Abstract
Drought has become a serious environmental problem affecting people all around the world as a result of rising atmospheric pollution and global warming. Through symbiosis with Arbuscular mycorrhizal fungus (AMF), plants may mitigate the impacts of drought stress on growth and development as well as physiological metabolism. As a pioneer plant for windbreak and sand fixation, the symbiosis between AMF and Ammodendron bifolium can improve its drought resistance, which is of great significance for species protection and desertification control. In this study, A. bifolium seedlings were used as the test subject in a pot experiment with four drought stress gradients and two inoculation treatments to examine the effects of water treatment and AMF inoculation on the growth of the seedlings. The results showed that drought stress significantly inhibited the growth indexes of A. bifolium seedlings such as the height, basal diameter, blades number, and biomass, and that inoculation with AMF could promote the growth of A. bifolium seedlings and help mitigate the damage caused by drought stress. Drought stress increased the antioxidant enzyme activity and proline (Pro) accumulation in A. bifolium plants, and AMF inoculation induced higher antioxidant enzyme activity and lower malondialdehyde (MDA) and Pro contents in A. bifolium seedlings compared to non-AMF-inoculated plants. Drought stress harmed the chloroplast structure, reduced the chlorophyll concentration, and decreased the photosynthetic efficiency in A. bifolium seedlings. The ability of AMF-inoculated plants to withstand drought was enhanced by increased levels of photosynthetic pigments, higher photosynthetic activity, and increased photosynthetic product accumulation in the roots. These results suggest that AMF inoculation can alleviate drought-induced damage by promoting plant growth and improving plant antioxidant, osmoregulation, and photosynthetic capacity. In the context of increasing drought due to global warming, AMF inoculation can be an excellent way to enhance A. bifolium drought resistance.
1. Introduction
Drought is one of the most damaging environmental factors that the world faces [1]. According to the data, as early as 2005, global dry zones already represented 65% of the entire global area [2]. Yet, as a result of global climate change and rising temperatures, droughts are becoming increasingly severe [3,4,5], with some studies indicating that more than one-third of all soils may be affected by drought in the future [6]. A lack of water is the most major limiting factor in plant growth in dry and semi-arid zones [7]. Drought stress accelerates leaf senescence or causes stomatal closure and chlorophyll decomposition in leaves [8], reducing the plant’s ability to trap light or interfering with electron transport [9,10], resulting in reduced photosynthesis and the decreased production and accumulation of energy materials [11]. Furthermore, when exposed to drought stress, plants produce significant levels of reactive oxygen species (ROS) [12], resulting in an oxidative burst and oxidative damage processes such as membrane lipid peroxidation, which causes serious plant damage [13]. At the same time, drought stress reduces the plant’s own water potential [14] and nutrient uptake [15,16], which has a negative impact on the plant’s regular growth and development as well as important activities [17].
Plants can increase their drought tolerance when they are subjected to the impacts of drought stress by developing a symbiotic interaction with AMF [3,18]. Mycorrhiza is a reciprocal symbiosis formed by fungi and plant roots [19]. Arbuscular mycorrhizal fungi (AMFs), which are widely dispersed in inter-root soils of various plant species, are a type of mycorrhizal fungi [20] and can form a symbiosis with 80% of plants [21], which is essential for plant drought tolerance. It was shown that AMF colonizes plant roots and develops a strong mycelial network that extends into the soil outside of the root zone, increasing the plant’s root uptake area, enhancing the water and mineral element uptake and utilization by the host plant [22,23,24], fostering plant growth [25,26], and consequently enhancing the plant’s capacity to withstand drought stress [27]. At the level of photosynthetic activity, AMF inoculation can enhance plant photosynthetic activity by enhancing the leaf photosynthetic pigment content [28], leaf photosynthetic rate and stomatal conductance [29], and plant photosystem complex activity [30,31]. By raising the activity of antioxidant enzymes, including catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) [32,33], AMF inoculation can lessen the damage caused by reactive oxygen species (ROS) to plants at the physiological and biochemical level [34,35]. It can also diminish the formation of MDA in plants under drought stress [36]. Moreover, inoculation with AMF can enhance Pro accumulation and significantly enhance osmoregulation in plants to increase their ability to withstand drought stress.
Previous research has established the positive impacts of AMF inoculation on numerous economically vital crops, including improving growth and nutrient uptake in maize and wheat [37], enhancing antioxidant capacity in lettuce [38], and promoting photosynthetic capacity in tomato [31]. Despite this, it is yet to be determined whether these outcomes are also applicable to drought-tolerant plants growing within specialized environments, such as deserts. Given the current realities of global warming and increasing drought, finding ways to further enhance the drought resistance of sandy plants and guarantee their survival is a crucial issue that must be considered. Ammodendron bifolium is a desert deciduous shrub of the genus Ammodendron in the Fabaceae family and is a preferred tree species for wind prevention and sand fixation [39]. A. bifolium is only distributed in China in the Takelmohuur Desert in Huocheng County, Ili, Xinjiang, and is endangered [40]. The microbial community present in the roots of A. bifolium, as a legume, significantly contributes to its adaptation in the unique desert environment. Therefore, in the context of global warming and increasingly severe drought, we supposed that inoculation with AMF could improve the drought tolerance of A. bifolium plants to adapt to a harsher survival environment in the future, thus providing a theoretical basis for the conservation of A. bifolium. We supposed that: (1) drought stress affects A. bifolium growth and biomass accumulation, while AMF inoculation can mitigate this effect to some extent; (2) drought stress reduces A. bifolium photosynthesis, AMF-colonized plants have a higher chlorophyll content and PS II maximum quantum yield (Fv/Fm) compared to uninoculated plants; (3) drought stress increases antioxidant capacity in A. bifolium, and AMF colonization magnifies this effect. In this study, we analyzed the main growth morphology, physiological, and biochemical mechanisms, including the seedling morphological characteristics, photosynthesis, antioxidant enzyme system, and osmoregulatory indexes, to elucidate the potential mechanisms of AMF to improve the drought tolerance of A. bifolium seedlings under drought stress. This study provides a theoretical basis for the conservation of A. bifolium.
2. Materials and Methods
2.1. Experimental Materials
For this experiment, we used 3-month-old A. bifolium seedlings, and the seeds were harvested from Takr Mohur Desert (GPS coordinate position: 43°50′34″~44°09′00″ N, 80°27′00″~80°51′28″ E) A. bifolium plants in 2018. After disinfection of the seed surface with 1% NaClO solution, the seed coat was cut with a surgical blade to promote water absorption and germination. The cultivation substrate was Gurbantunggut desert (GPS coordinate position: 44°15′~46°50′ N, 84°50′~91°20′ E) sand. After digging back from the desert, impurities were sieved out with a 0.45 mm soil sieve and they were then sterilized using an electric thermostatic blast dryer (DHG-9240A) at 121 °C for 3 h, cooled to room temperature, and awaited application. The basic physicochemical properties of the test cultivation substrate were as follows: pH: 7.8, salinity: 0.7 g/kg, organic matter: 42.23‰, ammonia N: 32.46 mg/kg, nitrate N: 24.18 mg/kg, available P: 7.80 mg/kg, available K: 335.96 mg/kg, total N: 0.15 g/kg, total P: 1.39 g/kg, total K: 3.92 g/kg, and field water holding capacity: 24.43%. The AMF were a mixture of Rhizophagus intraradices and Claroideoglomus etunicatum, both purchased from the “Bank of Glomeromycota in China” (BGC) of the Institute of Plant Nutrition and Resources, Beijing Academy of Agriculture and Forestry. During the experiment, no other fungi were inoculated except AMF.
2.2. Experimental Design
The experiment was conducted in an indoor planting room from April to September 2022. Seedlings of full-grown A. bifolium seeds were selected for seedling cultivation on 1 April 2022. Seedlings in a good and consistent growth condition were selected for transplanting and acclimatization on 25th April 2022. The soil drought stress experiment was set up with four water treatments: normal water supply (80–85% relative soil water content, denoted as CK), light drought (60–65% relative soil water content, denoted as LD), moderate drought (40–45% relative soil water content, denoted as MD), and severe drought (20–25% relative soil water content, denoted as SD). During the experimental period, distilled water was used to water the seedlings every night at 21:00 using the “weighing method” to replenish the water lost by plant uptake and water evaporation daily, and the relative soil water content of each drought stress gradient was kept within the set range. Two inoculation treatments were set up: AMF inoculation (denoted as A) and no AMF inoculation (denoted as O). A total of 8 treatments were obtained by random combination. The experiments were conducted while ensuring uniformity in all other factors, except for two specific factors, namely soil moisture content and AMF inoculation. The abbreviations of the experimental groups are listed below (Table 1):

Table 1.
Abbreviated table of treatments for the duress test.
2.3. Sample Measurement
2.3.1. AMF in Root Colonization
AMF mycorrhizal colonization was detected using the ink vinegar method [41]: root segments (1 cm in length and <2 mm in diameter) were initially fixed using FAA fixative for 24 h, followed by hyalinization and acidification using 20% KOH and 5% acetic acid solutions, respectively. Staining was subsequently accomplished by immersing the roots in an ink vinegar solution (5% acetic acid: pure blue ink, mixed at a ratio of 95:5 by volume), with thorough rinsing and decolorization using acidified distilled water. Further staining was achieved utilizing Sudan IV staining solution, after which, again, distilled water was used for rinsing, decolorization, and observation under the microscope. The calculation formula is as follows:
Mycorrhizal colonization rate (%) = mycorrhizal root segments/number of total root segments observed × 100
2.3.2. Plant Growth Parameters
The original plant height, basal diameter, and number of blades were measured and recorded before drought stress (3 July 2022) using a meter ruler and vernier calipers. The diameter of the plant base was determined at a point 1 cm above the soil surface, and the plant height was measured from the plant base diameter to the highest point of the plant. Additionally, the data were recorded once more after drought stress using the same method. Five seedlings were selected as replicates for each group.
2.3.3. Physiological and Biochemical Parameters
A 10% tissue homogenate was prepared by accurately weighing 0.1 g of fresh leaves using 0.9% saline as the homogenization medium. After centrifugation at 3500 r/min for 10 min, the supernatant was left for index determination. The amount of enzyme catalyzing 1 μg of substrate per minute per mg of histone at 37 °C was defined as one unit of enzyme activity. SOD activity, POD activity, and CAT activity were calculated by detecting the absorbance changes at 560 nm, 240 nm, and 405 nm using the nitrogen blue tetrazolium method [42], guaiacol method, and ammonium molybdate method [42], respectively [43]. Pro and MDA were determined using commercial kits (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) and were performed according to the instructions inside the kit. Three seedlings were selected from each group and three samples were taken from a total of nine replicates.
2.3.4. Photosynthesis Index
After the drought treatment, 0.1 g of fresh leaves was accurately weighed to determine the leaf chlorophyll concentration using the “ethanol-acetone extraction method” [44], and the leaf carotenoid concentration using 80% acetone was used as the extraction solution [45]. The morphology of the chloroplasts was observed using transmission electron microscopy. Chlorophyll fluorescence parameters were measured in the leaves of A. bifolium using a basic modulated chlorophyll fluorometer (JUNIOR-PAM), selecting the 4th–5th leaf from the top to the bottom for the measurement and taking 6 leaves per treatment as the replicates.
2.3.5. The Soluble Sugars (SS), Starch Content, and Non-Structural Carbohydrates (NSC)
Samples of roots, stems, and leaves were collected separately and ground to powder form, 80% ethanol was then used to separate the soluble sugar (SS) from starch in the samples, and the SS content and starch content were determined by visible light spectrophotometry [46]. Finally, the total soluble sugars (T-SS), total starch (T-Starch), and non-structural carbohydrates (NSC) contents were calculated. Three seedlings were selected from each group and three samples were taken from a total of nine replicates.
T-SS = SSRoot + SSStem + SSLeaf
T-Starch = StarchRoot + StarchStem + StarchLeaf
NSC contents = SS contents + Starch contents
2.4. Statistical Analysis
The experimental data were organized using Microsoft Excel 2021 software, and a one-way analysis of variance (Duncan) was performed using IBM SPSS Statistics 27 (version number:27.0.1.0), with the significance level set at p < 0.05. The results of the data analysis were expressed as the mean ± standard error, and graphs were made using Origin 2021 software (version number:9.90.225).
3. Results
3.1. AMF Inoculation in the Root System of A. bifolium Seedlings under Drought Stress
As shown in Figure 1, mycorrhizal structures, such as vesicles and hyphae, were clearly visible in the roots of AMF-inoculated A. bifolium plants, but not in the roots of non-AMF-inoculated A. bifolium plants. Meanwhile, the infestation rate of AMF in the root system of A. bifolium decreased with the increase in drought stress; compared to the normal moisture gradient, each drought gradient was reduced by 9.13%, 26.35%, and 48.76% in turn.
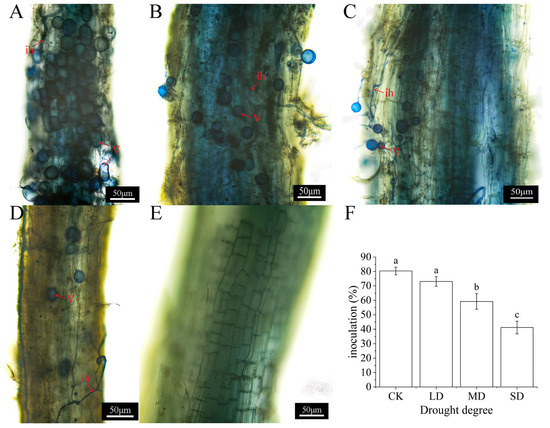
Figure 1.
AMF inoculation in the root system of A. bifolium seedlings under drought stress. Note: (A–D) represent AMF colonization under normal moisture, light drought, moderate drought, and severe drought stresses inoculated with AMF, respectively; (E) represents the root colonization of A. bifolium without AMF inoculation; (F) represents the effect of different drought stresses on AMF infestation rate. “ih” represents intraradical hyphae. “V” represents vesicles. Different lowercase letters indicate significant differences in AMF infestation rates under different treatments at the 5% level according to the Duncan test.
3.2. Effect of AMF Inoculation on the Plant Growth Morphology Indicators of A. bifolium Seedlings under Drought Stress
As shown in Figure 2, compared with the pre-drought period, the growth rate of the height of each group of A. bifolium plants after drought was 91.85%, 81.04%, 40.35%, 17.37%, 90.69%, 44.23%, 33.69%, and 12.25%; the growth rate of the basal diameter was 8.71%, 2.98%, 1.04%, 2.61%, 8.10%, 6.24%, 8.10%, 6.24%, and 12.25%; the growth rate of the leaf number was 86.27%, 63.81%, 48.11%, −24.21%, 75.00%, 24.69%, 13.10%, 2.81%, and 1.51%; and leaf yellowing and wilting occurred under severe drought, with a negative leaf number growth with or without AMF inoculation. Compared with non-inoculated plants, inoculation with AMF showed a higher plant height, basal diameter, and blades number, indicating that inoculation with AMF could promote the growth and new leaf germination of A. bifolium plants. The plant height and blades number were significantly higher under light drought after AMF inoculation, but the difference in the basal diameter was not significant. The blades number increased significantly under moderate drought, but the differences in the plant height and basal diameter were not significant. The differences in the plant height, basal diameter, and number of blades were not significant in all groups under severe drought. It can be concluded that drought stress had an inhibitory effect on the plant height, basal diameter growth, and new leaf generation, and the degree of inhibition gradually increased with the increase in drought stress. The inoculation of AMF could alleviate the damage caused by drought stress to a certain extent, and the alleviation effect was more obvious under light and moderate drought stress.
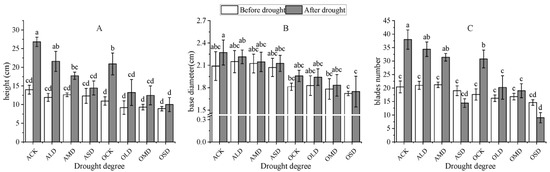
Figure 2.
Effect of AMF inoculation on the height, base diameter, and blades number of A. bifolium seedlings under drought stress. Note: (A) represents plant height; (B) represents plant basal diameter; and (C) represents the plant blades number. ACK-OSD indicates each treatment group of the experiment (as in Table 1). Different lowercase letters indicate significant differences at the 5% level according to the Duncan test in plant height, basal diameter, or blades number of A. bifolium seedlings under different treatments.
As shown in Table 2, drought stress reduced the fresh and dry weights of the roots, stems, and leaves of A. bifolium seedlings compared to normal water treatment, indicating that drought stress reduces the biomass accumulation of A. bifolium plants. The AMF-inoculated plants had a higher biomass accumulation compared to the non-AMF-inoculated plants.

Table 2.
Effect of AMF inoculation on the biomass of A. bifolium seedlings under normal irrigation and drought stress.
3.3. Effect of AMF Inoculation on the Antioxidant Enzymes, Malondialdehyde, and Osmoregulatory Substances of A. bifolium Seedlings under Drought Stress
As shown in Figure 3, drought stress increased the antioxidant enzyme activity of A. bifolium compared to the normal watering treatment, and the higher the degree of drought, the greater the increase, indicating that drought stress led to an increase in the antioxidant capacity of the plants. Compared to the non-AMF plants, the SOD activity increased by 15.12%, 11.52%, 6.81%, and 0.83% in the four water gradients, with significant differences (p < 0.05). The POD activity increased by −1.55%, 38.66%, 70.66%, and 35.88%, respectively, with no significant difference in the control group at the 5% level. It can be seen that the inoculation of AMF can increase the antioxidant enzyme activity of A. bifolium leaves under drought stress.
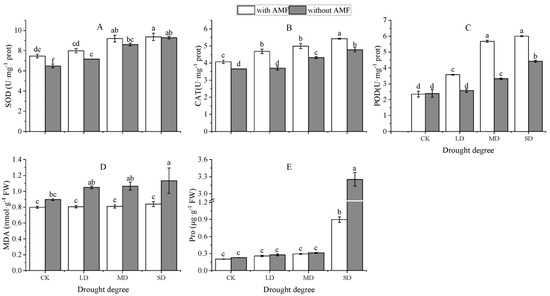
Figure 3.
Effect of AMF inoculation on the antioxidant enzymes, MDA, and osmoregulatory substances of A. bifolium seedlings under drought stress. Note: (A) represents the SOD activity; (B) represents the CAT activity; (C) represents the POD activity; (D) represents the MDA content; (E) represents the Pro content. CK, LD, MD, and SD represent control, light drought, moderate drought, and severe drought, respectively; AMF represents AMF-inoculated plants, without AMF represents non-AMF-inoculated plants. Different lowercase letters indicate significant differences at the 5% level according to the Duncan test in the antioxidant enzymes of A. bifolium seedlings under different treatments.
Drought stress resulted in the accumulation of malondialdehyde (MDA) and proline (Pro) in the leaves of A. bifolium. The difference in the MDA of AMF-inoculated plants under each drought gradient was not significant at the 5% level compared to normal water treatment, while the MDA of non-AMF-inoculated plants was significantly higher (p < 0.05) in the severe drought group compared to the control group. Compared to non-AMF-inoculated plants, the MDA content of A. bifolium blades was significantly lower after AMF inoculation, decreasing by 11.08%, 23.37%, 24.03%, and 26.08% in that order. The leaf Pro content was significantly higher under drought stress compared to the normal water group. Compared to the non-inoculated group, the leaf Pro content of the AMF-inoculated A. bifolium was lower, decreasing by 11.57%, 7.58%, 5.97%, and 72.45% in that order, with highly significant differences under severe drought stress (p < 0.01).
3.4. Effect of AMF Inoculation on the Chloroplast Morphology, Photosynthetic Pigment Content, and Chlorophyll Fluorescence Parameters of A. bifolium Seedlings under Drought Stress
As shown in Figure 4, the chloroplasts of normal-growing leaves of A. bifolium seedlings had clear outlines and complete morphological structures, the phenomenon of stromal wall separation did not appear, the chloroplasts had normal morphological structures, the outer capsule was clearly visible, and the individuals were independent and distributed against the cell membrane at the cell edges. Under light drought stress, the chloroplasts of the leaves of A. bifolium were still distributed against the wall, but the “agglomeration phenomenon” began to appear among the individuals. Under moderate and severe drought stress with AMF, the “agglomeration phenomenon” became more serious or chimeric, and chloroplasts began to move toward the middle of the cell and were no longer distributed against the wall. Compared with no AMF inoculation, the effect of AMF inoculation on chloroplast cell changes under normal water and light drought was not obvious, but under moderate drought stress, the outer capsule of the chloroplasts without AMF inoculation was blurred and invisible, indicating that the morphological structure of the chloroplasts had been severely damaged at this time, while the leaf chloroplasts of AMF-inoculated plants were agglomerated, but the individual outline remained clear. Under severe drought stress, non-AMF-inoculated A. bifolium leaves showed an outflow of cytoplasm and severe cell vacuolation, while for those inoculated with AMF, the chloroplasts suffered some damage and agglomeration seemed to appear, but it did not enter into disintegration and cell vacuolation, indicating that inoculation with AMF had some alleviating effect.
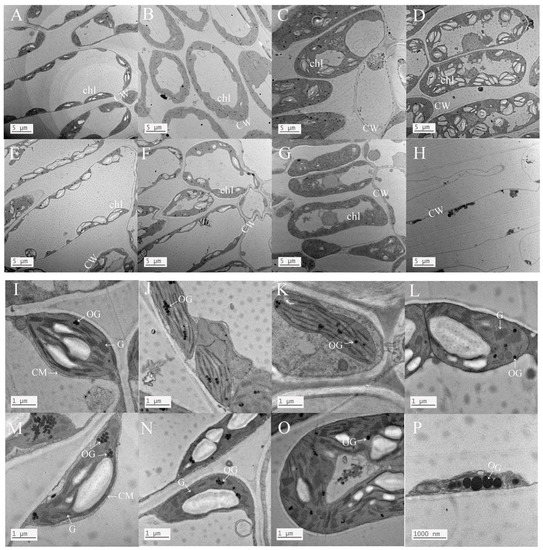
Figure 4.
Effect of AMF inoculation on the chloroplast morphology of A. bifolium seedlings under drought stress. Note: (A–H) represents the changes in chloroplasts in ACK-OSD groups; (I–P) represents the ultrastructural changes in chloroplasts in ACK-OSD groups. CW represents cell wall, chl represents chloroplast, CM represents cell membrane, G represents grana, and OG represents osmiophilic granules.
As shown in Figure 5, drought stress reduced chlorophyll a, chlorophyll b, and the total chlorophyll content in the leaves of A. bifolium compared to normal moisture treatment, and the greater the degree of drought, the more severe the damage to chlorophyll in the leaves of A. bifolium. Under the same water gradient, compared with non-AMF plants, chl. a, chl. b, and the total chl. of A. bifolium leaves inoculated with AMF increased significantly; chl. a increased by 33.37%, 14.30%, 18.78%, and 12.06%, and chl. b increased by 34.78%, 15.10%, 11.31%, and 9.29%, respectively. The total chl. improved by 33.76%, 14.53%, 16.58%, and 11.24%, respectively. The carotenoid (Car) content of the leaves of A. bifolium increased significantly with the increase in drought stress. Compared to uninoculated plants, Car was reduced by 13.58%, 10.73%, 20.82%, and 13.56% in AMF-inoculated plants in that order, respectively. Drought stress reduced the maximum quantum yield (Fv/Fm) and actual light quantum yield (Y(II)) of PSII in A. bifolium leaves; this reduction was proportional to the degree of drought. Compared with AMF inoculation, the Fv/Fm of non-inoculated plants decreased by 1.74%, 3.04%, 2.27%, and 2.16%, respectively. Y(II) decreased by 0.77%, 2.03%, 1.53%, and 7.94%, respectively. This indicates that inoculation of AMF can appropriately alleviate the damage to the photosynthetic system of A. bifolium by drought stress.
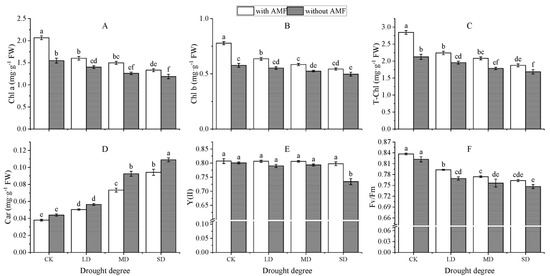
Figure 5.
Effect of AMF inoculation on the photosynthetic pigment content and chlorophyll fluorescence parameters of A. bifolium seedlings under drought stress. Note: (A) represents chlorophyll a content (Chl. a); (B) represents chlorophyll b content (Chl. b); (C) represents total chlorophyll content (T-Chl.); (D) represents carotenoid content (Car); (E) represents actual photochemical quantum yield of PS II (Y(II)); (F) represents maximum photochemical quantum yield of PS II (Fv/Fm). Different lowercase letters indicate significant differences at the 5% level according to the Duncan test in Chl. a, Chl. b, T-Chl., Car, Y(II), and Fv/Fm of A. bifolium seedlings under different treatments.
3.5. Effect of AMF Inoculation on the Soluble Sugars, Starch, and Non-Structural Carbohydrates of A. bifolium Seedlings under Drought Stress
As shown in Figure 6, drought stress promoted the accumulation of the total SS content in A. bifolium plants compared to normal water conditions (except for the OLD group, which was slightly lower than the OCK group; the difference was not significant). The SS content of inoculated AMF A. bifolium reached its maximum under mild drought stress, and each gradient increased 3.11-, 1.62-, and 1.08-fold in turn compared with the control group. Drought stress increased the total starch content of the plants compared to normal moisture treatment. The AMF-inoculated plants had a higher starch content compared to the non-AMF-inoculated plants, increasing by 8.96%, 52.26%, 42.30%, and 27.81% in that order.
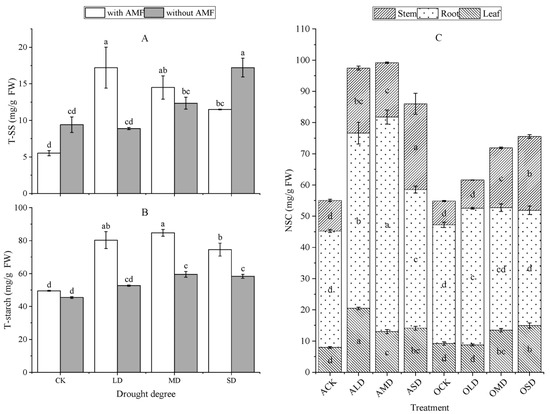
Figure 6.
Effect of AMF inoculation on the SS, starch, and non-structural carbohydrates of A. bifolium seedlings under drought stress. Note: (A) represents total soluble sugar content (T-SS); (B) represents total starch content (T-starch); (C) represents non-structural carbohydrates (NSC). Different lowercase letters indicate significant differences in T-SS, T-starch, and NSC contents of A. bifolium seedlings under different treatments at the 5% level according to the Duncan test.
Drought stress increased the plant NSC content compared to normal water treatment. The root system of A. bifolium was the main storage location for NSC, followed by the stem and leaves. Compared with normal moisture conditions, the NSC content of the root system increased by 50.51%, 84.36%, and 19.13% for each drought stress inoculated with AMF, and by 15.16%, 3.14%, and −2.73% for plants without AMF, respectively. The greater the degree of drought stress, the lower the NSC increase in the root system, and the overall increase in AMF-inoculated plants was higher than that in non-inoculated plants. Compared with the non-AMF plants, the NSC content of the roots of AMF-inoculated A. bifolium increased by −1.91%, 28.21%, 75.34%, and 20.13% in that order. This indicates that drought stress promotes NSC accumulation in A. bifolium plants, and the roots of A. bifolium inoculated with AMF can accumulate more NSC under drought stress.
4. Discussion
Water is an important factor influencing plant growth and physiological metabolism [47], but water scarcity impairs plant growth and development [48]. Drought stress significantly inhibited the plant height, basal diameter, leaf number growth, and biomass accumulation of A. bifolium in this experiment, indicating that drought stress inhibited the normal growth and development and morphological establishment of A. bifolium. This may be due to the lack of water causing the plant to suffer from nutrient uptake. Moreover, at all water gradients, the growth status of AMF-inoculated plants was better than that of non-inoculated plants, indicating that AMF inoculation had a significant growth-promoting effect on A. bifolium, which could be attributed to the increased nutrient and water uptake promoted by AMF inoculation [49]. These findings reflect the role of inoculation with AMF to alleviate the effects of drought stress [35] and improve the drought tolerance of A. bifolium. Notably, the reduction in the leaf number due to severe drought may be due to reduced leaf formation and increased abscission, a phenomenon that may be related to the redistribution of plant carbon under drought stress [50]; this is explored further below in the context of photosynthesis.
Plants mitigate oxidative damage caused by drought stress through the regulation of their own antioxidant enzyme systems [51,52]. Drought stress in the present experiment did increase the activities of various antioxidant enzymes in the leaves of A. bifolium, which is consistent with the study of Zhang et al. [53]. This is a self-protection mechanism of the plant against the accumulation of reactive oxygen species under drought stress. The inoculation of AMF further increased the activities of antioxidant enzymes in A. bifolium leaves, which is consistent with [54,55,56], and it also corroborated the changes in the MDA content in this experiment; MDA is a class of lipid peroxides produced during membrane lipid peroxidation in plants, which can reflect the degree of cell damage induced by stress [57]. In this experiment, drought stress led to the accumulation of MDA in the leaves of A. bifolium, especially under severe drought stress in plants without AMF inoculation, which was significantly higher than that under normal water conditions, which was corroborated by the degradation of the chloroplast outer membrane and the vacuolization of cells in the leaf flesh cells under this treatment. It was thought that MDA accumulation could be caused by damage to the organelle membrane system, such as the chloroplast membrane. However, the MDA contents of AMF-inoculated plants were not significantly different under each drought stress gradient, and all of them were significantly lower than the MDA contents of non-AMF-inoculated plants, indicating that AMF inoculation could alleviate the production of MDA under drought stress. Therefore, both in terms of increasing the antioxidant enzyme activity and enhancing the ability of the plant itself to scavenge reactive oxygen species, and in terms of MDA production after oxidative damage to plant cells, it can be shown that inoculation with AMF alleviated oxidative damage and improved the tolerance of drought stress in A. bifolium, which is consistent with the results of previous studies [35,56].
Pro is a major osmoregulatory substance [58,59] that is often accumulated to maintain plant osmotic balance when plants are subjected to environmental stresses such as drought stress [60,61,62]. According to previous research, drought stress increases the Pro content of A. bifolium, which helps the plant withstand drought [63]. However, even so, it should be noted that Pro accumulation not only has an “adaptive” significance for the plant, but it also characterizes the structural damage of plant cells under stress [64]. Under severe drought stress, a large amount of Pro was accumulated in the leaves of A. bifolium, and the leaf Pro content of uninoculated AMF plants reached 3.25301 mg/g. This phenomenon can no longer simply characterize the degree of drought stress to which A. bifolium is subjected, but it rather indicates that the drought stress injury to uninoculated AMF A. bifolium under severe drought has exceeded some limit, or that such an ultra-high concentration of Pro accumulation also causes some damage to the plant [65,66]. In contrast, the Pro accumulation in AMF-inoculated plants was relatively low, suggesting that AMF inoculation could improve the drought tolerance of A. bifolium and alleviate the damage caused by drought (including severe drought stress) to A. bifolium plants [67]. This phenomenon may be related to the fact that AMF inoculation effected either a decrease in the glutamate synthesis pathway or an increase in Pro catabolism [59,68], and it is important to further investigate the changes related to the expression of key enzymes and genes for Pro synthesis in A. bifolium leaves in future studies as a way to visualize the mechanism by which AMF promotes improved osmoregulation in A. bifolium to adapt to drought.
Photosynthesis, as the most basic physiological activity of plants, is quite sensitive to the environment [54], and photosynthetic pigments are the basis for photosynthesis in plants [69,70]. The degradation of chlorophyll in the leaves of A. bifolium in the present study due to drought stress is consistent with the studies of [71,72], which may be due to drought stress that reduces the nutrient uptake in A. bifolium, resulting in the absence of essential elements for chlorophyll synthesis, leading to a decrease in the chlorophyll content [73]; on the other hand, this deregulation may be due to chloroplast membrane peroxidation and chlorophyll degradation due to the production of reactive oxygen species under drought stress [74]. Additionally, inoculation with AMF can alleviate this phenomenon to a certain extent, which is consistent with the results obtained from studies on apple [75] and tomato [76]. However, it may be due to the fact that AMF inoculation enlarges the root absorption area of the plant, which promotes the absorption of nutrient elements and meets the elements required for chlorophyll synthesis, and thus facilitates the synthesis of chlorophyll in leaves [77]. Moreover, AMF increased the activity of the antioxidant enzyme system of A. bifolium leaves, reduced the oxidative damage to the chloroplast membrane of A. bifolium leaves, and reduced the degradation of chlorophyll, which is in agreement with the study of Jadrane et al. [78]. Carotenoids are one of the important antioxidants in plants, besides being auxiliary pigments for light absorption [79]. The Car content was appropriately increased under drought stress in this experiment, which both improved the light-trapping capacity of leaf chloroplasts and protected chlorophyll from an excessive oxidative decomposition, which is important for maintaining the plant’s autogenous photosynthetic capacity. However, the chlorophyll content of A. bifolium leaves decreased under severe drought stress, and the carotenoid content increased significantly and accumulated excessively, which morphologically showed that the leaves of plants in the severe drought group turned yellow and withered severely [80], indicating that severe drought stress caused severe damage to the photosynthetic pigments of A. bifolium leaves, which is consistent with the study of Anjum et al. [8]. This also corroborates the results of the experiment that drought stress caused severe damage to the chloroplast morphological structure of the leaves.
Chlorophyll fluorescence is a probe of photosynthesis that has been widely used in plant physiological studies [81]. In this study, drought stress reduced the Fv/Fm and Y(II) of PSII in the leaves of A. bifolium, indicating that drought stress reduced the activity of the PSII complex in the leaves of A. bifolium and also reduced the photosynthetic efficiency of the leaves, which is consistent with the studies of Xu et al., and Abid et al. [82,83]. Fv/Fm is highly sensitive to environmental stress, and a decrease in Fv/Fm indicates that the plant is under stress [54,84]. In the present study, Fv/Fm was higher in AMF-inoculated A. bifolium under the same drought stress, and high Fv/Fm indicated a higher photosynthetic efficiency in AMF-inoculated plants [85], which directly reflects the function of AMF in mitigating damage to the photosystem of A. bifolium by drought stress, which is consistent with the study of Chareesri et al. [86].
Under normal growth conditions, plants use most of the C fixed by photosynthesis for metabolic activities, such as respiration and biomass building, and a small fraction is retained in the form of nonstructural carbon compounds, which includes NSC [87]. Additionally, survival is ensured by adjusting carbon allocation when plants are subjected to unfavorable conditions such as drought stress [88]. The partitioning of NSC, as products of plant photosynthesis [89], in roots, stems, and leaves plays an important role in the plant response to drought [90,91]. NSC consists mainly of starch and SS [92]. Drought stress in the present study resulted in the increased accumulation of the SS content in A. bifolium, which is involved in the osmoregulation of the plant, which is consistent with the study of Zhang et al. [67]. However, SS accumulation in the experiment did not show a simple positive relationship with the degree of drought stress, probably because SS played different major roles under different drought stresses. Plants under mild drought stress first accumulate SS to improve osmoregulation [93], and changes in the SS content as the degree of drought stress increases may be related to the involvement of SS in the plant carbon metabolism or as nutrients [90,94]. For example, the inoculation of the AMF A. bifolium SS content started to decrease under moderate drought, which may have been due to the fact that the SS content increases in the threshold range under drought stress to maintain a cellular osmotic balance, and excess SS is converted into the form of starch for storage, which helps to improve the plant’s adaptation to drought stress [95].
In addition, as a preferred tree species for wind breaking and sand fixing, the special living environment of A. bifolium leads to the root system as the main storage organ of NSC to ensure root growth and the water uptake capacity. The increase in the root NSC content under drought stress to ensure root growth and improve its own water uptake for hydraulic recovery may be an adaptive mechanism of A. bifolium in the face of drought stress, but this adaptation was broken under severe drought stress: the overall NSC of uninoculated AMF plants increased under severe drought, but the root NSC decreased, which may be the result of severe drought stress causing the stem of A. bifolium to form the woody embolism [94], which affects plant C transport as a result [90]. In contrast, the root systems of AMF-inoculated plants were still able to accumulate more NSC under severe drought, indicating that AMF inoculation can appropriately mitigate the effects of drought stress on plant C transport, ensure the flow of photosynthetic products from the source to reservoir under stress, and improve the plants’ adaptation to drought stress.
As a natural desert plant, the A. bifolium is inherently more drought-resistant. Drought will become more common in many areas as a result of global warming, and AMF inoculation may be an important way to ensure normal growth and development or to further improve the drought tolerance of A. bifolium to resist drought and maintain the normal growth and development of the autochthonous population under environmental changes.
5. Conclusions
Drought stress reduces development and photosynthesis while increasing antioxidant capacity and osmoregulation. AMF can create a healthy symbiosis in A. bifolium root system colonization, although the colonization rate reduces under drought stress. Inoculation of AMF could promote A. bifolium growth by increasing plant height, basal diameter, leaf number, and biomass; increasing the antioxidant enhancement effect of drought stress on A. bifolium; mitigating chloroplast damage caused by drought stress; promoting chlorophyll accumulation; increasing photosystem activity to mitigate the effect of drought stress on photosynthesis; and promoting root NSC accumulation to facilitate plant adaptation to drought.
Author Contributions
Methodology, L.W.; investigation, Y.Z. and S.L.; writing—original draft preparation, L.W.; writing—review and editing, X.J.; visualization, L.W.; supervision, H.L.; project administration, H.L.; funding acquisition, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
Project of Key Laboratory of Special Species Conservation and Regulatory Biology in Xinjiang Uygur Autonomous Region (No. XJDX1414-2023-04). Xinjiang Uygur Autonomous Region University Student Innovation and Entrepreneurship Training Program (No. 202210762010). The National Natural Science Foundation of China (No. 32160090).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huan, G.; Yan, N.C.; Ya, Q.P.; Suo, M.W.; Ai, K.B. Sodium chloride facilitates the secretohalophyte Atriplex canescens adaptation to drought stress. Plant Physiol. Biochem. 2020, 150, 99–108. [Google Scholar] [CrossRef]
- Huang, J.; Yu, H.; Guan, X.; Wang, G.Y.; Guo, R.X. Accelerated dryland expansion under climate change. Nat. Clim. Change 2015, 6, 166–171. [Google Scholar] [CrossRef]
- Chen, W.; Meng, P.; Feng, H.; Wang, C.Y. Effects of arbuscular mycorrhizal fungi on growth and physiological performance of Catalpa bungei C.A.Mey. under Drought Stress. Forests 2020, 11, 1117. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, J.; Han, J.; Zhang, T.; Yang, S.S.; Wang, J.W.; Prodhan, F.A.; Yao, F.M. Projected increases in global terrestrial net primary productivity loss caused by drought under climate change. Earth’s Future 2022, 10, e2022EF002681. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, J.; Wang, F.; Wang, L.; Xu, Z.C. Morpho-physiological and proteomic responses to water stress in two contrasting tobacco varieties. Sci. Rep. 2019, 9, 18523. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.X.; Wang, X.B.; Qiu, L.J. Research progress and perspective on drought stress in legumes: A review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef]
- Amiri, R.; Nikbakht, A.; Etemadi, N.; Sabzalian, M.R. Nutritional status, essential oil changes and water-use efficiency of rose geranium in response to arbuscular mycorrhizal fungi and water deficiency stress. Symbiosis 2016, 73, 15–25. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and developmental responses of crop plants under drought stress: A review. Zemdirb. Agric. 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Tari, D.B.; Fathi, A. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Tang, H.; Hassan, M.U.; Feng, L.; Nawaz, M.; Shah, A.N.; Qari, S.H.; Liu, Y.; Miao, J.Q. The critical role of arbuscular mycorrhizal fungi to improve drought tolerance and nitrogen use efficiency in crops. Front. Plant Sci. 2022, 13, 919166. [Google Scholar] [CrossRef]
- Sadak, M.S.; Abd El-Hameid, A.R.; Zaki, F.S.A.; Dawood, M.G.; El-Awadi, M.E. Physiological and biochemical responses of soybean (Glycine max L.) to cysteine application under sea salt stress. Bull. Natl. Res. Cent. 2019, 44, 5692–5699. [Google Scholar] [CrossRef]
- Bhattacharjee, S. ROS and oxidative stress: Origin and implication. In Reactive Oxygen Species in Plant Biology; Springer: New Delhi, India, 2019; pp. 1–31. [Google Scholar] [CrossRef]
- Islam, S.M.N.; Paul, N.; Rahman, M.M.; Ashraful, H.M.; Rohman, M.M.; Golam, M.M. Salicylic acid application mitigates oxidative damage and improves the growth performance of barley under drought stress. Phyton-Int. J. Exp. Bot. 2023, 92, 1513–1537. [Google Scholar] [CrossRef]
- Umami, M.; Parker, L.M.; Arndt, S.K. The impacts of drought stress and phytophthora cinnamomi infection on short-term water relations in two year-old Eucalyptus obliqua. Forests 2021, 12, 109. [Google Scholar] [CrossRef]
- Barzana, G.; Carvajal, M. Genetic regulation of water and nutrient transport in water stress tolerance in roots. J. Biotechnol. 2020, 324, 134–142. [Google Scholar] [CrossRef]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Boldt, J.K. Effects of Drought on Nutrient Uptake and the Levels of Nutrient-Uptake Proteins in Roots of Drought-Sensitive and -Tolerant Grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Fadiji, A.E.; Yadav, A.N.; Santoyo, G.; Babalola, O.O. Understanding the plant-microbe interactions in environments exposed to abiotic stresses: An overview. Microbiol. Res. 2023, 271, 127368. [Google Scholar] [CrossRef]
- Guo, X. The role of arbuscular mycorrhiza in sustainable environment and agriculture. In Biofertilizers for Sustainable Agriculture and Environment; Springer: Cham, Switzerland, 2019; pp. 501–520. [Google Scholar] [CrossRef]
- Feijen, F.A.A.; Vos, R.A.; Nuytinck, J.; Merckx, V.S.F.T. Evolutionary dynamics of mycorrhizal symbiosis in land plant diversification. Sci. Rep. 2018, 8, 10698. [Google Scholar] [CrossRef]
- Liang, S.M.; Zhang, F.; Zou, Y.N.; Kuca, K.; Wu, Q.S. Metabolomics analysis reveals drought responses of trifoliate orange by arbuscular mycorrhizal fungi with a focus on terpenoid profile. Front. Plant Sci. 2021, 12, 740524. [Google Scholar] [CrossRef]
- Ma, J.; Wang, W.; Yang, J.; Qin, S.F.; Yang, Y.S.; Sun, C.Y.; Gen, P.; Zeeshan, M.; Liao, H.L.; Liu, L.; et al. Mycorrhizal symbiosis promotes the nutrient content accumulation and affects the root exudates in maize. BMC Plant Biol. 2022, 22, 64. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P.; Alyemeni, M.N.; Alsahli, A.A.; Ahmad, P. Arbuscular mycorrhiza in combating abiotic stresses in vegetables: An eco-friendly approach. Saudi J. Biol. Sci. 2021, 28, 1465–1476. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L.X. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Puschel, D.; Bitterlich, M.; Rydlova, J.; Jansa, J. Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: A Gordian knot of roots and hyphae. Mycorrhiza 2020, 30, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Bharti, A. Salicylic acid improves arbuscular mycorrhizal symbiosis, and chickpea growth and yield by modulating carbohydrate metabolism under salt stress. Mycorrhiza 2018, 28, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Asrar, A.W.; Elhindi, K.M. Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2011, 18, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.L.; He, J.D.; Zou, Y.N.; Wu, Q.S. Mycorrhiza-released glomalin-related soil protein fractions contribute to soil total nitrogen in trifoliate orange. Plant Soil Environ. 2020, 66, 183–189. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Al-Sadi, A.M.; Alharbi, S.A.; Datta, R.; Zuan, A.T.K. Biochar and Arbuscular mycorrhizal fungi mediated enhanced drought tolerance in Okra (Abelmoschus esculentus) plant growth, root morphological traits and physiological properties. Saudi J. Biol. Sci. 2021, 28, 5490–5499. [Google Scholar] [CrossRef]
- Abdel-Salam, E.; Alatar, A.; El-Sheikh, M.A. Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J. Biol. Sci. 2018, 25, 1772–1780. [Google Scholar] [CrossRef]
- Mirshad, P.P.; Puthur, J.T. Arbuscular mycorrhizal association enhances drought tolerance potential of promising bioenergy grass (Saccharum arundinaceum retz.). Envron. Monit. Assess. 2016, 188, 425. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreno, A.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef]
- Saboor, A.; Ali, M.A.; Hussain, S.; El, E.H.A.; Hussain, S.; Ahmed, N.G.A.; Sayyed, R.Z.; Fahad, S.; Danish, S. Zinc nutrition and arbuscular mycorrhizal symbiosis effects on maize (Zea mays L.) growth and productivity. Saudi J. Biol. Sci. 2021, 28, 6339–6351. [Google Scholar] [CrossRef]
- Bahraminia, M.; Zarei, M.; Ronaghi, A.; Sepehri, M.; Hassan, E. Ionomic and biochemical responses of maize plant (Zea mays L.) inoculated with Funneliformis mosseae to water-deficit stress. Rhizosphere 2020, 16, 100269. [Google Scholar] [CrossRef]
- Al-Arjani, A.F.; Hashem, A.; Abd Allah, E.F. Arbuscular mycorrhizal fungi modulates dynamics tolerance expression to mitigate drought stress in Ephedra foliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Liu, C.; Gao, Y.; Han, L.H.; Chu, H.L. Arbuscular mycorrhizal fungi contribute to reactive oxygen species homeostasis of Bombax ceiba L. under drought stress. Front. Microbiol. 2022, 13, 991781. [Google Scholar] [CrossRef] [PubMed]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef]
- Papoui, E.; Bantis, F.; Kapoulas, N.; Ipsilantis, I.; Koukounaras, A. A Sustainable Intercropping System for Organically Produced Lettuce and Green Onion with the Use of Arbuscular Mycorrhizal Inocula. Horticulturae 2022, 8, 466. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J.M. Enhanced Drought Stress Tolerance by the Arbuscular Mycorrhizal Symbiosis in a Drought-Sensitive Maize Cultivar Is Related to a Broader and Differential Regulation of Host Plant Aquaporins than in a Drought-Tolerant Cultivar. Front. Plant Sci. 2017, 8, 1056. [Google Scholar] [CrossRef]
- Zhu, Y.L.; She, X.P. Evaluation of the plant-growth-promoting abilities of endophytic bacteria from the psammophyte Ammodendron bifolium. Can. J. Microbiol. 2018, 64, 253–264. [Google Scholar] [CrossRef]
- Zhu, Y. Isolation and identification of Ammodendron bifolium endophytic bacteria and the action mechanism of selected isolates-induced seed germination and their effects on host osmotic-stress tolerance. Arch. Microbiol. 2019, 201, 431–442. [Google Scholar] [CrossRef]
- Vierheilig, C.; Wyss, P. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef]
- Naz, T.; Mazhar Iqbal, M.; Tahir, M.; Hassan, M.M.; Rehmani, M.I.A.; Zafar, M.I.; Ghafoor, U.; Qazi, M.A.; EL Sabagh, A.; Sakran, M.I. Foliar application of potassium mitigates salinity stress conditions in spinach (Spinacia oleracea L.) through reducing nacl toxicity and enhancing the activity of antioxidant enzymes. Horticulturae 2021, 7, 566. [Google Scholar] [CrossRef]
- Ni, J.; Wang, Q.J.; Shah, F.A.; Liu, W.B.; Wang, D.D.; Huang, S.W.; Fu, S.L.; Wu, L.F. Exogenous Melatonin Confers Cadmium Tolerance by Counterbalancing the Hydrogen Peroxide Homeostasis in Wheat Seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Zhou, J.; Shang, R.; Wang, Y.; Jing, P. Comparative study on several determination methods of chlorophyll content in plants. IOP Conf. Ser. Mater. Sci. Eng. 2020, 730, 012066. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Lv, Y.-J.; Zhang, Y.; Peng, X.Y.; Liu, Y.G.; Rong, L.S. Repair capacity of perennial ryegrass (Lolium perenne L.) based on arbuscular mycorrhizal fungi on the in uranium contaminated soil. IOP Conf. Ser. Earth Environ. Sci. 2019, 330, 032034. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Tang, X.; Wang, G.Y.; Wu, S.J.; Li, X.X.; Huang, X.F.; Qu, T.T.; Chen, J.F.; Tang, X.M. Effect of larginine on maintaining storage quality of the white button mushroom (Agaricus bisporus). Food Bioprocess Technol. 2019, 12, 563–574. [Google Scholar] [CrossRef]
- Zardak, S.G.; Dehnavi, M.M.; Salehi, A.; Majid, G. Effects of using arbuscular mycorrhizal fungi to alleviate drought stress on the physiological traits and essential oil yield of fennel. Rhizosphere 2018, 6, 31–38. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.; Ke, W.; Cai, M.L.; Chen, G.X.; Peng, C.L. Responses of Sphagneticola trilobata, Sphagneticola calendulacea and Their Hybrid to Drought Stress. Int. J. Mol. Sci. 2021, 22, 11288. [Google Scholar] [CrossRef] [PubMed]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse-grown lettuce. J. Agric. Food Chem. 2011, 59, 5504–5515. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.K.; Clifford, S.C.; Wanek, W.; Jones, H.G.; Popp, M. Physiological and morphological adaptations of the fruit tree Ziziphus rotundifolia in response to progressive drought stress. Tree Physiol. 2001, 21, 705–715. [Google Scholar] [CrossRef]
- Zou, Y.N.; Wu, Q.S.; Kuca, K. Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol. 2021, 23 (Suppl. S1), 50–57. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; Mohamed, M.E.A.; Abdelhalim, I.G.; Dalia, S.T.; Abdullah, A.A.D.; El-Sayed, E.E.; Abdelghafar, M.A.E.; Yaser, M.H. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Xu, D.; Zhou, H.; Zhang, N.N.; Cui, Z.Y. Effects of drought and host on the growth of Santalum album seedlings in pot culture. Int. J. Mol. Sci. 2022, 23, 11241. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, H.; Li, H. Arbuscular mycorrhizal fungi improve growth, photosynthetic activity, and chlorophyll fluorescence of Vitis vinifera L. cv. ecolly under drought stress. Agronomy 2022, 12, 1563. [Google Scholar] [CrossRef]
- Jerbi, M.; Labidi, S.; Laruelle, F.; Tisserant, B.; Dalpé, Y.; Lounès, H.S.A.; Ben, J.F. Contribution of native and exotic arbuscular mycorrhizal fungi in improving the physiological and biochemical response of hulless barley (Hordeum vulgare ssp. nudum L.) to Drought. J. Soil Sci. Plant Nutr. 2022, 22, 2187–2204. [Google Scholar] [CrossRef]
- Langeroodi, A.R.S.; Osipitan, O.A.; Radicetti, E.; Mancinelli, R. To what extent arbuscular mycorrhiza can protect chicory (Cichorium intybus L.) against drought stress. Sci. Hortic. 2020, 263, 109109. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Threeprom, W.; Tisarum, R.; Samphumphuang, T.; Chungloo, D.; Chaum, S. Matching of nitrogen enhancement and photosynthetic efficiency by arbuscular mycorrhiza in maize (Zea mays L.) in relation to organic fertilizer type. Plants 2022, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, Y.; Yadav, V.; Zhao, W.; He, Y.P.; Zhang, X.; Wei, C.H. Drought-induced proline is mainly synthesized in leaves and transported to roots in watermelon under water deficit. Hortic. Plant J. 2022, 8, 615–626. [Google Scholar] [CrossRef]
- Alotaibi, M.O.; Saleh, A.M.; Sobrinho, R.L.; Sheteiwy, M.S.; ElSawah, A.M.; Mohammed, A.E.; AbdElgawad, H. Arbuscular mycorrhizae mitigate aluminum toxicity and regulate proline metabolism in plants grown in acidic soil. J. Fungi 2021, 7, 531. [Google Scholar] [CrossRef]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Stella, C.; Donald, F.B. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.S. Proline metabolism and molecular cloning of AmP5CS in the mangrove Avicennia marina under heat stress. Ecotoxicology 2020, 29, 698–706. [Google Scholar] [CrossRef]
- Siddique, A.; Kandpal, G.; Kumar, P. Proline accumulation and its defensive role under diverse stress condition in plants: An overview. J. Pure Appl. Microbiol. 2018, 12, 1655–1659. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef] [PubMed]
- Zegaoui, Z.; Planchais, S.; Cabassa, C.; Reda, D.; Ouzna, A.B.; Pierre, C. Variation in relative water content, proline accumulation and stress gene expression in two cowpea landraces under drought. J. Plant Physiol. 2017, 218, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F.; Ali, E.F. Evaluation of proline functions in saline conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammari, M.Z.F.; Al-Jboory, W.S.H. Effect of amino acid proline on some growth characteristics of cowpea which exposed to drought stress. J. Phys. Conf. Ser. 2021, 1879, 022024. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Xu, G.; Zhou, L.W.; Li, Y.G. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Zenia insignis seedlings under drought stress. New For. 2018, 50, 593–604. [Google Scholar] [CrossRef]
- Cheng, S.; Zou, Y.N.; Kuca, K.; Hashem, A.; Abd, A.E.F.; Wu, Q.S. Elucidating the Mechanisms Underlying Enhanced Drought Tolerance in Plants Mediated by Arbuscular Mycorrhizal Fungi. Front. Microbiol. 2021, 12, 809473. [Google Scholar] [CrossRef]
- Ghadirnezhad, S.S.R.; Fathi, A.; Taghavi, G.F.; Amiri, E.; Pessarakli, M. Plants’ responses under drought stress conditions: Effects of strategic management approaches—A review. J. Plant Nutr. 2023, 46, 2198–2230. [Google Scholar] [CrossRef]
- Ebadi, S.M.; Sam Daliri, M.; Mousavi, S.A.A.; Mirmazloum, I. Changes in morpho-physiological traits of rice cultivars upon different fertilization regimes. J. Plant Nutr. 2022, 45, 2801–2815. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Tomasetto, F.; Yan, W.Q.; Tan, Z.F.; Liu, J.; Jiang, J.M. Non-destructive measurements of toona sinensis chlorophyll and nitrogen content under drought stress using near infrared spectroscopy. Front. Plant Sci. 2021, 12, 809828. [Google Scholar] [CrossRef]
- Aili, Y.; Chen, X.; Gao, W.; Wang, H.O.; Dawuti, M.; Ma, X.D. Response of Alhagi sparsifolia seedlings to AMF inoculation and nitrogen addition under drought stress. Atmosphere 2023, 14, 466. [Google Scholar] [CrossRef]
- Hosseyni Moghaddam, M.S.; Safaie, N.; Soltani, J.; Hagh, D.N. Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops. Plant Physiol. Biochem. 2021, 160, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Maleki Asayesh, Z.; Arzani, K.; Mokhtassi-Bidgoli, A.; Abdollahi, H. Enzymatic and non-enzymatic response of grafted and ungrafted young European pear (Pyrus communis L.) trees to drought stress. Sci. Hortic. 2023, 310, 111745. [Google Scholar] [CrossRef]
- Huang, D.; Ma, M.; Wang, Q.; Zhang, M.X.; Jing, G.Q.; Li, C.; Ma, F.W. Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol. Biochem. 2020, 149, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Nandhitha, G.; Nithila, S. Impact of drought on chlorophyll, soluble protein, abscisic acid, yield and quality characters of contrasting genotypes of tomato (Solanum lycopersicum). Br. J. Appl. Sci. Technol. 2017, 21, 1–10. [Google Scholar] [CrossRef]
- Asadi, M.; Rasouli, F.; Amini, T.; Hassanpouraghdam, M.B.; Souri, S.; Skrovankova, S.; Mlcek, J.; Ercisli, S. Improvement of photosynthetic pigment characteristics, mineral content, and antioxidant activity of lettuce (Lactuca sativa L.) by arbuscular mycorrhizal fungus and seaweed extract foliar application. Agronomy 2022, 12, 1943. [Google Scholar] [CrossRef]
- Jadrane, I.; Al Feddy, M.N.; Dounas, H.; Kouisni, L.; Aziz, F.; Ouahmane, L. Inoculation with selected indigenous mycorrhizal complex improves Ceratonia siliqua’s growth and response to drought stress. Saudi J. Biol. Sci. 2021, 28, 825–832. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Q.; Wang, Y.; Qin, L.L.; Shi, Y.C.; Wang, X.R.; Wang, R. NtDREB-1BL1 enhances carotenoid biosynthesis by regulating phytoene synthase in Nicotiana tabacum. Genes 2022, 13, 1134. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Guo, Y.; Ma, Y.L.; Yang, M.; Fu, R.Q.; Sun, Y.P. Elevated CO2 delayed yellowing by maintaining chlorophyll biosynthesis and inhibiting chlorophyll degradation and carotenoid accumulation of postharvest broccoli. Postharvest Biol. Technol. 2022, 194, 112089. [Google Scholar] [CrossRef]
- Liu, B.; Liang, J.; Tang, G.; Wang, X.F.; Liu, F.C.; Zhao, D.C. Drought stress affects on growth, water use efficiency, gas exchange and chlorophyll fluorescence of Juglans rootstocks. Sci. Hortic. 2019, 250, 230–235. [Google Scholar] [CrossRef]
- Xu, J.; Guo, L.; Liu, L. Exogenous silicon alleviates drought stress in maize by improving growth, photosynthetic and antioxidant metabolism. Environ. Exp. Bot. 2022, 201, 104974. [Google Scholar] [CrossRef]
- Abid, G.; Mhamdi, M.; Mingeot, D.; Aouida, M.; Aroua, I.; Muhovski, Y.; Sassi, K.; Souissi, F.; Mannai, K.; Jebara, M. Effect of drought stress on chlorophyll fluorescence, antioxidant enzyme activities and gene expression patterns in faba bean (Vicia faba L.). Arch. Agron. Soil Sci. 2016, 63, 536–552. [Google Scholar] [CrossRef]
- Pena, R.; Robbins, C.; Corella, J.C.; Huita, M.; Masso, C.; Vanlauwe, B.; Signarbieux, C.; Rodriguez, A.; Sanders, I.R. Genetically different isolates of the arbuscular mycorrhizal fungus Rhizophagus irregularis induce differential responses to stress in cassava. Front. Plant Sci. 2020, 11, 596929. [Google Scholar] [CrossRef] [PubMed]
- Vishnuveni, M.; Chandrasekhar, C.N.; Jeyakumar, P.; Ravikesavan, R.; Sudhakar, D. Effect of drought stress on gas exchange, chlorophyll and yield characters of pearl millet genotypes. Int. J. Agric. Sci. 2019, 11, 8582–8585. [Google Scholar]
- Chareesri, A.; De Deyn, G.B.; Sergeeva, L.; Polthanee, A.; Kuyper, T.W. Increased arbuscular mycorrhizal fungal colonization reduces yield loss of rice (Oryza sativa L.) under drought. Mycorrhiza 2020, 30, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Vilalta, J.M.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Hartmann, H.; Adams, H.D.; Hammond, W.M.; Hoch, G.; Simon, M.L.; Erin, W.; Zaehle, S. Identifying differences in carbohydrate dynamics of seedlings and mature trees to improve carbon allocation in models for trees and forests. Environ. Exp. Bot. 2018, 152, 7–18. [Google Scholar] [CrossRef]
- Zhang, G.; Maillard, P.; Mao, Z.; Brancheriau, L.; Engel, J.N.; Gérard, B.; Fortunel, C.; Maeght, J.L.; Martínez, V.J.; Ramel, M.; et al. Non-structural carbohydrates and morphological traits of leaves, stems and roots from tree species in different climates. BMC Res. Notes 2022, 15, 251. [Google Scholar] [CrossRef]
- Tomasella, M.; Petrussa, E.; Petruzzellis, F.; Andrea, N.; Valentino, C. The possible role of non-structural carbohydrates in the regulation of tree hydraulics. Int. J. Mol. Sci. 2019, 21, 144. [Google Scholar] [CrossRef]
- He, W.; Liu, H.; Qi, Y.; Liu, F.; Zhu, X.R. Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob. Change Biol. 2020, 26, 3627–3638. [Google Scholar] [CrossRef]
- Santos, M.; Barros, V.; Lima, L.; Gabriella, F.; Mauro, G.S. Whole plant water status and non-structural carbohydrates under progressive drought in a Caatinga deciduous woody species. Trees 2021, 35, 1257–1266. [Google Scholar] [CrossRef]
- Regier, N.; Streb, S.; Cocozza, C.; Schaub, M.; Cherubini, P.; Zeeman, S.C.; Frey, B. Drought tolerance of two black poplar (Populus nigra L.) clones: Contribution of carbohydrates and oxidative stress defence. Plant Cell Environ. 2009, 32, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Secchi, F.; Pagliarani, C.; Zwieniecki, M.A. The functional role of xylem parenchyma cells and aquaporins during recovery from severe water stress. Plant Cell Environ. 2017, 40, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).