Abstract
The endocannabinoid system has been shown to be involved in various skin functions, such as melanogenesis and the maintenance of redox balance in skin cells exposed to UV radiation, as well as barrier functions, sebaceous gland activity, wound healing and the skin’s immune response. In addition to the potential use of cannabinoids in the treatment and prevention of skin cancer, cannabinoid compounds and derivatives are of interest as potential systemic and topical applications for the treatment of various inflammatory, fibrotic and pruritic skin conditions. In this context, cannabinoid compounds have been successfully tested as a therapeutic option for the treatment of androgenetic alopecia, atopic and seborrhoeic dermatitis, dermatomyositis, asteatotic and atopic eczema, uraemic pruritis, scalp psoriasis, systemic sclerosis and venous leg ulcers. This review provides an insight into the current literature on cannabinoid compounds as potential medicines for the treatment of skin diseases.
1. Introduction
Numerous studies have shown that the use of cannabinoids to treat skin diseases has potential benefits for patients. In a recent review paper, we pointed out the effects of cannabinoid substances on malignant changes of the skin such as melanoma and squamous cell carcinoma [1]. Many publications in recent years suggest that cannabinoids could also play a useful role for patients as systemic and topical applications in inflammatory, allergic, fibrotic and pruritic skin diseases as well as in skin care. For this reason, these effects could be of particular importance to dermatologists. The use of cannabinoids in the context of skin diseases has continued to gain attention with increasing commercial interest [2].
The prominent role of the endocannabinoid system in skin homeostasis was demonstrated in a comprehensive study in 2007, which found a strong influence of cannabinoid receptors on the pathogenesis of allergic contact dermatitis [3]. This pioneering study led to an avalanche of publications on the role of cannabinoid receptors in the skin, the regulation of cannabinoid-triggered receptors in pathophysiological conditions of the skin, and the possibility of testing cannabinoid-based drugs for numerous skin diseases. Meanwhile, the endocannabinoid system has been implicated in various physiological processes of the skin, such as melanogenesis, maintenance of redox balance in response to ultraviolet (UV) radiation, wound healing, barrier functions, control of immunological sensitivity, sebaceous gland functions and hair growth. Due to the multiple effects of cannabinoid-activated receptors in the skin, the cutaneous endocannabinoid system was appropriately termed the “c(ut)annabinoid” system in a recent comprehensive review of cannabinoid effects in the skin [4].
Skin conditions where the efficacy of different cannabinoids has been successfully tested in in vitro and in vivo models include atopic dermatitis [5], psoriasis [6,7] and dermatomyositis [8], an idiopathic inflammatory myopathy. In the latter report, the cannabinoid receptor 2 (CB2) agonist lenabasum (Figure 1), a 9-carbon 1,1-dimethylheptyl side-chain analogue of the tetrahydrocannabinol (THC) metabolite THC-11-oic acid (also known as JBT-101, ajulemic acid, formerly anabasum), was discovered as a potential treatment for dermatomyositis, which has since been designated as an orphan drug by the European Medicines Agency (EMA) [9]. Moreover, the same cannabinoid was granted orphan drug designation by the EMA for the treatment of systemic sclerosis (scleroderma) as a typical fibrotic disease [10]. Particularly in connection with research into the antifibrotic properties of cannabinoids, a trend in drug design is already emerging towards cannabinoid substances that, chemically modified, interact with other receptors in addition to the classic cannabinoid targets or are specially formulated. This is especially evident with the non-intoxicating phytocannabinoid cannabidiol (CBD; Figure 1) [11], the antifibrotic CBD aminoquinone VCE-004.8 (Figure 1), a dual receptor agonist at the CB2 receptor and peroxisome proliferation-activated receptor γ (PPARγ) [12,13], and EHP-101, a lipidic formulation of VCE-004.8 [14,15].
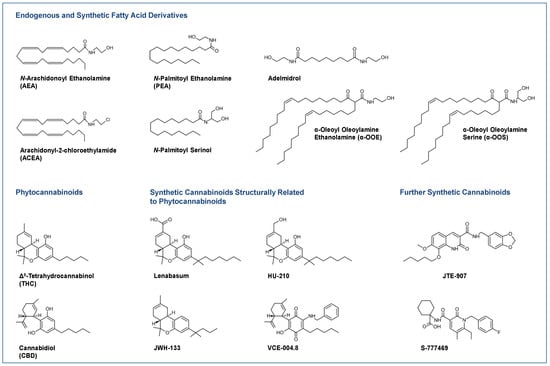
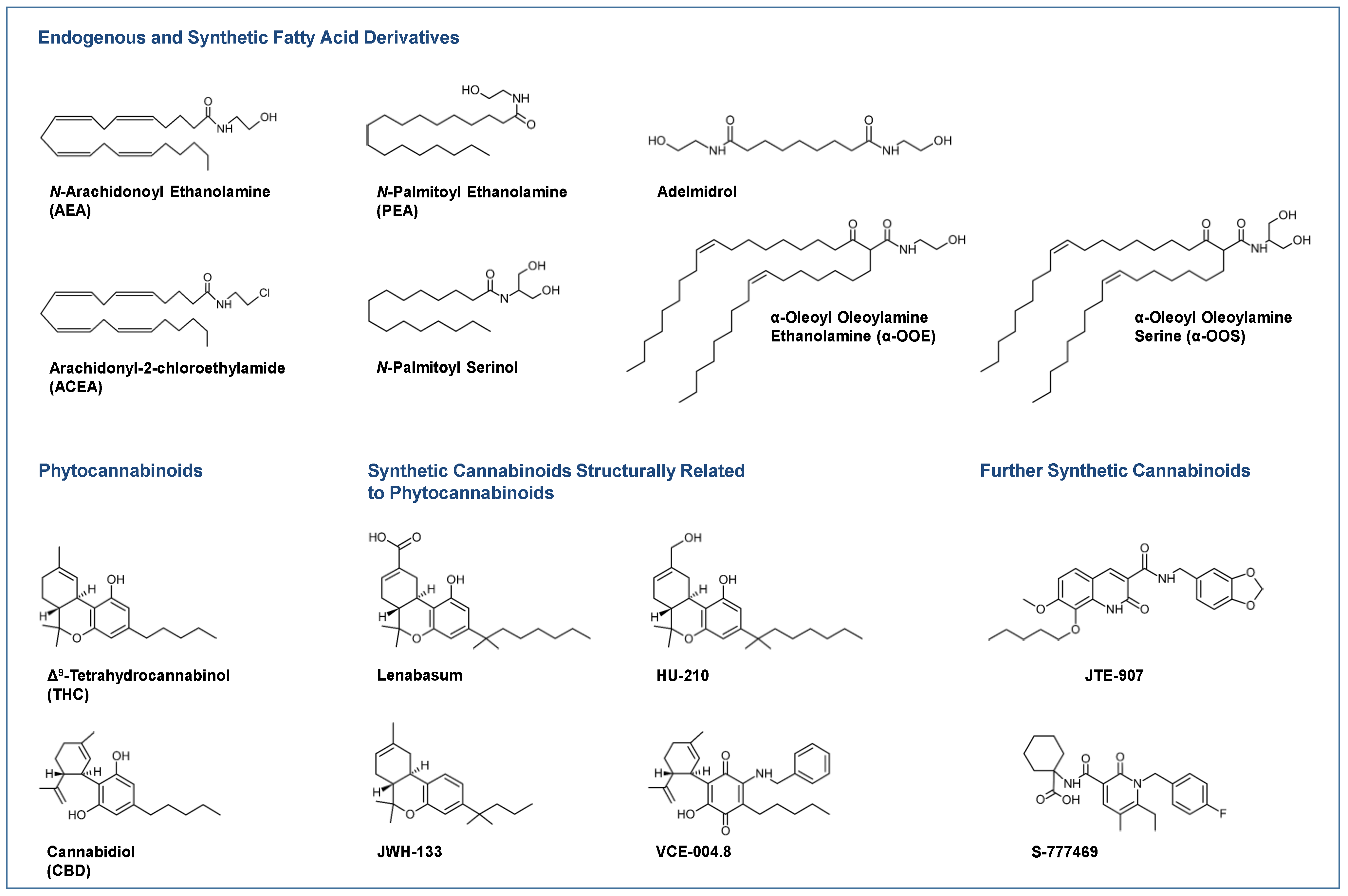
Figure 1.
Selected endocannabinoids and phytocannabinoids and their derivatives currently under investigation or in use for the treatment of skin diseases.
Most clinical studies have focused on the effects of CBD in various dosage forms and N-palmitoyl ethanolamine (PEA; Figure 1) for symptom relief in atopic dermatitis [16,17,18] and other diseases. For some diseases, there are only case reports on the potential efficacy of cannabinoids, such as in epidermolysis bullosa [19,20], pyoderma gangrenosum [21,22], pruritus due to cholestatic liver disease [23] and lichen simplex chronicus [24]. Figure 1 shows a collection of chemical formulae of the endocannabinoids, endocannabinoid-like substances and phytocannabinoids and their derivatives that are currently being studied or used for the treatment of skin diseases.
The aim of the present review is to summarise the currently available knowledge on the effect of cannabinoids on the pathogenesis of various selected skin diseases in the context of the respective existing pharmacotherapy. The presentations of the pharmacotherapeutic options currently used for the individual indications provide an insight into the drugs competing with cannabinoids and are intended to further identify potential combination partners in cannabinoid therapy that may be able to achieve synergistic effects in upcoming clinical trials. To this end, the components of the endocannabinoid system are first described in general terms, and then their distribution and function in the skin and their regulation under pathophysiological conditions are outlined. Finally, the individual preclinical and clinical results of the meanwhile numerous studies on the effects of cannabinoids in skin diseases are presented.
2. The Endocannabinoid System: A Brief Overview
The endocannabinoid system includes the endocannabinoid receptors, their endogenous agonists and the enzymes that synthesise and degrade the endocannabinoids.
2.1. Classic Cannabinoid Receptors
Long before the discovery of cannabinoid receptors, in the year 1978, Mechoulam and Carlini wrote “We believe that cannabinoids act on specific receptors.” [25]. However, that was more than a decade before the first cannabinoid receptor was actually cloned. Thus, the cannabinoid receptors, CB1 and CB2, a class of heptahelical pertussis toxin-sensitive Gi/o protein-coupled membrane receptors were discovered in the early 1990s [26,27]. Δ9-tetrahydrocannabinol (THC; Figure 1), the major psychoactive constituent of Cannabis sativa L. has been shown to act as a full agonist at the CB2 receptor and as a partial agonist at the CB1 receptor and can therefore be classified as a phytocannabinoid. In contrast, another major constituent of cannabis, the non-intoxicating CBD, exhibits a much weaker affinity for cannabinoid receptors [28] and has been reported to act as a negative allosteric modulator of the CB1 receptor [29]. The complex mechanisms of action and the underlying interference with various receptors on which CBD exerts its effects are discussed below.
2.2. Endocannabinoids
N-arachidonoyl ethanolamine (AEA, anandamide; Figure 1) and 2-arachidonoyl glycerol (2-AG) were the first arachidonic acid derivatives described as endogenous agonists at cannabinoid receptors [30,31]. Concerning receptor interaction modes, AEA was found to be a partial agonist at the CB1 receptor with an affinity comparable to THC [32,33] and a weak agonist at the CB2 receptor [34], whereas 2-AG exhibits full agonistic properties at the CB2 receptor [33,34].
As early as 1998, it was hypothesised that dopamine molecules are packaged and maintained as N-acyl dopamines in the dense nuclear vesicles of the chemoreceptor cells of the carotid body, with arachidonic acid as a possible cis-unsaturated fatty acid forming the acyl backbone [35]. From this group of initially still hypothetical endogenous substances, N-arachidonoyl dopamine (NADA) was synthesised in 2000 and investigated to what extent it shares with AEA the same transport and degradation mechanisms as well as properties for binding to cannabinoid receptors. Hereby, it was found that NADA does not bind to the corresponding dopamine receptors, but has a high affinity to the cannabinoid receptor CB1 [36]. Later, NADA was isolated from mammalian brain tissue, with the highest concentrations found in the striatum, hippocampus and cerebellum [37]. Around this time, 2-arachidonoyl glyceryl ether (2-AGE, noladin ether), a structural ether analogue of 2-AG with higher stability than 2-AG that preferentially binds to the CB1 receptor, was also discovered in porcine brain [38]. Another lipid of the endocannabinoid system is O-arachidonoyl ethanolamine (virodhamine), an ester derivative of arachidonic acid and ethanolamine, which has been detected in rat brain and human hippocampus and acts as an antagonist at the CB1 receptor and full agonist at the CB2 receptor [39]. Finally, it was recently discovered that pentadecanoylcarnitine, an endogenous metabolite of the essential fatty acid pentadecanoic acid found in bottlenose dolphin serum, is a fully potent CB1 and CB2 receptor agonist [40]. However, the significance of this endocannabinoid for the human body has not yet been clarified.
In addition, “endocannabinoid-like substances” such as PEA, oleoylethanolamide (OEA), stearoylethanolamide (SEA) and linoleoylethanolamide (LEA) lack binding affinity to cannabinoid receptors but have the highest concentrations in the human brain, with PEA being the most abundant (50%), followed by OEA (23. 6%) and SEA (13.9%), while AEA and 2-AG account for only 7.7% and 4.8%, respectively [41]. As recently reviewed, the mechanism of action of these fatty acids includes interference with receptors of the extended endocannabinoid system, and they have been found to share the corresponding synthesis and degradation enzymes with endocannabinoids (for review see [42]). According to some studies, PEA can enhance the effect of AEA in the sense of an “entourage effect” by down-regulating the expression and activity of the AEA-degrading enzyme fatty acid amide hydrolase (FAAH) [43] and causing a probably positive allosteric modulation of the AEA target transient receptor potential vanilloid 1 (TRPV1) [44]. Both endocannabinoid-degrading enzymes and other cannabinoid targets are discussed in more detail in the following chapters. As with PEA, the endocannabinoid-like compound SEA has been shown to enhance the effects of AEA by inhibiting FAAH [45]. A similar effect has also been described for 2-AG derivatives such as linoleoyl glycerol and palmitoyl glycerol, which likewise do not bind to cannabinoid receptors but act as entourage compounds for 2-AG by enhancing its effect at least partly by inhibiting inactivation of 2-AG [46].
2.3. Enzymes Involved in Biosynthesis and Degradation of Endocannabinoids
The enzymes responsible for the synthesis of endocannabinoids are, in the case of AEA, N-acylphosphatidylethanolamine-hydrolysing phospholipase D (NAPE-PLD) and, in the case of 2-AG, mainly phospholipase C or diacylglycerol lipases α and β (for review see [47]). The most important enzyme for the degradation of AEA is the already mentioned FAAH [48]. 2-AG is metabolised by two main pathways, namely via monoacylglycerol lipase (MAGL) or via the α/β-hydrolase domain (ABHD) containing enzymes ABHD6 and ABHD12 [49]. The endogenous hydrolysis and thus the biological inactivation of PEA is catalysed by the enzyme cysteine hydrolase N-acylethanolamine acid amidase (NAAA), which is mainly found in macrophages and B-lymphocytes [50,51].
2.4. Further Receptor Targets of Cannabinoids
Other receptor targets involved in cannabinoid action include ion channels of the transient receptor potential (TRP) family, such as TRPV1, which is activated, e.g., by AEA [52] and CBD [53]. Moreover, 2-AGE not only acts as a CB receptor agonist, but also has partial TRPV1 agonistic properties [38,54]. Furthermore, NADA has been described as a potent “endovanilloid”, i.e., an endogenous agonist at TRPV1 [37]. Likewise, the minor phytocannabinoids cannabigerol (CBG), cannabichromene (CBC), tetrahydrocannabivarin (THCV) and cannabigerolic acid (CBGA) were recently shown to stimulate TRPV1 [55]. TRPV2 was described as a target of CBD [56] and THC [57]. A number of phytocannabinoids were also tested for possible activation of TRPV3 and TRPV4. In these tests, CBD and THCV activated TRPV3-dependent Ca2+ currents, while cannabigerovarin (CBGV) and CBGA were able to sensitise TRPV3. Cannabidivarin (CBDV) and THCV stimulated TRPV4-mediated Ca2+ currents, while CBGA, CBGV, cannabinol (CBN) and CBG sensitised TRPV4 [58]. Finally, CBD and the minor phytocannabinoids CBC, CBN, CBDV, CBGV and THCV have been found to activate TRP channels of ankyrin type-1 (TRPA1) and the TRP channel of melastatin type-8 (TRPM8) [57].
A further target triggered by cannabinoid compounds is the G protein-coupled receptor 55 (GPR55), already discussed as a putative type 3 cannabinoid receptor [59], which can promote skin carcinogenesis [60]. GTPγS binding assays showed that GPR55 is coupled to Gα13 and mediates activation of the small G proteins ras homolog family member A (rhoA), cell division control protein 42 homolog (cdc42) and ras-related C3 botulinum toxin substrate 1 (rac1) [61]. In this pioneering study, GPR55 was found to be activated by AEA, 2-AG, 2-AGE, virodhamine, THC, abnormal CBD (abn-CBD; synthetic regioisomer of CBD), CP 55,940 (synthetic non-selective agonist with high affinity for CB1 and CB2 receptors) and PEA, but inhibited by CBD. The activating properties of THC and AEA were also confirmed in an investigation using the increase in intracellular calcium as an indicator of GPR55 activity [62]. Other cannabinoids with GPR55-activating properties here were the hydrolysis-stable AEA analogue R(+)-methanandamide and the aminoalkylindole JWH-015, which is known to be a specific CB2 agonist, while CP 55,940, 2-AG, PEA, virodhamine and abn-CBD did not significantly increase intracellular calcium levels [62]. Using a β-arrestin green fluorescent protein biosensor as readout of agonist-mediated receptor activation, a further study demonstrated that CP 55,940 acts as a GPR55 antagonist/partial agonist, while the CB1 receptor antagonists AM-251 and SR141716A (rimonabant) are GPR55 agonists [63]. As another G protein-coupled receptor, GPR119 was found to be activated by OEA [64].
Finally, there are several cannabinoids that have been shown to modulate GPR18, as summarised in a recent review [65]. In this context, it is interesting to note that it was already shown in 2006 that N-arachidonoyl glycine (NAGly; carboxylic metabolite of AEA) mediates effects such as an increase in calcium levels and a decrease in cAMP levels in GPR18-transfected cells compared to mock-transfected cells [66]. An GPR18-activating effect was later confirmed for N-palmitoyl glycine, an endogenous arachidonoyl amide, in the dorsal root ganglion cell line F11 [67]. In another report, NAGly was demonstrated to cause activation of mitogen-activated protein kinases (MAPK) p42/44 in GPR18-overexpressing HEK293 cells, as the most potent full agonist of a number of cannabinoids [68]. Other cannabinoids described as GPR18 full agonists in this study were abn-CBD, THC and AEA. The GPR18-mediated induction of MAPK activity was confirmed by other authors who focused on G-protein coupling of GPR18 and found that pertussis toxin completely blocked MAPK activity induced by abn-CBD and NAGly, but not by THC, demonstrating the involvement of multiple signal transduction pathways in GPR18 activation [69].
An important class of receptors triggered by cannabinoids is the group of peroxisome proliferator-activated receptors (PPAR), which are involved in skin inflammation, keratinocyte proliferation and differentiation, metabolism and oxidative stress response, as recently reviewed [70]. Of these, PPARα becomes activated by OEA [71] and PEA [72,73]. Furthermore, THC, although lacking binding affinity to PPARα, was found to upregulate and thereby enhance the transcriptional activity of PPARα [74]. Using luciferase reporter assays, another report demonstrated that PPARα transcriptional activity is increased by the non-selective cannabinoid receptor agonist WIN 55,212-2 as well as by AEA, OEA, virodhamine and 2-AGE [75]. PPARγ was found to be activated by THC [76] and CBD [77]. Further cannabinoids with PPARγ-activating properties include AEA, 2-AG and NADA, as well as the synthetic cannabinoids HU-210 (Figure 1), WIN 55,212-2, CP 55,940 (for review see [78]) and lenabasum [79]. Increased activity and intracellular concentration of PPARγ was also induced by THC and JWH-015 in another study [80]. Finally, cannabidiolic acid (CBDA) has been shown to cause abrogation of PPARβ/δ-related signalling [81] and THC has been found to cause PPARβ/δ-mediated suppression of PPARα function [82].
3. The Endocannabinoid System in the Skin
3.1. Distribution of Components of the Endocannabinoid System in the Skin
3.1.1. Classic Endocannabinoid System
The presence of the endocannabinoid system in skin tissue was demonstrated in the mid-1990s by the discovery of the endocannabinoid AEA in rat skin, where it was found in concentrations similar to those in rat brain [83]. Later, endocannabinoids were shown to cause tonic activation of local cannabinoid receptors in rat skin [84]. Among others, AEA and 2-AG were also found in cells derived from sweat glands [85] and in the hair follicle [86].
Classic cannabinoid receptors were detected in many cell types of the skin, such as nerve fibre bundels of the skin, mast cells, macrophages, epidermal keratinocytes, epithelial cells of hair follicles, sebocytes and myoepithelial cells of the eccrine sweat glands [87]. Regarding the differential distribution of cannabinoid receptors in human skin tissue, Ständer et al. reported, that the CB2 receptor is expressed in basal keratinocytes, whereas CB1 receptors are present in keratinocytes of the stratum spinosum and granulosum, and that both cannabinoid receptors are detectable in nerve fibres of the epidermis, in small unmyelinated subepidermal nerves and in large myelinated nerves of the dermis [87]. Other skin structures with detectable CB1 receptor expression include differentiated sebaceous gland cells and epithelial cells of the infundibulum and inner root sheath of hair follicles, while CB2 receptors were found in undifferentiated cells of these niches. It is worth noting, however, that in another report the CB2 receptor could not be detected in human hair follicles by immunohistochemistry and quantitative RT-PCR [86]. The authors of the latter report detected CB1 receptors in the epithelium of human scalp hair follicles mainly in the outer root sheath, but not in the fibroblasts of the dermal papilla of the hair follicle. In a further study, cannabinoid receptors were detected in the sebaceous gland epithelium of normal human scalp sections [88]. In addition, both cannabinoid receptors were found in cells derived from sweat glands [85].
The enzymes that synthesise and degrade endocannabinoids (NAPE-PLD, DAGL, FAAH and MAGL) were also detected in human sweat glands [85]. A similarly complete endowment of the endocannabinoid system has also been demonstrated in normal human epidermal melanocytes [89]. Remarkably, that there are currently no data on the expression of enzymes that synthesise and degrade endocannabinoids in the hair follicle, although endocannabinoids have been detected here [86].
3.1.2. Extended Endocannabinoid System
Other cannabinoid-triggered receptors that have been detected in skin tissue are ion channels of the TRP family. For example, TRPV4 was found in the secretory cells of the eccrine sweat glands [90]. In addition to the two cannabinoid receptors, human keratinocytes have also been shown to express TRPV1 [55]. Moreover, TRPV3 was detected in the hair follicles [91]. Finally, TRPV1–4 but not TRPM8 and TRPA1 were found in a human sweat gland epithelial cell line [85].
Among PPARs, PPARα was found to be predominantly expressed in the epithelial compartment in basal keratinocytes and hair papilla cells [92]. Others detected PPARα, δ and γ in epidermis and hair follicle [93,94,95] as well as in the sebaceous gland [96]. Human keratinocytes have also been shown to express PPARα/γ/δ receptors and GPR55 [55]. In addition, PPARγ is present in primary cultured human sebocytes [97] as well as in fibroblasts from human skin biopsies [98]. Finally, GPR119 was detected in the sebaceous gland [99].
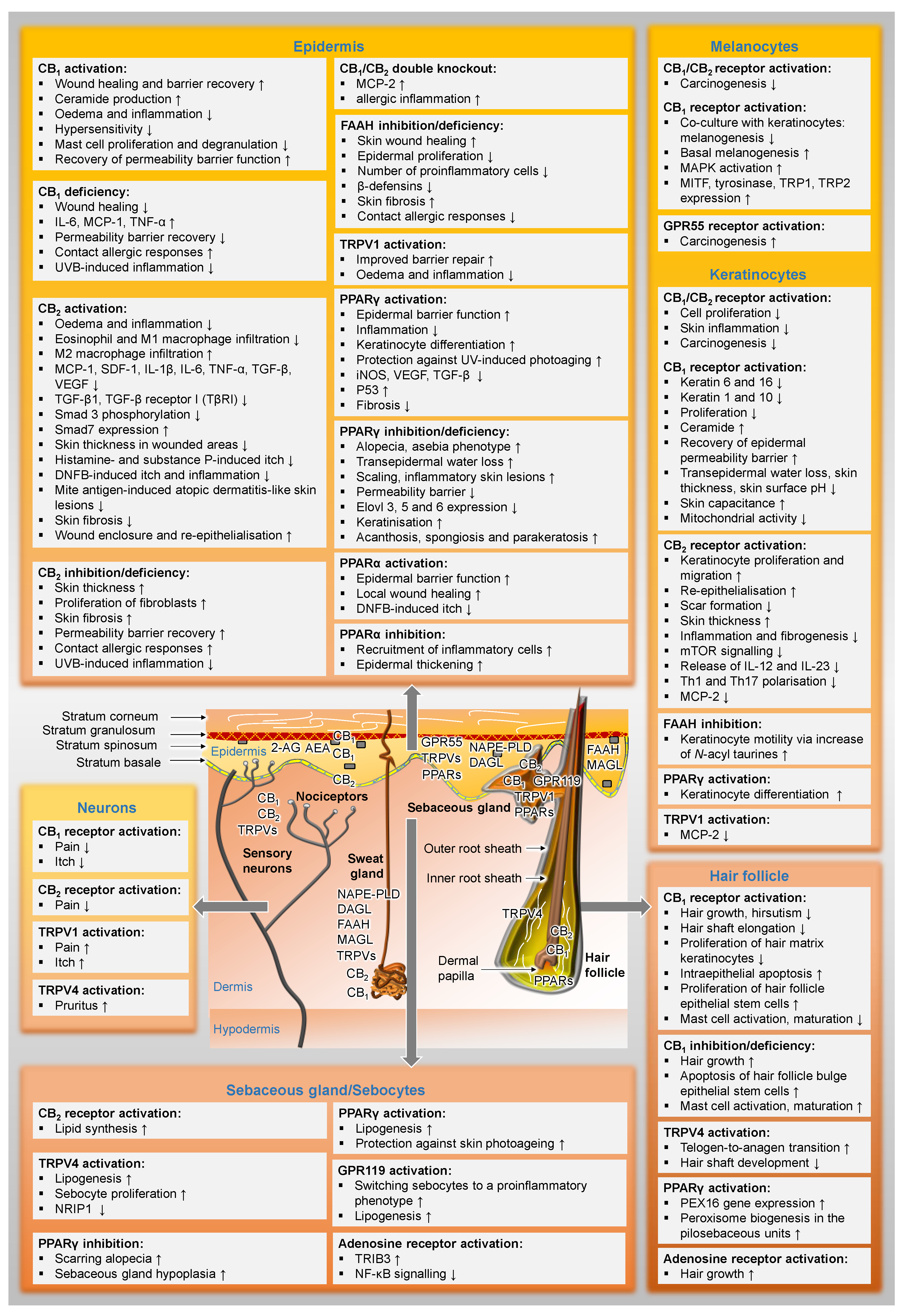
Figure 2 gives an overview of the distribution of cannabinoid-influenced receptors in the skin and already presents selected functional effects of these receptor activations or inhibitions in preparation for the following chapters.
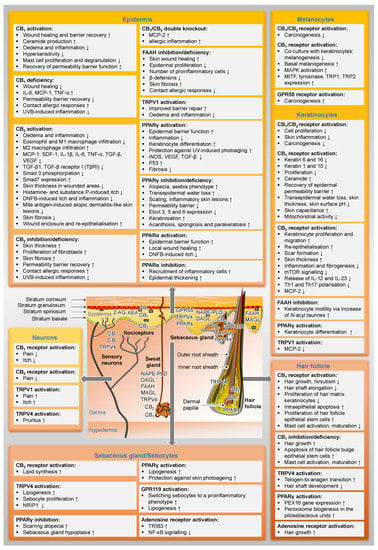
Figure 2.
Distribution of the elements of the endocannabinoid system in the skin and their functional effects. In the text boxes, the regulations proven in the literature have been indicated. The term deficiency always refers to the fact that results from experiments with knockout animals are included here. ↑, upregulated; ↓, downregulated; AEA, N-arachidonoyl ethanolamine (anandamide); 2-AG, 2-arachidonoyl glycerol; CB1, cannabinoid receptor 1; CB2, cannabinoid receptor 2; DAGL, diacylglycerol lipase; DNFB, 1-fluoro-2,4-dinitrobenzene; Elovl 3, 5 and 6, elongation of very-long-chain fatty acids-like group of enzymes; GPR, G protein-coupled receptor; FAAH, fatty acid amide hydrolase; IL, interleukin; iNOS, inducible nitric oxide synthase; MAGL, monoacylglycerol lipase; MAPK, mitogen-activated protein kinases; MCP, monocyte chemoattractant protein; MITF, microphthalmus-associated transcription factor; mTOR, mammalian target of rapamycin; NAPE-PLD, N-acylphosphatidylethanolamine-hydrolysing phospholipase D; NF-κB, nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells; NRIP1, nuclear receptor interacting protein-1; PEX16, peroxin 16; PPAR, peroxisome proliferator-activated receptor; SDF-1, stromal cell-derived factor-1; Smad, small mothers against decapentaplegic homolog; TGF-β, transforming growth factor-β; TNF-α, tumour necrosis factor-α; TRIB3, tribbles homolog 3; TRP, tyrosinase-related protein; TRPV, transient receptor potential vanilloid; UVB, ultraviolet B radiation; VEGF, vascular endothelial growth factor.
3.2. The Endocannabinoid System as a Regulator of Skin Homeostasis
3.2.1. Influence on Melanogenesis
As melanogenesis plays a crucial role in protecting the skin from UV radiation and oxidative stress, a recent study focused on cannabinoids as a protective pharmacotherapeutic option for the treatment of skin diseases caused by external factors. Here, CBD was found to stimulate melanogenesis via CB1 receptors by activating p38 and p42/44 MAPK and subsequently increasing the expression of microphthalmus-associated transcription factor (MITF), tyrosinase, tyrosinase-related protein 1 and 2 (TRP1, TRP2), all of which are involved in melanogenesis [100]. On the other hand, the CB1 agonist arachidonyl-2-chloroethylamide (ACEA; Figure 1) was shown to decrease melanogenesis in the melanoma cell line SK-Mel-1 co-cultured with the keratinocyte cell line HaCat under both basal and UVB irradiation stimulated conditions by activating the CB1 receptor [101]. An induction of melanin synthesis was also confirmed in primary human melanocytes treated with 1 µM of the cannabinoids R(+)-methanandamide, AEA, ACEA and 2-AG, while the CB2 agonist JWH133 did not increase melanogenesis [89]. Using siRNA transfections targeting CB1 and receptor antagonists, the latter work demonstrated that R(+)-methanandamide triggers melanogenesis via CB1 receptor activation and a p38- and p42/44-MAPK-mediated pathway involving tyrosinase expression via the master regulator MITF. In addition, a recent study examined the effects of THC and CBD on human epidermal melanocytes derived from light and dark pigmented cells. In light pigmented cells, both cannabinoids increased melanin synthesis and dendriticity, as evidenced by an increase in total dendrite length [102], a surrogate measure of melanosome secretion as a step in the melanogenesis cycle. However, in dark pigmented cells, none of the cannabinoids significantly altered dendriticity, although they retained their melanin synthesis-inducing effect, which in the case of THC was faintly inhibited by the CB1 receptor antagonist SR141716. Interestingly, the authors found an increased production of reactive oxygen species (ROS) by both cannabinoids in light pigmented cells, which was not observed in dark pigmented cells. These findings suggest that individuals with dark pigmented melanocytes may be protected from UV light-induced oxidative stress after treatment with THC and CBD, whereas in individuals with light pigmented skin, increased skin pigmentation is associated with higher oxidative stress when exposed to THC or CBD.
3.2.2. Influence on Wound Healing
An essential endogenous process in which the endocannabinoid system appears to play an important role is skin wound healing. In this context, numerous preclinical studies confirm the effects of cannabinoid receptor modulation on the signaling pathways responsible for re-epithelialization and scar formation. Thus, it can be assumed that the endocannabinoid system is an integral part of this physiological process. The involvement of the endocannabinoid system in wound healing processes accordingly forms the basis for the use of cannabinoids in wound care, as recently outlined [103].
Cannabinoid Receptor Knockout Models
The importance of the role of cannabinoid receptors is particularly evident in the knockout model. During early wound healing, CB1 knockout mice exhibit an impaired wound closure response associated with increased levels of interleukin (IL)-6, tumour necrosis factor (TNF)-α and monocyte chemoattractant protein (MCP)-1/CC-chemokine ligand (CCL)2 [104]. In the latter investigation, knockout of CB2 was found to increase IL-6 and TNF-α without affecting tissue regeneration.
Role of CB2 Receptor Activation—Results from In Vivo Experiments
A study using a mouse skin incision wound model found that activation of the CB2 receptor by the selective CB2 receptor agonists GP1a (1-(2,4-dichlorophenyl)-6-methyl-N-piperidin-1-yl-4H-indeno [1,2-c]pyrazole-3-carboxamide) and JWH-133 (Figure 1) reduces the infiltration of M1 macrophages and increases the infiltration of M2 macrophages during skin wound healing, thereby reducing skin inflammation [105]. In this context, a separate investigation by the same group observed accelerated re-epithelialisation after treatment of mice with GP1a [106]. In the latter study, the authors described that activation of the CB2 receptor reduced neutrophil and macrophage infiltration and increased the expression of MCP-1/CCL2, stromal cell-derived factor (SDF)-1, IL-6, IL-1β, TNF-α, transforming growth factor (TGF)-β1 and vascular endothelial growth factor (VEGF)-A, but increased keratinocyte proliferation and migration, ultimately leading to accelerated re-epithelialisation of wounds and reduced scar formation [106]. In a model using BALB/c mice, GP1a was used again to reduce the number of collagen I-positive fibroblast cells, resulting in a substantial decrease in skin thickness in the wounded areas. During skin wound healing, levels of TGF-β1 and TGF-β receptor I (TβRI) were decreased by GP1a and increased by the CB2 receptor antagonist AM-630, suggesting that TGF-β signalling is involved in CB2 receptor action. As downstream mediators of the canonical TGF-β signalling pathway, phosphorylation of small mothers against decapentaplegic homolog (Smad) 3 was downregulated by GP1a and increased by AM-630, while Smad7 was increased by GP1a in skin samples [107]. Another in vivo study using a mouse model of skin excision wounds showed that a GP1a-containing gel using triglycerol monostearate hydrogel prepared by a specific method [108] was able to reduce inflammation and fibrogenesis and to promote wound enclosure and re-epithelialisation [109]. In addition, the CB2 receptor agonist β-caryophyllene was found to enhance re-epithelialisation in a mouse model of cut wound repair due to increased cell proliferation and cell migration from intact skin near the wound towards the wound centre [110]. Interestingly, this observation was only made in female mice, while β-caryophyllene did not result in faster wound closure in male mice. When β-caryophyllene was combined with the CB2 receptor antagonist AM-630, the effect was partially but not significantly reduced. However, in this study, the CB2 agonist JWH133 also significantly promoted re-epithelialisation compared to the controls. When considered as a whole, CB2 receptor activation appears to be an effective strategy to achieve wound healing effects.
Effects of ACEA and Adelmidrol
Interestingly, there is little research specifically describing the effects of CB1 receptor activation on wound healing. However, the enhancement of wound healing by CB1 activation is supported by a study reporting that activation of the CB1 receptor by ACEA reduced the levels of keratin 6 and 16 in an organ culture of human skin [111]. The latter keratins are functionally important proteins for the regulation of epithelial wound healing, but on the other hand are upregulated in psoriasis [111]. In addition, the PEA-like cannabimimetic adelmidrol (Figure 1) was reported to stimulate skin wound healing in a diabetic mouse model [112]. However, this study did not investigate which receptors mediate this effect. Since adelmidrol has been described as a PPARγ agonist [113], it seems likely that this receptor is involved.
Role of FAAH—Results from In Vivo Experiments
A comprehensive study on the involvement of FAAH in wound healing processes demonstrated that genetic or pharmacological disruption of FAAH activity accelerates skin wound healing in vivo and stimulates human keratinocyte motility [114]. Accordingly, healing of excision wounds was accelerated in mice lacking the FAAH gene compared to wild-type control mice. However, the authors of the latter study reported that the mechanism of action was related to the epidermal growth factor receptor (EGFR) and an increase in intracellular calcium levels in response to TRPV1 rather than to the modulation of cannabinoid receptors. The authors of the latter study also identified the amides of long-chain fatty acids with taurine (N-acyl taurines) as substrates of FAAH and mediators of the observed accelerated wound healing.
Role of the Endocannabinoid System in Cell Migration—Results from In Vitro Experiments
Numerous investigations conducted with skin-derived cells have provided more detailed insight into the mechanisms of wound healing by cannabinoids. In one study, THC was reported to induce migration of human primary mesenchymal stem cells, which served as in vitro system to demonstrate the regenerative effects of cannabinoids, via CB1 receptor-dependent activation of p42/44 MAPK [115]. A similar effect on stem cells has been observed for CBD, but mediated via activation of the CB2 receptor and antagonisation of GPR55, leading to downstream activation of p42/44 MAPK, which ultimately increased migratory capacity [116]. Furthermore, using the same cells, it was reported that inhibition of FAAH by URB597 and N-arachidonoyl serotonin (AA-5HT) also led to increased stem cell migration via activation of p42/44 MAPK, which in turn resulted in downstream activation of PPARα [117]. One caveat, however, is that the cells used in these studies were not derived from skin tissue but from underlying adipose tissue. Another study reported that a hydrophobic extract of flax fibres containing CBD activates skin cell matrix remodelling and promotes fibroblast and keratinocyte migration as crucial parameters of wound healing [118]. Regarding the effect of cannabinoids on cell migration, primary cultured fibroblasts and keratinocytes from wild-type C57BL/6 mice exposed to β-caryophyllene showed higher chemotactic responses, while β-caryophyllene displayed no such effects in cells from CB2 knockout mice [110].
Interestingly, the enhanced wound healing by cannabinoids in skin wounds and the associated cannabinoid-induced migration of human keratinocytes and stem cells seem to be in contrast to their effect on malignant skin cells. For example, in melanoma cells, the CB2 agonist JWH-133 has been shown to lead to inhibition of transendothelial migration [119]. In another work, a standardised cannabis extract also exhibited a migration-inhibiting effect on melanoma cells [120]. The differences in this regard could be due to the fact that, for example, the CB2 receptor is upregulated in melanoma tissue [121] and therefore corresponding agonists achieve greater efficacy in malignant tissue in some studies. Moreover, cannabinoid compounds often exert different effects at higher concentrations than at lower ones. For the endocannabinoid-like substance OEA, for example, it could be shown that above a concentration of 2 µM, the migration of melanoma cells is inhibited, while below this concentration, migration is increased [122]. On the other hand, a recent study found that CBD and THC at a concentration of 5 µM each did not significantly alter the migration of A375 melanoma cells [123], suggesting that the effects on skin cancer motility depend on the cannabinoid used. Considering that genetic deletion of the CB1 receptor resulted in reduced rather than increased migration of melanoma cell lines [124], it is tempting to speculate that the cannabinoid receptor-mediated effect on the migration of normal physiological skin cells is in fact no different from the effect on the migration of skin cancer migration.
On the Path to Clinical Use
The finding that cannabinoids offer preclinical benefits in models of scarring in wound healing processes as well as in diabetic wound healing suggests that cannabinoids could be used in cosmetic support therapy for scar-free wound healing as well as in the critical treatment of diabetic wounds. More and more promising new formulations are being developed. Thus, a CBD-containing hydrogel dressing based on the ion-crosslinked interaction between Zn2+ ions and the alginate polymer was recently reported to accelerate wound healing in vivo [125]. Based on the promising preclinical results, corresponding cannabinoid effects have meanwhile been investigated in patients with venous leg ulcers [126]. Here, liposomal formulations with a mixture of CBD, THC and other substances proved to be beneficial. Details can be found below in chapter 5.
3.2.3. Influence on Cutaneous Barrier Function
Recent studies have also shown that cannabinoid receptors are involved in important barrier functions, the dysregulation of which is in turn a crucial factor in the pathophysiology of diseases such as psoriasis and acne. Accordingly, CB1 receptor knockout mice were found to exhibit delayed permeability barrier recovery compared to wild-type mice, while CB2 receptor knockout mice had accelerated barrier recovery [127]. Consistent with these findings on the role of the CB1 receptor, a recent study reported that acute barrier disruption induced by repeated tape stripping and acute inflammation induced by TPA were inhibited by the newly synthesised CB1 agonist α-oleoyl-oleoylamine serine (α-OOS) [128]. Another study has also adressed the role of endocannabinoid reuptake and degradation on skin barrier function and skin inflammation in vivo [129]. Here, WOL067-531, an inhibitor of endocannabinoid reuptake with no appreciable effect on FAAH activity, inhibited barrier repair functions [129]. Methodologically, the latter study was designed so that barrier disruption was likewise achieved by tape stripping and skin inflammation was achieved by a patch test with sodium dodecyl sulphate, which is used as a positive reference control in such experiments [130]. In the experiments on skin inflammation, a reduction in epidermal proliferation and the number of proinflammatory cells as well as a reduction in β-defensins by WOL067-531 and additionally by the selective FAAH inhibitor, WOBE440, was observed [129]. β-Defensins are an important component of the antimicrobial barrier and defence [131].
A positive influence of cannabinoid compounds has also been observed where inflammatory stimuli disrupt epidermal permeability barrier function, as demonstrated for N-palmitoyl serinol (Figure 1), a derivative of 2-palmitoyl glycerol [132]. Here, N-palmitoyl serinol increased total ceramide production in human epidermal keratinocytes, which are a critical structural component of the epidermal permeability barrier, in a CB1 receptor-dependent manner [132]. Results beyond skin cell analysis have confirmed the effect of cannabinoids on cellular ceramide production [133,134]. In a recent study, CBD was also found to increase the expression of aquaporin 3 (AQP3) in the skin of mice, which plays an important role in water retention in the skin and is therefore likely responsible for the moisturising effect of CBD [135]. Here, CBD-induced AQP3 expression was associated with increased skin water content without any change in transepidermal water loss, indicating the maintenance of normal barrier function. However, this study did not investigate the extent to which cannabinoid-triggered receptors contribute to this effect.
Since cannabinoids have a wide range of interactions with PPARs, as described above, the results of the studies on the role of PPARs on barrier function are also relevant. In this context, an investigation revealed that PPARγ knockout mice exhibit a disruption of the permeability barrier [136]. The latter is associated with gene regulations related to lipid metabolism and cutaneous lipid barrier function, such as the downregulation of enzymes of the very long chain fatty acid group (Elovl 3, 5 and 6), which are important factors of the lipid permeability barrier of the stratum corneum [137,138]. In addition, PPARα was found to protect epidermal barrier function (for review see [139]). However, although there are many studies on PPARγ- and PPARα agonists that do not belong to the cannabinoid group, it is not yet clear to what extent cannabinoids influence the barrier function of the skin via this receptor. Furthermore, based on current data, it is difficult to clearly determine which receptor plays the most important role in cannabinoid-mediated homeostasis of skin barrier function. However, it is tempting to speculate that cannabinoids with a network of different receptors may favour the stability of skin function in certain circumstances.
3.2.4. Influence on Sebocyte Biology
Cannabinoids may also play a crucial role in the function of the sebaceous glands, which have an intense lipid metabolism. The main product of the sebaceous gland, sebum, is an oily substance enriched with lipids, mainly consisting of triglycerides, free fatty acids, wax esters, cholesterol and squalene, which protects the skin (for review see [138]). In this context, endocannabinoids were found to upregulate the expression of key genes involved in lipid synthesis via CB2 in the human SZ95 sebocyte cell line, which does not express CB1 receptors [88]. In addition, inhibition of endocannabinoid uptake has been shown to have anti-inflammatory effects and to increase sebum production [140]. Among further cannabinoid receptor targets, PPARs are classical lipid regulators in sebocytes, as studied by others (for review see [141]). PPARα and PPARγ were found to stimulate sebum production [142], stimulate sebocyte proliferation and inhibit terminal differentiation and apoptosis, thereby preventing the release of acne-associated lipids [143], a property that serves as a rationale for activation of these PPARs in the treatment of acne. Consistent with this notion, PPARγ knockout mice even exhibited complete absence of sebaceous glands [144]. In contrast to the signalling pathways leading to the potentially beneficial effects described above, the TRPV3 receptor can lead to an unfavourable reduction in sebocyte lipogenesis. Thus, activation of TRPV3 resulted in inhibition of lipogenesis and stimulated the production of the proinflammatory cytokines IL-1, IL-6, IL-8 and TNF-α in human sebaceous gland-derived SZ95 sebocytes [145]. A similar increase in proinflammatory cytokines after TRPV3 activation has also been observed in keratinocytes [146], suggesting that it is a cell type-independent general mechanism of this receptor. However, the extent to which the above-mentioned TRPV3-activating cannabinoids can limit their own anti-inflammatory effect via TRPV3 activation has not yet been investigated in skin cells.
With regard to the effect of cannabinoids on sebocyte function, a recent review even suggests that cannabinoids may act in the context of Parkinson’s disease treatment by both reducing the pathogenesis associated with Parkinson’s disease and inhibiting seborrheic dermatitis, which is a common symptom of Parkinson’s disease [147].
3.2.5. Influence on Hair Follicle Biology
Yet, another function attributed to the endocannabinoid system in the skin may be the control of hair growth. Accordingly, one study reported that AEA and THC inhibit hair shaft elongation and proliferation of hair matrix keratinocytes and stimulate apoptosis of cultured human hair follicles in an organ culture system [86]. In addition, a recent investigation using microdissected hair follicles cultured in toto to preserve the intact epithelial stem cell niche demonstrated that CB1 receptor activation induces the proliferation of hair follicle epithelial progenitor cells ex vivo [148]. The authors also found here that intrafollicular knockout of the CB1 receptor or repeated treatment with a CB1 antagonist leads to a significant reduction in the number of human epithelial stem cells in the hair follicle and that CB1 knockout mice have fewer epithelial stem cells in the bulge. The study therefore concludes that tonic CB1 activation is necessary for the survival of human hair follicle stem cells. Considering opposing effects of CB1 receptor activation and inhibition, it can be concluded that CB1 agonists could help reduce unwanted hair growth as CB1 receptor activation triggers apoptosis-induced premature catagenic regression of hair follicles, while CB1 antagonists could be used as a therapeutic option for alopecia [86].
In recent years, the influence of PPARs on hair growth has also been increasingly studied. In this context, PPARγ knockout mice were found to lead to upregulation of genes involved in inflammation and keratinisation, with increased epidermal acanthosis, spongiosis and parakeratosis. In addition, loss of PPARγ in the epidermis has been linked to the regulation of other marker genes associated with the asebia phenotype, increased transepidermal water loss, alopecia, dandruff and inflammatory skin lesions [136]. Here, in PPARγ knockout mice, an aseptic skin phenotype with alopecia was observed, which is consistent with the results from other authors [144]. Furthermore, tissue-specific deletion of PPARγ in bulb stem cells resulted in scarring alopecia and sebaceous gland hypoplasia resembling human lichen planopilaris, a scarring (cicatricial) alopecia in which PPARγ is also downregulated [149]. The latter study also demonstrated that non-cannabinoid receptor agonists at the PPARγ, i.e., ciglitazone, rosiglitazone, pioglitazone and troglitazone, induce peroxin 16 (PEX16) gene expression in keratinocytes of the outer root sheath. PEX16 gene product deficencies were found to be associated with peroxisomes disappearance (for review see [150]) that is a marker of the lymphocytic cicatricial or scarring alopecia disease, lichen planopilaris. Therefore, PPARγ as a target of various endo- and exocannabinoids could be an important parameter for hair follicle homeostasis.
3.2.6. Influence on the Photoexposed Epithelium
In an earlier review article of our group on the anticarcinogenic effects of cannabinoids on skin cancer, the preventive effects of cannabinoid compounds on sunlight-induced carcinogenesis were already discussed [1]. The basis of these effects is, as already stated above in connection with UV light-induced melanogenesis, the protective function of the endocannabinoid system in the case of physicochemically induced skin damage by UV light. However, a study on this revealed that UV irradiation was more likely to induce papillomas in wild-type mice than in CB1 and CB2 double-knockout mice, which accordingly showed a lower susceptibility to the inflammatory response triggered by UV light with a markedly attenuated activation of the transcription factor Nuclear Factor-κB (NF-κB), a decreased production of TNF-α and a far more resistant epidermis. Consequently, the results of this work suggested that cannabinoid receptors act as oncogenes in this process [151]. Nevertheless, several studies have now reached the consensus that CBD among cannabinoid compounds reduces the harmful effects of UV radiation. Accordingly, some more detailed proteomic analyses revealed a protective regulatory profile of CBD in human skin fibroblasts in this context. This was later confirmed in vivo using UV-irradiated rat skin, where CBD caused proteostasis in keratinocytes and reversed or greatly reduced regulations associated with UV light-induced inflammation [152]. In line with this notion, another study showed that CBD increased the activity of antioxidant enzymes such as superoxide dismutase and thioredoxin reductase in keratinocytes exposed to UV radiation [153]. Besides CBD, CBG was also shown to inhibit the release of proinflammatory cytokines from UV light-exposed human skin fibroblasts and normal human epidermal keratinocytes [154]. However, this study did not address the role of cannabinoid-triggered receptors in this process.
An example of a disease related to chronic UV light exposure is actinic keratosis, a premalignant intraepithelial skin lesion that develops in light-exposed skin areas and for which cannabinoids may be helpful. The expression of FAAH and TRPV1 in the actinic keratosis cell line HT-297.T has been described previously [155]. Furthermore, a study reported downregulation of CB1 receptor expression in 9 out of 9 samples from patients with seborrhoeic keratosis [156], a skin disease similar to actinic keratosis characterised by cellular dysfunction of mast cells [157], whose activities have been shown to be regulated by endocannabinoids [158]. However, there are no studies on the use of cannabinoid compounds in actinic keratosis. Current therapies include 5-fluorouracil, photodynamic therapy, imiquimod, chemical peeling with trichloroacetic acid and diclofenac gel [159]. Cannabinoids could usefully expand this limited pharmacological armamentarium.
3.2.7. Influence on Cutaneous Pain
A further important regulatory circuit, largely determined by the endocannabinoid system, is the control of pain transmission and perception in the skin. Thus, more than two decades ago, AEA was found to reduce pain behaviour in response to chemical damage to the skin via the CB1 receptor, suggesting a possible use of cannabinoids in painful skin conditions [84]. A supporting factor contributing to pain relief in this study was the release of PEA, which surprisingly activated peripheral CB2 receptors here. Accordingly, treatment with cannabinoids may have a general benefit for patients with painful skin conditions. As shown in various case reports, e.g., in epidermolysis bullosa and pyoderma gangrenosum (see Section 4.8. and Section 4.9.), the analgesic effect of cannabinoids in particular is an essential factor that improves the patients’ quality of life.
3.2.8. Influence on Keratinisation
There are a number of regulations of keratins by cannabinoids that have been demonstrated so far. In a review on the effects of neuroendocrine regulation of keratin expression on pathological conditions, keratin 6, 16 and 17 are described as mediators of psoriasis, lichen planus, pachyonychia congenita, but also as beneficial parameters in wound healing [160]. Other keratins that will play a role in the following are keratin 1 and 10, which are likewise associated with psoriasis and lichen planus, but also with epidermolytic ichthyosis [160].
What is known about the role of cannabinoid receptors in keratin regulation is that the CB1 agonist ACEA decreased the expression of keratin 6 and 16 in organ-cultured human skin, which was associated with reduced proliferation of human epidermal keratinocytes [111]. Here, the proliferation of keratinocytes and the downregulation of keratin 6 by ACEA was partially inhibited by a CB1 receptor antagonist. Topical CBD, on the other hand, tested for its suitability as a therapeutic option for keratin disorders, increased keratinocyte proliferation and keratins 16 and 17 [161]. However, this study did not investigate the cannabinoid-driven receptors that trigger these regulations. Considering these results and the fact that a disease such as psoriasis is characterised by hyperproliferation of keratinocytes, the authors of the latter study considered that the use of CBD in psoriasis should be taken with caution. Another study reported a reduction in keratin 10 by treatment of differentiating keratinocytes with CBD that was reversed by the CB1 antagonist SR141716 [162]. Consistent with this, AEA was found to downregulate keratin 1 and 10 in differentiating HaCaT keratinocytes via activation of the CB1 receptor [163]. The authors concluded that the endocannabinoid system significantly controls epidermal differentiation, with CB1 receptor activation inhibiting keratinocyte differentiation, which was also observed after treatment of differentiating keratinocytes with 2-AG, NADA and ACEA. These results also confirm an earlier study by the research group in which endogenous AEA levels in differentiating keratinocytes were reduced by an increase in FAAH expression, which was accompanied by reduced formation of cornified envelopes as a marker of keratinocyte differentiation [164].
3.2.9. Influence on Skin Ageing Processes
Early work on the involvement of cannabinoid receptors in skin ageing already showed that CB1 knockout mice exhibited clear signs of earlier skin ageing with reduced width of the subdermal fat layer compared to wild-type mice [165]. Later work using knockout mice has shown that a lack of CB1 receptors in the skin is associated with accelerated skin ageing due to increased production of ROS, a decrease in antioxidant defenses and a pro-inflammatory environment [166]. This finding could thus provide the basis for the use of topical cannabinoid applications to reduce skin ageing. In the cited study, collagen levels in the skin of CB1 knockout mice were decreased, suggesting that the CB1 receptor may be an endogenous “anti-ageing receptor”. However, it should be noted that these are animal experiments on a diabetes model, the transferability of which to humans would still have to be proven.
A recent study has further demonstrated that the CB1 receptor on the outer mitochondrial membrane in human epidermal keratinocytes is a negative regulator of mitochondrial activity in the human epidermis, the activation of which could reduce excessive mitochondrial ROS production and thus exert a protective function against skin ageing or photodestruction [167]. In addition, extracts derived from Cannabis sativa have recently been shown to have an antioxidant capacity associated with an increase in superoxide dismutase, free radical scavenging, reduced elastase and collagenase activity, as well as in vivo water binding and a long-lasting moisturising effect [168]. However, this study did not address the role of cannabinoid receptors, and for the moisturising effect, the authors assume that cannabinoids act like an occlusive film on the skin surface and retain water.
3.3. Regulation of the Endocannabinoid System in Skin Diseases
3.3.1. Endocannabinoids and Classic Cannabinoid Receptors
The role of the elements of the endocannabinoid system becomes even clearer when one considers how dynamically endocannabinoids and cannabinoid receptors are regulated in the context of skin diseases. A recent study revealed that AEA and 2-AG were elevated 2- to 4-fold in fibrotic mouse skin compared to control skin [169]. Induction of 2-AG has also been demonstrated to be detectable following 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ear inflammation of mice [170] and in a mouse model of contact dermatitis [171]. In another report, higher concentrations of AEA and 2-AG were also found in the plasma of patients with psoriasis vulgaris and psoriatic arthritis [172]. In addition, AEA and 2-AG have been reported to be reduced in keratinocytes from psoriasis patients, while PEA levels are increased [7]. Finally, in the model for 1-fluoro-2,4-dinitrobenzene (DNFB)-induced contact dermatitis, AEA and 2-AG were both shown to be upregulated in exposed ear samples [3]. Upregulation of 2-AG has also been demonstrated in skin lesions of mite antigen-induced atopic dermatitis in mice [5].
Upregulation of the CB1 receptor was detected in granulocytes of psoriatic arthritis patients, while the CB2 receptor appeared to be upregulated only in granulocytes of psoriasis vulgaris patients [172]. In skin biopsies from psoriasis and atopic dermatitis patients, another recent comprehensive investigation focusing on mRNA profiles of itchy lesional skin found both cannabinoid receptors downregulated here [173]. Interestingly, there is a veterinary study on this topic that has shown that both cannabinoid receptors are upregulated in the skin of dogs with atopic dermatitis compared to the skin of healthy dogs [174]. However, no quantitative assessment was performed in this study. The focus here was on the distribution of cannabinoid receptors in the individual components of the skin, with strong CB1 immunoreactivity observed in the keratinocytes of the suprabasal spinous layers of the epidermis and strong CB2 immunoreactivity in the basal, suprabasal and granular layers of the epidermis in dogs with atopic dermatitis. In addition, it was observed that CB1 receptor immunoreactivity was significantly downregulated in human epidermis at sites of seborrhoeic keratosis compared to the marginal lesion, leading to a subsequent upregulation of stem cell factor (SCF), which activated mast cells and increased the severity of seborrhoeic keratosis. In this context, endocannabinoids have been defined as important neuroendocrine regulators for the maintenance of skin homeostasis [156]. In a recent report, CB2 receptor expression was observed to be increased in dermatomyositis in certain cell types found in the skin, such as dendritic cells, B-cells, T-cells and macrophages, which produce IL-31, IL-4, interferon (IFN)-γ and IFN-β, compared to healthy skin [8]. Another investigation showed that cannabinoid CB2 receptors are upregulated in neutrophils, macrophages and myofibroblasts in a time-dependent manner during skin wound healing in mice [175]. Moreover, a recent study using primary cultures of adult human fibroblasts revealed that TGF-β, a profibrotic mediator, significantly increases the expression of CB1 [176] and CB2 receptors [177], suggesting that both receptors may play a role in fibrotic diseases of the skin and thus represent potential targets for pharmacotherapeutic interventions. Moreover, in mice in the above-mentioned model of streptozotocin-induced type 1 diabetes associated with accelerated skin ageing, the CB1 receptor has been shown to be downregulated, which was accompanied by higher expression of pro-inflammatory markers [166]. In the in vivo model of DNFB-induced contact dermatitis, the CB1 receptor was further found to be downregulated and the CB2 receptor upregulated in the affected ear tissues [3]. Finally, for the CB2 receptor, upregulation was detected in psoriatic skin lesions in a mouse model of imiquimod-induced psoriasis [178].
In general, it is difficult to assess whether the regulations of these receptors are causally related to the disease or represent epiphenomena or are counter-regulations to maintain homeostasis. The known regulations of endocannabinoids and cannabinioid receptors in the context of skin diseases can only ever hint at functional tendencies. In order to derive more precise functional implications, it would actually first be necessary to qualitatively and quantitatively assign the regulations in the respective disease context to the individual components and cell types of the skin through immunohistochemical analyses of skin sections. In any case, the current knowledge about the regulations already illustrates the close involvement of these receptors in physiological and pathophysiological processes.
Table 1 summarises selected regulations of elements of the classic endocannabinoid system associated with pathogenic changes of the skin.

Table 1.
Regulation of endocannabinoids and cannabinoid receptors in tissue, plasma or cells from biopsies of skin diseases.
3.3.2. Extended Endocannabinoid System
Considering the role of cannabinoid targets beyond the classic cannabinoid receptors CB1 and CB2, such as TRP channels, PPARs, GPR55, GPR119 and FAAH, there are numerous other proven or suspected mechanisms of cannabinoid action in skin diseases. There are also multiple findings on how these targets are regulated in skin diseases.
Table 2 provides an overview of the regulation of these cannabinoid-modulated receptors of the extended endocannabinoid system under the indicated pathophysiological alterations.

Table 2.
Regulation of cannabinoid-modulated receptors in human skin tissue biopsies or plasma.
TRP Channels
The regulations of the individual TRP channels appear to be complex and differentiated, with some upregulated and others downregulated in a pathophysiological context. Analysis of skin biopsies revealed that TRPV2 was downregulated in itchy skin regions of psoriasis patients, while FAAH1, TRPV1 and TRPV3 were upregulated. In contrast, in skin biopsies from patients with atopic dermatitis, TRPV1 and TRPV2 were upregulated and TRPV3 and TRPM8 were downregulated [173]. Immunohistochemical analyses confirmed these data for selected regulations, including TRPV1, which is upregulated in both atopic dermatitis and psoriasis biopsies, suggesting that this cannabinoid target may represent an underappreciated mediator of itch as a parameter of a common “itch-scriptome” match [173]. In this context, TRPV1 was also found to contribute to adverse pain-mediating effects in an in vitro model of psoralen UVA (PUVA) therapy used to treat vitiligo, psoriasis, eczema and mycosis fungoides [189].
Based on studies indicating interaction of various phytocannabinoids with TRP channels [58], the authors of a recent publication hypothesised that phytocannabinoids could be a therapeutic option for the treatment of rosacea, in which such ion channels play an important role in pathogenesis [190]. Consistent with this notion, TRPV channels are dynamically regulated in different subtypes of rosacea. Accordingly, TRPV1, 2 and 3 appear to be upregulated in erythematotelangiectatic rosacea and TRPV2 and 3 in papulopustular rosacea [179]. In phymatous rosacea, on the other hand, TRPV1, 3 and 4 are upregulated, while TRPV2 is downregulated [179]. In line with the upregulation of TRPV1 in dysregulated skin, TRPV1 antagonists have been reported to reduce itching behaviours [191] and improve barrier repair [191,192]. In summary, although TRPV2 was downregulated in pruritic skin regions of psoriasis and phymatous rosacea and TRPV3 was downregulated in skin biopsies from patients with atopic dermatitis, TRP channels appear to be upregulated in most skin disease studies. Therefore, it is reasonable to assume that TRP channels may play a role in symptoms, especially pain. In view of this, it cannot be ruled out that cannabinoids that act agonistically on TRPV1, such as CBD, may partially attenuate their beneficial effects elicited by other receptors.
TRPV4 has been found to mediate dry-skin-induced pruritis [193] and chronic pruritis [180]. Accordingly, in the latter investigation, TRPV4 expression was found to be significantly increased in skin biopsies from patients with chronic idiopathic pruritus compared to skin from healthy control subjects.
Finally, a possible involvement of TRPV3 downregulation has been suggested in patients with middle ear cholesteatoma. Middle ear cholesteatoma is a chronic purulent inflammation of the middle ear with bone destruction as another disease involving epidermal cells, which in most cases results from the ingrowth of squamous epithelium from the external auditory canal into the middle ear [194].
GPR55, GPR119 and GPR18
A recent study reported that higher levels of GPR55 are found in granulocytes from psoriasis and psoriatic arthritis patients than in the control group [172], suggesting that cannabinoids with GPR55-antagonising effects, as is the case with CBD [61], may be beneficial for these indications. In addition, GPR119, which can be activated by OEA, has been found to be downregulated in the sebaceous glands of acne patients [99]. In the latter study, the OEA/GPR119 pathway was described as an essential mechanism of seborrhoea and acne, with OEA causing a lipogenic effect, increasing cellular granulation and switching sebocytes to a proinflammatory phenotype, suggesting pharmacological blockade of GPR119 as a potential treatment option [99]. However, the fact that prolipogenic GPR119 is downregulated in the sebaceous glands of acne patients seems to contradict the acne-promoting effect of GPR119. Here, the authors suspect an over-activation of the receptor in the early and not in the late stages of acne or a downregulation due to secondary compensatory mechanisms in response to excessive acneogenic sebum production.
Regarding the effect of cannabinoids at GPR18 in skin diseases, there are currently no data on a possible pharmacological effect, although both GPR18 [195] and the endogenously synthesised ligand N-palmitoyl glycine [67] have been detected in skin tissue.
PPARs
An mRNA profiling analysis of skin biopsies from patients with atopic dermatitis and psoriasis revealed that PPARα and PPARγ are downregulated in the itchy skin regions of psoriasis patients, while PPARα remains unchanged in patients with atopic dermatitis and PPARγ increases by 24% [173]. Downregulation of both PPAR subtypes was confirmed in skin biopsies from patients with systemic sclerosis [184]. Another study confirmed the downregulation of PPARγ in explanted fibroblasts of lesional skin from patients with systemic sclerosis [98]. In addition, PPARγ has been reported to be downregulated in UV-irradiated damaged skin [185], in systemic lupus erythematosus patients with skin lesions [187] and in psoriatic and atopic lesions [186]. An investigation of 100 acne patients compared to 100 healthy subjects further found that the Pro12Ala polymorphism of the PPARγ gene, which results in decreased PPARγ transcriptional activity, is associated with a lower risk of acne vulgaris [196]. In line with the importance of PPARγ in atopic diseases, another study suggested PPARγ activation as a potential therapeutic option for the treatment of allergic rhinitis [197].
With respect to PPARα, its expression has been found to be reduced in eczematous skin from patients with atopic dermatitis compared to skin from non-atopic donors [182]. Likewise, PPARα has been shown to be downregulated in affected skin regions in melasma [183] and in human skin lesion with actinic keratosis compared to healthy skin [181]. Interesting new aspects could also result from veterinary findings. For example, it has been shown that in cats with eosinophilic dermatitis, PPARα accumulates in the basal and upper cells of the hyperplasmatic epidermis and in keratinocytes adjacent to the ulcer in affected skin compared to healthy controls [92].
Finally, one study reported that PPARδ appears upregulated in the lesions of patients with psoriasis [188] which may therefore serve as another possible target effected by cannabinoids.
4. Selected Skin Diseases—Pharmacotherapy and Effect of Cannabinoids
Several data on cannabinoid effects have now been collected using in vitro and in vivo models of skin diseases such as androgenetic alopecia, atopic dermatitis, allergic contact dermatitis, psoriasis, acne, systemic sclerosis, dermatomyositis, epidermolysis bullosa and pyoderma gangrenosum, acute inflammation, post-herpetic neuralgia and various keratin diseases. Since the diseases discussed below for which cannabinoids have been tested almost all have inflammation as a common feature, each of which has a different significance for the development and progression of the corresponding diseases, some historical background on the anti-inflammatory effects of cannabinoids will be presented first. Since pruritus is symptomatic in most of these diseases, the following remarks on the pharmacotherapeutic possibilities of cannabinoids in the specific diseases are also preceded by a short section on the general antipruritic effect of cannabinoids.
- A Brief Historical Overview on Anti-Inflammatory Effects of Cannabinoids
Consistent with this exposed homeostatic function of the endocannabinoid system in the skin, numerous publications have described a potential therapeutic value of cannabinoids in the treatment of inflammatory skin diseases. An important part of the anti-inflammatory action of cannabinoids is their effect on various cells of the immune system, which was summarised in a recently published review [198].
The first descriptions of the use of cannabis may date back to ancient Egyptian sources, where cannabis was probably used as an anti-inflammatory option to treat skin swelling [199]. Well documented in ancient writings is the use of cannabis for burns by Gaius Plinius Secundus (AD 23/24–79) [200]. The use of cannabis for burns appears again and again over the centuries, for example in 1698 with Nicholas Lémery, who recommended hemp for the treatment of burns [201]. Although the analgesic effect of cannabinoids in burns is still a subject of scientific discussion today [202], the use of cannabinoid compounds for this indication has rather faded into the background.
Insights into mechanisms of anti-inflammatory effects of cannabinoids were gained long before the discovery of cannabinoid receptors. Thus, experiments from the early 1970s already demonstrated an anti-oedematous and anti-arthritic effect of THC [203]. Later experiments testing the anti-oedema effect in rats showed that the inhibitory effect of THC was limited to oedema induced by carrageenan, kaolin and sodium urate, while hydrocortisone and aspirin were effective against a wider range of oedemas, including those induced by histamine and bradykinin. This suggests a different antiphlogistic mode of action of THC compared to steroidal and non-steroidal anti-inflammatory drugs [204]. The inhibitory effect of cannabinoids on inflammatory pain has also been demonstrated in several other reports [205,206,207].
Interestingly, the earliest published comprehensive data on anti-inflammatory and anti-allergic effects of lipids belonging to the endocannabinoid system mainly refer to the endocannabinoid-like substance PEA and date back to the 1950s [208]. These findings were later substantiated in animal experiments, which showed that PEA reduces oedema [209,210] and inhibits mast cell activation [211]. Other results of the latter study were a PEA-induced reduction in extravasation caused by passive cutaneous anaphylaxis and a reduction in oedema in various animal models. Only later was it recognised that PEA exerts its anti-inflammatory effect via activation of PPARγ [72]. A clinical study was able to prove the benefit of PEA in temporomandibular joint inflammatory pain [212]. As a result of this and other studies, a recent meta-analysis concluded that PEA may be a useful treatment option for pain, including inflammatory pain [213]. The effect of PEA on the skin, which has now been tested in several clinical studies on skin diseases, will be discussed later.
A number of publications prove that the activation of the CB2 receptor causes an inhibition of the immune response. For the CB2 receptor agonist O-1966, a mechanism was described that could cause such an effect via the increase of the two potent anti-inflammatory parameters IL-10 and regulatory T cells (Treg) [214]. The anti-inflammatory effect of CB2 agonists could also be confirmed in other studies [215,216]. An atypical cannabinoid that has been shown to reduce inflammatory processes is the CB2 receptor agonist lenabasum, which reduces the release of IL-1β from peripheral blood mononuclear cells (PBMCs) in vitro [217] and causes a reduction in inflammatory pain in a rat model of neuropathic and inflammatory pain [218]. In terms of mechanisms of action, lenabasum was found to inhibit IL-8 promoter activity in a PPARγ-dependent manner [79], confirming the antifibrotic and anti-inflammatory effects of lenabasum via PPARγ shown by other authors [219]. In contrast, another publication investigating the inhibition of IL-6 production by lenabasum in human monocyte-derived macrophages as a potential strategy for the treatment of patients with rheumatoid arthritis and systemic lupus erythematosus reported that this effect occurs in a PPARγ-independent manner [220]. Some studies have reported that lenabasum not only blocks inflammatory processes, but also accelerates the resolution of inflammation when it has already occurred. As a novel anti-inflammatory and proresolving drug, lenabasum is currently under clinical investigation for several diseases [221,222]. One mechanism of action of lenabasum is to support the release of free arachidonic acid after activation of the CB2 receptor and phospholipase A2. As a result, higher concentrations of 15-deoxy-Δ12,14-prostaglandin (PG) J2 are formed via cyclooxygenase-2 (COX-2), causing apoptosis and the resolution of chronic inflammation via eleavated caspase-3 activity. Secondly, a metabolic pathway mediated by lipoxygenase is also thought to mediate lenabasum-triggered production of anti-inflammatory and proresolving lipoxin A4 and other proresolving mediators [222].
There are also studies that have investigated the involvement of the CB1 receptor in pro-inflammatory effects. Thus, using microdissected human scalp hair follicles in the anagen VI stage of the hair cycle, a comprehensive study reported that inhibition of CB1 receptors with AM-251 induces degranulation of mast cells in the connective tissue sheath, which was inhibited by AEA and ACEA [158]. Further results from this work showed that inhibition or silencing of the CB1 receptor stimulated the maturation of mast cells from resident progenitor cells, which was associated with an upregulation of SCF.
Moreover, THC, AEA and NAGly have been found to act as full agonists at GPR18 [68], a receptor that has been detected in naïve endothelium as well as in infiltrating leukocytes of inflamed skin [195] and has furthermore been described as receptor of the resolvin D2 [223], a potent immunoresolvent. However, so far it has not been investigated to what extent cannabinoids can cause an inflammation-resolving effects in the skin via a direct activation of GRP18.
- A Brief Overview on Anti-Pruritic Effects of Cannabinoids
Already almost two decades ago, a case report was published on three patients suffering from treatment-resistant cholestatic related pruritus, in which treatment with THC resulted in an antipruritic effect of about 4 to 6 h [23]. In the meantime, a number of well-documented effects of cannabinoids and their derivatives on skin diseases associated with itching have been described in the literature. The importance of the endocannabinoid system in the development of pruritus was shown in a study using pharmacological and genetic approaches in a murine pruritus model. Here, subcutaneous administration of the mast cell degranulator compound 48/80 triggered an intense, concentration-dependent scratch reaction, which was reduced by THC in a CB1 receptor-dependent manner [224]. Furthermore, genetic deletion of FAAH in this study resulted in a reduction in scratching behaviour. This reduction in scratching behaviour was mediated independently of hypomotility. In another study, WIN 55,212-2 was shown to have an antipruritic effect by reducing serotonin-induced scratching behaviour in mice [225]. An antipruritic effect was also found for S-777469 (Figure 1), a selective CB2 receptor agonist containing a 3-carbamoyl-2-pyridone system with carboxylic acid at the 3-position [226]. In animal experiments, itching in mice was induced by histamine or substance P and suppressed by S-777469, which was reversed by a CB2 receptor antagonist [227]. Interestingly, some studies have also tested options that indirectly target the endocannabinoid system rather than acting directly on cannabinoid receptors. Accordingly, in an in vivo study, a significant decrease in both groin and overall itch was found with a reduction in skin symptom severity following allergen challenge in atopic beagles after treatment with WOL067-531, a topical endocannabinoid membrane transporter inhibitor [228].
When considering the extended endocannabinoid system, it is striking that TRP channels such as TRPV1 and TRPA1 play an important role in itch signal transduction, as highlighted in a systematic review on this topic [229]. In addition, scratching behaviour in response to glucosylsphingosine was decreased in TRPV4 knockout mice [230], and GSK1016790A, a TRPV4 channel-specific agonist, induced acute itch in mice [231]. For this reason, one can speculate whether cannabinoids also counteract their antipruritic effect via their TRP-activating properties. This would be the case with the TRPV4-activating or -sensitising cannabinoids CBDV, THCV, CBGA, CBGV, CBN and CBG [58], as well as with a higher concentration of CBD (50 µM), which also activates TRPV4 [232], and with TRPV1-activating cannabinoids such as AEA [52], CBD [53], 2-AGE [38,54], NADA [37] as well as CBG, CBC, THCV and CBGA [55]. However, as far as we know, there are no studies yet that have investigated the role of these TRP channels in the effect of cannabinoids on skin itch.
In the following chapters, the antipruritic effect of cannabinoids in numerous diseases such as allergic contact dermatitis, atopic dermatitis, psoriasis and neurogenic flare reactions will be addressed.
4.1. Androgenetic Alopecia
4.1.1. Current Therapies of Androgenetic Alopecia
Androgenetic alopecia is a hereditary thinning of the hair with a polygenic pattern of inheritance [233], which in susceptible individuals is triggered dominantly by androgens [234,235]. Approximately half of the population develop this feature before the age of 50 [236]. Currently, there are three common drugs used to treat androgenetic alopecia, namely minoxidil and the two 5α-reductase inhibitors finasteride and dutasteride, which, according to a recent meta-analysis, are roughly equivalent in terms of average change in hair number after treatment [237]. However, the optimal treatment of androgenetic alopecia is still a matter of debate due to variable adverse effects and relatively unstable results [238]. Another option for the treatment of androgenetic alopecia is a minimum triple injection of platelet-rich plasma, which was considered a safe and effective alternative procedure for the treatment of hair loss instead of minoxidil and finasteride in a recent systematic review [239]. Although androgenetic alopecia is primarily related to androgen metabolism, the results of some studies suggest microinflammation with perifollicular lymphocytic infiltration associated with this condition [240,241], in which case the anti-inflammatory effects of cannabinoids would also come into play as a mechanism of action.
4.1.2. Preclinical Findings on the Effect of Cannabinoids in Androgenetic Alopecia
Among the cannabinoid compounds, CBD has been shown to enhance hair growth (for review see [242]). As a possible mechanism of action, a recent investigation reported that CBD restores testosterone-induced loss of β-catenin in human dermal papilla cells, which is downregulated in alopecia by ubiquitination and subsequent proteasomal degradation [243]. However, the latter study did not address the involvement of CBD-triggered receptors. For CBD, which also has a negative allosteric effect on the CB1 receptor [29] and should therefore actually promote hair growth via this mechanism, a complex mechanism besides the modulation of the cannabinoid receptors has been demonstrated: this cannabinoid inhibits hair shaft development at a concentration of 50 µM via TRPV4 agonism, but enhances it at submicromolar concentrations via activation of the adenosine receptor [232]. Interestingly, in a study investigating the effect of newly synthesised thienyl-substituted pyrazole carboxamide derivatives as CB1 receptor antagonists against obesity, it was found that a compound (“compound 3”) structurally mimicking rimonabant induced exceptionally strong hair growth in C57BL/6J mice [244], supporting the assumption that CB1 receptor antagonists increase hair growth accordingly [86].
4.2. Atopic Dermatitis
4.2.1. Current Therapies of Atopic Dermatitis
Atopic dermatitis is the most prevalent inflammatory skin disease in developed countries, with symptoms including skin redness, peeling, lichenification and pruritus that interfere with daily life and especially sleep [245]. Characteristic of atopic dermatitis is hypertrophy of sensory nerve fibres [246] and release of calcitonin gene-related peptide (CGRP) as a critical mediator of itch and flare-ups occurring in infiltrating inflammatory cells in the late phase of the skin reaction [247]. In addition, histamine receptor 4 is upregulated on CD4-positive T cells from patients with atopic dermatitis, which is associated with the induction of IL-31, a major mediator of itch [248].
Symptom treatment of mild forms of atopic dermatitis is currently carried out with emollients and low potency topical steroids, while severe types are treated with oral corticosteroids, cyclosporine (for review see [249]) and other immunosuppressants such as azathioprine [250], methotrexate [251,252] and the calcineurin inhibitors tacrolimus and pimecrolimus, which were approved as topical treatment options for atopic dermatitis about two decades ago [253]. An important biologic for the treatment of atopic dermatitis is dupilumab, an IL-4 receptor-α inhibitor that blocks both IL-4 and IL-13 activity and is approved for the treatment of moderate-to-severe atopic dermatitis in patients 6 years of age and older (for review see [254]). Other biologics currently under investigation for the treatment of atopic dermatitis include the IL-13 inhibitors lebrikizumab and tralokinumab [255], with the latter agent now approved in Europe. Another target is IL-31, for which the antibody nemolizumab is currently under investigation for the treatment of moderate-to-severe atopic dermatitis [256]. In addition, several clinical trials are addressing the potential of biologics that block IL-33 (etokimab) and Th22/IL-22 (fezakinumab) signalling pathways or target TNF Receptor Superfamily Member 4 (TNFRSF4, OX40) with ligands that prevent differentiation of naïve CD4-positive cells into inflammatory Th2 memory cells (for review see [257]). A further therapeutic strategy is the use of crisaborole, a topical anti-inflammatory phosphodiesterase-4 (PDE4) inhibitor approved by the US Food and Drug Administration (FDA) for the treatment of atopic dermatitis [258], which is superior even to topical immunomodulators such as tacrolimus and pimecrolimus [259]. Since the Janus kinase/signal transducers and activators of transcription (JAK/STAT) signalling pathway is associated with the development of atopic dermatitis, inhibitors of this pathway have been discovered as a possible pharmacotherapeutic option, of which baricitinib has been approved by the EMA for moderate-to-severe atopic dermatitis in adult patients (for review see [254]). A small molecule JAK1 inhibitor approved by the EMA for the treatment of moderate-to-severe atopic dermatitis in patients 12 years of age and older is upadacitinib, which recently showed a faster onset of action than dupilumab in a phase III study [260]. Furthermore, recently, the topical JAK inhibitor delgotinib was approved in Japan for the treatment of atopic dermatitis [261] and is currently in clinical trials for the European market [262]. In addition, combinations of immunomodulators and H1 antihistamines have been widely used to relieve pruritus in patients with moderate-to-severe atopic dermatitis [263].
4.2.2. Preclinical Findings on the Effect of Cannabinoids in Atopic Dermatitis
In the context of hypersensitive skin reactions, a study using a mouse model of Th2-type contact hypersensitivity with pathophysiological features of mouse atopic-like dermatitis found that animals in which the CB1 receptor was knocked out showed a hypersensitivity reaction as measured by ear swelling, transepidermal water loss, Th2-type skin inflammatory reactions and serum IgE levels, all of which are appropriately characteristic of atopic dermatitis [264]. Furthermore, JTE-907 (Figure 1), a CB2 receptor inverse agonist, reduced itch-related responses in a murine model of atopic dermatitis to an extent comparable to that of tacrolimus [265]. In another work, the CB2 receptor agonist S-777469 showed an inhibitory effect on mite antigen-induced atopic dermatitis-like skin lesions in NC/Nga mice [5], with the latter exhibiting reduced eosinophil infiltration after treatment with S-77469. Collectively, these studies show conflicting results regarding the role of the CB2 receptor in atopic dermatitis, as both the CB2 receptor antagonist/inverse agonist JTE-907 and the CB2 agonist S-777469 produced positive effects. Thus, the role of the CB2 receptor seems to be a complex one here.
As a further mechanism of action one investigation found AEA and THC at submicromolar concentration were able to inhibit CGRP release from isolated rat and mouse skin preparations in a CB1 receptor-dependent way [266]. In addition, AEA and the newly synthesised CB1 agonists α-oleoyl oleoylamine ethanolamine (α-OOE; Figure 1) and α-OOS (Figure 1) suppressed mast cell proliferation, inhibited the release of inflammatory mediators and prevented the increase of skin fold thickness in mice with oxazolone-induced atopic dermatitis mice. These findings thus point to a crucial role of the CB1 receptor in modulating antigen-dependent IgE-mediated mast cell activation [267]. In another study, the symptoms of oxazolone-induced atopic dermatitis were inhibited by α-OOS [128], as evidenced by α-OOS-reduced transepidermal water loss, skinfold thickness and skin surface pH, while skin capacitance was increased. Also worth mentioning in this context is a recent prospective, randomised, double-blind, placebo-controlled study in dogs with atopic dermatitis in which a mixed oil based on CBD and CBD acid was tested [268]. No effect of treatment on IL-6, -8, -31, -34 and MCP-2 serum levels or skin lesions could be detected, although an improvement in itching was observed.
4.3. Allergic Contact Dermatitis
4.3.1. Current Therapies of Allergic Contact Dermatitis
Allergic contact dermatitis is a type IV hypersensitivity reaction triggered by contact allergens. The impairment of the skin barrier here leads to increased permeability and penetration of allergens and triggers innate signalling pathways required for the activation of the adaptive T-cell response during the sensitisation phase, which is accompanied by the release of proinflammatory cytokines [269]. Although a recent review referred contact dermatitis as a “great imitator” that mimic many skin diseases such as atopic dermatitis, lichen planus and angioedema [270], a recent meta-analysis found no overall association between atopic dermatitis and contact sensitisation [271]. Concerning therapeutic measures, suspected allergens or cross-allergens should be avoided. In addition, topical glucocorticoids are the primary basis for treatment. Topical calcineurin inhibitors are indicated for prolonged use [270]. In contact dermatitis of the hand, which has an overall prevalence of 5.2% in men and 10.6% in women in Northern Europe [272], retinoids such as acitretin are an option for chronic symptoms [273]. It has also been shown that contact dermatitis of the hand can be treated long-term with oral cyclosporine and azathioprine [270], although the latter has only been tested in patients with the rare chronic actinic dermatitis [274].
4.3.2. Preclinical Findings on the Effect of Cannabinoids in Allergic Contact Dermatitis
The crucial role of cannabinoid receptors in contact dermatitis was shown in animal models of DNFB-induced ear inflammation in C57BL/6J mice serving as a model of cutaneous allergic contact hypersensitivity, in which a cannabinoid receptor double knockout induced greater sensitivity to inflammatory conditions accompanied by increased expression of MCP-2 [3]. Cannabinoid receptor activation here decreased Toll-like receptor 3 (TLR3) ligand polyinosinic polycytidylic acid (poly-(I:C))-induced MCP-2 expression in the keratinocyte cell line HaCaT. Stimulation with poly-(I:C) serves as an in vitro model for contact dermatitis in this context. In addition, the latter study demonstrated a reduced allergic response after DNFB exposure in FAAH knockout mice. In another investigation, the CB2 receptor agonist S-777469 was shown to exert an inhibitory effect on DNFB-induced ear inflammation in dermatitis of Balb/c mice [5]. A contribution of TRPV1 to the anti-inflammatory properties of PEA was discovered, as PEA inhibits DNFB-induced ear inflammation in mice via a TRPV1-dependent mechanism [275]. The same group later found that CBD decreases MCP-2, IL-6, IL-8 and TNF-α via CB2 and TRPV1 in human keratinocytes stimulated with poly-(I:C) [276]. The latter investigation further reported other cannabinoids such as CBC, CBG, THCV and CBGV to inhibit MCP-2, IL-6 and IL-8. Further work found that PEA reduced the number of ear scratches in mice exposed to DNFB, which was abolished by CB2 and PPARα antagonists [277]. In this context, it should be mentioned that the rationale for the use of PEA in diseases such as allergic contact dermatitis lies not only in its endocannabinoid effect, but also in the fact that it is an essential component of the stratum corneum, which accordingly compensates for deficiencies when applied topically in diseased skin [278]. Finally, in a recent work, newly developed CB2 receptor agonists were tested for their symptom-reducing properties in atopic contact dermatitis. Here, in agreement with the previously shown data, the CB2 receptor full agonist “8g” was also demonstrated to reduce poly-(I:C)-induced MCP-2 expression in HaCaT cells [279].
In contrast to the beneficial effects of CB2 receptor activation described above, another study found that the CB2 receptor antagonist SR144528 inhibited the expression of pro-inflammatory cytokines, reduced the recruitment of eosinophils and attenuated ear swelling in a mouse model of oxazolone-induced contact dermatitis [171]. Consistent with this, the CB2 receptor inverse agonist JTE-907 showed anti-inflammatory properties in the carrageenin-induced paw oedema test in mice [280] and inhibited DNFB-induced ear swelling of mice [281,282].
Just as in the case of atopic dermatitis presented above, it is difficult to assess the extent to which activation or inhibition of the CB2 receptor brings perspective benefits for patients with allergic contact dermatitis. Particularly difficult in this context are findings that show on one occasion that CB2 knockout mice exhibit greater ear swelling in the DNFB-induced model of allergic contact dermatitis [3] and on another occasion that the CB2 knockout mice show less ear swelling in an IgE-dependent three-phase cutaneous response in the mouse ear [282]. The authors of the first mentioned study therefore suggested that CB2 antagonism may be beneficial initially but detrimental with chronic blockade [3].
An important role in skin homeostasis has likewise been evidenced for the cannabinoid target PPARα, which is a major trigger of skin inflammation [283]. In this context, PPARα-deficient mice were reported to exhibit greater epidermal thickening and increased recruitment of inflammatory cells from the skin compared to their wild-type counterparts [182]. Consistent with these results and the above-mentioned functional role of PPARα in the beneficial effect of PEA on atopic contact dermatitis [277], inhibition of endogenous PEA degradation was shown to improve symptoms in the DNFB-induced murine dermatitis model via PPARα [284]. Application of the NAAA inhibitor ARN077, which resulted in a large increase in PEA, caused a reduction in pathological skin thickening, scratching behaviour, a reduction in circulating IL-4, IL-5 and IgE levels, and an increase in IFN-γ. A comparable reduction in skin thickening by ARN077 could no longer be observed in PPARα knockout animals. Finally, the crucial role of NAAA was demonstrated in this study by the fact that DNFB-treated NAAA knockout mice showed significantly less skin thickening and greatly reduced scratching behaviour compared to wild-type littermates.
Finally, topical N-palmitoyl serinol, previously described as a CB1 agonist [132], was found to improve the epidermal permeability barrier and partially counteract transepidermal water loss and reduced hydration of the stratum corneum in DNFB-treated mice [285]. However, the latter study only speculated on a possible role of cannabinoid receptors in the N-palmitoyl serinol effect presented, without investigating it experimentally.
4.4. Psoriasis
4.4.1. Current Therapies of Psoriasis
Psoriasis is an immune cell-mediated systemic inflammation that occurs in relapses and is characterised by recurrent exacerbations and remissions of thickened, erythematous and scaly plaques and other comorbidities [286]. An essential feature of psoriasis is the proliferation and keratinisation of epidermal cells (for review see [287]). Dendritic cells here over-secrete TNF-α and IL-23, leading to the differentiation of naïve T cells into the Th17 subtype, which under healthy conditions triggers the maintenance and recruitment of neutrophils for immune defence [288]. These cells exhibit increased production of IL-17 and IL-22. As a result, TNF-α and IL-17 cause epidermal hyperplasia and increased recruitment of inflammatory cells such as neutrophils. In addition, IL-17 increases the production of the proinflammatory cytokines IL-6 and IL-8 in keratinocytes [289].
The pharmacotherapy of psoriasis has some similarities with the treatment of atopic dermatitis, e.g., the use of immunosuppressants such as calcineurin inhibitors for mild-to-moderate facial psoriasis and intertriginous psoriasis [290] as well as topical corticosteroids such as betamethasone [291], which not only have an anti-inflammatory effect but also decrease keratinocyte proliferation and induce immunosuppression [292]. However, a whole range of small molecules and biologics have been approved specifically for different types of psoriasis.
Among topical treatments for psoriasis, there are several options for mild-to-moderate forms such as the use of dithranol, also known as anthralin, which inhibits epidermal hyperproliferation and the number of neutrophils in the epidermis and dermis [293]. Other recommended drugs for mild-to-moderate plaque psoriasis include vitamin D analogs such as calcipotriol, tacalcitol, maxacalcitol, paricalcitol and becocalcidiol [286]. One group of drugs that is still controversial in this context because of their side effects are the retinoids that are also used to treat acne, such as topically applied tazarotene [294]. Even more critical are systemically administered retinoids such as acitretin, which are recommended for psoriasis because retinoid acid receptor activation reduces STAT signalling, cell proliferation and epidermal differentiation, and inhibits scaling and thickness of psoriatic plaques [295]. Acitretin is recommended for treating moderate-to-severe psoriasis, but is clinically limited by a number of adverse side effects [296]. Other systemic treatment options include immunosuppressive therapies with methotrexate and cyclosporine A [286]. Although the mechanism of action is still unclear, fumaric acid esters such as dimethyl fumarate are used in Europe for treatment of moderate-to-severe psoriasis [297]. In addition, the treatment of moderate-to-severe psoriasis shares further common features with the treatment of atopic dermatitis, such as the use of PDE4 inhibitors like apremilast, which is only approved for psoriasis in Europe, and crisaborole, which is only approved for atopic dermatitis in Europe [298], as well as JAK inhibitors [299] and various immunosuppressants [300,301].
Finally, there are currently a plethora of biologics that can interrupt the pathophysiological cascade of advanced psoriasis. These include some biologics approved for the treatment of rheumatic diseases, such as the TNF-α blockers adalimumab, etanercept, infliximab and certolizumab [302]. Other treatment options for psoriasis are biologics that target IL-23, the key factor that triggers the activation of the Th17 inflammatory pathway. IL-23 consists of a p19 subunit linked to a p40 subunit, sharing it with IL-12, which triggers Th1 differentiation and proliferation. Therefore, antibodies targeting these factors have emerged as a treatment option for psoriasis. A currently approved antibody against the p40 subunit of IL-23/IL-12 is ustekinumab [303]. Other biologics for the treatment of psoriasis include antibodies against the p19 subunit of IL-23 such as guselkumab [304], tildrakizumab [305] and risankizumab [306]. An alternative option for the treatment of psoriasis is antibodies that directly interfere with the downstream Th17 pathway triggered by IL-23, resulting in increased release of IL-17A, -17F, -22, -26 and -29. A further relevant biologic in this context is ixekizumab, a humanised IgG4 monoclonal antibody that binds with high affinity to IL-17A/F heterodimers, thereby inhibiting the inflammatory processes involved in psoriasis [307]. Other IL-17-directed antibodies are secukinumab [308] and brodalumab, both of which bind to IL-17A [309].
Thus, even in the treatment of psoriasis, the newer substances are less small molecules and more biologics that act on the immune system and inflammatory markers.
4.4.2. Preclinical Findings on the Effect of Cannabinoids in Psoriasis
An important factor and reason for the use of cannabinoids in psoriatic skin is their property to inhibit the proliferation of keratinocytes. This antiproliferative effect on keratinocytes was observed for the CB2 agonist JWH-015 and the compound BML190 [6], which has been described as an inverse agonist at the CB2 receptor [310,311,312]. A comparable antiproliferative effect was reported for the non-selective cannabinoid receptor agonist HU-210, in whose actions, however, cannabinoid receptors did not play a mediating role [6]. Instead, the authors of the study suspected PPARγ involvement in the described effect. Experiments with cultured normal human epidermal keratinocytes from human skin samples and HaCaT cells revealed that AEA sequentially initiates a signalling mechanism leading to activation of CB1, TRPV1, calcium influx and finally cell death, thereby regulating epidermal homeostasis, which in turn prevents the initiation or exacerbation of chronic hyperproliferative, pruritic and/or proinflammatory skin diseases [313]. In another study, using CB2 knockout mice, the CB2 receptor was shown to play an important protective role in imiquimod-induced psoriasis through immunosuppression, mainly by regulating the proliferation and differentiation of CD4-positive T cells and inhibiting the expression of cytokines and chemokines [178]. Accordingly, in these animal studies, symptom exacerbation was observed in CB2 knockout mice and in animals treated with the CB2 antagonist AM-630, while symptom relief was recorded in animals treated with the CB2 agonist JWH-133. In another report, AEA was noted to reduce the proinflammatory T-cell response via CB1 receptor-induced upregulation of the mammalian target of rapamycin (mTOR) inhibitor FK506-binding protein (FKBP)12, leading to downstream inhibition of the release of IL-12 and IL-23 from keratinocytes, which results in reduced Th1 and Th17 polarisation intercellularly [314]. These CB1 receptor-dependent regulations correspond to the regulatory profile required for the treatment of psoriasis.
In a recent report, CBD was found to reduce redox imbalance in UV-irradiated keratinocytes isolated from human skin tissue by decreasing ROS levels, increasing vitamin A and E, and specifically increasing the efficiency of the thioredoxin reductase system. Thioredoxin reductase is an enzyme whose activity is increased in keratinocytes from psoriasis patients. In this context, CBD increased thioredoxin reductase activity in keratinocytes from healthy individuals but decreased it in keratinocytes from psoriasis patients and UVB-irradiated samples [7]. In another recent publication, the lipidomic profile of keratinocytes from healthy individuals and patients with psoriasis was studied with and without UVB light in the presence and absence of CBD [315]. The study found that in the keratinocytes of patients with psoriasis, phosphatidylcholines, phosphatidylinositols, phosphatidylserines and ether-linked phosphoethanolamine are reduced and sphingomyelins are upregulated, while UVB, as used in UVB phototherapy, causes upregulation of phosphatidylcholine species, phosphatidylcholine plasmalogens and ether-linked phosphoethanolamine. The study revealed that CBD induced apoptosis in keratinocytes from psoriatic patients. However, in keratinocytes from patients irradiated with UVB, CBD enhanced the antioxidant properties and prevented the loss of transepidermal water. It is noteworthy that in the above studies, no experiments with receptor antagonists were performed to prove the causal involvement or non-involvement of receptors in the effects triggered by cannabinoids. However, Jarocka-Karpowicz et al. [7] showed that CBD increased AEA levels, upregulated CB1 receptors in UV-irradiated keratinocytes from healthy and psoriasis patients, increased CB2 receptors in keratinocytes from healthy and psoriasis patients and decreased CB2 expression in UV-irradiated keratinocytes from psoriasis patients. TRPV1 was also upregulated by CBD here, but only in keratinocytes that were not exposed to UV light. In addition, a massive accumulation of CBD in the membranes of the keratinocytes was found, which was further enhanced by UVB irradiation. Recently, CBD has also been reported to activate antioxidant metabolic pathways in keratinocytes and to modulate pathways involved in keratinocyte differentiation, skin development and epidermal cell differentiation, with the latter effect occuring via nuclear export and proteasomal degradation of the transcriptional repressor BTB Domain And CNC Homolog (BACH)1 [161]. However, in this study, topical CBD (0.1% or 1%) resulted in hyperproliferation of keratinocytes and wound healing of the skin of mice, indicating a harmful rather than beneficial effect of CBD on psoriasis.
4.5. Acne
4.5.1. Current Therapies of Acne
Acne is a chronic inflammatory disease affecting the pilosebaceous unit. Symptoms include hyperseborrhoea due to increased androgen levels, inflammation and hyperkeratinisation of follicular keratinocytes [316]. The Global Burden of Disease Study identified acne vulgaris as the eighth most common skin disease, with an estimated worldwide prevalence (for all age groups) of 9.38% [317]. In the age group between 12 and 25 years, 85% of people in the United States develop acne [318]. Acne can occur non-inflammatory as an open or closed comedo, but also as an inflammatory form. Inflammatory forms of acne are usually papular or pustular acne caused by Cutibacterium acnes and are treated with, e.g., macrolides, clindamycin and tetracyclines (for review see [319]). In addition, Staphylococcus epidermis and Malassezia furfur can promote inflammation and induce follicular epidermal proliferation [320]. During puberty, sebum secretion is increased by dihydrotestosterone, which causes hyperproliferation of the follicular epidermis, leading to sebum retention [320]. Genetic variants associated with acne thus include genes that influence inflammatory responses, such as genes encoding TNF family proteins, and genes that influence sebaceous gland functions, particularly cytochrome p450 subtype 17A1 and the gene encoding follistatin [321].
Currently used therapies include topical and systemic options. Besides the antibiotics mentioned above, topical formulations contain retinoids such as retinoic acid, adapalene and tretinoin as comedolytic agents [322]. Azelaic acid is used as an antimicrobial and comedolytic substance that acts as a competitive inhibitor of 5α-reductase, thereby inhibiting the conversion of testosterone into 5α-dihydrotestosterone [323]. In addition, azelaic acid has the properties of an anti-skin-forming drug that inhibits the formation of comedones [324]. Topical salicylic acid is used for seborrhoea and comedonal acne and for pigmentation after acne has healed [325]. Consistent with its effect on sebocyte function, salicylic acid attenuates sebocyte lipogenesis by down-regulating the adenosine monophosphate-activated protein kinase (AMPK)/sterol response element-binding protein-1 (SREBP-1) pathway [326]. Another topical option is the use of dapsone, which was recently described as effective in a meta-analysis, with no significant difference in side effects compared with other drugs [327]. The reason why the safety of dapsone was questioned were reports showing that dapsone caused haemolytic anaemia in patients with glucose-6-phosphate dehydrogenase deficiency [328]. Systemic therapies further include isotretinoin [329]. Other options target hormone receptors with oral contraceptives containing low-dose oestrogen in combination with cyproterone acetate as antiandrogen [330]. Reduced production of androgens and inhibition of the effects of testosterone may eventually be achieved by spironolactone in male patients, as recently studied [331].
4.5.2. Preclinical Findings on the Effect of Cannabinoids in Acne
Several studies have shown that cannabinoids affect sebocytes, especially their inflammatory properties, and could therefore be a therapeutic option for skin conditions such as acne. In this context, CBD has been reported to exert homeostatic and anti-inflammatory effects on sebocytes [332]. Here, the authors found that CBD inhibited lipogenic effects and sebocyte proliferation in vitro via activation of TRPV4 and mediated anti-inflammatory effects in an adenosine A2a receptor-dependent manner via upregulation of the endoplasmic reticulum stress-associated factor pseudokinase tribbles homolog 3 (TRIB3) and inhibition of NF-κB signalling. In another study, the same group investigated the phytocannabinoids CBC, CBDV, CBG, CBGV and THCV for their effects on human sebocyte functions. All of the above cannabinoids caused a decrease in lipopolysaccharide (LPS)-induced release of the proinflammatory cytokines IL-6 and IL-8 and a decrease in mRNA expression of IL-1α, IL-1β and TNF-α. Among these, CBC, CBDV and THCV inhibited arachidonic acid-induced lipogenesis and THCV also reduced sebocyte proliferation [333]. The anti-inflammatory properties of topically applied phytocannabinoids such as CBC, CBCV, CBD, CBDV, ∆8-THCV, Δ8-THC and THC were further confirmed in the Croton oil mouse ear dermatitis test, measured as inhibition of the oedematous response [334]. Moreover, preclinical studies revealed a possible anti-acne effect of hemp seed hexane extracts due to their anti-microbial, anti-lipogenic and collagen-promoting properties [335]. Finally, some studies have focused on the effect of cannabinoids against bacterial-induced inflammation. In this context, CBD was found to inhibit inflammation induced by extracellular vesicles of Cutibacterium acnes [336]. In line with this notion, CBD and CBG were found to inhibit the release of IL-8 and IL-1 from normal human epidermal keratinocytes exposed to Cutibacterium acnes [154]. Moreover, CBD was recently reported to have selective activity against a subset of Gram-negative bacteria [337]. Collectively, the antimicrobial effect of various cannabinoids may play a role in the effect on acne, which would need to be further investigated in future studies.
4.6. Systemic Sclerosis
4.6.1. Current Therapies of Systemic Sclerosis
Systemic sclerosis, also called scleroderma, is a rare connective tissue disease with autoimmune character, which is mainly characterised by fibrosis of the skin, but also of the internal organs such as the lungs [338]. As the cannabinoid lenabasum has recently received orphan drug designation for the treatment of systemic sclerosis by the EMA [10], a more detailed description of the pathogenesis and pharmacotherapy of this disease is given here. Systemic sclerosis is characterised by fibroblast dysfunction with increased deposition of extracellular matrix proteins and small vessel vasculopathy leading to tissue hypoxia and autoantibody production [339]. Conventional therapy for scleroderma includes a variety of pharmacological interventions targeting a wide range of symptoms. These include, for example, vasoactive agents to control Raynaud’s disease as a typical symptom of scleroderma and to reduce the risk of amputation in the long term [340], as well as agents indicated to support healing, especially of digital ulcers, and to improve symptoms of pulmonary hypertension. Particularly difficult to treat are musculoskeletal damage, asthenia, pruritus and calcinosis, as well as skin fibrosis, whose pharmacological treatment options are currently limited to a few agents such as immunosuppressants [341]. Treatment of lung fibrosis with specific antifibrotic drugs is currently limited to blocking fibroblast growth factor receptor (FGFR) and platelet-derived growth factor (PDGF) and VEGF receptors by nintedanib [342], a triple angiokinase inhibitor [343]. Another critical symptom is the sclerodermal renal crisis, which is treated with angiotensin-converting enzyme (ACE) inhibitors [344]. As systemic sclerosis also affects internal organs, the therapy goes far beyond the conventional therapies of skin diseases with, e.g., cytostatics, anti-inflammatory substances and immunomodulators, which is why a complete description of the pharmacotherapy of systemic sclerosis is omitted here. However, the therapy of skin symptoms is similar to that of other inflammatory-immunological skin diseases. Topical therapies include tacrolimus ointments, potent corticosteroids and the vitamin D derivative calcipotriol. Systemic pharmacotherapies involve corticosteroids, but also mycophenolate mofetil, azathioprin, methotrexate, cyclophosphamide, rituximab as B-cell depletion therapy, nintedanib as a tyrosine kinase inhibitor, tocilizumab as an anti-IL-6 therapy and abatacept as an anti-cytotoxic T-cell lymphocyte-associated inhibitor (CTLA) 4 therapy [338]. The current challenge in systemic sclerosis is the treatment of skin fibrosis and other pathologies for which there is still no effective therapy, as recently noted [345].
4.6.2. Preclinical Findings on Effects of Cannabinoids in Skin Fibrosis/Systemic Sclerosis
In the following chapter, bleomycin-induced fibrosis is listed at various points as a model system for systemic sclerosis, which in this context is a suitable model for the transfer of knowledge from animals to humans, although in its original form it is also an extended model for pulmonary fibrosis [346].
In an attempt to evaluate the effects of cannabinoids on fibrosis in an in vivo model, a corresponding effect was investigated in a mouse model of diffuse systemic sclerosis by injections of hypochlorite, which induced characteristic skin and lung fibrosis [347]. Here, the application of the non-selective cannabinoid agonist WIN 55,212-2 or the selective CB2 agonist JWH-133 reduced skin and lung fibrosis as well as fibroblast proliferation and the development of autoantibodies such as anti-DNA topoisomerase 1. The antifibrotic effect of WIN 55,212-2 was confirmed in the mouse model of bleomycin-induced systemic sclerosis [348]. Consistent with these findings, CB2 knockout mice exhibited increased skin thickness and collagen content in the skin and lungs, as well as increased proliferation of fibroblasts, suggesting that the CB2 receptor mediates an endogenous antifibrotic effect in the skin and lungs [347]. In line with this, another study reported that CB2 knockout mice are more sensitive to bleomycin-induced skin fibrosis [349].
In a further investigation, the novel CBD aminoquinone VCE-004.8, a dual PPARγ and CB2 receptor agonist, was shown to confer antifibrotic properties, such as reduction of differentiation of mouse embryonic fibroblasts into myofibroblasts. This was demonstrated by a reduction in α-smooth muscle actin (α-SMA) and inhibition of skin thickness, collagen accumulation around blood vessels, dermal mast cell degranulation and macrophage infiltration in the mouse model of bleomycin-induced fibrosis [12,13]. Using the same experimental model, EHP-101, a lipid formulation of VCE-004.8, was shown to inhibit skin and lung fibrosis and downregulate the expression of several fibrosis and inflammatory markers [14]. In a subsequent study, again based on the same experimental system, the authors revealed relief of skin fibrosis, reduction in skin thickness, recovery of lipoatrophy, reduction in Ashcroft score and dermal collagen content, and prevention of collagen accumulation around vessels with daily oral administration of EHP-101 (20 mg/kg body weight) or lenabasum (5 mg/kg body weight) [15]. In general, EHP-101 and lenabasum decreased the expression of genes involved in the inflammatory and fibrotic markers of systemic sclerosis, but EHP-101 was additionally able to upregulate factors related to angiogenesis and vasculogenesis. The property of EHP-101 to inhibit fibrosis in various tissues of mice was later also confirmed in a model of angiotensin II-induced fibrosis [350]. In the bleomycin-induced skin fibrosis model, celastrol, a natural compound isolated from the traditional Chinese medicinal plant Tripterygium wilfordii Hook F with the properties of a CB2 receptor agonist, and WIN 55,212-2 were found to be effective against skin fibrosis [351]. Celastrol and WIN 55,212-2 reduced skin thickness and α-SMA, a biomarker for myofibroblasts that accumulates in the skin during systemic sclerosis, and decreased mRNA levels of several inflammatory and fibrosis markers such as TNF-α, collagen 1α and TGF-β. In general, celastrol was superior to WIN 55,212-2 in all inhibitions of systemic sclerosis markers. In addition, WIN 55,212-2 was reported to reduce the deposition of extracellular matrix in fibroblasts from patients with diffuse systemic sclerosis and to counteract their transdifferentiation into myofibroblasts and resistance to apoptosis as typical cellular abnormalties in systemic sclerosis [352]. These effects were not inhibited by cannabinoid receptor antagonists.
In one study, lenabasum was found to prevent skin fibrosis in a mouse model in which constitutively active TβRI was overexpressed [219]. Interestingly, the latter study was able to provide data showing that lenabasum reduces the amount of pro-collagen type I propeptide in the cell culture medium of fibroblasts from patients with diffuse cutaneous systemic sclerosis as a marker of collagen synthesis in a PPARγ-dependent manner. Notably, data from animal studies have shown that deletion of PPARγ leads to increased susceptibility to bleomycin-induced skin fibrosis, suggesting that PPARγ suppresses fibrogenesis [353].
The role of the CB1 receptor in fibrotic diseases appears to be more in mediating a profibrotic effect. Accordingly, CB1 receptor activation has been linked with the development of pulmonary fibrosis [354,355,356]. Consistent with these findings, silencing of FAAH was associated with increased skin fibrosis in a mouse model of bleomycin-induced fibrosis [357]. In this study, the CB1 antagonist AM-281 reversed the profibrotic effects of FAAH inhibition, while the CB2 antagonist AM-630 enhanced fibrosis, indicating an opposite unfavourable effect of the CB1 receptor and a favourable effect of the CB2 receptor. In another study, also using the bleomycin-induced fibrosis model, similar results were obtained with CB1 receptor knockout mice that did not develop fibrosis, whereas activation of CB1 with ACEA increased leukocyte infiltration and enhanced the fibrotic response to bleomycin [358]. In a recent report, the peripherally restricted CB1/inducible nitric oxide synthase (iNOS) hybrid inhibitor zevaquenabant (MRI-1867) was found to reduce bleomycin-induced fibrosis in mice deficient in the multi-drug resistance gene 1 (MDR1), which encodes the drug efflux transporter p-glycoprotein (P-gp), but not in mice with intact MDR1 expression [169]. Consistent with the beneficial role of the CB2 receptor, another recent investigation reported that a dermal formulation of the CB2 receptor agonist GP1a reduced fibrosis in a mouse model of excitional wound healing [109].
4.7. Dermatomyositis
4.7.1. Current Therapy of Dermatomyositis
Dermatomyositis belongs to the idiopathic inflammatory myopathies. Symptoms of this disease include fatigue, reduced mobility, dysphagia, dysphonia, calcinosis and dyspnoea due to weakened oesophageal and respiratory muscles [359]. Dermatomyositis may be accompanied by interstitial lung disease and is then associated with increased mortality [360]. However, there are also clinically amyopathic dermatomyositis subgroups of patients with the typical skin features of dermatomyositis such as heliotrope rash, facial erythema, Gottron’s papules and nailfold capillary abnormalities [361]. Treatment of this type of dermatomyositis involves a regimen that starts with topical steroids and calcineurin inhibitors and, depending on the response to therapy, includes further pharmacotherapeutic measures such as the use of hydroxychloroquine, methotrexate, intravenous immunoglobulins and mycophenolate mofetil. In addition, JAK inhibitors such as tofacitinib and biologics such as rituximab are also used [362].
4.7.2. Preclinical Findings on Effects of Cannabinoids in Dermatomyositis
Reference has already been made to the orphan drug designation of lenabasum in the treatment of dermatomyositis [9]. The substance, which acts as a CB2 receptor agonist, suppressed the production of TNF-α, IFN-α and IFN-β in PBMCs of dermatomyositis patients [363]. In the context of dermatomyositis, lenabasum was also able to inhibit the expression of IL-31 in skin lesions, a cytokine upregulated in dermatomyositis patients suffering from pruritus, and to downregulate the release of IL-31 into the supernatants of PBMCs obtained from dermatomyositis patients and activated by the Toll-like receptor (TLR) 9 agonist CpG oligodeoxynucleotide [364]. In a recent publication, extensive profiling of dermatomyositis skin was performed and lenabasum was found to downregulate CD4-positive T cells, IFN-β, IFN-γ, IL-31 and pathophysiological CB2 receptor expression, while a similar effect was not observed in healthy skin [8].
4.8. Epidermolysis Bullosa
4.8.1. Current Therapies of Epidermolysis Bullosa
Epidermolysis bullosa comprises a group of rare hereditary skin diseases characterised by marked fragility of the skin, which is why the disease is also called butterfly wing disease. It is predominantly caused by dominant-negative mutations in the genes encoding the keratins 5 or 14 [365]. There are four subtypes, namely epidermolysis bullosa simplex, junctional epidermolysis bullosa, dystrophic epidermolysis bullosa and Kindler syndrome [366]. Treatment of epidermolysis bullosa is usually supportive with wound care and relief of itching or pain [367]. Experimental approaches to the treatment of epidermolysis bullosa include gene therapies, cell-based therapies and protein therapies with recombinant collagen VII [367]. Pharmacological options currently being investigated include diacerein, a prodrug that after activation inhibits the plasma membrane-bound IL-1-converting enzyme, which can inhibit blistering of the skin [368]. Other pharmacological options have been derived from the treatment of atopic dermatitis, such as the reduction of blister formation after treatment with the PDE4 inhibitor apremilast [369]. Benefits for epidermolysis bullosa patients have also been shown for antipruritic therapies with dupilumab [370]. There is a body of research and anecdotal evidence for many other therapies, but the treatment of epidermolysis bullosa will be a challenge for future generations, in which cannabinoids could play a role that should not be underestimated.
4.8.2. Case Reports on Cannabinoid Effects on Epidermolysis Bullosa
In relation to epidermolysis bullosa, where cannabinoids have been applied topically, case reports and retrospective observations showed promising effects. For example, in one observational study, topical CBD oil was found to reduce the symptoms of epidermolysis bullosa in three cases (a 6-month-old boy, a 3-year-old girl and a 10-year-old boy), which was associated with less concomitant analgesic medication, fewer blisters and a shorter healing time for active blisters [22]. A recent case report further found that a CBD oil formulation improved pain scores in a 21-year-old woman suffering from recessive dystrophic epidermolysis bullosa [371]. Here, CBD was administered at a dose of 124 mg (3.1 mg/kg body weight) twice daily. According to another case report, a sublingually administered THC/CBD combination in three patients with epidermolysis bullosa (a 64-year-old woman, a 41-year-old man and a 36-year-old man) also led to an improvement in pain scores, a reduction in itching and a reduction in the pain medication taken [19]. In this study, patient 1 received sublingual doses of a cannabinoid-based oil that were stepwise increased to 2.5 mg CBD and 1.625 mg THC, four times daily. Patient 2 reached the maximum dose of 3 mg CBD and 1.95 mg THC four times daily. Patient 3 ingested dried flowers of the female cannabis plant by inhalation and only later ingested an unspecified amount of the cannabinoid oil.
The results of a recently published international anonymous cross-sectional study on the use of medical cannabinoids showed an improvement in pain with a simultaneous reduction in pain medication, as well as a reduction of pruritus and an improvement of wound healing effects in patients with epidermolysis bullosa who used cannabinoid-based medications [20]. In this study, 71 surveys of patients taking cannabinoid-based medicines were evaluated. Both THC- and CBD-containing products were used, as well as those containing only CBD or only THC. However, a recently registered phase II/III study on the efficacy and safety of a CBD cream in epidermolysis bullosa patients was withdrawn [372]. Some hope rests on the use of CBD in this context. As noted in a commentary related to the use of cannabinoids in epidermolysis bullosa, it would be time “to start planning robust studies in patients with epidermolysis bullosa”, as the effects of pain are generally under-recognised in people suffering from epidermolysis bullosa [373].
4.9. Pyoderma Gangrenosum
4.9.1. Current Therapies of Pyoderma Gangrenosum
Pyoderma gangrenosum is a painful ulcerative dermatosis associated with an autoimmune dysfunction characterised by dysregulation of neutrophil function and contribution of the Th17 immune axis which most frequently affects the lower extremity and appears associated with other diseases such as inflammatory bowel disease (34–65%), polyarthritis (16–19%) and hematologic disorders (12–20%) [374]. As pyoderma gangrenosum is an immunological disease, pharmacotherapy has a similar pattern to atopic dermatitis and psoriasis. Accordingly, the pharmacological management includes topical corticosteroids and tacrolimus, systemic treatment with corticosteroids, cyclosporine A, tacrolimus, azathioprine, methotrexate, mycophenolate mofetil as well as targeted biological therapies with anti-TNF-α treatments such as infliximab, adalimumab, etanercept, and IL-12/23 treatment with ustekinumab (for review see [375]). Biological treatment options for pyoderma gangrenosum furthermore include strategies from rheumatic treatment such as targeting IL-1 with anakinra, canakinumab and gevokizumab. Further cytostatic drugs applied in treatment of pyoderma gangrenosum include systemic therapies with cyclophosphamide, sulfasalazine and chlorambucil as well as antibiotics such as minocycline. A therapeutic problem associated with pyoderma gangrenosum is the treatment of wound-related pain requiring, for example, topical opioids which are associated with reduced likelihood of healing in patients with chronic wounds [376].
4.9.2. Case Report on Cannabinoid Effects on Pyoderma Gangrenosum
A prospective case series of pyoderma gangrenosum [21] reported improvements, particularly in pain management, observed in three patients (a 50-year-old woman, a 76-year-old man and a 60-year-old woman) treated with cannabinoids. In one case, the topical oil ARGYLE™ (THC 5 mg/mL + CBD 6 mg/mL; 1.0 mL applied daily to the wound bed) and in the other two cases, the oil Bedrolite™ (THC 7 mg/mL + CBD 9 mg/mL; 0.5 to 1.0 mL applied twice daily to the wound bed plus once to three times daily for breakthrough pain) was used. Here, the case report showed an analgesic effect of topical medicinal cannabis associated with reduced opioid consumption, suggesting that cannabinoid therapies may also be beneficial and useful as topical applications for patients with pain-related skin conditions.
4.10. Beneficial Effects of Cannabinoids in Other Disorders
4.10.1. Acute Inflammation
In an in vivo assay to identify cannabinoid substances that have an inhibitory effect on TPA-induced inflammation, a model of acute inflammation with erythema, edema and polymorphonuclear leukocyte infiltration [377], a series of 23 different analogues of synthetic cannabinoids were tested [378]. Among them, the topically applied naphthoylindoles JWH-018, -122 and -210 showed the strongest inhibitory effect on the formation of oedema on the ear of mice. In a recent in vivo study, it was also found that the topical formulation of CBD and PEA reduced TPA-induced ear oedema by 51.27% at 24 h and 65.69% at 48 h (in the control group, the reduction was 26.32% at 24 h and 44.21% at 48 h), suggesting that such a combined formulation has a remarkable effect on inflamed skin tissue [379]. These result are further supported by in vitro studies using a novel selective CB2 agonist, the 1,8-naphthyridine-2(1H)-one-3-carboxamide derivative JT11, that showed CB2 receptor-dependent inhibition of LPS-induced p42/44 MAPK and NF-κB activation, as well as inhibition of LPS-induced release of TNF-α, IL-1β, IL-6 and IL-8 from human PBMCs, with the described effects partially inhibited by the CB2 antagonist SR144528 [380].
4.10.2. Keratin Disorders
The results of experiments focusing on the influence of cannabinoids on keratin synthesis, as already mentioned in Section 3.2.8, suggest that the use of certain cannabinoids may be beneficial in the treatment of diseases associated with keratin dysregulation, as is the case with a genodermatosis such as pachyonychia congenita [381] or a rare disease such as cicatricial alopecia (for review see [382]). On this basis, there has already been speculation about the extent to which endocannabinoid analogues might be helpful in the treatment of pachyonychia congenita [160]. A hyperkeratosis disorder, epidermolytic ichthyosis, a rare genodermatosis caused by mutations in keratins 1 or 10, has a profile of keratin dysregulation that may be counter-regulated by cannabinoids, as recently shown in an image correlation describing that ACEA upregulates the expression of keratin 10 in human epidermis and decreases the expression of keratin 1 in human skin organ culture [383]. However, to confirm the assumption that cannabinoids could help in such diseases, clinical studies would have to provide clarity.
4.10.3. Scars and Keloids
Other work suggests a possible topical application of cannabinoids for the prevention or treatment of hypertrophic scars or keloids. Accordingly, the CB1 receptor agonist ACEA significantly increased collagen deposition in primary cultures of adult human fibroblasts obtained from TGF-β-treated human abdominal skin, while the CB1 antagonist AM-251 decreased α-SMA expression, collagen content and fibroblast differentiation [176]. Under the same experimental conditions, the authors also found that the CB2 receptor agonist JWH-133 decreased α-SMA expression and collagen content in TGF-β-stimulated cells, but not in resting cells. On the other hand, the CB2 receptor antagonist AM-630 also decreased α-SMA expression and collagen content in both resting and TGF-β-stimulated cells, but at higher concentrations (≥3 µM) [177]. The authors concluded that the effects produced by JWH-133 show potential for the treatment of hypertrophic scars or keloids in humans.
5. Clinical Trials with Cannabinoids
The above preclinical data has led to numerous clinical studies on the possible effects of cannabinoids on skin diseases that have been conducted and are still being conducted, with no posted results to date. One advantage of cannabinoids seems to be that they are relatively safe. For example, topical administration of a CBD-enriched ointment has recently been shown to be safe and effective in patients with inflammatory skin conditions [384]. In this study, no irritant or allergic reactions were observed during treatment. However, as this was an anecdotal, retrospective study without a blinded vehicle control group, the observations made here on the effects of cannabinoids on symptom relief cannot be generalised. Another non-randomised controlled trial conducted in two centres in Australia and New Zealand, looking at the safety of the transdermal CBD formulation ZYN002 in the context of the treatment of developmental disorders and epileptic encephalopathies, concluded that this CBD gel was safe and well tolerated [385]. Measures of this trial also included skin examination. In addition, an ongoing study is investigating the pharmacokinetic and pharmacodynamic effects of 5 different topically applied CBD-containing products, in which cognitive performance, working memory and psychomotor performance are also being interrogated [386]. In the following, clinical studies on the effect of cannabinoids in various diseases are listed alphabetically according to the corresponding disease.
5.1. Acne Vulgaris
CBD has been repeatedly discussed as a remedy for acne due to its anti-inflammatory properties [387,388]. As a consequence of the preclinical results, BTX 1503, a transdermal gel formulation containing synthetic CBD, is currently under clinical evaluation for the treatment of moderate-to-severe acne vulgaris [389]. The results of this study have not yet been published. Interestingly, however, in a single-blind comparative study to investigate tolerability and reduction of sebum and erythema, healthy men were treated twice daily for 12 weeks with a cream containing 3% cannabis seed extract on one cheek, with a control cream applied to the other cheek. The authors reported a reduction in the sebum and redness content of the skin on the sides treated with cannabis seed extract, suggesting a possible use of this extract for the treatment of acne, seborrhoea, papules and pustules [390]. Expanding the therapeutic options for acne would be a valuable step in treatment, because the treatment of acne with isotretinoin, for example, is known to cause serious side effects such as hypertriglyceridaemia, hepatotoxicity and mood disorders, e.g., depression and psychosis [391,392], which is why the status of these drugs has repeatedly been critically questioned [393].
5.2. Acute Inflammation
In a recent study investigating the anti-inflammatory effect of lenabasum, healthy male volunteers were injected with E. coli killed by UV light into the dermis, where they induced a strong local infiltration of neutrophils. Thereby, lenabasum showed a similar anti-inflammatory efficacy to prednisolone in terms of inhibiting neutrophil infiltration [221]. Furthermore, using inflammatory exudate from the injection site, lenabasum was found to accelerate the resolution of the inflammation by inhibiting chemoattractant leukotriene B4 and antiphagocytic prostanoids (PGE2, PGF2α and thromboxane B2), while maintaining levels of the vasodilatory prostacyclin and having only a modest effect on the regulation of cytokines/chemokines that trigger acute inflammation. Instead, the proresolving factors lipoxin A4 and B4 as well as resolvin D1 and D3 were upregulated. At the local site of acute inflammation, lenabasum caused a dose-dependent increase in microvascular hyperaemia, which, however, was not oedematous, painful or inflammatory and was not accompanied by an increase in neutrophils or macrophages or increased biosynthesis of nitric oxide. The authors concluded that this effect was due to an aspirin-like action based on inhibition of vasoconstrictive thromboxane while maintaining prostacyclin levels.
5.3. Androgenetic Alopecia
A recent study found that daily application of a topical hemp oil formulation containing 3–4 mg of CBD and minimal amounts of other cannabinoids over a six-month period reduced hair loss associated with androgenetic alopecia [394]. The authors of the study announced that they will conduct a follow-up study with topical hemp oil extracts that contain high levels of THCV, CBDV and CBD. This study is now registered [395] and is also investigating, as a case series with 40 participants, the effect of two dosages (500 mg or 1000 mg of hemp oil per dispenser, which then results in either 1.7 mg or 3.4 mg of hemp extract per day) on androgenetic alopecia. Also worth mentioning is a case report of a 52-year-old man with alopecia areata showing that the application of cannabis oil in the area of hair loss helped the hair to grow again [396].
5.4. Asteatotic Eczema
A skin condition for which cannabinoids have also been tested is asteatotic eczema, also known as eczema craquelé or xerotic eczema. This is an itchy dermatitis with dry, scaly, inflamed skin that becomes cracked and fissured in advanced stages of the disease [397] and is the most common eczema of older people [398]. Treatment of asteatotic eczema consists of hydrating the skin through the use of lotions with high oil content. Pharmacological interventions are limited to topical corticosteroids such as fluocinolone, triamcinolone and betamethasone or topical pimecrolimus [399].
A monocentric randomised controlled trial concluded that regular use of a topical PEA/AEA emollient in patients with asteatotic eczema can improve both physicochemical and neuroimmuno-endocrine skin functions [400]. Symptoms improved by the PEA/AEA emollient included an increase in the 5 Hz current perception threshold and a reduction of itch, as well as improvements in skin surface hydration and transepidermal water loss, although the latter did not reach statistical significance compared with the PEA/AEA-free emollient.
5.5. Atopic Dermatitis
Two studies published in 2007 focused on the effect of PEA and adelmidrol in the treatment of atopic dermatitis [17,401]. The result of a study with 25 children and 18 adults was that a cream containing PEA caused a faster healing of a neurodermatitis flare-up on the respective treated side of the body and prolonged the mean time until the occurrence of another flare [17]. In another pilot study of 20 patients with mild atopic dermatitis, 2% adelmidrol caused improvement of erythema and pruritus in 60% of patients after 10–15 days, with complete resolution observed in 80% of patients after 4 weeks of treatment [401]. In a recent study on the effects of CBD (BTX 1204) on atopic dermatitis [16], CBD did not meet primary endpoints [402]. A more recent investigation confirmed the lack of effect of CBD but showed that JW-100, a novel CBD and aspartame formulation, resulted in significant improvements in atopic dermatitis after 14 days of topical application [18]. Patients with mild-to-moderate atopic dermatitis benefited particularly, as half of them had no or almost no symptoms after treatment. The authors confirmed here the effects that had previously been observed in a pilot study with a smaller group of patients [403]. In the latter study, 67% of the 16 patients who completed the study reported a decrease in itching and 50% noted an improvement in eczema of more than 60%. In another work analysing the effects and ingredients of hemp seed oil, patients with atopic dermatitis reported a decrease in skin dryness and itching, as well as a reduction in skin medication after using hemp seed oil [404]. In this study, however, the authors concluded that this effect was due to the high content of polyunsaturated fatty acids in hemp seed oil rather than specific cannabinoid effects. The CB2 receptor agonist S-777469 is also being investigated for the treatment of atopic dermatitis [405]. This double-blind, randomised, placebo-controlled, phase Ib/IIa, multiple-dose study evaluated the safety, tolerability, pharmacokinetics and pharmacodynamics of S-777469 in patients with mild-to-moderate atopic dermatitis. As secondary outcome, a possible antipruritic and anti-inflammatory effect was assessed. However, the results of this study have not yet been published. Another single-blind clinical trial conducted in 20 healthy volunteers addressed a possible anti-inflammatory effect of CBG in a sodium lauryl sulphate-induced skin irritation model of contact dermatitis. CBG here showed improvement over placebo in reducing transepidermal water loss and redness [154].
5.6. Atopic Eczema
A multinational, multicentre, observational, non-controlled, prospective cohort study of 2456 patients with mild-to-moderate atopic eczema who used an emollient containing PEA (ATOPA study), initiated as early as 2004, found that this formulation resulted in significant relief of skin dryness, excoriation, lichenification, scaling, erythema and pruritus [406]. Notably, the latter study did not include a placebo control. Another investigation is currently being prepared to investigate the size of eczema and its severity under the influence of a topical cream containing 1.5% PEA traded as Levagen®+ [407].
5.7. Dermatomyositis
A double-blind, randomised, placebo-controlled phase II study investigated the safety, tolerability and efficacy of lenabasum in 22 patients with dermatomyositis [408]. As a result of this study, the authors observed a decrease in the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) in the lenabasum-treated group compared to the placebo group, which was significant at day 113 [409]. The company has meanwhile announced the follow-up multicentre, randomised, double-blind, placebo-controlled phase III study “DETERMINE study of lenabasum for the treatment of dermatomyositis” to further investigate the efficacy and safety of lenabasum in dermatomyositis with 176 participants [410]. This study has already been officially completed, but the results have not yet been published. Initial results from this phase III double-blind study in dermatomyositis showed that although lenabasum did not meet the primary and secondary efficacy endpoints, there were significant beneficial effects in the Total Improvement Score (TIS) with enhancement in muscle strength in patients with muscle weakness and significant favourable outcome in the CDASI with reduction in rash in patients without muscle weakness [411]. Notably, lenabasum has been granted an orphan drug designation status by the EMA for the treatment of dermatomyositis [9].
5.8. Hidradenitis Suppurativa
Hidradenitis suppurativa is a chronic, recurrent, inflammatory skin disease in which hair follicles in apocrine gland areas of the body are inflamed and manifest as painful nodules, abscesses, fistulas and scarring [412]. Antibiotics such as topical clindamycin are the first-line therapies here. Other options include oral antibiotics, retinoids, dapsone, oral contraceptives, oral immunomodulators and anti-TNF-α therapy [413]. A randomised, double-blind, placebo-controlled phase Ⅱ study, apparently interrupted prematurely in the recruitment phase by the COVID-19 pandemic, was designed to investigate the efficacy and safety of a cannabis oil in the treatment of 40 patients with hidradenitis suppurativa [414]. The basis of this study is the analgesic effect of cannabinoids, which is associated with a reduction in CGRP release and is therefore also mentioned as such in a review article on topically applicable analgesics in the context of hidradenitis suppurativa [415].
5.9. Neurogenic Flare Reaction
An early investigation addressed the effect of the peripherally delivered cannabinoid agonist HU-210 on histamine-induced inflammatory and pruritic responses in human skin of healthy volunteers. To this end, HU-210 was administered by skin patch or dermal microdialysis. In this study, the effects of HU-210 on vasodilatation and pruritus were measured after administration of histamine by iontophoresis or microdialysis [416]. To evaluate protein plasma extravasation, simultaneous administration of HU-210 and histamine was performed by dermal microdialysis, whereas in the analysis of vasodilatation and pruritus HU-210 was applied via a patch. HU-210 decreased itch rating, local blood flow and axon reflex flare response, while protein plasma extravasation was enhanced by HU-210 after histamine application. The fact that itch was diminished without reducing plasma extravasation of proteins suggests, according to the authors, that the relief of itch is not due to an antihistaminergic property of HU-210.
5.10. Postherpetic Neuralgia
Postherpetic neuralgia is an adverse effect appearing in herpes zoster cases with burning pain which is difficult to treat. Pain treatment includes non-steroidal anti-inflammatory drugs, opioid analgesics, carbamazepine, gabapentin and pregabalin [417]. In an open-labeled observational study, 5 of 8 patients reported a mean pain reduction of 87.8% after use of a cream containing PEA [418].
5.11. Pruritus (Chronic)
The effects of PEA have also been investigated in patients with chronic pruritus [419]. This prospective, randomised, controlled, open-label, non-interventional study on dry skin-induced chronic pruritus also examined the antipruritic effect of PEA compared to a PEA-free body lotion that served as a vehicle control. In this study, the authors did not find a significant difference between the lotion with and without PEA in terms of improvement of pruritus and quality of life.
5.12. Pruritis (Uremic)
In one study, 21 patients with uremic pruritis due to end-stage renal failure were tested with a cream containing structured physiological lipids and endogenous cannabinoids (AEA and PEA; patented as Physiogel AI® cream) applied twice daily for a period of three weeks. In 8 patients the itching was completely eliminated and in 17 patients a complete reduction of xerosis was observed [420]. However, in this study the PEA group was not compared with a placebo group and no information was given on the amounts of AEA and PEA administered.
5.13. Psoriasis
Unlike other diseases, there are no solid clinical studies in the literature that directly address the effect of cannabinoids on patients’ psoriatic skin symptoms. Only CBD was studied, but it could not relieve psoriatic arthritis pain compared to placebo [421]. Furthermore, a case report was published in which a 33-year-old patient with psoriasis after failure of therapeutic application of hydrocortisone and triamcinolone acetonide cream successfully used a combination of cannabinoid-containing applications, consisting of THC distillate cream with medium-chain triglyceride oil, beeswax and sunflower lecithin and THC soap infused with a scent-free hemp soap, and a hair oil containing THC distillate dissolved in jojoba oil. The symptoms were improved after 2 days, and this effect lasted permanently when the THC-containing products were used as needed [422]. A spontaneous, anecdotal, retrospective study showed that CBD ointment also improved the Psoriasis Area and Severity Index (PASI) at day 90 from baseline, although the study design excluded a comparison with placebo [384].
5.14. Scalp Inflammation (Scalp Psoriasis/Seborrhoeic Dermatitis)
A recently published study of 50 subjects with mild-to-moderate scalp psoriasis or seborrhoeic dermatitis found that a CBD-containing shampoo significantly reduced both the severity and symptoms of scalp inflammation within two weeks with excellent tolerability [423]. However, it must be mentioned that this study did not include a comparison or placebo control with, for example, a cannabinoid-free shampoo, which significantly affects the conclusions drawn from this study.
5.15. Systemic Sclerosis
Further clinical trials were conducted on the effect of lenabasum in diffuse cutaneous systemic sclerosis, where lenabasum exhibited antifibrotic and anti-inflammatory activity [424,425,426]. In addition to a well-tolerated safety trial in which dizziness was the most common adverse effect, treatment with lenabasum was associated with improvement in several efficacy assessments of overall disease, skin involvement and patient-reported function. Lenabasum has recently received orphan drug status for the treatment of systemic sclerosis [10].
5.16. Urticaria (Chronic Spontaneous)
A recently registered, single-centre, placebo-controlled, open-label phase IIa trial is designed to investigate the safety, tolerability and efficacy of CBD as a potential treatment for patients with chronic spontaneous urticaria [427], another skin disorder in which histamine and other proinflammatory mediators involved in pathogenesis are released. However, the results of this study are not yet available.
5.17. Venous Leg Ulcers
Finally, data from a recent small open-label trial suggest that cannabinoids are a therapeutic option for rapid wound closure of previously non-healing venous leg ulcers [126]. This study tested the wound healing properties of cannabinoids in 14 patients with ulcers due to chronic venous hypertension and existing venous insufficiency. The test preparations were VS-12 and VS-14, which are hyaluronic acid and aloe vera gel-based (VS-12) and liposomal-based formulations of a mixture of CBD, THC, quercetin, diosmin, hesperidin and β-caryophyllene, respectively, applied to the wound bed and periwound area. Completely epithelialised wound closure was achieved in 11 of 14 patients within a median of 34 days, which is optimistic for larger studies that are definitely needed on this topic.
The clinical studies presented in chapter 5 for the individual indications are summarised in Table 3, differentiated for PEA, CBD and lenabasum.

Table 3.
Clinical studies on the 3 most important compounds in the investigation of cannabinoid effects on patients’ skin.
6. Conclusions
Based on the current publications, it can be summarised that cannabinoid compounds have great potential in the treatment of skin diseases, both as topical applications and as systemic medications. It can also be assumed that other effects of cannabinoids, such as the improvement of impaired sleep that has been outlined in a recent systematic review [429], could have a positive influence on the quality of life of patients with painful and itchy skin diseases who often also suffer from sleep disorders. However, this effect of cannabinoids has not yet been investigated in patients with such skin diseases.
Since there are indications of possible effects of cannabinoids in several skin diseases other than those studied in patients so far, it can be further assumed that the potential of cannabinoids is far from exhausted and that further indications will be tested in the coming years. Accordingly, preclinical studies of cannabinoid-mediated molecular biological effects and mechanisms suggest that they may be effective in conditions such as rosacea, actinic keratosis, epidermolytic ichthyosis, hypertrophic scars, keloids, pachyonychia congenita, cicatricial alopecia or hidradenitis suppurativa. Robust clinical trials are needed in these areas.
Since some studies have already shown that, depending on skin type and gender, partly opposite effects can occur due to cannabinoids, clinical studies should primarily include larger patient collectives that allow for corresponding subgroup analyses. Accurate and quantitatively robust analysis of the clinical effects of cannabinoids on skin conditions is also important in that many cannabinoid-based products are now freely available on the market and, accordingly, are also used by patients for various treatments, including skin conditions [430], which in turn may be associated with risks.
From the currently available data, it appears that cannabinoid receptor agonists not only have positive effects, but that in some cases such as androgenetic alopecia or fibrotic diseases, CB1 receptor antagonists rather than agonists are indicated. Activation of cannabinoid receptors also leads to increased sebum production in the skin and may therefore be detrimental to the development of acne. In special cases such as fibrosis and systemic sclerosis, on the other hand, dual CB2/PPARγ agents such as lenabasum are more suitable cannabinoids. In some diseases, such as atopic dermatitis, the role of cannabinoid receptors must also be critically evaluated due to conflicting data. Due to these complex effects of agonists and antagonists at different cannabinoid-sensitive receptors on the skin, it is accordingly important to further investigate the role of these receptors in basic research.
The difficulty for the future development of new cannabinoid-based medicines also arises from the fact that in many publications it unfortunately remains open which receptors mediate the corresponding effects or whether a specific cannabinoid effect or a purely physicochemical fatty acid effect is involved in the improvement of skin structure and symptom relief. For this reason, too, further preclinical basic research is important. Only a more comprehensive understanding of the role of individual receptors of the endocannabinoid system in skin diseases will allow the targeted use of specific cannabinoids or even their chemical modifications to provide the optimal medication for each disease.
What can be stated with certainty today is that a major advantage of cannabinoids is their interaction with a variety of receptors, which can accordingly lead to improvements in skin diseases via multimodal mechanisms of action. However, the findings published so far seem to be far from a comprehensive understanding of the role of the PPAR, TRP, GPR55 and GPR119 receptors in connection with the effects of cannabinoids on the skin. In this context, based on current data, CBD appears to have an exceptionally attractive interaction profile with various target structures that is pharmacotherapeutically useful for patients with a variety of skin diseases. In addition, CBD has a relatively safe risk-benefit ratio, although its possible harmful influence on psoriasis is controversial.
Finally, the possibility of intervening pharmacotherapeutically with active substances in systems whose target structures contribute to a natural homeostasis of skin functions is of great interest for the therapy of skin diseases. The endocannabinoid system clearly belongs to these systems.
Author Contributions
Conceptualisation, B.H. and R.R.; methodology, B.H. and R.R.; software, B.H. and R.R.; validation, B.H. and R.R.; formal analysis, B.H. and R.R.; investigation, B.H. and R.R.; resources, B.H.; data curation, B.H. and R.R.; writing—original draft preparation, B.H. and R.R.; writing—review and editing, B.H. and R.R.; visualisation, R.R.; supervision, B.H.; project administration, B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The corresponding author’s (B.H.) preclinical experimental work on the effects of cannabinoids and chemotherapeutic agents is funded by the German Research Foundation (DFG, HI 813/9-1), the joint research project ONKOTHER-H (supported by the European Social Fund, reference: ESF/14-BM-A55-0002/18, and the Ministry of Education, Science, and Culture of Mecklenburg-Vorpommern, Germany), and the joint research projects RESPONSE FV18 (reference: 03ZZ0928A) and RESPONSE TV3 (reference: 03ZZ0933A) of the Federal Ministry of Education and Research (BMBF), Germany. Moreover, the authors would like to thank Felix Wittig, Jessica Pichl and Jana Spaller for their support in preparing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramer, R.; Wendt, F.; Wittig, F.; Schäfer, M.; Boeckmann, L.; Emmert, S.; Hinz, B. Impact of Cannabinoid Compounds on Skin Cancer. Cancers 2022, 14, 1769. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Kirchhof, M.G. Dermatology-Related Uses of Medical Cannabis Promoted by Dispensaries in Canada, Europe, and the United States. J. Cutan. Med. Surg. 2019, 23, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Karsak, M.; Gaffal, E.; Date, R.; Wang-Eckhardt, L.; Rehnelt, J.; Petrosino, S.; Starowicz, K.; Steuder, R.; Schlicker, E.; Cravatt, B.; et al. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science 2007, 316, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid Signaling in the Skin: Therapeutic Potential of the “C(ut)annabinoid” System. Molecules 2019, 24, 918. [Google Scholar] [CrossRef]
- Haruna, T.; Soga, M.; Morioka, Y.; Imura, K.; Furue, Y.; Yamamoto, M.; Hayakawa, J.; Deguchi, M.; Arimura, A.; Yasui, K. The Inhibitory Effect of S-777469, a Cannabinoid Type 2 Receptor Agonist, on Skin Inflammation in Mice. Pharmacology 2017, 99, 259–267. [Google Scholar] [CrossRef]
- Wilkinson, J.D.; Williamson, E.M. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J. Dermatol. Sci. 2007, 45, 87–92. [Google Scholar] [CrossRef]
- Jarocka-Karpowicz, I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Effects on Phospholipid Metabolism in Keratinocytes from Patients with Psoriasis Vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef]
- Maddukuri, S.; Patel, J.; Diaz, D.A.; Chen, K.L.; Wysocka, M.; Bax, C.; Li, Y.; Ravishankar, A.; Grinnell, M.; Zeidi, M.; et al. Cannabinoid type 2 receptor (CB2R) distribution in dermatomyositis skin and peripheral blood mononuclear cells (PBMCs) and in vivo efects of LenabasumTM. Arthritis Res. Ther. 2022, 24, 12. [Google Scholar] [CrossRef]
- EU Designation Number: EU/3/18/2070. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3182070 (accessed on 15 June 2022).
- EU Designation Number: EU/3/16/1808. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3161808 (accessed on 15 June 2022).
- Vanti, G.; Grifoni, L.; Bergonzi, M.C.; Antiga, E.; Montefusco, F.; Caproni, M.; Bilia, A.R. Development and optimisation of biopharmaceutical properties of a new microemulgel of cannabidiol for locally-acting dermatological delivery. Int. J. Pharm. 2021, 607, 121036. [Google Scholar] [CrossRef]
- Del Río, C.; Navarrete, C.; Collado, J.A.; Bellido, M.L.; Gómez-Cañas, M.; Pazos, M.R.; Fernández-Ruiz, J.; Pollastro, F.; Appendino, G.; Calzado, M.A.; et al. The cannabinoid quinol VCE-004.8 alleviates bleomycin-induced scleroderma and exerts potent antifibrotic effects through peroxisome proliferator-activated receptor-γ and CB2 pathways. Sci. Rep. 2016, 6, 21703. [Google Scholar] [CrossRef]
- Palomares, B.; Ruiz-Pino, F.; Navarrete, C.; Velasco, I.; Sánchez-Garrido, M.A.; Jimenez-Jimenez, C.; Pavicic, C.; Vazquez, M.J.; Appendino, G.; Bellido, M.L.; et al. VCE-004.8, A Multitarget Cannabinoquinone, Attenuates Adipogenesis and Prevents Diet-Induced Obesity. Sci. Rep. 2018, 8, 16092. [Google Scholar] [CrossRef] [PubMed]
- García-Martín, A.; Garrido-Rodríguez, M.; Navarrete, C.; Del Río, C.; Bellido, M.L.; Appendino, G.; Calzado, M.A.; Muñoz, E. EHP-101, an oral formulation of the cannabidiol aminoquinone VCE-004.8, alleviates bleomycin-induced skin and lung fibrosis. Biochem. Pharmacol. 2018, 157, 304–313. [Google Scholar] [CrossRef] [PubMed]
- García-Martín, A.; Garrido-Rodríguez, M.; Navarrete, C.; Caprioglio, D.; Palomares, B.; DeMesa, J.; Rollland, A.; Appendino, G.; Muñoz, E. Cannabinoid derivatives acting as dual PPARγ/CB2 agonists as therapeutic agents for systemic sclerosis. Biochem. Pharmacol. 2019, 163, 321–334. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine, ClinicalTrials.gov. Study of the Safety, Tolerability and Efficacy of BTX 1204 in Patients with Moderate Atopic Dermatitis. ClinicalTrials.gov Identifier: NCT03824405. Available online: https://clinicaltrials.gov/ct2/show/NCT03824405?term=CBD&cond=Skin+Diseases&draw=1&rank=15 (accessed on 15 June 2022).
- Del Rosso, J.Q. Use of a palmitoylethanolamide-containing nonsteroidal cream for treating atopic dermatitis: Impact on the duration of response and time between flares. Cosmetic Dermatol. 2007, 20, 208–211. [Google Scholar]
- Gao, Y.; Li, Y.; Tan, Y.; Liu, W.; Ouaddi, S.; McCoy, J.; Kovacevic, M.; Situm, M.; Stanimirovic, A.; Li, M.; et al. Novel cannabidiol aspartame combination treatment (JW-100) significantly reduces ISGA score in atopic dermatitis: Results from a randomized double-blinded placebo-controlled interventional study. J. Cosmet. Dermatol. 2021, 21, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Schräder, N.H.B.; Duipmans, J.C.; Molenbuur, B.; Wolff, A.P.; Jonkman, M.F. Combined tetrahydrocannabinol and cannabidiol to treat pain in epidermolysis bullosa: A report of three cases. Br. J. Dermatol. 2019, 180, 922–924. [Google Scholar] [CrossRef]
- Schräder, N.H.B.; Gorell, E.S.; Stewart, R.E.; Duipmans, J.C.; Harris, N.; Perez, V.A.; Tang, J.Y.; Wolff, A.P.; Bolling, M.C. Cannabinoid use and effects in patients with epidermolysis bullosa: An international cross-sectional survey study. Orphanet J. Rare Dis. 2021, 16, 377. [Google Scholar] [CrossRef] [PubMed]
- Maida, V.; Corban, J. Topical Medical Cannabis: A New Treatment for Wound Pain-Three Cases of Pyoderma Gangrenosum. J. Pain Symptom Manag. 2017, 54, 732–736. [Google Scholar] [CrossRef]
- Chelliah, M.P.; Zinn, Z.; Khuu, P.; Teng, J.M.C. Self-initiated use of topical cannabidiol oil for epidermolysis bullosa. Pediatr. Dermatol. 2018, 35, e224–e227. [Google Scholar] [CrossRef]
- Neff, G.W.; O’Brien, C.B.; Reddy, K.R.; Bergasa, N.V.; Regev, A.; Molina, E.; Amaro, R.; Rodriguez, M.J.; Chase, V.; Jeffers, L.; et al. Preliminary observation with dronabinol in patients with intractable pruritus secondary to cholestatic liver disease. Am. J. Gastroenterol. 2002, 97, 2117–2119. [Google Scholar] [CrossRef]
- Laborada, J.; Cohen, P.R. Cutaneous Squamous Cell Carcinoma and Lichen Simplex Chronicus Successfully Treated with Topical Cannabinoid Oil: A Case Report and Summary of Cannabinoids in Dermatology. Cureus 2022, 14, e23850. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Carlini, E.A. Toward drugs derived from cannabis. Naturwissenschaften 1978, 65, 174–179. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Mackie, K.; Devane, W.A.; Hille, B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol. Pharmacol. 1993, 44, 498–503. [Google Scholar]
- Sugiura, T.; Kodaka, T.; Nakane, S.; Miyashita, T.; Kondo, S.; Suhara, Y.; Takayama, H.; Waku, K.; Seki, C.; Baba, N.; et al. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J. Biol. Chem. 1999, 274, 2794–2801. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Kishimoto, S.; Miyashita, T.; Nakane, S.; Kodaka, T.; Suhara, Y.; Takayama, H.; Waku, K. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J. Biol. Chem. 2000, 275, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Pokorski, M.; Matysiak, Z. Fatty acid acylation of dopamine in the carotid body. Med. Hypotheses 1998, 50, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Melck, D.; Bobrov, M.; Gretskaya, N.M.; Bezuglov, V.V.; De Petrocellis, L.; Di Marzo, V. N-acyl-dopamines: Novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem. J. 2000, 351, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Bisogno, T.; Trevisani, M.; Al-Hayani, A.; De Petrocellis, L.; Fezza, F.; Tognetto, M.; Petros, T.J.; Krey, J.F.; Chu, C.J.; et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 8400–8405. [Google Scholar] [CrossRef] [PubMed]
- Hanus, L.; Abu-Lafi, S.; Fride, E.; Breuer, A.; Vogel, Z.; Shalev, D.E.; Kustanovich, I.; Mechoulam, R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 3662–3665. [Google Scholar] [CrossRef]
- Porter, A.C.; Sauer, J.-M.; Knierman, M.D.; Becker, G.W.; Berna, M.J.; Bao, J.; Nomikos, G.G.; Carter, P.; Bymaster, F.P.; Leese, A.B.; et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002, 301, 1020–1024. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Reiner, J.; Jensen, E.D. Pentadecanoylcarnitine is a newly discovered endocannabinoid with pleiotropic activities relevant to supporting physical and mental health. Sci. Rep. 2022, 12, 13717. [Google Scholar] [CrossRef]
- Maccarrone, M.; Attinà, M.; Cartoni, A.; Bari, M.; Finazzi-Agrò, A. Gas chromatography-mass spectrometry analysis of endogenous cannabinoids in healthy and tumoral human brain and human cells in culture. J. Neurochem. 2001, 76, 594–601. [Google Scholar] [CrossRef]
- Schwarz, R.; Ramer, R.; Hinz, B. Targeting the endocannabinoid system as a potential anticancer approach. Drug Metab. Rev. 2018, 50, 26–53. [Google Scholar] [CrossRef]
- Di Marzo, V.; Melck, D.; Orlando, P.; Bisogno, T.; Zagoory, O.; Bifulco, M.; Vogel, Z.; De Petrocellis, L. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem. J. 2001, 358, 249–255. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Bisogno, T.; Ligresti, A.; Bifulco, M.; Melck, D.; Di Marzo, V. Effect on cancer cell proliferation of palmitoylethanolamide, a fatty acid amide interacting with both the cannabinoid and vanilloid signalling systems. Fundam. Clin. Pharmacol. 2002, 16, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Pauselli, R.; Di Rienzo, M.; Finazzi-Agrò, A. Binding, degradation and apoptotic activity of stearoylethanolamide in rat C6 glioma cells. Biochem. J. 2002, 366, 137–144. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.-H.; Vogel, Z.; Bisogno, T.; De Petrocellis, L.; Di Marzo, V.; Mechoulam, R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 1998, 353, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 2009, 60, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, D.G.; Chin, S.A. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem. Pharmacol. 1993, 46, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Sun, Y.-X.; Okamoto, Y.; Araki, N.; Tonai, T.; Ueda, N. Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J. Biol. Chem. 2005, 280, 11082–11092. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Pontis, S.; Mengatto, L.; Armirotti, A.; Chiurchiù, V.; Capurro, V.; Fiasella, A.; Nuzzi, A.; Romeo, E.; Moreno-Sanz, G.; et al. A Potent Systemically Active N-Acylethanolamine Acid Amidase Inhibitor that Suppresses Inflammation and Human Macrophage Activation. ACS Chem. Biol. 2015, 10, 1838–1846. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sørgård, M.; Di Marzo, V.; Julius, D.; Högestätt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452–457. [Google Scholar] [CrossRef]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Duncan, M.; Millns, P.; Smart, D.; Wright, J.E.; Kendall, D.A.; Ralevic, V. Noladin ether, a putative endocannabinoid, attenuates sensory neurotransmission in the rat isolated mesenteric arterial bed via a non-CB1/CB2 Gi/o linked receptor. Br. J. Pharmacol. 2004, 142, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, C.; Tortolani, D.; Standoli, S.; Angelucci, C.B.; Fanti, F.; Leuti, A.; Sergi, M.; Kadhim, S.; Hsu, E.; Rapino, C.; et al. Effects of Rare Phytocannabinoids on the Endocannabinoid System of Human Keratinocytes. Int. J. Mol. Sci. 2022, 23, 5430. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Neeper, M.P.; Liu, Y.; Hutchinson, T.L.; Lubin, M.L.; Flores, C.M. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J. Neurosci. 2008, 28, 6231–6238. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Orlando, P.; Moriello, A.S.; Aviello, G.; Stott, C.; Izzo, A.A.; Di Marzo, V. Cannabinoid actions at TRPV channels: Effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 2012, 204, 255–266. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, J.; Lehmann, C. GPR55—A putative “type 3” cannabinoid receptor in inflammation. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 297–302. [Google Scholar] [CrossRef]
- Pérez-Gómez, E.; Andradas, C.; Flores, J.M.; Quintanilla, M.; Paramio, J.M.; Guzmán, M.; Sánchez, C. The orphan receptor GPR55 drives skin carcinogenesis and is upregulated in human squamous cell carcinomas. Oncogene 2013, 32, 2534–2542. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.-O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- Lauckner, J.E.; Jensen, J.B.; Chen, H.-Y.; Lu, H.-C.; Hille, B.; Mackie, K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA 2008, 105, 2699–2704. [Google Scholar] [CrossRef]
- Kapur, A.; Zhao, P.; Sharir, H.; Bai, Y.; Caron, M.G.; Barak, L.S.; Abood, M.E. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J. Biol. Chem. 2009, 284, 29817–29827. [Google Scholar] [CrossRef]
- Overton, H.A.; Babbs, A.J.; Doel, S.M.; Fyfe, M.C.T.; Gardner, L.S.; Griffin, G.; Jackson, H.C.; Procter, M.J.; Rasamison, C.M.; Tang-Christensen, M.; et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006, 3, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, G.; Simcocks, A.; Hryciw, D.H.; Hutchinson, D.S.; McAinch, A.J. G protein coupled receptor 18: A potential role for endocannabinoid signaling in metabolic dysfunction. Mol. Nutr. Food Res. 2016, 60, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Hasegawa, H.; Inoue, A.; Muraoka, M.; Miyazaki, T.; Oka, K.; Yasukawa, M. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem. Biophys. Res. Commun. 2006, 347, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Rimmerman, N.; Bradshaw, H.B.; Hughes, H.V.; Chen, J.S.-C.; Hu, S.S.-J.; McHugh, D.; Vefring, E.; Jahnsen, J.A.; Thompson, E.L.; Masuda, K.; et al. N-palmitoyl glycine, a novel endogenous lipid that acts as a modulator of calcium influx and nitric oxide production in sensory neurons. Mol. Pharmacol. 2008, 74, 213–224. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Page, J.; Dunn, E.; Bradshaw, H.B. Δ9-Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 2012, 165, 2414–2424. [Google Scholar] [CrossRef] [PubMed]
- Console-Bram, L.; Brailoiu, E.; Brailoiu, G.C.; Sharir, H.; Abood, M.E. Activation of GPR18 by cannabinoid compounds: A tale of biased agonism. Br. J. Pharmacol. 2014, 171, 3908–3917. [Google Scholar] [CrossRef]
- Blunder, S.; Pavel, P.; Minzaghi, D.; Dubrac, S. PPARdelta in Affected Atopic Dermatitis and Psoriasis: A Possible Role in Metabolic Reprograming. Int. J. Mol. Sci. 2021, 22, 7354. [Google Scholar] [CrossRef]
- Fu, J.; Gaetani, S.; Oveisi, F.; Lo Verme, J.; Serrano, A.; Rodríguez de Fonseca, F.; Rosengarth, A.; Luecke, H.; Di Giacomo, B.; Tarzia, G.; et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature 2003, 425, 90–93. [Google Scholar] [CrossRef]
- Lo Verme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The nuclear receptor peroxisome proliferator-activated receptor-α mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef]
- Sarnelli, G.; Gigli, S.; Capoccia, E.; Iuvone, T.; Cirillo, C.; Seguella, L.; Nobile, N.; D’Alessandro, A.; Pesce, M.; Steardo, L.; et al. Palmitoylethanolamide Exerts Antiproliferative Effect and Downregulates VEGF Signaling in Caco-2 Human Colon Carcinoma Cell Line Through a Selective PPAR-α-Dependent Inhibition of Akt/mTOR Pathway. Phytother. Res. 2016, 30, 963–970. [Google Scholar] [CrossRef]
- Takeda, S.; Ikeda, E.; Su, S.; Harada, M.; Okazaki, H.; Yoshioka, Y.; Nishimura, H.; Ishii, H.; Kakizoe, K.; Taniguchi, A.; et al. Δ9-THC modulation of fatty acid 2-hydroxylase (FA2H) gene expression: Possible involvement of induced levels of PPARα in MDA-MB-231 breast cancer cells. Toxicology 2014, 326, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Alexander, S.P.H.; Garle, M.J.; Gibson, C.L.; Hewitt, K.; Murphy, S.P.; Kendall, D.A.; Bennett, A.J. Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism. Br. J. Pharmacol. 2007, 152, 734–743. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Tarling, E.J.; Bennett, A.J.; Kendall, D.A.; Randall, M.D. Novel time-dependent vascular actions of Δ9-tetrahydrocannabinol mediated by peroxisome proliferator-activated receptor gamma. Biochem. Biophys. Res. Commun. 2005, 337, 824–831. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Sun, Y.; Bennett, A.J.; Randall, M.D.; Kendall, D.A. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur. J. Pharmacol. 2009, 612, 61–68. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007, 152, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, H.; Burstein, S.H.; Zurier, R.B.; Chen, J.D. Activation and binding of peroxisome proliferator-activated receptor γ by synthetic cannabinoid ajulemic acid. Mol. Pharmacol. 2003, 63, 983–992. [Google Scholar] [CrossRef]
- Vara, D.; Morell, C.; Rodríguez-Henche, N.; Diaz-Laviada, I. Involvement of PPARγ in the antitumoral action of cannabinoids on hepatocellular carcinoma. Cell Death Dis. 2013, 4, e618. [Google Scholar] [CrossRef]
- Hirao-Suzuki, M.; Takeda, S.; Koga, T.; Takiguchi, M.; Toda, A. Cannabidiolic acid dampens the expression of cyclooxygenase-2 in MDA-MB-231 breast cancer cells: Possible implication of the peroxisome proliferator-activated receptor β/δ abrogation. J. Toxicol. Sci. 2020, 45, 227–236. [Google Scholar] [CrossRef]
- Hirao-Suzuki, M.; Takeda, S.; Watanabe, K.; Takiguchi, M.; Aramaki, H. Δ9-Tetrahydrocannabinol upregulates fatty acid 2-hydroxylase (FA2H) via PPARα induction: A possible evidence for the cancellation of PPARβ/δ-mediated inhibition of PPARα in MDA-MB-231 cells. Arch. Biochem. Biophys. 2019, 662, 219–225. [Google Scholar] [CrossRef]
- Felder, C.C.; Nielsen, A.; Briley, E.M.; Palkovits, M.; Priller, J.; Axelrod, J.; Nguyen, D.N.; Richardson, J.M.; Riggin, R.M.; Koppel, G.A.; et al. Isolation and measurement of the endogenous cannabinoid receptor agonist, anandamide, in brain and peripheral tissues of human and rat. FEBS Lett. 1996, 393, 231–235. [Google Scholar] [CrossRef]
- Calignano, A.; La Rana, G.; Giuffrida, A.; Piomelli, D. Control of pain initiation by endogenous cannabinoids. Nature 1998, 394, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Czifra, G.; Szöllősi, A.G.; Tóth, B.I.; Demaude, J.; Bouez, C.; Breton, L.; Bíró, T. Endocannabinoids regulate growth and survival of human eccrine sweat gland-derived epithelial cells. J. Investig. Dermatol. 2012, 132, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Telek, A.; Bíró, T.; Bodó, E.; Tóth, B.I.; Borbíró, I.; Kunos, G.; Paus, R. Inhibition of human hair follicle growth by endo- and exocannabinoids. FASEB J. 2007, 21, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Schmelz, M.; Metze, D.; Luger, T.; Rukwied, R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J. Dermatol. Sci. 2005, 38, 177–188. [Google Scholar] [CrossRef]
- Dobrosi, N.; Tóth, B.I.; Nagy, G.; Dózsa, A.; Géczy, T.; Nagy, L.; Zouboulis, C.C.; Paus, R.; Kovács, L.; Bíró, T. Endocannabinoids enhance lipid synthesis and apoptosis of human sebocytes via cannabinoid receptor-2-mediated signaling. FASEB J. 2008, 22, 3685–3695. [Google Scholar] [CrossRef]
- Pucci, M.; Pasquariello, N.; Battista, N.; Di Tommaso, M.; Rapino, C.; Fezza, F.; Zuccolo, M.; Jourdain, R.; Finazzi Agrò, A.; Breton, L.; et al. Endocannabinoids stimulate human melanogenesis via type-1 cannabinoid receptor. J. Biol. Chem. 2012, 287, 15466–15478. [Google Scholar] [CrossRef]
- Fujii, N.; Kenny, G.P.; Amano, T.; Honda, Y.; Kondo, N.; Nishiyasu, T. Evidence for TRPV4 channel induced skin vasodilatation through NOS, COX, and KCa channel mechanisms with no effect on sweat rate in humans. Eur. J. Pharmacol. 2019, 858, 172462. [Google Scholar] [CrossRef]
- Borbíró, I.; Lisztes, E.; Tóth, B.I.; Czifra, G.; Oláh, A.; Szöllosi, A.G.; Szentandrássy, N.; Nánási, P.P.; Péter, Z.; Paus, R.; et al. Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J. Investig. Dermatol. 2011, 131, 1605–1614. [Google Scholar] [CrossRef]
- Miragliotta, V.; Ricci, P.L.; Albanese, F.; Pirone, A.; Tognotti, D.; Abramo, F. Cannabinoid receptor types 1 and 2 and peroxisome proliferator-activated receptor-α: Distribution in the skin of clinically healthy cats and cats with hypersensitivity dermatitis. Vet. Dermatol. 2018, 29, 316-e111. [Google Scholar] [CrossRef]
- Billoni, N.; Buan, B.; Gautier, B.; Collin, C.; Gaillard, O.; Mahé, Y.F.; Bernard, B.A. Expression of peroxisome proliferator activated receptors (PPARs) in human hair follicles and PPAR alpha involvement in hair growth. Acta Derm. Venereol. 2000, 80, 329–334. [Google Scholar]
- Westergaard, M.; Henningsen, J.; Svendsen, M.L.; Johansen, C.; Jensen, U.B.; Schrøder, H.D.; Kratchmarova, I.; Berge, R.K.; Iversen, L.; Bolund, L.; et al. Modulation of keratinocyte gene expression and differentiation by PPAR-selective ligands and tetradecylthioacetic acid. J. Investig. Dermatol. 2001, 116, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Schmuth, M.; Moosbrugger-Martinz, V.; Blunder, S.; Dubrac, S. Role of PPAR, LXR, and PXR in epidermal homeostasis and inflammation. Biochim. Biophys. Acta 2014, 1841, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Alestas, T.; Ganceviciene, R.; Fimmel, S.; Müller-Decker, K.; Zouboulis, C.C. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J. Mol. Med. 2006, 84, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Wang, T.-T.; Quan, J.-H.; Li, S.-J.; Zhang, M.-F.; Liao, P.-Y.; Fan, Y.-M. Sox9 facilitates proliferation, differentiation and lipogenesis in primary cultured human sebocytes. J. Dermatol. Sci. 2017, 85, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ghosh, A.K.; Sargent, J.L.; Komura, K.; Wu, M.; Huang, Q.-Q.; Jain, M.; Whitfield, M.L.; Feghali-Bostwick, C.; Varga, J. PPARγ downregulation by TGFß in fibroblast and impaired expression and function in systemic sclerosis: A novel mechanism for progressive fibrogenesis. PLoS ONE 2010, 5, e13778. [Google Scholar] [CrossRef] [PubMed]
- Markovics, A.; Angyal, Á.; Tóth, K.F.; Ádám, D.; Pénzes, Z.; Magi, J.; Pór, Á.; Kovács, I.; Törőcsik, D.; Zouboulis, C.C.; et al. GPR119 Is a Potent Regulator of Human Sebocyte Biology. J. Investig. Dermatol. 2020, 140, 1909–1918.e8. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.S.; Kim, Y.-J.; Kim, M.O.; Kang, M.; Oh, S.W.; Nho, Y.H.; Park, S.-H.; Lee, J. Cannabidiol upregulates melanogenesis through CB1 dependent pathway by activating p38 MAPK and p42/44 MAPK. Chem. Biol. Interact. 2017, 273, 107–114. [Google Scholar] [CrossRef]
- Magina, S.; Esteves-Pinto, C.; Moura, E.; Serrão, M.P.; Moura, D.; Petrosino, S.; Di Marzo, V.; Vieira-Coelho, M.A. Inhibition of basal and ultraviolet B-induced melanogenesis by cannabinoid CB1 receptors: A keratinocyte-dependent effect. Arch. Dermatol. Res. 2011, 303, 201–210. [Google Scholar] [CrossRef]
- Goenka, S. Comparative Study of Δ9-Tetrahydrocannabinol and Cannabidiol on Melanogenesis in Human Epidermal Melanocytes from Different Pigmentation Phototypes: A Pilot Study. J. Xenobiot. 2022, 12, 131–144. [Google Scholar] [CrossRef]
- Weigelt, M.A.; Sivamani, R.; Lev-Tov, H. The therapeutic potential of cannabinoids for integumentary wound management. Exp. Dermatol. 2021, 30, 201–211. [Google Scholar] [CrossRef]
- Ruhl, T.; Lippold, E.F.; Christer, T.; Schaefer, B.; Kim, B.-S.; Beier, J.P. Genetic deletion of the cannabinoid receptors CB1 and CB2 enhances inflammation with diverging effects on skin wound healing in mice. Life Sci. 2021, 285, 120018. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ren, P.; Wang, Q.; Jiang, S.-K.; Zhang, M.; Li, J.-Y.; Wang, L.-L.; Guan, D.-W. Cannabinoid 2 receptor attenuates inflammation during skin wound healing by inhibiting M1 macrophages rather than activating M2 macrophages. J. Inflamm. 2018, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Zhao, R.; Li, J.-Y.; Li, S.-S.; Liu, M.; Wang, M.; Zhang, M.-Z.; Dong, W.-W.; Jiang, S.-K.; Zhang, M.; et al. Pharmacological activation of cannabinoid 2 receptor attenuates inflammation, fibrogenesis, and promotes re-epithelialization during skin wound healing. Eur. J. Pharmacol. 2016, 786, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Wang, L.-L.; Liu, M.; Jiang, S.-K.; Zhang, M.; Tian, Z.-L.; Wang, M.; Li, J.-Y.; Zhao, R.; Guan, D.-W. Cannabinoid CB₂ receptors are involved in the regulation of fibrogenesis during skin wound repair in mice. Mol. Med. Rep. 2016, 13, 3441–3450. [Google Scholar] [CrossRef]
- Zhao, Z.; Shen, J.; Zhang, L.; Wang, L.; Xu, H.; Han, Y.; Jia, J.; Lu, Y.; Yu, R.; Liu, H. Injectable postoperative enzyme-responsive hydrogels for reversing temozolomide resistance and reducing local recurrence after glioma operation. Biomater. Sci. 2020, 8, 5306–5316. [Google Scholar] [CrossRef]
- Zhao, C.; Dong, Y.; Liu, J.; Cai, H.; Li, Z.; Sun, X.; Yin, W.; Ma, J.; Liu, H.; Li, S. An enzyme-responsive Gp1a-hydrogel for skin wound healing. J. Biomater. Appl. 2021, 36, 714–721. [Google Scholar] [CrossRef]
- Koyama, S.; Purk, A.; Kaur, M.; Soini, H.A.; Novotny, M.V.; Davis, K.; Kao, C.C.; Matsunami, H.; Mescher, A. Beta-caryophyllene enhances wound healing through multiple routes. PLoS ONE 2019, 14, e0216104. [Google Scholar] [CrossRef]
- Ramot, Y.; Sugawara, K.; Zákány, N.; Tóth, B.I.; Bíró, T.; Paus, R. A novel control of human keratin expression: Cannabinoid receptor 1-mediated signaling down-regulates the expression of keratins K6 and K16 in human keratinocytes in vitro and in situ. PeerJ 2013, 1, e40. [Google Scholar] [CrossRef]
- Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Gugliandolo, E.; Peritore, A.F.; Di Paola, R.; Cuzzocrea, S. Topical Application of Adelmidrol + Trans-Traumatic Acid Enhances Skin Wound Healing in a Streptozotocin-Induced Diabetic Mouse Model. Front. Pharmacol. 2018, 9, 871. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Di Paola, R.; Cordaro, M.; Gugliandolo, E.; Casili, G.; Morittu, V.M.; Britti, D.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a palmitoylethanolamide analogue, as a new pharmacological treatment for the management of acute and chronic inflammation. Biochem. Pharmacol. 2016, 119, 27–41. [Google Scholar] [CrossRef]
- Sasso, O.; Pontis, S.; Armirotti, A.; Cardinali, G.; Kovacs, D.; Migliore, M.; Summa, M.; Moreno-Sanz, G.; Picardo, M.; Piomelli, D. Endogenous N-acyl taurines regulate skin wound healing. Proc. Natl. Acad. Sci. USA 2016, 113, E4397–E4406. [Google Scholar] [CrossRef] [PubMed]
- Lüder, E.; Ramer, R.; Peters, K.; Hinz, B. Decisive role of P42/44 mitogen-activated protein kinase in Δ9-tetrahydrocannabinol-induced migration of human mesenchymal stem cells. Oncotarget 2017, 8, 105984–105994. [Google Scholar] [CrossRef] [PubMed]
- Schmuhl, E.; Ramer, R.; Salamon, A.; Peters, K.; Hinz, B. Increase of mesenchymal stem cell migration by cannabidiol via activation of p42/44 MAPK. Biochem. Pharmacol. 2014, 87, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Wollank, Y.; Ramer, R.; Ivanov, I.; Salamon, A.; Peters, K.; Hinz, B. Inhibition of FAAH confers increased stem cell migration via PPARα. J. Lipid Res. 2015, 56, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- Styrczewska, M.; Kostyn, A.; Kulma, A.; Majkowska-Skrobek, G.; Augustyniak, D.; Prescha, A.; Czuj, T.; Szopa, J. Flax Fiber Hydrophobic Extract Inhibits Human Skin Cells Inflammation and Causes Remodeling of Extracellular Matrix and Wound Closure Activation. Biomed Res. Int. 2015, 2015, 862391. [Google Scholar] [CrossRef]
- Haskó, J.; Fazakas, C.; Molnár, J.; Nyúl-Tóth, Á.; Herman, H.; Hermenean, A.; Wilhelm, I.; Persidsky, Y.; Krizbai, I.A. CB2 receptor activation inhibits melanoma cell transmigration through the blood-brain barrier. Int. J. Mol. Sci. 2014, 15, 8063–8074. [Google Scholar] [CrossRef]
- Vaseghi, G.; Taki, M.J.; Javanmard, S.H. Standardized Cannabis sativa extract attenuates tau and stathmin gene expression in the melanoma cell line. Iran. J. Basic Med. Sci. 2017, 20, 1178–1181. [Google Scholar]
- Zhao, Z.; Yang, J.; Zhao, H.; Fang, X.; Li, H. Cannabinoid receptor 2 is upregulated in melanoma. J. Cancer Res. Ther. 2012, 8, 549–554. [Google Scholar] [CrossRef]
- Sailler, S.; Schmitz, K.; Jäger, E.; Ferreiros, N.; Wicker, S.; Zschiebsch, K.; Pickert, G.; Geisslinger, G.; Walter, C.; Tegeder, I.; et al. Regulation of circulating endocannabinoids associated with cancer and metastases in mice and humans. Oncoscience 2014, 1, 272–282. [Google Scholar] [CrossRef]
- Hohmann, U.; Walsleben, C.; Ghadban, C.; Kirchhoff, F.; Dehghani, F.; Hohmann, T. Interaction of Glia Cells with Glioblastoma and Melanoma Cells under the Influence of Phytocannabinoids. Cells 2022, 11, 147. [Google Scholar] [CrossRef]
- Carpi, S.; Fogli, S.; Polini, B.; Montagnani, V.; Podestà, A.; Breschi, M.C.; Romanini, A.; Stecca, B.; Nieri, P. Tumor-promoting effects of cannabinoid receptor type 1 in human melanoma cells. Toxicol. Vitro 2017, 40, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Qi, J.; Hu, L.; Ouyang, D.; Wang, H.; Sun, Q.; Lin, L.; You, L.; Tang, B. A cannabidiol-containing alginate based hydrogel as novel multifunctional wound dressing for promoting wound healing. Biomater. Adv. 2022, 134, 112560. [Google Scholar] [CrossRef] [PubMed]
- Maida, V.; Shi, R.B.; Fazzari, F.G.T.; Zomparelli, L. Topical cannabis-based medicines—A novel adjuvant treatment for venous leg ulcers: An open-label trial. Exp. Dermatol. 2021, 30, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Roelandt, T.; Heughebaert, C.; Bredif, S.; Giddelo, C.; Baudouin, C.; Msika, P.; Roseeuw, D.; Uchida, Y.; Elias, P.M.; Hachem, J.-P. Cannabinoid receptors 1 and 2 oppositely regulate epidermal permeability barrier status and differentiation. Exp. Dermatol. 2012, 21, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, B.; Park, B.M.; Jeon, J.E.; Lee, S.H.; Mann, S.; Ahn, S.K.; Hong, S.-P.; Jeong, S.K. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int. J. Dermatol. 2015, 54, e401–e408. [Google Scholar] [CrossRef]
- Proksch, E.; Soeberdt, M.; Neumann, C.; Kilic, A.; Abels, C. Modulators of the endocannabinoid system influence skin barrier repair, epidermal proliferation, differentiation and inflammation in a mouse model. Exp. Dermatol. 2019, 28, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Mehling, A.; Chkarnat, C.; Degwert, J.; Ennen, J.; Fink, E.; Matthies, W.; Roethlisberger, R.; Rossow, U.; Schnitker, J.; Tronnier, H.; et al. Interlaboratory studies with a proposed patch test design to evaluate the irritation potential of surfactants. Contact Dermat. 2010, 62, 157–164. [Google Scholar] [CrossRef]
- Hinrichsen, K.; Podschun, R.; Schubert, S.; Schröder, J.M.; Harder, J.; Proksch, E. Mouse beta-defensin-14, an antimicrobial ortholog of human beta-defensin-3. Antimicrob. Agents Chemother. 2008, 52, 1876–1879. [Google Scholar] [CrossRef]
- Shin, K.-O.; Kim, S.; Park, B.D.; Uchida, Y.; Park, K. N-Palmitoyl Serinol Stimulates Ceramide Production through a CB1-Dependent Mechanism in In Vitro Model of Skin Inflammation. Int. J. Mol. Sci. 2021, 22, 8302. [Google Scholar] [CrossRef]
- Ramer, R.; Weinzierl, U.; Schwind, B.; Brune, K.; Hinz, B. Ceramide is involved in R(+)-methanandamide-induced cyclooxygenase-2 expression in human neuroglioma cells. Mol. Pharmacol. 2003, 64, 1189–1198. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Sánchez, C.; Cortés, M.L.; Del Gómez Pulgar, T.; Izquierdo, M.; Guzmán, M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Shiseki, M.; Yoshida, R.; Tabata, K.; Kimura, R.; Watanabe, T.; Kon, R.; Sakai, H.; Kamei, J. Cannabidiol Application Increases Cutaneous Aquaporin-3 and Exerts a Skin Moisturizing Effect. Pharmaceuticals 2021, 14, 879. [Google Scholar] [CrossRef] [PubMed]
- Konger, R.L.; Derr-Yellin, E.; Zimmers, T.A.; Katona, T.; Xuei, X.; Liu, Y.; Zhou, H.-M.; Simpson, E.R.; Turner, M.J. Epidermal PPARγ Is a Key Homeostatic Regulator of Cutaneous Inflammation and Barrier Function in Mouse Skin. Int. J. Mol. Sci. 2021, 22, 8634. [Google Scholar] [CrossRef] [PubMed]
- de Guzman Strong, C.; Wertz, P.W.; Wang, C.; Yang, F.; Meltzer, P.S.; Andl, T.; Millar, S.E.; Ho, I.-C.; Pai, S.-Y.; Segre, J.A. Lipid defect underlies selective skin barrier impairment of an epidermal-specific deletion of Gata-3. J. Cell Biol. 2006, 175, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A. Epidermal surface lipids. Dermatoendocrinology 2009, 1, 72–76. [Google Scholar] [CrossRef]
- Furue, K.; Mitoma, C.; Tsuji, G.; Furue, M. Protective role of peroxisome proliferator-activated receptor α agonists in skin barrier and inflammation. Immunobiology 2018, 223, 327–330. [Google Scholar] [CrossRef]
- Zákány, N.; Oláh, A.; Markovics, A.; Takács, E.; Aranyász, A.; Nicolussi, S.; Piscitelli, F.; Allarà, M.; Pór, Á.; Kovács, I.; et al. Endocannabinoid Tone Regulates Human Sebocyte Biology. J. Investig. Dermatol. 2018, 138, 1699–1706. [Google Scholar] [CrossRef]
- Tóth, B.I.; Oláh, A.; Szöllosi, A.G.; Czifra, G.; Bíró, T. “Sebocytes’ makeup”: Novel mechanisms and concepts in the physiology of the human sebaceous glands. Pflugers Arch. 2011, 461, 593–606. [Google Scholar] [CrossRef]
- Trivedi, N.R.; Cong, Z.; Nelson, A.M.; Albert, A.J.; Rosamilia, L.L.; Sivarajah, S.; Gilliland, K.L.; Liu, W.; Mauger, D.T.; Gabbay, R.A.; et al. Peroxisome proliferator-activated receptors increase human sebum production. J. Investig. Dermatol. 2006, 126, 2002–2009. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Zouboulis, C.C.; Ochsendorf, F.; Müller, J.; Thaçi, D.; Bernd, A.; Kaufmann, R.; Kippenberger, S. Peroxisome proliferator-activated receptor activators protect sebocytes from apoptosis: A new treatment modality for acne? Br. J. Dermatol. 2011, 164, 182–186. [Google Scholar] [CrossRef]
- Sardella, C.; Winkler, C.; Quignodon, L.; Hardman, J.A.; Toffoli, B.; Giordano Attianese, G.M.P.; Hundt, J.E.; Michalik, L.; Vinson, C.R.; Paus, R.; et al. Delayed Hair Follicle Morphogenesis and Hair Follicle Dystrophy in a Lipoatrophy Mouse Model of Pparg Total Deletion. J. Investig. Dermatol. 2018, 138, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Szántó, M.; Oláh, A.; Szöllősi, A.G.; Tóth, K.F.; Páyer, E.; Czakó, N.; Pór, Á.; Kovács, I.; Zouboulis, C.C.; Kemény, L.; et al. Activation of TRPV3 Inhibits Lipogenesis and Stimulates Production of Inflammatory Mediators in Human Sebocytes-A Putative Contributor to Dry Skin Dermatoses. J. Investig. Dermatol. 2019, 139, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Szöllősi, A.G.; Vasas, N.; Angyal, Á.; Kistamás, K.; Nánási, P.P.; Mihály, J.; Béke, G.; Herczeg-Lisztes, E.; Szegedi, A.; Kawada, N.; et al. Activation of TRPV3 Regulates Inflammatory Actions of Human Epidermal Keratinocytes. J. Investig. Dermatol. 2018, 138, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Rietcheck, H.R.; Maghfour, J.; Rundle, C.W.; Husayn, S.S.; Presley, C.L.; Sillau, S.H.; Liu, Y.; Leehey, M.A.; Dunnick, C.A.; Dellavalle, R.P. A Review of the Current Evidence Connecting Seborrheic Dermatitis and Parkinson’s Disease and the Potential Role of Oral Cannabinoids. Dermatology 2020, 237, 872–877. [Google Scholar] [CrossRef]
- Sugawara, K.; Zákány, N.; Tiede, S.; Purba, T.; Harries, M.; Tsuruta, D.; Bíró, T.; Paus, R. Human epithelial stem cell survival within their niche requires “tonic” cannabinoid receptor 1-signalling-Lessons from the hair follicle. Exp. Dermatol. 2021, 30, 479–493. [Google Scholar] [CrossRef]
- Karnik, P.; Tekeste, Z.; McCormick, T.S.; Gilliam, A.C.; Price, V.H.; Cooper, K.D.; Mirmirani, P. Hair follicle stem cell-specific PPARγ deletion causes scarring alopecia. J. Investig. Dermatol. 2009, 129, 1243–1257. [Google Scholar] [CrossRef]
- Heiland, I.; Erdmann, R. Biogenesis of peroxisomes. Topogenesis of the peroxisomal membrane and matrix proteins. FEBS J. 2005, 272, 2362–2372. [Google Scholar] [CrossRef]
- Zheng, D.; Bode, A.M.; Zhao, Q.; Cho, Y.-Y.; Zhu, F.; Ma, W.-Y.; Dong, Z. The cannabinoid receptors are required for ultraviolet-induced inflammation and skin cancer development. Cancer Res. 2008, 68, 3992–3998. [Google Scholar] [CrossRef]
- Atalay, S.; Gęgotek, A.; Wroński, A.; Domigues, P.; Skrzydlewska, E. Therapeutic application of cannabidiol on UVA and UVB irradiated rat skin. A proteomic study. J. Pharm. Biomed. Anal. 2021, 192, 113656. [Google Scholar] [CrossRef]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef]
- Perez, E.; Fernandez, J.R.; Fitzgerald, C.; Rouzard, K.; Tamura, M.; Savile, C. In Vitro and Clinical Evaluation of Cannabigerol (CBG) Produced via Yeast Biosynthesis: A Cannabinoid with a Broad Range of Anti-Inflammatory and Skin Health-Boosting Properties. Molecules 2022, 27, 491. [Google Scholar] [CrossRef] [PubMed]
- Arlen, P.A.; Wu, X. The expression of cannabinoid-related genes in multiple disease cell lines. Appl. Cell Biol. 2020, 8, 11–20. [Google Scholar]
- Yamanaka-Takaichi, M.; Sugawara, K.; Sumitomo, R.; Tsuruta, D. The Mast Cell-SCF-CB1 Interaction Is a Key Player in Seborrheic Keratosis. J. Histochem. Cytochem. 2020, 68, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.V.; Miot, H.A. Actinic keratosis: A clinical and epidemiological revision. An. Bras. Dermatol. 2012, 87, 425–434. [Google Scholar] [CrossRef]
- Sugawara, K.; Bíró, T.; Tsuruta, D.; Tóth, B.I.; Kromminga, A.; Zákány, N.; Zimmer, A.; Funk, W.; Gibbs, B.F.; Zimmer, A.; et al. Endocannabinoids limit excessive mast cell maturation and activation in human skin. J. Allergy Clin. Immunol. 2012, 129, 726–738.e8. [Google Scholar] [CrossRef]
- Piquero-Casals, J.; Morgado-Carrasco, D.; Gilaberte, Y.; Del Rio, R.; Macaya-Pascual, A.; Granger, C.; López-Estebaranz, J.L. Management Pearls on the Treatment of Actinic Keratoses and Field Cancerization. Dermatol. Ther. 2020, 10, 903–915. [Google Scholar] [CrossRef]
- Ramot, Y.; Paus, R. Harnessing neuroendocrine controls of keratin expression: A new therapeutic strategy for skin diseases? Bioessays 2014, 36, 672–686. [Google Scholar] [CrossRef]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de La Vega, L.; Muñoz, E. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef]
- Pucci, M.; Rapino, C.; Di Francesco, A.; Dainese, E.; D’Addario, C.; Maccarrone, M. Epigenetic control of skin differentiation genes by phytocannabinoids. Br. J. Pharmacol. 2013, 170, 581–591. [Google Scholar] [CrossRef]
- Paradisi, A.; Pasquariello, N.; Barcaroli, D.; Maccarrone, M. Anandamide regulates keratinocyte differentiation by inducing DNA methylation in a CB1 receptor-dependent manner. J. Biol. Chem. 2008, 283, 6005–6012. [Google Scholar] [CrossRef]
- Maccarrone, M.; Di Rienzo, M.; Battista, N.; Gasperi, V.; Guerrieri, P.; Rossi, A.; Finazzi-Agrò, A. The endocannabinoid system in human keratinocytes. Evidence that anandamide inhibits epidermal differentiation through CB1 receptor-dependent inhibition of protein kinase C, activation protein-1, and transglutaminase. J. Biol. Chem. 2003, 278, 33896–33903. [Google Scholar] [CrossRef] [PubMed]
- Bilkei-Gorzo, A.; Drews, E.; Albayram, Ö.; Piyanova, A.; Gaffal, E.; Tueting, T.; Michel, K.; Mauer, D.; Maier, W.; Zimmer, A. Early onset of aging-like changes is restricted to cognitive abilities and skin structure in Cnr1⁻/⁻ mice. Neurobiol. Aging 2012, 33, 200.e11–200.e22. [Google Scholar] [CrossRef] [PubMed]
- Leal, E.C.; Moura, L.I.F.; Pirzgalska, R.M.; Marques-da-Silva, D.; Ledent, C.; Köfalvi, A.; Carvalho, E. Diabetes and Cannabinoid CB1 receptor deficiency promote similar early onset aging-like changes in the skin. Exp. Gerontol. 2021, 154, 111528. [Google Scholar] [CrossRef] [PubMed]
- Oláh, A.; Alam, M.; Chéret, J.; Kis, N.G.; Hegyi, Z.; Szöllősi, A.G.; Vidali, S.; Bíró, T.; Paus, R. Mitochondrial energy metabolism is negatively regulated by cannabinoid receptor 1 in intact human epidermis. Exp. Dermatol. 2020, 29, 616–622. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Bujak, T.; Ziemlewska, A.; Nizioł-Łukaszewska, Z. Positive Effect of Cannabis sativa L. Herb Extracts on Skin Cells and Assessment of Cannabinoid-Based Hydrogels Properties. Molecules 2021, 26, 802. [Google Scholar] [CrossRef]
- Zawatsky, C.N.; Park, J.K.; Abdalla, J.; Kunos, G.; Iyer, M.R.; Cinar, R. Peripheral Hybrid CB1R and iNOS Antagonist MRI-1867 Displays Anti-Fibrotic Efficacy in Bleomycin-Induced Skin Fibrosis. Front. Endocrinol. 2021, 12, 744857. [Google Scholar] [CrossRef]
- Oka, S.; Yanagimoto, S.; Ikeda, S.; Gokoh, M.; Kishimoto, S.; Waku, K.; Ishima, Y.; Sugiura, T. Evidence for the involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation in mouse ear. J. Biol. Chem. 2005, 280, 18488–18497. [Google Scholar] [CrossRef]
- Oka, S.; Wakui, J.; Ikeda, S.; Yanagimoto, S.; Kishimoto, S.; Gokoh, M.; Nasui, M.; Sugiura, T. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J. Immunol. 2006, 177, 8796–8805. [Google Scholar] [CrossRef]
- Ambrożewicz, E.; Wójcik, P.; Wroński, A.; Łuczaj, W.; Jastrząb, A.; Žarković, N.; Skrzydlewska, E. Pathophysiological Alterations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients. Cells 2018, 7, 159. [Google Scholar] [CrossRef]
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. J. Investig. Dermatol. 2018, 138, 1311–1317. [Google Scholar] [CrossRef]
- Campora, L.; Miragliotta, V.; Ricci, E.; Cristino, L.; Di Marzo, V.; Albanese, F.; Della Federica Valle, M.; Abramo, F. Cannabinoid receptor type 1 and 2 expression in the skin of healthy dogs and dogs with atopic dermatitis. Am. J. Vet. Res. 2012, 73, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-L.; Yu, T.-S.; Li, X.-N.; Fan, Y.-Y.; Ma, W.-X.; Du, Y.; Zhao, R.; Guan, D.-W. Cannabinoid receptor type 2 is time-dependently expressed during skin wound healing in mice. Int. J. Legal Med. 2012, 126, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Correia-Sá, I.B.; Carvalho, C.M.; Serrão, P.V.; Machado, V.A.; Carvalho, S.O.; Marques, M.; Vieira-Coelho, M.A. AM251, a cannabinoid receptor 1 antagonist, prevents human fibroblasts differentiation and collagen deposition induced by TGF-β—An in vitro study. Eur. J. Pharmacol. 2021, 892, 173738. [Google Scholar] [CrossRef] [PubMed]
- Correia-Sá, I.; Carvalho, C.; Machado, V.A.; Carvalho, S.; Serrão, P.; Marques, M.; Vieira-Coelho, M.A. Targeting cannabinoid receptor 2 (CB2) limits collagen production-An in vitro study in a primary culture of human fibroblasts. Fundam. Clin. Pharmacol. 2022, 36, 89–99. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Ge, W.; Chen, C.; Huang, Y.; Jin, Z.; Zhan, M.; Duan, X.; Liu, X.; Kong, Y.; et al. CB2R Deficiency Exacerbates Imiquimod-Induced Psoriasiform Dermatitis and Itch Through the Neuro-Immune Pathway. Front. Pharmacol. 2022, 13, 790712. [Google Scholar] [CrossRef]
- Sulk, M.; Seeliger, S.; Aubert, J.; Schwab, V.D.; Cevikbas, F.; Rivier, M.; Nowak, P.; Voegel, J.J.; Buddenkotte, J.; Steinhoff, M. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J. Investig. Dermatol. 2012, 132, 1253–1262. [Google Scholar] [CrossRef]
- Luo, J.; Feng, J.; Yu, G.; Yang, P.; Mack, M.R.; Du, J.; Yu, W.; Qian, A.; Zhang, Y.; Liu, S.; et al. Transient receptor potential vanilloid 4-expressing macrophages and keratinocytes contribute differentially to allergic and nonallergic chronic itch. J. Allergy Clin. Immunol. 2018, 141, 608–619.e7. [Google Scholar] [CrossRef]
- Nijsten, T.; Geluyckens, E.; Colpaert, C.; Lambert, J. Peroxisome proliferator-activated receptors in squamous cell carcinoma and its precursors. J. Cutan. Pathol. 2005, 32, 340–347. [Google Scholar] [CrossRef]
- Staumont-Sallé, D.; Abboud, G.; Brénuchon, C.; Kanda, A.; Roumier, T.; Lavogiez, C.; Fleury, S.; Rémy, P.; Papin, J.-P.; Bertrand-Michel, J.; et al. Peroxisome proliferator-activated receptor alpha regulates skin inflammation and humoral response in atopic dermatitis. J. Allergy Clin. Immunol. 2008, 121, 962–968.e6. [Google Scholar] [CrossRef]
- Lee, D.J.; Lee, J.; Ha, J.; Park, K.-C.; Ortonne, J.-P.; Kang, H.Y. Defective barrier function in melasma skin. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1533–1537. [Google Scholar] [CrossRef]
- Ruzehaji, N.; Frantz, C.; Ponsoye, M.; Avouac, J.; Pezet, S.; Guilbert, T.; Luccarini, J.-M.; Broqua, P.; Junien, J.-L.; Allanore, Y. Pan PPAR agonist IVA337 is effective in prevention and treatment of experimental skin fibrosis. Ann. Rheum. Dis. 2016, 75, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Jin, X.-J.; Kim, Y.K.; Oh, I.K.; Kim, J.E.; Park, C.-H.; Chung, J.H. UV decreases the synthesis of free fatty acids and triglycerides in the epidermis of human skin in vivo, contributing to development of skin photoaging. J. Dermatol. Sci. 2010, 57, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, D.; El Tawdy, A.M.; Metwally, D.; Manar, A.; Rashed, L. Estimation of peroxisome proliferators—Activated receptor γ gene expression in inflammatory skin diseases: Atopic dermatitis and psoriasis. Our Dermatol. Online 2014, 5, 107–112. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Luo, S.; Zhan, Y.; Wang, J.; Zhao, R.; Li, Y.; Zeng, J.; Lu, Q. Increased Expression of PPAR-γ Modulates Monocytes Into a M2-Like Phenotype in SLE Patients: An Implicative Protective Mechanism and Potential Therapeutic Strategy of Systemic Lupus Erythematosus. Front. Immunol. 2020, 11, 579372. [Google Scholar] [CrossRef] [PubMed]
- Westergaard, M.; Henningsen, J.; Johansen, C.; Rasmussen, S.; Svendsen, M.L.; Jensen, U.B.; Schrøder, H.D.; Staels, B.; Iversen, L.; Bolund, L.; et al. Expression and localization of peroxisome proliferator-activated receptors and nuclear factor κB in normal and lesional psoriatic skin. J. Investig. Dermatol. 2003, 121, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Babes, A.; Kichko, T.I.; Selescu, T.; Manolache, A.; Neacsu, C.; Gebhardt, L.; Reeh, P.W. Psoralens activate and photosensitize Transient Receptor Potential channels Ankyrin type 1 (TRPA1) and Vanilloid type 1 (TRPV1). Eur. J. Pain 2021, 25, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Garbutcheon-Singh, K.B.; Smith, S.D. Cannabinoids interaction with transient receptor potential family and implications in the treatment of rosacea. Dermatol. Ther. 2021, 34, e15162. [Google Scholar] [CrossRef]
- Yun, J.-W.; Seo, J.A.; Jang, W.-H.; Koh, H.J.; Bae, I.-H.; Park, Y.-H.; Lim, K.-M. Antipruritic effects of TRPV1 antagonist in murine atopic dermatitis and itching models. J. Investig. Dermatol. 2011, 131, 1576–1579. [Google Scholar] [CrossRef]
- Denda, M.; Sokabe, T.; Fukumi-Tominaga, T.; Tominaga, M. Effects of skin surface temperature on epidermal permeability barrier homeostasis. J. Investig. Dermatol. 2007, 127, 654–659. [Google Scholar] [CrossRef]
- Lee, W.-J.; Shim, W.-S. Cutaneous Neuroimmune Interactions of TSLP and TRPV4 Play Pivotal Roles in Dry Skin-Induced Pruritus. Front. Immunol. 2021, 12, 772941. [Google Scholar] [CrossRef]
- Do, B.H.; Koizumi, H.; Ohbuchi, T.; Kawaguchi, R.; Suzuki, H. Expressions of TRPVs in the cholesteatoma epithelium. Acta Otolaryngol. 2017, 137, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.P.; Colas, R.A.; George, M.J.; Flint, J.D.; Dalli, J.; Richard-Loendt, A.; de Maeyer, R.P.; Serhan, C.N.; Gilroy, D.W. Pro-resolving mediators promote resolution in a human skin model of UV-killed Escherichia coli-driven acute inflammation. JCI Insight 2018, 3, e94463. [Google Scholar] [CrossRef] [PubMed]
- Amr, K.; Abdel-Hameed, M.; Sayed, K.; Nour-Edin, F.; Abdel Hay, R. The Pro12Ala polymorphism of the gene for peroxisome proliferator activated receptor-gamma is associated with a lower Global Acne Grading System score in patients with acne vulgaris. Clin. Exp. Dermatol. 2014, 39, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Zhang, Y.L.; Han, D.H.; Kim, D.Y.; Rhee, C.S. Antiallergic Function of KR62980, a Peroxisome Proliferator-Activated Receptor-γ Agonist, in a Mouse Allergic Rhinitis Model. Allergy Asthma Immunol. Res. 2015, 7, 256–264. [Google Scholar] [CrossRef]
- Angelina, A.; Pérez-Diego, M.; López-Abente, J.; Palomares, O. The Role of Cannabinoids in Allergic Diseases: Collegium Internationale Allergologicum (CIA) Update 2020. Int. Arch. Allergy Immunol. 2020, 181, 565–584. [Google Scholar] [CrossRef]
- Russo, E.B. History of cannabis and its preparations in saga, science, and sobriquet. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef]
- Russo, E.B. The Pharmacological History of Cannabis. In Handbook of Cannabis, 1st ed.; Pertwee, R.G., Ed.; Oxford University Press: Oxford, UK, 2014; pp. 23–43. ISBN 9780199662685. [Google Scholar]
- Lemery, N. Dictionaire ou Traite Universel Des Drogues Simples; Jean Hofhout: Rotterdam, The Netherlands, 1727. [Google Scholar]
- Johanek, L.M.; Simone, D.A. Activation of peripheral cannabinoid receptors attenuates cutaneous hyperalgesia produced by a heat injury. Pain 2004, 109, 432–442. [Google Scholar] [CrossRef]
- Sofia, R.D.; Nalepa, S.D.; Harakal, J.J.; Vassar, H.B. Anti-edema and analgesic properties of Δ9-tetrahydrocannabinol (THC). J. Pharmacol. Exp. Ther. 1973, 186, 646–655. [Google Scholar]
- Duane Sofia, R.; Nalepa, S.D.; Vassar, H.B.; Knobloch, L.C. Comparative anti-phlogistic activity of Δ9-tetrahydrocannabinol, hydrocortisone and aspirin in various rat paw edema models. Life Sci. 1974, 15, 251–260. [Google Scholar] [CrossRef]
- Kehl, L.J.; Hamamoto, D.T.; Wacnik, P.W.; Croft, D.L.; Norsted, B.D.; Wilcox, G.L.; Simone, D.A. A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain 2003, 103, 175–186. [Google Scholar] [CrossRef]
- Clayton, N.; Marshall, F.H.; Bountra, C.; O’Shaughnessy, C.T. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain 2002, 96, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Hanus, L.; Breuer, A.; Tchilibon, S.; Shiloah, S.; Goldenberg, D.; Horowitz, M.; Pertwee, R.G.; Ross, R.A.; Mechoulam, R.; Fride, E. HU-308: A specific agonist for CB2, a peripheral cannabinoid receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 14228–14233. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, F.A.; Jacob, T.A.; Ganley, O.H.; Ormond, R.E.; Meisinger, M.A.P. The identification of N-(2-hydroxyethyl)-palmitamide as a naturally occurring anti-inflammatory agent. J. Am. Chem. Soc. 1957, 79, 5577–5578. [Google Scholar] [CrossRef]
- Costa, B.; Conti, S.; Giagnoni, G.; Colleoni, M. Therapeutic effect of the endogenous fatty acid amide, palmitoylethanolamide, in rat acute inflammation: Inhibition of nitric oxide and cyclo-oxygenase systems. Br. J. Pharmacol. 2002, 137, 413–420. [Google Scholar] [CrossRef]
- Benvenuti, F.; Lattanzi, F.; de Gori, A.; Tarli, P. Attivita’ di alcuni derivati della palmitoiletanolamide sull’edema da carragenina nella zampa di ratto [Activity of some derivatives of palmitoylethanolamide on carragenine-induced edema in the rat paw]. Boll. Soc. Ital. Biol. Sper. 1968, 44, 809–813. [Google Scholar]
- Mazzari, S.; Canella, R.; Petrelli, L.; Marcolongo, G.; Leon, A. N-(2-Hydroxyethyl)hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation. Eur. J. Pharmacol. 1996, 300, 227–236. [Google Scholar] [CrossRef]
- Marini, I.; Bartolucci, M.L.; Bortolotti, F.; Gatto, M.R.; Bonetti, G.A. Palmitoylethanolamide versus a nonsteroidal anti-inflammatory drug in the treatment of temporomandibular joint inflammatory pain. J. Orofac. Pain 2012, 26, 99–104. [Google Scholar]
- Artukoglu, B.B.; Beyer, C.; Zuloff-Shani, A.; Brener, E.; Bloch, M.H. Efficacy of Palmitoylethanolamide for Pain: A Meta-Analysis. Pain Physician 2017, 20, 353–362. [Google Scholar]
- Robinson, R.H.; Meissler, J.J.; Fan, X.; Yu, D.; Adler, M.W.; Eisenstein, T.K. A CB2-Selective Cannabinoid Suppresses T-Cell Activities and Increases Tregs and IL-10. J. Neuroimmune Pharmacol. 2015, 10, 318–332. [Google Scholar] [CrossRef]
- Ke, P.; Shao, B.-Z.; Xu, Z.-Q.; Wei, W.; Han, B.-Z.; Chen, X.-W.; Su, D.-F.; Liu, C. Activation of Cannabinoid Receptor 2 Ameliorates DSS-Induced Colitis through Inhibiting NLRP3 Inflammasome in Macrophages. PLoS ONE 2016, 11, e0155076. [Google Scholar] [CrossRef]
- Tomar, S.; Zumbrun, E.E.; Nagarkatti, M.; Nagarkatti, P.S. Protective role of cannabinoid receptor 2 activation in galactosamine/lipopolysaccharide-induced acute liver failure through regulation of macrophage polarization and microRNAs. J. Pharmacol. Exp. Ther. 2015, 353, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Zurier, R.B.; Rossetti, R.G.; Burstein, S.H.; Bidinger, B. Suppression of human monocyte interleukin-1β production by ajulemic acid, a nonpsychoactive cannabinoid. Biochem. Pharmacol. 2003, 65, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, V.A.; Aslan, S.; Safaei, R.; Vaughan, C.W. Effect of the cannabinoid ajulemic acid on rat models of neuropathic and inflammatory pain. Neurosci. Lett. 2005, 382, 231–235. [Google Scholar] [CrossRef]
- Gonzalez, E.G.; Selvi, E.; Balistreri, E.; Akhmetshina, A.; Palumbo, K.; Lorenzini, S.; Lazzerini, P.E.; Montilli, C.; Capecchi, P.L.; Lucattelli, M.; et al. Synthetic cannabinoid ajulemic acid exerts potent antifibrotic effects in experimental models of systemic sclerosis. Ann. Rheum. Dis. 2012, 71, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; Atez, F.; Rossetti, R.G.; Skulas, A.; Patel, R.; Zurier, R.B. Suppression of human macrophage interleukin-6 by a nonpsychoactive cannabinoid acid. Rheumatol. Int. 2008, 28, 631–635. [Google Scholar] [CrossRef]
- Motwani, M.P.; Bennett, F.; Norris, P.C.; Maini, A.A.; George, M.J.; Newson, J.; Henderson, A.; Hobbs, A.J.; Tepper, M.; White, B.; et al. Potent Anti-Inflammatory and Pro-Resolving Effects of Anabasum in a Human Model of Self-Resolving Acute Inflammation. Clin. Pharmacol. Ther. 2018, 104, 675–686. [Google Scholar] [CrossRef]
- Burstein, S. Molecular Mechanisms for the Inflammation-Resolving Actions of Lenabasum. Mol. Pharmacol. 2021, 99, 125–132. [Google Scholar] [CrossRef]
- Chiang, N.; Dalli, J.; Colas, R.A.; Serhan, C.N. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 2015, 212, 1203–1217. [Google Scholar] [CrossRef]
- Schlosburg, J.E.; Boger, D.L.; Cravatt, B.F.; Lichtman, A.H. Endocannabinoid modulation of scratching response in an acute allergenic model: A new prospective neural therapeutic target for pruritus. J. Pharmacol. Exp. Ther. 2009, 329, 314–323. [Google Scholar] [CrossRef]
- Gercek, O.Z.; Oflaz, B.; Oguz, N.; Demirci, K.; Gunduz, O.; Ulugol, A. Role of Nitric Oxide in the Antipruritic Effect of WIN 55,212-2, a Cannabinoid Agonist. Basic Clin. Neurosci. 2020, 11, 473–480. [Google Scholar] [CrossRef]
- Odan, M.; Ishizuka, N.; Hiramatsu, Y.; Inagaki, M.; Hashizume, H.; Fujii, Y.; Mitsumori, S.; Morioka, Y.; Soga, M.; Deguchi, M.; et al. Discovery of S-777469: An orally available CB2 agonist as an antipruritic agent. Bioorg. Med. Chem. Lett. 2012, 22, 2803–2806. [Google Scholar] [CrossRef] [PubMed]
- Haruna, T.; Soga, M.; Morioka, Y.; Hikita, I.; Imura, K.; Furue, Y.; Yamamoto, M.; Imura, C.; Ikeda, M.; Yamauchi, A.; et al. S-777469, a novel cannabinoid type 2 receptor agonist, suppresses itch-associated scratching behavior in rodents through inhibition of itch signal transmission. Pharmacology 2015, 95, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Marsella, R.; Ahrens, K.; Sanford, R.; Trujillo, A.; Massre, D.; Soeberdt, M.; Abels, C. Double blinded, vehicle controlled, crossover study on the efficacy of a topical endocannabinoid membrane transporter inhibitor in atopic Beagles. Arch. Dermatol. Res. 2019, 311, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Lucaciu, O.C.; Connell, G.P. Itch sensation through transient receptor potential channels: A systematic review and relevance to manual therapy. J. Manip. Physiol. Ther. 2013, 36, 385–393. [Google Scholar] [CrossRef]
- Sanjel, B.; Kim, B.-H.; Song, M.-H.; Carstens, E.; Shim, W.-S. Glucosylsphingosine evokes pruritus via activation of 5-HT2A receptor and TRPV4 in sensory neurons. Br. J. Pharmacol. 2022, 179, 2193–2207. [Google Scholar] [CrossRef]
- Yan, J.; Ye, F.; Ju, Y.; Wang, D.; Chen, J.; Zhang, X.; Yin, Z.; Wang, C.; Yang, Y.; Zhu, C.; et al. Cimifugin relieves pruritus in psoriasis by inhibiting TRPV4. Cell Calcium 2021, 97, 102429. [Google Scholar] [CrossRef]
- Szabó, I.L.; Lisztes, E.; Béke, G.; Tóth, K.F.; Paus, R.; Oláh, A.; Bíró, T. The Phytocannabinoid (-)-Cannabidiol Operates as a Complex, Differential Modulator of Human Hair Growth: Anti-Inflammatory Submicromolar versus Hair Growth Inhibitory Micromolar Effects. J. Investig. Dermatol. 2020, 140, 484–488.e5. [Google Scholar] [CrossRef]
- Küster, W.; Happle, R. The inheritance of common baldness: Two B or not two B? J. Am. Acad. Dermatol. 1984, 11, 921–926. [Google Scholar] [CrossRef]
- Thiers, B.H.; Dobson, R.L. (Eds.) Pathogenesis of Skin Disease; Churchill Livingstone: New York, NY, USA, 1986. [Google Scholar]
- Hamilton, J.B. Male hormone stimulation is prerequisite and an incitant in common baldness. Am. J. Anat. 1942, 71, 451–480. [Google Scholar] [CrossRef]
- Hamilton, J.B. Patterned loss of hair in man; types and incidence. Ann. N. Y. Acad. Sci. 1951, 53, 708–728. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mays, R.R.; Dotzert, M.S.; Versteeg, S.G.; Shear, N.H.; Piguet, V. Efficacy of non-surgical treatments for androgenetic alopecia: A systematic review and network meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2112–2125. [Google Scholar] [CrossRef]
- Motofei, I.G.; Rowland, D.L.; Baconi, D.L.; Tampa, M.; Sârbu, M.-I.; Păunică, S.; Constantin, V.D.; Bălălău, C.; Păunică, I.; Georgescu, S.R. Androgenetic alopecia; drug safety and therapeutic strategies. Expert Opin. Drug Saf. 2018, 17, 407–412. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic Review of Platelet-Rich Plasma Use in Androgenetic Alopecia Compared with Minoxidil®, Finasteride®, and Adult Stem Cell-Based Therapy. Int. J. Mol. Sci. 2020, 21, 2702. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.A. Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. J. Am. Acad. Dermatol. 1993, 28, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, B.; Somiah, S.; Sacchidanand, S.A.; Biligi, D.S.; Palo, S. Evaluation of Perifollicular Inflammation of Donor Area during Hair Transplantation in Androgenetic Alopecia and its Comparison with Controls. Int. J. Trichology 2013, 5, 73–76. [Google Scholar] [PubMed]
- Gupta, A.K.; Talukder, M. Cannabinoids for skin diseases and hair regrowth. J. Cosmet. Dermatol. 2021, 20, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Ryu, J.-M.; Na, H.-H.; Jung, H.-S.; Kim, B.; Park, J.-S.; Ahn, B.-S.; Kim, K.-C. Regulatory Effect of Cannabidiol (CBD) on Decreased β-Catenin Expression in Alopecia Models by Testosterone and PMA Treatment in Dermal Papilla Cells. J. Pharmacopunct. 2021, 24, 68–75. [Google Scholar] [CrossRef]
- Srivastava, B.K.; Soni, R.; Patel, J.Z.; Joharapurkar, A.; Sadhwani, N.; Kshirsagar, S.; Mishra, B.; Takale, V.; Gupta, S.; Pandya, P.; et al. Hair growth stimulator property of thienyl substituted pyrazole carboxamide derivatives as a CB1 receptor antagonist with in vivo antiobesity effect. Bioorg. Med. Chem. Lett. 2009, 19, 2546–2550. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Prim. 2018, 4, 1. [Google Scholar] [CrossRef]
- Tobin, D.; Nabarro, G.; de La Faute, H.B.; van Vloten, W.A.; van der Putte, S.C.; Schuurman, H.-J. Increased number of immunoreactive nerve fibers in atopic dermatitis. J. Allergy Clin. Immunol. 1992, 90, 613–622. [Google Scholar] [CrossRef]
- Kay, A.B. Calcitonin gene-related peptide– and vascular endothelial growth factor-positive inflammatory cells in late-phase allergic skin reactions in atopic subjects. J. Allergy Clin. Immunol. 2011, 127, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Mommert, S.; Gschwandtner, M.; Zwingmann, K.; Stark, H.; Werfel, T. The histamine H4 receptor is functionally expressed on TH2 cells. J. Allergy Clin. Immunol. 2009, 123, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Chun, P.I.F.; Lehman, H. Current and Future Monoclonal Antibodies in the Treatment of Atopic Dermatitis. Clin. Rev. Allergy Immunol. 2020, 59, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Meggitt, S.J.; Gray, J.C.; Reynolds, N.J. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: A double-blind, randomised controlled trial. Lancet 2006, 367, 839–846. [Google Scholar] [CrossRef]
- Roekevisch, E.; Schram, M.E.; Leeflang, M.M.G.; Brouwer, M.W.D.; Gerbens, L.A.A.; Bos, J.D.; Spuls, P.I. Methotrexate versus azathioprine in patients with atopic dermatitis: 2-year follow-up data. J. Allergy Clin. Immunol. 2018, 141, 825–827.e10. [Google Scholar] [CrossRef]
- Gerbens, L.A.A.; Hamann, S.A.S.; Brouwer, M.W.D.; Roekevisch, E.; Leeflang, M.M.G.; Spuls, P.I. Methotrexate and azathioprine for severe atopic dermatitis: A 5-year follow-up study of a randomized controlled trial. Br. J. Dermatol. 2018, 178, 1288–1296. [Google Scholar] [CrossRef]
- Hong, C.-H.; Gooderham, M.; Bissonnette, R. Evidence Review of Topical Calcineurin Inhibitors for the Treatment of Adult Atopic Dermatitis. J. Cutan. Med. Surg. 2019, 23, 5S–10S. [Google Scholar] [CrossRef]
- Nogueira, M.; Torres, T. Janus Kinase Inhibitors for the Treatment of Atopic Dermatitis: Focus on Abrocitinib, Baricitinib, and Upadacitinib. Dermatol. Pract. Concept. 2021, 11, e2021145. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Gori, N.; Maurelli, M.; Gisondi, P.; Caldarola, G.; De Simone, C.; Peris, K.; Girolomoni, G. Biological agents targeting interleukin-13 for atopic dermatitis. Expert Opin. Biol. Ther. 2022, 22, 651–659. [Google Scholar] [CrossRef]
- Sidbury, R.; Alpizar, S.; Laquer, V.; Dhawan, S.; Abramovits, W.; Loprete, L.; Krishnaswamy, J.K.; Ahmad, F.; Jabbar-Lopez, Z.; Piketty, C. Pharmacokinetics, Safety, Efficacy, and Biomarker Profiles During Nemolizumab Treatment of Atopic Dermatitis in Adolescents. Dermatol. Ther. 2022, 12, 631–642. [Google Scholar] [CrossRef]
- Schneider, S.; Li, L.; Zink, A. The New Era of Biologics in Atopic Dermatitis: A Review. Dermatol. Pract. Concept. 2021, 11, e2021144. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Tom, W.L.; Lebwohl, M.G.; Blumenthal, R.L.; Boguniewicz, M.; Call, R.S.; Eichenfield, L.F.; Forsha, D.W.; Rees, W.C.; Simpson, E.L.; et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J. Am. Acad. Dermatol. 2016, 75, 494–503.e6. [Google Scholar] [CrossRef] [PubMed]
- Thom, H.; Cheng, V.; Keeney, E.; Neary, M.P.; Eccleston, A.; Zang, C.; Cappelleri, J.C.; Cha, A.; Thyssen, J.P. Matching-Adjusted Indirect Comparison of Crisaborole Ointment 2% vs. Topical Calcineurin Inhibitors in the Treatment of Patients with Mild-to-Moderate Atopic Dermatitis. Dermatol. Ther. 2022, 12, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Teixeira, H.D.; Simpson, E.L.; Costanzo, A.; de Bruin-Weller, M.; Barbarot, S.; Prajapati, V.H.; Lio, P.; Hu, X.; Wu, T.; et al. Efficacy and Safety of Upadacitinib vs Dupilumab in Adults with Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2021, 157, 1047–1055. [Google Scholar] [CrossRef]
- Dhillon, S. Delgocitinib: First Approval. Drugs 2020, 80, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Molin, S. Delgocitinib in atopic dermatitis. Drugs Today 2021, 57, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Sher, L.G.; Chang, J.; Patel, I.B.; Balkrishnan, R.; Fleischer, A.B. Relieving the pruritus of atopic dermatitis: A meta-analysis. Acta Derm. Venereol. 2012, 92, 455–461. [Google Scholar] [CrossRef]
- Gaffal, E.; Glodde, N.; Jakobs, M.; Bald, T.; Tüting, T. Cannabinoid 1 receptors in keratinocytes attenuate fluorescein isothiocyanate-induced mouse atopic-like dermatitis. Exp. Dermatol. 2014, 23, 401–406. [Google Scholar] [CrossRef]
- Maekawa, T.; Nojima, H.; Kuraishi, Y.; Aisaka, K. The cannabinoid CB2 receptor inverse agonist JTE-907 suppresses spontaneous itch-associated responses of NC mice, a model of atopic dermatitis. Eur. J. Pharmacol. 2006, 542, 179–183. [Google Scholar] [CrossRef]
- Engel, M.A.; Izydorczyk, I.; Mueller-Tribbensee, S.M.; Becker, C.; Neurath, M.F.; Reeh, P.W. Inhibitory CB1 and activating/desensitizing TRPV1-mediated cannabinoid actions on CGRP release in rodent skin. Neuropeptides 2011, 45, 229–237. [Google Scholar] [CrossRef]
- Nam, G.; Jeong, S.K.; Park, B.M.; Lee, S.H.; Kim, H.J.; Hong, S.-P.; Kim, B.; Kim, B.-W. Selective Cannabinoid Receptor-1 Agonists Regulate Mast Cell Activation in an Oxazolone-Induced Atopic Dermatitis Model. Ann. Dermatol. 2016, 28, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Loewinger, M.; Wakshlag, J.J.; Bowden, D.; Peters-Kennedy, J.; Rosenberg, A. The effect of a mixed cannabidiol and cannabidiolic acid based oil on client-owned dogs with atopic dermatitis. Vet. Dermatol. 2022, 33, 329-e77. [Google Scholar] [CrossRef] [PubMed]
- Jakasa, I.; Thyssen, J.P.; Kezic, S. The role of skin barrier in occupational contact dermatitis. Exp. Dermatol. 2018, 27, 909–914. [Google Scholar] [CrossRef]
- Li, Y.; Li, L. Contact Dermatitis: Classifications and Management. Clin. Rev. Allergy Immunol. 2021, 61, 245–281. [Google Scholar] [CrossRef] [PubMed]
- Hamann, C.R.; Hamann, D.; Egeberg, A.; Johansen, J.D.; Silverberg, J.; Thyssen, J.P. Association between atopic dermatitis and contact sensitization: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Smit, H.A.; Burdorf, A.; Coenraads, P.J. Prevalence of hand dermatitis in different occupations. Int. J. Epidemiol. 1993, 22, 288–293. [Google Scholar] [CrossRef]
- Ruzicka, T.; Larsen, F.G.; Galewicz, D.; Horváth, A.; Coenraads, P.J.; Thestrup-Pedersen, K.; Ortonne, J.P.; Zouboulis, C.C.; Harsch, M.; Brown, T.C.; et al. Oral alitretinoin (9-cis-retinoic acid) therapy for chronic hand dermatitis in patients refractory to standard therapy: Results of a randomized, double-blind, placebo-controlled, multicenter trial. Arch. Dermatol. 2004, 140, 1453–1459. [Google Scholar] [CrossRef]
- Murphy, G.M.; Maurice, P.D.; Norris, P.G.; Morris, R.W.; Hawk, J.L. Azathioprine treatment in chronic actinic dermatitis: A double-blind controlled trial with monitoring of exposure to ultraviolet radiation. Br. J. Dermatol. 1989, 121, 639–646. [Google Scholar] [CrossRef]
- Petrosino, S.; Cristino, L.; Karsak, M.; Gaffal, E.; Ueda, N.; Tüting, T.; Bisogno, T.; de Filippis, D.; D’Amico, A.; Saturnino, C.; et al. Protective role of palmitoylethanolamide in contact allergic dermatitis. Allergy 2010, 65, 698–711. [Google Scholar] [CrossRef]
- Petrosino, S.; Verde, R.; Vaia, M.; Allarà, M.; Iuvone, T.; Di Marzo, V. Anti-inflammatory Properties of Cannabidiol, a Nonpsychotropic Cannabinoid, in Experimental Allergic Contact Dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef]
- Vaia, M.; Petrosino, S.; de Filippis, D.; Negro, L.; Guarino, A.; Carnuccio, R.; Di Marzo, V.; Iuvone, T. Palmitoylethanolamide reduces inflammation and itch in a mouse model of contact allergic dermatitis. Eur. J. Pharmacol. 2016, 791, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L. A nonsteroidal lamellar matrix cream containing palmitoylethanolamide for the treatment of atopic dermatitis. J. Drugs Dermatol. 2010, 9, 334–338. [Google Scholar] [PubMed]
- Mugnaini, C.; Rabbito, A.; Brizzi, A.; Palombi, N.; Petrosino, S.; Verde, R.; Di Marzo, V.; Ligresti, A.; Corelli, F. Synthesis of novel 2-(1-adamantanylcarboxamido)thiophene derivatives. Selective cannabinoid type 2 (CB2) receptor agonists as potential agents for the treatment of skin inflammatory disease. Eur. J. Med. Chem. 2019, 161, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, H.; Suzuki, H.; Ueda, Y.; Kaya, T.; Inaba, T. In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J. Pharmacol. Exp. Ther. 2001, 296, 420–425. [Google Scholar] [PubMed]
- Ueda, Y.; Miyagawa, N.; Wakitani, K. Involvement of cannabinoid CB2 receptors in the IgE-mediated triphasic cutaneous reaction in mice. Life Sci. 2007, 80, 414–419. [Google Scholar] [CrossRef]
- Ueda, Y.; Miyagawa, N.; Matsui, T.; Kaya, T.; Iwamura, H. Involvement of cannabinoid CB2 receptor-mediated response and efficacy of cannabinoid CB2 receptor inverse agonist, JTE-907, in cutaneous inflammation in mice. Eur. J. Pharmacol. 2005, 520, 164–171. [Google Scholar] [CrossRef]
- Sheu, M.Y.; Fowler, A.J.; Kao, J.; Schmuth, M.; Schoonjans, K.; Auwerx, J.; Fluhr, J.W.; Man, M.-Q.; Elias, P.M.; Feingold, K.R. Topical peroxisome proliferator activated receptor-α activators reduce inflammation in irritant and allergic contact dermatitis models. J. Investig. Dermatol. 2002, 118, 94–101. [Google Scholar] [CrossRef]
- Sasso, O.; Summa, M.; Armirotti, A.; Pontis, S.; de Mei, C.; Piomelli, D. The N-Acylethanolamine Acid Amidase Inhibitor ARN077 Suppresses Inflammation and Pruritus in a Mouse Model of Allergic Dermatitis. J. Investig. Dermatol. 2018, 138, 562–569. [Google Scholar] [CrossRef]
- Wen, S.; Ye, L.; Liu, D.; Yang, B.; Man, M.-Q. Topical N-palmitoyl serinol, a commensal bacterial metabolite, prevents the development of epidermal permeability barrier dysfunction in a murine model of atopic dermatitis-like skin. Can. J. Vet. Res. 2021, 85, 201–204. [Google Scholar]
- Timis, T.-L.; Florian, I.-A.; Vesa, S.-C.; Mitrea, D.R.; Orasan, R.-I. An updated guide in the management of psoriasis for every practitioner. Int. J. Clin. Pract. 2021, 75, e14290. [Google Scholar] [CrossRef]
- Ogawa, E.; Sato, Y.; Minagawa, A.; Okuyama, R. Pathogenesis of psoriasis and development of treatment. J. Dermatol. 2018, 45, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Liu, T.; Zhou, W.-Y.; Zhuang, Y.; Peng, L.; Zhang, J.-Y.; Yin, Z.-N.; Mao, X.; Guo, G.; Shi, Y.; et al. Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol. 2012, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, M.B.; Koomen, C.W.; de Waal Malefyt, R.; Wierenga, E.A.; Bos, J.D. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J. Investig. Dermatol. 1998, 111, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Korman, N.J.; Prater, E.F.; Wong, E.B.; Rupani, R.N.; Kivelevitch, D.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.R.; et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J. Am. Acad. Dermatol. 2021, 84, 432–470. [Google Scholar] [CrossRef] [PubMed]
- Katz, H.I.; Prawer, S.E.; Medansky, R.S.; Krueger, G.G.; Mooney, J.J.; Jones, M.L.; Samson, C.R. Intermittent corticosteroid maintenance treatment of psoriasis: A double-blind multicenter trial of augmented betamethasone dipropionate ointment in a pulse dose treatment regimen. Dermatologica 1991, 183, 269–274. [Google Scholar] [CrossRef]
- Menter, A.; Korman, N.J.; Elmets, C.A.; Feldman, S.R.; Gelfand, J.M.; Gordon, K.B.; Gottlieb, A.; Koo, J.Y.M.; Lebwohl, M.; Lim, H.W.; et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J. Am. Acad. Dermatol. 2009, 60, 643–659. [Google Scholar] [CrossRef]
- Benezeder, T.; Painsi, C.; Patra, V.; Dey, S.; Holcmann, M.; Lange-Asschenfeldt, B.; Sibilia, M.; Wolf, P. Dithranol targets keratinocytes, their crosstalk with neutrophils and inhibits the IL-36 inflammatory loop in psoriasis. eLife 2020, 9, e56991. [Google Scholar] [CrossRef]
- Weinstein, G.D.; Koo, J.Y.M.; Krueger, G.G.; Lebwohl, M.G.; Lowe, N.J.; Menter, M.A.; Lew-Kaya, D.A.; Sefton, J.; Gibson, J.R.; Walker, P.S. Tazarotene cream in the treatment of psoriasis: Two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J. Am. Acad. Dermatol. 2003, 48, 760–767. [Google Scholar] [CrossRef]
- Lan, J.; Li, Y.; Wen, J.; Chen, Y.; Yang, J.; Zhao, L.; Xia, Y.; Du, H.; Tao, J.; Li, Y.; et al. Acitretin-Conjugated Dextran Nanoparticles Ameliorate Psoriasis-like Skin Disease at Low Dosages. Front. Bioeng. Biotechnol. 2021, 9, 816757. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Panduri, S.; Dini, V.; Tonini, A.; Gualtieri, B.; Romanelli, M. Optimizing acitretin use in patients with plaque psoriasis. Dermatol. Ther. 2017, 30, e12453. [Google Scholar] [CrossRef]
- Atwan, A.; Ingram, J.R.; Abbott, R.; Kelson, M.J.; Pickles, T.; Bauer, A.; Piguet, V. Oral fumaric acid esters for psoriasis: Abridged Cochrane systematic review including GRADE assessments. Br. J. Dermatol. 2016, 175, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Afra, T.P.; Razmi, T.M.; Dogra, S. Apremilast in Psoriasis and Beyond: Big Hopes on a Small Molecule. Indian Dermatol. Online J. 2019, 10, 1–12. [Google Scholar] [PubMed]
- Bachelez, H.; van de Kerkhof, P.C.M.; Strohal, R.; Kubanov, A.; Valenzuela, F.; Lee, J.-H.; Yakusevich, V.; Chimenti, S.; Papacharalambous, J.; Proulx, J.; et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: A phase 3 randomised non-inferiority trial. Lancet 2015, 386, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Gelfand, J.M.; Connor, C.; Armstrong, A.W.; Cordoro, K.M.; Davis, D.M.R.; Elewski, B.E.; Gordon, K.B.; Gottlieb, A.B.; Kaplan, D.H.; et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J. Am. Acad. Dermatol. 2020, 82, 1445–1486. [Google Scholar] [CrossRef] [PubMed]
- Akhyani, M.; Chams-Davatchi, C.; Hemami, M.R.; Fateh, S. Efficacy and safety of mycophenolate mofetil vs. methotrexate for the treatment of chronic plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1447–1451. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Soliman, A.M.; Betts, K.A.; Wang, Y.; Gao, Y.; Puig, L.; Augustin, M. Comparative Efficacy and Relative Ranking of Biologics and Oral Therapies for Moderate-to-Severe Plaque Psoriasis: A Network Meta-analysis. Dermatol. Ther. 2021, 11, 885–905. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Shin, D.B.; Alavi, A.; Torigian, D.A.; Werner, T.; Papadopoulos, M.; Takeshita, J.; Noe, M.H.; Dey, A.K.; Playford, M.P.; et al. A Phase IV, Randomized, Double-Blind, Placebo-Controlled Crossover Study of the Effects of Ustekinumab on Vascular Inflammation in Psoriasis (the VIP-U Trial). J. Investig. Dermatol. 2020, 140, 85–93.e2. [Google Scholar] [CrossRef]
- Nogueira, M.; Torres, T. Guselkumab for the treatment of psoriasis—Evidence to date. Drugs Context 2019, 8, 212594. [Google Scholar] [CrossRef]
- Drerup, K.A.; Seemann, C.; Gerdes, S.; Mrowietz, U. Effective and Safe Treatment of Psoriatic Disease with the Anti-IL-23p19 Biologic Tildrakizumab: Results of a Real-World Prospective Cohort Study in Nonselected Patients. Dermatology 2021, 238, 615–619. [Google Scholar] [CrossRef]
- Gooderham, M.; Pinter, A.; Ferris, L.K.; Warren, R.B.; Zhan, T.; Zeng, J.; Soliman, A.M.; Kaufmann, C.; Kaplan, B.; Photowala, H.; et al. Long-term, durable, absolute Psoriasis Area and Severity Index and health-related quality of life improvements with risankizumab treatment: A post hoc integrated analysis of patients with moderate-to-severe plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 855–865. [Google Scholar] [CrossRef]
- Giunta, A.; Ventura, A.; Chimenti, M.S.; Bianchi, L.; Esposito, M. Spotlight on ixekizumab for the treatment of moderate-to-severe plaque psoriasis: Design, development, and use in therapy. Drug Des. Devel. Ther. 2017, 11, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Bruin, G.; Loesche, C.; Nyirady, J.; Sander, O. Population Pharmacokinetic Modeling of Secukinumab in Patients with Moderate to Severe Psoriasis. J. Clin. Pharmacol. 2017, 57, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, A.C.; Warren, R.B. Brodalumab in psoriasis: Evidence to date and clinical potential. Drugs Context 2019, 8, 212570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, P.; Cole, R.B.; Wang, G. In vitro metabolism of indomethacin morpholinylamide (BML-190), an inverse agonist for the peripheral cannabinoid receptor (CB2) in rat liver microsomes. Eur. J. Pharm. Sci. 2010, 41, 163–172. [Google Scholar] [CrossRef]
- Maybruck, B.T.; Lam, W.C.; Specht, C.A.; Ilagan, M.X.G.; Donlin, M.J.; Lodge, J.K. The Aminoalkylindole BML-190 Negatively Regulates Chitosan Synthesis via the Cyclic AMP/Protein Kinase A1 Pathway in Cryptococcus neoformans. mBio 2019, 10, e02264-19. [Google Scholar] [CrossRef]
- Urasaki, Y.; Beaumont, C.; Workman, M.; Talbot, J.N.; Hill, D.K.; Le, T.T. Fast-Acting and Receptor-Mediated Regulation of Neuronal Signaling Pathways by Copaiba Essential Oil. Int. J. Mol. Sci. 2020, 21, 2259. [Google Scholar] [CrossRef]
- Tóth, B.I.; Dobrosi, N.; Dajnoki, A.; Czifra, G.; Oláh, A.; Szöllosi, A.G.; Juhász, I.; Sugawara, K.; Paus, R.; Bíró, T. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J. Investig. Dermatol. 2011, 131, 1095–1104. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Rapino, C.; Talamonti, E.; Leuti, A.; Lanuti, M.; Gueniche, A.; Jourdain, R.; Breton, L.; Maccarrone, M. Anandamide Suppresses Proinflammatory T Cell Responses In Vitro through Type-1 Cannabinoid Receptor-Mediated mTOR Inhibition in Human Keratinocytes. J. Immunol. 2016, 197, 3545–3553. [Google Scholar] [CrossRef]
- Łuczaj, W.; Dobrzyńska, I.; Wroński, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Cannabidiol-Mediated Changes to the Phospholipid Profile of UVB-Irradiated Keratinocytes from Psoriatic Patients. Int. J. Mol. Sci. 2020, 21, 6592. [Google Scholar] [CrossRef]
- Degitz, K.; Placzek, M.; Borelli, C.; Plewig, G. Pathophysiology of acne. J. Dtsch. Dermatol. Ges. 2007, 5, 316–323. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef] [PubMed]
- Zaenglein, A.L. Acne Vulgaris. N. Engl. J. Med. 2018, 379, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am. J. Clin. Dermatol. 2019, 20, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Sutaria, A.H.; Masood, S.; Schlessinger, J. Acne Vulgaris; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Heng, A.H.S.; Say, Y.-H.; Sio, Y.Y.; Ng, Y.T.; Chew, F.T. Gene variants associated with acne vulgaris presentation and severity: A systematic review and meta-analysis. BMC Med. Genom. 2021, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- See, J.-A.; Goh, C.L.; Hayashi, N.; Suh, D.H.; Casintahan, F.A. Optimizing the use of topical retinoids in Asian acne patients. J. Dermatol. 2018, 45, 522–528. [Google Scholar] [CrossRef]
- Stamatiadis, D.; Bulteau-Portois, M.C.; Mowszowicz, I. Inhibition of 5 alpha-reductase activity in human skin by zinc and azelaic acid. Br. J. Dermatol. 1988, 119, 627–632. [Google Scholar] [CrossRef]
- Sieber, M.A.; Hegel, J.K.E. Azelaic acid: Properties and mode of action. Skin Pharmacol. Physiol. 2014, 27 (Suppl. 1), 9–17. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H.; Xia, J.; Liu, L.; Liu, G.J.; Sang, H.; Peinemann, F. Topical azelaic acid, salicylic acid, nicotinamide, sulphur, zinc and fruit acid (alpha-hydroxy acid) for acne. Cochrane Database Syst. Rev. 2020, 5, CD011368. [Google Scholar]
- Lu, J.; Cong, T.; Wen, X.; Li, X.; Du, D.; He, G.; Jiang, X. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp. Dermatol. 2019, 28, 786–794. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Sun, L.; Liu, H.; Zhang, F. Efficacy and safety of dapsone gel for acne: A systematic review and meta-analysis. Ann. Palliat. Med. 2022, 11, 611–620. [Google Scholar] [CrossRef]
- Rogers, L.R.; Oppelt, P.; Nayak, L. Hemolytic anemia associated with dapsone PCP prophylaxis in GBM patients with normal G6PD activity. Neuro Oncol. 2020, 22, 892–893. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, M. Making sense of the effects of the cumulative dose of isotretinoin in acne vulgaris. Int. J. Dermatol. 2016, 55, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Arowojolu, A.O.; Gallo, M.F.; Lopez, L.M.; Grimes, D.A. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst. Rev. 2012, 6, CD004425. [Google Scholar]
- Han, J.J.; Faletsky, A.; Barbieri, J.S.; Mostaghimi, A. New Acne Therapies and Updates on Use of Spironolactone and Isotretinoin: A Narrative Review. Dermatol. Ther. 2021, 11, 79–91. [Google Scholar] [CrossRef]
- Oláh, A.; Tóth, B.I.; Borbíró, I.; Sugawara, K.; Szöllõsi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E.; et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J. Clin. Investig. 2014, 124, 3713–3724. [Google Scholar] [CrossRef]
- Oláh, A.; Markovics, A.; Szabó-Papp, J.; Szabó, P.T.; Stott, C.; Zouboulis, C.C.; Bíró, T. Differential effectiveness of selected non-psychotropic phytocannabinoids on human sebocyte functions implicates their introduction in dry/seborrhoeic skin and acne treatment. Exp. Dermatol. 2016, 25, 701–707. [Google Scholar] [CrossRef]
- Tubaro, A.; Giangaspero, A.; Sosa, S.; Negri, R.; Grassi, G.; Casano, S.; Della Loggia, R.; Appendino, G. Comparative topical anti-inflammatory activity of cannabinoids and cannabivarins. Fitoterapia 2010, 81, 816–819. [Google Scholar] [CrossRef]
- Jin, S.; Lee, M.-Y. The ameliorative effect of hemp seed hexane extracts on the Propionibacterium acnes-induced inflammation and lipogenesis in sebocytes. PLoS ONE 2018, 13, e0202933. [Google Scholar] [CrossRef]
- Jiang, Z.; Jin, S.; Fan, X.; Cao, K.; Liu, Y.; Wang, X.; Ma, Y.; Xiang, L. Cannabidiol Inhibits Inflammation Induced by Cutibacterium acnes-Derived Extracellular Vesicles via Activation of CB2 Receptor in Keratinocytes. J. Inflamm. Res. 2022, 15, 4573–4583. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Dowson, C.; Simpson, N.; Duffy, L.; O’Reilly, S. Innate Immunity in Systemic Sclerosis. Curr. Rheumatol. Rep. 2017, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Bobeica, C.; Niculet, E.; Tatu, A.L.; Craescu, M.; Vata, D.; Statescu, L.; Iancu, A.V.; Musat, C.L.; Draganescu, M.L.; Onisor, C.; et al. Old and new therapeutic strategies in systemic sclerosis (Review). Exp. Ther. Med. 2022, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P. Advances in pathogenesis and treatment of systemic sclerosis. Clin. Med. 2016, 16, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Barsotti, S.; Orlandi, M.; Codullo, V.; Di Battista, M.; Lepri, G.; Della Rossa, A.; Guiducci, S. One year in review 2019: Systemic sclerosis. Clin. Exp. Rheumatol. 2019, 37, 3–14. [Google Scholar]
- Hilberg, F.; Roth, G.J.; Krssak, M.; Kautschitsch, S.; Sommergruber, W.; Tontsch-Grunt, U.; Garin-Chesa, P.; Bader, G.; Zoephel, A.; Quant, J.; et al. BIBF 1120: Triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008, 68, 4774–4782. [Google Scholar] [CrossRef]
- Bütikofer, L.; Varisco, P.A.; Distler, O.; Kowal-Bielecka, O.; Allanore, Y.; Riemekasten, G.; Villiger, P.M.; Adler, S. ACE inhibitors in SSc patients display a risk factor for scleroderma renal crisis-a EUSTAR analysis. Arthritis Res. Ther. 2020, 22, 59. [Google Scholar] [CrossRef]
- Ghosh, A.K. Pharmacological activation of PPAR-γ: A potential therapy for skin fibrosis. Int. J. Dermatol. 2021, 60, 376–383. [Google Scholar] [CrossRef]
- Liu, T.; de Los Santos, F.G.; Phan, S.H. The Bleomycin Model of Pulmonary Fibrosis. Methods Mol. Biol. 2017, 1627, 27–42. [Google Scholar]
- Servettaz, A.; Kavian, N.; Nicco, C.; Deveaux, V.; Chéreau, C.; Wang, A.; Zimmer, A.; Lotersztajn, S.; Weill, B.; Batteux, F. Targeting the cannabinoid pathway limits the development of fibrosis and autoimmunity in a mouse model of systemic sclerosis. Am. J. Pathol. 2010, 177, 187–196. [Google Scholar] [CrossRef]
- Balistreri, E.; Garcia-Gonzalez, E.; Selvi, E.; Akhmetshina, A.; Palumbo, K.; Lorenzini, S.; Maggio, R.; Lucattelli, M.; Galeazzi, M.; Distler, J.W.H. The cannabinoid WIN55, 212–2 abrogates dermal fibrosis in scleroderma bleomycin model. Ann. Rheum. Dis. 2011, 70, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Akhmetshina, A.; Dees, C.; Busch, N.; Beer, J.; Sarter, K.; Zwerina, J.; Zimmer, A.; Distler, O.; Schett, G.; Distler, J.H.W. The cannabinoid receptor CB2 exerts antifibrotic effects in experimental dermal fibrosis. Arthritis Rheum. 2009, 60, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- García-Martín, A.; Navarrete, C.; Garrido-Rodríguez, M.; Prados, M.E.; Caprioglio, D.; Appendino, G.; Muñoz, E. EHP-101 alleviates angiotensin II-induced fibrosis and inflammation in mice. Biomed. Pharmacother. 2021, 142, 112007. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, S.; Zhang, Q.; Yi, C.; He, J.; Ye, X.; Liu, M.; Lu, W. Celastrol is a novel selective agonist of cannabinoid receptor 2 with anti-inflammatory and anti-fibrotic activity in a mouse model of systemic sclerosis. Phytomedicine 2020, 67, 153160. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, E.; Selvi, E.; Balistreri, E.; Lorenzini, S.; Maggio, R.; Natale, M.-R.; Capecchi, P.-L.; Lazzerini, P.-E.; Bardelli, M.; Laghi-Pasini, F.; et al. Cannabinoids inhibit fibrogenesis in diffuse systemic sclerosis fibroblasts. Rheumatology 2009, 48, 1050–1056. [Google Scholar] [CrossRef]
- Kapoor, M.; McCann, M.; Liu, S.; Huh, K.; Denton, C.P.; Abraham, D.J.; Leask, A. Loss of peroxisome proliferator-activated receptor gamma in mouse fibroblasts results in increased susceptibility to bleomycin-induced skin fibrosis. Arthritis Rheum. 2009, 60, 2822–2829. [Google Scholar] [CrossRef]
- Cinar, R.; Park, J.K.; Zawatsky, C.N.; Coffey, N.J.; Bodine, S.P.; Abdalla, J.; Yokoyama, T.; Jourdan, T.; Jay, L.; Zuo, M.X.G.; et al. CB1 R and iNOS are distinct players promoting pulmonary fibrosis in Hermansky-Pudlak syndrome. Clin. Transl. Med. 2021, 11, e471. [Google Scholar] [CrossRef]
- Cinar, R.; Gochuico, B.R.; Iyer, M.R.; Jourdan, T.; Yokoyama, T.; Park, J.K.; Coffey, N.J.; Pri-Chen, H.; Szanda, G.; Liu, Z.; et al. Cannabinoid CB1 receptor overactivity contributes to the pathogenesis of idiopathic pulmonary fibrosis. JCI Insight 2017, 2, e92281. [Google Scholar] [CrossRef]
- Bronova, I.; Smith, B.; Aydogan, B.; Weichselbaum, R.R.; Vemuri, K.; Erdelyi, K.; Makriyannis, A.; Pacher, P.; Berdyshev, E.V. Protection from Radiation-Induced Pulmonary Fibrosis by Peripheral Targeting of Cannabinoid Receptor-1. Am. J. Respir. Cell Mol. Biol. 2015, 53, 555–562. [Google Scholar] [CrossRef]
- Palumbo-Zerr, K.; Horn, A.; Distler, A.; Zerr, P.; Dees, C.; Beyer, C.; Selvi, E.; Cravatt, B.F.; Distler, O.; Schett, G.; et al. Inactivation of fatty acid amide hydrolase exacerbates experimental fibrosis by enhanced endocannabinoid-mediated activation of CB1. Ann. Rheum. Dis. 2012, 71, 2051–2054. [Google Scholar] [CrossRef]
- Marquart, S.; Zerr, P.; Akhmetshina, A.; Palumbo, K.; Reich, N.; Tomcik, M.; Horn, A.; Dees, C.; Engel, M.; Zwerina, J.; et al. Inactivation of the cannabinoid receptor CB1 prevents leukocyte infiltration and experimental fibrosis. Arthritis Rheum. 2010, 62, 3467–3476. [Google Scholar] [CrossRef]
- Okogbaa, J.; Batiste, L. Dermatomyositis: An Acute Flare and Current Treatments. Clin. Med. Insights Case Rep. 2019, 12, 1179547619855370. [Google Scholar] [CrossRef]
- Fujisawa, T. Management of Myositis-Associated Interstitial Lung Disease. Medicina 2021, 57, 347. [Google Scholar] [CrossRef]
- Dressler, F.; Maurer, B. Dermatomyositis und juvenile Dermatomyositis. Z. Rheumatol. 2022; epub ahead of print. [Google Scholar] [CrossRef]
- Rodríguez-Tejero, A.; López-Espadafor, B.; Montero-Vílchez, T.; Sánchez-Díaz, M.; Arias-Santiago, S.; Molina-Leyva, A. Treatment challenges in clinically amyopathic dermatomyositis: A case series and review of new therapeutic options for skin involvement. Dermatol. Ther. 2021, 34, e14942. [Google Scholar] [CrossRef]
- Robinson, E.S.; Alves, P.; Bashir, M.M.; Zeidi, M.; Feng, R.; Werth, V.P. Cannabinoid Reduces Inflammatory Cytokines, Tumor Necrosis Factor-α, and Type I Interferons in Dermatomyositis In Vitro. J. Investig. Dermatol. 2017, 137, 2445–2447. [Google Scholar] [CrossRef]
- Kim, H.J.; Zeidi, M.; Bonciani, D.; Pena, S.M.; Tiao, J.; Sahu, S.; Werth, V.P. Itch in dermatomyositis: The role of increased skin interleukin-31. Br. J. Dermatol. 2018, 179, 669–678. [Google Scholar] [CrossRef]
- Atkinson, S.D.; McGilligan, V.E.; Liao, H.; Szeverenyi, I.; Smith, F.J.D.; Moore, C.B.T.; McLean, W.H.I. Development of allele-specific therapeutic siRNA for keratin 5 mutations in epidermolysis bullosa simplex. J. Investig. Dermatol. 2011, 131, 2079–2086. [Google Scholar] [CrossRef]
- Baardman, R.; Yenamandra, V.K.; Duipmans, J.C.; Pasmooij, A.M.G.; Jonkman, M.F.; van den Akker, P.C.; Bolling, M.C. Novel insights into the epidemiology of epidermolysis bullosa (EB) from the Dutch EB Registry: EB more common than previously assumed? J. Eur. Acad. Dermatol. Venereol. 2021, 35, 995–1006. [Google Scholar] [CrossRef]
- Hou, P.-C.; Wang, H.-T.; Abhee, S.; Tu, W.-T.; McGrath, J.A.; Hsu, C.-K. Investigational Treatments for Epidermolysis Bullosa. Am. J. Clin. Dermatol. 2021, 22, 801–817. [Google Scholar] [CrossRef]
- Wally, V.; Lettner, T.; Peking, P.; Peckl-Schmid, D.; Murauer, E.M.; Hainzl, S.; Hintner, H.; Bauer, J.W. The pathogenetic role of IL-1β in severe epidermolysis bullosa simplex. J. Investig. Dermatol. 2013, 133, 1901–1903. [Google Scholar] [CrossRef]
- Castela, E.; Tulic, M.K.; Rozières, A.; Bourrat, E.; Nicolas, J.-F.; Kanitakis, J.; Vabres, P.; Bessis, D.; Mazereeuw, J.; Morice-Picard, F.; et al. Epidermolysis bullosa simplex generalized severe induces a T helper 17 response and is improved by apremilast treatment. Br. J. Dermatol. 2019, 180, 357–364. [Google Scholar] [CrossRef]
- Shehadeh, W.; Sarig, O.; Bar, J.; Sprecher, E.; Samuelov, L. Treatment of epidermolysis bullosa pruriginosa-associated pruritus with dupilumab. Br. J. Dermatol. 2020, 182, 1495–1497. [Google Scholar] [CrossRef]
- Welponer, T.; Diem, A.; Nahler, G.; Laimer, M. Purified oral cannabidiol for pain management in severe recessive dystrophic epidermolysis bullosa. Indian J. Dermatol. Venereol. Leprol. 2022, 88, 551–552. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine, ClinicalTrials.gov. The Efficacy and Safety of 3% Cannabidiol (CBD) Cream in Patients with Epidermolysis Bullosa: A Phase II/III Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT04613102?term=CBD&cond=Skin+Diseases&draw=1&rank=6 (accessed on 15 June 2022).
- Martinez, A.E. Time to drop the stigma: Cannabinoids are drugs that may alleviate pain in people with epidermolysis bullosa. Br. J. Dermatol. 2019, 180, 711–712. [Google Scholar] [CrossRef]
- DeFilippis, E.M.; Feldman, S.R.; Huang, W.W. The genetics of pyoderma gangrenosum and implications for treatment: A systematic review. Br. J. Dermatol. 2015, 172, 1487–1497. [Google Scholar] [CrossRef]
- Alavi, A.; French, L.E.; Davis, M.D.; Brassard, A.; Kirsner, R.S. Pyoderma Gangrenosum: An Update on Pathophysiology, Diagnosis and Treatment. Am. J. Clin. Dermatol. 2017, 18, 355–372. [Google Scholar] [CrossRef]
- Shanmugam, V.K.; Couch, K.S.; McNish, S.; Amdur, R.L. Relationship between opioid treatment and rate of healing in chronic wounds. Wound Repair Regen. 2017, 25, 120–130. [Google Scholar] [CrossRef]
- Stanley, P.L.; Steiner, S.; Havens, M.; Tramposch, K.M. Mouse skin inflammation induced by multiple topical applications of 12-O-tetradecanoylphorbol-13-acetate. Skin Pharmacol. 1991, 4, 262–271. [Google Scholar] [CrossRef]
- Nakajima, J.; Nakae, D.; Yasukawa, K. Structure-dependent inhibitory effects of synthetic cannabinoids against 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and skin tumour promotion in mice. J. Pharm. Pharmacol. 2013, 65, 1223–1230. [Google Scholar] [CrossRef]
- Rundle, C.W.; Rietcheck, H.R.; Maghfour, J.; Dercon, S.; Fernandez, J.; Lio, P.; Dellavalle, R.P.; Fujita, M.; Yardley, H. Anti-inflammatory Effect of Cannabidiol and Palmitoylethanolamide Containing Topical Formulation on Skin in a 12-O-Tetradecanoylphorbol-13-Acetate-Induced Dermatitis Model in Mice. Dermatitis 2021, 33, 277–281. [Google Scholar] [CrossRef]
- Capozzi, A.; Caissutti, D.; Mattei, V.; Gado, F.; Martellucci, S.; Longo, A.; Recalchi, S.; Manganelli, V.; Riitano, G.; Garofalo, T.; et al. Anti-Inflammatory Activity of a CB2 Selective Cannabinoid Receptor Agonist: Signaling and Cytokines Release in Blood Mononuclear Cells. Molecules 2021, 27, 64. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.J.D.; Liao, H.; Cassidy, A.J.; Stewart, A.; Hamill, K.J.; Wood, P.; Joval, I.; van Steensel, M.A.M.; Björck, E.; Callif-Daley, F.; et al. The genetic basis of pachyonychia congenita. J. Investig. Dermatol. Symp. Proc. 2005, 10, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.J.; Paus, R. The pathogenesis of primary cicatricial alopecias. Am. J. Pathol. 2010, 177, 2152–2162. [Google Scholar] [CrossRef]
- Ramot, Y.; Oláh, A.; Paus, R. Cover Image: Neuroendocrine treatment of inherited keratin disorders by cannabinoids? Br. J. Dermatol. 2018, 178, 1469. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Laurino, C.; Vadalà, M. A therapeutic effect of cbd-enriched ointment in inflammatory skin diseases and cutaneous scars. Clin. Ter. 2019, 170, e93–e99. [Google Scholar] [PubMed]
- Scheffer, I.E.; Hulihan, J.; Messenheimer, J.; Ali, S.; Keenan, N.; Griesser, J.; Gutterman, D.L.; Sebree, T.; Sadleir, L.G. Safety and Tolerability of Transdermal Cannabidiol Gel in Children with Developmental and Epileptic Encephalopathies: A Nonrandomized Controlled Trial. JAMA Netw. Open 2021, 4, e2123930. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine, ClinicalTrials.gov. The Pharmacokinetics and Pharmacodynamics of Hemp-based Topical Cannabinoid Products. ClinicalTrials.gov Identifier: NCT04741477. Available online: https://clinicaltrials.gov/ct2/show/NCT04741477?term=cannabinoid&draw=4&rank=7 (accessed on 15 June 2022).
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. The Anti-Inflammatory Effects of Cannabidiol (CBD) on Acne. J. Inflamm. Res. 2022, 15, 2795–2801. [Google Scholar] [CrossRef]
- Spleman, L.; Sinclair, R.; Freeman, M.; Davis, M.; Gebauer, K. 1061 The safety of topical cannabidiol (CBD) for the treatment of acne. J. Investig. Dermatol. 2018, 138, S180. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine, ClinicalTrials.gov. Evaluation of BTX 1503 in Patients with Moderate to Severe Acne Vulgaris”. ClinicalTrials.gov Identifier: NCT03573518. Available online: https://clinicaltrials.gov/ct2/show/NCT03573518?term=CBD&cond=Skin+Diseases&draw=1&rank=10 (accessed on 15 June 2022).
- Ali, A.; Akhtar, N. The safety and efficacy of 3% Cannabis seeds extract cream for reduction of human cheek skin sebum and erythema content. Pak. J. Pharm. Sci. 2015, 28, 1389–1395. [Google Scholar]
- Kurokawa, I.; Danby, F.W.; Ju, Q.; Wang, X.; Xiang, L.F.; Xia, L.; Chen, W.; Nagy, I.; Picardo, M.; Suh, D.H.; et al. New developments in our understanding of acne pathogenesis and treatment. Exp. Dermatol. 2009, 18, 821–832. [Google Scholar] [CrossRef]
- Ellis, C.N.; Krach, K.J. Uses and complications of isotretinoin therapy. J. Am. Acad. Dermatol. 2001, 45, S150–S157. [Google Scholar] [CrossRef] [PubMed]
- Rigopoulos, D.; Larios, G.; Katsambas, A.D. The role of isotretinoin in acne therapy: Why not as first-line therapy? facts and controversies. Clin. Dermatol. 2010, 28, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Satino, J. Hair Regrowth with Cannabidiol (CBD)-rich Hemp Extract—A Case Series. Cannabis 2021, 4, 53–59. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine, ClinicalTrials.gov. Androgenetic Alopecia Treatment Using Varin and Cannabidiol Rich Topical Hemp Oil: A Case Series (Hair Regrowth). ClinicalTrials.gov Identifier: NCT04842383. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04842383 (accessed on 15 June 2022).
- Buranakarn, V. Alopecia Areata Treatment with Extracted Cannabis Oil: Case Study 52-year-old Asian Male. Int. Jounal Sci. Innov. Technol. 2019, 2, 51–56. [Google Scholar]
- Specht, S.; Persaud, Y. Asteatotic Eczema; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Polat, M.; İlhan, M.N. Dermatological Complaints of the Elderly Attending a Dermatology Outpatient Clinic in Turkey: A Prospective Study over a One-year Period. Acta Dermatovenerol. Croat. 2015, 23, 277–281. [Google Scholar]
- Schulz, P.; Bunselmeyer, B.; Bräutigam, M.; Luger, T.A. Pimecrolimus cream 1% is effective in asteatotic eczema: Results of a randomized, double-blind, vehicle-controlled study in 40 patients. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 90–94. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, X.-M.; Guichard, A.; Tan, Y.-M.; Qian, C.-Y.; Yang, L.-J.; Humbert, P. N-palmitoylethanolamine and N-acetylethanolamine are effective in asteatotic eczema: Results of a randomized, double-blind, controlled study in 60 patients. Clin. Interv. Aging 2014, 9, 1163–1169. [Google Scholar] [CrossRef]
- Pulvirenti, N.; Nasca, M.R.; Micali, G. Topical adelmidrol 2% emulsion, a novel aliamide, in the treatment of mild atopic dermatitis in pediatric subjects: A pilot study. Acta Dermatovenerol. Croat. 2007, 15, 80–83. [Google Scholar]
- Reuters. Available online: https://www.reuters.com/article/brief-botanix-pharmaceuticals-says-btx-1-idUSFWN2BH1HT (accessed on 15 June 2022).
- Maghfour, J.; Rietcheck, H.R.; Rundle, C.W.; Runion, T.M.; Jafri, Z.A.; Dercon, S.; Lio, P.; Fernandez, J.; Fujita, M.; Dellavalle, R.P.; et al. An Observational Study of the Application of a Topical Cannabinoid Gel on Sensitive Dry Skin. J. Drugs Dermatol. 2020, 19, 1204–1208. [Google Scholar] [CrossRef]
- Callaway, J.; Schwab, U.; Harvima, I.; Halonen, P.; Mykkänen, O.; Hyvönen, P.; Järvinen, T. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J. Dermatolog. Treat. 2005, 16, 87–94. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine, ClinicalTrials.gov. A Phase Ib/IIa, Double-Blind, Randomized Study to Assess the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of S-777469 in Subjects with Atopic Dermatitis. ClinicalTrials.gov Identifier: NCT00697710. Available online: https://clinicaltrials.gov/ct2/show/NCT00697710?term=cannabinoid&cond=skin&draw=1&rank=6 (accessed on 15 June 2022).
- Eberlein, B.; Eicke, C.; Reinhardt, H.-W.; Ring, J. Adjuvant treatment of atopic eczema: Assessment of an emollient containing N-palmitoylethanolamine (ATOPA study). J. Eur. Acad. Dermatol. Venereol. 2008, 22, 73–82. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine, ClinicalTrials.gov. The Effectiveness of a Topical Palmitoylethanolamide (PEA) Formulation (Levagen+) for Reducing Symptoms of Eczema. ClinicalTrials.gov Identifier: NCT05003453. Available online: https://clinicaltrials.gov/ct2/show/NCT05003453?term=palmitoylethanolamide&draw=2&rank=7 (accessed on 15 June 2022).
- U.S. National Library of Medicine, ClinicalTrials.gov. Safety, Tolerability, and Efficacy of JBT-101 in Subjects with Dermatomyositis. ClinicalTrials.gov Identifier: NCT02466243. Available online: https://clinicaltrials.gov/ct2/show/NCT02466243?term=lenabasum&draw=1&rank=5 (accessed on 15 June 2022).
- Werth, V.P.; Hejazi, E.; Pena, S.M.; Haber, J.; Zeidi, M.; Reddy, N.; Okawa, J.; Feng, R.; Bashir, M.M.; Gebre, K.; et al. Safety and Efficacy of Lenabasum, a Cannabinoid Receptor Type 2 Agonist, in Patients with Dermatomyositis with Refractory Skin Disease: A Randomized Clinical Trial. J. Investig. Dermatol. 2022, 142, 2651–2659.e1. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine, ClinicalTrials.gov. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial to Evaluate Efficacy and Safety of Lenabasum in Dermatomyositis (DETERMINE). ClinicalTrials.gov Identifier: NCT03813160. Available online: https://clinicaltrials.gov/ct2/show/NCT03813160?cond=Dermatomyositis&draw=2&rank=6 (accessed on 15 June 2022).
- Werth, V.; White, B.; Dgetluck, N.; Hally, K.; Constantine, S.; Aggarwal, R.; Fiorentino, D.; Lundberg, I.E.; Oddis, C.V. OP0162 EFFICACY AND SAFETY OF LENABASUM IN THE PHASE 3 DETERMINE TRIAL IN DERMATOMYOSITIS. Ann. Rheum. Dis. 2022, 81, 106–107. [Google Scholar] [CrossRef]
- Amat-Samaranch, V.; Agut-Busquet, E.; Vilarrasa, E.; Puig, L. New perspectives on the treatment of hidradenitis suppurativa. Ther. Adv. Chronic Dis. 2021, 12, 20406223211055920. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.R.; Collier, F.; Brown, D.; Burton, T.; Burton, J.; Chin, M.F.; Desai, N.; Goodacre, T.E.E.; Piguet, V.; Pink, A.E.; et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br. J. Dermatol. 2019, 180, 1009–1017. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Study to Investigate the Efficacy and Safety of Cannabis Oil for the Treatment of Subjects with Hidradenitis Suppurativa. ClinicalTrials.gov Identifer: NCT03929835. Available online: https://clinicaltrials.gov/ct2/show/NCT03929835 (accessed on 15 June 2022).
- Scheinfeld, N. Topical treatments of skin pain: A general review with a focus on hidradenitis suppurativa with topical agents. Dermatol. Online J. 2014, 20, 13030. [Google Scholar] [CrossRef]
- Dvorak, M.; Watkinson, A.; McGlone, F.; Rukwied, R. Histamine induced responses are attenuated by a cannabinoid receptor agonist in human skin. Inflamm. Res. 2003, 52, 238–245. [Google Scholar] [CrossRef]
- Wassilew, S.W. Zoster-associated neuralgias. J. Dtsch. Dermatol. Ges. 2006, 4, 871–879, quiz 880-1. [Google Scholar] [CrossRef]
- Phan, N.Q.; Siepmann, D.; Gralow, I.; Ständer, S. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J. Dtsch. Dermatol. Ges. 2010, 8, 88–91. [Google Scholar] [CrossRef]
- Visse, K.; Blome, C.; Phan, N.Q.; Augustin, M.; Ständer, S. Efficacy of Body Lotion Containing N-palmitoylethanolamine in Subjects with Chronic Pruritus due to Dry Skin: A Dermatocosmetic Study. Acta Derm. Venereol. 2017, 97, 639–641. [Google Scholar] [CrossRef]
- Szepietowski, J.C.; Szepietowski, T.; Reich, A. Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: A preliminary study. Acta Dermatovenerol. Croat. 2005, 13, 97–103. [Google Scholar] [PubMed]
- Vela, J.; Dreyer, L.; Petersen, K.K.; Arendt-Nielsen, L.; Duch, K.S.; Kristensen, S. Cannabidiol treatment in hand osteoarthritis and psoriatic arthritis: A randomized, double-blind, placebo-controlled trial. Pain 2022, 163, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.J.; Momeni, K.; Kogan, M. Topical Cannabinoids for the Management of Psoriasis Vulgaris: Report of a Case and Review of the Literature. J. Drugs Dermatol. 2020, 19, 795. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, C.; Tosti, A. Efficacy and Tolerability of a Shampoo Containing Broad-Spectrum Cannabidiol in the Treatment of Scalp Inflammation in Patients with Mild to Moderate Scalp Psoriasis or Seborrheic Dermatitis. Skin Appendage Disord. 2020, 6, 355–361. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine, ClinicalTrials.gov. Trial to Evaluate Efficacy and Safety of Lenabasum in Diffuse Cutaneous Systemic Sclerosis (RESOLVE-1). ClinicalTrials.gov Identifier: NCT03398837. Available online: https://clinicaltrials.gov/ct2/show/NCT03398837?term=cannabinoid&cond=skin&draw=1&rank=8 (accessed on 15 June 2022).
- Spiera, R.; Hummers, L.; Chung, L.; Frech, T.M.; Domsic, R.; Hsu, V.; Furst, D.E.; Gordon, J.; Mayes, M.; Simms, R.; et al. Safety and Efficacy of Lenabasum in a Phase II, Randomized, Placebo-Controlled Trial in Adults with Systemic Sclerosis. Arthritis Rheumatol. 2020, 72, 1350–1360. [Google Scholar] [CrossRef]
- Hinchcliff, M. Lenabasum for Skin Disease in Patients with Diffuse Cutaneous Systemic Sclerosis. Arthritis Rheumatol. 2020, 72, 1237–1240. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine, ClinicalTrials.gov. Study to Evaluate the Safety, Tolerability and Efficacy of Cannabidiol (CBD) as a Steroid-sparing Therapy in Chronic Spontaneous Urticaria (CSU) Patients. ClinicalTrials.gov Identifier: NCT04439955. Available online: https://clinicaltrials.gov/ct2/show/NCT04439955?term=CBD&cond=Skin+Diseases&draw=1&rank=4 (accessed on 15 June 2022).
- Mallard, A.; Briskey, D.; Richards, A.; Mills, D.; Rao, A. The Effect of Orally Dosed Levagen+™ (palmitoylethanolamide) on Exercise Recovery in Healthy Males-A Double-Blind, Randomized, Placebo-Controlled Study. Nutrients 2020, 12, 596. [Google Scholar] [CrossRef]
- AminiLari, M.; Wang, L.; Neumark, S.; Adli, T.; Couban, R.J.; Giangregorio, A.; Carney, C.E.; Busse, J.W. Medical cannabis and cannabinoids for impaired sleep: A systematic review and meta-analysis of randomized clinical trials. Sleep 2022, 45, 234. [Google Scholar] [CrossRef]
- Moltke, J.; Hindocha, C. Reasons for cannabidiol use: A cross-sectional study of CBD users, focusing on self-perceived stress, anxiety, and sleep problems. J. Cannabis Res. 2021, 3, 5. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).