Cystoid Macular Lesions in Inherited Retinal Diseases: Prevalence, Characteristics, and Genetic Associations in a Hungarian Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Ophthalmic Examination
2.2. Genetic Testing
2.3. Statistical Analysis
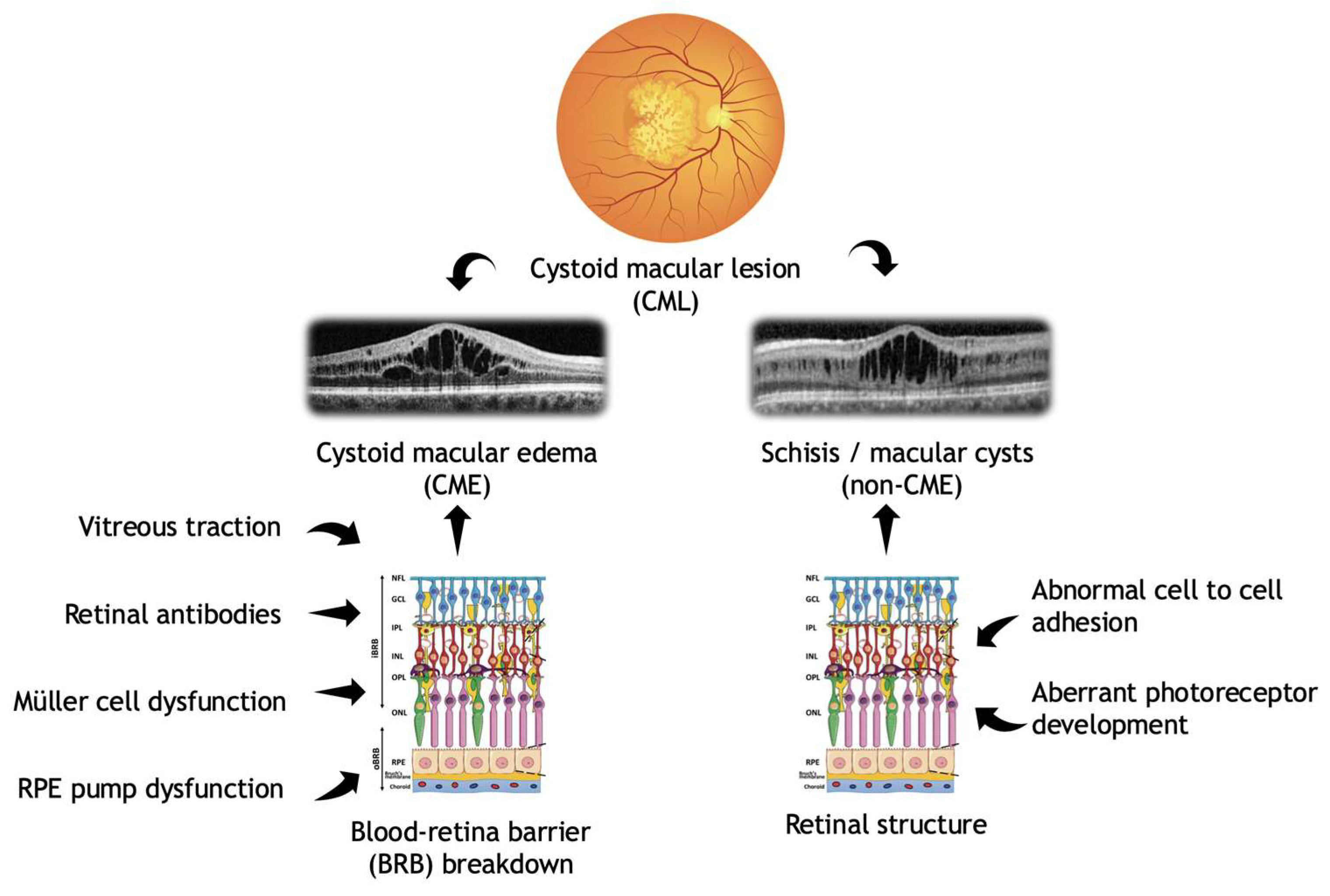
3. Results
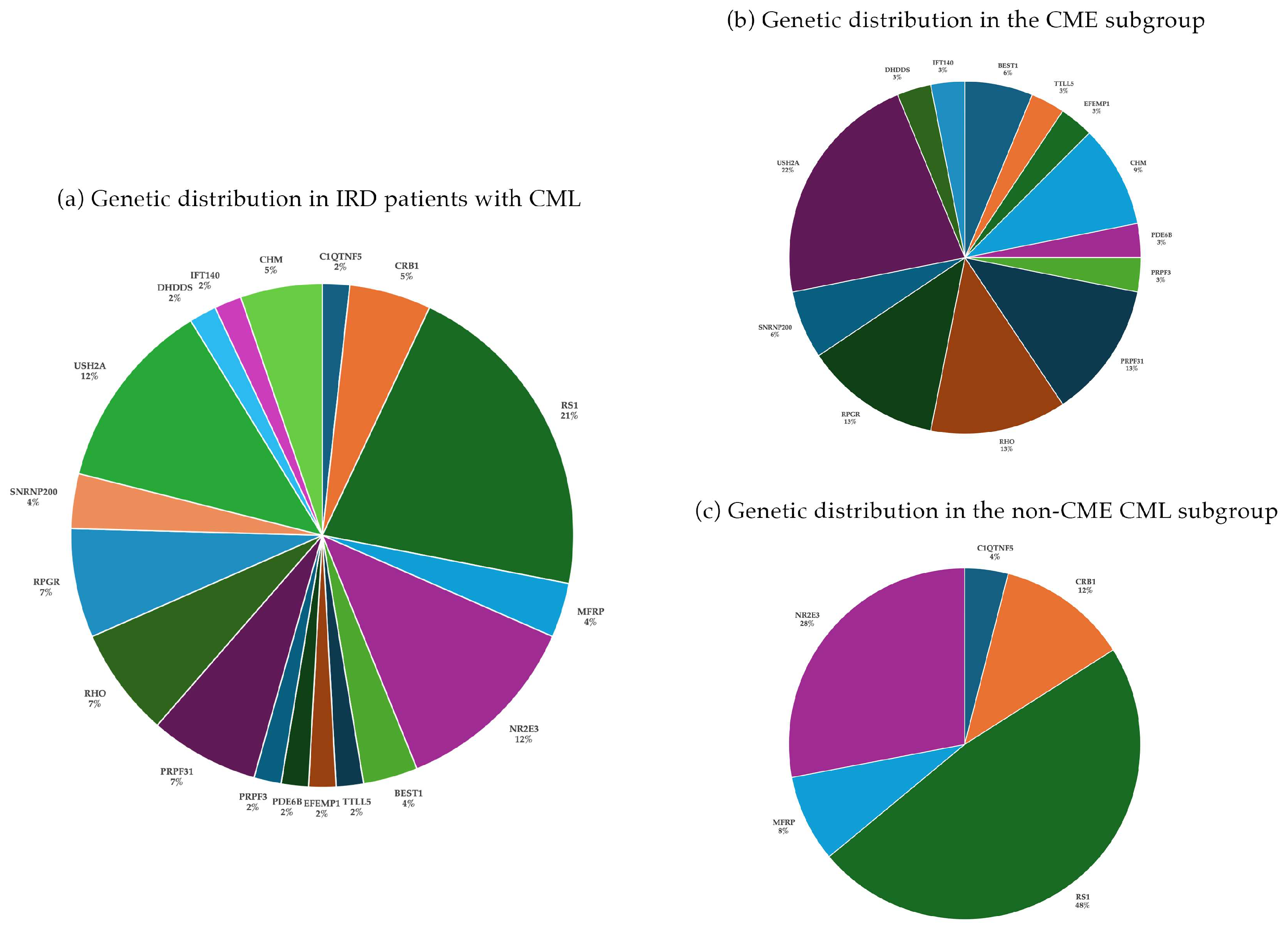
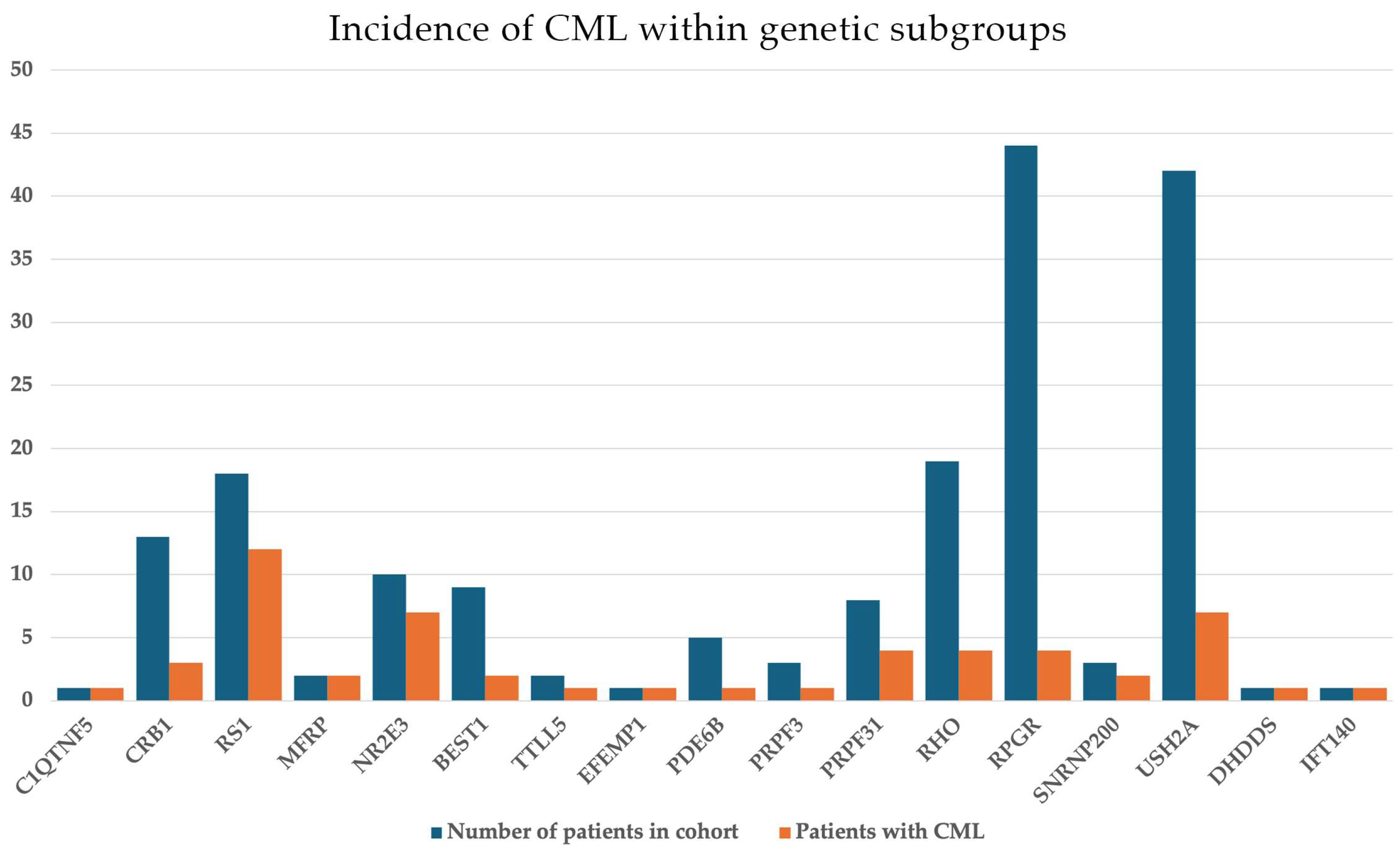
3.1. IRD Cohort: General Findings
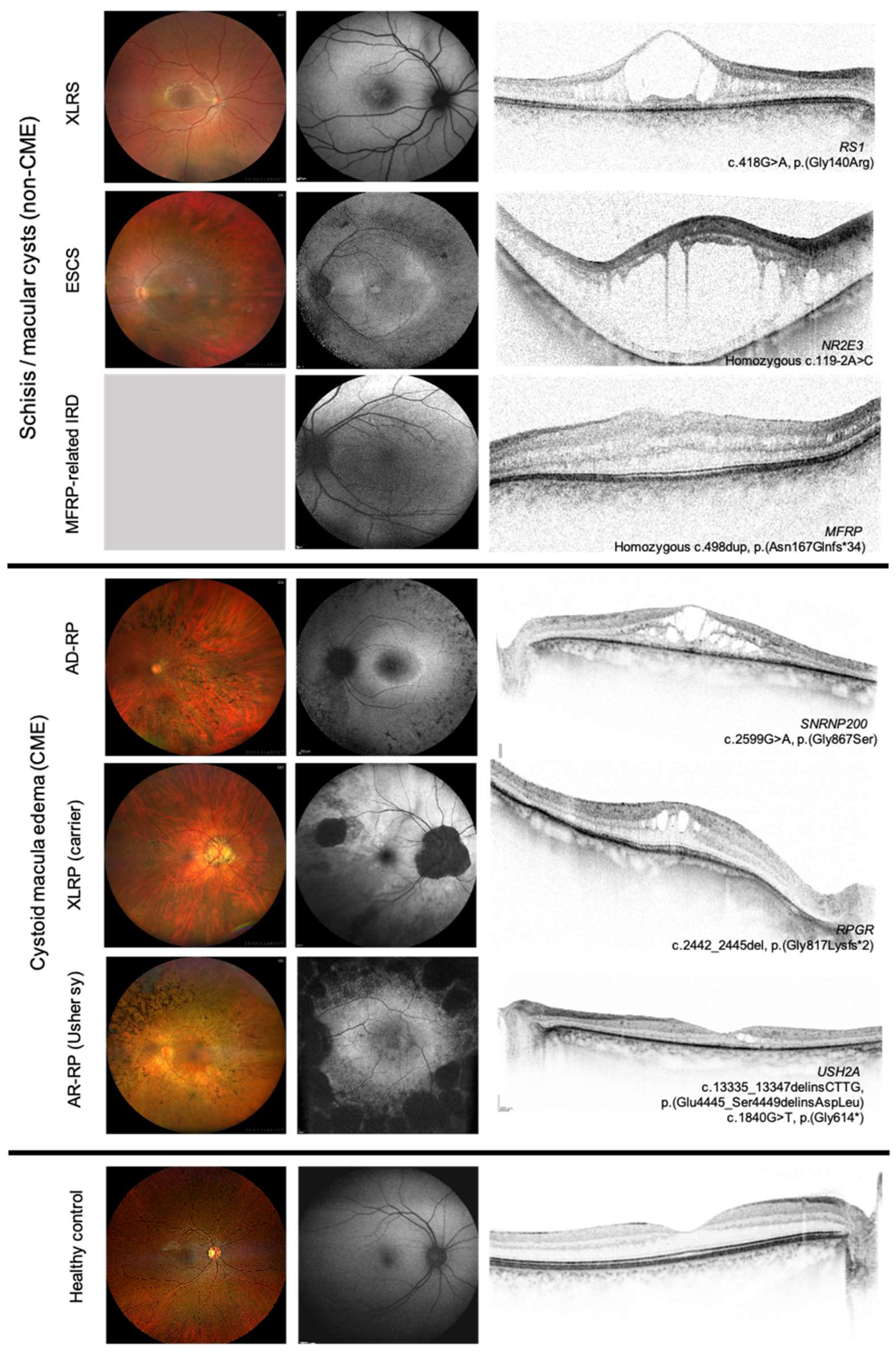
3.2. CME Cohort
3.3. Non-CME CML (Schisis-like) Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | autosomal dominant |
| AR | autosomal recessive |
| BCVA | best corrected visual acuity |
| BRB | blood retina barrier |
| CAI | carbonic anhydrase inhibitors |
| CME | cystoid macular edema |
| CML | cystoid macular lesion |
| CRT | central retinal thickness |
| ESCS | enhanced S-cone syndrome |
| EZ | ellipsoid zone |
| FA | fluorescein angiography |
| ILM | internal limiting membrane |
| INL | inner nuclear layer |
| IRD | inherited retinal disease |
| IVTA | intravitreal triamcinolone acetonide |
| LP | likely pathigenic |
| NGS | next generation sequencing |
| OCT | optical coherence tomography |
| OCTA | OCT angiography |
| OD | right eye |
| OS | left eye |
| ONL | outer nuclear layer |
| OPL | outer plexiform layer |
| P | pathogenic |
| RP | retinitis pigmentosa |
| RPE | retinal pigment epithelium |
| SD | standard deviation |
| VEGF | vascular endothelial growth factor |
| VUS | variant of uncertain significance |
| XLRP | X-linked retinitis pigmentosa |
| XLRS | X-linked retinoschisis |
References
- Sahel, J.-A.M.; Audo, I. Clinical characteristics and current therapies for inherited retinal degenerations. Cold Spring Harb. Perspect. Med. 2014, 5, a017111. [Google Scholar] [CrossRef]
- Bennett, J.; Marshall, K.A.; McCague, S.; Ashtari, M.; DiStefano-Pappas, J.; Elci, O.U.; Chung, D.C.; Sun, J.; Wright, J.F.; Cross, D.R.; et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: A follow-on phase 1 trial. Lancet 2016, 388, 661–672. [Google Scholar] [CrossRef]
- den Hollander, A.I.; Roepman, R.; Koenekoop, R.K.; Cremers, F.P.M. Leber congenital amaurosis: Genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 2008, 27, 391–419. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Cideciyan, A.V.; Ratnakaram, R.; Heon, E.; Schwartz, S.B.; Roman, A.J.; Peden, M.C.; Aleman, T.S.; Boye, S.L.; Sumaroka, A.; et al. Gene therapy for Leber congenital amaurosis caused by RPE65 mutations: Safety and efficacy in 15 children and adults followed up to 3 years. Arch. Ophthalmol. 2012, 130, 9–24. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Y.; Li, J.; Zhang, W.; Wang, J.; Lewis, R.A.; Wong, L.J. Retinal diseases caused by mutations in genes not specifically associated with the clinical diagnosis. PLoS ONE 2016, 11, e0165405. [Google Scholar] [CrossRef]
- Salvatore, S.; Fishman, G.A.; Genead, M.A. Treatment of cystic macular lesions in hereditary retinal dystrophies. Surv. Ophthalmol. 2013, 58, 560–584. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, N.A.; Khanna, Y. Neuroanatomy, Retina. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hajali, M.; Fishman, G.A.; Anderson, R.J. The prevalence of cystoid macular oedema in retinitis pigmentosa patients determined by optical coherence tomography. Br. J. Ophthalmol. 2008, 92, 1065–1068. [Google Scholar] [CrossRef]
- Richards, S.A.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Strupaitė, R.; Ašoklis, L.; Cimbalistienė, L.; Utkus, A. X-linked juvenile retinoschisis: Phenotypic and genetic characterization. Int. J. Ophthalmol. 2018, 11, 1875–1878. [Google Scholar] [CrossRef]
- Sieving, P.A.; Iannaccone, A.; Hoang, S. X-linked congenital retinoschisis. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2025. [Google Scholar]
- Sauer, C.G.; Gehrig, A.; Warneke-Wittstock, R.; Marquardt, A.; Ewing, C.C.; Gibson, A.; Lorenz, B.; Jurklies, B.; Weber, B.H.F. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat. Genet. 1997, 17, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Gaudric, A.; Ayuso, C.; Vignal, C.; Couturier, A.; Boulanger-Scemama, É.; Tadayoni, R.; Cohen, S.Y. Non-vasogenic cystoid maculopathies. Prog. Retin. Eye Res. 2022, 91, 101092. [Google Scholar] [CrossRef]
- Milam, A.H.; Radu, R.A.; Cideciyan, A.V.; Barakat, M.R.; Tang, W.; Gupta, N.; Aleman, T.S.; Wright, A.F.; Stone, E.M.; Sheffield, V.C.; et al. The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 473–478. [Google Scholar] [CrossRef]
- Hood, D.C.; Cideciyan, A.V.; Roman, A.J.; Jacobson, S.G. Enhanced S-cone syndrome: Evidence for an abnormally large number of S cones. Vis. Res. 1995, 35, 1473–1481. [Google Scholar] [CrossRef]
- Audo, I.; Manayath, G.J.; Robson, A.G.; Hawlina, M.; Vaclavik, V.; Sandbach, J.M.; Neveu, M.; Hogg, C.R.; Hunt, D.M.; Moore, A.T.; et al. Phenotypic variation in enhanced S-cone syndrome. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2082–2093. [Google Scholar] [CrossRef]
- Schorderet, D.F.; Escher, P. NR2E3 mutations in enhanced S-cone sensitivity syndrome (ESCS), Goldmann-Favre syndrome (GFS), clumped pigmentary retinal degeneration (CPRD), and retinitis pigmentosa (RP). Hum. Mutat. 2009, 30, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.B.; Jacobson, S.G.; Cideciyan, A.V.; Swiderski, R.; Streb, L.M.; Searby, C.; Beck, G.; Hockey, R.; Hanna, D.B.; Gorman, S.; et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat. Genet. 2000, 24, 127–131. [Google Scholar] [CrossRef]
- Gabrielle, P.-H.; Faivre, L.; Audo, I.; Zanlonghi, X.; Dollfus, H.; Thiadens, A.A.H.J.; Zeitz, C.; Mancini, G.M.S.; Perdomo, Y.; Mohand-Saïd, S.; et al. Cystoid maculopathy is a frequent feature of Cohen syndrome-associated retinopathy. Sci. Rep. 2021, 11, 16412. [Google Scholar] [CrossRef] [PubMed]
- Strong, S.L.; George, M.; Michaelides, M. Retinitis pigmentosa-associated cystoid macular oedema: Pathogenesis and avenues of intervention. Br. J. Ophthalmol. 2017, 101, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, A.; Stroh, E.; Manayath, G.J.; Al-Zuhaibi, S.; Levin, A.V. Macular cysts in retinal dystrophy. Curr. Opin. Ophthalmol. 2011, 22, 332–339. [Google Scholar] [CrossRef]
- Hirakawa, H.; Gomi, T. Optical coherence tomography of cystoid macular edema associated with retinitis pigmentosa. Am. J. Ophthalmol. 1999, 128, 185–191. [Google Scholar] [CrossRef]
- Battaglia Parodi, M.; Staurenghi, G.; Triolo, G.; Riccieri, F.; Pierro, L.; Gagliardi, M.; Bandello, F. Correlation of SD-OCT findings and visual function in patients with retinitis pigmentosa. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, Y.; Mitamura-Aizawa, S.; Katome, T.; Naito, T.; Hagiwara, A.; Kumagai, K.; Yamamoto, S. Photoreceptor impairment and restoration on optical coherence tomographic image. J. Ophthalmol. 2013, 2013, 518170. [Google Scholar] [CrossRef]
- Jia, Y.; Bailey, S.T.; Hwang, T.S.; McClintic, S.M.; Gao, S.S.; Pennesi, M.E.; Flaxel, C.J.; Lauer, A.K.; Wilson, D.J.; Hornegger, J.; et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc. Natl. Acad. Sci. USA 2015, 112, E2395–E2402. [Google Scholar] [CrossRef]
- Iovino, C.; Costa, C.M.; Pisani, D.; Damiano, L.; Di Iorio, V.; Testa, F.; Simonelli, F. Clinical applications of optical coherence tomography angiography in inherited retinal diseases: An up-to-date review of the literature. J. Clin. Med. 2023, 12, 3170. [Google Scholar] [CrossRef]
- Tao, Z.; Bu, S.; Liang, L.; Yang, Y.; She, K.; Lu, F. Visual acuity-related outer retinal structural parameters on swept source optical coherence tomography and angiography in XLRS patients and carriers. Transl. Vis. Sci. Technol. 2023, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Adackapara, C.A.; Stelzer, J.S.; Dibernardo, C.W.; Melia, B.M.; Dagnelie, G. Prevalence of cystoid macular edema and stability in OCT retinal thickness in eyes with retinitis pigmentosa during a 48-week lutein trial. Retina 2008, 28, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Xiao, X.; Peng, X. Management of cystoid macular edema in retinitis pigmentosa: A systematic review and meta-analysis. Front. Med. 2022, 9, 895208. [Google Scholar] [CrossRef]
- Jimenez-Davila, H.J.; Pacheco-Avalanche, C.R.A.; Klufas, M.A. A review of retinitis pigmentosa. Rev. Ophthalmol. 2022. [Google Scholar]
- Kim, C.; Chong, H.; Yu, H.G. Association of p.P347L in the rhodopsin gene with early-onset cystoid macular edema in patients with retinitis pigmentosa. Ophthalmic Genet. 2012, 33, 96–99. [Google Scholar] [CrossRef]
- Pisani, D.; Korb, M.; Di Iorio, V.; Cantile, L.; Madonna, E.; Niccolò, A.; Colucci, R.; De Benedictis, A.; Melillo, P.; Testa, F.; et al. Cystoid macular edema in patients with retinitis pigmentosa and its genetic association. In Proceedings of the ARVO Annual Meeting, Seattle, WA, USA, 5–9 May 2024. [Google Scholar]
- Gallo, B.; Capelli, R.; De Benedictis, A.; Brunetti-Pierri, R.; Melillo, P.; Rossi, S.; Testa, F.; Azzolini, C.; Della Corte, M.; Simonelli, F. Prevalence of macular abnormalities assessed by optical coherence tomography in patients with Usher syndrome. In Proceedings of the ARVO Annual Meeting, Orlando, FL, USA, 25–29 September 2016. [Google Scholar]
- Arrigo, A.; Aragona, E.; Battaglia Parodi, M.; Bandello, F. Quantitative approaches in multimodal fundus imaging: State of the art and future perspectives. Prog. Retin. Eye Res. 2023, 92, 101111. [Google Scholar] [CrossRef]
- Bakthavatchalam, M.L.; Prakash, F.H.; Rong, S.S.; Ng, D.S.; Brelen, M.E. Treatment of cystoid macular edema secondary to retinitis pigmentosa: A systematic review. Surv. Ophthalmol. 2018, 63, 329–339. [Google Scholar] [CrossRef] [PubMed]
- The Retinoschisis Consortium. Functional implications of the spectrum of mutations found in 234 cases with X-linked juvenile retinoschisis. Hum. Mol. Genet. 1998, 7, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Retinal Physician. Hereditary Retinal Degenerations and Cystoid Macular Edema. Available online: https://www.retinalphysician.com/issues/2017/october/hereditary-retinal-degenerations-and-cystoid-macular-edema/ (accessed on 1 October 2017).
- Ruff, A.; Tezel, A.; Tezel, T.H. Anatomical and Functional Correlates of Cystic Macular Edema in Retinitis Pigmentosa. PLoS ONE 2022, 17, e0276629. [Google Scholar] [CrossRef] [PubMed]
| Group | Patients (n) | % | Mean BCVA (Decimal ± SD) | Mean CRT (µm ± SD) | Correlation Between BCVA and CRT (rS) |
|---|---|---|---|---|---|
| CME | 32 | 56% | 0.43 ± 0.3 | 401 ± 182 | 0.36 |
| Non-CME | 25 | 44% | 0.40 ± 0.2 | 465 ± 258 | −0.05 |
| Total cohort | 57 | 100% | 0.42 ± 0.26 | 430 ± 220 | 0.34 |
| Inheritance Pattern | Patients (n) | % of Total | Bilateral CML n (%) | Unilateral CML n (%) |
|---|---|---|---|---|
| Autosomal Dominant | 14 | 24.6% | 9 (64.3%) | 5 (35.7%) |
| Autosomal Recessive | 24 | 42.1% | 13 (54.2%) | 11 (45.8%) |
| X-linked | 19 | 33.3% | 14 (73.7%) | 5 (26.3%) |
| Total cohort | 57 | 100% | 36 (63.1%) | 21 (36.9%) |
| Patient ID | Gene | Age (ys) | Gender | BCVA OD | BCVA OS | CRT OD (µm) | CRT OS (µm) | Genetic Variants | ACMG Classification |
|---|---|---|---|---|---|---|---|---|---|
| 26 | DHDDS | 48 | F | 0.5 | 0.9 | 409 | 376 | c.124A>G, p.(Lys42Glu) homozygous | P |
| 27 | IFT140 | 43 | M | 0.5 | 322 | c.1565G>A, p.(Gly522Glu) c.3788C>T, p.(Pro1263Leu) | P VUS | ||
| 28 | PDE6B | 32 | M | 0.15 | 0.016 | 265 | 262 | c.385G>A, p.(Glu129Lys) homozygous | P |
| 29 | USH2A | 32 | F | 1.0 | 1.0 | 349 | 365 | c.8682-9A>G c.2299del, p.(Glu767Serfs*21) | P P |
| 30 | USH2A | 37 | F | 0.3 | 0.2 | 421 | 547 | c.11048-2A>G c.7595-2144A>G | P P |
| 31 | USH2A | 27 | M | 0.6 | 0.6 | 754 | 762 | c.7595-2144A>G c.582del, p.(Leu194Phefs*5) | P LP |
| 32 | USH2A | 67 | M | 0.3 | 319 | c.13335_13347delinsCTTG, c.1840G>T, p.(Gly614*) | P LP | ||
| 33 | USH2A | 21 | F | 0.6 | 0.7 | 507 | 591 | c.11864G>A, p.(Trp3955*) c.9424G>T, p.(Gly3142*) | P P |
| 34 | USH2A | 30 | F | 0.8 | 0.6 | 366 | 345 | c.11864G>A, p.(Trp3955*) c.(4627+1_4628-1)_(4987+1_4988-1)del | P P |
| 35 | USH2A | 62 | F | 0.01 | 197 | c.11864G>A, p.(Trp3955*) c.14621C>G, p.(Ser4874*) | P LP | ||
| 36 | RPGR | 51 | M | 0.1 | 184 | c.2293G>T, p.(Glu765*) hemizygous | P | ||
| 37 | RPGR | 23 | M | 0.25 | 565 | c.1415-9A>G hemizygous | P | ||
| 38 | RPGR | 59 | F | 0.3 | 0.4 | 587 | 524 | c.3067G>A, p.(Gly1023Arg) heterozygous | VUS |
| 39 | RPGR | 46 | M | 0.25 | 0.3 | 277 | 296 | c.2237_2238del, p.(Glu746Glyfs*23) hemizygous | P |
| 40 | SNRNP200 | 41 | F | 0.5 | 0.4 | 483 | 553 | c.2599G>A, p.(Gly867Ser) heterozygous | VUS |
| 41 | SNRNP200 | 44 | F | 0.016 | 334 | c.2599G>A, p.(Gly867Ser) heterozygous | VUS | ||
| 42 | RHO | 19 | M | 0.6 | 0.6 | 834 | 838 | c.1040C>T, p.(Pro347Leu) heterozygous heterozygous | P |
| 43 | RHO | 58 | M | 1.0 | 0.3 | 349 | 308 | c.541G>A, p.(Glu181Lys) heterozygous | P |
| 44 | RHO | 31 | F | 0.2 | 0.2 | 525 | 935 | c.50C>T, p.(Thr17Met) heterozygous | P |
| 45 | RHO | 50 | M | 0.2 | 0.1 | 274 | 304 | c.512C>T, p.(Pro171Leu) heterozygous | P |
| 46 | PRPF31 | 30 | F | 0.8 | 0.7 | 262 | 322 | c.1040del, p.(Leu347Argfs*16) heterozygous | P |
| 47 | PRPF31 | 53 | F | 0.12 | 372 | c.469C>T, p.(Gln157*) heterozygous | P | ||
| 48 | PRPF31 | 54 | F | 0.5 | 0.6 | 381 | 376 | c.1040del, p.(Leu347Argfs*16) heterozygous | P |
| 49 | PRPF31 | 54 | F | 0.016 | 234 | c.58G>T, p.(Gly20*) heterozygous | LP | ||
| 50 | PRPF3 | 21 | F | 0.7 | 1.0 | 396 | 336 | c.1481C>T, p.(Thr494Met) heterozygous | P |
| 51 | BEST1 | 40 | F | 0.25 | 198 | c.203A>G, p.(Tyr68Cys) heterozygous | LP | ||
| 52 | BEST1 | 31 | F | 0.5 | 0.5 | 208 | 217 | c.920C>A, p.(Thr307Asn) heterozygous | LP |
| 53 | CHM | 36 | M | 0.5 | 401 | c.525_526del, p.(Glu177Lysfs*6) hemizygous | P | ||
| 54 | CHM | 47 | M | 0.3 | 1 | 494 | 382 | c.534del, p.(Glu179Lysfs*18) hemizygous | LP |
| 55 | CHM | 38 | M | 0.5 | 292 | c.1153del, p.(Gln385Serfs*24) hemizygous | P | ||
| 56 | EFEMP1 | 49 | F | 0.1 | 172 | c.1033C>T, p.(Arg345Trp) heterozygous | P | ||
| 57 | TTLL5 | 50 | M | 0.2 | 119 | c.2132_2135dup, p.(Met712Ilefs*15) c.1058A>G, p.(Asp353Gly) | P VUS |
| Patient ID | Gene | Age (ys) | Gender | BCVA OD | BCVA OS | CRT OD (µm) | CRT OS (µm) | Genetic Variants | ACMG Classification |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MFRP | 20 | M | 0.3 | 0.25 | 476 | 485 | c.498dup, p.(Asn167Glnfs*34) homozygous | P |
| 2 | MFRP | 4 | M | 0.3 | 740 | c.498dup, p.(Asn167Glnfs*34) homozygous | P | ||
| 3 | C1QTNF5 | 72 | M | 0.002 | 185 | c.489C>A, p.(Ser163Arg) heterozygous | P | ||
| 4 | RS1 | 15 | M | 0.4 | 0.50 | 709 | 686 | c.214G>A, p.(Glu72Lys) hemizygous | P |
| 5 | RS1 | 10 | M | 0.3 | 0.25 | 312 | 593 | c.574C>T, p.(Pro192Ser) hemizygous | P |
| 6 | RS1 | 10 | M | 0.6 | 0.50 | 392 | 391 | c.214G>A, p.(Glu72Lys) hemizygous | P |
| 7 | RS1 | 7 | M | 0.4 | 0.25 | 443 | 485 | c.214G>A, p.(Glu72Lys) hemizygous | P |
| 8 | RS1 | 30 | M | 0.2 | 0.50 | 219 | 702 | c.665dup, p.(Cys223Valfs*41) hemizygous | P |
| 9 | RS1 | 27 | M | 0.4 | 0.4 | 334 | 323 | c.665dup, p.(Cys223Valfs*41) hemizygous | P |
| 10 | RS1 | 20 | M | 0.15 | 0.125 | 483 | 792 | c.214G>A, p.(Glu72Lys) hemizygous | P |
| 11 | RS1 | 24 | M | 0.5 | 0.5 | 268 | 306 | c.527T>C, p.(Phe176Ser) hemizygous | P |
| 12 | RS1 | 19 | M | 0.7 | 0.7 | 379 | 341 | c.522+1G>A hemizygous | P |
| 13 | RS1 | 5 | M | 0.6 | 441 | c.527T>C, p.(Phe176Ser) hemizygous | LP | ||
| 14 | RS1 | 19 | M | 0.8 | 0.4 | 561 | 618 | c.574C>T, p.(Pro192Ser) hemizygous | P |
| 15 | RS1 | 76 | M | 0.25 | 0.32 | 449 | 528 | c.610C>A, p.(Leu204Met) hemizygous | VUS |
| 16 | NR2E3 | 63 | F | 0.25 | 0.3 | 200 | 265 | c.62T>G, p.(Leu21Arg) homozygous | P |
| 17 | NR2E3 | 69 | F | 0.25 | 257 | c.119-2A>C homozygous | P | ||
| 18 | NR2E3 | 67 | F | 0.6 | 0.8 | 347 | 278 | c.227G>A, p.(Arg76Gln) c.352G>C, p.(Val118Leu) | P LP |
| 19 | NR2E3 | 37 | F | 0.016 | 0.1 | 1614 | 1093 | c.119-2A>C homozygous | P |
| 20 | NR2E3 | 32 | M | 0.3 | 1.0 | 336 | 328 | c.481del, p.(Thr161Hisfs*18) c.932G>A, p.(Arg311Gln) | P P |
| 21 | NR2E3 | 30 | F | 0.6 | 0.9 | 446 | 417 | c.227G>A, p.(Arg76Gln) homozygous | P |
| 22 | NR2E3 | 76 | M | 0.5 | 451 | c.227G>A, p.(Arg76Gln) homozygous | P | ||
| 23 | CRB1 | 28 | F | 0.1 | 347 | c.2843G>A, p.(Cys948Tyr) c.2101C>T, p.(Pro701Ser) | P VUS | ||
| 24 | CRB1 | 32 | M | 0.02 | 245 | c.2843G>A, p.(Cys948Tyr) c.2101C>T, p.(Pro701Ser) | P VUS | ||
| 25 | CRB1 | 44 | F | 0.5 | 291 | c.498_506del, p.(Ile167_Gly169del) c.2843G>A, p.(Cys948Tyr) | P P |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asboth, B.; Sanrocco, A.; Besztercei, B.; Lesch, B.; Takacs, A.; Vamos, R.; Varsanyi, B.; Vegh, A.; Knezy, K.; Szabo, V.; et al. Cystoid Macular Lesions in Inherited Retinal Diseases: Prevalence, Characteristics, and Genetic Associations in a Hungarian Cohort. Genes 2025, 16, 1212. https://doi.org/10.3390/genes16101212
Asboth B, Sanrocco A, Besztercei B, Lesch B, Takacs A, Vamos R, Varsanyi B, Vegh A, Knezy K, Szabo V, et al. Cystoid Macular Lesions in Inherited Retinal Diseases: Prevalence, Characteristics, and Genetic Associations in a Hungarian Cohort. Genes. 2025; 16(10):1212. https://doi.org/10.3390/genes16101212
Chicago/Turabian StyleAsboth, Barbara, Alessandra Sanrocco, Barbara Besztercei, Balazs Lesch, Agnes Takacs, Rita Vamos, Balazs Varsanyi, Andras Vegh, Krisztina Knezy, Viktoria Szabo, and et al. 2025. "Cystoid Macular Lesions in Inherited Retinal Diseases: Prevalence, Characteristics, and Genetic Associations in a Hungarian Cohort" Genes 16, no. 10: 1212. https://doi.org/10.3390/genes16101212
APA StyleAsboth, B., Sanrocco, A., Besztercei, B., Lesch, B., Takacs, A., Vamos, R., Varsanyi, B., Vegh, A., Knezy, K., Szabo, V., Nagy, Z. Z., & Zobor, D. (2025). Cystoid Macular Lesions in Inherited Retinal Diseases: Prevalence, Characteristics, and Genetic Associations in a Hungarian Cohort. Genes, 16(10), 1212. https://doi.org/10.3390/genes16101212






