Abstract
In most animals, transient increases of extracellular ATP (ATPe) are used for physiological signaling or as a danger signal in pathological conditions. ATPe dynamics are controlled by ATP release from viable cells and cell lysis, ATPe degradation and interconversion by ecto-nucleotidases, and interaction of ATPe and byproducts with cell surface purinergic receptors and purine salvage mechanisms. Infection by protozoan parasites may alter at least one of the mechanisms controlling ATPe concentration. Protozoan parasites display their own set of proteins directly altering ATPe dynamics, or control the activity of host proteins. Parasite dependent activation of ATPe conduits of the host may promote infection and systemic responses that are beneficial or detrimental to the parasite. For instance, activation of organic solute permeability at the host membrane can support the elevated metabolism of the parasite. On the other hand ecto-nucleotidases of protozoan parasites, by promoting ATPe degradation and purine/pyrimidine salvage, may be involved in parasite growth, infectivity, and virulence. In this review, we will describe the complex dynamics of ATPe regulation in the context of protozoan parasite–host interactions. Particular focus will be given to features of parasite membrane proteins strongly controlling ATPe dynamics. This includes evolutionary, genetic and cellular mechanisms, as well as structural-functional relationships.
Keywords:
parasite; membrane proteins; host–parasite interaction; transport; pathogenesis; evolution 1. Introduction
Adenosine 5′ triphosphate, ATP, is usually described as an essential energy source for cells. ATP hydrolysis provides the energy required for many chemical reactions of metabolism, acts as a precursor of a number of essential enzyme cofactors, such as nicotinamide adenine dinucleotide (NAD) and coenzyme A, and is the source of the phosphoryl group in most kinase-mediated phosphorylation reactions [1,2]. ATP is also used to synthetize cyclic adenosine monophosphate (cAMP), a major second messenger involved in the regulation of numerous cellular processes.
A less known function for ATP is to act as an extracellular signaling molecule, allowing cells, tissues, and organisms to communicate [3]. In addition, several studies during the last 30 years show that extracellular ATP (ATPe) is involved in modulating a vast array of cellular functions, such as neurotransmission, secretion of pro-inflammatory mediators, apoptosis, chemotaxis, cell differentiation, and vasodilatation [3].
In cells and organisms, ATPe is subjected to a sophisticated system of regulation. At least four processes are required to understand ATPe regulation at the cellular level: (1) ATPe, and/or some of its breakdown products can interact with specific purinergic receptors (P receptors), thus triggering ionotropic and/or metabotropic cellular responses [3]; (2) intracellular ATP can be released by cell rupture (cytolysis) or by regulated mechanisms involving membrane transport proteins [4,5]; (3) ATPe can be metabolized by specific membrane bound enzymes collectively called ecto-nucleotidases [6,7]; (4) full dephosphorylation of ATPe and other extracellular nucleotides leads to accumulation of nucleosides, which promotes uptake of these molecules by nucleoside salvage mechanisms [8]. The mechanisms involved in ATPe regulation are usually cell type and species specific, and may often change according to the pathophysiological context [3]. The picture turns even more complex during parasite infection of targeted hosts [9,10].
At the evolutionary level, a continuous crosstalk exists between the parasite and the host leading to adaptive changes allowing parasites to invade, grow and survive inside the host, in an attempt to subvert host defense mechanisms to ensure development of the infection [11].
Such changes may include adapting host molecules, metabolic pathways and transporters for their specific needs, and remodeling host membranes to avoid clearance. Moreover, parasites undergo successive stages during their life cycle and may alternate between different hosts and tissues, resulting in diverse combinations of host–parasite interactions. At the physiological level, on the other hand, molecular, cellular and systemic adjustments can be relatively fast.
In humans, while transient increases of ATPe are involved in physiological signaling, strong increases of ATPe concentration can occur in seconds, and serve as a danger signal in pathological conditions, triggering a complex immunological and inflammatory response aimed at recognizing and controlling intracellular pathogens [10,12]. In this context, infection by protozoan parasites has been shown to alter host ATPe dynamics, by promoting changes in at least one of the above-mentioned mechanisms controlling ATPe concentration. Protozoans may display their own set of proteins directly altering ATPe dynamics or control the activity of host proteins. Parasite-dependent activation of ATPe conduits [13,14] of the host may promote infection and systemic responses that are beneficial or detrimental to the parasite [15,16,17], while protozoan ecto-nucleotidases can affect parasite growth, infectivity and virulence [18,19]. Both actions on ATP efflux and ATPe hydrolysis mediate cellular responses by altering the availability of nucleotide ligands involved in purinergic signaling of the host.
In this review, a special focus is given to protozoan proteins involved in mechanistic interactions that regulate the homeostasis of ATPe and byproducts as well as the consequences of this regulation for the parasite and the host. When information on specific protozoan proteins is scarce, a brief description of related groups was included.
2. Purinergic Signaling
Signaling by P receptors appeared early in evolution as a form of chemical intercellular communication, possibly with the first unicellular eukaryotic cells [2]. Further evolution in multicellular organisms resulted in the development of multiple classes of P receptors. Cloning of P receptors [20] allowed for the production of polyclonal antisera for immunohistochemical studies of their expression and distribution, showing that most receptors were located on the cell surface, though in a few cases intracellular immunostaining was observed (see [21]).
The P indicates that these receptors can be activated by purine or pyrimidine nucleotides (P2) or by the nucleoside adenosine (P1). P2 receptors are separated into two major subfamilies, P2X and P2Y. Several comprehensive reviews summarize the accumulated knowledge on these proteins [3,22]. Cloned subtypes include seven P2X receptors (P2X1–7), eight P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11–14) and four P1 receptors (A1, A2A, A2B, and A3) [23,24]. P2Y and P1 receptors are metabotropic G-protein coupled receptors. Binding of di- or trinucleotides (P2Y) or adenosine (P1) triggers second messengers which in turn will activate specific protein kinases coupled to downstream signaling mechanisms [3].
As described in Section 4, extracellular metabolism of nucleotides facilitated by membrane bound ecto-nucleotidases will enable the accumulation of several purine and pyrimidine di- and trinucleotides and nucleosides. The effective concentration of these molecules at the cell surface will produce metabolic and physiological responses by interacting with specific subtypes of P2Y and P1 receptors [25,26].
In contrast to P2Y and P1, P2X receptors are ionic channels activated only by ATPe, its only natural ligand [22]. In this section, we will focus mainly on P2X receptors, since these are assumedly the first to appear in protozoa, according to the limited but consistent genomic, pharmacological and biophysical information [2,27]. Most P2X channels are cation-selective and discriminate poorly among Na+, K+, and Ca2+, the main diffusible cations of eukaryotes. Transmembrane transport of these ions is an important signaling mechanism, because it may alter the plasma membrane potential as well as local ion concentrations [20].
Transcripts and/or proteins of P2X subunits have been found in most mammalian tissues and are being increasingly discovered in several non-vertebrate species [28]. They exhibit a common topology with two transmembrane domains, a large ATPe binding loop, and intracellular N-and C-termini of variable lengths [29]. The ecto-domain is glycosylated and includes ten Cys residues conserved among all vertebrate receptors, bound in five disulphide bridges contributing to the tertiary structure of the receptor [20].
P2X receptors are able to form homo-and heterotrimeric complexes [30,31] around a central pore [32,33], and may be regulated by post-translational glycosylation and phosphorylation [34]. The trimeric structure was confirmed by atomic force microscopy, electron microscopy, single particle analysis, and by crystallization of the P2X4 subtype from zebrafish [28], which agrees well with ion conductance recordings suggesting that three molecules of ATP are required for channel gating [35,36].
2.1. P2X Receptors. Evolution and Early Appearance in Protozoa
As mentioned earlier, seven mammalian P2X cDNAs have been cloned [28]. Based on sequence identity and pharmacological profile, most receptors of vertebrate species seem to be orthologs of these receptors but low sequence homology has made difficult to determine potential homologues in non-vertebrate organisms. Currently, no prokaryotic P2X receptor has been identified, suggesting that structurally different ATP receptors evolved in bacteria [28,37]. P2X receptors appear to be absent in several invertebrates such as Caenorhabditis elegans, Drosophila melanogaster, Anopheles gambiae, and Apis mellifera [28], although extracellular nucleotides induce various cellular responses in tissues of these animals [38]. On the other hand, P2X-like receptors were identified in the unicellular algae Ostreococus tauri [39]. In vascular plants, although genomic sequence-based searches for canonical P2 receptors failed to detect candidate ATP receptors [40], ATPe and other extracellular nucleotides are able to trigger several cellular and systemic responses [41]. Recently, a lectin receptor kinase was found in Arabidopsis thaliana, which binds ATPe with high affinity and is required for ATPe-induced intracellular Ca2+ increase [40].
Alignment of P2X proteins from a diverse range of species suggests that the P2X1-7 receptor subtypes resulted from a lineage-specific gene expansion [42]. Similar but independent lineage specific expansions of P2X receptor subtypes may have occurred in amoeba Dicthiosthelium discoideum and algae O. tauri. D. discoideum possesses five P2X-like sequences, while O. tauri exhibits an identified P2X receptor together with three further open reading frames encoding proteins assumed to be distantly related to P2X receptors [43].
The protein sequences, kinetics and pharmacology of protozoan P2X-like receptors do not seem to correspond to a specific P2X receptor subtype [39,43,44,45], which is not surprising considering that development of the seven mammalian P2X receptor genes appeared to be a relatively recent phenomenon occurring after the branching between vertebrates and invertebrates [28]. Sensing nucleotides has adaptive value for protozoans, since they control a wide range of different processes like cilia beating, swimming, and chemotaxia in Paramecium [46] and Tetrahymena [47]; induction of parasitosis in Trypanosoma cruzi [48]; vacuole contraction in Amoeba proteus [49]; changes in the cytoskeletal organisation in Physarum [50]; and phagocytosis in Dicthiosthelium [42].
2.1.1. A Brief Tale of Three Protozoans
Monosiga brevicollis
Choanoflagellates are among the closest unicellular relatives of animals and provide important insights into the origin and diversity of animal phyla. These protozoa constitute the major component of aquatic microbial foodwebs [27,51]. The genome of the marine choanoflagellate, Monosiga brevicollis, has been sequenced in 2008 [52]. Because of horizontal gene transfer, >400 genes are likely derived from algae and prokaryotes, accounting for ≈4% of Monosiga nuclear genome [52]. Interestingly, among the 42 Mb genome containing approximately 9200 predicted protein coding genes, a P2X-like receptor (named MBP2X) was detected. When expressed in human embryonic kidney cells (HEK-293), MBP2X forms a functional ATP activated ion channel, which may be implicated in flagella driven swimming or feeding of this organism [27], though a biophysical/phamacological characterization remains to be performed.
Dictyostelium discoideum
A P2X-like gene encoding protein DDB0168616 appeared in D. discoideum [43], an amoeboid species whose genome was fully sequenced in 2005 [53].
Expression of a humanized version of this cDNA in HEK-293 cells showed that this gene (denoted as D. discoideum p2xA) encoded a membrane ion channel (DdP2X) activated by micromolar concentrations of ATPe as well as slow-degradable ATP analogues [43]. In HEK-293 cells, ATPe elicited whole-cell currents in a dose-dependent manner, with kinetic properties resembling human P2X2 or P2X4 receptors, although typical purinergic blockers did not inhibit the response [43]. On the other hand, site-directed mutagenesis revealed partial conservation of structure–function relations with P2X receptors of higher organisms. For example, as in mammalian P2X receptors, two lysine residues in the receptor ecto-domain contribute to ATP binding and a C-terminus YXXXK motif is involved in receptor stabilization at the plasma membrane [33,43]. Moreover, expression of the recombinant receptor in mammalian cells suggests conservation of trimer formation—similar to homologs of vertebrates—by DdP2X [43]. A green fluorescent protein (GFP)-tagged version of DdP2X was localized to the intracellular membranes of D. discoideum, mostly in the contractile vacuole, a specialized organelle in osmoregulation. Thus, DdP2X functions on intracellular organelles, a feature observed in mammal cells after constitutive or ligand-activated endocytosis of surface receptors [21]. DdP2X is oriented with the ATP binding site facing the vacuole lumen, thereby being in principle able to sense changes in luminal ATP. Targeted disruption of DdP2X resulted in cells being unable to regulate cell volume when challenged by hypotonicity [43]. A similar P2X dependent regulation of cell volume can be found in human red blood cells (RBCs), where surface located P2X receptors subtypes control cell volume by modulating the net flux of cations [54,55].
Interestingly, although DdP2X is strictly intracellular, D. discoideum also exhibits purinergic signaling induced by extracellular purine nucleotides [42], i.e, exposure to ATPe or ADPe trigger changes in intracellular Ca2+ content, which are comparable to the role for P2 receptors in vertebrate cells [56]. More recently, Sivaramakrishnan and Fountain (2015, [57]) showed that Dictyostelium uses ATPe to regulate cell volume. In most eukaryotic cells from multicellular organisms, swelling leads to intracellular ATP release. The accumulated ATPe, and/or its metabolic byproducts, interacts with P receptors mediating a decrease of cell volume [25,26]. Although a similar response sequence is observed in D. discoideum challenged by hypotonicity, the genome of this protozoan yields no information suggesting a putative cell surface P2 receptor [57].
Plasmodium falciparum, a Parasitic Protozoon
Plasmodium falciparum is the most dangerous etiological agent of human malaria [58]. After entering into the human body, P. falciparum first undergoes a developmental stage in the liver before invading RBCs, where the parasite grows and matures [59]. Classical antimalarial therapy is directed against the intraerythrocytic stage [60], which produces all symptoms of the disease [59]. The merozoite (parasite invading phenotype) invades the RBC and grows and replicates within the parasitophorous vacuole (PV), undergoing development through well-characterized stages of ring, trophozoite, and schizont. Subsequently, the infected RBC ruptures, releasing new merozoites that in turn infect more RBCs [59].
Sequencing of the parasite genome [61] revealed approximately 60% of the predicted genes not found in other organisms, thus hampering the search for homologous genes. This genomic diversity is in part due to the extreme evolutionary divergence of the phylum Apicomplexa, but also to species-specific features, with proteins in P. falciparum differing from those of the other closely related Plasmodia species [62].
Plasmodia spp. employ unique proteins involved in highly specialized processes, such as passage through blood vessels, evasion of the host immune system, and invasion and development within host cells [63]. To facilitate intracellular growth, P. falciparum hijacks host organelles for nutrient uptake, adapts the parasitophorous vacuole membrane (PVM) and the host membrane by exporting many proteins, and increasing erythrocyte plasma membrane (EPM) permeability to a wide variety of organic and inorganic solutes [64,65,66] (see Figure 1).
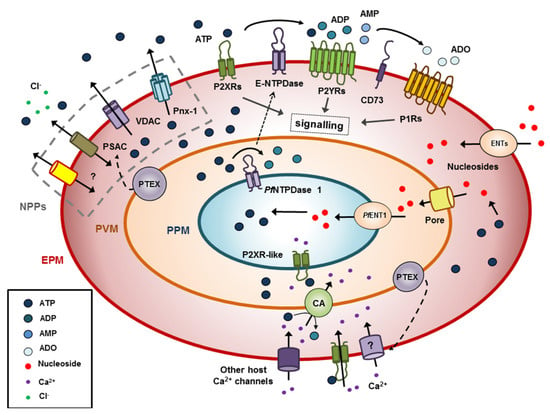
Figure 1.
Extracellular ATP (ATPe) dynamics of Plasmodium falciparum infected erythrocytes. The parasite induces a general increase of solute permeability at the host cell membrane (EPM; erythrocyte plasma membrane), via new permeability pathways (NPPs), which includes an increase of ATP efflux mediated by several proteins like pannexin 1 (Pnx-1), anionic channels such as voltage-dependent anion channel (VDAC) coupled to partner proteins, generic solute pores as plasmodial surface anion channel (PSAC) and novel parasite proteins yet to be discovered. ATPe hydrolysis by red blood cells (RBCs) E-NTPDases (ecto-nucleoside triphosphate diphosphohydrolases) is extremely low but strongly increases under infection, though participation of parasite NTPDases at the host membrane remains to be proven. Still, ATPe and its metabolic byproducts should be able to activate functional P1 and P2 receptors of the host, triggering intracellular signaling. In uninfected RBCs, part of this signaling was shown to affect ATP efflux. On the other hand, accumulation of nucleosides (due to hydrolase activities of E-NTPDases and CD73) will promote their uptake by host equilibrative nucleotide transporters (ENT). Nucleosides will then transverse both unspecific vacuolar pores (Pore) of the parasitophorous vacuole membrane (PVM) and PfENT1 of the parasite plasma membrane (PPM) to gain access to the parasite. Nucleosides will then serve as substrates in the parasite pathway for de novo synthesis of nucleotides. The parasite traslocon PTEX was shown to transport parasite protein(s) to the host membrane, EPM, upregulating Ca2+ uptake. Once in the RBC cytoplasm, Ca2+ may be transported through the parasitophorous vacuole (PV) by a calcium ATPase transporter, potentially reaching the parasite cytoplasm through a P2X-like receptor conductance. On the other hand PTEX may be involved in the translocation of parasite proteins to the NPPs, as e.g., PSAC.
The challenge for the parasite is to widely and unspecifically raise membrane solute traffic but excluding Na+, whose permeability should be kept low to avoid RBC lysis. Different proteins were proposed to be involved in these new permeability pathways (NPPs) induced by parasite infection. Among them, in the last years new experimental data support a crucial role for the plasmodial surface anion channel (PSAC) [66,67,68], associated with different proteins from Clag and RhopH families [67]. However, an alternative, non-exclusive hypothesis states that infection activates host proteins or protein complexes, which were expressed but inactive or weakly active in the uninfected RBC [69].
Amongst inorganic ions with increased permeability, Ca2+ uptake proved to be the most critical for intracellular parasite performance [63], since the P. falciparum expresses multiple Ca2+ effector proteins like calmodulin, Ca2+-dependent protein kinases, phosphatases such as calcineurin, and other Ca2+-binding proteins [70]. Early works have shown that both parasite invasion and the propagation of in vivo infected RBCs cultures required the presence of extracellular calcium [71,72]. At rest, P. falciparum, as most eukaryotic cells, exhibits submicromolar intracellular Ca2+ concentration [63]. However, intracellular Ca2+ concentration is highly increased when isolated parasites are treated with ATP in the presence of extracellular Ca2+, thus suggesting an activation of Ca2+ influx. Moreover, experiments in Ca2+ free medium abrogated the response, indicating that the parasite possesses an ATP-gated calcium channel [15], a form of primitive P2X receptor (Figure 1). Similar responses were obtained with Plasmodium yoelii and Plasmodium berghei (two species infecting rodents) [73]. Unlike in the case of D. discoideum DdP2X receptor, a set of blockers (IP5I, PPADS, KN-62, TNP-ATP, and Suramin) known to act on cloned vertebrates P receptors significantly inhibited the rise in ATPe-dependent intracellular Ca2+ concentration. Surprisingly, extracellular UTP also induced intracellular Ca2+ deregulation in the parasite, a featured not observed in P2X1-7 receptors [15].
Several lines of evidence suggest that such P2X-like receptor of P. falciparum can be important for parasite invasion to RBCs: (1) ATPe and Ca2+, but not other divalent cations triggered parasite invasion [15,63] and ATPe-dependent intracellular Ca2+ concentration increase is required to ensure the progress of the parasite life cycle [70]. (2) Under in vitro culture of RBCs with parasites, removing endogenous ATPe by apyrase or pre-incubation with P2X blockers, drastically impairs parasite invasion [15]. Interestingly in this context, exogenous ATP does not improve the efficiency of RBC infection, suggesting that the putative P2X receptor should exhibit a relative high affinity for ATPe. Alternatively, ATP affinity could be moderate, but receptor activation is possible in light of experiments showing an increased ATP efflux from infected RBCs throughout the parasite life cycle, which correlated with elevated ATPe concentrations in infected RBCs cultures with P. falciparum [14,74] and plasma samples from humans [75]. Once inside the RBC, P. falciparum is enclosed in a highly permeable PVM, and exposed to the RBC cytoplasm containing a very low intracellular Ca2+ concentration (<100 nM), which is much lower than in other cell types, and at least 3 orders lower than the millimolar Ca2+ concentration of the plasma. A very low Ca2+ concentration environment set by the host is incompatible with parasite functions and survival and may hamper a potential role of parasite P2X-like receptor in modulating ATP-dependent calcium influx [76]. In line with this notion, Gazarini et al. [76] nicely demonstrated that P. falciparum and Plasmodium chabaudi exhibit an active mechanism for Ca2+ accumulation in the immediate parasite microenvironment, with concentrations of the cation amounting to 40 µM. This is much lower than plasma concentrations of the cation, but nevertheless 100–1000-fold higher than that inside the parasite and in the RBC cytosol [77,78]. Thus, a Ca2+ gradient does exist between the relatively low intracellular Ca2+ concentration of the parasite inside the PV and its external milieu, making a P2X-like receptor dependent Ca2+ uptake in the parasite functionally relevant (Figure 1).
In addition, P. falciparum can activate Ca2+ influx of the RBC host. Early 45Ca2+ flux studies indicated that net Ca2+ entry into infected RBCs was approximately 20 times faster than into uninfected cells [79], which agrees well with the reported elevated Ca2+ conductance of patched membranes from infected RBCs [80]. This increased accumulation rate could not be explained by inhibition of the Ca2+ pump, a cell membrane ATPase that keeps the Ca2+ of uninfected RBCs very low. More recent pharmacological studies suggested that the observed elevated Ca2+ permeability was mediated by a new mechanism activated in the infected host cell [71]. I.e., when RBCs were infected with a parasite knocked down for PTEX (a translocon for export of parasite proteins into the host cell), Ca2+ uptake was highly reduced. Moreover, a cell-based high-throughput screen for specific Ca2+ transport inhibitors confirmed a protein-mediated uptake mechanism on infected cells distinct from the low capacity Ca2+ carrier of RBCs [79] (Figure 1).
2.2. P receptors of the Host and Parasite Infection
Host organisms have evolved several mechanisms to control infection, while protozoa parasites display several adaptations to evade host defense. P receptors of the host are often involved in this fight [9,10,81].
Toxoplasma infects practically all mammalian cells [82]. Although most infections are asymptomatic, they can cause serious problems when the immune system is compromised as with pregnant women, AIDS patients or during congenital transmission [83,84]. Similarly to Plasmodia, invasion results in the establishment of a subcellular PV. Success of the parasite depends on its ability to rapidly reproduce inside the PV and then for its progeny to escape from the membranes of the vacuole and that of the host cell, so as to invade more cells [85,86]. Following infection, the parasite inhibits the fusion between lysosomes and the PV, maintaining the extravacuolar medium at a neutral pH, thus allowing parasite survival [82]. However, ATPe ligation of P2X7 receptors restores that fusion, acidifying the PV and leading to elimination of Toxoplasma gondii in macrophages [87,88]. In addition, activation of P2X7 receptors in Toxoplasma infected macrophages increases the production of reactive oxygen species [87,89] and induces apoptosis [88], another two host defense responses.
To the extent that host strategies require an efficient, fully functional P2X7 receptor, polymorphisms at the P2X7 gene were shown to influence the susceptibility to toxoplasmosis in certain human populations [90]. In particular, macrophages from people with the 1513C loss-of-function single nucleotide polymorphism are less effective to kill T. gondii after treatment with ATP than macrophages from people with the 1315C wild-type allele [88]. Thus P2X7 receptor activation of specific polymorphic variants may influence the outcome of infection, no matter the route of transmission or parasite genotype, which varies considerably worldwide [90]. In line, macrophages from P2X7 receptor knock-out mice are not able to eliminate the parasite as successfully as macrophages from wild-type mice [88].
Parasites may counteract P2X7 receptor mediated attack by secreting ecto-nucleotidases of the E-NTPDase (ecto-nucleoside triphosphate diphosphohydrolase) family (see Section 4) before and after host cell entry [91,92], to promote ATPe hydrolysis and therefore a decrease in P2X7 receptor activation. As analyzed in Section 4, virulent T. gondii strains express the enzyme TgNTPDase 1, which possess typical signal peptides for secretion and displays high ATPase activity [92,93]. At least theoretically, TgNTPDase1 should be able to facilitate a decrease of ATPe concentration, thus lowering P2X7 receptor activity and dampening the inflammatory response. While this reasoning is still speculative, compelling evidence has revealed an important role for E-NTPDases in P2X7 receptor activity by controlling the effective ATP concentration at the cell surface, and the availability of ATPe during infection-induced inflammation [94].
In addition to downregulation of P2X7 receptor activity, an increase ATPe hydrolysis by TgNTPDase1 may have consequences on downstream purinergic signaling, by facilitating the accumulation of extracellular AMP, and further degradation to adenosine by host ecto-5′-nucleotidase. Elevated adenosine, in turn, may activate host P1 receptors. In contrast to ATPe, adenosine has anti-inflammatory and immunosuppressive effects on immune cells [95,96]. Although neutrophils are recruited during T. gondii infection [97], activation of P1-A2A receptors on these cells by extracellular adenosine was associated with inhibition of activation and migration [98]. Moreover, infection with T. gondii is lethal in mice deficient in 5′-ecto-nucleotidase, unless a potent P1 agonist is administrated [99], and mice deficient in the adenosine receptor P1-A2A are more susceptible to infection than wild type counterparts. Thus, production of adenosine by host 5′-ecto-nucleotidase, probably facilitated by TgNTPDase 1 degradation of ATPe, leads to increased susceptibility of mice to T. gondii infection. Several strategies of Leishmania are different from those of Toxoplasma. Leishmania species are the ethiological agents of leishmaniases. Parasites are transmitted through the bites of more than 90 different species of phebotomine sandflies, and have developed several strategies to survive within the host by evading the immune response.
After invading macrophages by phagocytosis, Leishmania can survive within an acid pH environment inside the phagolysosomes. In addition, Leishmania interferes with the production of reactive oxygen and nitrogen species by phagocytes [100]. Macrophages infected with Leishmania amazonensis exhibit higher expression of P2X7 receptors than noninfected cells [101]. ATP treatment reduced parasite load [101,102] though not in macrophages isolated from P2X7 knockout mice [102].
In addition to P2X7 receptor modulation, infection with L. amazonensis up-regulates the expression of P2Y2 and P2Y4 metabotropic receptors, which can be activated by either ATP or UTP [103]. This is again a host antimicrobial response, since UTP decreases parasite load [104]. P2Y2,4 receptors signaling involves intracellular Ca2+ mobilization, probably inducing apoptosis of infected cells as well as production of reactive oxygen and nitrogen species [104].
As in the case of Toxoplasma infection, effects of adenosine on Leishmania parasitism were also reported [105]. Patients with visceral leishmaniasis present higher concentrations of adenosine in serum than healthy people [106]. For mice infected with Leishmania braziliensis, administration of adenosine increased lesion development and tissue parasitism [107], while blockage of P1-A2B receptors decreased lesion size and parasitic load [107]. In a murine model of Leishmania infantum infection, P1-A2A receptor signaling reduced activation and migration of neutrophils, allowing the establishment of infection [108].
An additional mechanism for regulation of host P2 receptors could involves desensitization, which occurs in P2X1, P2X3, and P2Y1 receptors [21] by long exposure to their natural ligands. Considering that parasite infection alters the kinetic profiles of extracellular nucleotide accumulation, it may well regulate P2 receptors activity by affecting P2 desensitization. For instance, platelets activation of P2Y1 by extracellular ADP highly mediates aggregation [109]. During P. falciparum infection, strong ATP efflux of infected RBCs, together with a highly increased ATPe hydrolysis by upregulated ecto-nucleotidases [14], should promote a strong enhancement of ADPe in the blood stream. Under these conditions, platelets P2Y1 receptor may desensitize, and thus undergo a refractory state characterized by the inability to aggregate or change shape in response to extracellular ADP. Changes in ATPe concentration may also affect desensitization of P2X1 receptor, causing abnormalities in blood coagulation [109]. In patients with malaria, both hyperaggregation and defective aggregation were described [110], though the contribution of extracellular nucleotides in this regulation needs to be confirmed.
3. Transport of ATP
In cells and organisms multiple compartments exist from where ATP can be transported, depending on the effective concentration, the available transmembrane ATP gradients (the driving force for conductive ATP flux) and the capacity and kinetics of several proteins allowing ATP permeation.
Different cells from unicellular and multicellular organisms release ATP and other nucleotides (ADP, UTP, and UDP) and adenosine during mechanical injury, necrosis, apoptosis, as well as under various mechanical and chemical stimuli, such as shear stress, hypotonic swelling, stretching, and different Ca2+-mobilizing agonists [111,112,113,114].
Most non-lytic mechanisms of ATP release were studied in mammals, where conduits for regulated vesicular exocytosis or conductive nucleotide release via ion channels and transporters were identified [54,55,113]. In principle, two main classes of plasma membrane channels have been associated with ATP conductive activity: (1) Cl− channels such as the calcium homeostasis modulator 1 (CALHM1; [5]) channel, maxi-anion channels, volume-regulated anion channels (VRAC, also known as volume-sensitive outwardly rectifying anion channel), and tweety [113,115,116]; and (2) pore forming connexins and pannexin-1 (pnx-1). In addition, P2X7 receptor, alone or in association with pnx-1, is known to progressively generate a large membrane pore that allows ATP permeation [117]. Other conduits such as the voltage-dependent anion channel (VDAC) were shown to interact with partner proteins, like the 18-kDa-tranlocator protein (TSPO) and the adenine nucleotide transporter (ANT). Thus, it soon become established that, although most Cl- channels are able to permeate ATP to different extents [118,119], ATP transport may require these channels to operate as part of multimolecular complex systems [120].
Evidence of ATP transport conduits of protozoa is scarce. In D. discoideum, ATP is released following a hyposmotic challenge. Pharmacological evidence suggests an exocytotic mechanism, but the implicated mechanisms were not identified [57]. D. discoideum appears to harbour an ATP translocation mechanism to mediate ATP accumulation within the vacuole lumen. However, this is not the source of the observed ATP release, since knockout cells with impaired contractile vacuole fusion exhibited no inhibition of ATPe accumulation [57]. Irrespective of the mechanism, the resulting osmotically induced accumulation of ATPe could signal auto/paracrinally through a putative cell surface P2-like receptor [15].
Microsporidians are eukaryotic unicellular organisms living as obligate intracellular parasites of vertebrates and invertebrates. Initially thought to be protozoans, recent molecular studies indicate that the phylum Microspora should be more closely related to Fungi kingdom [121]. Recently, microsporidians have been reported to parasite humans, especially those immunologically compromised [122]. These organisms have undergone massive gene loss in the transition to obligate intracellular parasitism. Some of these genes encode proteins involved in purine and pyrimidine biosynthesis, and the oxidative synthesis of ATP. On the other hand, glycolysis is highly down regulated [123]. Thus, replicating parasites must import ATP from the host cell, using some kind of transport system.
Microsporidian genome shows genes encoding for nucleotide transporters (NTTs) capable of transporting ATP [124]. Three NTTs are located to the plasma membrane, while one is localized to the mitosome, a remnant mitochondrial organelle [125]. Genome sequences of several microsporidian species contain one or more of these transporters, probably acquired by lateral gene transfer from intracellular bacterial pathogens [124], enabling early microsporidians to take up host ATPe for their growth and biosynthesis processes. Expression in Escherichia coli suggests that NTTs display high affinity for ATP, while binding competition assays show that other nucleotides can be transported as well. Clearly in this case, ATPe of microsporidian is the cytosolic ATP of the host. Signaling of ATP might not be important in this case, but the energetic need for nucleotide salvage.
A similar strategy is used by protists living as endosymbionts of amoeba and paramecia, although ATP transport involves an ATP/ADP exchange mechanism [126].
Genes coding for these exchangers were found in several species of Rickettsiae and Chlamydiae, promoting import of host ATP through the prokaryotic cell membrane, which is otherwise impermeable for relatively large anionic nucleotides, in exchange for ADP export into the host cytosol [126]. ATP-ADP exchange was also verified when expressed in a heterologous bacterial system.
Although in mammals such an ATP/ADP exchanger occurs exclusively in the inner mitochondrial membrane, ANT has been observed in the plasma membranes of RBCs [120,127]. As we mentioned previously, ANT may physically associate with VDAC and other proteins at the RBC cell membrane, to form a conduit capable of exporting ATP to the extracellular milieu [120].
In Plasmodia, ATP and other organic anions were proposed to be transported by the NPPs, though the molecular identity of this pathway is a matter of debate. However, it was recently reported that drug ligands modulating VDAC activity were able to induce ATP release from RBCs [120,128], and that P. falciparum infection induced changes in the affinity of RBC membranes for these drug ligands [69].
In protozoan parasites, use of host ATP is qualitatively different to microsporidians and protists parasites, in that ATP and other nucleotides are first dephosphorylated by ecto-nucleotidases to nucleosides, purines, and pyrimidines. Specific nucleoside transporters allow uptake of these compounds into the parasite, which can then be used to resynthesize ATP and nucleic acids (see Section 5).
In many cells and organisms, hypoxia and anoxia constitute potential stimuli promoting regulated ATP release by vesicular and conductive mechanisms [129,130,131,132]. In human RBCs, hypoxia-induced ATP release is mediated by pnx-1 [133], although other stimuli can activate different ATP conduits in these cells [54,55,128].
Interestingly in this context, Leishmania promastigotes (the flagellated phenotype) were shown to induce non-lytic release of intracellular ATP, but not of other nucleotides, when challenged by anaerobiosis [134]. The accumulated ATPe may have various fates. On the one hand, Leishmania or host derived ATPe might activate parasite killing by activation of P2X7 receptors of macrophages [101]. Alternatively, ATPe may be degraded by Leishmania NTPDases, as part of a mechanism for purine salvage ([135]; see Section 5).
In addition, ATPe may participate in the phosphorylation of extracellular proteins, affecting the interaction of the parasite with the host’s immune system. Accordingly, exogenous ATP was shown to phosphorylate added histone and several parasite surface proteins, including tubulin [136].
4. Ecto-Nucleotidases
Ecto-nucleotidases are plasma membrane-associated nucleotidases that usually display their active site to the extracellular space, although cleaved and soluble extracellular isoforms may occur [137]. The currently known ecto-nucleotidases include members of the E-NTPDase family (ecto-nucleoside triphosphate diphosphohydrolases), hydrolyzing extracellular nucleoside 5′ di-and triphosphates, E-NPP (ecto-nucleotide pyrophosphatase/phosphodiesterases), which can hydrolyze pyrophosphate 5′-monodiester bonds in ATP and dinucleoside polyphosphates; ecto-alkaline phosphatases, acting as non-specific phosphomonoesterases; and ecto-5′-nucleotidases, also called CD73, which hydrolyse only nucleosides monophosphates [6]. Extracellular adenosine can thereafter be either inactivated by cell surface associated adenosine deaminase (ADA) and purine nucleoside phosphorylase (PNP; [114,138,139]) or transported into the cell by equilibrative or Na+-dependent nucleoside transporters in order to replenish the intracellular nucleotide pool, as discussed in Section 5 [140,141].
In different organisms, the relative contribution of different ecto-nucleotidases to the regulation of extracellular nucleotides depends not only on the availability and preference of substrates, but also on their cell and tissue distribution [34]. Hydrolysis of ATPe and other extracellular di- and trinucleotides is mainly promoted by different E-NTPDase subtypes, which are ubiquitously expressed in eukaryotes [6,142]. To date there are eight members of this family. NTPDases 1, 2, 3, and 8 are expressed as cell surface locate enzymes, with the catalytic site facing the extracellular milieu. NTPDases 5 and 6 are intracellular, but can be proteolitically cleaved and secreted, while NTDPases 4 and 7 are intracellular with the active site facing the lumen of organelles [6,114,137].
All E-NTPDases are highly glycosylated, integral membrane proteins, which require intracellular Ca2+ or Mg2+ for enzyme activity [114,143]. All members share five highly conserved sequences of amino acids called apyrase conserved regions (ACRs), which are essential for enzyme function [137,144,145,146,147], and may exist either as monomers or homo-oligomers [6,137,147]. Site-directed mutagenesis studies of conserved amino acid residues in ACRs regions show that, depending on the amino acid being altered, site mutations resulted in inactivation or activation of enzyme activity [147,148], while significant changes in the kinetic features are possible with only a few changes in the amino acid sequence of the ACRs [148].
The different subtypes of E-NTPDases differ in their preference for nucleotide 5′-tri-and nucleotide 5′-diphosphates, displaying broad substrate specificity towards different purine and pyrimidine di- and trinucleotides, with Km values ranging from 50 to 200 µM [7]. These values should be taken with caution, since the catalytic properties and nucleotide preferences obtained in vitro with enzymes purified to different extents and with different detergents may not match the in vivo kinetic features of membrane bound enzymes. In COS7 cells transfected with cDNA coding for human and murine E-NTPDases 1, 2, 3, and 8, most apparent Km values for these enzymes were in the low micromolar range [143].
For each system and a specific metabolic status, the rate of nucleotide to nucleoside conversion is relevant, since it determines the effective time a given nucleotide is available to interact with specific P receptors before being locally hydrolyzed by ecto-nucleotidases. In several cell types [149,150], E-NTPDases may act in concert with the 5′-ecto-nucleotidase to achieve the sequential and complete dephosphorylation of ATPe to adenosine. The 1-8 classification of the E-NTPDase family was mainly derived from studies on animal tissues [151], but—as we will see below—evolutionary earlier forms of these enzymes may differ in various structural and kinetic aspects. The gene family has members in various protozoan species [92,144], as well as in plants and yeast [92,137]. Sequencing of several genomes of protozoan parasites revealed the presence of genes encoding putative NTPDases [92]; [152]. However, as pointed out by Nakaar et al. ([153], see also [92]), it is sometimes difficult to clarify which gene encoding a putative NTPDase, or else another nucleotidase, is responsible for the observed nucleotide hydrolysis.
A few well-known protozoan models will be analyzed below, with particular focus on pathogenic species of genera Toxoplasma, Trypanosoma, and Leishmania.
In the last 25 years, evidence has been accumulating that expression of E-NTPDase genes is required for virulence of many pathogens [93,154,155,156,157]. Thus it appears likely that protozoan NTPDases, by altering the concentration of ATPe and other extracellular nucleotides accumulating on the cell surface of hosts may interfere with P signaling to suppress inflammatory responses and evade immune reactions [154].
4.1. Toxoplasma gondii
T. gondii encodes three sequences compatible with E-NTPDases on its genome [84], although cDNA analysis showed that only genes for TgNTPDases 1 and 2 [92], also termed NTPDases 3 and 1, respectively [84], are transcribed and translated. Amino acid sequences of both enzymes contain the canonical five ACRs, demonstrating that they are members of the E-NTPDase family. Despite 97% identity, both enzymes displayed different affinities for di- and trinucleotides, with TgNTPDase 1 exhibiting more than four-fold higher ATPase activity than TgNTPDase 2 [84,93,158]. Most virulent strains of T. gondii possess the gene encoding TgNTPDase 1, but avirulent strains carry only the gene encoding TgNTPDase2 [93,159]. Early studies showed that TgNTPDase 1 inhibition by antisense RNA compromised parasite replication, while pre-treatment of parasites with a monoclonal antibody inhibiting TgNTPDases 1 and 2 compromised invasion of the parasites into host cells, suggesting that NTPDase activity (one or both isoforms) is essential for parasite function [84,153,158].
Antibodies raised to recombinant NTPDases enabled localization of the enzymes within the parasite and during infection of host. Accordingly, TgNTPDases 1 and 2 are secreted into the lumen of the PV [160,161]. TgNTPDase 1 has only low levels of expression in the bradyzoite form of the parasite (the form responsible for chronic host infection) but is highly expressed in secretory granules of the actively replicating tachyzoite form [92,158].
Whether TgNTDPase activity contributes to the invasion of the parasite, and/or egress from the host, remains controversial [92,153,162]. As ATPe from the host can activate specific P2X7 receptors [12] to promote inflammation, we might assume that T. gondii NTPDases might likely interfere with the host inflammatory response. However, the intracellular location of TgNTPDases and their millimolar Km values [84] argue against this hypothesis. Alternatively, a role in purine salvage has been proposed. This is because TgNTPDase2 hydrolyzes ATP and ADP at similar rates. If coupled to host 5′-ecto-nucleotidase activity and other downstream ecto-enzymes, it may produce adenosine and by-products capable of being transported to the parasite (see Section 5) [163].
In the closely related Neospora caninum, NTPase (NcNTPDase) lacks nucleoside diphosphate hydrolase activity and appears to be more abundant in virulent isolates, suggesting a role in the pathogenicity of neosporosis, which causes abortion in cattle and neuromuscular disorders in canids [164]. Protein dynamics, secretion, and mRNA expression profiles and enzyme immunolocalization during the tachyzoite lytic cycle was compatible with NcNTPase, similarly to TgNTPDase, being localized in dense granules and the PVM throughout the lytic cycle. Up-regulation and secretion of NcNTPDase occurred during the egress and early invasion phases.
4.2. Trypanosoma cruzi
T. cruzi is the ethiological agent of Chagas disease, commonly spread to humans and more than 100 species of mammals by hematophagous triatomine insects. It is a cause of morbidity and mortality not only in endemic areas of North, Central, and South America but also among immigrants now residing in non-endemic areas of the world [165]. Up to date there are no vaccines or effective treatment for the disease [166]. Vector control is the most effective method of prevention. Blood screening is necessary to prevent infection through transfusion and organ transplantation [167].
Parasites circulate through the blood of the host in the form of trypomastigotes. When the triatomine insect bites and feeds on infected blood, the trypomastigote matures and differentiates to the epimastigote form, which attaches to the intestinal wall and divides. Three to four weeks later epimastigotes convert to infective trypomastigotes that move to the hindgut of the insect. Transmission to the new host occurs when parasites from insect feces enter cells at bite wound sites [165]. Parasite initially replicates within local tissues before disseminating in the bloodstream, where they can infect at distal sites [165].
T. cruzi encodes a single predicted NTPDase (TcNTPDase) containing all five ACRs. The fact that a protein on the parasite surface reacted with a specific antibody anti TcNTPDase suggests the surface localization of the enzyme, although a predicted N-terminal signal peptide may allow the enzyme to be secreted [168].
A recombinant form of TcNTPDase promoted hydrolysis of ATP and ADP, with an ATPase:ADPase ratio of approximately 2, i.e., similar to that of E-NTPDase 1 of mammalian models [166]. However, although E-NTPDase activity was clearly shown by exposing intact live parasites to extracellular nucleotides, problems arise when trying to assign the experimentally measured ecto-nucleotidase activities to the identity of the enzymes. For example, the ATPase:ADPase ratio was shown to vary during passage in culture, changing from 2:1 for trypomastigotes to 1:1 for epimastigotes. Moreover ARL67156, a generic ecto-nucleotidase inhibitor, partially inhibited ATP diphosphohydrolase activity, but failed to inhibit recombinant TcNTPDase [166]. On the other hand, gadolinium and suramin caused decreased infectivity in vitro and decreased virulence in a mouse model of disease [92,166], but these compounds may also interfere with ATP transport mechanisms and P receptor signaling ([157,159,169,170], see also [171]). Nevertheless, the fact that ecto-nucleotidase activities could be measured in vitro using different nucleotides (GDP, GTP, UDP, and UTP) suggests the participation of one or more NTPDases [172,173]. Moreover anti-TcNTPDase 1 polyclonal antiserum was shown to partially inhibit ATPDase activity and infectivity of trypomastigotes [166]. In addition to NTDPase activities, in vitro experiments showed that T. brucei has the ability to hydrolyse extracellular AMP to adenosine, indicating 5′-ecto-nucleotidase activity in this parasite [174].
More recently, Santos et al. [166] showed that high ratio of ATP/ADP extracellular hydrolysis is important to maintain the capacity of parasites to infect mammalian Vero cells lines (carcinoma-derived African green monkey fibroblast cells). Moreover, inhibition of parasite NTPDase modulated the infectivity and virulence in mice, suggesting that this enzyme might facilitate T. cruzi infection.
In contrast to T. cruzi, T. brucei is an extracellular pathogen that replicates in the bloodstream of mammals [175], causing African sleeping sickness. The genome of T. brucei encodes 2 predicted NTPDases containing N-terminal signal peptides, implying secretion by the parasite [176]. When intact parasites were exposed to exogenous nucleotides, hydrolysis of ATP, GTP, CTP, UTP, and ADP was found. In principle, the availability of efficient RNAi systems in T. brucei could be used to assess the importance of these enzymes in virulence [177].
4.3. Leishmania
The genome of five Leishmania species possesses two predicted NTPDases, containing the five ACRs [178] but no kinetic characterization of these enzymes is available. One putative E-NTPDase exhibits a predicted N-terminal transmembrane domain, compatible with anchoring of the enzyme on the membrane of the cell or that of an intracellular organelle. Another putative Leishmania NTPDase has a predicted N-terminal signal peptide, suggesting a potential secretion of the protein that could be responsible for extracellular nucleotide hydrolysis [179]. While it is not confirmed that the observed enzyme activity is due to members of the NTPDase family, the kinetic characterization is in principle consistent with the presence of NTPDases, although the enzymes identified in L. tropica and L. amazonensis cannot utilize Ca2+ instead of Mg2+, which is unusual for the E-NTPDase family [156,180].
In several Leishmania species ATP can be hydrolysed to adenosine at the cell surface, indicating the presence of NTPDase and 5′-ecto-nucleotidase activity [135]. Nucleotidase activity is higher in virulent strains than avirulent strains and is increased more than 10-fold in the obligate intracellular amastigote stage [155,156]. On the other hand, treatment of parasites with anti-CD39 antibody (i.e., cloned E-NTPDase 1) reduced the interaction of the parasites with mouse peritoneal macrophages [156], further suggesting a role for an NTPDase in pathogenesis. Moreover, the expression of ecto-nucleotidases in Leishmania is linked to lesion size upon infection, with high expression of these enzymes associated to decreased immune response and bigger lesions [18].
4.4. Plasmodium falciparum
Recently Borges-Pereira et al. [181] provided the first characterization of an NTPDase of P. falciparum. In this species, the genome predicts a gene encoding a putative E-NTPDase (Pf apyrase, PF3D7_1431800). ATPase activity as well as mRNA levels were confirmed at all stages of development, i.e., rings, trophozoites and schizonts. In isolated parasites, ATPe hydrolysis was inhibited by well-known E-NTPDase inhibitors, which strongly impaired parasite development. Expression of a N-terminal PfNTPDase-GFP chimera suggested that the NTPDase is expressed throughout the asexual cycle, but localization appears to change from the endoplasmic reticulum to the digestive vacuole as the parasite develops. Since the chimera lacks the C-terminal transmembrane sequence, the exact localization of the enzyme awaits confirmation [181]. Enzyme kinetics results suggest that PfNTPDase might translocate to the host membrane. This is because uninfected RBCs have a very low ATPe hydrolysis rate by ecto-ATPase activity, while infected RBC displayed several fold higher values at all stages [14]. Moreover, ecto-ATPase activity of trophozoite infected RBCs is approximately 400-fold higher than similar activity of uninfected cells, both at low nanomolar as well as high micromolar ATP concentrations [14]. This finding supports the hypothesis that infected cells express higher levels of functional ecto-nucleotidases than uninfected cells. Up-regulation of host E-NTPDase is unlikely, since mature RBCs lack nucleus and thus the synthesis of new proteins is restricted to the translation of pre-existing traces of mRNA. Elevated host E-NTDPase may be partly explained by a decreased degradation rate of the infected RBCs and/or posttranslational modifications. In this line, we observed before [14] that trophozoite infected RBCs can highly produce nitric oxide, which modulates host ATPe hydrolysis rate, probably by S-nitrosylation of E-NTPDase. Alternatively, PfNTPDase or an unknown parasite nucleotidase, might account to the observed elevated ecto-ATPase activity of infected cells.
An evolutionary tree depicting the evolution of NTPDases is shown in Figure 2 (sequence alignment of NTPDases are given in Figure S1). Evolution of NTPDases from humans and other vertebrates shows a clear separation of subtypes locating at the cell surface (i.e., NTPDases 1–3, 8) or intracellularly (i.e., NTPDases 4–7), which correlates well with the topological and structural differences between the two groups [6]. In fact, hNTPDase1 and hNTPDase2 proved sufficiently related to form catalytically active protein chimeras [6].
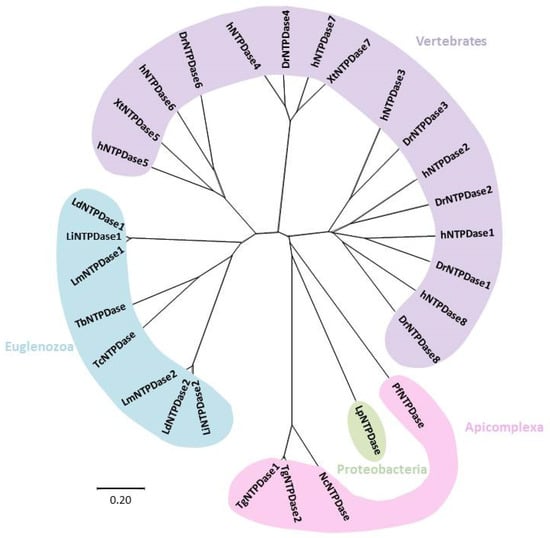
Figure 2.
Radial phylogenetic tree from amino acid sequences of NTPDases. NTPDases from protozoan species are shown, together with NTPDases from other vertebrate species and a bacterium. The tree was generated using the ClustalW sequence alignment tool, with the resulting tree being built with the MEGA-X software, using the Neighbor-Joining method. Visualization was done with the program Figtree. The tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site. NTPDases and their accession numbers are listed as follows. Human NTPDases: hNTPDase 1: NP_001767, hNTPDase2:NP_982293, hNTPDase3:NP_982293, hNTPDase4:NP_004892, hNTPDase5:NP_001240, hNTPDase6:NP_001238, hNTPDase7:NP_065087, hNTPDase8:NP_001028285. Danio rerio NTPDases: DrNTPDase 2:54261809, DrNTPDase 3: ABR15509, DrNTPDase4:50539906, DrNTPDase 6:62955697, DrNTPDase 8:268837940. Xenopus tropicalis NTPDases: XtNTPDase5:301618468, XtNTPDase7:62859996. Protozoan NTPDases for Plasmodium falciparum (Pf): PfNTPDase:AAN36910; Toxoplasma gondii (Tg): TgNTPDase 1: Q27893, TgNTPDase 2:Q27895; Toxoplasma brucei (Tc): TbNTPDase: XP_847211; Leishmania major (Lm): LmNTPDase1: XP_001681917, LmNTPDase2:CAJ02396; Leishmania donovani (Ld): LdNTPDase1:CBZ32820.1, LdNTPDase2:CBZ32136.1; Leishmania infantum (Li): LiNTPDase1:XP_001464341, LiNTPDase2:XP_001463665; Trypanosoma cruzi (Tc): TcNTPDase: AA575599; Neospora caninum (Nc): NcNTPDase: BAA31454. Aligments of aminoacid sequences of NTPDases are given in Supplementary Figure S1.
Similarly to vertebrates, all protozoan ecto-nucleotidases are denoted in the tree as NTPDases, as they contain the five ACRs. As expected, species from Euglenozoa (Trypanosoma and Leishmania) showed evolutionary proximity, and the same is true for species from Apicomplexa (Toxoplasma, Neospora and Plasmodium). Toxoplasma and Neospora NTPDases are consistently grouped, as TgNTPases 1 and 2 share 97% identity, while N. caninum exhibit 69% identity with TgNTPDase 1 [92]. Most bacteria lack NTPDases, but NTPDase from Legionella pneumophila, a pathogenic bacterium, was possibly acquired by horizontal transfer [6].
5. Nucleoside Transport
Although ATPe and other extracellular nucleotides are usually not taken up by cells, dephosphorylated nucleoside derivatives interact with specific transporters to enable intracellular uptake via specific nucleoside transporter proteins (NTs).
There are two different gene families of NTs [182]. In humans, the SLC28 gene family encodes Na+ dependent concentrative nucleoside transporters, with three isoforms (CNT1–3) [182], whereas the SLC29 gene family encodes nucleoside transporters comprising four isoforms in humans called equilibrative nucleotide transporters (ENT) 1–4. ENTs are glycosylated, possess 11 transmembrane helices with cytoplasmatic N-and extracellular C-termini, a large extracellular loop between transmembrane domains 1 and 2, and a large intracellular loop connecting transmembrane domains 6 and 7. First thought as being exclusively equilibrative transporters of nucleosides, later findings show that some family members also transport nucleobases whereas others act as proton-dependent concentrative transporters [183,184,185,186].
Recycling of nucleosides and nucleobases by NTs is essential for the synthesis of nucleotides and nucleic acids, intracellular signaling pathways (e.g., cAMP, cGMP), and phospholipid synthesis. In particular, adenosine uptake can modulate P signaling, in that it decreases the effective adenosine concentration at the cell surface, thereby terminating its action on P1 receptors [187]. Nucleoside uptake and subsequent intracellular anabolic reactions can also improve the energetic state of a cell, which sometimes translates into higher intracellular ATP concentrations. This is especially important for conductive ATP release (see Section 3), where intracellular ATP acts as the main force driving the efflux of this nucleotide [113] by protozoan and host transporters.
Following the first molecular characterizations of ENTs in human tissues (1997, in [188]), the identification of homologous proteins by functional cloning and genome analysis has revealed that the family is widely distributed in eukaryotes [188]. In several protozoa, nucleoside and nucleobase transporters are ENT family members displaying the canonical eleven transmembrane domains [189].
Structure–function relationships of parasitic protists have been reviewed recently [189]. Two NTs, LdNT1 and 2, were found in cell membrane of Leishmania donovani, transporting adenosine and pyrimidine nucleosides (LdNT1) and inosine and guanosine nucleosides (LdNT2) [189]. Interestingly, these transports exhibited a 100-fold higher affinity for their substrates than mammalian NTs, thus enabling an efficient use of intracellular nucleosides from the host. Expression in Xenopus oocytes showed LdNTs to use a proton gradient for secondary active transport of nucleosides, i.e., a concentrative mechanism qualitatively different from passive equilibrative transport of mammalian ENTs. Mutational analysis showed that a single amino acid substitution can affect substrate specificity, as substitution G183D in a transmembrane domain decreased adenosine transport, while G183A impair pyrimidine transport [189].
In Trypanosoma brucei, ENT transporters, include TbNT1 transporting adenine and adenosine and TbNT2-10 transporting purine nucleosides and nucleobases [183,190,191].
A low affinity adenosine ENT transporter was found in T. gondii, which may couple well with ecto-nucleotidases, since TgNTPDase 1 (see Section 4) promotes ATP and ADP hydrolysis at similar rates, producing AMP, which may then be a substrate of ecto 5′-nucleotidases producing adenosine, which can then be scavenged by ENT for growth [84].
Purine Salvage of Parasitic Protozoa
Most genetic and molecular studies on ENTs have been done in parasitic protozoa, which usually lack the early ATP-dependent steps in the de novo biosynthesis of purines [192] and hence import the missing substrates using ENTs. The need to grow rapidly imposes an energetic burden to these organisms, which require ATP as a source of energy and large amounts of purines for DNA and RNA synthesis. Several reports showed that functional ENTs transporters allow parasites to salvage a wide range of nucleoside and nucleobases from the host, later used to synthetize nucleotides within the parasite [183,193]. Proteins participating in the transport and metabolism of purine nucleosides differ considerably between the human host cells and protozoa, making this pathway a potential target for drug development [8,194,195]. In contrast, parasitic protozoa are capable of synthesizing pyrimidines, with the exception of Giardia lamblia, Tritrichomonas foetus, and Trichomonas vaginalis [196].
In mature human RBCs infected with Plasmodia, nucleoside transport and metabolism is more complex, as these host cells lack purine and pyrimidine biosynthetic pathways [197], and therefore largely depend on nucleoside uptake by human ENTs (hENTs). During the intraerythrocytic life cycle of the parasite, purine transport activity of hENT is complemented by the activation of NPPs, thus ensuring the entrance of nucleosides—and other nutrients—in the cytosol. Hypoxanthine, inosine and adenosine are transported across the PV through unspecific pores [8,198], and then taken up through NTs located at the plasma membrane of the parasite. Although four putative transporters (PfNT1-4) were identified in P. falciparum genome, isoform 1 is mainly used for purine salvage [192]. Accordingly, protein mass spectrometry shows that PfENT1 is expressed at all parasite stages, while PfENT1 knockout parasites are not viable in RBC cultures containing micromolar concentrations of purines similar to those found in plasma [192]. Moreover, genetic disruption analysis confirmed that the PfNT1 gene is essential for purine nucleoside uptake and parasite survival. Once inside the parasite, a series of enzymes such as N-ribosyltransferases and phosphotransferases will metabolize host purines to supply parasite needs.
6. Summary and Conclusions
ATPe dynamics of protozoan parasites was studied in terms of the proteins involved in purinergic signaling, transport and metabolism of ATP and purine salvage, with special focus on the role of protozoan proteins. Existing data show that the relative contribution of parasites versus host proteins in mediating these processes is still controversial.
Although extracellular nucleotides are known to mediate a wide range of metabolic and physiological processes in these organisms, only a few protozoan P2X-like receptors were described, with the most studied case being the DdP2X receptor of D. discoideum. A gene was identified encoding a P2X-like protein. Site directed mutagenesis studies, together with assessment of whole cell currents showed conservation of structure and function of a typical P2X channel. DdP2X appears to localize exclusively in an intracellular vacuole, with the ATP binding site in the lumen [57]. It remains to be seen if this intracellular localization of the receptor, possibly sensing and reacting to intravacuolar ATP, is also observed in other protozoa. Adaptation of the parasite host sometimes required up-regulation and/or activation of P2 receptors, especially P2X7 receptor involved in antimicrobial responses. In addition, polymorphisms of P2X7 receptor were identified in humans that influence the outcome of infection to toxoplasmosis, no matter the parasite genotype [90].
Only in the case of protozoan ecto-nucleotidases there is ample molecular, kinetic, and cellular information from various protozoan species, both studied in isolated conditions or interacting with their host. In most studied species, predicted NTPDases encoded in the genomes were found. Enzymes were shown to either remain attached to the parasite membrane as ecto-enzymes, or be released to their extracellular milieu. Parasite NTPDases contributed differently to ATPe regulation depending on substrate availability, the kinetic characteristics of the enzyme(s) and the localization and interaction with the host. In general, NTPDase activity seems to correlate with the virulence strength of the parasite strain. A role for these enzymes is clearly shown in Leishmania, where antisense RNA and antibodies raised against parasite NTPDases impaired replication and compromised host invasion [84,153,158]. Whether parasite NTPDase activity interferes with the host inflammatory response and plays a role in purine salvage should be studied in more detail.
ATPe and by products not only signal through purinergic signaling, but following ecto-nucleotidase activity they can be degraded sequentially to nucleosides and purines. In the metabolic competition of parasites and their hosts, parasites developed high performance purine salvage mechanisms, which are especially important for apicomplexan purine auxotrophs [192]. Two main strategies evolved in protozoa. First, as shown in L. donovani NTs, nucleoside uptake is driven actively, by means of a proton gradient [183,185]. This is unlike mammalian NTs taking purines by facilitated diffusion, which makes their activity dependent on building a favorable transmembrane gradient. Second, parasite NTs may have a several fold higher affinity for their substrates than mammalian counterparts [189].
An illustrative example of the multiple interactions of ATPe dynamics of P. falciparum infected human RBCs is shown in Figure 1, where both parasite and the host are purine auxotrophs.
For these and yet to discover protozoan proteins, bioinformatic tools, the design of specific ligands as well as co-immunoprecipitation studies are required to identify putative interacting proteins forming functional enzymes, receptors and transporters. Moreover, trafficking of parasite proteins to the host cell membrane deserves further investigation [68]. From a medical perspective, high throughput screening of potent and specific inhibitors against targeted parasite proteins might aid in finding drugs that kill parasites effectively, and have appropriate absorption, distribution, metabolism, and safety in humans [68].
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4425/10/1/16/s1, Figure S1: Sequence alignment of NTPDases used to build the evolutionary tree of Figure 2.
Author Contributions
Conceptualization, P.J.S.; Resources, P.J.S.; Writing—original draft preparation, N.L., Z.B., J.S., P.J.S.; Writing—review and editing, N.L., Z.B, C.L.A., M.F.L.D., J.S., M.A.O., V.H., P.J.S; Visualization, N.L., Z.B, C.L.A., M.F.L.D., J.S., M.A.O., V.H., P.J.S; Supervision, C.L.A., P.J.S.; Project administration, M.F.L.D., P.J.S.; Funding acquisition, M.F.L.D. and P.J.S.
Funding
This research was funded by Consejo Nacional de Investigaciones Científicas y Técnicas (Grant PIP 112 20110100639 and Grant PDTS/CIN 2014 193); Grant ECOS Sud A15S01; the Universidad de Buenos Aires (Grant 200201701001 52BA) and the Agencia Nacional de Promoción Científica y Técnica (Grant PICT 2014-0327, Grant PICT 2016-1041).
Acknowledgments
M.F.L.D., C.L.A., J.S., V.H., and P.J.S. are career researchers at Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). M.A.O. is full professor at Paris Diderot University. N.L. and Z.B. are doctoral students from Universidad de Buenos Aires. We thank Karina Alleva and Martin Noguera for technical advice on building the phylogenetic tree.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Ramdani, G.; Langsley, G. ATP, an extracellular signaling molecule in red blood cells: A messenger for malaria? Biomed. J. 2014, 37, 284–292. [Google Scholar] [PubMed]
- Burnstock, G.; Verkhratsky, A. Purinergic Signalling and the Nervous System; Springer: Heidelberg, Germany, 2012; ISBN 9783642288630. [Google Scholar]
- Burnstock, G. Purinergic signalling: From discovery to current developments. Exp. Physiol. 2014, 99, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Banerjee, J.; Leung, C.T.; Peterson-Yantorno, K.; Stamer, W.D.; Civan, M.M. Mechanisms of ATP release, the enabling step in purinergic dynamics. Cell. Physiol. Biochem. 2011, 28, 1135–1144. [Google Scholar] [CrossRef]
- Taruno, A. ATP release channels. Int. J. Mol. Sci. 2018, 19, 808. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: Functional implications and measurement of activities. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 473–497. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.P.; Bridges, D.J.; Burchmore, R.J.S. Purine and pyrimidine transport in pathogenic protozoa: From biology to therapy. FEMS Microbiol. Rev. 2005, 29, 987–1020. [Google Scholar] [CrossRef]
- Coutinho-Silva, R.; Corrêa, G.; Sater, A.A.; Ojcius, D.M. The P2X7 receptor and intracellular pathogens: A continuing struggle. Purinergic Signal. 2009, 5, 197–204. [Google Scholar] [CrossRef]
- Coutinho-Silva, R.; Ojcius, D.M. Role of extracellular nucleotides in the immune response against intracellular bacteria and protozoan parasites. Microbes Infect. 2012, 14, 1271–1277. [Google Scholar] [CrossRef]
- Lebrun, M.; Blanchard, N. Editorial overview: Host–microbe interactions: Parasites. Curr. Opin. Microbiol. 2017, 40, viii–xi. [Google Scholar] [CrossRef]
- Savio, L.E.B.; de Mello, P.A.; da Silva, C.G.; Coutinho-Silva, R. The P2X7 receptor in inflammatory diseases: Angel or demon? Front. Pharmacol. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, C.; Shumilina, E.; Bobballa, D.; Brand, V.B.; Mahmud, H.; Lang, F.; Huber, S.M. The Plasmodium falciparum-induced anion channel of human erythrocytes is an ATP-release pathway. Pflugers Arch. Eur. J. Physiol. 2009, 457, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.L.; Schachter, J.; de Sá Pinheiro, A.A.; de Souza Silva, L.; Verstraeten, S.V.; Persechini, P.M.; Schwarzbaum, P.J. Regulation of extracellular ATP in human erythrocytes infected with Plasmodium falciparum. PLoS ONE 2014, 9, e96216. [Google Scholar] [CrossRef] [PubMed]
- Levano-Garcia, J.; Dluzewski, A.R.; Markus, R.P.; Garcia, C.R.S. Purinergic signalling is involved in the malaria parasite Plasmodium falciparum invasion to red blood cells. Purinergic Signal. 2010, 6, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Tanneur, V.; Duranton, C.; Brand, V.B.; Sandu, C.D.; Akkaya, C.; Kasinathan, R.S.; Gachet, C.; Sluyter, R.; Barden, J.A.; Wiley, J.S.; et al. Purinoceptors are involved in the induction of an osmolyte permeability in malaria-infected and oxidized human erythrocytes. FASEB J. 2006, 20, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Fleck, S.L.; Birdsall, B.; Babon, J.; Dluzewski, A.R.; Martin, S.R.; Morgan, W.D.; Angov, E.; Kettleborough, C.A.; Feeney, J.; Blackman, M.J.; et al. Suramin and suramin analogues inhibit merozoite surface protein-1 secondary processing and erythrocyte invasion by the malaria parasite Plasmodium falciparum. J. Biol. Chem. 2003, 278, 47670–47677. [Google Scholar] [CrossRef]
- de Souza, M.C.; De Assis, E.A.; Saar Gomes, R.; De Almeida Marques-Da-Silva, E.; Melo, M.N.; Lopes Rangel Fietto, J.; Crocco Alfonso, L.C.; Afonso, C. The influence of ecto-nucleotidases on Leishmania amazonensis infection and immune response in C57B/6 mice. Acta Trop. 2010, 115, 262–269. [Google Scholar] [CrossRef]
- Leite, P.M.; Gomes, R.S.; Figueiredo, A.B.; Serafim, T.D.; Tafuri, W.L.; de Souza, C.C.; Moura, S.A.L.; Fietto, J.L.R.; Melo, M.N.; Ribeiro-Dias, F.; et al. Ecto-nucleotidase activities of promastigotes from Leishmania (Viannia) braziliensis relates to parasite infectivity and disease clinical outcome. PLoS Negl. Trop. Dis. 2012, 6, e1850. [Google Scholar] [CrossRef]
- Khakh, B.S.; Alan North, R. P2X receptors as cell-surface ATP sensors in health and disease. Nature 2006, 442, 527–532. [Google Scholar] [CrossRef]
- Burnstock, G. Blood cells: An historical account of the roles of purinergic signalling. Purinergic Signal. 2015, 11, 411–434. [Google Scholar] [CrossRef]
- Coddou, C.; Yan, Z.; Obsil, T.; Huidobro-Toro, J.P.; Stojilkovic, S.S. Activation and regulation of purinergic P2X receptor channels. Pharmacol. Rev. 2011, 63, 641–683. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Ralevic, V.; Burnstock, G. Receptor for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- Espelt, M.V.; de Tezanos Pinto, F.; Alvarez, C.L.; Alberti, G.S.; Incicco, J.; Leal Denis, M.F.; Davio, C.; Schwarzbaum, P.J. On the role of ATP release, ectoATPase activity, and extracellular ADP in the regulatory volume decrease of Huh-7 human hepatoma cells. Am. J. Physiol. Cell Physiol. 2013, 304, C1013–C1026. [Google Scholar] [CrossRef]
- Pafundo, D.E.; Alvarez, C.L.; Krumschnabel, G.; Schwarzbaum, P.J. A volume regulatory response can be triggered by nucleosides in human erythrocytes, a perfect osmometer no longer. J. Biol. Chem. 2010, 285, 6134–6144. [Google Scholar] [CrossRef] [PubMed]
- Fountain, S.J. Primitive ATP-activated P2X receptors: Discovery, function and pharmacology. Front. Cell. Neurosci. 2013, 7, 2007–2013. [Google Scholar] [CrossRef]
- Kaczmarek-Hájek, K.; Lörinczi, É.; Hausmann, R.; Nicke, A. Molecular and functional properties of P2X receptors-recent progress and persisting challenges. Purinergic Signal. 2012, 8, 375–417. [Google Scholar] [CrossRef]
- Newbolt, A.; Stoop, R.; Virginio, C.; Surprenant, A.; North, R.A.; Buell, G.; Rassendren, F. Membrane topology of an ATP-gated ion channel (P2X receptor). J. Biol. Chem. 1998, 273, 15177–15182. [Google Scholar] [CrossRef] [PubMed]
- Abbracchio, M.P.; Burnstock, G.; Verkhratsky, A.; Zimmermann, H. Purinergic signalling in the nervous system: An overview. Trends Neurosci. 2009, 32, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Y. Insights into the channel gating of P2X receptors from structures, dynamics and small molecules. Acta Pharmacol. Sin. 2016, 37, 44–55. [Google Scholar] [CrossRef]
- Nicke, A.; Bäumert, H.G.; Rettinger, J.; Eichele, A.; Lambrecht, G.; Mutschler, E.; Schmalzing, G. P2X1 and P2X3 receptors form stable trimers: A novel structural motif of ligand-gated ion channels. EMBO J. 1998, 17, 3016–3028. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, S.; Jiang, L.H.; Penna, A.; North, R.A.; Rassendren, F. Identification of a trafficking motif involved in the stabilization and polarization of P2X receptors. J. Biol. Chem. 2004, 279, 29628–29638. [Google Scholar] [CrossRef] [PubMed]
- Volonté, C.; D’Ambrosi, N. Membrane compartments and purinergic signalling: The purinome, a complex interplay among ligands, degrading enzymes, receptors and transporters. FEBS J. 2009, 276, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Sachs, F. Single channel properties of P2X 2 purinoceptors. J. Gen. Physiol. 1999, 113, 695–720. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-H.; Spelta, V.; Bo, X.; Surprenant, A.; North, R.A. Status survey of the forest leopard (Panthera pardus Linnaeus, 1758) in Nepal. Subunit Arrangement P2X Receptors. 2003, 23, 8903–8910. [Google Scholar]
- Alvarez, C.L.; Corradi, G.; Lauri, N.; Marginedas-Freixa, I.; Leal Denis, M.F.; Enrique, N.; Mate, S.M.; Milesi, V.; Ostuni, M.A.; Herlax, V.; et al. Dynamic regulation of extracellular ATP in Escherichia coli. Biochem. J. 2017, 474, 1395–1416. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Burnstock, G. Biology of purinergic signalling: Its ancient evolutionary roots, its omnipresence and its multiple functional significance. BioEssays 2014, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Fountain, S.J.; Cao, L.; Young, M.T.; North, R.A. Permeation properties of a P2X receptor in the green algae Ostreococcus tauri. J. Biol. Chem. 2008, 283, 15122–15126. [Google Scholar] [CrossRef] [PubMed]
- Hefetz, A.; Keeling, C.I.; Winston, M.L.; Slessor, K.N.; Nielsen, J.; Lanfear, R.; Liebig, J.; Millar, J.G.; Hanks, L.M.; Suarez, A.V.; et al. Identification of a plant receptor for extracellular ATP. Science 2014, 343, 290–295. [Google Scholar]
- Clark, G.; Roux, S.J. Apyrases, extracellular ATP and the regulation of growth. Curr. Opin. Plant Biol. 2011, 14, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, M.J.; Ludlow, M.J. Purinergic signalling in Dictyostelium discoideum. Ph.D. Thesis, University of Leicester, Leicester, UK, 2008. [Google Scholar]
- Fountain, S.J.; Parkinson, K.; Young, M.T.; Cao, L.; Thompson, C.R.L.; North, R.A. An intracellular P2X receptor required for osmoregulation in Dictyostelium discoideum. Nature 2007, 448, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.; Ohno, Y. Neighboring glycine residues are essential for P2X2 receptor/channel function. Eur. J. Pharmacol. 1999, 370, 247–248. [Google Scholar] [CrossRef]
- Agboh, K.C.; Webb, T.E.; Evans, R.J.; Ennion, S.J. Functional characterization of a P2X receptor from Schistosoma mansoni. J. Biol. Chem. 2004, 279, 41650–41657. [Google Scholar] [CrossRef] [PubMed]
- Hayasi, M.; Takahashi, M. Ciliary adenosinetriphosphatase from a slow swimming mutant of Paramecium caudatum. J. Biol. Chem. Biol. Chem. 1979, 254, 11561–11565. [Google Scholar]
- Kim, M.Y.; Kuruvilla, H.G.; Raghu, S.; Hennessey, T.M. ATP reception and chemosensory adaptation in Tetrahymena thermophila. J. Exp. Biol. 1999, 202, 407–416. [Google Scholar] [PubMed]
- Inverso, J.A.; Song, Y.; Santos-Buch, C.A. Plasma membrane ATP receptors in Trypanosoma cruzi trypomastigotes. Receptor 1995, 5, 197–206. [Google Scholar] [PubMed]
- Pothier, F.; Forget, J.; Sullivan, R.; Couillard, P. ATP and the contractile vacuole in Amoeba proteus: Mechanism of action of exogenous ATP and related nucleotides. J. Exp. Zool. 1987, 243, 379–387. [Google Scholar] [CrossRef]
- Uyeda, T.Q.P.; Furuya, M. ATP-induced relative movement between microfilaments and microtubules in myxomycete flagellates. Protoplasma 1987, 140, 190–192. [Google Scholar] [CrossRef]
- Yue, J.; Sun, G.; Hu, X.; Huang, J. The scale and evolutionary significance of horizontal gene transfer in the choanoflagellate Monosiga brevicollis. BMC Genom. 2013, 14, 729. [Google Scholar] [CrossRef]
- King, N.; Westbrook, M.J.; Young, S.L.; Kuo, A.; Abedin, M.; Chapman, J.; Fairclough, S.; Hellsten, U.; Isogai, Y.; Letunic, I.; et al. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 2008, 451, 783–788. [Google Scholar] [CrossRef]
- Eichinger, I.; Pachebat, J.A.; Glöckner, G.; Rajandream, M.A.; Sucgang, R.; Berriman, M.; Song, J.; Olsen, R.; Szafranski, K.; Xu, Q.; et al. The genome of the social amoeba Dictyostelium discoideum. Nature 2005, 435, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Leal Denis, M.F.; Incicco, J.J.; Espelt, M.V.; Verstraeten, S.V.; Pignataro, O.P.; Lazarowski, E.R.; Schwarzbaum, P.J. Kinetics of extracellular ATP in mastoparan 7-activated human erythrocytes. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4692–4707. [Google Scholar] [CrossRef] [PubMed]
- Leal Denis, M.F.; Alvarez, H.A.; Lauri, N.; Alvarez, C.L.; Chara, O.; Schwarzbaum, P.J. Dynamic regulation of cell volume and extracellular ATP of human erythrocytes. PLoS ONE 2016, 11, e0158305. [Google Scholar] [CrossRef] [PubMed]
- Surprenant, A.; North, R.A. Signaling at Purinergic P2X Receptors. Annu. Rev. Physiol. 2009, 71, 333–359. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, V.; Fountain, S.J. Evidence for extracellular ATP as a stress signal in a single-celled organism. Eukaryot. Cell 2015, 14, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, N.; Mueller, S.N.; Heath, W.R. Cerebral malaria in mouse and man. Front. Immunol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Cowman, A.F.; Tonkin, C.J.; Tham, W.H.; Duraisingh, M.T. The molecular basis of erythrocyte invasion by malaria parasites. Cell Host Microbe 2017, 22, 232–245. [Google Scholar] [CrossRef]
- Ashley, E.A.; Phyo, P.; Woodrow, C.J. Seminar Malaria. Lancet 2018, 6736, 1–14. [Google Scholar]
- Gardner, M.J.; Hall, N.; Fung, E.; White, O.; Berriman, M.; Hyman, R.W.; Carlton, J.M.; Pain, A.; Nelson, K.E.; Bowman, S.; et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 2002, 419, 498–511. [Google Scholar] [CrossRef]
- Frech, C.; Chen, N. Genome comparison of human and non-human malaria parasites reveals species subset-specific genes potentially linked to human disease. PLoS Comput. Biol. 2011, 7, e1002320. [Google Scholar] [CrossRef]
- Budu, A.; Garcia, C.R.S. Generation of second messengers in Plasmodium. Microbes Infect. 2012, 14, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.G.; Cooke, B.M.; Cowman, A.F.; Tilley, L. Malaria parasite proteins that remodel the host erythrocyte. Nat. Rev. Microbiol. 2009, 7, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Tilley, L.; Sougrat, R.; Lithgow, T.; Hanssen, E. The twists and turns of Maurer’s cleft trafficking in P. falciparum-infected erythrocytes. Traffic 2008, 9, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.A. Why do malaria parasites increase host erythrocyte permeability? Trends Parasitol. 2014, 30, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Balabaskaran-nina, P.; Nguitragool, W.; Saggu, G.S.; Schureck, M.A.; Desai, A. CLAG3 Self-associates in malaria parasites and quantitatively determines nutrient uptake channels at the host membrane. Am. Soc. Microbiol. 2018, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chalapareddy, S.; Desai, S.A. Malaria parasite proteins involved in nutrient channels at the host erythrocyte membrane: Advances and questions for future research. Int. J. Curr. Multidiscip. Stud. 2017, 3, 619–623. [Google Scholar] [PubMed]
- Marginedas-Freixa, I.; Hattab, C.; Bouyer, G.; Halle, F.; Chene, A.; Lefevre, S.D.; Cambot, M.; Cueff, A.; Schmitt, M.; Gamain, B.; et al. TSPO ligands stimulate ZnPPIX transport and ROS accumulation leading to the inhibition of P. falciparum growth in human blood. Sci. Rep. 2016, 6, 33516. [Google Scholar] [CrossRef]
- Brochet, M.; Billker, O. Calcium signalling in malaria parasites. Mol. Microbiol. 2016, 100, 397–408. [Google Scholar] [CrossRef]
- Zipprer, E.M.; Neggers, M.; Kushwaha, A.; Rayavara, K.; Desai, S.A. A kinetic fluorescence assay reveals unusual features of Ca++ uptake in Plasmodium falciparum-infected erythrocytes. Lipids Health Dis. 2014, 13, 184. [Google Scholar] [CrossRef]
- Wasserman, M.; Alarcón, C.; Mendoza, P.M. Effects of Ca++ depletion on the asexual cell cycle of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 1982, 31, 711–717. [Google Scholar] [CrossRef]
- Cruz, L.; Juliano, M.; Budu, A.; Juliano, L.; Holder, A.A.; Blackman, M.J.; Garcia, C.R. Extracellular ATP triggers proteolysis and cytosolic Ca2+ rise in Plasmodium berghei and Plasmodium yoelii malaria parasites. Malar. J. 2012, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Kanaani, J.; Ginsburg, H. Compartment analysis of ATP in malaria-infected erythrocytes. Biochem. Int. 1988, 17, 451–459. [Google Scholar] [PubMed]
- Gorman, M.W.; Feigl, E.O.; Buffington, C.W. Human plasma ATP concentration. Clin. Chem. 2007, 53, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Gazarini, M.L.; Thomas, A.P.; Pozzan, T.; Garcia, C.R.S. Calcium signaling in a low calcium environment: How the intracellular malaria parasite solves the problem. J. Cell Biol. 2003, 161, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Adovelande, J.; Bastide, B.; Délèze, J.; Schrével, J. Cytosolic free calcium in Plasmodium falciparum-infected erythrocytes and the effect of verapamil: A cytofluorimetric study. Exp. Parasitol. 1993, 76, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.R.; Dluzewski, A.R.; Catalani, L.H.; Burting, R.; Hoyland, J.; Mason, W.T. Calcium homeostasis in intraerythrocytic malaria parasites. Eur. J. Cell Biol. 1996, 71, 409–413. [Google Scholar] [PubMed]
- Kushwaha, A.K.; Apolis, L.; Ito, D.; Desai, S.A. Increased Ca++ uptake by erythrocytes infected with malaria parasites: Evidence for exported proteins and novel inhibitors. Cell. Microbiol. 2018, 20, e12853. [Google Scholar] [CrossRef]
- Schlesinger, P.H.; Krogstad, D.J.; Desai, S.A.; McCleskey, E.W. A novel pathway for Ca++ entry into Plasmodium falciparum-infected blood cells. Am. J. Trop. Med. Hyg. 1996, 54, 464–470. [Google Scholar]
- Miller, C.M.; Boulter, N.R.; Fuller, S.J.; Zakrzewski, A.M.; Lees, M.P.; Saunders, B.M.; Wiley, J.S.; Smith, N.C. The role of the P2X7 receptor in infectious diseases. PLoS Pathog. 2011, 7, e1002212. [Google Scholar] [CrossRef]
- Sibley, L.D. Toxoplasma gondii: Perfecting an intracellular life style. Traffic 2003, 4, 581–586. [Google Scholar] [CrossRef]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Krug, U.; Zebisch, M.; Krauss, M.; Sträter, N. Structural insight into activation mechanism of Toxoplasma gondii nucleoside triphosphate diphosphohydrolases by disulfide reduction. J. Biol. Chem. 2012, 287, 3051–3066. [Google Scholar] [CrossRef] [PubMed]
- Mercier, C.; Dubremetz, J.-F.; Rauscher, B.; Lecordier, L.; Sibley, L.D.; Cesbron-Delauw, M.-F. Biogenesis of nanotubular network in Toxoplasma parasitophorous vacuole induced by parasite proteins. Mol. Biol. Cell 2002, 13, 2397–2409. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, J.; Carruthers, V.B. Host cell manipulation by the human pathogen Toxoplasma gondii. Cell Mol Life Sci. 2008, 65, 1900–1915. [Google Scholar]
- Corrêa, G.; Marques da Silva, C.; de Abreu Moreira-Souza, A.C.; Vommaro, R.C.; Coutinho-Silva, R. Activation of the P2X7 receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect. 2010, 12, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Lees, M.P.; Fuller, S.J.; Mcleod, R.; Boulter, N.R.; Catherine, M.; Zakrzewski, A.M.; Mui, E.J.; Witola, W.H.; Coyne, J.J.; Hargrave, C.; et al. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages1. J Immunol. 2010, 184, 7040–7046. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Souza, A.C.A.; Almeida-da-Silva, C.L.C.; Rangel, T.P.; Rocha, G.D.C.; Bellio, M.; Zamboni, D.S.; Vommaro, R.C.; Coutinho-Silva, R. The P2X7 receptor mediates Toxoplasma gondii control in macrophages through canonical NLRP3 inflammasome activation and reactive oxygen species production. Front. Immunol. 2017, 8, 1257. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, S.E.; Peixoto-Rangel, A.L.; Hargrave, A.C.; Roubaix, L.A.D.; Mui, E.J.; Boulter, N.R.; Miller, E.N.; Fuller, S.J.; Wiley, J.S.; Castellucci, L.; et al. Evidence for associations between the purinergic receptor P2X7 (P2RX7) and toxoplasmosis. Genes Immun. 2010, 11, 374–383. [Google Scholar] [CrossRef]
- Melo, M.B.; Jensen, K.D.C.; Saeij, J.P.J. Toxoplasma gondii effectors are master regulators of the inflammatory response. Trends Parasitol. 2011, 27, 487–495. [Google Scholar] [CrossRef]
- Sansom, F.M. The role of the NTPDase enzyme family in parasites: What do we know, and where to from here? Parasitology 2012, 139, 963–980. [Google Scholar] [CrossRef]
- Asai, T.; Miura, S.; Sibley, L.D.; Okabayashi, H.; Takeuchi, T. Biochemical and molecular characterization of nucleoside triphosphate hydrolase isozymes from the parasitic protozoan Toxoplasma gondii. J. Exp. Psychol. Gen. 1995, 270, 11391–11397. [Google Scholar] [CrossRef]
- Coutinho-Silva, R.; Morandini, A.; Savio, L.B. The role of P2X7 receptor in infectious inflammatory diseases and the influence of ectonucleotidases. Biomed. J. 2014, 37, 169. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Costales, M.G.; Cavanaugh, C.; Williams, K. Extracellular adenosine generation in the regulation of pro-inflammatory responses and pathogen colonization. Biomolecules 2015, 5, 775–792. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.L.; Sarti, A.C.; Di Virgilio, F. Extracellular nucleotides and nucleosides as signalling molecules. Immunol. Lett. 2018. [Google Scholar] [CrossRef] [PubMed]
- Dunay, I.R.; Sibley, L.D. Monocytes mediate mucosal immunity to Toxoplasma gondii. Curr. Opin. Immunol. 2010, 22, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Säve, S.; Mohlin, C.; Vumma, R.; Persson, K. Activation of adenosine A2Areceptors inhibits neutrophil transuroepithelial migration. Infect. Immun. 2011, 79, 3431–3437. [Google Scholar] [CrossRef] [PubMed]
- Mahamed, D.A.; Toussaint, L.E.; Bynoe, M.S. CD73-generated adenosine is critical for immune regulation during Toxoplasma gondii infection. Infect. Immun. 2015, 83, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Denkers, E.Y.; Butcher, B.A. Sabotage and exploitation in macrophages parasitized by intracellular protozoans. Trends Parasitol. 2005, 21, 35–41. [Google Scholar] [CrossRef]
- Chaves, S.P.; Torres-Santos, E.C.; Marques, C.; Figliuolo, V.R.; Persechini, P.M.; Coutinho-Silva, R.; Rossi-Bergmann, B. Modulation of P2X7purinergic receptor in macrophages by Leishmania amazonensis and its role in parasite elimination. Microbes Infect. 2009, 11, 842–849. [Google Scholar] [CrossRef]
- Chaves, M.M.; Marques-da-Silva, C.; Monteiro, A.P.T.; Canetti, C.; Coutinho-Silva, R. Leukotriene B4 modulates P2X7 receptor-mediated Leishmania amazonensis elimination in murine macrophages. J. Immunol. 2014, 192, 4765–4773. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Balasubramanian, R.; Deflorian, F.; Gao, Z.-G. G protein-coupled adenosine (P1) and P2Y receptors: Ligand design and receptor interactions. Purinergic Signal. 2012, 8, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Marques-da-Silva, C.; Chaves, M.M.; Chaves, S.P.; Figliuolo, V.R.; Meyer-Fernandes, J.R.; Corte-Real, S.; Lameu, C.; Ulrich, H.; Ojcius, D.M.; Rossi-Bergmann, B.; et al. Infection with Leishmania amazonensis upregulates purinergic receptor expression and induces host-cell susceptibility to UTP-mediated apoptosis. Cell. Microbiol. 2011, 13, 1410–1428. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Rai, A.K. Dependence of Leishmania parasite on host derived ATP: An overview of extracellular nucleotide metabolism in parasite. J. Parasit. Dis. 2018. [Google Scholar] [CrossRef]
- Rai, A.K.; Thakur, C.P.; Velpandian, T.; Sharma, S.K.; Ghosh, B.; Mitra, D.K. High concentration of adenosine in human visceral leishmaniasis despite increased ADA and decreased CD73. Parasite Immunol. 2011, 33, 632–636. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Marques-da-Silva, E.; de Oliveira, J.C.; Figueiredo, A.B.; de Souza Lima Júnior, D.; Carneiro, C.M.; Rangel Fietto, J.L.; Crocco Afonso, L.C. Extracellular nucleotide metabolism in Leishmania: Influence of adenosine in the establishment of infection. Microbes Infect. 2008, 10, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.H.F.; Sacramento, L.A.; Quirino, G.F.S.; Ferreira, M.D.; Benevides, L.; Santana, A.K.M.; Cunha, F.Q.; Almeida, R.P.; Silva, J.S.; Carregaro, V. Leishmania infantum parasites subvert the host inflammatory response through the adenosine A2A receptor to promote the establishment of infection. Front. Immunol. 2017, 8, 815. [Google Scholar] [CrossRef] [PubMed]
- Mahaut-smith, M.P.; Jones, S.; Evans, R.J. The P2X1 receptor and platelet function. Purinergic Signal. 2011, 7, 341–356. [Google Scholar] [CrossRef]
- Angchaisuksiri, P. Coagulopathy in malaria. Thromb. Res. 2014, 133, 5–9. [Google Scholar] [CrossRef]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. NIH Public Access 2014, 509, 310–317. [Google Scholar] [CrossRef]
- Manohar, M.; Hirsh, M.I.; Chen, Y.; Woehrle, T.; Karande, A.A.; Junger, W.G. ATP release and autocrine signaling through P2X4 receptors regulate T cell activation. J. Leukoc. Biol. 2012, 92, 787–794. [Google Scholar] [CrossRef]
- Lazarowski, E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012, 8, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta Mol. Cell Res. 2008, 1783, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, H.A.; Leipziger, J. ATP release from non-excitable cells. Purinergic Signal. 2009, 5, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Sabirov, R.Z.; Merzlyak, P.G. Plasmalemmal VDAC controversies and maxi-anion channel puzzle. Biochim. Biophys. Acta Biomembr. 2012, 1818, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G. ATP release through pannexon channels. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2015, 370, 20140191. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.M. Purinoceptor signaling in malaria-infected erythrocytes. Microbes Infect. 2012, 14, 779–786. [Google Scholar] [CrossRef]
- Sabirov, R.Z.; Okada, Y. ATP release via anion channels. Purinergic Signal. 2005, 1, 311–328. [Google Scholar] [CrossRef]
- Marginedas-Freixa, I.; Alvarez, C.L.; Moras, M.; Leal Denis, M.F.; Hattab, C.; Halle, F.; Bihel, F.; Mouro-Chanteloup, I.; Lefevre, S.D.; Le Van Kim, C.; et al. Human erythrocytes release ATP by a novel pathway involving VDAC oligomerization independent of pannexin-1. Sci. Rep. 2018, 8, 11384. [Google Scholar] [CrossRef]
- Capella-Gutierrez, S.; Marcet-Houben, M.; Gabaldón, T. Phylogenomics supports microsporidia as the earliest diverging clade of sequenced fungi. BMC Biol. 2012, 10, 47. [Google Scholar] [CrossRef]
- Didier, E.S.; Weiss, L.M. Microsporidiosis: Not just in AIDS patients. Curr. Opin. Infect. Dis. 2011, 24, 490–495. [Google Scholar] [CrossRef]
- Nakjang, S.; Williams, T.A.; Heinz, E.; Watson, A.K.; Foster, P.G.; Sendra, K.M.; Heaps, S.E.; Hirt, R.P.; Embley, T.M. Reduction and expansion inmicrosporidian genome evolution: New insights from comparative genomics. Genome Biol. Evol. 2013, 5, 2285–2303. [Google Scholar] [CrossRef] [PubMed]
- Dean, P.; Sendra, K.M.; Williams, T.A.; Watson, A.K.; Major, P.; Nakjang, S.; Kozhevnikova, E.; Goldberg, A.V.; Kunji, E.R.S.; Hirt, R.P.; et al. Transporter gene acquisition and innovation in the evolution of Microsporidia intracellular parasites. Nat. Commun. 2018, 9, 1709. [Google Scholar] [CrossRef] [PubMed]
- Heinz, E.; Hacker, C.; Dean, P.; Mifsud, J.; Goldberg, A.V.; Williams, T.A.; Nakjang, S.; Gregory, A.; Hirt, R.P.; Lucocq, J.M.; et al. Plasma membrane-located purine nucleotide transport proteins are key components for host exploitation by microsporidian intracellular parasites. PLoS Pathog. 2014, 10, e1004547. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Esser, S.; Linka, N.; Collingro, A.; Beier, C.L.; Neuhaus, H.E.; Wagner, M.; Horn, M. ATP/ADP translocases: A common feature of obligate intracellular amoebal symbionts related to Chlamydiae and Rickettsiae. J. Bacteriol. 2004, 186, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, G.; Cueff, A.; Egée, S.; Kmiecik, J.; Maksimova, Y.; Glogowska, E.; Gallagher, P.G.; Thomas, S.L.Y. Erythrocyte peripheral type benzodiazepine receptor/voltage-dependent anion channels are upregulated by Plasmodium falciparum. Blood 2011, 118, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Marginedas-Freixa, I.; Alvarez, C.L.; Moras, M.; Hattab, C.; Bouyer, G.; Chene, A.; Lefevre, S.D.; Le Van Kim, C.; Bihel, F.; Schwarzbaum, P.J.; et al. Induction of ATP Release, PPIX Transport, and cholesterol uptake by human red blood cells using a new family of TSPO ligands. Int. J. Mol. Sci. 2018, 19, 3098. [Google Scholar] [CrossRef] [PubMed]
- Lutz, P.L.; Kabler, S. Release of adenosine and ATP in the brain of the freshwater turtle (Trachemys scripta) during long-term anoxia. Brain Res. 1997, 769, 281–286. [Google Scholar] [CrossRef]
- Clarke, T.C.; Williams, O.J.S.; Martin, P.E.M.; Evans, W.H. ATP release by cardiac myocytes in a simulated ischaemia model. Inhibition by a connexin mimetic and enhancement by an antiarrhythmic peptide. Eur. J. Pharmacol. 2009, 605, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Lim To, W.K.; Kumar, P.; Marshall, J.M. Hypoxia is an effective stimulus for vesicular release of ATP from human umbilical vein endothelial cells. Placenta 2015, 36, 759–766. [Google Scholar] [CrossRef]
- Rajani, V.; Zhang, Y.; Jalubula, V.; Rancic, V.; SheikhBahaei, S.; Zwicker, J.D.; Pagliardini, S.; Dickson, C.T.; Ballanyi, K.; Kasparov, S.; et al. Release of ATP by pre-Bötzinger complex astrocytes contributes to the hypoxic ventilatory response via a Ca2+-dependent P2Y1 receptor mechanism. J. Physiol. 2018, 596, 3245–3269. [Google Scholar] [CrossRef]
- Sridharan, M.; Adderley, S.P.; Bowles, E.A.; Egan, T.M.; Stephenson, A.H.; Ellsworth, M.L.; Sprague, R.S. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. AJP Hear. Circ. Physiol. 2010, 299, H1146–H1152. [Google Scholar] [CrossRef] [PubMed]
- Maguire, M.H.; Lukas, M.C.; Rettie, J.F. Adenine Nucleotide salvage synthesis pathways of adenosine salvage in the rat heart. Biochim. Biophys. Acta 1972, 262, 108–115. [Google Scholar] [CrossRef]
- Paes-Vieira, L.; Gomes-Vieira, A.L.; Meyer-Fernandes, J.R. NTPDase activities: Possible roles on Leishmania spp. infectivity and virulence. Cell Biol. Int. 2018, 42, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Saha, A.K.; Mukhopadhyay, N.K.; Glew, R.H. A cyclic nucleotide-independent protein kinase in Leishmania donovani. Biochem. J. 1986, 240, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Robson, S.C.; Sévigny, J.; Zimmermann, H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006, 2, 409–430. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Pacheco, R.; Gatell, J.M.; Gallart, T.; Lluis, C. Enzymatic and extraenzymatic role of adenosine deaminase 1 in T-cell-dendritic cell contacts and in alterations of the immune function. Crit. Rev. Immunol. 2007, 27, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G.; Samburski, S.S.; Jalkanen, S. Soluble purine-converting enzymes circulate in human blood and regulate extracellular ATP level via counteracting pyrophosphatase and phosphotransfer reactions. FASEB J. 2003, 17, 1328–1330. [Google Scholar] [CrossRef]
- Boison, D. Adenosine kinase: Exploitation for therapeutic gain. Pharmacol. Rev. 2013, 65, 906–943. [Google Scholar] [CrossRef]
- Virtanen, S.S.; Kukkonen-Macchi, A.; Vainio, M.; Elima, K.; Harkonen, P.L.; Jalkanen, S.; Yegutkin, G.G. Adenosine Inhibits tumor cell invasion via receptor-independent mechanisms. Mol. Cancer Res. 2014, 12, 1863–1874. [Google Scholar] [CrossRef]
- Plesner, L. Ecto-ATPases: Identities and functions. Int. Rev. Cytol. 1995, 158, 141–214. [Google Scholar]
- Kukulski, F.; Levesque, S.A.; Lavoie, E.G.; Lecka, J.; Bigonnesse, F.; Knowles, A.F.; Robson, S.C.; Kirley, T.L.; Sevigny, J. Erratum: Comparative hrydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. (Purinergic Signalling (2005) vol. 1 (2) (193-204)). Purinergic Signal. 2005, 1, 293. [Google Scholar] [CrossRef]
- Handa, M.; Guidotti, G. Purification and cloning of a soluble ATP-diphosphohydrolase (Apyrase) from potato tubers (Solanum tuberosum). Biochem. Biophys. Res. Commun. 1996, 218, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Schulte Am Esch, J.; Sévigny, J.; Kaczmarek, E.; Siegel, J.B.; Imai, M.; Koziak, K.; Beaudoin, A.R.; Robson, S.C. Structural elements and limited proteolysis of CD39 influence ATP diphosphohydrolase activity. Biochemistry 1999, 38, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Choi, L.E.; Guidotti, G. N-linked oligosaccharides affect the enzymatic activity of CD39: Diverse interactions between seven N-linked glycosylation sites. Mol. Biol. Cell 2005, 16, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Knowles, A.F. The GDA1_CD39 superfamily: NTPDases with diverse functions. Purinergic Signal. 2011, 7, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Kirley, T.L.; Crawford, P.A.; Smith, T.M. The structure of the nucleoside triphosphate diphosphohydrolases (NTPDases) as revealed by mutagenic and computational modeling analyses. Purinergic Signal. 2006, 2, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Mandapathil, M.; Hilldorfer, B.; Szczepanski, M.J.; Czystowska, M.; Szajnik, M.; Ren, J.; Lang, S.; Jackson, E.K.; Gorelik, E.; Whiteside, T.L. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J. Biol. Chem. 2010, 285, 7176–7186. [Google Scholar] [CrossRef]
- Heine, P.; Braun, N.; Sévigny, J.; Robson, S.C.; Servos, J.; Zimmermann, H. The C-terminal cysteine-rich region dictates specific catalytic properties in chimeras of the ectonucleotidases NTPDase1 and NTPDase2. Eur. J. Biochem. 2001, 268, 364–373. [Google Scholar] [CrossRef]
- Zimmermann, H. Ectonucleotidases: Some recent developments and a note on nomenclature. Drug Dev. Res. 2001, 52, 44–56. [Google Scholar] [CrossRef]
- EMBL. SMART-Simple Modular Architecture Research Tool; 1996–2014 Health Sciences Library System, University of Pittsburgh: Pittsburgh, PA, USA.
- Nakaar, V.; Samuel, B.U.; Ngo, E.O.; Joiner, K.A. Targeted reduction of nucleoside triphosphate hydrolase by antisense RNA inhibits Toxoplasma gondii proliferation. J. Biol. Chem. 1999, 274, 5083–5087. [Google Scholar] [CrossRef]
- Sansom, F.M.; Robson, S.C.; Hartland, E.L. Possible effects of microbial ecto-nucleoside triphosphate diphosphohydrolases on host-pathogen interactions. Microbiol. Mol. Biol. Rev. 2008, 72, 765–781. [Google Scholar] [CrossRef] [PubMed]
- Berrêdo-Pinho, M.; Peres-Sampaio, C.E.; Chrispim, P.P.M.; Belmont-Firpo, R.; Lemos, A.P.; Martiny, A.; Vannier-Santos, M.A.; Meyer-Fernandes, J.R. A Mg-dependent ecto-ATPase in Leishmania amazonensis and its possible role in adenosine acquisition and virulence. Arch. Biochem. Biophys. 2001, 391, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.M.; Martins-Duarte, E.S.; Ferraro, R.B.; Fonseca de Souza, A.L.; Gomes, M.T.; Lopes, A.H.C.S.; Vannier-Santos, M.A.; Santos, A.L.S.; Meyer-Fernandes, J.R. Leishmania amazonensis: Biological and biochemical characterization of ecto-nucleoside triphosphate diphosphohydrolase activities. Exp. Parasitol. 2006, 114, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Sansom, F.M.; Newton, H.J.; Crikis, S.; Cianciotto, N.P.; Cowan, P.J.; d’Apice, A.J.F.; Hartland, E.L. A bacterial ecto-triphosphate diphosphohydrolase similar to human CD39 is essential for intracellular multiplication of Legionella pneumophila. Cell. Microbiol. 2007, 9, 1922–1935. [Google Scholar] [CrossRef] [PubMed]
- Nakaar, V.; Beckers, C.J.M.; Polotsky, V.; Joiner, K.A. Basis for substrate specificity of the Toxoplasma gondii nucleoside triphosphate hydrolase. Mol. Biochem. Parasitol. 1998, 97, 209–220. [Google Scholar] [CrossRef]
- Silverman, J.A.; Qi, H.; Riehl, A.; Beckers, C.; Nakaar, V.; Joiner, K.A. Induced activation of the Toxoplasma gondii nucleoside triphosphate hydrolase leads to depletion of host cell ATP levels and rapid exit of intracellular parasites from infected cells. J. Biol. Chem. 1998, 273, 12352–12359. [Google Scholar] [CrossRef] [PubMed]
- Bermudes, D.; Peck, K.R.; Afifi, M.A.; Beckers, C.J.M.; Joiner, K.A. Tandemly repeated genes encode nucleoside triphosphate hydrolase isoforms secreted into the parasitophorous vacuole of Toxoplasma gondii. J. Biol. Chem. 1994, 269, 29252–29260. [Google Scholar] [PubMed]
- Sibley, L.D.; Niesman, I.R.; Asai, T.; Takeuchi, T. Toxoplasma gondii: Secretion of a potent nucleoside triphosphate hydrolase into the parasitophorous vacuole. Exp. Parasitol. 1994, 79, 301–311. [Google Scholar] [CrossRef]
- Kikuchi, T.; Furuta, T.; Kojima, S. Membrane localization and demonstration of isoforms of nucleoside triphosphate hydrolase from Toxoplasma gondii. Parasitology 2001, 122, S0031182000007101. [Google Scholar] [CrossRef]
- Montalbetti, N.; Leal Denis, M.F.; Pignataros, O.P.; Kobatake, E.; Lazarowski, E.R.; Schwarzbaum, P.J. Homeostasis of extracellular ATP in human erythrocytes. J. Biol. Chem. 2011, 286, 38397–38407. [Google Scholar] [CrossRef]
- Pastor-Fernández, I.; Regidor-Cerrillo, J.; Álvarez-García, G.; Marugán-Hernández, V.; García-Lunar, P.; Hemphill, A.; Ortega-Mora, L.M. The tandemly repeated NTPase (NTPDase) from Neospora caninum is a canonical dense granule protein whose RNA expression, protein secretion and phosphorylation coincides with the tachyzoite egress. Parasites Vectors 2016, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, F.; Machado, F.S.; Burleigh, B.A.; Jelicks, L.A.; Scherer, E.; Mukherjee, S.; Lisanti, M.P.; Weiss, L.M.; Garg, N.J.; Tanowitz, H.B. Mechanisms of Trypanosoma cruzi persistence in Chagas disease. Cell Microbiol. 2013, 14, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.F.; Pôssa, M.A.S.; Bastos, M.S.; Guedes, P.M.M.; Almeida, M.R.; DeMarco, R.; Verjovski-Almeida, S.; Bahia, M.T.; Fietto, J.L.R. Influence of ecto-nucleoside triphosphate diphosphohydrolase activity on Trypanosoma cruzi infectivity and virulence. PLoS Negl. Trop. Dis. 2009, 3, e387. [Google Scholar] [CrossRef] [PubMed]
- WHO Chagas Disease. American Trypanosomiasis. Available online: https://www.who.int/chagas/en/ (accessed on 19 December 2018).
- Fietto, J.L.R.; DeMarco, R.; Nascimento, I.P.; Castro, I.M.; Carvalho, T.M.U.; De Souza, W.; Bahia, M.T.; Alves, M.J.M.; Verjovski-Almeida, S. Characterization and immunolocalization of an NTP diphosphohydrolase of Trypanosoma cruzi. Biochem. Biophys. Res. Commun. 2004, 316, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Schnurr, M.; Then, F.; Galambos, P.; Scholz, C.; Siegmund, B.; Endres, S.; Eigler, A. Extracellular ATP and TNF- synergize in the activation and maturation of human dendritic cells. J. Immunol. 2000, 165, 4704–4709. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H. Extracellular metabolism of ATP and other nucleotides. Naunyn. Schmiedebergs. Arch. Pharmacol. 2000, 362, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.C.; Novaes, R.D.; Cupertino, M.C.; Bastos, D.S.S.; Klein, R.C.; Silva, E.A.M.; Fietto, J.L.R.; Talvani, A.; Bahia, M.T.; Oliveira, L.L. Concomitant benznidazole and suramin chemotherapy in mice infected with a virulent strain of Trypanosoma cruzi. Antimicrob. Agents Chemother. 2015, 59, 5999–6006. [Google Scholar] [CrossRef] [PubMed]
- Bisaggio, D.F.R.; Peres-Sampaio, C.E.; Meyer-Fernandes, J.R.; Souto-Padrón, T. Ecto-ATPase activity on the surface of Trypanosoma cruzi and its possible role in the parasite-host cell interaction. Parasitol. Res. 2003, 91, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Mariotini-Moura, C.; Bastos, M.S. e.; de Castro, F.F.; Trindade, M.L.; de Souza Vasconcellos, R.; Neves-do-Valle, M.A.A.; Moreira, B.P.; de Freitas Santos, R.; de Oliveira, C.M.; Cunha, L.C.S.; et al. Trypanosoma cruzi nucleoside triphosphate diphosphohydrolase 1 (TcNTPDase-1) biochemical characterization, immunolocalization and possible role in host cell adhesion. Acta Trop. 2014, 130, 140–147. [Google Scholar] [CrossRef]
- de Souza Leite, M.; Thomaz, R.; Fonseca, F.V.; Panizzutti, R.; Vercesi, A.E.; Meyer-Fernandes, J.R. Trypanosoma brucei brucei: Biochemical characterization of ecto-nucleoside triphosphate diphosphohydrolase activities. Exp. Parasitol. 2007, 115, 315–323. [Google Scholar] [CrossRef]
- Matthews, K.R. The developmental cell biology of Trypanosoma brucei. J. Cell Sci. 2005, 118, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Berriman, M.; Ghedin, E.; Hertz-Fowler, C.; Blandin, G.; Renauld, H.; Bartholomeu, D.C.; Lennard, N.J.; Caler, E.; Hamlin, N.E.; Haas, B.; et al. The genome of the African trypanosome Trypanosoma brucei. Science 2005, 309, 416–422. [Google Scholar] [CrossRef]
- Balaña-Fouce, R.; Reguera, R.M. RNA interference in Trypanosoma brucei: A high-throughput engine for functional genomics in trypanosomatids? TRENDS Parasitol. 2007, 23, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Peacock, C.S.; Seeger, K.; Harris, D.; Murphy, L.; Ruiz, J.C.; Quail, M.A.; Peters, N.; Adlem, E.; Tivey, A.; Aslett, M.; et al. 2008 Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat. Genet. 2008, 39, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Sansom, F.M.; Ralton, J.E.; Sernee, M.F.; Cohen, A.M.; Hooker, D.J.; Hartland, E.L.; Naderer, T.; McConville, M.J. Golgi-located NTPDase1 of Leishmania major is required for lipophosphoglycan elongation and normal lesion development whereas secreted NTPDase2 is dispensable for virulence. PLoS Negl. Trop. Dis. 2014, 8, e3402. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Fernandes, J.R.; Dutra, P.M.L.; Rodrigues, C.O.; Saad-Nehme, J.; Lopes, A.H.C.S. Mg-dependent ecto-ATPase activity in Leishmania tropica. Arch. Biochem. Biophys. 1997, 341, 40–46. [Google Scholar] [CrossRef]
- Borges-Pereira, L.; Meissner, K.A.; Wrenger, C.; Garcia, C.R.S. Plasmodium falciparum GFP-E-NTPDase expression at the intraerythrocytic stages and its inhibition blocks the development of the human malaria parasite. Purinergic Signal. 2017, 13, 267–277. [Google Scholar] [CrossRef]
- Dos Santos-Rodrigues, A.; Grañé-Boladeras, N.; Bicket, A.; Coe, I.R. Nucleoside transporters in the purinome. Neurochem. Int. 2014, 73, 229–237. [Google Scholar] [CrossRef]
- Landfear, S.M.; Ullman, B.; Carter, N.S.; Sanchez, M.A. Nucleoside and nucleobase transporters in parasitic protozoa. Eukaryot. Cell 2004, 3, 245–254. [Google Scholar] [CrossRef]
- Yao, S.Y.M.; Ng, A.M.L.; Cass, C.E.; Baldwin, S.A.; Young, J.D. Nucleobase transport by human equilibrative nucleoside transporter 1 (hENT1). J. Biol. Chem. 2011, 286, 32552–32562. [Google Scholar] [CrossRef]
- Landfear, S.M. Nutrient transport and pathogenesis in selected parasitic protozoa. Eukaryot. Cell 2011, 10, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Boswell-Casteel, R.C.; Hays, F.A. Equilibrative nucleoside transporters—A review. Nucleosides Nucleotides Nucleic Acids 2016, 36, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Löffler, M.; Morote-Garcia, J.C.; Eltzschig, S.A.; Coe, I.R.; Eltzschig, H.K. Physiological roles of vascular nucleoside transporters. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Hyde, R.J.; Cass, C.E.; Young, J.D.; Baldwin, S.A. The ENT family of eukaryote nucleoside and nucleobase transporters: Recent advances in the investigation of structure/function relationships and the identification of novel isoforms. Mol. Membr. Biol. 2001, 18, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Dean, P.; Major, P.; Nakjang, S.; Hirt, R.P.; Embley, T.M. Transport proteins of parasitic protists and their role in nutrient salvage. Front. Plant Sci. 2014, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.A.; Tryon, R.; Green, J.; Boor, I.; Landfear, S.M. Six related nucleoside/nucleobase transporters from Trypanosoma brucei exhibit distinct biochemical functions. J. Biol. Chem. 2002, 277, 21499–21504. [Google Scholar] [CrossRef] [PubMed]
- Mäser, P.; Sütterlin, C.; Kralli, A.; Kaminsky, R. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science 1999, 285, 242–244. [Google Scholar]
- Carter, N.S.; Mamoun, C.B.; Liu, W.; Silva, E.O.; Landfear, S.M.; Goldberg, D.E.; Ullman, B. Isolation and functional characterization of the PfNT1 nucleoside transporter gene from Plasmodium falciparum. J. Biol. Chem. 2000, 275, 10683–10691. [Google Scholar] [CrossRef]
- Carter, N.S.; Landfear, S.M.; Ullman, B. Nucleoside transporters of parasitic protozoa. Trends Parasitol. 2001, 17, 142–145. [Google Scholar] [CrossRef]
- Berg, M.; Van der Veken, P.; Goeminne, A.; Haemers, A.; Augustyns, K. Inhibitors of the purine salvage pathway: A valuable approach for antiprotozoal chemotherapy? Curr. Med. Chem. 2010, 17, 2456–2481. [Google Scholar] [CrossRef]
- Cassera, M.B.; Zhang, Y.; Hazleton, K.Z.; Schramm, V.L. Purine and pyrimidine pathways as targets in Plasmodium falciparum. Curr. Top. Med. Chem. 2011, 4511, 2103–2115. [Google Scholar] [CrossRef]
- Hassan, H.F.; Coombs, G.H. Purine and pyrimidine metabolism in parasitic protozoa. FEMS Microbiol. Lett. 1988, 54, 47–83. [Google Scholar]
- Riegelhaupt, P.M.; Cassera, M.B.; Fröhlich, R.F.G.; Hazleton, K.Z.; Hefter, J.J.; Schramm, V.L.; Akabas, M.H. Transport of purines and purine salvage pathway inhibitors by the Plasmodium falciparum equilibrative nucleoside transporter PfENT1. Mol. Biochem. Parasitol. 2010, 169, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.C.; Beckers, C.J.M.; Joiner, K.A. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii funtions as a molecular sieve. Cell Biol. 1994, 91, 509–513. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).