Abstract
Phosphonate-based fungicides are believed to control fungal diseases while also supplying nutrients to plants. However, opinions differ on whether they truly serve as nutrients for plants, and the residues of their transformation products have not yet been thoroughly evaluated or mathematically characterized. To address this gap, this study analyzed data from a two-factorial experiment investigating the effects of Agrifos 400 (potassium phosphonate) application. The experiment involved two soil types: red basalt soil and an organically enriched soil. Three-month-old pepper plants (Piper nigrum L.) were treated with Agrifos at application intervals of 10 and 20 days. The soils were inoculated with pathogenic Pythium spp., known to cause root rot diseases in plants. The soil chemical concentrations were analyzed every ten days, while plant growth parameters (height and leaf numbers) were recorded weekly. A mathematical model describing the fate of Agrifos transformation products was developed and parameterized using this experimental data. The results from the two-month experiment indicated that Agrifos did not enhance plant growth during this period. However, it led to a dramatic increase in soil phosphate (PO43−) levels, which could pose environmental risks. Despite this, the developed mathematical model demonstrated strong explanatory power, accurately capturing the observed data trends. Consequently, future research should consider integrating this model into broader biogeochemical cycle simulations, particularly those that incorporate chemical transport through soil water. Such integration would support more accurate predictions of the long-term environmental impacts of phosphonate-based products like Agrifos.
1. Introduction
Phosphites have been considered an alternative for controlling phytoparasitic organisms, demonstrating effectiveness against protozoa, oomycetes, fungi, bacteria, and nematodes [1,2]. Phosphite (H3PO3) differs from phosphate in that it represents a non-nutrient form of the essential element phosphorus (P) [3,4,5,6,7]. The term “phosphite” generally refers to the alkali metal salts of phosphorous acid (H3PO3), commonly known as phosphonates [8]. Recognized for its fungicidal properties, phosphite has been extensively studied for its ability to control various plant pathogens. Consequently, phosphite has long been incorporated into commercial fungicides and liquid fertilizers that are not registered as fungicides, with potassium phosphite products such as AgriFos, NutriPhite, ProPhyt, and K-Phite being widely used sources [9].
The efficacy of phosphite-based (Phi) fungicides in controlling pathogens has been widely demonstrated in various plant species at both the field and molecular levels [10]. Previous publications focused on the uptake and translocation of phosphite in plants such as potato and tomato, particularly in the context of controlling diseases like root rot caused by pathogens such as Phytophthora or Pythium [11,12]. However, the sustainability of using this plant protection chemical is questioned due to environmental concerns. Industrial and agricultural sources of these products significantly contribute to environmental phosphonate levels through wastewater, fungicides, and fertilizer use [13]. Understanding how these chemicals interact with soil and plants over time is essential for making informed decisions about their use and potential long-term consequences. Up to now, the fate of chemical substances released by these fungicides in the soil environment has not been fully explored [14].
The conversion of Phi into phosphate (Pi) in soil under aerobic conditions was investigated by Adams and Conrad (1953) [15]. They measured phosphonate indirectly by comparing phosphate levels before and after iodine oxidation. Their findings showed a decrease in phosphonate residues from 1.95 mg to 0.97 mg (as P2O3 equivalents per 25 g of soil) between weeks 4 and 16, while phosphate residues increased from 9.5 mg to 10.4 mg, indicating the conversion of phosphonate into phosphate in the soil. Later, Ouimette et al. (1989) [16] measured phosphonate concentrations in soil and plant tissues after application to avocado trees. Their results showed high levels of phosphonate in both soil and avocado tissues, with residues persisting for four weeks in the soil and eight weeks in avocado tissues. The persistence of phosphonate in avocado tissues eight weeks after chemical treatment provides evidence that it is not easily oxidized into phosphate in plant tissues.
Phosphonate solutions are resistant to oxidation at temperatures up to 60 °C and within a pH range of 1.5 to 7.6, which may explain their persistence in plants and their antifungal effects against Phytophthora-induced root rot [17]. However, in soil, phosphonate levels decline within four weeks of application [16]. This reduced persistence is attributed to microbial activity, as soil microorganisms can absorb, metabolize, and convert phosphonate into phosphate [15].
Phosphite (Phi) transformation in soil begins with bacterial absorption and assimilation, where bacteria oxidize Phi for energy and phosphate (Pi) production, an essential nutrient in ecosystems. Some bacteria can use Phi as their sole phosphorus source, but they prefer Pi. Phi also acts as an intermediate in hypophosphite oxidation and can be converted by prokaryotes such as Escherichia coli, Klebsiella aerogenes, Agrobacterium tumefaciens, and certain Pseudomonas and Rhizobium species. However, it was said that Phi is a poor phosphorus source for plants, as its conversion to Pi in the soil is too slow to provide significant agricultural benefits [6].
In plants, the amount of applied Phi is directly proportional to the Phi concentration detected in various plant parts and is inversely proportional to the development of wilt disease agents [12]. The highest phosphonate concentrations are observed at the Phytophthora inoculation site [18]. In cocoa beans from treated trees, phosphonate levels can reach up to 100 mg/kg of fresh weight [19]. Therefore, farmers and agronomists must balance the need for phosphite in pathogen control with the goal of minimizing chemical residues in crops and soil. This challenge necessitates optimizing crop yields while reducing pesticide use, making it essential to evaluate the impact of farming practices on agricultural pollution.
This presents a challenge in predicting and quantifying the dynamics and concentrations of chemical residues in the environment when fungicides are used. Data collected from cultivation practices or experiments reflect only the conditions at the time of recording and do not continuously capture temporal trends. In this regard, mathematical models can be useful. These models integrate the nutrient pool size, flux, and dynamics across crop systems, providing practical support to farmers in optimizing nutrient efficiency across different environments, management practices, crop types, and timeframes [20,21]. For decades, pesticide residue behavior in the environment has been modeled across various spatial and temporal scales with varying levels of complexity. Pesticide fate in the environment involves complex processes across air [22], plants [23,24], soil [25,26], and water [27,28]. Widely used for pesticide registration, these models provide a cost-effective and extensive alternative to field and laboratory experiments for predicting pesticide behavior.
Vietnam is among the world’s leading pepper exporters due to its tropical climatic conditions, which are suitable for black pepper cultivation. However, the country’s typically humid conditions, especially during the rainy season, also promote the growth of harmful fungi that cause plant diseases. Among these, root rot is the most severe disease affecting black pepper in Vietnam [29]. Root rot disease can severely affect crop yields, with losses of up to 40–50%, resulting in significant economic consequences across Southeast Asia, particularly in Vietnam [30]. In Vietnamese black pepper cultivation areas, three primary pathogens have been identified: Phytophthora spp., Pythium spp., and Fusarium spp. [29]. To manage these diseases, phosphonate-based fungicides such as Agrifos 400 are widely used. According to a field investigation conducted by the author (unpublished data), 35% of pepper farmers in one pepper cultivation area (Trang Bom District) reported using Agrifos 400. Agrifos 400 is a commercial formulation containing 400 g/L of phosphonate, provided as a mixture of two salts: KH2PO3 and K2HPO3. In the soil, these compounds break down into potassium (K+) and phosphite (H2PO3−) ions. Once absorbed by the plant, these ions are transported bidirectionally within plant tissues. Residues in the soil undergo various chemical and physical processes that can potentially raise pollution concerns.
This study aims to assess the nutritional role of the fungicide Agrifos in pepper cultivation and to investigate the fate of its transformation products in soil. Additionally, it seeks to develop a comprehensive mathematical model that simulates the behavior of Agrifos transformation products in soil, using parameters derived from experimental observations.
2. Materials and Methods
2.1. Experimental Greenhouse Trials with Agrifos
The experiment was conducted in a greenhouse over a two-month period. A total of 120 healthy, 3-month-old pepper plants were assigned to 6 treatment conditions. Details of the treatments are described in Table 1, and part of the data from this experiment was previously published [31]. In this experiment, young pepper plants were first exposed to pathogens and then treated with Agrifos fungicide through soil drenching. Each plant was placed in an individual plastic pot to prevent cross-contamination through water flow. A fungal-contaminated solution was applied to the soil in pots containing young pepper plants (50 mL per pot). The Pythium spp. used for contamination was isolated from infected field samples— including the roots, leaves, and branches of pepper plants—collected in Trang Bom District, a major pepper-growing region in southern Vietnam.

Table 1.
The dose and frequency of fungicide application in the treatments.
The timing of fungicide applications was evaluated, with Agrifos (1000 ppm) applied at 10-day intervals (the recommended frequency, labeled as the full dose) and 20-day intervals (twice the recommended interval, labeled as the half dose). Control treatments received no fungicide. Two soil types were used: red basalt soil transported from Trang Bom District (labeled as red soil), and a commercial soil consisting of soil enriched with organic matter (labeled as mixed soil). The physical and chemical properties of these soils are discussed in the next section.
The plant height and number of leaves were recorded once a week. Soil samples (three replicates per treatment) were collected for chemical analysis before and during the experiment.
2.2. Sampling Methods, Sample Preparation, and Analysis
During the experiment, soil samples were collected from plant pots, ensuring coverage from both the surface and deeper layers. The sampling process followed a randomized complete block design with three replicates. As Agrifos degraded into K+ and phosphite, which then oxidizes into phosphate in the soil, the soil PO43− levels were measured alongside K to monitor their concentrations over time.
All soil samples were analyzed at the laboratory of the National Institute of Applied Mechanics and Informatics, Vietnam Academy of Science and Technology. Soil pHKCl levels were measured using a 1 M KCl solution at a 1:5 ratio. Up to 10 g of dried, sieved soil was mixed with 50 mL of KCl, stirred for 2 h, and settled for 1 h. The pH was measured potentiometrically using a calibrated glass electrode after the suspension was stirred twice [32]. The soil texture was analyzed using the pipette method on a 20 g sample. The organic matter was removed with H2O2, followed by boiling with HCl and washing to a pH of 6. The sample was mixed with sodium pyrophosphate and water for 8 h and then transferred through a 0.2 mm sieve. Coarse sand was dried at 105 °C, while aliquots for “total (silt + clay)” and “clay” were taken, dried, and weighed. The percentages of coarse sand, clay, silt, and fine sand were then calculated [33,34]. To determine organic matter (OM), 1.0 g of dried soil was mixed with K2Cr2O7 and H2SO4 in a flask, swirled, and left to react for 30 min. Water was added for clarity, followed by H3PO4 and a diphenylamine indicator. The solution was then titrated with (NH4)2Fe(SO4)2 until a sharp green color indicated the endpoint [35]. For Fe, Al, and K analyses, 0.5 g of dried soil was digested with nitric and hydrofluoric acids using microwave digestion at 180 °C. After cooling, the digestate was diluted to 25 mL. A blank solution was prepared similarly, and element concentrations were measured using ICP-OES. The availability of Al in the soils was extracted using a 1 M KCl solution. This was followed by a 1:2 soil to solution ratio and shaking for 1 h on an end-over-end shaker at 50 rpm, then centrifuging at 2000 rpm for 15 min. The Al concentration in all of the extracts was then determined by inductively coupled plasma atomic emission spectrometry (ICP-OES). Nitrogen in the soil was determined using the Kjeldahl method. A 1 g soil sample was digested with a Kjeldahl catalyst and sulfuric acid at 350–380 °C until clear. After cooling, water was added, and the sample was distilled with NaOH to convert NH4+ to NH3. The NH3 was captured in boric acid with Tashiro’s indicator and titrated with HCl until the color changed from green to violet. The nitrogen content was calculated based on the HCl volume and concentration (ISO 11261:1995).
2.3. Statistical Data Analysis
The plant height increment was calculated by subtracting the initial height from the subsequent measurement. Plant growth parameters and soil chemical data were analyzed using descriptive statistics and a three-way analysis of variance (ANOVA; R software version 4.4.1). The Tukey Honest Significant Difference (HSD) test was used to compare treatments at p < 5%.
2.4. Mathematical Model Development
Figure 1 illustrates the metabolic pathways of Agrifos 400 after its application to the soil environment. Upon application, Agrifos metabolizes into phosphite (PO33−) and potassium (K+). These transformation products follow two pathways:
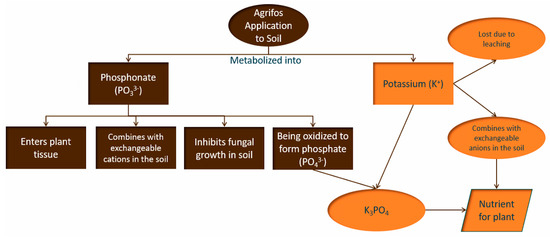
Figure 1.
The metabolic pathways of Agrifos 400 after its application to the soil environment.
Pathway 1: Phosphite (PO33−) enters the plant tissue, interacts with exchangeable cations in the soil, and partially inhibits fungal growth. It also undergoes oxidation, converting into phosphate (PO43−), some of which combines with K+ to form potassium phosphate (K3PO4).
Pathway 2: Potassium (K+) interacts with exchangeable anions in the soil, contributing to plant nutrition, while some is lost through leaching.
A mathematical model was developed to represent the main processes involved in the fate of Agrifos, as described above. In this model, the following are applicable:
Equation (1) expresses the presence of the fungicide (Agrifos 400) in the soil. The first term represents its transformation into phosphite and potassium. The second term corresponds to the application rate.
The fate of phosphite (H2PO3−) is described in Equation (2), where the first term represents the dissolution of Agrifos, the second term represents the conversion of phosphite to phosphate by microorganisms, and the third term expresses the uptake by plant roots.
Equation (3) describes the fate of H2PO4 in the liquid soil phase. Following microbial conversion from phosphite (the 1st term), it undergoes sorption to and desorption from the soil (the 2nd and 3rd terms) and is uptaken by the plant roots (the 4th term).
Equation (4) is describes PO43− in the solid soil phase: the 1st term: sorption, the 2nd term: desorption.
Equation (5) describes the fate of potassium in soil: the first term represents the contribution from the fungicide, the second term accounts for precipitation, and the third term reflects the loss by leaching.
Equation (6) describes the precipitated phosphate.
Equation (7) is the time course of microbial activity in soil following application.
State variables:
: fungicide Agrifos 400
: phosphonite (H2PO3−)
H2PO3− was calculated according to M.E. Fenn and M.D. Coffey (1984) [36], 1 g/L (1000 ppm) of H3PO3 contains 12.7 meq/L of PO33−.
: phosphate H2 PO43− in solid phase (bound to soil particles)
: phosphate in liquid phase (available for plants)
: potassium (K)
: precipitated phosphate
: microbial activity as function of time
Parameters:
: binding capacity of soil for phosphate
: rate constant for decay of fungicide to phosphonate and potassium
: rate constant for adsorption
: rate constant for desorption
: maximum uptake rate of phosphonate by plant roots with Michalis constant K
: maximum uptake rate of phosphate by plant roots with Michalis constant K
: amount of Agrifos applied at time ti
: Dirac function
: microbial activity before application
: maximum microbial activity
: growth rate of microbial activity
The model parameters were estimated using nonlinear regression techniques, achieving a model efficiency of R2 = 0.76. The regression problem was addressed by integrating an ordinary differential equation (ODE) solver within the Optimization Toolbox of MATLAB R2021b.
3. Results and Discussion
3.1. Chemical Residues in the Soil
3.1.1. Initial Chemicals in the Soil
Table 2 provides summary statistics of the physical and chemical properties of the mixed and red soils used in the experiment. The mixed soil has a higher clay content, whereas the red soil has a more balanced texture with a higher sand content.

Table 2.
A statistical summary of the physical and chemical properties of mixed and red soils used in the experiment.
In terms of nutrient availability, the red soil contains more iron, potassium, phosphate, and organic matter; however, it is more acidic, with a lower pH and higher exchangeable aluminum content. The mixed soil, with its higher clay content, may retain more moisture.
3.1.2. The Total Potassium in the Soil
Because Agrifos 400 was the sole input in this experiment aside from water application, the measured concentrations of potassium (K) and phosphate (PO43−) during the experiment were therefore attributed to the release of these elements from the applied Agrifos, in addition to any pre-existing chemical constituents in the soils.
Figure 2 illustrates the time course of the potassium (K) concentration during the experiment. Initially, potassium levels were higher in red soil compared to mixed soil; however, over time, the potassium concentration in mixed soil surpassed that in red soil. By day 20, the potassium levels had declined to nearly zero, with red soil showing a more rapid decrease than the mixed soil. In the mixed soil, treatments T5 and T8 exhibited a slight increase in potassium levels after day 40. This modest rise—approximately 0.2% after each application—suggests that Agrifos contributes a small amount of potassium to the soil. The overall decline in potassium content across treatments is likely due to the daily nutritional demands of young pepper plants, which exceed the amount of exchangeable potassium released by Agrifos 400. Previous studies also support the conclusion that potassium phosphonate contributes minimally to plant potassium nutrition [37,38,39].
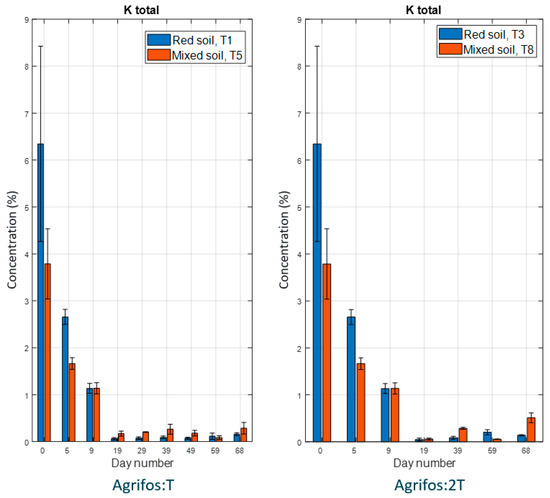
Figure 2.
The time course of the potassium concentration in the experiment. Error bars represent the standard error of the mean.
The potassium concentration declined rapidly in the first ten days and then stabilized at low levels. The difference between Agrifos full-dose and half-dose conditions may indicate variations in potassium retention or release due to different soil types and treatment levels. A. Raheb and A. Heidari (2012) [40] reported that different forms of potassium (K) were positively associated with organic carbon (OC), electrical conductivity (EC), and clay content. Soils rich in organic matter and clay tend to retain more K, but in cases of low soil pH (pH < 5.5), increased K leaching can occur. This explains the differences in K content between red soil and mixed soil in this study. Due to the higher organic matter (OM) content in the red soil (6.98%) compared to the mixed soil (4.71%), the K levels in the red soil were initially higher during the first ten days of the experiment. However, after this period, the K content in the red soil became lower than in the mixed soil. This shift is attributed to the lower pH of red soil (pH_KCl = 4.70 ± 0.154) compared to mixed soil (pH_KCl = 5.5 ± 0.31), which enhanced the K leaching process. In contrast, the higher clay content of mixed soil (81.4%) helped retain K. Potassium in the mixed soil, on the other hand, may be more readily available for plant uptake because the attraction between potassium ions and organic matter particles is relatively weak [41].
3.1.3. Phosphate in the Soil
Figure 3 shows the time course of phosphate concentration in the experimental soils. An increase in phosphate content was observed across all four treatments from day 19 onward, suggesting that phosphite undergoes oxidation to phosphate. This result aligns with previous studies on the microbial-driven transformation of phosphonate into phosphate in soil. Various microorganisms have developed the ability to utilize phosphite as a phosphorus source by catalyzing its oxidation to phosphate. This process, known as assimilatory phosphite oxidation (APO), plays a crucial role in phosphorus cycling within soil ecosystems [15,42,43,44,45].
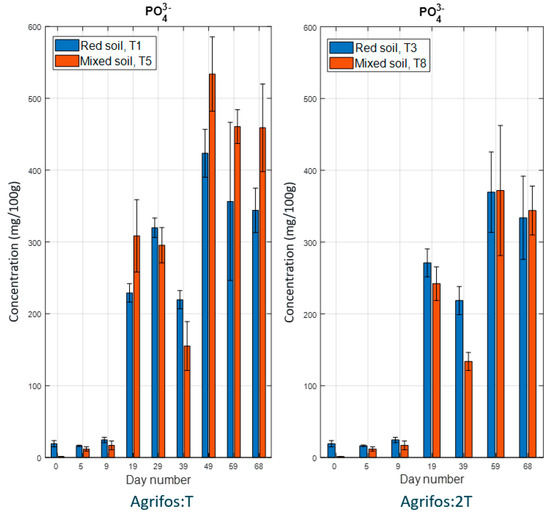
Figure 3.
The time course of the phosphate concentration in the experiment. Error bars represent the standard error of the mean.
The results from the one-way ANOVA indicated that the levels of PO43− in different soil types did not differ significantly across the Agrifos treatments. This suggests that the potassium phosphonate applied was not fully oxidized into phosphate, and that the release of PO43− from Agrifos is influenced more by the microbial population than by the amount of Agrifos applied. A previous study by Michael D. Coffey (1986) [46] estimated that the half-life of phosphonate in soil is approximately 16 weeks when potassium phosphonate was tested on two types of clay soils.
Between the two soils, PO43− levels in the red soil remained higher than in the mixed soil until day 39. This may be due to the higher organic matter content in red soil, which could enhance microbial activity and accelerate the oxidation of phosphite (PO33−) into phosphate (PO43−), particularly within 20–30 days after the application of Agrifos 400.
After day 39, PO43− levels in the red soil became lower than in the mixed soil. This can be attributed to the higher presence of Al3+ in red soil compared to mixed soil (Table 2), making it more acidic (lower pH). As a result, a portion of PO43− may have been absorbed by plants or leached away, while another portion precipitated with Al3+, rendering it unavailable to plants. This phenomenon can lead to phosphorus deficiency stress, a common characteristic of plants growing in acidic soils [47].
It is important to note that a portion of PO33− enters the plant before being converted into PO43−. Another possible explanation for the lower PO43− levels in red soil is that mixed soil, with its higher clay content, is more compact and less permeable. In contrast, red soil has a more balanced texture with a higher sand content, which improves water and nutrient transport, making it more readily available for plant uptake.
3.2. Modelling the Fate of Agrifos-Released Chemicals in the Soil
Figure 4 and Figure 5 illustrate the simulated results (dashed lines) for K and PO43− kinetics in the soils of four treatments (T1, T3, T5, and T8), alongside the corresponding experimental data (points). The model accurately predicts the behavior of K and PO43− content, as demonstrated by the strong agreement between simulated and observed values.
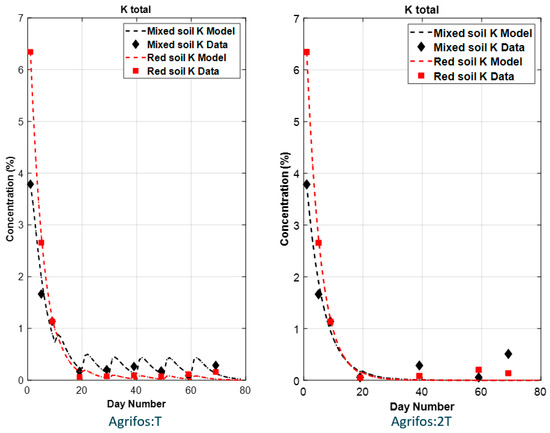
Figure 4.
Simulated results (curves) vs. data points of potassium in experimental soils.
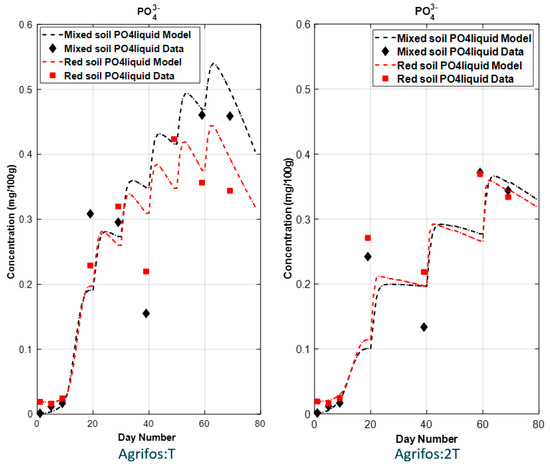
Figure 5.
Simulated results (curves) vs. data points of PO43− in experimental soils.
For potassium, in both full and half-dose treatments, the initial potassium concentration is high but decreases over time (Figure 4). The decline follows a similar pattern in both soil types, with the full-dose treatment maintaining slightly higher concentrations at later time points compared to the half-dose treatment. The modeling results clearly illustrate the continuous trend of K kinetics, where concentrations rapidly decline due to various soil chemical processes, as was described above.
The fate of simulated PO43− (Figure 5) shows a time delay in the increase in PO43− concentration in the soil. This delay is attributed to the gradual rise in microbial activity responsible for converting PO33− to PO43−. As these microbial communities expand over time, fueled by the input of PO33−, the conversion rate accelerates. This explains why higher PO43− concentrations were observed in the full-dose applications (T1 and T5) compared to the half-dose applications (T3 and T8).
The experimental data (Figure 2 and Figure 3) reveal fluctuating levels of PO43− and K across different sampling intervals, making it difficult to comprehensively characterize their kinetics through observation alone. This highlights the important role of mathematical modeling in capturing such variability. The simulated K and PO43− curves illustrate the capability of mathematical equations to effectively represent the kinetics of soil nutrients, including processes such as plant uptake, leaching, and chemical interactions with other soil ions.
Although the simulated results demonstrate a good fit to the experimental data, some deviations persist, particularly for PO43−. These discrepancies may originate from the complex kinetics of K and PO43− in soils, which are influenced by various physical and chemical properties [48]. Key factors affecting K and PO43− dynamics include extractable iron (Fe) and aluminum (Al) oxides [49,50], clay content [50,51], sand content [52], organic carbon [53], soil pH [54], and calcium carbonate [55].
The current model primarily simulates the pathways of K and PO33− released by Agrifos. To improve its predictive accuracy, future developments should integrate additional factors which affect K and PO43− dynamics, as well as the chemical reactions and transport of matter through soil water.
3.3. Advantages and Challenges of Using Phosphonate-Based Fungicides
3.3.1. The Growth Pattern of Young Pepper Plants in the Experiment
Table 3 shows descriptive statistical data for the plant height, height increment, and leaf number from all treatments. The height of young pepper plants at the start exhibited a wide variation, ranging from 15 cm to 75 cm, and occurred in treatment T1, while treatment T8 had the highest average and median plant heights (41.3 cm and 40.4 cm, respectively). After two months (at the end of the experiment), the data revealed that the lowest and highest plant heights were still found in treatment T1; however, the highest average growth occurred in treatment V3 (control treatment), which also had the highest median height.

Table 3.
Descriptive statistical data for the plant height, height increment, and leaf number in the experiment.
Regarding plant height increments, after two months, the lowest increment was observed in treatment T1 (0 cm), while the highest increment occurred in the control treatment V1 (18 cm). Across all treatments, the lowest average height increment was recorded in T1 (4.8 cm), and the highest average was in V3 (12.6 cm).
For the leaf number, at the start, all plants across treatments had between five and seven leaves per plant. After two months, the highest leaf number was recorded in treatments T5 and V1 (14 leaves), while treatment T1 showed the lowest leaf count.
It was observed that young pepper plants developed better under low doses of Agrifos (half-dose and no-Agrifos treatments) compared to those receiving the full dose (Figure 6 and Figure 7). Additionally, both the plant height increment and leaf number were higher in the mixed soil treatment (V3) than in the red soil treatment (V1). This aligns with expected plant growth patterns, as mixed soil, with its higher clay content, better moisture and nutrient retention, and more neutral pH (cf. Table 2), provides more favorable growing conditions.
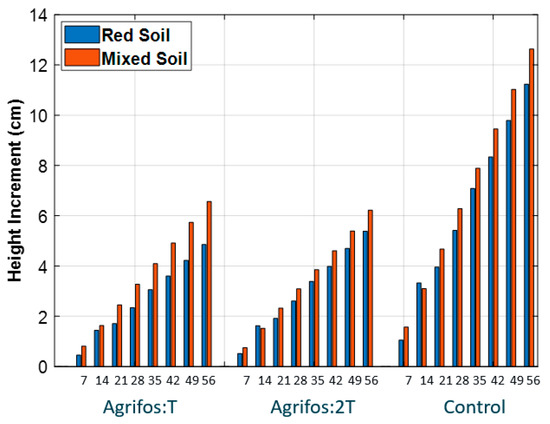
Figure 6.
The time course (day) of the plant height increment across the treatments. The figure shows that mixed soil promotes greater height growth in plants compared to red soil. Furthermore, the height increment in the control treatments (no fungicide application) is higher than in the treatments with fungicide application.
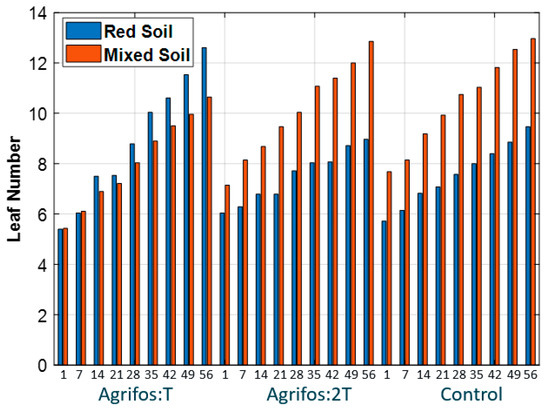
Figure 7.
The time course (day) of the leaf number across the treatments. Similar to the plant height increment pattern, mixed soil promotes better leaf development than red soil across all treatments, regardless of fungicide application. The negative impact appears stronger with a full dose than with a half dose, reinforcing the idea that the Agrifos fungicide may inhibit plant vegetative growth.
Another notable issue is that, while the results show that V1 < V3 for height increment, they indicate that V1 > V3 for leaf number. This discrepancy may be due to the leaf number being influenced by more factors than the height increment, as suggested by the analysis of variance (ANOVA) results presented in Table 4.

Table 4.
A summary of the results of the ANOVAs: **: p < 10−3 (very strong effect), -: p ≥ 0.05 (not statistically significant).
3.3.2. Benefits and Limitations of Phosphonate-Based Fungicide Application in Agriculture
The better growth of the plants in the control treatments (without Agrifos application) compared to those receiving the fungicide suggests that Agrifos 400 does not promote plant growth. This result is consistent with previous studies reporting little to no fertilizing effect from potassium or calcium phosphites [4,14,56,57,58,59,60], although some researchers have suggested that phosphite (Phi) may promote plant growth [61,62,63,64].
The mechanism of Phi inhibiting plant growth was that phosphonate salts are generally more soluble than their corresponding phosphate salts and are rapidly absorbed by plants once introduced into the soil [65]. Due to their structural similarity, phosphonates are taken up by root and leaf tissues via phosphate transporters involved in phosphate uptake, facilitating absorption through both soil and foliar applications [66]. Following uptake, phosphonate is initially transported through the xylem and subsequently redistributed via the phloem, where its movement follows the plant’s natural source–sink dynamics, leading to its accumulation in leaves, flowers, and fruits. In phosphate-starved cells, phosphonate accumulates in the cytoplasm, preventing phosphate efflux from the vacuole [4]. This inhibition can exacerbate phosphate starvation symptoms and accelerate programmed cell death [65]. McDonald (2001) [7] suggested that the application of phosphite (Phi) in agriculture may worsen the effects of phosphorus (P) deficiency by causing phosphate (Pi)-deficient plant cells to falsely perceive an adequate Pi supply, despite extremely low intracellular Pi levels. Phi appears to disrupt the molecular signaling pathways that regulate plant perception and response to Pi deficiency.
Rather than viewing phosphite (Phi) as a nutritional source, Malusà and Tosi (2005) [67] suggested that phosphonates should be classified as biostimulants. They enhance plant performance by activating molecular, biochemical, and physiological responses, rather than functioning as direct nutrients. Phi salts contain a higher phosphorus concentration (39%) compared to inorganic phosphate (Pi) fertilizers based on H2PO4−, which typically contain 32%. Additionally, Phi salts are more soluble than their Pi counterparts, improving their absorption through both leaves and roots. Once inside plant cells, Phi can trigger biochemical and structural defense mechanisms [68,69].
For this reason, phosphites are primarily recognized for their fungicidal properties rather than their role as fertilizers. As a fungicide, Agrifos has demonstrated effectiveness in protecting pepper plants from foot rot [31,65,70].
It should be noted that the dose and frequency of phosphonate-based fungicide application are critically important, as the concentration of PO43− in the soil increased following Agrifos application in this study. An excessive rise in PO43− levels can lead to several soil-related issues, such as affecting cation and anion exchange capacities. For instance, the fixation of cations such as Ca2+, Fe3+, and Al3+ can reduce the soil’s cation exchange capacity, while the strong competition between phosphate and other anions, such as sulfate (SO42−), can impair the exchange and uptake of these nutrients. Furthermore, high concentrations of PO43− in the soil often lead to phosphate fixation, decreasing its bioavailability to plants. Excess phosphate can also leach into water bodies, contributing to eutrophication, which promotes algal blooms and degrades water quality. To address these concerns, Bao et al. (2021) [31] recommended applying fungicides sequentially and at reduced frequency to effectively protect pepper plants from foot rot disease while minimizing negative impacts on plant growth and environmental quality.
4. Conclusions
This study examined experimental data on the alternating application of the fungicide Agrifos (potassium phosphonate) and developed a mathematical model to simulate the behavior of its transformation products in the soil. The findings indicate that Agrifos did not enhance pepper growth during the two-month experiment. Moreover, the intensive application of Agrifos led to increased concentrations of PO43− in the soil, which could negatively impact soil quality and surrounding water systems. This study also demonstrated the effectiveness of mathematical models in capturing chemical transformations and interactions within the soil environment, highlighting their value in providing a comprehensive understanding of these processes. A key conclusion from this research is the importance of integrating the developed model into broader simulations of biogeochemical cycles, particularly to account for chemical transport through soil water.
Funding
This research received no external funding.
Data Availability Statement
For access to the data used in this analysis, please contact the author.
Acknowledgments
I acknowledge Ho Chi Minh City University of Technology (HCMUT), VNU-HCM for supporting this study.
Conflicts of Interest
The author declares no conflict of interest.
References
- Juárez, M.G.Y.; Tafoya, F.A.; Ruvalcaba, L.P.; Alcaraz, T.d.J.V.; Angulo, T.P.G.; López, R.M. Efecto in vitro de fosfito de potasio sobre Athelia rolfsii y Pythium aphanidermatum. Rev. Mex. Cienc. Agric. 2018, 9, 1532–1538. [Google Scholar] [CrossRef][Green Version]
- Morales-Morales, E.J.; Martínez-Campos, Á.R.; López-Sandoval, J.A.; González, A.M.C.; Rubí-Arriaga, M. Phosphites and their applications in agriculture. Rev. Mex. Cienc. Agric. 2022, 13, 2. [Google Scholar]
- Chang, E.G.B. Phosphite, phosphate, and their interactions in soil and turfgrass. Grass Res. 2023, 3, 13. [Google Scholar] [CrossRef]
- Varadarajan, D.K.; Karthikeyan, A.S.; Matilda, P.D.; Raghothama, K.G. Phosphite, an Analog of Phosphate, Suppresses the Coordinated Expression of Genes under Phosphate Starvation. Plant Physiol. 2002, 129, 1232–1240. [Google Scholar] [CrossRef]
- Dguest, D.; Grant, B. The complex action of phosphonates as antifungal agents. Biol. Rev. 1991, 66, 159–187. [Google Scholar] [CrossRef]
- McDonald, A.E.; Grant, B.R.; Plaxton, W.C. Phosphite (Phosphorous acid): Its Relevance in the Environment and Agriculture and Influence on Plant Phosphate Starvation Response. J. Plant Nutr. 2001, 24, 1505–1519. [Google Scholar] [CrossRef]
- McDonald, A.E.; Niere, J.O.; Plaxton, W.C. Phosphite disrupts the acclimation of Saccharomyces cerevisiae to phosphate starvation. Can. J. Microbiol. 2001, 47, 969–978. [Google Scholar] [CrossRef]
- Rickard, D.A. Review of phosphorus acid and its salts as fertilizer materials. J. Plant Nutr. 2000, 23, 161–180. [Google Scholar] [CrossRef]
- Hua, G.K.H.; Ji, P.; Culbreath, A.K.; Ali, M.E. Comparative Study of Phosphorous-Acid-Containing Products for Managing Phytophthora Blight of Bell Pepper. Agronomy 2022, 12, 1293. [Google Scholar] [CrossRef]
- Achary, V.M.M.; Ram, B.; Manna, M.; Datta, D.; Bhatt, A.; Reddy, M.K.; Agrawal, P.K. Phosphite: A novel P fertilizer for weed management and pathogen control. Plant Biotechnol. J. 2017, 15, 1493–1508. [Google Scholar] [CrossRef]
- Perumal, R.; Kumar, K.S.; Babu, S.M.; Bhagavannarayana, G. Optical characterization of ferroelectric glycinium phosphite single crystals. J. Alloys Compd. 2010, 490, 342–349. [Google Scholar] [CrossRef]
- Borza, T.; Schofield, A.; Sakthivel, G.; Bergese, J.; Gao, X.; Rand, J.; Wang-Pruski, G. Ion chromatography analysis of phosphite uptake and translocation by potato plants: Dose-dependent uptake and inhibition of Phytophthora infestans development. Crop Prot. 2014, 56, 74–81. [Google Scholar] [CrossRef]
- Nader, W.; Zahm, A.; Jaschik, J. Phosphonic acid in plant-based food and feed products—Where does it come from? Food Control 2023, 150, 109701. [Google Scholar] [CrossRef]
- Hthao, T.B.; Yamakawa, T. Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator? Soil Sci. Plant Nutr. 2009, 55, 228–234. [Google Scholar] [CrossRef]
- Adams, F.; Conrad, J.P. Transition of Phosphite to Phosphate in Soils. Soil Sci. 1953, 75, 361. Available online: https://journals.lww.com/soilsci/fulltext/1953/05000/transition_of_phosphite_to_phosphate_in_soils.4.aspx (accessed on 20 February 2025). [CrossRef]
- Ouimette, D.G.; Coffey, M.D. Phosphonate Levels in Avocado (Persea americana) Seedlings and Soil Following Treatment with Fosetyl-Al or Potassium Phosphonate. Am. Phytopathol. Soc. 1989, 73, 3. [Google Scholar] [CrossRef]
- Robertson, H.E.; Boyer, P.D. Orthophosphite as a buffer for biological studies. Arch. Biochem. Biophys. 1956, 62, 396–401. [Google Scholar] [CrossRef]
- Griffith, J.M.; Akins, L.A.; Grant, B.R. Properties of the phosphate and phosphite transport systems of Phytophthora palmivora. Arch. Microbiol. 1989, 152, 430–436. [Google Scholar] [CrossRef]
- McMahon, P.J.; Purwantara, A.; Wahab, A.; Imron, M.; Lambert, S.; Keane, P.J.; Guest, D.I. Phosphonate applied by trunk injection controls stem canker and decreases Phytophthora pod rot (black pod) incidence in cocoa in Sulawesi. Australas. Plant Pathol. 2010, 39, 170–175. [Google Scholar] [CrossRef]
- Carberry, P.; Hochman, Z.; McCown, R.; Dalgliesh, N.; Foale, M.; Poulton, P.; Hargreaves, J.; Hargreaves, D.; Cawthray, S.; Hillcoat, N.; et al. The FARMSCAPE approach to decision support: Farmers’, advisers’, researchers’ monitoring, simulation, communication and performance evaluation. Agric. Syst. 2002, 74, 141–177. [Google Scholar] [CrossRef]
- McCown, R.L. A cognitive systems framework to inform delivery of analytic support for farmers’ intuitive management under seasonal climatic variability. Agric. Syst. 2012, 105, 7–20. [Google Scholar] [CrossRef]
- Bedos, C.; Génermont, S.; Le Cadre, E.; Garcia, L.; Barriuso, E.; Cellier, P. Modelling pesticide volatilization after soil application using the mechanistic model Volt’Air. Atmos. Environ. 2009, 43, 3630–3639. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.Q. Foliar uptake of pesticides: Present status and future challenge. Pestic. Biochem. Physiol. 2007, 87, 1–8. Available online: https://api.semanticscholar.org/CorpusID:83610657 (accessed on 20 February 2025). [CrossRef]
- Fantke, P.; Wieland, P.; Wannaz, C.; Friedrich, R.; Jolliet, O. Dynamics of pesticide uptake into plants: From system functioning to parsimonious modeling. Environ. Model. Softw. 2013, 40, 316–324. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, W.; He, Z.; Zhang, L. Stochastic modelling of soil moisture dynamics in a grassland of Qilian Mountain at point scale. Sci. China Ser. D Earth Sci. 2007, 50, 1844–1856. [Google Scholar] [CrossRef]
- Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J.; Mejuto, J.-C.; García-Río, L. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar] [CrossRef]
- Holvoet, K.M.A.; Seuntjens, P.; Vanrolleghem, P.A. Monitoring and modeling pesticide fate in surface waters at the catchment scale. Ecol. Modell. 2007, 209, 53–64. [Google Scholar] [CrossRef]
- Baran, N.; Lepiller, M.; Mouvet, C. Agricultural diffuse pollution in a chalk aquifer (Trois Fontaines, France): Influence of pesticide properties and hydrodynamic constraints. J. Hydrol. 2008, 358, 56–69. [Google Scholar] [CrossRef]
- Truong, N.-V.; Burgess, L.W.; Liew, E.C.Y. Prevalence and aetiology of Phytophthora foot rot of black pepper in Vietnam. Australas. Plant Pathol. 2008, 37, 431–442. [Google Scholar] [CrossRef]
- Nguyen, V.L. Spread of Phytophthora capsici in Black Pepper (Piper nigrum) in Vietnam. Engineering 2015, 7, 506–513. [Google Scholar] [CrossRef]
- Le, B.V.Q.; Nguyen, A.; Richter, O.; Nguyen, T.T. Comparison of Frequentist and Bayesian Generalized Linear Models for Analyzing the Effects of Fungicide Treatments on the Growth and Mortality of Piper Nigrum. Agronomy 2021, 11, 2524. [Google Scholar] [CrossRef]
- Al-Busaidi, A.; Cookson, P.; Yamamoto, T. Methods of pH determination in calcareous soils: Use of electrolytes and suspension effect. Soil Res. 2005, 43, 541–545. [Google Scholar] [CrossRef]
- Robinson, G.W. A new method for the mechanical analysis of soils and other dispersions. J. Agric. Sci. 1922, 12, 306–321. [Google Scholar] [CrossRef]
- Rząsa, S.; Owczarzak, W. Methods for the granulometric analysis of soil for science and practice. Pol. J. Soil Sci. 2013, 46, 1. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and A Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. Available online: https://journals.lww.com/soilsci/fulltext/1934/01000/an_examination_of_the_degtjareff_method_for.3.aspx (accessed on 20 February 2025). [CrossRef]
- Fenn, M.E.; Coffey, M.D. Studies on the In Vitro and In Vivo Antifungal Activity of Fosetyl-Al and Phosphorous Acid. Dis. Control. Pest Manag. 1984, 74, 5. [Google Scholar] [CrossRef]
- Rawat, J.; Pandey, N.; Saxena, J. Role of Potassium in Plant Photosynthesis, Transport, Growth and Yield. In Role of Potassium in Abiotic Stress; Iqbal, N., Umar, S., Eds.; Springer Nature: Singapore, 2022; pp. 1–14. [Google Scholar] [CrossRef]
- Vinas, M.; Mendez, J.C.; Jiménez, V.M. Effect of foliar applications of phosphites on growth, nutritional status and defense responses in tomato plants. Sci. Hortic. 2020, 265, 109200. [Google Scholar] [CrossRef]
- Çalişkan, B.; Çalişkan, A.C. Potassium Nutrition in Plants and Its Interactions with Other Nutrients in Hydroponic Culture. In Potassium; Asaduzzaman, M., Asao, T., Eds.; IntechOpen: Rijeka, Croatia, 2017; p. Ch. 2. [Google Scholar] [CrossRef]
- Raheb, A.; Heidari, A. Effects of clay mineralogy and physico-chemical properties on potassium availability under soil aquic conditions. J. Soil Sci. Plant Nutr. 2012, 12, 747–761. [Google Scholar] [CrossRef]
- Schulte, E.E.; Kelling, K.A. Soil and Applied Potassium. In Soil and Applied Potassium (A2521) by the University of Wisconsin-Madison’s Division of Extension; University of Wisconsin: Madison, WI, USA, 2002. [Google Scholar]
- Casida, L.E. Microbial Oxidation and Utilization of Orthophosphite During Growth. J. Bacteriol. 1960, 80, 237–241. [Google Scholar] [CrossRef]
- George, M.; Konetzka, W.A. Bacterial Oxidation of Orthophosphite. J. Bacteriol. 1966, 91, 578–582. [Google Scholar] [CrossRef]
- Foster, T.L.; Winans, L.; Helms, S.J. Anaerobic utilization of phosphite and hypophosphite by Bacillus sp. Appl. Environ. Microbiol. 1978, 35, 937–944. [Google Scholar] [CrossRef]
- Martínez, A.; Osburne, M.S.; Sharma, A.K.; DeLong, E.F.; Chisholm, S.W. Phosphite utilization by the marine picocyanobacterium Prochlorococcus MIT9301. Environ. Microbiol. 2012, 14, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Coffey, M.D. Systemic Fungicides and the Control of Oomycetes. Annu. Rev. Phytopathol. 1986, 24, 311–338. [Google Scholar] [CrossRef]
- Zelinová, V.; Huttová, J.; Mistrík, I.; Pal’ove-Balang, P.; Tamás, L. Impact of Aluminum on Phosphate Uptake and Acid Phosphatase Activity in Root Tips of Lotus Japonicus. J. Plant Nutr. 2009, 32, 1633–1641. [Google Scholar] [CrossRef]
- Zhou, J.M.; Huang, P.M. Kinetics of potassium release from illite as influenced by different phosphates. Geoderma 2007, 4, 221–228. [Google Scholar] [CrossRef]
- Freese, D.; van der Zee, S.E.A.T.M.; van Riemsdijk, W.H. Comparison of different models for phosphate sorption as a function of the iron and aluminium oxides of soils. J. Soil Sci. 1992, 43, 729–738. [Google Scholar] [CrossRef]
- Torrent, J. Rapid and Slow Phosphate Sorption by Mediterranean Soils: Effect of Iron Oxides. Soil Sci. Soc. Am. J. 1987, 51, 78–82. [Google Scholar] [CrossRef]
- Johnston, A.E. Soil fertility and soil organic matter. In Advances in Soil Organic Matter Research: The Impact on Agriculture and the Environment; The Royal Society of Chemistry: Melksham, UK, 1991. [Google Scholar]
- Leclerc, M.L.; Nolin, M.C.; Cluis, D.; Simard, R.R. Grouping soils of the Montreal Lowlands (Quebec) according to fertility and P sorption and desorption characteristics. Can. J. Soil Sci. 2001, 81, 71–83. [Google Scholar] [CrossRef]
- Daly, K.; Jeffrey, D.; Tunney, H. The effect of soil type on phosphorus sorption capacity and desorption dynamics in Irish grassland soils. Soil Use Manag. 2001, 17, 12–20. [Google Scholar] [CrossRef]
- BARROW, N.J. A mechanistic model for describing the sorption and desorption of phosphate by soil. J. Soil Sci. 1983, 34, 733–750. [Google Scholar] [CrossRef]
- Ige, D.V.; Akinremi, O.O.; Flaten, D.N.; Ajiboye, B.; Kashem, M.A. Phosphorus sorption capacity of alkaline Manitoba soils and its relationship to soil properties. Can. J. Soil Sci. 2005, 85, 417–426. [Google Scholar] [CrossRef]
- Carswell, C.; Grant, B.R.; Theodorou, M.E.; Harris, J.; Niere, J.O.; Plaxton, W.C. The Fungicide Phosphonate Disrupts the Phosphate-Starvation Response in Brassica nigra Seedlings. Plant Physiol. 1996, 110, 105–110. [Google Scholar] [CrossRef]
- Carswell, M.C.; Grant, B.R.; Plaxton, W.C. Disruption of the phosphate-starvation response of oilseed rape suspension cells by the fungicide phosphonate. Planta 1997, 203, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Förster, H.; Adaskaveg, J.E.; Kim, D.H.; Stanghellini, M.E. Effect of Phosphite on Tomato and Pepper Plants and on Susceptibility of Pepper to Phytophthora Root and Crown Rot in Hydroponic Culture. Plant Dis. 1998, 82, 1165–1170. [Google Scholar] [CrossRef]
- Ticconi, C.A.; Delatorre, C.A.; Abel, S. Attenuation of Phosphate Starvation Responses by Phosphite in Arabidopsis. Plant Physiol. 2001, 127, 963–972. [Google Scholar] [CrossRef]
- Thao, H.T.B.; Yamakawa, T.; Myint, A.K.; Sarr, P.S. Effects of phosphite, a reduced form of phosphate, on the growth and phosphorus nutrition of spinach (Spinacia oleracea L.). Soil Sci. Plant Nutr. 2008, 54, 761–768. [Google Scholar] [CrossRef]
- Albrigo, G. Effects of foliar applications of urea or Nutriphite on flowering and yields of Valencia orange trees. Agric. Food Sci. 1999, 112, 1–4. Available online: https://api.semanticscholar.org/CorpusID:73712679 (accessed on 20 February 2025).
- Lovatt, B.; Mikkelsen, R.L. Phosphite Fertilizers: What Are They? Can You Use Them? What Can They Do? Better Crops 2006, 90, 4. Available online: https://api.semanticscholar.org/CorpusID:52994032 (accessed on 20 February 2025).
- Rossall, S.; Qing, C.; Paneri, M.; Bennett, M.; Swarup, R. A ‘growing’ role for phosphites in promoting plant growth and development. ISHS Acta Hortic. 2016, 1148, 61–68. [Google Scholar] [CrossRef]
- Mohammed, U.; Davis, J.; Rossall, S.; Swarup, K.; Czyzewicz, N.; Bhosale, R.; Foulkes, J.; Murchie, E.H.; Swarup, R. Phosphite treatment can improve root biomass and nutrition use efficiency in wheat. Front. Plant Sci. 2022, 13, 1017048. [Google Scholar] [CrossRef]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. Biostimulant activity of phosphite in horticulture. Sci. Hortic. 2015, 196, 82–90. [Google Scholar] [CrossRef]
- Nussaume, L.; Kanno, S.; Javot, H.; Marin, E.; Nakanishi, T.M.; Thibaud, M.-C. Phosphate Import in Plants: Focus on the PHT1 Transporters. Front. Plant Sci. 2011, 2, 83. [Google Scholar] [CrossRef] [PubMed]
- Malusà, E.; Tosi, L. Phosphorous acid residues in apples after foliar fertilization: Results of field trials. Food Addit. Contam. 2005, 22, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, V.; Viteri, R.; Rubio, C.; Tovar, D. Effects of potassium phosphite in combination with the fungicide metalaxyl mancozeb in the control of downy mildew (Peronospora destructor berk) in bulb onion (Allium cepa L.). Rev. Natl. Fac. Agron. Medellin. 2012, 65, 6317–6325. [Google Scholar]
- Yáñez-Juárez, M.G.; López-Orona, C.A.; Ayala-Tafoya, F.; Partida-Ruvalcaba, L.; de Jesús Velázquez-Alcaraz, T.; Medina-López, R. Phosphites as alternative for the management of phytopathological problems. Rev. Mex. Fitopatol. 2018, 36, 79–94. [Google Scholar]
- Truong, N.V.; Burgess, L.W.; Liew, E.C.Y. Greenhouse and field evaluations of potassium phosphonate: The control of Phytophthora foot rot of black pepper in Vietnam. Arch. Phytopathol. Plant Prot. 2012, 45, 724–739. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).