Anaerobic Digestion of Municipal Sewage Sludge Integrated with Brewery Wastewater Treatment: Importance of Temperature and Mixing Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. Operation of the Plant-Scale Anaerobic Digesters
2.2. Batch Tests Operation
2.2.1. Substrates and Inoculum
2.2.2. Batch Anaerobic Reactors and Operations
2.3. Analytical Methods
3. Results and Discussion
3.1. Plant-Scale Anaerobic Digestion Performance of Municipal Sewage Sludge without/with the Involvement of Brewery Wastewater
3.2. Effects of Brewery Wastewater Mixing Ratio and Temperature on Anaerobic Co-Digestion of Municipal Sewage Sludge
3.2.1. Biogas Production Process
3.2.2. The Variation in Organic Matter, Ammonia and Phosphate Concentrations in the Reactors
3.3. Microbial Community
4. Conclusions
- (1)
- Within a certain range (0–20%), an increased ratio of brewery wastewater is beneficial to increase biogas production. The highest biogas production was achieved when the mixing ratio was 20%, which was 44.6% higher than that without brewery wastewater. However, a further increase in the mixing ratio could lead to the acidification of the anaerobic system, thus inhibiting biogas production.
- (2)
- With increasing temperature, the biogas production effect of the reactor with a high mixing ratio of brewery wastewater was significantly improved, indicating that high temperature is beneficial for improving the synergistic anaerobic digestion effect between brewery wastewater and municipal sewage sludge.
- (3)
- The anaerobic co-digestion of municipal sewage sludge with brewery wastewater was accompanied by the release of ammonia nitrogen and the removal of phosphate, which was also influenced by the mixing ratio and the temperature.
- (4)
- The microbial community structure was significantly changed with the introduction of brewery wastewater. With the increasing mixing ratio of brewery wastewater, Firmicutes gradually become dominant instead of Chloroflexi. Meanwhile, the proportion of Methanothrix was significantly reduced, and Methanolinea and Methanosarcina gradually dominated.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Török, Á.; Szerletics, Á.; Jantyik, L. Factors influencing competitiveness in the global beer trade. Sustainability 2020, 12, 5957. [Google Scholar] [CrossRef]
- Qin, R. The characteristics of beer industrial wastewater and its influence on the environment. IOP Conf. Ser. Earth Environ. Sci. 2018, 170, 032068. [Google Scholar] [CrossRef]
- Akunna, J.C. Anaerobic treatment of brewery wastes. In Brewing Microbiology: Managing Microbes, Ensuring Quality and Valorising Waste; Hill, A.E., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Elsevier Ltd.: Oxford, UK, 2015; pp. 407–424. [Google Scholar]
- Simate, G.S.; Cluett, J.; Iyuke, S.E.; Musapatika, E.T.; Ndlovu, S.; Walubita, L.F.; Alvarez, A.E. The treatment of brewery wastewater for reuse: State of the art. Desalination 2011, 273, 235–247. [Google Scholar] [CrossRef]
- Olajire, A.A. The brewing industry and environmental challenges. J. Clean. Prod. 2020, 256, 102817. [Google Scholar] [CrossRef]
- Bochmann, G.; Drosg, B.; Fuchs, W. Anaerobic digestion of thermal pretreated brewers’ spent grains. Environ. Prog. Sustain. Energy 2015, 34, 1092–1096. [Google Scholar] [CrossRef]
- Sturm, B.; Hugenschmidt, S.; Joyce, S.; Hofacker, W.; Roskilly, A.P. Opportunities and barriers for efficient energy use in a medium-sized brewery. Appl. Therm. Eng. 2013, 53, 397–404. [Google Scholar] [CrossRef]
- Werkneh, A.A.; Beyene, H.D.; Osunkunle, A.A. Recent advances in brewery wastewater treatment; approaches for water reuse and energy recovery: A review. Environ. Sustain. 2019, 2, 199–209. [Google Scholar] [CrossRef]
- Jaiyeola, A.T.; Bwapwa, J.K. Treatment technology for brewery wastewater in a water-scarce country: A review. S. Afr. J. Sci. 2016, 112, 1–8. [Google Scholar] [CrossRef][Green Version]
- Meshksar, M.; Roostaee, T.; Rahimpour, M.R. Membrane technology for brewery wastewater treatment. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Cassano, A., Rastogi, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 289–303. [Google Scholar]
- Wang, S.G.; Liu, X.W.; Gong, W.X.; Gao, B.Y.; Zhang, D.H.; Yu, H.Q. Aerobic granulation with brewery wastewater in a sequencing batch reactor. Bioresour. Technol. 2007, 98, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Corsino, S.F.; di Biase, A.; Devlin, T.R.; Munz, G.; Torregrossa, M.; Oleszkiewicz, J.A. Effect of extended famine conditions on aerobic granular sludge stability in the treatment of brewery wastewater. Bioresour. Technol. 2017, 226, 150–157. [Google Scholar] [CrossRef]
- Bakare, B.F.; Shabangu, K.; Chetty, M. Brewery wastewater treatment using laboratory scale aerobic sequencing batch reactor. S. Afr. J. Chem. Eng. 2017, 24, 128–134. [Google Scholar] [CrossRef]
- Forssell, P.; Kontkanen, H.; Schols, H.A.; Hinz, S.; Eijsink, V.G.; Treimo, J.; Robertson, J.A.; Waldron, K.W.; Faulds, C.B.; Buchert, J. Hydrolysis of brewers’ spent grain by carbohydrate degrading enzymes. J. Inst. Brew. 2008, 114, 306–314. [Google Scholar] [CrossRef]
- Arantes, M.K.; Alves, H.J.; Sequinel, R.; da Silva, E.A. Treatment of brewery wastewater and its use for biological production of methane and hydrogen. Int. J. Hydrogen Energy 2017, 42, 26243–26256. [Google Scholar] [CrossRef]
- Chen, H.; Chang, S.; Guo, Q.; Hong, Y.; Wu, P. Brewery wastewater treatment using an anaerobic membrane bioreactor. Biochem. Eng. J. 2016, 105, 321–331. [Google Scholar] [CrossRef]
- Shao, X.; Peng, D.; Teng, Z.; Ju, X. Treatment of brewery wastewater using anaerobic sequencing batch reactor (ASBR). Bioresour. Technol. 2008, 99, 3182–3186. [Google Scholar] [CrossRef]
- Shrestha, P.M.; Malvankar, N.S.; Werner, J.J.; Franks, A.E.; Elena-Rotaru, A.; Shrestha, M.; Liu, F.; Nevin, K.P.; Angenent, L.T.; Lovley, D.R. Correlation between microbial community and granule conductivity in anaerobic bioreactors for brewery wastewater treatment. Bioresour. Technol. 2014, 174, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Xu, D.; Hao, B.; Liu, L.; Wang, S.; Wu, Z. Thermochemical methods for the treatment of municipal sludge. J. Clean. Prod. 2021, 311, 127811. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Liu, K.; Hu, L.; Yang, J.; Wang, X.; Song, Z.; Yang, Y.; Tang, M.; Wang, R. Bacterial community composition of internal circulation reactor at different heights for large-scale brewery wastewater treatment. Bioresour. Technol. 2021, 331, 125027. [Google Scholar] [CrossRef] [PubMed]
- Rulkens, W. Sewage sludge as a biomass resource for the production of energy: Overview and assessment of the various options. Energy Fuels 2008, 22, 9–15. [Google Scholar] [CrossRef]
- Feng, L.; Luo, J.; Chen, Y. Dilemma of sewage sludge treatment and disposal in China. Environ. Sci. Technol. 2015, 49, 4781–4782. [Google Scholar] [CrossRef]
- Chen, H.; Yan, S.H.; Ye, Z.L.; Meng, H.J.; Zhu, Y.G. Utilization of urban sewage sludge: Chinese perspectives. Environ. Sci. Pollut. Res. 2012, 19, 1454–1463. [Google Scholar] [CrossRef]
- Sosnowski, P.; Wieczorek, A.; Ledakowicz, S. Anaerobic co-digestion of sewage sludge and organic fraction of municipal solid wastes. Adv. Environ. Res. 2003, 7, 609–616. [Google Scholar] [CrossRef]
- Murto, M.; Björnsson, L.; Mattiasson, B. Impact of food industrial waste on anaerobic co-digestion of sewage sludge and pig manure. J. Environ. Manag. 2004, 70, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Fonoll, X.; Astals, S.; Dosta, J.; Mata-Alvarez, J. Anaerobic co-digestion of sewage sludge and fruit wastes: Evaluation of the transitory states when the co-substrate is changed. Chem. Eng. J. 2015, 262, 1268–1274. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Dong, J.; Chen, M.; He, Y.; Liu, H.; Li, Y.; Liu, H. Hydrolase production via food waste fermentation and its application to enhance anaerobic digestion of sewage sludge. Fermentation 2023, 9, 526. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal. Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Mudzanani, K.; van Heerden, E.; Mbhele, R.; Daramola, M.O. Enhancement of biogas production via co-digestion of wastewater treatment sewage sludge and brewery spent grain: Physicochemical characterization and microbial community. Sustainability 2021, 13, 8225. [Google Scholar] [CrossRef]
- Rasmeni, Z.Z.; Madyira, D.M.; Matheri, A.N. Optimum loading ratio for co-digested wastewater sludge and brewery spent yeast. Energy Rep. 2022, 8, 1141–1149. [Google Scholar] [CrossRef]
- Yin, Z.; Xie, L.; Zhou, Q. Effects of sulfide on the integration of denitrification with anaerobic digestion. J. Biosci. Bioeng. 2015, 120, 426–431. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Ripoll, V.; Agabo-García, C.; Perez, M.; Solera, R. Improvement of biomethane potential of sewage sludge anaerobic co-digestion by addition of “sherry-wine” distillery wastewater. J. Clean. Prod. 2020, 251, 119667. [Google Scholar] [CrossRef]
- Hao, J.; Wang, H. Volatile fatty acids productions by mesophilic and thermophilic sludge fermentation: Biological responses to fermentation temperature. Bioresour. Technol. 2015, 175, 367–373. [Google Scholar] [CrossRef]
- Ripoll, V.; Agabo-García, C.; Solera, R.; Perez, M. Modelling of the anaerobic semi-continuous co-digestion of sewage sludge and wine distillery wastewater. Environ. Sci. Water Res. Technol. 2020, 6, 1880–1889. [Google Scholar] [CrossRef]
- Ripoll, V.; Solera, R.; Perez, M. Kinetic modelling of anaerobic co-digestion of sewage sludge and Sherry-wine distillery wastewater: Effect of substrate composition in batch bioreactor. Fuel 2022, 329, 125524. [Google Scholar] [CrossRef]
- Cheng, J.; Hua, J.; Kang, T.; Meng, B.; Yue, L.; Dong, H.; Li, H.; Zhou, J. Nanoscale zero-valent iron improved lactic acid degradation to produce methane through anaerobic digestion. Bioresour. Technol. 2020, 317, 124013. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.Z.; Pereira, M.A.; Alves, J.I.; Smidt, H.; Stams, A.M.; Alves, M.M. Anaerobic microbial LCFA degradation in bioreactors. Water Sci. Technol. 2008, 57, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.H.; Chen, C.; Huang, C.; Lee, D.J. Glucose fermentation with biochar-amended consortium: Microbial consortium shift. Bioengineered 2020, 11, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, J.; Cao, X.; Cheng, Y. Characterization of functional microbial communities involved in different transformation stages in a full-scale printing and dyeing wastewater treatment plant. Biochem. Eng. J. 2018, 137, 162–171. [Google Scholar] [CrossRef]
- Tukanghan, W.; Hupfauf, S.; Gómez-Brandón, M.; Insam, H.; Salvenmoser, W.; Prasertsan, P.; Cheirsilp, B.; Sompong, O. Symbiotic Bacteroides and Clostridium-rich methanogenic consortium enhanced biogas production of high-solid anaerobic digestion systems. Bioresour. Technol. Rep. 2021, 14, 100685. [Google Scholar] [CrossRef]
- Cao, Z.; Huang, X.; Wu, Y.; Wang, D.; Du, W.; Zhang, J.; Yang, Q.; Kuang, Z.; Chen, Z.; Li, X. Tonalide facilitates methane production from anaerobic digestion of waste activated sludge. Sci. Total Environ. 2021, 779, 146195. [Google Scholar] [CrossRef]
- Roopnarain, A.; Rama, H.; Ndaba, B.; Bello-Akinosho, M.; Bamuza-Pemu, E.; Adeleke, R. Unravelling the anaerobic digestion ‘black box’: Biotechnological approaches for process optimization. Renew. Sustain. Energy Rev. 2021, 152, 111717. [Google Scholar] [CrossRef]
- Deng, P.; Yan, X.; Liu, R.; Ding, T.; Chen, J.; Wu, Z. Effect of cow dung on anaerobic digestion characteristics of poplar fuel ethanol wastewater. Biomass Convers. Biorefinery 2022, 1–11. [Google Scholar] [CrossRef]
- Mathai, P.P.; Dunn, H.M.; Venkiteshwaran, K.; Zitomer, D.H.; Maki, J.S.; Ishii, S.; Sadowsky, M.J. A microfluidic platform for the simultaneous quantification of methanogen populations in anaerobic digestion processes. Environ. Microbiol. 2019, 21, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
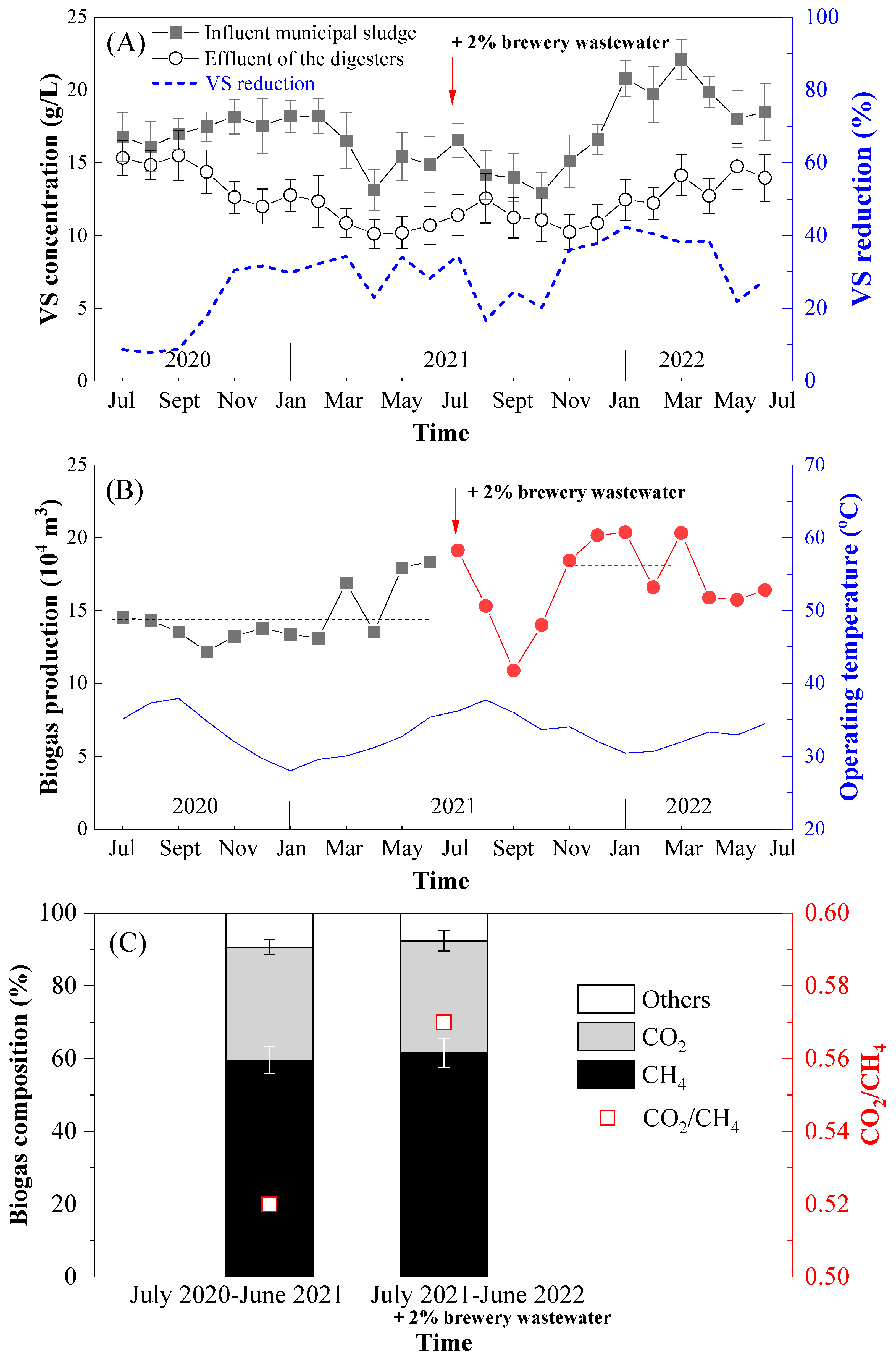



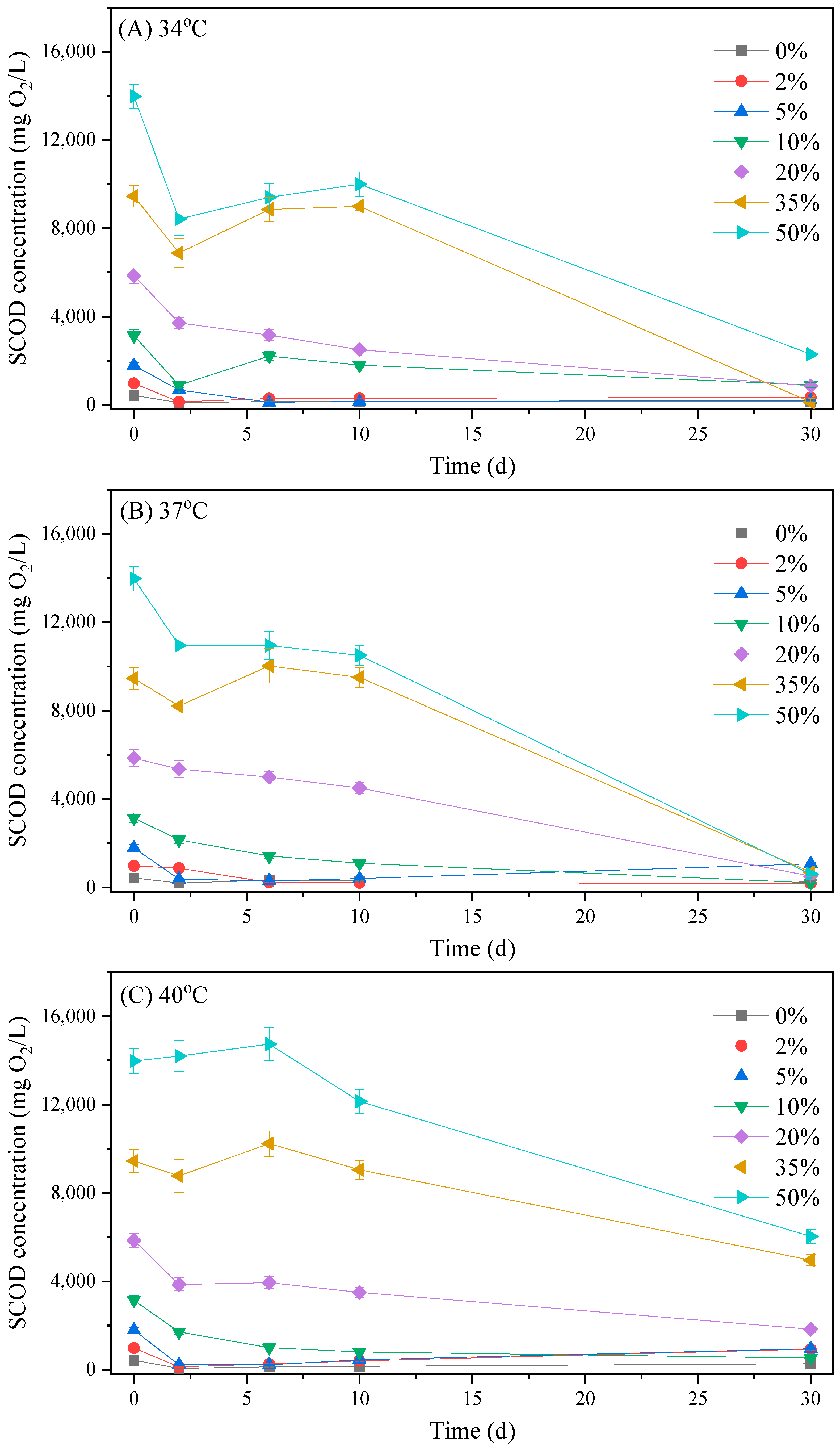

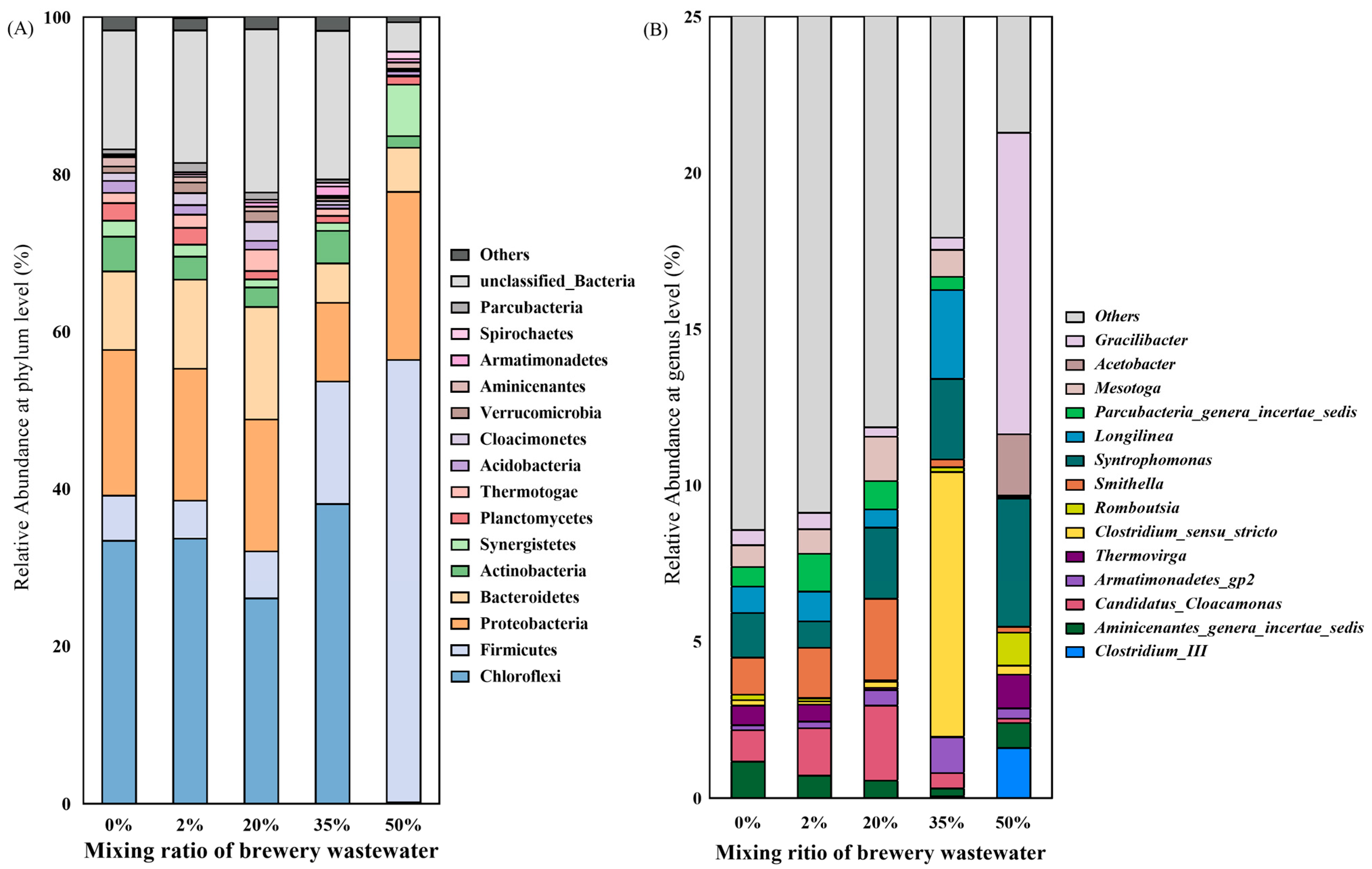

| Parameter | Primary Sludge | Excess Activated Sludge | Brewery Wastewater |
|---|---|---|---|
| TS (g/L) | 35.5 ± 5.6 | 38.5 ± 3.8 | 25.3 ± 5.1 |
| VS (g/L) | 16.7 ± 3.9 | 19.6 ± 2.2 | 19.5 ± 4.2 |
| SCOD (mg/L) | 312.2 ± 52.3 | 126.4 ± 18.9 | 30,576 ± 6547 |
| NH4+-N (mg/L) | 93.8 ± 13.5 | 1.25 ± 0.2 | 95.6 ± 35.7 |
| PO43−-P (mg/L) | 37.1 ± 2.4 | 0.6 ± 0.2 | 85.6 ± 18.5 |
| pH | 6.4 ± 0.2 | 6.8 ± 0.2 | 5.0 ± 0.7 |
| Parameter | Municipal Sewage Sludge | Brewery Wastewater | Inoculum |
|---|---|---|---|
| TS (g/L) | 37.5 ± 3.5 | 19.2 ± 3.4 | 42.1 ± 3.2 |
| VS (g/L) | 16.2 ± 1.6 | 18.1 ± 1.9 | 27.5 ± 2.4 |
| SCOD (mg/L) | 239 ± 18 | 28,851 ± 485 | 423 ± 52 |
| Carbohydrate (mg/L) | 12.8 ± 1.3 | 23,700 ± 520 | 11.5 ± 1.8 |
| NH4+-N (mg/L) | 45.0 ± 3.4 | 153.8 ± 15.5 | 440.3 ± 27.2 |
| PO43−-P (mg/L) | 47.8 ± 3.1 | 94.3 ± 3.9 | 24.2 ± 1.6 |
| Alkilinity (mg/L) | 1567.2 ± 92.7 | 999.6 ± 56.4 | 112.9 ± 1.5 |
| pH | 6.5 ± 0.3 | 4.3 ± 0.3 | 7.8 ± 0.2 |
| Mixing Ratio | 0% | 2% | 5% | 10% | 20% | 35% | 50% |
|---|---|---|---|---|---|---|---|
| SCOD/NH4+-N | 5.3 ± 0.2 | 17.2 ± 0.6 | 33.1 ± 1.4 | 55.5 ± 2.8 | 89.3 ± 3.6 | 123.4 ± 3.8 | 146.3 ± 3.4 |
| DF | Sum of Squares | Mean Square | F Value | p Value | |
|---|---|---|---|---|---|
| Temperature | 2 | 184.94148 | 92.47074 | 0.09721 | 0.90807 |
| Mixing ratio | 6 | 13,564.24872 | 2260.70812 | 2.37656 | 0.09517 |
| Interaction | 12 | 11,415.01938 | 951.25161 | ||
| Model | 20 | 25,164.20957 | 1258.21048 | 1.80672 | 0.17148 |
| Error | 0 | 0 | -- | ||
| Corrected Total | 20 | 25,164.20957 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Yin, Z.; Gu, R.; Liu, C.; Xu, N.; Sun, Z.; Jing, L.; Niu, X. Anaerobic Digestion of Municipal Sewage Sludge Integrated with Brewery Wastewater Treatment: Importance of Temperature and Mixing Ratio. Water 2023, 15, 2902. https://doi.org/10.3390/w15162902
Zhang W, Yin Z, Gu R, Liu C, Xu N, Sun Z, Jing L, Niu X. Anaerobic Digestion of Municipal Sewage Sludge Integrated with Brewery Wastewater Treatment: Importance of Temperature and Mixing Ratio. Water. 2023; 15(16):2902. https://doi.org/10.3390/w15162902
Chicago/Turabian StyleZhang, Wei, Zhixuan Yin, Ruihuan Gu, Changqing Liu, Nan Xu, Zhifu Sun, Lu Jing, and Xinyuan Niu. 2023. "Anaerobic Digestion of Municipal Sewage Sludge Integrated with Brewery Wastewater Treatment: Importance of Temperature and Mixing Ratio" Water 15, no. 16: 2902. https://doi.org/10.3390/w15162902
APA StyleZhang, W., Yin, Z., Gu, R., Liu, C., Xu, N., Sun, Z., Jing, L., & Niu, X. (2023). Anaerobic Digestion of Municipal Sewage Sludge Integrated with Brewery Wastewater Treatment: Importance of Temperature and Mixing Ratio. Water, 15(16), 2902. https://doi.org/10.3390/w15162902








