1. Introduction
Soybean (
Glycine max (L.) Merrill) is one of the world’s main agricultural commodities and is cultivated in approximately 60 countries. In Brazil, this crop is planted in an area of more than 40 million hectares and is the main export product [
1] (FAO, 2024). Companies have made large investments in technologies such as biotechnology, remote sensing, mineral nutrition, and irrigation for soybean cultivation due to the global importance of this legume. Increasing yields with sustainable production practices is essential for the supply of food and energy in the coming years [
2,
3] (Mizik and Gyarmati, 2021; Pagano and Miransari, 2016).
The irrigated area in Brazil is more than eight million hectares, and one of the irrigation methods is the central pivot sprinkler [
4] (Tang et al., 2021). Light supplementation in soybeans with light-emitting diodes (LEDs) installed in the central irrigation pivot is under study, with the purpose of improving environmental conditions to increase crop yield and quality. Light supplementation in soybeans in the field is capable of increasing yield [
5] (Lemes et al., 2021), also influencing the quality of soybeans [
6] (Zhang et al., 2020).
Supplementary light is a technology capable of increasing productivity and food quality [
7] (Taulavuori et al., 2017). LEDs can increase the contents of phenols, isoflavones, and antioxidant factors in soybeans [
8] (Azad et al., 2018). Additionally, manipulating light quality using LEDs allows for changes in the photochemical efficiency of photosystem II, the photochemical extinction coefficient, the electron transport rate, and the structure of chloroplasts [
9] (Gao et al., 2020).
Light supplementation with LEDs interfere with many plant attributes. However, the responses are relative to the species involved and the quality of light [
10] (Bian et al., 2018). LEDs interfere with plant growth speed, germination rate, and the number of stomata, chlorophylls, and carotenoids [
11] (Kowalczyk et al., 2022). The balance between red LEDs and blue LEDs influences the activity of enzymes such as superoxide dismutase, catalase, and peroxidase [
12,
13] (Simlat et al., 2016; Su et al., 2014). These enzymes are related to the tolerance and selectivity of soybean plants to herbicides [
14,
15] (Guan et al., 2020; Moldes et al., 2008). It is likely, therefore, that the supplementary supply of light influences the mode of action of herbicides used in soybeans, as these products act in different metabolic pathways. Furthermore, supplementation can influence the weed community in the area, which will indirectly influence the effectiveness of herbicides.
The herbicides used in soybeans act on routes that are directly or indirectly affected by light supplementation. The formation route of aromatic amino acids is affected by the herbicide glyphosate [
16] (Steinrücken and Amrhein, 1980). The biosynthesis of branched amino acids is interrupted by the action of the herbicide diclosulam [
17] (Shimizu et al., 2002). The herbicide s-metolachlor inhibits the biosynthesis of flavonoids, anthocyanins, and very long-chain fatty acids [
18] (Böger, 2003). The herbicides fomesafen and flumioxazin inhibit protoporphyrinogen oxidase in the chlorophyll formation pathway within chloroplasts [
19] (Zhou et al., 2007). Finally, the herbicide clethodim inhibits the enzyme acetyl-coenzyme A carboxylase and destroys cell membranes [
20] (Lichtenthaler, 2014).
Studies on light supplementation in the field, with LED panels installed on the central irrigation pivot, are still in their infancy. The objective of this work was to evaluate the effect of herbicides on weed control and the qualitative and quantitative attributes of soybeans grown under light supplementation.
2. Material and Methods
2.1. Experimental Setup
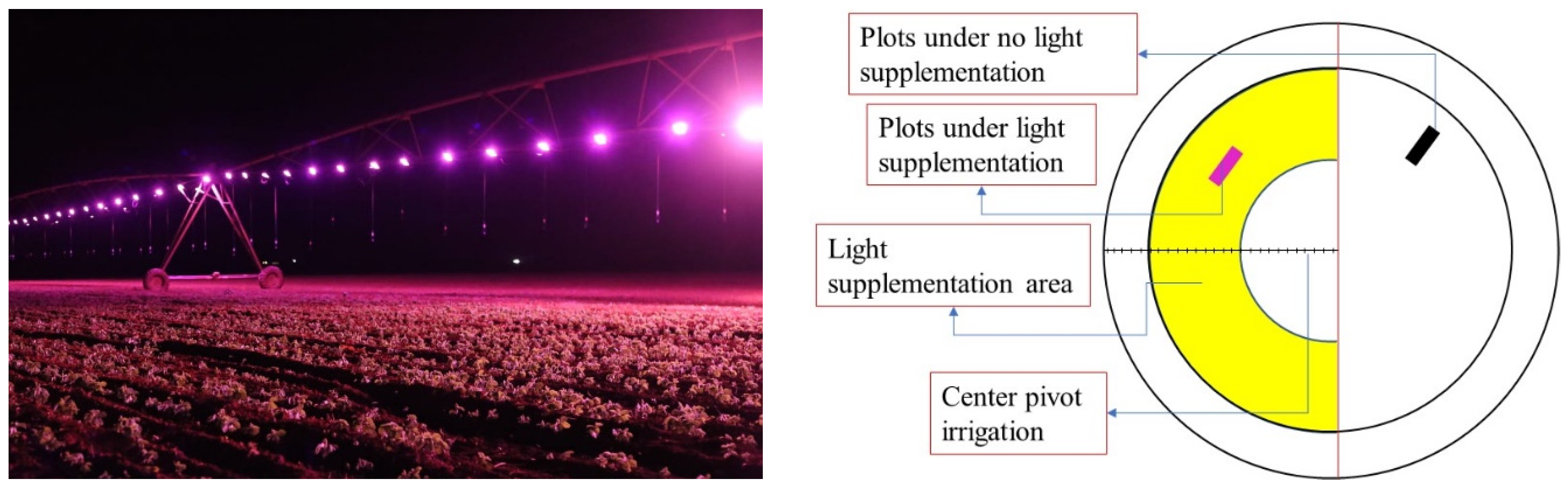
The experiment was set up in January 2022 on a farm located in Monte Carmelo, MG, Brazil (18°57′04″ S and 47°25′38″ W). Treatments were distributed in plots measuring 15 m2, with six soybean rows spaced 0.5 m apart and 5.0 m long. The experiment was set up under a central pivot in two environments: with light supplementation and without light supplementation. In the area where the experiment was carried out, 333 mm of rain accumulated between soybean planting and harvesting. Monthly accumulated rainfall was 2.3 mm in January, 175.5 mm in February, 134.5 mm in March, 17.5 mm in April, and 3.5 mm in May. Temperature values varied between 15.4 °C and 33.5 °C, with an average of 23.7 °C. Temperatures ranged from 18.7 °C to 33.5 °C in January, from 17.6 °C to 33.3 °C in February, from 18.9 °C to 33.1 °C in March, from 15.7 °C to 31.3 °C in April, and from 15.4 °C to 30.8 °C in May.
LED panels were installed (each panel had a power range of 50 to 200 Watts) on the central pivot, 4.0 m above the ground, which provided red (59%), green (33%), and blue (8%) light every day for approximately 20 min, over the experimental area (at an approximate speed of 250 mh
−1), in the period between 8:00 pm and 5:00 am. The light supplementation process consumed 0.06 W h
−1m
−2, and the LEDs provided a luminous flux of approximately 30 Lux for the plants. The supply of light coincied with the turning of the pivot (
Figure 1).
There was wheat straw in the area. Before planting soybeans, the soil was prepared through plowing and harrowing. 320 kg ha
−1 of a formula containing NPK 04:14:08 was applied to the planting line, after soil analysis (
Table 1). Soil analyses were carried out at the Soil and Limestone Analysis Laboratory (LABAS) at the Federal University of Uberlândia.
The treatments were distributed across four blocks, with plots subdivided in space. All herbicides (
Table 2) were applied in environments with and without light supplementation (
Figure 1). Herbicides were used before planting and 30 days after planting, when soybeans were at the V4 stage of development [
21] (Fehr and Caviness, 1977).
For spraying, a knapsack sprayer was used, with a constant pressure of 2.02 Kgf maintained by CO2 and monitored by pressure gauges. The spray bar is 1.5 m and has two Teejet 11002 AI tips, with an application range of 2.0 m. 240 L ha−1 of spray volume was used.
The soybean cultivar used was Brasmax challenge RR8472, with a population of 350 thousand plants ha−1. Products registered for the crop were used throughout the area to control pests and diseases. Likewise, irrigation was the same across plots, with supplementation and without supplementation.
2.2. Measurements and Observations
The number of weeds was evaluated at 34 and 60 DAP (days after planting). For this, a 0.5 × 0.5 m2 square was positioned in the middle of each plot, and the weeds were counted. Weed biomass was evaluated at 90 DAP. For this, inside the plot, in an area of 0.5 × 0.5 m, the weed plants were cut close to the ground and sent to the Phytotechnics Laboratory (LAFIT) at the Federal University of Uberlândia, where they were subjected to drying in an oven at 70 °C for 72 h.
Weed control was evaluated at 35 DAP. For this, grades from 0 to 10 were assigned to each plot according to the weed population and the effects of herbicides. Grade 10 indicated completely dead plants, and grade 0 represented healthy plants without injuries from herbicides [
22] (EWRC, 1964).
Chlorophyll a and chlorophyll b contents were measured in the second fully expanded trefoil with a portable electronic meter (ClorofiLOG CFL-1030—Falker, Porto Alegre, RS, Brazil) at 35 DAP. The plants were evaluated between 2:00 pm and 4:00 pm.
Physiological evaluations were carried out at 35 DAP on the second completely expanded trefoil to determine the variables: initial fluorescence (Fo), maximum photochemical efficiency of photosystem II (Fv/Fm = ratio of variable fluorescence over maximum fluorescence), and electron transport rate per reaction center (ETo/RC). Assessments were carried out at night (between 22:00 and 01:00). Fo was determined before the leaf received a saturating light pulse (1000 µmol m−2 s−1). A modulated fluorometer (Walz, Germany) was used.
The number of days until flowering and until reaching the R8 stage were also determined. Flowering was considered achieved when 50% of the plants had at least one flower.
At the harvest point stage (R8), soybean plants were evaluated in relation to the height of insertion of the first pod in relation to the soil. This assessment was carried out on the 30 central plants within each plot. Afterward, the central 4.8 m2 of each plot was cut with pruning shears, and the harvested material was sent to the laboratory to determine the number of pods per plant, the number of grains per pod, and productivity (kg ha−1) at 13% humidity.
Soybeans were evaluated in relation to macronutrient concentration (Nitrogen, Phosphorus, and Potassium). For this, 100 g of grains with a humidity of 13% were ground, and, subsequently, 1.0 g of the ground sample was subjected to nitrogen sulfur digestion to determine N and nitro-perchloric digestion. To determine P, a UV light spectrophotometer was used. To determine K, a flame photometer was used. The Perkin–Elmer (1968) methodology was followed [
23]. Nutrient analysis of soybeans was carried out at the Soil and Limestone Analysis Laboratory (LABAS) at the Federal University of Uberlândia.
2.3. Data Analysis
All data were subjected to evaluation for homogeneity of variance and normality of errors. The Shapiro–Wilk and Levene tests were used. Afterward, the data were subjected to analysis of variance (α ≤ 0.05), and means were compared using the Tukey test. Excel 2019, R-4.3.3, and SigmaPlot 12.5 programs were used.
3. Results
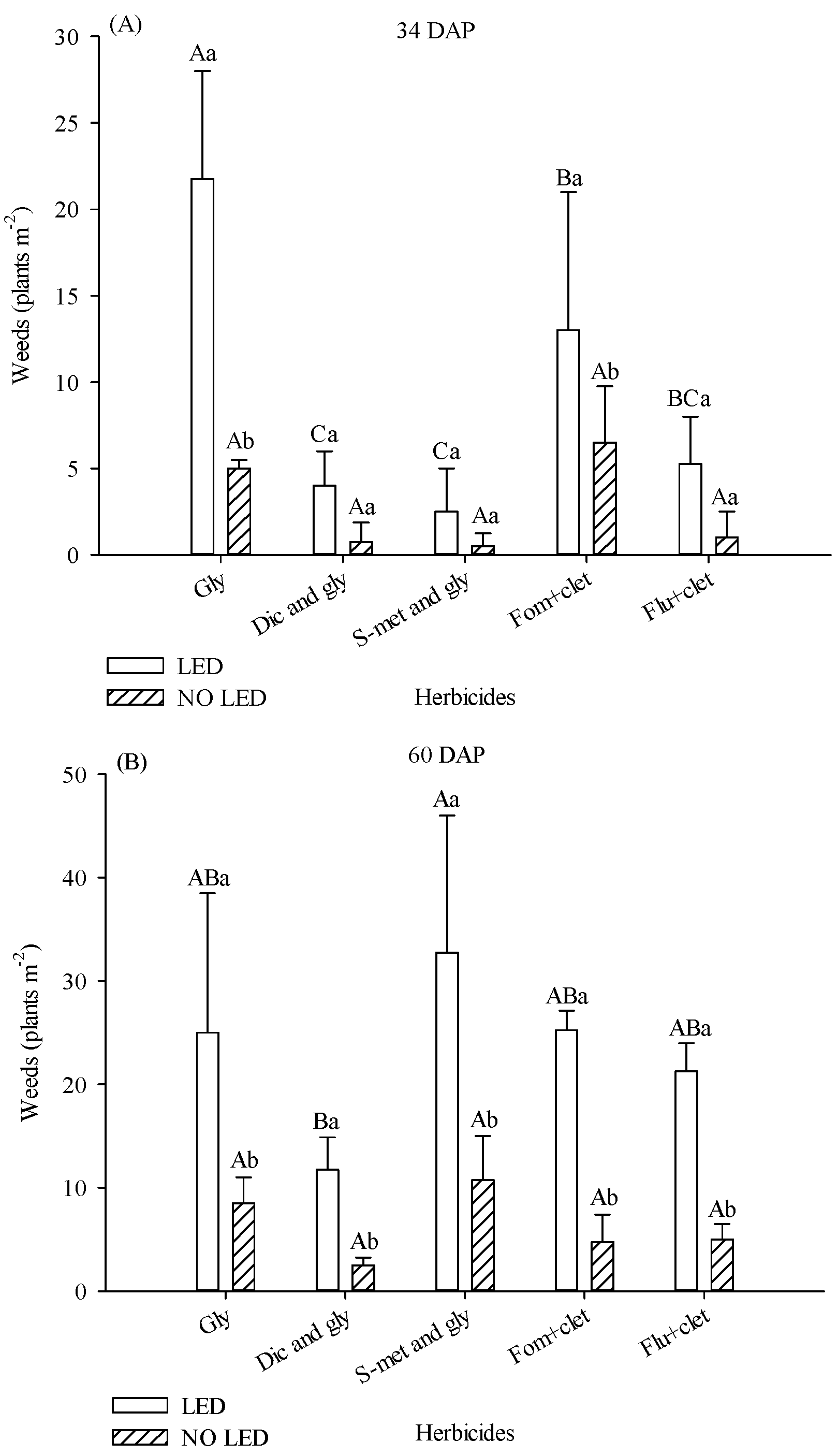
Herbicides and light supplementation influenced weed density. At 34 DAP, the number of weeds was higher in the area under supplementation. For the plots treated with glyphosate and fomesafen + clethodim, in the area with supplementation, the weed population was 77% and 50% higher, respectively (
Figure 2A). In the presence of light supplementation, treatments with diclosulam and glyphosate and s-metolachlor and glyphosate provided lower weed averages (
Figure 2A).
At 60 DAP, the number of weeds in all areas increased. Across all herbicides, there were more weeds in the supplemented plots (
Figure 2B). In the presence of LED, the treatment with diclosulam and glyphosate had fewer weeds (
Figure 2B).
The main weed species observed in the area were Eleusine indica, Amaranthus deflexus, Cyperus rotundus, Galinsoga parviflora, Nicandra physalodes, Oxalis latifolia, and Portulaca oleraceae.
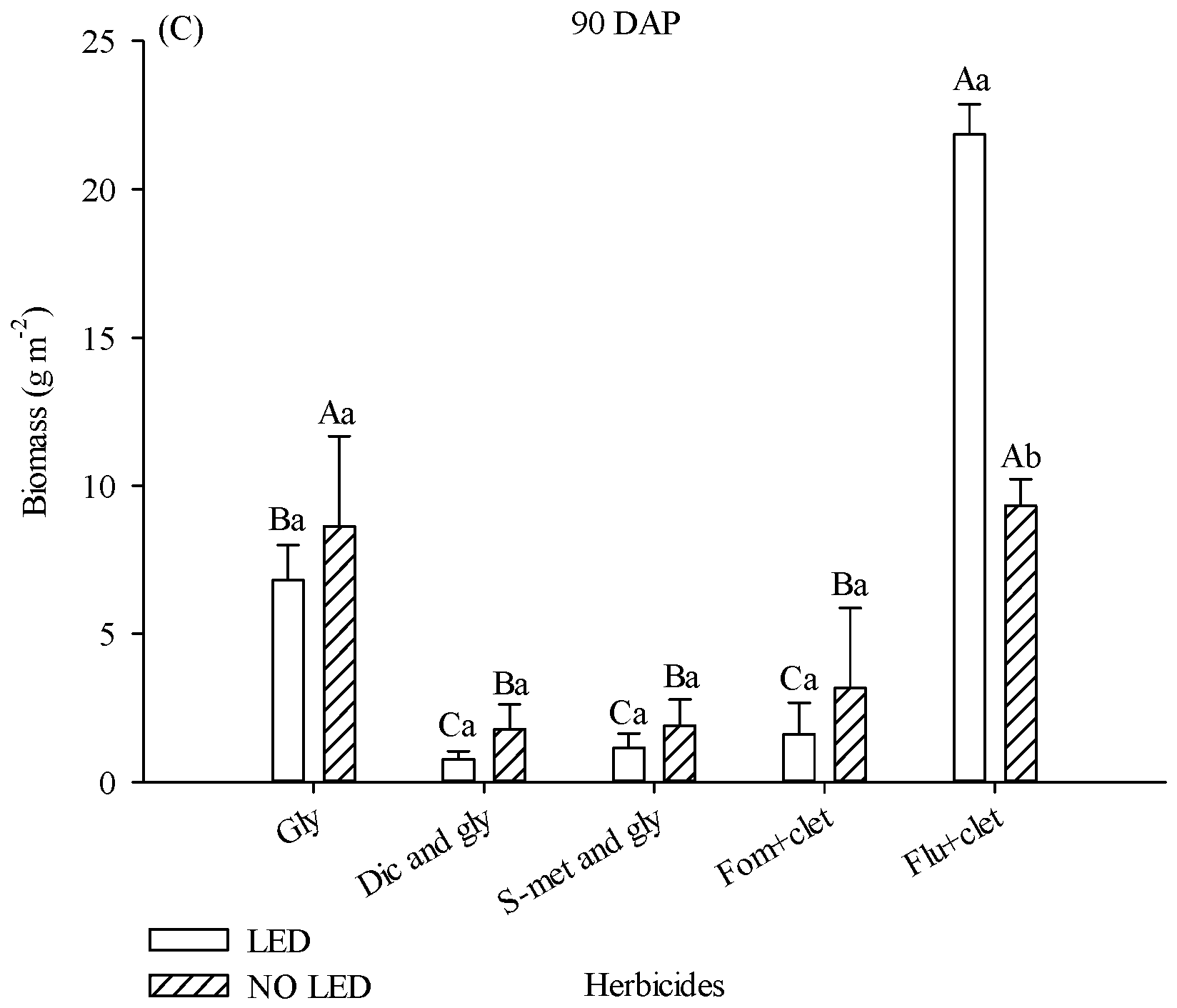
The biomass of the aerial part of weed plants collected at 90 DAP was also influenced by light supplementation and herbicides. In plots where flumioxazin + clethodim was applied, weed biomass was 67% higher in plots with supplementation (
Figure 2C). Although weed density was numerically much higher in the area treated with Fom + clet than in plots treated with S-met and gly without LED at 34 DAP, there was no statistical difference between treatments. With or without the presence of LED, treatments with diclosulam and glyphosate, s-metolachlor and glyphosate, and fomesafen + clethodim promoted lower values of weed biomass (
Figure 2C).
There was no interaction between herbicides and light supplementation for weed control. The average control in areas with supplementation was 10% lower than in areas without supplementation. In general, weed control was lower only in the glyphosate treatment (
Table 3).
Regarding the chlorophyll content of soybean plants, there was no significant interaction between herbicides and light supplementation (
Table 4). In general, higher values of chlorophyll
a,
b, and total were observed in treatments with fomesafen + clethodim, and lower values were observed in the treatment with sequential application of s-metolachlor and glyphosate, regardless of supplementation (
Table 4). When taking into account only the treatment with light supplementation, the general averages of chlorophyll
a and total chlorophyll contents were higher with LED light than the treatment without light, regardless of the herbicide used. For chlorophyll
b, there was no difference between supplementing or not with light (
Table 4).
The variables initial chlorophyll fluorescence (F0) and maximum quantum yield of PSII (Fv/Fm) were not influenced by the treatments (
Table 5).
The electron transport rate was the same across the herbicide treatments. Only in the general average of light supplementation was there a difference, with a higher value for plots with supplementation (
Table 6).
It was observed that soybean flowered less quickly (59 DAP) and reached the R8 stage earlier in the area without light supplementation. These stages were not influenced by herbicides (
Table 7).
Only light supplementation had an influence on the insertion height of the first pod. Plants under LED had a higher average insertion height of the first pod than plants without light supplementation (
Table 8).
Herbicides and light supplementation influenced the number of pods per plant and grains per pod, but there was no interaction between the factors. Light supplementation increased soybean yield indicators. Soybean plants under supplementation produced 23% more pods per plant and 26% more grains per pod. The number of grains per pod was higher in plants treated with flumioxazin + clethodim compared to diclosulam and glyphosate. The number of pods per plant was higher in the plots with glyphosate and diclosulam and glyphosate (
Table 9).
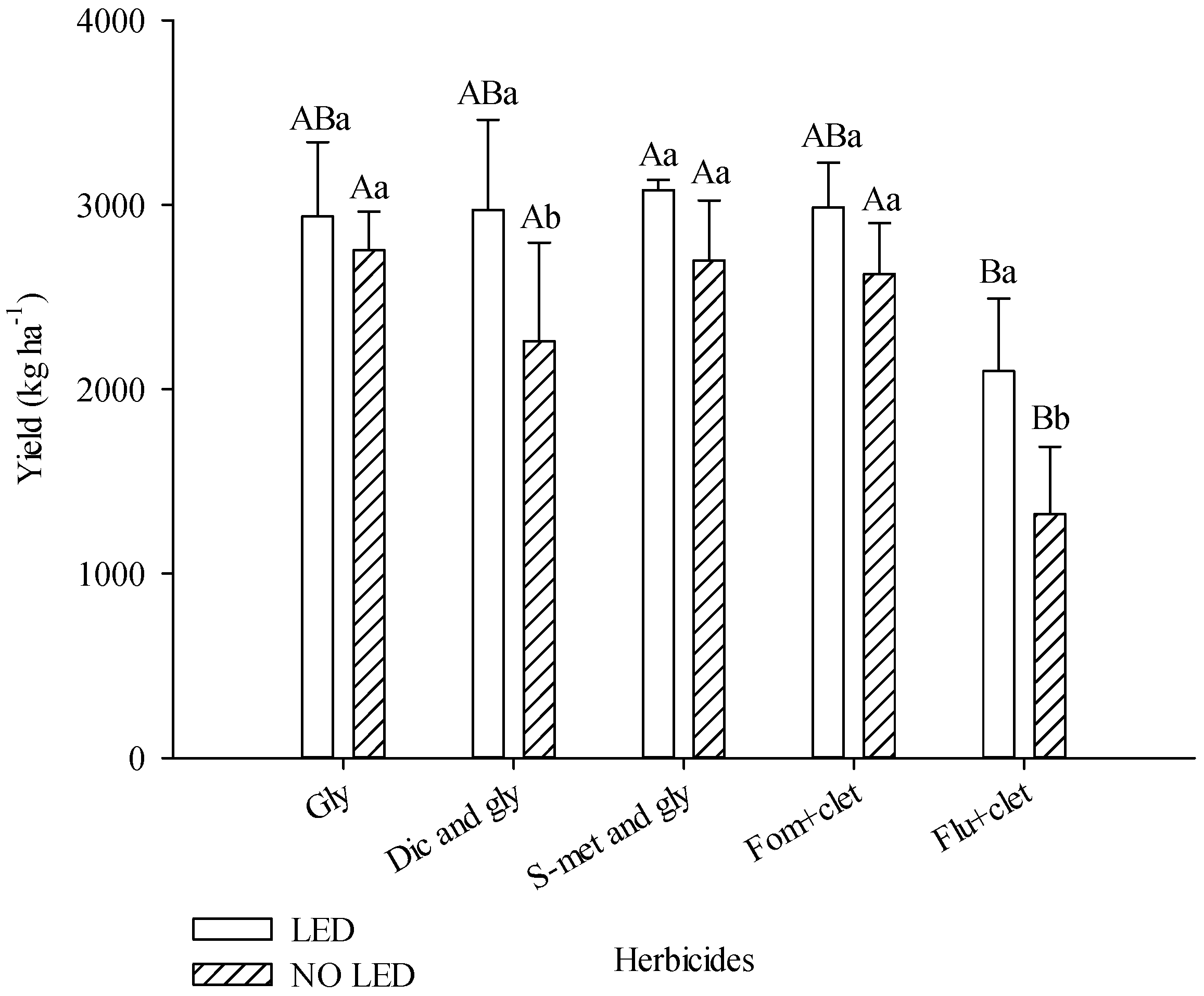
Light supplementation increased soybean productivity. In plots with LED, productivity was 21% higher. The plots with diclosulam and glyphosate and flumioxazin + clethodim produced 31% and 59%, respectively, more when grown under light supplementation. In plots without light supplementation, flumioxazin + clethodim provided lower productivity compared to other herbicides. In plots with light supplementation, the s-metolachlor and glyphosate treatments provided greater yield compared to other herbicide treatments (
Figure 3).
Regarding the concentration of macronutrients in soybeans, there was no effect of treatments on N and P. The herbicides influenced the concentration of K in the grains but had no effect on supplementation. The mixture containing fomesafen + clethodim reduced the average K concentration in soybeans by 12% compared to other herbicides (
Table 10).
4. Discussion
The higher density of weeds in areas with light supplementation may be related to the effect of light on germination parameters. Providing light in the form of LED can increase the percentage of germination, and species respond differently according to light lengths [
12] (Simlat et al., 2016). Exposing seeds to light, after preparing the area with plowing and harrowing, exposes more seeds to natural or artificial light. In this research, exposure to artificial light occurred for 20 min. However, it is known that red light (predominant in LED panels) increases seed germination rates [
24] (Lal and Sachan, 2017).
The characteristics provided by light supplementation had a greater influence on weed emergence than on biomass. At 90 DAP, weed biomass was higher only in the treatment with flumioxazin + clethodim. Flumioxazin is harmful to soybeans if applied post-emergence [
25] (Shaner, 2014). Intoxicated soybean plants compete inefficiently and allow weeds to accumulate biomass. However, it is possible that the harmful effect of flumioxazin on soybeans was reduced in plots with supplementation, as the decrease in soybean productivity in plots with flumioxazin was more severe in the area without supplementation (
Figure 3). As light supplementation is related to better qualitative and productive indicators in crops, it is possible that LED acts by reducing the toxic effect of the herbicide on soybeans. Fertilization and irrigation are examples of mitigating herbicide injuries to crops [
26,
27] (Barroso et al., 2022; France et al., 2022).
Light supplementation interferes with the effectiveness of herbicides indirectly, as it increases the population of weeds. In the control evaluation, the effectiveness of the herbicides in the plots under supplementation was lower (
Table 3). However, this evaluation occurred just five days after the application of post-emergence herbicides. It is possible that the effectiveness of the products was even lower in the following days, as the number of plants increased in the plots without supplementation. The lower growth of soybeans is also a factor that provides more conditions for weed plants to germinate. In this work, there are indicators that soybean plants grew more under supplementation.
Light supplementation interacted with herbicides, influencing weed emergence, as seen for diclosulam and s-metolachlor. These herbicides had their residual period impaired by supplementation (
Figure 2B). These herbicides are pre-emergent used in soybean areas, and both have a residual effect, controlling the emergence of weeds for a longer period. Farmers apply the products and wait for a residual period of close to 50 days [
28,
29] (Long and Wu, 2014; Pandolfo et al., 2016).
According to the characteristics of this soybean plantation, before halfway through the crop cycle, the plants grow and prevent light from reaching the soil. The time between planting and closing the row depends on many factors. Therefore, the decrease in the number of weeds in the evaluation at 60 DAP may have occurred due to the increase in soil cover provided by the vegetative growth of soybeans. Thus, good agricultural practices reduce the incidence of weeds in the area [
30,
31] (Holt, 1991; Van Acker, 2009).
Furthermore, lower weed biomass in treatments containing diclosulam and glyphosate, s-metolachlor and glyphosate, and fomesafen + clethodim indicate that these herbicides, regardless of the presence or absence of light, were more efficient in reducing weed growth.
Pre-emergent herbicides have been very important for controlling weeds in soybean areas [
32] (Salomão et al., 2018). The main weed in the area was
Eleusine indica. However, there were also
Amaranthus deflexus,
Cyperus rotundus,
Galinsoga parviflora,
Nicandra physalodes,
Oxalis latifolia, and
Portulaca oleraceae, which are greatly influenced by soil preparation [
31] (Van Acker, 2009). Thus, in plots treated with pre-emergent herbicides (s-metolachlor and diclosulam), despite the control being the same as treatments without pre-emergents (except glyphosate alone), lower weed germination values were observed. Additionally, lower average control in areas with supplementation may be related to the photodegradation of some of the herbicides used, such as flumioxazin and fomesafen [
33,
34] (Gong et al., 2017; Li et al., 2019).
Chlorophylls
a and
b are green pigments present in plant cells and indicators of the intensity of photosynthesis [
35] (Roca et al., 2024). The amount of pigments is related to the plant organism’s reaction to growth conditions, such as the presence of herbicides [
36] (Zheryakov et al., 2021).
The increase in chlorophyll
a and total chlorophyll values in soybean plants under light supplementation may be related to the quality of the LED. In this experiment, LEDs were used that provide red (59%), green (33%), and blue (8%) lights. Different photosynthetic pigments absorb different spectrums of light. Blue light is generally considered beneficial for the formation of chlorophyll
a [
37] (Marsac and Houmard, 1993). Blue light improves gene expression, which regulates chlorophyll synthesis [
38] (Wang et al., 2009). The red and blue light spectra are in accordance with the peak area of the absorption spectrum of chlorophyll, and the absorption percentage of blue and red light by plant leaves is about 90% [
39] (Terashima et al., 2009). Therefore, red and blue light strongly influence plant development and physiology and provide a more effective rate of photosynthesis [
40] (Hogewoning et al., 2010). It has been reported that blue light is beneficial for pigment accumulation [
41] (Kurilčik et al., 2008) and that it can reverse the inhibition response induced by red light [
42] (Sood et al., 2005). As there was no increase in chlorophyll
b, the increase in total chlorophyll is related to higher concentrations of chlorophyll
a.
Under stress-free conditions, light energy is absorbed by chlorophyll and used in photochemical processes. However, a proportion of this energy is released in the form of heat and fluorescence. When electron transport in the photosynthetic system is partially or completely blocked due to a stress factor, the intensity of emitted fluorescence increases, reflecting less use of photosynthetically active radiation by plants [
43] (Rastogi et al., 2019).
The initial fluorescence of chlorophyll (F0) is the fluorescence observed when QA (PS2 primary electron acceptor quinone) is completely oxidized, and the PS2 reaction center is open, a situation imminent to the activation of photochemical reactions [
44] (Mouget and Tremblin, 2002). Thus, F0 is independent of photochemical events, and its increase may be a consequence of damage to the PS2 reaction center or a reduction in the capacity to transfer excitation energy from the antenna to the reaction center [
35] (Baker and Rosenqvst, 2004). The Fv/Fm ratio is an estimate of the maximum quantum efficiency of PS2 photochemical activity, when all PS2 reaction centers are open [
45] (Baker and Rosenqvst, 2004). This relationship has been used to detect disturbances in the photosynthetic system caused by stress, as its decrease indicates a decline in the photochemical efficiency of PS2 and a disturbance of the photosynthetic apparatus [
46] (Percival and Fraser, 2001). All herbicides maintained significantly equal values for these variables, indicating that the treatments did not cause stress levels to the point of interfering with soybean photosynthesis. Furthermore, plants with Fv/Fm values between 0.75 and 0.85 quantum-1 electrons have their photosynthetic apparatus intact, and those with values lower than 0.75 quantum-1 electrons have their photosynthetic potential reduced [
47] (Reis and Campostrini, 2011), indicating that the cultivar used in this work presented adequate quantum yield values.
A higher electron transport rate in the presence of greater luminosity means that electron transport is occurring normally. If this value is low, it means that the excess light has damaged the photosynthetic apparatus [
48] (Maxwell and Johnson, 2000). Leaves exposed to higher light invest fewer resources in the formation of antenna complexes (they have a smaller amount of antenna complex II) and increase the levels of electron transporters (cytochrome, plastoquinone, plastocyanin, ferredoxin) and ATPase complexes per unit of chlorophyll [
49] (Freitas, 2016). This investment in carrier proteins in soybean leaves is reflected in higher values of the electron transport rate [
49] (Freitas, 2006).
Soybean is sensitive to photoperiod, and this characteristic is important for local adaptation. Suitable cultivars will make the most of the growing season in the target region [
50] (Lin et al., 2021). Adaptation is generally limited to a narrow range of latitudes, mainly because it is based on specific sensitivity to photoperiod [
50] (Lin et al., 2021). Generally speaking, in the tropics, the shorter the photoperiod, the faster the phenological development of soybeans [
50] (Lin et al., 2021). In this way, artificial light may have extemded this photoperiod, increasing the time to flowering and arrival at the R8 stage. On the other hand, in plots where there was no supplementation, the photoperiod was shorter, promoting shorter phenological development time.
Higher values of the average insertion height of the first pod in plants under light supplementation may be related to the effect of light on the phenological development of soybeans. Long days delay reproductive development and physiological maturity, prolonging the post-flowering phase and the duration of the critical period [
51] (Nico et al., 2015). Furthermore, another factor that influences the height of insertion of the first pod is the shading capacity of the area by the soybean population. Normally, with greater plant biomass, the insertion height of the first pod is higher. Thus, indirectly, supplementation also influences this indicator. The insertion height of the first pod is related to the efficiency of the harvester and consequently, to the crop yield. Therefore, in this experiment, losses with mechanical harvesting would be greater in plots without light supplementation [
52] (Kuzbakova et al., 2022).
The post-flowering photoperiod and its sensitivity positively affect soybean yield indicators [
50] (Lin et al., 2021), as seen by the increase in the number of grains per pod, number of pods per plant, and productivity. These factors regulate the length of the critical period for determining seed number and thus capturing resources such as radiation [
53,
54] (Kantolic and Slafer, 2005; Kantolic et al., 2013). Furthermore, the post-flowering photoperiod directly affects the partition between vegetative and reproductive structures [
51,
55] (Nico et al., 2015; Han et al., 2006) and meristem activity by modifying the number of nodes per plant [
51] (Nico et al., 2015). As the number of nodes is positively related to the number of pods, seeds, and yield [
56,
57] (Board et al., 1999; Egli, 2013), the effect of a longer photoperiod on nodes per m
2 will increase productivity. Additionally, the long photoperiod during post-flowering delays the onset of elongation of the first fruits within a node, prolongs flowering, and concomitantly, more flowers and fruits are produced at that node, usually on the lateral racemes [
51] (Nico et al., 2015).
The lower soybean productivity observed in plots without supplementation and with diclosulam may be related to the loss of the residual period of this herbicide and the control spectrum. Diclosulam is recommended to control eudicot plants. However, in the area, the most common weed was Eleusine indica, considered the main weed in soybean areas in Brazil today [
58,
59] (Araújo et al., 2023; Guo et al., 2023). The better conditions that light supplementation provided to the weeds may have impaired the residual effect of diclosulam. The same effect was not observed for plots with s-metolachlor, as this product is residual and recommended for controlling Eleusine indica [
25] (Shaner, 2014).
Regarding the effect of treatments on nutrient accumulation, a minor effect was observed only in relation to potassium. This nutrient is completely mobile and does not form organic compounds in plants. Therefore, it is always moving from the soil to the tissues in ionic form and can be an indicator of nutritional disturbance [
60] (Wakeel and Ishfaq, 2022). The decrease in potassium content in soybeans in the presence of fomesafen + clethodim may be related to the mechanism of action of these herbicides when applied together. Herbicides have selectivity for the crop. However, when applied at the same time, they can harm some indicators. The detrimental effect of tank herbicide mixtures on grain potassium content is reported by Younesabadi et al. (2013) [
61] and Abdel-Wahab et al. (2022) [
62]. Finally, the same effect was not observed for the mixture of flumioxazin + clethodim, as in these plots, soybean was more affected by the effect of flumioxazin and by the greater biomass of weeds in the area without supplementation. Thus, this effect outweighed the possible decrease in potassium content since soybeans produced less in these plots, and the content is relative to productivity.