Abstract
As the global population experiences a notable surge in aging demographics, the need to understand the intricate molecular pathways exacerbated by age-related stresses, including epigenetic dysregulation, becomes a priority. Epigenetic mechanisms play a critical role in driving age-related diseases through altered gene expression, genomic instability, and irregular chromatin remodeling. In this review, we focus on histones, a central component of the epigenome, and consolidate the key findings of histone loss and genome-wide redistribution as fundamental processes contributing to aging and senescence. The review provides insights into novel histone expression profiles, nucleosome occupancy, disruptions in higher-order chromatin architecture, and the emergence of noncanonical histone variants in the aging cellular landscape. Furthermore, we explore the current state of our understanding of the molecular mechanisms of histone deficiency in aging cells. Specific emphasis is placed on highlighting histone degradation pathways in the cell and studies that have explored potential strategies to mitigate histone loss or restore histone levels in aging cells. Finally, in addressing future perspectives, the insights gained from this review hold profound implications for advancing strategies that actively intervene in modulating histone expression profiles in the context of cellular aging and identifying potential therapeutic targets for alleviating a multitude of age-related diseases.
Keywords:
histones; nucleosome occupancy; aging; senescence; epigenetics; histone degradation; histone variant 1. Introduction
With demographic shifts toward aging populations, the incidence of age-related diseases such as cancer, diabetes, cardiovascular disorders, osteoarthritis, and macular degeneration is on the rise in many countries [1,2,3]. There is a massive unmet need to significantly improve our fundamental scientific understanding of aging biology in order to develop new therapeutic approaches to age-related diseases. Achieving this goal hinges on decoding the intricate molecular mechanisms of aging and identifying new experimental models to mitigate or reverse age-associated phenotypes.
Aging is a complex and multifactorial process characterized by a combination of aging hallmarks that contribute to declines at the molecular, cellular, and systemic levels in an organism. The hallmarks of aging, as outlined by López-Otín et al. [4], are categorized into three groups: primary hallmarks, antagonistic hallmarks, and integrative hallmarks. The primary hallmarks of aging that are deleterious to the cell include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, and disabled macroautophagy [5,6,7,8,9,10,11]. The antagonistic hallmarks represent a more intricate response of the cell to aging and include deregulated nutrient sensing, cellular senescence, and mitochondrial dysfunction. The cellular nutrient sensing pathways that adapt to the nutrition availability change with age. Deregulated nutrient sensing is closely associated with mitochondrial dysfunction, peroxisomal dysfunction, and the deregulation of protein synthesis and glucose, nucleotide, and lipid metabolisms [12,13]. Mitochondrial dysfunction can also induce oxidative stress, which can initially benefit the cell by eliciting a protective gene response but may prove detrimental in the long run [14,15]. Collaborating closely with mitochondria, peroxisomes play a crucial role in preserving the oxidative balance within the cell [16]. While the peroxisomes typically generate elevated levels of reactive oxygen species and reactive nitrogen species, they also are armed with a battery of antioxidant enzymes and nonenzymatic free radical scavengers [17]. In addition to regulating ROS metabolism, peroxisomes perform crucial functions in lipid metabolism. However, a growing body of evidence indicates a decline in peroxisomal function with age, linking oxidative stress and disrupted lipid metabolism resulting from dysregulated peroxisomal function to various age-related diseases [17,18]. The accumulation of cholesterol is associated with peroxisomal dysfunction, and cholesterol oxide derivatives, known as oxysterols, are known to induce significant dysfunction in organelles, especially mitochondria. Oxysterols play pivotal roles in the disruption of redox homeostasis, inflammatory status, lipid metabolism, and ultimately, cell death induction during aging [19,20]. While specific phytosterols, also known as “plant cholesterol”, have been identified for their health benefits, recent research indicates that imbalances in these diet-derived phytosterols have substantial implications in neurodegeneration and cognitive decline [21]. Thus, antagonistic hallmarks seem to be beneficial to the cell when meticulously regulated but tend to become deleterious only at high levels. Integrative hallmarks arise when damage from primary and antagonistic aging accumulates, leading to stem cell exhaustion, altered intercellular communication, chronic inflammation, and dysbiosis [22,23,24]. Although these hallmarks appear to be independent entities, they are integrative, and a complex interplay of different regulatory pathways is inherent in driving the complex biological process of aging.
Earlier studies proposed that the accumulated burden of somatic postnatal mutations is the primary cause of aging [25,26,27]. As various theories on aging, particularly those centered on genetic changes within cells, evolved, a growing body of evidence also underscored the importance of epigenetic factors in the aging process [8,28,29]. While an organism’s genome maintains a relatively steady state throughout its lifetime, the epigenome undergoes extensive reprogramming. Thus, there is a growing interest in research challenging the prevailing idea that genetic aberrations primarily drive the aging process [30]. There are significant data to support the notion that normal cellular aging may occur despite fewer mutations, and conversely, cells with a higher mutation rate do not necessarily display premature aging [31,32,33]. The mounting evidence from yeast to humans indicates that the breakdown of epigenetic information, rather than genetic mutations, plays a pivotal role in aging [30,34,35,36]. The dysregulation of the cellular epigenome during aging and senescence is a complex phenomenon that manifests through various elements, including global histone levels, histone positioning on the DNA sequence, post-translational modifications (PTMs) of histones, histone variants, DNA methylation, and noncoding RNAs.
Eukaryotes have evolved a complex genome architecture composed of DNA and histones that synergistically regulate cell function and differentiation. The fundamental architecture of the chromatin, known as “beads-on-a-string”, refers to the nucleosome core particle. This nucleosome core comprises 147 base pairs of genomic DNA wrapped around a core histone octamer made of H2A, H2B, H3, and H4 [37]. The linker histone H1 stabilizes the chromatin by binding to specific sites on the DNA, anchoring the nucleosomes and facilitating the formation of higher-order chromatin structures. This compaction of chromatin around the core and linker histones is a key mechanism in eukaryotes to package and organize their genome efficiently. The higher-order chromatin structure can be classified as euchromatin and heterochromatin, depending on the level of compaction. Euchromatin corresponds to the less condensed regions of chromatin that allow for easy access to regulatory molecules such as transcription factors. On the other hand, heterochromatin represents transcriptionally inactive and highly condensed chromatin sites. The organization of these two distinct chromatin regions is closely associated with the histone content and organization of the nucleosome landscape. Nucleosomes are highly dynamic, both in their composition and positioning on the genome [38]. The genome is constantly reorganized through nucleosome eviction and histone sliding processes that are mediated by factors such as chromatin remodeling enzymes, histone chaperones, and polymerases [38,39,40]. Furthermore, nucleosomes in transcriptionally active or repressed sites carry multiple PTMs on histone proteins, which ultimately define the epigenomic state of the cell [41,42]. Among the vast repertoire of histone PTMs, acetylation, methylation, phosphorylation, and ubiquitination are the main modifications regulating the chromatin structure and gene transcription [41,43,44]. The accumulating evidence has shown that age-related dysregulation of the epigenome involves the breakdown of the nucleosome landscape, leading to changes in the organization of nuclear components, activation of previously silenced genes, and aberrant gene expression patterns [5,8].
Here, we will review recent findings that demonstrate the importance of histone expression profiles that occur in aging and examine how histone loss and nucleosome repositioning impact the chromatin architecture and numerous other critical homeostatic cellular processes. While some outstanding reviews in the last decade have shed light on histone modifications and their roles in aging [45,46,47,48], our emphasis is on exploring the breakdown of epigenetic information, specifically, histone loss, altered nucleosome occupancy, and the increased expression of noncanonical histone variants within the context of aging and senescence. Alterations in the abundance and composition of histones, along with histone PTMs, have been associated with changes in chromatin organization and gene expression patterns during aging. Histones, as key players in epigenetic regulation, have been implicated in the pathogenesis of various age-related diseases, including cancer and neurodegenerative disorders [49]. Evidence of aberrant histone modifications and changes in the chromatin structure is frequently observed in disease models characterized by premature aging, such as Hutchinson–Gilford progeria syndrome and Werner syndrome, emphasizing the relevance of histones in the context of age-related pathologies [50,51]. Gaining a deeper understanding of the intricate interplay between histone depletion and cellular aging is crucial for deciphering the underlying mechanisms that govern the fundamental aging-associated pathways and identifying potential therapeutic targets for various age-related pathologies.
2. Age-Related Histone Loss and Altered Nucleosome Occupancy in Non-Mammalian Models
For over 30 years, scientists have been studying global histone loss and its implications in cellular viability and aging in various yeast models. Early studies by Grunstein et al. provided compelling evidence that the depletion of histones H4 and H2B in yeast led to transcriptional defects, the disruption of the chromatin structure, and cell cycle arrest [52,53]. Subsequent investigations showed that the loss of histones H3 or H4 in yeast altered the transcriptional landscape with the derepression of many previously silenced genes [54,55]. Perhaps the most radical observation made by Hu et al. revealed an ~50% global depletion of histones and nucleosome repositioning in budding yeast during replicative aging. Histone removal from nucleosomes significantly enhanced DNA accessibility to transcriptional machinery, precipitating a sharp derepression of gene expression. Consequently, the yeast cells showed an overall upregulation in gene transcription and an increased genomic instability [56]. Similar to yeast studies, an age-dependent loss of histone H3 was reported in Caenorhabditis elegans when worm lysates were examined from young and old adults [57]. Thus, the mounting evidence from studies using yeast strains and other nonmammalian models via conditional histone knockouts or replicative aging has significantly enhanced our scientific understanding of the biologic effects of histone loss, altered nucleosome occupancy, differential gene expression, and cellular senescence (Table 1).

Table 1.
Histone loss and changes in nucleosome occupancy in yeast aging.
3. Histone Loss in Mammalian Models of Replicative and Chronological Aging
Mammalian cells progressively succumb to the adverse effects of aging assessed through two primary modalities: replicative life span and chronological life span [62,63]. Comprehensive investigations into these aging processes in yeast have demonstrated the emergence of aging hallmarks similar to those found in mammals [62,63,64]. Hence, evidence of the age-related effects on histone expression profiling of yeast cells captured the attention of researchers, prompting further investigations into histone levels in mammalian cells. An early study of histone depletion in human cells using pulse-chase labeling revealed a gradual decrease in H1 linker histones in senescent human diploid fibroblast cultures. This reduction was linked to a decline in the biosynthesis of H1 histone with progressive in vitro aging [65]. Houde and colleagues [66] provide a novel perspective on H1 expression changes in cultured human fibroblasts that exhibit a progressive cell cycle elongation. Their findings revealed that cells in early (28–35 mean population doubling; MPD) and late (65–70 MPD) passages, maintained in a confluent state at 0 and 6 weeks, exhibited a similar shift in the gene expression of H1 variants. Notably, this involved a reduction in H1B and a concurrent increase in H10 and H1A. These data suggest that changes in the expression levels of histone H1 variants occurred as a function of time of density-dependent growth rather than replicative age. Building on Mitsui et al.’s [65] discovery, subsequent studies focused on in vitro and in vivo aging in human skin fibroblasts and discovered a reduction in the nucleosome occupancy and an altered chromatin structure with aging [67,68]. While histone expression profiles in aging were being investigated in yeast, it took considerable time before Karsleder and colleagues delved into the correlation between histone levels and mammalian aging [69]. Their study using human diploid fibroblast (HDF) cell lines IMR90 and WI38 as models for replicative senescence revealed a significant decrease in histones H3 and H4 (43% and 47%, respectively) of late passage cells. The dysregulation of histone expression was primarily attributed to stress signals associated with telomere shortening. The telomeric region, affected by age-associated cellular damage, led to a DNA damage response and progressive genome-wide epigenetic changes with successive cell cycles that ultimately impacted histone biosynthesis. Chronic replicative stress only affected the H3 and H4 histones in HDFs, while the H1, H2A, and H2B histones remained unchanged [69]. Although the concept of histone loss in aging was initially suggested in actively replicating cells, Liu et al. studied the histone patterns of quiescent muscle stem cells (QSC) in young (2 months) and aged (24 months) mice and found a comparable decrease in the histone levels of H1, H2B, H3, and H4 [36]. This finding was quite significant in that histone loss also occurred during chronological aging in a quiescent cell type with minimal turnover.
While some aging models demonstrate a selective loss of canonical histones, recent research in mammals has revealed a global reduction in histone levels associated with aging. In activated naive CD4+ T cells from both young (20–35 years) and old (65–85 years) adults, a transcriptome analysis via RNA-seq showed that aged T cells tended to have globally reduced expressions of core histone genes compared to young cells, and this decrease was dependent on the cell cycle state of the activated, aged T cells. The global downregulation of histones, confirmed at both the mRNA and protein levels, was observed to delay the S-phase progression in aged T cells, thereby triggering a replication-stress response [70]. Similarly, a global loss of histones was observed in aged retinal pigment epithelium (RPE) cells, where all five canonical histones were depleted. To provide a comprehensive perspective, Dubey et al. assessed the histone expression in mouse RPE cells undergoing chronological aging and primary human RPE cell lines that reached senescence through replicative aging. In the RPE cells obtained from young (2–3 months) and aged (20–24 months) mice, a substantial reduction was observed in all core and linker histones. Furthermore, a replicative aging model using primary human RPE cells revealed a gradual decrease in the histone levels as the cells aged [71]. Together, these studies demonstrate that both actively proliferating and postmitotic cells in a quiescent state develop histone loss with aging. Also, a decreased histone expression in aged cells may be global or specific depending on the cell type and experimental model.
Much like aging, diverse senescence pathways can contribute to epigenetic dysregulation in cells [72]. Cellular senescence is an innate stress response, wherein cells enter a stable and irreversible state of cell cycle arrest even under optimal growth conditions. Senescent cells exhibit distinct changes in their morphology, chromatin structure, gene expression, and the emergence of a senescence-associated secretory phenotype (SASP), a chronic inflammatory state [73,74]. These alterations collectively cause the cell to exit the cell cycle permanently. Senescence is induced by multiple mechanisms including replicative senescence, senescence due to DNA damage, stress-induced senescence, and programmed developmental senescence [73,75]. In human RPE cells that attained replicative exhaustion and entered the senescent state, there was a substantial decrease in all canonical histones [71]. Furthermore, histone loss was observed in two different senescent models utilizing human umbilical vein endothelial cells (HUVECs) [76]. In the first model, senescence was induced through natural passaging until the cells reached a point of replicative exhaustion, while the second model involved prolonged exposure to TNF-alpha over 26 days. In both instances, senescent cells displayed a reduction in many histone isoforms along with the downregulation of genes responsible for regulating the cell cycle and DNA repair mechanisms. Regardless of the upstream stimuli prompting senescence, the process typically converges in downstream effects, with histone depletion emerging as one of the significant consequences. These findings underscore the importance of histone depletion as a critical factor driving the process of cellular senescence [76].
While the acquisition of senescence has been viewed as an alternative pathway to prevent cancer, the prolonged accumulation of senescent cells can paradoxically promote cancer development [77,78]. The emergence of SASP factors during senescence can impact the surrounding cells via alterations of the cellular microenvironments, establishing chronic inflammation and fostering conditions conducive to cancer [79]. Interestingly, histone depletion associated with aging and senescence has been inversely linked to malignancy, suggesting that histone loss can facilitate cell proliferation arrest, ultimately contributing to tumor suppression [80]. Unlike histone loss, histone mutations, known as oncohistones, are implicated in promoting cancers [81]. However, given that many age-dependent changes in the cellular epigenome resemble those observed in cancer, the epigenetic reprogramming occurring during aging may predispose individuals to cancer development [82]. Therefore, gaining a deeper understanding of age-related epigenomic changes holds the potential to elucidate the underlying causes of cancer.
4. Alterations in Nucleosome Landscape in Aging
The age-related loss of histone expression appears to be a widespread phenomenon observed in organisms ranging from yeast to mammals. However, there is limited research on how the distribution of nucleosomes across the genome changes as organisms age (Table 2). Celona et al. studied the impacts of histone loss and alterations in the nucleosome occupancy in yeast and mammalian cells. Their proposed model suggested that the loss of nucleosomes predominantly occurs in specific localized regions [60]. Mammalian cells with ablated high mobility group box 1 (HMGB1), a DNA-binding protein, showed a significant decrease in histones and nucleosomes. Their findings demonstrated that the shRNA- and siRNA-mediated ablation of HMGB1 in HeLa cells, or its yeast equivalent (Nhp6a/b), did not compromise the cell viability despite the histone loss. However, the HMGB1-deficient cells displayed a greater sensitivity to DNA damage, underwent a global increase in transcription, and showed specific alterations in the transcriptional profile of certain genes. This study proposed a chromatin model in which the loss of nucleosomes within the cells is not widespread, but rather localized, particularly in regions that inherently exhibit a lower tendency to form nucleosomes [60]. This model aligns with the genomic DNA code regulating nucleosome positioning, as postulated by Kaplan et al., and emphasizes the role of DNA sequence information in governing the arrangement of nucleosomes [83]. Thus, chromatin regions characterized by an innately lower tendency for histone deposition are more histone deficient. It is important to note that this study primarily aimed to unravel the fundamental genome organization under conditions of limited numbers of nucleosomes without delving into the aging aspect.
Similar to the findings of Celona and colleagues, a subsequent study demonstrated that rather than a global redistribution of nucleosomes, aging causes nucleosome loss at specific sites [84]. Bochkis et al. used high-throughput methods such as RNA-seq, MNase-seq, and ChIP-seq to map nucleosome changes in young (3 months) and aged (21 months) mouse livers. They discovered age-related differences in the nucleosome distribution, which in turn influenced the accessibility of chromatin to transcriptional factors. A reduction in the nucleosome density correlated with the elevated transcription of key genes involved in lipid metabolic pathways in aged livers, potentially leading to metabolic dysfunction and hepatic steatosis. Furthermore, there were specific sites with an increased nucleosome occupancy with age, including the serum response factor (SRF) gene that impacted its target genes involved in liver proliferation, lipid metabolism, and histone expression. The interrogation of histone isoforms revealed an irregular expression pattern displaying both upregulation and downregulation within aged hepatic tissue [84]. Although this study focused on the decline in SRF activity in the aging liver due to altered nucleosome occupancy, it is important to note that other mechanisms have been reported for repressed SRF activity including nuclear exclusion, protein kinase C-δ induced phosphorylation, and the inactivation of SRF in senescent cells [85,86]. In line with Bochkis et al.’s research, Chen and colleagues investigated H3 nucleosomal profiles in chronologically aged mouse tissues [87]. A comprehensive H3 nucleosomal map was generated using ChIP-Seq data from four tissues (heart, liver, cerebellum, and olfactory bulb) and one primary cell type (primary neural stem cells obtained from the subventricular zone) in mice from different age groups (3, 12, and 29 months). While they did not find significant changes in the global H3 levels, localized alterations in the H3 occupancy were observed in all the four tissue types and cultured neural stem cells during aging. These changes can manifest as an increased or decreased occupancy in specific regions with subsequent chromatin remodeling in aging tissues. Notably, the distal regions located 5–500 kb away from the annotated transcriptional start sites exhibited a significant remodeling of H3 occupancy, both upstream and downstream. The sites proximal to genes, particularly within intronic regions, consistently demonstrated robust nucleosome enrichment. Within aging chromatin, the distinct changes observed in distally located nucleosomes suggest a differential occupancy of nucleosomes in regulatory elements, particularly the forkhead transcription factors, which are critical regulators of DNA remodeling. Additionally, the repositioning of nucleosomes triggered transcriptional changes in inflammatory transcription factors such as STAT6 and IRF8. Together, these studies suggest that compared to the observations in actively replicating yeast and mammalian cells, histone depletion in chronologically aging tissues is relatively less and limited to specific genomic sites [87].

Table 2.
Histone depletion and nucleosome redistribution in human and other mammalian models.
Table 2.
Histone depletion and nucleosome redistribution in human and other mammalian models.
| Part B | Histone Alteration | Organism or Cell Line | Reason for Histone Alterations | Cellular Alterations | Reference |
|---|---|---|---|---|---|
| 1 | Histone H1 and histone H4 | Human fetal lung fibroblast (TIG-1) | Replicative senescence | Age-related increase in nuclear proteins; decrease in histone H1 biosynthesis in senescent fibroblasts | Mitsui et al., 1980 [65] |
| 2 | Reduction in nucleosome occupancy | Human fibroblast | In vitro and in vivo aging of human diploid fibroblasts | Changes in chromatin structure | Ishimi et al., 1987 [67] |
| 3 | Reduction in nucleosome occupancy | Human donor fibroblast | In vitro and in vivo aging of human diploid fibroblasts | Changes in chromatin structure and cytoskeletal elements | Macieira-Coelho, 1991 [68] |
| 4 | Loss of histone H1 | Human fibroblasts WI-38, MRC-5, IMR-90, and BJ | Cellular senescence induced by retroviral expression of oncogenic Ras (oncogene-induced senescence) | Exhibit unique chromatin condensation, termed senescence-associated heterochromatic foci (SAHF); loss of linker histone H1 accompanied by increased chromatin-bound high mobility group A2 (HMGA2) competitor protein | Funayama, 2006 [88] |
| 5 | Histone H3 and H4 | Human diploid fibroblast IMR90 and WI38, fibroblast from young (9 y.o.) and 92 y.o. individual | Replicative aging in cell culture models and normal aging in primary cells | Downregulation of histone regulatory factors and histone chaperones (SLBP, Asf1a, Asf1b, CAF1-p150, and CAF1-p60); several altered histone modifications; accumulation of DNA damage and DNA damage response; telomere dysfunction | O’Sullivan et al., 2010 [69] |
| 6 | H1, H2A, H2B, H3, H4, and the variant histone H2AX | HeLa Hmgb1−/− | HMGB1 knockdown HeLa cells were generated using shRNA/siRNA | Increased global transcription and altered transcriptome profile in cells undergoing histone depletion | Celona et al., 2011 [60] |
| 7 | Altered histone expression and changes in nucleosome occupancy | Liver tissue from young (3 months) and old (21 months) C57BL6 mouse | Age-dependent changes | Age-related activation of lipogenesis and inflammatory genes; nucleosome occupancy changes with age selectively repress or derepress genes involved in lipid metabolism; histone isoforms are differentially regulated | Bochkis et al., 2014 [84] |
| 8 | HIST1H3D, HIST1H3E, and HIST4H4 | CD8+ T cells from healthy young (22–40 yr) and old (65+ yr) individuals | Closed chromatin associated with aging | Age-associated changes to chromatin accessibility | Ucar et al., 2017 [89] |
| 9 | Altered histone H3 expression and changes in H3 nucleosome occupancy | Tissues (i.e., heart, liver, cerebellum, and olfactory bulb) and one primary cell type (i.e., primary neural stem cell cultures from the subventricular zone) from 3-, 12-, and 29-month-old mice | Chronological aging | Age-related alterations in nucleosome positioning exhibit localized changes in genomic loci linked to DNA remodeling factors; these changes also extend their influence to the regulation of inflammatory genes | Chen et al., 2020 [87] |
| 10 | H4 depletion and degradation of nucleosomes | Human fibroblasts IMR90, CRL-1474, and HEK-293T | Senescence-mediated H4 loss; proteasome-mediated H4 degradation | H4 and H3 concentrations are reduced at the promoter regions of cell cycle inhibitor genes, SASP-related genes, and anti-apoptotic genes | Lin et al., 2020 [90] |
| 11 | Global loss of core histones | Human naive CD4+ T cell from young (20–35 years) and old (65–85 years) adults | Age-related reduction of miR-181ab1 and consequent increase of its target histone deacetylase, SIRT1, leads to deacetylation of histone gene promoters and downregulation of histone genes | Replication stress, delayed S-phase progression, and activation of proinflammatory pathways | Kim et al., 2021 [70] |
| 12 | Histone reduction | Human fetal lung IMR90 diploid fibroblasts, neonatal human epidermal melanocytes | Senescent cells form cytoplasmic chromatin fragments (CCFs), and proteolysis of CCFs depletes total histones in a lysosome-dependent manner | Genomic DNA damage; deterioration of nuclear integrity; CCFs transition to the cytoplasm and subsequent histone loss from CCFs | Ivanov et al., 2013 [80] |
| 13 | H1, H2A, H2B, H3, and H4 | Primary human umbilical vein endothelial cells (HUVECs) | Replicative senescence and inflammatory cytokine TNF-α-mediated senescence | Cell cycle arrest due to decline in cell cycle and mitosis regulatory factors; deterioration of DNA repair mechanisms; loss of chromatin architecture | Kandhaya-Pillai et al., 2023 [76] |
5. Multiple Mechanisms of Histone Loss during Aging
Although aging is associated with histone loss and the reorganization of nucleosomes, the effects of histone depletion, nucleosome repositioning, and the factors driving histone reduction are still largely uncharted territories [4]. Studies in yeast offered critical insights into the genetic and epigenetic mechanisms behind the age-related dysregulation of histone expression. These mechanisms included telomere loss, DNA damage, changes in histone chaperones, reduced histone production, and alterations in histone marks, all of which affected the chromatin structure [91]. Despite limited research in mammals, similar causal links between decreasing histone levels and various genetic and epigenetic factors have been established in aging cells and tissues (Figure 1). For instance, the experiments on lung fibroblast cell lines undergoing replicative aging revealed that chronic exposure to DNA damage signals, such as those arising from telomere shortening, impacted histone biosynthesis. [69]. The presence of short telomeres and the consequent DNA damage signaling resulted in the depletion of the stem–loop binding protein (SLBP), a stabilizer of histone mRNA. This, in turn, reduced the biosynthesis of H3 and H4 and destabilized Asf1, a histone chaperone, leading to genome-wide epigenetic changes that amplified the damage signal during repeated cell cycles. This self-enforced regulatory loop constantly changed the chromatin environment at the telomeres, facilitating the incursion of damage markers beyond the threshold of cell tolerance, ultimately leading to irreversible cell cycle exit and senescence. The ectopic expression of telomerase enzymes in aging cells to increase the telomere length normalized the histone expression levels similar to those of young cells. Furthermore, this process partially restored SLBP and ASF1 expression, likely by alleviating the DNA damage signaling associated with short telomeres [69].
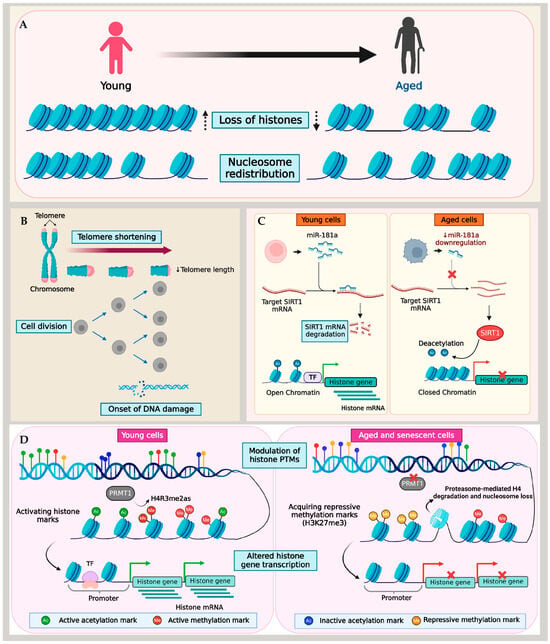
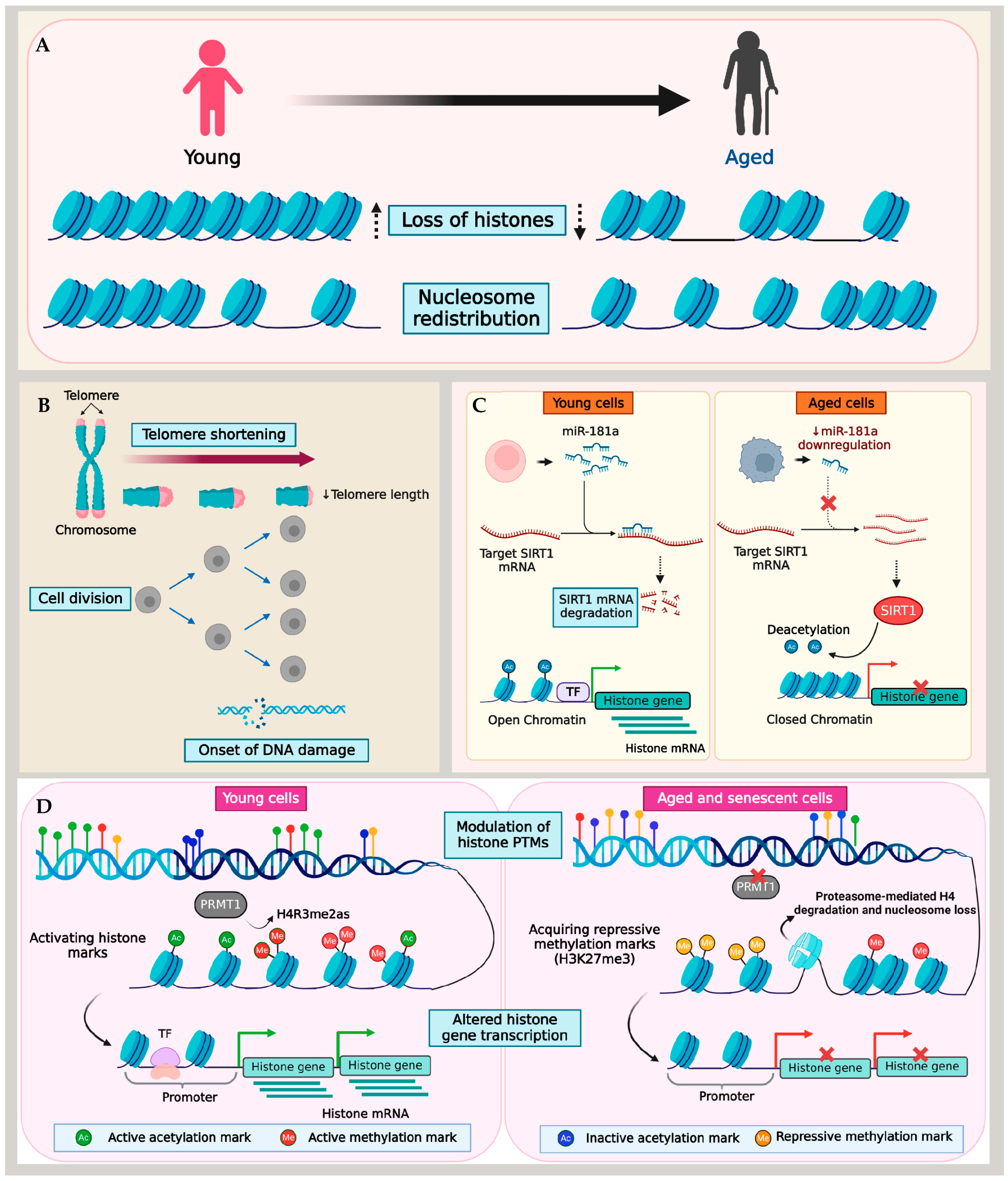
Figure 1.
Mechanism of histone loss in aging and senescence. (A) Aging and senescence are characterized by the loss of histones and nucleosome redistribution. Different models show histone loss due to (B) telomere shortening and DNA damage, (C) miRNA-mediated changes to histone transcription, and (D) the imbalance of activating and repressive histone PTMs.
Telomere shortening and dysfunction are hallmarks of cellular aging and senescence. Despite their crucial role in maintaining chromosomal stability, the regulatory mechanisms governing telomeres during cellular aging remain poorly understood. Situated at the termini of linear chromosomes, telomeres form complexes of TTAGGG nucleotide repeats and proteins that regulate their functions, shielding them from recognition by the cell’s repair mechanism as double-stranded DNA breaks (DSBs). These proteins, including the telomerase (TERT) enzyme, histones, and the Shelterin complex, are critical for regulating the telomere length and preventing chromosomal end fusion [92,93].
Telomeres typically exhibit a heterochromatin structure, and a widespread phenomenon known as the telomere position effect results in low expression levels or the transcriptional silencing of genes within or near telomeres [94]. Additionally, telomeres tend to spatially organize at the nuclear periphery, a zone of transcriptional repression, in a cell cycle-dependent manner [95,96,97], and therefore, experience the transcriptional repression of genes. However, as cells approached senescence, a spatial overlap of lamina intranuclear structures with telomeres was observed [98]. During senescence, as the nuclear lamina’s organization gets disrupted, telomeres tend to form large aggregates lacking TERT. These telomere aggregates accumulated histone γ-H2AX, a classical marker of DSBs and telomere shortening, in senescent cells [98].
Furthermore, the formation of senescence-associated heterochromatin foci (SAHF), representing facultative heterochromatin domains, correlates with telomere shortening in cells entering senescence [99,100]. The SAHF contain domains with di- or tri-methylated lysine 9 of histone H3 (H3K9me2/3), a histone H2A variant (macroH2A), and heterochromatin protein 1 (HP1) proteins [100,101,102]. Additionally, epigenetic modifications such as histone methylation in the telomere region and TERT demethylation in humans play significant roles in maintaining the heterochromatin, transcriptional silencing at telomeres, and telomerase inactivation. Preserving the telomere structure and ensuring transcriptional silencing are critical to preventing premature aging [103,104].
Telomere shortening assumes that each successive cell division acts as a mitotic counting mechanism, eventually leading cells to attain replicative senescence [4,105]. In contrast, cells in a quiescent state transition into senescence despite negligible telomere shortening [106,107]. Therefore, the primary causes of age-associated histone loss in quiescent cells are likely driven by mechanisms beyond telomere dysfunction. Muscle stem cells provide a well-studied model for cellular quiescence and aging [108,109]. Liu and colleagues reported an epigenetic mechanism for histone loss in aging quiescent muscle stem cells (QSCs). Using the ChIP-Seq approach, the QSCs derived from young and aged mice were profiled for histone methylation marks, including H3K4me3, H3K27me3, and H3K36me3. As the QSCs underwent aging, there was an overall reduction in the expression of histone genes that acquired H3K27me3, a transcription repressive mark, at their transcription start sites. This finding underscores the critical role played by the repressive methylation mark in the regulation of histones within QSCs, simultaneously emphasizing the involvement of histone PTMs in histone regulation.
A similar methylation regulatory mechanism of histones was reported in fibroblast cell lines [90]. In this study, histone expression was examined in IMR90 fibroblast cells after inducing premature senescence via protein arginine methyltransferase 1 (PRMT1) knockdown. PRMT1 is the predominant arginine methyltransferase in humans, which mediates the asymmetric dimethylation of histone H4 at arginine 3 (H4R3me2as), a critical modification essential for histone H4 stability. Studies over the past decades have demonstrated the reduced expression of PRMT1 in replicative aging and senescent cells [110,111]. Lin et al. demonstrated that premature senescence in fibroblasts induced by PRMT1 knockdown caused a reduction in PRMT1-mediated H4 dimethylation, leading to the destabilization of H4. Consequently, H4 showed increased binding to PA200, causing the ubiquitin-independent degradation of H4 by PA200-capped proteasomes [90,112] (described further in Section 7). A similar H4 degradation was also observed in fibroblasts exposed to different senescence-associated signals such as oxidative stress, DNA damage, or oncogenic signaling. Importantly, H4 degradation preceded the depletion of other histones, resulting in a reduced nucleosomal occupancy and impacted cell proliferation by enhancing the transcription of genes responsible for cell cycle inhibition, senescence-related genes, and apoptosis regulators [90]. It is critical to note that different histone marks have contrasting roles in regulating genes in the context of aging and lifespan across species and even within different tissues of an organism [47,113]. Therefore, recognizing the precise functions of various histone PTMs in aging tissues is critical for unraveling the complexity of epigenetic processes in aging.
Though a large part of the age-associated histone depletion in cells appears to stem from altered histone PTM marks, telomere dysfunction, and DNA damage, a recent study reported the miRNA-mediated downregulation of histones in aged T cells. Initially, Ucar et al. detected a significant compaction of chromatin in several histone genes (HIST1H3D, HIST1H3E, and HIST4H4) and histone modifiers in T cells with age [89]. By combining ATAC-seq and RNA-seq data, their study showed that immune cells undergo alterations in their epigenome as individuals age. This involves the closing of the chromatin structure at the promoter and enhancer regions of genes that are actively expressed, including histones. This finding was consistent with the reduced expression of core histones demonstrated in a subsequent study by Kim et al. using T cells from young (20–35 years) and old (65–85 years) adults [70]. Naive T cells from aged individuals with a reduced miR-181a level as well as miR-181ab1-deficient murine T cells in an actively dividing state showed the downregulation of histone expression, which induced replication stress and a consequential slow-down of the cell cycle. In the absence of miR-181a, its target molecule SIRT1—a histone deacetylase enzyme—binds directly to the histone promoter region. This interaction led to a reduction in histone acetylation, thereby inducing the repression of histone genes [70].
Although different studies provide insight into the ramifications of age-related genetic and epigenetic alterations of the nucleosome landscape, a key factor is that this mechanism is cell-type dependent. Interestingly, Ivanov and colleagues reported that histone loss in senescent cells occurred in both fibroblasts and epidermal melanocytes [80]. Cells approaching senescence and those already in a senescent state undergo significant stress, marked by changes in both the morphology and physiology. Genomic DNA damage, particularly DSBs, along with the deterioration of nuclear integrity in senescent cells, leads to damaged DNA fragments leaving the nucleus. Ivanov et al. identified these fragments in the cytoplasm as cytoplasmic chromatin fragments (CCFs), which are strongly positive for γ-H2AX and H3K27me3. In the cytoplasm, an autophagic proteolytic activity targets these CCFs, progressively depleting the total histones in a process dependent on the lysosomal activity [80]. This process of lysosomal ubiquitin-mediated histone proteolysis, elucidated by Ivanov and colleagues in senescent cells, represents an important mechanism of post-translational histone degradation. Together, these studies have enhanced our understanding of the intricate genetic and epigenetic pathways that control histone downregulation in the context of aging. Despite these insights, a consensus on the mechanism or function of histone loss during aging remains elusive, indicating the need for further research in this area.
6. Age-Associated Replacement of Canonical Isoforms by Histone Variants
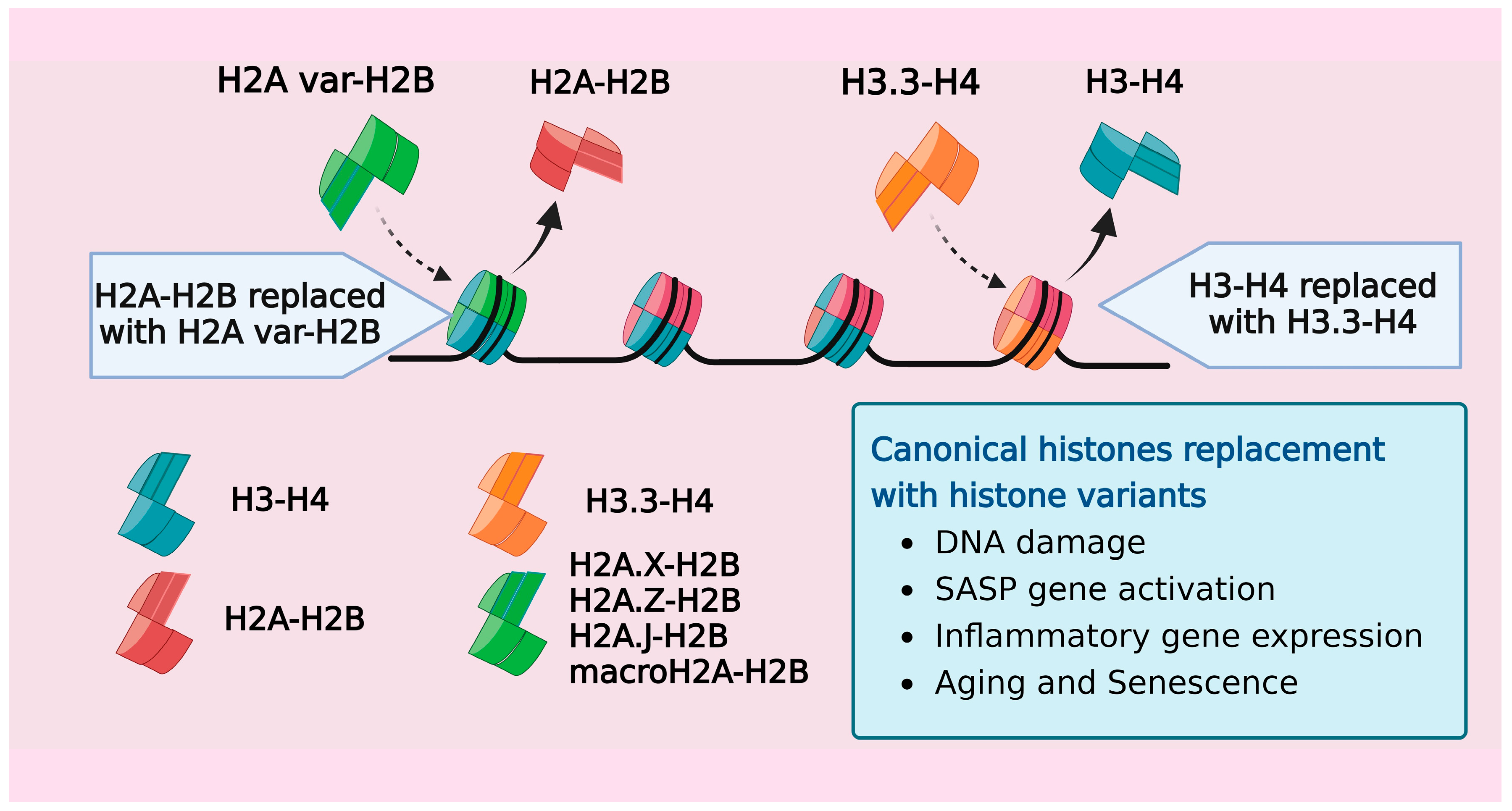
Besides the canonical histones that mediate higher-order chromatin organization, mammals possess replication-independent, noncanonical variant histones (all canonical histones and their variants from humans are listed in Table 3). These variants contribute to creating distinct nucleosome structures and functions. Given the widespread distribution and multifaceted roles of histone variants, it is only natural that they exert their influence not only on normal physiological functions, but also on aging, senescence, and various pathogenic states. [114]. Many studies have revealed that replacing canonical histones with their variant counterparts impacts the chromatin dynamics and reprograms the transcriptome and epigenome of the cell [115,116]. One of the earliest studies reporting this phenomenon discovered the progressive accumulation of the H3.3 variant in rodent brains over a span of 150 days, with a concurrent decrease in the levels of canonical H3.1 and H3.2 histones [117]. H3.3, an evolutionarily highly conserved variant, has been increasingly recognized for its role in regulating developmental processes within organisms [118,119,120]. The depletion of H3.3 had detrimental effects on embryonic Mus musculus and Xenopus, underlining its critical role in maintaining the genome integrity during tissue maturation [118,120,121,122,123]. The interaction of the H3.3 variant with histone chaperone complexes like HIRA and ATRX/DAXX facilitated its incorporation at important chromatin regions such as protein-coding genes, telomeres, and pericentric chromatin. The HIRA complex-mediated deposition of the H3.3 variant in HeLa cells was identified as a potential cellular repair mechanism to fill DNA regions lacking nucleosomes, thereby reinstating nucleosomal organization [124].
Several reports demonstrated that H3.3 accumulated in aged and senescent cells, effectively replacing the H3.1 and H3.2 canonical histones. In the somatic tissues of mice, including the liver, kidney, heart, and brain, histone H3.3 progressively replaces the canonical histones, achieving near-saturation levels of neuronal nucleosomes by mid-adolescence [125,126,127]. A similar pattern was observed in the human brain, where H3.3 constituted >93% of the total H3 pool across individuals aged 14 to 72 years [125]. The accumulation of H3.3 was accompanied by an altered methylation landscape of tissue, reflecting the epigenetic plasticity in response to the association of different histone isoforms with the genome [126,127]. Furthermore, in fibroblasts and melanocytes, the proteolytic cleavage of H3.3 led to the integration of its cleaved product, known as H3.3cs1, into the nucleosome structure. Both H3.3 and its derivative H3.3cs1 induced senescence by suppressing genes linked to cell proliferation [128].
With the vast repertoire of H2A variants expressed in mammalian cells, they mainly account for the nucleosome diversity [129]. Histone H2A has functionally distinct variants like H2A.B, H2A.X, H2A.Z, and macroH2A that play a significant role in maintaining the nucleosome. Studies demonstrating the accumulation of H2A variants, macroH2A, H2A.Z, and γ-H2AX in the context of aging and senescence revealed the impact these variants have on the transcription and regulation of senescence biomarkers, including SASP genes [130,131,132]. H2A.Z, a promoter of heterochromatin, is increased in the hippocampi of aging mice. Conversely, the removal of H2A.Z from nucleosomes enhanced the gene expression of learning-associated genes [130]. Senescent cells, showing γ-H2AX accumulation as a result of persistent DNA damage, were found to regulate p53-dependent senescent growth arrest and senescence-associated extracellular inflammatory signaling [132,133]. Senescent fibroblast cells harbored elevated levels of the H2B type 1-K variant, but the accumulation was exclusive to cells with persistent DNA damage [134]. Thus, DNA damage-associated variant histone exchange within senescent cells likely serves to maintain the genome integrity by nucleosome deposition in damaged chromatin regions. The histone H2A variant, H2A.J, also accumulated in fibroblasts undergoing replicative senescence and supplanted canonical H2A expression in cells with persistent DNA damage [135,136]. The H2A.J variant was found to regulate inflammatory gene expression, as the depletion or overexpression of H2A.J regulated essential SASP genes either negatively or positively, respectively [136]. Similarly, a gradual buildup of the H2A.J variant was observed in epidermal keratinocytes from donors between 18–90 years, correlating to their chronological age [137]. The H1 histones are a diverse class comprising eleven variants in mammals [138,139], and of these, H1.0, a replication-independent variant, is increased in terminally differentiated cells [140]. Several reports over the last decade have highlighted the importance of H1.0 and other H1 variants in the regulation of the heterochromatin structure and genome remodeling.
These studies illustrate that the replacement of canonical histones by variants with age occurs in a cell-type-specific or context-specific manner [141]. In various cellular processes, studies have shown that certain histone variants possess unique functions and are vital for the survival of particular organisms [142,143]. Mutations in these variants can lead to severe defects, although they might be nonessential for other organisms [142,143,144,145]. The regulated deposition of histone variants plays a crucial role in preserving the senescent state of the cell, acting as a barrier against the development of cancer [128,146]. Mutations in these histone variants can result in severe defects and are commonly observed in association with various cancers [146]. The intricate interplay between these histone variants in senescent cells underscores their significance in maintaining genomic integrity, preventing uncontrolled cell proliferation, and potentially transforming into cancerous cells. Additionally, the sequence of the genome and the organization of chromatin influence the pattern of incorporation and tissue specificity of histone variants [147,148]. Hence, in the aging process, biological factors such as DNA damage, SASP activation, and the expression of inflammation genes likely contribute to the integration of specific variants into the nucleosome (Figure 2).
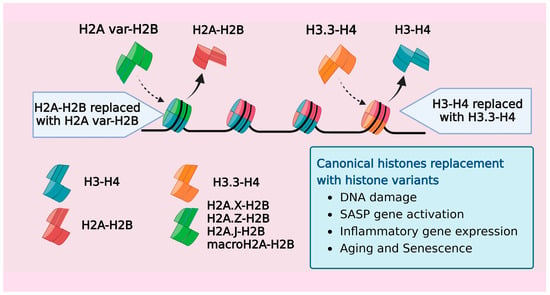
Figure 2.
A schematic representation showing the exchange of canonical histones with variants over a gene. Histone exchange involves the replacement of both H2A and H3 for their respective histone variants in response to age-related stress stimuli.

Table 3.
List of human histones and histone variants [149].
Table 3.
List of human histones and histone variants [149].
| Human H1 Histones | ||||
|---|---|---|---|---|
| Variant Symbol | Previous Symbol | HGNC Gene Symbol | HGNC ID | HGNC Gene Name |
| H1.0 | H1F0 | H1-0 | HGNC:4714 | H1.0 linker histone |
| H1.1 | HIST1H1A | H1-1 | HGNC:4715 | H1.1 linker histone, cluster member |
| H1.2 | HIST1H1C | H1-2 | HGNC:4716 | H1.2 linker histone, cluster member |
| H1.3 | HIST1H1D | H1-3 | HGNC:4717 | H1.3 linker histone, cluster member |
| H1.4 | HIST1H1E | H1-4 | HGNC:4718 | H1.4 linker histone, cluster member |
| H1.5 | HIST1H1B | H1-5 | HGNC:4719 | H1.5 linker histone, cluster member |
| H1.6 | HIST1H1T | H1-6 | HGNC:4720 | H1.6 linker histone, cluster member |
| H1.7 | H1FNT | H1-7 | HGNC:24893 | H1.7 linker histone |
| H1.8 | H1FOO | H1-8 | HGNC:18463 | H1.8 linker histone |
| NA | HILS1 | H1-9P | HGNC:30616 | H1.9 linker histone, pseudogene |
| H1.10 | H1FX | H1-10 | HGNC:4722 | H1.10 linker histone |
| NA | HIST1H1PS1 | H1-12P | HGNC:19163 | H1.12 linker histone, cluster member pseudogene |
| Human H2A Histones | ||||
| Variant Symbol | Previous Symbol | HGNC Gene Symbol | HGNC ID | HGNC Gene Name |
| H2A | HIST1H2AA | H2AC1 | HGNC:18729 | H2A clustered histone 1 |
| NA | HIST1H2APS1 | H2AC2P | HGNC:18720 | H2A clustered histone 2, pseudogene |
| NA | HIST1H2APS2 | H2AC3P | HGNC:18804 | H2A clustered histone 3, pseudogene |
| H2A | HIST1H2AB | H2AC4 | HGNC:4734 | H2A clustered histone 4 |
| NA | HIST1H2APS5 | H2AC5P | HGNC:4728 | H2A clustered histone 5, pseudogene |
| H2A | HIST1H2AC | H2AC6 | HGNC:4733 | H2A clustered histone 6 |
| H2A | HIST1H2AD | H2AC7 | HGNC:4729 | H2A clustered histone 7 |
| H2A | HIST1H2AE | H2AC8 | HGNC:4724 | H2A clustered histone 8 |
| NA | HIST1H2APS3 | H2AC9P | HGNC:18805 | H2A clustered histone 9, pseudogene |
| NA | HIST1H2APS4 | H2AC10P | HGNC:4732 | H2A clustered histone 10, pseudogene |
| H2A | HIST1H2AG | H2AC11 | HGNC:4737 | H2A clustered histone 11 |
| H2A | HIST1H2AH | H2AC12 | HGNC:13671 | H2A clustered histone 12 |
| H2A | HIST1H2AI | H2AC13 | HGNC:4725 | H2A clustered histone 13 |
| H2A | HIST1H2AJ | H2AC14 | HGNC:4727 | H2A clustered histone 14 |
| H2A | HIST1H2AK | H2AC15 | HGNC:4726 | H2A clustered histone 15 |
| H2A | HIST1H2AL | H2AC16 | HGNC:4730 | H2A clustered histone 16 |
| H2A | HIST1H2AM | H2AC17 | HGNC:4735 | H2A clustered histone 17 |
| H2A | HIST2H2AA3 | H2AC18 | HGNC:4736 | H2A clustered histone 18 |
| H2A | HIST2H2AA4 | H2AC19 | HGNC:29668 | H2A clustered histone 19 |
| H2A | HIST2H2AC | H2AC20 | HGNC:4738 | H2A clustered histone 20 |
| H2A | HIST2H2AB | H2AC21 | HGNC:20508 | H2A clustered histone 21 |
| H2A | HIST3H2A | H2AC25 | HGNC:20507 | H2A clustered histone 25 |
| H2A.Z.1 | H2AFZ | H2AZ1 | HGNC:4741 | H2A.Z variant histone 1 |
| H2A.Z.2 | H2AFV | H2AZ2 | HGNC:20664 | H2A.Z variant histone 2 |
| macroH2A.1 | H2AFY | MACROH2A1 | HGNC:4740 | macroH2A.1 histone |
| macroH2A.2 | H2AFY2 | MACROH2A2 | HGNC:14453 | macroH2A.2 histone |
| H2A.X | H2AFX | H2AX | HGNC:4739 | H2A.X variant histone |
| H2A.J | H2AFJ | H2AJ | HGNC:14456 | H2A.J histone |
| H2A.B | H2AFB1 | H2AB1 | HGNC:22516 | H2A.B variant histone 1 |
| H2A.B | H2AFB2 | H2AB2 | HGNC:18298 | H2A.B variant histone 2 |
| H2A.B | H2AFB3 | H2AB3 | HGNC:14455 | H2A.B variant histone 3 |
| H2A.P | HYPM | H2AP | HGNC:18417 | H2A.P histone |
| NA | NA | H2AQ1P | HGNC:53962 | H2A.Q variant histone 1, pseudogene |
| H2A.L | NA | H2AL1Q | HGNC:53959 | H2A.L variant histone 1Q |
| NA | NA | H2AL1MP | HGNC:53961 | H2A.L variant histone 1 M, pseudogene |
| H2A.L | NA | H2AL3 | HGNC:53960 | H2A.L variant histone 3 |
| Human H2B Histones | ||||
| Variant Symbol | Previous Symbol | HGNC Gene Symbol | HGNC ID | HGNC Gene Name |
| H2B | HIST1H2BA | H2BC1 | HGNC:18730 | H2B clustered histone 1 |
| NA | HIST1H2BPS1 | H2BC2P | HGNC:18719 | H2B clustered histone 2, pseudogene |
| H2B | HIST1H2BB | H2BC3 | HGNC:4751 | H2B clustered histone 3 |
| H2B | HIST1H2BC | H2BC4 | HGNC:4757 | H2B clustered histone 4 |
| H2B | HIST1H2BD | H2BC5 | HGNC:4747 | H2B clustered histone 5 |
| H2B | HIST1H2BE | H2BC6 | HGNC:4753 | H2B clustered histone 6 |
| H2B | HIST1H2BF | H2BC7 | HGNC:4752 | H2B clustered histone 7 |
| H2B | HIST1H2BG | H2BC8 | HGNC:4746 | H2B clustered histone 8 |
| H2B | HIST1H2BH | H2BC9 | HGNC:4755 | H2B clustered histone 9 |
| H2B | HIST1H2BI | H2BC10 | HGNC:4756 | H2B clustered histone 10 |
| H2B | HIST1H2BJ | H2BC11 | HGNC:4761 | H2B clustered histone 11 |
| H2B | HIST1H2BK | H2BC12 | HGNC:13954 | H2B clustered histone 12 |
| H2B | HIST1H2BL | H2BC13 | HGNC:4748 | H2B clustered histone 13 |
| H2B | HIST1H2BM | H2BC14 | HGNC:4750 | H2B clustered histone 14 |
| H2B | HIST1H2BN | H2BC15 | HGNC:4749 | H2B clustered histone 15 |
| NA | HIST1H2BPS2 | H2BC16P | HGNC:4754 | H2B clustered histone 16, pseudogene |
| H2B | HIST1H2BO | H2BC17 | HGNC:4758 | H2B clustered histone 17 |
| H2B | HIST2H2BF | H2BC18 | HGNC:24700 | H2B clustered histone 18 |
| NA | HIST2H2BD | H2BC19P | HGNC:20517 | H2B clustered histone 19, pseudogene |
| NA | HIST2H2BC | H2BC20P | HGNC:20516 | H2B clustered histone 20, pseudogene |
| H2B | HIST2H2BE | H2BC21 | HGNC:4760 | H2B clustered histone 21 |
| H2B | HIST3H2BB | H2BC26 | HGNC:20514 | H2B clustered histone 26 |
| NA | HIST3H2BA | H2BC27P | HGNC:20515 | H2B clustered histone 27, pseudogene |
| H2B.K | H2BE1 | H2BK1 | HGNC:53833 | H2B.K variant histone 1 |
| NA | H2BP4 | H2BL1P | HGNC:54442 | H2B.L histone variant 1, pseudogene |
| H2B.W | H2BFWT | H2BW1 | HGNC:27252 | H2B.W histone 1 |
| H2B.W | H2BFM | H2BW2 | HGNC:27867 | H2B.W histone 2 |
| NA | NA | H2BW3P | HGNC:44390 | H2B.W histone 3, pseudogene |
| NA | H2BFXP | H2BW4P | HGNC:25757 | H2B.W histone 4, pseudogene |
| H2B.N | NA | H2BN1 | HGNC:56200 | H2B.N variant histone 1 |
| H2B | H2BFS | H2BC12L | HGNC:4762 | H2B clustered histone 12 like |
| Human H3 Histones | ||||
| Variant Symbol | Previous Symbol | HGNC Gene Symbol | HGNC ID | HGNC Gene Name |
| H3.1 | HIST1H3A | H3C1 | HGNC:4766 | H3 clustered histone 1 |
| H3.1 | HIST1H3B | H3C2 | HGNC:4776 | H3 clustered histone 2 |
| H3.1 | HIST1H3C | H3C3 | HGNC:4768 | H3 clustered histone 3 |
| H3.1 | HIST1H3D | H3C4 | HGNC:4767 | H3 clustered histone 4 |
| NA | NA | H3C5P | HGNC:54427 | H3 clustered histone 5, pseudogene |
| H3.1 | HIST1H3E | H3C6 | HGNC:4769 | H3 clustered histone 6 |
| H3.1 | HIST1H3F | H3C7 | HGNC:4773 | H3 clustered histone 7 |
| H3.1 | HIST1H3G | H3C8 | HGNC:4772 | H3 clustered histone 8 |
| NA | HIST1H3PS1 | H3C9P | HGNC:18982 | H3 clustered histone 9, pseudogene |
| H3.1 | HIST1H3H | H3C10 | HGNC:4775 | H3 clustered histone 10 |
| H3.1 | HIST1H3I | H3C11 | HGNC:4771 | H3 clustered histone 11 |
| H3.1 | HIST1H3J | H3C12 | HGNC:4774 | H3 clustered histone 12 |
| H3.2 | HIST2H3D | H3C13 | HGNC:25311 | H3 clustered histone 13 |
| H3.2 | HIST2H3C | H3C14 | HGNC:20503 | H3 clustered histone 14 |
| H3.2 | HIST2H3A | H3C15 | HGNC:20505 | H3 clustered histone 15 |
| H3.3 | H3F3, H3F3A | H3-3A | HGNC:4764 | H3.3 histone A |
| H3.3 | H3F3B | H3-3B | HGNC:4765 | H3.3 histone B |
| H3.4 | HIST3H3 | H3-4 | HGNC:4778 | H3.4 histone, cluster member |
| H3.5 | H3F3C | H3-5 | HGNC:33164 | H3.5 histone |
| NA (H3.6) | H3F3AP6 | H3P16 | HGNC:42982 | H3 histone pseudogene 16 |
| H3.7 | HIST2H3PS2 | H3-7 | HGNC:32060 | H3.7 histone (putative) |
| NA (H3.8) | H3F3AP5 | H3P44 | HGNC:42981 | H3 histone pseudogene 44 |
| H3.Y.1 | NA | H3Y1 | HGNC:43735 | H3.Y histone 1 |
| H3.Y.2 | NA | H3Y2 | HGNC:43734 | H3.Y histone 2 |
| cenH3 | NA | CENPA | HGNC:1851 | Centromere protein A |
| Human H4 Histones | ||||
| Variant Symbol | Previous Symbol | HGNC Gene Symbol | HGNC ID | HGNC Gene Name |
| H4 | HIST1H4A | H4C1 | HGNC:4781 | H4 clustered histone 1 |
| H4 | HIST1H4B | H4C2 | HGNC:4789 | H4 clustered histone 2 |
| H4 | HIST1H4C | H4C3 | HGNC:4787 | H4 clustered histone 3 |
| H4 | HIST1H4D | H4C4 | HGNC:4782 | H4 clustered histone 4 |
| H4 | HIST1H4E | H4C5 | HGNC:4790 | H4 clustered histone 5 |
| H4 | HIST1H4F | H4C6 | HGNC:4783 | H4 clustered histone 6 |
| H4 | HIST1H4G | H4C7 | HGNC:4792 | H4 clustered histone 7 |
| H4 | HIST1H4H | H4C8 | HGNC:4788 | H4 clustered histone 8 |
| H4 | HIST1H4I | H4C9 | HGNC:4793 | H4 clustered histone 9 |
| NA | HIST1H4PS1 | H4C10P | HGNC:4786 | H4 clustered histone 10, pseudogene |
| H4 | HIST1H4J | H4C11 | HGNC:4785 | H4 clustered histone 11 |
| H4 | HIST1H4K | H4C12 | HGNC:4784 | H4 clustered histone 12 |
| H4 | HIST1H4L | H4C13 | HGNC:4791 | H4 clustered histone 13 |
| H4 | HIST2H4A | H4C14 | HGNC:4794 | H4 clustered histone 14 |
| H4 | HIST2H4B | H4C15 | HGNC:29607 | H4 clustered histone 15 |
| H4 | HIST4H4 | H4C16 | HGNC:20510 | H4 histone 16 |
7. Multilevel Regulation of Histone Degradation
Cells have developed robust mechanisms to synthesize core and linker histones abundantly during the S-phase of the cell cycle, ensuring a sufficient supply during chromatin duplication [150,151,152,153]. The regulation of histone biosynthesis occurs at various levels encompassing histone gene transcription, mRNA stability, translation efficiency, and a wide array of histone PTMs [154,155]. Extensive reviews have delved into these processes that have significantly enhanced our understanding of histone metabolism and its correlation to cell cycle checkpoints [156,157]. Following synthesis, histone density is regulated at both the post-transcriptional and post-translational levels, and aging studies have demonstrated the vital roles these regulatory mechanisms play in histone depletion [36,70,90]. Here, we will explore the final frontier of histone regulation, mainly focusing on histone degradation at both the mRNA and protein levels. This phenomenon is observed under specific cellular conditions such as extensive chromatin remodeling, the formation of relaxed chromatin structures with increased access to DNA, DNA damage response, and a surplus of histones. Histone degradation, documented across various developmental stages such as spermiogenesis, embryogenesis, senescence, and aging, plays a pivotal role in meticulously controlling the histone pool. This regulation is vital for maintaining the equilibrium required in the dynamic processes of chromatin assembly and disassembly.
7.1. Histone mRNA Degradation
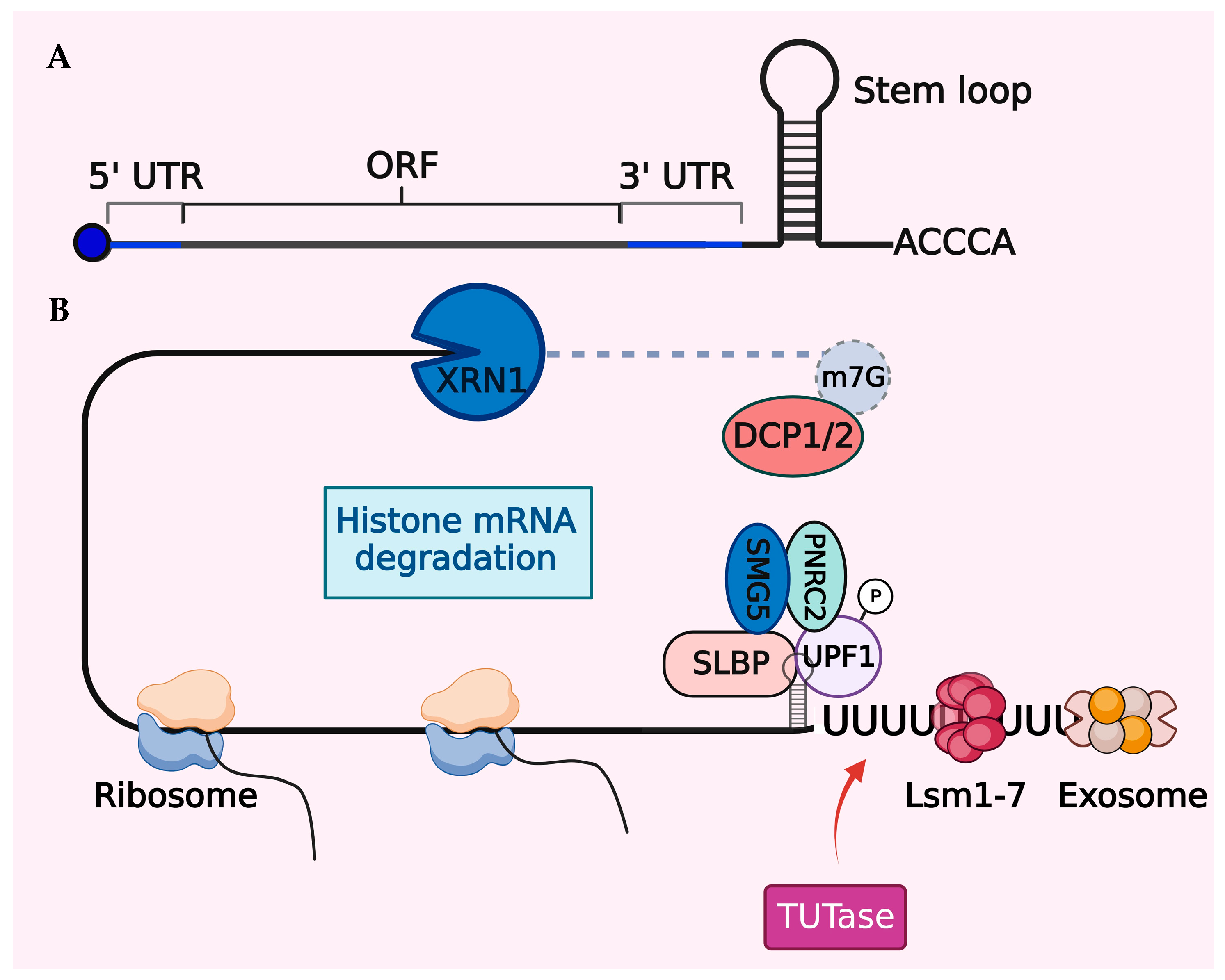
In mammalian cells, there is a substantial increase (~35-fold) in histone transcription at the G1/S transition phase of the cell cycle, followed by rapid histone mRNA degradation [151,158,159]. Typically, histone mRNAs lack poly-A tails and instead have a conserved 25–26 nucleotide sequence at their 3′ ends, forming a unique stem–loop structure crucial for swift mRNA decay at the end of the S-phase (Figure 3). The stem–loop binding protein (SLBP) specifically binds to the stem–loop structure and plays a central role in the post-transcriptional processing, translation, and degradation of histone mRNAs [156,160,161]. Cells employ a potent feedback inhibition mechanism to regulate their histone pool. Soluble histones are central to this mechanism, as they enable the degradation of histone mRNAs, coinciding with the inhibition of DNA synthesis [158,162,163]. Initially, the nuclear autoantigenic sperm protein (NASP), a histone chaperone, shields these soluble histones from degradation, protecting them right from their synthesis until they are integrated into the nucleosome unit [164,165]. However, an excess of soluble histones can disturb the SLBP-3’ stem–loop interaction, ultimately resulting in mRNA degradation through the action of the exonuclease 3′hExo [166,167].
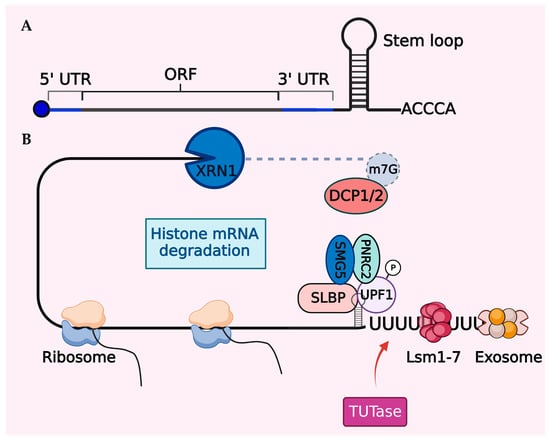
Figure 3.
Degradation mechanism of histone mRNA. (A) Schematic illustration of histone mRNA showing the cap on the 5′ end, the short 5′ and 3′ UTRs, and the stem–loop at the 3′ end. (B) Following translation termination, phosphorylated Upf1, a crucial factor for nonsense-mediated mRNA decay (NMD), is recruited to the 3′ end of histone mRNA and subsequently, Upf1 interacts with other NMD cofactors such as SMG5 and PNRC2. Histone mRNA degradation occurs through decapping with DCP1/2 and 5′→3′ cleavage by XRN1 from the 5′ end. Additionally, TUTase-mediated oligouridylation targets histone mRNA for 3′→5′ degradation by the exosome.
Similar to histones, SLBP also follows a cell cycle-regulated pattern of expression. SLBP interacts with phosphorylated upstream frameshift 1 (UPF1), an important RNA decay factor [168]. This interaction forms the SLBP–UPF1 complex, which recruits SMG5 and PNRC2 (proline-rich nuclear receptor coregulatory protein 2), initiating histone mRNA decapping by DCP1A/2 enzymes [169]. Subsequently, degradation occurs through a bidirectional 5′-to-3′ XRN1-mediated exoribonucleolytic activity and 3′-to-5′ exosome degradation [166,170,171]. A prerequisite for histone mRNA decay is the TUTase enzyme-, TUT7-, and/or TUT4-mediated modification of the 3′ end by oligourydilation [166,170]. The TUT enzymes provide a scaffold for the Lsm 1–7 heptamer complex, which along with SLBP, coordinates histone mRNA degradation through a distinct set of nucleases [172,173]. In mammals, these decay mechanisms for histone mRNAs can occur simultaneously or independently to regulate histones.
7.2. Histone Protein Degradation
The abundance of histones in a cell is also regulated through proteolytic degradation, a process facilitated by multiple pathways such as through proteases, the autophagy–lysosomal system, and the proteasome complex (Figure 4). The histone proteins integrated into nucleosomes exhibit a remarkable stability, with half-lives ranging from several days to months, but this is significantly influenced by cellular proliferation and the differentiation status [174,175]. Nevertheless, soluble histones not bound to chromatin undergo rapid degradation at the end of the S-phase. Therefore, the maintenance of homeostasis in the histone pool results from the synergistic degradation of free and chromatin-bound histones through the proteasomal and autophagy–lysosomal systems.
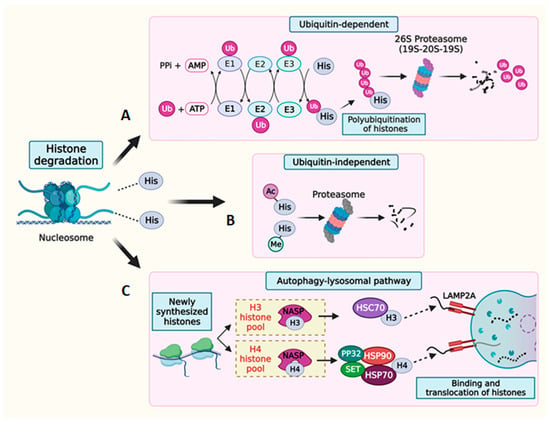
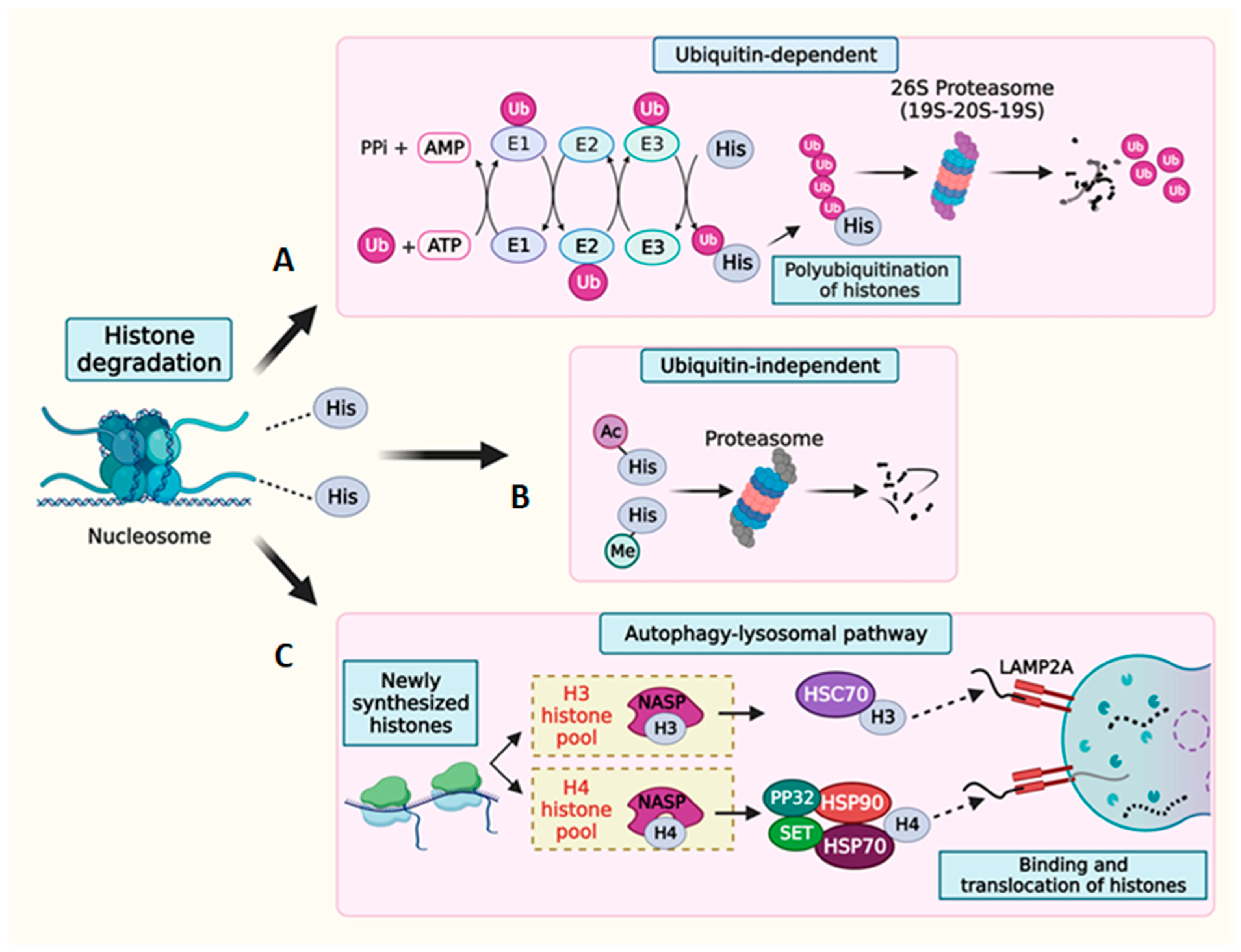
Figure 4.
Degradation of histone proteins. Schematic representation of histone degradation via (A) ubiquitin-dependent or (B) ubiquitin-independent pathways. (C) Schematic representation of histone degradation mediated by the autophagy–lysosomal system. The NASP protein stabilizes a reservoir of soluble histones H3-H4 by protecting them from degradation. Chaperone-mediated autophagy involves the direct uptake of cytosolic chaperone-bound H3 and H4 into lysosomes via a translocation complex consisting of LAMP2A monomers.
Histones are channeled through both ubiquitin-dependent and ubiquitin-independent pathways for proteasome-mediated degradation [176]. Ubiquitination constitutes an important histone PTM wherein a highly conserved, 76-amino-acid ubiquitin tag typically conjugates to lysine residues of the target histone proteins [177]. This modification serves as a signal for protein degradation through the proteasome complex [178,179]. The ubiquitination process involves a coordinated cascade of enzymes, including E1 ubiquitin-activating enzymes, E2 ubiquitin-conjugating enzymes, and E3 ubiquitin ligases [180]. Sequential rounds of ubiquitination on the lysine residues of the initial histone-conjugated ubiquitin result in the polyubiquitination of histone substrates, thereby facilitating the binding of polyubiquitin chains to the ubiquitin receptor on the proteasome complex [181,182]. The proteasome itself is a large, hollow, multisubunit complex that exists in two primary forms within cells: the 20S and 26S species. The 20S proteasome is a four-stacked ring structure and houses six active protease sites within its central cavity, making it the catalytic core of this complex. The openings to this cavity exclusively admit denatured proteins that are systematically degraded into smaller peptides. Various regulatory particles (RP) are positioned at one or both ends of the 20S proteasome, carrying out diverse functions including substrate recognition and their subsequent translocation to the catalytic core [183,184]. A well-known RP, 19S, complexes with the 20S core to form the 26S proteasome. Depending on their intracellular localization, different RPs such as PA28γ, PA200 (primarily located in the nucleus), and PA28α and β (predominantly cytosolic) associate with the 20S core and assume critical roles in facilitating protein deubiquitination, denaturation, and translocation into the 20S proteasomal core, eventually leading to degradation. Histone degradation by ubiquitin tagging is known to occur in various cellular contexts. Histone turnover is a critical mechanism that regulates DNA accessibility during transcription and replication, DNA damage repair, and the excess accumulation of histones [185]. When histones undergo ubiquitin-dependent degradation, it is often accompanied by the addition or removal of histone PTM marks, revealing an intricate link between the ubiquitin-dependent degradation process and the regulation of epigenetic marks on the histones. Studies have shown that denaturation and deubiquitination are prerequisites for a polyubiquitinated protein’s entry into the proteasome core for degradation [186]. However, the proteasome-mediated degradation of denatured proteins can also occur independently of the ubiquitin tag. This ubiquitin-independent degradation of histones is observed in response to DNA damage and spermiogenesis [112,187]. The 20S proteasome core, associated with RP PA200, specifically degrades the core histones bearing specific PTM marks [112].
Cells also utilize the autophagy pathway to degrade excess histones, safeguarding themselves from potential detrimental effects. Chaperone-mediated autophagy (CMA) has been identified as the primary pathway responsible for maintaining optimal histone levels, specifically by breaking down newly synthesized H3 and H4 histones [188]. During the maturation process of newly formed histones, a series of steps ensure their proper folding and dimerization. In mammals, the NASP chaperone plays a crucial role in regulating the soluble histone pool of H3 and H4. NASP is a cell cycle-regulated protein that shows a bidirectional modulation of the histone reservoir. A precisely regulated interaction between NASP and its cochaperones—HSC70 (for H3) and HSP70/HSP90/PP32/Set (for H4)—targets histones for lysosomal degradation through the lysosomal surface receptor protein LAMP2A [188,189].
8. Histone Complementation in Cells Experiencing Histone Loss
Budding yeast is a versatile organism well-suited for studying eukaryotic aging due to its finite lifespan and genetic manipulability. Studies using yeast models have shown that as cells age, they experience a significant loss of histones. Feser and colleagues demonstrated that elevating the histone expression in aging yeast cells extended their lifespan effectively [59]. Mutant yeast strains lacking specific histone chaperone genes like asf1, rtt109, and hir1 exhibited distinct histone mRNA levels. Short-lived mutants (asf1 and rtt109) displayed diminished histone levels, while hir1 mutants, marked by increased histone levels, exhibited prolonged lifespans. Furthermore, the overexpression of histone genes using a high copy number plasmid in both wild-type and yeast mutants with a shorter lifespan resulted in an increased cellular longevity. Although this discovery seemed promising for addressing histone loss in aging and senescent cells, a subsequent yeast study highlighted the adverse effects, including genomic instability and cytotoxicity associated with histone overexpression [190]. Persistent histone production beyond the S-phase resulted in aberrant cell division, defective chromosome segregation, whole genome duplications, and reduced histone variant incorporation into the chromatin [191,192,193]. Further research in yeast by Yu and colleagues demonstrated an intriguing correlation between histone dosage and replicative lifespan. It was observed that histone biosynthesis surpassed requisite levels in young cells but gradually declined with age [194]. Despite these insights into yeast, it remains unclear whether histone overexpression promotes longevity and addresses aging challenges in mammalian cells. Understanding the cellular sensitivity to histone levels and the consequences of aberrant histone expression in mammalian models becomes crucial in light of the contrasting effects observed in yeast.
Histones are basic proteins and, in excess, can disrupt cellular functions, causing defects in mitotic chromosome segregation through their interaction with negatively charged molecules [195,196]. Therefore, maintaining an optimal histone supply is crucial for cellular homeostasis. Histone biosynthesis is tightly coordinated with DNA replication, involving a wide array of regulatory molecules to maintain a nucleosomal balance in actively proliferating or quiescent cells [155,197]. In a recent study, Kim et al. demonstrated that aged T cells exhibited reduced histone levels due to the elevated levels of SIRT1, a histone deacetylase [70]. Inhibiting SIRT1 in aged T cells resulted in an increased histone gene expression, restored the cell cycle progression, and mitigated the replication-stress response. Additionally, an in vivo mouse model of T-cell aging revealed an enhanced T-cell activation during the murine antiviral response upon SIRT1 inhibition. Thus, modulating the SIRT1 activity to replenish histone levels appeared to have a positive impact by restoring cellular functionality. In a subsequent study by Yang et al. the authors used a transgenic mouse model called ‘inducible changes to the epigenome’ (ICE) that expresses I-PpoI endonuclease in a tamoxifen-inducible manner. The ICE mice allowed for the introduction of DSBs in 19 noncoding regions in the genome with no evidence of mutations or immediate deleterious effects. However, at ten months postinduction, the ICE mice displayed aging characteristics accompanied by alterations in epigenetic patterns and transcriptional activity at the histone level. Using this model, the researchers effectively demonstrated that by inducing the expression of Yamanaka factors (OSK), they could successfully reprogram the epigenetic profile to resemble a more youthful state, thereby restoring the normal histone expression [30]. Together, these studies showed that certain regulatory factors play a central role in the expression of histone genes, and tweaking the expression of these master regulators could attenuate or accentuate the histone levels [60,198,199,200,201].
Recent research in mammalian models demonstrated the restoration of histone expression via treatment with pharmacological compounds, including rapamycin, metformin, and resveratrol. These drugs are increasingly recognized for their geroprotective and anti-aging properties, as evidenced by clinical studies demonstrating improved outcomes in age-related pathologies [202]. In a recent study by Lu et al., a robust upregulation of H3 and H4 histones was observed with the administration of rapamycin, an inhibitor of the protein kinase mTORC1 [203]. The study found that rapamycin induced an increased histone expression in the intestinal enterocytes of drosophila and also alleviated the age-associated decline in a mouse model by promoting gut health and homeostasis. Rapamycin-treated enterocytes exhibited a chromosomal rearrangement, redistribution of heterochromatin domains, and activation of autophagy. Although the primary focus of this study was on the effects of rapamycin-induced histone upregulation in Drosophila, it also highlighted similar outcomes in mouse intestinal autophagy, gut health, and lifespan. The restoration of histones using rapamycin in this study identified the mTORC1–histone axis as a critical prolongevity mechanism in mammals.
Another study comparing the transcriptome of muscle progenitor cells (MPC) derived from old and young participants showed the reduced expression of several histone isoforms [204]. However, in older participants who ingested metformin for two weeks, there was partial restoration of histone levels and improved function of myoblasts. Although this study did not delve into the precise cellular mechanisms reversing histone loss, metformin ingestion altered the transcriptional profile of old MPCs, significantly upregulating the histone genes. Similarly, in IMR90 fibroblast cells experiencing H4 histone loss due to H2O2-induced senescence, the restoration of H4 and delayed onset of senescence were observed within 24 h of treatment with rapamycin, metformin, and resveratrol [90]. While this study did not elaborate on the mechanism of H4 renewal, previous reports suggested that rapamycin prevented the degradation of ubiquitinylated substrates like histones by binding to the proteasome subunit. Collectively, these findings suggest that the response of histone genes to these anti-aging drugs could be effective in regulating molecules that are at the crossroads of aging and age-related histone loss.
9. Conclusions and Future Perspectives
The loss of histones and changes in nucleosome occupancy, among other classical epigenomic biomarkers like the global decline in genomic DNA methylation, heterochromatin loss, altered patterns of histone PTMs, and deregulation of noncoding RNAs are recognized as hallmarks of mammalian aging and senescence [4]. This review provides a comprehensive overview of the changing histone profile and nucleosome occupancy in the aging cell epigenome, drawing insights from human, animal, and cell-based models. Systematic studies in yeast have demonstrated the functional significance of histone loss and restoration in cellular aging and senescence. However, unraveling the mechanisms behind histone loss in aging mammals, especially humans, poses technical challenges due to the complex encoding of histones by various gene clusters distributed across multiple chromosomal locations. As an added layer of regulatory control, histone expression is a multitiered process involving numerous transcription factors and chaperones. Furthermore, the redistribution and histone profiles of nucleosomes exert varied effects on cellular aging and longevity through the differential regulation of the transcriptome and epigenomic landscape across species, and even within tissues of the same species. Despite these challenges, it is critical to determine (1) potential cellular mechanisms maintaining histone levels and identify key factors contributing to age-related histone depletion and genetic instability and (2) whether changes in the histone expression could be leveraged to mitigate the effects of aging and extend the lifespans of multicellular organisms.
Several recent technological advances offer promising avenues for addressing these challenges. Recent advances in genome editing, like the CRISPR–Cas9 technology, open the possibility of a systematic, targeted manipulation of histone regulatory genes in mammalian systems to determine the functional requirements of various regulatory factors. While the use of geroprotective drugs like metformin, rapamycin, and resveratrol presents a promising approach for rejuvenating aging cells experiencing histone depletion in model organisms, the critical question remains whether these drugs can achieve sufficient selectivity among different histone variants and PTMs to effectively address age-related pathologies. A substantial gap in our understanding pertains to the efficacy of various geroprotectors across diverse human tissues. Existing data are largely derived from a limited number of studies, predominantly utilizing mouse models or in vitro cell cultures, which may not accurately reflect the pathological conditions in humans [205,206]. The targeting of epigenetic factors and markers of senescence has been at the core of several clinical trials, but precise clinical trials targeting histones or histone regulatory molecules have not been performed yet (NCT03353597, NCT00242255, NCT06065241, and NCT05392582). An evaluation of the clinical efficacy of these geroprotective drugs is crucial, as Juricic et al. recently demonstrated in Drosophila that an increased histone expression is due to the “memory effect” of chronic rapamycin treatment [207]. Rapamycin is known for its ability to inhibit the mechanistic target of rapamycin (mTOR) pathway, which is involved in cellular processes such as growth, proliferation, and survival. The “memory effect” suggests that the changes induced by rapamycin, particularly in the regulation of mTOR-related processes, continue to influence the cellular behavior or function even when the drug is no longer present. As clinical trials of drugs like rapamycin are already underway and currently being tested in the context of aging, these are attractive options for targeting histone loss and genomic instability. Given that aging is a continuous process that unfolds over many years in humans, with cells having variable turnover times, tracking histone changes in specific cell types becomes imperative for a better understanding of how histones contribute to each stage of aging. The continuing advances in RNA-seq technologies, particularly single-cell omics sequencing, provide a higher resolution for dissecting epigenetic characteristics during aging and provide new leads for investigating the heterogeneity of aged cells. The creation of a spatiotemporal atlas detailing histone recruitment or eviction from the genome across multiple mammalian tissues can provide deeper insight into the epigenetic changes that are causal to aging.
Author Contributions
All authors contributed to the conceptualization, writing, and revision of this review manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the NIH (R01EY028206 and K08EY021757; Mark E. Kleinman), the BrightFocus AMD Research Award (Mark E. Kleinman), the American Federation for Aging Research (Mark E. Kleinman), and the International Retinal Research Foundation (Sushil K. Dubey).
Acknowledgments
All figures were created using Biorender.com.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- United Nations. World Population Ageing 2020 Highlights; United Nations Department of Economic and Social Affairs: New York, NY, USA, 2020. [Google Scholar]
- Jaul, E.; Barron, J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front. Public Health 2017, 5, 335. [Google Scholar] [CrossRef]
- Swenor, B.K.; Ehrlich, J.R. Ageing and vision loss: Looking to the future. Lancet Glob. Health 2021, 9, e385–e386. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tyler, J.K. Epigenetics and aging. Sci. Adv. 2016, 2, e1600584. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodero, S.; Fernandez-Morera, J.L.; Menendez-Torre, E.; Calvanese, V.; Fernandez, A.F.; Fraga, M.F. Aging genetics and aging. Aging Dis. 2011, 2, 186–195. [Google Scholar] [PubMed]
- Wheeler, H.E.; Kim, S.K. Genetics and genomics of human ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Booth, L.N.; Brunet, A. The Aging Epigenome. Mol. Cell 2016, 62, 728–744. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- van der Rijt, S.; Molenaars, M.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Integrating the Hallmarks of Aging Throughout the Tree of Life: A Focus on Mitochondrial Dysfunction. Front. Cell Dev. Biol. 2020, 8, 594416. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Schulz, T.J.; Zarse, K.; Voigt, A.; Urban, N.; Birringer, M.; Ristow, M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007, 6, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Ahuir, A.; Manzanares-Estreder, S.; Proft, M. Pro- and Antioxidant Functions of the Peroxisome-Mitochondria Connection and Its Impact on Aging and Disease. Oxid. Med. Cell Longev. 2017, 2017, 9860841. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O.; Van Veldhoven, P.P. Aging, age-related diseases and peroxisomes. Subcell. Biochem. 2013, 69, 45–65. [Google Scholar] [CrossRef]
- Terlecky, S.R.; Koepke, J.I.; Walton, P.A. Peroxisomes and aging. Biochim. Biophys. Acta 2006, 1763, 1749–1754. [Google Scholar] [CrossRef]
- Vigne, S.; Pot, C. Implication of Oxysterols and Phytosterols in Aging and Human Diseases. Adv. Exp. Med. Biol. 2024, 1440, 231–260. [Google Scholar] [CrossRef]
- Zarrouk, A.; Vejux, A.; Mackrill, J.; O’Callaghan, Y.; Hammami, M.; O’Brien, N.; Lizard, G. Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res. Rev. 2014, 18, 148–162. [Google Scholar] [CrossRef]
- Spinedi, M.; Clark, C.; Zullo, L.; Kerksiek, A.; Pistis, G.; Castelao, E.; von Gunten, A.; Preisig, M.; Lütjohann, D.; Popp, J. Cholesterol-metabolism, plant sterols, and long-term cognitive decline in older people—Effects of sex and APOEe4. iScience 2024, 27, 109013. [Google Scholar] [CrossRef]
- Blokzijl, F.; de Ligt, J.; Jager, M.; Sasselli, V.; Roerink, S.; Sasaki, N.; Huch, M.; Boymans, S.; Kuijk, E.; Prins, P.; et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature 2016, 538, 260–264. [Google Scholar] [CrossRef]
- Mogilenko, D.A.; Shpynov, O.; Andhey, P.S.; Arthur, L.; Swain, A.; Esaulova, E.; Brioschi, S.; Shchukina, I.; Kerndl, M.; Bambouskova, M.; et al. Comprehensive Profiling of an Aging Immune System Reveals Clonal GZMK+ CD8+ T Cells as Conserved Hallmark of Inflammaging. Immunity 2021, 54, 99–115.e112. [Google Scholar] [CrossRef] [PubMed]
- Bosco, N.; Noti, M. The aging gut microbiome and its impact on host immunity. Genes. Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Szilard, L. On the Nature of the Aging Process. Proc. Natl. Acad. Sci. USA 1959, 45, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Failla, G. The aging process and cancerogenesis. Ann. N. Y. Acad. Sci. 1958, 71, 1124–1140. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.A.; Charlesworth, B. A genetic analysis of senescence in Drosophila. Nature 1994, 367, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L. Evolutionary theories of ageing applied to long-lived organisms. Exp. Gerontol. 2001, 36, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Benayoun, B.A.; Pollina, E.A.; Brunet, A. Epigenetic regulation of ageing: Linking environmental inputs to genomic stability. Nat. Rev. Mol. Cell Biol. 2015, 16, 593–610. [Google Scholar] [CrossRef]
- Yang, J.H.; Hayano, M.; Griffin, P.T.; Amorim, J.A.; Bonkowski, M.S.; Apostolides, J.K.; Salfati, E.L.; Blanchette, M.; Munding, E.M.; Bhakta, M.; et al. Loss of epigenetic information as a cause of mammalian aging. Cell 2023, 186, 305–326.e327. [Google Scholar] [CrossRef]
- De Majo, F.; Martens, L.; Hegenbarth, J.C.; Ruhle, F.; Hamczyk, M.R.; Nevado, R.M.; Andres, V.; Hilbold, E.; Bar, C.; Thum, T.; et al. Genomic instability in the naturally and prematurely aged myocardium. Proc. Natl. Acad. Sci. USA 2021, 118, e2022974118. [Google Scholar] [CrossRef]
- Kaya, A.; Lobanov, A.V.; Gladyshev, V.N. Evidence that mutation accumulation does not cause aging in Saccharomyces cerevisiae. Aging Cell 2015, 14, 366–371. [Google Scholar] [CrossRef]
- Narayanan, L.; Fritzell, J.A.; Baker, S.M.; Liskay, R.M.; Glazer, P.M. Elevated levels of mutation in multiple tissues of mice deficient in the DNA mismatch repair gene Pms2. Proc. Natl. Acad. Sci. USA 1997, 94, 3122–3127. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Gotta, M.; Sinclair, D.A.; Mills, K.; McNabb, D.S.; Murthy, M.; Pak, S.M.; Laroche, T.; Gasser, S.M.; Guarente, L. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell 1997, 89, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.A.; Mills, K.; Guarente, L. Accelerated aging and nucleolar fragmentation in yeast sgs1 mutants. Science 1997, 277, 1313–1316. [Google Scholar] [CrossRef]
- Liu, L.; Cheung, T.H.; Charville, G.W.; Hurgo, B.M.; Leavitt, T.; Shih, J.; Brunet, A.; Rando, T.A. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep. 2013, 4, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Luger, K.; Mader, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 1997, 389, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Gaullier, G.; Luger, K. Nucleosome structure and dynamics are coming of age. Nat. Struct. Mol. Biol. 2019, 26, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.K.M.; Pugh, B.F. Understanding nucleosome dynamics and their links to gene expression and DNA replication. Nat. Rev. Mol. Cell Biol. 2017, 18, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Gurard-Levin, Z.A.; Quivy, J.P.; Almouzni, G. Histone chaperones: Assisting histone traffic and nucleosome dynamics. Annu. Rev. Biochem. 2014, 83, 487–517. [Google Scholar] [CrossRef]
- Stillman, B. Histone Modifications: Insights into Their Influence on Gene Expression. Cell 2018, 175, 6–9. [Google Scholar] [CrossRef]
- Allahverdi, A.; Yang, R.; Korolev, N.; Fan, Y.; Davey, C.A.; Liu, C.F.; Nordenskiold, L. The effects of histone H4 tail acetylations on cation-induced chromatin folding and self-association. Nucleic Acids Res. 2011, 39, 1680–1691. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Swygert, S.G.; Peterson, C.L. Chromatin dynamics: Interplay between remodeling enzymes and histone modifications. Biochim. Biophys. Acta 2014, 1839, 728–736. [Google Scholar] [CrossRef]
- McCauley, B.S.; Dang, W. Histone methylation and aging: Lessons learned from model systems. Biochim. Biophys. Acta 2014, 1839, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, Q.; Xie, L. Histone Modifications in Aging: The Underlying Mechanisms and Implications. Curr. Stem Cell Res. Ther. 2018, 13, 125–135. [Google Scholar] [CrossRef]
- Peleg, S.; Feller, C.; Ladurner, A.G.; Imhof, A. The Metabolic Impact on Histone Acetylation and Transcription in Ageing. Trends Biochem. Sci. 2016, 41, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Bainor, A.J.; David, G. Chapter 7—The Dynamics of Histone Modifications During Aging. In Epigenomics in Health and Disease; Fraga, M.F., Fernández, A.F., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 145–162. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Fan, X.G.; Tang, D. Release and activity of histone in diseases. Cell Death Dis. 2014, 5, e1370. [Google Scholar] [CrossRef] [PubMed]
- Shumaker, D.K.; Dechat, T.; Kohlmaier, A.; Adam, S.A.; Bozovsky, M.R.; Erdos, M.R.; Eriksson, M.; Goldman, A.E.; Khuon, S.; Collins, F.S.; et al. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc. Natl. Acad. Sci. USA 2006, 103, 8703–8708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, J.; Suzuki, K.; Qu, J.; Wang, P.; Zhou, J.; Liu, X.; Ren, R.; Xu, X.; Ocampo, A.; et al. Aging stem cells. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science 2015, 348, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Chang, M.; Kim, U.J.; Grunstein, M. Histone H2B repression causes cell-cycle-specific arrest in yeast: Effects on chromosomal segregation, replication, and transcription. Cell 1987, 48, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.J.; Han, M.; Kayne, P.; Grunstein, M. Effects of histone H4 depletion on the cell cycle and transcription of Saccharomyces cerevisiae. EMBO J. 1988, 7, 2211–2219. [Google Scholar] [CrossRef]
- Gossett, A.J.; Lieb, J.D. In vivo effects of histone H3 depletion on nucleosome occupancy and position in Saccharomyces cerevisiae. PLoS Genet. 2012, 8, e1002771. [Google Scholar] [CrossRef]
- Wyrick, J.J.; Holstege, F.C.; Jennings, E.G.; Causton, H.C.; Shore, D.; Grunstein, M.; Lander, E.S.; Young, R.A. Chromosomal landscape of nucleosome-dependent gene expression and silencing in yeast. Nature 1999, 402, 418–421. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, K.; Xia, Z.; Chavez, M.; Pal, S.; Seol, J.H.; Chen, C.C.; Li, W.; Tyler, J.K. Nucleosome loss leads to global transcriptional up-regulation and genomic instability during yeast aging. Genes. Dev. 2014, 28, 396–408. [Google Scholar] [CrossRef]
- Ni, Z.; Ebata, A.; Alipanahiramandi, E.; Lee, S.S. Two SET domain containing genes link epigenetic changes and aging in Caenorhabditis elegans. Aging Cell 2012, 11, 315–325. [Google Scholar] [CrossRef]
- Dang, W.; Steffen, K.K.; Perry, R.; Dorsey, J.A.; Johnson, F.B.; Shilatifard, A.; Kaeberlein, M.; Kennedy, B.K.; Berger, S.L. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature 2009, 459, 802–807. [Google Scholar] [CrossRef]
- Feser, J.; Truong, D.; Das, C.; Carson, J.J.; Kieft, J.; Harkness, T.; Tyler, J.K. Elevated histone expression promotes life span extension. Mol. Cell 2010, 39, 724–735. [Google Scholar] [CrossRef]
- Celona, B.; Weiner, A.; Di Felice, F.; Mancuso, F.M.; Cesarini, E.; Rossi, R.L.; Gregory, L.; Baban, D.; Rossetti, G.; Grianti, P.; et al. Substantial histone reduction modulates genomewide nucleosomal occupancy and global transcriptional output. PLoS Biol. 2011, 9, e1001086. [Google Scholar] [CrossRef] [PubMed]
- Platt, J.M.; Ryvkin, P.; Wanat, J.J.; Donahue, G.; Ricketts, M.D.; Barrett, S.P.; Waters, H.J.; Song, S.; Chavez, A.; Abdallah, K.O.; et al. Rap1 relocalization contributes to the chromatin-mediated gene expression profile and pace of cell senescence. Genes Dev. 2013, 27, 1406–1420. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Shadel, G.S.; Kaeberlein, M.; Kennedy, B. Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab. 2012, 16, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Janssens, G.E.; Veenhoff, L.M. Evidence for the hallmarks of human aging in replicatively aging yeast. Microb. Cell 2016, 3, 263–274. [Google Scholar] [CrossRef]
- Denoth Lippuner, A.; Julou, T.; Barral, Y. Budding yeast as a model organism to study the effects of age. FEMS Microbiol. Rev. 2014, 38, 300–325. [Google Scholar] [CrossRef]
- Mitsui, Y.; Sakagami, H.; Murota, S.; Yamada, M. Age-related decline in histone H1 fraction in human diploid fibroblast cultures. Exp. Cell Res. 1980, 126, 289–298. [Google Scholar] [CrossRef]
- Houde, M.; Shmookler Reis, R.J.; Goldstein, S. Proportions of H1 histone subspecies in human fibroblasts shift during density-dependent growth arrest independent of replicative senescence. Exp. Cell Res. 1989, 184, 256–261. [Google Scholar] [CrossRef]
- Ishimi, Y.; Kojima, M.; Takeuchi, F.; Miyamoto, T.; Yamada, M.; Hanaoka, F. Changes in chromatin structure during aging of human skin fibroblasts. Exp. Cell Res. 1987, 169, 458–467. [Google Scholar] [CrossRef]
- Macieira-Coelho, A. Chromatin reorganization during senescence of proliferating cells. Mutat. Res. 1991, 256, 81–104. [Google Scholar] [CrossRef]
- O’Sullivan, R.J.; Kubicek, S.; Schreiber, S.L.; Karlseder, J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat. Struct. Mol. Biol. 2010, 17, 1218–1225. [Google Scholar] [CrossRef]
- Kim, C.; Jin, J.; Ye, Z.; Jadhav, R.R.; Gustafson, C.E.; Hu, B.; Cao, W.; Tian, L.; Weyand, C.M.; Goronzy, J.J. Histone deficiency and accelerated replication stress in T cell aging. J. Clin. Investig. 2021, 131, e143632. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Dubey, R.; Jung, K.S.; Prajapati, S.; Tian, W.; Kleinman, M.E. Age-related global histone loss and altered histone acetylation in retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2023, 64, 4444. [Google Scholar]
- Zhu, X.; Chen, Z.; Shen, W.; Huang, G.; Sedivy, J.M.; Wang, H.; Ju, Z. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: The regulation and intervention. Signal Transduct. Target. Ther. 2021, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Schmeer, C.; Kretz, A.; Wengerodt, D.; Stojiljkovic, M.; Witte, O.W. Dissecting Aging and Senescence-Current Concepts and Open Lessons. Cells 2019, 8, 1446. [Google Scholar] [CrossRef] [PubMed]
- Kandhaya-Pillai, R.; Miro-Mur, F.; Alijotas-Reig, J.; Tchkonia, T.; Schwartz, S.; Kirkland, J.L.; Oshima, J. Key elements of cellular senescence involve transcriptional repression of mitotic and DNA repair genes through the p53-p16/RB-E2F-DREAM complex. Aging 2023, 15, 4012–4034. [Google Scholar] [CrossRef]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 2022, 22, 340–355. [Google Scholar] [CrossRef]
- Domen, A.; Deben, C.; Verswyvel, J.; Flieswasser, T.; Prenen, H.; Peeters, M.; Lardon, F.; Wouters, A. Cellular senescence in cancer: Clinical detection and prognostic implications. J. Exp. Clin. Cancer Res. 2022, 41, 360. [Google Scholar] [CrossRef]
- Ivanov, A.; Pawlikowski, J.; Manoharan, I.; van Tuyn, J.; Nelson, D.M.; Rai, T.S.; Shah, P.P.; Hewitt, G.; Korolchuk, V.I.; Passos, J.F.; et al. Lysosome-mediated processing of chromatin in senescence. J. Cell Biol. 2013, 202, 129–143. [Google Scholar] [CrossRef]
- Amatori, S.; Tavolaro, S.; Gambardella, S.; Fanelli, M. The dark side of histones: Genomic organization and role of oncohistones in cancer. Clin. Epigenetics 2021, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Gautrey, H.E.; van Otterdijk, S.D.; Cordell, H.J.; Newcastle 85+ Study Core, T.; Mathers, J.C.; Strathdee, G. DNA methylation abnormalities at gene promoters are extensive and variable in the elderly and phenocopy cancer cells. FASEB J. 2014, 28, 3261–3272. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.; Moore, I.K.; Fondufe-Mittendorf, Y.; Gossett, A.J.; Tillo, D.; Field, Y.; LeProust, E.M.; Hughes, T.R.; Lieb, J.D.; Widom, J.; et al. The DNA-encoded nucleosome organization of a eukaryotic genome. Nature 2009, 458, 362–366. [Google Scholar] [CrossRef]
- Bochkis, I.M.; Przybylski, D.; Chen, J.; Regev, A. Changes in nucleosome occupancy associated with metabolic alterations in aged mammalian liver. Cell Rep. 2014, 9, 996–1006. [Google Scholar] [CrossRef]
- Wheaton, K.; Riabowol, K. Protein kinase C delta blocks immediate-early gene expression in senescent cells by inactivating serum response factor. Mol. Cell Biol. 2004, 24, 7298–7311. [Google Scholar] [CrossRef]
- Ding, W.; Gao, S.; Scott, R.E. Senescence represses the nuclear localization of the serum response factor and differentiation regulates its nuclear localization with lineage specificity. J. Cell Sci. 2001, 114, 1011–1018. [Google Scholar] [CrossRef]
- Chen, Y.; Bravo, J.I.; Son, J.M.; Lee, C.; Benayoun, B.A. Remodeling of the H3 nucleosomal landscape during mouse aging. Transl. Med. Aging 2020, 4, 22–31. [Google Scholar] [CrossRef]
- Funayama, R.; Saito, M.; Tanobe, H.; Ishikawa, F. Loss of linker histone H1 in cellular senescence. J. Cell Biol. 2006, 175, 869–880. [Google Scholar] [CrossRef]
- Ucar, D.; Marquez, E.J.; Chung, C.H.; Marches, R.; Rossi, R.J.; Uyar, A.; Wu, T.C.; George, J.; Stitzel, M.L.; Palucka, A.K.; et al. The chromatin accessibility signature of human immune aging stems from CD8+ T cells. J. Exp. Med. 2017, 214, 3123–3144. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Li, H.; Liu, J.; Hu, Q.; Zhang, S.; Zhang, N.; Liu, L.; Dai, Y.; Cao, D.; Li, X.; et al. Arginine hypomethylation-mediated proteasomal degradation of histone H4-an early biomarker of cellular senescence. Cell Death Differ. 2020, 27, 2697–2709. [Google Scholar] [CrossRef] [PubMed]
- Hauer, M.H.; Seeber, A.; Singh, V.; Thierry, R.; Sack, R.; Amitai, A.; Kryzhanovska, M.; Eglinger, J.; Holcman, D.; Owen-Hughes, T.; et al. Histone degradation in response to DNA damage enhances chromatin dynamics and recombination rates. Nat. Struct. Mol. Biol. 2017, 24, 99–107. [Google Scholar] [CrossRef]
- de Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Bar, C.; Blasco, M.A. Telomeres and telomerase as therapeutic targets to prevent and treat age-related diseases. F1000Research 2016, 5, F1000 Faculty Rev-89. [Google Scholar] [CrossRef]
- Gottschling, D.E.; Aparicio, O.M.; Billington, B.L.; Zakian, V.A. Position effect at S. cerevisiae telomeres: Reversible repression of Pol II transcription. Cell 1990, 63, 751–762. [Google Scholar] [CrossRef]
- Andrulis, E.D.; Neiman, A.M.; Zappulla, D.C.; Sternglanz, R. Perinuclear localization of chromatin facilitates transcriptional silencing. Nature 1998, 394, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Galy, V.; Olivo-Marin, J.C.; Scherthan, H.; Doye, V.; Rascalou, N.; Nehrbass, U. Nuclear pore complexes in the organization of silent telomeric chromatin. Nature 2000, 403, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Weierich, C.; Brero, A.; Stein, S.; von Hase, J.; Cremer, C.; Cremer, T.; Solovei, I. Three-dimensional arrangements of centromeres and telomeres in nuclei of human and murine lymphocytes. Chromosome Res. 2003, 11, 485–502. [Google Scholar] [CrossRef]
- Raz, V.; Vermolen, B.J.; Garini, Y.; Onderwater, J.J.; Mommaas-Kienhuis, M.A.; Koster, A.J.; Young, I.T.; Tanke, H.; Dirks, R.W. The nuclear lamina promotes telomere aggregation and centromere peripheral localization during senescence of human mesenchymal stem cells. J. Cell Sci. 2008, 121, 4018–4028. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Narita, M.; Krizhanovsky, V.; Nunez, S.; Chicas, A.; Hearn, S.A.; Myers, M.P.; Lowe, S.W. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell 2006, 126, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Nunez, S.; Heard, E.; Narita, M.; Lin, A.W.; Hearn, S.A.; Spector, D.L.; Hannon, G.J.; Lowe, S.W. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 2003, 113, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Kreiling, J.A.; Tamamori-Adachi, M.; Sexton, A.N.; Jeyapalan, J.C.; Munoz-Najar, U.; Peterson, A.L.; Manivannan, J.; Rogers, E.S.; Pchelintsev, N.A.; Adams, P.D.; et al. Age-associated increase in heterochromatic marks in murine and primate tissues. Aging Cell 2011, 10, 292–304. [Google Scholar] [CrossRef]
- Zhang, R.; Poustovoitov, M.V.; Ye, X.; Santos, H.A.; Chen, W.; Daganzo, S.M.; Erzberger, J.P.; Serebriiskii, I.G.; Canutescu, A.A.; Dunbrack, R.L.; et al. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev. Cell 2005, 8, 19–30. [Google Scholar] [CrossRef]
- Kozak, M.L.; Chavez, A.; Dang, W.; Berger, S.L.; Ashok, A.; Guo, X.; Johnson, F.B. Inactivation of the Sas2 histone acetyltransferase delays senescence driven by telomere dysfunction. EMBO J. 2010, 29, 158–170. [Google Scholar] [CrossRef]
- Smeal, T.; Claus, J.; Kennedy, B.; Cole, F.; Guarente, L. Loss of transcriptional silencing causes sterility in old mother cells of S. cerevisiae. Cell 1996, 84, 633–642. [Google Scholar] [CrossRef]
- Harley, C.B. Telomere loss: Mitotic clock or genetic time bomb? Mutat. Res. 1991, 256, 271–282. [Google Scholar] [CrossRef]
- Munro, J.; Steeghs, K.; Morrison, V.; Ireland, H.; Parkinson, E.K. Human fibroblast replicative senescence can occur in the absence of extensive cell division and short telomeres. Oncogene 2001, 20, 3541–3552. [Google Scholar] [CrossRef] [PubMed]
- Marthandan, S.; Priebe, S.; Hemmerich, P.; Klement, K.; Diekmann, S. Long-term quiescent fibroblast cells transit into senescence. PLoS ONE 2014, 9, e115597. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; Gutarra, S.; Garcia-Prat, L.; Rodriguez-Ubreva, J.; Ortet, L.; Ruiz-Bonilla, V.; Jardi, M.; Ballestar, E.; Gonzalez, S.; Serrano, A.L.; et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 2014, 506, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Tumpel, S.; Rudolph, K.L. Quiescence: Good and Bad of Stem Cell Aging. Trends Cell Biol. 2019, 29, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Wang, T.; Lin, X.W.; Denlinger, D.L.; Xu, W.H. Reactive oxygen species extend insect life span using components of the insulin-signaling pathway. Proc. Natl. Acad. Sci. USA 2017, 114, E7832–E7840. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Lee, E.; Lee, J.; Oh, S.; Kim, S. Down-regulation of asymmetric arginine methylation during replicative and H2O2-induced premature senescence in WI-38 human diploid fibroblasts. J. Biochem. 2008, 144, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.X.; Ma, S.; Han, X.; Luo, Z.Y.; Zhu, Q.Q.; Chiba, T.; Xie, W.; Lin, K.; Qiu, X.B. Proteasome activator PA200 maintains stability of histone marks during transcription and aging. Theranostics 2021, 11, 1458–1472. [Google Scholar] [CrossRef]
- Yi, S.J.; Kim, K. New Insights into the Role of Histone Changes in Aging. Int. J. Mol. Sci. 2020, 21, 8241. [Google Scholar] [CrossRef]
- Maze, I.; Noh, K.M.; Soshnev, A.A.; Allis, C.D. Every amino acid matters: Essential contributions of histone variants to mammalian development and disease. Nat. Rev. Genet. 2014, 15, 259–271. [Google Scholar] [CrossRef]
- Akiyama, T.; Suzuki, O.; Matsuda, J.; Aoki, F. Dynamic replacement of histone H3 variants reprograms epigenetic marks in early mouse embryos. PLoS Genet. 2011, 7, e1002279. [Google Scholar] [CrossRef] [PubMed]
- Santenard, A.; Ziegler-Birling, C.; Koch, M.; Tora, L.; Bannister, A.J.; Torres-Padilla, M.E. Heterochromatin formation in the mouse embryo requires critical residues of the histone variant H3.3. Nat. Cell Biol. 2010, 12, 853–862. [Google Scholar] [CrossRef]
- Pina, B.; Suau, P. Changes in histones H2A and H3 variant composition in differentiating and mature rat brain cortical neurons. Dev. Biol. 1987, 123, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.W.; Shibata, Y.; Starmer, J.; Yee, D.; Magnuson, T. Histone H3.3 maintains genome integrity during mammalian development. Genes Dev. 2015, 29, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Nashun, B.; Hill, P.W.; Smallwood, S.A.; Dharmalingam, G.; Amouroux, R.; Clark, S.J.; Sharma, V.; Ndjetehe, E.; Pelczar, P.; Festenstein, R.J.; et al. Continuous Histone Replacement by Hira Is Essential for Normal Transcriptional Regulation and De Novo DNA Methylation during Mouse Oogenesis. Mol. Cell 2015, 60, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Szenker, E.; Lacoste, N.; Almouzni, G. A developmental requirement for HIRA-dependent H3.3 deposition revealed at gastrulation in Xenopus. Cell Rep. 2012, 1, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, M.D.; Frederick, B.; Hoff, H.; Tang, Y.; Schultz, D.C.; Singh Rai, T.; Grazia Vizioli, M.; Adams, P.D.; Marmorstein, R. Ubinuclein-1 confers histone H3.3-specific-binding by the HIRA histone chaperone complex. Nat. Commun. 2015, 6, 7711. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.W.; Elsaesser, S.J.; Noh, K.M.; Stadler, S.C.; Allis, C.D. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc. Natl. Acad. Sci. USA 2010, 107, 14075–14080. [Google Scholar] [CrossRef]
- Elsaesser, S.J.; Allis, C.D. HIRA and Daxx constitute two independent histone H3.3-containing predeposition complexes. Cold Spring Harb. Symp. Quant. Biol. 2010, 75, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ray-Gallet, D.; Woolfe, A.; Vassias, I.; Pellentz, C.; Lacoste, N.; Puri, A.; Schultz, D.C.; Pchelintsev, N.A.; Adams, P.D.; Jansen, L.E.; et al. Dynamics of histone H3 deposition in vivo reveal a nucleosome gap-filling mechanism for H3.3 to maintain chromatin integrity. Mol. Cell 2011, 44, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Maze, I.; Wenderski, W.; Noh, K.M.; Bagot, R.C.; Tzavaras, N.; Purushothaman, I.; Elsasser, S.J.; Guo, Y.; Ionete, C.; Hurd, Y.L.; et al. Critical Role of Histone Turnover in Neuronal Transcription and Plasticity. Neuron 2015, 87, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Tvardovskiy, A.; Schwammle, V.; Kempf, S.J.; Rogowska-Wrzesinska, A.; Jensen, O.N. Accumulation of histone variant H3.3 with age is associated with profound changes in the histone methylation landscape. Nucleic Acids Res. 2017, 45, 9272–9289. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Occean, J.R.; Melters, D.P.; Shi, C.; Wang, L.; Stransky, S.; Doyle, M.E.; Cui, C.Y.; Delannoy, M.; Fan, J.; et al. A hyper-quiescent chromatin state formed during aging is reversed by regeneration. Mol. Cell 2023, 83, 1659–1676. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.F.; Young, A.R.; Wang, Z.; Wu, H.A.; Panda, T.; Kou, Y.; Kapoor, A.; Hasson, D.; Mills, N.R.; Ma’ayan, A.; et al. Histone H3.3 and its proteolytically processed form drive a cellular senescence programme. Nat. Commun. 2014, 5, 5210. [Google Scholar] [CrossRef]
- Oberdoerffer, P.; Miller, K.M. Histone H2A variants: Diversifying chromatin to ensure genome integrity. Semin. Cell Dev. Biol. 2023, 135, 59–72. [Google Scholar] [CrossRef]
- Stefanelli, G.; Azam, A.B.; Walters, B.J.; Brimble, M.A.; Gettens, C.P.; Bouchard-Cannon, P.; Cheng, H.M.; Davidoff, A.M.; Narkaj, K.; Day, J.J.; et al. Learning and Age-Related Changes in Genome-wide H2A.Z Binding in the Mouse Hippocampus. Cell Rep. 2018, 22, 1124–1131. [Google Scholar] [CrossRef]
- Chen, H.; Ruiz, P.D.; McKimpson, W.M.; Novikov, L.; Kitsis, R.N.; Gamble, M.J. MacroH2A1 and ATM Play Opposing Roles in Paracrine Senescence and the Senescence-Associated Secretory Phenotype. Mol. Cell 2015, 59, 719–731. [Google Scholar] [CrossRef]
- Rodier, F.; Coppe, J.P.; Patil, C.K.; Hoeijmakers, W.A.; Munoz, D.P.; Raza, S.R.; Freund, A.; Campeau, E.; Davalos, A.R.; Campisi, J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009, 11, 973–979. [Google Scholar] [CrossRef]
- Sedelnikova, O.A.; Horikawa, I.; Zimonjic, D.B.; Popescu, N.C.; Bonner, W.M.; Barrett, J.C. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell Biol. 2004, 6, 168–170. [Google Scholar] [CrossRef]
- Contrepois, K.; Mann, C.; Fenaille, F. H2B Type 1-K Accumulates in Senescent Fibroblasts with Persistent DNA Damage along with Methylated and Phosphorylated Forms of HMGA1. Proteomes 2021, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Isermann, A.; Mann, C.; Rube, C.E. Histone Variant H2A.J Marks Persistent DNA Damage and Triggers the Secretory Phenotype in Radiation-Induced Senescence. Int. J. Mol. Sci. 2020, 21, 9130. [Google Scholar] [CrossRef] [PubMed]
- Contrepois, K.; Coudereau, C.; Benayoun, B.A.; Schuler, N.; Roux, P.F.; Bischof, O.; Courbeyrette, R.; Carvalho, C.; Thuret, J.Y.; Ma, Z.; et al. Histone variant H2A.J accumulates in senescent cells and promotes inflammatory gene expression. Nat. Commun. 2017, 8, 14995. [Google Scholar] [CrossRef] [PubMed]
- Rube, C.E.; Baumert, C.; Schuler, N.; Isermann, A.; Schmal, Z.; Glanemann, M.; Mann, C.; Scherthan, H. Human skin aging is associated with increased expression of the histone variant H2A.J in the epidermis. NPJ Aging Mech. Dis. 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Happel, N.; Doenecke, D. Histone H1 and its isoforms: Contribution to chromatin structure and function. Gene 2009, 431, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ponte, I.; Romero, D.; Yero, D.; Suau, P.; Roque, A. Complex Evolutionary History of the Mammalian Histone H1.1-H1.5 Gene Family. Mol. Biol. Evol. 2017, 34, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Sekeri-Pataryas, K.E.; Sourlingas, T.G. The differentiation-associated linker histone, H1.0, during the in vitro aging and senescence of human diploid fibroblasts. Ann. N. Y. Acad. Sci. 2007, 1100, 361–367. [Google Scholar] [CrossRef]
- Nishibuchi, I.; Suzuki, H.; Kinomura, A.; Sun, J.; Liu, N.A.; Horikoshi, Y.; Shima, H.; Kusakabe, M.; Harata, M.; Fukagawa, T.; et al. Reorganization of damaged chromatin by the exchange of histone variant H2A.Z-2. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 736–744. [Google Scholar] [CrossRef]
- Faast, R.; Thonglairoam, V.; Schulz, T.C.; Beall, J.; Wells, J.R.; Taylor, H.; Matthaei, K.; Rathjen, P.D.; Tremethick, D.J.; Lyons, I. Histone variant H2A.Z is required for early mammalian development. Curr. Biol. 2001, 11, 1183–1187. [Google Scholar] [CrossRef]
- van Daal, A.; Elgin, S.C. A histone variant, H2AvD, is essential in Drosophila melanogaster. Mol. Biol. Cell 1992, 3, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.D.; Gorovsky, M.A. Histone H2A.Z has a conserved function that is distinct from that of the major H2A sequence variants. Nucleic Acids Res. 2000, 28, 3811–3816. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, B.; Gorovsky, M.A. Essential and nonessential histone H2A variants in Tetrahymena thermophila. Mol. Cell Biol. 1996, 16, 4305–4311. [Google Scholar] [CrossRef] [PubMed]
- Rai, T.S.; Cole, J.J.; Nelson, D.M.; Dikovskaya, D.; Faller, W.J.; Vizioli, M.G.; Hewitt, R.N.; Anannya, O.; McBryan, T.; Manoharan, I.; et al. HIRA orchestrates a dynamic chromatin landscape in senescence and is required for suppression of neoplasia. Genes Dev. 2014, 28, 2712–2725. [Google Scholar] [CrossRef] [PubMed]
- Talbert, P.B.; Henikoff, S. Histone variants--ancient wrap artists of the epigenome. Nat. Rev. Mol. Cell Biol. 2010, 11, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Bonisch, C.; Hake, S.B. Histone H2A variants in nucleosomes and chromatin: More or less stable? Nucleic Acids Res. 2012, 40, 10719–10741. [Google Scholar] [CrossRef]
- Seal, R.L.; Denny, P.; Bruford, E.A.; Gribkova, A.K.; Landsman, D.; Marzluff, W.F.; McAndrews, M.; Panchenko, A.R.; Shaytan, A.K.; Talbert, P.B. A standardized nomenclature for mammalian histone genes. Epigenetics Chromatin 2022, 15, 34. [Google Scholar] [CrossRef]
- Osley, M.A. The regulation of histone synthesis in the cell cycle. Annu. Rev. Biochem. 1991, 60, 827–861. [Google Scholar] [CrossRef]
- Marzluff, W.F.; Wagner, E.J.; Duronio, R.J. Metabolism and regulation of canonical histone mRNAs: Life without a poly(A) tail. Nat. Rev. Genet. 2008, 9, 843–854. [Google Scholar] [CrossRef]
- Gunesdogan, U.; Jackle, H.; Herzig, A. Histone supply regulates S phase timing and cell cycle progression. eLife 2014, 3, e02443. [Google Scholar] [CrossRef]
- Armstrong, C.; Spencer, S.L. Replication-dependent histone biosynthesis is coupled to cell-cycle commitment. Proc. Natl. Acad. Sci. USA 2021, 118, e2100178118. [Google Scholar] [CrossRef] [PubMed]
- Hogan, A.K.; Foltz, D.R. Reduce, Retain, Recycle: Mechanisms for Promoting Histone Protein Degradation versus Stability and Retention. Mol. Cell Biol. 2021, 41, e0000721. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Huang, J.; Chen, W.; Tang, J.; Xu, C.; Yu, Q.; Cheng, Y.; Ma, L.; Yu, X.; Li, S. Regulation of DNA replication-coupled histone gene expression. Oncotarget 2017, 8, 95005–95022. [Google Scholar] [CrossRef] [PubMed]
- Marzluff, W.F.; Koreski, K.P. Birth and Death of Histone mRNAs. Trends Genet. 2017, 33, 745–759. [Google Scholar] [CrossRef]
- Ewen, M.E. Where the cell cycle and histones meet. Genes Dev. 2000, 14, 2265–2270. [Google Scholar] [CrossRef]
- Armstrong, C.; Passanisi, V.J.; Ashraf, H.M.; Spencer, S.L. Cyclin E/CDK2 and feedback from soluble histone protein regulate the S phase burst of histone biosynthesis. Cell Rep. 2023, 42, 112768. [Google Scholar] [CrossRef]
- Harris, M.E.; Bohni, R.; Schneiderman, M.H.; Ramamurthy, L.; Schumperli, D.; Marzluff, W.F. Regulation of histone mRNA in the unperturbed cell cycle: Evidence suggesting control at two posttranscriptional steps. Mol. Cell Biol. 1991, 11, 2416–2424. [Google Scholar] [CrossRef]
- Dominski, Z.; Zheng, L.X.; Sanchez, R.; Marzluff, W.F. Stem-loop binding protein facilitates 3′-end formation by stabilizing U7 snRNP binding to histone pre-mRNA. Mol. Cell Biol. 1999, 19, 3561–3570. [Google Scholar] [CrossRef]
- Sanchez, R.; Marzluff, W.F. The stem-loop binding protein is required for efficient translation of histone mRNA in vivo and in vitro. Mol. Cell Biol. 2002, 22, 7093–7104. [Google Scholar] [CrossRef] [PubMed]
- Peltz, S.W.; Ross, J. Autogenous regulation of histone mRNA decay by histone proteins in a cell-free system. Mol. Cell Biol. 1987, 7, 4345–4356. [Google Scholar] [CrossRef] [PubMed]
- Kaygun, H.; Marzluff, W.F. Translation termination is involved in histone mRNA degradation when DNA replication is inhibited. Mol. Cell Biol. 2005, 25, 6879–6888. [Google Scholar] [CrossRef]
- Bao, H.; Carraro, M.; Flury, V.; Liu, Y.; Luo, M.; Chen, L.; Groth, A.; Huang, H. NASP maintains histone H3-H4 homeostasis through two distinct H3 binding modes. Nucleic Acids Res. 2022, 50, 5349–5368. [Google Scholar] [CrossRef]
- Finn, R.M.; Browne, K.; Hodgson, K.C.; Ausio, J. sNASP, a histone H1-specific eukaryotic chaperone dimer that facilitates chromatin assembly. Biophys. J. 2008, 95, 1314–1325. [Google Scholar] [CrossRef]
- Mullen, T.E.; Marzluff, W.F. Degradation of histone mRNA requires oligouridylation followed by decapping and simultaneous degradation of the mRNA both 5′ to 3′ and 3′ to 5′. Genes. Dev. 2008, 22, 50–65. [Google Scholar] [CrossRef]
- Meaux, S.A.; Holmquist, C.E.; Marzluff, W.F. Role of oligouridylation in normal metabolism and regulated degradation of mammalian histone mRNAs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20180170. [Google Scholar] [CrossRef]
- Kim, Y.K.; Maquat, L.E. UPFront and center in RNA decay: UPF1 in nonsense-mediated mRNA decay and beyond. RNA 2019, 25, 407–422. [Google Scholar] [CrossRef]
- Choe, J.; Ahn, S.H.; Kim, Y.K. The mRNP remodeling mediated by UPF1 promotes rapid degradation of replication-dependent histone mRNA. Nucleic Acids Res. 2014, 42, 9334–9349. [Google Scholar] [CrossRef] [PubMed]
- Slevin, M.K.; Meaux, S.; Welch, J.D.; Bigler, R.; Miliani de Marval, P.L.; Su, W.; Rhoads, R.E.; Prins, J.F.; Marzluff, W.F. Deep sequencing shows multiple oligouridylations are required for 3′ to 5′ degradation of histone mRNAs on polyribosomes. Mol. Cell 2014, 53, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Hoefig, K.P.; Rath, N.; Heinz, G.A.; Wolf, C.; Dameris, J.; Schepers, A.; Kremmer, E.; Ansel, K.M.; Heissmeyer, V. Eri1 degrades the stem-loop of oligouridylated histone mRNAs to induce replication-dependent decay. Nat. Struct. Mol. Biol. 2013, 20, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Ha, M.; Chang, H.; Kwon, S.C.; Simanshu, D.K.; Patel, D.J.; Kim, V.N. Uridylation by TUT4 and TUT7 marks mRNA for degradation. Cell 2014, 159, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Garneau, N.L.; Wilusz, J.; Wilusz, C.J. The highways and byways of mRNA decay. Nat. Rev. Mol. Cell Biol. 2007, 8, 113–126. [Google Scholar] [CrossRef]
- Commerford, S.L.; Carsten, A.L.; Cronkite, E.P. Histone turnover within nonproliferating cells. Proc. Natl. Acad. Sci. USA 1982, 79, 1163–1165. [Google Scholar] [CrossRef]
- Pina, B.; Martinez, P.; Simon, L.; Suau, P. Differential kinetics of histone H1(0) accumulation in neuronal and glial cells from rat cerebral cortex during postnatal development. Biochem. Biophys. Res. Commun. 1984, 123, 697–702. [Google Scholar] [CrossRef]
- Shmueli, M.D.; Sheban, D.; Eisenberg-Lerner, A.; Merbl, Y. Histone degradation by the proteasome regulates chromatin and cellular plasticity. FEBS J. 2022, 289, 3304–3316. [Google Scholar] [CrossRef]
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef] [PubMed]
- Glickman, M.H.; Ciechanover, A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar] [CrossRef] [PubMed]
- Ciechanover, A.; Orian, A.; Schwartz, A.L. Ubiquitin-mediated proteolysis: Biological regulation via destruction. Bioessays 2000, 22, 442–451. [Google Scholar] [CrossRef]
- Komander, D.; Rape, M. The ubiquitin code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Chau, V.; Tobias, J.W.; Bachmair, A.; Marriott, D.; Ecker, D.J.; Gonda, D.K.; Varshavsky, A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science 1989, 243, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Thrower, J.S.; Hoffman, L.; Rechsteiner, M.; Pickart, C.M. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000, 19, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K. The proteasome: Overview of structure and functions. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 12–36. [Google Scholar] [CrossRef]
- Bard, J.A.M.; Goodall, E.A.; Greene, E.R.; Jonsson, E.; Dong, K.C.; Martin, A. Structure and Function of the 26S Proteasome. Annu. Rev. Biochem. 2018, 87, 697–724. [Google Scholar] [CrossRef]
- Arrieta, A.; Vondriska, T.M. Nucleosome proteostasis and histone turnover. Front. Mol. Biosci. 2022, 9, 990006. [Google Scholar] [CrossRef]
- de Poot, S.A.H.; Tian, G.; Finley, D. Meddling with Fate: The Proteasomal Deubiquitinating Enzymes. J. Mol. Biol. 2017, 429, 3525–3545. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.X.; Pang, Y.; Liu, C.H.; Haratake, K.; Du, B.Y.; Ji, D.Y.; Wang, G.F.; Zhu, Q.Q.; Song, W.; Yu, Y.; et al. Acetylation-mediated proteasomal degradation of core histones during DNA repair and spermatogenesis. Cell 2013, 153, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Hormazabal, J.; Saavedra, F.; Espinoza-Arratia, C.; Martinez, N.W.; Cruces, T.; Alfaro, I.E.; Loyola, A. Chaperone mediated autophagy contributes to the newly synthesized histones H3 and H4 quality control. Nucleic Acids Res. 2022, 50, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Tirgar, R.; Davies, J.P.; Plate, L.; Nordman, J.T. The histone chaperone NASP maintains H3-H4 reservoirs in the early Drosophila embryo. PLoS Genet. 2023, 19, e1010682. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Liang, D.; Gajjalaiahvari, U.R.; Kabbaj, M.H.; Paik, J.; Gunjan, A. Excess histone levels mediate cytotoxicity via multiple mechanisms. Cell Cycle 2010, 9, 4236–4244. [Google Scholar] [CrossRef] [PubMed]
- Maya Miles, D.; Penate, X.; Sanmartin Olmo, T.; Jourquin, F.; Munoz Centeno, M.C.; Mendoza, M.; Simon, M.N.; Chavez, S.; Geli, V. High levels of histones promote whole-genome-duplications and trigger a Swe1(WEE1)-dependent phosphorylation of Cdc28(CDK1). eLife 2018, 7, e35337. [Google Scholar] [CrossRef] [PubMed]
- Au, W.C.; Crisp, M.J.; DeLuca, S.Z.; Rando, O.J.; Basrai, M.A. Altered dosage and mislocalization of histone H3 and Cse4p lead to chromosome loss in Saccharomyces cerevisiae. Genetics 2008, 179, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.G.; Mellone, B.G.; Partridge, J.F.; Richardson, W.; Hamilton, G.L.; Allshire, R.C.; Pidoux, A.L. Plasticity of fission yeast CENP-A chromatin driven by relative levels of histone H3 and H4. PLoS Genet. 2007, 3, e121. [Google Scholar] [CrossRef]
- Yu, R.; Sun, L.; Sun, Y.; Han, X.; Qin, L.; Dang, W. Cellular response to moderate chromatin architectural defects promotes longevity. Sci. Adv. 2019, 5, eaav1165. [Google Scholar] [CrossRef]
- Meeks-Wagner, D.; Hartwell, L.H. Normal stoichiometry of histone dimer sets is necessary for high fidelity of mitotic chromosome transmission. Cell 1986, 44, 43–52. [Google Scholar] [CrossRef]
- Morillo-Huesca, M.; Maya, D.; Munoz-Centeno, M.C.; Singh, R.K.; Oreal, V.; Reddy, G.U.; Liang, D.; Geli, V.; Gunjan, A.; Chavez, S. FACT prevents the accumulation of free histones evicted from transcribed chromatin and a subsequent cell cycle delay in G1. PLoS Genet. 2010, 6, e1000964. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.M.; Stromme, C.B.; Huang, H.; Patel, D.J.; Groth, A. Histone chaperone networks shaping chromatin function. Nat. Rev. Mol. Cell Biol. 2017, 18, 141–158. [Google Scholar] [CrossRef]
- Ghule, P.N.; Xie, R.L.; Medina, R.; Colby, J.L.; Jones, S.N.; Lian, J.B.; Stein, J.L.; van Wijnen, A.J.; Stein, G.S. Fidelity of histone gene regulation is obligatory for genome replication and stability. Mol. Cell Biol. 2014, 34, 2650–2659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; McKillop-Smith, S.; Muller, B. The human histone gene expression regulator HBP/SLBP is required for histone and DNA synthesis, cell cycle progression and cell proliferation in mitotic cells. J. Cell Sci. 2004, 117, 6043–6051. [Google Scholar] [CrossRef] [PubMed]
- Barcaroli, D.; Bongiorno-Borbone, L.; Terrinoni, A.; Hofmann, T.G.; Rossi, M.; Knight, R.A.; Matera, A.G.; Melino, G.; De Laurenzi, V. FLASH is required for histone transcription and S-phase progression. Proc. Natl. Acad. Sci. USA 2006, 103, 14808–14812. [Google Scholar] [CrossRef]
- Yoo, Y.; Park, S.Y.; Jo, E.B.; Choi, M.; Lee, K.W.; Hong, D.; Lee, S.; Lee, C.R.; Lee, Y.; Um, J.Y.; et al. Overexpression of Replication-Dependent Histone Signifies a Subset of Dedifferentiated Liposarcoma with Increased Aggressiveness. Cancers 2021, 13, 3122. [Google Scholar] [CrossRef]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.H. Epigenetic regulation of aging: Implications for interventions of aging and diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef]
- Lu, Y.X.; Regan, J.C.; Esser, J.; Drews, L.F.; Weinseis, T.; Stinn, J.; Hahn, O.; Miller, R.A.; Gronke, S.; Partridge, L. A TORC1-histone axis regulates chromatin organisation and non-canonical induction of autophagy to ameliorate ageing. eLife 2021, 10, e62233. [Google Scholar] [CrossRef]
- Mahmassani, Z.S.; McKenzie, A.I.; Petrocelli, J.J.; de Hart, N.M.; Reidy, P.T.; Fix, D.K.; Ferrara, P.J.; Funai, K.; Drummond, M.J. Short-term metformin ingestion by healthy older adults improves myoblast function. Am. J. Physiol. Cell Physiol. 2021, 320, C566–C576. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.S.; Aleksic, S.; Berger, D.M.; Sierra, F.; Kuchel, G.A.; Barzilai, N. Geroscience-guided repurposing of FDA-approved drugs to target aging: A proposed process and prioritization. Aging Cell 2022, 21, e13596. [Google Scholar] [CrossRef] [PubMed]
- Elliehausen, C.J.; Anderson, R.M.; Diffee, G.M.; Rhoads, T.W.; Lamming, D.W.; Hornberger, T.A.; Konopka, A.R. Geroprotector drugs and exercise: Friends or foes on healthy longevity? BMC Biol. 2023, 21, 287. [Google Scholar] [CrossRef] [PubMed]
- Juricic, P.; Lu, Y.X.; Leech, T.; Drews, L.F.; Paulitz, J.; Lu, J.; Nespital, T.; Azami, S.; Regan, J.C.; Funk, E.; et al. Long-lasting geroprotection from brief rapamycin treatment in early adulthood by persistently increased intestinal autophagy. Nat. Aging 2022, 2, 824–836. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).