Abstract
Perfluoroalkyl and polyfluoroalkyl substances (PFASs) are a class of synthetic organic compounds with extremely high chemical stability and environmental persistence that are widely used in the industrial sector and in consumer goods. Their strong C-F bonds make them difficult to degrade, meaning they can migrate through the atmosphere and settle over long distances, posing long-term risks to the global ecological environment and human health. This article systematically reviews the classification, physicochemical properties, concentration levels, spatial distribution, migration and transformation behaviors, and health and ecological impacts of PFASs in the atmosphere, along with related analytical detection techniques and pollution control methods. Studies show that short-chain PFASs are more likely to migrate through the atmosphere due to their high water solubility and volatility, while long-chain PFASs tend to be adsorbed onto particulate matter and display stronger bioaccumulation. Although atmospheric research on PFASs lags behind that focused on their dynamics in water and soil, the existing data still reveal a difference in their distribution and regional pollution characteristics in the gas and particle phases. Toxicological studies have confirmed that PFAS exposure is associated with liver injury, immunosuppression, developmental toxicity, and cancer risk and can threaten ecological security through the food chain. Currently, governance technologies are confronted with the challenges of low efficiency and high cost. In the future, it will be necessary to combine multi-media models, new analytical techniques, and international collaboration to promote the development of source control and innovative governance strategies.
1. Introduction
Perfluoroalkyl and polyfluoroalkyl substances (PFASs) are a class of synthetic fluorine-containing organic compounds that have been widely used in the industrial sector and in consumer goods (such as non-stick pan coatings, waterproof textiles, fire-fighting foams, etc.) since the middle of the 20th century. Their strong C-F bonds endow them with extremely high chemical and thermal stability but also make them difficult to degrade in the environment, meaning they are often referred to as “permanent chemicals”. In recent years, the problem presented by PFAS pollution has become increasingly serious worldwide, especially in industrial-intensive areas and remote regions where their presence has been detected. In addition to direct emissions into the atmosphere, PFASs in water bodies and the soil can also volatilize into the atmosphere in the form of vapor [1] because of their high mobility [2] and chemical stability [3], whether in a gaseous [4] or a granular state [4]. This means that they can migrate through the atmosphere and be transported to areas far from emission sources [5]. Epidemiological and toxicological studies have shown that PFAS exposure is associated with a variety of adverse health effects, such as liver and kidney diseases [6], immunotoxicity [7], and developmental toxicity [8], while the inhalation of PFASs can inhibit the function of pulmonary surfactants and cause acute toxicity [9]. Therefore, the atmosphere is the main site for the migration and transformation of PFASs, representing an important exposure route for their impact on human health [10]. It is therefore of great significance for understanding the dynamics of PFASs and the harm they pose to the environment, ecology, and human beings. Regarding PFASs in the atmosphere, previous studies have performed source distribution and health analysis of PFASs in water, soil, and the atmosphere, but there is a lack of systematic reviews focused on screening, evaluation, and control. The purpose of this article is to systematically review the classification and properties of PFASs in the tropospheric near-surface atmosphere; review the concentration levels and distribution characteristics of PFASs in gas and particle phases across different regions of China and compare them with findings from other countries; discuss the migration and transformation mechanisms that drive the persistence and long-range transport of PFASs in the atmosphere; evaluate toxicological evidence regarding the impacts of atmospheric PFASs on human, animal, and plant health; summarize the sampling and analytical techniques used for detecting atmospheric PFASs; review the current strategies for pollution control and identify their challenges; and finally provide a forward-looking perspective on research directions and policy implications for atmospheric PFAS management. By focusing on the situation in China and placing it within a global context, this paper seeks to highlight both the regional characteristics of atmospheric PFAS pollution and its broader implications for environmental health and regulatory control.
2. Classification and Properties of PFASs
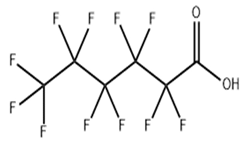
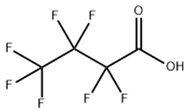
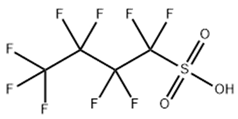
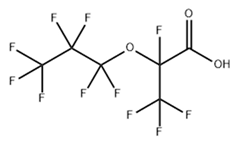
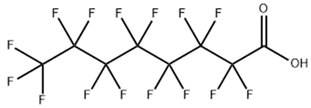
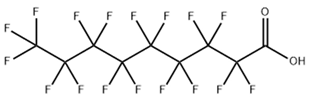
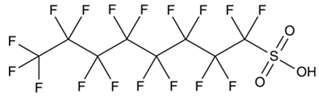
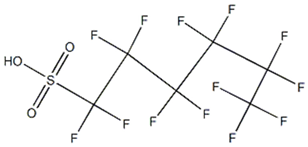
Based on their functional groups and structures, PFASs (Figure 1) can be categorized into three main types: perfluoroalkyl substances, polyfluoroalkyl substances, and other derivatives.
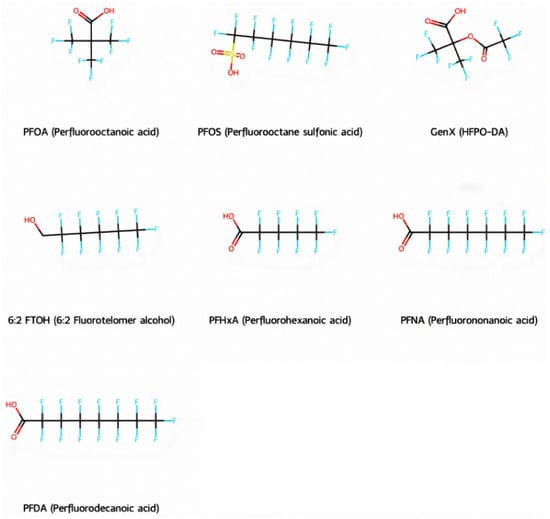
Figure 1.
Molecular structural formulas of some typical PFAS compounds.
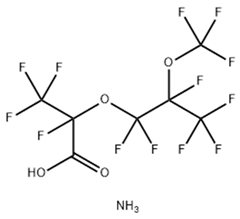
According to carbon chain length, PFASs are divided into long-chain and short-chain compounds. Long-chain PFASs include perfluorocarboxylic acids (PFCAs) and perfluorosulfonic acids (PFSAs), with carbon chain lengths ≥ 7 (PFCAs) and ≥6 (PFSAs), such as PFOA (perfluorooctanoic acid, C8, PFCA) and PFOS (perfluorooctane sulfonic acid, C8, PFSA). Short-chain PFASs, developed as alternatives to long-chain compounds, have carbon chain lengths ≤ 6 (PFCAs) or ≤5 (PFSAs) and include PFBA (perfluorobutanoic acid, C4, PFCA), PFBS (perfluorobutane sulfonic acid, C4, PFSA), and GenX chemicals (hexafluoropropylene oxide dimer, C6, as a PFOA substitute). Some abbreviations related to FTOH substances include 1H,1H,2H,2H-perfluoro-1-octanol (6:2FTOH) and 1H,1H,2H, 2H-perfluoro-1-decanol (8:2FTOH).
The compounds listed in Table 1 are difficult to degrade due to the high stability of their C-F bonds, meaning that they can be widely diffused through atmospheric transport, the water cycle, and other pathways, posing long-term risks to ecosystems and human health. The table clearly shows that PFASs generally have extremely high chemical and thermal stability, enabling them to persist in the environment for a long time [11]. Their physical properties, such as their boiling and melting points, are relatively high, while their water solubility varies due to differences in chain length and structure [12]. However, the melting and boiling points of different types of PFASs vary significantly. For instance, experimental data from the new alternative GenX (HFPO-DA) indicate that its melting point is approximately −98 °C, while its boiling point is about 64.7 °C [13]. These values were directly obtained from the Chemical Safety Data Sheet (SDS) and can provide reliable physicochemical parameter support for subsequent research. In contrast, the boiling points of traditional long-chain PFASs (such as PFOA and PFOS) are generally above 150 °C, while that of some compounds is difficult to determine due to their extremely high thermal stability [14]. Therefore, it can be considered that GenX is more prone to migration in terms of physical and chemical properties, while other long-chain PFASs exhibit stronger environmental persistence. From the classification provided in the table, we can see that short-chain PFASs usually have higher water solubility and lower bioaccumulation, while long-chain PFASs are more persistent and more easily accumulate in organisms [15].

Table 1.
Common PFASs and their properties.
3. Concentration Levels and Behavior of PFASs in the Atmosphere
3.1. Concentration Levels of Atmospheric PFASs
Up to now, research on the environmental pollution characteristics of PFASs in China and other countries has mainly focused on water bodies and soil, while studies on PFASs in the atmosphere are relatively scarce. The atmosphere is vital for human survival, and atmospheric transport is also one of the important pathways for PFAS transport. The distribution of PFASs (Table 2) in the gas and particle phases affects their form and deposition mode in the atmosphere. However, compared with water bodies and sediments, research on monitoring PFASs in the atmosphere is still very limited. On the one hand, most PFASs have low volatility, and only a few precursor compounds such as FTOHs and sulfonamide ethanol can exist in the atmosphere in a gaseous or granular form, thereby limiting the number of target species that can be monitored. On the other hand, the concentration of PFASs (Figure 2) in the atmosphere is usually at the pg/m3 level, posing extremely high requirements for sampling and detection sensitivity. In addition, PFASs are prone to complex photochemical transformations in the atmosphere, generating multiple intermediates and stable end products, which increase the uncertainty of monitoring and traceability. Although there is already evidence indicating that long-distance atmospheric transmission is an important pathway for the global distribution of PFASs, the current related monitoring data still display fragmented and unsystematic characteristics, causing atmospheric research to lag significantly behind that focused on other environmental media [16]. In previous atmospheric research on PFASs, reports from outside China have mainly focused on developed coastal countries such as northwest Europe, the United Kingdom, Germany, and the United States. However, this research has been ongoing for over a decade, lacking long-term and sustained monitoring and detection. Research on PFASs in the atmosphere in China has accelerated in the past decade, but related studies remain limited, with the main research sites including coastal cities such as Tianjin, Yantai, and Xiamen, as well as some areas in the South China Sea or near pollution sources such as factories.

Table 2.
The concentration levels of PFASs in the atmosphere, reported in the literature from China and other countries.
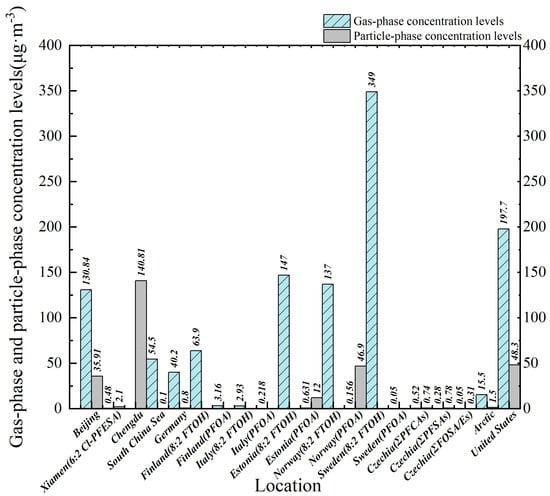
Figure 2.
Atmospheric PFASs concentration levels in various regions.
On a global scale, previous studies have performed sampling from 2010 to 2021, and the spatial scope includes both China and international regions, such as Europe, and domestic areas of China like Beijing, the South China Sea, and remote regions like the Arctic. China has carried out more than twenty atmospheric PFAS monitoring projects, with studies related to the granular phase being predominant, as the sampling techniques used (such as solid-phase adsorbents and high-volume air sampling) are mainly applicable to the analysis of PFASs in particulate matter, while less attention has been given to gas-phase PFASs [25]. In a study focused on Xiamen, the concentration of particulate PFASs was significant (approximately 4.11–67.41 pg/m3, with an average of 26.56 pg/m3), highlighting the status of particulate matter as the main carrier of atmospheric PFASs [26]. Multi-phase sampling in Shijiazhuang and a structural gas/particle distribution study in Beijing further confirmed that most PFASs tend to be adsorbed onto particulate matter (distribution coefficient < 0), while the specific gravity of the gas phase is relatively low [27,28]. FTOHs in the northern South China Sea are the most significant group of PFASs, accounting for 95.2% to 99.3% of all PFASs. The low concentrations of short-lived PFASs, such as FTAs and FASEs, indicate that their sources may be related to long-distance transmission. The Asian continent is an important source of PFASs in the atmosphere of the northern South China Sea, with the concentration of PFASs being significantly higher in samples affected by continental airflows than in samples not subject to this effect. The concentration of PFASs in the atmospheric particle phase from the northern South China Sea is extremely low, further confirming that they mainly exist in a gaseous form in the atmosphere.
In a study [29] conducted in the southwestern suburbs of Chengdu, China, Zhang Yun et al. studied the concentration level of PFASs in atmospheric particulate matter during winter. Their research results showed that among the 25 types of PFASs identified, a total of 10 were found in this area, with the total concentration (∑10PFASs) ranging from 4.58 to 647.59 pg·m−3 and the average value being 140.81 pg·m−3. Among these PFASs, the concentration of PFBA was the highest, with an average of 133.18 pg·m−3, contributing 69% of the total. Next was PFOA, with an average concentration of 2.98 pg·m−3. In contrast, the concentrations of PFHpA and 6:2 FTSA were relatively low, at 1.36 pg·m−3 and 1.22 pg·m−3, respectively. These concentrations were comparable to the PFAS levels in Beijing (157 pg·m−3) and Zhejiang [30] (251.93 pg·m−3), although the concentration of PFBA was significantly higher than that in Beijing (1.22 pg·m−3) and Xiamen (2.4 pg·m−3). This research also found that the concentration of short-chain PFASs (such as PFBA) was relatively high, which might be related to the use of substitutes for long-chain PFASs and local industrial emissions. In the research conducted by North China Electric Power University in Beijing, PFOA contributed the most to the overall PFAS concentration, accounting for 37.4% in the gas phase and 36.7% in the particulate phase, followed by PFHpA, which accounted for 36.4% in the gas phase and 24.7% in the granular phase.
Research on the gas-phase concentration levels of PFASs is relatively scarce in some countries, with most studies focusing on the contamination characteristics of PFASs in the particulate phase. It is worth noting that although the concentration levels in New Jersey and the Arctic region differ significantly, the underlying source mechanisms complement each other: the former represents a typical industrial and urban emission source area, while the latter represents a distant subsidence zone of long-distance atmospheric transmission. Combining the concentration characteristics of these two regions for discussion can not only help us to better explain the regional distribution differences in PFASs, but also highlight their global migration potential. In recent research [31] conducted in the Americas, Ying Yao et al. found that in the urban atmosphere of northern New Jersey, USA, the concentration of PFASs in the gas phase (197.7 ± 47.9 pg·m−3) was higher than that in the particle phase (48.3 ± 47.9 pg·m−3), indicating a higher risk to human health from inhalation. Short-chain alternatives to PFASs, such as perfluoroheptanoic acid, were found to have a concentration in the gas phase (142.6 ± 28.0 pg·m−3) that was higher than the maximum PFAS concentration level set out by the US EPA’s regulations. Research on the distribution of PFAS gas particles was also carried out in the Czech Republic, and it was found that short-chain PFCAs (PFPeA, PFHxA) are distributed in both the gas phase and the particle phase, with a relatively low particle-phase fraction (θ), averaging 0.30–0.65. Long-chain PFCAs (PFDA, PFUnDA) tend to exist in the granular phase, with θ increasing with the length of the carbon chain, and the maximum θ can reach 0.65–0.74. The average θ of PFOA is relatively low, at 0.14 ± 0.33, PFOS mainly exists in the particulate phase, with θ = 0.80 ± 0.29, and PFDS mainly exists in the gas phase, with θ = 0.12 ± 0.29. Other PFSAs (PFBS, PFHxS) are relatively evenly distributed in the two phases, with θ = 0.38–0.65. FOSA/Es mainly exist in the gas phase, with θ = 0.03–0.22, and among them, the θ of FOSA is the lowest, θ = 0.03 ± 0.09, while that of EtFOSE is slightly higher, θ = 0.22 ± 0.38. It can be seen from this that the distribution behavior of PFASs in the gas phase and the granular phase varies depending on the compound type and chain length. Short-chain PFCAs and FOSA/Es tend to exist in the gas phase, while long-chain PFCAs and PFOS tend to exist in the granular phase. In research conducted on the Arctic region, the PFASs in the atmosphere were mainly found to be in the gaseous phase (91%), with 8:2 FTOH being the main component. The concentration of FOSAs in the particle phase is relatively high, while the concentration of FTA is relatively low due to its anion characteristics. In research conducted in the regions of Northern Europe where skiing is prevalent, the particle phase distribution of PFASs was divided into two parts—among the inhalable particles (<100 μm), PFHxA accounted for 45%, PFTDA for 16%, and PFOA for 14%, while among the lung-penetrable particles (<4 μm), PFHxA accounted for 28%, PFTDA for 27%, and PFOA for 16%. The gas phase mainly consists of FTOHs, especially 8:2 FTOH, which almost entirely exists in the gas phase and has not been detected in the particle phase. PFHxA and PFOA are dominant in the particle phase, with relatively high concentrations, and are found to be carbon chain homologs. The data obtained from this research indicate that the concentration of PFASs that ski waxing technicians are exposed to (at the μg·m−3 level) is significantly higher than that for the general population (at the ng·m−3 level) by an order of magnitude. The above data indicate that ski waxing technicians are exposed to high concentrations of PFASs in both the gaseous and granular phases, especially 8:2 FTOH and PFCAs, posing a significant risk to occupational health. The following are some abbreviations that appear in the table: MeFOSE: 2-(N-methylperfluoro-1-octanesulfonamido)-ethanol; EtFOSA: N-ethyl-perfluoro-octane Sulfonamide; MDL: method detection limit. All concentrations have been converted to pg·m−3 for comparability (1 μg·m−3 = 1 × 106 pg·m−3; 1 ng·m−3 = 1 × 103 pg·m−3).
3.2. Migration and Transformation Processes of PFASs in the Atmosphere and Their Persistence in the Atmosphere
PFASs mainly enter the atmosphere through industrial emissions and volatilization from consumer products and can exist in a gaseous or particulate form. Among them, short-chain substances with strong volatility can migrate over long distances, while long-chain compounds are mostly adsorbed on particulate matter. PFASs spread globally through atmospheric circulation and condense and settle in cold regions under the influence of temperature. Eventually, they return to the surface through dry or wet deposition, and some may be released a second time. During this migration process, PFASs may undergo photodegradation or oxidative transformation. Their distribution is jointly regulated by the characteristics of the compound and meteorological conditions, leading to persistent pollution and even accumulation in remote areas.
3.2.1. Migration Process of PFASs in the Atmosphere
The long-distance atmospheric transport of PFASs is an important factor influencing their distribution in a region. Many PFASs, such as FTOHs and PFCAs, have relatively high vapor pressures and can exist in gaseous form in the atmosphere and be transported over long distances. For example, the atmospheric retention time of 8:2 FTOH can reach 80 days, which is sufficient for its global spread. The global production of 8:2 FTOH is approximately 5 × 106 kg per year, of which 40% is produced in North America [32]. In the abovementioned study on the Arctic, the average ratio of 8:2, 10:2, 6:2, and 12:2 FTOHs in the atmosphere was 4.8:0.9:1.0:0.4. This ratio was higher than that in urban areas (Toronto: 1.1:0.2:1.0), indicating that FTOHs mainly reached the Arctic through long-distance (Figure 3) atmospheric transport, demonstrating that this is a key factor affecting the distribution of PFASs in the Arctic [20]. Recent research in a factory in the Netherlands [33] showed that PFASs such as HFPO-DA (GenX) can be transported over long distances through the atmosphere, with model predictions showing that HFPO-DA can still be detected thousands of kilometers away from the Dordrecht factory—for example, the annual deposition in Reykjavik, Iceland, is 0.5–2.4 ng·m−2yr−1. This research also found that wind direction is one of the influencing factors in PFAS concentration—when the wind blows from the direction of the factory, the PFAS concentration in the air increases significantly.
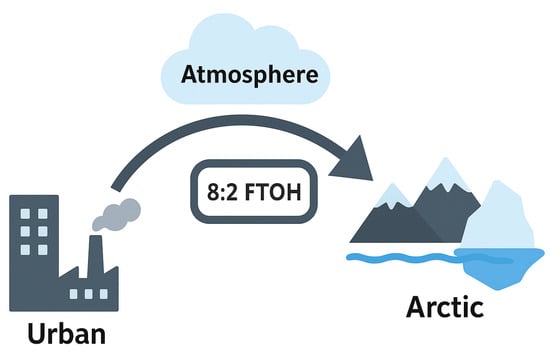
Figure 3.
A simple transmission path diagram for 8:2 FTOH in the Arctic.
The dry deposition of PFASs is one of the most important ways in which they are removed from the atmosphere and enter soil and water bodies. In the abovementioned study on a Dutch factory, during the deposition process, PFASs settled from the atmosphere onto the surface through both dry and wet deposition. The model showed that the daily deposition amount of HFPO-DA in the area close to the factory (within 556 m) reached 1 µg·m−2yr−1, gradually decreasing as the distance increases. There are significant differences in the dry deposition rates of PFASs. Long-chain PFCAs and PFSAs, due to their extremely low vapor pressure and strong hydrophobicity, mainly adsorb on fine atmospheric particulate matter, and their dry deposition rates are relatively low, usually within the range of 0.1–0.5 cm·s−1 [20]. Short-chain PFCAs have a slightly higher dry deposition rate due to their higher water solubility and the fact that some of them exist in a gaseous form. Although volatile precursors mainly exist in a gaseous form, their dry deposition rates are controlled by the exchange interface process with the surface (vegetation, water bodies, etc.), and the range of the variation rate is relatively large (~0.1–1.0 cm·s−1) [34]. PFASs mainly exist in gaseous and particulate states in the atmosphere, and their distribution between these states is a dynamic equilibrium process. The gas/particle distribution of PFASs in the atmosphere is influenced by factors such as temperature, relative humidity, and the nature of the particulate matter. Under conditions of low temperature or high humidity, PFASs are more inclined to adsorb onto the surface of particulate matter and then settle through gravity, as well as directly settling from the gaseous state onto the ground, thereby increasing their deposition rate in the atmosphere. Generally speaking, shorter chain PFASs (6:2 FTOH) are more likely to exist in a gaseous form, while longer chain compounds (PFOA) tend to adsorb onto particulate matter [20]. The dry deposition rate of PFASs adsorbed on particulate matter is affected by its particle size, density, and atmospheric turbulence. Larger particulate matter (such as PM10) settles faster, while smaller particulate matter like PM2.5 may remain suspended in the atmosphere for a longer period of time. Wet deposition is also an important way in which PFASs are removed from the atmosphere, mainly including deposition onto the ground through precipitation (rain, snow, and fog) [35]. The wet deposition process is influenced by the intensity of precipitation, the concentration of PFASs in the atmosphere, and the nature of aerosols. A previous study showed that the median concentration range of PFASs after wet deposition was 2.4 to 4.5 ng·L−1, and there was no significant difference in concentration among different sites, indicating that PFASs, especially ionic PFASs, are widely distributed in the atmosphere [36].
3.2.2. The Transformation Process of PFASs in the Atmosphere
PFASs undergo photochemical transformation in the atmosphere. Although PFASs have extremely high chemical stability, their environmental behavior is still significantly affected by their chain length—for instance, some short-chain PFASs containing fluorine, such as perfluorobutyric acid, may undergo photolysis under strong ultraviolet light, generating low-fluorinated organic acids or other small-molecule compounds. However, the efficiency of this transformation process is very low, mainly involving short-chain PFASs, while long-chain PFASs like perfluorooctanoic acid rarely undergo photolysis [35]. The current research consensus indicates that the reaction with ·OH radicals in the atmosphere represents the most important degradation pathway for neutral PFAS precursors [37]. Their atmospheric lifetime (τ) can be estimated according to the formula *τ = 1/(k·OH·[OH])*, where k·OH is the second-order rate constant of the reaction with ·OH, and [·OH] is the average concentration of atmospheric ·OH radicals (usually taken as 1.0 × 106 molecules·cm−3). Based on kinetic parameters determined in the laboratory, the estimated atmospheric half-lives of the main precursors are as follows:
Fluorinated alcohol (FTOHs): The k·OH of the reactions between 6:2 FTOH, 8:2 FTOH, and 10:2 FTOH and ·OH is approximately (1.0–1.5) × 10−12 cm3·molecule−1·s−1 [38]. Based on this, their atmospheric half-life is estimated to be approximately 10 to 20 days. This is in the same order of magnitude as those inferred from field studies for 6:2 FTOH (~50 days), 8:2 FTOH (~80 days), and 10:2 FTOH (~70 days) [39], verifying that ·OH oxidation is the dominant degradation mechanism.
Sulfonamides (FOSA/FOSE): Taking N-methylperfluorooctane sulfonamide ethanol (MeFOSE) as an example, its k·OH is relatively high, at approximately 5.8 × 10−12 cm3·molecule−1·s−1 [40], and thus its atmospheric half-life is shorter, at about 2 to 3 days.
Straight-chain PFCAs/PFSAs: The direct photolysis or reaction of PFOA, PFOS, and other ionic substances with ·OH is extremely slow, meaning they have an atmospheric half-life of tens of days or even longer. Their final removal is therefore more dependent on physical deposition processes rather than chemical transformation [41].
In summary, short-lived precursors can undergo significant transformation during regional-scale transport, while long-lived precursors and end products have the ability to undergo global-scale migration. This explains why these substances can also be detected in remote polar environments.
In addition, PFASs undergo atmospheric chemical reactions in the atmosphere. The core structure of PFASs is the perfluoroalkyl chain (CF3[CF2]n−), which has extremely high chemical stability, meaning that they are not easily biodegraded or chemically degraded in the natural environment. Some studies have indicated that the chemical bond energy of PFASs such as PFOA and PFOS is extremely high, making them almost non-degradable under natural conditions [35]. Under specific chemical conditions, PFASs may undergo limited chemical transformations. For instance, PFASs may react with hydroxyl radicals (OH·) in the atmosphere to form fluorine-containing carboxylic acids or other intermediate products [32]. However, the rate of this reaction is very slow, and the degree of transformation is limited. Short-chain PFASs have a higher vapor pressure and are more likely to volatilize into the atmosphere, thus having a stronger transport potential, reflecting the trend of short-chain PFASs replacing long-chain PFASs [42]. In addition, chemical reactions on the surface of particulate matter may also lead to the partial transformation of PFASs, for instance by undergoing oxidation reactions with other pollutants such as ozone and free radicals to form more degradable compounds [43].
PFASs also undergo biotransformation in the atmosphere. Although they rarely undergo biodegradation in the atmosphere because their chemical structure lacks the active sites required for microbial degradation, some studies [44] have shown that certain microorganisms may carry out a limited transformation of PFASs under specific conditions. For instance, in the literature, researchers used a mixed microbial culture (acclimated with ethanol as the carbon source) to conduct a degradation experiment on 8:2 FTOH under aerobic conditions, while setting up a sterile control to eliminate the influence of non-biodegradable factors. However, this transformation process is very rare in the atmospheric environment due to a lack of microorganisms and suitable environmental conditions [45]. PFASs have high hydrophobicity and oleophobicity and can combine with proteins and lipids in organisms, leading to their accumulation within the body. This bioaccumulation further increases the persistence of PFASs in the environment, as they can be transferred through the food chain and accumulate in high-trophic organisms [42].
3.2.3. Limitations of Laboratory Studies Versus Real Atmospheric Conditions
Although numerous studies have investigated the migration, transformation, and removal of PFASs under laboratory conditions, it is important to note that these results may not fully reflect their actual behavior in the atmosphere. Laboratory experiments are often carried out under controlled settings, where factors such as temperature, humidity, radiation, and the presence of co-pollutants are simplified or isolated. In contrast, the real atmosphere is a far more complex system, with dynamic meteorological conditions, diverse aerosol compositions, and multiple competing chemical and physical processes. These environmental variables can significantly alter the persistence, partitioning, and degradation efficiency of PFASs. Therefore, while laboratory results are valuable in elucidating potential mechanisms, their direct extrapolation to atmospheric scenarios should be treated with caution. Future research should strengthen the integration of laboratory experiments, field monitoring, and atmospheric modeling to bridge this gap and provide more realistic assessments of PFAS behavior and risks in the environment.
4. Toxicity of PFASs in the Atmosphere
4.1. Hazards of PFASs in the Atmosphere to Human Health and Assessment Methods
PFASs in the atmospheric environment not only enter the human body through the respiratory pathway but have also been found to have significant systemic and reproductive development toxicity effects, even at relatively low concentrations (Table 3). In [46], the FTOH level in indoor air reached 250–82,300 pg/m3 and the adult inhalation intake was 1.04–14.1 ng/kg·day, which was significantly higher than the dust exposure level, highlighting the importance of air inhalation. In animal experiments, the inhaled LC50 of PFOS was 5.2 mg/L and that of PFOA was 980 ng/m3, both of which led to systemic damage to the respiratory tract, liver, etc. [47]. Most crucially, atmospheric exposure to PFASs has been closely associated with thyroid dysfunction, immunosuppression, decreased sperm quality, and fetal developmental toxicity [48], supporting the view that it is a key pollutant with both environmental and health risks.

Table 3.
Summary of effects.
PFASs are carcinogenic, with epidemiological studies [54] showing that PFOA exposure is associated with an increased risk of testicular cancer, kidney cancer, non-Hodgkin’s lymphoma, etc. Recently [55], Hong et al. systematically analyzed the effects of PFASs on the occurrence and development of hepatocellular carcinoma (HCC) and their potential molecular mechanisms. In this study, these authors analyzed transcriptome data from multiple public databases, including core targets TCGA-LIHC (n = 355), LIRI (n = 202), LICA (n = 152), CHCC (n = 159), GSE14520 (n = 221), and GSE54236 (n = 78) (where “n” represents the sample size and serves as the foundation of the research), to identify differentially expressed genes (DEGs) associated with PFAS exposure and HCC. Through differential gene expression analysis, the researchers identified 174 target genes that are commonly associated with PFAS and HCC. These genes are significantly enriched in metabolic signaling pathways and are involved in processes such as lipid metabolism, glucose metabolism, and drug metabolism. Among these target genes, the researchers further screened out six core target genes (APOA1, ESR1, IGF1, PPARGC1A, SERPINE1 and PON1) by integrating machine learning and protein–protein interaction (PPI) network analysis. These genes may play an important role in the progression and prognosis of HCC. For instance, the expression level of APOA1 in normal tissues is significantly higher than that in tumor tissues, and it is associated with the overall survival (OS) of HCC patients. Furthermore, as verified by RT-qPCR and immunohistochemistry (IHC), the expression levels of these core target genes in tumor tissues were significantly lower than those in normal tissues (p < 0.05). The researchers also developed a survival risk model for PFAS-related HCC (PFASRHSig), which integrates 10 machine learning algorithms and 101 algorithm combinations to select 14 genes from 41 genes with prognostic value to construct a risk score. In the TCGA-LIHC training dataset and five validation datasets, the overall survival of patients in the high-risk group was significantly lower than that in the low-risk group (p < 0.001). The diagnostic accuracy of this model was evaluated by means of ROC-AUC analysis. AUC stands for area under the ROC curve, indicating the accuracy of the model’s prediction, and is a core metric for evaluating the performance of machine learning models. The AUC values of survival rates at 1 year, 3 years, and 5 years were all relatively high in the different cohorts. For example, they were 0.728, 0.708, and 0.732, respectively, in the TCGA-LIHC cohort. In addition, through molecular docking simulation, the researchers found that PFAS compounds have a strong binding affinity with these six core target proteins, with binding energies ranging from −5.3 to −7.6 kcal·mol−1. This indicates that these interactions are spontaneous and may play an important role in the occurrence of PFAS-induced liver cancer. The results of the analysis of the molecular pathways related to hepatocellular carcinoma (HCC) obtained through bioinformatics modeling indicated that PFAS exposure might induce abnormal expression associated with oxidative stress, inflammatory response, and the PPAR signaling pathway. Although these findings mainly originated from the analysis of cell and transcriptome databases and the dose levels were higher than the actual concentrations in the atmospheric environment, the findings remain important. Based on existing epidemiological evidence, it can be speculated that long-term, low-dose exposure to atmospheric PFASs leads to their continuous accumulation in the human body and may increase the risk of HCC through a similar molecular mechanism. Therefore, bioinformatics modeling provides a molecular-level explanation for the potential health effects of low-level atmospheric exposure and helps to establish a mechanism chain from atmospheric concentration levels to disease risks.
PFASs can interfere with the endocrine system. PFASs such as PFOS and PFOA may disrupt the function of thyroid hormones, affecting metabolism, growth, and development. Some studies [51] have shown that PFASs are associated with abnormal thyroid hormone levels, especially in pregnant women and children, who are more susceptible. PFOA, PFOS, and 6:2 Cl-PFESA can cross the blood–cerebrospinal fluid barrier (BCSFB), and although the permeability is relatively low (e.g., 0.84% for 6:2 Cl-PFESA and 1.07% for PFOA), their persistent presence in the cerebrospinal fluid indicates that they have the ability to enter the central nervous system [56]. Among these, specific data have also demonstrated that PFOA and PFOS were detected in human thyroid tissues, with median concentrations of 2 ng·g−1 and 5.3 ng·g−1, respectively [57]. PFASs may also interfere with lipid and glucose metabolism by activating the peroxisome proliferator-activated receptor (PPAR) pathway, increasing the risk of diabetes. It was observed that PFOA, PFOS, and their substitute 6:2 Cl-PFESA were all found at high levels in the serum and could cross the blood–cerebrospinal fluid barrier to varying degrees, thus entering the central nervous system. Among these compounds, the ability of 6:2 Cl-PFESA to activate the PPAR signaling pathway was significantly higher than that of traditional PFASs, showing a stronger potential for metabolic interference. The authors of [53] point out that the biological activity of such new substitutes may be even higher than that of the “previous generation” of pollutants which have been phased out, suggesting that their potential health risks cannot be ignored. Further clinical analysis showed that the cerebrospinal fluid permeability of PFASs was significantly positively correlated with the serum glucose concentration. In particular, the R of PFOA and 6:2 Cl-PFESA with the glucose level was β = 0.320 (95% CI: 0.108, 0.532) and β = 0.392 (95% CI: 0.130, 0.654), respectively, indicating a positive linear relationship. This discovery supports the idea that PFASs may be involved in the occurrence mechanism of metabolic diseases such as diabetes by influencing glucose metabolism [56].
PFASs can also suppress the immune system. PFAS exposure is associated with immunosuppression, which may reduce the vaccine antibody response and increase the risk of infection. Studies have found that the level of PFASs is positively correlated with inflammatory markers such as C-reactive protein (CRP) in the serum, indicating that they may promote inflammatory responses. Regarding the relationship between CRP levels and the permeability of the blood–brain barrier, current research mainly indicates a statistical correlation rather than a strict causal relationship. Atmospheric exposure to PFASs may indirectly lead to an increase in CRP by inducing an inflammatory response and may affect blood–brain barrier function, but this mechanism still lacks direct support from animal experiments and longitudinal population studies. Future research needs to combine experimental verification and epidemiological data to further clarify the role played by atmospheric PFAS exposure in inflammatory responses and neurotoxicity risks. Chronic inflammatory states are closely related to various endocrine diseases, such as hypothyroidism, diabetes, polycystic ovary syndrome, etc., and chronic inflammation or immune activation induced by PFASs may be an important way in which they indirectly interfere with hormone secretion [56]. Experiments have also shown that PFASs can cause thymus atrophy and a decrease in lymphocyte levels, affecting immune function [57], particularly related to the liver and kidneys, which are important organs in the immune system. It has been found in the literature that the concentration of PFOS is relatively high in the liver (with a median of 41.9 ng·g−1), while that of PFOA is relatively high in the bones (with a median of 20.9 ng·g−1). These accumulations may potentially interfere with the immune system.
Exposure of the human body to atmospheric PFASs can cause toxicity in the nervous system. Exposure to PFASs during the developmental period may lead to neurobehavioral abnormalities, such as a decline in learning and memory ability and spontaneous behavioral changes. In the serum, PFOA (7.4 ng·mL−1), PFOS (6.8 ng·mL−1), and 6:2 Cl-PFESA (6.2 ng·mL−1) are the main PFASs present, accounting for 79% of the total PFAS burden. Furthermore, in the CSF, PFOA (0.078 ng·mL−1), PFOS (0.028 ng·mL−1), and 6:2 Cl-PFESA (0.051 ng·mL−1) are the main PFASs observed, accounting for 73% of the total PFAS burden. PFASs can penetrate the blood–brain barrier, accumulate in brain tissue, and interfere with the development of neurons [56]. The permeability of PFASs (RPFAS) was positively correlated with the brain barrier permeability index RAlb (CSF/serum albumin ratio) (r2 > 0.6, p < 0.001), indicating that the integrity of the brain barrier is the main determinant of PFAS permeability. The level of CRP in the serum was positively correlated with the RPFAS value of the main PFASs, indicating that inflammation may cause PFASs to cross the brain barrier. For example, for PFOA, for every additional logarithmic unit of CRP, the RPFAS increases by 0.109 (95% CI: 0.036, 0.182).
PFASs in the atmosphere can also be toxic for reproduction and development. PFASs may affect reproductive health, such as reducing sperm quality and interfering with sex hormone levels [52]. Exposure among pregnant women may lead to fetal growth restriction and decreased birth weight [57]. This research also found that the accumulation of PFASs is related to age, and the concentration of PFASs in people over 60 years old is generally higher than that in other age groups. Smoking habits have a certain impact on the accumulation of PFASs in the lungs. The accumulation of PFASs in the lungs of smokers is lower than that of non-smokers. Some PFASs, such as 6:2 Cl-PFESA, have a stronger activating effect on the PPAR signaling pathway than PFOS and have higher potential developmental toxicity [56]. In the liver and brain, the concentration of PFHxA is the highest (with a median of 68.3 ng·g−1 in the liver and 141 ng·g−1 in the brain), while in the bones, PFOA is the main contributing substance (with a median of 20.9 ng·g−1). The concentration of accumulated PFASs is the highest in lung tissue, but PFOS and PFOA are more common in the liver and bones.
The assessment of the human health risks posed by PFASs in the atmosphere requires comprehensive consideration of factors such as exposure levels, receptors, toxicological characteristics, and dose–response relationships. The health risks posed by PFASs in the atmosphere to the human body are not a single additive effect, but rather a synergistic effect of different compounds, meaning they may be much higher than the estimated value. For the human body, air and dust intake through breathing is the main source of PFAS. The action processes of the two routes, skin contact exposure and respiratory intake, involve many parameters, making it difficult to accurately measure the level of PFAS intake and exposure. It is worth noting that there are currently no specific exposure concentration limits for atmospheric PFASs, but there are toxicological parameters that can provide a preliminary reference for assessment. The U.S. Environmental Protection Agency (EPA) has set oral reference doses (RFDS) for perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) at 2.0 × 10−5 and 2.0 × 10−5 mg/kg·day, respectively (based on the 2016 Health Recommendations) [50]. The Poison and Disease Registry (ATSDR) [49] of the United States has proposed a more conservative minimum risk level (MRL) for PFOA, PFOS, perfluorohexane sulfonic acid (PFHxS), and perfluorononanoic acid (PFNA) ranging between 2 × 10−6 and 3 × 10−6 mg/kg·day. Suppose an adult (70 kg) inhales 20 m3 of air daily. Under the condition of complete absorption, calculated based on the ATSDR MRL of PFOA (3 × 10−6 mg/kg·day), the corresponding atmospheric concentration is approximately 10,500 pg/m3. The observational data summarized in this review show that the concentration of PFASs in the air in most areas is far below this range. However, in some areas near pollution sources (such as the industrial area of New Jersey), the observed concentration may approach or exceed these reference levels, indicating potential health risks. As there is currently a lack of systematic efforts to set atmospheric exposure limits, future research and risk management should focus on this aspect.
4.2. The Impact of PFASs in the Atmosphere on Ecology
4.2.1. The Toxic Mechanism of PFASs in the Atmosphere on Plants
PFASs in the atmosphere can affect photosynthesis by influencing the integrity of plant cell membranes. For instance, PFASs can interact with phospholipids in plant cell membranes, altering the fluidity and permeability of these membranes, thereby influencing the synthesis of photosynthetic pigments and the efficiency of photosynthetic electron transport chains [58]. PFASs may also damage the structure and function of photosynthetic organs by inducing oxidative stress responses within plant cells. For instance, PFOS (perfluorooctane sulfonate) can increase the reactive oxygen species level within plant cells, leading to damage to the photosynthetic membrane and the inactivation of photosystem II [59].
PFASs in the atmosphere can also affect plants’ nutrient absorption and transport by binding to receptors on the surface of plant cells. For instance, PFASs can compete for binding sites with ion channels on these cells, thereby inhibiting the absorption of nutrients such as calcium and magnesium. PFASs may also affect the absorption of water and nutrients by plants by altering the morphology and function of their root systems. For instance, PFASs can inhibit the growth of plant roots, resulting in a reduction in the surface area of the roots and thereby lowering the efficiency of plants in absorbing nutrients from the soil [60].
4.2.2. Hazards to Animal Health
The concentration level of PFASs in the atmosphere is closely related to their cumulative effect within organisms. Birds can be indirectly exposed to PFASs in the air through respiration and the food chain. Precursors (such as FTOHs and FOSAs) can be converted into more persistent PFOS in the body, leading to its accumulation. Previous studies have shown that the LOAEL of PFOS in birds is approximately 50–100 ng·g−1 wet weight [61]. In field investigations, air deposition and insect feeding are considered key sources. Similarly, the exposure of marine mammals is mainly achieved through deposition at the atmosphere–ocean interface and food chain transfer. For instance, in the Arctic region [62], despite the lack of direct emission sources, PFAS concentrations of tens to hundreds of ng·g−1 have been detected in polar bears and seals [63], which is closely related to the cumulative effect of long-distance atmospheric transport. Therefore, linking the concentration levels in the air with data for within organisms can help to reveal the health hazards posed by PFASs to animals.
Exposure to PFASs may lead to impaired immune system function in animals, with studies showing that exposure to PFOS can affect the immune system of birds, causing liver and spleen enlargement and influencing the development of the wings and brain of embryos [64]. PFASs may also have a negative impact on the reproductive system of animals, with PFOS exposure reducing the hatching rate of birds, especially under exposure to high concentrations [65]. In addition, exposure to PFASs may affect the normal development of embryos and larvae, with studies finding that exposure to PFOA increases the incidence of leg deformities in poultry embryos and inhibits the development of yellow feathers [66]. Finally, exposure to PFASs may lead to liver damage, manifested as liver enlargement and abnormal liver function, as studies have shown that exposure to PFOS and PFOA can cause pathological changes in the livers of birds [67].
The assessment of PFASs’ impact is based on the toxicity reference value (TRV) and the minimum observed effect level (LOAEL). Studies have shown that the minimum observed effect level (LOAEL) of PFOS in birds is 100 ng·g−1 [68]. The framework for the use of the TRV is explicitly stated in the risk assessment guidelines of Health Canada [69] and USGS [70] and has been applied in the recent EPA Human Health Toxicity Assessment document [71,72]. A concentration higher than this may lead to a decrease in the hatching rate [65]. The toxicity reference value (TRV) of PFOS is 1.7 μg·mL−1, and the predicted no-effect concentration (PNEC) is 1 μg·mL−1 [73]. The toxicity reference value of PFOA is relatively low, but its effect on embryonic development is more significant at high concentrations [66].
4.2.3. The Pathways Through Which PFASs in the Atmosphere Affect Ecological Risks
- (1)
- Widespread distribution in the environment: PFASs are ubiquitous in the air, dust, water bodies, and living organisms, and indoor environments (such as carpets and textiles) are significant sources of pollution [34]. Firstly, for long-distance transmission and accumulation, PFASs are transported through the atmosphere and ocean to remote areas such as the Arctic and islands in the Indian Ocean. These substances accumulate in the marine ecosystem and may have long-term effects on marine life [74]. Secondly, studies have shown that plastic pollution can serve as an important carrier for PFASs, which can adhere to plastic particles and spread to remote ecosystems through marine plastic pollution [75].
- (2)
- Food chain transmission: PFASs accumulate through aquatic and terrestrial food chains, reaching high levels in predators at high trophic levels, thus affecting the survival and reproduction of wild animals such as fish and birds [57]. Studies have shown that PFASs have a significant biological amplification effect in the marine food chain, especially in seabirds and marine mammals [73], and accumulate in the marine ecosystem, potentially having long-term effects on marine life [74]. Studies have also shown that the concentration of PFASs in seabird eggs may affect the development and hatching success rate of embryos [76].
5. Sample Collection Methods and Sampling Techniques of PFASs in the Atmospheric Environment
The sampling methods for PFASs in the air mainly include active air sampling methods and passive sampling methods.
Active air sampling methods involve using an air extraction device to pass air through a filter membrane, effectively collecting PFASs attached to particulate matter. These methods can simultaneously collect PFASs in both the gas phase and the particulate phase and have a relatively high sampling efficiency. The gas-phase PFASs are enriched onto polyethylene and other films through a vacuum pump and then captured by adsorbents such as PUF or XAD resin (where PUF stands for polyurethane foam and XAD is a non-polar macroporous resin). This method has the advantages of controllable flow and high sampling efficiency and is suitable for short-term and quantitative research. During the sampling process, a high-flow sampling pump, a glass fiber filter membrane, and an adsorption cylinder should be used, and the flow velocity should be calibrated in combination with the flowmeter. After collection, the sample needs to be refrigerated and stored and quantitatively detected through solvent extraction and LC-MS/GC-MS analysis (Figure 4).
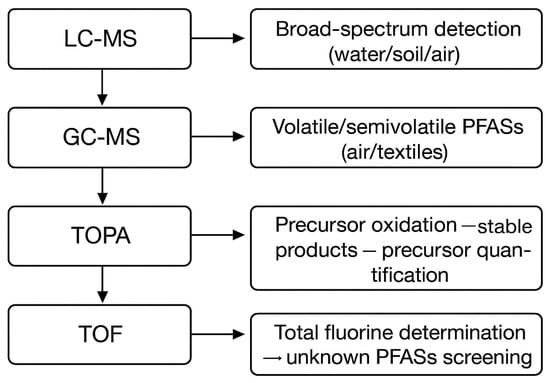
Figure 4.
The principle and application of the analytical methods for the detection technology of PFASs.
- (1)
- Gaseous PFASs: Collected using polyurethane foam (PUF) or activated carbon adsorption tubes. Avoid using sampling equipment containing fluorine materials.
- (2)
- Particulate matter: PM2.5/PM10 is collected through a quartz-fiber filter membrane and then analyzed by solvent extraction.
The passive sampling method [77] has shown great potential in monitoring pollutants. By integrating sampling and analysis, the sensitivity is greatly improved, and through continuous deployment for several days to several weeks, the time-weighted average (TWA) concentration of pollutants over a period of time can be obtained. These methods can thus be used for the detection of trace or ultra-trace pollutants in the environment.
Passive samplers are divided into two types: diffusion samplers and penetration samplers. Adsorbents come in various types, mainly determined by the substance to be tested. Common passive sampling techniques include semi-permeable membrane devices, chemical traps, integrated samplers for polar organic substances, ceramic dosimeters, and solid-phase microextraction and thin-film diffusion gradient technology.
The analysis and detection of PFASs mainly rely on liquid chromatography–tandem mass spectrometry (LC-MS) and gas chromatography–mass spectrometry (GC-MS) techniques (Table 4). LC-MS separates PFASs through liquid chromatography and adopts the negative-ion mode of the electrospray ion source for tandem mass spectrometry analysis. For liquid chromatography (LC), when the sample passes through the chromatographic column, different components are separated due to their varying interactions with the stationary phase. In mass spectrometry (MS), the separated components enter the mass spectrometer, are ionized, and are detected and quantified according to the mass-to-charge ratio (m/z). Common ionization methods include electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI). LC-MS displays high sensitivity and selectivity and is widely used in the detection of various PFASs in water, soil, and biological samples. Separation is carried out by taking advantage of the difference in the distribution coefficients of solutes between the stationary phase and the mobile phase. When a sample solution containing multiple components enters the chromatographic column, due to the differences in adsorption, dissolution, distribution, and other characteristics of each component between the stationary phase and the mobile phase, their movement speeds in the chromatographic column are different, thus achieving the separation of each component.

Table 4.
The principle and application of the analytical methods for the detection technology of PFASs.
GC-MS is suitable for the analysis of volatile or semi-volatile PFASs such as fluorinated alkanols and fluorinated sulfonamides. The samples need to undergo derivatization treatment before gas phase separation and then are detected by means of mass spectrometry. This technique is often used for the analysis of PFASs in the atmosphere. In gas chromatography (GC), after the sample is vaporized, it is carried into the chromatographic column by the carrier gas and separated based on the differences in boiling point and polarity. In mass spectrometry (MS), the separated components are ionized by electron bombardment (EI) or chemical ionization (CI) to generate characteristic ion fragments. Volatile or semi-volatile compounds (such as FTOH, FOSA, etc.) are separated by GC and detected by MS. GC-MS can directly detect PFASs in indoor and outdoor air, while also being able to analyze and detect volatile PFASs in paper and textiles. However, GC-MS is not suitable for ionic or thermally unstable PFASs, as the sensitivity of this technique is usually lower than that of LC-MS. GC-MS is therefore a supplementary technology specifically designed for the analysis of volatile PFASs.
Other auxiliary technologies include TOPA and TOF. TOPA is the process of converting unknown PFAS precursors into detectable final products using strong oxidants such as persulfates. TOF is used to determine the total fluorine content by means of combustion ion chromatography (CIC) or particle-induced gamma-ray emission (PIGE). Both of these methods enable the quantitative analysis of PFAS precursors in environmental samples, filling the gap in the reference standards.
6. Pollution Control and Treatment of PFASs in the Atmosphere
6.1. The Particularity of PFAS Control in the Atmosphere
The treatment of PFASs in the atmosphere has significant particularities and challenges, mainly due to their unique physicochemical properties, environmental behaviors, and technical limitations. The text above also described why PFASs have chemical stability and persistence, and their long-distance migration and global distribution. The diverse emission sources and complex existence forms of PFASs are important reasons for its particularity. It is not only the dispersion of sources: industrial emissions, consumer product volatilization, waste incineration, etc., that makes monitoring and control difficult, but PFASs also coexist in gaseous, particle-adsorbed, and water-soluble ionic forms, and require the coordinated treatment of multiple technologies. The text above also mentioned that precursors in the atmosphere can generate more stable end products through photolysis or oxidation, which further increases the complexity of governance. Some of the pollution control technologies introduced below have limited efficiency for nanoscale PFASs in the atmosphere. Traditional dust removal or adsorption technologies need to be refined into advanced methods such as efficient catalytic degradation or low-temperature plasma. Finally, it is not difficult to see that the pollution control and treatment strategies for atmospheric PFASs have the challenge of cross-border supervision, and international collaboration is still needed to manage the emission sources, especially for industrial emissions and the use of consumer products containing PFASs. Therefore, the governance of atmospheric PFASs requires a full-chain strategy of “source–process–end”, emphasizing the combination of forward-looking monitoring and innovative technologies.
6.2. Common Methods for Controlling Atmospheric PFASs Pollution
Although controlling atmospheric PFASs pollution is difficult, the following technologies show potential breakthroughs.
- (1)
- Physical methods (adsorption, filtration) [79]
Physical methods include activated carbon adsorption, ion exchange resins, and new types of adsorption, etc. Activated carbon (AC) is the most widely studied adsorbent and has a good adsorption effect on long-chain PFASs (PFOS and PFOA). Both granular activated carbon (GAC) and powdered activated carbon (PAC) can effectively remove PFASs, but they need to be replaced or regenerated frequently. Ion exchange resins (such as the Amberlite series) have a relatively high removal efficiency for PFASs, especially for negatively charged PFASs (PFOS and PFOA). The resin can be regenerated through salt solutions such as NaCl and methanol. Other adsorbents include molecularly imprinted polymers (MIPs), carbon nanotubes (MWCNTs), magnetic mesoporous carbon–nitrogen materials (MMCN), etc. Some of these materials exhibit highly efficient adsorption capabilities under specific conditions. This method has a relatively high removal efficiency for particulate PFASs. For instance, when treating long-chain PFASs (such as PFOS) with anion exchange resin (IEX), the removal rate can be as high as or exceed 92%, while the effect on short-chain PFASs is relatively poor [80]. This method is particularly efficient in removing granular contamination, but its ability to act on gas-phase PFASs is significantly insufficient.
- (2)
- Chemical methods (advanced oxidation, thermal decomposition) [81]
The main chemical methods are photocatalysis and thermal decomposition. Photolysis and photocatalysis utilize ultraviolet irradiation to degrade PFASs through direct photolysis or photochemical oxidation. Direct photolysis has a poor effect on PFASs and requires specific wavelengths (such as VUV < 190 nm), while the combination of chemical reagents/catalysts and ultraviolet light can effectively degrade it. For example, the UV–Fenton process has a certain removal and defluorination effect on PFOA. However, this method has a poor effect on PFOS, and the degradation and defluorination efficiency is relatively low. The C-F bond of PFASs in the atmosphere is more difficult to be completely broken. Low-temperature plasma (NTP) can generate highly active free radicals (·OH, O3) to decompose PFASs, but it has relatively high energy consumption. Thermal decomposition is essentially the incineration of PFASs: high temperatures (600–1000 °C) can completely decompose PFASs, but it may produce harmful by-products (such as hydrogen fluoride and greenhouse gases), and it also requires the installation of corresponding tail-gas treatment equipment, which is costly. Chemical methods have shown high degradation efficiency for both gas-phase and granular PFASs. For instance, the treatment of PFOA by UV–Fenton showed that the removal rate reached 87.9% within 1 h, and the total removal rate exceeded 95% after 5 h, with the fluoride removal efficiency rising to 53.2% [82]. Another one employs a UV/S + I system, achieving a removal rate of over 99.7% for various PFSAs and PFCAs, with a fluoride removal efficiency exceeding 90% [83]. However, these processing procedures may generate by-products such as short-chain PFASs or fluorine-containing intermediates. Non-thermal plasma technology has recently been used in experiments to degrade PFAS substitutes such as ADONA and GenX, and the degradation pathways and by-products have been preliminarily described. However, the toxicity of the final products remains unknown [84].
- (3)
- Biological methods (microbial degradation) [85]
Microbial degradation of PFASs usually begins with the enzymatic attack of the C-F bond by reductase for reductive defluorination, where fluorine atoms are replaced by hydrogen to generate intermediate products such as some fluorinated hydrocarbons. However, due to the fluorine shielding around the carbon–carbon bonds in PFASs molecules, this process is hindered, making it difficult for microorganisms to utilize PFASs as a carbon source and energy. Many microbial strains have demonstrated certain capabilities in the degradation of PFASs. For example, Acidimicrobium sp. A6 can degrade PFOA and PFOS by 60% within 100 days under anaerobic conditions, and produce transformation products such as PFBA and PFPeA. In another study [86], this strain enabled PFOA to degrade by 50% within 150 days. Under aerobic conditions, Pseudomonas aeruginosa strain HJ4 can reduce the concentration of PFOS by 67% within 48 days, and the transformation products are PFBS and PFHxS. Gordonia sp. Strain NB4-1Y can degrade 99.9% of 6:2 FTSA within 7 days and generate various transformation products. These data indicate that different microorganisms have varying degradation capabilities and transformation products for different PFASs. At present, this observation is mainly based on laboratory conditions, and although microbial transformation has some degradation capacity, its efficiency and practicality are significantly limited. A review points out that microbial degradation of PFAS is often accompanied by long reaction times, extremely low degradation rates and complex unknown by-products. To date, there is no completely defluorinated microbial system that can be applied in the real environment [87].
7. Conclusions and Prospects
7.1. Conclusions
Through a review and analysis of the classification and properties, pollution characteristics, and migration processes of PFASs in the atmosphere, along with related toxicological analysis and assessment, detection techniques, and pollution control measures, the following conclusions are obtained.
7.1.1. Key Points of the Current Research Status of PFASs
PFASs are known as “permanent chemicals” due to the presence of strong C-F bonds and exhibit significant persistence and mobility in the atmosphere. Short-chain PFASs are more likely to exist in a gaseous form and be transmitted over long distances, while long-chain PFASs are more often adsorbed onto particulate matter and undergo stronger bioaccumulation. Monitoring results show that there are significant differences in atmospheric PFAS concentrations, both globally and among regions in China. For instance, the particulate phase ∑PFAS concentrations in Beijing and Chengdu reached 157 pg·m−3 and 140.81 pg·m−3, respectively, with short-chain PFBA accounting for as much as 69% of the total burden in Chengdu. The proportion of FTOHs in the South China Sea gas phase exceeds 95%, indicating that the input of continental air currents contributes significantly to this. In contrast, the concentration of gas-phase PFASs (197.7 ± 47.9 pg·m−3) in New Jersey, USA, exceeded that of the granular phase (48.3 ± 47.9 pg·m−3), indicating that short-chain substitutes have become a major source of risk in North America. Studies conducted in the polar regions and Northern Europe have also found that the gaseous phase accounts for as much as 91% of the overall burden, reflecting the long-distance migration ability of PFASs. Overall, the southern coastal areas of China are mainly affected by short-chain gaseous substances, while the concentration of particulate substances is higher in the north and around industrial cities. In some cities (such as Zhejiang, 251.93 pg·m−3), the concentration has approached or exceeded that of developed countries in Europe and America.
Toxicological evidence indicates that atmospheric PFASs pose significant risks to humans, animals, and plants. In humans, PFOA is associated with an increased risk of liver cancer, testicular cancer, and kidney cancer. Animal experiments have shown that a PFOS inhalation LC50 of 5.2 mg·L−1 and a PFOA inhalation concentration of 980 ng·m−3 can cause respiratory and liver damage. Epidemiological studies have also found that PFOA and PFOS can cross the blood–brain barrier, accumulating to 2 ng·g−1 and 5.3 ng·g−1, respectively, in thyroid tissue, and are associated with glucose metabolism disorders (β ≈ 0.320–0.392, p < 0.05) and immunosuppression. In terms of ecology, the minimum observed effect level (LOAEL) of PFOS on birds is 100 ng·g−1. Exceeding this value will significantly reduce the hatching rate. Plants, on the other hand, show impaired photosynthesis and inhibited root development in response to PFASs. Overall, atmospheric PFASs pose a lasting threat to humans and ecosystems through multiple pathways and multi-organ effects.
Although detection methods such as LC-MS/GC-MS and treatment methods such as physical adsorption, chemical oxidation, and microbial degradation have been continuously advanced, the existing technologies are limited by their sensitivity, efficiency, and cost, meaning that the efficient control of atmospheric PFASs is difficult to achieve.
7.1.2. Regional Perspectives (Urban/Coastal/Industrial/Polar)
Summary of regional characteristics: As mentioned above, existing research has revealed the distribution differences of atmospheric PFASs in different regions: in urban areas, short-chain PFASs and indoor and outdoor exposure are prominent issues. Coastal areas are significantly affected by the exchange between the atmosphere and the ocean interface. Point-source emissions from industrial zones lead to local high-concentration accumulation. The monitoring results in polar and remote areas highlight its long-distance migration capability. These differences provide important regional references for subsequent research and governance.
7.2. Prospects for Future Research
At present, scholars around the world are breaking through traditional cognitive limitations through multi-media coupling models and ultra-high-resolution mass spectrometry technology. Furthermore, policymakers are highly concerned about the challenges that cross-media circulation poses to the management of “permanent chemicals”. Current research on PFASs in the atmosphere focuses on four core directions: (1) the construction of long-distance atmospheric migration mechanisms and global diffusion models; (2) assessing the environmental behavior and secondary pollution risks of new substitutes; (3) establishing the dynamics of gas/particle distribution and the influence of interfacial reactions on the fate of PFASs; and (4) the development of traceability technology based on isotope fingerprints and regional contribution analysis. It is suggested that future research focus on the following directions:
- (1)
- Developing predictive models incorporating multi-phase interactions and deposition processes. Future models should move beyond simple transport models to integrate dynamic gas/particle partitioning, interfacial chemical reactions, and dry/wet deposition processes. This will enable the more accurate prediction of the environmental fate of PFASs, from emission sources to ultimate sinks in remote areas, and support the development of targeted mitigation strategies.
- (2)
- Proposing targeted mitigation methods for specific sources. Research should focus on developing efficient and economically feasible control technologies for major emission sources. This includes (1) end-of-pipe treatment technologies for industrial exhaust gases, such as non-thermal plasma coupled with catalysts or customized adsorbents for volatile precursors; (2) source control strategies, such as finding alternatives to PFASs in consumer products that are prone to volatile release; and (3) exploring remediation techniques for secondary sources, such as suppressing the re-release of PFASs from contaminated soils into the atmosphere.
- (3)
- Strengthening long-term atmospheric monitoring systems and building a comprehensive monitoring network covering typical regions (industrial, urban, coastal, remote). Research should utilize both active and passive sampling techniques to obtain large-scale, long-term temporal and spatial data, clarifying regional pollution trends and providing a robust data foundation for model validation and policy formulation.
- (4)
- Improving exposure and health risk assessment frameworks, integrating atmospheric concentration data with multi-pathway exposure models (inhalation, dust ingestion, dermal contact) to establish a composite risk assessment system, and strengthening epidemiological and toxicological mechanistic studies to clarify the dose–response relationship and pathogenic mechanisms of low-concentration, long-term exposure to atmospheric PFASs.
- (5)
- Enhancing institutional and global collaboration, promoting the improvement of policies and regulations covering the entire lifecycle of PFASs, reducing transboundary pollution through global agreements, and strengthening international cooperation in scientific research, data sharing, and risk management to jointly address the challenges posed by these persistent pollutants.
Regionalization Outlook:
- (1)
- Urban areas: Strengthen long-term air monitoring and residents’ exposure risk assessment, and explore the inclusion of PFASs in the existing air quality regulatory framework.
- (2)
- Coastal areas: Focus on the material exchange and deposition fluxes at the atmosphere–ocean interface, and evaluate their input effects on marine ecosystems in combination with models.
- (3)
- Industrial zone: Improve source apportioning and emission inventories, and focus on developing control and alternative assessment technologies suitable for industrial waste gas.
- (4)
- Polar and remote regions: Establish long-term observation stations, combine models to verify the long-distance atmospheric transmission mechanism, and reveal its accumulation and risks in the original ecosystem.
Author Contributions
Conceptualization, S.T., X.L. and Y.L. (Yanju Liu); investigation, H.Y. and S.T.; resources, X.L. and Y.L. (Yanju Liu); data curation, H.Y. and Y.L. (Ying Liang); writing—original draft preparation, S.T. and H.Y.; writing—review and editing, S.T. and H.Y.; supervision, Y.L. (Yanju Liu). All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Beijing Natural Science Foundation (8222044 and 8232025), the Beijing Academy of Science and Technology Innovation Foster (25CB003-06), and the Beijing Academy of Science and Technology Innovation Project (25CA004).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the main text.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jones, K.C. Persistent Organic Pollutants (POPs) and Related Chemicals in the Global Environment: Some Personal Reflections. Environ. Sci. Technol. 2021, 55, 9400–9412. [Google Scholar] [CrossRef] [PubMed]
- Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Scheringer, M.; Vierke, L.; et al. Strategies for grouping per-and polyfluoroalkyl substances (PFAS) to protect human and environmental health. Environ. Sci. Process. Impacts 2020, 22, 1444–1460. [Google Scholar] [CrossRef] [PubMed]
- Roscales, J.L.; Vicente, A.; Ryan, P.G.; González-Solís, J.; Jiménez, B. Spatial and Interspecies Heterogeneity in Concentrations of Perfluoroalkyl Substances (PFASs) in Seabirds of the Southern Ocean. Environ. Sci. Technol. 2019, 53, 9855–9865. [Google Scholar] [CrossRef]
- Thackray, C.P.; Selin, N.E.; Young, C.J. A Global atmospheric chemistry model for the fate and transport of PFCA and their precursors. Environ. Sci. Process. Impacts 2020, 22, 285–293. [Google Scholar] [CrossRef]
- MacInnis, J.J.; Lehnherr, I.; Muir, D.C.G.; St Pierre, K.A.; St Louis, V.L.; Spencer, C.; De Silva, A.O. Fate and Transport of Perfluoroalkyl Substances from Snowpacks into a Lake in the High Arctic of Canada. Environ. Sci. Technol. 2019, 53, 10753–10762. [Google Scholar] [CrossRef]
- Bassler, J.; Ducatman, A.; Elliott, M.; Wen, S.; Wahlang, B.; Barnett, J.; Cave, M.C. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ. Pollut. 2019, 247, 1055–1063. [Google Scholar] [CrossRef]
- Dewitt, J.C.; Blossom, S.J.; Schaider, L.A. Exposure to per-fluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: Epidemiological and toxicological evidence. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Apelberg, B.J.; Witter, F.R.; Herbstman, J.B.; Calafat, A.M.; Halden, R.U.; Needham, L.L.; Goldman, L.R. Cord Serum Concentrations of Perfluoro octane Sulfonate (PFOS) and Perfluorooctanoate (PFOA) in Relation to Weight and Size at Birth. Environ. Health Perspect. 2007, 115, 1670–1676. [Google Scholar] [CrossRef]
- Sørli, J.B.; Låg, M.; Ekeren, L.; Perez-Gil, J.; Haug, L.S.; Da Silva, E.; Matrod, M.N.; Gützkow, K.B.; Lindeman, B. Per- and polyfluoroalkyl substances (PFASs) modify lung surfactant function and pro-inflammatory responses in human bronchial epithelial cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2020, 62, 104656. [Google Scholar] [CrossRef]
- Styler, S.A.; Myers, A.L.; Donaldson, D.J. Heterogeneous Photooxidation of Fluorotelomer Alcohols: A New Source of Aerosol-Phase Perfluorinated Carboxylic Acids. Environ. Sci. Technol. 2013, 47, 6358–6367. [Google Scholar] [CrossRef]
- Sima, M.W.; Jaffé, P.R. A critical review of modeling Poly-and Perfluoroalkyl Substances (PFAS) in the soil-water environment. Sci. Total Environ. 2020, 757, 143793. [Google Scholar] [CrossRef]
- Zhao, N.; Zhao, M.; Liu, W.; Jin, H. Atmospheric Particulate Represents A Source of C8-C12 Perfluoroalkyl Carboxylates and 10:2 Fluorotelomer Alcohol in Tree Bark. Environ. Pollut. 2021, 273, 116475. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Isotope Laboratories. Safety Data Sheet: Sodium Tetrafluoro-2-(Heptafluoropropoxy)Propanoate (HFPO-DA, GenX), version 3.0; CLM-11543-S; Cambridge Isotope Laboratories: Tewksbury, MA, USA, 2021.
- U.S. Environmental Protection Agency. Technical Fact Sheet—Perfluorooctanoic Acid (PFOA) and Perfluoro octane Sulfonate (PFOS); EPA 505-F-21-001; U.S. Environmental Protection Agency: Washington, DC, USA, 2021. [Google Scholar]
- Chen, H.; Yao, Y.; Zhao, Z.; Wang, Y.; Wang, Q.; Ren, C.; Wang, B.; Sun, H.; Alder, A.C. Multimedia Distribution and Transfer of Per-and Polyfluoroalkyl Substances (PFASs) Surrounding Two Fluorochemical Manufacturing Facilities in Fuxin, China. Environ. Sci. Technol. 2018, 52, 8263–8271. [Google Scholar] [CrossRef] [PubMed]
- Evich, M.G.; Davis, M.J.B.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, Z.; Möller, A.; Mi, W.; Wolschke, H.; Ebinghaus, R. Atmospheric concentrations and gas/particle partitioning of neutral poly-and perfluoroalkyl substances in northern German coast. Atmos. Environ. 2014, 95, 207–213. [Google Scholar] [CrossRef]
- Lai, S.; Song, J.; Song, T.; Huang, Z.; Zhang, Y.; Zhao, Y.; Liu, G.; Zheng, J.; Mi, W.; Tang, J.; et al. Neutral polyfluoroalkyl substances in the atmosphere over the northern South China Sea. Environ. Pollut. 2016, 214, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Paragot, N.; Bečanová, J.; Karásková, P.; Prokeš, R.; Klánová, J.; Lammel, G.; Degrendele, C. Multi-year atmospheric concentrations of per-and polyfluoroalkyl substances (PFASs) at a background site in central Europe. Environ. Pollut. 2020, 265, 114851. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, Z.; Mi, W.; Möller, A.; Wolschke, H.; Ebinghaus, R. Neutral Poly-/perfluoroalkyl Substances in Air and Snow from the Arctic. Sci. Rep. 2015, 5, 8912. [Google Scholar] [CrossRef]
- Nilsson, H.; Kärrman, A.; Rotander, A.; van Bavel, B.; Lindström, G.; Westberg, H. Professional ski waxers’ exposure to PFAS and aerosol concentrations in gas phase and different particle size fractions. Environ. Sci. Process. Impacts 2013, 15, 814–822. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wu, J.; Dong, B.; Wang, F.; Hu, D.; Zhang, Y.; Peng, L. Pollution characteristics, atmospheric source regions and health impacts of some perfluorinated and polyfluoroalkyl compounds in the atmosphere. Environ. Chem. 2025, 44, 1254–1263. (In Chinese) [Google Scholar]
- Yang, S.; Chen, H.; Gai, N.; Lu, G.; Zheng, Y.; Shao, P.; Yang, Y. Beijing perfluorinated alkyl compounds in atmospheric particulate matter of particle size distribution. J. Test. 2018, 5, 549–557. (In Chinese) [Google Scholar]
- Liu, C.; Ma, X.; Li, Q.; Wang, X. Pollution Characteristics and Evolution Trends of total/Polyfluoride Compounds in the Jiulong River Estuary—Xiamen Sea Area. Mar. Environ. Sci. 2024, 43, 559–571. (In Chinese) [Google Scholar]
- Wang, Q.; Ruan, Y.; Lin, H.; Lam, P.K.S. Review on perfluoroalkyl and polyfluoroalkyl substances (PFASs) in the Chinese atmospheric environment. Sci. Total Environ. 2020, 737, 139804. [Google Scholar] [CrossRef]
- Wang, S.; Lin, X.; Li, Q.; Li, Y.; Yamazaki, E.; Yamashita, N.; Wang, X. Particle size distribution, wet deposition and scavenging effect of per- and polyfluoroalkyl substances (PFASs) in the atmosphere from a subtropical city of China. Sci. Total Environ. 2022, 823, 153528. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Cui, J.; Shi, Y.; Cai, Y. Occurrence and Fate of Per- and Polyfluoroalkyl Substances (PFAS) in Atmosphere: Size-Dependent Gas-Particle Partitioning, Precipitation Scavenging, and Amplification. Environ. Sci. Technol. 2024, 58, 9283–9291. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wu, J.; Dong, B.; Wang, F.; Hu, D.; Zhang, Y.; Bo, Y.; Peng, L. Evidences for the influence from key chemical structures of per- and polyfluoroalkyl substances on their environmental behaviors. J. Hazard. Mater. 2024, 471, 134383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, C.; Yan, T.; Liu, W.; Sun, J.; Fang, S. Study on the Pollution Characteristics of Typical New Pollutants in Winter Atmospheric Particulate Matter in the Southwest Suburbs of Chengdu City. China Environ. Sci. 2025, 45, 2983–2991. (In Chinese) [Google Scholar]
- Li, B.; Chen, J.; Liu, Z.; Wang, J.; He, S. Analysis of pollution characteristics and health risk assessment of perfluorinated compounds in PM2.5 atmospheric particulate matter in Zhejiang Province. Environ. Sci. 2022, 43, 639–648. (In Chinese) [Google Scholar]
- Yao, Y.; Wang, X.; Liu, F.; Zhang, W.; Artigas, F.J.; Gao, Y. Distributions and partitioning of airborne Per- and Polyfluoroalkyl Substances (PFAS) in urban atmosphere of Northern New Jersey. Sci. Total Environ. 2025, 970, 179037. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.A.; Martin, J.W.; De Silva, A.O.; Mabury, S.A.; Hurley, M.D.; Sulbaek Andersen, M.P.; Wallington, T.J. Degradation of Fluorotelomer Alcohols:? A Likely Atmospheric Source of Perfluorinated Carboxylic Acids. Environ. Sci. Technol. 2004, 38, 3316–3321. [Google Scholar] [CrossRef]
- Dalmijn, J.; Shafer, J.J.; Benskin, J.P.; Salter, M.E.; Johansson, J.H.; Cousins, I.T. HFPO-DA and Other PFAS in Air Downwind of a Fluoropolymer Production Plant in the Netherlands: Measurements and Modeling. Environ. Sci. Technol. 2025, 59, 8662–8672. [Google Scholar] [CrossRef]
- Shoeib, M.; Harner, T.; Wilford, B.H.; Jones, K.C.; Zhu, J. Perfluorinated Sulfonamides in Indoor and Outdoor Air and Indoor Dust: Occurrence, Partitioning, and Human Exposure. Environ. Sci. Technol. 2005, 39, 6599–6606. [Google Scholar] [CrossRef]
- Faust, J.A. PFAS on atmospheric aerosol particles: A review. Environ. Sci. Process. Impacts 2023, 25, 133–150. [Google Scholar] [CrossRef]
- Xia, C.; Capozzi, S.L.; Romanak, K.A.; Lehman, D.C.; Dove, A.; Richardson, V.; Greenberg, T.; McGoldrick, D.; Venier, M. The Ins and Outs of Per-and Polyfluoroalkyl Substances in the Great Lakes: The Role of Atmospheric Deposition. Environ. Sci. Technol. 2024, 58, 11. [Google Scholar] [CrossRef]
- Ellis, D.A.; Martin, J.W.; Mabury, S.A.; Hurley, M.D.; Andersen, M.P.S.; Wallington, T.J. Atmospheric Lifetime of Fluorotelomer Alcohols. Environ. Sci. Technol. 2003, 37, 3816–3820. [Google Scholar] [CrossRef]
- Hurley, M.D.; Wallington, T.J.; Andersen, M.P.S.; Ellis, D.A.; Martin, J.W.; Mabury, S.A. Atmospheric Chemistry of Fluorinated Alcohols: Reaction with Cl Atoms and OH Radicals and Atmospheric Lifetimes. J. Phys. Chem. A 2004, 108, 1973–1979. [Google Scholar] [CrossRef]
- Piekarz, A.M.; Primbs, T.; Field, J.A.; Barofsky, D.F.; Simonich, S. Semivolatile Fluorinated Organic Compounds in Asian and Western U.S. Air Masses. Environ. Sci. Technol. 2007, 41, 8248–8255. [Google Scholar] [CrossRef] [PubMed]
- D’eon, J.C.; Hurley, M.D.; Wallington, T.J.; Mabury, S.A. Atmospheric Chemistry of N-methyl Perfluoro butane Sulfonamidoethanol, C4F9SO2N(CH3)CH2CH2OH: Kinetics and Mechanism of Reaction with OH. Environ. Sci. Technol. 2006, 40, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (US). Toxicological Profile for Perfluoroalkyls; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2021; Chapter 5, Potential for Human Exposure. Available online: https://www.ncbi.nlm.nih.gov/books/NBK592133/ (accessed on 30 July 2025).
- Shi, Y.; Zhang, B.; Zheng, Z.; Cai, Y. Research Progress on the Existence and Behavior of Perfluorinated and polyfluoroalkyl Substances in the Atmospheric Environment. Environ. Sci. Res. 2022, 35, 2037–2046. (In Chinese) [Google Scholar]
- Gar Alalm, M.; Boffito, D.C. Mechanisms and pathways of PFAS degradation by advanced oxidation and reduction processes: A critical review. Chem. Eng. J. 2022, 450, 138352. [Google Scholar] [CrossRef]
- Dinglasan, M.J.; Ye, Y.; Edwards, E.A.; Mabury, S.A. Fluorotelomer alcohol biodegradation yields poly- and perfluorinated acids. Environ. Sci. Technol. 2004, 38, 2857. [Google Scholar] [CrossRef]
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, fate and transport of perfluoro carboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, Y.; Sun, H.; Chang, S.; Zhu, L.; Alder, A.C.; Kannan, K. Per- and Polyfluoroalkyl Substances (PFASs) in Indoor Air and Dust from Homes and Various Microenvironments in China: Implications for Human Exposure. Environ. Sci. Technol. 2018, 52, 3156–3166. [Google Scholar] [CrossRef] [PubMed]
- Peritore, A.F.; Gugliandolo, E.; Cuzzocrea, S.; Crupi, R.; Britti, D. Current Review of Increasing Animal Health Threat of Per- and Polyfluoroalkyl Substances (PFAS): Harms, Limitations, and Alternatives to Manage Their Toxicity. Int. J. Mol. Sci. 2023, 24, 11707. [Google Scholar] [CrossRef]
- Ajana, R.; Rachoń, D.; Gałęzowska, G. Reproductive toxicity of per- and polyfluoroalkyl substances. Environ. Toxicol. Pharmacol. 2025, 117, 104740. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Perfluoroalkyls. Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services. 2021. Available online: https://www.atsdr.cdc.gov (accessed on 30 July 2025).
- U.S. EPA. Health Effects Support Document for Perfluorooctanoic Acid (PFOA); EPA 822-R-16-002; U.S. Environmental Protection Agency: Washington, DC, USA, 2016. [Google Scholar]
- Ballesteros, V.; Costa, O.; Iñiguez, C.; Fletcher, T.; Ballester, F.; Lopez-Espinosa, M.J. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: A systematic review of epidemiologic studies. Environ. Int. 2017, 99, 15–28. [Google Scholar] [CrossRef]
- Li, L.; Guo, Y.; Ma, S.; Wen, H.; Li, Y.; Qiao, J. Association between exposure to per- and perfluoroalkyl substances (PFAS) and reproductive hormones in human: A systematic review and meta-analysis. Environ. Res. 2024, 241, 117553. [Google Scholar] [CrossRef]
- Hong, J.; Du, K.; Jin, H.; Chen, Y.; Jiang, Y.; Zhang, W.; Chen, D.; Zheng, S.; Cao, L. Evidence of promoting effects of 6:2 Cl-PFESA on hepatocellular carcinoma proliferation in humans: An ideal alternative for PFOS in terms of environmental health? Environ. Int. 2024, 186, 108582. [Google Scholar] [CrossRef]
- Steenland, K.; Winquist, A. PFAS and cancer, a scoping review of the epidemiologic evidence. Environ. Res. 2021, 194, 110690. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, D.; Liu, Z.; Chen, Y.; Wang, Y.; Li, J. Decoding per- and polyfluoroalkyl substances (PFAS) in hepatocellular carcinoma: A multi-omics and computational toxicology approach. J. Transl. Med. 2025, 23, 504. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, Y.; Cui, Q.; Yao, B.; Wang, J.; Dai, J. Penetration of PFASs across the blood cerebrospinal fluid barrier and its determinants in humans. Environ. Sci. Technol. 2018, 52, 13553–13561. [Google Scholar] [CrossRef] [PubMed]
- Pérez, F.; Nadal, M.; Navarro-Ortega, A.; Fàbrega, F.; Domingo, J.L.; Barceló, D.; Farré, M. Accumulation of perfluoroalkyl substances in human tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Kang, Y.; Fei, Z.; Cao, J. The interaction of perfluoro octane sulfonate with hemoglobin: Influence on protein stability. Chem. Biol. Interact. 2016, 254, 1–10. [Google Scholar] [CrossRef]
- Qu, B.; Zhao, H.; Zhou, J. Toxic effects of perfluoro octane sulfonate (PFOS) on wheat (Triticum aestivum L.) plant. Chemosphere 2010, 79, 555–560. [Google Scholar] [CrossRef]
- Blaine, A.C.; Rich, C.D.; Sedlacko, E.M.; Hundal, L.S.; Kumar, K.; Lau, C.; Mills, M.A.; Harris, K.M.; Higgins, C.P. Perfluoroalkyl acid distribution in various plant compartments of edible crops grown in biosolids-amended soils. Environ. Sci. Technol. 2014, 48, 7858–7865. [Google Scholar] [CrossRef]
- Dennis, N.M.; Subbiah, S.; Karnjanapiboonwong, A.; Dennis, M.L.; McCarthy, C.; Salice, C.J.; Anderson, T.A. Species- and tissue-specific avian chronic toxicity values for perfluoro octane sulfonate (PFOS) and a binary mixture of PFOS and perfluoro hexane sulfonate. Environ. Toxicol. Chem. 2021, 40, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Butt, C.M.; Mabury, S.A.; Kwan, M.; Wang, X.; Muir, D.C. Spatial trends of perfluoroalkyl compounds in ringed seals (Phoca hispida) from the Canadian Arctic. Environ. Toxicol. Chem. 2008, 27, 542–553. [Google Scholar] [CrossRef]
- Houde, M.; De Silva, A.O.; Muir, D.C.G.; Letcher, R.J. Monitoring of perfluorinated compounds in aquatic biota: An updated review. Environ. Sci. Technol. 2011, 45, 7962–7973. [Google Scholar] [CrossRef]
- Peden-Adams, M.M.; Stuckey, J.E.; Gaworecki, K.M.; Berger-Ritchie, J.; Bryant, K.; Jodice, P.G.; Scott, T.R.; Ferrario, J.B.; Guan, B.; Vigo, C.; et al. Developmental toxicity in white leghorn chickens following in ovo exposure to perfluoro octane sulfonate (PFOS). Reprod. Toxicol. 2009, 27, 307–318. [Google Scholar] [CrossRef]
- Molina, E.D.; Balander, R.; Fitzgerald, S.D.; Giesy, J.P.; Kannan, K.; Mitchell, R.; Bursian, S.J. Effects of air cell injection of perfluoro octane sulfonate before incubation on development of the white leghorn chicken (Gallus domesticus) embryo. Environ. Toxicol. Chem. 2010, 25, 227–232. [Google Scholar] [CrossRef]
- Yanai, J.; Dotan, S.; Goz, R.; Pinkas, A.; Seidler, F.J.; Slotkin, T.A.; Zimmerman, F. Exposure of developing chicks to perfluorooctanoic acid induces defects in prehatch and early post hatch development. J. Toxicol. Environ. Health 2008, 71, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Newsted, J.L.; Jones, P.D.; Coady, K.; Giesy, J.P. Avian Toxicity Reference Values for Perfluoro octane Sulfonate. Environ. Sci. Technol. 2005, 39, 9357–9362. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Perfluoroalkyls; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2021. [Google Scholar]
- Health Canada. Toxicological Reference Values (TRVs); Government of Canada Publications: Ottawa, ON, Canada, 2021. [Google Scholar]
- U.S. Geological Survey (USGS). Guide to PFAS Sampling within Natural Resource Damage Assessment and Restoration; U.S. Department of the Interior: Reston, VA, USA, 2024. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Human Health Toxicity Assessment for Perfluoro octane Sulfonic Acid (PFOS); U.S. EPA: Washington, DC, USA, 2024. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Human Health Toxicity Assessment for Perfluorooctanoic Acid (PFOA); U.S. EPA: Washington, DC, USA, 2024. [Google Scholar]
- Braune, B.M.; Letcher, R.J. Perfluorinated Sulfonate and Carboxylate Compounds in Eggs of Seabirds Breeding in the Canadian Arctic: Temporal Trends (1975–2011) and Interspecies Comparison. Environ. Sci. Technol. 2013, 47, 616–624. [Google Scholar] [CrossRef]
- Yamashita, N.; Taniyasu, S.; Petrick, G.; Wei, S.; Gamo, T.; Lam, P.K.; Kannan, K. Perfluorinated acids as novel chemical tracers of global circulation of ocean waters. Chemosphere 2008, 70, 1247–1255. [Google Scholar] [CrossRef]
- Bouwman, H.; Evans, S.W.; Cole, N.; Choong Kwet Yive, N.S.; Kylin, H. The flip-or-flop boutique: Marine debris on the shores of St Brandon’s rock, an isolated tropical atoll in the Indian Ocean. Mar. Environ. Res. 2016, 114, 58–64. [Google Scholar] [CrossRef]
- Van der Schyff, V.; Kwet Yive, N.S.C.; Polder, A.; Cole, N.C.; Bouwman, H. Perfluoroalkyl substances (PFAS) in tern eggs from St. Brandon’s Atoll, Indian Ocean. Mar. Pollut. Bull. 2020, 154, 111061. [Google Scholar] [CrossRef]
- Dong, F. Research on DGT Sampling Method and Application of Perfluorinated/Polyfluoroalkyl Compounds. Master’s Thesis, Dalian University of Technology, Dalian, China, 2023. (In Chinese). [Google Scholar]
- Al Amin, M.; Sobhani, Z.; Liu, Y.; Dharmaraja, R.; Chadalavada, S.; Naidu, R.; Chalker, J.M.; Fang, C. Recent advances in the analysis of per-and polyfluoroalkyl substances (PFAS)—A review. Environ. Technol. Innov. 2020, 19, 100879. [Google Scholar] [CrossRef]
- Merino, N.; Qu, Y.; Deeb, R.A.; Hawley, E.L.; Hoffmann, M.R.; Mahendra, S. Degradation and Removal Methods for Perfluoroalkyl and Polyfluoroalkyl Substances in Water. Environ. Eng. Sci. 2016, 33, 615–649. [Google Scholar] [CrossRef]
- Tshangana, C.S.; Nhlengethwa, S.T.; Glass, S.; Denison, S.; Kuvarega, A.T.; Nkambule, T.T.I.; Mamba, B.B.; Alvarez, P.J.J.; Muleja, A.A. Technology status to treat PFAS-contaminated water and limiting factors for their effective full-scale application. NPJ Clean Water 2025, 8, 41. [Google Scholar] [CrossRef]
- Nzeribe, B.N.; Crimi, M.; Thagard, S.M.; Holsen, T.M. Physico-Chemical Processes for the Treatment of Per-And Polyfluoroalkyl Substances (PFAS): A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 866–915. [Google Scholar] [CrossRef]
- Tang, H.; Xiang, Q.; Lei, M.; Yan, J.; Zhu, L.; Zou, J. Efficient degradation of perfluorooctanoic acid by UV–Fenton process. Chem. Eng. J. 2012, 184, 156–162. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Gao, J.; Yu, Y.; Men, Y.; Gu, C.; Liu, J. Accelerated Degradation of Perfluoro sulfonates and Perfluoro carboxylates by UV/Sulfite + Iodide: Reaction Mechanisms and System Efficiencies. Environ. Sci. Technol. 2022, 56, 3699–3709. [Google Scholar] [CrossRef] [PubMed]
- Topolovec, B.; Jovanovic, O.; Puac, N.; Skoro, N.; Lumbaque, E.C.; Petrovic, M. Plasma water treatment for PFAS: Study of degradation of perfluorinated substances and their byproducts by using cold atmospheric pressure plasma jet. J. Environ. Chem. Eng. 2024, 12, 112979. [Google Scholar] [CrossRef]
- Zeeshan, M.; Tabraiz, S.; Hashmi, S.I.; Iqbal, A.; Dittmann, D.; Abbas, Z.; MacLeod, C.L.; Ruhl, A.S. A comprehensive overview on the occurrence and removal of per-and polyfluoroalkyl substances through adsorption and biodegradation. Bioresour. Technol. Rep. 2025, 29, 102077. [Google Scholar] [CrossRef]
- Yang, S.-H.; Shi, Y.; Strynar, M.; Chu, K.-H. Desulfonation and defluorination of 6:2 fluorotelomer sulfonic acid (6:2 FTSA) by Rhodococcus jostii RHA1: Carbon and sulfur sources, enzymes, and pathways. J. Hazard. Mater. 2022, 423, 127052. [Google Scholar] [CrossRef]
- Douna, B.K.; Yousefi, H. Removal of PFAS by Biological Methods. Asian Pac. J. Environ. Cancer 2023, 6, 53–68. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).