Impact of Pre- and Post-Emergence Herbicides on Controlling Predominant Weeds at Late-Rainy Season Sugarcane Plantations in Northeastern Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
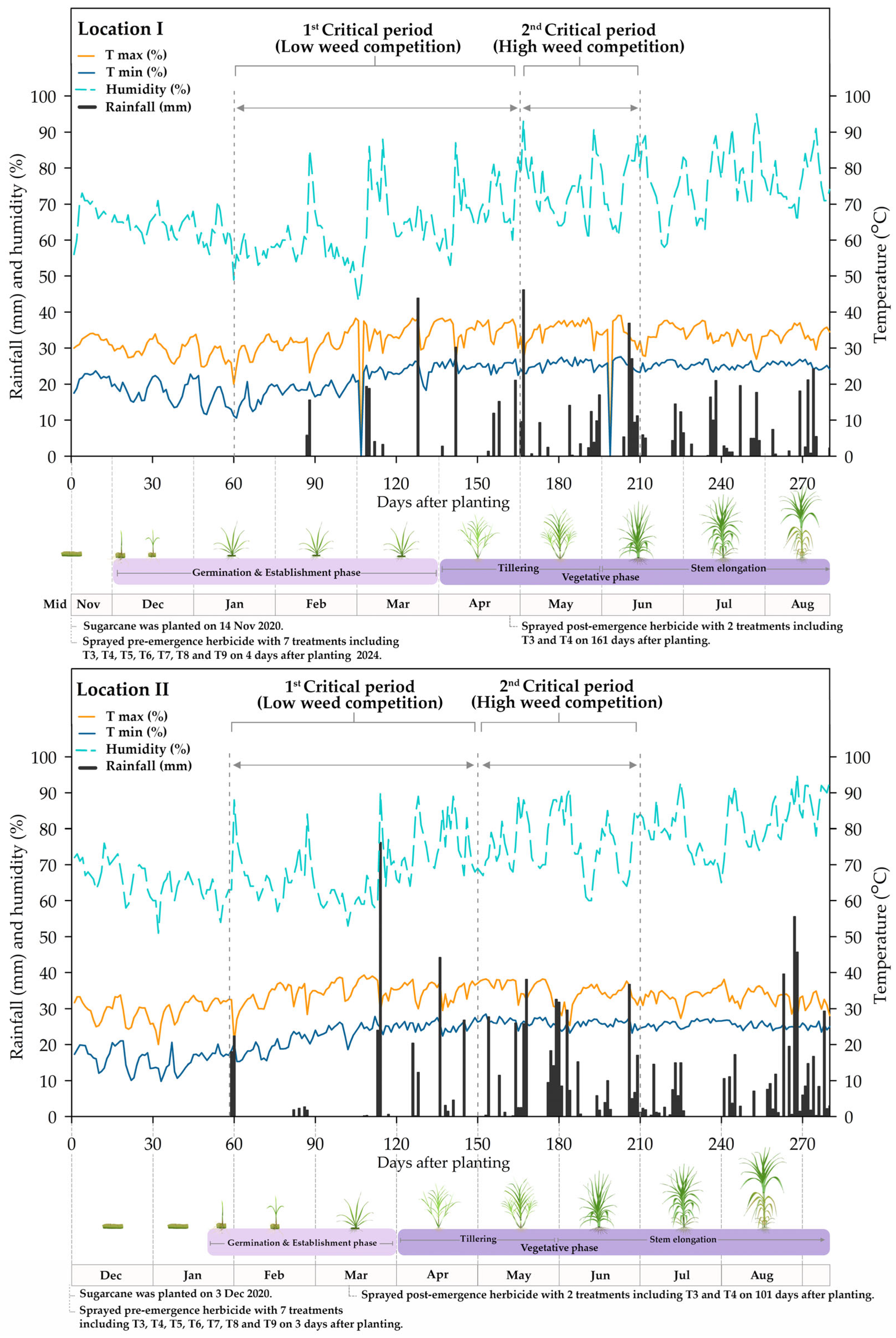
2.2. Weather Data
2.3. Experimental Design and Plant Material
2.4. Data Collection
2.4.1. Weed Data
2.4.2. Herbicide Phytotoxicity of Sugarcane
2.4.3. Yield and Yield Components
2.4.4. Production Costs and Net Profit
2.5. Statistical Analysis
3. Results
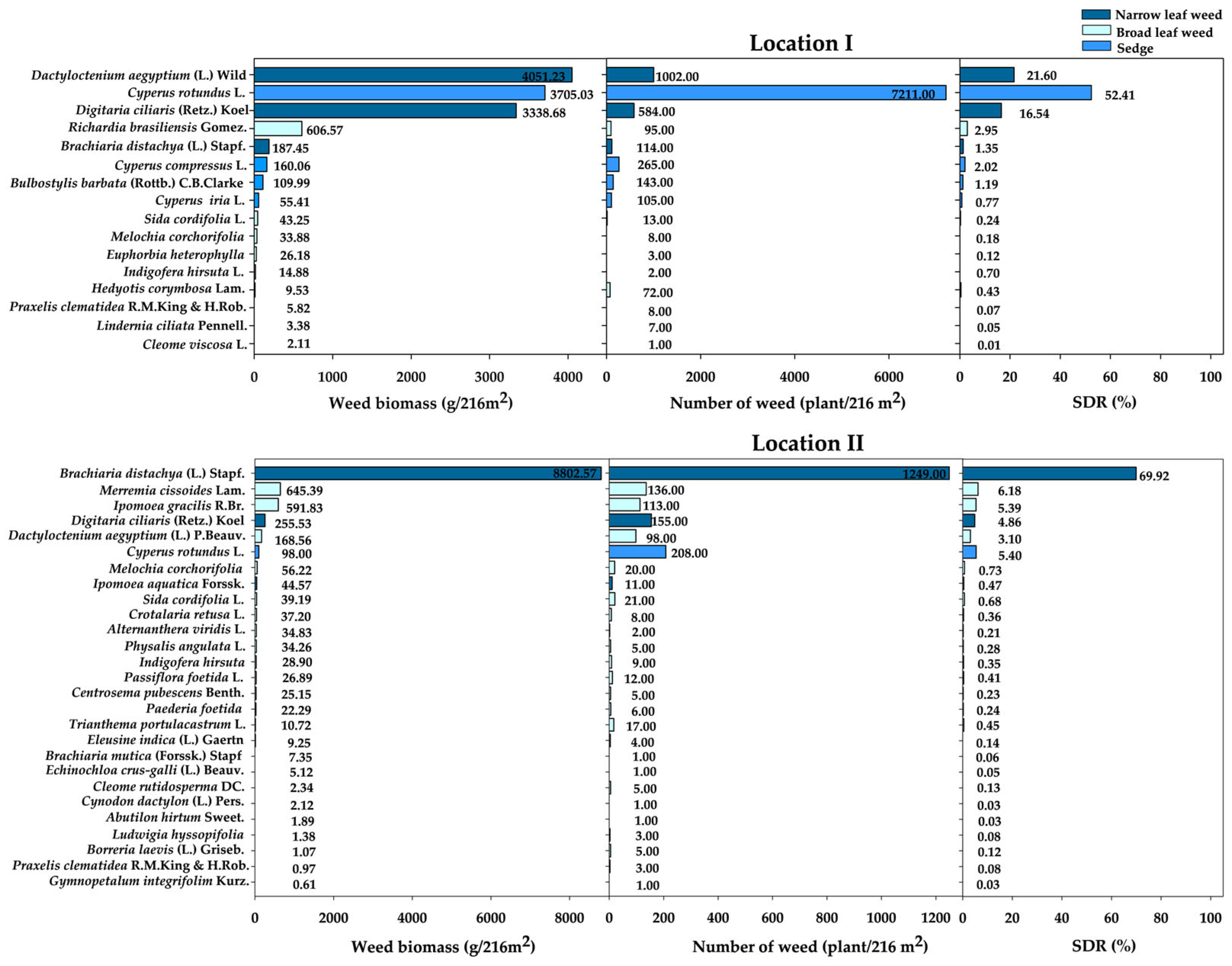
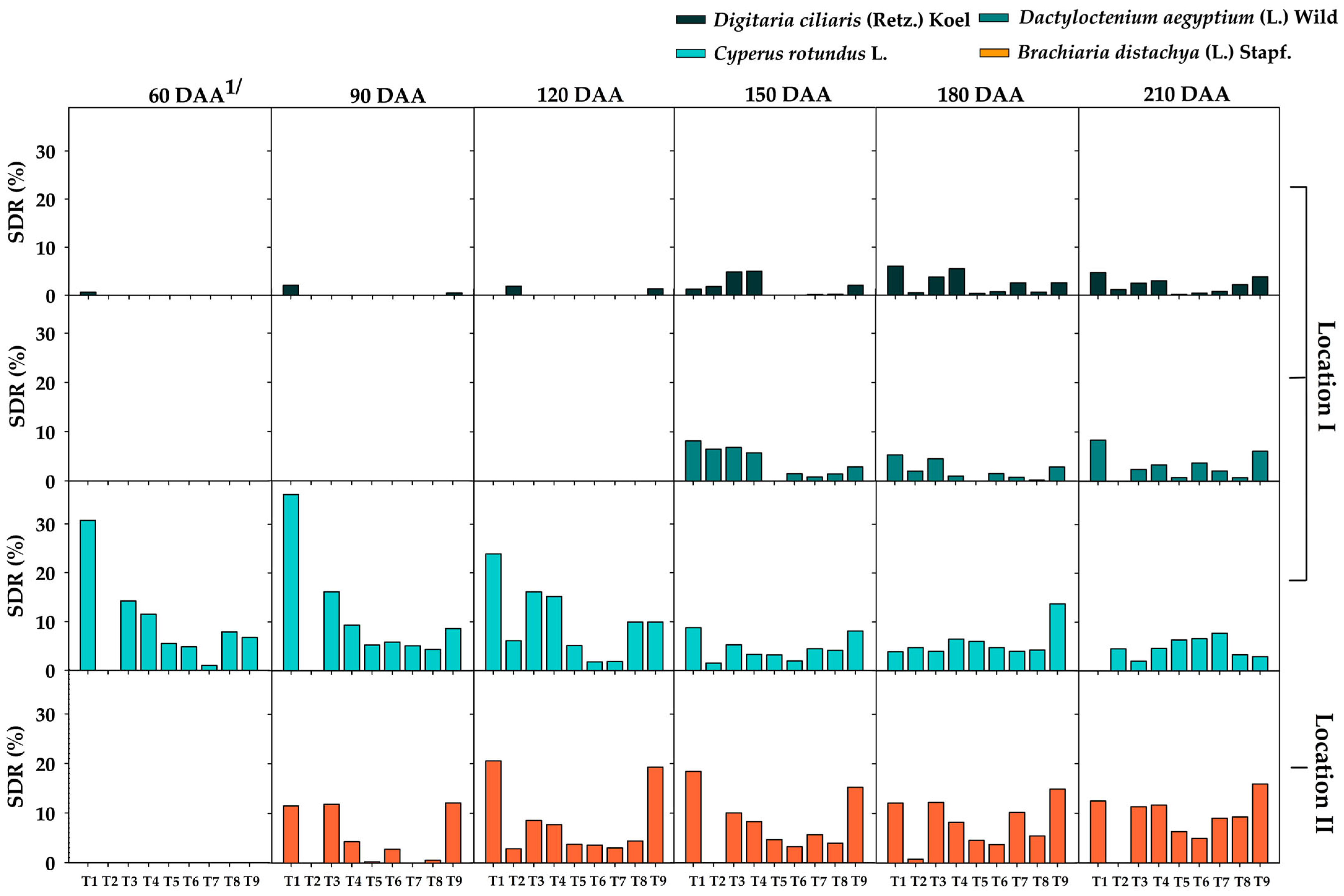
3.1. Weed Species, Abundance, and Summed Dominance Ratio (SDR)
The Predominant Weed Species in Locations I and II
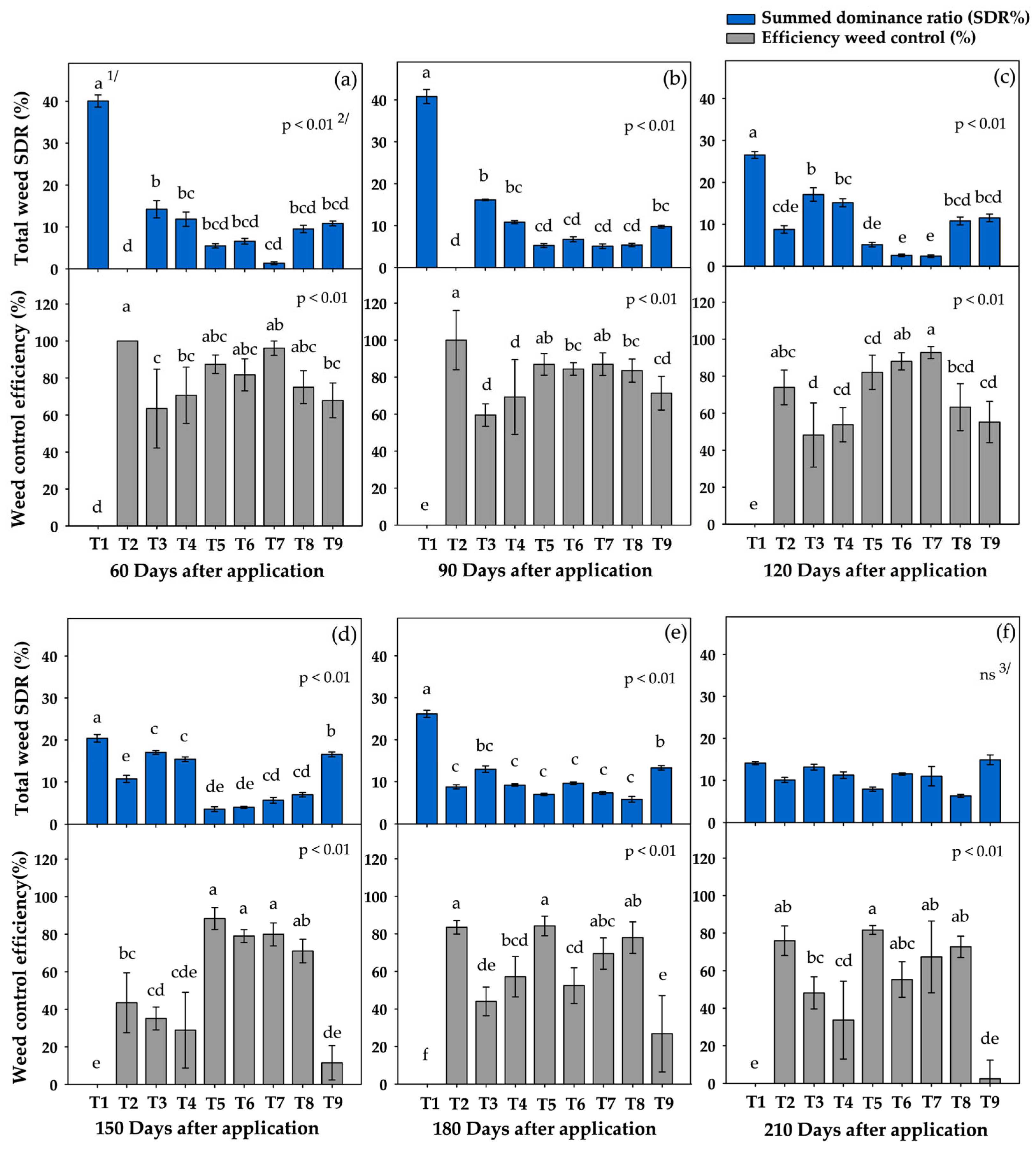
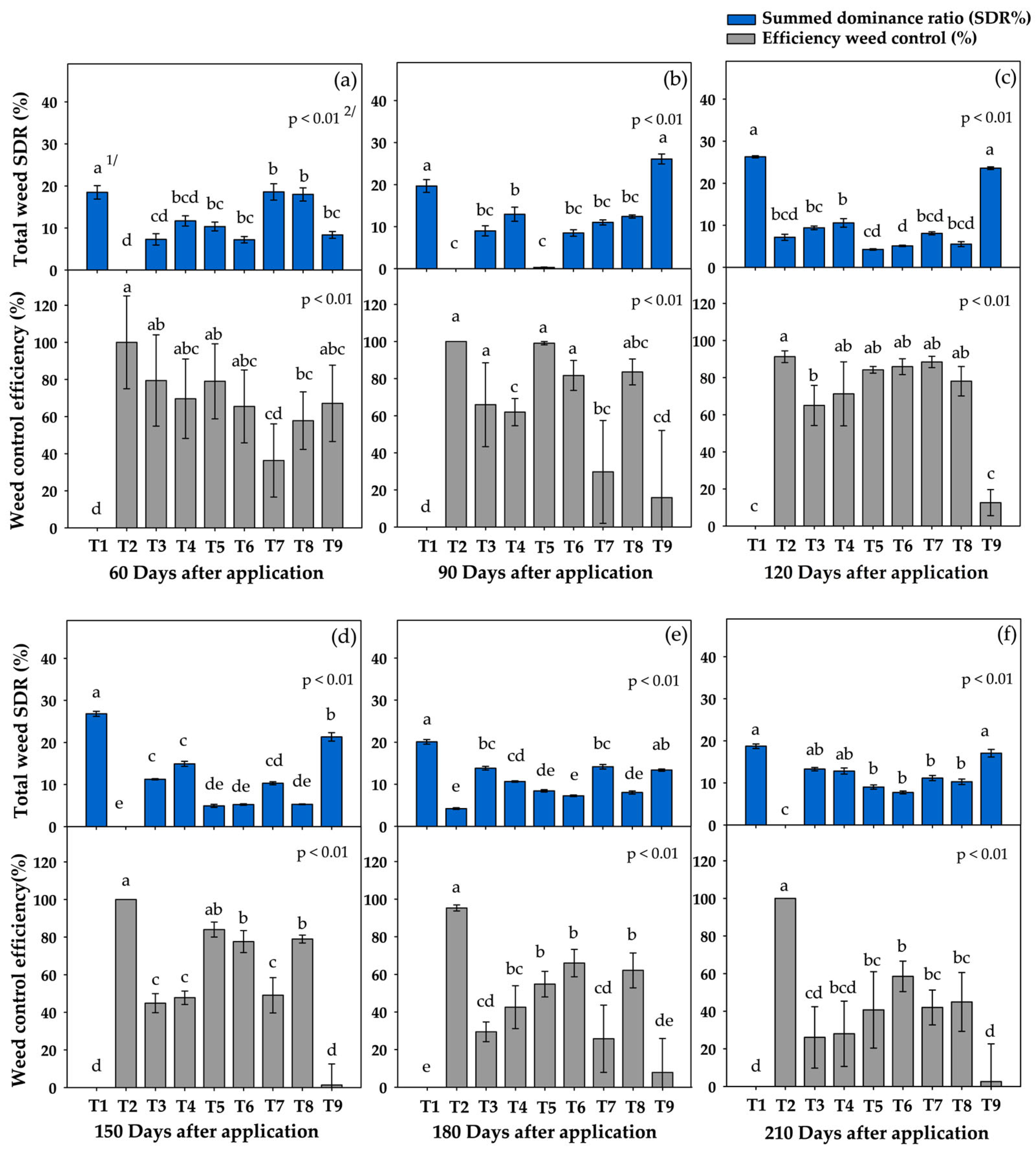
3.2. Weed Control Efficiency and SDR of Total Weeds
3.3. Summed Dominance Ratio (SDR) During the Critical Period
3.4. Herbicide Phytotoxicity to Sugarcane
3.5. Sugarcane Yield, Yield Components, and Economics
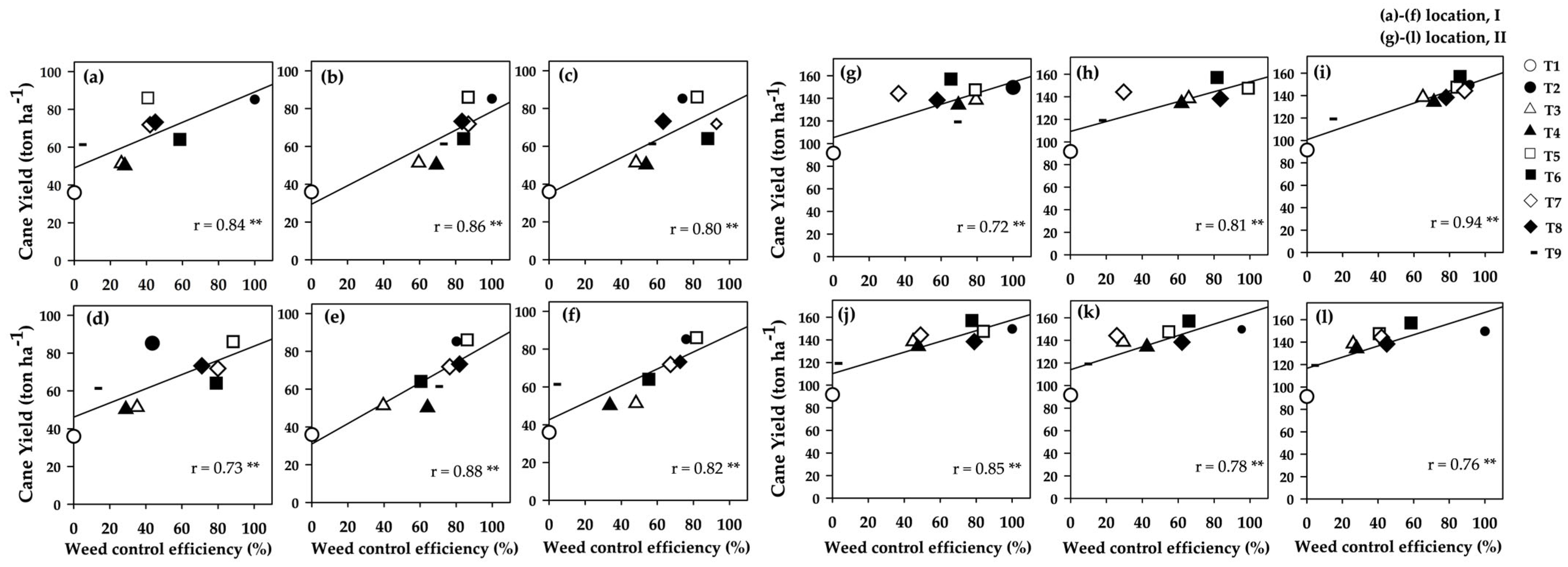
3.6. Correlations Between Weed Control Efficiency and Sugarcane Yield
4. Discussion
4.1. Dominant Weeds in Sugarcane Fields
4.2. Assessment of Weed Control Effectiveness
4.3. Toxicity of Herbicides to Sugarcane
4.4. Crop Yield and Its Components
4.4.1. Difference in Sugarcane Yield Between Locations I and II
4.4.2. Impact of Weed Control on Sugarcane Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, P.A.; Virdia, H.M.; Parmar, V.T.; Patel, V.D.; Patel, D.K. Effect of weed management on weeds and yield of sugarcane (Saccharum Hy. sp.) under South Gujarat condition. J. Pharm. Innov. 2024, 13, 13–18. [Google Scholar]
- Mobarak, O.M.M.; Zohdy, N.O.M.; Abdelaziz, Y.M. Impact of Sugarcane (Saccharum officinarum L.) Genotypes and Different Weed Control Treatments on Weeds, Sugarcane Productivity and Quality. Environ. Sci. 2019, 19, 93–105. [Google Scholar] [CrossRef]
- Zimdahl, R.L. Fundamentals of Weed Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Boston, MA, USA, 2007. [Google Scholar]
- Yang, W.; Li, Z.; Wang, J.; Wu, P.; Zhang, Y. Crop Yield, Nitrogen Acquisition and Sugarcane Quality as Affected by Interspecific Competition and Nitrogen Application. Field Crops Res. 2013, 146, 44–50. [Google Scholar] [CrossRef]
- Christina, M.; Chevalier, L.; Viaud, P.; Schwartz, M.; Chetty, J.; Ripoche, A.; Versini, A.; Jourdan, C.; Auzoux, S.; Mansuy, A. Intercropping and Weed Cover Reduce Sugarcane Roots Colonization in Plant Crops as a Result of Spatial Root Distribution and the Co-Occurrence of Neighboring Plant Species. Plant Soil 2025, 506, 39–55. [Google Scholar] [CrossRef]
- Guerra, S.P.S.; Denadai, M.S.; Spadim, E.R. Sugarcane Biorefinery, Technology and Perspectives. In Sugarcane: Biorefinery, Technology, and Perspectives; Santos, F., Rabelo, S.C., de Matos, M., Eichlier, P., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 49–65. [Google Scholar] [CrossRef]
- Office of the Cane and Sugar Board. Sugarcane Plantation Areas in Thailand Crop Year 2024/25 Report. Office of the Cane and Sugar Board, Ministry of Industry. Crops and Livestock Products. Available online: https://www.ocsb.go.th/wp-content/uploads/2023/06 (accessed on 24 May 2025).
- Sedtha, S.; Pramanik, M.; Szabo, S.; Wilson, K.; Park, K.S. Climate Change Perception and Adaptation Strategies to Multiple Climatic Hazards: Evidence from the Northeast of Thailand. J. Environ. Dev. 2023, 48, 100906. [Google Scholar] [CrossRef]
- Khumla, N.; Sakuanrungsirikul, S.; Punpee, P.; Hamarn, T.; Chaisan, T.; Soulard, L.; Songsri, P. Sugarcane Breeding, Germplasm Development and Supporting Genetics Research in Thailand. Sugar Tech 2022, 24, 193–209. [Google Scholar] [CrossRef]
- Singh, M.; Thapa, R.; Kukal, M.S.; Irmak, S.; Mirsky, S.; Jhala, A.J. Effect of Water Stress on Weed Germination, Growth Characteristics, and Seed Production: A Global Meta-Analysis. Weed Sci. 2022, 70, 621–640. [Google Scholar] [CrossRef]
- Qaderi, M.M. Environmental Regulation of Weed Seed Dormancy and Germination. Seeds 2023, 2, 259–277. [Google Scholar] [CrossRef]
- Hirani, A.K.; Korav, S.; Rajanna, G.A.; Elansary, H.O.; Mahmoud, E.A. Determination of Critical Crop-Weed Competition Period: Impact on Growth, Nutrient Dynamics and Productivity of Green Gram (Vigna radiata). Heliyon 2024, 10, e36855. [Google Scholar] [CrossRef]
- Temel, N.; Torun, H.; Tangolar, S.; Tangolar, S.; Karaman, Y.; Tursun, N. The Effects of Organic and Inorganic Mulching on Weed Flora and Composition in Irrigated and Non-Irrigated Vineyards. Weed Res. 2025, 65, e70020. [Google Scholar] [CrossRef]
- Beluci, L.R.; Bacha, A.L.; Barroso, A.A.M.; Alves, P.L.C.A. One-Eye-Set Sugarcane Susceptibility to Weed Interference. An. Acad. Bras. Ciênc. 2018, 90, 3513–3523. [Google Scholar] [CrossRef]
- Reis, F.C.; Victória Filho, R.; Andrade, M.T.; Barroso, A.A.M. Use of Herbicides in Sugarcane in the São Paulo State. Planta Daninha 2019, 37, e019184227. [Google Scholar] [CrossRef]
- Hadary, M.H.; Chung, G. Herbicides—A Double Edged Sword. In Herbicides—Current Research and Case Studies in Use; Price, A.J., Kelton, J.A., Eds.; InTech: Rijeka, Croatia, 2013; pp. 621–652. [Google Scholar] [CrossRef][Green Version]
- Duke, S.O. Overview of Herbicide Mechanisms of Action. Environ. Health Perspect. 1990, 87, 263. [Google Scholar] [CrossRef] [PubMed]
- Janiya, J.D.; Moody, K. Weed Populations in Transplanted and Wet—Seeded Rice as Affected by Weed Control Method. Trop. Pest Manag. 1989, 35, 8–11. [Google Scholar] [CrossRef]
- Aekrathok, P.; Songsri, P.; Jongrungklang, N.; Gonkhamdee, S. Efficacy of Post-Emergence Herbicides Against Important Weeds of Sugarcane in North-East Thailand. Agronomy 2021, 11, 429. [Google Scholar] [CrossRef]
- Jantho, L. Impact of Thailand’s Import Bans of the Three Agricultural Subtraces; Paraquat, Chlorpyrifos, Glyphosate to the Thai Agricultural Industry and Compliance with Article 20 of the General Agreement on Tariffs and Trade. Huachiew Chalermprakiet Law J. 2024, 14, 56–70. [Google Scholar]
- Takano, H.K.; Beffa, R.; Preston, C.; Westra, P.; Dayan, F.E. A Novel Insight into the Mode of Action of Glufosinate: How Reactive Oxygen Species Are Formed. Photosynth. Res. 2020, 144, 361–372. [Google Scholar] [CrossRef]
- Simon, W.R. Impact of Glufosinate on Sugarcane Yield and Yield Components When Applied Post-Directed and Its Efficacy in Controlling Emerged Itchgrass (Rottboellia cochinchinensis). Master’s Thesis, Louisiana State University and Agricultural and Mechanical College, Louisiana, LA, USA, 2025. [Google Scholar]
- Shaner, D.L. Herbicide Mechanism of Action. In Herbicide Handbook, 10th ed.; Wilcut, J., LeBaron, H., Eds.; Weed Science Society of America: Lawrence, KS, USA, 2014; pp. 1–514. [Google Scholar]
- Guerra, N.; Oliveira Jr, R.S.; Constantin, J.; Oliveira Neto, A.M.; Puton, G.; Garrido, T.H.P. Influence of Precipitation and Sugarcane Straw in Aminocyclopyrachlor and Indaziflam Control Efficiency. Planta Daninha 2015, 33, 535–542. [Google Scholar] [CrossRef]
- Ghirardello, G.A.; Araújo, L.D.S.; Baccin, L.C.; Dotta, M.A.; Souza, R.O.; Silva, A.F.M.; Silva, G.S.D.; Victoria Filho, R. Selectivity Index of Indaziflam to Sugarcane Cv. IACSP95-5000 in Two Soil Textures. Rev. Fac. Nac. Agron. Medellín 2021, 74, 9530–9539. [Google Scholar] [CrossRef]
- Sebastian, D.J.; Nissen, S.J.; Westra, P.; Shaner, D.L.; Butters, G. Influence of Soil Properties and Soil Moisture on the Efficacy of Indaziflam and Flumioxazin on Kochia scoparia L. Pest Manag. Sci. 2017, 73, 444–451. [Google Scholar] [CrossRef]
- Onwuchekwa-Henry, C.B.; Coe, R.; Ogtrop, F.V.; Roche, R.; Tan, D.K.Y. Efficacy of Pendimethalin Rates on Barnyard Grass (Echinochloa crus-galli (L.) Beauv) and Their Effect on Photosynthetic Performance in Rice. Agronomy 2023, 13, 582. [Google Scholar] [CrossRef]
- Alshallash, K.S. Effect of Pendimethalin, Trifluralin, and Terbutryn on Lolium Multiflorum Growing with Barley during Pre-Emergence Stage. Ann. Agric. Sci. 2014, 59, 239–242. [Google Scholar] [CrossRef]
- de Oliveira, G.F.P.B.; Langaro, A.C.; Araujo, A.L.S.; Pimpinato, R.F.; Tornisielo, V.L.; de Pinho, C.F. Sorption and Desorption of Pendimethalin Alone and Mixed with Adjuvant in Soil and Sugarcane Straw. J. Environ. Sci. Health B 2020, 55, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.D.; Kim, H.J.; Chung, J.B.; Jeong, B.R. Loss of Pendimethalin in Runoff and Leaching from Turfgrass Land Under Simulated Rainfall. J. Agric. Food Chem. 2000, 48, 5376–5382. [Google Scholar] [CrossRef]
- Yakadri, M.; Rani, P.L.; Prakash, T.R.; Madhavi, M.; Mahesh, N. Weed Management in Zero Till-Maize. Indian J. Weed Sci. 2015, 47, 240–245. [Google Scholar]
- Negrisoli, R.M.; Odero, D.C.; MacDonald, G.E.; Sellers, B.A.; Laughinghouse, H.D. Sugarcane response and fall panicum (Panicum dichotomiflorum) control with topramezone and triazine herbicides. Weed Technol. 2020, 34, 241–249. [Google Scholar] [CrossRef]
- Rathika, S.; Ramesh, T.; Jagadeesan, R. Weed Management in Sugarcane: A Review. Pharma Innov. 2023, 12, 3883–3887. [Google Scholar]
- Brabham, C.; Lei, L.; Gu, Y.; Stork, J.; Barrett, M.; DeBolt, S. Indaziflam Herbicidal Action: A Potent Cellulose Biosynthesis Inhibitor. Plant Physiol. 2014, 166, 1177–1185. [Google Scholar] [CrossRef]
- Brunharo, C.A.D.C.G.; Carrijo, D.R.; Obara, F.E.B.; Nicolai, M.; Melo, M.S.C.D.; Sousa, P.R.D., Jr.; Christoffoleti, P.J. Eficácia Dos Herbicidas Imazapic e Amicarbazone Aplicado Em Diferentes Quantidades de Palha de Cana-de-Açúcar Para o Controle de Plantas Daninhas. Rev. Bras. Herbic. 2012, 11, 276. [Google Scholar] [CrossRef]
- Silva, B.; Almeida, R.; Salgado, T.; Alves, P.L. Efficacy of Imazapic, Halosulfuron and Sulfentrazone for Cyperus rotundus L. Control in Response to Weed Tuber Density. Afr. J. Agric. Res. 2014, 9, 3458–3464. [Google Scholar] [CrossRef]
- Melo, T.S.; Thiel, C.H.; da Silva, L.B.X.; Deuner, S.; Andres, A.; Ávila, G.E.; Pires, S.N.; Concenço, G. Cumulative potential and half-life of [imazapic + imazapyr] in lowland soils of Rio Grande Do Sul grown with clearfield® rice. J. Environ. Sci. Heal. Part B 2022, 57, 450–457. Available online: https://www.tandfonline.com/doi/full/10.1080/03601234.2022.2063613 (accessed on 11 September 2025). [CrossRef]
- Hussain, H.; Dom, N.C.; Md Rashid, R.I. Degradation of Imazapyr and Imazapic in Aqueous Solutions and Soil Under Direct Sunlight. MJAS 2017, 21, 1074–1079. [Google Scholar] [CrossRef]
- Mueller, T.C.; Boswell, B.W.; Mueller, S.S.; Steckel, L.E. Dissipation of Fomesafen, Saflufenacil, Sulfentrazone, and Flumioxazin from a Tennessee Soil Under Field Conditions. Weed Sci. 2014, 62, 664–671. [Google Scholar] [CrossRef]
- Dear, B.; Spencer, D.; Khan, M.; Higgins, T.J. The Tolerance of Three Transgenic Subterranean Clover (Trifolium subterraneum L.) Lines with the Bxn Gene to Herbicides Containing Bromoxynil. Aust. J. Agric. Res. 2003, 54, 203. [Google Scholar] [CrossRef]
- Ramalingam, S.P.; Chinnagounder, C.; Perumal, M.; Palanisamy, M.A. Evaluation of New Formulation of Oxyfluorfen (23.5% EC) for Weed Control Efficacy and Bulb Yield in Onion. Am. J. Plant Sci. 2013, 4, 890–895. [Google Scholar] [CrossRef]
- Albertson, P.L.; Grof, C.P. The effect of hexose upon Pol, Brix and calculated CCS in sugarcane: A potential for negative Pol bias in juice from actively growing cane. J. Am. Soc. Sugar Cane Technol. 2014, 24, 185–198. [Google Scholar]
- Office of the Cane and Sugar Board. Sugarcane Price Situation and Sugar Production Returns Year 2021/22 Report. Office of the Cane and Sugar Board, Ministry of Industry. Crops and Livestock Products. Available online: https://www.ocsb.go.th/wp-content/uploads/2023/03/13813-1585.pdf (accessed on 11 September 2024).
- Booth, B.D.; Murphy, S.D.; Swanton, C.J. Describing the Distribution and Abundance of Populations. In Weed Ecology in Natural and Agricultural Systems; CABI Books: Wallingford, UK, 2003; pp. 17–28. [Google Scholar] [CrossRef]
- Suwanarak, K. Weed in Sugarcane Fields. Thai J. Agric. Sci. 1994, 12, 222–230. [Google Scholar] [CrossRef]
- Zimdahl, R.L. Chapter 10—Methods of Weed Management. In Fundamentals of Weed Science, 5th ed.; Zimdahl, R.L., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 271–335. Available online: https://www.sciencedirect.com/book/9780128111437/fundamentals-of-weed-science (accessed on 11 September 2025).
- Meena, M.; Singh, R.K.; Meena, R.; Meena, R.N.; Meena, K. Pendimethalin Effects on Weed Dynamics, Crop Growth and Yield of Wheat. J. Plant Dev. Sci. 2022, 14, 433–435. Available online: https://www.researchgate.net/profile/Rajesh-Singh-31/publication/360699771_PENDIMETHALIN_EFFECTS_ON_WEED_DYNAMICS_CROP_GROWTH_AND_YIELD_OF_WHEAT (accessed on 11 September 2025).
- Pringgani, A.M.; Khonghinta, J.; Gonkhamdee, S.; Songsri, P.; Jongrungkl, N. Ground Covering Characteristics of Sugarcanes Using High-Angle Images and Their Relationship with Growth Destructive Sampling. Asian J. Plant Sci. 2023, 22, 434–443. [Google Scholar] [CrossRef]
- Dias, H.B.; Inman-Bamber, G.; Everingham, Y.; Sentelhas, P.C.; Bermejo, R.; Christodoulou, D. Traits for Canopy Development and Light Interception by Twenty-Seven Brazilian Sugarcane Varieties. Field Crops Res. 2020, 249, 107716. [Google Scholar] [CrossRef]
- Webster, T.M.; Grey, T.L.; Ferrell, J.A. Purple Nutsedge (Cyperus rotundus) Tuber Production and Viability Are Reduced by Imazapic. Weed Sci. 2017, 65, 97–106. [Google Scholar] [CrossRef]
- Ducar, J.T.; Wilcut, J.W.; Richburg, J.S. Weed Management in Imidazolinone-Resistant Corn with Imazapic. Weed Technol. 2004, 18, 1018–1022. [Google Scholar] [CrossRef]
- Vinicius da Silva, P.; Malardo, R.; de Carvalho Dias, R.; Monquero, A.; Christoffoleti, J. Indaziflam: Control Effectiveness in Monocotyledonous and Eudicotyledonous Weeds as a Function of Herbicide Dose and Soil Texture. Pak. J. Biol. Sci. 2021, 24, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Dombro, A.; Raatz, L.; Bork, E.W. Indaziflam Provides Long-Term Reduction of Annual Brome Grass (Bromus Spp.) in Canada’s Mixedgrass Prairie. Rangel. Ecol. Manag. 2025, 98, 73–82. [Google Scholar] [CrossRef]
- Ramanathan, S.S.; Gannon, T.W.; Maxwell, P.J. Dose-Response of Five Weed Species to Indaziflam and Oxadiazon. Weed Technol. 2023, 37, 303–312. [Google Scholar] [CrossRef]
- Giraldeli, A.L.; Silva, A.F.M.; Baccin, L.C.; Araújo, L.d.S.; da Silva, G.S.; Pagenotto, A.C.V.; Filho, R.V. Chemical Management of Cyperus rotundus L. and Other Weeds at Sugarcane in PSS System. Biosci. J. 2021, 37, e37056. [Google Scholar] [CrossRef]
- Alves, P.L.; José, M.J.; Ferraudo, A. Soil Attributes and Efficiency of Sulfentrazone on Control of Purple Nutsedge (Cyperus rotundus L.). Sci. Agric. 2004, 61, 319–325. [Google Scholar] [CrossRef]
- Wehtje, G.R.; Walker, R.H.; Grey, T.L.; Hancock, H.G. Response of Purple (Cyperus rotundus) and Yellow Nutsedges (C. esculentus) to Selective Placement of Sulfentrazone. Weed Sci. 1997, 45, 382–387. [Google Scholar] [CrossRef]
- dos Santos, P.H.V.; Schedenffeldt, B.F.; Monquero, P.A. Control of Poaceae and Convolvulaceae Weed Species by Herbicides Applied to the Soil and Sugarcane Straw. Int. J. Agric. Biol. 2022, 27, 431–440. [Google Scholar]
- Amilcar, I.S.N.; Carlos, A.M.B.; Modesto, O.D.S.O.; Miguel, A.F.R.; Oscar, L.C.C.; Florencio, D.V.O.; Javier, I.B.V. Control of Broadleaved and Grass Weeds in Saccharum officinarum with the Use of Pre-Emergence Herbicides. Afr. J. Agric. Res. 2018, 13, 2232–2238. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, C.; Wang, Y.; Gao, Y.; Qin, J.; Wei, J. Herbicidal Activity Evaluation of Topramezone and Its Safety to Sugarcane. Sugar Tech 2022, 25, 698–704. [Google Scholar] [CrossRef]
- Ducca, A.S.; Vargas, P.D.; Sabaté, S.; Romero, E.R. Topramezone: New Herbicide Registered in Sugarcane for Post-Emergent Management of Cynodon dactylon in Tucumán, Argentina. Sugar Tech 2020, 22, 738–740. [Google Scholar] [CrossRef]
- Sondhia, S. Evaluating the Environmental Impact of Topramezone in Maize Fields: A Comprehensive Assessment of Risk, Degradation Kinetics, and Residue Dynamics. J. Food Compos. Anal. 2025, 147, 108022. [Google Scholar] [CrossRef]
- Loddo, D.; Carlesi, S.; Da Cunha, A.T. Germination of Chloris barbata, Cynodon dactylon, and Cyperus rotundus from Angola at Constant and Alternate Temperatures. Agronomy 2019, 9, 615. [Google Scholar] [CrossRef]
- Parsons, W.T.; Cuthbertson, E.G. Noxious Weeds of Australia. 2001. Available online: https://ci.nii.ac.jp/ncid/BA55577521 (accessed on 11 September 2025).
- Stoller, E.W.; Sweet, R.D. Biology and Life Cycle of Purple and Yellow Nutsedges (Cyperus rotundus and C. esculentus). Weed Technol. 1987, 1, 66–73. [Google Scholar] [CrossRef]
- Souza, R.; Dias, A.; Figueiredo, M.; Obara, F.E.B.; Christoffoleti, P. Growth of the Crabgrass Species Digitaria ciliaris and Digitaria nuda. Planta Daninha 2012, 30, 317–325. [Google Scholar] [CrossRef]
- Sandoval, J.R.; Rodríguez, P.A. Digitaria ciliaris (Southern Crabgrass). 2014. Available online: https://www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.18912 (accessed on 4 October 2025).
- Nawata, E.; Nagata, Y.; Sasaki, A.; Iwama, K.; Sakuratani, T. Mapping of Climatic Data in Northeast Thailand: Rainfall. Tropics 2005, 14, 191–201. [Google Scholar] [CrossRef]
- Fay, P.A.; Schultz, M.J. Germination, Survival, and Growth of Grass and Forb Seedlings: Effects of Soil Moisture Variability. Acta Oecologica 2009, 35, 679–684. [Google Scholar] [CrossRef]
- Basu, S.; Rao, Y.V. Environmental Effects and Management Strategies of the Herbicides. IJBSM 2020, 11, 518–535. [Google Scholar] [CrossRef]
- Carpenter, D.; Boutin, C. Sublethal Effects of the Herbicide Glufosinate Ammonium on Crops and Wild Plants: Short-Term Effects Compared to Vegetative Recovery and Plant Reproduction. Ecotoxicology 2010, 19, 1322–1336. [Google Scholar] [CrossRef]
- Mummery, D.; Battaglia, M. Significance of Rainfall Distribution in Predicting Eucalypt Plantation Growth, Management Options, and Risk Assessment Using the Process-Based Model CABALA. For. Ecol. Manag. 2004, 193, 283–296. [Google Scholar] [CrossRef]
- Qaisrani, Z.N.; Som-Ard, J.; Suwanlee, S.R.; Keawsomsee, S.; Homtong, N.; Ninsawat, S.; Asadullah; Mengal, A.N. Evaluating and Mapping Spatial Drought in Northeast Thailand: Utilizing Analytic Hierarchy Process and Random Forest Algorithms. Geocarto Int. 2025, 40, 2518413. [Google Scholar] [CrossRef]
- Bonini Da Luz, F.; Carvalho, M.L.; Aquino De Borba, D.; Schiebelbein, B.E.; Paiva De Lima, R.; Cherubin, M.R. Linking Soil Water Changes to Soil Physical Quality in Sugarcane Expansion Areas in Brazil. Water 2020, 12, 3156. [Google Scholar] [CrossRef]
- Smit, M.A.; Singels, A. The Response of Sugarcane Canopy Development to Water Stress. Field Crops Res. 2006, 98, 91–97. [Google Scholar] [CrossRef]
- Little, N.; DiTommaso, A.; Westbrook, A.; Ketterings, Q.; Mohler, C. Effects of Fertility Amendments on Weed Growth and Weed-Crop Competition: A Review. Weed Sci. 2021, 69, 132–146. [Google Scholar] [CrossRef]
- Romanowska, E.; Wasilewska-Dębowska, W. Light-Dependent Reactions of Photosynthesis in Mesophyll and Bundle Sheath Chloroplasts of C4 Plant Maize. How Our Views Have Changed in Recent Years. Acta Soc. Bot. Pol. 2022, 91, 1–18. [Google Scholar] [CrossRef]
- Wu, H.Y.; Liu, L.A.; Shi, L.; Zhang, W.F.; Jiang, C.-D. Photosynthetic Acclimation During Low-Light-Induced Leaf Senescence in Post-Anthesis Maize Plants. Photosynth. Res. 2021, 150, 313–326. [Google Scholar] [CrossRef]
- Mohamed, H.; Marzouk, E.E. Efficacy of Certain Herbicides for Controlling Weeds, Improvement Yield and Quality of Sugarcane Varieties. Egypt. J. Appl. Sci. 2019, 34, 152–171. [Google Scholar] [CrossRef]
- Ghodke, S.; Chaudhari, P.; Thorave, D. Integrated Weed Management in Preseasonal Sugarcane. Int. J. Chem. Stud. 2020, 8, 3183–3187. [Google Scholar] [CrossRef]
- Bhullar, M.S.; Kamboj, A.; Singh, G. Weed Management in Spring-Planted Sugarcane (Saccharum officinarum)-Based Intercropping Systems. Indian J. Agron. 2006, 51, 183–185. [Google Scholar] [CrossRef]
| Treatment | Active Ingredient and Formulation | Trade Name Manufactured | Doses (g a.i. ha−1) |
|---|---|---|---|
| No weeding (T1) | - | - | - |
| Hand weeding (T2) | - | - | - |
| Atrazine fb glufosinate ammonium (T3) | Atrazine 90% WG, Glufosinate ammonium 15% SL | BK Prim/Baka Company (Bangkok, Thailand), BastaX/BASF (Bangkok, Thailand) | 3000 fb 562.5 |
| Atrazine fb glufosinate ammonium (T4) | Atrazine 90% WG, Glufosinate ammonium 15% SL | BK Prim/Baka Company (Bangkok, Thailand), BastaX/BASF (Bangkok, Thailand) | 3000 fb 750.0 |
| Pendimethalin + imazapic (T5) | Pendimethalin 33% EC, Imazapic 24% SL | BK Ranger/BK Agro Company (Bangkok, Thailand), BKX/BK Agro Company (Bangkok, Thailand) | 825.0 + 75.0 |
| Indaziflam (T6) | Indaziflam 50% SC | B Kano/Baka Company (Bangkok, Thailand) | 62.5 |
| Sulfentrazone (T7) | Sulfentrazone 48% SC | Authority/Baka Company (Bangkok, Thailand) | 875.0 |
| Indaziflam + sulfentrazone (T8) | Indaziflam 50% SC, Sulfentrazone 48% SC | B Kano/Baka Company (Bangkok, Thailand), Authority/Baka Company (Bangkok, Thailand) | 46.88 + 750.0 |
| Topramezone (T9) | Topramezone 33.6% SC | Cleo/Chia Tai Company (Samut Prakarn, Thailand) | 52.5 |
| Treatment 1/ | Phytotoxicity of Herbicides to Sugarcane | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location I 2/ | Location II 3/ | ||||||||||||||||
| 0 | 1 | 7 | 14 | 21 | 30 | 0 | 1 | 7 | 14 | 21 | 30 | ||||||
| Days After Post-Emergence Application | Days After Post-Emergence Application | ||||||||||||||||
| T1 | 0.00 | 0.00 | c 4/ | 0.00 | b | 0.00 | b | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | b | 0.00 | b | 0.00 | 0.00 |
| T2 | 0.00 | 0.00 | c | 0.00 | b | 0.00 | b | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | b | 0.00 | b | 0.00 | 0.00 |
| T3 | 0.00 | 6.25 | b | 27.50 | a | 16.25 | a | 0.00 | 0.00 | 0.00 | 0.00 | 8.75 | a | 20.00 | a | 0.00 | 0.00 |
| T4 | 0.00 | 8.75 | a | 30.00 | a | 20.00 | a | 0.00 | 0.00 | 0.00 | 0.00 | 10.00 | a | 25.00 | a | 0.00 | 0.00 |
| T5 | 0.00 | 0.00 | c | 0.00 | b | 0.00 | b | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | b | 0.00 | b | 0.00 | 0.00 |
| T6 | 0.00 | 0.00 | c | 0.00 | b | 0.00 | b | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | b | 0.00 | b | 0.00 | 0.00 |
| T7 | 0.00 | 0.00 | c | 0.00 | b | 0.00 | b | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | b | 0.00 | b | 0.00 | 0.00 |
| T8 | 0.00 | 0.00 | c | 0.00 | b | 0.00 | b | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | b | 0.00 | b | 0.00 | 0.00 |
| T9 | 0.00 | 0.00 | c | 0.00 | b | 0.00 | b | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | b | 0.00 | b | 0.00 | 0.00 |
| F-test | ND 5/ | ** 6/ | ** | ** | ND | ND | ND | ND | ** | ** | ND | ND | |||||
| Treatment 1/ | Stalk Length (cm) | Number of Stalk (stalk ha−1) | Cane Yield (ton ha−1) | Sugar Yield (ton CCS ha−1) | CCS 7/ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location I 2/ | Location II 3/ | Location I | Location II | Location I | Location II | Location I | Location II | Location I | Location II | |||||||
| T1 | 211.51 | 339.42 | 28,125 | e 5/ | 54,688 | c | 35.98 | e | 91.49 | d | 4.78 | f | 12.37 | c | 13.50 | 13.50 |
| T2 | 240.31 | 337.83 | 52,214 | abc | 80,273 | a | 85.25 | ab | 149.65 | ab | 11.95 | a | 21.13 | a | 14.09 | 14.09 |
| T3 | 199.84 | 334.88 | 43,099 | bcd | 77,865 | a | 51.32 | d | 138.54 | abc | 7.53 | de | 20.44 | ab | 14.72 | 14.72 |
| T4 | 214.30 | 352.15 | 42,839 | cd | 74,414 | a | 50.27 | d | 134.24 | bc | 7.00 | e | 18.78 | ab | 14.31 | 14.10 |
| T5 | 246.46 | 320.25 | 56,641 | a | 76,563 | a | 86.05 | a | 147.56 | ab | 11.94 | a | 20.52 | ab | 13.89 | 13.89 |
| T6 | 223.54 | 344.55 | 52,979 | ab | 79,427 | a | 64.07 | cd | 157.07 | a | 8.28 | cde | 20.24 | ab | 12.93 | 12.93 |
| T7 | 217.81 | 331.95 | 54,297 | a | 78,906 | a | 71.84 | bc | 144.25 | ab | 9.71 | bc | 19.78 | ab | 13.75 | 13.75 |
| T8 | 232.47 | 340.08 | 57,031 | a | 78,386 | a | 73.24 | abc | 138.37 | abc | 10.52 | ab | 19.83 | ab | 14.34 | 14.34 |
| T9 | 232.44 | 332.83 | 39,063 | d | 64,193 | b | 61.39 | cd | 119.18 | c | 9.18 | bcd | 17.79 | b | 14.96 | 14.96 |
| F-test | ns | ns 4/ | ** | ** 6/ | ** | ** | ** | ** | ns | ns | ||||||
| Treatment 1/ | Cost of Production (USD ha−1) | Total Income (USD ha−1) | Net Profit (USD 4/ ha−1) | |||
|---|---|---|---|---|---|---|
| Location I 2/ | Location II 3/ | Location I | Location II | Location I | Location II | |
| T1 | 1440.08 | 1790.28 | 1377.52 | 3726.29 | −62.56 | 1936.02 |
| T2 | 2616.26 | 2941.65 | 3338.67 | 5992.32 | 722.41 | 3050.67 |
| T3 | 1685.43 | 2245.41 | 2022.56 | 5621.46 | 337.12 | 3376.05 |
| T4 | 1690.53 | 2227.98 | 1927.63 | 5429.03 | 237.10 | 3201.05 |
| T5 | 2081.60 | 2383.69 | 3380.11 | 6125.57 | 1298.51 | 3741.88 |
| T6 | 1769.44 | 2349.15 | 2301.03 | 6628.51 | 531.60 | 4279.36 |
| T7 | 1856.59 | 2267.14 | 2639.07 | 5985.56 | 782.48 | 3718.42 |
| T8 | 1888.01 | 2241.17 | 2931.44 | 5437.48 | 1043.44 | 3196.31 |
| T9 | 1775.92 | 2096.00 | 2490.93 | 4881.33 | 715.01 | 2785.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gongka, S.; Jongrungklang, N.; Songsri, P.; Chankaew, S.; Monkham, T.; Gonkhamdee, S. Impact of Pre- and Post-Emergence Herbicides on Controlling Predominant Weeds at Late-Rainy Season Sugarcane Plantations in Northeastern Thailand. Agronomy 2025, 15, 2341. https://doi.org/10.3390/agronomy15102341
Gongka S, Jongrungklang N, Songsri P, Chankaew S, Monkham T, Gonkhamdee S. Impact of Pre- and Post-Emergence Herbicides on Controlling Predominant Weeds at Late-Rainy Season Sugarcane Plantations in Northeastern Thailand. Agronomy. 2025; 15(10):2341. https://doi.org/10.3390/agronomy15102341
Chicago/Turabian StyleGongka, Sujittra, Nakorn Jongrungklang, Patcharin Songsri, Sompong Chankaew, Tidarat Monkham, and Santimaitree Gonkhamdee. 2025. "Impact of Pre- and Post-Emergence Herbicides on Controlling Predominant Weeds at Late-Rainy Season Sugarcane Plantations in Northeastern Thailand" Agronomy 15, no. 10: 2341. https://doi.org/10.3390/agronomy15102341
APA StyleGongka, S., Jongrungklang, N., Songsri, P., Chankaew, S., Monkham, T., & Gonkhamdee, S. (2025). Impact of Pre- and Post-Emergence Herbicides on Controlling Predominant Weeds at Late-Rainy Season Sugarcane Plantations in Northeastern Thailand. Agronomy, 15(10), 2341. https://doi.org/10.3390/agronomy15102341









