Abstract
While the effects of elevated CO2 (eCO2) on crops have been extensively studied, cultivar-specific responses and impacts under higher CO2 concentrations (>800 μmol/mol) remain unclear. Here, we addressed these two aspects to reveal the effects of eCO2 (approximately 900 μmol/mol) on the yield and quality of three wheat cultivars, Chinese spring (CS), Chuanmai 44 and Neimai 9. The results indicated the net photosynthetic rate (Pn) and water use efficiency (WUEi) of the three cultivars significantly increased under 900 μmol/mol CO2 concentration. Elevated CO2 increased the hundred-grain weight (HGW) of Chuanmai 44 (+32.51%, p < 0.05) and Neimai 9 (+8.47% p < 0.05), but had little effect on HGW of CS. CO2 elevation significantly increased the N content in the grain of CS (+7.27%, p < 0.05). Elevated CO2 enhanced amino acid biosynthesis in CS but suppressed it in Chuanmai 44 and Neimai 9. No significant changes in grain mineral concentrations occurred in CS and Chuanmai 44 under eCO2 conditions. Neimai 9 demonstrated significant decreases in K and Mg, with non-significant reductions in other elements. The effects of eCO2 on grain yield and quality were closely linked to cultivars. This study will provide insights for understanding effects of CO2 concentration and cultivar interactions on crop growth and selecting wheat cultivar to cope with future climate change.
1. Introduction
Carbon dioxide (CO2), a critical atmospheric component accounting for approximately 0.04% of atmospheric composition, serves as the primary substrate for plant photosynthesis. Its major sources include respiration in plants and animals, microbial decomposition of organic matter, and combustion of fossil fuels in industrial processes. It is indisputable that atmospheric CO2 concentrations have been steadily increasing over time. According to a study by the Intergovernmental Panel on Climate Change (IPCC), CO2 concentration has reached 413.92 μmol/mol, with further predictions of 936 μmol/mol (RCP8.5) by the year 2100 [1].
Atmospheric CO2 concentration constitutes a primary environmental regulator for photosynthesis in C3 plants. Elevated CO2 (eCO2) levels directly influence crop photosynthetic rates, stomatal conductance, growth development, and ultimately yield [2]. The projected continuous rise in CO2 concentration will persistently impact global agricultural production in the foreseeable future [3]. As one of the three major cereals in the world, wheat (Triticum aestivum L.) is an important source of starch and energy, providing a large number of nutrients for humans, including protein, free amino acids and mineral elements. Extensive studies have suggested that the growth and grain yield of wheat have been significantly influenced by high atmospheric CO2 concentration because of its photosynthesis-enhancing effects. Thousand-grain weight (TGW) often increased under eCO2, indicating positive effects on grain quality in terms of milling quality and hence economic value [4]. Long-term FACE experiments in Arizona in the United States and in Beijing in China have shown that eCO2 could increase the yield of winter wheat by 25–28%, even under persistent moderate-drought conditions. The yield increase surpassed the reported 11–19% gain under irrigation [5,6]. In experiments using FACE technology under realistic field conditions, aboveground biomass and yield increased by 10–16% (550 versus 380 μmol/mol CO2) when given ample nutrients and water [7]. When CO2 levels were further increased to 600 μmol/mol, it still had a promoting effect on wheat yield [8]. However, some studies suggested that the increase of CO2 concentration may not significantly enhance crop yields. As demonstrated by Tan et al. [9], eCO2 concentrations up to 600 μmol/mol failed to offset the adverse effects of warming (+2.5~3.0 °C) on the growth and yield of winter wheat. A FACE experiment conducted across five different countries showed that the increase in CO2 concentration (550 μmol/mol) had no significant effect on the yield of durum wheat and bread wheat, which may be attributed to the adaptation of photosynthetic capacity to high CO2 concentrations [10], a phenomenon known as “photosynthetic adaptation”. Most studies have found that elevated atmospheric CO2 concentrations have a negative effect on crop grain quality. When CO2 levels rise to 550 μmol/mol, the contents of various amino acids tended to decrease overall [4]. The composition of proteinogenic amino acids was also found to change in the study of Högy et al. (2009) [11], but no clear response pattern of different types of amino acids was observed under CO2 enrichment [11].
Elevated CO2 concentrations generally reduced mineral element content in wheat [12]. K, Mo and Pb increased, while Mn, Fe, Ca and Si decreased, suggesting that adjustments in agricultural practices may be required to retain current grain quality standards [13]. Högy et al. [4] found that elevated CO2 concentrations (500 μmol/mol) significantly reduced the concentration of macro-elements in wheat grains except K and P, among which Ca and Mg decreased by 9.7% and 4.8%, respectively. The study also observed declines in micronutrient concentrations, with Fe and Zn decreasing by 18.3% and 13.1%, respectively. Elevated CO2 concentrations altered the entire stoichiometry of plants, resulting in lower concentrations of most elements [14]. The majority of studies have shown that eCO2 led to decreased nitrogen concentration and increased carbon concentration in crops [15]. However, recent research has found that when CO2 rise to 800 μmol/mol, wheat grain carbon concentrations decreased, possibly because higher CO2 concentrations inhibited carbohydrate transport from leaves to grains, resulting in a decrease in grain carbon concentrations [16].
Although the effects of elevated CO2 concentration on wheat yield and grain quality have been well studied, most of them focus on a specific wheat variety. In fact, distinct wheat cultivars exhibit differential responses to elevated CO2 concentrations. For instance, spring wheat cultivar demonstrated more pronounced photosynthetic enhancement under high CO2 conditions, indicating significant genotype × CO2 interaction effects on wheat physiology [17]. An intriguing question is whether genotypic variation in leaf morphology and spike architecture mediates differential responses of wheat cultivars to eCO2, and how such genotype-specific physiological adaptations manifest under eCO2. Current understanding of these genotype × eCO2 interactions remain limited. Furthermore, most existing studies on the effect of CO2 concentration on wheat yield and grain quality have been conducted at concentrations ≤ 800 μmol/mol. Under projected atmospheric CO2 trajectories, the impacts of higher CO2 levels (>800 μmol/mol) on wheat productivity and grain quality remain poorly understood, representing a critical knowledge gap in climate change research. We hypothesize that elevated CO2 concentration does not always lead to yield increase or quality reduction, as its effects are cultivar-dependent, which requires verification.
To this end, the present study employed three wheat cultivars (Chinese spring, Chuanmai 44, and Neimai 9) as experimental materials to investigate how eCO2 (~900 μmol/mol) influenced yield and quality parameters across different genotypes. The findings will provide critical insights for understanding effects of CO2 concentration and cultivar interactions on crop growth and selecting suitable wheat cultivar to cope with future climate change, so as to increase wheat yield and improve its grain quality.
2. Materials and Methods
2.1. Experimental Set-Up
The experiment was carried out from January 2022 to June 2022 in a climate-controlled greenhouse at College of Life Sciences, China West Normal University. Three wheat cultivars, namely Chinese spring (CS), Chuanmai 44 and Neimai 9, were selected as materials. Chinese spring was internationally recognized as genetic material. Chuanmai 44 and Neimai 9 were the predominant wheat cultivars extensively grown in the southwestern wheat-growing region of China. Compared to CS, Chuanmai 44 and Neimai 9 exhibited comparatively broad leaves, long spikes and smaller plant heights (~85 cm). They were grown in two climate cells with an ambient CO2 concentration (aCO2, 410 μmol/mol) and elevated CO2 concentration (eCO2, 900 μmol/mol), respectively. Each CO2 treatment was repeated three times. Wheat seeds (one seeds per pot) were sown in plastic pots (8.5 cm in height and 10.0 cm in diameter) filled with clay loam soil and surface soil (0–20 cm) of nearby cropland, with a pH (1:5 soil: water) of 5.8–6.3, and contained 1.51% organic carbon (C) and 0.18% total N. To avoid nutrient deficiency, all plants were fertilized with 200 mL standard fertilizer per pot twice from the tillering stage to grain filling stage (stock solution with macronutrient: NPK 14-3-23 + Mg and micronutrient: B 0.23%, Cu 0.14%, Fe 1.32%, Mn 0.5%, Mo 0.05%, Zn 0.18%; diluted 100- fold when applying) [16]. In each greenhouse cell, 90 pots were established and arranged randomly, with thirty replicates for each cultivar. The other climate conditions were set as 24/16 ± 2 °C day/night air temperature, 65% relative humidity (RH) and a 16 h photoperiod with a photosynthetic active radiation (PAR) > 300 μmol m−2 s−1 supplied by LED lamps.
The CO2 was supplied from high-pressure cylinders containing pure liquid CO2. Flexible tubing connected the CO2 cylinders to PVC pipes inside the growth chambers. A branch rotameter was used to quantify the CO2 flow rate, and a blower delivered the gas into the growth chambers. The CO2 concentration inside the chambers was continuously monitored every six seconds using a CO2 analyzer (GMT222, Vaisala, Helsinki, Finland), and the flow rate from the cylinders was adjusted to maintain the target concentration, with fluctuations kept within ±10%. To avoid excessive heat buildup due to prolonged enclosure and to avoid hypoxia-induced growth inhibition, the lids above the growth chamber were equipped with 45° inward-angled ventilation openings. The greenhouse maintained excellent airflow conditions. CO2 enrichment was applied daily from 09:00 to 18:00 (accounting for negligible photosynthetic activity at night), and the lid was opened the rest of the time. The elevated CO2 treatment continued from the tillering stage to the filling stage.
2.2. Photosynthetic Index Determination
At the beginning of the filling stage, leaf gas exchange rates were measured on flag leaves in the morning between 9.00 and 11.00 a.m., including the net photosynthetic rate (Pn), stomatal conductance (Gs) and transpiration rate (Tr), using a portable photosynthetic system (Li- 6400, Li- Cor, Lincoln, NE, USA). Measurements were performed on one flag leaf per pot under 1450 μmol m−2 s−1 photon flux density at 25 °C leaf temperature and at [CO2] of 410 μmol/mol for aCO2 and 900 μmol/mol for eCO2 treatment, respectively. Intrinsic water use efficiency (WUEi) was calculated as the Pn:Gs ratio. To avoid edge effects, 15 plants of each cultivar located at the center of the climate cells and showing healthy growth were selected. A total of 45 plants from three replicates were used for analysis, and each plant was measured 3 times.
2.3. Yield-Related Trait Measurement
After maturation, the aboveground parts of the wheat plants were harvested to measure yield components including spike length, spikelet number, grain number on the main spike, tiller number, grain weight per plant, grain length and width, and hundred-grain weight. A total of 15 plants of each cultivar located at the center of the climate cells and showing healthy growth were selected. A total of 45 plants from three replicates were used for trait measurement.
2.4. Determination of Grain Quality Traits
The grain quality parameters, including C, N, amino acid, and mineral concentrations, were analyzed.
The main spikes of 15 healthy plants of each cultivar located at the center of each of the climate cells were selected. Three biological and three technical replicates were performed. The wheat seeds were oven-dried at 105 °C to a constant weight, then grounded using a ball mill (Mixer Mill 400, Retsch, Haan, Germany) before being sieved through a 60-mesh screen. According to the national standard for the determination of amino acids in food (GB5009.124-2016 [18]), the sieved samples were treated with an acid or alkali hydrolysis method, and then the amino acid content was determined by S433D amino acid analyzer (Sykam, Furstenfeldbruck, Germany). The concentrations of carbon and nitrogen were analyzed using the Vario MICRO cube elemental analyzer (Elementar, Langenselbold, Hessen, Germany). The concentrations of macronutrients (Ca, K, Mg) and micronutrients (Cu, Fe, Mn, Zn) in grain were determined by AA-7000 atomic absorption spectrometry (Shimadzu, Kyoto, Japan) after microwave-assisted digestion with 70% HNO3 and 15% H2O2.
2.5. Data Analysis
Two-way analysis of variance (ANOVA) was performed to analyze the effects of [CO2] and [Cultivar] and their interactions on grain yield and quality parameters using the SPSS 27.0 software. All data were expressed as mean ± standard error. The standard errors were calculated using the ANOVA table’s pooled error term. Treatments were compared using Tukey’s tests at p = 0.05. After the data were standardized, principle component analysis (PCA) was applied to comprehensively evaluate the variation in yield and quality characteristics among wheat cultivars under different CO2 concentrations. The histograms were made using Origin 2021 software.
3. Results
3.1. Leaf Gas Exchange
Pn and WUEi were significantly increased, while Tr and Gs decreased significantly under eCO2 in all three wheat cultivars compared with those under aCO2. And the magnitude of change in different cultivars varied, indicating an interactive effect of [CO2] and [Cultivar] (Figure 1).
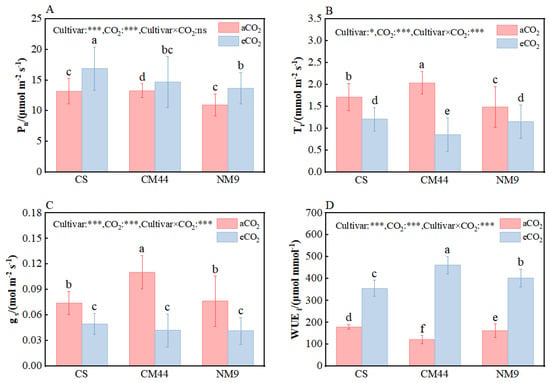
Figure 1.
Leaf gas exchange and water use efficiency of three wheat cultivars under different CO2 growth environments. Note: net photosynthetic rate (Pn) (A), transpiration rate (Tr) (B), stomatal conductance (Gs) (C), intrinsic water use efficiency (WUEi) (D). Different letters on the columns indicate statistically significant difference between the treatments according to Tukey’s test at p < 0.05. Error bars indicate standard error of the means (SE). The effects of CO2 concentration, cultivar and their interactions are presented. * and ***, respectively, represent the significant levels when p < 0.05 and p < 0.001; “ns” represents non-significant difference. Abbreviations: CS, Chinese spring; CM44, Chuanmai 44; NM9, Neimai 9.
3.2. Grain Yield Components
Elevated CO2 (eCO2) significantly enhanced grain length in both CS and Neimai 9, as well as grain width in Chuanmai 44. Notably, eCO2 induced a significant elevation in the length-to-width ratio of Neimai 9 grains, resulting in more slender grains. Conversely, Chuanmai 44 exhibited a substantial reduction in grain length-to-width ratio under eCO2 treatment, leading to a more rounded grain shape (Table 1). These findings demonstrated genotype-specific modulation of grain morphology by eCO2, with distinct effects on dimensional parameters across different wheat cultivars.

Table 1.
Effects of eCO2 on grain length and width of three wheat cultivars.
Under eCO2 conditions, the main spike length of CS showed a modest increase, while tiller number was markedly enhanced. However, significant reductions were observed in main panicle spikelet number, fertile spikelet number per main spike, and grain number per spike. Notably, yield-related parameters, including grain weight per panicle and hundred-grain weight (HGW), remained statistically unchanged. Except for the significant increase in HGW, the other indexes of Chuanmai 44 decreased significantly. Neimai 9 exhibited a marked increase in HGW alongside significant decreases in main panicle spikelet number, fertile spikelet number, and total grains per panicle. Remaining agronomic traits including spike length, tiller number, and grain weight per panicle exhibited no statistically significant alterations (Table 2). The PCA plot illustrate distinct clustering patterns among the three wheat cultivars (Figure 2A) and the separation of yield indicators under two CO2 concentrations (Figure 2B). Figure 2A demonstrate clear spatial segregation of the three cultivars, consistent with ANOVA results, indicating divergent responses of yield component traits to eCO2 across genotypes. In Figure 2B, all yield indicators except HGW exhibited vector orientations aligned with or proximal to the aCO2 treatment, while HGW uniquely associated with the eCO2 treatment. The differential clustering patterns and vector alignments collectively underscored genotype-specific plasticity in balancing grain weight enhancement against reductions in other yield-related parameters under CO2 enrichment.

Table 2.
Effects of eCO2 on yield-related traits of three wheat cultivars.
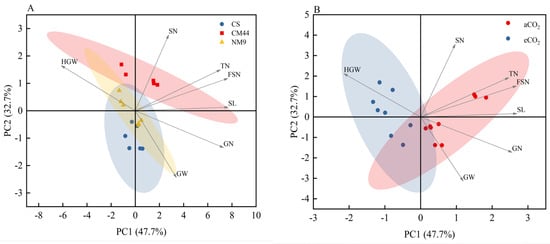
Figure 2.
Principal component analysis (PCA) analysis of yield-related traits (A) in three wheat cultivars and (B) under different CO2 conditions. The contributions of each PCA axis (PC1 and PC2) are indicated on the graph. Abbreviations: CS, Chinese spring; CM44, Chuanmai 44; NM9, Neimai 9.
3.3. C and N Concentrations in Grain
Elevated CO2 (~900 μmol/mol) showed differential effects on carbon content, nitrogen content, and C:N ratio in CS, Chuanmai 44, and Neimai 9. Grain carbon content remained statistically unchanged across all three cultivars (p > 0.05). Nitrogen content exhibited an increasing trend, with a significant elevation (7.27%) observed exclusively in CS (p < 0.05). The C:N ratio showed significant reductions in all cultivars (p < 0.05), with CS displaying the most pronounced decrease (7.10%) (Table 3).

Table 3.
Effects of eCO2 on carbon and nitrogen content in grains of three wheat cultivars.
3.4. Grain Amino Acid Content
Analysis of amino acid profiles under eCO2 conditions revealed distinct genotype-specific patterns (Table 4). In cultivar CS, 14 amino acids showed significant increases (p < 0.05), except proline and glutamic acid. Contrastingly, Chuanmai 44 exhibited declining trends in 15 amino acids, with significant reductions (p < 0.05) in tryptophan, glutamic acid, glycine, leucine, and phenylalanine, despite marginal elevation in methionine. Neimai 9 demonstrated reduced levels in 14 amino acids, with significant decreases (p < 0.05) in aspartic acid, serine, glutamic acid, glycine, valine, leucine, and phenylalanine, while proline showed slight accumulation. Collectively, eCO2 enhanced amino acid biosynthesis in CS but suppressed it in Chuanmai 44 and Neimai 9.

Table 4.
Effects of eCO2 on amino acid content in grains of three wheat cultivars.
PCA (Figure 3) further elucidated these responses. Figure 3A displays extensive overlap in amino acid clustering across cultivar, paralleled by CO2-concentration-dependent intermixing in Figure 3B. This spatial convergence aligns with Table 4 findings, collectively demonstrating significant CO2 × genotype interactive effects (p < 0.05) on amino acid metabolism.
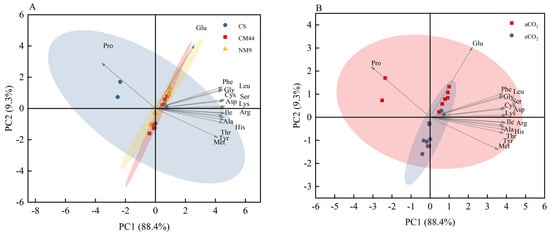
Figure 3.
Principal component analysis (PCA) of the amino acid concentrations (A) in three wheat cultivars and (B) under different CO2 conditions. The contributions of each PCA axis (PC1 and PC2) are indicated on the graph. Abbreviations: CS, Chinese spring; CM44, Chuanmai 44; NM9, Neimai 9.
3.5. Grain Mineral Concentration
The effects of eCO2 (~900 μmol/mol) on grain mineral composition across cultivars CS, Chuanmai 44, and Neimai 9 are summarized in Table 5. No statistically significant changes in Ca, Cu, K, Mg, Zn, Fe, or Mn concentrations were detected in CS and Chuanmai 44 under eCO2 conditions. Neimai 9 demonstrated significant decreases in K and Mg (p < 0.05), with non-significant reductions in other elements. PCA ordination revealed high varietal dependency in mineral responses (Figure 4A). The intermixed clustering among cultivars and CO2 treatments, coupled with divergent parameter vector orientations, confirmed strong varietal effects and significant genotype × CO2 interactions. Figure 4B further illustrates bidirectional regulation: overlapping clusters between CO2 treatments with parameter vectors distributed across both aCO2 and eCO2 axes, quantitatively demonstrating cultivar-specific mineral reallocation patterns. These findings established that eCO2 differentially modulated grain mineral homeostasis.

Table 5.
Effects of eCO2 on the content of mineral elements in grains of three wheat cultivars.
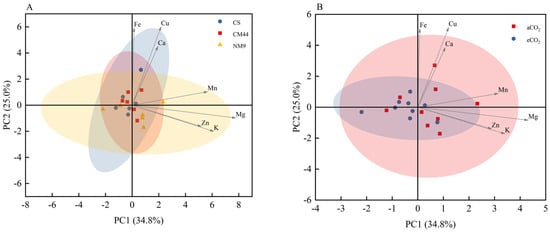
Figure 4.
Principal component analysis (PCA) of the mineral element concentrations (A) in three wheat cultivars and (B) under different CO2 conditions. The contributions of each PCA axis (PC1 and PC2) are indicated on the graph. Abbreviations: CS, Chinese spring; CM44, Chuanmai 44; NM9, Neimai 9.
4. Discussion
4.1. Elevated CO2 Levels Changed Plant Gas Exchange and Improved Water Use Efficiency
The physiological impacts of eCO2 concentrations on angiosperms are well-documented, particularly regarding enhanced Pn and WUEi, though species-specific response patterns exist [17,19,20]. In this study, under eCO2 concentrations (~900 μmol/mol), all three wheat cultivars displayed significant Pn enhancement (p < 0.05), but this varied among cultivars (CS: +28.95%, Chuanmai 44: +10.60%, Neimai 9: +24.80%), suggesting that there may be differential stomatal regulation strategies under eCO2 [21]. Another relatively consistent response to eCO2 was an increase in WUEi. Our data showed the WUEi of three cultivars was enhanced by eCO2 in the order of Chuanmai 44 (+279.20%) > Neimai 9 (+148.63%) > CS (+97.82%). These differences in physiological response to eCO2 among different wheat cultivars revealed that the underlying biochemical and molecular mechanisms of their response to CO2 enrichment may be different, which warrants further exploration.
4.2. Elevated CO2 Levels Increased the Hundred-Grain Weight (HGW) but Decreased Individual Plant Yield
Theoretically, synergistic increases in Pn and WUEi should drive biomass accumulation and yield enhancement. Both open-top chamber (OTC) and Free-Air CO2 Enrichment (FACE) experiments confirmed eCO2-mediated yield increased in crops [22,23]. In the present study, under ~900 μmol/mol CO2, Chuanmai 44 and Neimai 9 exhibited significant hundred-grain weight (HGW) increases (+32.51% and +8.47%, p < 0.05), which was consistent with previous studies [24,25]. The increase in HGW with eCO2 may be due to increased grain length and/or grain width [26]. Our research found that eCO2 significantly elongated the grain in Neimai 9 (+2.89%, p < 0.05) or broadened the grain in Chuanmai 44 (+9.03%, p < 0.05), whereas that of CS remained relatively stable (Table 1). This partially accounted for the observed increase in HGW in Neimai 9 and Chuanmai 44, in contrast to the unaltered HGW in CS. Additionally, it could also be related to the higher starch content in grains [27], as the density of starch was significantly greater than that of structural polysaccharides and proteins [28]. Of course, this needs further verification.
However, for Chuanmai 44 and Neimai 9, critical yield components displayed contrasting trends: significant reductions (p < 0.05) occurred in main spike length (Chuanmai 44: −6.80%, Neimai 9: −3.44%), spikelets per spike (−3.64% and −3.64%), fertile spikelets (−21.87% and −14.22%), grains per spike (−31.87% and −14.42%), and single-plant spike weight (−24.71% and −7.35%). Chuanmai 44 additionally showed decreased tiller number (−30.29%, p < 0.05), whereas Neimai 9 maintained stable tillering. These results are inconsistent with many previous studies [29]. These physiological trade-offs between grain filling and architectural parameters ultimately constrain whole-plant productivity, revealing a complex balance between eCO2-driven photosynthetic gains and source–sink regulation [22,30,31].
This study was conducted under controlled greenhouse conditions with two applications of standard fertilizer during the tillering initiation phase and heading stage. All environmental parameters, including temperature and water supply, were maintained consistent across treatments, eliminating confounding stressors such as high-temperature or drought conditions. While eCO2 would theoretically enhance wheat yield through CO2 fertilization effects, the three investigated cultivars—particularly Chuanmai 44 and Neimai 9—exhibited significant reductions in key yield components, including spikelet per main spike, fertile spikelet per main spike, and grains per main spike (Table 2). This paradoxical response could be attributed to two primary factors. First, the responsiveness to eCO2 varies significantly across plant genotypes [32,33]. The plant type and structure were supposed to have an important effect on source–sink relations, and an ideal plant type characterized by more vertical upper leaves and more horizontal lower leaves has been proposed [34,35]. Compared to CS, Chuanmai 44 and Neimai 9 have relatively larger leaves, which often droop vertically under eCO2 conditions later in their growth period. This somewhat affected the source of individual stems, thereby affecting the source–sink relationship. Second, the concentration-dependent nature of CO2 fertilization benefits was only effective within a specific threshold range [36]. From the jointing to grain-filling stages, three cultivars were continuously exposed to hyper-elevated CO2 concentrations. We have also observed that such supra-optimal CO2 levels accelerated plant growth and developmental processes, inducing premature phenological transitions. The entire phenological periods of Chuanmai 44 and Neimai 9 were shortened by an average of 5~6 days, while no significant effect was observed on CS. This temporal compression reduced the duration for dry matter accumulation and truncated critical reproductive phases, including grain filling and pollination, thereby directly compromising yield formation [37]. These findings demonstrate that CO2 fertilization effects are constrained by both genetic determinants and concentration thresholds, with hyper-elevated CO2 (>900 μmol/mol) overriding potential benefits through maladaptive phenological shifts.
4.3. Elevated CO2 Concentrations Negatively Affect Wheat Grain Quality, but These Impacts Exhibit Genotype-Dependent Variation
Numerous studies have established that eCO2 concentrations lead to significant reductions in nitrogen content across crop species [38], though the underlying mechanisms remain incompletely resolved. A prevailing hypothesis attributes this phenomenon to CO2-induced photosynthetic enhancement, which drives accelerated biomass accumulation while soil nitrogen availability fails to match the amplified nutrient demands of CO2-stimulated growth, resulting in nitrogen “dilution” within plant tissues [39]. Contrasting with these established patterns, our study revealed divergent responses under ~900 μmol/mol CO2 exposure: the nitrogen content in CS grains exhibited a significant increase (+7.27%, p < 0.05), while Chuanmai 44 and Neimai 9 maintained stable nitrogen levels (+1.65% and +1.52%, respectively, p > 0.05). This indicated that the eCO2 effect on the C metabolism and N assimilation occurred in a highly genotype-specific manner. These three wheat cultivars may have a high nitrogen uptake efficiency, and sufficient nitrogen supplementation throughout the growth cycle may counteract CO2-driven nitrogen depletion through enhanced soil nitrogen uptake efficiency [40].
The content of amino acids and mineral elements in grains serves as a critical parameter for evaluating wheat grain quality [41]. Previous studies have demonstrated that elevated atmospheric CO2 concentrations reduce grain amino acid content [4,11] and diminish levels of macronutrients and micronutrients such as iron, zinc, magnesium, and calcium [42]. In this study, under CO2 enrichment at 900 μmol/mol, the total amino acid content in CS grains showed a significant increase, whereas Chuanmai 44 and Neimai 9 exhibited widespread reductions in major amino acids with statistical significance (Table 4). A similar divergence was observed for mineral elements. All seven mineral concentrations in CS and Chuanmai 44 remained stable. For Neimai 9, only K (−31.03%) and Mg (−32.41%) showed a significant decline, while the reductions in other elements were not insignificant. These findings demonstrated substantial genotypic variation in CO2 responsiveness of amino acid and mineral profiles, challenging the prevailing paradigm that eCO2 universally degraded crop grain quality [43]. Notably, the HGW of CS remained unchanged under eCO2, contrasting with significant increases in Chuanmai 44 and Neimai 9. This contrast highlights a typical inverse correlation between yield enhancement and quality maintenance under eCO2 [44], emphasizing the metabolic trade-offs inherent in carbon–nutrient allocation.
The detrimental effects of eCO2 on crop quality are now well-documented. The “carbohydrate dilution” hypothesis posited that CO2-driven biomass accumulation dilutes nutrient concentrations, but the dilution was not selective [45]. Another mechanism implicated that eCO2 diminished the transpiration rate, thereby weakening the mass flow of transpiration-driven mineral nutrients, resulting in a parallel downward trend for all mineral elements [46,47]. We observed divergent trends across wheat cultivars and inconsistent patterns within the same cultivar—some nutrients declined while others remained stable or even increased. This variability aligned with emerging reports of similar anomalies in other crops [48], collectively underscoring the insufficiency of current models to fully explain CO2–nutrient dynamics [49]. Further research on the mechanism needs to be carried out, which can not only improve plant physiology, but also provide a theoretical reference for high-quality crop breeding.
5. Conclusions
Our data indicated that the gas exchange rates, yields, and quality of three cultivars vary under the influence of eCO2. The Pn and WUEi of the three cultivars significantly increased. Elevated CO2 increased the HGW of Chuanmai 44 and Neimai 9 in contrast to the unaltered HGW in CS, but decreased individual plant yield. CO2 elevation significantly or slightly increased the N content in the grains of the three cultivars. Elevated CO2 enhanced amino acid biosynthesis in CS but suppressed it in Chuanmai 44 and Neimai 9. No statistically significant changes in grain mineral concentrations were detected in CS and Chuanmai 44 under eCO2 conditions. Neimai 9 demonstrated significant decreases in K and Mg, with non-significant reductions in other elements.
Future research should prioritize integrating CO2 elevation with other climatic factors (e.g., temperature, water availability) to identify adaptive genotypes. We posit that integrating conventional cross-breeding techniques with molecular marker-assisted selection and gene-editing technologies (e.g., genomic selection, CRISPR, variable-rate fertilization) will enable the development of climate-resilient wheat cultivars.
Author Contributions
Conceptualization, Y.W., C.K., Z.Y. and Y.Z.; supervision, S.W., Z.Y. and C.K.; methodology, S.W., C.K., Z.Y., Y.W. and Y.Z.; software, Y.W., Y.Z. and S.L.; guidance for experiment, Z.Y., Y.W., S.L. and Y.Z.; test sample processing and collection, S.L. and Y.Z.; experimental operation, Y.Z. and S.L.; writing—original draft preparation, Y.Z. and S.W.; writing—review and editing, S.W., C.K. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China West Normal University National General Cultivation Project (no. 19B039).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. IPCC Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Dakora, D.F.; Li, H.; Zhao, J. Exploring the impacts of elevated CO2 on food security: Nutrient assimilation, Plant Growth, and Crop Quality. Engineering 2025, 44, 234–244. [Google Scholar] [CrossRef]
- Thomey-Michell, L.; Slattery-Rebecca, A.; Köhler-Iris, H.; Bernacchi-Carl, J.; Ort, D.R. Yield response of field-grown soybean exposed to heat waves under current and elevated [CO2]. Glob. Change Biol. 2019, 25, 4352–4368. [Google Scholar] [CrossRef] [PubMed]
- Högy, P.; Fangmeier, A. Effects of elevated atmospheric CO2 on grain quality of wheat. J. Cereal Sci. 2008, 48, 580–591. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob. Change Biol. 2021, 27, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Shuai, J.; Hui, J. Interactive effects of elevated carbon dioxide and water on the growth and development of winter wheat. Chin. J. Agrometeorol. 2013, 34, 31–37. [Google Scholar]
- Long, S.P.; Ainsworth, E.A.; Leakey, A.D.B.; Nösberger, J.; Ort, D.R. Food for thought: Lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 2006, 312, 1918–1921. [Google Scholar] [CrossRef]
- Parmar, R.; Gupta, S.C.; Kollah, B.; Agarwal, K.; Devi, M.H.; Mittal, I.; Chicham, S.; Trivedi, S.K.; Yadav, S.S.; Khambalkar, P.A.; et al. Influence of elevated CO2 and temperature on yield attributes of rice and wheat in central India. J. Exp. Agric. Int. 2024, 46, 374–395. [Google Scholar] [CrossRef]
- Tan, K.; Zhou, G.; Lv, X.; Guo, J.P.; Ren, S.X. Combined effects of elevated temperature and CO2 enhance threat from low temperature hazard to winter wheat growth in North China. Sci. Rep. 2018, 8, 4336. [Google Scholar] [CrossRef]
- Tcherkez, G.; Mariem, S.B.; Larraya, L.; García-Mina, J.M.; Zamarreño, A.M.; Paradela, A.; Cui, J.; Badeck, F.W.; Meza, D.; Rizza, F.; et al. Despite minimal effects on yield, elevated CO2 has concurrent effects on leaf and grain metabolism in wheat. J. Exp. Bot. 2020, 71, 5990–6003. [Google Scholar] [CrossRef]
- Högy, P.; Wieser, H.; Köhler, P.; Schwadorf, K.; Breuer, J.; Erbs, M.; Weber, S.; Fangmeier, A. Does elevated atmospheric CO2 allow for sufficient wheat grain quality in the future? J. Appl. Bot. Food Qual. 2009, 82, 114–121. [Google Scholar]
- Marcos-Barbero, E.L.; Pérez, P.; Martínez-Carrasco, R.; Arellano, J.B.; Morcuende, R. Genotypic variability on grain yield and grain nutritional quality characteristics of wheat grown under elevated CO2 and high temperature. Plants 2021, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Högy, P.; Wieser, H.; Köhler, P.; Schwadorf, K.; Breuer, J.; Franzaring, J.; Muntifering, R.; Fangmeier, A. Effects of elevated CO2 on grain yield and quality of wheat, results from a 3-year free-air CO2 enrichment experiment. Plant Biol. 2009, 11, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Loladze, I. Rising atmospheric CO2 and human nutrition, toward globally imbalanced plant stoichiometry? Trends Ecol. Evol. 2002, 17, 457–461. [Google Scholar] [CrossRef]
- Taub, D.R.; Wang, X.Z. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J. Integr. Plant Biol. 2008, 50, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Z.; Li, X.N.; Zhong, Y.Y.; Blennow, A.; Liang, K.H.; Liu, F.L. Effects of elevated CO2 on grain yield and quality in five wheat cultivars. J. Agron. Crop Sci. 2022, 208, 733–745. [Google Scholar] [CrossRef]
- Barnes, J.D.; Ollerenshaw, J.H.; Whitfield, C.P. Effects of elevated CO2 and/or O3 on growth, development and physiology of wheat (Triticum aestivum L.). Glob. Change Biol. 2010, 1, 129–142. [Google Scholar] [CrossRef]
- GB5009.124-2016; National Food Safety Standard—Determination of Amino Acids in Foods. China Standards Press: Beijing, China, 2016.
- Lv, D.; Xing, Q.J.; Wang, T.L.; Song, J.C.; Duan, R.N.; Hao, X.Y.; Zong, Y.Z.; Zhang, D.S.; Shi, X.R.; Zhao, Z.G.; et al. Elevated CO2 concentration enhances plant growth, photosynthesis, and ion homeostasis of soybean under salt-alkaline stress. Environ. Exp. Bot. 2024, 228, 106000. [Google Scholar] [CrossRef]
- Li, S.L.; Fang, L.; Hegelund, J.N.; Liu, F.L. Elevated CO2 modulates plant hydraulic conductance through regulation of PIPs under progressive soil drying in tomato plants. Front. Plant Sci. 2021, 12, 666066. [Google Scholar] [CrossRef]
- Du, B.; Shukla, M.K.; Du, T. A meta-analysis of crop leaf gas exchange responses to elevated CO2 and water deficits using optimal stomatal theory. Environ. Exp. Bot. 2025, 232, 106107. [Google Scholar] [CrossRef]
- Jablonski, L.M.; Wang, X.; Curtis, P.S. Plant reproduction under elevated CO2 conditions, a meta-analysis of reports on 79 crop and wild species. New Phytol. 2002, 156, 9–26. [Google Scholar] [CrossRef]
- Kimball, B.A. Crop responses to elevated CO2 and interactions with H2O, N, and temperature. Curr. Opin. Plant Biol. 2016, 31, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.X.; Zhu, T.; Zhang, Y.; Ke, X.R.; Sun, W.J.; Hu, Z.H.; Zhu, X.G.; Shen, H.H.; Huang, Y.; Tang, Y.H. Elevated CO2 enhances dynamic photosynthesis in rice and wheat. Front. Plant Sci. 2021, 12, 727374. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Ulfa, A.; Shokat, S.; Liu, S.Q.; Zhu, X.C.; Liu, F.L. Responses of carbohydrate metabolism enzymes in leaf and spike to CO2 elevation and nitrogen fertilization and their relations to grain yield in wheat. Environ. Exp. Bot. 2019, 164, 149–156. [Google Scholar] [CrossRef]
- Rahman, S.; Copeland, L.; Atwell, B.J.; Roberts, T.H. Elevated CO2 differentially affects the properties of grain from wild and domesticated rice. J. Cereal Sci. 2021, 100, 103227. [Google Scholar] [CrossRef]
- Robinson, E.A.; Ryan, G.D.; Newman, J.A. A meta-analytical review of the effects of elevated CO2 on plant-arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 2012, 194, 321–336. [Google Scholar] [CrossRef]
- Lee, C.J.; Moon, T.W. Structural characteristics of slowly digestible starch and resistant starch isolated from heat–moisture treated waxy potato starch. Carbohydr. Polym. 2015, 125, 200–205. [Google Scholar] [CrossRef]
- Fan, F.F.; Liu, M.M.; Li, N.N.; Guo, Y.; Yuan, H.Y.; Si, F.F.; Cheng, M.M.; Chen, G.L.; Cai, M.; Li, N.W.; et al. Gain-of-function allele of HPY1 coordinates source and sink to increase grain yield in rice. Sci. Bull. 2023, 68, 2155–2159. [Google Scholar] [CrossRef]
- Smith, M.R.; Rao, I.M.; Merchant, A. Source-sink relationships in crop plants and their influence on yield development and nutritional quality. Front. Plant Sci. 2018, 9, 1889. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Souza, L.; Yokoyama, R.; Sonnewald, U.; Fernie, A.R. Understanding source–sink interactions, progress in model plants and translational research to crops. Mol. Plant 2023, 16, 96–121. [Google Scholar] [CrossRef]
- Różewicz, M.; Grabiński, J.; Wyzińska, M. Effect of strip-till and cultivar on photosynthetic parameters and grain yield of winter wheat. Int. Agrophysics. 2024, 38, 279–291. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhang, S.Y.; Qian, H.Y.; Shen, C.B.; Hu, S.J.; Zhang, W.J.; Wang, Y.; Huang, S.; Wang, S.H.; Liu, Z.H.; et al. Variation in a single allele drives divergent yield responses to elevated CO2 between rice subspecies. Nat. Commun. 2025, 16, 376. [Google Scholar] [CrossRef] [PubMed]
- Asseng, S.; Kassiea, B.T.; Labrab, M.H.; Amadorb, C.; Calderini, D.F. Simulating the impact of source–sink manipulations in wheat. Field Crops Res. 2017, 202, 47–56. [Google Scholar] [CrossRef]
- Long, S.P.; Zhu, X.G.; Naidu, S.L.; Ort, D.R. Can improvement in photo synthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef]
- Sugiura, D.; Wang, Y.; Kono, M.; Mizokami, Y. Exploring the responses of crop photosynthesis to CO2 elevation at the molecular, physiological, and morphological levels toward increasing crop production. Crop Environ. 2024, 3, 75–83. [Google Scholar] [CrossRef]
- Yang, K.; Huang, Y.; Yang, J.G.; Lv, C.H.; Sun, W.J.; Hu, Z.H.; You, C.Y.; Yu, L.F. Do rice growth and yield respond similarly to abrupt and gradual increase in atmospheric CO2? Sci. Total Environ. 2024, 906, 167658. [Google Scholar] [CrossRef]
- Tausz, N.; Norton, R.M.; Tausz-Posch, S.; Löw, M.; Seneweera, S.; O’Leary, G.; Armstrong, R.; Fitzgerald, G.J. Can additional N fertiliser ameliorate the elevated CO2-induced depression in grain and tissue N concentrations of wheat on a high soil N background? J. Agron. Crop Sci. 2017, 203, 574–583. [Google Scholar] [CrossRef]
- Gifford, R.M.; Barrett, D.J.; Lutze, J.L. The effects of elevated [CO2] on the C, N and C, P mass ratios of plant tissues. Plant Soil 2000, 224, 1–14. [Google Scholar] [CrossRef]
- Sułek, A.; Cacak-Pietrzak, G.; Różewicz, M.; Nieróbca, A.; Grabiński, J.; Studnicki, M.; Sujka, K.; Dziki, D. Effect of production technology intensity on the grain yield, protein content and amino acid profile in common and durum wheat grain. Plants 2023, 12, 364. [Google Scholar] [CrossRef]
- Akar, T.; Cengiz, M.F.; Tekin, M. A comparative study of protein and free amino acid contents in some important ancient wheat lines. Qual. Assur. Saf. Crops Foods 2019, 11, 1–10. [Google Scholar] [CrossRef]
- Erbs, M.; Manderscheid, R.; Jansen, G.; Seddig, S.; Pacholski, A.; Hans-Joachim, W. Effects of free-air CO2 enrichment and nitrogen supply on grain quality parameters and elemental composition of wheat and barley grown in a crop rotation. Agric. Ecosyst. Environ. 2009, 136, 59–68. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.B. Crop nitrogen demand and grain protein concentration of spring and winter wheat. Agron. J. 2003, 95, 260–265. [Google Scholar] [CrossRef]
- Li, Y.S.; Yu, Z.H.; Jin, J.; Zhang, Q.Y.; Wang, G.H.; Liu, C.K.; Wu, J.J.; Wang, C.; Liu, X.B. Impact of elevated CO2 on seed quality of soybean at the fresh edible and mature stages. Front. Plant Sci. 2018, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Bernacchi, C.J.; Kimball, B.A.; Quarles, D.R.; Long, S.P.; Ort, D.R. Decreases in stomatal conductance of soybean under open-air elevation of [CO2] are closely coupled with decreases in ecosystem evapotranspiration. Plant Physiol. 2007, 143, 134–144. [Google Scholar] [CrossRef]
- Ploschuk, E.L.; Bado, L.A.; Salinas, M.; Wassner, D.F.; Windauer, L.B.; Insausti, P. Photosynthesis and fluorescence responses of Jatropha curcas to chilling and freezing stress during early vegetative stages. Environ. Exp. Bot. 2014, 102, 18–26. [Google Scholar] [CrossRef]
- McGrath, J.M.; Lobell, D.B. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant Cell Environ. 2013, 36, 697–705. [Google Scholar] [CrossRef]
- Uddling, J.; Broberg, M.C.; Feng, Z.Z.; Pleijel, H. Crop quality under rising atmospheric CO2. Curr. Opin. Plant Biol. 2018, 45, 262–267. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).