Abstract
Drought is a key abiotic stress that confines agriculture development worldwide. Silicon (Si) is commonly considered to be a valuable element for resistance against drought and for sustainable agriculture. To investigate the morpho-physiological and biochemical characteristics of Gerbera jamesonii plants, a pot experiment was conducted under greenhouse conditions and exposed to water stress (60% FC) and well-watered (100% FC) conditions. Foliar application of Si was carried out after ten days (48 days after sowing) of drought treatment and was repeated weekly, while well-water was regarded as control. Water deficiency significantly abridged the morphological attributes, pigments, and stress-related metabolites and negatively affected the photosynthetic apparatus in drought-stressed gerbera plants. However, Si supplementation by 40 mg L−1 produced increased leaf area (31%), stem length (25%), flower diameter (22%), plant fresh biomass (17%), total chlorophyll (48%), and concentration of carotenoids (54%) in water-stressed plants. Similarly, the accretion of a total free amino acid (41%) and the activities of peroxidase, catalase, superoxide dismutase, ascorbate peroxidase, glycinebetaine, total soluble proteins, total free proline, and malondialdehyde were enhanced by 44%, 31%, 53%, 33%, 330%, 61%, 51%, and 66%, respectively, under drought stress in comparison with control conditions. Meanwhile, the photosynthetic rate (89%), the transpiration rate (12%), and stomatal conductance (55%) were significantly enhanced in water-deficit gerbera leaves with Si supplementation. This study proposes that the foliar application of Si is a viable and convenient method of improving the performance of elegant gerbera flower plants in regions of the world that are facing severe water deficiency.
1. Introduction
Silicon (Si) is the second-greatest copious mineral component existing in soil and silicon dioxide comprises around 50–70% of soil mass [1]. Along with different ecological functions, Si has multifaceted roles in plant mechanisms and in arbitrating relations with other organisms and the environment [2]. Si concentrations range from 0.1–10% of the dry biomass in a plant [3]. Although Si is not a crucial mineral substance, its advantageous effects have been established in many horticultural crops, such as cucumber, melons, tomatoes, and potatoes [4,5], particularly under stress conditions. Si plays a vital role in improving pest and disease resistance in plants, relieving the stress of heavy metal and UV radiations, enhancing drought resistance and nutrition deficiency tolerance, encouraging the growth of plants, and advancing photosynthetic activities and flower quality [6].
Climate models project decreasing rainfall frequency along with increasing temperatures that will increase the gap between actual and potential yields [7]. Plants are often subjected to environmental restraints and water deficiency is the most important abiotic stress that limits crop production worldwide [8,9]. Drought, a recurrent spectacle with significant influences on biodiversity, is a major extensive climatic extreme and will diminish crop yields by up to 30% by 2025, compared with current yields [10]. In parts of the world, the water resource’s potential to swell landscapes and enhance the cultivation of horticultural crops is vulnerable [11]. The distribution of water for the floricultural industry is rivalled by other necessities, such as human consumption, urban management, and agriculture, and must be utilized properly and with extreme efficacy [12]. Experts claimed that the drylands on earth will enhance by 30%, and drier summers along with decreased rainfall are predicted to disturb, primarily, southern Asia, southern Europe, and northern and southern Africa [13].
Private gardens and public green areas are attractive and striking due to the presence of colorful bedding plants [14]. These plants can suffer from drought constraints due to less water availability, especially when they have a small root system and are grown in pots [15]. Bedding plants always face a great risk of undergoing water stress during the nursery stage, due to their cultivation in limited spaces, which hinders the growth of roots and exposes the plants to severe drought stress. Additionally, substrate hydraulic conductivity (which is often organic), which is used for the cultivation of bedding plants, reduces speedily with minute variations in the content of water in the substrate [16]. This makes a plant’s water extraction very problematic, particularly when the water concentration in the substrate is stumpy. Plants may exhibit various response processes to water-deficiency, morphologically as well as physiologically [17]. At the whole-plant level, some plants enhance fresh root growth and dry weight to increase the uptake of water, thereby preserving the plant water state and safeguarding photosynthetic activities under drought stress [11]. During the nursery phase of bedding plant production, this adaptation response may be impossible, due to limited water availability and limited root growth in small pots [14].
Drought-stress exposure causes morphological variations in the shoots that smaller plant leaves produce, and older leaves drop down to minimize transpiration and, ultimately, water loss [18]. The leaf-area decline decreases photosynthesis and plant carbon gain. Photosynthesis declines significantly in plants that are under drought stress because of their high sensitivity to drought conditions [19]. Drought stress causes osmotic stress, harming enzymatic activity and the structure of perilous macromolecules [20]. Under drought stress, plants show an unevenness between generation and scavenging of reactive oxygen species (ROS), such as H2O2. Peroxidase (POX), catalase (CAT), and dismutase (SOD) are major ROS detoxification enzymes, while carotenoids are key non-enzymatic antioxidant metabolites [21]. The undue generation of ROS causes a range of destructive impacts, such as leakage of coupled electrolyte and peroxidation of lipids [22]. Although numerous experiments have studied the effect of water stresses in the alteration of photosynthesis in bedding plants [23], there is limited experiment-based information related to morpho-biochemical and physiological adjustments to drought by bedding plants.
Gerbera (Gerbera jamesonii) is one of the world’s most popular ornamental plants and is extensively used as a bedded plant, a potted plant, and a cut flower in a variety of climatic conditions [24]. It belongs to the Asteraceae family and, due to its attractive color variation, its stem length, its floral diameter, and its long adaptability for culture [25], gerbera is an ideal choice for cultivation in greenhouses in several countries. In the cut flower industry, it ranks fifth after roses, carnations, mums, and tulips [26].
Numerous studies have indicated that Si application to crops elevates their drought tolerance [8,27]. Therefore, crop plants use Si as a quasi-essential element for the mitigation of the effects of drought. The impact of Si application under drought stress, enhancing plant growth and biomass, has been observed in several ornamental species such as Callistemon [28], Bougainvillea [29], Pittosporum, Spiraea [30], and Chrysanthemum [31]. In these previous studies, the useful effects of Si were mainly due to its role in enhancing the production of antioxidants, upholding photosynthetic processes, and delaying plant senescence [32]. In addition to its increased-antioxidant activities, Si has been shown to increase redial hydraulic conductivity and to arbitrate stress tolerance in gerbera plants exposed to drought conditions [33]. Al-Maitah [34] argued that Si supplementation decreased osmotic stresses in plant cells and encouraged root activities, resulting in enhanced nutrient uptake and improved growth, as well as improved quality of gerbera flowers.
Due to the increasing frequency of drought that severely affects the growth and development of plants, analyzing the impacts of drought stress on plants provides a basis for hypothesizing about the impacts of future climate change on the production of plant species. Silicon provides strength to ornamental plants by making plant tissues strong and rigid under abiotic stress [34]. With this in mind, an experiment was conducted to investigate the impact of Si, under water-deficient conditions, on the important floricultural plant gerbera. In this study, we hypothesize that Si foliar application positively affects morphological, physiological, and biochemical mechanisms, thus increasing the performance of Gerbera jamesonii under drought stress.
2. Materials and Methods
2.1. Plant Collection, Experimental Treatments, and Layout
The present study was conducted from late October to mid-March 2021 in a greenhouse at the Research Area of the Horticultural Department, The Islamia University of Bahawalpur, Pakistan (29°22′42″ N, 71°45′53″ E). There was a natural photoperiod (approximately 14 h light), temperature (day/night) of 29/20 °C, and relative humidity of 75%.
Gerbera jamesonii cv. Ruby red nurseries at four-leaf stages were purchased from the local nursery and transplanted in terracotta pots (diameter of 18 cm; depth of 25 cm) filled with sandy loam soil (one plant per pot). The electrical conductivity and pH of the soil were 0.97 dS m−1 and 7.88, respectively. Arrangement of the pots was carried out following a two-factor factorial setup with a completely randomized design that was replicated four times. There were four treatments in the experiment, i.e., well-watered with tap water spray (W), foliar spray of Si under well-watered conditions (W + Si), drought stress with tap water spray (D), and foliar spray of Si with drought stress conditions (D + Si). Each treatment consisted of eight pots; there were 3.2 kg soil per pot.
Treatments to counteract drought stress, i.e., 60% FC (drought stress) and 100% FC (well-watered), were applied to every pot. A soil moisture sensor (ML3 ThetaProbe, Delta-T Devices Co., Burwell, UK) was fixed and used to determine the applied water quantity. Foliar treatment of Si at 40 mg Si L−1 was prepared by utilizing sodium metasilicate (Na2SiO3; MW = 122.06; Sigma–Aldrich, St. Louis, MO, USA). The first Si foliar spray was carried out after 10 days of applying the drought-stress treatment during the vegetative stage, and there were four sprays at weekly intervals. The spraying was carried out in the early morning (07:00 to 08:00 a.m.) via a compression layer with a capacity of 1 L. The pots were irrigated daily in a measured quantity to maintain the respective FCs during the experimental period.
2.2. Measurement of Morphological Parameters
Plant morphological parameters, such as the number of leaves, were recorded after the flowers reached full bloom. The stem length and the diameter of the flowers were noted with the help of digital caliper. For plant biomass, the plants were uprooted and washed with deionized water and oven dried at 70 °C for 48 h. Dry plant biomass was measured via electric balance (Sartorius, Basic, Bavaria, Germany). The leaf area was estimated with a leaf-area meter (Delta-T, Ltd., Cambridge, UK).
2.3. Determination of Amino Acids and Antioxidants Enzyme Activity
For the estimation of total free amino acids (TFA), fresh leaves (1.0 g) were used according to the suggestions of Hamilton and Van Slyke [35]. With the help of a spectrophotometer, the antioxidant enzymes, such as ascorbate peroxidase (APX), superoxide dismutase (SOD), catalase (CAT), and peroxidase (POX), were estimated. For this purpose, a 1.0 g fresh leaf sample was standardized in 50 mM buffer of phosphate (7.0 pH) and dithiothreitol (DTT), as explained by Dixit et al. [36]. The SOD and APX activities were estimated by following the procedure of Giannopolitis and Ries [37] and Cakmak [38], respectively. The methodology suggested by Zhang et al. [39] was adopted for the measurement of CAT and POX activities.
2.4. Detection of Pigments
About 1.0 g of fresh leaf sample was chosen for each treatment and sliced into 0.5 cm sections. Extraction was done at 4 °C overnight in 80% acetone (10 mL) for the determination of carotenoid (CAR) and levels of chlorophyll (Chl), following the procedures of Davis [40] and Arnon [41]. With the help of the formula, the Chla, Chlb, total Chl, and CAR concentrations were calculated after estimating the absorbance of the supernatant on a spectrophotometer (Hitachi, U-2800) at 645, 652, 663, and 480 nm.
where V is the sample volume, OD is the optical density indicating the wavelength at which the readings were recorded, W is the sample weight, and Acar = (OD 480) + 0.114 (OD 663) − 0.638 (OD 645); Emax 100 cm = 2500
Chla (mg g−1 FW) = [12.7 (OD 663) − 2.69 (OD 645)] × V/1000 × W
Chlb (mg g−1 FW) = [22.9 (OD 645) − 4.68 (OD 663)] × V/1000 × W
Chlt (mg g−1 FW) = [20.2 (OD 645) + 8.02 (OD 663)] × V/1000 × W
CAR (µg g−1 FW) = A car/Emax 100.
2.5. Determination of Glycinebetaine (GB) and Total Soluble Proteins
Glycinebetine (GB) was estimated via a 500 mg leaf sample (dry form) with 0.5% toluene (10 mL), which was kept overnight at 4 °C. A filtrate of about 1.0 mL was reacted with H2SO4 (1.0 mL). The extract (0.5 mL) and the solution of Kl3 (200 µL) were cooled. The 1–2 di-chloroethane (5 mL) and de-ionized H2O (2.8 mL) were added. The absorbance of the organic layer was determined by the spectrophotometer at λ 365 nm. Applying the method of Grieve and Grattan [42], the concentration of GB was confirmed by a curve. Moreover, fresh leaf (500 mg) was used for the estimation of soluble protein and extracted in an ice bath along with 10 mL potassium phosphate (50 mM). The aliquot was centrifuged at 4 °C for 15 min at 10,000× g. Applying the method of Bradford [43], the contents of protein in the extract were estimated.
2.6. Free Proline and MDA Contents Estimation
The concentration of free proline was estimated by using a fresh leaf sample (1.0 g) that was homogenized in 5 mL of 3% aqueous sulfosalicylic acid, as described by Ahmad et al. [44], for the determination of proline contents with the help of the spectrophotometer (Jenway, Staffordshire, UK). The malondialdehyde (MDA) value was estimated by using a sample of leaf in 5 mL 1.0% trichloroacetic acid (w/v), as proposed by Li et al. [45].
2.7. Determination of Photosynthetic Attribute
Photosynthetic synthetic attributes, such as the rate of photosynthesis and transpiration and stomatal conductance, were determined by using an infrared gas analyzer (LCA-4, Analytical Development Company, Hoddesdon, UK).
2.8. Statistical Analysis
With the help of computer software STATISTIX (version 8.1), the analysis of variance (ANOVA) procedure was adopted for statistical analysis of the collected data. The treatment means were compared, statistically, by the LSD at a 5% probability.
3. Results
3.1. Morphological Attributes
Drought stress (60% FC) noticeably reduced leaf numbers and the areas of gerbera by 11% and 38%, respectively, compared with well-water spray (control). The supplementation of Si enhanced both leaf characteristics by 7% and 8% in well-watered conditions and by 12% and 31% in drought-stress conditions (Figure 1a,b). A similar trend was noted with elevated stem length (29%) and flower diameter (22%), as Si supplementation increased both parameters under drought-stress conditions. There were 25% and 22% reductions in stem length and flower diameter, respectively, in water-deficient plants, as compared to control (Figure 1c,d). Foliar spray of Si enhanced plant fresh weight by 24% under controlled conditions and by 17% in drought-stress conditions (Figure 1e). Plant dry weight was also enhanced by 8% in drought-stressed plants (without Si supplementation), but Si foliar spray reduced dry weight by 17% with 60% FC and increased dry weight by 38% with 100% FC (Figure 1f).
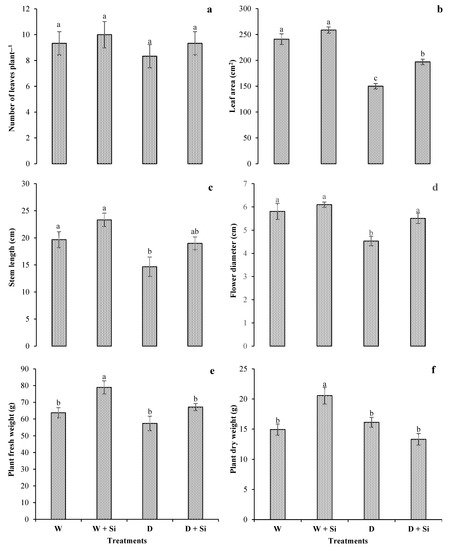
Figure 1.
Influence of Si supplementation on the number of leaves (a), the leaf area (b), the stem length (c), the flower diameter (d), the plant fresh weight (e), and the plant dry weight (f) of Gerbera jamesonii under water-deficient conditions. Each bar shows a mean of four replicates ± SE. Dissimilar lettering illustrates significant statistical differences at p ≤ 0.05 after applying LSD test.
3.2. Total Free Amino Acids and Antioxidants Enzyme
Statistically, the impact of drought stress significantly (p < 0.05) enhanced the accumulation of TFA by 130% compared with well-watered conditions (control), whereas Si foliar spray increased TFA contents by 40% under drought-stress conditions (Figure 2a). The antioxidant enzymatic activities were significantly enhanced under drought-stress conditions. For plants at 60% FC, Si foliar application increased POX, CAT, SOD, and APX by 44% (Figure 2b), 31% (Figure 2c), 53% (Figure 2d), and 33% (Figure 2e), respectively, compared with well-watered spray (control).
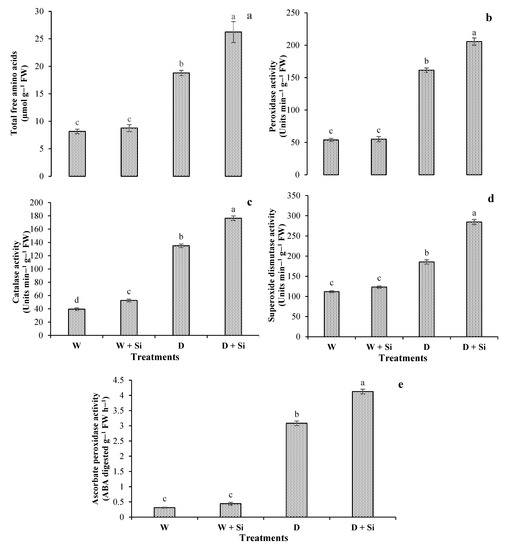
Figure 2.
Impact of foliar Si supplementation on accrual of osmoprotectants and activities of antioxidant enzymes, total free amino acids (a), peroxidase (b), catalase (c), superoxide dismutase (d) and ascorbate peroxidase (e) of Gerbera jamesonii under water-deficient conditions. Each bar shows a mean of four replicates ± SE. Dissimilar lettering illustrates significant statistical differences at p ≤ 0.05 after applying LSD test.
3.3. Pigments
Exposure of plants to water-deficient conditions significantly (p < 0.05) decreased the Chla (50%), Chlb (33%), Chlt (48%), and CAR (57%), compared with plants in well-watered conditions (control). However, gerbera plants with Si foliar spray showed increases of 44%, 51%, 48%, and 54% in leaf Chla (Figure 3a), Chlb (Figure 3b), Chlt (Figure 3c), and CAR (Figure 3d), respectively, compared with those irrigated under water-stressed regimes.
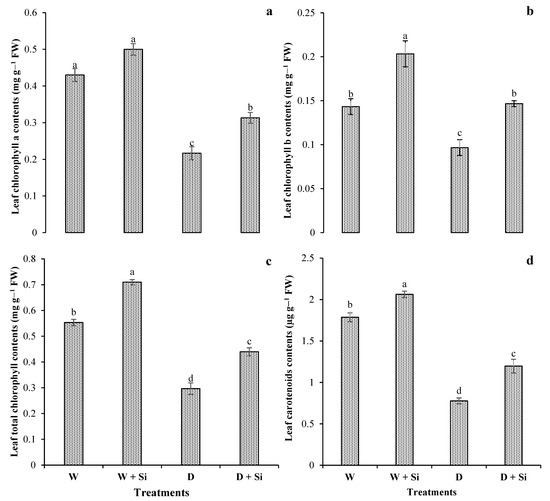
Figure 3.
Impact of foliar Si supplementation on leaf photosynthetic pigments, chlorophyll a (a), chlorophyll b (b), total chlorophyll (c), and carotenoid contents (d) of Gerbera jamesonii under water-deficient conditions. Each bar shows a mean of four replicates ± SE. Dissimilar lettering illustrates significant statistical differences at p ≤ 0.05 after applying LSD test.
3.4. GB and Soluble Proteins
The application of Si significantly (p < 0.05) enhanced GB concentration in both well-watered and water-stressed regimes. There was an increase of 112% and 330% under well-watered and water-deficit conditions, respectively (Figure 4a). Drought treatment without Si spray reduced GB contents by 49%, compared with plants in well-watered conditions (control). A similar trend was also recorded for total soluble protein, as Si application enhanced total soluble protein by 24% (well-watered conditions) and 61% (drought-stress conditions), respectively, compared with well-watered irrigated plants (Figure 4b).

Figure 4.
Effect of foliar Si supplementation on glycinebetaine (a) and total soluble proteins (b) of Gerbera jamesonii under water-deficient conditions. Each bar shows a mean of four replicates ± SE. Dissimilar lettering illustrates significant statistical differences at p ≤ 0.05 after applying LSD test.
3.5. Free Proline and MDA
Free proline contents were significantly enhanced under drought-stressed gerbera plants, compared with well-watered irrigated plants. Proline levels increased by 38% and 51%, respectively, due to Si supplementation in both well-watered and water-stressed gerbera plants (Figure 5a). However, without Si foliar spray, drought-stressed (60% FC) plants showed 83% proline enhancement, compared with plants with 100% FC. MDA concentration also showed that Si supplementation was effectively enhanced by 66% (drought-stress conditions) and 20% (well-watered conditions), while the plants under drought stress reduced MDA level by 13%, compared with well-watered (control) plants (Figure 5b).
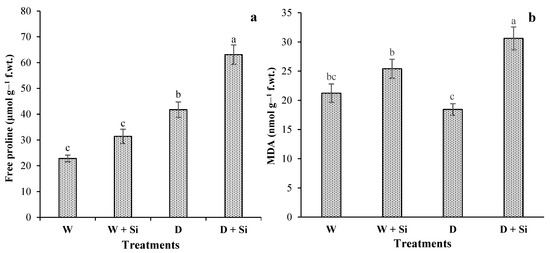
Figure 5.
Impact of foliar Si supplementation on free proline (a) and MDA (b) of Gerbera jamesonii under water-deficient conditions. Each bar shows a mean of four replicates ± SE. Dissimilar lettering illustrates significant statistical differences at p ≤ 0.05 after applying LSD test.
3.6. Photosynthetic Attributes
Drought-stress conditions markedly abridged photosynthetic attributes such as A, E, and gs by 38%, 7%, and 27%, respectively, in well-watered conditions. The supplementation of Si enhanced A (28% and 89%), E (9% and 12%), and gs (29% and 55%) with 100% FC and 60% FC conditions, respectively (Figure 6a–c).
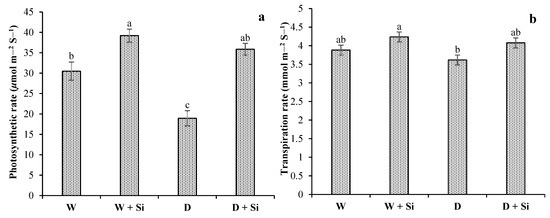
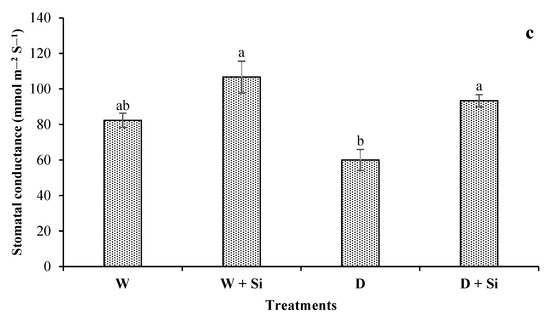
Figure 6.
Impact of foliar Si supplementation on photosynthetic rate (a), transpiration rate (b), and stomatal conductance (c) of Gerbera jamesonii under water-deficient conditions. Each bar shows an average of four replicates ± SE. Dissimilar lettering illustrates significant statistical differences at p ≤ 0.05 after applying LSD test.
4. Discussion
Water availability is the most important factor in the cultivation of ornamental plants, especially those with shallow root systems [14]. In the nursery stage, the generalized container usage, often of small volume, determines the impact of root constraints [46]. In cities, it is imperative to choose plant types that survive under drought conditions without losing their plant quality [47]. Bedding ornamental plants are sensitive to drought stress. In these plants, the first response to water shortage is the reduction in plant growth. In the present experiment, water-deficient conditions noticeably decreased external quality traits such as the number of leaves, the leaf area, the stem length, the flower diameter, and the plant biomass, i.e., the fresh and dry weight of the plant. All of these parameters are very important, and market value is often determined by these external quality traits in both potted plants and cut flowers [48]. Previous experiments revealed that plant growth parameters in Zinnia [14] and Adonis species [49] decreased significantly in drought-stress conditions Water status and the production of biomass are supposed to be the standards for evaluating plant behavior in response to osmotic stress [50]. Abridged biomass accumulation results from water deficiency because of the reduction in leaf biomass, which is caused by the decrease in the number and sizes of the leaves [51]. In situations of drought stress, the reduction in plant growth and biomass is linked to a decrease in the expansion and elongation of cells, as the diminished absorption of water leads to a decrease in the production of metabolites to uphold the regular activities of cells [47].
The supplementation of Si under both well-watered and drought-stress conditions markedly bettered the growth of gerbera plants. The application of Si is known to arbitrate biochemical and physiological processes to enhance biomass accumulation in plants [52]. Similarly, in the current study, Si augmented morphological growth characteristics under water-deficit conditions. Kamenidou et al. [33] showed increased gerbera flower diameter and stem length when Si was applied under a hydroponic nutrient solution. Hameed et al. [53] proposed that Si supplementation enhances the protein contents in plant seedlings that provide amino acids and energy to counter the harmful effects of drought stress. This study confirms the results of Zomorrodi et al. [48], who stated that Si supplementation offers improvement in the ornamental quality of flowering plants, independently of water accessibility, under drought-stress conditions.
Silicon foliar application remarkably enhanced the accrual of TFA, which assisted water-deficient gerbera to maintain a level of water. This effect can be contingent on data showing that Si arouses the activity of amylase to boost the decomposition of starch during drought stress. Previous studies on the Zinnia elegance [14] and Iris species [54] are concordant with our results, suggesting that acquaintance with drought stress results in the biosynthesis and accretion of the amino acids that vigorously take part in the adjustment of osmotic potential under drought-stress conditions. The augmented antioxidant enzymatic activities show increased production of reactive oxygen species (ROS) under water-deficient conditions [55]. The enzymes are responsible for O2− and H2O2 detoxification and assist in averting the highly poisonous HO− formation [56]. A significant increment in POX, CAT, SOD, and APX production by Si application provides an additional indication that Si controls the spontaneous dismutation of O2− and H2O2, or can be responsible directly for the quenching of O2− and OH− in cells [57]. Early reports on ornamental sunflower [56], Rosa damascena [11], and gerbera [24] also proposed a rise in antioxidant activity in plants supplemented by Si during drought stress. The current study is in agreement about the improvement of CAT and POX capabilities to scavenge H2O2 from the chloroplast [58], due to Si supplementation. In scavenging ROS, the antioxidant enzymes—especially APX and SOD—have a major role. APX acts directly on H2O2 molecules and converts them to water. SOD carries out the dismutation reaction by dropping the O2− molecule to H2O2: APX converts H2O2 to water, thus achieving the ROS removal [59]. The capacity of antioxidant enzyme activities in ROS scavenging and minimizing harmful impact may be related to the drought resistance of plants [60].
Chlorophylls are indicative of stressful conditions of a plant under abiotic stress. Biosynthesis and the conservation of photosynthetic pigments are latent predictors of drought resistance in plants [61]. The intensity of the green color on leaves generally assists as a vital gauge of plant health [48]. The current study showed that plants under water-deficient conditions (60% FC) were severely affected and caused a noticeable decrease in Chla, Chlb, Chlt, and CAR concentrations, compared with plants with 100% FC. This reduction in chlorophyll pigments might be linked to the decline in methionine and cysteine concentrations under drought stress, which are key constituents of chloroplast target proteins [62]. A shortage of water supply results in oxidative impairment or ROS overproduction, which diminishes chlorophyll because of photo-oxidative injury to pigments [63]. Carotenoids (non-enzymatic antioxidant) are major metabolites for the appropriate functioning of photosynthetic apparatus during fluctuations of light intensity [64]. Furthermore, drought-stressed plants are associated with decreased CAR contents and the elevation of antioxidant enzymatic activity [48]. The foliar application of Si substantially reduces the damage to photosynthetic pigments and significantly enhances the carotenoid contents in tissues of leaves under drought-stress conditions. Carotenoids transmit photochemical energy to chlorophyll to facilitate photosynthesis [65]. These reports accord with the findings of previous studies in other ornamental bedding plants, such as marigold [66], Zinnia [14], and sunflower [67]. Beneficial Si impacts are associated with activities of antioxidant enzyme upregulation that protect the structure of chloroplast to maintain chlorophyll contents under drought-stress conditions [60].
In plants, for the management of the lethal impacts of ROS, a defensive mechanism is started for drought-stress resistance, including the production of solutes such as GB [68]. In the present study, Si supplementation significantly increased GB under both drought-stress and well-watered conditions. The elevated contents of this osmolyte in plants might be responsible for various processes such as the integrity of the membrane, ROS detoxification, and osmotic adjustment for drought resistance in plants [69]. In plants, the total accumulation of soluble protein content varies from species to species [56]. In the present study, total soluble proteins were remarkably enhanced by the addition of Si in both well-watered and drought-stressed plants. The increase in soluble proteins might be one of the prime mechanisms for the mitigation by Si of drought-stress injury [70]. Similar reports were provided by Rastogi et al. [71], who found an increased soluble-protein concentration in strawberry leaves that was induced by Si supplementation, which elevates photosynthetic activities under abiotic stress.
Plants under water-deficient conditions accrue compatible solutes, such as proline, for facilitating water absorption, minimizing cell damage, and enhancing the osmotic potential of cells [72]. The accrual of proline is a general marker of water-deficiency tolerance and permits osmotic change that results in cell dehydration avoidance and water retention [73]. In our study, drought-stressed plants showed remarkably higher proline concentrations than well-watered (control) irrigated plants of gerbera. Proline accumulation under abiotic strain in several crops has been linked to tolerance of stress; proline content has been higher in stress-tolerant plants than in stressed-sensitive plant species [74]. MDA (a producer of lipid peroxidation) has been regarded as a gauge of oxidative injury [56]. It is generally considered the greatest physiological component of drought resistance in plants [75]. The present study revealed a reduction in MDA contents under drought-stress conditions; however, with Si supplementation, MDA contents increased in gerbera plants under both water-deficient and well-watered conditions. Enhanced MDA concentrations with Si supplements under drought-stress conditions showed a strong encouraging effect on membrane protection from abiotic stress injury [76]. These findings from the current experiment are in contrast to the recommendations of Zahedi et al. [77], who stated that Si supplementation reduced MDA contents with increased antioxidant enzymatic activities. The increase in MDA contents during drought conditions also showed that, despite the existence of an antioxidant mechanism, drought stress may cause peroxidation of lipids in the leaves of plants [78].
The rates of gas exchange are severely affected by drought-stress conditions. The primary site of stress in the photosynthetic apparatus of plants is extremely delicate in response to water shortage; as a result, photosynthetic activity is decreased, due to the closure of stomata and complicated non-gassing impacts [79]. In the present research, the photosynthetic rate (A), the transpiration rate (E), and stomatal conductance (gs) were remarkably reduced in water-deficit plants, compared with well-watered and irrigated plants. As water-deficient plants decrease A, E, and gs, the application of Si can enhance water-use efficiency by reducing photosynthetic attributes. Nevertheless, the supplementation of Si considerably improved these reductions in gerbera leaves. It can be speculated that the application of Si permitted water-deficient plants to retain higher chlorophyll contents and water-use efficiency, thereby improving photosynthesis. Improvement in the gs level by Si supplementation showed that regulation of gaseous exchange (CO2 and water) can allow plants under well-watered growth conditions to enhance their CO2 uptake and, thereafter, increase photosynthesis [77]. Toscano and Romano [14] argued that the supplementation of Si enhanced the photosynthetic enzymatic activities that are involved in the Si-induced regulation of photosynthesis in Zinnia under drought stress.
5. Conclusions
As an effective quasi-vital element, Si plays a key part in elevating the drought tolerance of elegant gerbera plants. Positive impacts of exogenous Si supplementation on the morphological attributes and the plant biomass were recorded to be linked with Si-mediated improvement in physiological processes, including the maintenance of turgor by the accrual of osmolytes, such as free amino acids, and enhancement of chlorophyll and carotenoid contents. Silicon diminishes the injury caused by drought stress through different biochemical processes, including the stimulation of antioxidant enzymatic machinery and the uplifting of the stress-related metabolites under drought-stress conditions. Water-deficient plants severely reduced soluble proteins and gaseous exchange rates, but the addition of Si might alleviate such adversative effects. The addition of Si helped plants to increase nutrient availability and improved the performance of underwater photosynthetic apparatus applications.
Author Contributions
Conceptualization, methodology, validation, and formal analysis: M.A., F.N., M.R., H.T.A., M.S., A.M. and S.K.; writing—original draft preparation, writing—review and editing, visualization, supervision, and funding acquisition: M.V., E.A.M., R.C., H.O.E., R.C., E.R. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The Researchers Supporting Project number (RSP2023R118), King Saud University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data in this publication are available.
Acknowledgments
The authors would like to thank the Researchers Supporting Project number (RSP2023R118), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Yin, J.L.; Jia, J.H.; Lian, Z.Y.; Hu, Y.H.; Guo, J.; Huo, H.Q.; Zhu, Y.X.; Gong, H.J. Silicon enhances the salt tolerance of cucumber through increasing polyamine accumulation and decreasing oxidative damage. Ecotoxicol. Environ. Saf. 2019, 169, 8–17. [Google Scholar] [CrossRef]
- Gao, H.; Wenying, Y.; Xiaoqing, Y.; Jiahui, L.; Xiwu, S.; Maoxiang, S.; Yuansong, X.; Futian, P. Silicon enhances the drought resistance of peach seedlings by regulating hormone, amino acid, and sugar metabolism. BMC Plant Biol. 2022, 22, 422. [Google Scholar] [CrossRef] [PubMed]
- Seifikalhor, M.; Niknam, V.; Aliniaeifard, S.; Didaran, F.; Tsaniklidis, G.; Fanourakis, D.; Teymoorzadeh, M.; Mousavi, S.H.; Bosacchi, M.; Li, T. The regulatory role of γ-aminobutyric acid in chickpea plants depends on drought tolerance and water scarcity level. Sci. Rep. 2022, 12, 7034. [Google Scholar] [CrossRef]
- Ali, N.; Schwarzenberg, A.; Yvin, J.-C.; Hosseini, S.A. Regulatory Role of Silicon in Mediating Differential Stress Tolerance Responses in Two Contrasting Tomato Genotypes under Osmotic Stress. Front. Plant Sci. 2018, 9, 1475. [Google Scholar] [CrossRef]
- Ahsan, M.; Nafees, M.; Amin, M.; Nawaz, F.; Tufail, A.; Sardar, H.; Shokralla, S.; Mahmoud, E.A.; El-Sabrout, A.M.; Elansary, H.O. Nutrients Uptake and Accumulation in Plant Parts of Fragrant Rosa Species Irrigated with Treated and Untreated Wastewater. Plants 2022, 11, 1260. [Google Scholar] [CrossRef]
- Schwalm, C.R.; Anderegg, W.R.L.; Michalak, A.M.; Fisher, J.B.; Biondi, F.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Wolf, A.; et al. Global patterns of drought recovery. Nature 2017, 548, 202–205. [Google Scholar] [CrossRef]
- Hessini, K.; Wasli, H.; Al-Yasi, H.M.; Ali, E.F.; Issa, A.A.; Hassan, F.A.S.; Siddique, K.H.M. Graded Moisture Deficit Effect on Secondary Metabolites, Antioxidant, and Inhibitory Enzyme Activities in Leaf Extracts of Rosa damascena Mill. var. trigentipetala. Horticulturae 2022, 8, 177. [Google Scholar] [CrossRef]
- Jafari, S.; Garmdareh, S.E.H.; Azadegan, B. Effects of drought stress on morphological, physiological, and biochemical characteristics of stock plant (Matthiola incana L.). Sci. Hortic. 2019, 253, 128–133. [Google Scholar] [CrossRef]
- IPCC. Intergovernmental panel on climate change. In 5th Assessment Report, WGII, Climate Change 2014: Impacts, Adaptation, and Vulnerability; Cambridge University Press: Cambridge, UK, 2014; Available online: http://www.ipcc.ch/report/ar5/wg2/ (accessed on 16 July 2018).
- Toscano, S.; Romano, D. Morphological, Physiological, and Biochemical Responses of Zinnia to Drought Stress. Horticulturae 2021, 7, 362. [Google Scholar] [CrossRef]
- Heidari, Z.; Nazarideljou, M.J.; Danesh, Y.R.; Khezrinejad, N. Morphophysiological and Biochemical Responses of Zinnia elegans to Different Irrigation Regimes in Symbiosis with Glomus mosseae. Int. J. Hortic. Sci. 2016, 3, 19–32. [Google Scholar] [CrossRef]
- Talbi, S.; Rojas, J.A.; Sahrawy, M.; Rodríguez-Serrano, M.; Cárdenas, K.E.; Debouba, M.; Sandalio, L.M. Effect of drought on growth, photosynthesis and total antioxidant capacity of the saharan plant Oudeneya africana. Environ. Exp. Bot. 2020, 176, 104099. [Google Scholar] [CrossRef]
- Cal, A.J.; Sanciangco, M.; Rebolledo, M.C.; Luquet, D.; Torres, R.O.; McNally, K.L.; Henry, A. Leaf morphology, rather than plant water status, underlies genetic variation of rice leaf rolling under drought. Plant Cell Environ. 2019, 42, 1532–1544. [Google Scholar] [CrossRef]
- Nemali, K.S.; Bonin, C.; Dohleman, F.G.; Stephens, M.; Reeves, W.R.; Nelson, D.E.; Castiglioni, P.; Whitsel, J.E.; Sammons, B.; Silady, R.A.; et al. Physiological responses related to increased grain yield under drought in the first biotechnology-derived drought-tolerant maize. Plant Cell Environ. 2015, 38, 1866–1880. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Yousefzadeh, K.; Houshmand, S.; Shiran, B.; Mousavi-Fard, S.; Zeinali, H.; Nikoloudakis, N.; Gheisari, M.M.; Fanourakis, D. Joint Effects of Developmental Stage and Water Deficit on Essential Oil Traits (Content, Yield, Composition) and Related Gene Expression: A Case Study in Two Thymus Species. Agronomy 2022, 12, 1008. [Google Scholar] [CrossRef]
- Ahmadi-Majd, M.; Mousavi-Fard, S.; Nejad, A.R.; Fanourakis, D. Carbon nanotubes in the holding solution stimulate flower opening and prolong vase life in carnation. Chem. Biol. Technol. Agric. 2022, 9, 15. [Google Scholar] [CrossRef]
- Ahmadi-Majd, M.; Nejad, A.R.; Mousavi-Fard, S.; Fanourakis, D. Postharvest application of single, multi-walled carbon nanotubes and nanographene oxide improves rose keeping quality. J. Hortic. Sci. Biotechnol. 2021, 97, 346–360. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S.; Siddique, K.H.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Uzma, J.; Talla, S.K.; Madam, E.; Mamidala, P. Assessment of Salinity Tolerance Deploying Antioxidant Defense Systems in Gerbera Jamesonii. Biosci. Biotechnol. Res. Asia 2022, 19, 243–254. [Google Scholar] [CrossRef]
- Hajizadeh, H.S.; Asadi, M.; Zahedi, S.M.; Hamzehpour, N.; Rasouli, F.; Helvacı, M.; Alas, T. Silicon dioxide-nanoparticle nutrition mitigates salinity in gerbera by modulating ion accumulation and antioxidants. Folia Hortic. 2021, 33, 91–105. [Google Scholar] [CrossRef]
- da Silva, D.P.C.; Paiva, P.D.D.O.; Herrera, R.C.; Porto, J.M.P.; dos Reis, M.V.; Paiva, R. Effectiveness of silicon sources for in vitro development of gerbera. Plant Cell Tissue Organ Cult. 2020, 141, 77–85. [Google Scholar] [CrossRef]
- Amin, M.; Ahmad, R.; Ali, A.; Hussain, I.; Mahmood, R.; Aslam, M.; Lee, D.J. Influence of Silicon Fertilization on Maize Performance under Limited Water Supply. Silicon 2016, 10, 177–183. [Google Scholar] [CrossRef]
- Álvarez, S.; Sánchez-Blanco, M.J. Comparison of individual and combined effects of salinity and deficit irrigation on physiological, nutritional and ornamental aspects of tolerance in Callistemon laevis plants. J. Plant Physiol. 2015, 185, 65–74. [Google Scholar] [CrossRef]
- Cirillo, C.; De Micco, V.; Rouphael, Y.; Balzano, A.; Caputo, R.; De Pascale, S. Morpho-anatomical and physiological traits of two Bougainvillea genotypes trained to two shapes under deficit irrigation. Trees 2017, 31, 173–187. [Google Scholar] [CrossRef]
- Elansary, H.O.; Salem, M.Z.M. Morphological and physiological responses and drought resistance enhancement of ornamental shrubs by trinexapac ethyl application. Sci. Hortic. 2015, 189, 1–11. [Google Scholar] [CrossRef]
- Hajipour, H.; Zohreh, J. Effect of foliar application of silicon on physiological responses of chrysanthemum (Dendranthema × grandiflorum) at two different growth stages. J. Ornam. Plants 2015, 6, 39–47. [Google Scholar]
- Hosseini, S.A.; Maillard, A.; Hajirezaei, M.R.; Ali, N.; Schwarzenberg, A.; Jamois, F.; Yvin, J.-C. Induction of Barley Silicon Transporter HvLsi1 and HvLsi2, increased silicon concentration in the shoot and regulated Starch and ABA Homeostasis under Osmotic stress and Concomitant Potassium Deficiency. Front. Plant Sci. 2017, 8, 1359. [Google Scholar] [CrossRef] [PubMed]
- Kamenidou, S.; Cavins, T.J.; Marek, S. Silicon supplements affect floricultural quality traits and elemental nutrient concentrations of greenhouse produced gerbera. Sci. Hortic. 2010, 123, 390–394. [Google Scholar] [CrossRef]
- Al-Maitah, S.S. Silicon mitigate salinity stress on gerbera cut flower. Nat. App. Sci. 2022, 37, 2. [Google Scholar]
- Hamilton, P.B.; Van Slyke, D.D. Amino acids determination with ninhydrin. J. Biol. Chem. 1943, 150, 231–233. [Google Scholar] [CrossRef]
- Dixit, V.; Pandey, V.; Shyam, R. Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J. Exp. Bot. 2001, 52, 1101–1109. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase I. Occurrence in higher plants. Plant Physiol. 1997, 59, 309–314. [Google Scholar] [CrossRef]
- Cakmak, I. Activity of ascorbate-dependent H2O2-scavenging enzymes and leaf chlorosis are enhanced in magnesium- and potassium-deficient leaves, but not in phosphorus-deficient leaves. J. Exp. Bot. 1994, 45, 1259–1266. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Yang, X.; Xia, Z. Effects of Sodium Selenite and Germination on the Sprouting of Chickpeas (Cicer arietinum L.) and Its Content of Selenium, Formononetin and Biochanin A in the Sprouts. Biol. Trace Elem. Res. 2012, 146, 376–380. [Google Scholar] [CrossRef]
- Davies, B. “Carotenoids”, in Chemistry and Biochemistry of Plant Pigments; Goodwin, T.W., Ed.; Academic Press: London, UK, 1976; pp. 38–165. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annu. Rev. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ahmad, P.; John, R.; Sarwat, M.; Umar, S. Responses of proline, lipid peroxidation and antioxidative enzymes in two varieties of Pisum sativum L. under salt stress. Int. J. Plant. Prod. 2008, 2, 353–366. [Google Scholar] [CrossRef]
- Li, G.; Wan, S.; Zhou, J.; Yang, Z.; Qin, P. Leaf chlorophyll fluorescence, hyperspectral reflectance, pigments content, malondialdehyde and proline accumulation responses of castor bean (Ricinus communis L.) seedlings to salt stress levels. Ind. Crops Prod. 2010, 31, 13–19. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Tribulato, A.; Romano, D. Leaf physiological and anatomical responses of Lantana and Ligustrum species under different water availability. Plant Physiol. Biochem. 2018, 127, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Chyliński, W.K.; Łukaszewska, A.J.; Kutnik, K. Drought response of two bedding plants. Acta Physiol. Plant. 2007, 29, 399–406. [Google Scholar] [CrossRef]
- Zomorrodi, N.; Nejad, A.R.; Mousavi-Fard, S.; Feizi, H.; Tsaniklidis, G.; Fanourakis, D. Potency of Titanium Dioxide Nanoparticles, Sodium Hydrogen Sulfide and Salicylic Acid in Ameliorating the Depressive Effects of Water Deficit on Periwinkle Ornamental Quality. Horticulturae 2022, 8, 675. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Y.; Yu, S.; Huang, Y.; Liu, H.; Chen, W.; He, X. Effects of drought stress on growth, physiology and secondary metabolites of Two Adonis species in Northeast China. Sci. Hortic. 2020, 259, 108795. [Google Scholar] [CrossRef]
- Damalas, C.A. Improving drought tolerance in sweet basil (Ocimum basilicum) with salicylic acid. Sci. Hortic. 2019, 246, 360–365. [Google Scholar] [CrossRef]
- Hessini, K.; Issaoui, K.; Ferchichi, S.; Saif, T.; Abdelly, C.; Siddique, K.H.; Cruz, C. Interactive effects of salinity and nitrogen forms on plant growth, photosynthesis and osmotic adjustment in maize. Plant Physiol. Biochem. 2019, 139, 171–178. [Google Scholar] [CrossRef]
- Bukhari, M.A.; Ahmad, Z.; Ashraf, M.Y.; Afzal, M.; Nawaz, F.; Nafees, M.; Jatoi, W.N.; Malghani, N.A.; Shah, A.N.; Manan, A. Silicon Mitigates Drought Stress in Wheat (Triticum aestivum L.) through Improving Photosynthetic Pigments, Biochemical and Yield Characters. Silicon 2020, 13, 4757–4772. [Google Scholar] [CrossRef]
- Hameed, A.; Farooq, T.; Hameed, A.; Sheikh, M.A. Silicon mediated priming induces acclimation to mild water-deficit stress by altering physio-biochemical attributes in wheat plants. Front. Plant Sci. 2021, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Sarvandi, S.; Ehtesham, A.N.; Rezaei, A.N.; Azimi, M.H. Morpho-physiological responses of some iris cultivars under drought and salinity stresses. J. Agric. Sci. Tech. 2020, 22, 535–546. [Google Scholar]
- Nawaz, F.; Naeem, M.; Ashraf, M.Y.; Tahir, M.N.; Zulfiqar, B.; Salahuddin, M.; Shabbir, R.N.; Aslam, M. Selenium Supplementation Affects Physiological and Biochemical Processes to Improve Fodder Yield and Quality of Maize (Zea mays L.) under Water Deficit Conditions. Front. Plant Sci. 2016, 7, 1438. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.; Zulfiqar, H.; Farooq, M.A.; Ali, S.; Tufail, A.; Kanwal, S.; Shaheen, M.R.; Sajid, M.; Gul, H.; Jamal, A.; et al. Strigolactone (GR24) Application Positively Regulates Photosynthetic Attributes, Stress-Related Metabolites and Antioxidant Enzymatic Activities of Ornamental Sunflower (Helianthus annuus cv. Vincent’s Choice) under Salinity Stress. Agriculture 2022, 13, 50. [Google Scholar] [CrossRef]
- Farman, M.; Nawaz, F.; Majeed, S.; Javeed, H.M.R.; Ahsan, M.; Ahmad, K.S.; Aurangzaib, M.; Bukhari, M.A.; Shehzad, M.A.; Hussain, M.B. Silicon Seed Priming Combined with Foliar Spray of Sulfur Regulates Photosynthetic and Antioxidant Systems to Confer Drought Tolerance in Maize (Zea mays L.). Silicon 2022, 14, 7901–7917. [Google Scholar] [CrossRef]
- Khoshbakht, D.; Asghari, M.R.; Haghighi, M. Effects of foliar applications of nitric oxide and spermidine on chlorophyll fluorescence, photosynthesis and antioxidant enzyme activities of citrus seedlings under salinity stress. Photosynthetica 2018, 56, 1313–1325. [Google Scholar] [CrossRef]
- Amiri, R.; Nikbakht, A.; Etemadi, N. Alleviation of drought stress on rose geranium [Pelargonium graveolens (L.) Herit.] in terms of antioxidant activity and secondary metabolites by mycorrhizal inoculation. Sci. Hortic. 2015, 197, 373–380. [Google Scholar] [CrossRef]
- Dehghanipoodeh, S.; Ghobadi, C.; Baninasab, B.; Gheysari, M.; Shiranibidabadi, S. Effect of Silicon on Growth and Development of Strawberry under Water Deficit Conditions. Hortic. Plant J. 2018, 4, 226–232. [Google Scholar] [CrossRef]
- Naeem, M.; Naeem, M.S.; Ahmad, R.; Ahmad, R. Foliar-applied calcium induces drought stress tolerance in maize by manipulating osmolyte accumulation and antioxidative responses. Pak. J. Bot. 2017, 49, 427–434. [Google Scholar]
- Parveen, A.; Liu, W.; Hussain, S.; Asghar, J.; Perveen, X.Y. Silicon priming regulates morpho-physiological growth and oxidative metabolism in maize under drought stress. Plants 2019, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.; Tissue, D.T.; Cazzonelli, C.I. Leaf-age dependent response of carotenoid accumulation to elevated CO2 in Arabidopsis. Arch. Biochem. Biophys. 2018, 647, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Seif, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Shomali, A.; Fanourakis, D.; Li, T.; Woltering, E. Monochromatic red light during plant growth decreases the size and improves the functionality of stomata in chrysanthemum. Funct. Plant Biol. 2021, 48, 515. [Google Scholar] [CrossRef] [PubMed]
- Asrar, A.-W.A.; Elhindi, K.M. Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2011, 18, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kiani, S.P.; Maury, P.; Sarrafi, A.; Grieu, P. QTL analysis of chlorophyll fluorescence parameters in sunflower (Helianthus annuus L.) under well-watered and water-stressed conditions. Plant Sci. 2008, 175, 565–573. [Google Scholar] [CrossRef]
- Parveen, A.; Saleem, M.H.; Kamran, M.; Haider, M.Z.; Chen, J.-T.; Malik, Z.; Rana, M.S.; Hassan, A.; Hur, G.; Javed, M.T.; et al. Effect of Citric Acid on Growth, Ecophysiology, Chloroplast Ultrastructure, and Phytoremediation Potential of Jute (Corchorus capsularis L.) Seedlings Exposed to Copper Stress. Biomolecules 2020, 10, 592. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Souri, Z.; Khanna, K.; Karimi, N.; Ahmad, P. Silicon and Plants: Current Knowledge and Future Prospects. J. Plant Growth Regul. 2020, 14, 1–20. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Pan, K.; Li, W.; Zhang, L.; Shen, X.; Liu, L.; Deng, M. Responses of the antioxidant defense system to drought stress in the leaves of Fargesia denudata seedlings, the staple food of the giant panda. Russ. J. Plant Physiol. 2019, 3, 374–383. [Google Scholar] [CrossRef]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhris, M.; Ben Abdallah, F. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2009, 67, 345–352. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.; Wang, L.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Babaei, K.; Moghaddam, M.; Farhadi, N.; Pirbalouti, A.G. Morphological, physiological and phytochemical responses of Mexican marigold (Tagetes minuta L.) to drought stress. Sci. Hortic. 2021, 284, 110116. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The Role of Silicon in Higher Plants under Salinity and Drought Stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Hosseini, M.S.; Hoveizeh, N.F.; Kadkhodaei, S.; Vaculík, M. Comparative morphological, physiological and molecular analyses of drought-stressed strawberry plants affected by SiO2 and SiO2-NPs foliar spray. Sci. Hortic. 2023, 309, 111686. [Google Scholar] [CrossRef]
- Oraee, A.; Tehranifar, A.; Nezami, A.; Shoor, M. Effects of drought stress on cold hardiness of non-acclimated viola (Viola × wittrockiana ‘Iona Gold with Blotch’) in controlled conditions. Sci. Hortic. 2018, 238, 98–106. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).