Abstract
Basic leucine zipper (bZIP) transcription factors play pivotal roles in plant secondary metabolism, influencing the production of bioactive compounds that determine the medicinal value of plants. Despite their significance, a comprehensive genomic overview of bZIPs in non-model medicinal species remains limited. Here, we present the first genome-wide identification and characterization of the bZIP family in Lycium ruthenicum Murr. (black wolfberry), revealing 63 members grouped into 13 subfamilies. These genes showed conserved bZIP domains, distinct exon–intron architectures, and promoter cis-elements related to light, hormones and stress responses. Family expansion occurred through tandem (LrbZIP6-LrbZIP9 cluster) and segmental duplications under purifying selection (Ka/Ks < 1). Collinearity analysis revealed closer relationships with Solanaceae species than Arabidopsis thaliana, with LrbZIP10 and LrbZIP11 as conserved orthologs. Expression profiling identified tissue-specific patterns: LrbZIP17 showed broad expression while LrbZIP14 was fruit-specific. qRT-PCR confirmed floral-preferential (LrbZIP1, LrbZIP10, LrbZIP15, LrbZIP17, LrbZIP50) and root-specific (LrbZIP54, LrbZIP55) expression. The co-occurrence of light/hormone-responsive elements and high LrbZIP expression in anthocyanin-rich tissues suggests their regulatory roles in bioactive compound biosynthesis. This study provides foundational genomic resources for understanding L. ruthenicum bZIP evolution and identifies candidate genes for molecular breeding to enhance medicinal compound production.
1. Introduction
The basic leucine zipper (bZIP) transcription factors represent a highly conserved and multifunctional regulatory gene family that plays pivotal roles in plant growth, development, and environmental adaptation [,]. Characterized by a 60–80 amino acid bZIP domain, these proteins contain two functionally distinct regions: a basic region that specifically recognizes DNA elements containing ACGT core sequences (such as the G-box CACGTG) for target gene binding [,], and a leucine zipper region that mediates protein dimerization through hydrophobic amino acid interactions [,]. This distinctive modular architecture enables bZIP proteins to form diverse homodimers or heterodimers, thereby significantly expanding their functional versatility within transcriptional regulatory networks.
Comparative genomic studies have revealed substantial variation in the bZIP transcription factor family size across plant species, ranging from 45 members in Ziziphus jujuba Mill. to 227 in Triticum aestivum [,]. This remarkable divergence primarily stems from species-specific whole-genome duplication events and localized tandem duplication events, which collectively shape the composition and expansion of bZIP gene families during evolution [,]. From a functional evolutionary perspective, bZIP transcription factors serve as central regulators in plant stress responses by integrating multiple signaling pathways, particularly demonstrating crucial roles in abscisic acid (ABA)-mediated drought and salinity tolerance mechanisms [,,]. Previous studies have identified that the pepper bZIP transcription factor CaADBZ1 (Capsicum annuum ABA and Dehydration-Induced bZIP transcription factor 1) plays a pivotal role in enhancing dehydration stress tolerance by positively regulating ABA sensitivity and dehydration-responsive gene expression []. Similarly, AtbZIP17 and AtbZIP60 confer salt tolerance through the endoplasmic reticulum stress signaling pathway [,], while soybean GmbZIP71-4 increases submergence sensitivity by negatively modulating ABA biosynthesis []. Recent advances have significantly expanded our understanding of bZIP functions, demonstrating their widespread involvement in regulating specialized metabolic pathways in plants. Notably, the photomorphogenesis regulator HY5 (ELONGATED HYPOCOTYL 5) promotes far-red light-induced anthocyanin accumulation by directly activating Myeloblastosis (MYB)-type transcription factors [,]. Furthermore, overexpression of grape VlbZIP30 significantly enhances lignin deposition in grapevines by binding to G-box cis-elements in the promoters of lignin biosynthesis (VvPRX N1) and drought-responsive (VvNAC17) genes, consequently improving cold tolerance []. These groundbreaking discoveries not only broaden the potential applications of bZIP transcription factors in plant metabolic engineering but also provide theoretical frameworks for understanding the molecular mechanisms underlying the co-evolution of environmental adaptation and secondary metabolism in plants.
bZIP transcription factors have been confirmed to regulate the biosynthesis of key secondary metabolites in medicinal plants, such as flavonoids, terpenoids, and alkaloids [,]. These metabolites not only serve as the core material basis for the pharmacological activities of medicinal plants but also provide important support for the formation of their environmental adaptability and resource value. In the process of mediating plant responses to external abiotic stresses, this class of transcription factors can precisely regulate the accumulation of specific bioactive components, thus becoming core targets for deciphering the molecular mechanisms underlying secondary metabolism in medicinal plants and assisting in the breeding of high-quality varieties []. Although the functional research on bZIP transcription factors in model plants such as Arabidopsis thaliana has been relatively thorough, systematic identification and functional characterization of these factors in medicinal species remain scarce []. This has largely restricted the precise improvement of the yield of beneficial secondary metabolites in medicinal plants and the process of breeding elite varieties. Lycium ruthenicum Murr., as an important medicinal plant with both ecological and economic values, its fruits are rich in various bioactive compounds including polysaccharides, anthocyanins (with an average content of 500 mg per 100 g fresh weight), flavonoids, vitamins, and essential amino acids, and possess extremely high medicinal and health-care values [,,]. However, systematic studies on the bZIP gene family in L. ruthenicum are still lacking. Investigating the evolutionary characteristics, expression patterns, and functional association of bZIP genes with bioactive compound biosynthesis will not only enhance our understanding of the regulatory networks underlying plant secondary metabolism but also provide novel targets and strategies for molecular breeding in black wolfberry. In this study, we conducted a genome-wide analysis of the 63 bZIP family members in L. ruthenicum, examining their phylogenetic relationships, gene structures, interspecies collinearity, and tissue-specific expression profiles. Quantitative real-time PCR (qRT-PCR) was employed to determine the relative expression levels of bZIP genes across different tissues, laying the foundation for elucidating the molecular mechanisms by which bZIP transcription factors regulate the biosynthesis of bioactive compounds in black wolfberry.
2. Materials and Methods
2.1. Identification of the LrbZIP Family
Based on previous studies, we acquired the reference genome data of L. ruthenicum (https://doi.org/10.6084/m9.figshare.26550406, accessed on 13 March 2025) []. Using the Hidden Markov Models of the bZIP_1 domain (PF00170) from the Pfam protein database (http://pfam.xfam.org/), we performed a genome-wide scan of L. ruthenicum. with HMMER v3.4 software [] to preliminarily identify candidate members of the bZIP gene family. To enhance the reliability of candidate genes, we further validated these candidates by integrating the conserved domains database (CDD, https://www.ncbi.nlm.nih.gov/cdd/, accessed on 13 March 2025) and the SMART database (http://smart.embl-heidelberg.de/, accessed on 13 March 2025), thereby selecting genes containing complete bZIP domains. These genes were systematically named according to their chromosomal positions. Using the ProtParam module (https://web.expasy.org/protparam/, accessed on 13 March 2025) in Expasy, we comprehensively analyzed the physicochemical properties of all L. ruthenicum bZIP-encoded proteins, including pI, molecular weight, amino acid count, total numbers of positively and negatively charged residues, and GRAVY. Based on the annotation information of all members of the LrbZIP family, we utilized the MG2C v2 tool (http://mg2c.iask.in/mg2c_v2.0/, accessed on 13 March 2025) to generate a distribution map of these genes across the chromosomes, providing a comprehensive visualization of their genomic localization.
2.2. Phylogenetic Tree, Gene Structure, and Conserved Motif Analysis
The protein sequences of A. thaliana were retrieved from the Ensembl Plants database (https://plants.ensembl.org/index.html, accessed on 13 March 2025), and the previously identified bZIP family member sequences of A. thaliana were extracted. Using MEGA 11 software [], multiple sequence alignment of the bZIP protein sequences from L. ruthenicum and A. thaliana was performed, with the ClustalW algorithm employed to optimize the alignment results. A phylogenetic tree was constructed using the Neighbor-Joining (NJ) method, and the reliability of the branches was assessed with 1000 bootstrap replicates. Based on the classification criteria of the A. thaliana bZIP family, the LrbZIP family genes were systematically grouped to reveal their potential evolutionary relationships. For further analysis of conserved domains, the conserved domain sequences were extracted from the aligned protein sequences and visualized using WebLogo 3 (https://weblogo.threeplusone.com/), which graphically illustrates the conservation and variability of amino acid sites within the bZIP domain. Additionally, the annotation information of all LrbZIP family members was extracted, and gene structure diagrams were generated using the gene structure display server (GSDS, https://gsds.gao-lab.org/), allowing for a comparison of gene structure features among different subfamily members. Furthermore, conserved motif analysis was conducted using the MEME online tool v5.5.8 (https://meme-suite.org/meme/, accessed on 13 March 2025), with parameters set to identify a maximum of 10 motifs and a motif width of 6–50 amino acids, systematically uncovering functionally relevant conserved motifs. The 1.5 kb upstream sequences of the coding regions of all LrbZIP family genes were submitted to the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 13 March 2025) for the prediction of cis-acting regulatory elements.
2.3. Gene Replication and Collinearity Analysis
Using MCScanX v1.0.0 software [] combined with BLAST v2.2.20 alignment results, we screened collinear gene pairs based on the following criteria: (1) the alignment length must cover ≥70% of the longer gene sequence; (2) the sequence identity of the aligned regions must be ≥70%; (3) for closely linked gene clusters, only one duplication event was counted to avoid redundant statistical representation. Protein sequences of Capsicum annuum and Solanum lycopersicum were obtained from the Ensembl Plants database (https://plants.ensembl.org/index.html, accessed on 13 March 2025), and orthologous genes of the LrbZIP family across species were identified using InParanoid v4 []. The duplication events and orthologous relationships of the LrbZIP family were visualized with Circos v0.69-9 [], providing an intuitive representation of their genomic distribution and evolutionary connections. Additionally, the Ka and Ks of collinear gene pairs were calculated using PAML v4.3 [] to evaluate the evolutionary selection pressure acting on these gene pairs.
2.4. Gene Expression Profiling
To comprehensively investigate the tissue-specific expression patterns of the bZIP transcription factor family in L. ruthenicum, this study obtained raw RNA-seq data from the national center for biotechnology information (NCBI) database (Project PRJNA505629) encompassing nine critical developmental stages. The dataset includes samples from roots, stems, leaves, flowers, and fruits representing five distinct developmental phases (S1: 9–12 days after the flowering; S2: 14–19 days after the flowering; S3: 20–26 days after the flowering; S4: 30–37 days after the flowering; S5: 34–45 days after the flowering). Quality control of the raw sequencing data was performed using Trimmomatic v0.39 [] to remove low-quality reads and adapter sequences. Subsequently, a reference genome index was constructed using Hisat2 v2.1.0 [], and the quality-controlled sequencing reads were aligned to the reference genome. The alignment results were output in SAM format and then converted to BAM format and sorted using Samtools v1.19.2 []. Following this, transcript assembly and expression quantification were conducted on the sorted BAM files using StringTie v2.2.1 []. Finally, the quantification results were visualized using the R package pheatmap v1.0.12 [] to intuitively display gene expression patterns and their variations across different samples.
2.5. qRT-PCR Analysis
Based on prior transcriptome analysis, this study selected seven candidate genes from the LrbZIP family for quantitative validation. Total RNA was extracted from leaves, flowers, roots, and fruits at five distinct developmental stages using the Eastep® Super Total RNA Extraction Kit (Promega, Madison, WI, USA). Following quality verification, cDNA synthesis was performed with the All-in-One First-Strand Synthesis MasterMix (Yugong Biotech, Jiangsu, China). Quantitative real-time PCR (qRT-PCR) was conducted on the QuantStudioTM 5 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA) using F488 SYBR Green PCR Master Mix (Yugong Biotech, Jiangsu, China). The amplification protocol consisted of an initial denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 3 s and 60 °C for 30 s, with three technical replicates performed for each sample to ensure data reliability. For data analysis, LrEF1-α (GenBank: JX427553.1) served as the reference gene, and relative expression levels of target genes were calculated using the 2−ΔCt method. Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Least Significant Difference (LSD) post hoc tests in the R v4.4.1 software environment, with gene expression levels compared across seven distinct tissue types (root, leaf, flower, and five fruit developmental stages). Data visualization was implemented through the ggplot2 package [].
3. Results
3.1. Identification and Characterization of the bZIP Transcription Factor Family in L. ruthenicum
Using HMMER software, we scanned the entire genome of L. ruthenicum and identified 63 members of the bZIP gene family. By extracting and visualizing the amino acid sequences of the conserved bZIP domains, we found that these domains include a basic DNA-binding region and an adjacent leucine zipper structure, which are highly consistent with those of the A. thaliana bZIP family (Figure S1) []. These structural features are crucial for the dimerization of bZIP proteins. Genomic distribution analysis showed that the 63 LrbZIP genes are distributed across all 12 chromosomes of L. ruthenicum, with chromosome 6 harboring the highest density (10 genes), while chromosomes 4, 5, and 7 contain only 2 genes each (Figure S2, Table S1). The encoded proteins range from 152 amino acids (LrbZIP8 and LrbZIP9) to 849 amino acids (LrbZIP19), with an average length of 373 amino acids. The smallest member, LrbZIP62, has a molecular weight of 17.67 kDa, while the largest member, LrbZIP19, has a molecular weight of 93.15 kDa. The theoretical isoelectric point (pI) ranged from 5.0 (LrbZIP7) to 9.95 (LrbZIP55), indicating that these family members may function under both acidic and alkaline conditions. The grand average of hydropathy (GRAVY) analysis showed that all LrbZIP proteins are hydrophilic, with LrbZIP18 exhibiting the strongest hydrophilicity. Subcellular localization predictions indicated that all LrbZIP family members are localized in the nucleus, consistent with the central role of bZIP transcription factors in gene expression regulation.
3.2. Phylogenetic Classification of LrbZIP Genes
To investigate the classification and evolutionary relationships of the LrbZIP gene family, we constructed a phylogenetic tree using the Neighbor-Joining (NJ) method based on the full-length amino acid sequences of bZIP family members from A. thaliana and L. ruthenicum (Figure 1). Based on phylogenetic analysis and following the established classification framework of the 13 subgroups defined in the A. thaliana bZIP transcription factor family, the LrbZIP members were systematically categorized. Their nomenclature adheres to the conventional designations used for homologous subgroups in Arabidopsis, specifically A to K, M, and S. Among these, subgroup I contained the largest number of members, with 13 genes, while subgroups J and M did not include any LrbZIP members. Additionally, subgroups B, H, and K each contained only one member, specifically LrbZIP4, LrbZIP22, and LrbZIP17, respectively. This classification provides a crucial foundation for further research into the functional divergence and evolutionary mechanisms of the LrbZIP gene family.
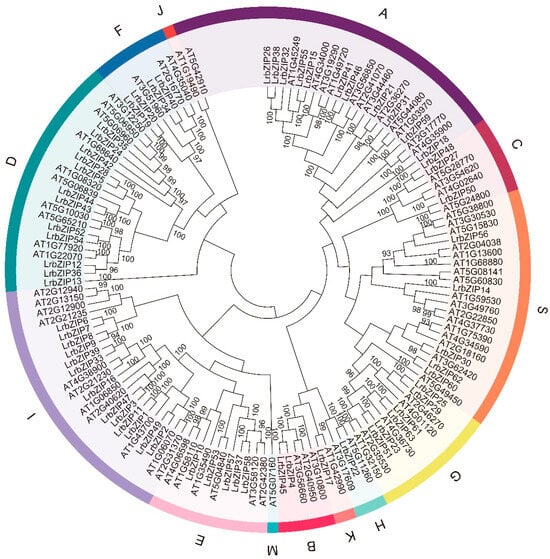
Figure 1.
Unrooted phylogenetic tree of bZIP proteins from L. ruthenicum and A. thaliana. The bZIP genes are classified into subfamilies A–K, M, and S.
3.3. Gene Structure and Motif Analysis of the LrbZIP Family
To elucidate the evolutionary, functional, and regulatory characteristics of the LrbZIP family, we conducted an in-depth analysis of the intron-exon gene structures and conserved motifs of LrbZIP members. The results revealed that LrbZIP24 and LrbZIP35, which contain the highest number of exons, both belong to subgroup D, while all members of subgroup G possess only four exons (Figure 2b). Using the MEME software, we predicted 10 conserved motifs, among which Motif 1 was identified as the bZIP conserved domain and was present in 59 genes. According to the phylogenetic grouping results, members within each subfamily share identical conserved motifs, while certain motifs are unique to specific subfamilies (Figure 2c). For example, motif 8 and motif 10 are exclusively found in subgroup A, whereas the combination of motif 1, motif 9, motif 6, motif 2, motif 5, and motif 3 is unique to subgroup D. These findings indicate that genes within the same subfamily typically exhibit similar intron-exon structures and conserved motif compositions, while significant differences exist between different subfamilies.
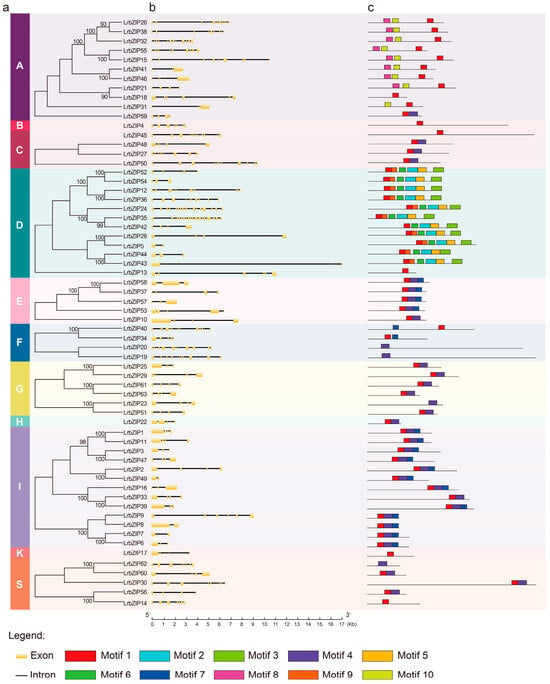
Figure 2.
Phylogenetic relationships, gene structures, and conserved protein motifs of LrbZIP genes. (a) NJ phylogenetic tree of LrbZIP family members. The letters A–I, K, and S represent the subgroup classifications corresponding to the homologous subfamilies previously defined in Arabidopsis thaliana. (b) Exon–intron organization. Yellow boxes represent coding sequences (CDSs), and gray lines between boxes indicate introns. (c) Distribution and composition of conserved protein motifs in LrbZIP members.
3.4. Cis-Acting Elements in LrbZIP Gene Promoters
To gain deeper insights into the functional characteristics of LrbZIP family members, we performed cis-acting element prediction on the 1500 bp upstream promoter regions, identifying various regulatory elements associated with development, tissue specificity, light responsiveness, stress response, and hormone signaling (Table S2). In terms of development and tissue specificity, we detected the AC-I element related to xylem-specific expression, CAT-box and CCGTCC-box elements involved in meristem-specific activation, and the GCN4-motif associated with endosperm expression. Among light-responsive elements, significant enrichment of G-box, GATA-motif, and I-box elements suggests that LrbZIP genes may extensively participate in light signal transduction. For stress responses, we identified LTR and Wun-motif elements related to low-temperature and wound responses, respectively, along with adenosine-uridine-rich sequence elements (AREs) which plays a crucial role in anaerobic induction. Hormone-responsive elements included ABA response elements (ABREs), gibberellin response elements (GAREs, GARE-motif), and CGTCA-motif (methyl jasmonate response). These findings collectively indicate that the LrbZIP gene family likely participates in the regulation of diverse biological processes through a complex network of cis-acting elements.
3.5. Gene Duplication and Collinearity Analysis
To elucidate the expansion mechanisms and evolutionary characteristics of the LrbZIP gene family, we systematically analyzed gene duplication events and conducted cross-species collinearity studies. The duplication analysis revealed a tandemly duplicated gene cluster composed of LrbZIP6, LrbZIP7, LrbZIP8, and LrbZIP9 on chromosome 1, along with one pair of tandem duplications each on chromosomes 10 and 12 (Figure 3). Additionally, four pairs of segmental duplication events were identified, namely LrbZIP5 on chromosome 1 and LrbZIP28 on chromosome 6, LrbZIP12 on chromosome 2 and LrbZIP36 on chromosome 7, as well as LrbZIP33/LrbZIP35 on chromosome 6 and LrbZIP39/LrbZIP42 on chromosome 8. Among these, chromosome 6 showed the highest frequency of segmental duplication events. Calculation of the nonsynonymous substitution rate (Ka)/synonymous substitution rate (Ks) ratios (0.04–0.70) for duplicated gene pairs demonstrated that all pairs were under strong purifying selection (Ka/Ks < 1), indicating functional conservation during evolution (Table S3).
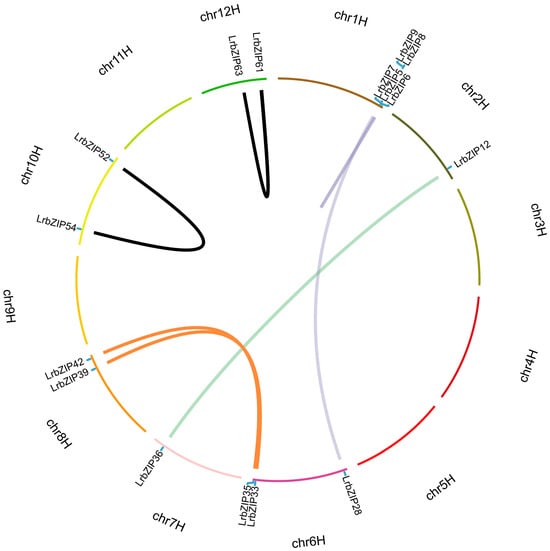
Figure 3.
Chromosomal distribution and gene duplication events of LrbZIP genes.
Further cross-species collinearity analysis identified 31, 36, and 42 orthologous gene pairs between LrbZIPs and C. annuum, S. lycopersicum, and A. thaliana, respectively (Figure 4). Notably, L. ruthenicum showed significantly more orthologous pairs with Solanaceae species than with A. thaliana. Distinct collinearity patterns were observed across chromosomes and species. Specifically, chromosomes 1 and 2 exhibited the most extensive collinearity with C. annuum, while in comparison with S. lycopersicum, besides chromosome 2, chromosome 6 also showed highly significant collinearity. Chromosome 6 consistently demonstrated the most prominent collinearity pattern even in analyses with A. thaliana, suggesting its distinctive conservation features in cross-species comparisons. Remarkably, LrbZIP10 and LrbZIP11 retained conserved orthologs across all three species. All orthologous gene pairs exhibited Ka/Ks values below 1, further validating strong functional constraints acting on the bZIP gene family during evolution (Table S4). These findings not only clarify the molecular mechanisms driving LrbZIP family expansion through tandem and segmental duplications but also reveal its highly conserved evolutionary features within Solanaceae species, providing critical theoretical foundations for functional evolution studies.
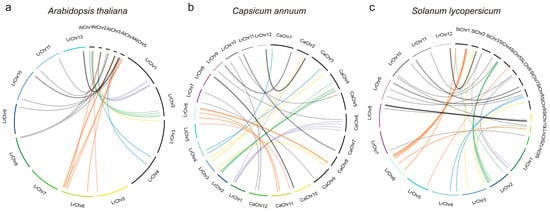
Figure 4.
Synteny analysis of LrbZIP genes with C. annuum, S. lycopersicum, and A. thaliana. (a) A. thaliana (b) C. annuum (c) S. lycopersicum.
3.6. Tissue-Specific Expression Patterns of LrbZIP Genes and qRT-PCR Analysis
To investigate the tissue-specific expression patterns of the LrbZIP gene family, we systematically analyzed the expression profiles of LrbZIP genes using RNA-seq data from roots, stems, leaves, flowers, and five developmental stages of fruits (Figure 5). The results revealed that five genes showed no expression in any tissues (FPKM < 1), while 34.9% of LrbZIP genes were highly expressed across all nine examined tissues. Notably, LrbZIP17 displayed the highest expression levels in root, stem, leaf, fruit s1, fruit s2, and fruit s5, with its expression in flowers being second only to LrbZIP10. Homology analysis identified LrbZIP17 as an ortholog of AtbZIP60 [], a plant-specific RIP-regulated bZIP transcription factor participating in endoplasmic reticulum stress response. Furthermore, LrbZIP14 exhibited specifically high expression during fruit ripening but showed low or no expression in other tissues, suggesting its potential crucial role in the fruit maturation process. These tissue-specific expression patterns provide valuable insights into the functional diversification of LrbZIP gene family during L. ruthenicum growth and development.
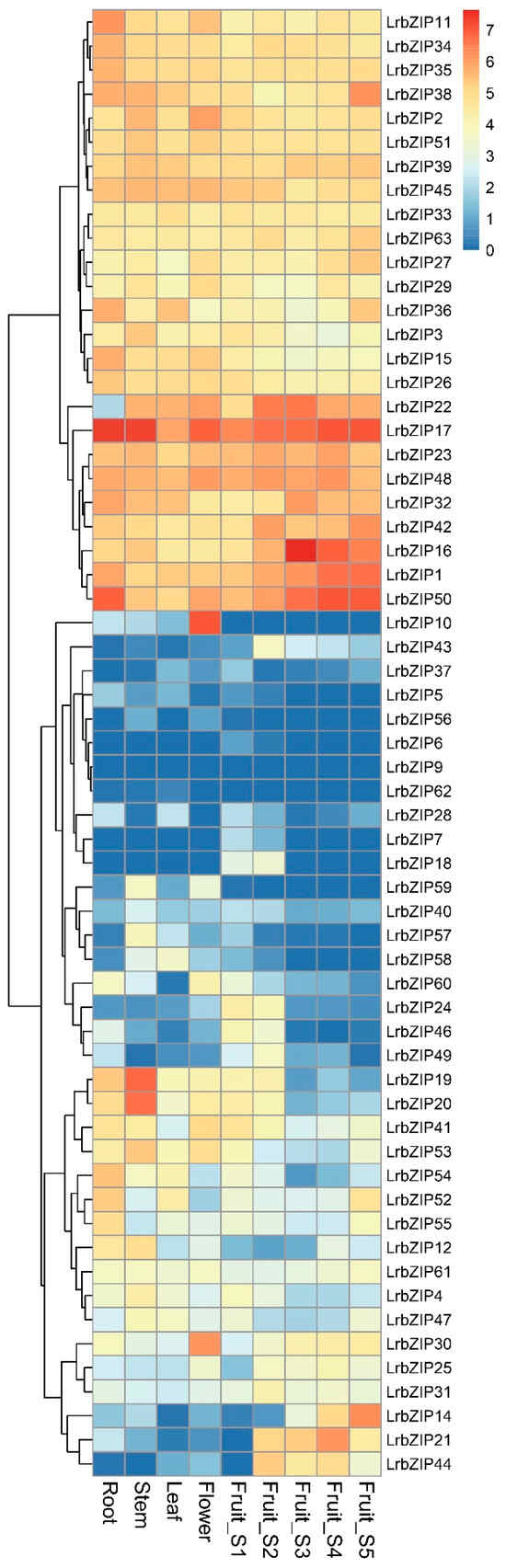
Figure 5.
Expression profiles of LrbZIP genes across different tissues or developmental stages in L. ruthenicum. FPKM values were log2(FPKM + 1) transformed for normalization.
Based on preliminary analysis of transcriptome sequencing data, this study systematically validated the expression patterns of seven LrbZIP transcription factor genes in roots, leaves, flowers, and five developmental stages of fruits using qRT-PCR technology, with LrEF1-α (GenBank: JX427553.1) serving as the reference gene and relative expression levels calculated using the 2−ΔCt method. The experimental results were consistent with the trends observed in the transcriptome data (Figure 6). Notably, LrbZIP1, LrbZIP10, LrbZIP15, LrbZIP17, and LrbZIP50 all exhibited significantly high expression in floral organs, with LrbZIP1 showing the strongest expression level, suggesting that these genes may collectively participate in regulating key biological processes during floral organ development. In contrast, LrbZIP54 and LrbZIP55 displayed distinct tissue-specific high expression patterns in roots. Regarding the dynamic analysis of fruit development, the expression levels of LrbZIP10, LrbZIP15, and LrbZIP54 were generally low or undetectable across the five fruit developmental stages (S1–S5), indicating that they may not be directly involved in fruit development regulation. In comparison, LrbZIP17 and LrbZIP50 maintained relatively stable expression levels during fruit development, implying their potential roles in sustained regulatory functions throughout fruit ripening.
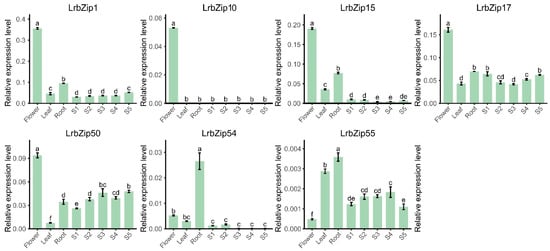
Figure 6.
Expression levels of seven LrbZIP genes in flowers, leaves, roots, and five fruit developmental stages. Lowercase letters above columns indicate significant differences (p < 0.05, LSD test). LSD, least significant difference.
4. Discussion
L. ruthenicum, an important medicinal plant within the Solanaceae family’s Lycium genus, exhibits significantly higher anthocyanin content in its fruits compared to common wolfberry varieties, endowing it with substantial research and application value []. As the primary bioactive compounds, anthocyanins not only determine the characteristic purple-black pigmentation of the fruits but have also emerged as a research focus due to their remarkable pharmacological properties, including antioxidant, anti-inflammatory, and hypolipidemic activities [,]. Current studies have demonstrated that anthocyanin biosynthesis is tightly regulated by a sophisticated, multi-layered control network encompassing transcriptional regulation []. Among various transcription factor families, the basic leucine zipper (bZIP) family has attracted considerable attention owing to its large number of members and functional diversity. Notably, bZIP transcription factors are key regulators of plant antioxidant defense systems. They can be induced by oxidative stress and subsequently activate the expression of both enzymatic antioxidants (e.g., peroxidases) and non-enzymatic antioxidant biosynthesis pathways, thereby enhancing reactive oxygen species (ROS) scavenging capacity and improving plant stress tolerance [,]. Furthermore, bZIP transcription factors play pivotal roles in critical biological processes such as plant growth and development, abiotic stress responses, and secondary metabolite biosynthesis, significantly contributing to plant environmental adaptation and quality trait formation [,].
Genome-wide analysis identified 63 bZIP transcription factor genes in L. ruthenicum This repertoire size is comparatively smaller than documented bZIP family members in other plant species: Solanum lycopersicum (69) [], A. thaliana (78) [], Malus domestica (114) [], and hexaploid T. aestivum (191) []. Such variation likely reflects differential evolutionary selection pressures and functional diversification of this transcription factor family across species. Our analysis revealed that 93% of LrbZIP proteins contain the canonical bZIP domain (Motif 1), comprising both a basic DNA-binding region and a leucine zipper structure. These structural features show high conservation with their Arabidopsis bZIP counterparts, suggesting evolutionary preservation of dimerization and DNA-binding capabilities []. Notably, cis-element analysis of LrbZIP promoters identified significant enrichment of light-responsive elements (e.g., G-box) and hormone-responsive elements (e.g., ABRE). This finding aligns with established reports that anthocyanin biosynthesis is induced by both light and hormones (such as ABA) [,]. Together, these results suggest that LrbZIP transcription factors may regulate anthocyanin accumulation by perceiving and integrating these environmental signals.
Phylogenetic analysis using the neighbor-joining method (bootstrap value = 1000) classified the 63 LrbZIP transcription factors in L. ruthenicum into 13 evolutionary subgroups (A–K, M, S) based on the established classification system for A. thaliana bZIP proteins. Among these, subgroup I was the largest, comprising 13 members (20.6%), whereas subgroups J and M were entirely absent—a distribution pattern distinct from that observed in Arabidopsis []. Gene structure analysis revealed that LrbZIP genes exhibit considerable exon number variation (2–18 exons per gene), with highly conserved exon–intron architectures within each subgroup. Notably, subgroup D was characterized by a unique multi-exon structure. Furthermore, MEME motif analysis identified subgroup-specific conserved motif combinations (e.g., motif8–motif10 in subgroup A), providing strong structural support for the phylogenetic classification. In Arabidopsis, subgroup I members are preferentially expressed in vascular tissues (e.g., cambium and xylem) and are functionally linked to secondary growth regulation [,]. Consistently, our RNA-seq data showed that seven LrbZIPs subgroup I genes exhibited high expression (FPKM > 20) in stem tissues. These findings suggest that this subgroup may play a crucial role in vascular development and functional maintenance in L. ruthenicum, offering novel insights into the molecular mechanisms underlying desert plant adaptation to extreme environments.
The bZIP family is one of the largest known transcription factor families. Tandem duplications (such as the LrbZIP6-9 cluster on chromosome 1) and segmental duplications together have driven the expansion of the LrbZIP family. Consistent with the results in species such as Populus trichocarpa [], Solanum tuberosum [], and Betula platyphylla [], the Ka/Ks values of all duplication events within the bZIP family of L. ruthenicum are less than 1 (ranging from 0.04 to 0.70), indicating a strong purifying selection pressure. This is beneficial for family members to maintain their core functions during evolution. The number of orthologous gene pairs between L. ruthenicum and Solanaceae plants (such as C. annuum and S. lycopersicum) is significantly greater than that between L. ruthenicum and A. thaliana. Moreover, LrbZIP10/11 retains orthologous genes in all three plants. Notably, LrbZIP10 exhibits consistently high expression levels in floral tissues, as confirmed by both transcriptomic analysis and qRT-PCR validation. These genes may have conserved regulatory functions in Solanaceae plants, such as in fruit development or secondary metabolism.
5. Conclusions
In this study, we conducted a comprehensive genome-wide analysis of the bZIP transcription factor family in L. ruthenicum, identifying 63 LrbZIP genes and systematically characterizing their phylogenetic relationships, gene structures, conserved motifs, chromosomal distribution, duplication events, and expression patterns. Our findings provide valuable insights into the evolutionary dynamics and functional diversification of this important gene family in L. ruthenicum.
Phylogenetic analysis classified the LrbZIP family into 13 subgroups, revealing both conserved and lineage-specific features compared to A. thaliana. Gene structure and motif analyses demonstrated subgroup-specific conservation, supporting functional specialization within the family. Notably, promoter analysis identified abundant cis-elements associated with light responsiveness, hormone signaling, and stress adaptation, suggesting that LrbZIP genes may play key roles in environmental adaptation and secondary metabolism regulation.
Gene duplication analysis revealed that both tandem and segmental duplications contributed to LrbZIP family expansion, with strong purifying selection maintaining functional conservation. Collinearity analysis highlighted closer evolutionary relationships between L. ruthenicum and other Solanaceae species (C. annuum, S. lycopersicum) than with A. thaliana, with LrbZIP10 and LrbZIP11 emerging as particularly conserved orthologs.
Expression profiling revealed tissue-specific patterns, with some genes (e.g., LrbZIP17) showing broad expression across multiple tissues, while others (e.g., LrbZIP14) exhibited fruit-specific expression, suggesting specialized roles in fruit development and ripening. The high expression of several LrbZIP genes in flowers implies potential functions in floral organ development.
These results collectively enhance our understanding of bZIP transcription factors in L. ruthenicum and provide a foundation for future functional studies, particularly regarding their roles in anthocyanin biosynthesis and stress responses. The conserved LrbZIP genes identified here represent promising targets for molecular breeding aimed at improving fruit quality and stress resistance in this economically important medicinal plant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15112619/s1, Figure S1. Sequence features of LrbZIP proteins. Figure S2. Chromosomal distribution of LrbZIP genes. Table S1. Protein sequence information of LrbZIP genes identified in this study. Table S2. Characteristics of cis-acting regulatory elements in the promoter regions of LrbZIP genes. Table S3. Segmental and tandem duplication events of LrbZIP genes. Table S4. Ka/Ks ratios of LrbZIP genes in L. ruthenicum compared with A. thaliana, C. annuum, and S. lycopersicum.
Author Contributions
T.L.: conceptualization, writing—original draft, formal analysis, visualization. Z.C.: methodology, software, data curation. C.H.: validation, investigation, resources. L.H., Y.Y.: writing—original draft, formal analysis, visualization. Y.C.: project administration, writing—review and editing; X.N.: writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key Research and Development projects of Ningxia Hui Autonomous Region (Grant Nos. 2022BBF01001 and 2022BBF02008).
Data Availability Statement
The original data presented in the study are openly available in the NCBI Database under accession number PRJNA845109.
Acknowledgments
We are grateful to the High-Performance Computing platform at Northwest A&F University for their assistance with data processing.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABA | Abscisic Acid |
| ABREs | ABA Response Elements |
| ANOVA | Analysis of Variance |
| AREs | Adenosine-uridine-rich Sequence Elements |
| BLAST | Basic Local Alignment Search Tool |
| bZIP | Basic Leucine Zipper |
| CaADBZ1 | Capsicum annuum ABA and Dehydration-Induced bZIP Transcription Factor 1 |
| CDSs | Coding Sequences |
| GAREs | Gibberellin Response Elements |
| GRAVY | Grand Average of Hydropathy |
| HY5 | ELONGATED HYPOCOTYL5 |
| Ka | Nonsynonymous Substitution Rate |
| Ks | Synonymous Substitution Rate |
| LSD | Least Significant Difference |
| MEME | Multiple Em for Motif Elicitation |
| NCBI | National Center for Biotechnology Information |
| NJ | Neighbor-Joining |
| PCR | Polymerase Chain Reaction |
| pI | Isoelectric point |
| qRT-PCR | Quantitative Real-time Reverse Transcription PCR |
| ROS | Reactive Oxygen Species |
References
- Landschulz, W.H.; Johnson, P.F.; McKnight, S.L. The leucine zipper: A hypothetical structure common to a new class of DNA binding proteins. Science 1988, 240, 1759–1764. [Google Scholar] [CrossRef]
- Liang, Y.; Xia, J.; Jiang, Y.; Bao, Y.; Chen, H.; Wang, D.; Zhang, D.; Yu, J.; Cang, J. Genome-wide identification and analysis of bZIP gene family and resistance of TaABI5 (TabZIP96) under freezing stress in wheat (Triticum aestivum). Int. J. Mol. Sci. 2022, 23, 2351. [Google Scholar] [CrossRef]
- Izawa, T.; Foster, R.; Chua, N.-H. Plant bZIP protein DNA binding specificity. J. Mol. Biol. 1993, 230, 1131–1144. [Google Scholar] [CrossRef]
- Jakoby, M.; Weisshaar, B.; Dröge-Laser, W.; Vicente-Carbajosa, J.; Tiedemann, J.; Kroj, T.; Parcy, F. bZIP transcription factors in Arabidopsis. Trends Plant Sci. 2002, 7, 106–111. [Google Scholar] [CrossRef]
- Kouzarides, T.; Ziff, E. Leucine zippers of fos, jun and GCN4 dictate dimerization specificity and thereby control DNA binding. Nature 1989, 340, 568–571. [Google Scholar] [CrossRef]
- Dröge-Laser, W.; Snoek, B.L.; Snel, B.; Weiste, C. The Arabidopsis bZIP transcription factor family—An update. Curr. Opin. Plant Biol. 2018, 45, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, W.; Li, H.; Wang, Y.; Li, D.; Xue, C.; Liu, Z.; Liu, M.; Zhao, J. Genome-wide analysis of the bZIP gene family in Chinese jujube (Ziziphus jujuba Mill.). BMC Genom. 2020, 21, 483. [Google Scholar] [CrossRef] [PubMed]
- Nijhawan, A.; Jain, M.; Tyagi, A.K.; Khurana, J.P. Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiol. 2008, 146, 333. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, D.; Jia, L.; Huang, X.; Ma, G.; Wang, S.; Zhu, M.; Zhang, A.; Guan, M.; Lu, K. Genome-wide identification and structural analysis of bZIP transcription factor genes in Brassica napus. Genes 2017, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Fujita, M.; Satoh, R.; Maruyama, K.; Parvez, M.M.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 2005, 17, 3470–3488. [Google Scholar] [CrossRef]
- Zong, W.; Tang, N.; Yang, J.; Peng, L.; Ma, S.; Xu, Y.; Li, G.; Xiong, L. Feedback regulation of ABA signaling and biosynthesis by a bZIP transcription factor targets drought-resistance-related genes. Plant Physiol. 2016, 171, 2810–2825. [Google Scholar] [CrossRef]
- Liu, H.; Tang, X.; Zhang, N.; Li, S.; Si, H. Role of bZIP transcription factors in plant salt stress. Int. J. Mol. Sci. 2023, 24, 7893. [Google Scholar] [CrossRef]
- Choi, J.; Lim, C.W.; Lee, S.C. Role of pepper bZIP transcription factor CaADBZ1 in abscisic acid signalling and drought stress response. Physiol. Plant. 2025, 177, e70159. [Google Scholar] [CrossRef] [PubMed]
- LIU, J.X.; Srivastava, R.; Howell, S.H. Stress-induced expression of an activated form of AtbZIP17 provides protection from salt stress in Arabidopsis. Plant Cell Environ. 2008, 31, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Koizumi, N. An Arabidopsis transcription factor, AtbZIP60, regulates the endoplasmic reticulum stress response in a manner unique to plants. Proc. Natl. Acad. Sci. USA 2005, 102, 5280–5285. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Huo, X.; Xu, J.; Li, Y.; Zhu, H.; Yu, Y.; Tang, L.; Wang, X. A soybean bZIP transcription factor is involved in submergence resistance. Biochem. Biophys. Res. Commun. 2024, 722, 150151. [Google Scholar] [CrossRef]
- Han, H.; Wang, C.; Yang, X.; Wang, L.; Ye, J.; Xu, F.; Liao, Y.; Zhang, W. Role of bZIP transcription factors in the regulation of plant secondary metabolism. Planta 2023, 258, 13. [Google Scholar] [CrossRef]
- Liu, C.C.; Chi, C.; Jin, L.J.; Zhu, J.; Yu, J.Q.; Zhou, Y.H. The bZip transcription factor HY5 mediates CRY1a-induced anthocyanin biosynthesis in tomato. Plant Cell Environ. 2018, 41, 1762–1775. [Google Scholar] [CrossRef]
- Tu, M.; Wang, X.; Yin, W.; Wang, Y.; Li, Y.; Zhang, G.; Li, Z.; Song, J.; Wang, X. Grapevine VlbZIP30 improves drought resistance by directly activating VvNAC17 and promoting lignin biosynthesis through the regulation of three peroxidase genes. Hortic. Res. 2020, 7, 150. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, X.; Lv, Z.; Lu, X.; Shen, Q.; Zhang, L.; Zhu, M.; Wang, G.; Sun, X.; Liao, Z. A basic leucine zipper transcription factor, AabZIP1, connects abscisic acid signaling with artemisinin biosynthesis in Artemisia annua. Mol. Plant 2015, 8, 163–175. [Google Scholar] [CrossRef]
- Chang, C.; Liu, Z.; Wang, Y.; Tang, Z.; Yu, F. A bZIP transcription factor, CaLMF, mediated light-regulated camptothecin biosynthesis in Camptotheca acuminata. Tree Physiol. 2019, 39, 372–380. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Zhang, L.; Li, Y.; Duan, J.A. Lycium ruthenicum studies: Molecular biology, Phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, B.; Wen, H.; Tao, Y.; Shao, Y. Phytochemical profiles, nutritional constituents and antioxidant activity of black wolfberry (Lycium ruthenicum Murr.). Ind. Crops Prod. 2020, 154, 112692. [Google Scholar] [CrossRef]
- Zheng, J.; Ding, C.; Wang, L.; Li, G.; Shi, J.; Li, H.; Wang, H.; Suo, Y. Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Food Chem. 2011, 126, 859–865. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Shi, T.; Luo, Q.; Chen, Y.; Guo, S.; Tian, W.; An, W.; Zhao, J.; Yin, Y. High-quality genome of black wolfberry (Lycium ruthenicum Murr.) provides insights into the genetics of anthocyanin biosynthesis regulation. Hortic. Res. 2025, 12, uhae298. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- O’brien, K.P.; Remm, M.; Sonnhammer, E.L. Inparanoid: A comprehensive database of eukaryotic orthologs. Nucleic Acids Res. 2005, 33, D476–D480. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Kovaka, S.; Zimin, A.V.; Pertea, G.M.; Razaghi, R.; Salzberg, S.L.; Pertea, M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019, 20, 278. [Google Scholar] [CrossRef]
- Kolde, R.; Kolde, M.R. Package ‘pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Wickham, H. Data analysis. In ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016; pp. 189–201. [Google Scholar]
- Iwata, Y.; Fedoroff, N.V.; Koizumi, N. Arabidopsis bZIP60 is a proteolysis-activated transcription factor involved in the endoplasmic reticulum stress response. Plant Cell 2008, 20, 3107–3121. [Google Scholar] [CrossRef]
- Liu, P.; Li, W.; Hu, Z.; Qin, X.; Liu, G. Isolation, purification, identification, and stability of anthocyanins from Lycium ruthenicum Murr. LWT—Food Sci. Technol. 2020, 126, 109334. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, C.; Zhong, W.; Shu, Y.; Zhang, Y.; Yang, D. Antibacterial effect and mechanism of anthocyanin from Lycium ruthenicum Murr. Front. Microbiol. 2022, 13, 974602. [Google Scholar] [CrossRef]
- Tao, L.; Hao, F.; Fei, P.; Chen, D.; Fan, H.; Zhao, S.; Wang, Y.; Li, B.; Ma, Y.; Zhao, X. Advance on Traditional Uses, Phytochemistry and Pharmacology of Lycium ruthenicum Murr. Pharm. Chem. J. 2022, 56, 844–861. [Google Scholar] [CrossRef]
- Sunil, L.; Shetty, N.P. Biosynthesis and regulation of anthocyanin pathway genes. Appl. Microbiol. Biotechnol. 2022, 106, 1783–1798. [Google Scholar] [CrossRef]
- Yang, S.; Xu, K.; Chen, S.; Li, T.; Xia, H.; Chen, L.; Liu, H.; Luo, L. A stress-responsive bZIP transcription factor OsbZIP62 improves drought and oxidative tolerance in rice. BMC Plant Biol. 2019, 19, 260. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Kim, H.; Shim, D.; Jang, S.; Yamaoka, Y.; Shin, S.; Yamano, T.; Kajikawa, M.; Jin, E.; Fukuzawa, H. The Chlamydomonas bZIP transcription factor BLZ8 confers oxidative stress tolerance by inducing the carbon-concentrating mechanism. Plant Cell 2022, 34, 910–926. [Google Scholar] [CrossRef]
- Chen, M.; Cao, X.; Huang, Y.; Zou, W.; Liang, X.; Yang, Y.; Wang, Y.; Wei, J.; Li, H. The bZIP transcription factor MpbZIP9 regulates anthocyanin biosynthesis in Malus ‘Pinkspire’ fruit. Plant Sci. 2024, 342, 112038. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Sotomayor, P.; Chávez Montes, R.A.; Silvestre-Vañó, M.; Herrera-Ubaldo, H.; Greco, R.; Pablo-Villa, J.; Galliani, B.M.; Diaz-Ramirez, D.; Weemen, M.; Boutilier, K. Altered expression of the bZIP transcription factor DRINK ME affects growth and reproductive development in Arabidopsis thaliana. Plant J. 2016, 88, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fu, F.; Zhang, H.; Song, F. Genome-wide systematic characterization of the bZIP transcriptional factor family in tomato (Solanum lycopersicum L.). BMC Genom. 2015, 16, 771. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Meng, D.; Li, M.; Cheng, L. Genome-wide identification and expression analysis of the bZIP gene family in apple (Malus domestica). Tree Genet. Genomes 2016, 12, 82. [Google Scholar] [CrossRef]
- An, X.; Tan, T.; Zhang, X.; Guo, X.; Zhu, Y.; Song, Z.; Wang, D. Effects of light intensity on endogenous hormones and key enzyme activities of anthocyanin synthesis in blueberry leaves. Horticulturae 2023, 9, 618. [Google Scholar] [CrossRef]
- Li, Z.; Ahammed, G.J. Hormonal regulation of anthocyanin biosynthesis for improved stress tolerance in plants. Plant Physiol. Biochem. 2023, 201, 107835. [Google Scholar] [CrossRef]
- Pyo, H.; Demura, T.; Fukuda, H. Vascular cell expression patterns of Arabidopsis bZIP group I genes. Plant Biotechnol. 2006, 23, 497–501. [Google Scholar] [CrossRef]
- Zhao, K.; Chen, S.; Yao, W.; Cheng, Z.; Zhou, B.; Jiang, T. Genome-wide analysis and expression profile of the bZIP gene family in poplar. BMC Plant Biol. 2021, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, K.; Bahramnejad, B.; Fatemi, S. Genome-wide identification and characterization of the bZIP gene family in potato (Solanum tuberosum). Plant Gene 2020, 24, 100257. [Google Scholar] [CrossRef]
- Dong, W.; Xie, Q.; Liu, Z.; Han, Y.; Wang, X.; Xu, R.; Gao, C. Genome-wide identification and expression profiling of the bZIP gene family in Betula platyphylla and the functional characterization of BpChr04G00610 under low-temperature stress. Plant Physiol. Biochem. 2023, 198, 107676. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).