Abstract
A large population in Africa, particularly West Africa, depends on leafy vegetables such as red amaranth (Amaranthus cruentus), Lagos spinach (Celosia argentea), and African eggplant (Solanum macrocarpon) as affordable and readily available sources of nutrition. These vegetables are rich sources of phenolics, minerals, vitamins, and bioactive compounds, contributing significantly to dietary nutrition and providing an important source of revenue for farmers. However, the temperature rise due to climate change threatens their availability and nutritional value. This study assessed the effects of temperature regimes (23, 30, and 40 °C) on the growth and quality of these vegetables under greenhouse conditions for 48 (A. cruentus and C. argentea) and 54 (S. macrocarpon) days after sowing by measuring biomass (leaf, stem, shoot, root dry weight, root/shoot and leaf area), photosynthetic parameters, pigments, sugars, mineral content, antioxidant activity, total phenolic compounds, total flavonoids, and free amino acids. Temperature significantly affected biomass, with A. cruentus and C. argentea showing declines of 13.5–32.2% and 5.1–27.8%, respectively, at 40 °C compared to 23 °C, indicating sensitivity to heat stress. Photosynthetic rates increased with a rise from 23 to 30 °C by 2.1–29.2% across all species. Sugar contents remained generally stable, except for notable decreases in glucose and soluble sugars by 43.3% and 40.5%, respectively, in C. argentea between 30 and 40 °C, and a 52.6% reduction in starch in S. macrocarpon from 23 to 40 °C. Mineral nutrient responses varied by species; however, they exhibited similar increases in nitrogen and phosphorus, as well as decreases in calcium and manganese, at higher temperatures. Notably, antioxidant capacity and total phenolic compounds declined significantly in C. argentea (8.1% and 8.0%) and S. macrocarpon (4.7% and 13.3%). In contrast, free amino acid contents increased by 35.2% and 28.8% in A. cruentus and S. macrocarpon, respectively. It was concluded that A. cruentus and C. argentea suffer reduced growth and nutrients at 40 °C, while S. macrocarpon maintains biomass but has some biochemical declines; antioxidant capacity and phenolics drop at high temperatures, free amino acids rise, and 30 °C is optimal for all three.
1. Introduction
Climate change has increased the global average temperature in Africa over the past half-century and is anticipated to exceed the worldwide mean increase during the twenty-first century [1,2]. Its impacts on agriculture pose a challenge to crop yields, livestock productivity, and food security [3]. Rising temperatures, altered precipitation patterns, and increased frequency of extreme weather events disrupt agricultural systems globally [4]. The projected rise in temperature is expected to harm global food security, particularly vegetable production. This is because vegetables are more susceptible to environmental extremes, which could reduce global food production [5].
Leafy vegetables are essential components of the diet in many households across Africa, particularly in Nigeria, serving as a significant source of nutrients. They play important roles in remote areas, where they provide essential protein, minerals, fiber, vitamins, and other nutrients often lacking in daily diets [6]. Amaranthus cruentus L. (A. cruentus) and Celosia argentea (C. argentea) belong to the Amaranthaceae family. The leaves and seeds of A. cuentus are nutrient-rich, containing vitamins, proteins, minerals, and dietary fibers [7]. Various parts of the plant, which include stalks and leaves, contain phytochemicals that contribute to human and animal nutrition [8,9]. The plant is rich in polyphenols, including flavonoids and hydroxycinnamic acids, which are well known for their antioxidant properties [10,11]. The plant C. argentea is abundant in calcium, proteins, iron, and vitamins A and C. It also contains bioactive compounds such as anthocyanins, betalains, and betaxanthins, which increase its health benefits and dietary significance [12,13]. Traditionally, the plant is used as a remedy for snake bites, and its flowers, seeds, and roots are used in the treatment of conditions such as gonorrhea, eczema, dysentery, muscle disorders, and diarrhea [12]. Solanum macrocarpon L. is a member of the family Solanaceae. They contain high amounts of dietary fiber and various vitamins, as well as minerals such as potassium and calcium. Antioxidants such as flavonoids, phenolics, and tocopherols are present in S. macrocarpon, which help combat harmful radicals linked to diseases like cancer and Alzheimer’s [14,15]
Plant development is said to be influenced by the ambient temperature surrounding the plant [16]. Exposure to temperatures above the normal threshold level of adaptation will lead to high-temperature stress, which is expected to cause permanent harm to plants [17]. Impacts of this stress caused by high temperature on crops could vary; these effects could range from poor plant establishment and germination, reduced photosynthesis, leaf senescence, cellular damage, cell death, impairment of chlorophyll biosynthesis, induced sterility, protein denaturation, fluidity of membranes, enzyme inactivation, and inhibition of protein synthesis [18]. Research indicates that elevated temperatures cause significant variations in plant morphology, physiology, and chemical composition [18]. All these changes happen owing to a disruption in plant metabolism caused by the increased generation of reactive oxygen species (ROS), including hydrogen peroxide, superoxide ions, hydroxyl ions, and singlet oxygen, which are harmful molecules that lead to oxidative damage to different cellular structures and macromolecules such as RNA and DNA [19]. Plants use a variety of antioxidant enzymes, including catalase, glutathione reductase, ascorbate peroxidase, peroxidase, and superoxide dismutase, as well as non-enzymatic antioxidants like ascorbic acid, alpha-tocopherol, and carotenoids, to combat ROS [20].
The brief life cycle and high moisture content of vegetables make them particularly prone to elevated temperatures [21]. For instance, exposure of chili peppers to temperatures above 37 °C leads to a significant decrease in the morphological characteristics of harvested fruits, including fruit weight, diameter, and the number of seeds per fruit [22]. The vegetative and reproductive development of grain amaranth plants were adversely affected by high temperature [23]. During the reproductive stage of chickpeas, temperatures above 35 °C led to a 39% loss due to pollen sterility [24]. Increased temperatures of 30 °C and 35 °C resulted in a decrease of approximately 31–63% in the shoot crude protein content, as well as fresh and dry weight of cowpeas [25]. Peas showed a reduction in yield when exposed to 32 °C [26]. Soybean plants exposed to 35 °C showed reduced growth, resulting in a 29.5% decrease in seed yield [27]. High temperatures cause a minimum reduction of at least 18% in photosynthesis, 26.74% in chlorophyll contents, and 30.15% in photochemical activity in Chinese cabbage [28]. Productivity and tuber yield of potato plants decrease by at least 24% when grown above their optimum temperature range [29,30].
The cultivation of A. cruentus L., C. argentea L., and S. macrocarpon holds significant nutritional and economic value in Nigeria and West Africa, where they serve as vital sources of essential micronutrients, vitamins, minerals, and health-promoting phytochemicals. These species contribute meaningfully to dietary diversity and food security, supporting both household nutrition and livelihoods. Despite their nutritional and economic importance, little is known about how these African leafy vegetables respond to high-temperature stress. Previous studies on A. cruentus are fragmented, crop-specific, and rarely consider physiological and biochemical responses together, while C. argentea and S. macrocarpon are particularly underexplored. This study fills that gap by providing one of the first integrative, comparative assessments of biomass, gas exchange, chlorophylls, mineral composition, sugar-related traits, and biochemical metabolites under controlled high-temperature stress, highlighting novel species-specific heat tolerance strategies in these leafy vegetables.
This research aims to establish a resilient vegetable production program to ensure nutritional and financial security for vegetable producers in the event of adverse effects from high-temperature events. This research will assess the impact of different temperature regimes, particularly high temperatures, on the growth, yield, and quality of A. cruentus, C. argentea, and S. macrocarpon, including sugar content, total flavonoids, mineral contents, total phenolic compounds, free amino acids, and antioxidant capacity.
2. Materials and Methods
2.1. Experimental Set-Up and Plant Materials
The experiment was carried out at the research greenhouse of the Institut für Bio- und Geowissenschaften (IBG-2: Pflanzenwissenschaften), Forschungszentrum GmbH, Jülich, Germany. Seeds of A. cruentus, C. argentea, and S. macrocarpon were all procured from a professional supplier in Nigeria, Figure 1. Seeds were germinated for 6 days in VM 800 soil substrate, containing 70% organic material (raised bog peat, decomposition degree H2–H5), perlite, raw clay, calcic lime, and NPK (Mg) fertilizer with trace nutrients (organic matter 35%, pH 5.8, EC 0.8 g/L KCl). After germination, seedlings were transferred to 35-cell trays with the same substrate. After 11 days, uniform seedlings were transplanted into individual 11 × 11 cm pots filled with ED 73 soil substrate, composed of 70% organic material, raw clay, carbonate lime, and NPK (Mg) fertilizer with minor amounts of nitrogen, phosphate, potassium oxide, and magnesium. The study was set up in digitally controlled growth chambers with the air and root zone temperatures maintained at day temperatures of room temperatures (RT) of around 23 °C (RT), 30 °C, and 40 °C, and with night temperatures of 18 °C,22 °C, and 22 °C (16/8 h) each. Relative humidity was maintained at 60 ± 5%, and photosynthetically active radiation (PAR) was provided by an automated sodium fluorescent lamp supplying 160 µmol m−2 s−1 of PAR. Watering was performed via sub-irrigation every two days, or more frequently as needed for high-temperature treatments to avoid water stress. The soil and leaf temperatures were also measured.

Figure 1.
The three species, A. cruentus, C. argentea, and S. macrocarpon, used for this study.
2.2. Experimental Design
The experiment was arranged in a completely randomized design with three temperature treatments: day temperatures of room temperatures (RT) of around 23 °C (RT), 30 °C, and 40 °C and with night temperatures of 18 °C, 22 °C, and 22 °C, (16/8 h) each, with five replicates for the biochemical analysis and ten replicates for the biomass out of which five were used after drying, for the mineral element analyses; there were one hundred and thirty plants in total. The temperature treatments were applied 4 days after transplanting and continued until harvest, which occurred 48 days after sowing for A. cruentus and C. argentea, and 54 days for S. macrocarpon. Plants were watered every two days, or more frequently for those under high-temperature conditions, using deionized water to prevent desiccation.
2.3. Harvest, Plant Biomass, and Leaf Area Measurement
A. cruentus and C. argentea were harvested 48 days after sowing, while S. macrocarpon was harvested 54 days after sowing. Fresh samples were used for biomass and leaf area measurements, while dried samples were used for determining biomass and mineral content. Plant samples were partitioned into leaves, stems, and roots, and fresh and dried weights were recorded. The plant roots were cleansed by washing and weighed. The dry weight was obtained by oven-drying the plants at 60 °C for 5 days. The leaf area measurements were carried out using the Li-3100C area meter (LI-COR Biosciences, Lincoln, NE, USA). During harvest, leaves were detached from the whole plant and placed on the meter’s lower transparent belt. The leaf area was read across the scanning bed. Additionally, some fresh tissues were snap-frozen using liquid nitrogen and maintained in cold storage for later biochemical analysis.
2.4. Gas Exchange Parameters Measurement
Gas exchange measurements were conducted using a LI-6400 portable photosynthesis system (LI-COR Biosciences, Lincoln, NE, USA) equipped with a standard leaf chamber. The most recently matured leaf of each plant was selected for measurement. Measurements were performed under controlled conditions: light intensity (photosynthetically active radiation, PAR) was maintained at 600 µmol m−2 s−1, CO2 concentration at 400 µmol mol−1, a leaf temperature of 21 °C, and relative humidity ranging from 50% to 60%. Measurements were taken one week before harvest, with five plants per treatment. The parameters measured were net photosynthetic rate, stomatal conductance, and transpiration rate, which were recorded once steady-state conditions were reached.
2.5. Mineral Nutrient Analyses
Leaf samples were dried, finely ground, and digested using microwave-assisted extraction with a combination of hydrogen peroxide, hydrofluoric acid, and nitric acid. The resulting digests were analyzed for elemental concentrations of Fe, C, K, N, P, Ca, Zn, and Mg using inductively coupled plasma optical emission spectrometry (ICP-OES; Elan 6000, PerkinElmer Sciex; Agilent 7500ce, Planitz, Germany), following the procedure outlined by He et al. [31].
2.6. Carbohydrate (Soluble Sugars and Starch) Content Determination
Fructose, glucose, and sucrose contents were quantified using enzymatic assays described by Viola and Davies [32], with modifications reported by He et al. [31]. For each sample, approximately 45–60 milligrams of frozen, homogenized plant tissue underwent sequential extraction with 400 microliters of 80% ethanol, followed by 400 microliters of 50% ethanol. After each extraction, samples were incubated at 80 °C for 15 min and centrifuged at 13,200 rpm for 10 min. The clear supernatants were retrieved and placed on ice. To ensure thorough extraction, the pellet was repeatedly treated with 200 µL of 80% ethanol until it was visibly lacking coloration. The retrieved supernatants were pooled and either analyzed immediately or preserved at −80 °C for future use. Sugar concentrations were measured spectrophotometrically at 340 nm using a microplate reader (Synergy™ 2 Multi-Mode, BioTek, Winooski, VT, USA). Measurements were carried out in two replicates, with outcomes expressed in mg/g FW. The remaining pellets following sugar extraction served as material for subsequent starch quantification. For starch analysis, the residual pellets from the sugar extraction were used. The pellets were first rinsed with distilled water and then autoclaved for 90 min at 120 °C. Starch was enzymatically broken down to glucose by incubating the samples at 37 °C in a sodium acetate buffer containing α-amylase and amyloglucosidase enzymes from Roche Diagnostics (Basel, Switzerland). Quantification of starch content was carried out using the same enzymatic assay applied for glucose determination. All samples were analyzed twice, and the values were expressed as mg/g FW.
2.7. Extraction of Plant Metabolites
The extraction procedure followed the protocol described by López-Hidalgo et al. [33], with minor adjustments. An amount of 2 mL of 80% ethanol (cold at 4 °C) was added to 100–120 mg of frozen tissue, 10–20 mg of lyophilized sample, and homogenized in a bead mill for 30 s (three intervals of 10 s, maintaining low temperatures) and kept on ice. Samples were centrifuged at 10,000× g for 10 min at 4 °C. The resulting supernatant was transferred into a 2.0 mL Eppendorf tube for subsequent biochemical compound analysis.
2.8. Chlorophylls and Carotenoid Analysis
Supernatants from the metabolite extract from 2.7 were collected and diluted at a ratio of 1:1 for chlorophyll determination. The contents of chlorophyll a, chlorophyll b, and carotenoids were measured following the procedure described by Lichtenthaler [34]. For each sample, 150 µL of the diluted extract or a blank solution (80% [v/v] ethanol) was added to a 96-well microplate. Absorbance was then measured at 470, 649, and 664 nm using a microplate reader. Pigment concentrations were determined using standard equations: chlorophyll a (µg/mL) = 13.36 × A664 − 5.19 × A649; chlorophyll b (µg/mL) = 27.43 × A649 − 8.12 × A664; and total carotenoids (µg/mL) = (1000 × A470 − 2.13 × chlorophyll a − 97.63 × chlorophyll b)/209. The absorbance of each sample value was measured in duplicate for reliability.
2.9. Total Phenolic Compounds
The Folin–Ciocalteu (F–C) reagent was used to quantify total phenolic content, according to the procedure outlined by Ainsworth and Gillespie [35], with slight modifications. A volume of 200 microliters of 10% F–C reagent was mixed with 100 microliters of the sample supernatant in a microcentrifuge tube and incubated at room temperature (RT) for 2 min. Subsequently, 800 microliters of 700 mM sodium carbonate were transferred into the mixture, followed by thorough mixing. The mixture was subjected to dark incubation at room temperature for 2 h. After incubation, the tubes were centrifuged at 10,000× g for 1 min. An amount of 150 µL of the supernatant from each sample, standard, or blank (prepared with 80% ethanol instead of extract) was transferred into a clear 96-well microplate, and absorbance was read at 720 nm using a microplate reader. Total phenolic compounds were calculated using a gallic acid calibration curve (0, 2.5, 5.0, 25, 50, 100, 150, 250 mg/L) and expressed in mM gallic acid equivalents. Each absorbance measurement was performed in duplicate.
2.10. Total Flavonoids Determination
The aluminum chloride colorimetric assay was employed to estimate total flavonoid content, following the modified procedure of Huang et al. [36]. A 100 µL aliquot of the supernatant was transferred into a 1.5 mL microcentrifuge tube and mixed with 300 µL of methanol by vortexing. To this mixture, 20 microliters of 10% aluminum chloride solution and 20 microliters of 1 M potassium acetate were added, followed by thorough mixing. Seven hundred microliters of methanol were added to reach the final volume, and the reaction mixture was incubated for 30 min at room temperature. To prepare the blank, 100 microliters of supernatant were diluted in 1 milliliter of methanol, without reagents. After incubation, 150 microliters of the mixture, standard, or blank were transferred into a 96-well clear microplate. The measurement of absorbance at 415 nm was performed using a plate reader. The flavonoid concentration was determined using a quercetin standard curve (0, 10, 20, 40, 60, 80, 100, 150, 200 mg/mL) and expressed as mg quercetin equivalents per ml. All measurements were performed in duplicate for accuracy.
2.11. Free Amino Acid Determination
Free amino acid content was measured using a modified protocol from López-Hidalgo et al. [33]. For each sample, 150 µL of diluted supernatant (1:1, 80% ethanol) was combined with 75 µL of ninhydrin reagent in a 1.5 mL microcentrifuge tube and thoroughly mixed. The mixture was then heated in a heat block at 100 °C for 10 min. A blank was prepared using 150 µL of 80% ethanol instead of the sample extract. After heating, the tubes were immediately cooled on ice. After cooling, 375 µL of 95% ethanol was added to each tube. From each reaction mixture (sample, standard, or blank), 150 µL was pipetted into a clear 96-well microplate, and the absorbance was recorded at 440, 520, and 570 nm [37]. The concentration of free amino acids was determined using a calibration curve developed with L-proline and L-glycine standards at concentrations of 0, 0.03125, 0.0625, 0.125, 0.25, 0.5, and 1 mg/mL. The results were presented as mg/mL of an equal mixture of L-proline and L-glycine equivalents. Each sample was analyzed in duplicate for accuracy. The ninhydrin reagent used in this analysis can be prepared following the formulation described by Moore and Stein [38].
2.12. Antioxidant Capacity
Antioxidant capacity was assessed using the cupric ion reducing antioxidant capacity (CUPRAC) method, with modifications from the procedure outlined by Apak et al. [39]. The reaction mixture was formulated by combining 300 µL of 10 mM Cu(II) solution, 300 µL of 7.5 mM neocuproine, 300 µL of 1 M ammonium acetate buffer (NH4Ac), 60 µL of the sample supernatant, and 270 µL of distilled water to reach a volume of 1230 µL. The solution was gently mixed and left to react for 1 h at room temperature. After incubation, 150 µL of the reaction mixture was transferred to a well in a clear 96-well microplate, and absorbance was recorded at 450 nm. A blank was prepared using the same reagents but replacing the sample with distilled water (totaling 330 µL). Antioxidant capacity was quantified as millimolar gallic acid equivalents (mM GAE), calculated from a standard curve generated using gallic acid concentrations of 0, 2.5, 5.0, 25, 50, 100, 150, and 250 mg/L. Each assay was performed in duplicate to ensure the consistency of the results.
2.13. Statistical Analysis
Five biological replicates were measured for each parameter at each temperature level. The Shapiro–Wilk test was applied to verify the normality of the data and confirm suitability for analysis of variance (ANOVA). Statistical analyses were performed in R. Differences among temperature treatments were evaluated using ANOVA, and mean separations were conducted with Tukey’s HSD test at p ≤ 0.05. The Bonferroni correction was applied to adjust for multiple comparisons.
3. Results
3.1. Biomass and Leaf Area
Temperature had a highly significant effect (p ≤ 0.001) on the biomass accumulation and leaf area of the three species (Table 1). After applying the Bonferroni correction for multiple comparisons, the adjusted significance threshold was 0.008. In A. cruentus, both room temperature (23 °C) and 30 °C enhanced biomass production, resulting in higher leaf, stem, shoot, and root dry weights, along with an improved root-to-shoot ratio. Although the leaf area at 30 °C exceeded that at RT by 7%, root-to-shoot ratio and root dry weight were 11.8% and 13.3% lower, respectively, compared to 23 °C. Exposure to 40 °C, however, resulted in a substantial decline in biomass production in A. cruentus, with reductions ranging from 13% in leaf area to 32.2% in root dry weight. In C. argentea, a moderate increase in temperature to 30 °C significantly improved all measured growth parameters, including stem, shoot, and root dry weights, root-to-shoot ratio, and leaf area, with increases ranging from 0.1% to 11.4% relative to 23 °C. Conversely, at 40 °C, there was a marked decline in plant biomass, with reductions of up to 27.8% when compared with 23 °C. For S. macrocarpon, an increase in temperature up to 30 °C positively influenced growth. This led to improvements in leaf, stem, and shoot growth, as well as in root development, root-to-shoot ratio, and leaf area. Relative to 23 °C, these increases reached up to 20.5%. At 40 °C, S. macrocarpon exhibited an increase in stem dry weight (11.75%) and leaf area (6.9%) compared to 23 °C, while leaf and shoot dry weights remained the same between 23 °C and 40 °C (Supplementary Figure S1). After applying the Bonferroni correction for multiple comparisons (adjusted significance threshold p = 0.008), the significance of all biomass and leaf area parameters remained unchanged. These results reveal species-specific differences in heat tolerance, with moderately high temperature (30 °C) enhancing growth across all species, while extreme temperatures predominantly reduced biomass.

Table 1.
Leaf dry weight, stem dry weight, shoot dry weight, root dry weight, root/shoot ratio, and leaf area per plant of A. cruentus, C. argentea, and S. macrocarpon under different temperature regimes (mean ± standard deviation).
3.2. Gas Exchange Parameters Measurements
Temperature treatments had a significant effect on gas exchange parameters in the three species (Supplementary Table S1). In A. cruentus, exposure to 40 °C led to an increase of 2.1% in net photosynthetic rate compared to plants at room temperature (23 °C), while a notable decrease of 10% was observed at 30 °C (Figure 2). Stomatal conductance and transpiration rate in A. cruentus also increased at 40 °C by 23.2% and 29.2%, respectively, relative to 23 °C. Conversely, at 30 °C, reductions of 2.6% in stomatal conductance and 3.4% in transpiration rate were recorded. In C. argentea, net photosynthesis remained statistically unchanged across all temperature treatments. However, both stomatal conductance and transpiration rate increased by at least 16% at 30 °C and 40 °C compared to 23 °C. The S. macrocarpon plants showed consistently positive responses to rising temperatures. At 40 °C, net photosynthesis, stomatal conductance, and transpiration rate increased by 3%, 9.7%, and 20%, respectively, compared to 23 °C, indicating a greater tolerance to elevated temperatures (Figure 2). However, after applying the Bonferroni correction (p = 0.017), the significance of net photosynthesis in A. cruentus and C. argentea, and stomatal conductance in S. macrocarpon, was no longer statistically significant. An enhanced gas exchange performance was observed for A. cruentus and S. macrocarpon at high temperature, while C. argentea maintained stable photosynthesis but increased stomatal conductance and transpiration rate at high temperatures.
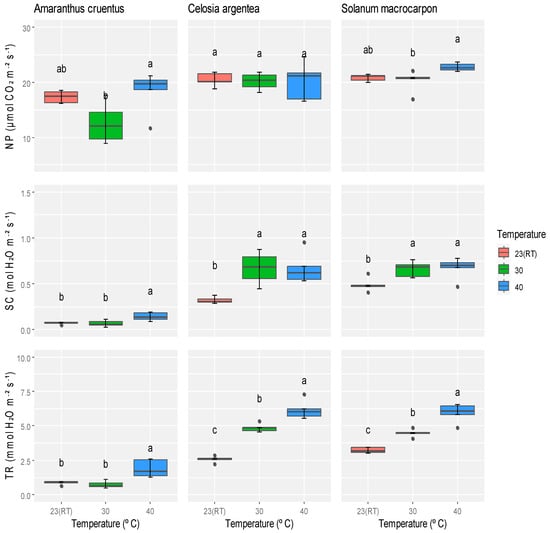
Figure 2.
Effect of different temperatures on the NP (net photosynthesis), SC (stomatal conductance), and TR (transpiration rate) of A. cruentus, C. argentea, and S. macrocarpon. Means marked with the same letter in a row indicate no statistically significant difference.
3.3. Chlorophylls and Carotenoids
Temperature treatments had differential effects on chlorophyll and carotenoid contents among the three species (Supplementary Table S2). In A. cruentus, temperature treatments did not significantly affect the chlorophyll a, chlorophyll b, total chlorophyll, or carotenoid contents (p ≥ 0.05). However, chlorophyll b content at 30 °C was slightly higher than at other temperatures, exceeding the value at 40 °C by 6.9%. In C. argentea, temperature had a significant effect on chlorophyll b (p ≤ 0.001) and total chlorophyll (p ≤ 0.01), but not on chlorophyll a or carotenoids. The highest pigment concentrations in C. argentea were observed at 30 °C, and the lowest were recorded at room temperature (23 °C), with chlorophyll a, chlorophyll b, and total chlorophyll contents increasing by 4.9%, 8.2%, and 5.8%, respectively. For S. macrocarpon, temperature significantly influenced all chlorophyll parameters (chlorophyll a, b, and total chlorophyll; p ≤ 0.05), but did not affect carotenoid content. Similar to C. argentea, the chlorophyll contents in S. macrocarpon peaked at 30 °C and were lowest at room temperature, with differences of 8.2%, 7.3%, and 7.9% for chlorophyll a, chlorophyll b, and total chlorophyll, respectively (Figure 3). After Bonferroni correction (p = 0.0125), the chlorophyll b effect in S. macrocarpon was no longer significant. These results indicate that moderately high temperature (30 °C) enhances chlorophyll accumulation in C. argentea and S. macrocarpon, while A. cruentus chlorophyll levels remain largely stable across all temperatures.
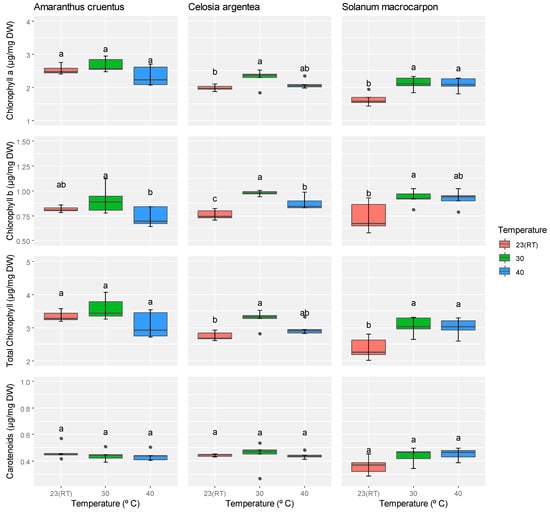
Figure 3.
Effect of different temperatures on the chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid content of A. cruentus, C. argentea, and S. macrocarpon. Means marked with the same letter in a row indicate no statistically significant difference.
3.4. Soluble Sugars and Starch (Carbohydrate)
Temperature treatments had no significant effect on the glucose, fructose, total soluble sugar, or starch contents in A. cruentus (p ≥ 0.05), though sucrose content was significantly affected (p ≤ 0.05; Table 2). However, sucrose levels in A. cruentus at 40 °C were 43.5% lower than those at room temperature (23 °C). In C. argentea, glucose and total soluble sugar contents were significantly influenced by temperature (p ≤ 0.05), whereas fructose, sucrose, and starch levels remained unaffected. The lowest glucose and sucrose concentrations in C. argentea were observed at 40 °C, with reductions of 34.4% and 20.5%, respectively, compared to room temperature. For S. macrocarpon, temperature had no significant impact on glucose, fructose, sucrose, or total soluble sugar levels. However, starch content was significantly reduced at 40 °C (p ≤ 0.05), with a 55% decrease compared to room temperature (Supplementary Figure S2). After applying the Bonferroni correction (adjusted α = 0.001), the effects of temperature on glucose and soluble sugar contents in C. argentea were no longer statistically significant. These findings suggest a species-specific sensitivity of carbohydrate metabolism to high temperature, with sucrose in A. cruentus and starch in S. macrocarpon being particularly affected.

Table 2.
Glucose, fructose, sucrose, soluble sugar, and starch content of A. cruentus, C. argentea, and S. macrocarpon under different temperatures (mean ± standard deviation).
3.5. Mineral Elements
Temperature treatment had a significant influence on the mineral composition of the three species. In A. cruentus, the contents of nitrogen, magnesium, iron, manganese, phosphorus, zinc, sodium, calcium, and potassium differed significantly (p ≤ 0.05, p ≤ 0.01, p ≤ 0.001). Carbon and sulfur contents did not show significant variation (p ≥ 0.05) (Table 3). For C. argentea, temperature had a significant effect on carbon, nitrogen, iron, sodium, calcium, and potassium (p ≤ 0.05, p ≤ 0.01, p ≤ 0.001). Sulfur, magnesium, manganese, phosphorus, and zinc contents were unaffected by temperature changes (Table 3). In S. macrocarpon, carbon, nitrogen, manganese, phosphorus, calcium, and potassium contents were significantly affected by temperature (p ≤ 0.05, p ≤ 0.01, p ≤ 0.001), whereas sulfur, magnesium, zinc, and sodium contents did not differ significantly (p ≥ 0.05) (Table 3). Specifically, in A. cruentus, increasing the temperature to 40 °C resulted in higher nitrogen (6.7%), phosphorus (8%), zinc (14.5%), sodium (20%), and potassium (15.9%) contents compared to 23 °C. Conversely, magnesium, iron, manganese, and calcium contents decreased by 23.4%, 2.1%, 12.4%, and 19.7%, respectively. The carbon and sulfur contents remained unchanged across all temperature treatments (Supplementary Figures S3 and S4). In C. argentea, nitrogen, iron, phosphorus, and potassium contents increased by up to 35.7% at 40 °C, whereas carbon, sodium, and calcium contents decreased by up to 19.8%. Sulfur, magnesium, manganese, and zinc contents did not vary significantly (Supplementary Figures S3 and S4). For S. macrocarpon, carbon and manganese contents increased between 23 and 30 °C but declined at 40 °C by 0.2% and 9.6%, respectively, relative to 23 °C. Phosphorus content was highest at 40 °C, increasing by 7.7% compared to 23 °C. Nitrogen content peaked at 40 °C and was lowest at 30 °C, with a difference of 6.8%. The calcium content decreased by 11% at 40 °C compared to 30 °C, but was unchanged between 23 °C and 30 °C. The potassium content was lowest at 30 °C, with similar values at 23 °C and 40 °C. Across all temperatures, the sulfur, magnesium, iron, zinc, and sodium remained stable (Supplementary Figures S3 and S4). With the Bonferroni correction (p = 0.0045), significance for carbon in C. argentea and S. macrocarpon, and manganese and nitrogen in S. macrocarpon, was lost. These results indicate that temperature stress affects nutrient allocation differently among species, with specific minerals increasing or decreasing depending on the species and temperature level.

Table 3.
Element concentrations (mg/gDW) of A. cruentus, C. argentea, and S. macrocarpon under different temperature regimes (mean ± standard deviation).
3.6. Antioxidant Capacity, Total Phenolic Compounds, Total Flavonoids, and Free Amino Acids
Temperature did not significantly affect the antioxidant capacity, total phenolic compounds, or total flavonoid content in A. cruentus (p > 0.05); however, a significant influence was observed on free amino acid levels (Supplementary Table S3). Notably, free amino acid content in A. cruentus increased by 35.2% at 40 °C compared to room temperature (23 °C). In C. argentea, temperature had a significant effect on both antioxidant capacity and total phenolic compounds (p ≤ 0.05), while total flavonoids and free amino acids remained unaffected. At 40 °C, antioxidant capacity and total phenolic content decreased by 8.1% and 8.0%, respectively, relative to room temperature. For S. macrocarpon, antioxidant capacity, total phenolics, and free amino acid content were significantly influenced by temperature, whereas total flavonoid content showed no significant change. Antioxidant capacity in S. macrocarpon was highest at room temperature and lowest at 30 °C, where it declined by 12.7%. Similarly, the phenolic content decreased by 11.9% and 13.3% at 30 °C and 40 °C, respectively, compared to room temperature (23 °C). In contrast, free amino acid content increased by 28.8% at 40 °C (Figure 4). Bonferroni correction (p = 0.0125) rendered the antioxidant capacity of S. macrocarpon non-significant. From these results, it can be observed that free amino acids generally increase at higher temperatures, whereas antioxidant capacity and phenolic compounds showed variable trends among the species.
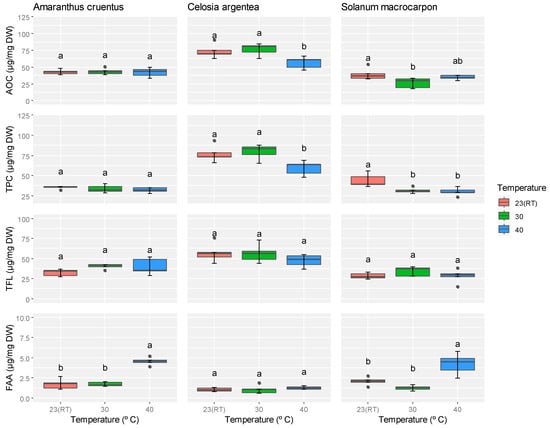
Figure 4.
Effect of different temperatures on the antioxidant capacity, total phenolic compounds, total flavonoids, and free amino acids content of A. cruentus, C. argentea, and S. macrocarpon. Means marked with the same letter in a row indicate no statistically significant difference.
4. Discussion
High-temperature stress poses a major challenge to plant growth and development worldwide. Numerous studies have demonstrated that various environmental factors can impact the growth and development of fruits and vegetables [40,41]. In this study, we examined how different temperature regimes impact the growth, yield, bioactive components, mineral elements, and carbohydrate content of three vegetable species.
Temperature is a key factor influencing plant growth, development, and biomass allocation. The growth reduction observed at 40 °C in A. cruentus and C. argentea corroborates the existing literature, which shows that heat stress disrupts critical physiological functions. The growth reduction observed at 40 °C in A. cruentus and C. argentea supports the existing literature, which shows that heat stress disrupts critical physiological functions [42]. Heat stress also accelerates water loss and reduces membrane stability [43], leading to oxidative stress through excessive production of reactive oxygen species (ROS). These ROS cause lipid peroxidation and protein oxidation, which compromise cellular function and result in reduced biomass accumulation [44,45]. Similar reductions in growth attributes due to high temperature have been reported in other vegetable species. For example, Wang et al. [46] found that increasing temperatures (35, 40, and 45 °C) markedly reduced growth in water spinach, with 30 °C causing a less pronounced decline. Likewise, Jahan et al. [47] observed reduced biomass accumulation in tomato seedlings under thermal stress, attributing this to metabolic disturbances. Comparable findings have been reported in okra, potato, melon, and tomato [48,49,50]. The responses of A. cruentus and C. argentea in the current study are consistent with these reports, highlighting their sensitivity to heat-induced physiological stress. On the other hand, S. macrocarpon demonstrated a comparatively higher degree of thermotolerance. Although 30 °C supported optimal growth, the improved stem dry weight and leaf area at 40 °C suggest that S. macrocarpon may possess adaptive traits that promote resilience under moderately elevated temperatures. Mild heat stress may trigger shoot elongation and leaf expansion as avoidance strategies before cellular damage occurs [51]. High temperature also affects root growth and architecture, which are essential for water and nutrient uptake. Heat stress increases transpirational demand while often limiting root development, thereby reducing resource acquisition [51]. Heat stress has been shown to reduce root biomass in water spinach [46], spinach [52], and wheat [44]. Significant alterations in root architecture have also been observed in tomato, pepper, and watermelon during heat stress [53,54]. In the present study, the decline in root-to-shoot ratio and root dry weight at 40 °C across all three species further supports the view that heat stress compromises root development, resulting in a lower root-to-shoot ratio and ultimately reducing the shoot biomass of the plant [55,56].
As a major environmental factor, temperature plays a central role in shaping plant physiological functions. Photosynthesis, in particular, is recognized as one of the plant metabolic processes most sensitive to temperature changes [57]. In this study, the net photosynthetic rate in A. cruentus and S. macrocarpon increased with rising temperature. This trend may reflect the ability of these plants to acclimate their physiological processes to function within a more favorable temperature range under thermal stress, as reported in previous studies [58,59]. For example, spinach plants grown in growth chambers at 30/25 °C exhibited higher photosynthetic rates compared to those grown at 15/10 °C [60]. This is in contrast to the common expectation that extreme temperatures inhibit photosynthesis, as reported by Wahid et al. [61] and Ahmad and Prasad [62]. They noted that most photosynthetic systems are hindered above 38 °C. However, the elevated photosynthesis at 40 °C in these species may reflect adaptive thermotolerance mechanisms, such as enhanced stomatal opening and transpiration-mediated leaf cooling, which have been described in heat-tolerant cultivars of tomato [63]. In addition, the increased net photosynthesis observed at higher temperatures in this study may be ascribed to the adequate water provided to the vegetable plants during the high-temperature treatment. Past research by Wahid et al. [64] reported that the presence of moisture can help plants retain water in their tissues during periods of high-temperature stress. Conversely, an inadequate water supply can negatively affect plant survival. Meanwhile, C. argentea exhibited no significant differences in net photosynthetic rate across the three temperatures, suggesting a relatively stable photosynthetic performance within this range. Furthermore, stomatal conductance was higher at 30 °C and 40 °C than at 23 °C for all three vegetables. Increased stomatal conductance under heat stress can facilitate evaporative cooling and gas exchange, mitigating the negative effects of thermal stress [63]. This behavior aligns with studies on tomato, in which heat stress led to increased stomatal activity, helping to preserve chlorophyll content and maintain photosynthetic performance [63]. Furthermore, transpiration rates increased significantly with rising temperature, being highest at 40 °C and lowest at 23 °C, further highlighting the role of transpirational cooling in thermal adaptation [65]. Consistent with the findings of Lambers et al. [66], it has been observed that plants tend to enhance their transpiration rates as temperatures rise. This is owed to the presence of significant water vapor gradients and the plants’ requirement for cooling, ensuring an adequate water supply. Research conducted by Hannachi et al. and Faiz et al. [67,68] found that under stress conditions, the stomatal conductance and transpiration rate increased in Solanum melongena. Interestingly, while high temperature is typically associated with impaired photosynthetic pigment integrity and reduced photosynthesis [69,70], A. cruentus and S. macrocarpon appear to compensate through physiological plasticity. Chloroplasts play a crucial role in these responses by initiating heat stress signaling when damaged [71,72], which may lead to activation of protective mechanisms, such as upregulation of heat shock proteins or antioxidants. However, the lower photosynthetic rate at 30 °C in A. cruentus and S. macrocarpon suggests that this temperature may trigger partial stress without activating the full heat adaptation mechanisms observed at 40 °C. Our findings demonstrate that while extreme heat often impairs photosynthetic efficiency [73,74], certain plant species or cultivars can sustain or even enhance photosynthetic activity at high temperatures through increased stomatal conductance and transpiration.
High-temperature stress significantly inhibits chlorophyll biosynthesis, and chlorophyll content therefore serves as a reliable physiological indicator for assessing thermal stress responses in plants [75]. Among the photosynthetic pigments, chlorophyll a, chlorophyll b, and carotenoids are particularly sensitive to elevated temperatures [76,77]. Numerous studies have reported substantial pigment degradation under heat stress in various crops, including water spinach [46], wheat [78], tomato [48], pea [79], and maize [80]. These reductions are typically associated with oxidative damage, which destabilizes cellular membranes and activates pigment-degrading enzymes such as peroxidase and chlorophyllase [70,81]. In the present study, A. cruentus exhibited no significant changes in chlorophyll a, chlorophyll b, total chlorophyll, or carotenoid levels across the temperature range (23 °C to 40 °C), and a similar lack of significance was observed for chlorophyll b in S. macrocarpon. This relative stability suggests that A. cruentus has a high degree of pigment-related thermal tolerance. Such stability may be attributed to inherent protective mechanisms, including enhanced antioxidant capacity or the presence of thermally stable pigment–protein complexes [82,83]. Similar thermal resilience in pigment content has been documented in heat-tolerant tomato cultivars, where chlorophyll levels remained largely unaffected by high temperatures [84]. In contrast, C. argentea and S. macrocarpon showed higher chlorophyll accumulation at 30 °C compared to 23 °C. This suggests that moderately high temperatures promote pigment biosynthesis in these species, consistent with reports that cooler temperatures suppress pigment formation due to reduced metabolic and enzymatic activity [85]. Moreover, although chlorophyll pigments are known to be heat-sensitive [86,87,88], S. macrocarpon did not exhibit a pigment reduction at 40 °C. This apparent stability may be indicative of short-term acclimation, partial heat tolerance, or the activation of protective responses such as antioxidant upregulation or osmolyte accumulation, which help preserve pigment integrity under moderate heat stress [89,90]. Furthermore, carotenoid levels were not significantly affected by temperature in any of the three species examined. Given the well-established role of carotenoids in photoprotection and the mitigation of oxidative stress [91,92], this temperature insensitivity may suggest that carotenoid biosynthesis is less thermally regulated in these species. It may also indicate that existing carotenoid concentrations were sufficient to confer photoprotection across all tested temperatures. This finding contrasts with previous reports of temperature-induced carotenoid degradation in other crops [89,93], highlighting the species-specific nature of pigment regulation under heat stress.
Heat stress strongly influences sugar metabolism in plants, although the response varies among species and genotypes [94]. In this study, the glucose, fructose, sucrose, total soluble sugars, and starch of C. argentea and A. cruentus (except for sucrose) remained stable across temperature treatments, indicating strong metabolic regulation under heat stress. However, the sucrose content of A. cruentus declined at 40 °C, suggesting that extreme or prolonged heat may impair sucrose biosynthesis or transport. This finding is consistent with reports that heat stress can disrupt carbon allocation by reducing photosynthetic yield and increasing respiratory demand [95,96]. Previous studies have shown similar patterns. For example, high temperatures reduce sugar content in tomato pollen before anthesis, lowering fruit set and total sugar accumulation [97,98,99]. In contrast, heat-tolerant genotypes can maintain or increase total soluble sugars under thermal stress, making sugar regulation a potential marker of thermotolerance [84]. Accumulation of soluble sugars under heat stress has also been observed in crops such as strawberry [100], tomato [101], Catharanthus roseus [102], and pepper [103], where it helps mitigate oxidative damage and maintain osmotic balance [104,105]. In C. argentea, glucose and total soluble sugars peaked at 30 °C but declined at 40 °C. This suggests that 30 °C may be optimal for carbohydrate metabolism, while higher temperatures promote sugar degradation, as observed in cucumber and watermelon above 35 °C [106,107]. For S. macrocarpon, levels of glucose, fructose, sucrose, and total soluble sugars remained stable across temperatures, indicating moderate resilience of sugar metabolism under heat stress. However, starch content was significantly higher at 23 °C compared to 30 °C and 40 °C, suggesting suppressed starch biosynthesis or increased breakdown to meet higher respiratory demands at elevated temperatures [108]. Together, these findings highlight that while A. cruentus and S. macrocarpon maintain stable soluble sugar levels under heat stress; they may rely on different metabolic adjustments than species that accumulate sugars as a primary stress response [89].
Temperature treatment influenced the mineral composition of all three species. In A. cruentus, nitrogen, phosphorus, zinc, sodium, and potassium contents increased at 40 °C compared to 23 °C, while magnesium, iron, manganese, and calcium decreased, and carbon and sulfur remained stable. Similar patterns were observed in C. argentea, where nitrogen, iron, phosphorus, and potassium increased, but sodium and calcium decreased under higher temperatures. In S. macrocarpon, the effects were more variable: phosphorus peaked at 40 °C, while calcium decreased at 40 °C. Sulfur, magnesium, zinc, carbon, manganese, nitrogen, and sodium contents were mostly unaffected in both C. argentea and S. macrocarpon. These species-specific trends in our current study are consistent with previous findings that mineral nutrient accumulation can be strongly modified by heat stress. For instance, Netshimbupfe et al. [109] reported that Amaranthus species tend to show reduced K, Ca, and nitrate contents at 40 °C compared to moderate temperatures, indicating a threshold of heat sensitivity. Similar reductions in essential minerals have been observed in tomato (Solanum lycopersicum), lentil (Lens culinaris), and cucumber (Cucumis sativus) under heat stress, with significant decreases in Fe, Zn, Ca, and Mg contents [110,111,112]. The increase in nitrogen and phosphorus at higher temperatures in A. cruentus, C. argentea, and S. macrocarpon suggests that these species may partially compensate for heat stress by enhancing nitrogen assimilation and phosphorus uptake. However, the concurrent decline in calcium and magnesium (by A. cruentus) aligns with reports linking heat stress to reduced root uptake capacity and impaired membrane stability due to increased electrolyte leakage and decreased relative water content [56,113]. Unlike macro-elements such as nitrogen and phosphorus, the stable carbon content in all three species and the sulfur content in A. cruentus and C. argentea indicate that some mineral elements may be less responsive to temperature fluctuations. This observation agrees with findings in other leafy vegetables, such as lettuce and arugula, where iron and sulfur were not consistently affected by elevated temperatures [114,115]. The mineral nutrient responses to heat stress in our study were not uniform and varied according to the species, mineral type, and severity.
The biochemical responses observed across the three species indicate species-specific adaptive strategies to heat stress. In A. cruentus, antioxidant capacity, TPC, and TFL were not significantly affected by temperature. However, FAA levels increased at 40 °C. This indicates that A. cruentus may maintain a stable phenolic profile while shifting toward amino acid-mediated protection. Such a shift aligns with the known osmoprotective, redox-buffering, and signaling roles of free amino acids [116,117]. In C. argentea, antioxidant capacity and TPC declined at 40 °C, while TFL and FAA remained unchanged. This suggests that its antioxidant defense system is more vulnerable to heat stress. Similar reductions in polyphenols were observed in water spinach at high temperature [89]. A decline in phenolics may weaken the plant’s defense against oxidative damage, especially if enzymatic antioxidant systems are also impaired, as shown in tomato [118] and cucumber [119]. A more complex response was observed in S. macrocarpon. Antioxidant capacity was not significantly affected, but it peaked at 23 °C and declined to its lowest level at 30 °C. TPC also declined at both 30 °C and 40 °C. At the same time, FAA levels increased at 40 °C. This suggests a compensatory response, where the plant shifts from phenolic-based antioxidant defenses toward amino acid-mediated tolerance. A similar reduction in phenolic content under high temperature was also reported in beet [120]. Our findings indicate that phenolic declines and amino acid increases represent adaptive metabolic reprogramming. Some species rely on stable phenolic defenses, while others increase amino acid accumulation to cope with stress. Such variation supports earlier findings that antioxidant responses depend on both the type of stress and the species’ metabolic plasticity [109,121]. Moreover, the reduction observed in the TPC and AOC in C. argentea and S. macrocarpon may diminish their health-promoting properties. Since phenolics and antioxidants help protect the body against oxidative stress and chronic diseases, consuming vegetables with lower levels of these compounds could provide less protection [122,123]. Furthermore, the increase in FAA under heat stress suggests its role as an osmo-protective metabolite that helps regulate ion balance, stomatal conductance, and enzyme activity [124]. FAAs, including proline and others, act as compatible solutes and reservoirs of carbon and nitrogen, supporting both stress tolerance and recovery after stress [117]. Their accumulation at 40 °C in the present study therefore reflects a metabolic shift toward osmotic adjustment and stress signaling, consistent with their established role in plant adaptation to heat [125].
5. Conclusions
This study demonstrated that Amaranthus cruentus, Celosia argentea, and Solanum macrocarpon respond differently to rising temperatures. While A. cruentus and C. argentea showed reduced biomass and some declines in chlorophylls, sugars, and mineral elements at 40 °C, S. macrocarpon maintained biomass and gas exchange parameters (stomatal conductance, net photosynthesis, and transpiration rate) but showed reductions in some biochemical qualities. Antioxidant capacity and total phenolics generally declined at higher temperatures, whereas free amino acids increased for A. cruentus and S. macrocarpon. However, 30 °C appeared to be the most favorable temperature for maintaining growth, photosynthetic performance, and nutritional quality across all three species, suggesting that moderate warmth supports optimal production while minimizing heat stress. Providing sufficient water to plants, particularly at 40 °C, helps prevent desiccation caused by drought stress. Further studies should explore these species-specific responses to heat stress to develop targeted management strategies that maintain yield and nutritional quality under rising temperatures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15092057/s1, Figure S1: Effect of different temperatures on the leaf dry weight (LDW), Stem dry weight (SDW), shoot dry weight (SHDW), root dry weight (RDW), root/shoot, and leaf area per plant of A. cruentus, C. argentea, and S. macrocarpon; Figure S2: Effect of different temperatures on the glucose, fructose, sucrose, soluble sugars, and starch content of A. cruentus, C. argentea, and S. macrocarpon; Figure S3: Effect of different temperatures on the carbon, nitrogen, sulfur, magnesium, iron, and manganese content of A. cruentus, C. argentea, and S. macrocarpon; Figure S4: Effect of different temperatures on the phosphorus, zinc, sodium, calcium, and potassium content of A. cruentus, C. argentea, and S. macrocarpon; Table S1: Effect of different temperatures on the net photosynthesis, stomatal conductance, and transpiration rate of A. cruentus, C. argentea, and S. macrocarpon (mean ± standard deviation); Table S2: Chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid content of A.cruentus. C. argentea and S. macrocarpon under different temperatures (mean ± standard deviation); Table S3: Antioxidant capacity, total phenolic compounds, total flavonoids, and free amino acid content of A. cruentus, C. argentea, and S. macrocarpon under different temperatures (mean ± standard deviation).
Author Contributions
Conceptualization, O.R.I., O.C.A. and A.J.K.; Funding acquisition, O.R.I., O.C.A., A.J.K. and U.S.; Formal analysis, O.R.I. and F.H.; Investigation, O.R.I. Methodology, O.R.I., F.H., B.T. and A.J.K.; Project administration, A.J.K.; Supervision, A.J.K., O.C.A. and F.H.; Visualization, O.R.I.; Writing—original draft, O.R.I.; Writing—review and editing, O.R.I., F.H., B.T., A.J.K. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Academic Exchange Service (DAAD) under the Research Grants—Binationally Supervised Doctoral Degrees/Cotutelle, 2022/23 program (Grant No. 57588368).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We gratefully acknowledge Katharina Wolter-Heinen, Beate Uhlig, Thorsten Brehm, and Jana Kelm (IBG-2, Forschungszentrum Jülich, Germany) for their invaluable guidance and assistance with greenhouse operations and laboratory procedures. We also extend our appreciation to the technical assistance provided by ZEA-3 at Forschungszentrum Jülich in conducting the mineral analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Change, I.C. Mitigation of climate change. Contrib. Work. Group III Fifth Assess. Rep. Intergov. Panel Clim. Change 2014, 1454, 147. [Google Scholar] [CrossRef]
- Maino, M.R.; Emrullahu, D. Climate Change in Sub-Saharan Africa Fragile States: Evidence from Panel Estimations; International Monetary Fund: Washington, DC, USA, 2022. [Google Scholar]
- Ahmed, M.; Asim, M.; Ahmad, S.; Aslam, M. Climate change, agricultural productivity, and food security. In Global Agricultural Production: Resilience to Climate Change; Springer: Berlin/Heidelberg, Germany, 2023; pp. 31–72. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.S.; Singh, M.; Ranjan, J. Impact of climate change on vegetable production and adaptation measures. In Abiotic Stress Management for Resilient Agriculture; Springer: Singapore, 2017; pp. 413–428. [Google Scholar] [CrossRef]
- Imathiu, S. Neglected and underutilized cultivated crops with respect to indigenous African leafy vegetables for food and nutrition security. J. Food Secur. 2021, 9, 115–125. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Protein, dietary fiber, minerals, antioxidant pigments and phytochemicals, and antioxidant activity in selected red morph Amaranthus leafy vegetable. PLoS ONE 2019, 14, e0222517. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; Grusak, M.A. Minerals, vitamin C, phenolics, flavonoids and antioxidant activity of Amaranthus leafy vegetables. J. Food Compos. Anal. 2017, 58, 33–39. [Google Scholar] [CrossRef]
- Sarker, U.; Hossain, M.M.; Oba, S. Nutritional and antioxidant components and antioxidant capacity in green morph Amaranthus leafy vegetable. Sci. Rep. 2020, 10, 1336. [Google Scholar] [CrossRef]
- Karamać, M.; Gai, F.; Longato, E.; Meineri, G.; Janiak, M.A.; Amarowicz, R.; Peiretti, P.G. Antioxidant activity and phenolic composition of amaranth (Amaranthus caudatus) during plant growth. Antioxidants 2019, 8, 173. [Google Scholar] [CrossRef]
- Ma, J.; Sun, G.; Shah, A.M.; Fan, X.; Li, S.; Yu, X. Effects of different growth stages of amaranth silage on the rumen degradation of dairy cows. Animals 2019, 9, 793. [Google Scholar] [CrossRef]
- Oluwole, S.O.; Ogun, M.L.; Adogba, N.P.; Fasuyi, D. Impacts of two different locations on the growth, proximate and mineral compositions of Celosia argentea L. and Amaranthus cruentus. Res. Anal. J. Appl. Res. 2020, 6, 2698–2705. [Google Scholar]
- Jimoh, M.; Okunlola, G.; Olatunji, O.; Olowolaju, E. Effects of Phosphorous Application on Growth Performance, Yield and Nutritional Value of Cockscomb (Celosia argentea L). J. Appl. Sci. Environ. Manag. 2020, 24, 1057–1061. [Google Scholar] [CrossRef]
- Ezechukwu, C.S.; Mbegbu, E.C.; Nwani, C.D.; Onoja, S.O.; Orji, E.A.; Ugwu, G.C.; Nnamonu, E.I.; Ugwu, G.N. Spermicidal and antioxidant potency of Solanum macrocarpon L. (African eggplant) leaf ethanol extract in albino rats. Comp. Clin. Pathol. 2024, 33, 367–377. [Google Scholar] [CrossRef]
- Khatoon, U.; Sharma, L.; Dubey, R. Assessment of bioactive compounds, antioxidative activity and quantification of phenols through HPLC in solanum species. Ethno Med 2018, 12, 87–95. [Google Scholar]
- Mcclung, C.R.; Lou, P.; Hermand, V.; Kim, J.A. The importance of ambient temperature to growth and the induction of flowering. Front. Plant Sci. 2016, 7, 1266. [Google Scholar] [CrossRef]
- Argosubekti, N. (Ed.) A review of heat stress signaling in plants. IOP Conf. Ser. Earth Environ. Sci. 2020, 484, 012041. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/484/1/012041/pdf (accessed on 15 April 2025). [CrossRef]
- Aslam, M.A.; Ahmed, M.; Hassan, F.-U.; Afzal, O.; Mehmood, M.Z.; Qadir, G.; Asif, M.; Komal, S.; Hussain, T. Impact of temperature fluctuations on plant morphological and physiological traits. In Building Climate Resilience in Agriculture: Theory, Practice and Future Perspective; Springer: Cham, Switzerland, 2022; pp. 25–52. [Google Scholar] [CrossRef]
- Garcia-Caparros, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and defence mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Thuy, T.L.; Kenji, M. Effect of high temperature on fruit productivity and seed-set of sweet pepper (Capsicum annuum L.) in the field condition. J. Agric. Sci. Technol. A B Hue Univ. J. Sci. 2015, 5, 515–520. [Google Scholar] [CrossRef]
- Reyes-Rosales, A.; Cabrales-Orona, G.; Martínez-Gallardo, N.A.; Sánchez-Segura, L.; Padilla-Escamilla, J.P.; Palmeros-Suárez, P.A.; De´lano-Frier, J.P. Identification of genetic and biochemical mechanisms associated with heat shock and heat stress adaptation in grain amaranths. Front. Plant Sci. 2023, 14, 1101375. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Gaur, P.; Raju, T.; Trethowan, R.; Tan, D. Field response of chickpea (Cicer arietinum L.) to high temperature. Field Crops Res. 2015, 172, 59–71. [Google Scholar] [CrossRef]
- Nevhulaudzi, T.; Ntushelo, K.; Kanu, S.A. Growth and nutritional responses of cowpea (cv. Soronko) to short-term elevated temperature. HortScience 2020, 55, 1495–1499. [Google Scholar] [CrossRef]
- Zanetta, C.U.; YRafii, M.; Jaafar, J.N.; Warkentin, T.D.; Waluyo, B.; Ramlee, S.I. Variability and assessment of interrelationships among yield and yield-related characters of pea accessions under the influence of high temperature. N. Z. J. Crop Hortic. Sci. 2023, 53, 870–888. [Google Scholar] [CrossRef]
- Bihter, O.; Bakal, H.; Gulluoglu, L.; Arioglu, H. The effects of high temperature at the growing period on yield and yield components of soybean [Glycine max (L.) Merr] varieties. Turk. J. Field Crops 2017, 22, 178–186. [Google Scholar] [CrossRef]
- Yuan, L.; Yuan, Y.; Liu, S.; Wang, J.; Zhu, S.; Chen, G.; Hou, J.; Wang, C. Influence of high temperature on photosynthesis, antioxidative capacity of chloroplast, and carbon assimilation among heat-tolerant and heat-susceptible genotypes of nonheading Chinese cabbage. HortScience 2017, 52, 1464–1470. [Google Scholar] [CrossRef]
- Hancock, R.D.; Morris, W.L.; Ducreux, L.J.; Morris, J.A.; Usman, M.; Verrall, S.R.; Fuller, J.; Simpson, C.G.; Zhang, R.; Hedley, P.E.; et al. Physiological, biochemical and molecular responses of the potato (Solanum tuberosum L.) plant to moderately elevated temperature. Plant Cell Environ. 2014, 37, 439–450. [Google Scholar] [CrossRef]
- Rykaczewska, K. The effect of high temperature occurring in subsequent stages of plant development on potato yield and tuber physiological defects. Am. J. Potato Res. 2015, 92, 339–349. [Google Scholar] [CrossRef]
- He, F.; Thiele, B.; Kraus, D.; Bouteyine, S.; Watt, M.; Kraska, T.; Schurr, U.; Kuhn, A.J. Effects of short-term root cooling before harvest on yield and food quality of Chinese broccoli (Brassica oleracea var. Alboglabra Bailey). Agronomy 2021, 11, 577. [Google Scholar] [CrossRef]
- Viola, R.; Davies, H. A microplate reader assay for rapid enzymatic quantification of sugars in potato tubers. Potato Res. 1992, 35, 55–58. [Google Scholar] [CrossRef]
- López-Hidalgo, C.; Meijón, M.; Lamelas, L.; Valledor, L. The Rainbow Protocol: A Sequential Method for Quantifying Pigments, Sugars, Free Amino Acids, Phenolics, Flavonoids, and MDA from a Small Amount of Sample; Wiley Online Library: Chichester, UK, 2021; Report No.: 0140-7791. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Huang, R.; Wu, W.; Shen, S.; Fan, J.; Chang, Y.; Chen, S.; Ye, X. Evaluation of colorimetric methods for quantification of citrus flavonoids to avoid misuse. Anal. Methods 2018, 10, 2575–2587. [Google Scholar] [CrossRef]
- Seracu, D.I. The study of UV and VIS absorption spectra of the complexes of amino acids with ninhydrin. Anal. Lett. 1987, 20, 1417–1428. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J. Biol. Chem. 1954, 211, 907–913. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Bindi, M.; Fibbi, L.; Miglietta, F. Free Air CO2 Enrichment (FACE) of grapevine (Vitis vinifera L.): II. Growth and quality of grape and wine in response to elevated CO2 concentrations. Eur. J. Agron. 2001, 14, 145–155. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Firoz, M.; Al-Khaishany, M.Y. Role of nanoparticles in plants. In Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Springer: Cham, Switzerland, 2015; pp. 19–35. [Google Scholar] [CrossRef]
- Blum, A. Plant Breeding for Stress Environments; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; Abou El-Yazied, A.; Alabdallah, N.M.; Hikal, M.; Mohammed, M.H.M.; Ibrahim, M.F.M.; et al. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Boyle, D.; Welti, R.; Jagadish, S.; Prasad, P. Decreased photosynthetic rate under high temperature in wheat is due to lipid desaturation, oxidation, acylation, and damage of organelles. BMC Plant Biol. 2018, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Wang, X.; Altaf, M.A.; Hao, Y.; Wang, Z.; Zhu, G. Effect of heat stress on root architecture, photosynthesis, and antioxidant profile of water spinach (Ipomoea aquatica Forsk) seedlings. Horticulturae 2023, 9, 923. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Pagamas, P.; Nawata, E. Sensitive stages of fruit and seed development of chili pepper (Capsicum annuum L. var. Shishito) exposed to high-temperature stress. Sci. Hortic. 2008, 117, 21–25. [Google Scholar] [CrossRef]
- Tang, R.; Niu, S.; Zhang, G.; Chen, G.; Haroon, M.; Yang, Q.; Rajora, O.P.; Qing, X. Physiological and growth responses of potato cultivars to heat stress. Botany 2018, 96, 897–912. [Google Scholar] [CrossRef]
- Heckathorn, S.A.; Giri, A.; Mishra, S.; Bista, D. Heat stress and roots. In Climate Change and Plant Abiotic Stress Tolerance; John Wiley & Sons Ltd.: Weinheim, Germany, 2013; pp. 109–136. [Google Scholar] [CrossRef]
- Khosa, Q.; uz Zaman, Q.; An, T.; Ashraf, K.; Abbasi, A.; Nazir, S.; Naz, R.; Chen, Y. Silicon-mediated improvement of biomass yield and physio-biochemical attributes in heat-stressed spinach (Spinacia oleracea). Crop Pasture Sci. 2022, 74, 230–243. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M.; Bie, X.; Huang, Y. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shu, H.; Hao, Y.; Mumtaz, M.A.; Lu, X.; Wang, Z. Melatonin affects the photosynthetic performance of pepper (Capsicum annuum L.) seedlings under cold stress. Antioxidants 2022, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.M.; Testerink, C. Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar] [CrossRef]
- Mishra, S.; Spaccarotella, K.; Gido, J.; Samanta, I.; Chowdhary, G. Effects of heat stress on plant-nutrient relations: An update on nutrient uptake, transport, and assimilation. Int. J. Mol. Sci. 2023, 24, 15670. [Google Scholar] [CrossRef]
- Tao, M.-Q.; Jahan, M.S.; Hou, K.; Shu, S.; Wang, Y.; Sun, J.; Guo, S. Bitter melon (Momordica charantia L.) rootstock improves the heat tolerance of cucumber by regulating photosynthetic and antioxidant defense pathways. Plants 2020, 9, 692. [Google Scholar] [CrossRef]
- Körner, C. Significance of Temperature in Plant Life. In Plant Growth and Climate Change; Wiley-Blackwell: Weinheim, Germany, 2006; pp. 48–69. [Google Scholar] [CrossRef]
- Rashid, F.A.A.; Crisp, P.A.; Zhang, Y.; Berkowitz, O.; Pogson, B.J.; Day, D.A.; Masle, J.; Dewar, R.C.; Whelan, J.; Atkin, O.K.; et al. Molecular and physiological responses during thermal acclimation of leaf photosynthesis and respiration in rice. Plant Cell Environ. 2020, 43, 594–610. [Google Scholar] [CrossRef]
- Yamori, W.; Noguchi, K.; Hanba, Y.T.; Terashima, I. Effects of internal conductance on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant Cell Physiol. 2006, 47, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Farooq, M.; Hussain, I.; Rasheed, R.; Galani, S. Responses and management of heat stress in plants. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2012; pp. 135–157. [Google Scholar] [CrossRef]
- Ahmad, P.; Prasad, M.N.V. Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2011; Available online: https://link.springer.com/book/10.1007/978-1-4614-0815-4 (accessed on 22 April 2025).
- Haque, M.S.; Husna, M.T.; Uddin, M.N.; Hossain, M.A.; Sarwar, A.K.M.G.; Ali, O.M.; Abdel Latef, A.A.H.; Hossain, A. Heat stress at early reproductive stage differentially alters several physiological and biochemical traits of three tomato cultivars. Horticulturae 2021, 7, 330. [Google Scholar] [CrossRef]
- Wahid, A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant Res. 2007, 120, 219–228. [Google Scholar] [CrossRef]
- Zhao, X.X.; Huang, L.K.; Zhang, X.Q.; Li, Z.; Peng, Y. Effects of heat acclimation on photosynthesis, antioxidant enzyme activities, and gene expression in orchardgrass under heat stress. Molecules 2014, 19, 13564–13576. [Google Scholar] [CrossRef]
- Lambers, H.; Oliveira, R.S.; Lambers, H.; Oliveira, R.S. Plant water relations. In Plant Physiological Ecology; Springer: Cham, Switzerland, 2019; pp. 187–263. [Google Scholar] [CrossRef]
- Hannachi, S.; Signore, A.; Adnan, M.; Mechi, L. Single and associated effects of drought and heat stresses on physiological, biochemical and antioxidant machinery of four eggplant cultivars. Plants 2022, 11, 2404. [Google Scholar] [CrossRef]
- Faiz, H.; Ayyub, C.M.; Khan, R.W.; Ahmad, R. Morphological, physiological and biochemical responses of eggplant (Solanum melongena L.) seedling to heat stress. Pak. J. Agric. Sci. 2020, 57, 371–380. Available online: http://bit.ly/4ewKIxw (accessed on 2 May 2025).
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. COMT1 silencing aggravates heat stress-induced reduction in photosynthesis by decreasing chlorophyll content, photosystem II activity, and electron transport efficiency in tomato. Front. Plant Sci. 2018, 9, 998. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and responses of chloroplasts to heat stress in plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.-Z.; Guo, F.-Q. Chloroplast retrograde regulation of heat stress responses in plants. Front. Plant Sci. 2016, 7, 398. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Altaf, M.A.; Kumar, A.; Kumar, R. Abiotic and biotic stress in horticultural crops: Insight into recent advances in the underlying tolerance mechanism. Front. Plant Sci. 2023, 14, 1212982. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B: Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.; Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, Q.; Hou, X.; Wang, J.; Chen, S.; Zhang, Q.; Wang, Z.; Yin, Y.; Liu, J. The effect of high-temperature stress on the physiological indexes, chloroplast ultrastructure, and photosystems of two herbaceous peony cultivars. J. Plant Growth Regul. 2023, 42, 1631–1646. [Google Scholar] [CrossRef]
- Rysiak, A.; Dresler, S.; Hanaka, A.; Hawrylak-Nowak, B.; Strzemski, M.; Kováčik, J.; Sowa, I.; Latalski, M.; Wójciak, M. High temperature alters secondary metabolites and photosynthetic efficiency in Heracleum sosnowskyi. Int. J. Mol. Sci. 2021, 22, 4756. [Google Scholar] [CrossRef] [PubMed]
- Tafesse, E.G.; Warkentin, T.D.; Shirtliffe, S.; Noble, S.; Bueckert, R. Leaf pigments, surface wax and spectral vegetation indices for heat stress resistance in pea. Agronomy 2022, 12, 739. [Google Scholar] [CrossRef]
- Feng, B.; Liu, P.; Li, G.; Dong, S.; Wang, F.; Kong, L.; Zhang, J.W. Effect of heat stress on the photosynthetic characteristics in flag leaves at the grain-filling stage of different heat-resistant winter wheat varieties. J. Agron. Crop Sci. 2014, 200, 143–155. [Google Scholar] [CrossRef]
- Georgieva, K.; Lichtenthaler, H. Photosynthetic response of different pea cultivars to low and high temperature treatments. Photosynthetica 2006, 44, 569–578. [Google Scholar] [CrossRef]
- Yüzbaşıoğlu, E.; Dalyan, E.; Akpınar, I. Changes in photosynthetic pigments, anthocyanin content and antioxidant enzyme activities of maize (Zea mays L.) seedlings under high temperature stress conditions. Trak. Univ. J. Nat. Sci. 2017, 18, 97–104. Available online: https://dergipark.org.tr/en/download/article-file/328798 (accessed on 2 May 2025).
- Jahan, M.S.; Shu, S.; Wang, Y.; Hasan, M.M.; El-Yazied, A.A.; Alabdallah, N.M.; Hajjar, D.; Altaf, M.A.; Sun, J.; Guo, S. Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in tomato through the modulation of ABA-and GA-mediated pathways. Front. Plant Sci. 2021, 12, 650955. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Camejo, D.; Jiménez, A.; Alarcón, J.J.; Torres, W.; Gómez, J.M.; Sevilla, F. Changes in photosynthetic parameters and antioxidant activities following heat-shock treatment in tomato plants. Funct. Plant Biol. 2006, 33, 177–187. [Google Scholar] [CrossRef]
- Zhou, R.; Kjær, K.H.; Rosenqvist, E.; Yu, X.; Wu, Z.; Ottosen, C.O. Physiological response to heat stress during seedling and anthesis stage in tomato genotypes differing in heat tolerance. J. Agron. Crop Sci. 2017, 203, 68–80. [Google Scholar] [CrossRef]
- Bhattacharya, A. Effect of low temperature stress on photosynthesis and allied traits: A review. In Physiological Processes in Plants Under Low Temperature Stress; Springer: Singapore, 2022; pp. 199–297. [Google Scholar] [CrossRef]
- Murkowski, A. Heat stress and spermidine: Effect on chlorophyll fluorescence in tomato plants. Biol. Plant. 2001, 44, 53–57. [Google Scholar] [CrossRef]
- Jeon, M.-W.; Ali, M.B.; Hahn, E.-J.; Paek, K.-Y. Photosynthetic pigments, morphology and leaf gas exchange during ex vitro acclimatization of micropropagated CAM Doritaenopsis plantlets under relative humidity and air temperature. Environ. Exp. Bot. 2006, 55, 183–194. [Google Scholar] [CrossRef]
- Song, L.; Guanter, L.; Guan, K.; You, L.; Huete, A.; Ju, W.; Zhang, Y. Satellite sun-induced chlorophyll fluorescence detects early response of winter wheat to heat stress in the Indian Indo-Gangetic Plains. Glob. Change Biol. 2018, 24, 4023–4037. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, X.; Han, X.; Chen, X.; Wang-Pruski, G. Physiological and transcriptomic responses of water spinach (Ipomoea aquatica) to prolonged heat stress. BMC Genom. 2020, 21, 533. [Google Scholar] [CrossRef]
- Kaushal, N.; Awasthi, R.; Gupta, K.; Gaur, P.; Siddique, K.H.; Nayyar, H. Heat-stress-induced reproductive failures in chickpea (Cicer arietinum) are associated with impaired sucrose metabolism in leaves and anthers. Funct. Plant Biol. 2013, 40, 1334–1349. [Google Scholar] [CrossRef]
- Young, A.J. The photoprotective role of carotenoids in higher plants. Physiol. Plant. 1991, 83, 702–708. [Google Scholar] [CrossRef]
- Sandmann, G. Antioxidant protection from UV-and light-stress related to carotenoid structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef]
- Moradpour, M.; Abdullah, S.N.A.; Namasivayam, P. The impact of heat stress on morpho-physiological response and expression of specific genes in the heat stress-responsive transcriptional regulatory network in Brassica oleracea. Plants 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Balfagón, D.; Gómez-Cadenas, A.; Mittler, R. Plant responses to climate change: Metabolic changes under combined abiotic stresses. J. Exp. Bot. 2022, 73, 3339–3354. [Google Scholar] [CrossRef]
- Timlin, D.; Lutfor Rahman, S.; Baker, J.; Reddy, V.; Fleisher, D.; Quebedeaux, B. Whole plant photosynthesis, development, and carbon partitioning in potato as a function of temperature. Agron. J. 2006, 98, 1195–1203. [Google Scholar] [CrossRef]
- Vasseur, F.; Pantin, F.; Vile, D. Changes in light intensity reveal a major role for carbon balance in Arabidopsis responses to high temperature. Plant Cell Environ. 2011, 34, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Harsh, A.; Sharma, Y.; Joshi, U.; Rampuria, S.; Singh, G.; Kumar, S.; Sharma, R. Effect of short-term heat stress on total sugars, proline and some antioxidant enzymes in moth bean (Vigna aconitifolia). Ann. Agric. Sci. 2016, 61, 57–64. [Google Scholar] [CrossRef]
- Raja, M.M.; Vijayalakshmi, G.; Naik, M.L.; Basha, P.O.; Sergeant, K.; Hausman, J.F.; Khan, P.S.S.V. Pollen development and function under heat stress: From effects to responses. Acta Physiol. Plant. 2019, 41, 47. [Google Scholar] [CrossRef]
- Mazzeo, M.F.; Cacace, G.; Iovieno, P.; Massarelli, I.; Grillo, S.; Siciliano, R.A. Response mechanisms induced by exposure to high temperature in anthers from thermo-tolerant and thermo-sensitive tomato plants: A proteomic perspective. PLoS ONE 2018, 13, e0201027. [Google Scholar] [CrossRef]
- Neocleous, D.; Vasilakakis, M. Antioxidant response of salt-treated strawberry plants to heat stress. Acta Hortic 2009, 838, 217–222. [Google Scholar] [CrossRef]
- Paupière, M.J.; Müller, F.; Li, H.; Rieu, I.; Tikunov, Y.M.; Visser, R.G.; Bovy, A.G. Untargeted metabolomic analysis of tomato pollen development and heat stress response. Plant Reprod. 2017, 30, 81–94. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in ecophysiology, osmolytes, and secondary metabolites of the medicinal plants of Mentha piperita and Catharanthus roseus subjected to drought and heat stress. Biomolecules 2019, 10, 43. [Google Scholar] [CrossRef]
- Li, J.; Xie, J.; Yu, J.; Lyv, J.; Zhang, J.; Ding, D.; Li, N.; Zhang, J.; Bakpa, E.P.; Yang, Y.; et al. Melatonin enhanced low-temperature combined with low-light tolerance of pepper (Capsicum annuum L.) seedlings by regulating root growth, antioxidant defense system, and osmotic adjustment. Front. Plant Sci. 2022, 13, 998293. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, W.; Bian, J.; Xie, H.; Li, Y.; Xu, C.; Ma, J.; Guo, S.; Chen, J.; Cai, X.; et al. Proteomics and phosphoproteomics of heat stress-responsive mechanisms in spinach. Front. Plant Sci. 2018, 9, 800. [Google Scholar] [CrossRef]
- Aydogan, C.; Turhan, E. Impact of heat stress on sucrose metabolism of watermelon. BIO Web Conf. 2024, 85, 01039. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; Hao, T.; Jin, H.; Zhang, H.; He, L.; Zhou, Q.; Huang, D.; Hui, D.; Yu, J. Effects of heat shock on photosynthetic properties, antioxidant enzyme activity, and downy mildew of cucumber (Cucumis sativus L.). PLoS ONE 2016, 11, e0152429. [Google Scholar] [CrossRef]
- Liu, P.; Guo, W.; Jiang, Z.; Pu, H.; Feng, C.; Zhu, X.; Peng, Y.; Kuang, A.; Little, C.R. Effects of high temperature after anthesis on starch granules in grains of wheat (Triticum aestivum L.). J. Agric. Sci. 2011, 149, 159–169. [Google Scholar] [CrossRef]
- Netshimbupfe, M.H.; Berner, J.; Van Der Kooy, F.; Oladimeji, O.; Gouws, C. Influence of drought and heat stress on mineral content, antioxidant activity, and bioactive compound accumulation in four African Amaranthus species. Plants 2023, 12, 953. [Google Scholar] [CrossRef]
- El Haddad, N.; Sanchez-Garcia, M.; Visioni, A.; Jilal, A.; El Amil, R.; Sall, A.T.; Lagesse, W.; Kumar, S.; Bassi, F.M. Crop wild relatives crosses: Multi-location assessment in durum wheat, barley, and lentil. Agronomy 2021, 11, 2283. [Google Scholar] [CrossRef]
- Giri, A.; Heckathorn, S.; Mishra, S.; Krause, C. Heat stress decreases levels of nutrient-uptake and assimilation proteins in tomato roots. Plants 2017, 6, 6. [Google Scholar] [CrossRef]
- Du, Y.; Tachibana, S. Effect of supraoptimal root temperature on the growth, root respiration, and sugar content of cucumber plants. Sci. Hortic. 1994, 58, 289–301. [Google Scholar] [CrossRef]
- Hniličková, H.; Hnilička, F.; Orsák, M.; Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ. 2019, 65, 90–96. Available online: https://www.agriculturejournals.cz/pdfs/pse/2019/02/06.pdf (accessed on 6 May 2025). [CrossRef]
- He, J.; See, X.E.; Qin, L.; Choong, T.W. Effects of root-zone temperature on photosynthesis, productivity, and nutritional quality of aeroponically grown salad rocket (Eruca sativa) vegetable. Am. J. Plant Sci. 2016, 7, 1993–2005. Available online: https://www.scirp.org/journal/paperinformation?paperid=71238 (accessed on 6 May 2025). [CrossRef]
- He, J.; Tan, C.; Qin, L. Root-zone heat priming effects on maximum quantum efficiency of PSII, productivity, root morphology and nutritional quality of two aeroponically grown leafy greens in a tropical greenhouse. Plants 2022, 11, 1684. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.-O.; Zhao, T.; Jiang, F.; Wu, Z. Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol. Plant. 2019, 41, 20. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Kundu, A. Sugars and sugar polyols in overcoming environmental stresses. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 71–101. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; Garcıa, P.C.; Lopez-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Balal, R.M.; Shahid, M.A.; Javaid, M.M.; Iqbal, Z.; Anjum, M.A.; Garcia-Sanchez, F.; Mattson, N.S. The role of selenium in amelioration of heat-induced oxidative damage in cucumber under high temperature stress. Acta Physiol. Plant. 2016, 38, 158. [Google Scholar] [CrossRef]
- Lee, S.G.; Choi, C.S.; Lee, H.J.; Jang, Y.A.; Lee, J.G. Effect of air temperature on growth and phytochemical content of beet and ssamchoo. Hortic. Sci. Technol. 2015, 33, 303–308. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Maghsoudlou, Y.; Asghari Ghajari, M.; Tavasoli, S. Effects of heat treatment on the phenolic compounds and antioxidant capacity of quince fruit and its tisane’s sensory properties. J. Food Sci. Technol. 2019, 56, 2365–2372. [Google Scholar] [CrossRef]
- Narra, F.; Piragine, E.; Benedetti, G.; Ceccanti, C.; Florio, M.; Spezzini, J.; Troisi, F.; Giovannoni, R.; Martelli, A.; Guidi, L. Impact of thermal processing on polyphenols, carotenoids, glucosinolates, and ascorbic acid in fruit and vegetables and their cardiovascular benefits. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13426. [Google Scholar] [CrossRef]
- Trovato, M.; Funck, D.; Forlani, G.; Okumoto, S.; Amir, R. Amino acids in plants: Regulation and functions in development and stress defense. Front. Plant Sci. 2021, 12, 772810. [Google Scholar] [CrossRef]
- Tiwari, Y.K. Proline as a key player in heat stress tolerance: Insights from maize. Discov. Agric. 2024, 2, 121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).