Spatiotemporal Variations in Seston C:N:P Stoichiometry in a Large Eutrophic Floodplain Lake (Lake Taihu): Do the Sources of Seston Explain Stoichiometric Flexibility?
Abstract
1. Introduction
2. Materials and Methods
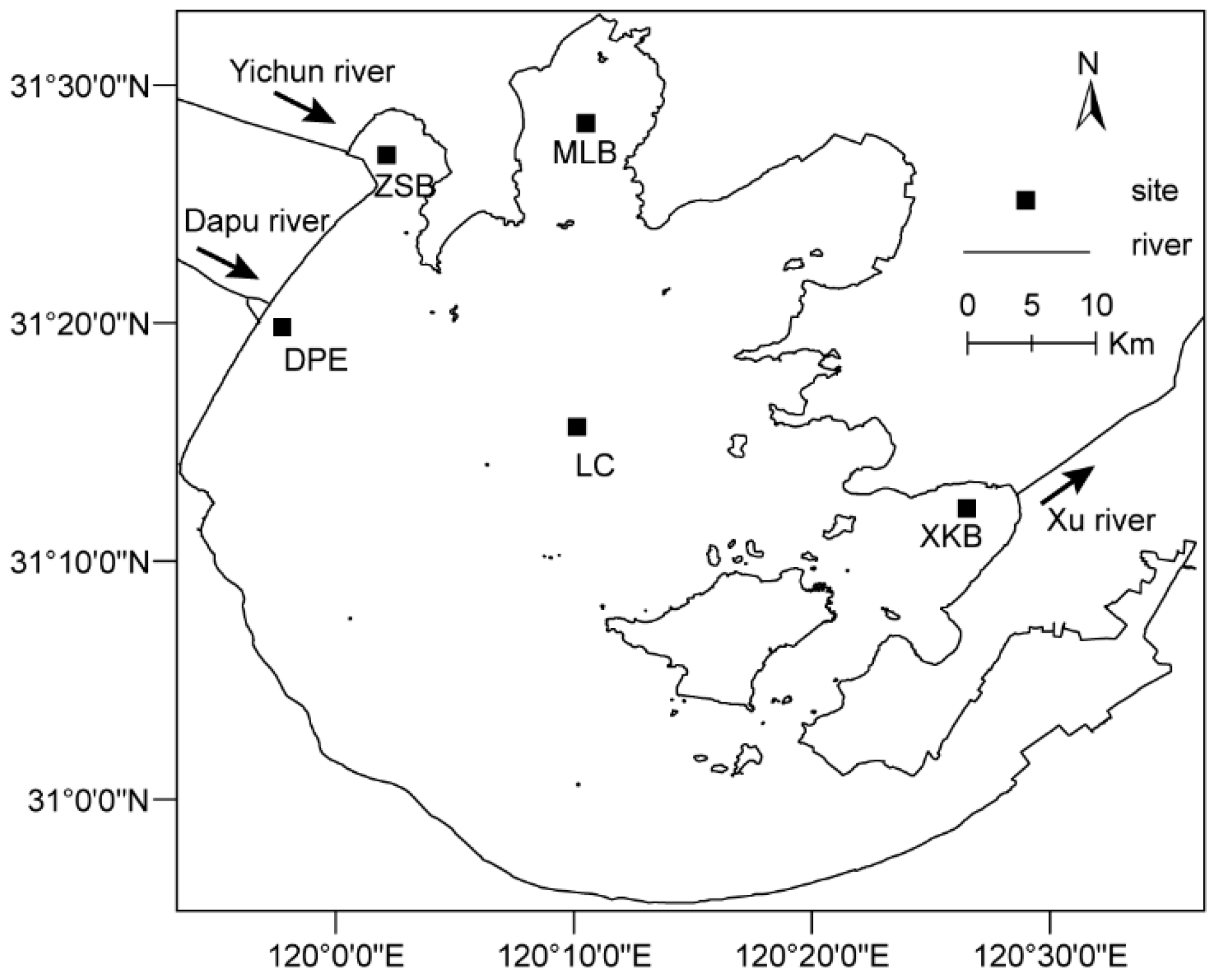
2.1. Study Sites
2.2. Fieldwork
2.3. Sample Processing and Analyses
2.4. Calculation of n-Alkane Proxies
2.5. Statistical Analysis
3. Results
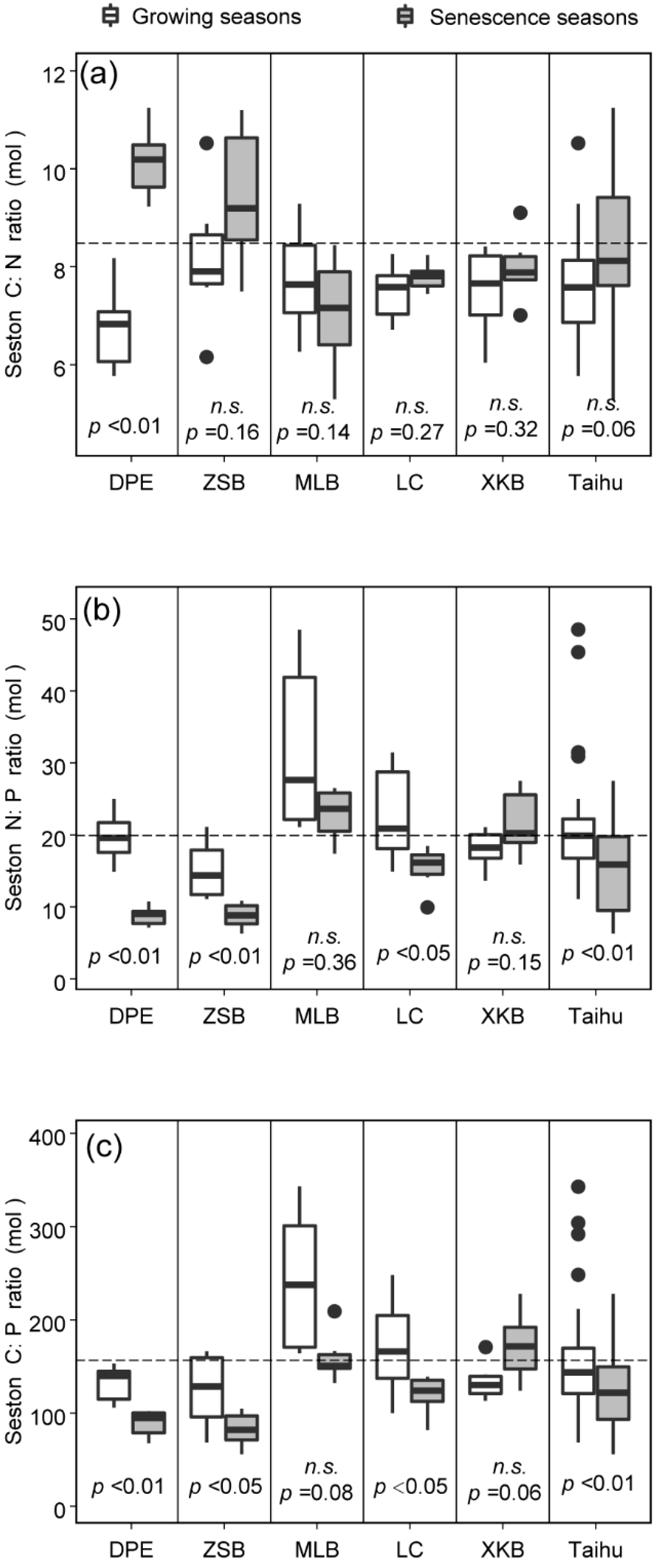
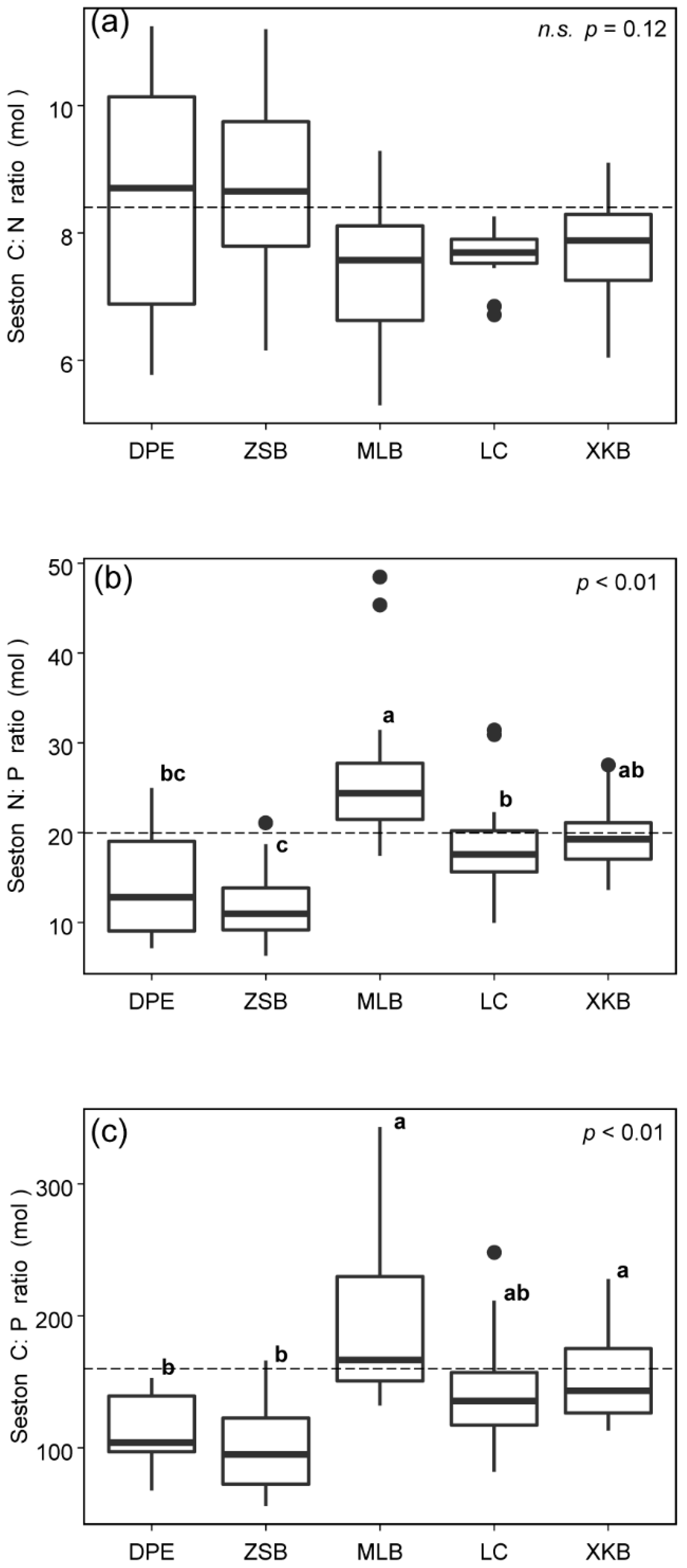
3.1. Spatiotemporal Variations in Seston C:N:P Stoichiometry
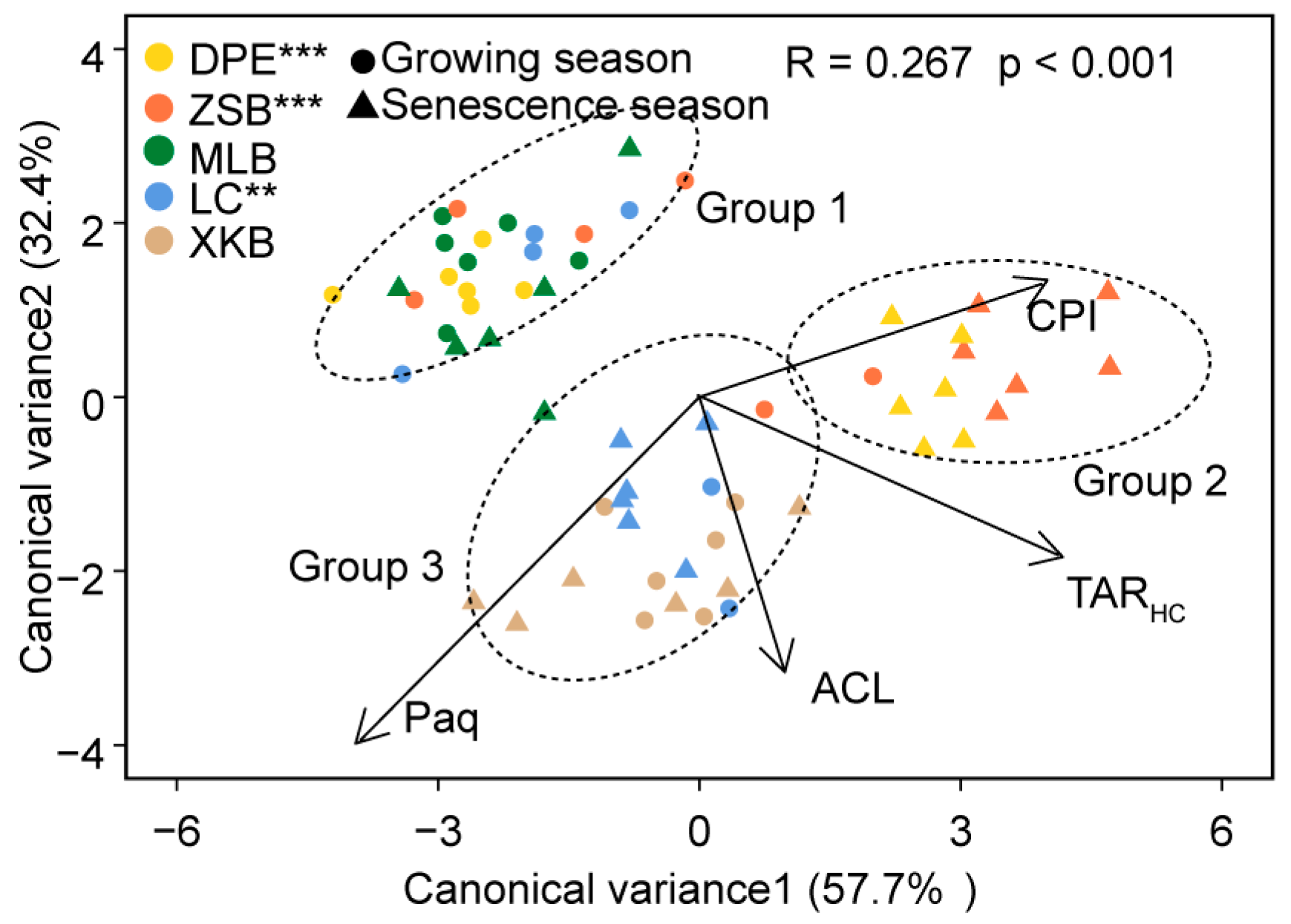
3.2. Discriminate Analysis of n-Alkane Proxies
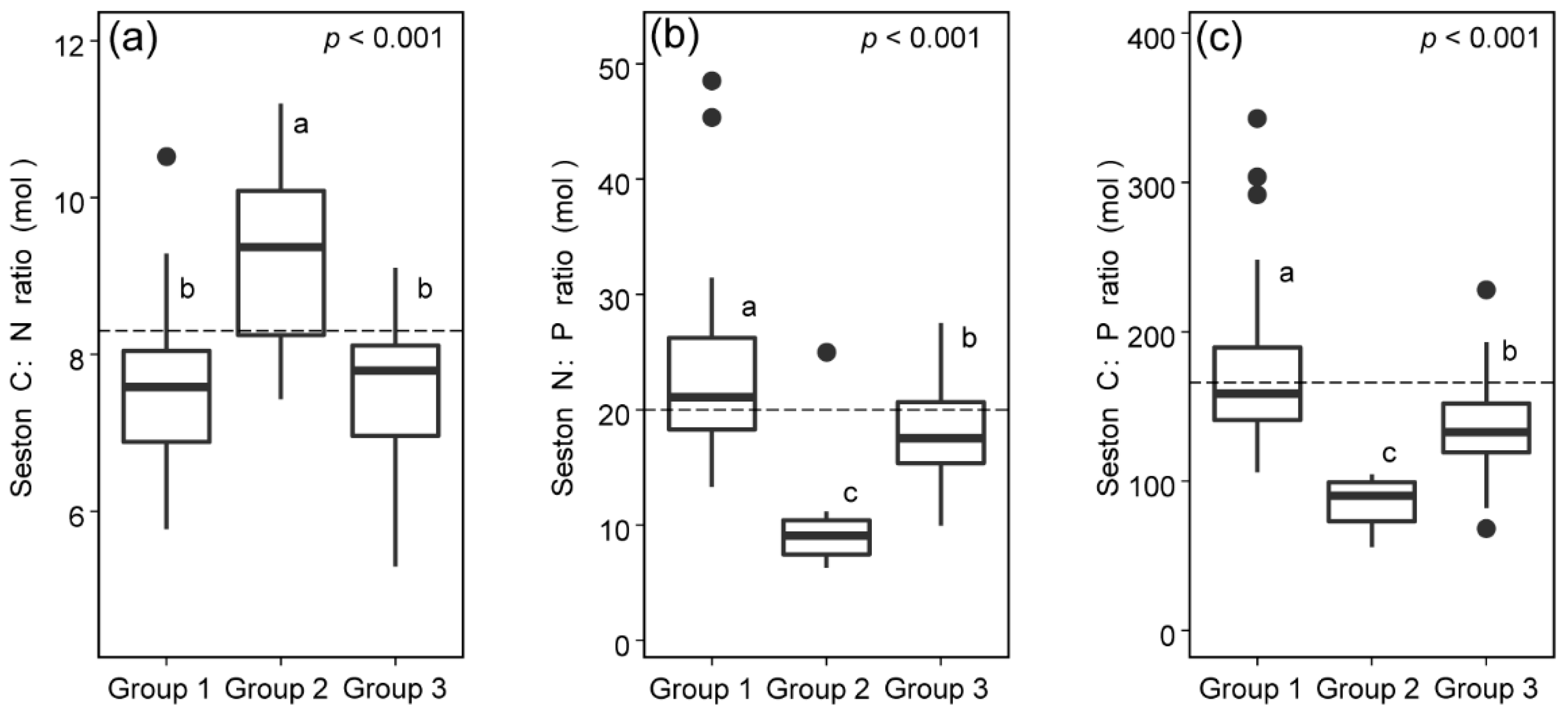
3.3. C:N:P Stoichiometric Ratios of Seston from Different Sources
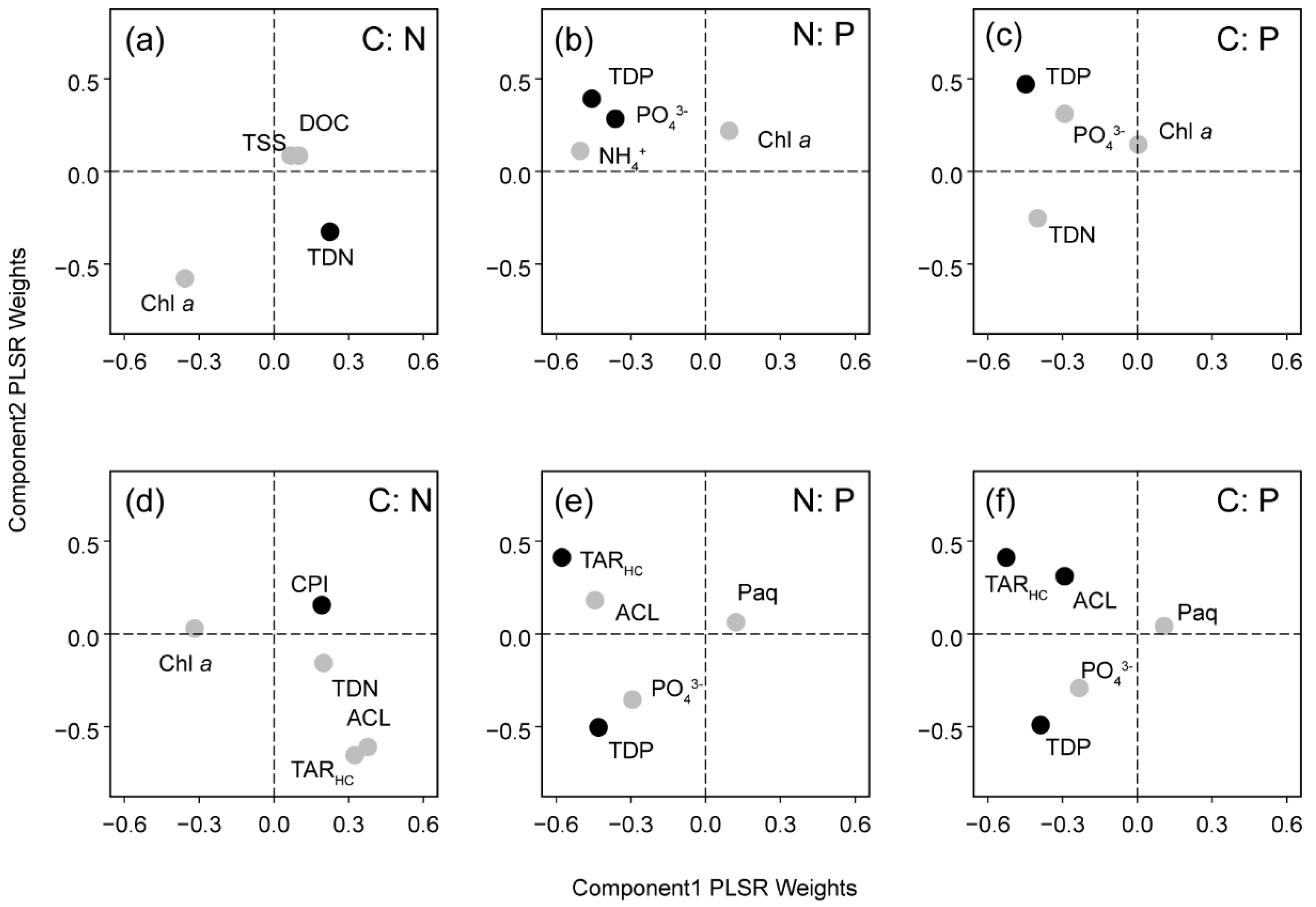
3.4. PLSR Analysis
4. Discussion
4.1. Application of Multiple n-Alkane Proxies as Complementary Source Indicators
4.2. Spatiotemporal Variations in Stoichiometric Ratios Corresponded with Changes in Seston Sources
4.3. The Role of Phosphorus in Influencing the Seston Stoichiometric Ratios
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heuer, H.; Smalla, K. Manure and sulfadiazine synergistically increased bacterial antibiotic resistance in soil over at least two months. Environ. Microbiol. 2007, 9, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Demi, L.M.; Benstead, J.P.; Rosemond, A.D.; Maerz, J.C. Experimental N and P additions alter stream macroinvertebrate community composition via taxon-level responses to shifts in detrital resource stoichiometry. Funct. Ecol. 2019, 33, 855–867. [Google Scholar] [CrossRef]
- He, H.; Han, Y.; Li, Q.; Jeppesen, E.; Li, K.; Yu, J.; Liu, Z. Crucian carp (Carassius carassius) strongly affect C/N/P stoichiometry of suspended particulate matter in shallow warm water eutrophic lakes. Water 2019, 11, 524. [Google Scholar] [CrossRef]
- Redfield, A. The biological control of the chemical factors in the enviroment. Am. Sci. 1958, 46, 205–221. [Google Scholar]
- Hecky, R.E.; Campbell, P.; Hendzel, L.L. The stoichiometry of carbon, nitrogen, and phosphorus in particulate matter of lakes and oceans. Limnol. Oceanogr. 1993, 38, 709–724. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef]
- Sterner, R.W.; Andersen, T.; Elser, J.J.; Hessen, D.O.; Hood, J.M.; McCauley, E.; Urabe, J. Scale-dependent carbon:nitrogen:phosphorus seston stoichiometry in marine and freshwaters. Limnol. Oceanogr. 2008, 53, 1169–1180. [Google Scholar] [CrossRef]
- Hessen, D.O.; van Donk, E.; Gulati, R. Seasonal seston stoichiometry: Effects on zooplankton in cyanobacteria-dominated lakes. J. Plankton Res. 2005, 27, 449–460. [Google Scholar] [CrossRef]
- Hessen, D.O.; Andersen, T.; Brettum, P.; Faafeng, B.A. Phytoplankton contribution to sestonic mass and elemental ratios in lakes: Implications for zooplankton nutrition. Limnol. Oceanogr. 2003, 48, 1289–1296. [Google Scholar] [CrossRef]
- Martiny, A.C.; Pham, C.T.A.; Primeau, F.W.; Vrugt, J.A.; Moore, J.K.; Levin, S.A.; Lomas, M.W. Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter. Nat. Geosci. 2013, 6, 279–283. [Google Scholar] [CrossRef]
- They, N.H.; Amado, A.M.; Cotner, J.B. Redfield Ratios in Inland Waters: Higher Biological Control of C:N:P Ratios in Tropical Semi-arid High Water Residence Time Lakes. Front. Microbiol. 2017, 8, 1505. [Google Scholar] [CrossRef] [PubMed]
- Omta, A.W.; Bruggeman, J.; Kooijman, S.A.L.M.; Dijkstra, H.A. Biological carbon pump revisited: Feedback mechanisms between climate and the Redfield ratio. Geophys. Res. Lett. 2006, 33, L14613. [Google Scholar] [CrossRef]
- Wilkinson, G.M.; Pace, M.L.; Cole, J.J. Terrestrial dominance of organic matter in north temperate lakes. Glob. Biogeochem. Cycles 2013, 27, 43–51. [Google Scholar] [CrossRef]
- Dong, X.; Anderson, N.J.; Yang, X.; Chen, X.; Shen, J. Carbon burial by shallow lakes on the Yangtze floodplain and its relevance to regional carbon sequestration. Glob. Chang. Biol. 2012, 18, 2205–2217. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, X.; Zhang, Y.; Zhang, Y.; Shi, K.; Tang, X.; Qin, B.; Podgorski, D.C.; Brookes, J.D.; Jeppesen, E. Response of dissolved organic matter optical properties to net inflow runoff in a large fluvial plain lake and the connecting channels. Sci. Total Environ. 2018, 639, 876–887. [Google Scholar] [CrossRef]
- Xu, X.; Li, W.; Fujibayashi, M.; Nomura, M.; Nishimura, O.; Li, X. Predominance of terrestrial organic matter in sediments from a cyanobacteria- blooming hypereutrophic lake. Ecol. Indic. 2015, 50, 35–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeppesen, E.; Liu, X.; Qin, B.; Shi, K.; Zhou, Y.; Thomaz, S.M.; Deng, J. Global loss of aquatic vegetation in lakes. Earth Sci. Rev. 2017, 173, 259–265. [Google Scholar] [CrossRef]
- Hessen, D.O. Determinants of seston C:P-ratio in lakes. Freshw. Biol. 2006, 51, 1560–1569. [Google Scholar] [CrossRef]
- Meyers, P.A.; Ishiwatari, R. Lacustrine organic geochemistry—An overview of indicators of organic matter sources and diagenesis in lake sediments. Org. Geochem. 1993, 20, 867–900. [Google Scholar] [CrossRef]
- Shi, L.; Huang, Y.; Lu, Y.; Chen, F.; Zhang, M.; Yu, Y.; Kong, F. Stocks and dynamics of particulate and dissolved organic matter in a large, shallow eutrophic lake (Taihu, China) with dense cyanobacterial blooms. J. Oceanol. Limnol. 2018, 36, 738–749. [Google Scholar] [CrossRef]
- Erga, S.R.; Haugen, S.B.; Bratbak, G.; Egge, J.K.; Heldal, M.; Mork, K.A.; Norland, S. Seasonal variations in C:N:Si:Ca:P:Mg:S:K:Fe relationships of seston from Norwegian coastal water: Impact of extreme offshore forcing during winter-spring 2010. Mar. Chem. 2017, 196, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Frigstad, H.; Andersen, T.; Hessen, D.O.; Naustvoll, L.J.; Johnsen, T.M.; Bellerby, R.G.J. Seasonal variation in marine C:N:P stoichiometry: Can the composition of seston explain stable Redfield ratios? Biogeosciences 2011, 8, 2917–2933. [Google Scholar] [CrossRef]
- Guo, W.; Ye, F.; Xu, S.; Jia, G. Seasonal variation in sources and processing of particulate organic carbon in the Pearl River estuary, South China. Estuar. Coast. Shelf Sci. 2015, 167, 540–548. [Google Scholar] [CrossRef]
- Tipping, E.; Somerville, C.J.; Luster, J. The C:N:P:S stoichiometry of soil organic matter. Biogeochemistry 2016, 130, 117–131. [Google Scholar] [CrossRef]
- Müller, P.J. C N ratios in Pacific deep-sea sediments: Effect of inorganic ammonium and organic nitrogen compounds sorbed by clays. Geochim. Cosmochim. Acta 1977, 41, 765–776. [Google Scholar] [CrossRef]
- Hesselsoe, M.; Füreder, S.; Schloter, M.; Bodrossy, L.; Iversen, N.; Roslev, P.; Nielsen, P.H.; Wagner, M.; Loy, A. Isotope array analysis of Rhodocyclales uncovers functional redundancy and versatility in an activated sludge. ISME J. 2009, 3, 1349–1364. [Google Scholar] [CrossRef]
- Sampei, Y.; Matsumoto, E. C/N ratios in a sediment core from Nakaumi Lagoon, southwest Japan—Usefulness as an organic source indicator—. Geochem. J. 2001, 35, 189–205. [Google Scholar] [CrossRef]
- Fang, J.; Wu, F.; Xiong, Y.; Li, F.; Du, X.; An, D.; Wang, L. Source characterization of sedimentary organic matter using molecular and stable carbon isotopic composition of n-alkanes and fatty acids in sediment core from Lake Dianchi, China. Sci. Total Environ. 2014, 473, 410–421. [Google Scholar] [CrossRef]
- Xu, F.L.; Yang, C.; He, W.; He, Q.S.; Li, Y.L.; Kang, L.; Liu, W.X.; Xiong, Y.Q.; Xing, B. Bias and association of sediment organic matter source apportionment indicators: A case study in a eutrophic Lake Chaohu, China. Sci. Total Environ. 2017, 581–582, 874–884. [Google Scholar] [CrossRef]
- Meyers, P.A. Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org. Geochem. 1997, 27, 213–250. [Google Scholar] [CrossRef]
- Ficken, K.; Li, B.; Swain, D.; Eglinton, G. An n-alkane proxy for the sedimentary input of submerged/floating freshwater aquatic macrophytes. Org. Geochem. 2000, 31, 745–749. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Y.; Liu, Z.; Yu, J.; Jin, M. Lipid biomarker evidence for determining the origin and distribution of organic matter in surface sediments of Lake Taihu, Eastern China. Ecol. Indic. 2017, 77, 397–408. [Google Scholar] [CrossRef]
- Silliman, J.E.; Meyers, P.A.; Bourbonniere, R.A. Record of postglacial organic matter delivery and burial in sediments of Lake Ontario. Org. Geochem. 1996, 24, 463–472. [Google Scholar] [CrossRef]
- Routh, J.; Hugelius, G.; Kuhry, P.; Filley, T.; Tillman, P.K.; Becher, M.; Crill, P. Multi-proxy study of soil organic matter dynamics in permafrost peat deposits reveal vulnerability to climate change in the European Russian Arctic. Chem. Geol. 2014, 368, 104–117. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, W.; Meyers, P.A.; Xie, S. Lipid biomarkers in the Zoigê-Hongyuan peat deposit: Indicators of Holocene climate changes in West China. Org. Geochem. 2007, 38, 1927–1940. [Google Scholar] [CrossRef]
- Tareq, S.M.; Tanoue, E.; Tsuji, H.; Tanaka, N.; Ohta, K. Hydrocarbon and elemental carbon signatures in a tropical wetland: Biogeochemical evidence of forest fire and vegetation changes. Chemosphere 2005, 59, 1655–1665. [Google Scholar] [CrossRef]
- Derrien, M.; Yang, L.; Hur, J. Lipid biomarkers and spectroscopic indices for identifying organic matter sources in aquatic environments: A review. Water Res. 2017, 112, 58–71. [Google Scholar] [CrossRef]
- Qin, B.; Xu, P.; Wu, Q.; Luo, L.; Zhang, Y. Environmental issues of Lake Taihu, China. Hydrobiologia 2007, 581, 3–14. [Google Scholar] [CrossRef]
- Tang, X.; Krausfeldt, L.E.; Shao, K.; Lecleir, G.R.; Stough, J.M.A.; Gao, G.; Boyer, G.L.; Zhang, Y.; Paerl, H.W.; Qin, B.; et al. Seasonal Gene Expression and the Ecophysiological Implications of Toxic Microcystis aeruginosa Blooms in Lake Taihu. Environ. Sci. Technol. 2018, 52, 11049–11059. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, X.; Qin, B. A critical review of the development, current hotspots, and future directions of Lake Taihu research from the bibliometrics perspective. Environ. Sci. Pollut. Res. 2016, 23, 12811–12821. [Google Scholar] [CrossRef]
- Prater, C.; Frost, P.C.; Howell, E.T.; Watson, S.B.; Zastepa, A.; King, S.S.E.; Vogt, R.J.; Xenopoulos, M.A. Variation in particulate C : N : P stoichiometry across the Lake Erie watershed from tributaries to its outflow. Limnol. Oceanogr. 2017, 62, S194–S206. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, K.; Liu, X.; Zhou, Y.; Qin, B. Lake topography and wind waves determining seasonal-spatial dynamics of total suspended matter in turbid Lake Taihu, China: Assessment using long-term high-resolution MERIS data. PLoS ONE 2014, 9, e98055. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Yang, G.; Ma, J.; Wu, T.; Li, W.; Liu, L.; Deng, J.; Zhou, J. Spatiotemporal Changes of Cyanobacterial Bloom in Large Shallow Eutrophic Lake Taihu, China. Front. Microbiol. 2018, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Bai, C.; Tang, X.; Dai, J.; Gong, Y.; Hu, Y.; Shao, K.; Zhou, L.; Gao, G. Characterization of bacterial and microbial eukaryotic communities associated with an ephemeral hypoxia event in Taihu Lake, a shallow eutrophic Chinese lake. Environ. Sci. Pollut. Res. 2018, 25, 31543–31557. [Google Scholar] [CrossRef] [PubMed]
- Clescerl, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Mehtods for the Examination of Water and Wastewater, 20th ed.; America Public Health Association: Washington, DC, USA, 1999. [Google Scholar]
- He, Y.; Zheng, Y.; Pan, A.; Zhao, C.; Sun, Y.; Song, M.; Zheng, Z.; Liu, Z. Biomarker-based reconstructions of Holocene lake-level changes at Lake Gahai on the northeastern Tibetan Plateau. Holocene 2014, 24, 405–412. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Moreno, L.; Torres, T.; Vegas, J.; Ruiz-Zapata, B.; García-Cortés, Á.; Galán, L.; Pérez-González, A. A 220 ka palaeoenvironmental reconstruction of the Fuentillejo maar lake record (Central Spain) using biomarker analysis. Org. Geochem. 2013, 55, 85–97. [Google Scholar] [CrossRef]
- Gao, G.; Zhu, G.; Qin, B.; Chen, J.; Wang, K. Alkaline phosphatase activity and the phosphorus mineralization rate of Lake Taihu. Sci. China Ser. D Earth Sci. 2006, 49, 176–185. [Google Scholar] [CrossRef]
- Mudge, S.M.; Duce, C.E. Identifying the source, transport path and sinks of sewage derived organic matter. Environ. Pollut. 2005, 136, 209–220. [Google Scholar] [CrossRef]
- Li, B.; Morris, J.; Martin, E.B. Model selection for partial least squares regression. Chemom. Intell. Lab. Syst. 2002, 64, 79–89. [Google Scholar] [CrossRef]
- Chong, I.G.; Jun, C.H. Performance of some variable selection methods when multicollinearity is present. Chemom. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Xu, H.; Paerl, H.W.; Qin, B.; Zhu, G.; Hall, N.S.; Wu, Y. Determining Critical Nutrient Thresholds Needed to Control Harmful Cyanobacterial Blooms in Eutrophic Lake Taihu, China. Environ. Sci. Technol. 2015, 49, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Qin, B.; Paerl, H.W.; Brookes, J.D.; Hall, N.S.; Shi, K.; Zhou, Y.; Guo, J.; Li, Z.; Xu, H.; et al. The persistence of cyanobacterial (Microcystis spp.) blooms throughout winter in Lake Taihu, China. Limnol. Oceanogr. 2016, 61, 711–722. [Google Scholar] [CrossRef]
- Veldboom, J.A.; Haro, R.J. Stoichiometric relationship between suspension-feeding caddisfly (Trichoptera: Brachycentridae) and seston. Hydrobiologia 2011, 675, 129–141. [Google Scholar] [CrossRef]
- Frost, P.C.; Kinsman, L.E.; Johnston, C.A.; Larson, J.H. Watershed discharge modulates relationships between landscape components and nutrient ratios in stream seston. Ecology 2009, 90, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Strokal, M.; Burek, P.; Kroeze, C.; Ma, L.; Janssen, A.B.G. Excess nutrient loads to Lake Taihu: Opportunities for nutrient reduction. Sci. Total Environ. 2019, 664, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Wu, H.P.; Hao, B.B.; Liu, G.H. Stoichiometric characteristics and responses of submerged macrophytes to eutrophication in lakes along the middle and lower reaches of the Yangtze River. Ecol. Eng. 2013, 54, 16–21. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; Hall, N.S.; Rossignol, K.L.; Joyner, A.R.; Zhu, G.; Qin, B. Nutrient limitation dynamics examined on a multi-annual scale in Lake Taihu, China: Implications for controlling eutrophication and harmful algal blooms. J. Freshw. Ecol. 2015, 30, 5–24. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Huang, B.; Wei, Z.B.; Miao, A.J.; Yang, L.Y. Nitrogen and phosphorus limitation of phytoplankton growth in different areas of Lake Taihu, China. J. Freshw. Ecol. 2015, 30, 113–127. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, H.; Li, Z.; Yuan, S.; Li, Q.; Ji, F.; Xiao, Y. Phosphorus sorption to suspended sediment in freshwater. Proc. Inst. Civ. Eng. Water Manag. 2017, 170, 231–242. [Google Scholar] [CrossRef]
- Güsewell, S.; Gessner, M.O. N:P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 2009, 23, 211–219. [Google Scholar] [CrossRef]
| Name | Formula | Diagnostic Values | Source Assignment | References |
|---|---|---|---|---|
| CPI | CPI > 5 CPI ≈ 1 | Terrestrial Algal | [32,37] | |
| ACL | ACL > 25.1 ACL < 23.0 | Terrestrial Algal | [28,36] | |
| TARHC | TARHC > 4 TARHC < 1 | Terrestrial Algal | [33,47] | |
| Paq | Paq > 0.6 Paq < 0.25 | Macrophytes Terrestrial | [31,32] |
| Ratios | Site | Season | Site × Season | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| C:N | 2.82 | 0.12 | 8.423 | 0.058 | 7.94 | <0.01 |
| N:P | 22.81 | <0.01 | 29.23 | <0.01 | 7.02 | <0.01 |
| C:P | 13.72 | <0.01 | 12.96 | <0.01 | 3.40 | <0.05 |
| Groups | Proxies Values | ANOSIM | ||||
|---|---|---|---|---|---|---|
| TARHC | CPI | ACL | Paq | Comparisons | Statistic R | |
| Group 1 | 0.53 ± 0.23 | 1.92 ± 0.61 | 20.71 ± 1.81 | 0.39 ± 0.06 | Group 1 vs. Group 2 | 0.556 *** |
| Group 2 | 11.34 ± 4.63 | 3.26 ± 0.65 | 27.03 ± 0.31 | 0.27 ± 0.04 | Group 2 vs. Group 3 | 0.368 *** |
| Group 3 | 10.22 ± 5.03 | 2.10 ± 0.33 | 26.50 ± 0.67 | 0.44 ± 0.09 | Group 3 vs. Group 1 | 0.221 *** |
| PLSR Models | No. of Optimum Components | Calibration | Cross-Validation | ||||
|---|---|---|---|---|---|---|---|
| R2 | RMSE | Bias | R2 | RMSE | Bias | ||
| Environmental variables | |||||||
| C:N | 2 | 0.27 | 0.12 | 0.000 | 0.16 | 0.16 | 0.004 |
| N:P | 3 | 0.38 | 0.37 | 0.000 | 0.35 | 0.39 | 0.007 |
| C:P | 3 | 0.35 | 0.34 | 0.000 | 0.29 | 0.36 | −0.005 |
| Environmental variables andn-alkane proxies | |||||||
| C:N | 3 | 0.38 | 0.11 | 0.000 | 0.34 | 0.12 | 0.000 |
| N:P | 3 | 0.62 | 0.21 | 0.000 | 0.59 | 0.23 | −0.007 |
| C:P | 4 | 0.66 | 0.22 | 0.000 | 0.60 | 0.23 | −0.004 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Bai, C.; Tang, X.; Dai, J.; Jiang, X.; Hu, Y.; Shao, K.; Gao, G. Spatiotemporal Variations in Seston C:N:P Stoichiometry in a Large Eutrophic Floodplain Lake (Lake Taihu): Do the Sources of Seston Explain Stoichiometric Flexibility? Water 2020, 12, 36. https://doi.org/10.3390/w12010036
Cai J, Bai C, Tang X, Dai J, Jiang X, Hu Y, Shao K, Gao G. Spatiotemporal Variations in Seston C:N:P Stoichiometry in a Large Eutrophic Floodplain Lake (Lake Taihu): Do the Sources of Seston Explain Stoichiometric Flexibility? Water. 2020; 12(1):36. https://doi.org/10.3390/w12010036
Chicago/Turabian StyleCai, Jian, Chengrong Bai, Xiangming Tang, Jiangyu Dai, Xingyu Jiang, Yang Hu, Keqiang Shao, and Guang Gao. 2020. "Spatiotemporal Variations in Seston C:N:P Stoichiometry in a Large Eutrophic Floodplain Lake (Lake Taihu): Do the Sources of Seston Explain Stoichiometric Flexibility?" Water 12, no. 1: 36. https://doi.org/10.3390/w12010036
APA StyleCai, J., Bai, C., Tang, X., Dai, J., Jiang, X., Hu, Y., Shao, K., & Gao, G. (2020). Spatiotemporal Variations in Seston C:N:P Stoichiometry in a Large Eutrophic Floodplain Lake (Lake Taihu): Do the Sources of Seston Explain Stoichiometric Flexibility? Water, 12(1), 36. https://doi.org/10.3390/w12010036






