Photodegradation of Methylene Blue and Crystal Violet by Zr-Modified Engelhard Titanium Silicate 10
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis of Na-K-Engelhard Titanium Silicate 10 (Na-K-ETS-10)
2.1.2. Functionalization of Na-K-ETS-10 with Zr
2.2. Methods of Characterization
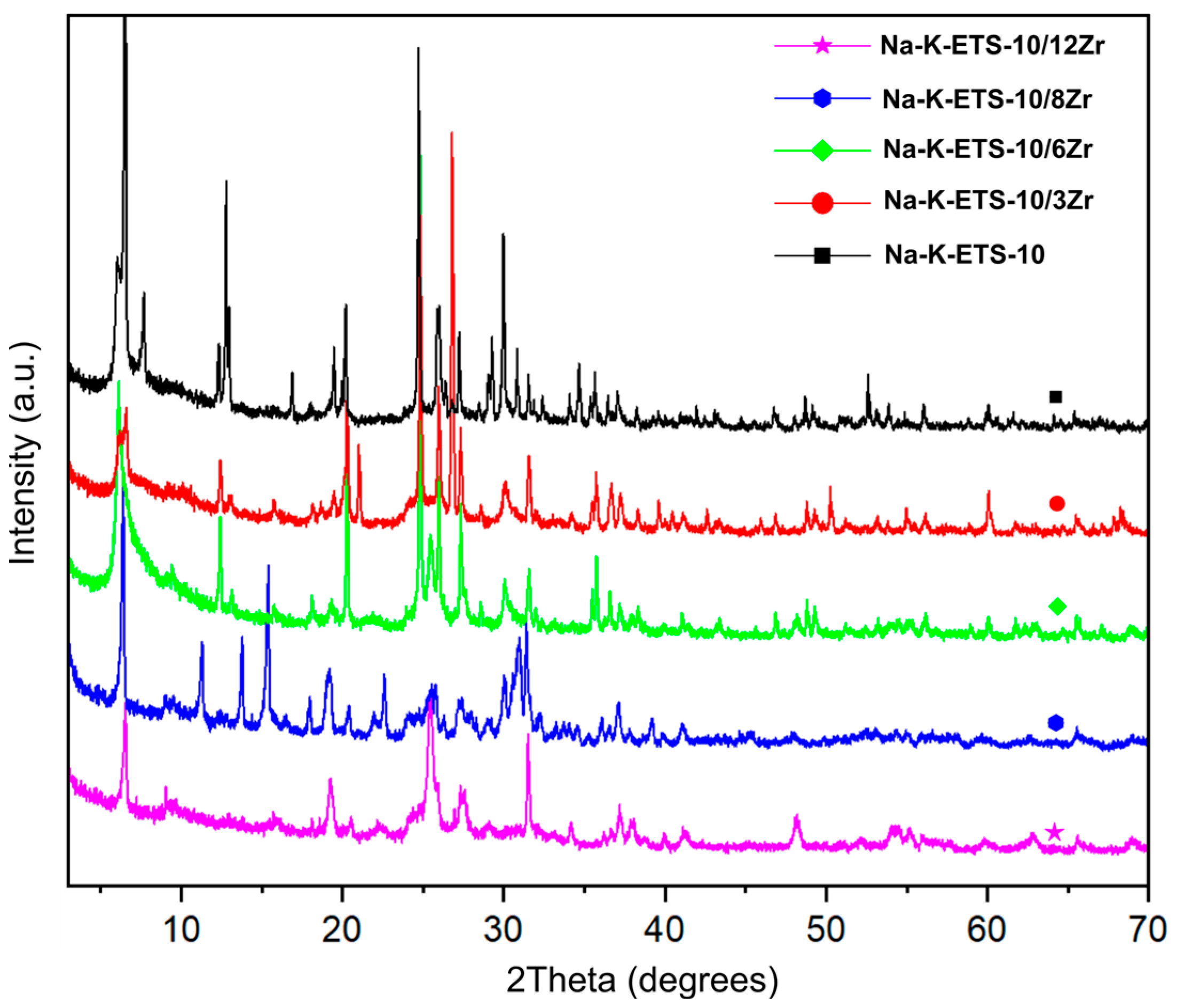
2.2.1. Powder X-ray Diffraction (PXRD) Analysis
2.2.2. Wavelength Dispersive X-ray Fluorescence (WDXRF) Spectroscopy
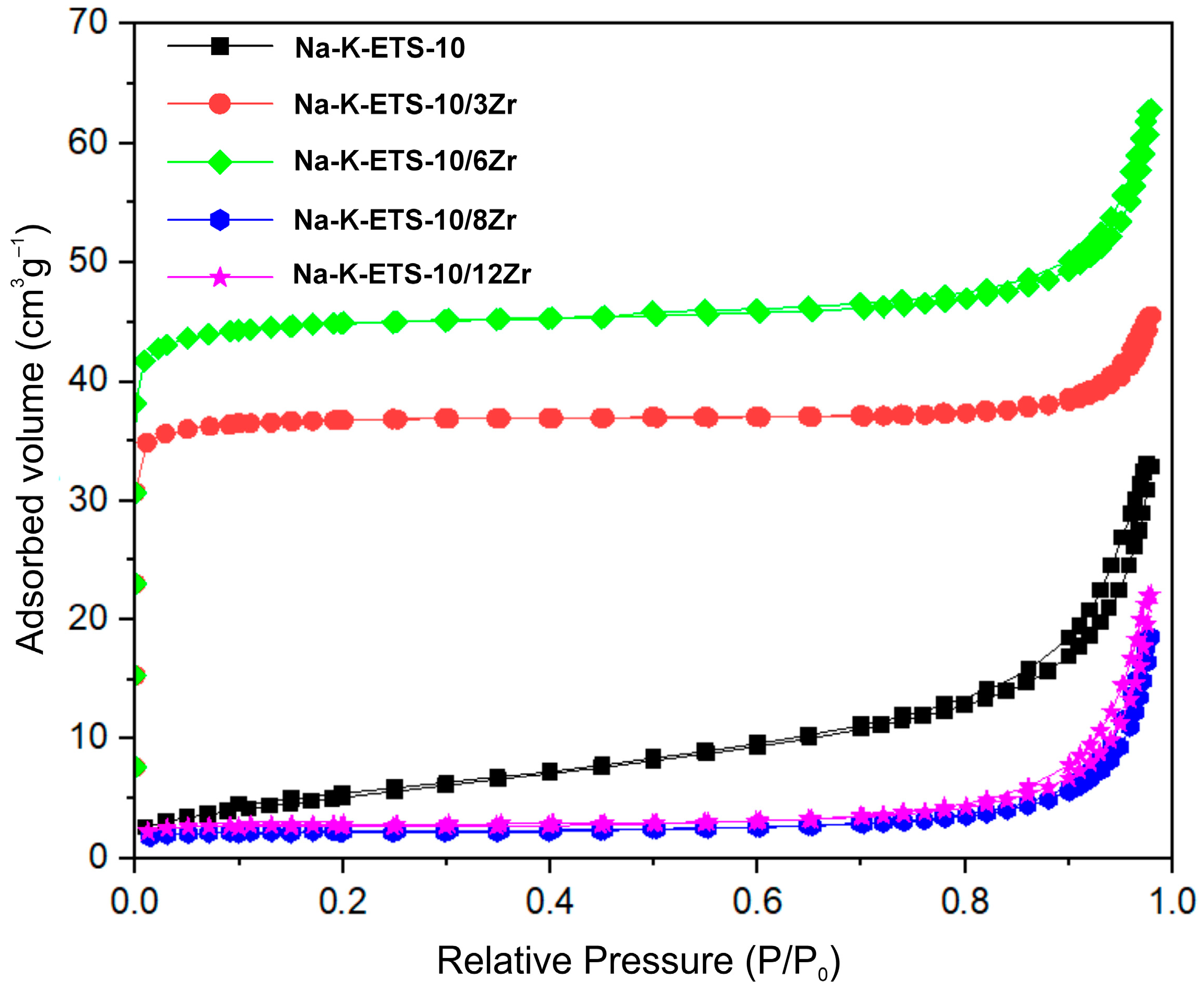
2.2.3. Specific Surface Area Analysis—N2 Physisorption
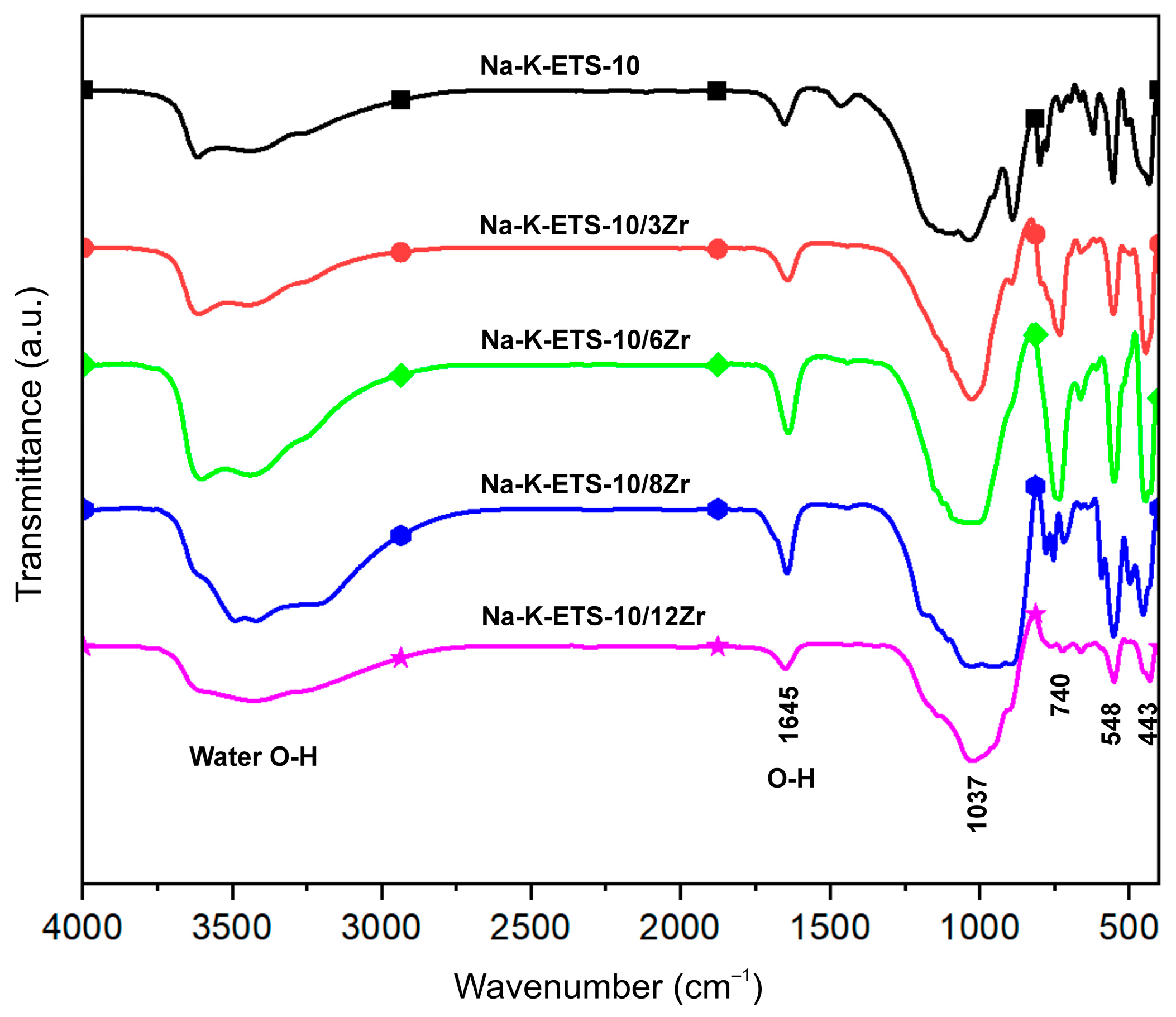
2.2.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.3. Photocatalytic Degradation Experiments
3. Results and Discussion
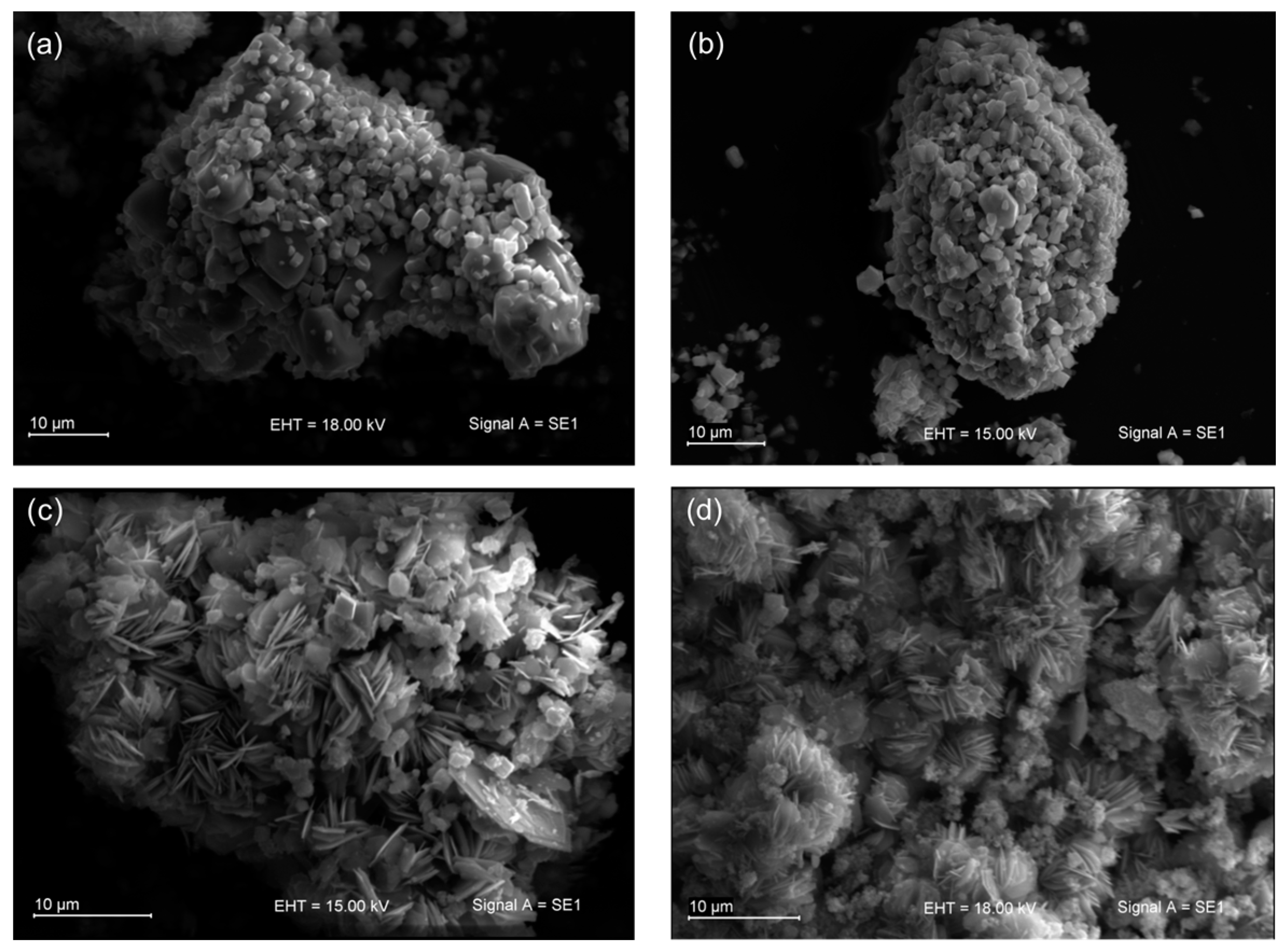
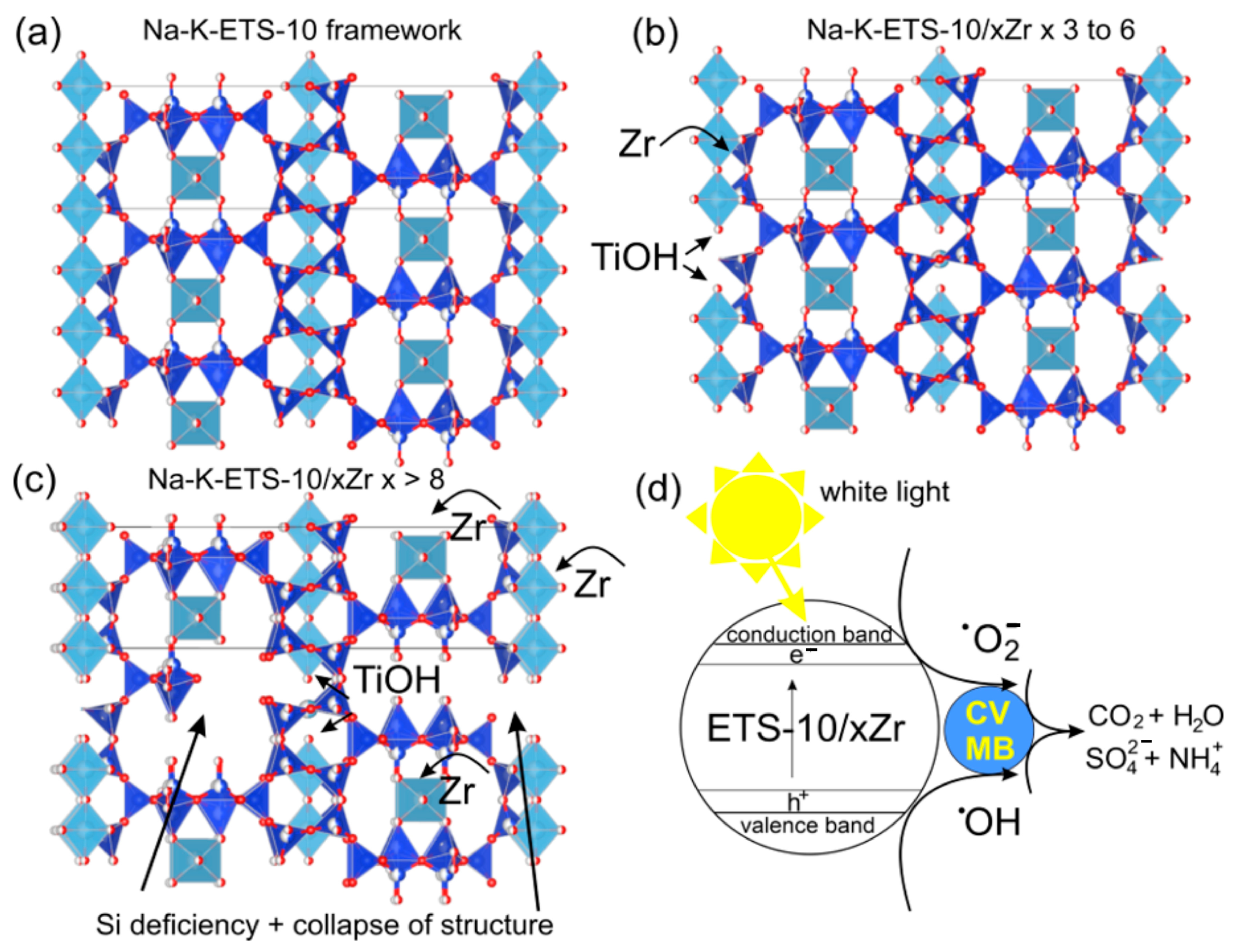
3.1. Physicochemical Characterization of Na-K-ETS-10/xZr Form
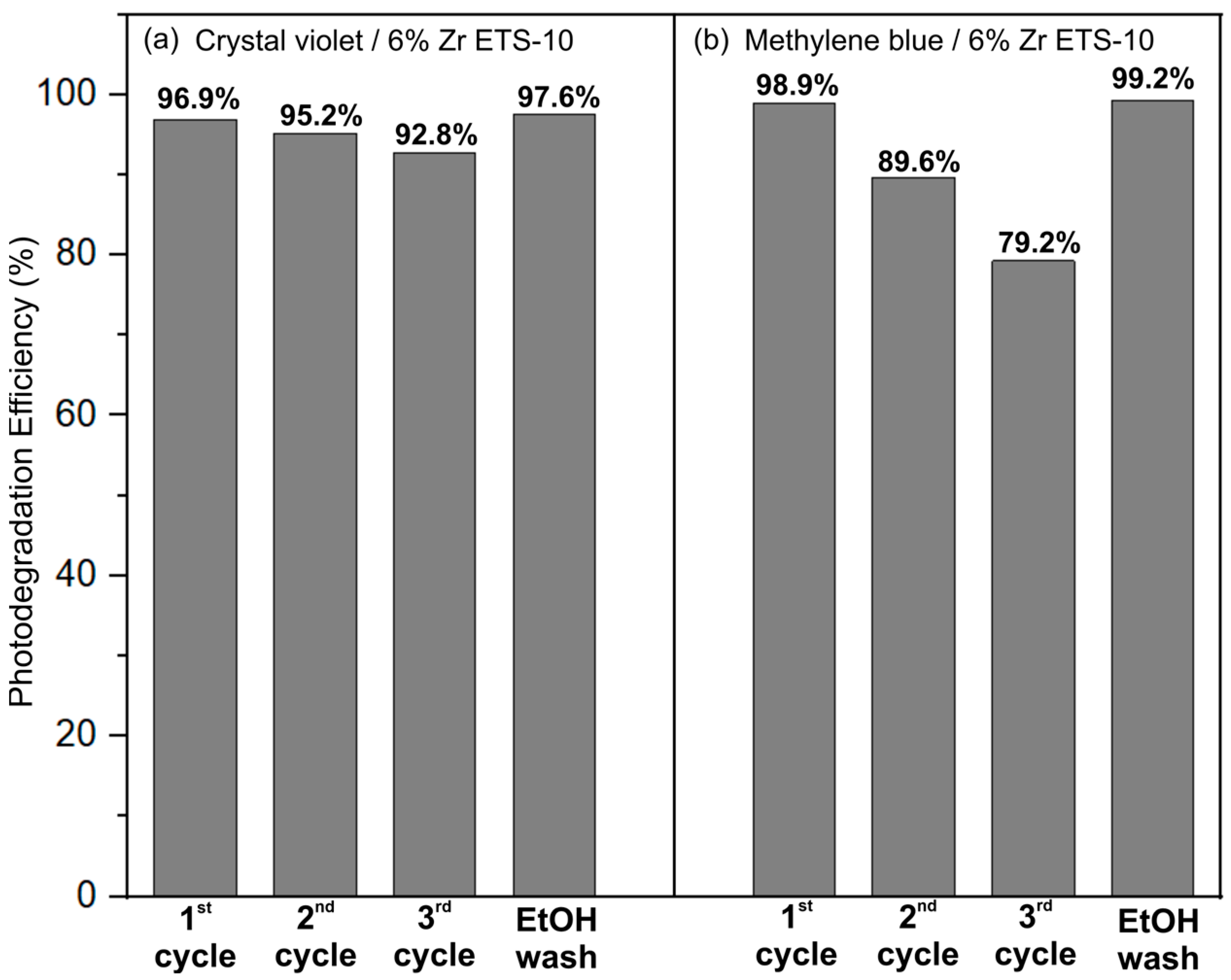
3.2. Photocatalytic Activity of Patterns of the Studied Samples
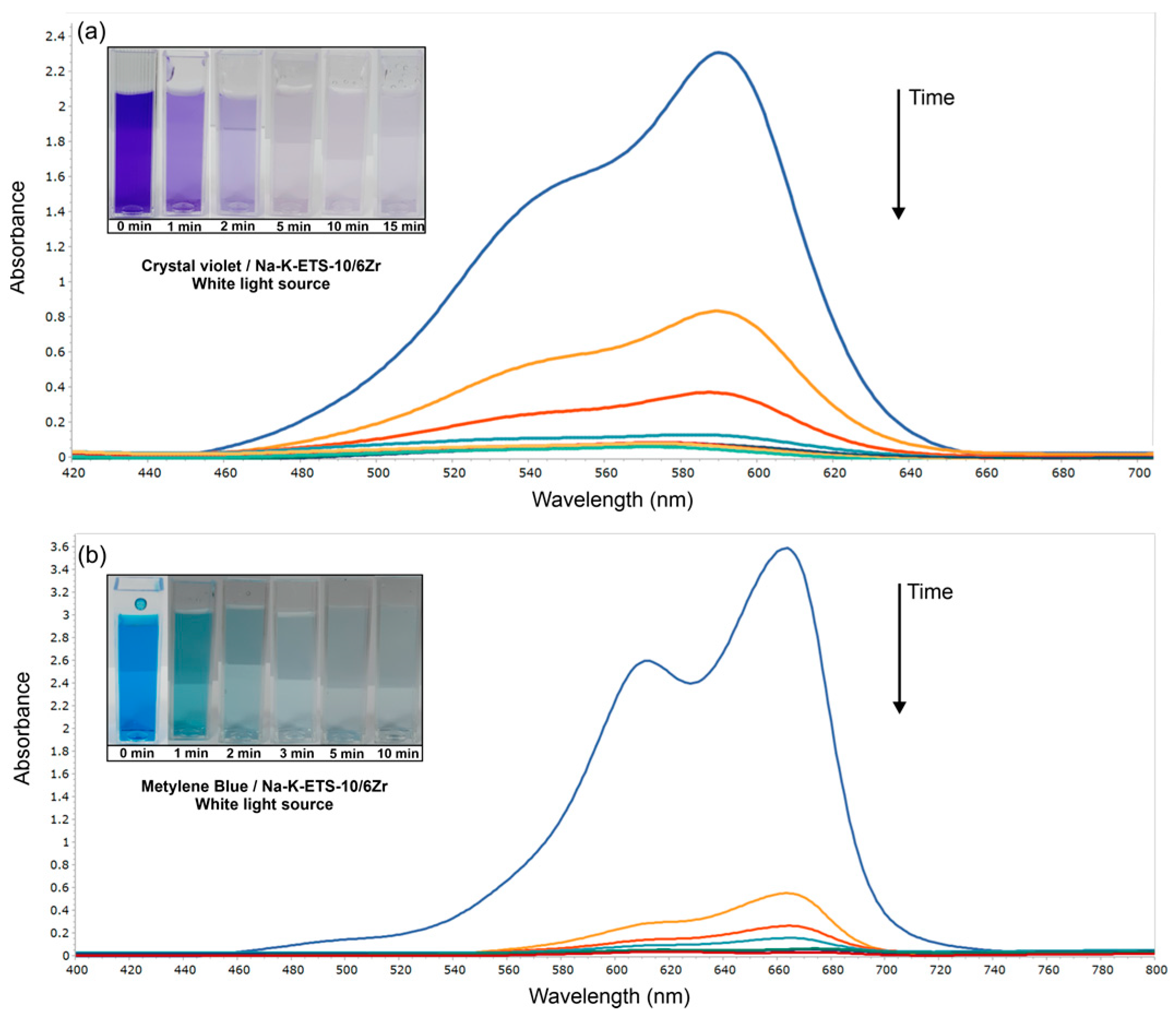
3.2.1. Photodegradation of Crystal Violet and Methylene Blue
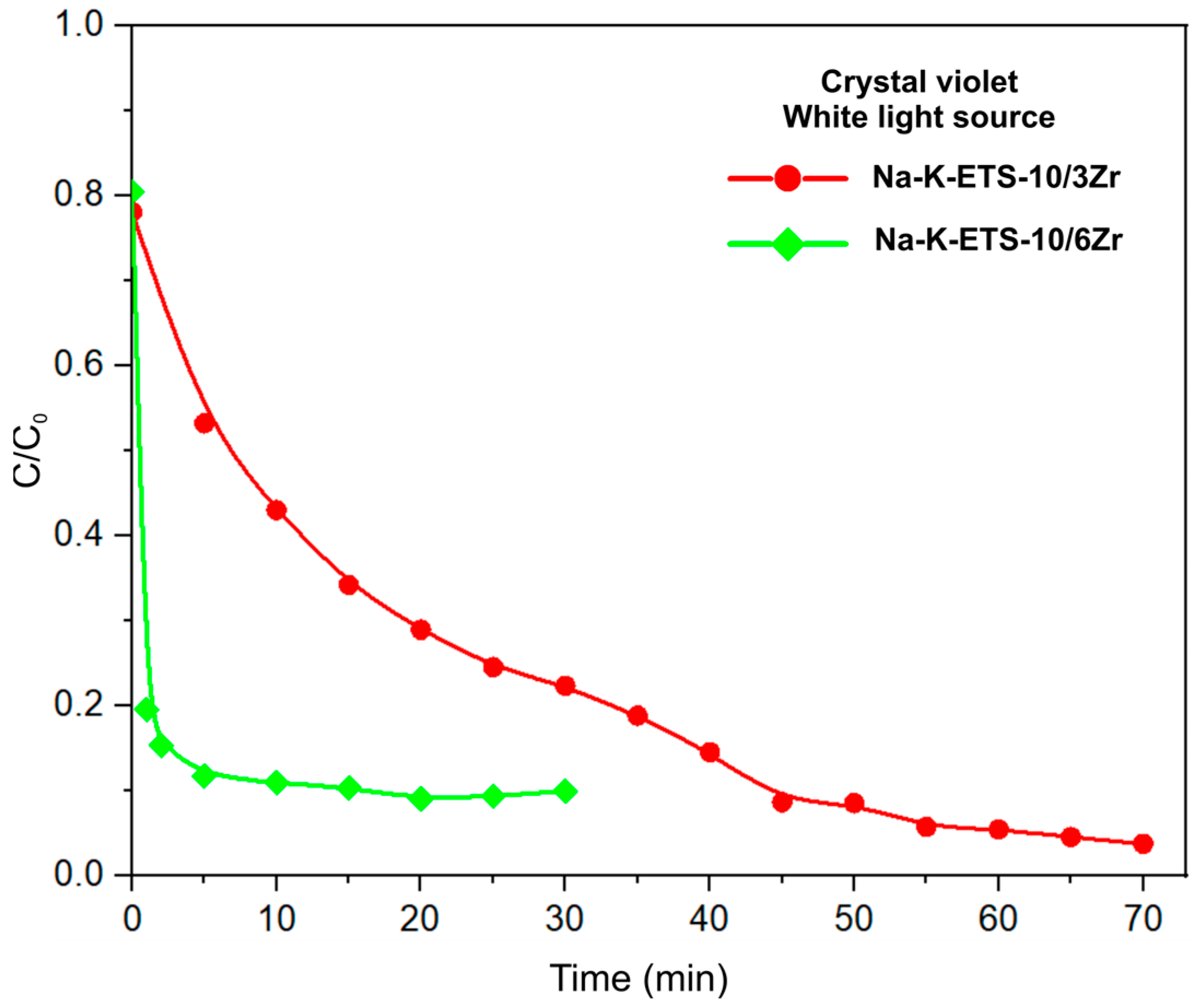
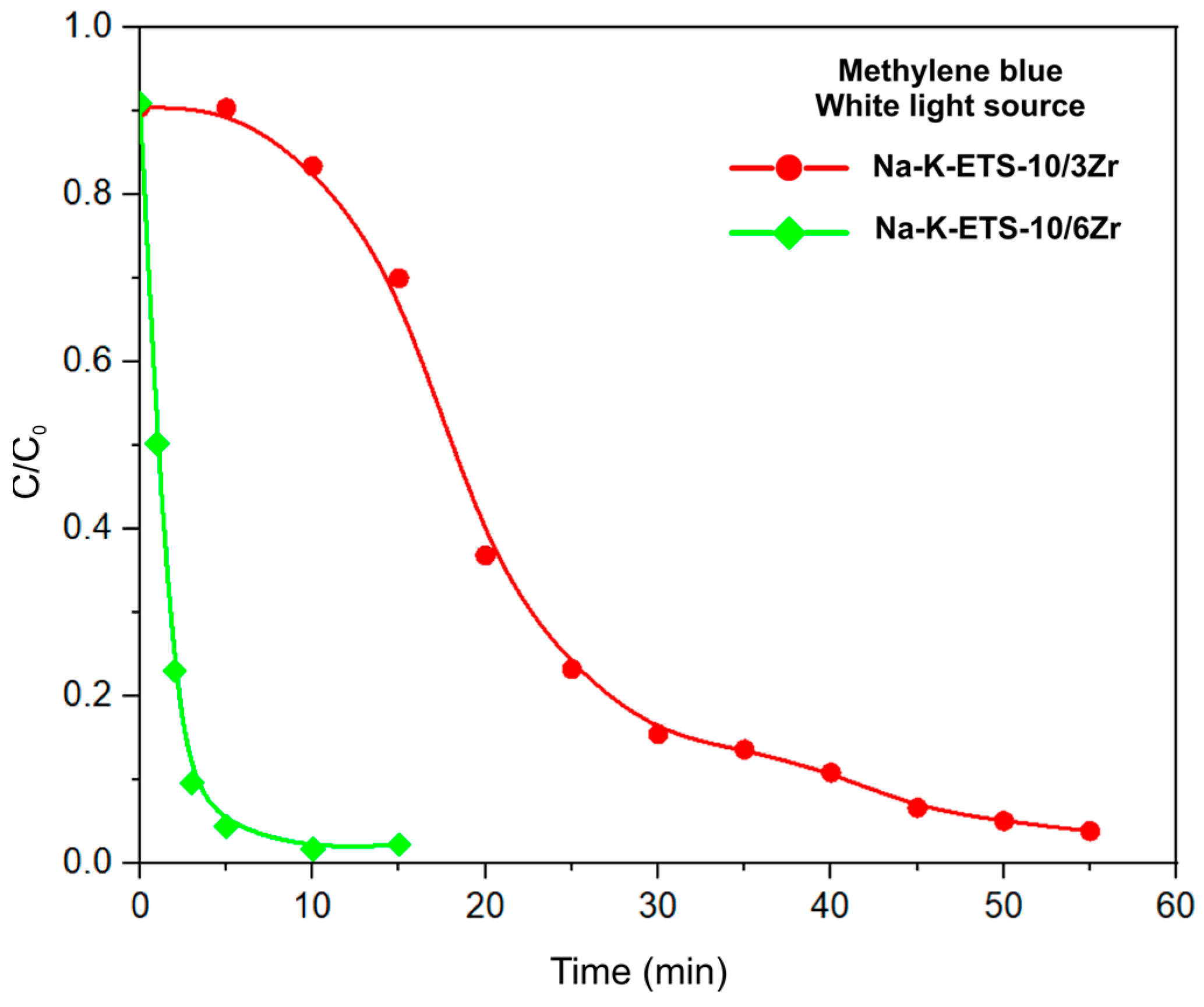
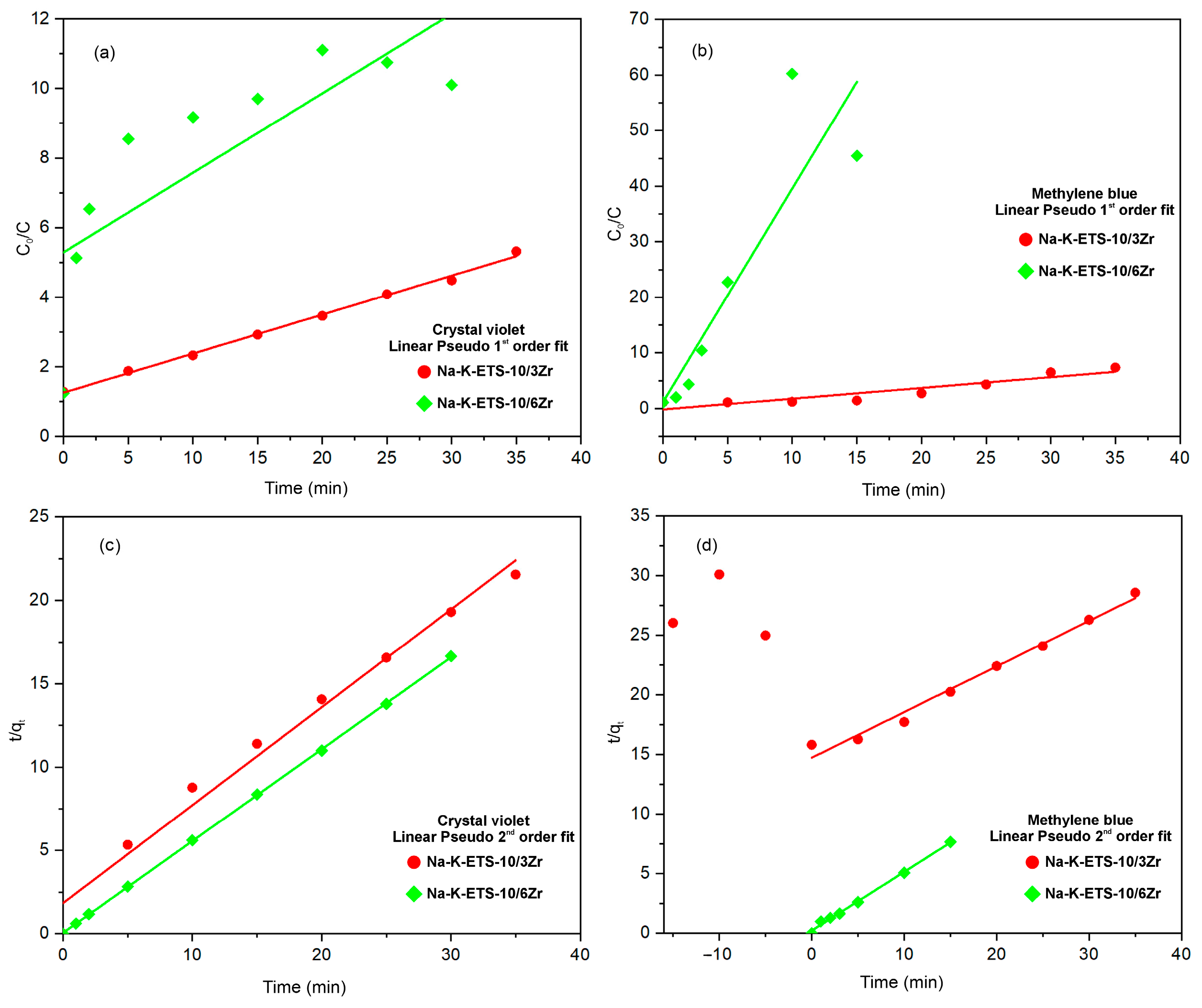
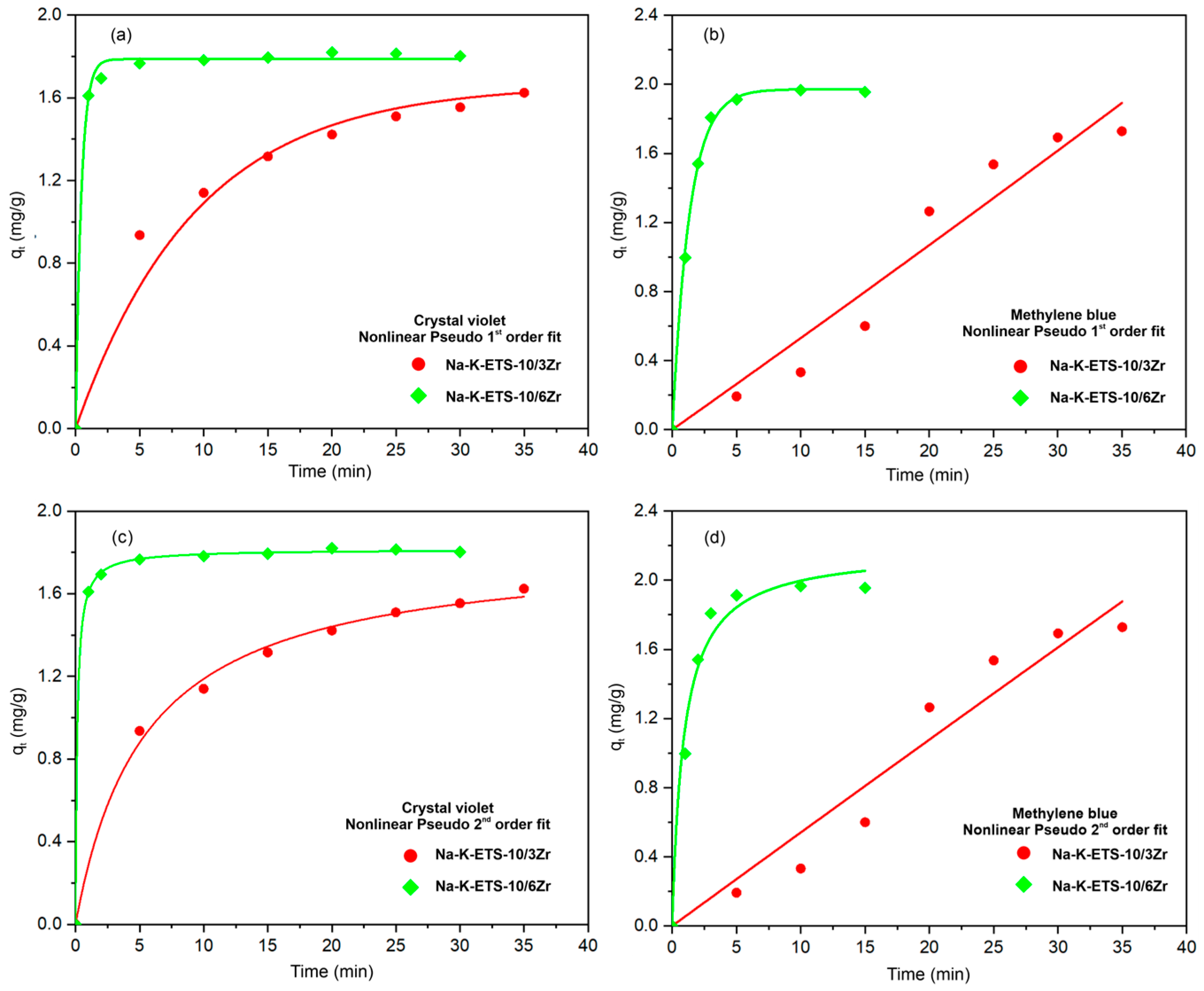
3.2.2. Kinetic Studies
3.2.3. Plausible Photodegradation Mechanism of MB and CV by Na-K-ETS-10/6Zr
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Guidelines for Drinking-Water Quality: First Addendum to the Fourth Edition; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Dettori, M.; Arghittu, A.; Deiana, G.; Castiglia, P.; Azara, A. The revised European Directive 2020/2184 on the quality of water intended for human consumption. A step forward in risk assessment, consumer safety and informative communication. Environ. Res. 2022, 209, 112773. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Haleem, A.; Shafiq, A.; Chen, S.-Q.; Nazar, M. A comprehensive review on adsorption, photocatalytic and chemical degradation of dyes and nitro-compounds over different kinds of porous and composite materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Soni, V. Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Reg. Stud. Mar. Sci. 2021, 45, 101802. [Google Scholar] [CrossRef]
- Iqbal, A.; Yusaf, A.; Usman, M.; Hussain Bokhari, T.; Mansha, A. Insight into the degradation of different classes of dyes by advanced oxidation processes; a detailed review. Int. J. Environ. Anal. Chem. 2023. [Google Scholar] [CrossRef]
- Carvalho, S.S.; Rodrigues, A.C.C.; Lima, J.F.; Carvalho, N.M. Photocatalytic degradation of dyes by mononuclear copper (II) complexes from bis-(2-pyridylmethyl) amine NNN-derivative ligands. Inorg. Chim. Acta 2020, 512, 119924. [Google Scholar] [CrossRef]
- Sangareswari, M.; Meenakshi Sundaram, M. Development of efficiency improved polymer-modified TiO2 for the photocatalytic degradation of an organic dye from wastewater environment. Appl. Water Sci. 2017, 7, 1781–1790. [Google Scholar] [CrossRef]
- Shanmugaratnam, S.; Selvaratnam, B.; Baride, A.; Koodali, R.; Ravirajan, P.; Velauthapillai, D.; Shivatharsiny, Y. SnS2/TiO2 nanocomposites for hydrogen production and photodegradation under extended solar irradiation. Catalysts 2021, 11, 589. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, X.; Chen, S.; Ashok, J.; Kawi, S. Oxygen-deficient wo3/tio2/cc nanorod arrays for visible-light photocatalytic degradation of methylene blue. Catalysts 2021, 11, 1349. [Google Scholar] [CrossRef]
- Vogt, E.T.; Whiting, G.T.; Chowdhury, A.D.; Weckhuysen, B.M. Zeolites and zeotypes for oil and gas conversion. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 2015; Volume 58, pp. 143–314. [Google Scholar]
- Durán, B.; Saldías, C.; Villarroel, R.; Hevia, S.A. In Situ Synthesis of CuO/Cu2O Nanoparticle-Coating Nanoporous Alumina Membranes with Photocatalytic Activity under Visible Light Radiation. Coatings 2023, 13, 179. [Google Scholar] [CrossRef]
- Noh, S.H.; Kim, S.D.; Chung, Y.J.; Park, J.W.; Moon, D.K.; Hayhurst, D.T.; Kim, W.J. The effects of (Na + K)/Na molar ratio and kinetic studies on the rapid crystallization of a large pored titanium silicate, ETS-10 using cost efficient titanium oxysulfate, TiOSO4 under stirring. Microporous Mesoporous Mater. 2006, 88, 197–204. [Google Scholar] [CrossRef]
- Kostov-Kytin, V.; Ferdov, S.; Kalvachev, Y.; Mihailova, B.; Petrov, O. Hydrothermal synthesis of microporous titanosilicates. Microporous Mesoporous Mater. 2007, 105, 232–238. [Google Scholar] [CrossRef]
- Pavel, C.; Vuono, D.; Catanzaro, L.; De Luca, P.; Bilba, N.; Nastro, A.; Nagy, J.B. Synthesis and characterization of the microporous titanosilicates ETS-4 and ETS-10. Microporous Mesoporous Mater. 2002, 56, 227–239. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Lippens, B.C.; De Boer, J. Studies on pore systems in catalysts: V. The t method. J. Catal. 1965, 4, 319–323. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Terzyk, A.P.; Gauden, P.A.; Rychlicki, G. Numerical Analysis of the Horvath–Kawazoe Equation—The Adsorption of Nitrogen, Argon, Benzene, Carbon Tetrachloride and Sulphur Hexafluoride. Adsorpt. Sci. Technol. 2002, 20, 295–305. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Khan, A.A.P.; Jibali, B.M. Removal of Congo red, methylene blue and Cr (VI) ions from water using natural serpentine. J. Taiwan Inst. Chem. Eng. 2018, 82, 102–116. [Google Scholar] [CrossRef]
- Krisnandi, Y.K.; Lachowski, E.E.; Howe, R.F. Effects of Ion Exchange on the Structure of ETS-10. Chem. Mater. 2006, 18, 928–933. [Google Scholar] [CrossRef]
- Anderson, M.; Terasaki, O.; Ohsuna, T.; Philippou, A.; MacKay, S.; Ferreira, A.; Rocha, J.; Lidin, S. Structure of the microporous titanosilicate ETS-10. Nature 1994, 367, 347–351. [Google Scholar] [CrossRef]
- Xiang, M.; Zhang, F.; Tong, L.; Wang, H.; Ding, Y.; Zhang, W.; Wu, Z.; Zhang, Z.; Wei, X.; Jiang, F. Development of the hierarchical ETS-10 zeolite catalyst for improving the aqueous-phase biomass hydrodeoxygenation activity. J. Mater. Sci. 2020, 55, 10505–10521. [Google Scholar] [CrossRef]
- Faroldi, B.; Irusta, S.; Cornaglia, L. Influence of La incorporation on the catalytic activity of Ru/ETS-10 catalysts for hydrogen production. Appl. Catal. A Gen. 2015, 504, 391–398. [Google Scholar] [CrossRef]
- Surolia, P.K.; Tayade, R.J.; Jasra, R.V. Photocatalytic degradation of nitrobenzene in an aqueous system by transition-metal-exchanged ETS-10 zeolites. Ind. Eng. Chem. Res. 2010, 49, 3961–3966. [Google Scholar] [CrossRef]
- Lv, L.; Zhou, J.; Su, F.; Zhao, X. Local structure changes of microporous titanosilicate ETS-10 upon acid treatment. J. Phys. Chem. C 2007, 111, 773–778. [Google Scholar] [CrossRef]
- Revellame, E.; Fortela, D.; Sharp, W.; Hernandez, R.; Zappi, M. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Tan, K.; Hameed, B. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Uddin, M.T.; Islam, M.A.; Mahmud, S.; Rukanuzzaman, M. Adsorptive removal of methylene blue by tea waste. J. Hazard. Mater. 2009, 164, 53–60. [Google Scholar] [CrossRef]
- Robati, D. Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J. Nanostruct. Chem. 2013, 3, 55. [Google Scholar] [CrossRef]
- Yuh-Shan, H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Kumbhar, P.; Narale, D.; Bhosale, R.; Jambhale, C.; Kim, J.-H.; Kolekar, S. Synthesis of tea waste/Fe3O4 magnetic composite (TWMC) for efficient adsorption of crystal violet dye: Isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2022, 10, 107893. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Ji, Y.; Ma, T.; Zhang, F.; Wang, Y.; Ci, M.; Chen, D.; Jiang, A.; Wang, W. Photocatalytic degradation of methylene blue with ZnO@C nanocomposites: Kinetics, mechanism, and the inhibition effect on monoamine oxidase A and B. NanoImpact 2019, 15, 100174. [Google Scholar] [CrossRef]
- Dagher, S.; Soliman, A.; Ziout, A.; Tit, N.; Hilal-Alnaqbi, A.; Khashan, S.; Alnaimat, F.; Qudeiri, J.A. Photocatalytic removal of methylene blue using titania-and silica-coated magnetic nanoparticles. Mater. Res. Express 2018, 5, 065518. [Google Scholar] [CrossRef]
- Trandafilović, L.V.; Jovanović, D.J.; Zhang, X.; Ptasińska, S.; Dramićanin, M. Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO: Eu nanoparticles. Appl. Catal. B Environ. 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Mohanty, S.; Moulick, S.; Maji, S.K. Adsorption/photodegradation of crystal violet (basic dye) from aqueous solution by hydrothermally synthesized titanate nanotube (TNT). J. Water Process Eng. 2020, 37, 101428. [Google Scholar] [CrossRef]
- Sahoo, C.; Gupta, A.; Pal, A. Photocatalytic degradation of Crystal Violet (CI Basic Violet 3) on silver ion doped TiO2. Dye. Pigment. 2005, 66, 189–196. [Google Scholar] [CrossRef]
- Yu, Z.; Chuang, S.S. Probing methylene blue photocatalytic degradation by adsorbed ethanol with in situ IR. J. Phys. Chem. C 2007, 111, 13813–13820. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, L.; Wang, H.; Yang, J.; Yuan, X.; Wang, H.; Liang, J.; Li, X.; Li, H.; Bu, Y. Oxygen Vacancies Modified TiO2/O-Terminated Ti3C2 Composites: Unravelling the Dual Effects between Oxygen Vacancy and High-Work-Function Titanium Carbide. Adv. Funct. Mater. 2023, 33, 2307702. [Google Scholar] [CrossRef]
- Wei, R.; Wang, H.; Jiang, L.; Yang, J.; Li, W.; Yuan, X.; Wang, H.; Liang, J.; Chen, Y.; Bu, Y. Molecular self-assembled synthesis of highly dispersed Co single-atom coordinated 2-methylimidazole modified carbon nitride for peroxymonosulfate activation. Chem. Eng. J. 2023, 471, 144494. [Google Scholar] [CrossRef]
- Wang, X.; Tang, W.; Jiang, L.; Feng, J.; Yang, J.; Zhou, S.; Li, W.; Yuan, X.; Wang, H.; Wang, J. Mechanism insights into visible light-induced crystalline carbon nitride activating periodate for highly efficient ciprofloxacin removal. Chem. Eng. J. 2023, 471, 144521. [Google Scholar] [CrossRef]
- Shaukat, A.; Farrukh, M.A.; Chong, K.-K.; Nawaz, R.; Qamar, M.T.; Iqbal, S.; Awwad, N.S.; Ibrahium, H.A. The Impact of Different Green Synthetic Routes on the Photocatalytic Potential of FeSnO2 for the Removal of Methylene Blue and Crystal Violet Dyes under Natural Sunlight Exposure. Catalysts 2023, 13, 1135. [Google Scholar] [CrossRef]
- Elashery, S.E.; Ibrahim, I.; Gomaa, H.; El-Bouraie, M.M.; Moneam, I.A.; Fekry, S.S.; Mohamed, G.G. Comparative Study of the Photocatalytic Degradation of Crystal Violet Using Ferromagnetic Magnesium Oxide Nanoparticles and MgO-Bentonite Nanocomposite. Magnetochemistry 2023, 9, 56. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Naushad, M.; Dhiman, P.; Thakur, B.; García-Peñas, A.; Stadler, F.J. Gum acacia-crosslinked-poly (acrylamide) hydrogel supported C3N4/BiOI heterostructure for remediation of noxious crystal violet dye. Materials 2022, 15, 2549. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.A.; Kim, H. Ag3PO4-Deposited TiO2@ Ti3C2 Petals for Highly Efficient Photodecomposition of Various Organic Dyes under Solar Light. Nanomaterials 2022, 12, 2464. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, R.; Peighambardoust, S.J.; Boffito, D.C.; Ramavandi, B. Sono-photocatalytic activity of cloisite 30B/ZnO/Ag2O nanocomposite for the simultaneous degradation of crystal violet and methylene blue dyes in aqueous media. Nanomaterials 2022, 12, 3103. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Belessiotis, G.V.; Elseman, A.M.; Mohamed, M.M.; Ren, Y.; Salama, T.M.; Mohamed, M.B.I. Magnetic TiO2/CoFe2O4 photocatalysts for degradation of organic dyes and pharmaceuticals without oxidants. Nanomaterials 2022, 12, 3290. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, K.; Bateni, A.; Sojoodi, N.; Ataabadi, M.R.; Behroozi, A.H.; Maleki, A.; You, Z. Magnetized inulin by Fe3O4 as a bio-nano adsorbent for treating water contaminated with methyl orange and crystal violet dyes. Sci. Rep. 2022, 12, 22034. [Google Scholar] [CrossRef]
- Abdel-Hady, E.; Mohamed, H.F.; Hafez, S.H.; Fahmy, A.M.; Magdy, A.; Mohamed, A.S.; Ali, E.O.; Abdelhamed, H.R.; Mahmoud, O.M. Textural properties and adsorption behavior of Zn–Mg–Al layered double hydroxide upon crystal violet dye removal as a low cost, effective, and recyclable adsorbent. Sci. Rep. 2023, 13, 6435. [Google Scholar] [CrossRef]
- Ali, N.; Khan, A.A.; Wakeel, M.; Khan, I.A.; Din, S.U.; Qaisrani, S.A.; Khan, A.M.; Hameed, M.U. Activation of Peroxymonosulfate by UV-254 nm Radiation for the Degradation of Crystal Violet. Water 2022, 14, 3440. [Google Scholar] [CrossRef]
- Algethami, J.S.; Hassan, M.S.; Alorabi, A.Q.; Alhemiary, N.A.; Fallatah, A.M.; Alnaam, Y.; Almusabi, S.; Amna, T. Manganese Ferrite–Hydroxyapatite Nanocomposite Synthesis: Biogenic Waste Remodeling for Water Decontamination. Nanomaterials 2022, 12, 1631. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, B.; Liu, D.; Cheng, L.; Zhang, Y.; Lu, D.; Yu, H.; Chen, A.; Yuan, S.; Chen, K. Morphological Control of Supported ZnO Nanosheet Arrays and Their Application in Photodegradation of Organic Pollutants. Nanomaterials 2023, 13, 443. [Google Scholar] [CrossRef] [PubMed]
- Gharbani, P.; Mehrizad, A.; Mosavi, S.A. Optimization, kinetics and thermodynamics studies for photocatalytic degradation of Methylene Blue using cadmium selenide nanoparticles. NPJ Clean Water 2022, 5, 34. [Google Scholar] [CrossRef]
- Qu, T.; Yao, X.; Owens, G.; Gao, L.; Zhang, H. A sustainable natural clam shell derived photocatalyst for the effective adsorption and photodegradation of organic dyes. Sci. Rep. 2022, 12, 2988. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Han, H.; Wang, D.; Chen, J.; Zhang, R.; Zuo, S.; Yao, C.; Kang, J.; Gui, H. C, N co-doped TiO2 hollow nanofibers coated stainless steel meshes for oil/water separation and visible light-driven degradation of pollutants. Sci. Rep. 2023, 13, 5716. [Google Scholar] [CrossRef] [PubMed]
- Berehe, B.A.; Assen, A.H.; Kumar, A.S.K.; Ulla, H.; Duma, A.D.; Chang, J.-Y.; Gedda, G.; Girma, W.M. Highly efficient visible light active ZnO/Cu-DPA composite photocatalysts for the treatment of wastewater contaminated with organic dye. Sci. Rep. 2023, 13, 16454. [Google Scholar] [CrossRef]
- Nutescu Duduman, C.; Gómez de Castro, C.; Apostolescu, G.A.; Ciobanu, G.; Lutic, D.; Favier, L.; Harja, M. Enhancing the TiO2-Ag Photocatalytic Efficiency by Acetone in the Dye Removal from Wastewater. Water 2022, 14, 2711. [Google Scholar] [CrossRef]
- Ahmad, Z.; Tahir, R.; Sajjad, N.; Batool, F.; Zada, N.; Ullah, H. Cleansing Water: Harnessing Trimetallic Nanoparticles in Sunlight to Degrade Methylene Blue Dye, Aiding Aquatic Contaminant Cleanup. Water 2023, 15, 3404. [Google Scholar] [CrossRef]
- Vallejo, W.; Cantillo, A.; Salazar, B.; Diaz-Uribe, C.; Ramos, W.; Romero, E.; Hurtado, M. Comparative study of ZnO thin films doped with transition metals (Cu and Co) for methylene blue photodegradation under visible irradiation. Catalysts 2020, 10, 528. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Li, W.; Yang, L.; Wu, H.; Mao, N. Photocatalytic Properties of a Novel Keratin char-TiO2 Composite Films Made through the Calcination of Wool Keratin Coatings Containing TiO2 Precursors. Catalysts 2021, 11, 1366. [Google Scholar] [CrossRef]
- Weon, S.H.; Han, J.; Choi, Y.-K.; Park, S.; Lee, S.H. Development of Blended Biopolymer-Based Photocatalytic Hydrogel Beads for Adsorption and Photodegradation of Dyes. Gels 2023, 9, 630. [Google Scholar] [CrossRef] [PubMed]
- Altaf, N.U.H.; Naz, M.Y.; Shukrullah, S.; Ghaffar, A.; Irfan, M.; Walczak, D.; Głowacz, A.; Mahnashi, M.H.; Rahman, S.; Królczyk, G. Concurrent synthesis and immobilization of Ag nanoparticles over TiO2 via plasma reduction for photocatalytic treatment of methyl blue in water. Materials 2021, 14, 6082. [Google Scholar] [CrossRef] [PubMed]


| Synthesis Batch Composition | Na, wt% | Si, wt% | K, wt% | Ti, wt% | Zr, wt% |
|---|---|---|---|---|---|
| Na-K-ETS-10/5Zr | 9.90 | 58.85 | 7.15 | 20.84 | 3.26 |
| Na-K-ETS-10/10Zr | 9.63 | 45.23 | 8.56 | 30.37 | 6.21 |
| Na-K-ETS-10/15Zr | 12.07 | 42.10 | 13.93 | 23.78 | 8.13 |
| Na-K-ETS-10/20Zr | 7.97 | 34.97 | 9.53 | 35.44 | 12.10 |
| Synthesized Sample Composition | Chemical Composition |
|---|---|
| Na-K-ETS-10 | Na 3.28 K 1.37 (Ti 3.48 Si 15.52 O 41.48) |
| Na-K-ETS-10/3 wt% Zr | Na 3.23 K 1.37 (Ti 3.27 Zr 0.27 Si 15.73 O 41.27) |
| Na-K-ETS-10/6 wt% Zr | Na 3.55 K 1.85 (Ti 5.37 Zr 0.58 Si 13.63 O 43.37) |
| Na-K-ETS-10/8 wt% Zr | Na 5.01 K 3.36 (Ti 4.71 Zr 0.85 Si 14.29 O 42.71) |
| Na-K-ETS-10/12 wt% Zr | Na 3.32 K 2.33 (Ti 7.08 Zr 1.27 Si 11.92 O 45.08) |
| Sample | SBET m2·g−1 | Vt cm3·g−1 | Average Pore Diameter nm |
|---|---|---|---|
| Na-K-ETS-10 | 19.53 | 0.052 | 10 |
| Na-K-ETS-10/3Zr | 118.00 | 0.054 | 2.1 |
| Na-K-ETS-10/6Zr | 144.56 | 0.064 | 2.3 |
| Na-K-ETS-10/8Zr | 7.35 | 0.003 | 7.8 |
| Na-K-ETS-10/12Zr | 9.15 | 0.003 | 7.7 |
| Sample | Pseudo-First-Order Crystal Violet | Pseudo-Second-Order Crystal Violet | Pseudo-First-Order Methylene Blue | Pseudo-Second-Order Methylene Blue |
|---|---|---|---|---|
| Na-K-ETS-10/3Zr | qt = 3.5146 k1 = 0.0032 R2 = 0.9964 | qe = 1.6985 k2 = 0.1876 R2 = 0.9828 | qt = 0.8414 k1 = 0.0055 R2 = 0.8650 | qe = 6.8121 k2 = 0.0099 R2 = 0.9851 |
| Na-K-ETS-10/6Zr | qt = 198.83 k1 = 0.0076 R2 = 0.6237 | qe = 0.0579 k2 = 5.2132 R2 = 0.9999 | qt = 3.0918 k1 = 0.2562 R2 = 0.8038 | qe = 2.0298 k2 = 1.0655 R2 = 0.9964 |
| Sample | Pseudo-First-Order Crystal Violet | Pseudo-Second-Order Crystal Violet | Pseudo-First-Order Methylene Blue | Pseudo-Second-Order Methylene Blue |
|---|---|---|---|---|
| Na-K-ETS-10/3Zr | qt = 5.2791 k1 = 0.0030 R2 = 0.9649 | qt = 1.8305 k2 = 0.1010 R2 = 0.9958 | qt = 7.54·10−16 k1 = 4.3·10−5 R2 = 0.9460 | qt = 160.0229 k2 = 2.12·10−6 R2 = 0.9458 |
| Na-K-ETS-10/6Zr | qt = 5.9701 k1 = 0.0743 R2 = 0.9971 | qt = 1.8150 k2 = 4.1875 R2 = 0.9998 | qt = 7.1800 k1 = 0.0497 R2 = 0.9986 | qt = 2.1794 k2 = 0.5020 R2 = 0.9822 |
| Catalyst | Degradation Efficiency (%) | Ref. | |
|---|---|---|---|
| Crystal Violet | Methylene Blue | ||
| FeSnO2 (Lawsonia inermis leaves) | 84.0 | 76.0 | [45] |
| MgO NPs | 99.2 | [46] | |
| MgO-Bentonite | 83.2 | [46] | |
| GA-cl-poly(acrylamide)@ C3N4/BiOI nanocomposite hydrogel | 88.0 | [47] | |
| Ag3PO4/TiO2@Ti3C2 | 99.2 | 94.4 | [48] |
| Closite 30B/ZnO/Ag2O | 99.2 | 98.4 | [49] |
| CoFe2O4/TiO2 | 83.0 | 95.0 | [50] |
| Fe3O4@inulin | 91.1 | [51] | |
| Zn–Mg–Al/LDH | 87.4 | [52] | |
| Peroxymonosulfate | 42.0 | [53] | |
| HAP–MnFe2O4 | 88.0 | [54] | |
| ZnO nanosheet arrays | 98.0 | [55] | |
| CdSe nanoparticles | 92.8 | [56] | |
| mussel shell | 99.6 | [57] | |
| TN450 coated | 90.6 | [58] | |
| ZnO/20%Cu-DPA | 87.0 | [59] | |
| TiO2-Ag | 97.3 | [60] | |
| Fe-Ni-Cd TMNP’s | 79.4 | [61] | |
| ZnO:Co5% | 62.6 | [62] | |
| R-WO3/R-TiO2/CC | 68.0 | [11] | |
| calcined keratin char-TiO2 composite film | 87.4 | [63] | |
| cellulose/TiO2 microbeads | 95.0 | [64] | |
| Ag/TiO2 | 90.9 | [65] | |
| ETS-10/6Zr | 96.1 | 97.8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarova, H.; Rusew, R.; Iliev, K.; Tsvetanova, L.; Barbov, B.; Shivachev, B. Photodegradation of Methylene Blue and Crystal Violet by Zr-Modified Engelhard Titanium Silicate 10. Water 2023, 15, 4186. https://doi.org/10.3390/w15234186
Lazarova H, Rusew R, Iliev K, Tsvetanova L, Barbov B, Shivachev B. Photodegradation of Methylene Blue and Crystal Violet by Zr-Modified Engelhard Titanium Silicate 10. Water. 2023; 15(23):4186. https://doi.org/10.3390/w15234186
Chicago/Turabian StyleLazarova, Hristina, Rusi Rusew, Kostadin Iliev, Liliya Tsvetanova, Borislav Barbov, and Boris Shivachev. 2023. "Photodegradation of Methylene Blue and Crystal Violet by Zr-Modified Engelhard Titanium Silicate 10" Water 15, no. 23: 4186. https://doi.org/10.3390/w15234186
APA StyleLazarova, H., Rusew, R., Iliev, K., Tsvetanova, L., Barbov, B., & Shivachev, B. (2023). Photodegradation of Methylene Blue and Crystal Violet by Zr-Modified Engelhard Titanium Silicate 10. Water, 15(23), 4186. https://doi.org/10.3390/w15234186







