Effects of Activated Carbon on Reduction in Pesticide Residues in Lettuce Grown in Soil Treated with Cyantraniliprole and Fluopyram
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Application of Pesticides and Activated Carbon
2.3. Greenhouse Experiments
2.4. Sample Preparation
2.5. LC-MS/MS Analyses and Method Validation
3. Results
3.1. Validation and Establishment of Methods
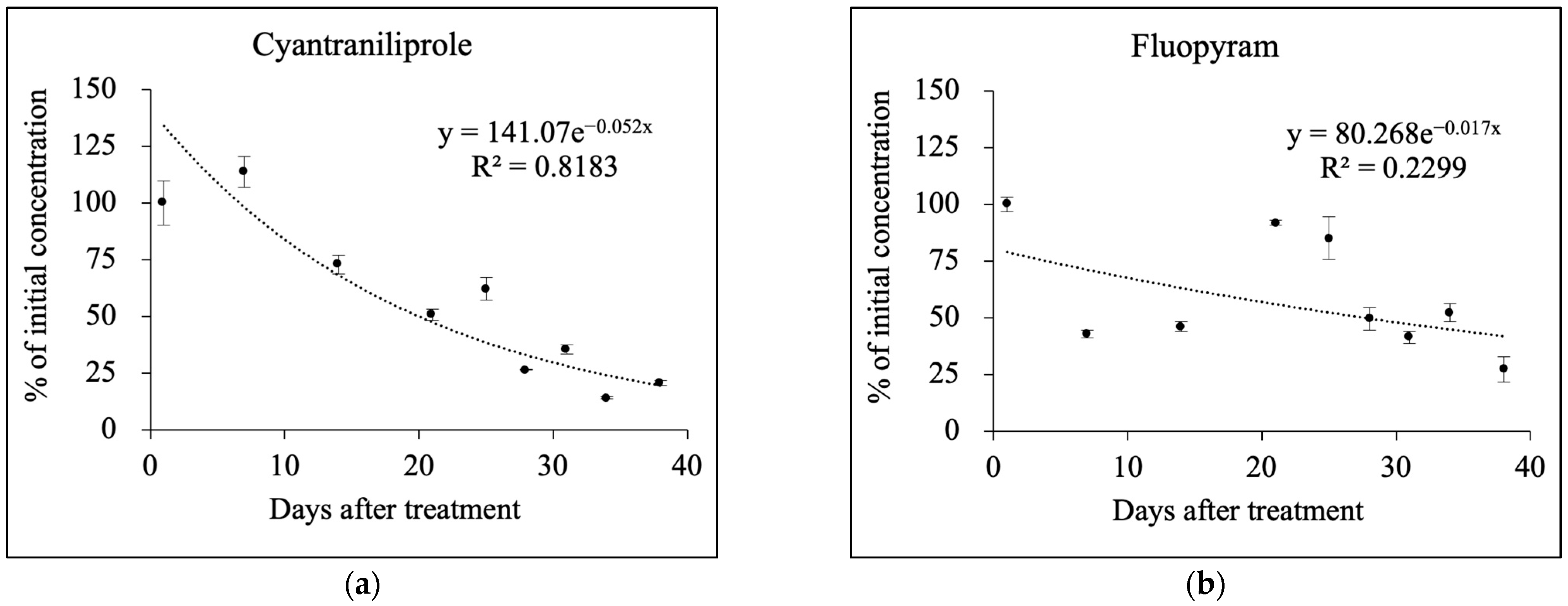
3.2. Pesticide Dissipation Patterns in Soil
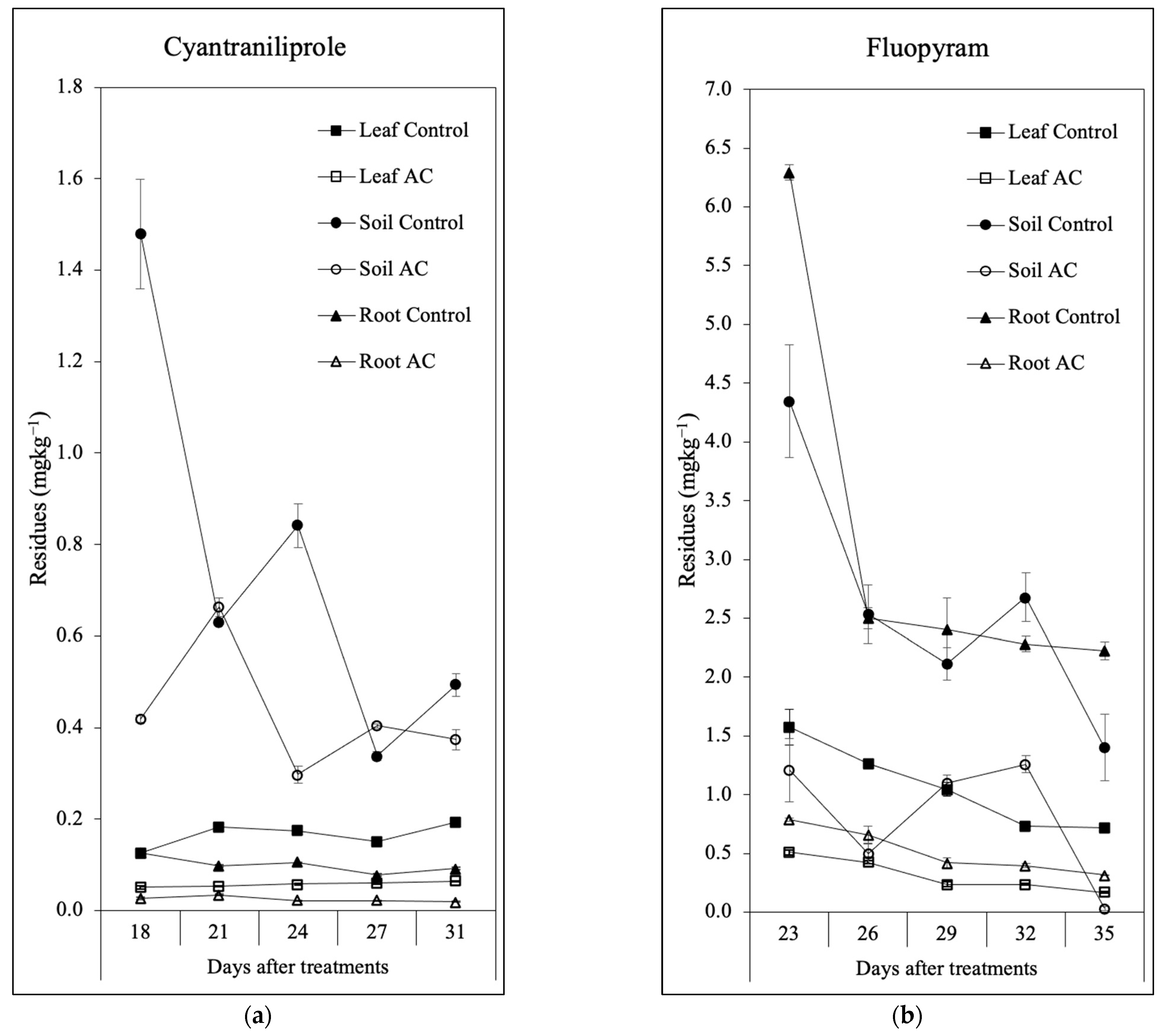
3.3. Dynamics of Pesticide Residues in Lettuce Tissues and Soil
3.4. Effects of the Activated Carbon on Pesticide Reduction in Lettuce
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogura, A.P.; Lima, J.Z.; Marques, J.P.; Massaro Sousa, L.; Rodrigues, V.G.S.; Espíndola, E.L.G. A review of pesticides sorption in biochar from maize, rice, and wheat residues: Current status and challenges for soil application. J. Environ. Manag. 2021, 300, 113753. [Google Scholar] [CrossRef] [PubMed]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Lim, D.J.; Kim, S.W.; Kim, Y.E.; Yoon, J.H.; Cho, H.J.; Shin, B.G.; Kim, H.Y.; Kim, I.S. Plant-Back Intervals of Imicyafos Based on Its Soil Dissipation and Plant Uptake for Rotational Cultivation of Lettuce and Spinach in Greenhouse. Agriculture 2021, 11, 495. [Google Scholar] [CrossRef]
- Park, D.W.; Kim, K.G.; Choi, E.A.; Kang, G.R.; Kim, T.S.; Yang, Y.S.; Moon, S.J.; Ha, D.R.; Kim, E.S.; Cho, B.S. Pesticide residues in leafy vegetables, stalk and stem vegetables from South Korea: A long-term study on safety and health risk assessment. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2016, 33, 105–118. [Google Scholar] [CrossRef]
- Park, B.K.; Kwon, S.H.; Yeom, M.S.; Joo, K.S.; Heo, M.J. Detection of pesticide residues and risk assessment from the local fruits and vegetables in Incheon, Korea. Sci. Rep. 2022, 12, 9613. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Selby, T.P.; Lahm, G.P.; Stevenson, T.M.; Hughes, K.A.; Cordova, D.; Annan, I.B.; Barry, J.D.; Benner, E.A.; Currie, M.J.; Pahutski, T.F. Discovery of cyantraniliprole, a potent and selective anthranilic diamide ryanodine receptor activator with cross-spectrum insecticidal activity. Bioorganic Med. Chem. Lett. 2013, 23, 6341–6345. [Google Scholar] [CrossRef]
- Tiwari, S.; Stelinski, L.L. Effects of cyantraniliprole, a novel anthranilic diamide insecticide, against Asian citrus psyllid under laboratory and field conditions. Pest Manag. Sci. 2013, 69, 1066–1072. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Kosmidou, G.; Gekas, A.; Kitsiou, F.; Eliopoulos, P.A.; Patakioutas, G. A Preliminary Analysis on the Insecticidal Effect of Cyantraniliprole against Stored-Product Pests. Appl. Sci. 2022, 12, 1297. [Google Scholar] [CrossRef]
- Lahm, G.P.; Cordova, D.; Barry, J.D. New and selective ryanodine receptor activators for insect control. Bioorganic Med. Chem. 2009, 17, 4127–4133. [Google Scholar] [CrossRef] [PubMed]
- Stansly, P.A.; Kostyk, B.; Riefer, R. Effect of rate and application method of Cyazypyr (HGW86) on control of silverleaf whitefly and southern armyworm in staked tomato, 2009. Arthropod Manag. Tests 2010, 35, E43. [Google Scholar] [CrossRef]
- Özşirvan, G.; Yalçın, M.; Turgut, N.; Tari, V.; Turgut, C. Insights into the uptake, translocation, and accumulation dynamics of cyantraniliprole and thiamethoxam seed coating pesticides in maize plants. Environ. Sci. Pollut. Res. 2024, 31, 44900–44907. [Google Scholar] [CrossRef]
- Zhang, C.; Fang, N.; Li, Y.; Wang, X.; He, H.; Jiang, J.; Tang, T.; Xu, Z.; Zhao, X.; Li, Y. Uptake, translocation and distribution of cyantraniliprole in rice planting system. J. Hazard. Mater. 2022, 436, 129125. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.D.; Portillo, H.E.; Annan, I.B.; Cameron, R.A.; Clagg, D.G.; Dietrich, R.F.; Watson, L.J.; Leighty, R.M.; Ryan, D.L.; McMillan, J.A.; et al. Movement of cyantraniliprole in plants after foliar applications and its impact on the control of sucking and chewing insects. Pest Manag. Sci. 2015, 71, 395–403. [Google Scholar] [CrossRef]
- Faske, T.R.; Hurd, K. Sensitivity of Meloidogyne incognita and Rotylenchulus reniformis to Fluopyram. J. Nematol. 2015, 47, 316–321. [Google Scholar]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- Beckerman, J. Detection of Fungicide Resistance. In Fungicides—Showcases of Integrated Plant Disease Management from Around the World; Nita, M., Ed.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Rathod, P.H.; Shah, P.G.; Parmar, K.D.; Kalasariya, R.L. The Fate of Fluopyram in the Soil–Water–Plant Ecosystem: A Review. Rev. Environ. Contam. Toxicol. 2022, 260, 1. [Google Scholar] [CrossRef]
- Naeem, H.; Ahmad, K.S. Lithospheric Based Eco-Decontamination and Fate Elucidation of Fungicide Fluopyram Containing Benzamides Background. 2022. Available online: https://www.researchsquare.com/article/rs-1184189/v1 (accessed on 9 April 2025).
- Gustafson, D.I. Groundwater ubiquity score: A simple method for assessing pesticide leachability. Environ. Toxicol. Chem. 1989, 8, 339–357. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J.; Mejuto, J.-C.; García-Río, L. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar] [CrossRef]
- Finizio, A.; Tosadori, A.; Di Guardo, A. Selecting a pesticide leaching indicator for integration into a decision support system at farm scale. Environ. Sci. Pollut. Res. 2025, 32, 6161–6171. [Google Scholar] [CrossRef]
- Manocha, S.; Manocha, L.M.; Joshi, P.; Patel, B.; Dangi, G.; Verma, N. Activated carbon from biomass. In Proceedings of the Carbon Materials 2012 (CCM12): Carbon Materials for Energy Harvesting, Environment, Nanoscience and Technology, Mumbai, India, 1–3 November 2013; pp. 120–123. [Google Scholar]
- Dong, L.H.; Liu, W.J.; Jiang, R.F.; Wang, Z.S. Physicochemical and porosity characteristics of thermally regenerated activated carbon polluted with biological activated carbon process. Bioresour. Technol. 2014, 171, 260–264. [Google Scholar] [CrossRef]
- Goswami, R.; Dey, A.K. Activated carbon from agricultural residues: A review. Desalination Water Treat. 2022, 278, 283–292. [Google Scholar] [CrossRef]
- Çeçen, F. Activated Carbon. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: New York, NY, USA, 2000; pp. 1–32. [Google Scholar]
- Ban, S.-E.; Lee, E.-J.; Yoon, J.; Lim, D.-J.; Kim, I.-S.; Lee, J.-W. Role of cellulose and lignin on biochar characteristics and removal of diazinon from biochar with a controlled chemical composition. Ind. Crops Prod. 2023, 200, 116913. [Google Scholar] [CrossRef]
- Yadav, A.K.; Sharma, U.C.; Rakshitha, K.; Maity, S. Production of activated carbon from coconut. In Recent Advances in Activated Carbon: Synthesis, Properties and Applications, 1st ed.; Min, H.S., Kusuma, H.S., Sharma, Y.C., Eds.; Green Chemical Innovations and Sustainability Series; CRC Press: Boca Raton, FL, USA, 2025; pp. 170–185. [Google Scholar]
- Zieliński, B.; Miądlicki, P.; Przepiórski, J. Development of activated carbon for removal of pesticides from water: Case study. Sci. Rep. 2022, 12, 20869. [Google Scholar] [CrossRef]
- Grant, G.A.; Fisher, P.R.; Barrett, J.E.; Wilson, P.C. Removal of Agrichemicals from Water Using Granular Activated Carbon Filtration. Water Air Soil Pollut. 2018, 230, 7. [Google Scholar] [CrossRef]
- Morales-Pérez, A.A.; Arias, C.; Ramírez-Zamora, R.-M. Removal of atrazine from water using an iron photo catalyst supported on activated carbon. Adsorption 2016, 22, 49–58. [Google Scholar] [CrossRef]
- Kim, Y.E.; Kim, S.W.; Lim, D.J.; Kim, I.S. Residues Analysis of Acetamiprid, Boscalid, Imidacloprid and Pyraclostrobin in the Minor Crop Mustard Green under Greenhouse Conditions for Evaluation of their Potentiality of PLS Violation. Korean J. Environ. Agric. 2020, 39, 214. [Google Scholar] [CrossRef]
- McGinley, J.; Healy, M.G.; Scannell, S.; Ryan, P.C.; Harmon O’Driscoll, J.; Mellander, P.-E.; Morrison, L.; Siggins, A. Field assessment of coconut-based activated carbon systems for the treatment of herbicide contamination. Chemosphere 2024, 349, 140823. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef]
- Lin, T.; Chen, X.; Wang, L.; Fang, H.; Li, M.; Li, Y.; Liu, H. Determination of new generation amide insecticide residues in complex matrix agricultural food by ultrahigh performance liquid chromatography tandem mass spectrometry. Sci. Rep. 2021, 11, 23208. [Google Scholar] [CrossRef]
- Sandín-España, P.; Mateo-Miranda, M.; López-Goti, C.; Seris-Barrallo, E.; Alonso-Prados, J.-L. Analysis of Pesticide Residues by QuEChERS Method and LC-MS/MS for a New Extrapolation of Maximum Residue Levels in Persimmon Minor Crop. Molecules 2022, 27, 1517. [Google Scholar] [CrossRef]
- Pihlström, T.; Fernández-Alba, A.R.; Amate, C.F.; Poulsen, M.E.; Hardebusch, B.; Anastassiades, M.; Lippold, R.; Cabrera, L.C.; de Kok, A.; ORegan, F. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed SANTE 11312/2021; Directorate-General for Health and Food Safety: Brussel, Belgium, 2021. [Google Scholar]
- Sun, C.; Bei, K.; Xu, Y.; Pan, Z. Effect of Biochar on the Degradation Dynamics of Chlorantraniliprole and Acetochlor in Brassica chinensis L. and Soil under Field Conditions. ACS Omega 2021, 6, 217–226. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, S.; Wang, D.; Cao, A. Biochar significantly reduced fumigant emissions and benefited germination and plant growth under field conditions. Environ. Pollut. 2022, 303, 119113. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, Z.; Zheng, L.; Zhang, H. Remediation of acetochlor-contaminated maize field soil using Serratia odorifera AC-1 fertilizer: Effects on soil microbial communities. Front. Microbiol. 2025, 16, 1510157. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Rasool, T.; Gani, K.M. A review of interactions of pesticides within various interfaces of intrinsic and organic residue amended soil environment. Chem. Eng. J. Adv. 2022, 11, 100301. [Google Scholar] [CrossRef]
- Damale, R.D.; Dutta, A.; Shaikh, N.; Pardeshi, A.; Shinde, R.; Babu, K.D.; Gaikwad, N.N.; Banerjee, K. Multiresidue analysis of pesticides in four different pomegranate cultivars: Investigating matrix effect variability by GC-MS/MS and LC-MS/MS. Food Chem. 2023, 407, 135179. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.T.; Ren, J.; Sharma, V.K.; Ma, X. Mutual Effects and Uptake of Organic Contaminants and Nanoplastics by Lettuce in Co-Exposure. ACS Agric. Sci. Technol. 2024, 4, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, N.; Lotteraner, L.; Sigmund, G.; Hofmann, T.; Krauss, M.; Mackenzie, K.; Georgi, A. Towards a better understanding of sorption of persistent and mobile contaminants to activated carbon: Applying data analysis techniques with experimental datasets of limited size. Water Res. 2025, 274, 123032. [Google Scholar] [CrossRef] [PubMed]
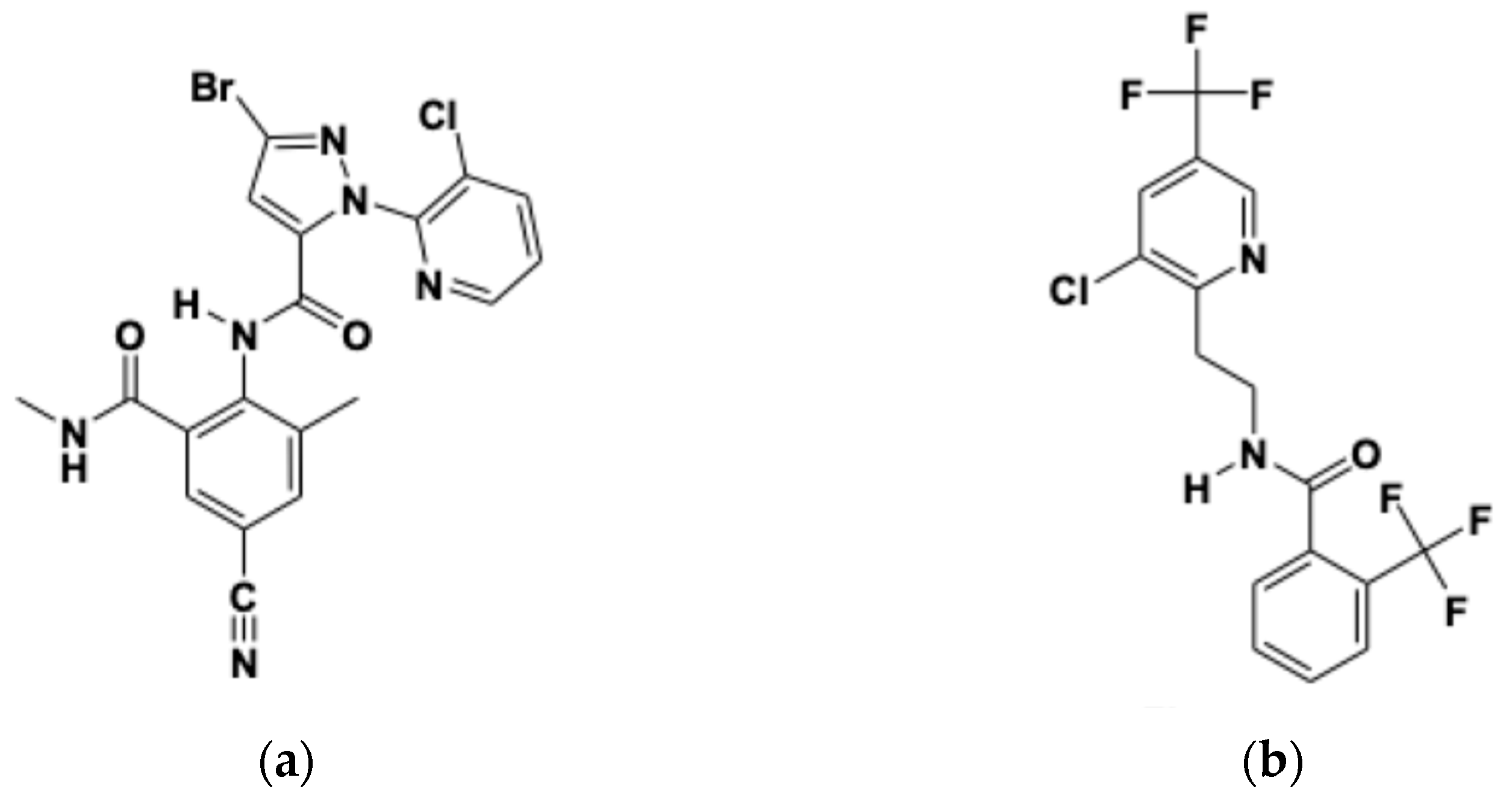

| Cyantraniliprole | Fluopyram | |
|---|---|---|
| Molecular formula | C19H14BrClN6O2 | C16H11ClF6N2O |
| Molecular weight (g/mol) | 473.71 | 396.71 |
| Water solubility (20 °C) | 12.33 mg/L | 16 mg/L |
| Log Kow (1) (Hydrophobicity) | 1.94 at 22 °C | 3.3 at pH 6.5 |
| Dissociation constant (pKa) | 8.8 (at 20 °C at pH 2–11) | 0.5 at 23 °C at pH 1 |
| DT50 (2) (days) in Soil | 32.4 | 118.8 |
| Koc (3) (binding affinity) | 155–266 mLg−1 | 233–400 mLg−1 |
| GUS (4) | 2.59 | 3.87 |
| C (%) | H (%) | O (%) | N (%) | S (%) | O/C | H/C | SBET (m2g−1) (1) | Vtotal (cm3g−1) (2) | MPD (nm) (3) |
|---|---|---|---|---|---|---|---|---|---|
| 81.30 | 0.91 | 3.98 | 0.48 | 0.74 | 0.05 | 0.01 | 1.5681 | 0.0137 | 35.044 |
| Soil Texture | Particle Size Distribution (%) | pH | Organic Matter (gkg−1) | Exchangeable Cation (cmolkg−1) | ||
|---|---|---|---|---|---|---|
| Sand | Silt | Clay | ||||
| Loam | 50.4 | 37.6 | 12.0 | 6.8 | 95.08 | 31.62 |
| Pesticide | Retention Time (min) | Precursor Ion (m/z) | Fragment Ion (m/z) | Declustering Potential (eV) | Collision Energy (eV) |
|---|---|---|---|---|---|
| Cyantraniliprole | 1.81 | 475.1 | 286.0 | 23 | 14 |
| 177.1 | 41 | ||||
| Fluopyram | 3.10 | 397.2 | 208.0 | 15 | 25 |
| 173.0 | 30 |
| Pesticide | Matrix | Matrix-Matched Calibration Equation | R2 | Matrix Effect (%) (1) | Ion Ratio Tolerance (%) (2) | LOQ (mgkg−1) (3) |
|---|---|---|---|---|---|---|
| Cyantraniliprole | Leaf | y = 6335x + 25.432 | 1.000 | 9.802 | 2.03 | 0.01 |
| Root | y = 10,099x + 87.699 | 1.000 | 37.903 | −2.80 | 0.01 | |
| Soil | y = 8837x + 17.708 | 1.000 | −4.692 | 3.62 | 0.01 | |
| Fluopyram | Leaf | y = 36,235x + 39.45 | 0.997 | −6.359 | 2.69 | 0.01 |
| Root | y = 21,540x + 14.574 | 0.996 | −44.335 | 1.42 | 0.01 | |
| Soil | y = 14,021x + 334.826 | 0.999 | −5.618 | −6.32 | 0.01 |
| Pesticide | Sample | Recovery (%) (1) | |
|---|---|---|---|
| LOQ (2) | 10 × LOQ | ||
| Cyantraniliprole | Leaf | 84.3 ± 3.6 | 105.5 ± 2.3 |
| Root | 73.6 ± 9.7 | 81.6 ± 1.5 | |
| Soil | 101.4 ± 10.4 | 113.7 ± 2.1 | |
| Fluopyram | Leaf | 94.6 ± 6.4 | 94.9 ± 4.5 |
| Root | 113.0 ± 2.1 | 104.7 ± 1.2 | |
| Soil | 77.7 ± 7.3 | 102.1 ± 2.9 | |
| Pesticide | DAT | Pesticide Reduction (%) (1) | |
|---|---|---|---|
| Leaf | Root | ||
| Cyantraniliprole | 18 | 59.6 ± 2.97 | 78.7 ± 2.13 |
| 21 | 71.3 ± 0.60 | 65.1 ± 1.62 | |
| 24 | 67.0 ± 1.02 | 79.7 ± 1.74 | |
| 27 | 59.7 ± 2.35 | 71.8 ± 0.53 | |
| 31 | 66.7 ± 0.78 | 79.3 ± 1.30 | |
| Fluopyram | 23 | 67.6 ± 1.59 | 87.5 ± 0.38 |
| 26 | 66.4 ± 1.62 | 73.8 ± 3.81 | |
| 29 | 77.3 ± 2.18 | 82.5 ± 2.24 | |
| 32 | 67.5 ± 1.19 | 82.6 ± 0.86 | |
| 35 | 76.4 ± 2.31 | 85.8 ± 0.27 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Lim, D.J.; Yoon, J.; Kim, I.S. Effects of Activated Carbon on Reduction in Pesticide Residues in Lettuce Grown in Soil Treated with Cyantraniliprole and Fluopyram. Agronomy 2025, 15, 2340. https://doi.org/10.3390/agronomy15102340
Kim SH, Lim DJ, Yoon J, Kim IS. Effects of Activated Carbon on Reduction in Pesticide Residues in Lettuce Grown in Soil Treated with Cyantraniliprole and Fluopyram. Agronomy. 2025; 15(10):2340. https://doi.org/10.3390/agronomy15102340
Chicago/Turabian StyleKim, Seon Hwa, Da Jung Lim, Jihyun Yoon, and In Seon Kim. 2025. "Effects of Activated Carbon on Reduction in Pesticide Residues in Lettuce Grown in Soil Treated with Cyantraniliprole and Fluopyram" Agronomy 15, no. 10: 2340. https://doi.org/10.3390/agronomy15102340
APA StyleKim, S. H., Lim, D. J., Yoon, J., & Kim, I. S. (2025). Effects of Activated Carbon on Reduction in Pesticide Residues in Lettuce Grown in Soil Treated with Cyantraniliprole and Fluopyram. Agronomy, 15(10), 2340. https://doi.org/10.3390/agronomy15102340





