Thymol Stimulates Lateral Root Formation via Regulating Endogenous Reactive Oxygen Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Treatment
2.2. Quantification of Lateral Root and LRP
2.3. Fluorescent Detection of ROS in LRP
2.4. Determination of ROS Content in Roots
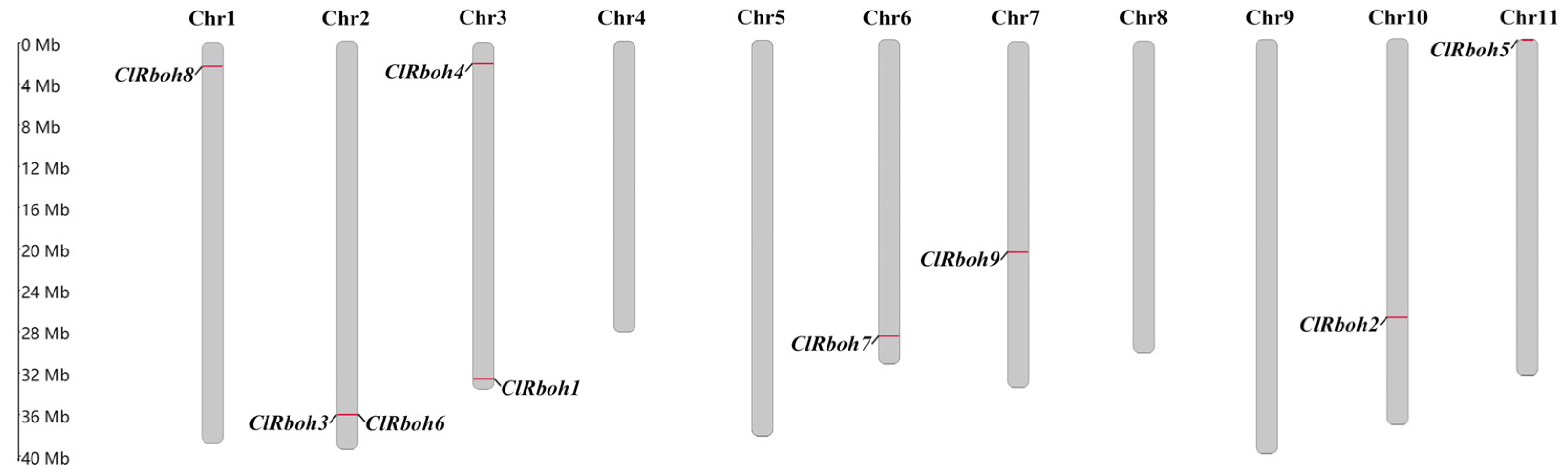
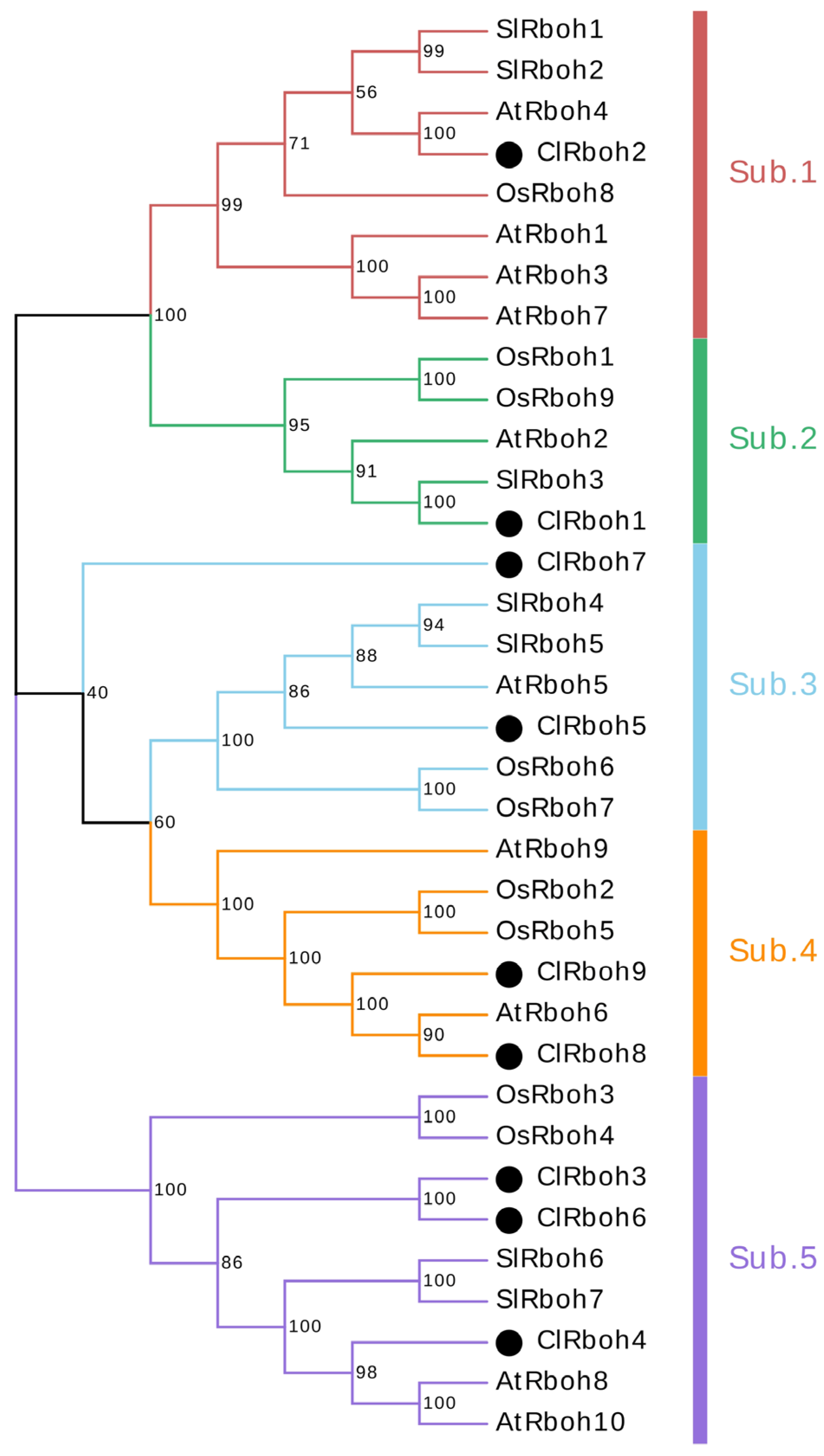
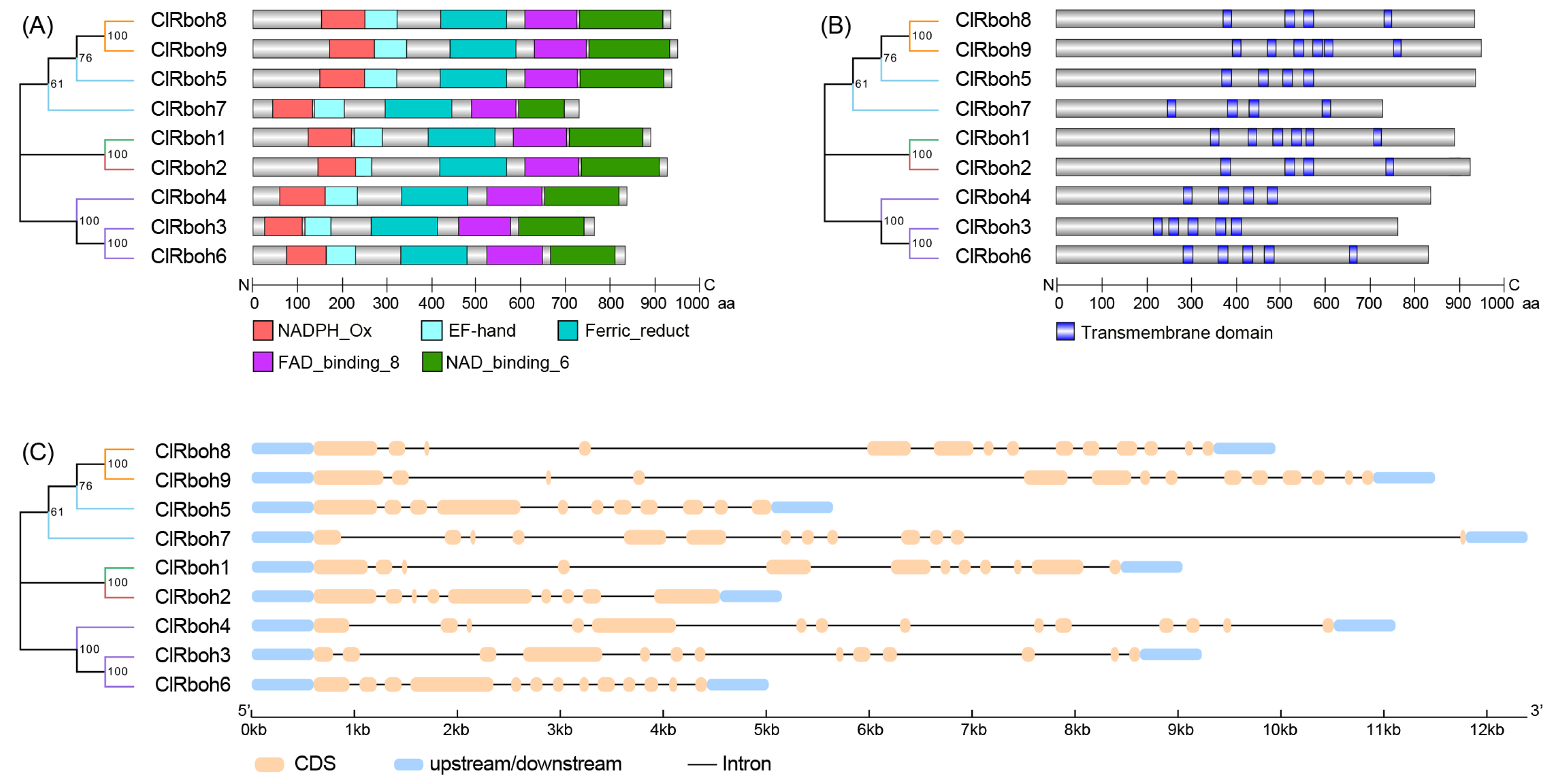
2.5. Analysis of ClRboh Gene Family in Watermelon
2.6. Quantification of Gene Transcripts
2.7. Statistical Analysis
3. Results
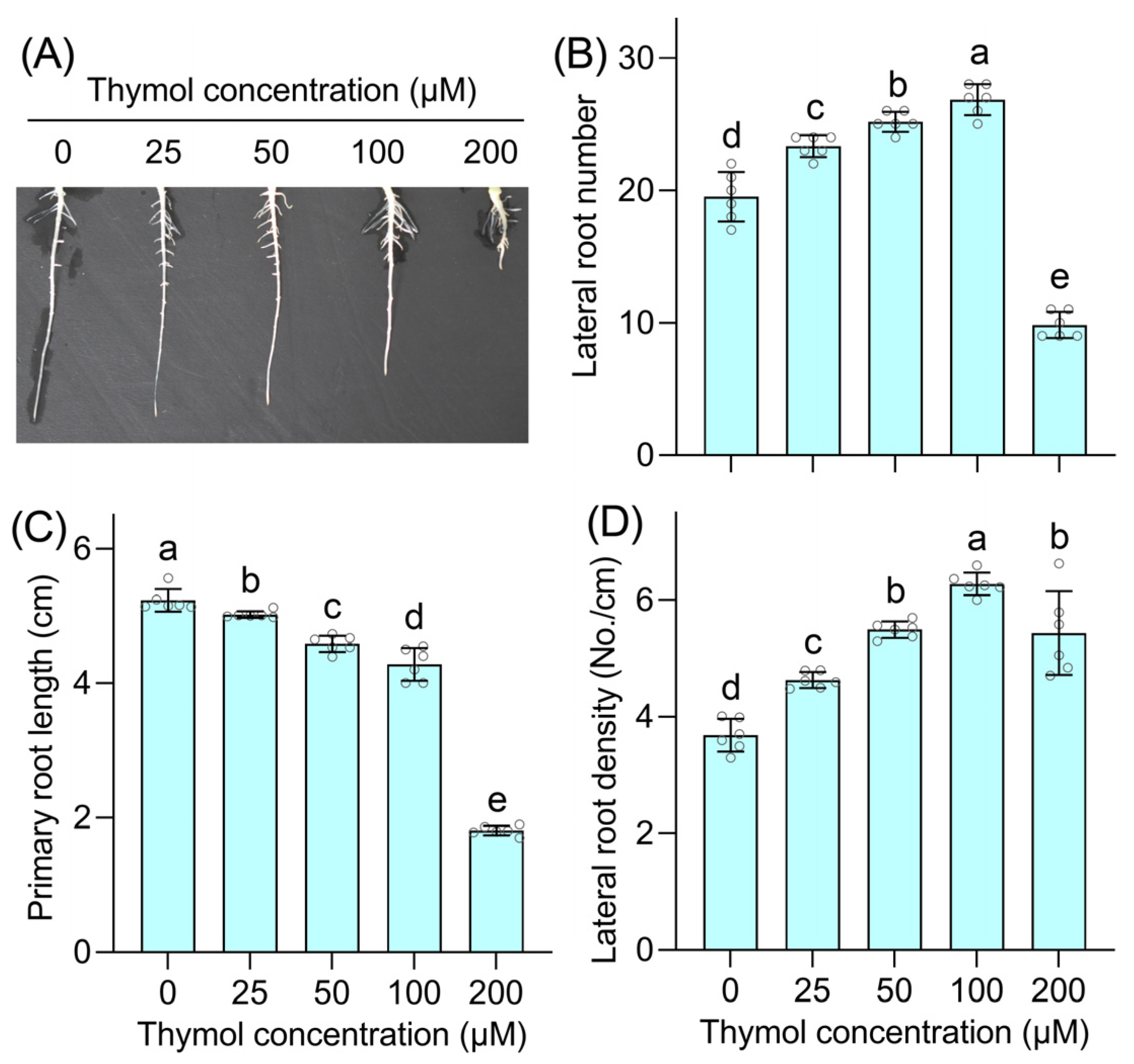
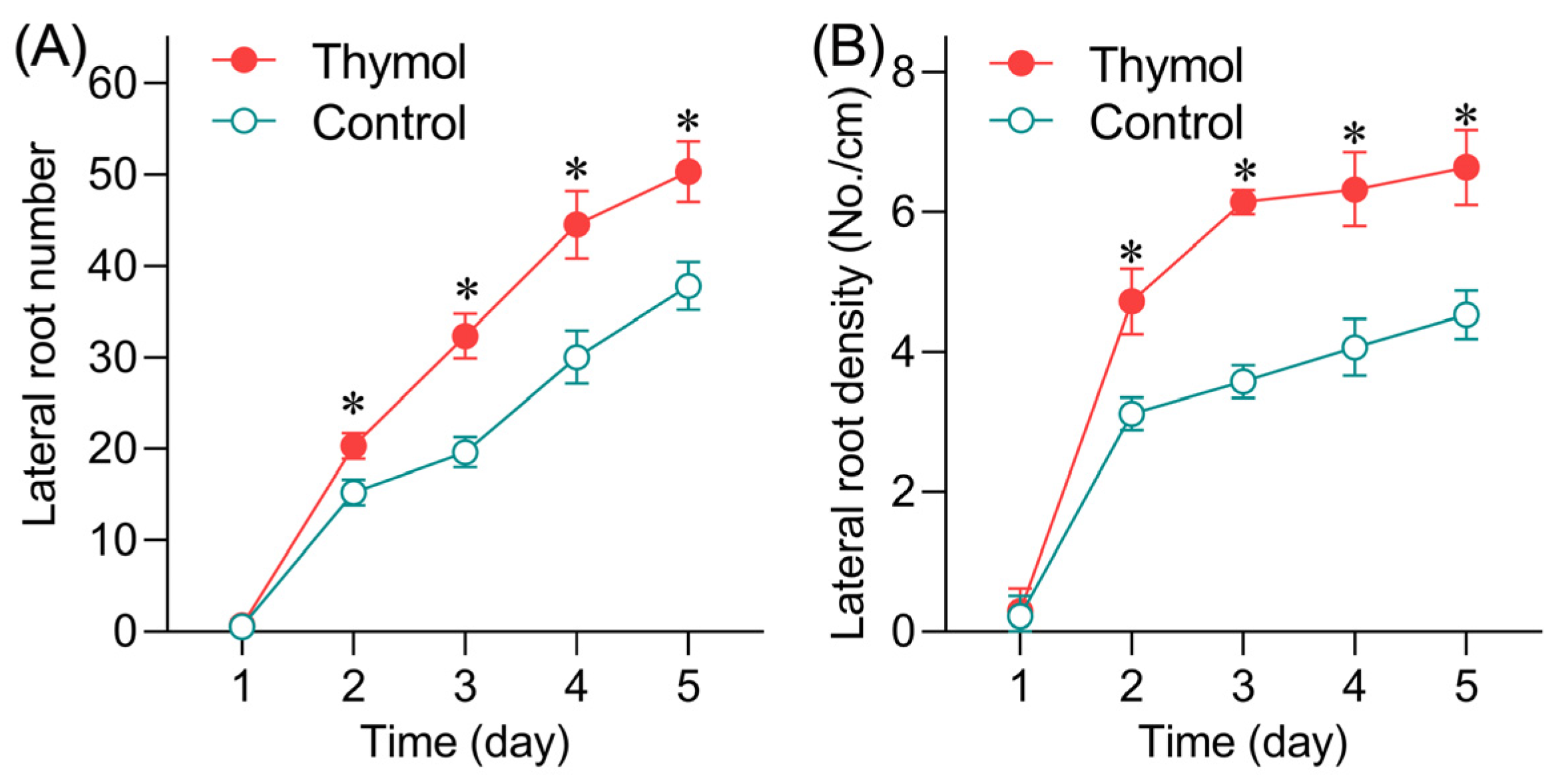
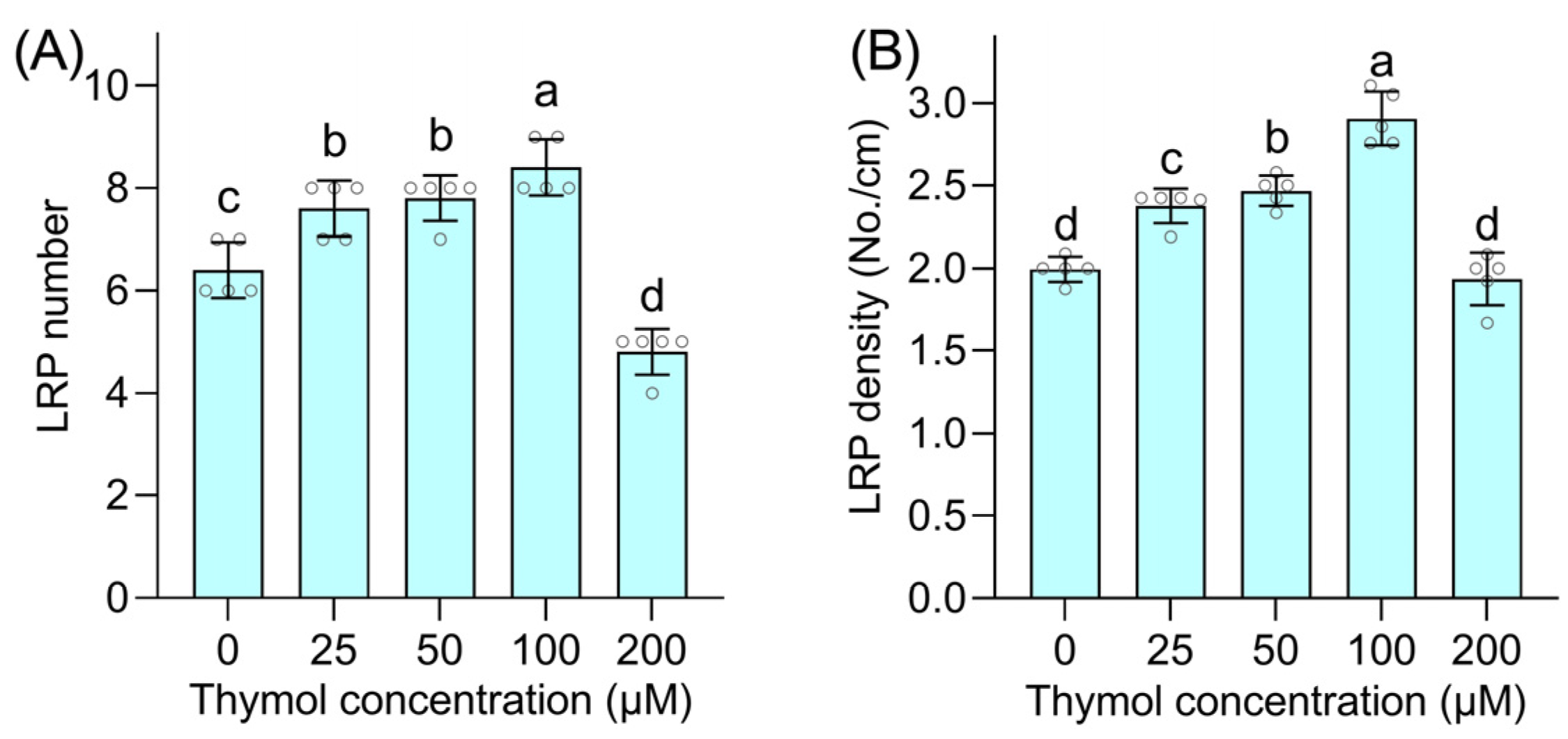
3.1. Thymol Promotes Lateral Root Formation and LRP Emergence
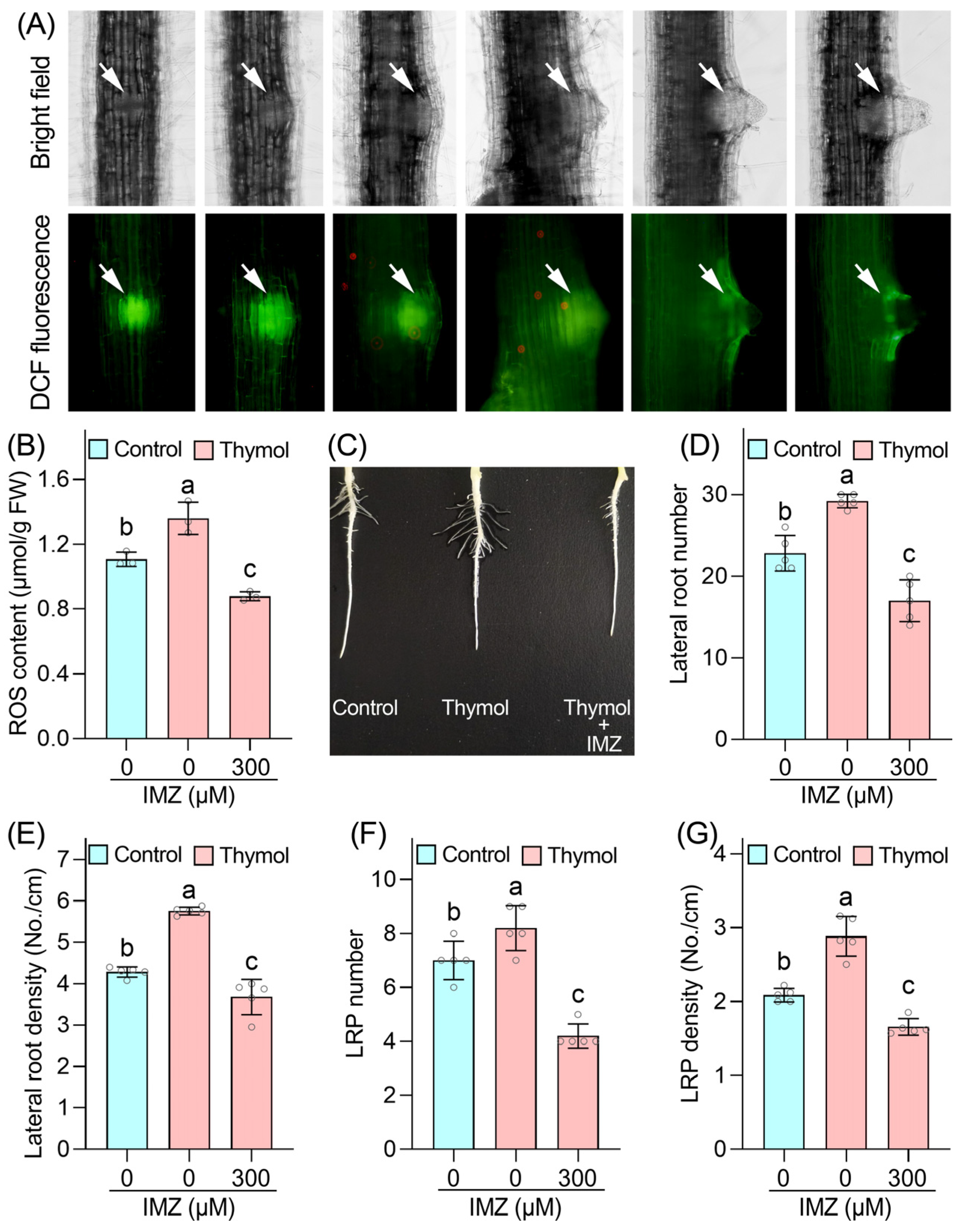
3.2. ROS Is Involved in Thymol-Promoted Lateral Root and LRP Formation
3.3. Identification of ClRboh Gene Family in Watermelon
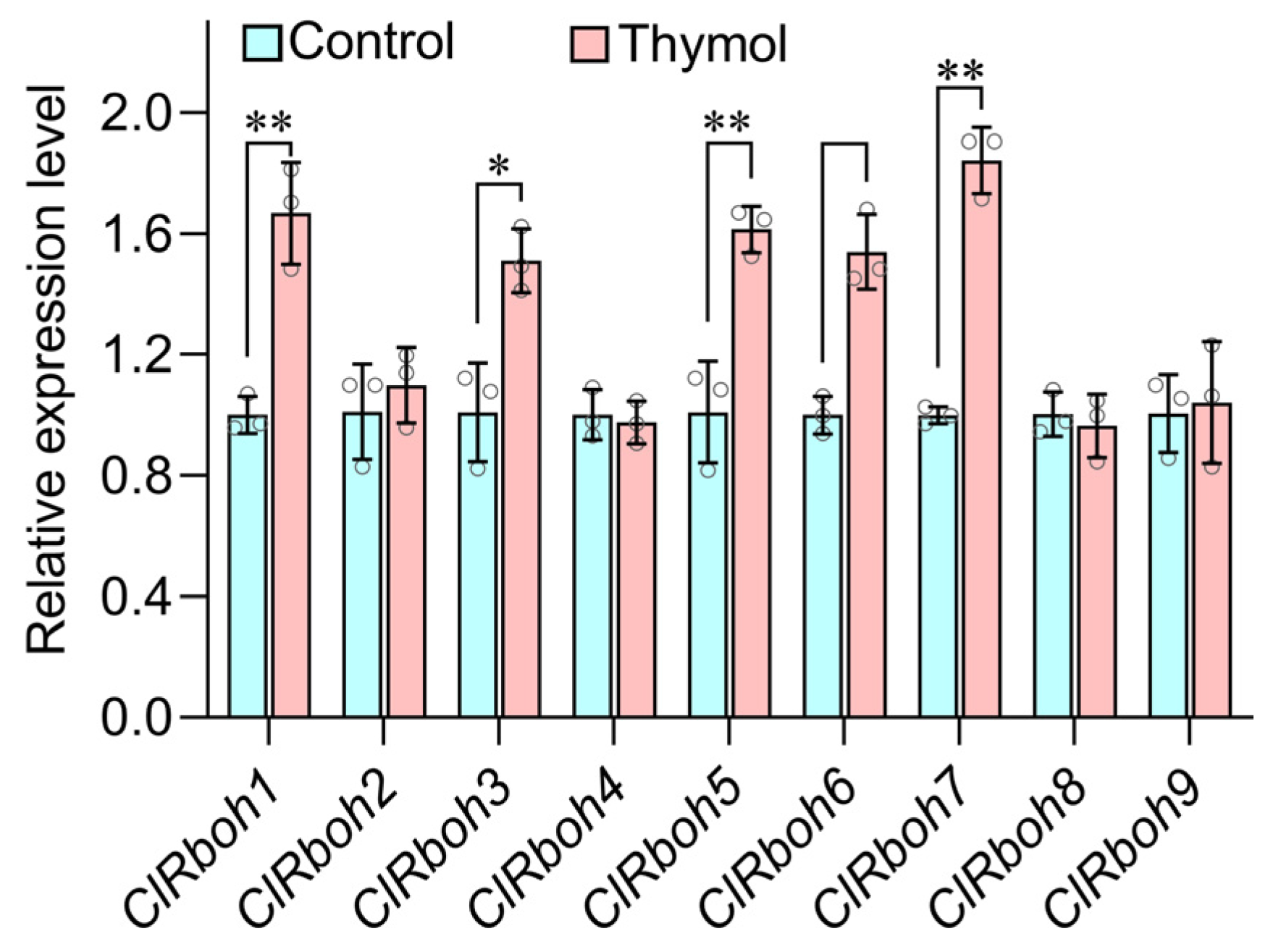
3.4. Thymol Upregulated the Expression of ClRbohs in Roots
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos Teixeira, J.A.; Tusscher, K.H.T. The systems biology of lateral root formation: Connecting the dots. Mol. Plant 2019, 12, 784–803. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, N.; Artner, C.; Benkova, E. Auxin-regulated lateral root organogenesis. Cold Spring Harb. Perspect. Biol. 2021, 13, a039941. [Google Scholar] [CrossRef] [PubMed]
- Correa-Aragunde, N.; Graziano, M.; Chevalier, C.; Lamattina, L. Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. J. Exp. Bot. 2006, 57, 581–588. [Google Scholar] [CrossRef]
- Li, H.; Chen, H.; Chen, L.; Wang, C. The role of hydrogen sulfide in plant roots during development and in response to abiotic stress. Int. J. Mol. Sci. 2022, 23, 1024. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.-Y.; Xuan, W.; Liu, Z.-Y.; Li, X.-N.; Zhao, N.; Xu, P.; Wang, Z.; Guan, R.-Z.; Shen, W.-B. Carbon monoxide promotes lateral root formation in rapeseed. J. Integr. Plant Biol. 2007, 49, 1070–1079. [Google Scholar] [CrossRef]
- Chen, Y.; Kao, C. Calcium is involved in nitric oxide- and auxin-induced lateral root formation in rice. Protoplasma 2012, 249, 187–195. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, Q.; Yu, X.; Huang, L.; Xu, S.; Wang, R.; Shen, W.; Shen, W. Hydrogen peroxide acts downstream of melatonin to induce lateral root formation. Ann. Bot. 2018, 121, 1127–1136. [Google Scholar]
- Fang, T.; Cao, Z.; Li, J.; Shen, W.; Huang, L. Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol. Biochem. 2014, 76, 44–51. [Google Scholar]
- Guo, K.; Xia, K.; Yang, Z.M. Regulation of tomato lateral root development by carbon monoxide and involvement in auxin and nitric oxide. J. Exp. Bot. 2008, 59, 3443–3452. [Google Scholar]
- Zhang, S.; Yu, R.; Yu, D.; Chang, P.; Guo, S.; Yang, X.; Liu, X.; Xu, C.; Hu, Y. The calcium signaling module CaM-IQM destabilizes IAA-ARF interaction to regulate callus and lateral root formation. Proc. Natl. Acad. Sci. USA 2022, 119, e2202669119. [Google Scholar] [CrossRef]
- Manzano, C.; Pallero-Baena, M.; Casimiro, I.; De Rybel, B.; Orman-Ligeza, B.; Van Isterdael, G.; Beeckman, T.; Draye, X.; Casero, P.; del Pozo, J.C. The emerging role of reactive oxygen species signaling during lateral root development. Plant Physiol. 2014, 165, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Yan, A.; Deng, J.; Xie, Y.; Liu, S.; Liu, D.; He, L.; Weng, J.; Xu, J. Evolutionary analysis of respiratory burst oxidase homolog (RBOH) genes in plants and characterization of ZmRBOHs. Int. J. Mol. Sci. 2023, 24, 3858. [Google Scholar] [CrossRef] [PubMed]
- Orman-Ligeza, B.; Parizot, B.; de Rycke, R.; Fernandez, A.; Himschoot, E.; Van Breusegem, F.; Bennett, M.J.; Périlleux, C.; Beeckman, T.; Draye, X. RBOH-mediated ROS production facilitates lateral root emergence in Arabidopsis. Development 2016, 143, 3328–3339. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Wang, Y.; Bai, H.; Li, H.; Zhang, C.; Chen, J.; Xu, W. Melatonin-ROS signal module regulates plant lateral root development. Plant Signal. Behav. 2021, 16, 1901447. [Google Scholar] [CrossRef]
- Terzic, D.; Popovic, V.; Malić, N.; Ikanović, J.; Rajičić, P.S.; Lončar, M.; Lončarević, V. Effects of long-term fertilization on yield of siderates and organic matter content of soil in the process of recultivation. J. Anim. Plant Sci. 2019, 29, 790–795. [Google Scholar]
- Lakić, Ž.; Popović, V.; Ćosić, M.; Antić, M. Genotypes variation of Medicago sativa (L.) seed yield components in acid soil under conditions of cross-fertilization. Genetika 2022, 54, 1–14. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Yadav, S.R.; Mochida, K.; Tran, L.-S.P. Plant growth regulators: True managers of plant life. Plant Cell Physiol. 2022, 63, 1757–1760. [Google Scholar]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef]
- Escobar, A.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arabian J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Koc, K.; Cerig, S.; Ucar, S.; Colak, S.; Bakir, M.; Erol, H.S.; Yildirim, S.; Hosseinigouzdagani, M.; Simsek Ozek, N.; Aysin, F.; et al. Gastroprotective effects of oleuropein and thymol on indomethacin-induced gastric ulcer in Sprague-Dawley rats. Drug Chem. Toxicol. 2020, 43, 441–453. [Google Scholar] [CrossRef]
- Magyar, J.; Szentandrássy, N.; Bányász, T.; Fülöp, L.; Varró, A.; Nánási, P.P. Effects of thymol on calcium and potassium currents in canine and human ventricular cardiomyocytes. Br. J. Pharmacol. 2002, 136, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Shi, Z.Q.; Hu, L.B.; Xu, X.F.; Han, F.X.; Zhou, L.G.; Chen, J. Thymol ameliorates cadmium-induced phytotoxicity in the root of rice (Oryza sativa) seedling by decreasing endogenous nitric oxide generation. J. Agric. Food Chem. 2017, 65, 7396–7405. [Google Scholar] [PubMed]
- Xu, L.; Song, J.Q.; Wang, Y.L.; Liu, X.H.; Li, X.L.; Zhang, B.; Li, A.J.; Ye, X.F.; Wang, J.; Wang, P. Thymol improves salinity tolerance of tobacco by increasing the sodium ion efflux and enhancing the content of nitric oxide and glutathione. BMC Plant Biol. 2022, 22, 31. [Google Scholar]
- Guo, K.; An, G.; Wang, N.; Pang, B.; Shi, Z.; Bai, H.; Zhang, L.; Chen, J.; Xu, W. Thymol ameliorates ammonium toxicity via repressing polyamine oxidase-derived hydrogen peroxide and modulating ammonium transporters in rice root. Food Prod. Process. Nutri. 2021, 3, 7. [Google Scholar] [CrossRef]
- Hu, X.; Wang, W.; Li, C.; Zhang, J.; Lin, F.; Zhang, A.; Jiang, M. Cross-talks between Ca2+/CaM and H2O2 in abscisic acid-induced antioxidant defense in leaves of maize plants exposed to water stress. Plant Growth Regul. 2008, 55, 183–198. [Google Scholar]
- Wang, H.; Taketa, S.; Miyao, A.; Hirochika, H.; Ichii, M. Isolation of a novel lateral-rootless mutant in rice (Oryza sativa L.) with reduced sensitivity to auxin. Plant Sci. 2006, 170, 70–77. [Google Scholar]
- Blum, M.; Andreeva, A.; Florentino, L.C.; Chuguransky, S.R.; Grego, T.; Hobbs, E.; Pinto, B.L.; Orr, A.; Paysan-Lafosse, T.; Ponamareva, I.; et al. InterPro: The protein sequence classification resource in 2025. Nucleic Acids Res. 2024, 53, D444–D456. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E. Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar]
- Hu, B.; Jin, J.P.; Guo, A.Y.; Zhang, H.; Luo, J.C.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A user-friendly online tool for drawing genetic maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Meena, D.C.; Birthal, P.S.; Kumara, T.M.K. Biostimulants for sustainable development of agriculture: A bibliometric content analysis. Discov. Agric. 2025, 3, 2. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Baffi, C.; Colla, G. A vegetal biopolymer-based biostimulant promoted root growth in melon while triggering brassinosteroids and stress-related compounds. Front. Plant Sci. 2018, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, H. Hydrogen peroxide is involved in cGMP modulating the lateral root development of Arabidopsis thaliana. Plant Signal. Behav. 2013, 8, e25052. [Google Scholar]
- Mei, Y.; Chen, H.; Shen, W.; Shen, W.; Huang, L. Hydrogen peroxide is involved in hydrogen sulfide-induced lateral root formation in tomato seedlings. BMC Plant Biol. 2017, 17, 162. [Google Scholar]
- Cui, W.; Zhu, D.; Shen, W.; Mei, Y.; Hu, D.; Shi, Y.; Ren, Y.; Shen, W.; Gu, Q.; Xu, D.; et al. Hydrogen peroxide is involved in β-Cyclodextrin-hemin complex-induced lateral root formation in tomato seedlings. Front. Plant Sci. 2017, 8, 1445. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Liu, F.; Wang, R.; Huang, L.; Shen, W. Hydrogen peroxide is involved in methane-induced tomato lateral root formation. Plant Cell Rep. 2019, 38, 377–389. [Google Scholar] [CrossRef]
- Eljebbawi, A.; Guerrero, Y.; Dunand, C.; Estevez, J.M. Highlighting reactive oxygen species as multitaskers in root development. iScience 2021, 24, 101978. [Google Scholar]
- Torres-Martínez, H.H.; Rodríguez-Alonso, G.; Shishkova, S.; Dubrovsky, J.G. Lateral root primordium morphogenesis in angiosperms. Front. Plant Sci. 2019, 10, 206. [Google Scholar]
- Xiao, T.T.; van Velzen, R.; Kulikova, O.; Franken, C.; Bisseling, T. Lateral root formation involving cell division in both pericycle, cortex and endodermis is a common and ancestral trait in seed plants. Development 2019, 146, dev182592. [Google Scholar]
- Zhou, H.; Huang, J.; Willems, P.; Van Breusegem, F.; Xie, Y. Cysteine thiol-based post-translational modification: What do we know about transcription factors? Trends Plant Sci. 2023, 28, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Kärkönen, A.; Kuchitsu, K. Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 2015, 112, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-L.; Li, W.-Y.; Miao, H.; Yang, S.-Q.; Li, R.; Wang, X.; Li, W.-Q.; Chen, K.-M. Comprehensive genomic analysis and expression profiling of the nox gene families under abiotic stresses and hormones in plants. Genome Biol. Evol. 2016, 8, 791–810. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, H.; Yang, K.; Wang, Y.; Yang, L.; Hu, L.; Liu, R.; Shi, Z. Melatonin facilitates lateral root development by coordinating PAO-derived hydrogen peroxide and Rboh-derived superoxide radical. Free Radic. Biol. Med. 2019, 143, 534–544. [Google Scholar] [CrossRef]
- Liu, H.; Lyu, H.-M.; Zhu, K.; Van de Peer, Y.; Cheng, Z.-M. The emergence and evolution of intron-poor and intronless genes in intron-rich plant gene families. Plant J. 2021, 105, 1072–1082. [Google Scholar] [CrossRef]
- Shen, Q.; Zhou, W.; Li, H.; Hu, L.; Mo, H. ROS involves the fungicidal actions of thymol against spores of Aspergillus flavus via the Induction of nitric oxide. PLoS ONE 2016, 11, e0155647. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, K.; Yin, N.; Ma, X.; Zhao, G.; Qiu, C.; Deng, G. Thymol mitigates lipopolysaccharide-induced endometritis by regulating the TLR4- and ROS-mediated NF-κB signaling pathways. Oncotarget 2017, 8, 20042–20055. [Google Scholar] [CrossRef]
- Prakash, V.; Vishwakarma, K.; Singh, V.P.; Rai, P.; Ramawat, N.; Tripathi, D.K.; Sharma, S. NO and ROS implications in the organization of root system architecture. Physiol. Plant 2020, 168, 473–489. [Google Scholar] [CrossRef]
- Lee, S.P.; Buber, M.T.; Yang, Q.; Cerne, R.; Cortés, R.Y.; Sprous, D.G.; Bryant, R.W. Thymol and related alkyl phenols activate the hTRPA1 channel. Br. J. Pharmacol. 2008, 153, 1739–1749. [Google Scholar] [CrossRef]
- Kurusu, T.; Kuchitsu, K.; Tada, Y. Plant signaling networks involving Ca2+ and Rboh/Nox-mediated ROS production under salinity stress. Front. Plant Sci. 2015, 6, 427. [Google Scholar] [CrossRef]
- Jamali, T.; Kavoosi, G.; Jamali, Y.; Mortezazadeh, S.; Ardestani, S.K. In-vitro, in-vivo, and in-silico assessment of radical scavenging and cytotoxic activities of Oliveria decumbens essential oil and its main components. Sci. Rep. 2021, 11, 14281. [Google Scholar]
- Zhang, C.; Bousquet, A.; Harris, J.M. Abscisic Acid and Lateral Root Organ Defective/Numerous Infections and Polyphenolics modulate root elongation via reactive oxygen species in Medicago truncatula. Plant Physiol. 2014, 166, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Karuppanapandian, T.; Juncheol, M.; Changsoo, K.; Manoharan, K.; Wook, K. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011, 5, 709–725. [Google Scholar]
- Ali, E.; Hussain, S.; Hussain, N.; Kakar, K.U.; Shah, J.M.; Zaidi, S.H.R.; Jan, M.; Zhang, K.; Khan, M.A.; Imtiaz, M. Tocopherol as plant protector: An overview of Tocopherol biosynthesis enzymes and their role as antioxidant and signaling molecules. Acta Physiol. Plant. 2022, 44, 20. [Google Scholar] [CrossRef]
| Gene | Accession No. | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|
| ClRboh1 | Cla97C03G067430 | CGGACGCAGAATATGAACCAA | GTGAGAGTACCAGAAGGCATTG |
| ClRboh2 | Cla97C10G195960 | GCCTCCTTCCGTCCTCCTTGTT | TGCGTCCGTATCCACCATGTCA |
| ClRboh3 | Cla97C02G046480 | GCCACATGCCTAAGCAACAA | CCAGTAACACCAGGAACACTTG |
| ClRboh4 | Cla97C03G052820 | GGGTGGGATAAGGCTTGGAAGA | GCATGTTGATGGAATCGGCTCT |
| ClRboh5 | Cla97C11G206420 | ACCTACAAGATGACGCCGCTCT | GTTCGCATCAGAGGCACCAGTG |
| ClRboh6 | Cla97C02G046490 | TAGATTCACGGCTGCGGATAT | CAGAAGCACTCCACTCCAGTA |
| ClRboh7 | Cla97C06G125190 | TCGCCGTCGTCGTTATCAA | GAACCGCTTCTCTGCCAATT |
| ClRboh8 | Cla97C01G002330 | CCAGCCAAGCATTGAGCCAGAA | AGCCGCACCCTTAGCCGTAA |
| ClRboh9 | Cla97C07G134970 | TTGTGGAGGTGACGCTTGATGT | GCTTCCGCCTTGAGTTCCTGAG |
| β-actin | Cla97C02G026960 | CCATGTATGTTGCCATCCAG | GGATAGCATGGGGTAGAGCA |
| Gene | Accession No. | Chr | CDS (bp) | Pr. Length (aa) | Molecular Weight (kD) | Theoretical pI | Transmembrane Domain |
|---|---|---|---|---|---|---|---|
| ClRboh1 | Cla97C03G067430 | 3 | 2670 | 889 | 101.30 | 9.27 | 6 |
| ClRboh2 | Cla97C10G195960 | 10 | 2781 | 926 | 104.29 | 9.15 | 4 |
| ClRboh3 | Cla97C02G046480 | 2 | 2292 | 763 | 88.14 | 8.59 | 5 |
| ClRboh4 | Cla97C03G052820 | 3 | 2511 | 836 | 96.22 | 9.07 | 4 |
| ClRboh5 | Cla97C11G206420 | 11 | 2811 | 936 | 106.41 | 8.76 | 4 |
| ClRboh6 | Cla97C02G046490 | 2 | 2499 | 832 | 95.67 | 8.69 | 5 |
| ClRboh7 | Cla97C06G125190 | 6 | 2190 | 729 | 84.07 | 9.06 | 4 |
| ClRboh8 | Cla97C01G002330 | 1 | 2805 | 934 | 106.50 | 9.25 | 4 |
| ClRboh9 | Cla97C07G134970 | 7 | 2850 | 949 | 108.35 | 9.02 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chen, J.; Hao, Y.; Li, Y.; Wang, Y.; Wang, L.; Lu, C.; Hu, L.; Yu, X. Thymol Stimulates Lateral Root Formation via Regulating Endogenous Reactive Oxygen Species. Agronomy 2025, 15, 784. https://doi.org/10.3390/agronomy15040784
Li J, Chen J, Hao Y, Li Y, Wang Y, Wang L, Lu C, Hu L, Yu X. Thymol Stimulates Lateral Root Formation via Regulating Endogenous Reactive Oxygen Species. Agronomy. 2025; 15(4):784. https://doi.org/10.3390/agronomy15040784
Chicago/Turabian StyleLi, Jiajun, Jian Chen, Yini Hao, Yong Li, Ya Wang, Liyuan Wang, Chuan Lu, Liangbin Hu, and Xiangyang Yu. 2025. "Thymol Stimulates Lateral Root Formation via Regulating Endogenous Reactive Oxygen Species" Agronomy 15, no. 4: 784. https://doi.org/10.3390/agronomy15040784
APA StyleLi, J., Chen, J., Hao, Y., Li, Y., Wang, Y., Wang, L., Lu, C., Hu, L., & Yu, X. (2025). Thymol Stimulates Lateral Root Formation via Regulating Endogenous Reactive Oxygen Species. Agronomy, 15(4), 784. https://doi.org/10.3390/agronomy15040784










