Residual Tumor Resection After Anti-PD-1 Therapy: A Promising Treatment Strategy for Overcoming Immune Evasive Phenotype Induced by Anti-PD-1 Therapy in Gastric Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. IHC Staining
2.3. Assessment of IHC Staining
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Patients Treated with Chemotherapy and Anti-PD-1 Therapy
3.2. Prognostic Factors
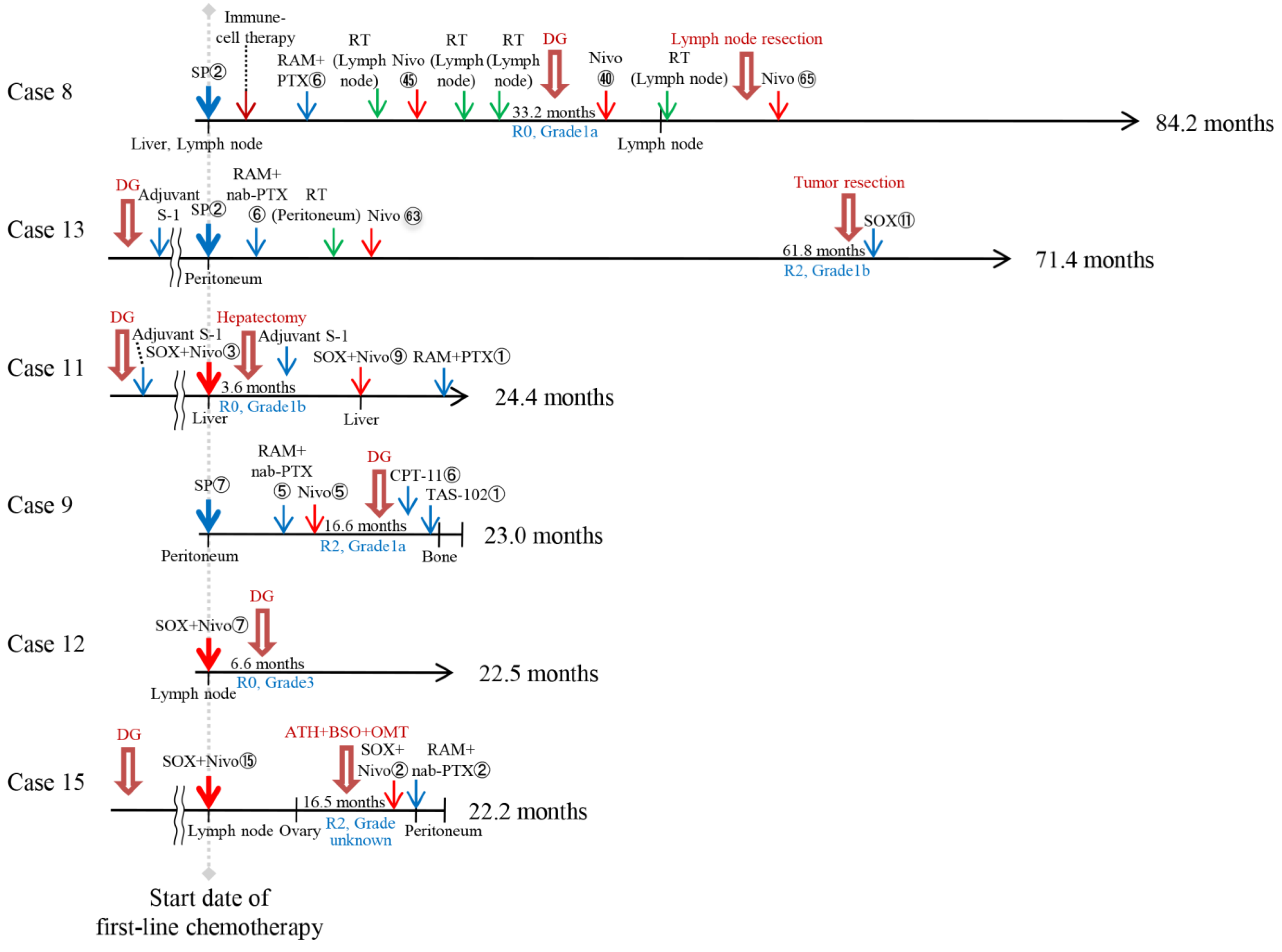
3.3. Characteristics of Patients in the TR After Chemotherapy or Anti-PD-1 Therapy
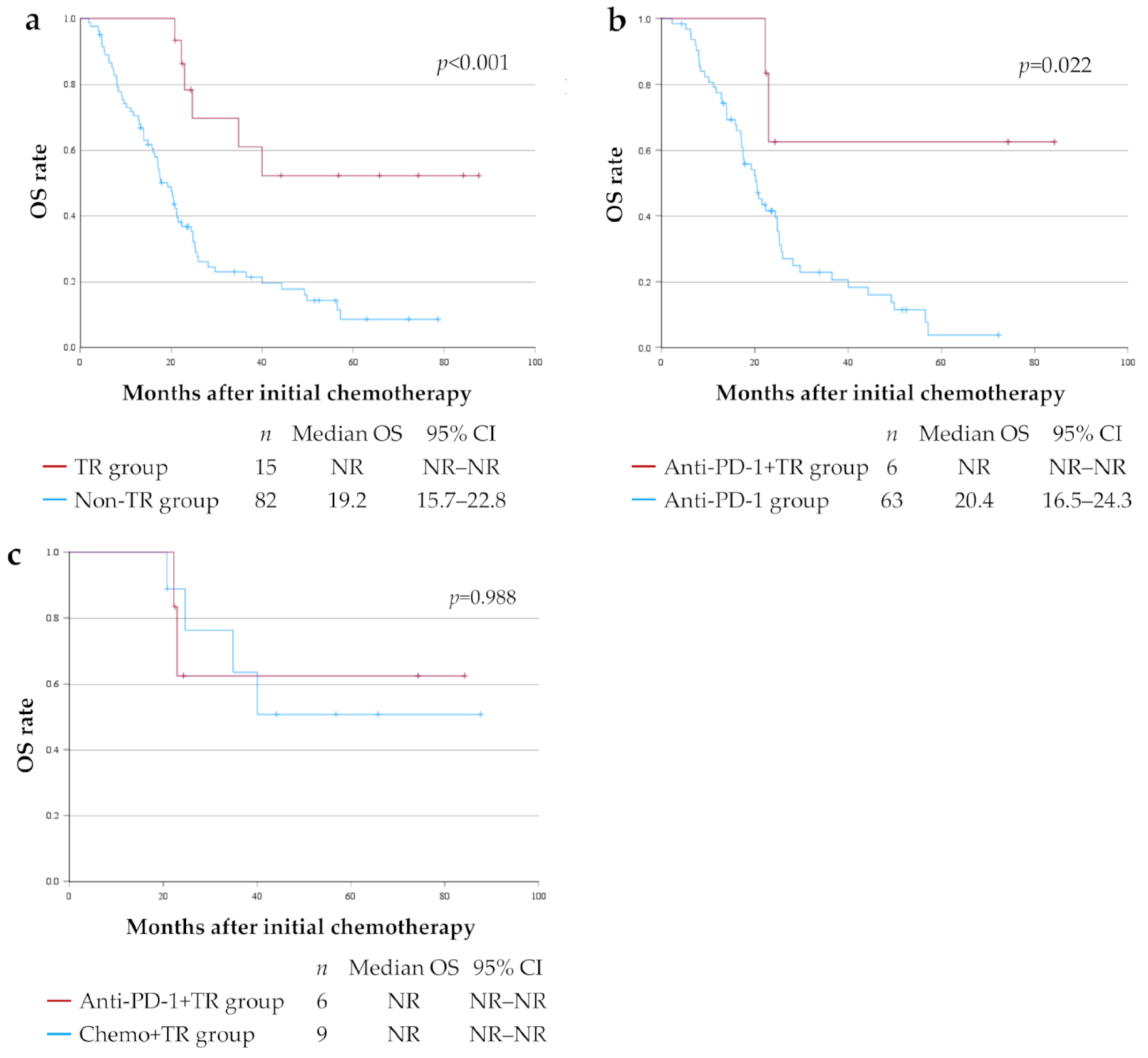
3.4. OS for Each Treatment Group
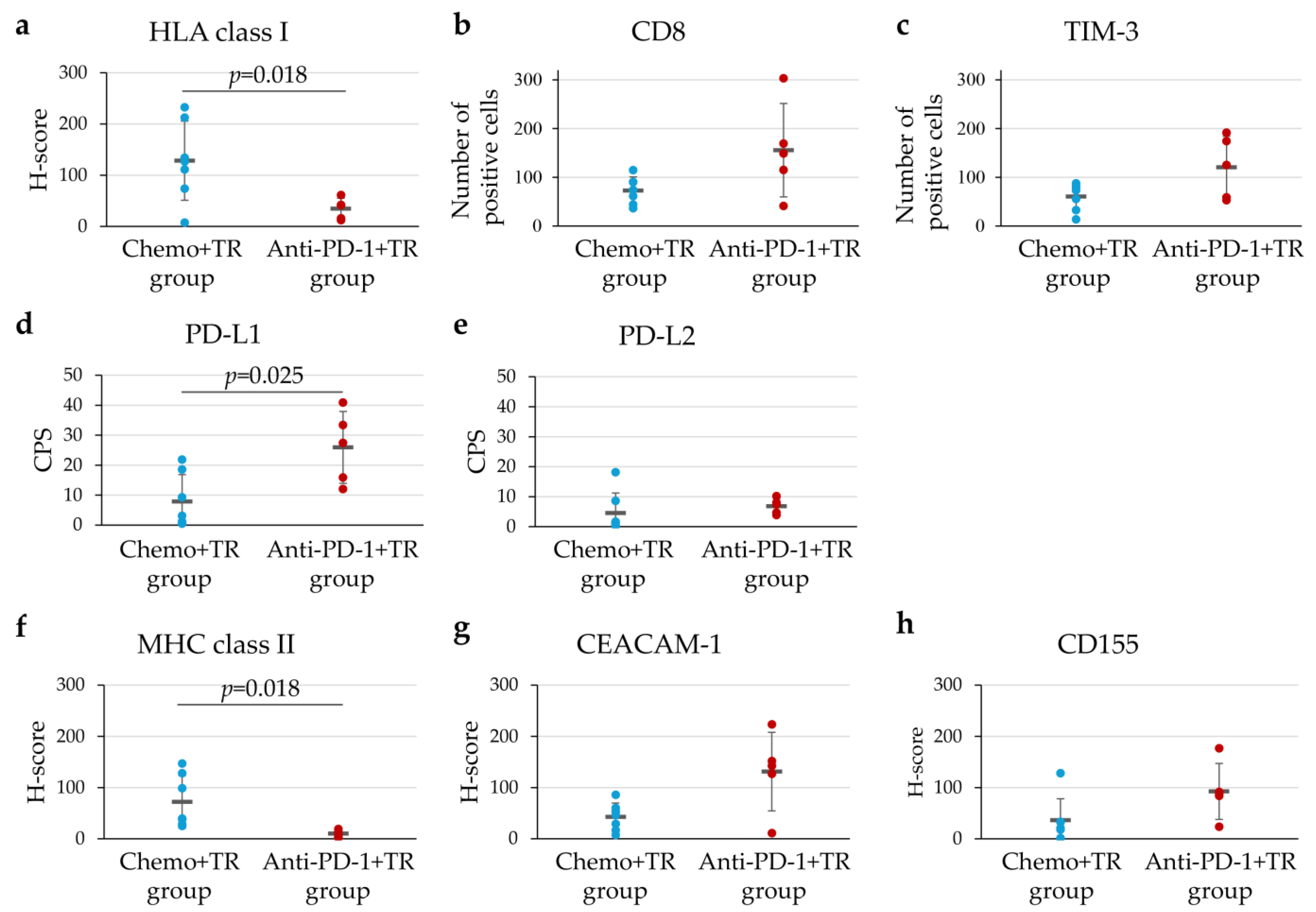
3.5. Characteristics of TIME in Unresectable Advanced or Recurrent G/GEJ Cancer Treated with Chemotherapy or Immunotherapy
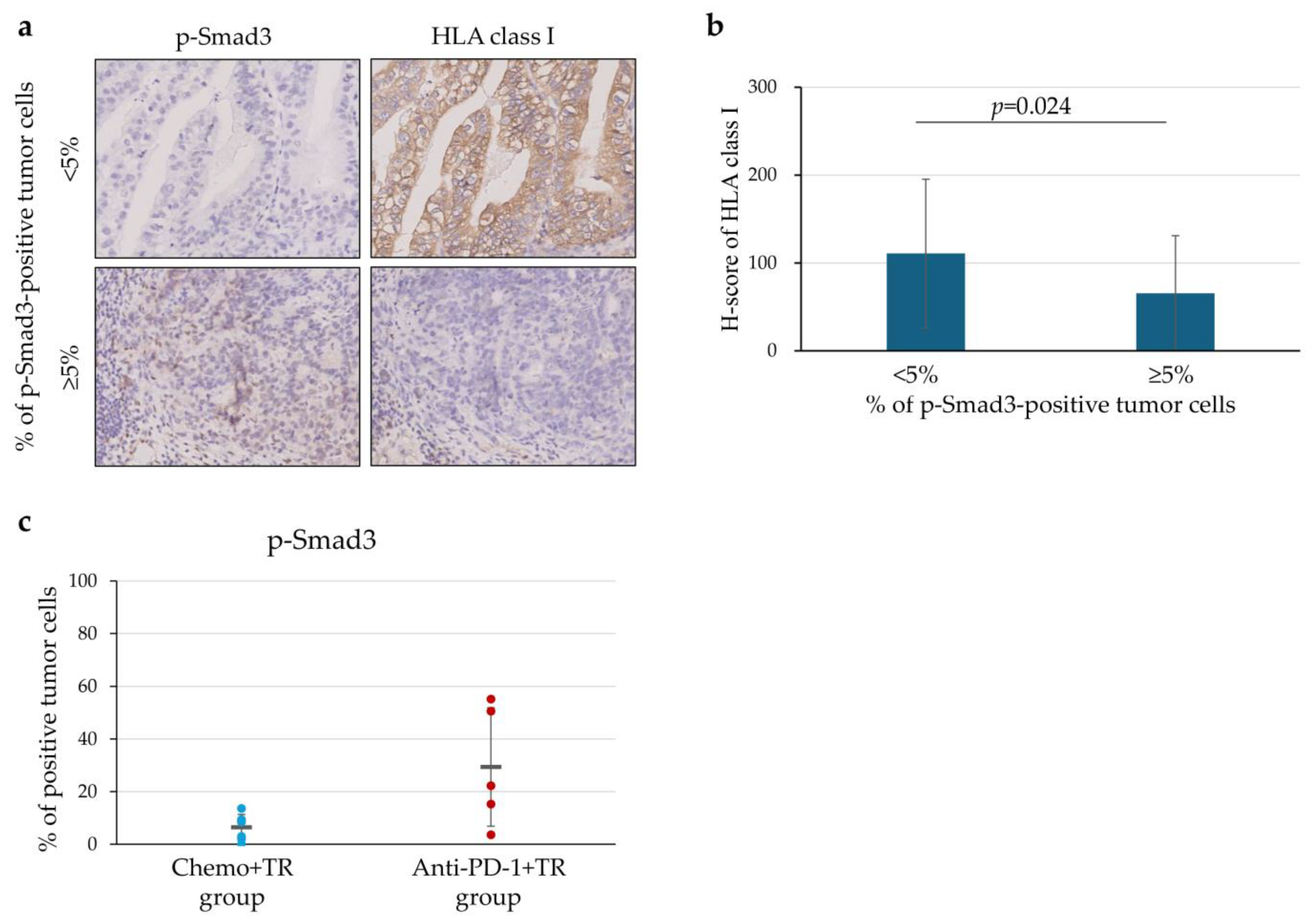
3.6. Downregulation of HLA Class I Expression in Relation to p-Smad3 on the Tumor Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Alb | Albumin |
| ALP | Alkaline phosphatase |
| CA19-9 | Carbohydrate antigen 19-9 |
| CEA | Carcinoembryonic antigen |
| CEACAM-1 | Carcinoembryonic antigen-related adhesion molecule-1 |
| CI | Confidence interval |
| CPS | Combined positive score |
| Cre | Creatinine |
| CT | Computed tomography |
| CTL | Cytotoxic T lymphocyte |
| ECOG-PS | Eastern Cooperative Oncology Group performance status |
| G/GEJ | Gastric/Gastroesophageal junction |
| HER2 | Human epidermal growth factor receptor 2 |
| HLA | Human leukocyte antigen |
| IFN | Interferon |
| IHC | Immunohistochemistry |
| LAG-3 | Lymphocyte activation gene 3 |
| MHC | Major histocompatibility complex |
| NLR | Neutrophil-to-lymphocyte ratio |
| ORR | Objective response rate |
| OS | Overall survival |
| PD-1 | Programmed death 1 receptor |
| PD-L1 | Programmed death ligand 1 |
| p-Smad3 | Phospho-Smad3 |
| SD | Standard deviation |
| TGF | Transforming growth factor |
| TIGIT | T-cell immunoglobulin and ITIM domain |
| TIM-3 | T cell immunoglobulin and mucin domain-3 |
| TIME | Tumor immune microenvironment |
| TLS | Tertiary lymphoid structures |
| TME | Tumor microenvironment |
| TR | Tumor resection |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Kang, Y.K.; Chen, L.T.; Ryu, M.H.; Oh, D.Y.; Oh, S.C.; Chung, H.C.; Lee, K.W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Rha, S.Y.; Oh, D.Y.; Yanez, P.; Bai, Y.; Ryu, M.H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.K.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Shitara, K.; Ajani, J.A.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Lordick, F.; Van Cutsem, E.; Gallego Plazas, J.; Huang, J.; et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: The randomized, phase 3 GLOW trial. Nat. Med. 2023, 29, 2133–2141. [Google Scholar] [CrossRef]
- Shitara, K.; Lordick, F.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yamaguchi, K.; Okumura, N.; Tanahashi, T.; Kodera, Y. Is conversion therapy possible in stage IV gastric cancer: The proposal of new biological categories of classification. Gastric Cancer 2016, 19, 329–338. [Google Scholar] [CrossRef]
- Choe, H.J.; Kim, J.W.; Han, S.H.; Lee, J.H.; Ahn, S.H.; Park, D.J.; Kim, J.W.; Kim, Y.J.; Lee, H.S.; Kim, J.H.; et al. Conversion Surgery in Metastatic Gastric Cancer and Cancer Dormancy as a Prognostic Biomarker. Cancers 2019, 12, 86. [Google Scholar] [CrossRef]
- Ohnuma, H.; Sato, Y.; Onoyama, N.; Hamaguchi, K.; Hayasaka, N.; Sato, M.; Murase, K.; Takada, K.; Miyanishi, K.; Murakami, T.; et al. Survival benefit of conversion surgery after intensive chemotherapy for unresectable metastatic gastric cancer: A propensity score-matching analysis. J. Cancer Res. Clin. Oncol. 2021, 147, 2385–2396. [Google Scholar] [CrossRef]
- Yoshida, K.; Yasufuku, I.; Terashima, M.; Young Rha, S.; Moon Bae, J.; Li, G.; Katai, H.; Watanabe, M.; Seto, Y.; Hoon Noh, S.; et al. International Retrospective Cohort Study of Conversion Therapy for Stage IV Gastric Cancer 1 (CONVO-GC-1). Ann. Gastroenterol. Surg. 2022, 6, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shklovskaya, E.; Lim, S.Y.; Carlino, M.S.; Menzies, A.M.; Stewart, A.; Pedersen, B.; Irvine, M.; Alavi, S.; Yang, J.Y.H.; et al. Transcriptional downregulation of MHC class I and melanoma de-differentiation in resistance to PD-1 inhibition. Nat. Commun. 2020, 11, 1897. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Mimura, K.; Nakajima, S.; Saito, K.; Min, A.K.T.; Okayama, H.; Saito, M.; Momma, T.; Saze, Z.; Ohtsuka, M.; et al. Immune escape mechanism behind resistance to anti-PD-1 therapy in gastrointestinal tract metastasis in malignant melanoma patients with multiple metastases. Cancer Immunol. Immunother. 2022, 71, 2293–2300. [Google Scholar] [CrossRef]
- Endo, E.; Okayama, H.; Saito, K.; Nakajima, S.; Yamada, L.; Ujiie, D.; Kase, K.; Fujita, S.; Endo, H.; Sakamoto, W.; et al. A TGFbeta-Dependent Stromal Subset Underlies Immune Checkpoint Inhibitor Efficacy in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Colorectal Cancer. Mol. Cancer Res. 2020, 18, 1402–1413. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- Mimura, K.; Kua, L.F.; Xiao, J.F.; Asuncion, B.R.; Nakayama, Y.; Syn, N.; Fazreen, Z.; Soong, R.; Kono, K.; Yong, W.P. Combined inhibition of PD-1/PD-L1, Lag-3, and Tim-3 axes augments antitumor immunity in gastric cancer-T cell coculture models. Gastric Cancer 2021, 24, 611–623. [Google Scholar] [CrossRef]
- Spranger, S.; Spaapen, R.M.; Zha, Y.; Williams, J.; Meng, Y.; Ha, T.T.; Gajewski, T.F. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl. Med. 2013, 5, 200ra116. [Google Scholar] [CrossRef]
- Tanaka, K.; Miyata, H.; Sugimura, K.; Kanemura, T.; Hamada-Uematsu, M.; Mizote, Y.; Yamasaki, M.; Wada, H.; Nakajima, K.; Takiguchi, S.; et al. Negative influence of programmed death-1-ligands on the survival of esophageal cancer patients treated with chemotherapy. Cancer Sci. 2016, 107, 726–733. [Google Scholar] [CrossRef]
- Sudo, S.; Kajiya, H.; Okano, S.; Sasaki, M.; Katsumata, Y.; Ohno, J.; Ikebe, T.; Hiraki, A.; Okabe, K. Cisplatin-induced programmed cell death ligand-2 expression is associated with metastasis ability in oral squamous cell carcinoma. Cancer Sci. 2020, 111, 1113–1123. [Google Scholar] [CrossRef]
- Axelrod, M.L.; Cook, R.S.; Johnson, D.B.; Balko, J.M. Biological Consequences of MHC-II Expression by Tumor Cells in Cancer. Clin. Cancer Res. 2019, 25, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; Bullock, B.L.; Neuwelt, A.J.; Poczobutt, J.M.; Kaspar, R.E.; Li, H.Y.; Kwak, J.W.; Hopp, K.; Weiser-Evans, M.C.M.; Heasley, L.E.; et al. Cancer Cell-Intrinsic Expression of MHC Class II Regulates the Immune Microenvironment and Response to Anti-PD-1 Therapy in Lung Adenocarcinoma. J. Immunol. 2020, 204, 2295–2307. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Zhu, C.; Kondo, Y.; Anderson, A.C.; Gandhi, A.; Russell, A.; Dougan, S.K.; Petersen, B.S.; Melum, E.; Pertel, T.; et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2015, 517, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gu, Y.; Yan, X.; Huo, C.; Wang, G.; Zhao, Y.; Teng, M.; Li, Y. Role of CD155/TIGIT in Digestive Cancers: Promising Cancer Target for Immunotherapy. Front. Oncol. 2022, 12, 844260. [Google Scholar] [CrossRef]
- Pitzalis, C.; Jones, G.W.; Bombardieri, M.; Jones, S.A. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 2014, 14, 447–462. [Google Scholar] [CrossRef]
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 307–325. [Google Scholar] [CrossRef]
- Ukita, M.; Hamanishi, J.; Yoshitomi, H.; Yamanoi, K.; Takamatsu, S.; Ueda, A.; Suzuki, H.; Hosoe, Y.; Furutake, Y.; Taki, M.; et al. CXCL13-producing CD4+ T cells accumulate in the early phase of tertiary lymphoid structures in ovarian cancer. JCI Insight 2022, 7, e157215. [Google Scholar] [CrossRef]
- Nakazawa, N.; Sohda, M.; Hosoi, N.; Watanabe, T.; Kumakura, Y.; Yamashita, T.; Tanaka, N.; Saito, K.; Kimura, A.; Kasuga, K.; et al. Conversion Surgery After Chemotherapy Plus Nivolumab as the First-Line Treatment for Unresectable Advanced or Recurrent Gastric Cancer and a Biomarker Study Using the Gustave Roussy Immune Score: A Multicenter Study. Ann. Surg. Oncol. 2024, 31, 9023–9029. [Google Scholar] [CrossRef]


| Characteristics | All Patients (n = 97) | Non-TR Group * (n = 82) | TR Group * (n = 15) | p-Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 67.3 ± 10.0 | 67.8 ± 9.3 | 64.2 ± 13.1 | 0.324 |
| Sex | ||||
| Male | 70 (72.2%) | 58 (70.7%) | 12 (80.0%) | 0.548 |
| Female | 27 (27.8%) | 24 (29.3%) | 3 (20.0%) | |
| Performance status | ||||
| 0, 1 | 95 (97.9%) | 80 (97.6%) | 15 (100%) | >0.999 |
| 2 | 2 (2.1%) | 2 (2.4%) | 0 (0%) | |
| Primary tumor site | ||||
| Stomach | 93 (95.9%) | 79 (96.3%) | 14 (93.3%) | 0.495 |
| GEJ | 4 (4.1%) | 3 (3.7%) | 1 (6.7%) | |
| Histological type | ||||
| Diffuse | 51 (52.6%) | 42 (51.2%) | 9 (60.0%) | 0.667 |
| Non-diffuse | 43 (44.3%) | 37 (45.1%) | 6 (40.0%) | |
| Unknown | 3 (3.1%) | 3 (3.7%) | 0 (0%) | |
| HER2 status, positive | 10 (10.3%) | 7 (8.5%) | 3 (20.0%) | 0.183 |
| Disease status at first-line chemotherapy | ||||
| Unresectable | 66 (68.0%) | 55 (67.1%) | 11 (73.3%) | 0.768 |
| Recurrent | 31 (32.0%) | 27 (32.9%) | 4 (26.7%) | |
| Number of non-curative factors | ||||
| 1 | 59 (60.8%) | 47 (57.3%) | 12 (80.0%) | 0.098 |
| ≥2 | 38 (39.2%) | 35 (42.7%) | 3 (20.0%) | |
| Number of chemotherapy regimens | ||||
| <3 | 40 (41.2%) | 35 (42.7%) | 5 (33.3%) | 0.499 |
| ≥3 | 57 (58.8%) | 47 (57.3%) | 10 (66.7%) | |
| Anti-PD-1 therapy | 74 (76.3%) | 63 (76.8%) | 11 (73.3%) | 0.749 |
| Nivolumab | 73 (98.6%) | 62 (98.4%) | 11 (100%) | |
| Pembrolizumab | 1 (1.4%) | 1 (1.6%) | 0 (0%) | |
| Laboratory data at first-line chemotherapy | ||||
| Alb (g/dL), mean ± SD | 3.77 ± 0.52 | 3.72 ± 0.51 | 4.05 ± 0.49 | 0.032 |
| ALP (U/L), mean ± SD | 127.2 ± 173.5 | 135.9 ± 187.4 | 79.6 ± 19.5 | 0.010 |
| Cre (mg/dL), mean ± SD | 0.861 ± 0.484 | 0.867 ± 0.522 | 0.825 ± 0.178 | 0.568 |
| NLR, mean ± SD | 3.14 ± 1.85 | 3.12 ± 1.86 | 3.23 ± 0.47 | 0.842 |
| CEA (ng/mL), mean ± SD | 78.58 ± 343.70 | 86.56 ± 370.57 | 34.96 ± 114.60 | 0.310 |
| CA19-9 (U/mL), mean ± SD | 1429.20 ± 8028.58 | 1656.33 ± 8719.19 | 187.52 ± 435.46 | 0.134 |
| Factors | Category | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | ||
| Age (years) | ≥65 (vs. <65) | 0.975 | 0.600–1.585 | 0.919 | |||
| Sex | Male (vs. Female) | 0.801 | 0.476–1.347 | 0.403 | |||
| Performance status | 0, 1 (vs. 2) | 0.390 | 0.095–1.610 | 0.193 | |||
| Primary tumor site | Stomach (vs. GEJ) | 0.552 | 0.199–1.527 | 0.252 | |||
| Histological type | Non-diffuse (vs. Diffuse) | 0.761 | 0.478–1.214 | 0.252 | |||
| HER2 status | Positive (vs. Negative) | 0.403 | 0.173–0.935 | 0.034 | 0.408 | 0.169–0.986 | 0.046 |
| * Disease status | Recurrent (vs. Unresectable) | 0.718 | 0.428–1.204 | 0.209 | |||
| Number of non-curative factors | 1 (vs. ≥2) | 0.832 | 0.521–1.328 | 0.440 | |||
| Number of chemotherapy regimens | ≥3 (vs. <3) | 0.641 | 0.395–1.042 | 0.073 | |||
| Anti-PD-1 therapy | Yes (vs. No) | 1.153 | 0.647–2.056 | 0.629 | |||
| # TR | Yes (vs. No) | 0.258 | 0.111–0.599 | 0.002 | 0.274 | 0.117–0.643 | 0.003 |
| Alb (g/dL) | ≥3.6 (vs. <3.6) | 0.604 | 0.371–0.985 | 0.043 | 0.606 | 0.377–1.007 | 0.062 |
| ALP (U/L) | <100 (vs. ≥100) | 1.264 | 0.774–2.065 | 0.350 | |||
| Cre (mg/dL) | <1.0 (vs. ≥1.0) | 0.917 | 0.519–1.621 | 0.765 | |||
| NLR | <3.0 (vs. ≥3.0) | 0.608 | 0.382–0.969 | 0.036 | 0.616 | 0.377–1.007 | 0.053 |
| Characteristics | All Patients (n = 15) | Chemo+TR Group (n = 9) | Anti-PD-1+TR Group (n = 6) | p-Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 64.2 ± 13.1 | 58.5 ± 13.7 | 72.8 ± 6.1 | 0.018 |
| Sex | ||||
| Male | 12 (80.0%) | 7 (77.8%) | 5 (83.3%) | >0.999 |
| Female | 3 (20.0%) | 2 (22.2%) | 1 (16.7%) | |
| Primary tumor site | ||||
| Stomach | 14 (93.3%) | 8 (88.9%) | 6 (100%) | >0.999 |
| GEJ | 1 (6.7%) | 1 (11.1%) | 0 (0%) | |
| Histological type | ||||
| Diffuse | 6 (40.0%) | 4 (44.4%) | 2 (33.3%) | >0.999 |
| Non-diffuse | 9 (60.0%) | 5 (55.6%) | 4 (66.7%) | |
| HER2 status, positive | 3 (20.0%) | 3 (33.3%) | 0 (0%) | 0.229 |
| Disease status at first-line chemotherapy | ||||
| Unresectable | 11 (73.3%) | 8 (88.9%) | 3 (50.0%) | 0.235 |
| Recurrent | 4 (26.7%) | 1 (11.1%) | 3 (50.0%) | |
| Number of non-curative factors | ||||
| 1 | 12 (80.0%) | 9 (100%) | 3 (50.0%) | 0.044 |
| ≥2 | 3 (20.0%) | 0 (0%) | 3 (50.0%) | |
| Residual tumor status | ||||
| R0 | 10 (66.7%) | 7 (77.8%) | 3 (50.0%) | 0.329 |
| R1, R2 | 5 (33.3%) | 2 (22.2%) | 3 (50.0%) | |
| Pathological tumor response | ||||
| Grade1a | 8 (53.3%) | 6 (66.7%) | 2 (33.3%) | 0.201 |
| Grade1b | 2 (13.3%) | 0 (0%) | 2 (33.3%) | |
| Grade2 | 1 (6.7%) | 1 (11.1%) | 0 (0%) | |
| Grade3 | 3 (20.0%) | 2 (22.2%) | 1 (16.7%) | |
| Unknown | 1 (6.7%) | 0 (0%) | 1 (16.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuida, H.; Mimura, K.; Nakajima, S.; Saito, K.; Hayashishita, S.; Takiguchi, C.; Nirei, A.; Kikuchi, T.; Hanayama, H.; Okayama, H.; et al. Residual Tumor Resection After Anti-PD-1 Therapy: A Promising Treatment Strategy for Overcoming Immune Evasive Phenotype Induced by Anti-PD-1 Therapy in Gastric Cancer. Cells 2025, 14, 1212. https://doi.org/10.3390/cells14151212
Matsuida H, Mimura K, Nakajima S, Saito K, Hayashishita S, Takiguchi C, Nirei A, Kikuchi T, Hanayama H, Okayama H, et al. Residual Tumor Resection After Anti-PD-1 Therapy: A Promising Treatment Strategy for Overcoming Immune Evasive Phenotype Induced by Anti-PD-1 Therapy in Gastric Cancer. Cells. 2025; 14(15):1212. https://doi.org/10.3390/cells14151212
Chicago/Turabian StyleMatsuida, Hajime, Kosaku Mimura, Shotaro Nakajima, Katsuharu Saito, Sohei Hayashishita, Chiaki Takiguchi, Azuma Nirei, Tomohiro Kikuchi, Hiroyuki Hanayama, Hirokazu Okayama, and et al. 2025. "Residual Tumor Resection After Anti-PD-1 Therapy: A Promising Treatment Strategy for Overcoming Immune Evasive Phenotype Induced by Anti-PD-1 Therapy in Gastric Cancer" Cells 14, no. 15: 1212. https://doi.org/10.3390/cells14151212
APA StyleMatsuida, H., Mimura, K., Nakajima, S., Saito, K., Hayashishita, S., Takiguchi, C., Nirei, A., Kikuchi, T., Hanayama, H., Okayama, H., Saito, M., Momma, T., Saze, Z., & Kono, K. (2025). Residual Tumor Resection After Anti-PD-1 Therapy: A Promising Treatment Strategy for Overcoming Immune Evasive Phenotype Induced by Anti-PD-1 Therapy in Gastric Cancer. Cells, 14(15), 1212. https://doi.org/10.3390/cells14151212







