environ. Sci. Technol. 2017, 51, 4503–4511. [Google Scholar] [CrossRef] [PubMed]
- Raj, T.; Verma, S.; Kumar, N.; Agrawal, R. Chapter 11—Role of Woody Biomass in Carbon Capture, Circular Bioeconomy, and Biomanufacturing. In Sustainable Biorefining of Woody Biomass to Biofuels and Biochemicals; Kumar, D., Kumar, S., Rajendran, K., Ray, R.C., Eds.; Applied Biotechnology Reviews; Woodhead Publishing: Cambridge, UK, 2023; pp. 291–318. ISBN 978-0-323-91187-0. [Google Scholar]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid—Liquid Separation of Animal Slurry in Theory and Practice. A Review. Agron. Sustain. Dev. 2010, 30, 153–180. [Google Scholar] [CrossRef]
- Loyon, L.; Guiziou, F.; Béline, F.; Peu, P. Gaseous Emissions (NH3, N2O, CH4, CO2) during Pig Slurry Biological Aerobic Treatment and Treatment by-Product Storages. Int. Congr. Ser. 2006, 1293, 299–302. [Google Scholar] [CrossRef]
- Lymperatou, A.; Rasmussen, N.B.; Gavala, H.N.; Skiadas, I.V. Improving the Anaerobic Digestion of Swine Manure through an Optimized Ammonia Treatment: Process Performance, Digestate and Techno-Economic Aspects. Energies 2021, 14, 787. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of Anaerobic Digestion on Digestate Nutrient Availability and Crop Growth: A Review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Sommer, S.G.; Christensen, M.L.; Schmidt, T.; Stoumann Jensen, L. Animal Manure Recycling; John Wiley & Sons Ltd.: London, UK, 2013. [Google Scholar]
- Regueiro, I.; Coutinho, J.; Fangueiro, D. Alternatives to Sulfuric Acid for Slurry Acidification: Impact on Slurry Composition and Ammonia Emissions during Storage. J. Clean. Prod. 2016, 131, 296–307. [Google Scholar] [CrossRef]
- Fuchs, A.; Dalby, F.R.; Liu, D.; Kai, P.; Feilberg, A. Improved Effect of Manure Acidification Technology for Gas Emission Mitigation by Substituting Sulfuric Acid with Acetic Acid. Clean. Eng. Technol. 2021, 4, 100263. [Google Scholar] [CrossRef]
- Ottosen, L.; Poulsen, H.; Nielsen, D.; Finster, K.; Nielsen, L.P.; Revsbech, N. Observations on Microbial Activity in Acidified Pig Slurry. Biosyst. Eng. 2009, 102, 291–297. [Google Scholar] [CrossRef]
- McCrory, D.F.; Hobbs, P.J. Additives to Reduce Ammonia and Odor Emissions from Livestock Wastes: A Review. J. Environ. Qual. 2001, 30, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Provolo, G.; Finzi, A.; Perazzolo, F.; Mattachini, G.; Riva, E. Effect of a Biological Additive on Nitrogen Losses from Pig Slurry during Storage. J. Environ. Qual. 2016, 45, 1460–1465. [Google Scholar] [CrossRef]
- Wheeler, E.F.; Adviento-Borbe, M.A.A.; Brandt, R.C.; Topper, P.A.; Topper, D.A.; Elliott, H.A.; Graves, R.E.; Wheeler, E.F.; Arlene, M.; Adviento-Borbe, A.; et al. Manure Amendments for Mitigation of Dairy Ammonia and Greenhouse Gas Emissions: Preliminary Screening. Agric. Eng. Int. CIGR J. 2001, 13, 1–14. [Google Scholar]
- Van der Stelt, B.; Temminghoff, E.J.M.; Van Vliet, P.C.J.; Van Riemsdijk, W.H. Volatilization of Ammonia from Manure as Affected by Manure Additives, Temperature and Mixing. Bioresour Technol 2007, 98, 3449–3455. [Google Scholar] [CrossRef]
- International VERA Secretariat. Verification of Environmental Technologies for Agricultural Production Vera Test Protocol Covers and Other Mitigation Technologies for Reduction of Gaseous Emissions from Stored Manure; International VERA Secretariat: Delft, The Netherlands, 2018. [Google Scholar]
- Peter, A.S. Effect of Acidification and Soil Injection of Animal Slurry on Ammonia and Odour Emission; Univerzita Veterinárskeho Lekárstva a Farmácie v Košiciach: Košice, Slovakia, 2013. [Google Scholar]
- Petersen, S.O.; Andersen, A.J.; Eriksen, J. Effects of Cattle Slurry Acidification on Ammonia and Methane Evolution during Storage. J. Environ. Qual. 2012, 41, 88–94. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of Animal Slurry—A Review. J. Envion. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef]
- Magrama Evaluación de Técnicas de Gestión de Deyecciones en Ganadería; Ministerio de Agricultura, Alimentación y Medio Am-biente, Centro de Publicaciones: Madrid, Spain, 2015; pp. 1–104.
- Wypych, G. Principles of Thermal Degradation. In PVC Degradation and Stabilization, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2015; pp. 79–165. [Google Scholar] [CrossRef]
- Kevin, K.P.R. Greenhouse Gas Fluxes—Recirculating Chamber Method; KBS LTER: East Lansing, MI, USA, 2019. [Google Scholar]
- Duchaufour, P. Precis de Pedologie; Masson: Paris, France, 1970. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Hjorth, M.; Cocolo, G.; Jonassen, K.; Abildgaard, L.; Sommer, S.G. Continuous In-House Acidification Affecting Animal Slurry Composition. Biosyst. Eng. 2015, 132, 56–60. [Google Scholar] [CrossRef]
- Shin, S.R.; Im, S.; Mostafa, A.; Lee, M.K.; Yun, Y.M.; Oh, S.E.; Kim, D.H. Effects of Pig Slurry Acidification on Methane Emissions during Storage and Subsequent Biogas Production. Water Res. 2019, 152, 234–240. [Google Scholar] [CrossRef]
- Overmeyer, V.; Trimborn, M.; Clemens, J.; Hölscher, R.; Büscher, W. Acidification of Slurry to Reduce Ammonia and Methane Emissions: Deployment of a Retrofittable System in Fattening Pig Barns. J. Environ. Manag. 2023, 331. [Google Scholar] [CrossRef] [PubMed]
- Ao, S.-I.; Gelman, L.; Hukins, D.W.L. Two-Stage Thermoacoustic Electricity Generator for Waste Heat Recovery. In Proceedings of the International Association of Engineers World Congress on Engineering: WCE 2016, London, UK, 29 June–1 July 2016; Imperial College London: London, UK, 2016. ISBN 9789881404800. [Google Scholar]
- Berg, W.; Brunsch, R.; Pazsiczki, I. Greenhouse Gas Emissions from Covered Slurry Compared with Uncovered during Storage. Agric. Ecosyst. Environ. 2006, 112, 129–134. [Google Scholar] [CrossRef]
- Berg, W.; Pazsiczki, I. Mitigation of Methane Emissions during Manure Storage. Int. Congr. Ser. 2006, 1293, 213–216. [Google Scholar] [CrossRef]
- Petersen, S.O.; Højberg, O.; Poulsen, M.; Schwab, C.; Eriksen, J. Methanogenic Community Changes, and Emissions of Methane and Other Gases, during Storage of Acidified and Untreated Pig Slurry. J. Appl. Microbiol. 2014, 117, 160–172. [Google Scholar] [CrossRef]
- Chen, J.; Wade, M.J.; Dolfing, J.; Soyer, O.S. Increasing Sulfate Levels Show a Differential Impact on Synthetic Communities Comprising Different Methanogens and a Sulfate Reducer. J. R. Soc. Interface 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Wolter, M.; Prayitno, S.; Schuchardt, F. Greenhouse Gas Emission during Storage of Pig Manure on a Pilot Scale. Bioresour. Technol. 2004, 95, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Misselbrook, T.; Hunt, J.; Perazzolo, F.; Provolo, G. Greenhouse Gas and Ammonia Emissions from Slurry Storage: Impacts of Temperature and Potential Mitigation through Covering (Pig Slurry) or Acidification (Cattle Slurry). J. Environ. Qual. 2016, 45, 1520–1530. [Google Scholar] [CrossRef]
- Eriksen, J.; Andersen, A.J.; Poulsen, H.V.; Adamsen, A.P.S.; Petersen, S.O. Sulfur Turnover and Emissions during Storage of Cattle Slurry: Effects of Acidification and Sulfur Addition. J. Environ. Qual. 2012, 41, 1633–1641. [Google Scholar] [CrossRef]
- Joubin, M. Animal Slurry Acidification: Effects of Slurry Characteristics, Use of Different Acids, Slurry PH Buffering. Available online: https://www.semanticscholar.org/paper/Animal-slurry-acidification%3A-effects-of-slurry-use-Joubin/ecbe959322d9931936c9a9cba79a806e764fae4d (accessed on 26 November 2023).
- Regueiro, I.; Coutinho, J.; Fangueiro, D. Comparison of Different Approaches for Ammonia Emissions Minimization by Acidification of Dairy and Pig Slurries. In Proceedings of the Ramiran 2013 Recycling of Agricultural and Industrial Residues in Agriculture, Versailles, France, 3–5 June 2013. [Google Scholar]
- Husted, S.; Jensen, L.S.; Jørgensen, S.S. Reducing Ammonia Loss from Cattle Slurry by the Use of Acidifying Additives: The Role of the Buffer System. J. Sci. Food Agric. 1991, 57, 335–349. [Google Scholar] [CrossRef]
- Kumari, M.; Chandel, M.K. Anaerobic Co-Digestion of Sewage Sludge and Organic Fraction of Municipal Solid Waste: Focus on Mix Ratio Optimization and Synergistic Effects. J. Environ. Manag. 2023, 345. [Google Scholar] [CrossRef]
- Pongsopon, M.; Woraruthai, T.; Anuwan, P.; Amawatjana, T.; Tirapanampai, C.; Prombun, P.; Kusonmano, K.; Weeranoppanant, N.; Chaiyen, P.; Wongnate, T. Anaerobic Co-Digestion of Yard Waste, Food Waste, and Pig Slurry in a Batch Experiment: An Investigation on Methane Potential, Performance, and Microbial Community. Bioresour. Technol. Rep. 2023, 21. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, N.; Liu, C.; Mi, T.; Li, J.; He, X.; Li, S.; Sun, Z.; Zhen, Y. Methanogenesis Pathways of Methanogens and Their Responses to Substrates and Temperature in Sediments from the South Yellow Sea. Sci. Total Environ. 2022, 815. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhang, X.; Xia, W.; Li, Z.; Wang, L.; Chen, Z.; Ge, S. Effect of Extreme PH Conditions on Methanogenesis: Methanogen Metabolism and Community Structure. Sci. Total Environ. 2023, 877. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Wang, R.; Jiang, Q.; Zhang, Y.; Peng, X. Anaerobic Methane Oxidation Coupled to Denitrification Is an Important Potential Methane Sink in Deep-Sea Cold Seeps. Sci. Total Environ. 2020, 748, 142459. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Li, L.; Wang, W.; Xue, S.; Liu, J. Electro-Stimulated Anaerobic Oxidation of Methane with Synergistic Denitrification by Adding AQS: Electron Transfer Mode and Mechanism. Environ. Res. 2023, 229, 115997. [Google Scholar] [CrossRef] [PubMed]
- Bertora, C.; Alluvione, F.; Zavattaro, L.; van Groenigen, J.W.; Velthof, G.; Grignani, C. Pig Slurry Treatment Modifies Slurry Composition, N2O, and CO2 Emissions after Soil Incorporation. Soil Biol. Biochem. 2008, 40, 1999–2006. [Google Scholar] [CrossRef]
- Ambrose, H.W.; Dalby, F.R.; Feilberg, A.; Kofoed, M.V.W. Additives and Methods for the Mitigation of Methane Emission from Stored Liquid Manure. Biosyst. Eng. 2023, 229, 209–245. [Google Scholar] [CrossRef]
- Pedersen, J.; Feilberg, A.; Nyord, T. Effect of Storage and Field Acidification on Emissions of NH3, NMVOC, and Odour from Field Applied Slurry in Winter Conditions. J. Environ. Manag. 2022, 310, 114756. [Google Scholar] [CrossRef]
- Blanes-Vidal, V.; Guàrdia, M.; Dai, X.R.; Nadimi, E.S. Emissions of NH3, CO2 and H2S during Swine Wastewater Management: Characterization of Transient Emissions after Air-Liquid Interface Disturbances. Atmos. Environ. 2012, 54, 408–418. [Google Scholar] [CrossRef]
- Ni, J.Q.; Heber, A.J.; Sutton, A.L.; Kelly, D.T. Mechanisms of Gas Releases from Swine Wastes. Trans. ASABE 2009, 52, 2013–2025. [Google Scholar] [CrossRef]
- Luo, H.; Zhuang, D.; Yang, J.; Liu, X.; Zhang, K.; Fu, X.; Jiang, B.; Xue, R.; Fan, L.; Chen, W.; et al. Carbon Dioxide and Methane Emission of Denitrification Bioreactor Filling Waste Sawdust and Industrial Sludge for Treatment of Simulated Agricultural Surface Runoff. J. Environ. Manag. 2021, 289, 112503. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Wang, L.; Chen, Y.; Zheng, X.; Su, Y.; Tao, X. Insight into a Direct Carbon Dioxide Effect on Denitrification and Denitrifying Bacterial Communities in Estuarine Sediment. Sci. Total Environ. 2018, 643, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.R.; Blanes-Vidal, V. Emissions of Ammonia, Carbon Dioxide, and Hydrogen Sulfide from Swine Wastewater during and after Acidification Treatment: Effect of PH, Mixing and Aeration. J. Environ. Manag. 2013, 115, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kai, P.; Pedersen, P.; Jensen, J.E.; Hansen, M.N.; Sommer, S.G. A Whole-Farm Assessment of the Efficacy of Slurry Acidification in Reducing Ammonia Emissions. Eur. J. Agron. 2008, 28, 148–154. [Google Scholar] [CrossRef]
- Eihe, P.; Vebere, L.L.; Grinfelde, I.; Pilecka, J.; Sachpazidou, V.; Grinberga, L. The Effect of Acidification of Pig Slurry Digestate Applied on Winter Rapeseed on the Ammonia Emission Reduction. In IOP Conference Series: Earth and Environmental Science, Proceedings of the XVI-th International Youth Science and Environmental Baltic Region Countries Forum, Gdansk, Poland, 7–9 October 2019; Institute of Physics Publishing: Bristol, UK, 2019; Volume 390, p. 390. [Google Scholar]
- Kupper, T.; Häni, C.; Neftel, A.; Kincaid, C.; Bühler, M.; Amon, B.; VanderZaag, A. Ammonia and Greenhouse Gas Emissions from Slurry Storage—A Review. Agric. Ecosyst. Environ. 2020, 300, 106963. [Google Scholar] [CrossRef]
- Silva, A.A.; Fangueiro, D.; Carvalho, M. Slurry Acidification as a Solution to Minimize Ammonia Emissions from the Combined Application of Animal Manure and Synthetic Fertilizer in No-Tillage. Agronomy 2022, 12, 265. [Google Scholar] [CrossRef]
- Snoek, D.J.W.; Stigter, J.D.; Ogink, N.W.M.; Groot Koerkamp, P.W.G. Sensitivity Analysis of Mechanistic Models for Estimating Ammonia Emission from Dairy Cow Urine Puddles. Biosyst. Eng. 2014, 121, 12–24. [Google Scholar] [CrossRef]
- Bian, X.; Wu, Y.; Li, J.; Yin, M.; Li, D.; Pei, H.; Chang, S.; Guo, W. Effect of Dissolved Oxygen on High C/N Wastewater Treatment in Moving Bed Biofilm Reactors Based on Heterotrophic Nitrification and Aerobic Denitrification: Nitrogen Removal Performance and Potential Mechanisms. Bioresour. Technol. 2022, 365, 128147. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, J.; Wang, M.; Wu, Y.; Zhang, J.; Wang, S.; Pan, Y.; Cao, G. Nitrogen Removal from Wastewater for Heterotrophic Nitrification-Aerobic Denitrification Bacterium with the Combination of Bacteriophage DEY7 and Fe Nanoparticles. Biochem. Eng. J. 2023, 191. [Google Scholar] [CrossRef]
- Li, Y.; Jones, D.L.; Chen, Q.; Ge, T.; Chadwick, D.R. Acidification and Anaerobic Digestion Change the Phosphorus Forms and Distribution in Particle Fractions of Cattle Slurry and Phosphorus Dynamics in Soil after Application. Biosyst. Eng. 2020, 200, 101–111. [Google Scholar] [CrossRef]
- Overmeyer, V.; Kube, A.; Clemens, J.; Büscher, W.; Trimborn, M. One-Time Acidification of Slurry: What Is the Most Effective Acid and Treatment Strategy? Agronomy 2021, 11, 1319. [Google Scholar] [CrossRef]
- Zhu, K.; El-Din, M.G.; Moawad, A.K.; Bromley, D. Physical and Chemical Processes for Removing Suspended Solids and Phosphorus from Liquid Swine Manure. Environ. Technol. 2004, 25, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Paz Pérez-Sangrador, M.; Cristina León-Cófreces, M.; Acítores-Benavente, M.; Cruz García-González, M. Solids and Nutrient Removal from Flushed Swine Manure Using Polyacrylamides. J. Environ. Manag. 2012, 93, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Umweltbundesamt GmbH. FINAL REPORT: Framework Service Contract ENV.D2/FRA/2012/0013 Support to the Implementation of the UWWTD: COD Substitution Scoping Study; Umweltbundesamt: Dessau-Roßlau, Germany, 2017. [Google Scholar]
- El Bied, O.; García-Valero, A.; Fechtali, T.; Faz, Á.; Acosta, J.A. Purification Performance of Filtration Process for Pig Slurry Using Marine Sands, Silty Loam Soils, Fly Ash and Zeolite. Agronomy 2021, 11, 1608. [Google Scholar] [CrossRef]
- Gerardi, M.H.; Lytle, B. BOD and TSS. In The Biology and Troubleshooting of Facultative Lagoons; Wiley: Hoboken, NJ, USA, 2015; pp. 195–198. [Google Scholar]
- Alexander, R.T.; Cordat, E.; Chambrey, R.; Dimke, H.; Eladari, D. Acidosis and Urinary Calcium Excretion: Insights from Genetic Disorders. J. Am. Soc. Nephrol. 2016, 27, 3511–3520. [Google Scholar] [CrossRef]
- Wu, R.; Yao, F.; Li, X.; Shi, C.; Zang, X.; Shu, X.; Liu, H.; Zhang, W. Manganese Pollution and Its Remediation: A Review of Biological Removal and Promising Combination Strategies. Microorganisms 2022, 10, 2411. [Google Scholar] [CrossRef] [PubMed]
- Chiumenti, A. Complete Nitrification–Denitrification of Swine Manure in a Full-Scale, Non-Conventional Composting System. Waste Manag. 2015, 46, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Michaud, L.; Blancheton, J.P.; Bruni, V.; Piedrahita, R. Effect of Particulate Organic Carbon on Heterotrophic Bacterial Populations and Nitrification Efficiency in Biological Filters. Aquac. Eng. 2006, 34, 224–233. [Google Scholar] [CrossRef]
- Ouyang, C.F.; Chiou, R.J.; Lin, C.T. The Characteristics of Nitrogen Removal by the Biofilter System. Water Res. Technol. 2000, 42, 137–147. [Google Scholar] [CrossRef]
- Moyo, L.B.; Simate, G.S.; Mutsatsa, T. Biological Acidification of Pig Manure Using Banana Peel Waste to Improve the Dissolution of Particulate Phosphorus: A Critical Step for Maximum Phosphorus Recovery as Struvite. Heliyon 2022, 8. [Google Scholar] [CrossRef]
- Tiangco, K.; De Luna, M.D.; Vilando, A.; Lu, M.-C. Removal and Recovery of Calcium from Aqueous Solutions by Fluidized-Bed Homogeneous Crystallization. Process Saf. Environ. Prot. 2019, 128, 307–315. [Google Scholar] [CrossRef]
- Therdkiattikul, N.; Ratpukdi, T.; Kidkhunthod, P.; Chanlek, N.; Siripattanakul-Ratpukdi, S. Manganese-Contaminated Groundwater Treatment by Novel Bacterial Isolates: Kinetic Study and Mechanism Analysis Using Synchrotron-Based Techniques. Sci. Rep. 2020, 10, 13391. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, I.A.; Zouboulis, A.I. Biological Treatment of Mn(II) and Fe(II) Containing Groundwater: Kinetic Considerations and Product Characterization. Water Res. 2004, 38, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, C.; Zhou, Z.; Cao, X.; Zhou, Y. Coupling between Nitrification and Denitrification as Well as Its Effect on Phosphorus Release in Sediments of Chinese Shallow Lakes. Water 2019, 11, 1809. [Google Scholar] [CrossRef]
- Li, B.; Yang, Y.; Chen, J.; Wu, Z.; Liu, Y.; Xie, S. Nitrifying Activity and Ammonia-Oxidizing Microorganisms in a Constructed Wetland Treating Polluted Surface Water. Sci. Total Environ. 2018, 628–629, 310–318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).