B7-H3 in Cancer Immunotherapy—Prospects and Challenges: A Review of the Literature
Abstract
1. Introduction
2. B7-H3 in Tumorigenesis
2.1. B7-H3 in the Regulation of Immune Responses
2.1.1. The Influence of B7-H3 on Immune Cell Infiltrations in Tumors
2.1.2. Relationship Between B7-H3 and Cytokine Secretion
2.1.3. B7-H3 and Regulatory T Cells
2.1.4. Macrophage Function
2.1.5. B7-H3 in the Regulation of NK Cell Function
2.1.6. B7-H3 and Other Immune Cell Subsets
2.2. B7-H3 and Its Non-Immune Functions in Tumorigenesis
3. B7-H3 in Tumors
3.1. The Role and Clinical Potential of B7-H3 in Melanoma
3.2. Brain Tumors—A Potent Direction for Anti-B7-H3 Therapies
3.2.1. Gliomas
3.2.2. Glioblastoma
3.2.3. Diffuse Intrinsic Pontine Glioma (DIPG)
3.2.4. Neuroblastoma
3.2.5. Medulloblastoma
3.2.6. Craniopharyngioma
3.3. B7-H3 in Lung Cancer—Clinical Utility and Options for Combined Therapy
3.3.1. Non-Small Cell Lung Cancer (NSCLC)
3.3.2. Small-Cell Lung Cancer (SCLC)
3.4. Breast Cancer—B7-H3 as a Potential Prognostic Marker and Therapeutic Target
3.5. Cervical Cancer—Molecular Mechanisms of B7-H3 Influence on the Disease
3.6. Ovarian Cancer—Prognostic Role of B7-H3 and Its Influence on Immune Responses
3.7. Prostate Cancer—Prognostic and Predictive Potential of B7-H3
3.8. Renal Cancer—Molecular Mechanisms, Possible Prognostic and Predictive Role, and Therapeutic Opportunities Related to B7-H3
3.9. Bladder Cancer and Urothelial Carcinoma (UCC)—Disease Aggressiveness and Therapeutic Options Related to B7-H3
3.10. Head and Neck Cancers—Conflicting Results and Potential Therapeutic Strategies Involving B7-H3
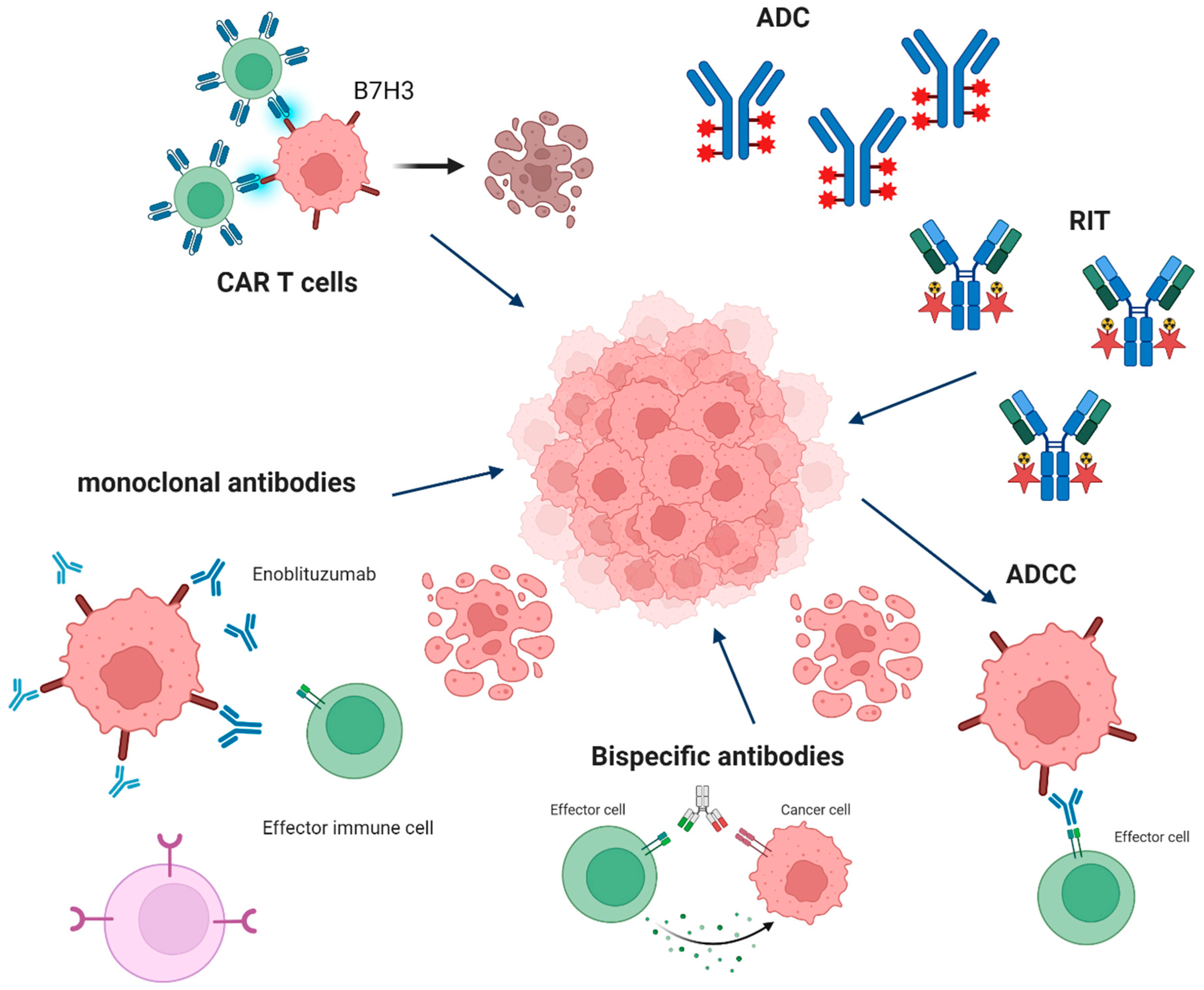
4. B7-H3 as a Target
4.1. CAR-T Therapy
4.2. ADC Therapy
4.3. ADCC Therapy
4.4. Monoclonal Antibody (mAb) Therapy
4.5. Radioimmunotherapy
4.6. Potential Mechanisms of Resistance to B7-H3-Targeted Therapies
4.7. Combined Therapies
5. Potential Biomarkers for B7-H3-Targeted Therapies
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, J.; Chen, S.; Wang, X.; Xi, Q.; Shen, H.; Zhang, R. Immune Checkpoint Inhibitors: Breakthroughs in Cancer Treatment. Cancer Biol. Med. 2024, 21, 451–472. [Google Scholar] [CrossRef]
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer Immunotherapy: The Beginning of the End of Cancer? BMC Med. 2016, 14, 73. [Google Scholar] [CrossRef]
- Schildberg, F.A.; Klein, S.R.; Freeman, G.J.; Sharpe, A.H. Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family. Immunity 2016, 44, 955–972. [Google Scholar] [CrossRef]
- Burke, K.P.; Chaudhri, A.; Freeman, G.J.; Sharpe, A.H. The B7:CD28 Family and Friends: Unraveling Coinhibitory Interactions. Immunity 2024, 57, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Bolandi, N.; Derakhshani, A.; Hemmat, N.; Baghbanzadeh, A.; Asadzadeh, Z.; Afrashteh Nour, M.; Brunetti, O.; Bernardini, R.; Silvestris, N.; Baradaran, B. The Positive and Negative Immunoregulatory Role of B7 Family: Promising Novel Targets in Gastric Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 10719. [Google Scholar] [CrossRef] [PubMed]
- Getu, A.A.; Tigabu, A.; Zhou, M.; Lu, J.; Fodstad, Ø.; Tan, M. New Frontiers in Immune Checkpoint B7-H3 (CD276) Research and Drug Development. Mol. Cancer 2023, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A Costimulatory Molecule for T Cell Activation and IFN-γ Production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef]
- Sun, M.; Richards, S.; Prasad, D.V.R.; Mai, X.M.; Rudensky, A.; Dong, C. Characterization of Mouse and Human B7-H3 Genes1. J. Immunol. 2002, 168, 6294–6297. [Google Scholar] [CrossRef]
- Kovaleva, O.V.; Gratchev, A.N.; Sokolov, N.Y.; Maslennikov, V.V.; Kuzmin, Y.B.; Gershtein, E.S.; Alferov, A.A.; Mamedli, Z.Z.; Stilidi, I.S.; Kushlinskii, N.E. Soluble B7-H3 in Colorectal Cancer. Bull. Exp. Biol. Med. 2023, 176, 87–90. [Google Scholar] [CrossRef]
- Flem-Karlsen, K.; Fodstad, Ø.; Tan, M.; Nunes-Xavier, C.E. B7-H3 in Cancer–Beyond Immune Regulation. Trends Cancer 2018, 4, 401–404. [Google Scholar] [CrossRef]
- Li, Y.; Guo, G.; Song, J.; Cai, Z.; Yang, J.; Chen, Z.; Wang, Y.; Huang, Y.; Gao, Q. B7-H3 Promotes the Migration and Invasion of Human Bladder Cancer Cells via the PI3K/Akt/STAT3 Signaling Pathway. J. Cancer 2017, 8, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hu, C.; Hui, K.; Jiang, X. Non-Immune Functions of B7-H3: Bridging Tumor Cells and the Tumor Vasculature. Front. Oncol. 2024, 14, 1408051. [Google Scholar] [CrossRef]
- Mielcarska, S.; Kot, A.; Kula, A.; Dawidowicz, M.; Sobków, P.; Kłaczka, D.; Waniczek, D.; Świętochowska, E. B7H3 in Gastrointestinal Tumors: Role in Immune Modulation and Cancer Progression: A Review of the Literature. Cells 2025, 14, 530. [Google Scholar] [CrossRef]
- Inamura, K.; Amori, G.; Yuasa, T.; Yamamoto, S.; Yonese, J.; Ishikawa, Y. Relationship of B7-H3 Expression in Tumor Cells and Tumor Vasculature with FOXP3+ Regulatory T Cells in Renal Cell Carcinoma. CMAR 2019, 11, 7021–7030. [Google Scholar] [CrossRef]
- Yim, J.; Koh, J.; Kim, S.; Song, S.G.; Ahn, H.K.; Kim, Y.A.; Jeon, Y.K.; Chung, D.H. Effects of B7-H3 Expression on Tumour-Infiltrating Immune Cells and Clinicopathological Characteristics in Non–Small-Cell Lung Cancer. Eur. J. Cancer 2020, 133, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, M.; Kobori, H.; Ritprajak, P.; Kamimura, Y.; Kozono, H.; Azuma, M. Triggering Receptor Expressed on Myeloid Cell-like Transcript 2 (TLT-2) Is a Counter-Receptor for B7-H3 and Enhances T Cell Responses. Proc. Natl. Acad. Sci. USA 2008, 105, 10495–10500. [Google Scholar] [CrossRef]
- Kobori, H.; Hashiguchi, M.; Piao, J.; Kato, M.; Ritprajak, P.; Azuma, M. Enhancement of Effector CD8+ T-Cell Function by Tumour-Associated B7-H3 and Modulation of Its Counter-Receptor Triggering Receptor Expressed on Myeloid Cell-like Transcript 2 at Tumour Sites. Immunology 2010, 130, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Lupu, C.; Eisenbach, C.; Lupu, A.; Kuefner, M.; Hoyler, B.; Stremmel, W.; Encke, J. Adenoviral B7-H3 Therapy Induces Tumor Specific Immune Responses and Reduces Secondary Metastasis in a Murine Model of Colon Cancer. Oncol. Rep. 2007, 18, 745–748. [Google Scholar] [CrossRef]
- Leitner, J.; Klauser, C.; Pickl, W.F.; Stöckl, J.; Majdic, O.; Bardet, A.F.; Kreil, D.P.; Dong, C.; Yamazaki, T.; Zlabinger, G.; et al. B7-H3 Is a Potent Inhibitor of Human T-Cell Activation: No Evidence for B7-H3 and TREML2 Interaction. Eur. J. Immunol. 2009, 39, 1754–1764. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Jia, L.; Kim, J.K.; Li, J.; Deng, P.; Zhang, W.; Krebsbach, P.H.; Wang, C.-Y. CD276 Expression Enables Squamous Cell Carcinoma Stem Cells to Evade Immune Surveillance. Cell Stem Cell 2021, 28, 1597–1613.e7. [Google Scholar] [CrossRef]
- Miyamoto, T.; Murakami, R.; Hamanishi, J.; Tanigaki, K.; Hosoe, Y.; Mise, N.; Takamatsu, S.; Mise, Y.; Ukita, M.; Taki, M.; et al. B7-H3 Suppresses Antitumor Immunity via the CCL2–CCR2–M2 Macrophage Axis and Contributes to Ovarian Cancer Progression. Cancer Immunol. Res. 2022, 10, 56–69. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, D.; Saw, P.E.; Song, E. Turning Cold Tumors Hot: From Molecular Mechanisms to Clinical Applications. Trends Immunol. 2022, 43, 523–545. [Google Scholar] [CrossRef]
- Ouyang, P.; Wang, L.; Wu, J.; Tian, Y.; Chen, C.; Li, D.; Yao, Z.; Chen, R.; Xiang, G.; Gong, J.; et al. Overcoming Cold Tumors: A Combination Strategy of Immune Checkpoint Inhibitors. Front. Immunol. 2024, 15, 1344272. [Google Scholar] [CrossRef]
- Miller, C.D.; Lozada, J.R.; Zorko, N.A.; Elliott, A.; Makovec, A.; Radovich, M.; Heath, E.I.; Agarwal, N.; Mckay, R.R.; Garje, R.; et al. Pan-Cancer Interrogation of B7-H3 (CD276) as an Actionable Therapeutic Target Across Human Malignancies. Cancer Res. Commun. 2024, 4, 1369–1379. [Google Scholar] [CrossRef]
- Liu, H.-J.; Du, H.; Khabibullin, D.; Zarei, M.; Wei, K.; Freeman, G.J.; Kwiatkowski, D.J.; Henske, E.P. mTORC1 Upregulates B7-H3/CD276 to Inhibit Antitumor T Cells and Drive Tumor Immune Evasion. Nat. Commun. 2023, 14, 1214. [Google Scholar] [CrossRef]
- Cattaneo, G.; Ventin, M.; Arya, S.; Kontos, F.; Michelakos, T.; Sekigami, Y.; Cai, L.; Villani, V.; Sabbatino, F.; Chen, F.; et al. Interplay between B7-H3 and HLA Class I in the Clinical Course of Pancreatic Ductal Adenocarcinoma. Cancer Lett. 2024, 587, 216713. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.-K.; Gajewska, B.U.; Okada, H.; Gronski, M.A.; Bertram, E.M.; Dawicki, W.; Duncan, G.S.; Bukczynski, J.; Plyte, S.; Elia, A.; et al. The B7 Family Member B7-H3 Preferentially down-Regulates T Helper Type 1-Mediated Immune Responses. Nat. Immunol. 2003, 4, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Li, J.; Liu, D.; Hong, S.; Qiao, Q.; Sun, Q.; Li, P.; Lyu, N.; Sun, T.; Xie, S.; et al. Tumor-Expressed B7-H3 Mediates the Inhibition of Antitumor T-Cell Functions in Ovarian Cancer Insensitive to PD-1 Blockade Therapy. Cell Mol. Immunol. 2020, 17, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chen, G.; Li, H.; Yang, S.; Xu, Y.; Pan, B.; Lai, W.; Chen, G.; Liao, W.; Zhang, X. B7-H3 Promotes Nasopharyngeal Carcinoma Progression by Regulating CD8+ T Cell Exhaustion. Immun. Inflamm. Dis. 2024, 12, e70005. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Yang, M.; Chen, Y.; Chen, W.; Wang, W. miR-34a Induces Immunosuppression in Colorectal Carcinoma through Modulating a SIRT1/NF-κB/B7-H3/TNF-α Axis. Cancer Immunol. Immunother. 2021, 70, 2247–2259. [Google Scholar] [CrossRef]
- Peuker, K.; Strigli, A.; Tauriello, D.V.F.; Hendricks, A.; von Schönfels, W.; Burmeister, G.; Brosch, M.; Herrmann, A.; Krüger, S.; Nitsche, J.; et al. Microbiota-Dependent Activation of the Myeloid Calcineurin-NFAT Pathway Inhibits B7H3- and B7H4-Dependent Anti-Tumor Immunity in Colorectal Cancer. Immunity 2022, 55, 701–717.e7. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hsu, T.-W.; Li, M.O. Immunity beyond Cancer Cells: Perspective from Tumor Tissue. Trends Cancer 2021, 7, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Barreto, J.B.; Andreu, P.; Vasquez, L.; Tawfik, D.; Kolhatkar, N.; Coussens, L.M. CD4+ T Cells Regulate Pulmonary Metastasis of Mammary Carcinomas by Enhancing Protumor Properties of Macrophages. Cancer Cell 2009, 16, 91–102. [Google Scholar] [CrossRef]
- Zhou, X.; Mao, Y.; Zhu, J.; Meng, F.; Chen, Q.; Tao, L.; Li, R.; Fu, F.; Liu, C.; Hu, Y.; et al. TGF-Β1 Promotes Colorectal Cancer Immune Escape by Elevating B7-H3 and B7-H4 via the miR-155/miR-143 Axis. Oncotarget 2016, 7, 67196–67211. [Google Scholar] [CrossRef]
- Ahrends, T.; Borst, J. The Opposing Roles of CD4+ T Cells in Anti-tumour Immunity. Immunology 2018, 154, 582–592. Available online: https://onlinelibrary.wiley.com/doi/10.1111/imm.12941 (accessed on 9 February 2025). [CrossRef]
- Mendes, A.A.; Lu, J.; Kaur, H.B.; Zheng, S.L.; Xu, J.; Hicks, J.; Weiner, A.B.; Schaeffer, E.M.; Ross, A.E.; Balk, S.P.; et al. Association of B7-H3 Expression with Racial Ancestry, Immune Cell Density, and Androgen Receptor Activation in Prostate Cancer. Cancer 2022, 128, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479.e10. [Google Scholar] [CrossRef]
- Mao, L.; Fan, T.-F.; Wu, L.; Yu, G.-T.; Deng, W.-W.; Chen, L.; Bu, L.-L.; Ma, S.-R.; Liu, B.; Bian, Y.; et al. Selective Blockade of B7-H3 Enhances Antitumour Immune Activity by Reducing Immature Myeloid Cells in Head and Neck Squamous Cell Carcinoma. J. Cell. Mol. Med. 2017, 21, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, P.; Li, W.-J.; Zhang, J.; Wang, G.-P.; Jiang, D.-F.; Chen, F.-P. LncRNA NEAT1 Sponges miR-214 to Regulate M2 Macrophage Polarization by Regulation of B7-H3 in Multiple Myeloma. Mol. Immunol. 2020, 117, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, H.; Gao, X.; Yu, G.; Zhang, X.; Jin, H.; Xu, R.; Wang, Z.; Zhang, G. High Expression of B7-H3 on Monocyte/Macrophages in Tumor Microenvironment Promotes Lung Cancer Progression by Inhibiting Apoptosis. Transl. Oncol. 2024, 41, 101874. [Google Scholar] [CrossRef]
- Huntington, N.D.; Vosshenrich, C.A.J.; Di Santo, J.P. Developmental Pathways That Generate Natural-Killer-Cell Diversity in Mice and Humans. Nat. Rev. Immunol. 2007, 7, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Ho, K.-H.; Huang, T.-W.; Shih, C.-M.; Hsu, S.-Y.; Liu, A.-J.; Chen, K.-C. A Regulatory Loop among CD276, miR-29c-3p, and Myc Exists in Cancer Cells against Natural Killer Cell Cytotoxicity. Life Sci. 2021, 277, 119438. [Google Scholar] [CrossRef]
- Pathania, A.S.; Chava, H.; Chaturvedi, N.K.; Chava, S.; Byrareddy, S.N.; Coulter, D.W.; Challagundla, K.B. The miR-29 Family Facilitates the Activation of NK-Cell Immune Responses by Targeting the B7-H3 Immune Checkpoint in Neuroblastoma. Cell Death Dis. 2024, 15, 428. [Google Scholar] [CrossRef]
- Xiong, G.; Chen, Z.; Liu, Q.; Peng, F.; Zhang, C.; Cheng, M.; Ling, R.; Chen, S.; Liang, Y.; Chen, D.; et al. CD276 Regulates the Immune Escape of Esophageal Squamous Cell Carcinoma through CXCL1–CXCR2 Induced NETs. J. Immunother. Cancer 2024, 12, e008662. [Google Scholar] [CrossRef]
- Hegde, S.; Leader, A.M.; Merad, M. MDSC: Markers, Development, States, and Unaddressed Complexity. Immunity 2021, 54, 875–884. [Google Scholar] [CrossRef]
- Asakawa, A.; Yoshimoto, R.; Kobayashi, M.; Izumi, N.; Maejima, T.; Deguchi, T.; Kubota, K.; Takahashi, H.; Yamada, M.; Ishibashi, S.; et al. The Comprehensive Characterization of B7-H3 Expression in the Tumor Microenvironment of Lung Squamous Cell Carcinoma: A Retrospective Study. Cancers 2024, 16, 2140. [Google Scholar] [CrossRef]
- Shao, L.; He, Q.; Wang, J.; He, F.; Lin, S.; Wu, L.; Gao, Y.; Ma, W.; Dong, J.; Yang, X.; et al. MicroRNA-326 Attenuates Immune Escape and Prevents Metastasis in Lung Adenocarcinoma by Targeting PD-L1 and B7-H3. Cell Death Discov. 2021, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Koumprentziotis, I.-A.; Theocharopoulos, C.; Foteinou, D.; Angeli, E.; Anastasopoulou, A.; Gogas, H.; Ziogas, D.C. New Emerging Targets in Cancer Immunotherapy: The Role of B7-H3. Vaccines 2024, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Brustmann, H.; Igaz, M.; Eder, C.; Brunner, A. Epithelial and Tumor-Associated Endothelial Expression of B7-H3 in Cervical Carcinoma: Relation with CD8+ Intraepithelial Lymphocytes, FIGO Stage, and Phosphohistone H3 (PHH3) Reactivity. Int. J. Gynecol. Pathol. 2015, 34, 187–195. [Google Scholar] [CrossRef] [PubMed]
- International Journal of Gynecological Pathology. Available online: https://journals.lww.com/intjgynpathology/abstract/2015/03000/epithelial_and_tumor_associated_endothelial.14.aspx (accessed on 27 February 2025).
- Ding, J.; Sun, Y.; Sulaiman, Z.; Li, C.; Cheng, Z.; Liu, S. Comprehensive Analysis Reveals Distinct Immunological and Prognostic Characteristics of CD276/B7-H3 in Pan-Cancer. Int. J. Gen. Med. 2023, 16, 367–391. [Google Scholar] [CrossRef] [PubMed]
- Durlanik, S.; Fundel-Clemens, K.; Viollet, C.; Huber, H.J.; Lenter, M.; Kitt, K.; Pflanz, S. CD276 Is an Important Player in Macrophage Recruitment into the Tumor and an Upstream Regulator for PAI-1. Sci. Rep. 2021, 11, 14849. [Google Scholar] [CrossRef]
- Deng, M.; Wu, D.; Zhang, Y.; Jin, Z.; Miao, J. MiR-29c Downregulates Tumor-Expressed B7-H3 to Mediate the Antitumor NK-Cell Functions in Ovarian Cancer. Gynecol. Oncol. 2021, 162, 190–199. [Google Scholar] [CrossRef]
- Liu, Y.; Sæter, T.; Vlatkovic, L.; Servoll, E.; Waaler, G.; Axcrona, U.; Giercksky, K.-E.; Nesland, J.M.; Suo, Z.-H.; Axcrona, K. Dendritic and Lymphocytic Cell Infiltration in Prostate Carcinoma. Histol. Histopathol. 2013, 28, 1621–1628. [Google Scholar] [CrossRef]
- Emaldi, M.; Rey-Iborra, E.; Marín, Á.; Mosteiro, L.; Lecumberri, D.; Øyjord, T.; Roncier, N.; Mælandsmo, G.M.; Angulo, J.C.; Errarte, P.; et al. Impact of B7-H3 Expression on Metastasis, Immune Exhaustion and JAK/STAT and PI3K/AKT Pathways in Clear Cell Renal Cell Carcinoma. Oncoimmunology 2024, 13, 2419686. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, L.; Tian, J.; Man, H.; Li, P.; Shan, B. High Expression of B7-H3 and CD163 in Cancer Tissues Indicates Malignant Clinicopathological Status and Poor Prognosis of Patients with Urothelial Cell Carcinoma of the Bladder. Oncol. Lett. 2018, 15, 6519–6526. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Martin-Orozco, N.; Zheng, P.; Li, J.; Zhang, P.; Tan, H.; Park, H.J.; Jeong, M.; Chang, S.H.; Kim, B.-S.; et al. Inhibition of the B7-H3 Immune Checkpoint Limits Tumor Growth by Enhancing Cytotoxic Lymphocyte Function. Cell Res. 2017, 27, 1034–1045. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Li, F.; Shen, Z.; Qiao, Y.; Li, L.; Liu, S.; Song, M.; Zhao, X.; Ren, F.; et al. Large-Scale Analysis Reveals the Specific Clinical and Immune Features of B7-H3 in Glioma. Oncoimmunology 2018, 7, e1461304. [Google Scholar] [CrossRef]
- Li, S.; Poolen, G.C.; van Vliet, L.C.; Schipper, J.G.; Broekhuizen, R.; Monnikhof, M.; Van Hecke, W.; Vermeulen, J.F.; Bovenschen, N. Pediatric Medulloblastoma Express Immune Checkpoint B7-H3. Clin. Transl. Oncol. 2022, 24, 1204–1208. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Zhong, K.; Jiang, C.; Wang, L.; Yuan, Z.; Nie, C.; Xu, J.; Guo, G.; Zhou, L.; et al. Frequent B7-H3 Overexpression in Craniopharyngioma. Biochem. Biophys. Res. Commun. 2019, 514, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, W.; Phillips, J.B.; Arora, R.; McClellan, S.; Li, J.; Kim, J.-H.; Sobol, R.W.; Tan, M. Immunoregulatory Protein B7-H3 Regulates Cancer Stem Cell Enrichment and Drug Resistance through MVP-Mediated MEK Activation. Oncogene 2019, 38, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.; Wu, Y.; Zhao, K.; Ye, Z.; Zhu, J.; Xu, X.; Zhao, X.; Xing, C. B7-H3 Promotes Gastric Cancer Cell Migration and Invasion. Oncotarget 2017, 8, 71725–71735. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Han, S.; Li, Y.; Qian, Q.; Zhang, Q.; Zhang, H.; Yang, Z.; Zhang, Y. B7-H3 Is Related to Tumor Progression in Ovarian Cancer. Oncol. Rep. 2017, 38, 2426–2434. [Google Scholar] [CrossRef]
- Zhong, C.; Tao, B.; Chen, Y.; Guo, Z.; Yang, X.; Peng, L.; Xia, X.; Chen, L. B7-H3 Regulates Glioma Growth and Cell Invasion Through a JAK2/STAT3/Slug-Dependent Signaling Pathway. Onco Targets Ther. 2020, 13, 2215–2224. [Google Scholar] [CrossRef]
- Tekle, C.; Nygren, M.K.; Chen, Y.-W.; Dybsjord, I.; Nesland, J.M.; Maelandsmo, G.M.; Fodstad, O. B7-H3 Contributes to the Metastatic Capacity of Melanoma Cells by Modulation of Known Metastasis-Associated Genes. Int. J. Cancer 2012, 130, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Kang, F.; Wang, L.; Jia, H.; Li, D.; Li, H.; Zhang, Y.; Sun, D. B7-H3 Promotes Aggression and Invasion of Hepatocellular Carcinoma by Targeting Epithelial-to-Mesenchymal Transition via JAK2/STAT3/Slug Signaling Pathway. Cancer Cell Int. 2015, 15, 45. [Google Scholar] [CrossRef]
- Xie, J.; Sun, M.; Zhang, D.; Chen, C.; Lin, S.; Zhang, G. Fibronectin Enhances Tumor Metastasis through B7-H3 in Clear Cell Renal Cell Carcinoma. FEBS Open Bio 2021, 11, 2977–2987. [Google Scholar] [CrossRef]
- Liao, H.; Ding, M.; Zhou, N.; Yang, Y.; Chen, L. B7-H3 Promotes the Epithelial-mesenchymal Transition of NSCLC by Targeting SIRT1 through the PI3K/AKT Pathway. Mol. Med. Rep. 2022, 25, 79. [Google Scholar] [CrossRef]
- Sutton, M.N.; Glazer, S.E.; Muzzioli, R.; Yang, P.; Gammon, S.T.; Piwnica-Worms, D. Dimerization of the 4Ig Isoform of B7-H3 in Tumor Cells Mediates Enhanced Proliferation and Tumorigenic Signaling. Commun. Biol. 2024, 7, 21. [Google Scholar] [CrossRef]
- Meng, F.; Yin, Z.; Lu, F.; Wang, W.; Zhang, H. Disruption of LPA-LPAR1 Pathway Results in Lung Tumor Growth Inhibition by Downregulating B7-H3 Expression in Fibroblasts. Thorac. Cancer 2023, 15, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shi, Y.; Wang, J. B7-H3 Regulates Glucose Metabolism in Neuroblastom via Stat3/c-Met Pathway. Appl. Biochem. Biotechnol. 2024, 196, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiong, J.; Sun, Y.; Gu, L.; Chen, Y.; Guo, Y.; Liu, C.; Sun, J. B7-H3 Promotes Angiogenesis in Rheumatoid Arthritis. Mol. Immunol. 2024, 165, 19–27. [Google Scholar] [CrossRef]
- Shen, B.; Mei, J.; Xu, R.; Cai, Y.; Wan, M.; Zhou, J.; Ding, J.; Zhu, Y. B7-H3 Is Associated with the Armored-Cold Phenotype and Predicts Poor Immune Checkpoint Blockade Response in Melanoma. Pathol.-Res. Pract. 2024, 256, 155267. [Google Scholar] [CrossRef]
- Wang, J.; Chong, K.K.; Nakamura, Y.; Nguyen, L.; Huang, S.K.; Kuo, C.; Zhang, W.; Yu, H.; Morton, D.L.; Hoon, D.S.B. B7-H3 Associated with Tumor Progression and Epigenetic Regulatory Activity in Cutaneous Melanoma. J. Investig. Dermatol. 2013, 133, 2050–2058. [Google Scholar] [CrossRef]
- Flem-Karlsen, K.; Tekle, C.; Andersson, Y.; Flatmark, K.; Fodstad, Ø.; Nunes-Xavier, C.E. Immunoregulatory Protein B7-H3 Promotes Growth and Decreases Sensitivity to Therapy in Metastatic Melanoma Cells. Pigment. Cell Melanoma Res. 2017, 30, 467–476. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, C.; Liu, Z.; Yang, M.; Tang, X.; Wang, Y.; Zheng, M.; Huang, J.; Zhong, K.; Zhao, S.; et al. B7-H3-Targeted CAR-T Cells Exhibit Potent Antitumor Effects on Hematologic and Solid Tumors. Mol. Ther. Oncolytics 2020, 17, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Duan, X.; Wang, J.; Tian, Q.; Ren, Y.; Chen, K.; Zhang, Z.; Li, Y.; Feng, Y.; Zhong, K.; et al. Lipid Nanoparticle Delivery System for mRNA Encoding B7H3-redirected Bispecific Antibody Displays Potent Antitumor Effects on Malignant Tumors. Adv. Sci. 2023, 10, e2205532. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9875623/#advs4743-sec-0020 (accessed on 12 February 2025). [CrossRef]
- Ventin, M.; Cattaneo, G.; Arya, S.; Jia, J.; Gelmi, M.C.; Sun, Y.; Maggs, L.; Ksander, B.R.; Verdijk, R.M.; Boland, G.M.; et al. Chimeric Antigen Receptor T Cell with an Inducible Caspase-9 Suicide Gene Eradicates Uveal Melanoma Liver Metastases via B7-H3 Targeting. Clin. Cancer Res. 2024, 30, 3243–3258. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Loo, D.; Baughman, J.E.; Yun, S.; Chen, F.; Moore, P.A.; Bonvini, E.; Vasselli, J.R.; Wigginton, J.M.; Cohen, R.B.; et al. A Phase 1 Study of Enoblituzumab in Combination with Pembrolizumab in Patients with Advanced B7-H3-Expressing Cancers. J. Clin. Oncol. 2016, 34, TPS3104. [Google Scholar] [CrossRef]
- Deng, J.; Ma, M.; Wang, D.; Zhu, H.; Hua, L.; Sun, S.; Chen, H.; Cheng, H.; Qian, Z.R.; Xie, Q.; et al. Expression and Clinical Significance of Immune Checkpoint Regulator B7-H3 (CD276) in Human Meningioma. World Neurosurg. 2020, 135, e12–e18. [Google Scholar] [CrossRef]
- Zhou, Z.; Luther, N.; Ibrahim, G.M.; Hawkins, C.; Vibhakar, R.; Handler, M.H.; Souweidane, M.M. B7-H3, a Potential Therapeutic Target, Is Expressed in Diffuse Intrinsic Pontine Glioma. J. Neurooncol. 2013, 111, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Theruvath, J.; Sotillo, E.; Mount, C.W.; Graef, C.M.; Delaidelli, A.; Heitzeneder, S.; Labanieh, L.; Dhingra, S.; Leruste, A.; Majzner, R.G.; et al. Locoregionally Administered B7-H3-Targeted CAR T Cells for Treatment of Atypical Teratoid/Rhabdoid Tumors. Nat. Med. 2020, 26, 712–719. [Google Scholar] [CrossRef]
- Gregorio, A.; Corrias, M.V.; Castriconi, R.; Dondero, A.; Mosconi, M.; Gambini, C.; Moretta, A.; Moretta, L.; Bottino, C. Small Round Blue Cell Tumours: Diagnostic and Prognostic Usefulness of the Expression of B7-H3 Surface Molecule. Histopathology 2008, 53, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chang, M.; Wang, Y.; Xing, B.; Ma, W. B7-H3 in Brain Malignancies: Immunology and Immunotherapy. Int. J. Biol. Sci. 2023, 19, 3762. [Google Scholar] [CrossRef] [PubMed]
- Baral, A.; Ye, H.X.; Jiang, P.C.; Yao, Y.; Mao, Y. B7-H3 and B7-H1 Expression in Cerebral Spinal Fluid and Tumor Tissue Correlates with the Malignancy Grade of Glioma Patients. Oncol. Lett. 2014, 8, 1195–1201. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, C.; Li, X.; Li, X.; Wu, Y.; Yang, T.; Hu, Y.; Liu, C.; Shen, H.; Guo, P.; et al. Generation of B7-H3 Isoform Regulated by ANXA2/NSUN2/YBX1 Axis in Human Glioma. J. Cell. Mol. Med. 2024, 28, e18575. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Zhang, C.; Liu, X.; Li, G.; Liu, S.; Sun, L.; Liang, J.; Hu, H.; Liu, Y.; et al. Genetic and Clinical Characterization of B7-H3 (CD276) Expression and Epigenetic Regulation in Diffuse Brain Glioma. Cancer Sci. 2018, 109, 2697–2705. [Google Scholar] [CrossRef]
- Digregorio, M.; Coppieters, N.; Lombard, A.; Lumapat, P.N.; Scholtes, F.; Rogister, B. The Expression of B7-H3 Isoforms in Newly Diagnosed Glioblastoma and Recurrence and Their Functional Role. Acta Neuropathol. Commun. 2021, 9, 59. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Marzese, D.M.; Wang, X.; Yang, Z.; Li, C.; Zhang, H.; Zhang, J.; Chen, C.C.; Kelly, D.F.; et al. B7H3 Regulates Differentiation and Serves as a Potential Biomarker and Theranostic Target for Human Glioblastoma. Lab. Investig. 2019, 99, 1117–1129. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Z.; Zhong, K.; Wang, Z.; Yang, N.; Tang, X.; Li, H.; Lu, Q.; Wu, Z.; Yuan, B.; et al. CXCL11-Armed Oncolytic Adenoviruses Enhance CAR-T Cell Therapeutic Efficacy and Reprogram Tumor Microenvironment in Glioblastoma. Mol. Ther. 2023, 31, 134–153. [Google Scholar] [CrossRef]
- Sakunrangsit, N.; Khuisangeam, N.; Inthanachai, T.; Yodsurang, V.; Taechawattananant, P.; Suppipat, K.; Tawinwung, S. Incorporating IL7 Receptor Alpha Signaling in the Endodomain of B7H3-Targeting Chimeric Antigen Receptor T Cells Mediates Antitumor Activity in Glioblastoma. Cancer Immunol. Immunother. 2024, 73, 98. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, M.; Zhang, Z.; Tang, X.; Chen, Y.; Peng, A.; Peng, X.; Tong, A.; Zhou, L. Interleukin-7-Loaded Oncolytic Adenovirus Improves CAR-T Cell Therapy for Glioblastoma. Cancer Immunol. Immunother. 2021, 70, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Mashouf, L.A.; Lim, M. CAR T Cell Therapy in Primary Brain Tumors: Current Investigations and the Future. Front. Immunol. 2022, 13, 817296. [Google Scholar] [CrossRef]
- Mao, Y.; Wei, D.; Fu, F.; Wang, H.; Sun, Z.; Huang, Z.; Wang, Y.; Zhang, G.; Zhang, X.; Jiang, B.; et al. Development of a MMAE-Based Antibody-Drug Conjugate Targeting B7-H3 for Glioblastoma. Eur. J. Med. Chem. 2023, 257, 115489. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, Y.; Fu, F.; Zhang, K.; Wang, Y.; Zhao, S.; Liu, Q.; Mu, H.; Zhang, X.; Miao, L. Radioimmunotherapy Targeting B7-H3 in Situ Glioma Models Enhanced Antitumor Efficacy by Reconstructing the Tumor Microenvironment. Int. J. Biol. Sci. 2023, 19, 4278–4290. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.; Kelly, M.E.; Yeboa, D.N.; Buerki, R.A.; Cioffi, G.; Balaji, S.; Ostrom, Q.T.; Kruchko, C.; Barnholtz-Sloan, J.S. Epidemiology of Brainstem High-Grade Gliomas in Children and Adolescents in the United States, 2000–2017. Neuro-Oncology 2021, 23, 990–998. [Google Scholar] [CrossRef]
- Pandey, K.; Wang, S.S.; Mifsud, N.A.; Faridi, P.; Davenport, A.J.; Webb, A.I.; Sandow, J.J.; Ayala, R.; Monje, M.; Cross, R.S.; et al. A Combined Immunopeptidomics, Proteomics, and Cell Surface Proteomics Approach to Identify Immunotherapy Targets for Diffuse Intrinsic Pontine Glioma. Front. Oncol. 2023, 13, 1192448. [Google Scholar] [CrossRef]
- Vitanza, N.A.; Wilson, A.L.; Huang, W.; Seidel, K.; Brown, C.; Gustafson, J.A.; Yokoyama, J.K.; Johnson, A.J.; Baxter, B.A.; Koning, R.W.; et al. Intraventricular B7-H3 CAR T Cells for Diffuse Intrinsic Pontine Glioma: Preliminary First-in-Human Bioactivity and Safety. Cancer Discov. 2023, 13, 114–131. [Google Scholar] [CrossRef]
- Park, J.A.; Cheung, N.V. Targets and Antibody Formats for Immunotherapy of Neuroblastoma. J. Clin. Oncol. 2020, 38, 1836–1848. [Google Scholar] [CrossRef]
- Majzner, R.G.; Theruvath, J.L.; Nellan, A.; Heitzeneder, S.; Cui, Y.; Mount, C.W.; Rietberg, S.P.; Linde, M.H.; Xu, P.; Rota, C.; et al. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin. Cancer Res. 2019, 25, 2560–2574. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Cheuk, A.T.; Wei, J.S.; Abdelmaksoud, A.; Chou, H.-C.; Milewski, D.; Kelly, M.C.; Song, Y.K.; Dower, C.M.; Li, N.; et al. An Optimized Bicistronic Chimeric Antigen Receptor against GPC2 or CD276 Overcomes Heterogeneous Expression in Neuroblastoma. J. Clin. Investig. 2022, 132, e155621. [Google Scholar] [CrossRef]
- Xiang, T.; Li, Y.; Liu, G.; Li, X. NR1D1-transactivated lncRNA NUTM2A-AS1 Promotes Chemoresistance and Immune Evasion in Neuroblastoma via Inhibiting B7-H3 Degradation. J. Cell Mol. Med. 2024, 28, e18360. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrans, Z.T.; Erbe, A.K.; Clemons, N.B.; Feils, A.S.; Medina-Guevara, Y.; Jeffery, J.J.; Barnhart, T.E.; Engle, J.W.; Sondel, P.M.; Hernandez, R. Targeting Both GD2 and B7-H3 Using Bispecific Antibody Improves Tumor Selectivity for GD2-Positive Tumors. BioRxiv 2024, 30. 2024.05.23.595624. [Google Scholar] [CrossRef]
- Kendsersky, N.M.; Lindsay, J.; Kolb, E.A.; Smith, M.A.; Teicher, B.A.; Erickson, S.W.; Earley, E.J.; Mosse, Y.P.; Martinez, D.; Pogoriler, J.; et al. The B7-H3-Targeting Antibody-Drug Conjugate M276-SL-PBD Is Potently Effective against Pediatric Cancer Preclinical Solid Tumor Models. Clin. Cancer Res. 2021, 27, 2938–2946. [Google Scholar] [CrossRef]
- Brignole, C.; Calarco, E.; Bensa, V.; Giusto, E.; Perri, P.; Ciampi, E.; Corrias, M.V.; Astigiano, S.; Cilli, M.; Loo, D.; et al. Antitumor Activity of the Investigational B7-H3 Antibody-Drug Conjugate, Vobramitamab Duocarmazine, in Preclinical Models of Neuroblastoma. J. Immunother. Cancer 2023, 11, e007174. [Google Scholar] [CrossRef]
- Kramer, K.; Pandit-Taskar, N.; Kushner, B.H.; Zanzonico, P.; Humm, J.L.; Tomlinson, U.; Donzelli, M.; Wolden, S.L.; Haque, S.; Dunkel, I.; et al. Phase 1 Study of Intraventricular 131I-Omburtamab Targeting B7H3 (CD276)-Expressing CNS Malignancies. J. Hematol. Oncol. 2022, 15, 165. [Google Scholar] [CrossRef]
- Goldstein, A.M.; Yuen, J.; Tucker, M.A. Second Cancers after Medulloblastoma: Population-Based Results from the United States and Sweden. Cancer Causes Control 1997, 8, 865–871. [Google Scholar] [CrossRef]
- Marques, R.F.; Moreno, D.A.; da Silva, L.; Leal, L.F.; de Paula, F.E.; Santana, I.; Teixeira, G.; Saggioro, F.; Neder, L.; Junior, C.A.; et al. Digital Expression Profile of Immune Checkpoint Genes in Medulloblastomas Identifies CD24 and CD276 as Putative Immunotherapy Targets. Front. Immunol. 2023, 14, 1062856. [Google Scholar] [CrossRef]
- Purvis, I.J.; Velpula, K.K.; Guda, M.R.; Nguyen, D.; Tsung, A.J.; Asuthkar, S. B7-H3 in Medulloblastoma-Derived Exosomes; A Novel Tumorigenic Role. Int. J. Mol. Sci. 2020, 21, 7050. [Google Scholar] [CrossRef] [PubMed]
- Mews, E.A.; Beckmann, P.; Patchava, M.; Wang, Y.; Largaespada, D.A.; Wagner, C.R. Multivalent, Bispecific αB7-H3-αCD3 Chemically Self-Assembled Nanorings Direct Potent T Cell Responses Against Medulloblastoma. ACS Nano 2022, 16, 12185–12201. [Google Scholar] [CrossRef]
- Shishido, K.; Purvis, I.J.; Velpula, K.K.; Venkataraman, S.; Vibhakar, R.; Asuthkar, S. Targeting B7-H3 through EZH2 Inhibition in MYC-positive Group 3 Medulloblastoma. Oncol. Rep. 2023, 49, 119. [Google Scholar] [CrossRef]
- Müller, H.L.; Merchant, T.E.; Warmuth-Metz, M.; Martinez-Barbera, J.-P.; Puget, S. Craniopharyngioma. Nat. Rev. Dis. Primers 2019, 5, 75. [Google Scholar] [CrossRef]
- Müller, H.L. Paediatrics: Surgical Strategy and Quality of Life in Craniopharyngioma. Nat. Rev. Endocrinol. 2013, 9, 447–449. [Google Scholar] [CrossRef]
- Coy, S.; Lee, J.S.; Chan, S.J.; Woo, T.; Jones, J.; Alexandrescu, S.; Wen, P.Y.; Sorger, P.K.; Ligon, K.L.; Santagata, S. Systematic Characterization of Antibody–Drug Conjugate Targets in Central Nervous System Tumors. Neuro Oncol. 2023, 26, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, J.; Wang, L.; Zhou, T.; Yang, J.; Tian, Z.; Yang, J.; Chen, H.; Tang, X.; Zhao, S.; et al. Expression and Clinical Significance of PD-L1, B7-H3, B7-H4 and VISTA in Craniopharyngioma. J. Immunother. Cancer 2020, 8, e000406. [Google Scholar] [CrossRef]
- Tang, M.; Chen, C.; Wang, G.; Wang, Y.; Zhang, Z.; Li, H.; Lu, Q.; Wang, Z.; Zhao, S.; Yang, C.; et al. Evaluation of B7-H3 Targeted Immunotherapy in a 3D Organoid Model of Craniopharyngioma. Biomolecules 2022, 12, 1744. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.W.; Mino-Kenudson, M. Immunotherapy in Non-Small Cell Lung Cancer: Steps towards More Effective Combination Therapies. Transl. Cancer Res. 2018, 24, 2653–2664. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, X.; Wu, S.; Du, R.; Zhu, Q.; Fang, H.; Zhang, X.; Zhang, C.; Zheng, W.; Yang, J.; et al. Overexpression of B7-H3 Correlates with Aggressive Clinicopathological Characteristics in Non-Small Cell Lung Cancer. Oncotarget 2016, 7, 81750–81756. [Google Scholar] [CrossRef] [PubMed]
- Altan, M.; Pelekanou, V.; Schalper, K.A.; Toki, M.; Gaule, P.; Syrigos, K.; Herbst, R.S.; Rimm, D.L. B7-H3 Expression in NSCLC and Its Association with B7-H4, PD-L1 and Tumor Infiltrating Lymphocytes. Clin. Cancer Res. 2017, 23, 5202–5209. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; Lu, X.; Huang, H.; Zhou, Y.; Lu, B.; Zhang, X. Diagnosis Value of Serum B7-H3 Expression in Non-Small Cell Lung Cancer. Lung Cancer 2009, 66, 245–249. [Google Scholar] [CrossRef]
- Liu, Z.; Pei, M.-M.; Liu, J.-X.; Shi, F.; Zhang, Y.; Zhao, D.-F.; Li, J.-M.; Guo, F.-R.; Yan, J.-J.; Liu, J.-Q.; et al. The Expressions and Significance of B7-H3 and CTLA-4 in the Clinical Stages of Non-Small-Cell Lung Cancer. Int. J. Clin. Exp. Pathol. 2019, 12, 3032–3041. [Google Scholar]
- Nie, J.; Yang, R.; Zhou, R.; Deng, Y.; Li, D.; Gou, D.; Zhang, Y. Circular RNA circFARSA Promotes the Tumorigenesis of Non-Small Cell Lung Cancer by Elevating B7H3 via Sponging miR-15a-5p. Cell Cycle 2022, 21, 2575–2589. [Google Scholar] [CrossRef]
- Li, F.; Chen, H.; Wang, D. Silencing of CD276 Suppresses Lung Cancer Progression by Regulating Integrin Signaling. J. Thorac. Dis. 2020, 12, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gümüş, Z.H.; Colarossi, C.; Memeo, L.; Wang, X.; Kong, C.Y.; Boffetta, P. SCLC: Epidemiology, Risk Factors, Genetic Susceptibility, Molecular Pathology, Screening, and Early Detection. J. Thorac. Oncol. 2023, 18, 31–46. [Google Scholar] [CrossRef]
- Qiu, M.; Xia, Q.; Chen, Y.; Fang, X.; Li, Q.; Zhu, L.; Jiang, X.; Xiong, Z.; Yang, S. The Expression of Three Negative Co-Stimulatory B7 Family Molecules in Small Cell Lung Cancer and Their Effect on Prognosis. Front. Oncol. 2021, 11, 600238. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Hausdorf, D.; Altan, M.; Velcheti, V.; Gettinger, S.N.; Herbst, R.S.; Rimm, D.L.; Schalper, K.A. Expression and Clinical Significance of PD-L1, B7-H3, B7-H4 and TILs in Human Small Cell Lung Cancer (SCLC). J. Immunother. Cancer 2019, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Kinoshita, S.; Ando, J.; Azusawa, Y.; Ishii, M.; Harada, S.; Mitsuishi, Y.; Asao, T.; Tajima, K.; Yamamoto, T.; et al. SCLC-J1, a Novel Small Cell Lung Cancer Cell Line. Biochem. Biophys. Rep. 2021, 27, 101089. [Google Scholar] [CrossRef]
- Fabrizio, F.P.; Muscarella, L.A.; Rossi, A. B7-H3/CD276 and Small-Cell Lung Cancer: What’s New? Transl. Oncol. 2023, 39, 101801. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J.; Wang, J.; Liu, Y.; Zhang, F.; Lin, W.; Gao, A.; Sun, M.; Wang, Y.; Sun, Y. B7-H3 Expression in Ductal and Lobular Breast Cancer and Its Association with IL-10. Mol. Med. Rep. 2013, 7, 134–138. [Google Scholar] [CrossRef]
- Zhao, B.; Li, H.; Xia, Y.; Wang, Y.; Wang, Y.; Shi, Y.; Xing, H.; Qu, T.; Wang, Y.; Ma, W. Immune Checkpoint of B7-H3 in Cancer: From Immunology to Clinical Immunotherapy. J. Hematol. Oncol. 2022, 15, 153. [Google Scholar] [CrossRef]
- Kim, G.-E.; Kim, N.I.; Park, M.H.; Lee, J.S. B7-H3 and B7-H4 Expression in Phyllodes Tumors of the Breast Detected by RNA in Situ Hybridization and Immunohistochemistry: Association with Clinicopathological Features and T-Cell Infiltration. Tumour Biol. 2018, 40, 1010428318815032. [Google Scholar] [CrossRef] [PubMed]
- Avci, O.; Çavdar, E.; İriağaç, Y.; Karaboyun, K.; Çelikkol, A.; Özçağlayan, T.İ.K.; Öznur, M.; Gürdal, S.Ö.; Şeber, E.S. Soluble B7H3 Level in Breast Cancer and Its Relationship with Clinicopathological Variables and T Cell Infiltration. Contemp. Oncol. 2022, 26, 27–31. [Google Scholar] [CrossRef]
- Bam, R.; Lown, P.S.; Stern, L.A.; Sharma, K.; Wilson, K.E.; Bean, G.R.; Lutz, A.M.; Paulmurugan, R.; Hackel, B.J.; Dahl, J.; et al. Efficacy of Affibody-Based Ultrasound Molecular Imaging of Vascular B7-H3 for Breast Cancer Detection. Clin. Cancer Res. 2020, 26, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Lin, X.; Huo, J.; Zhang, F. Clinicopathological Features and Prognostic Value of CD276 Expression in Breast Cancer: A Meta-Analysis. Asian J. Surg. 2023, 46, 5323–5329. [Google Scholar] [CrossRef]
- Joshi, V.; Beecher, K.; Lim, M.; Stacey, A.; Feng, Y.; Jat, P.S.; Duijf, P.H.G.; Simpson, P.T.; Lakhani, S.R.; McCart Reed, A.E. B7-H3 Expression in Breast Cancer and Brain Metastasis. Int. J. Mol. Sci. 2024, 25, 3976. [Google Scholar] [CrossRef]
- Shao, L.; Yu, Q.; Xia, R.; Zhang, J.; Gu, S.; Yu, D.; Zhuang, Z. B7-H3 on Breast Cancer Cell MCF7 Inhibits IFN-γ Release from Tumour-Infiltrating T Cells. Pathol. Res. Pract. 2021, 224, 153461. [Google Scholar] [CrossRef]
- Cheng, N.; Bei, Y.; Song, Y.; Zhang, W.; Xu, L.; Zhang, W.; Yang, N.; Bai, X.; Shu, Y.; Shen, P. B7-H3 Augments the pro-Angiogenic Function of Tumor-Associated Macrophages and Acts as a Novel Adjuvant Target for Triple-Negative Breast Cancer Therapy. Biochem. Pharmacol. 2021, 183, 114298. [Google Scholar] [CrossRef]
- Mei, J.; Cai, Y.; Zhu, H.; Jiang, Y.; Fu, Z.; Xu, J.; Chen, L.; Yang, K.; Zhao, J.; Song, C.; et al. High B7-H3 Expression with Low PD-L1 Expression Identifies Armored-Cold Tumors in Triple-Negative Breast Cancer. NPJ Breast Cancer 2024, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, H.-L.; Li, Z.-L.; Du, T.; Chen, Y.-H.; Wang, Y.; Ni, H.-H.; Zhang, K.-M.; Mai, J.; Hu, B.-X.; et al. FUT8-Mediated Aberrant N-Glycosylation of B7H3 Suppresses the Immune Response in Triple-Negative Breast Cancer. Nat. Commun. 2021, 12, 2672. [Google Scholar] [CrossRef]
- Liu, H.; Tekle, C.; Chen, Y.-W.; Kristian, A.; Zhao, Y.; Zhou, M.; Liu, Z.; Ding, Y.; Wang, B.; Mælandsmo, G.M.; et al. B7-H3 Silencing Increases Paclitaxel Sensitivity by Abrogating Jak2/Stat3 Phosphorylation. Mol. Cancer Ther. 2011, 10, 960–971. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Ning, H.; Dong, S.; Wang, G.; Sun, R. B7 Homolog 3 Induces Lung Metastasis of Breast Cancer through Raf/MEK/ERK Axis. Breast Cancer Res. Treat. 2022, 193, 405–416. [Google Scholar] [CrossRef]
- Fang, J.; Chen, F.; Liu, D.; Gu, F.; Chen, Z.; Wang, Y. Prognostic Value of Immune Checkpoint Molecules in Breast Cancer. Biosci. Rep. 2020, 40, BSR20201054. [Google Scholar] [CrossRef]
- Kim, N.I.; Park, M.H.; Cho, N.; Lee, J.S. Comparison of the Clinicopathologic Features and T-Cell Infiltration of B7-H3 and B7-H4 Expression in Triple-Negative Breast Cancer Subtypes. Appl. Immunohistochem. Mol. Morphol. 2022, 30, 246–256. [Google Scholar] [CrossRef]
- MacroGenics. A Phase 1 Dose Escalation Study of MGA271 in Refractory Cancer. 2022. Available online: https://adisinsight.springer.com/trials/700202499 (accessed on 24 July 2025).
- Second Affiliated Hospital of Guangzhou Medical University. A Single-Arm, Open, Exploratory Clinical Study Evaluating the Safety and Efficacy of EGFR/B7H3 CAR-T in Patients with EGFR/B7H3-Positive Advanced Solid Tumors (Lung and Triple-Negative Breast Cancer). 2024. Available online: https://clinicaltrials.gov/study/NCT05341492 (accessed on 24 July 2025).
- Huang, C.; Zhou, L.; Chang, X.; Pang, X.; Zhang, H.; Zhang, S. B7-H3, B7-H4, Foxp3 and IL-2 Expression in Cervical Cancer: Associations with Patient Outcome and Clinical Significance. Oncol. Rep. 2016, 35, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Zhang, Q.; Zhou, Y.; Kong, Y.; Yu, S.; Chen, J.; Zhang, Y.; Xiang, Y. Expression and Significance of Immune Checkpoints in Clear Cell Carcinoma of the Uterine Cervix. J. Immunol. Res. 2020, 2020, 1283632. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, H.; Lian, H.; Ke, L.; Zhao, L.; Wang, C.; Han, Q. CD276 (B7H3) Improve Cancer Stem Cells Formation in Cervical Carcinoma Cell Lines. Transl. Cancer Res. 2021, 10, 65–72. [Google Scholar] [CrossRef]
- Han, S.; Wang, Y.; Shi, X.; Zong, L.; Liu, L.; Zhang, J.; Qian, Q.; Jin, J.; Ma, Y.; Cui, B.; et al. Negative Roles of B7-H3 and B7-H4 in the Microenvironment of Cervical Cancer. Exp. Cell Res. 2018, 371, 222–230. [Google Scholar] [CrossRef]
- Roth, T.J.; Sheinin, Y.; Lohse, C.M.; Kuntz, S.M.; Frigola, X.; Inman, B.A.; Krambeck, A.E.; McKenney, M.E.; Karnes, R.J.; Blute, M.L.; et al. B7-H3 Ligand Expression by Prostate Cancer: A Novel Marker of Prognosis and Potential Target for Therapy. Cancer Res. 2007, 67, 7893–7900. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Figueiredo, I.; Gurel, B.; Neeb, A.; Seed, G.; Crespo, M.; Carreira, S.; Rekowski, J.; Buroni, L.; Welti, J.; et al. B7-H3 as a Therapeutic Target in Advanced Prostate Cancer. Eur. Urol. 2023, 83, 224–238. [Google Scholar] [CrossRef]
- Kang, N.; Xue, H.; Lin, Y.-Y.; Dong, X.; Classen, A.; Wu, R.; Jin, Y.; Lin, D.; Volik, S.; Ong, C.; et al. Influence of ADT on B7-H3 Expression during CRPC Progression from Hormone-Naïve Prostate Cancer. Cancer Gene Ther. 2023, 30, 1382–1389. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Y.; Zhao, Y.; Kim, J.J.; Li, H.; Meng, C.; Chen, F.; Zhang, J.; Mak, D.H.; Van, V.; et al. Immune Checkpoint B7-H3 Is a Therapeutic Vulnerability in Prostate Cancer Harboring PTEN and TP53 Deficiencies. Sci. Transl. Med. 2023, 15, eadf6724. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Song, Y.; Zhang, Y. Suppressive Function of Bone Marrow-Derived Mesenchymal Stem Cell-Derived Exosomal microRNA-187 in Prostate Cancer. Cancer Biol. Ther. 2022, 23, 1–14. [Google Scholar] [CrossRef]
- Tian, Y.; Shen, H.; Li, L.; Jia, X.; Liu, J.; Hu, Z.; Wang, L.; Tian, J. Enhancing Surgical Outcomes: Accurate Identification and Removal of Prostate Cancer with B7-H3-Targeted NIR-II Molecular Imaging. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2569–2582. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.J.; Ryu, H.W.; Shin, S.W.; Kim, E.J.; Shin, S.H.; Park, J.Y.; Kim, S.Y.; Hwang, C.S.; Na, J.-Y.; et al. B7-H3 Expression Is Associated with High PD-L1 Expression in Clear Cell Renal Cell Carcinoma and Predicts Poor Prognosis. Diagn. Pathol. 2023, 18, 36. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Zhao, H.; Cao, J.; Wang, S.; Li, H.; Savoldo, B.; Rao, E.; Dotti, G.; Du, H. Preclinical Assessment of the Efficacy of B7-H3 CAR-T in Renal Cell Carcinoma. Mol. Immunol. 2024, 176, 1–10. [Google Scholar] [CrossRef]
- Saeednejad Zanjani, L.; Madjd, Z.; Axcrona, U.; Abolhasani, M.; Rasti, A.; Asgari, M.; Fodstad, Ø.; Andersson, Y. Cytoplasmic Expression of B7-H3 and Membranous EpCAM Expression Are Associated with Higher Grade and Survival Outcomes in Patients with Clear Cell Renal Cell Carcinoma. Ann. Diagn. Pathol. 2020, 46, 151483. [Google Scholar] [CrossRef]
- Deng, W.; Wu, L.; Chen, L.; Wang, K.; Lin, N.; Zhu, L.; Chen, J. Development of B7-H3 Targeted CAR-T Cells for Renal Cell Carcinoma Therapy: In Vitro and in Vivo Efficacy. Clin. Transl. Oncol. 2024, 27, 2667–2678. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, J.; Zhang, G.; Fang, C.; Jiang, F.; Ma, S.; Hou, J. Expression and Significance of B7-H3 and Tie-2 in the Tumor Vasculature of Clear Cell Renal Carcinoma. Onco Targets Ther. 2017, 10, 5417–5424. [Google Scholar] [CrossRef]
- Özalp, F.R.; Yörükoğlu, K.; Yıldırım, E.Ç.; Uzun, M.; Semiz, H.S. Prognostic Value of B7-H3 Expression in Metastatic Renal Cell Carcinoma and Its Impact on Immunotherapy Response. BMC Cancer 2024, 24, 1471. [Google Scholar] [CrossRef]
- Mischinger, J.; Fröhlich, E.; Mannweiler, S.; Meindl, C.; Absenger-Novak, M.; Hutterer, G.C.; Seles, M.; Augustin, H.; Chromecki, T.F.; Jesche-Chromecki, J.; et al. Prognostic Value of B7-H1, B7-H3 and the Stage, Size, Grade and Necrosis (SSIGN) Score in Metastatic Clear Cell Renal Cell Carcinoma. Cent. Eur. J. Urol. 2019, 72, 23–31. [Google Scholar] [CrossRef]
- Zheng, Y.; Lu, Z.; Zhu, F.; Zhao, G.; Shao, Y.; Lu, B.; Ding, J.; Wang, G.; Fang, L.; Zheng, J.; et al. Therapeutic Vaccine Targeting Dual Immune Checkpoints Induces Potent Multifunctional CD8+ T Cell Anti-Tumor Immunity. Int. Immunopharmacol. 2024, 142, 113004. [Google Scholar] [CrossRef]
- Sun, H.; Gao, F.; Liu, Y.; Shao, J. Survival and Clinicopathological Significance of B7-H3 in Bladder Cancer: A Systematic Review and Meta-Analysis. BMC Urol. 2024, 24, 57. [Google Scholar] [CrossRef]
- Koyama, Y.; Morikawa, T.; Miyama, Y.; Miyakawa, J.; Kawai, T.; Kume, H.; Sawabe, M.; Ushiku, T. B7-H3 Expression in Upper Tract Urothelial Carcinoma Associates with Adverse Clinicopathological Features and Poor Survival. Pathol.-Res. Pract. 2020, 216, 153219. [Google Scholar] [CrossRef]
- Deol, E.S.; Nabavizadeh, R.; Lavoie, R.R.; Dumbrava, M.G.; Horjeti, E.; Thapa, P.; Cheville, J.C.; Frank, I.; Lucien, F. Role of B7-H3 in Predicting Response to Neoadjuvant Chemotherapy in Muscle-invasive Bladder Cancer. BJUI Compass 2024, 5, 1052–1058. [Google Scholar] [CrossRef]
- Azuma, T.; Sato, Y.; Ohno, T.; Azuma, M.; Kume, H. Serum Soluble B7-H3 Is a Prognostic Marker for Patients with Non-Muscle-Invasive Bladder Cancer. PLoS ONE 2020, 15, e0243379. [Google Scholar] [CrossRef]
- Xu, Z.L.; Zhang, Y.; Wang, L.; Li, F.; Man, H.W.; Li, P.F.; Shan, B.E. B7-H3 Promotes Malignant Progression of Muscle-invasive Bladder Cancer. Oncol. Rep. 2018, 40, 2722–2733. [Google Scholar] [CrossRef]
- Boschert, V.; Boenke, J.; Böhm, A.-K.; Teusch, J.; Steinacker, V.; Straub, A.; Hartmann, S. Differential Immune Checkpoint Protein Expression in HNSCC: The Role of HGF/MET Signaling. Int. J. Mol. Sci. 2024, 25, 7334. [Google Scholar] [CrossRef]
- Lin, W.; Xu, Y.; Gao, J.; Zhang, H.; Sun, Y.; Qiu, X.; Huang, Q.; Kong, L.; Lu, J.J. Multi-Omics Data Analyses Identify B7-H3 as a Novel Prognostic Biomarker and Predict Response to Immune Checkpoint Blockade in Head and Neck Squamous Cell Carcinoma. Front. Immunol. 2021, 12, 757047. [Google Scholar] [CrossRef]
- Borgmann, M.; Oetting, A.; Meyer, F.; Möckelmann, N.; Droste, C.; von Bargen, C.M.; Möller-Koop, C.; Witt, M.; Borgmann, K.; Rothkamm, K.; et al. The Prognostic Impact of B7-H3 and B7-H4 in Head and Neck Squamous Cell Carcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 3383–3393. [Google Scholar] [CrossRef]
- Zou, W.-Q.; Luo, W.-J.; Feng, Y.-F.; Liu, F.; Liang, S.-B.; Fang, X.-L.; Liang, Y.-L.; Liu, N.; Wang, Y.-Q.; Mao, Y.-P. Expression Profiles and Prognostic Value of Multiple Inhibitory Checkpoints in Head and Neck Lymphoepithelioma-Like Carcinoma. Front. Immunol. 2022, 13, 818411. [Google Scholar] [CrossRef] [PubMed]
- Sieviläinen, M.; Wirsing, A.M.; Hyytiäinen, A.; Almahmoudi, R.; Rodrigues, P.; Bjerkli, I.-H.; Åström, P.; Toppila-Salmi, S.; Paavonen, T.; Coletta, R.D.; et al. Evaluation Challenges in the Validation of B7-H3 as Oral Tongue Cancer Prognosticator. Head. Neck Pathol. 2021, 15, 469–478. [Google Scholar] [CrossRef]
- Liao, C.; An, J.; Tan, Z.; Xu, F.; Liu, J.; Wang, Q. Changes in Protein Glycosylation in Head and Neck Squamous Cell Carcinoma. J. Cancer 2021, 12, 1455–1466. [Google Scholar] [CrossRef]
- Katayama, A.; Takahara, M.; Kishibe, K.; Nagato, T.; Kunibe, I.; Katada, A.; Hayashi, T.; Harabuchi, Y. Expression of B7-H3 in Hypopharyngeal Squamous Cell Carcinoma as a Predictive Indicator for Tumor Metastasis and Prognosis. Int. J. Oncol. 2011, 38, 1219–1226. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Yang, X.; Zhang, F.; Wang, J.; Wu, Z.; Wanyan, C.; Meng, Q.; Gao, W.; Yang, X.; et al. LINC01123 Promotes Immune Escape by Sponging miR-214-3p to Regulate B7–H3 in Head and Neck Squamous-Cell Carcinoma. Cell Death Dis. 2022, 13, 109. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Z.; Luan, S.; Zheng, M.; Wang, Z.; Chen, Y.; Chen, X.; Tong, A.; Yang, H. Novel Bispecific Antibody-Drug Conjugate Targeting PD-L1 and B7-H3 Enhances Antitumor Efficacy and Promotes Immune-Mediated Antitumor Responses. J. Immunother. Cancer 2024, 12, e009710. [Google Scholar] [CrossRef]
- Lin, H.; Cheng, J.; Mu, W.; Zhou, J.; Zhu, L. Advances in Universal CAR-T Cell Therapy. Front. Immunol. 2021, 12, 744823. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, E.; Du, H.; Shou, P.; Song, F.; Suzuki, K.; Ahn, S.; Li, G.; Ferrone, S.; Su, L.; Savoldo, B.; et al. Preclinical Evaluation of B7-H3-Specific Chimeric Antigen Receptor T Cells for the Treatment of Acute Myeloid Leukemia. Clin. Cancer Res. 2021, 27, 3141–3153. [Google Scholar] [CrossRef]
- Haydar, D.; Houke, H.; Chiang, J.; Yi, Z.; Odé, Z.; Caldwell, K.; Zhu, X.; Mercer, K.S.; Stripay, J.L.; Shaw, T.I.; et al. Cell-Surface Antigen Profiling of Pediatric Brain Tumors: B7-H3 Is Consistently Expressed and Can Be Targeted via Local or Systemic CAR T-Cell Delivery. Neuro-Oncol. 2021, 23, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, N.A.; Ronsley, R.; Choe, M.; Seidel, K.; Huang, W.; Rawlings-Rhea, S.D.; Beam, M.; Steinmetzer, L.; Wilson, A.L.; Brown, C.; et al. Intracerebroventricular B7-H3-Targeting CAR T Cells for Diffuse Intrinsic Pontine Glioma: A Phase 1 Trial. Nat. Med. 2025, 31, 861–868. [Google Scholar] [CrossRef]
- Li, H.; Harrison, E.B.; Li, H.; Hirabayashi, K.; Chen, J.; Li, Q.-X.; Gunn, J.; Weiss, J.; Savoldo, B.; Parker, J.S.; et al. Targeting Brain Lesions of Non-Small Cell Lung Cancer by Enhancing CCL2-Mediated CAR-T Cell Migration. Nat. Commun. 2022, 13, 2154. [Google Scholar] [CrossRef]
- St. Jude Children’s Research Hospital. Loc3CAR: Locoregional Delivery of B7-H3-Specific Chimeric Antigen Receptor Autologous T Cells for Pediatric Patients with Primary CNS Tumors. 2024. Available online: https://connect.careboxhealth.com/en-US/partner/hope4atrt/trial/400799 (accessed on 24 July 2025).
- Mackall, C. Phase I Clinical Trial of Locoregionally (LR) Delivered Autologous B7-H3 Chimeric Antigen Receptor T Cells (B7-H3CART) in Adults with Recurrent Glioblastoma Multiforme (GBM). 2024. Available online: https://clinicaltrials.gov/study/NCT05474378 (accessed on 24 July 2025).
- UNC Lineberger Comprehensive Cancer Center A Phase I Study of Autologous CAR-T Cells Targeting the B7-H3 Antigen and Containing the Inducible Caspase 9 Safety Switch in Subjects with Refractory Pancreatic Ductal Adenocarcinoma (PDAC). 2024. Available online: https://clinicaltrials.gov/study/NCT06158139 (accessed on 24 July 2025).
- The First People’s Hospital of Lianyungang An Open, Single-Arm Clinical Study of Autologous T Cells (CAR-T) Targeting B7-H3 Chimeric Antigen Receptor Gene in the Treatment of Patients with Advanced Gastrointestinal Tumors. 2022. Available online: https://clinicaltrials.gov/study/NCT05515185 (accessed on 24 July 2025).
- PersonGen BioTherapeutics (Suzhou) Co., Ltd. Clinical Study of UTAA06 Injection in the Treatment of Relapsed/Refractory Acute Myeloid Leukemia. 2023. Available online: https://clinicaltrials.gov/study/NCT05731219 (accessed on 24 July 2025).
- PersonGen BioTherapeutics (Suzhou) Co., Ltd. An Open-Label, Dose-Escalation, and Dose-Expansion Phase I Trial of TAA06 Injection in Patients with Relapsed/Refractory Neuroblastoma. 2023. Available online: https://clinicaltrials.gov/study/NCT05562024 (accessed on 24 July 2025).
- UNC Lineberger Comprehensive Cancer Center. Phase I Study of Intraventricular Infusion of T Cells Expressing B7-H3 Specific Chimeric Antigen Receptors (CAR) in Subjects with Recurrent or Refractory Glioblastoma. 2024. Available online: https://clinicaltrials.gov/study/NCT05366179 (accessed on 24 July 2025).
- Meeus, F.; Funeh, C.N.; Awad, R.M.; Zeven, K.; Autaers, D.; De Becker, A.; Van Riet, I.; Goyvaerts, C.; Tuyaerts, S.; Neyns, B.; et al. Preclinical Evaluation of Antigen-Sensitive B7-H3-Targeting Nanobody-Based CAR-T Cells in Glioblastoma Cautions for on-Target, off-Tumor Toxicity. J. Immunother. Cancer 2024, 12, e009110. [Google Scholar] [CrossRef]
- Li, G.; Wang, H.; Wu, H.; Chen, J. B7-H3-Targeted CAR-T Cell Therapy for Solid Tumors. Int. Rev. Immunol. 2022, 41, 625–637. [Google Scholar] [CrossRef]
- Hay, K.A.; Hanafi, L.A.; Li, D.; Gust, J.; Liles, W.C.; Wurfel, M.M.; López, J.A.; Chen, J.; Chung, D.; Harju-Baker, S.; et al. Kinetics and Biomarkers of Severe Cytokine Release Syndrome after CD19 Chimeric Antigen Receptor–Modified T-Cell Therapy. Blood 2017, 130, 2295–2306. [Google Scholar] [CrossRef]
- Curran, K.J.; Margossian, S.P.; Kernan, N.A.; Silverman, L.B.; Williams, D.A.; Shukla, N.; Kobos, R.; Forlenza, C.J.; Steinherz, P.; Prockop, S.; et al. Toxicity and Response after CD19-Specific CAR T-Cell Therapy in Pediatric/Young Adult Relapsed/Refractory B-ALL. Blood 2019, 134, 2361–2368. [Google Scholar] [CrossRef]
- Zhou, W.-T.; Jin, W.-L. B7-H3/CD276: An Emerging Cancer Immunotherapy. Front. Immunol. 2021, 12, 701006. [Google Scholar] [CrossRef]
- Scribner, J.A.; Brown, J.G.; Son, T.; Chiechi, M.; Li, P.; Sharma, S.; Li, H.; De Costa, A.; Li, Y.; Chen, Y.; et al. Preclinical Development of MGC018, a Duocarmycin-Based Antibody–Drug Conjugate Targeting B7-H3 for Solid Cancer. Mol. Cancer Ther. 2020, 19, 2235–2244. [Google Scholar] [CrossRef]
- DS-7300a, a DNA Topoisomerase I Inhibitor, DXd-Based Antibody–Drug Conjugate Targeting B7-H3, Exerts Potent Antitumor Activities in Preclinical Models | Molecular Cancer Therapeutics | American Association for Cancer Research. Available online: https://aacrjournals.org/mct/article/21/4/635/689570/DS-7300a-a-DNA-Topoisomerase-I-Inhibitor-DXd-Based (accessed on 2 March 2025).
- Shenderov, E.; Mallesara, G.H.G.; Wysocki, P.J.; Xu, W.; Ramlau, R.; Weickhardt, A.J.; Zolnierek, J.; Spira, A.; Joshua, A.M.; Powderly, J.; et al. 620P MGC018, an Anti-B7-H3 Antibody-Drug Conjugate (ADC), in Patients with Advanced Solid Tumors: Preliminary Results of Phase I Cohort Expansion. Ann. Oncol. 2021, 32, S657–S659. [Google Scholar] [CrossRef]
- Daiichi Sankyo Co., Ltd. Phase 1, Open Label Study to Assess the Safety and Pharmacokinetics of DS-5573a in Japanese Patients with Advanced Solid Malignant Tumors. 2017. Available online: https://clinicaltrials.gov/study/NCT02192567 (accessed on 24 July 2025).
- Daiichi Sankyo. A Phase 3, Multicenter, Randomized, Open-Label Study of Ifinatamab Deruxtecan (I-DXd), a B7-H3 Antibody Drug Conjugate (ADC), Versus Treatment of Physician’s Choice (TPC) in Subjects with Relapsed Small Cell Lung Cancer (SCLC) (IDeate-Lung02). 2025. Available online: https://clinicaltrials.gov/study/NCT06203210 (accessed on 24 July 2025).
- Hansoh BioMedical R&D Company. ARTEMIS-007: A Phase 2 Study to Evaluate Efficacy and Safety of HS-20093 in Patients with Extensive Stage Small Cell Lung Cancer. 2024. Available online: https://adisinsight.springer.com/trials/700367566 (accessed on 24 July 2025).
- DualityBio Inc. A Phase 1/2a, Multicenter, Open-Label, First in Human Study to Assess the Safety, Tolerability, Pharmacokinetics, and Preliminary Antitumor Activity of DB-1311 in Subjects with Advanced/Metastatic Solid Tumors. 2024. Available online: https://www.clinicaltrials.gov/study/NCT05914116?term=NCT05914116&rank=1 (accessed on 24 July 2025).
- MacroGenics. A Phase 1/1b Dose Escalation and Cohort Expansion Study of MGC018 in Combination with Checkpoint Inhibitor in Participants with Advanced Solid Tumors. 2024. Available online: https://clinicaltrials.gov/study/NCT05293496 (accessed on 24 July 2025).
- Passaro, A.; Jänne, P.A.; Peters, S. Antibody-Drug Conjugates in Lung Cancer: Recent Advances and Implementing Strategies. J. Clin. Oncol. 2023, 41, 3747–3761. Available online: https://ascopubs.org/doi/10.1200/JCO.23.00013?url_ver=Z39.882003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 24 July 2025). [CrossRef]
- Donaghy, H. Effects of Antibody, Drug and Linker on the Preclinical and Clinical Toxicities of Antibody-Drug Conjugates. mAbs 2016, 8, 659–671. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4966843/ (accessed on 24 July 2025). [CrossRef] [PubMed]
- Masters, J.C.; Nickens, D.J.; Xuan, D.; Shazer, R.L.; Amantea, M. Clinical Toxicity of Antibody Drug Conjugates: A Meta-Analysis of Payloads. Investig. New Drugs 2018, 36, 121–135. [Google Scholar] [CrossRef]
- Feustel, K.; Martin, J.; Falchook, G.S. B7-H3 Inhibitors in Oncology Clinical Trials: A Review. J. Immunother. Precis. Oncol. 2024, 7, 53–66. [Google Scholar] [CrossRef]
- Narvekar, A.; Pardeshi, A.; Jain, R.; Dandekar, P. ADCC Enhancement: A Conundrum or a Boon to mAb Therapy? Biologicals 2022, 79, 10–18. [Google Scholar] [CrossRef]
- Loo, D.; Alderson, R.F.; Chen, F.Z.; Huang, L.; Zhang, W.; Gorlatov, S.; Burke, S.; Ciccarone, V.; Li, H.; Yang, Y.; et al. Development of an Fc-Enhanced Anti–B7-H3 Monoclonal Antibody with Potent Antitumor Activity. Clin. Cancer Res. 2012, 18, 3834–3845. [Google Scholar] [CrossRef]
- Singh, T.; Fatehi Hassanabad, M.; Fatehi Hassanabad, A. Non-Small Cell Lung Cancer: Emerging Molecular Targeted and Immunotherapeutic Agents. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188636. [Google Scholar] [CrossRef]
- Aggarwal, C.; Prawira, A.; Antonia, S.; Rahma, O.; Tolcher, A.; Cohen, R.B.; Lou, Y.; Hauke, R.; Vogelzang, N.; P Zandberg, D.; et al. Dual Checkpoint Targeting of B7-H3 and PD-1 with Enoblituzumab and Pembrolizumab in Advanced Solid Tumors: Interim Results from a Multicenter Phase I/II Trial. J. Immunother. Cancer 2022, 10, e004424. [Google Scholar] [CrossRef]
- Shenderov, E.; De Marzo, A.M.; Lotan, T.L.; Wang, H.; Chan, S.; Lim, S.J.; Ji, H.; Allaf, M.E.; Chapman, C.; Moore, P.A.; et al. Neoadjuvant Enoblituzumab in Localized Prostate Cancer: A Single-Arm, Phase 2 Trial. Nat. Med. 2023, 29, 888–897. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10921422/#T2 (accessed on 2 March 2025). [CrossRef]
- Shepard, H.M.; Phillips, G.L.; Thanos, C.D.; Feldmann, M. Developments in Therapy with Monoclonal Antibodies and Related Proteins. Clin. Med. 2017, 17, 220–232. [Google Scholar] [CrossRef]
- Michelakos, T.; Kontos, F.; Barakat, O.; Maggs, L.; Schwab, J.H.; Ferrone, C.R.; Ferrone, S. B7-H3 Targeted Antibody-Based Immunotherapy of Malignant Diseases. Expert. Opin. Biol. Ther. 2021, 21, 587–602. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8087627/#_ci93_ (accessed on 2 March 2025). [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455. [Google Scholar] [CrossRef]
- Lu, H.; Shi, T.; Wang, M.; Li, X.; Gu, Y.; Zhang, X.; Zhang, G.; Chen, W. B7-H3 Inhibits the IFN-γ-Dependent Cytotoxicity of Vγ9Vδ2 T Cells against Colon Cancer Cells. Oncoimmunology 2020, 9, 1748991. [Google Scholar] [CrossRef]
- Y-mAbs Therapeutics. A Phase I/II Dose-Escalation and Expansion Cohort Trial of Intracerebroventricular Radioimmunotherapy Using 177Lu-DTPA-Omburtamab in Pediatric and Adolescent Patients with Recurrent or Refractory Medulloblastoma. 2023. Available online: https://clinicaltrials.gov/study/NCT04167618 (accessed on 24 July 2025).
- Y-mAbs Therapeutics. A Multicenter Phase 2/3 Trial of the Efficacy and Safety of Intracerebroventricular Radioimmunotherapy Using 131I-Omburtamab for Neuroblastoma Central Nervous System/Leptomeningeal Metastases. 2024. Available online: https://clinicaltrials.gov/study/NCT03275402 (accessed on 24 July 2025).
- Y-mAbs Therapeutics. Phase I Study of Intraperitoneal Radioimmunotherapy with 131I-8H9 for Patients with Desmoplastic Small Round Cell Tumors and Other Solid Tumors Involving the Peritoneum. 2023. Available online: https://clinicaltrials.gov/study/NCT01099644 (accessed on 24 July 2025).
- Zhuang, X.; Shen, J.; Jia, Z.; Wu, A.; Xu, T.; Shi, Y.; Xu, C. Anti-B7-H3 Monoclonal Antibody Ameliorates the Damage of Acute Experimental Pancreatitis by Attenuating the Inflammatory Response. Int. Immunopharmacol. 2016, 35, 1–6. Available online: https://www.sciencedirect.com/science/article/pii/S1567576916300911?via%3Dihub (accessed on 24 July 2025). [CrossRef]
- Kasten, B.B.; Ferrone, S.; Zinn, K.R.; Buchsbaum, D.J. B7-H3-Targeted Radioimmunotherapy of Human Cancer. Curr. Med. Chem. 2020, 27, 4016. [Google Scholar] [CrossRef] [PubMed]
- Modak, S.; Zanzonico, P.; Grkovski, M.; Slotkin, E.K.; Carrasquillo, J.A.; Lyashchenko, S.K.; Lewis, J.S.; Cheung, I.Y.; Heaton, T.; LaQuaglia, M.P.; et al. B7H3-Directed Intraperitoneal Radioimmunotherapy with Radioiodinated Omburtamab for Desmoplastic Small Round Cell Tumor and Other Peritoneal Tumors: Results of a Phase I Study. J. Clin. Oncol. 2020, 38, 4283. [Google Scholar] [CrossRef]
- Kasten, B.B.; Arend, R.C.; Katre, A.A.; Kim, H.; Fan, J.; Ferrone, S.; Zinn, K.R.; Buchsbaum, D.J. B7-H3-Targeted 212Pb Radioimmunotherapy of Ovarian Cancer in Preclinical Models. Nucl. Med. Biol. 2017, 47, 23–30. [Google Scholar] [CrossRef]
- Agarwal, S.; Fang, L.; McGowen, K.; Yin, J.; Bowman, J.; Ku, A.T.; Alilin, A.N.; Corey, E.; Roudier, M.P.; True, L.D.; et al. Tumor-Derived Biomarkers Predict Efficacy of B7H3 Antibody-Drug Conjugate Treatment in Metastatic Prostate Cancer Models. J. Clin. Investig. 2023, 133, e162148. [Google Scholar] [CrossRef]
- Lake, J.A.; Woods, E.; Hoffmeyer, E.; Schaller, K.L.; Cruz-Cruz, J.; Fernandez, J.; Tufa, D.; Kooiman, B.; Hall, S.C.; Jones, D.; et al. Directing B7-H3 Chimeric Antigen Receptor T Cell Homing through IL-8 Induces Potent Antitumor Activity against Pediatric Sarcoma. J. Immunother. Cancer 2024, 12, e009221. [Google Scholar] [CrossRef]
- Yamada, Y.; Venkadakrishnan, V.B.; Mizuno, K.; Bakht, M.; Ku, S.-Y.; Garcia, M.M.; Beltran, H. Targeting DNA Methylation and B7-H3 in RB1-Deficient and Neuroendocrine Prostate Cancer. Sci. Transl. Med. 2023, 15, eadf6732. [Google Scholar] [CrossRef]
- Zhang, C.; Li, K.; Zhu, H.; Cheng, M.; Chen, S.; Ling, R.; Wang, C.; Chen, D. ITGB6 Modulates Resistance to Anti-CD276 Therapy in Head and Neck Cancer by Promoting PF4+ Macrophage Infiltration. Nat. Commun. 2024, 15, 7077. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, J.; Jia, W.; Li, L.; Li, Y.; Hu, J.; Luo, W.; Li, R.; Ye, D.; Lan, P. Histone Lactylation-Driven B7-H3 Expression Promotes Tumor Immune Evasion. Theranostics 2025, 15, 2338–2359. [Google Scholar] [CrossRef]
- Lu, H.; Ma, Y.; Wang, M.; Shen, J.; Wu, H.; Li, J.; Gao, N.; Gu, Y.; Zhang, X.; Zhang, G.; et al. B7-H3 Confers Resistance to Vγ9Vδ2 T Cell-Mediated Cytotoxicity in Human Colon Cancer Cells via the STAT3/ULBP2 Axis. Cancer Immunol. Immunother. 2020, 70, 1213–1226. [Google Scholar] [CrossRef]
- Alhamad, S.; Elmasry, Y.; Uwagboe, I.; Chekmeneva, E.; Sands, C.; Cooper, B.W.; Camuzeaux, S.; Salam, A.; Parsons, M. B7-H3 Associates with IMPDH2 and Regulates Cancer Cell Survival. Cancers 2023, 15, 3530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hong, M.; Zhao, D.; Li, W.; Yuan, X.; Wang, Y.; Li, H.; Yang, Y.; Jin, T.; Pan, J. Reprogramming the Tumor Immune Microenvironment with ICAM-1-Targeted Antibody—Drug Conjugates and B7-H3-CD3 Bispecific Antibodies. Adv. Sci. 2025, 12, 2415577. [Google Scholar] [CrossRef]
- Moore, G.L.; Zeng, V.G.; Diaz, J.E.; Bonzon, C.; Avery, K.N.; Rashid, R.; Qi, J.; Nam, D.H.; Jacinto, J.; Dragovich, M.A.; et al. A B7-H3–Targeted CD28 Bispecific Antibody Enhances the Activity of Anti–PD-1 and CD3 T-Cell Engager Immunotherapies. Mol. Cancer Ther. 2025, 24, 331–344. [Google Scholar] [CrossRef]
- You, G.; Lee, Y.; Kang, Y.-W.; Park, H.W.; Park, K.; Kim, H.; Kim, Y.-M.; Kim, S.; Kim, J.-H.; Moon, D.; et al. B7-H3×4-1BB Bispecific Antibody Augments Antitumor Immunity by Enhancing Terminally Differentiated CD8+ Tumor-Infiltrating Lymphocytes. Sci. Adv. 2021, 7, eaax3160. [Google Scholar] [CrossRef]
- Zhou, Z.Z.; Si, Y.; Zhang, J.; Chen, K.; George, A.; Kim, S.; Zhou, L.; Liu, X.M. A Dual-Payload Antibody–Drug Conjugate Targeting CD276/B7-H3 Elicits Cytotoxicity and Immune Activation in Triple-Negative Breast Cancer. Cancer Res. 2024, 84, 3848–3863. [Google Scholar] [CrossRef]
- Wang, K.; Osei-Hwedieh, D.O.; Walhart, T.A.; Hung, Y.P.; Wang, Y.; Cattaneo, G.; Ma, T.; Dotti, G.; Wang, X.; Ferrone, S.; et al. B7-H3 CAR-T Cell Therapy Combined with Irradiation Is Effective in Targeting Bulk and Radiation-Resistant Chordoma Cancer Cells. J. Immunother. Cancer 2025, 13, e009544. [Google Scholar] [CrossRef]
- Hsu, M.; Martin, T.C.; Vyas, N.S.; Desman, G.; Mendelson, K.; Horst, B.; Parsons, R.E.; Celebi, J.T. B7-H3 Drives Immunosuppression and Co-targeting with CD47 Is a New Therapeutic Strategy in Β-catenin Activated Melanomas. Pigment Cell Melanoma Res. 2023, 36, 407–415. Available online: https://onlinelibrary.wiley.com/doi/10.1111/pcmr.13091 (accessed on 24 July 2025). [CrossRef] [PubMed]
- Mielcarska, S.; Dawidowicz, M.; Kula, A.; Kiczmer, P.; Skiba, H.; Krygier, M.; Chrabańska, M.; Piecuch, J.; Szrot, M.; Ochman, B.; et al. B7H3 Role in Reshaping Immunosuppressive Landscape in MSI and MSS Colorectal Cancer Tumours. Cancers 2023, 15, 3136. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Zhao, J.; Gu, M.; Giscombe, R.; Lefvert, A.K.; Wang, X. B7-H3 and B7-H4 Expression in Non-Small-Cell Lung Cancer. Lung Cancer 2006, 53, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, H.; Cheng, X.; Liu, K.; Cai, D.; Zhao, R. Potential Therapeutic Targets of B7 Family in Colorectal Cancer. Front. Immunol. 2020, 11, 681. Available online: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2020.00681/full (accessed on 24 July 2025). [CrossRef] [PubMed]
- Zhang, T.; Jiang, B.; Zou, S.-T.; Liu, F.; Hua, D. Overexpression of B7-H3 Augments Anti-Apoptosis of Colorectal Cancer Cells by Jak2-STAT3. World J. Gastroenterol. 2015, 21, 1804–1813. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, T.; Zou, S.; Jiang, B.; Hua, D. B7-H3 Promotes Cell Migration and Invasion through the Jak2/Stat3/MMP9 Signaling Pathway in Colorectal Cancer. Mol. Med. Rep. 2015, 12, 5455–5460. [Google Scholar] [CrossRef] [PubMed]
| Cancer | Influence of B7-H3 Upregulation on the Immune Landscape | References | |
|---|---|---|---|
| Increase in | Decrease in | ||
| Lung cancer | PD-L1+ macrophages (lung squamous cell carcinoma) | CD8+ T-cell activity (lung adenocarcinoma) | [48,49] |
| Breast cancer | - | T-cells/CD8+ cells | [50] |
| Cervical cancer | - | CD8+ T-cells | [51,52] |
| Ovarian cancer | M2 macrophages | CD8+ T-cells, NK cells | [22,25,53,54,55] |
| Prostate cancer | Tregs (B7-H3 mRNA) | Dendritic cells, CD4+ T-cells | [37,56] |
| Renal cancer | Tregs, TAMs; T-cell exhaustion score | - | [15,57] |
| Bladder cancer | TAMs | - | [58] |
| Melanoma | - | CD8+ T-cells, NK cells | [59] |
| Gliomas | T-cell activation | T-cell anti-tumor activity | [60] |
| Neuroblastoma | TAMs | NK cells | [45] |
| Medulloblastoma | - | Tγδ cells, TILs | [61] |
| Craniopharyngioma | TAMs | TILs | [62] |
| Drug | Immunotherapy Type | Trial | Type of Study | Tumor Types | Status |
|---|---|---|---|---|---|
| TH027 | CAR T cells | NCT06951425 | Early phase I clinical trial | Relapsed/Refractory Solid Tumors | Not yet recruiting |
| MT027 | CAR T cells | NCT06912152 | Phase I clinical trial | Advanced Peritoneal Malignancies and Abdominal Metastatic Solid Tumors | Recruiting |
| Super Hi-TCR-T cells | CAR T cells | NCT06902389 | Phase I/II clinical trial | Advanced Hepatocellular Carcinoma | Not yet recruiting |
| GD2/B7-H3 CAR-T therapy | CAR T cells | NCT06836505 | Phase I/II clinical trial | Relapsed/refractory Neuroblastoma and Desmoplastic Small Round Cell Tumors | Recruiting |
| Allogeneic B7-H3 CAR-γδT-Cell Therapy | CAR T cells | NCT06825455 | Early phase I clinical trial | Advanced Solid Tumors | Not yet recruiting |
| MT027 | CAR T cells | NCT06742593 | Phase I clinical trial | Brain, Meninges, and Spinal Cord Metastatic Solid Tumors | Not yet recruiting |
| MT027 | CAR T cells | NCT06737146 | Phase I clinical trial | Recurrent or Progressive High-grade Glioma | Not yet recruiting |
| MT027 | CAR T cells | NCT06726564 | Phase I clinical trial | Pleural Malignant Tumors | Recruiting |
| WL276 CAR T Cells | CAR T cells | NCT06691308 | Early phase I clinical trial | CD276 Positive Recurrent or Progressive Glioblastoma | Not yet recruiting |
| Autologous B7-H3 CAR T Cells | CAR T cells | NCT06646627 | Phase I clinical trial | Recurrent Platinum-resistant Ovarian Tumors | Recruiting |
| CMD03 | CAR T cells | NCT06612645 | Phase I clinical trial | Relapse or Refractory Solid Tumors | Recruiting |
| QH104 Cell Injection | CAR T cells | NCT06592092 | Not applicable clinical trial | Meningeal Metastases of B7-H3+ Solid Tumors | Recruiting |
| Autologous B7-H3 CAR T Cells | CAR T cells | NCT06500819 | Phase I clinical trial | Relapsed/Refractory Solid Tumors | Recruiting |
| Anti-B7-H3 CAR T-cell injection (TX103) | CAR T cells | NCT06482905 | Phase I clinical trial | Recurrent or Progressive Grade 4 Glioma | Recruiting |
| UTAA06 Injection | CAR T cells | NCT06372236 | Phase I clinical trial | Advanced Malignant Solid Tumors | Recruiting |
| iC9-CAR.B7-H3 T Cells | CAR T cells | NCT06347068 | Phase I clinical trial | Relapsed/Refractory Triple-Negative Breast Cancer | Recruiting |
| iC9-CAR.B7-H3 T Cells | CAR T cells | NCT06305299 | Phase I clinical trial | Ovarian Cancer | Recruiting |
| B7-H3 specific CAR T-cell with IL-7Ra signaling domain | CAR T cells | NCT06221553 | Phase I clinical trial | Diffuse Intrinsic Pontine Glioma | Recruiting |
| iC9-CAR.B7-H3 T-cell infusion | CAR T cells | NCT06158139 | Phase I clinical trial | Pancreas Cancer, Resistant Cancer | Recruiting |
| Loc3CAR: Locoregional Delivery of B7-H3-CAR T Cells | CAR T cells | NCT05835687 | Phase I clinical trial | Pediatric Patients with Primary CNS Tumors | Recruiting |
| SC-CAR4BRAIN | CAR T cells | NCT05768880 | Phase I clinical trial | Pediatric Diffuse Intrinsic Pontine Glioma, Diffuse Midline Glioma, Recurrent/Refractory Central Nervous System Tumors | Recruiting |
| Targeted IL-13 Rα2 UCAR T-cell injection, Targeted B7-H3 UCAR T-cell injection | CAR T cells | NCT05752877 | Not applicable clinical trial | Advanced Glioma | Recruiting |
| UTAA06 injection | CAR T cells | NCT05731219 | Phase I clinical trial | Relapsed/Refractory Acute Myeloid Leukemia | Recruiting |
| UTAA06 Injection | CAR T cells | NCT05722171 | Phase I clinical trial | Relapsed/Refractory Acute Myeloid Leukemia | Unknown status |
| TAA06 injection | CAR T cells | NCT05562024 | Phase I clinical trial | Relapsed/Refractory Neuroblastoma | Recruiting |
| KT095 CAR-T injection | CAR T cells | NCT05515185 | Early phase I clinical trial | Advanced Gastrointestinal Tumors, Advanced Solid Tumor | Unknown status |
| B7-H3-CAR T cells | CAR T cells | NCT05474378 | Phase I clinical trial | Recurrent Glioblastoma Multiforme (GBM), Brain and Nervous System | Recruiting |
| B7-H3-CAR T cells | CAR T cells | NCT05366179 | Phase I clinical trial | Refractory Glioblastoma | Recruiting |
| EGFR/B7-H3 CAR-T | CAR T cells | NCT05341492 | Early phase I clinical trial | EGFR/ B7-H3-positive Advanced Lung Cancer, EGFR/ B7-H3-Positive Advanced Triple-Negative Breast Cancer | Recruiting |
| fhB7-H3.CAR-Ts | CAR T cells | NCT05323201 | Phase I/II clinical trial | Advanced Liver Cancer | Recruiting |
| B7-H3-CAR T cells | CAR T cells | NCT05241392 | Phase I clinical trial | Recurrent Glioblastoma | Active, not recruiting |
| fhB7-H3.CAR-Ts | CAR T cells | NCT05211557 | Phase I/II clinical trial | Recurrent Malignant Ovarian Cancer | Recruiting |
| TAA06 injection | CAR T cells | NCT05190185 | Phase I clinical trial | Advanced Solid Tumors | Unknown status |
| CD276 CART -cells | CAR T cells | NCT05143151 | Phase I/II clinical trial | Advanced Pancreatic Carcinoma | Recruiting |
| Autologous B7-H3 CAR T Cells | CAR T cells | NCT04897321 | Phase I clinical trial | Pediatric Solid Tumors | Recruiting |
| TILs and CAR-TILs targeting B7-H3, HER2, Mesothelin, PSCA, MUC1, Lewis-Y, GPC3, AXL, EGFR, Claudin18.2/6, ROR1, GD1 | CAR T cells | NCT04842812 | Phase I clinical trial | Advanced Solid Tumors | Recruiting |
| TAA06 injection | CAR T cells | NCT04692948 | Not applicable clinical trial | Relapsed/Refractory Acute Myeloid Leukemia | Unknown status |
| Autologous B7-H3 CAR T Cells | CAR T cells | NCT04670068 | Phase I clinical trial | Recurrent Epithelial Ovarian Cancer | Active, not recruiting |
| CD19CAR-CD3Zeta-4-1BB-Expressing Autologous T-Lymphocyte Cells | CAR T cells | NCT04544592 | Phase I/II clinical trial | Elapsed and/or Refractory B-Cell Acute Lymphoblastic Leukemia (B-ALL), B-Cell Non-Hodgkin Lymphoma (B-NHL) | Recruiting |
| Second-generation 4-1BBζ B7-H3-EGFRt-DHFR | CAR T cells | NCT04483778 | Phase I clinical trial | Recurrent/Refractory Solid Tumors in Children and Young Adults | Active, not recruiting |
| 4SCAR-276 | CAR T cells | NCT04432649 | Phase I/II clinical trial | Solid Tumors | Unknown status |
| B7-H3-CAR T cells | CAR T cells | NCT04385173 | Phase I clinical trial | Recurrent/Refractory Glioblastoma | Recruiting |
| SCRI-CARB7-H3(s) | CAR T cells | NCT04185038 | Phase I clinical trial | Diffuse Intrinsic Pontine Glioma/Diffuse Midline Glioma, Recurrent/Refractory Pediatric Central Nervous System Tumors | Recruiting |
| B7-H3-CAR T cells | CAR T cells | NCT04077866 | Phase I/II clinical trial | Recurrent/Refractory Glioblastoma | Recruiting |
| CAR T-cells targeting GPC3, Mesothelin, Claudin18.2, GUCY2C, B7-H3, PSCA, PSMA, MUC1, TGFβ, HER2, Lewis-Y, AXL, or EGFR | CAR T cells | NCT03198052 | Phase I clinical trial | Advanced cancer that expresses GPC3/Mesothelin/Claudin18.2/GUCY2C/B7-H3/PSCA/PSMA/MUC1/TGFβ/HER2/Lewis-Y/AXL/EGFR protein | Recruiting |
| Antigen-specific IgT cells | CAR T cells | NCT03170141 | Phase I clinical trial | Glioblastoma Multiforme | Enrolling by invitation |
| Drug | Immunotherapy Type | Trial | Type of Study | Tumor Types | Status |
|---|---|---|---|---|---|
| MHB088C | B7-H3 ADC | NCT06951243 | Phase II clinical trial | Extrapulmonary Neuroendocrine Cancer | Not yet recruiting |
| HS-20093 | B7-H3 ADC | NCT06825624 | Phase I clinical trial | Advanced Metastatic Colorectal Cancer | Recruiting |
| HS-20093 | B7-H3 ADC | NCT06699576 | Phase Ib clinical trial | Bone and Soft Tissue Sarcoma | Not yet recruiting |
| YL201 | B7-H3 ADC | NCT06629597 | Phase III clinical trial | Nasopharyngeal Carcinoma | Recruiting |
| HS-20117 | B7-H3 ADC | NCT06621563 | Phase I clinical trial | Advanced Solid Tumors | Recruiting |
| YL201 | B7-H3 ADC | NCT06612151 | Phase III clinical trial | Relapsed Small Cell Lung Cancer | Recruiting |
| BGB-C354 | B7-H3 ADC | NCT06422520 | Phase I clinical trial | Advanced Solid Tumors | Recruiting |
| YL201 | B7-H3 ADC | NCT06394414 | Phase I clinical trial | Advanced Solid Tumors | Recruiting |
| I-DXd in Combination with Atezolizumab with or without Carboplatin | B7-H3 ADC | NCT06362252 | Phase I/II clinical trial | Extensive Stage-Small Cell Lung Cancer | Recruiting |
| MGC026 | B7-H3 ADC | NCT06242470 | Phase I clinical trial | Advanced Solid Tumors | Recruiting |
| YL201 | B7-H3 ADC | NCT06241846 | Phase II clinical trial | Metastatic Castration-resistant Prostate Cancer | Recruiting |
| Ifinatamab Deruxtecan (I-DXd) | B7-H3 ADC | NCT06203210 | Phase III clinical trial | Relapsed Small Cell Lung Cancer (SCLC) | Recruiting |
| HS-20093 | B7-H3 ADC | NCT06112704 | Phase II clinical trial | Advanced Esophageal Carcinoma, Advanced Solid Tumors | Recruiting |
| HS-20093 | B7-H3 ADC | NCT06052423 | Phase II clinical trial | Extensive Stage Small Cell Lung Cancer (ES-SCLC) | Not yet recruiting |
| IBB0979 | B7-H3-IL10 immunocytokine | NCT05991583 | Phase I/II clinical trial | Advanced Malignant Tumors | Recruiting |
| DB-1311/BNT324 | B7-H3 ADC | NCT05914116 | Phase I/IIa clinical trial | Advanced/Metastatic Solid Tumors | Recruiting |
| Vobramitamab duocarmazine (MGC018)/Lorigerlimab (MGD019) | B7-H3 ADC/Bispecific DART® Protein Binding PD-1 and CTLA-4 | NCT05293496 | Phase I/Ib clinical trial | Relapsed/Refractory, Unresectable, Locally Advanced/Metastatic Solid Tumors | Active, not recruiting |
| Ifinatamab Deruxtecan (I-DXd) | B7-H3 ADC | NCT05280470 | Phase II clinical trial | Extensive-stage Small Cell Lung Cancer (ES-SCLC) | Active, not recruiting |
| HS-20093 | B7-H3 ADC | NCT05276609 | Phase I clinical trial | Advanced Solid Tumors | Unknown status |
| BNT116 | B7-H3 ADC | NCT05142189 | Phase I clinical trial | Advanced Non-small Cell Lung Cancer | Recruiting |
| Ifinatamab deruxtecan (I-DXd) | B7-H3 ADC | NCT04145622 | Phase I/II clinical trial | Advanced Solid Malignant Tumors | Recruiting |
| Drug | Immunotherapy Type | Trial | Type of Study | Tumor Types | Status |
|---|---|---|---|---|---|
| 131 I-omburtamab | Radioimmunotherapy | NCT04022213 | Phase II clinical trial | Desmoplastic Small Round Cell Tumor | Active, not recruiting |
| 131 I-omburtamab | Radioimmunotherapy | NCT04743661 | Phase II clinical trial | Recurrent Medulloblastoma, Recurrent Ependymoma | Active, not recruiting |
| 131 I-omburtamab | Radioimmunotherapy | NCT05064306 | Expanded access | Central Nervous System/Leptomeningeal Neoplasms in Children and Young Adults | Available |
| Trial | Type of Study | Study Design | Enrollment | Drug | Immunotherapy Type | Tumor Types | Conclusion |
|---|---|---|---|---|---|---|---|
| NCT02628535 | Phase I clinical trial | Open-label, dose escalation, cohort expansion, efficacy follow-up study | 67 | MGD009 | B7-H3- CD3 dual-affinity re-targeting (DART) protein | Advanced Solid Tumors | Study terminated—Business decision (not for safety reasons) AE:NA |
| NCT02982941 | Phase I clinical trial | Open-label, dose escalation, cohort expansion, and efficacy follow-up study | 25 | Enoblituzumab (MGA271) | IgG1κ B7-H3 monoclonal antibody, anti-B7-H3 ADCC | Pediatric Solid Tumors | Study completed AE:NA |
| NCT03406949 | Phase I clinical trial | Open-label, dose escalation, and cohort expansion study | 25 | MGD009/MGA012 | B7-H3- CD3 dual-affinity re-targeting (DART) protein with anti-PD-1 Antibody | Advanced Solid Tumors | Study completed AE:NA |
| NCT02381314 | Phase I clinical trial | Open-label, dose escalation, and cohort expansion study | 24 | Enoblituzumab (MGA271)/ Ipilimumab | IgG1κ B7-H3 monoclonal antibody, Anti-B7-H3 ADCC/CTLA-4 monoclonal antibody | Advanced Solid Tumors | Study completed AE:NA |
| NCT03729596 | Phase I/II clinical trial | Open-label, dose-escalation study | 143 | Vobramitamab duocarmazine (MGC018) with or without (MGA012) | Anti- B7-H3 ADC alone and in combination with anti-PD-1 antibody | Advanced Solid Tumors | Study terminated—Business decision (not for safety AE: In ≥10% of patients, neutropenia asthenia, palmar–plantar erythrodysesthesia, headache. |
| NCT04634825 | Phase II clinical trial | Open-label study | 62 | Enoblituzumab (MGA271) plus Retifanlimab (MGA012)/ Tebotelimab (MGD013) | Anti-B7-H3 ADCC plus Anti-PD-1 antibody/PD-1 X LAG-3 bispecific DART molecule | Head and Neck Squamous Cell Carcinoma | Study terminated—Safety concerns. AE: Retifanlimab cohort, In ≥10% of patients with hypothyroidism, fatigue, infusion-related reaction, and increased lipase level. Tebotelimab cohort, asthenia, infusion related reaction, increased N-terminal prohormone brain natriuretic peptide level, and headache. |
| NCT04630769 | Phase I clinical trial | Open-label study | 3 | Intraperitoneal FATE FT516 and Interleukin-2 (IL-2) with Enoblituzumab (MGA271) | Allogeneic natural killer (NK) cells, expressing CD16 (FT516) and IL-2 with Fc-optimized monoclonal antibody targeting B7-H3 | Ovarian, Fallopian Tube, and Primary Peritoneal Cancer | Study completed. AE: nausea, headache, abdominal pain, anemia, fatigue, decreased lymphocyte count, decreased white blood cell count, anorexia, and hypoalbuminemia. |
| NCT02192567 | Phase I clinical trial | Open-label, sequential dose escalation and expansion study | 19 | DS-5573a | Anti- B7-H3 ADC | Advanced Solid Tumors | Study terminated —Business decision (not for safety reasons) AE:NA |
| NCT01391143 | Phase I clinical trial | Open-label, multi-dose, single-arm, multi-center, dose-escalation study | 179 | Enoblituzumab (MGA271) | IgG1κ B7-H3 monoclonal antibody, Anti-B7-H3 ADCC | Solid Tumors | Study completed. Disease stabilization and tumor shrinkage (ranging from 2% to 69%) across various tumor types. MGA271 demonstrated good tolerance, with no dose-limiting toxicity. AE: Grade 3/4 E was observed in 6% of patients. |
| NCT02982941 | Phase I clinical trial | Open-label, dose escalation and cohort expansion study | 25 | Enoblituzumab (MGA271) | IgG1κ B7-H3 monoclonal antibody, Anti-B7-H3 ADCC | NB, sarcomas | Study completed. Results: NA AE: NA |
| NCT02475213 | Phase I clinical trial | Open-label, dose escalation study | 145 | Enoblituzumab (MGA271) in combination with Pembrolizumab or MGA012 | IgG1κ B7-H3 monoclonal antibody, anti-PD-1 antibody | Melanoma, HNSCC, NSCLCC, Urothelial Carcinoma | Study completed. Metastatic NSCLC 36% ORR, recurrent/metastatic HNSCC 33% ORR AE: acceptable safety profile. |
| NCT01502917 | Phase I clinical trial | Open-label, dose escalation, single-group assignment study | 50 | Radioactive iodine-labeled monoclonal antibody—124I-Omburtamab | Radioimmunotherapy | Central Nervous System Tumors | Study completed. AE: hemiparesis, dysarthria, ataxia, dysphagia, muscular weakness. |
| NCT00089245 | Phase I clinical trial | Open-label, single-arm study | 177 | Iodine I 131 MOAB 8H9 | Radioimmunotherapy | Central Nervous System Tumors | Study terminated. Median PFS 7.5 years. AE: headache, vomiting, fever, and biochemical abnormalities. |
| NCT01099644 | Phase I clinical trial | Open-label, dose escalation, single-group assignment study | 54 | 131I-omburtamab (131 I-8H9) | Radioimmunotherapy | Solid Tumors Involving the Peritoneum | Study terminated. AE: NA |
| NCT03275402 | Phase II/III clinical trial | Open-label, multicenter, single-group assignment study | 52 | 131I-omburtamab (131 I-8H9) | Murine IgG1 monoclonal antibody radiolabeled with iodine-131, radioimmunotherapy | Neuroblastoma Central Nervous System/Leptomeningeal Metastases | Study terminated. 3-year OS 0.65 (0.49 to 0.78). AE: lymphopenia, intracranial hemorrhage, and decreased platelet count. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielcarska, S.; Kot, A.; Dawidowicz, M.; Kula, A.; Sobków, P.; Kłaczka, D.; Waniczek, D.; Świętochowska, E. B7-H3 in Cancer Immunotherapy—Prospects and Challenges: A Review of the Literature. Cells 2025, 14, 1209. https://doi.org/10.3390/cells14151209
Mielcarska S, Kot A, Dawidowicz M, Kula A, Sobków P, Kłaczka D, Waniczek D, Świętochowska E. B7-H3 in Cancer Immunotherapy—Prospects and Challenges: A Review of the Literature. Cells. 2025; 14(15):1209. https://doi.org/10.3390/cells14151209
Chicago/Turabian StyleMielcarska, Sylwia, Anna Kot, Miriam Dawidowicz, Agnieszka Kula, Piotr Sobków, Daria Kłaczka, Dariusz Waniczek, and Elżbieta Świętochowska. 2025. "B7-H3 in Cancer Immunotherapy—Prospects and Challenges: A Review of the Literature" Cells 14, no. 15: 1209. https://doi.org/10.3390/cells14151209
APA StyleMielcarska, S., Kot, A., Dawidowicz, M., Kula, A., Sobków, P., Kłaczka, D., Waniczek, D., & Świętochowska, E. (2025). B7-H3 in Cancer Immunotherapy—Prospects and Challenges: A Review of the Literature. Cells, 14(15), 1209. https://doi.org/10.3390/cells14151209





