Effects of Acetylsalicylic Acid and Biosolids on Edaphic, Vegetative and Biochemical Parameters of Amelichloa caudata Under Water Shortage Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Conditions
2.2. Experimental Design and Treatments of Plants
2.3. Soil Characteristics
2.4. Vegetative Growth of Plants
2.5. Ion Content in Roots and Shoots of Plants
2.6. Malondialdehyde (MDA), Antioxidant Compounds and Osmolyte Quantification
2.7. Statistical Analysis
3. Results
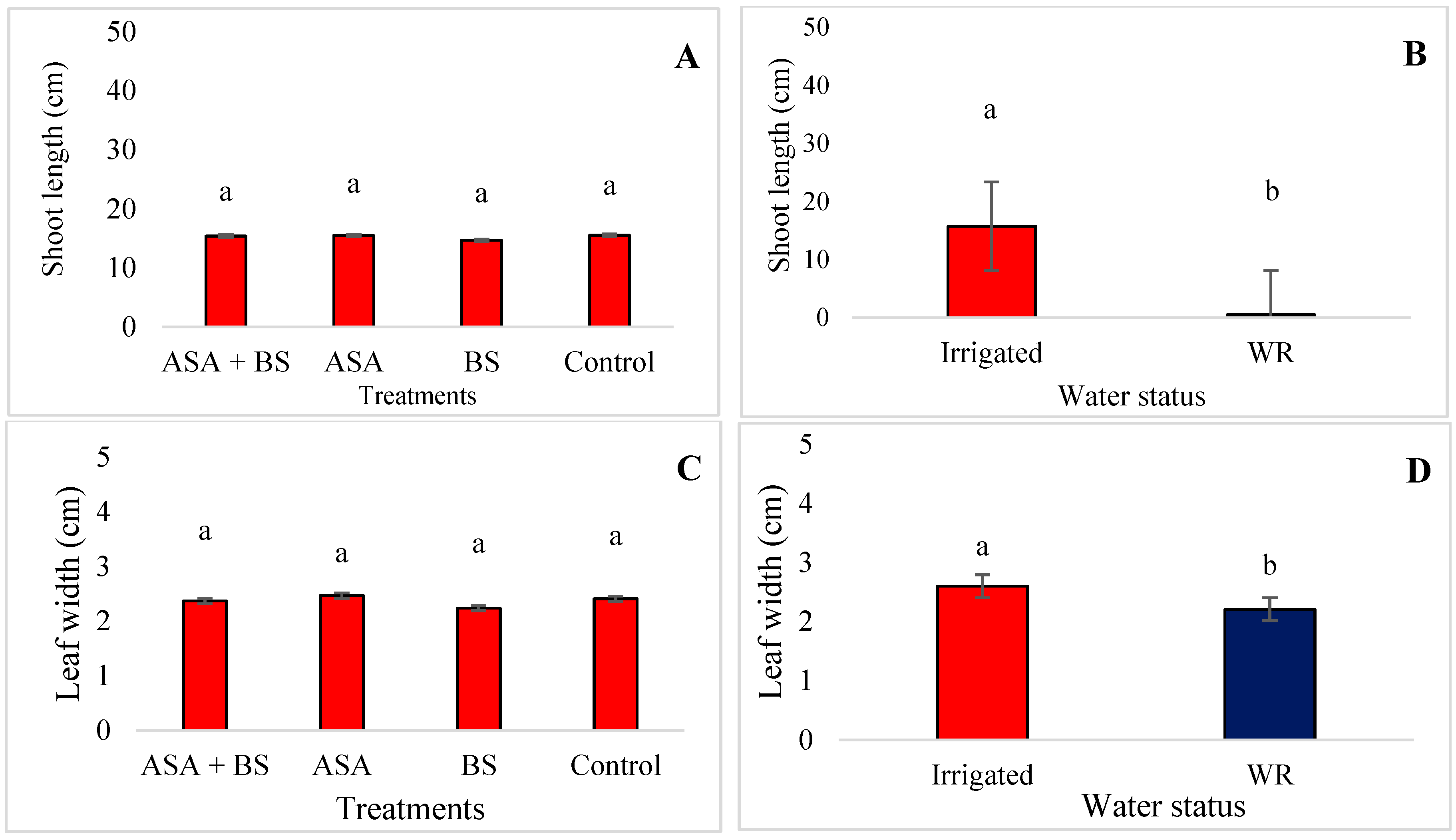
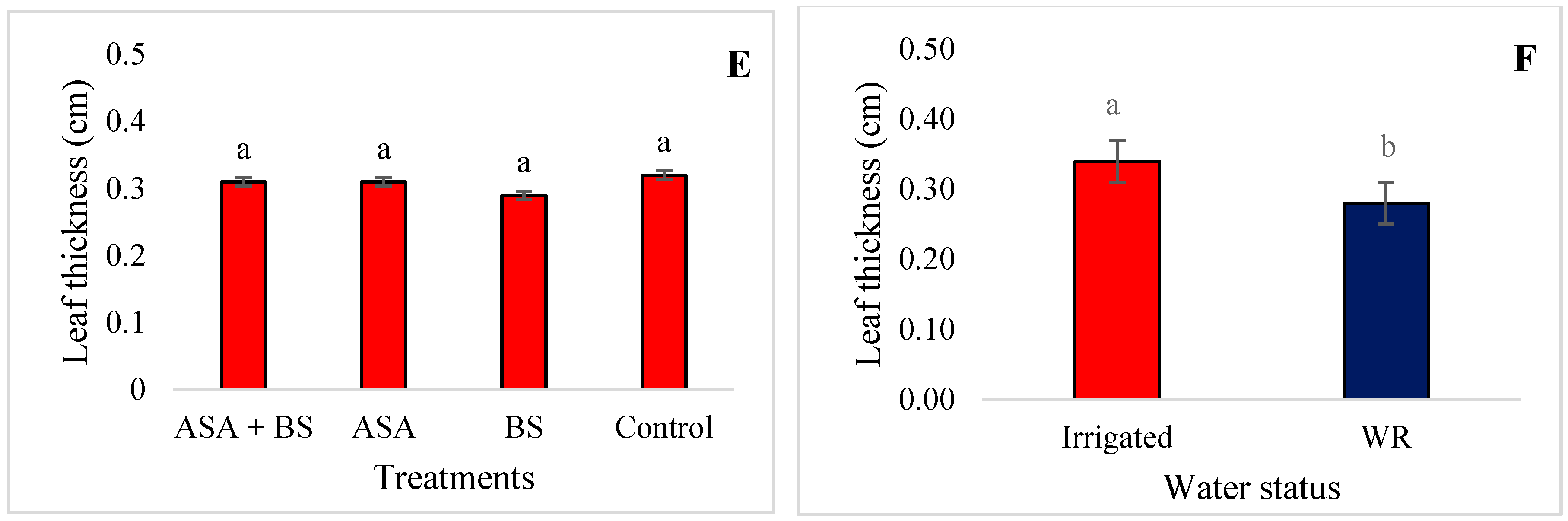
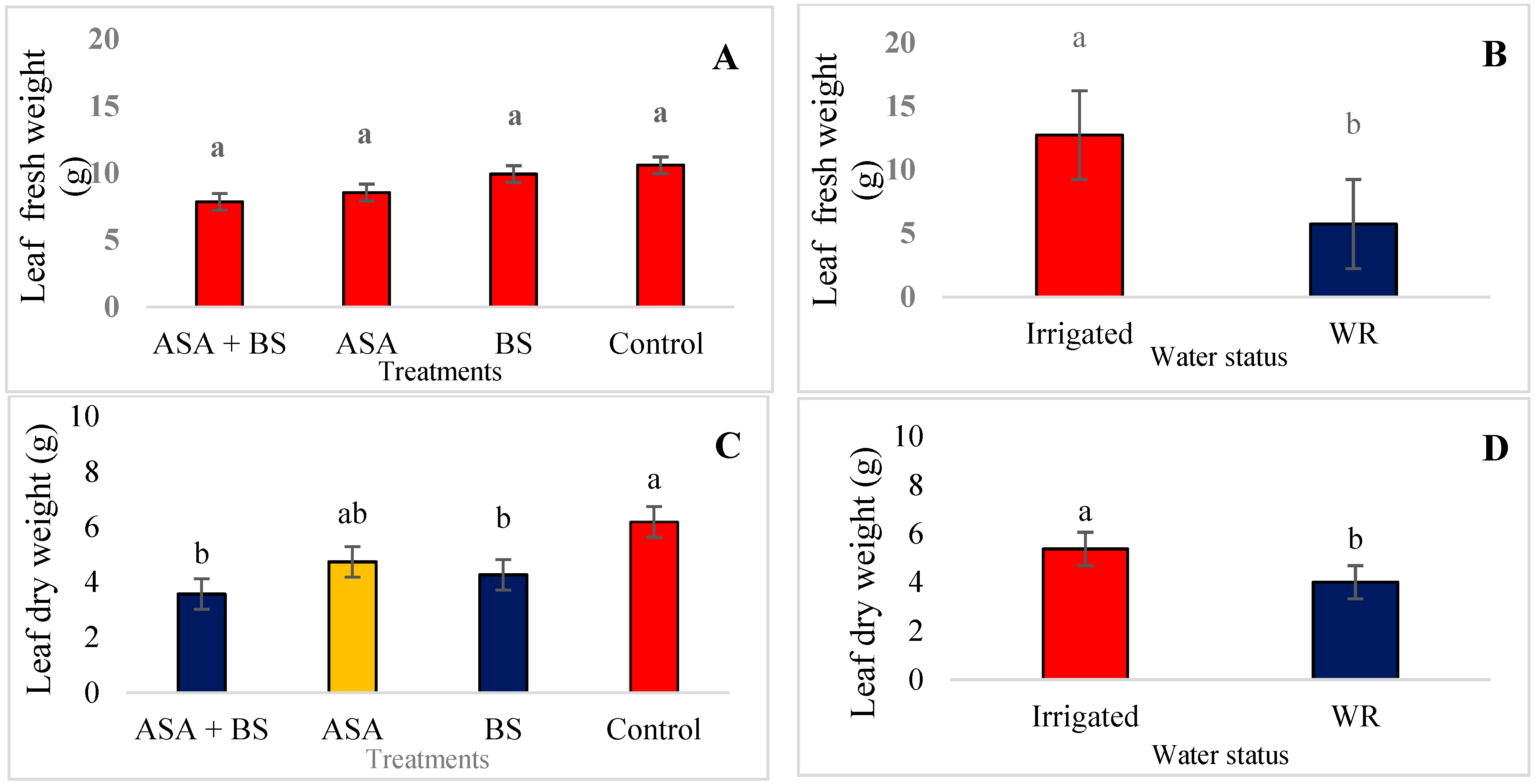
3.1. Vegetative Growth
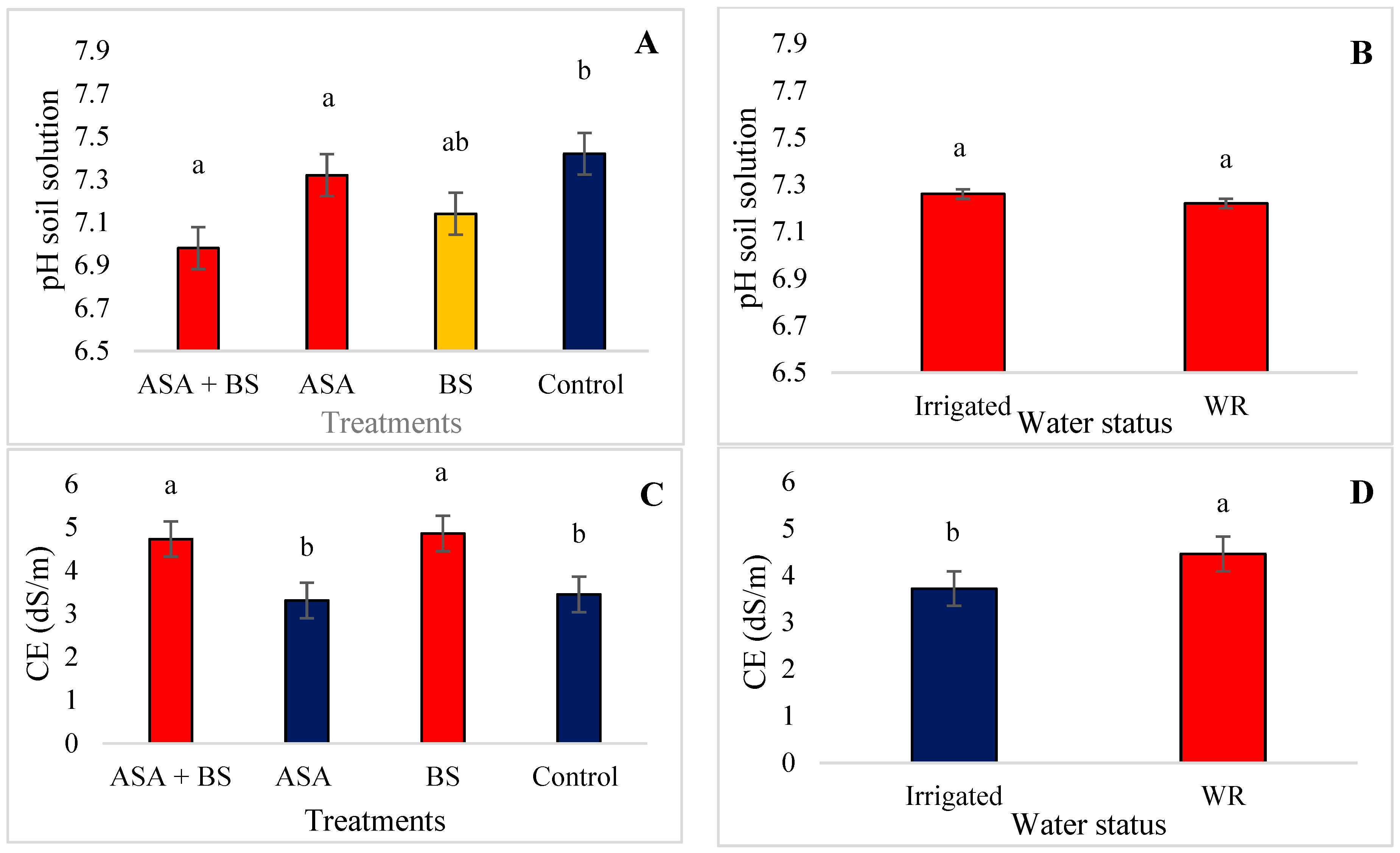
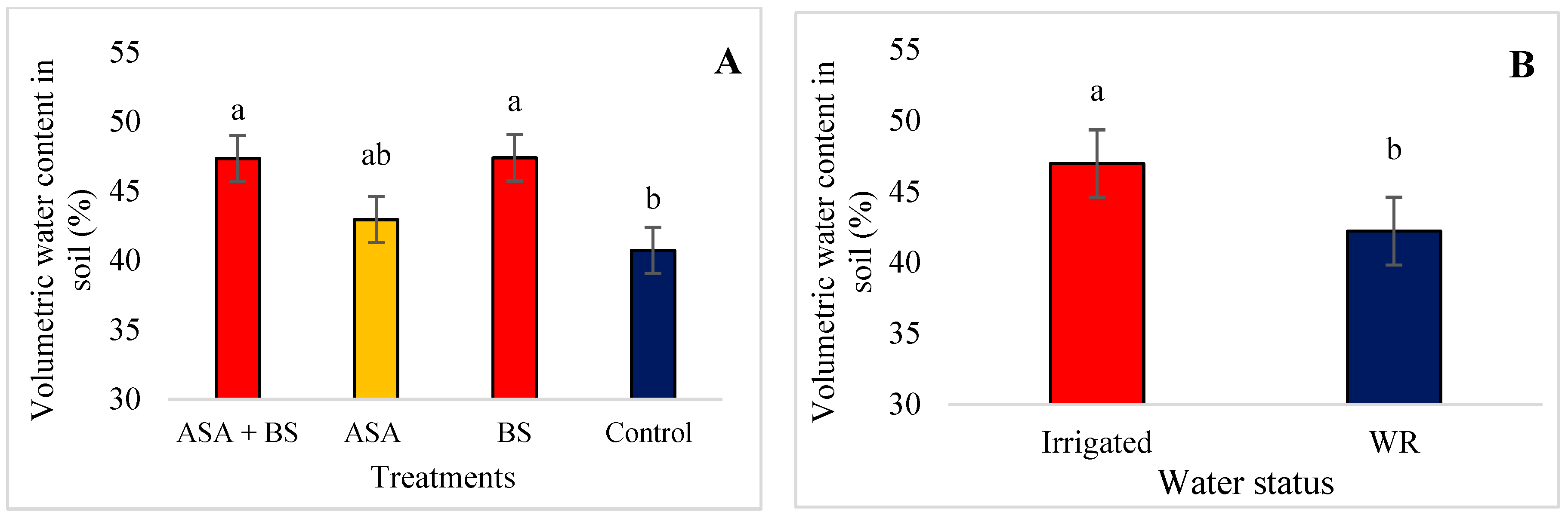
3.2. Soil and Water Status
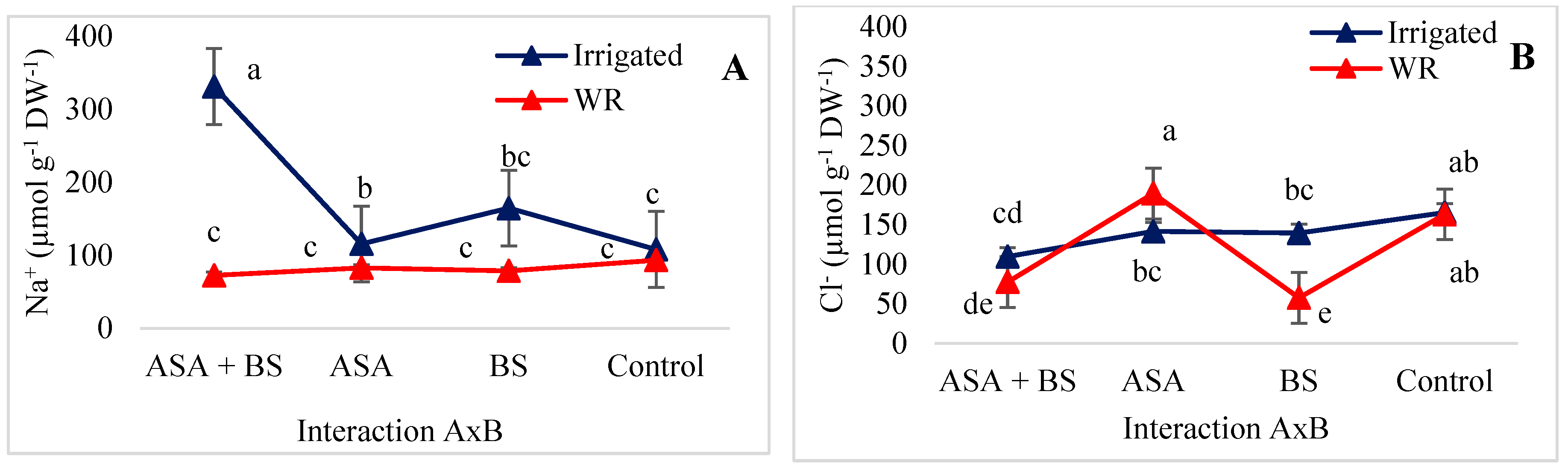
3.3. Ion Concentrations in Plants
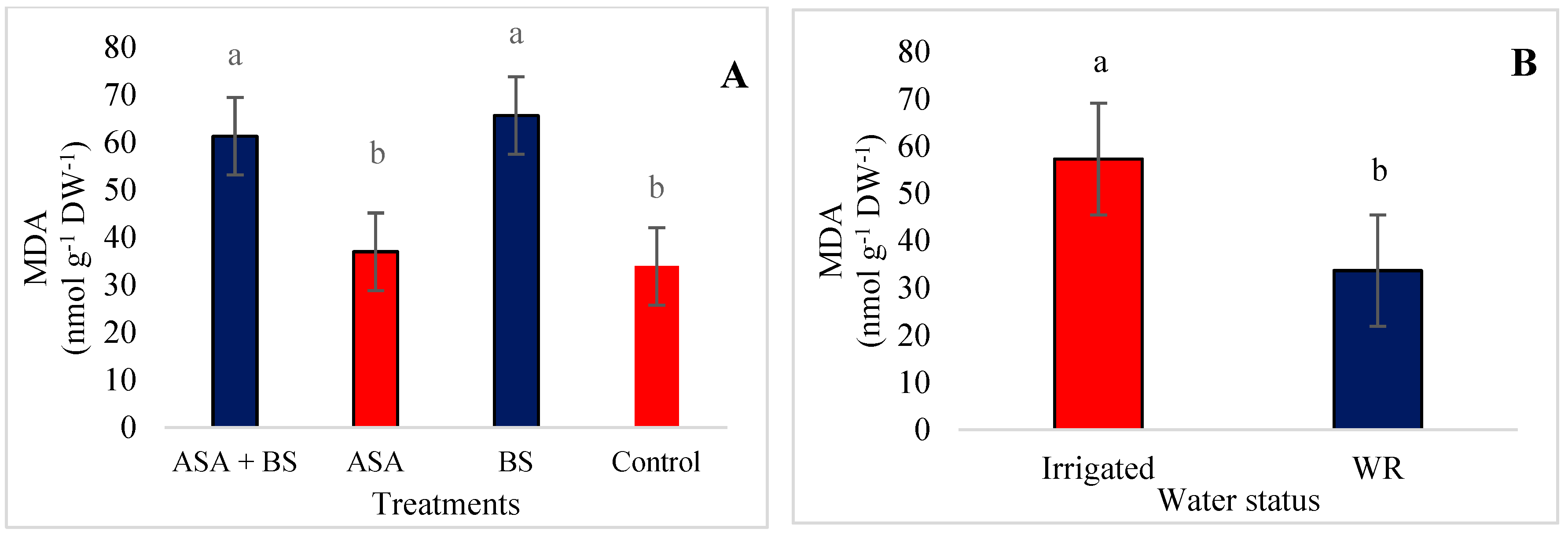
3.4. Malondialdehyde (MDA), Antioxidant Compounds and Osmolyte Quantification
4. Discussion
4.1. Effects of ASA and BSs on Plants
4.2. Soil and Water Status
4.3. Ion Concentrations in Plants
4.4. Biochemicals Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hood, R. Global Warming. In A Companion to Applied Ethics; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; pp. 674–684. [Google Scholar] [CrossRef]
- Araya-Osses, D.; Casanueva, A.; Román-Figueroa, C.; Uribe, J.M.; Paneque, M. Climate Change Projections of Temperature and Precipitation in Chile Based on Statistical Downscaling. Clim. Dyn. 2020, 54, 4309–4330. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Vaishnav, A.; Arya, S.S.; Choudhary, D.K. Plant Stress Mitigators: Action and Application; Springer Nature: Singapore, 2022; ISBN 9789811677595. [Google Scholar]
- Ilyas, M.; Nisar, M.; Khan, N.; Hazrat, A.; Khan, A.H.; Hayat, K.; Fahad, S.; Khan, A.; Ullah, A. Drought Tolerance Strategies in Plants: A Mechanistic Approach. J. Plant. Growth. Regul. 2021, 40, 926–944. [Google Scholar] [CrossRef]
- Popova, L.; Pancheva, T.; Uzunova, A. Salicylic Acid: Properties, Biosynthesis and Physiological Role. Bulg. J. Plant Physiol. 1997, 23, 85–93. [Google Scholar]
- Korkmaz, A.; Uzunlu, M.; Demirkiran, A.R. Treatment with Acetyl Salicylic Acid Protects Muskmelon Seedlings against Drought Stress. Acta Physiol. Plant. 2007, 29, 503–508. [Google Scholar] [CrossRef]
- Chattha, M.U.; Iqbal, L.; Khan, I.; Wang, L.H.; Nawaz, M.; Ali, B.; Fang, S.; Ul Haq, M.I.; Hassan, M.U.; Rasheed, A.; et al. Regulating Effects of Exogenous Salicylic Acid Application on Wheat Growth Under Saline and Heat Stress Conditions. Appl. Ecol. Environ. Res. 2024, 22, 1315–1337. [Google Scholar] [CrossRef]
- Kabiri, R.; Naghizadeh, M. Exogenous Acetylsalicylic Acid Stimulates’ Physiological Changes to Improve Growth, Yield and Yield Components of Barley under Water Stress Condition. J. Plant Physiol. Breed. 2015, 5, 35–45. [Google Scholar]
- Sierra, J.; Roig, N.; Martí, E.; Nadal, M.; Schuhmacher, M. Amendment of Soils with Composted Sewage Sludge. Long Term Effects on C and N Transformation. In Environmental Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2012; pp. 271–282. [Google Scholar] [CrossRef]
- Cieślik, B.M.; Namieśnik, J.; Konieczka, P. Review of Sewage Sludge Management- Standard, Regulations. J. Clean. Prod. 2015, 90, 1–15. [Google Scholar] [CrossRef]
- Sharma, B.; Sarkar, A.; Singh, P.; Pratap, R. Agricultural Utilization of Biosolids: A Review on Potential Effects on Soil and Plant Grown. Waste Manag. 2017, 64, 117–132. [Google Scholar] [CrossRef]
- Antilén, M.; Silva, K.; Acevedo, S.; Amiama, F.; Faúndez, M.; Knicker, H.; Pizarro, C. Characterization of Humic Acids Extracted from Biosolid Amended Soils. J. Soil. Sci. Plant Nutr. 2014, 14, 1005–1020. [Google Scholar] [CrossRef]
- Bettiol, W.; Ghini, R. Impacts of Sewage Sludge in Tropical Soil: A Case Study in Brazil. Appl. Env. Soil. Sci. 2011, 2011, 212807. [Google Scholar] [CrossRef]
- Lu, Q.; He, Z.L.; Stoffella, P.J. Land Application of Biosolids in the USA: A Review. Appl. Environ. Soil. Sci. 2012, 2012, 201462. [Google Scholar] [CrossRef]
- Adair, K.L.; Wratten, S.; Barnes, A.M.; Waterhouse, B.R.; Smith, M.; Lear, G.; Weber, P.; Pizey, M.; Boyer, S. Effects of Biosolids on Biodiesel Crop Yield and Belowground Communities. Ecol. Eng. 2014, 68, 270–278. [Google Scholar] [CrossRef]
- Donoso, S.; Peña-Rojas, K.; Pacheco, C.; Durán, S.; Santelices, R.; Mascaró, C. The Physiological and Growth Response of Acacia Caven under Water Stress and the Application of Different Levels of Biosolids. Cienc. Investig. Agrar. 2015, 42, 273–283. [Google Scholar] [CrossRef]
- Burkart, A. Evolution of Grasses and Grasslands in South America. Taxon 2010, 24, 53–66. [Google Scholar] [CrossRef]
- Elorza, M.S.; Ortiz, D.G.; Deltoro, V. La Flora Alóctona de La Comunidad Valenciana (España). Bot. Complut. 2011, 35, 97–130. [Google Scholar] [CrossRef]
- Galasso, G.; Conti, F.; Peruzzi, L.; Ardenghi, N.M.G.; Banfi, E.; Celesti-Grapow, L.; Albano, A.; Alessandrini, A.; Bacchetta, G.; Ballelli, S.; et al. An Updated Checklist of the Vascular Flora Alien to Italy. Plant Biosyst. 2018, 152, 556–592. [Google Scholar] [CrossRef]
- Molina, J.; González-Orenga, S.; Vicente, O.; Boscaiu, M.; Llinares, J.V.; Zambrano, F.; Santibáñez, C. Effect of Acetylsalicylic Acid and Ammonium Sulphate on Productive and Physiological Parameters in Amelichloa Caudata under Water Shortage Conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12645. [Google Scholar] [CrossRef]
- Verdugo, C.; Sánchez, P.; Santibáñez, C.; Urrestarazu, P.; Bustamante, E.; Silva, Y.; Gourdon, D.; Ginocchio, R. Efficacy of Lime, Biosolids, and Mycorrhiza for the Phytostabilization of Sulfidic Copper Tailings in Chile: A Greenhouse Experiment. Int. J. Phytoremediation 2011, 13, 107–125. [Google Scholar] [CrossRef]
- Weimberg, R. Solute Adjustments in Leaves of Two Species of Wheat at Two Different Stages of Growth in Response to Salinity. Physiol. Plant. 1987, 70, 381–388. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Bates, L.S.; Waldren, R.P.A.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 207, 205–207. [Google Scholar] [CrossRef]
- Balzarini, M.G.; Gonzalez, L.A.; Tablada, E.M.; Casanoves, F.; Di Rienzo, J.A.; Robledo, C.W. InfoStat Manual Del Usuario. 2008. Available online: https://www.infostat.com.ar/ (accessed on 20 March 2025).
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Poor, P.; Janda, T. Salicylic Acid: A Versatile Signaling Molecule in Plants. J. Plant Growth Regul. 2022, 41, 1887–1890. [Google Scholar] [CrossRef]
- Kang, G.; Li, G.; Guo, T. Molecular Mechanism of Salicylic Acid-Induced Abiotic Stress Tolerance in Higher Plants. Acta Physiol. Plant. 2014, 36, 2287–2297. [Google Scholar] [CrossRef]
- Elsisi, M.; Elshiekh, M.; Sabry, N.; Aziz, M.; Attia, K.; Islam, F.; Chen, J.; Abdelrahman, M. The genetic orchestra of salicylic acid in plant resilience to climate change induced abiotic stress: Critical review. Stress Biol. 2024, 4, 31. [Google Scholar] [CrossRef]
- Kang, G.; Li, G.; Xu, W.; Peng, X.; Han, Q.; Zhu, Y.; Guo, T. Proteomics Reveals the Effects of Salicylic Acid on Growth and Tolerance to Subsequent Drought Stress in Wheat. J. Proteome Res. 2012, 11, 6066–6079. [Google Scholar] [CrossRef]
- Kang, G.; Li, G.; Zheng, B.; Han, Q.; Wang, C.; Zhu, Y.; Guo, T. Proteomic Analysis on Salicylic Acid-Induced Salt Tolerance in Common Wheat Seedlings (Triticum aestivum L.). Biochim. Biophys. Acta 2012, 1824, 1324–1333. [Google Scholar] [CrossRef]
- Wang, H.; Tariq, L.; Yan, Y.; Bi, Y.; Song, F. NAC Transcription Factors Transcriptionally Fine-Tune Signal Homeostasis in Plant Systemic Acquired Resistance. Physiol. Plant. 2025, 177, e70123. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.M.; Cidade, L.C.; Gesteira, A.S.; Filho, M.A.C.; Filho, W.S.S.; Costa, M.G.C. Analysis of the NAC Transcription Factor Gene Family in Citrus Reveals a Novel Member Involved in Multiple Abiotic Stress Responses. Tree Genet. Genomes 2011, 7, 1123–1134. [Google Scholar] [CrossRef]
- Jin, H.; Huang, F.; Cheng, H.; Song, H.; Yu, D. Overexpression of the GmNAC2 Gene, an NAC Transcription Factor, Reduces Abiotic Stress Tolerance in Tobacco. Plant Mol. Biol. Rep. 2013, 31, 435–442. [Google Scholar] [CrossRef]
- Senaratna, T.; Touchell, D.; Bunn, E.; Dixon, K. Acetyl Salicylic Acid (Aspirin) and Salicylic Acid Induce Multiple Stress Tolerance in Bean and Tomato Plants. Plant Growth Regul. 2000, 30, 157–161. [Google Scholar] [CrossRef]
- Rihan, H. The Effect of Exogenous Applications of Salicylic Acid and Molybdenum on the Tolerance of Drought in Wheat. Agric. Res. Technol. 2017, 9, 555768. [Google Scholar] [CrossRef]
- Hussain, K.; Nawaz, K.; Majeed, A.; Khan, F.; Lin, F.; Ghani, A.; Raza, G.; Afghan, S.; Zia-ul-Hussnain, S.; Ali, K.; et al. Alleviation of Salinity Effects by Exogenous Applications of Salicylic Acid in Pearl Millet (Pennisetum glaucum (L.) R. Br.) Seedlings. Afr. J. Biotechnol. 2010, 9, 8602–8607. [Google Scholar]
- Ennajeh, M.; Vadel, A.M.; Cochard, H.; Khemira, H. Comparative Impacts of Water Stress on the Leaf Anatomy of a Drought-Resistant and a Drought-Sensitive Olive Cultivar. J. Hortic. Sci. Biotechnol. 2010, 85, 289–294. [Google Scholar] [CrossRef]
- Saska, P.; Skuhrovec, J.; Tylová, E.; Platková, H.; Tuan, S.J.; Hsu, Y.T.; Vítámvás, P. Leaf Structural Traits Rather than Drought Resistance Determine Aphid Performance on Spring Wheat. J. Pest Sci. 2021, 94, 423–434. [Google Scholar] [CrossRef]
- Chang, Z.; Zhuo, L.; Yu, F.; Zhang, X. Effects of Biosolids on Root Growth and Nitrogen Metabolism in Kentucky Bluegrass under Drought Stress. HortScience 2014, 49, 1205–1211. [Google Scholar] [CrossRef]
- Stavridou, E.; Giannakis, I.; Karamichali, I.; Kamou, N.N.; Lagiotis, G.; Madesis, P.; Emmanouil, C.; Kungolos, A.; Nianiou-obeidat, I.; Lagopodi, A.L. Biosolid-amended Soil Enhances Defense Responses in Tomato Based on Metagenomic Profile and Expression of Pathogenesis-related Genes. Plants 2021, 10, 2789. [Google Scholar] [CrossRef]
- Trippe, K.M.; Manning, V.A.; Reardon, C.L.; Klein, A.M.; Weidman, C.; Ducey, T.F.; Johnson, M.G. Phytostabilization of acidic mine tailings with biochar, biosolids, lime, and locally-sourced microbial inoculum: Do amendment mixtures influence plant growth, tailing chemistry, and microbial composition? Appl. Soil Ecol. 2021, 165, 103962. [Google Scholar] [CrossRef] [PubMed]
- Pampana, S.; Rossi, A.; Arduini, I. Biosolids Benefit Yield and Nitrogen Uptake in Winter Cereals without Excess Risk of n Leaching. Agronomy 2021, 11, 1482. [Google Scholar] [CrossRef]
- Sidhu, H.; O’Connor, G.; Kruse, J. Plant Toxicity and Accumulation of Biosolids-Borne Ciprofloxacin and Azithromycin. Sci. Total Environ. 2019, 648, 1219–1226. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of Anaerobic Digestion on Digestate Nutrient Availability and Crop Growth: A Review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Ondreičková, K.; Piliarová, M.; Klčová, L.; Žofajová, A.; Gubiš, J.; Horník, M.; Gubišová, M.; Hudcovicová, M.; Kraic, J. The Impact of Sewage Sludge on the Fungal Communities in the Rhizosphere and Roots of Barley and on Barley Yield. Open Life Sci. 2021, 16, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Gondek, M.; Weindorf, D.C.; Thiel, C.; Kleinheinz, G. Soluble Salts in Compost and Their Effects on Soil and Plants: A Review. Compos. Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Weggler-Beaton, K.; McLaughlin, M.J.; Graham, R.D. Salinity increases cadmium uptake by wheat and Swiss chard from soil amended with biosolids. Soil Res. 2000, 38, 37–46. [Google Scholar] [CrossRef]
- Kim, K.R.; Owens, G. Potential for Enhanced Phytoremediation of Landfills Using Biosolids—A Review. J. Environ. Manag. 2010, 91, 791–797. [Google Scholar] [CrossRef]
- López-Millán, A.F.; Sagardoy, R.; Solanas, M.; Abadía, A.; Abadía, J. Cadmium Toxicity in Tomato (Lycopersicon esculentum) Plants Grown in Hydroponics. Environ. Exp. Bot. 2009, 65, 376–385. [Google Scholar] [CrossRef]
- Jia, Y.; Tang, S.R.; Ju, X.H.; Shu, L.N.; Tu, S.X.; Feng, R.W.; Giusti, L. Effects of Elevated CO2 Levels on Root Morphological Traits and Cd Uptakes of Two Lolium Species under Cd Stress. J. Zhejiang Univ. Sci. B. 2011, 12, 313–325. [Google Scholar] [CrossRef]
- Muranaka, S.; Shimizu, K.; Kato, M. A salt-tolerant cultivar of wheat maintains photosynthetic activity by suppressing sodium uptake. Photosynthetica 2002, 40, 505–515. [Google Scholar]
- Brini, F.; Hanin, M.; Mezghani, I.; Berkowitz, G.A.; Masmoudi, K. Overexpression of Wheat Na+/H+ Antiporter TNHX1 and H+-Pyrophosphatase TVP1 Improve Salt- and Drought-Stress Tolerance in Arabidopsis thaliana Plants. J. Exp. Bot. 2007, 58, 301–308. [Google Scholar] [CrossRef]
- Dugasa, M.T.; Cao, F.; Ibrahim, W.; Wu, F. Differences in Physiological and Biochemical Characteristics in Response to Single and Combined Drought and Salinity Stresses between Wheat Genotypes Differing in Salt Tolerance. Physiol. Plant 2019, 165, 134–143. [Google Scholar] [CrossRef]
- Noreen, S.; Fatima, K.; Athar, H.U.R.; Ahmad, S.; Hussain, K. Enhancement of Physio-Biochemical Parameters of Wheat through Exogenous Application of Salicylic Acid under Drought Stress. J. Anim. Plant Sci. 2017, 27, 153–163. [Google Scholar]
- Kaydan, D.; Yagmur, M.; Okut, N. Effects of Salicylic Acid on the Growth and Some Physiological Characters in Salt Stressed Wheat (Triticum aestivum L.). Tarım Bilim. Der. 2007, 13, 1. [Google Scholar] [CrossRef]
- Jini, D.; Joseph, B. Physiological Mechanism of Salicylic Acid for Alleviation of Salt Stress in Rice. Rice Sci. 2017, 24, 97–108. [Google Scholar] [CrossRef]
- Yang, D.; Ni, R.; Yang, S.; Pu, Y.; Qian, M.; Yang, Y.; Yang, Y. Functional Characterization of the Amelichloa purpurea P5cs Gene under Drought Stress Conditions. Int. J. Mol. Sci. 2021, 22, 9599. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Alpaslan, M.; Cicek, N.; Guneri, E.; Eraslan, F.; Guzelordu, T. Effects of Exogenously Applied Salicylic Acid on the Induction of Multiple Stress Tolerance and Mineral Nutrition in Maize (Zea mays L.). Arch. Agron. Soil Sci. 2005, 51, 687–695. [Google Scholar] [CrossRef]
- Aldesuquy, H.S.; Abbas, M.A.; Abo-Hamed, S.A.; Elhakem, A.H. Does Glycine Betaine and Salicylic Acid Ameliorate the Negative Effect of Drought on Wheat by Regulating Osmotic Adjustment through Solutes Accumulation? Furthermore, osmotic. J. Plant Stress Physiol. 2013, 9, 5–22. [Google Scholar]
- Garg, N.; Chandel, S. Role of Arbuscular Mycorrhizal (AM) Fungi on Growth, Cadmium Uptake, Osmolyte, and Phytochelatin Synthesis in Cajanus cajan (L.) Millsp. Under NaCl and Cd Stresses. J. Plant Growth Regul. 2012, 31, 292–308. [Google Scholar] [CrossRef]
- Ziadi, N.; Gagnon, B.; Nyiraneza, J. Crop Yield and Soil Fertility as Affected by Papermill Biosolids and Liming By-Products. Can. J. Soil Sci. 2013, 93, 319–328. [Google Scholar] [CrossRef]
- Maftoun, M.; Moshiri, F.; Karimian, N.; Ronaghi, A.M. Effects of Two Organic Wastes in Combination with Phosphorus on Growth and Chemical Composition of Spinach and Soil Properties. J. Plant Nutr. 2004, 27, 1635–1651. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of Osmoprotectants in Improving Salinity and Drought Tolerance in Plants: A Review. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1995; ISBN 978-0-12-473542-2. [Google Scholar]
- Chen, H.; Yan, S.; Ye, Z.; Meng, H.; Zhu, Y. Utilization of Urban Sewage Sludge: Chinese Perspectives. Environ. Sci. Pollut. Res. 2012, 19, 1454–1463. [Google Scholar] [CrossRef]
- Torri, S.I.; Corrêa, R.S.; Renella, G. Soil Carbon Sequestration Resulting from Biosolids Application. Appl. Environ. Soil Sci. 2014, 2014, 821768. [Google Scholar] [CrossRef]
- Rajala, A. Plant Growth Regulators to Manipulate Oat Stands. Agric. Food Sci. 2004, 13, 186–197. [Google Scholar] [CrossRef]
- Brown, J.C.; Tiffin, L.O.; Holmes, R.S. Competition between chelating agents and roots as factor affecting absorption of iron and other ions by plant species. Plant Physiol. 1960, 35, 878. [Google Scholar] [CrossRef]
- Patel, J.A.; Vora, A.B. Free Proline Accumulation in Drought-Stressed Plants. Plant Soil 1985, 84, 427–429. [Google Scholar] [CrossRef]
- Johari-Pireivatlou, M.; Qasimov, N.; Maralian, H. Effect of Soil Water Stress on Yield and Proline Content of Four Wheat Lines. Afr. J. Biotechnol. 2010, 9, 036–040. [Google Scholar]
- Al Hassan, M.; López-Gresa, M.D.P.; Boscaiu, M.; Vicente, O. Stress Tolerance Mechanisms in Juncus: Responses to Salinity and Drought in Three Juncus Species Adapted to Different Natural Environments. Funct. Plant Biol. 2016, 43, 949–960. [Google Scholar] [CrossRef]
- Lee, B.R.; Islam, M.T.; Park, S.H.; Jung, H.I.; Bae, D.W.; Kim, T.H. Characterization of Salicylic Acid-mediated Modulation of the Drought Stress Responses: Reactive Oxygen Species, Proline, and Redox State in Brassica napus. Env. Exp. Bot. 2019, 157, 10. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Niazi, A.; Pessarakli, M.; Arvin, M.J. P5CS Expression Level and Proline Accumulation in the Sensitive and Tolerant Wheat Cultivars under Control and Drought Stress Conditions in the Presence/Absence of Silicon and Salicylic Acid. J. Plant Interact. 2018, 13, 461–471. [Google Scholar] [CrossRef]
- Ignatenko, A.; Talanova, V.; Repkina, N.; Titov, A. Exogenous Salicylic Acid Treatment Induces Cold Tolerance in Wheat through Promotion of Antioxidant Enzyme Activity and Proline Accumulation. Acta Physiol. Plant 2019, 41, 80. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Ma, J.; Li, X.-Y.; Li, Y.; Zhang, R.; Wang, R. Effects of Water Stress on Reactive Oxygen Species Generation and Protection System in Rice During Grain-Filling Stage. Agric. Sci. China 2010, 9, 633–641. [Google Scholar] [CrossRef]
- Shan, C.; Wang, B.; Sun, H.; Gao, S.; Li, H. H2S Induces No in the Regulation of AsA-GSH Cycle in Wheat Seedlings by Water Stress. Protoplasma 2020, 257, 1487–1493. [Google Scholar] [CrossRef]
- Mahouachi, J.; Argamasilla, R.; Gómez-Cadenas, A. Influence of Exogenous Glycine Betaine and Abscisic Acid on Papaya in Responses to Water-Deficit Stress. J. Plant Growth Regul. 2012, 31, 1–10. [Google Scholar] [CrossRef]
- Saruhan, N.; Saglam, A.; Kadioglu, A. Salicylic Acid Pretreatment Induces Drought Tolerance and Delays Leaf Rolling by Inducing Antioxidant Systems in Maize Genotypes. Acta Physiol. Plant. 2012, 34, 97–106. [Google Scholar] [CrossRef]
- La, V.H.; Lee, B.R.; Zhang, Q.; Park, S.H.; Islam, M.T.; Kim, T.H. Salicylic Acid Improves Drought-Stress Tolerance by Regulating the Redox Status and Proline Metabolism in Brassica rapa. Hortic. Environ. Biote. 2019, 60, 31–40. [Google Scholar] [CrossRef]
- Rai, G.K.; Magotra, I.; Khanday, D.M.; Choudhary, S.M.; Bhatt, A.; Gupta, V.; Rai, P.K.; Kumar, P. Boosting Drought Tolerance in Tomatoes through Stimulatory Action of Salicylic Acid Imparted Antioxidant Defense Mechanisms. Agronomy 2024, 14, 1227. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, D.; Ervin, E.H.; Evanylo, G.K.; Cataldi, D.; Li, J. Biosolids Impact Antioxidant Metabolism Associated with Drought Tolerance in Tall Fescue. HortScience 2012, 47, 1550–1555. [Google Scholar] [CrossRef]
- Mkhinini, M.; Helaoui, S.; Boughattas, I.; Amemou, C.; Banni, M. Earthworm Eisenia andrei Modulates Oxidative Stress in Bean Plants Vicia faba Irrigated with Treated Wastewater. Ecotoxicology 2020, 29, 1003–1016. [Google Scholar] [CrossRef]
- Marchuk, S.; Tait, S.; Sinha, P.; Harris, P.; Antille, D.L.; McCabe, B.K. Biosolids-derived fertilisers: A review of challenges and opportunities. Sci. Total Environ. 2023, 875, 162555. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Pang, S.; Yang, J.; Zhao, X.; Cao, J. Changes in soil microbial community structure following amendment of biosolids for seven years. Environ. Pollut. Bioavailab. 2019, 31, 24–31. [Google Scholar] [CrossRef]


| Block 1 | Block 2 | Block 3 | Block 4 | ||||
|---|---|---|---|---|---|---|---|
| Irrigated | WR | Irrigated | WR | Irrigated | WR | Irrigated | WR |
| T4 | T2 | T1 | T3 | T2 | T3 | T4 | T2 |
| T1 | T3 | T2 | T1 | T1 | T1 | T1 | T4 |
| T2 | T1 | T4 | T4 | T3 | T2 | T2 | T1 |
| T3 | T4 | T3 | T2 | T4 | T4 | T3 | T3 |
| Water Status | Treatments | Na+ (µmol g−1 DW−1). | Cl− (µmol g−1 DW−1) | K+ (µmol g−1 DW−1) |
|---|---|---|---|---|
| Irrigated | ASA + BS | 331.12 ± 17.89 | 109.76 ± 18.45 | 130.34 ± 10.71 |
| ASA | 115.52 ± 17.89 | 141.47 ± 15.97 | 136.24 ± 10.71 | |
| BS | 164.50 ± 17.89 | 139.35 ± 15.97 | 191.08 ± 55.53 | |
| Control | 108.08 ± 17.89 | 165.44 ± 15.97 | 195.05 ± 55.53 | |
| WR | ASA + BS | 72.63 ± 17.89 | 77.56 ± 15.97 | 120.49 ± 9.78 |
| ASA | 82.68 ± 17.89 | 189.33 ± 15.97 | 124.46 ± 9.78 | |
| BS | 78.71 ± 20.65 | 57.54 ± 15.97 | 127.30 ± 41.42 | |
| Control | 93.21 ± 17.89 | 163.05 ± 15.97 | 123.28 ± 41.42 | |
| Treatments (A) | *** | *** | n.s | |
| Water Status (B) | *** | n.s | n.s | |
| Interaction A × B | *** | *** | n.s |
| Water Condition | Treatments | TFC (mg eq. GA g−1 DW) | TF (catequin g−1 DW) | Pro (µmol g−1 DW−1) | MDA (nmol g−1 DW−1) |
|---|---|---|---|---|---|
| ASA + BS | 71.64 ± 13.23 | 3.53 ± 0.12 | 9.31 ± 4.40 | 81.60 ± 8.72 | |
| Irrigated | ASA | 67.12 ± 9.16 | 3.28 ± 0.11 | 8.89 ± 4.40 | 47.87 ± 8.72 |
| BS | 82.09 ± 13.23 | 3.73 ± 0.12 | 8.78 ± 4.40 | 6.26 ± 8.72 | |
| Control | 75.10 ± 13.23 | 3.01 ± 0.12 | 10.95 ± 4.40 | 37.79 ± 8.72 | |
| ASA + BS | 56.89 ± 16.01 | 2.85 ± 0.75 | 22.25 ± 5.45 | 41.65 ± 7.55 | |
| WR | ASA | 56.83 ± 9.16 | 2.60 ± 0.53 | 31.70 ± 5.87 | 26.10 ± 7.55 |
| BS | 57.92 ± 16.01 | 2.88 ± 0.75 | 25.09 ± 4.91 | 44.99 ± 10.68 | |
| Control | 57.88 ± 11.59 | 2.40 ± 0.53 | 30.03 ± 7.13 | 30.05 ± 7.55 | |
| Treatments (A) | n.s | n.s | n.s | ** | |
| Water Condition (B) | n.s | n.s | *** | * | |
| Interaction A × B | n.s | n.s | n.s | n.s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, J.; Silva-Romano, F.; Morar, I.M.; Boscaiu, M.; Santibáñez, C.; Llinares, J.V. Effects of Acetylsalicylic Acid and Biosolids on Edaphic, Vegetative and Biochemical Parameters of Amelichloa caudata Under Water Shortage Conditions. Agronomy 2025, 15, 785. https://doi.org/10.3390/agronomy15040785
Molina J, Silva-Romano F, Morar IM, Boscaiu M, Santibáñez C, Llinares JV. Effects of Acetylsalicylic Acid and Biosolids on Edaphic, Vegetative and Biochemical Parameters of Amelichloa caudata Under Water Shortage Conditions. Agronomy. 2025; 15(4):785. https://doi.org/10.3390/agronomy15040785
Chicago/Turabian StyleMolina, Julio, Fernando Silva-Romano, Irina M. Morar, Monica Boscaiu, Claudia Santibáñez, and Josep V. Llinares. 2025. "Effects of Acetylsalicylic Acid and Biosolids on Edaphic, Vegetative and Biochemical Parameters of Amelichloa caudata Under Water Shortage Conditions" Agronomy 15, no. 4: 785. https://doi.org/10.3390/agronomy15040785
APA StyleMolina, J., Silva-Romano, F., Morar, I. M., Boscaiu, M., Santibáñez, C., & Llinares, J. V. (2025). Effects of Acetylsalicylic Acid and Biosolids on Edaphic, Vegetative and Biochemical Parameters of Amelichloa caudata Under Water Shortage Conditions. Agronomy, 15(4), 785. https://doi.org/10.3390/agronomy15040785







