Abstract
Styrian hull-less pumpkins are valued for their use in health-promoting foods such as oils and snacks. Although deriving from Styria, they are now cultivated globally. Seeds of Styrian oil pumpkins are rich in lipids and represent a high-value product. Thus, seed yield and quality are traits of economic importance. These seed characteristics depend mostly on the cultivated variety and plant growing conditions. This study aimed to assess the variation in hull-less seed lipids of new hybrids of Styrian oil pumpkin developed recently for cultivation in moderate/colder climate zones of the north-eastern part of Poland. The study showed that the newly-developed hybrids have a relatively high content of total lipids in seeds, with a substantially higher share of linoleic acid (up to 63%). However, sterols, tocopherols, and squalene content and composition were similar to pumpkin seed oils produced from plants cultivated in other, warmer regions. This study provides insights into the chemical composition of Styrian pumpkin oil produced from hybrids grown under the relatively severe climatic conditions of north-eastern Poland.
1. Introduction
Pumpkin fruit preparations obtained from Cucurbita crop species have numerous positive effects on the human body and health [1,2,3] and can be classified as functional foods [4]. Recent reviews by Kaur et al. [3] and Sharma et al. [4,5] showed that this fruit has an abundance of bioactive compounds such as phytosterols, carotenoids, alkaloids, flavonoids, polyphenols, tannins, tocopherols, squalene, and cucurbitacins. Previous studies have accounted for numerous health benefits, including antidiabetic, antioxidant, anticarcinogenic, hypotensive, antiulcer, and antimicrobial activities. These activities appear to be likely since results are based on a significant amount of research, primarily conducted in recent decades. They indicate the health-promoting effects of pumpkin fruit extracts, oils, preparations, or preserves (more details are provided in the cited works). Pumpkin seed oil is particularly valuable due to large amounts of nutritionally valuable ingredients, such as rare types of phytosterols, accompanied by squalene, tocopherols, and carotenoids [6,7,8,9,10]. Linoleic acid predominates in the fatty acid composition, which accounts for 31 to 66% of all fatty acids. In addition to this acid, pumpkin seed lipids also contain significant shares of oleic acid (15–49%) and palmitic acid (10–13%) [11,12,13]. Variation in fatty acid composition of pumpkin seeds highly depends on climatic conditions. In general, a lower temperature during the last weeks of seed filling (even variation at a level of ca 1 °C) results in a significant shift from oleic to linoleic acid accumulation [14].
Cucurbita pepo L. pumpkins originate from the New World and were introduced to Europe soon after the discovery of the American continent [15,16]. Hull-less seed pumpkins, also called Styrian oil pumpkins (Cucurbita pepo L. subsp. pepo var. styriaca Greb.), are especially useful for the production of high-quality pumpkin oil [7,17,18]. They originate from Styria (currently Austria and Slovenia) and were developed based on spontaneous, single gene mutation of hull-less (“naked”) seeds, which appeared about 100 years ago [19]. The first oil-seed pumpkin cultivar “Gleisdorfer Ölkürbis” was developed from hull-less landrace, and its high seed-yield hybrids are now being employed [17,20,21]. The EU Commission declared Styrian Pumpkin Seed Oil as Protected Geographical Indication (P.G.I.) from the 1996 year [22]. In 2016, the acreage of the Styrian oil pumpkin reached a peak level of 39,450 ha in Austria [23]. It is worth mentioning that the average yield of pumpkin seeds strongly depends on the variety and weather conditions, ranging from approximately 400 kg/ha (under drought) up to 1000 kg/ha under optimal conditions, with an average yield of 500–600 kg/ha [7]. Styrian pumpkin cultivation has spread to other countries, such as Croatia, Serbia, Hungary, Romania, Lithuania, Poland, Germany, Ukraine, Iran, and Australia [19,23,24]. This spread to various countries was possible because different germplasms were selected and bred for acclimatization to the local climate, better agronomic characters, higher seed and oil yield, higher unsaturated fatty acid, and tocopherol contents [19].
Scientific studies indicate large fluctuations in the composition of pumpkin oils are dependent on genotype (different types of varieties in global cultivation), degree of seed maturation, and environmental conditions (cultivation in many climate zones and agricultural systems). To date, a small number of studies have determined the chemical composition of oil produced from Styrian pumpkin cultivated in colder climate zones, such as in Poland [8]. The present study aimed to show the variation in the chemical composition of pumpkin seed oil depending on newly developed hybrids, compared to the Junona variety registered in Poland, and the year of cultivation (2014 and 2015) in the climatic conditions of north-eastern Poland.
2. Materials and Methods
2.1. Plant Material
Styrian oil pumpkin (Cucurbita pepo L. subsp. pepo var. styriaca Greb.) variety Junona and seven single-cross hybrids were used in this study. Junona is a Polish open-pollinated variety with an entry date in the Polish vegetable varieties registry of 23 January 2003 [25]. Junona is characterized by indeterminate plant growth, slightly flattened round fruits with orange-green stripes, and olive-green hull-less seeds (Figure 1). Hybrids named 71, 73, 74, 75, 77, 78, and 79 were developed by crossing different inbred lines of hull-less seed pumpkins. All the inbred lines were obtained by several cycles of self-pollination at the Department of Plant Genetics, Breeding and Biotechnology, Warsaw University of Life Sciences, Poland (DPGBB-WULS). The seeds of hybrids were produced by hand cross-pollination of inbreds at the DPGBB-WULS Experimental Station “Wolica”. The seeds obtained in this manner were used to establish open-field experiments. Seeds of Junona were purchased from W. Legutko seed company (W. Legutko, Jutrosin, Poland).

Figure 1.
Fruits and seeds of Styrian oil pumpkin (Cucurbita pepo L. subsp. pepo var. styriaca Greb.) variety Junona. Bars: left photo = 10 cm and right photo = 1 cm.
To collect hull-less seeds for oil analyses, two open-field experiments were established at the production fields of the Szarłat company (Szarłat, Cibory Gałeckie, Poland). Two independent experiments were performed in 2014 and 2015 with average climate conditions from August to September shown in Table S1. In both experiments, for each hybrid/variety 60 plants were planted on a 100 m2 plot. Seeds of the hybrids and Junona were sown directly to the soil in the middle of May with 1 × 1.6 m. Standard agrotechnical methods were applied during plant cultivation [26]. At the end of September fruits were harvested. Seeds were extracted from the fruits and for each hybrid/variety a bulked seed sample was prepared. Seeds were collected and dried at 35–40 °C for 2 days. The main characteristics of the genotypes of Styrian oil pumpkins (Cucurbita pepo L. subsp. pepo var. styriaca Greb.) used in the study are presented in Table S2. All seed samples were placed in hermetic sterile plastic bags and stored at −18 °C until use.
2.2. Lipid Extraction From Seeds
Free lipid content was determined by the Soxhlet method according to the Polish Standard [27]. The total lipid content was determined by the extraction with a 2:1 (v/v) chloroform:methanol mixture according to the modified method described by Folch et al. [28], consisting of the extraction of 10 g milled seeds mixed with 150 mL of a 2:1 (v/v) chloroform:methanol mixture, which was shaken in a 500 mL Erlenmeyer flask by a magnetic stirrer for 45 min. The mixture was then centrifuged and the solid phase was re-extracted two times more with the same volume of extractant. The liquid phases were combined in a separatory funnel. Thirty-five milliliters of 10% sodium chloride were added and the mixture was gently shaken. After phase separation, the chloroform phase was dried with anhydrous sodium sulfate and filtered again. Finally, the extractant was evaporated to dryness under an N2 stream. The bound lipid content was presented as the difference between the total and free lipid content. All lipid classes were presented as percent of seed dry matter (DM). The total oil samples were transferred to dark glass bottles without headspace, sealed hermetically, and kept at −18 °C until use in chemical analysis.
2.3. Fatty Acid Composition of Pumpkin Seed Lipids
Fatty acid esters were prepared according to the procedure described in the work of Zadernowski and Sosulski [29]. The fatty acids were methylated by heating their solution in a chloroform:methanol:sulfuric acid mixture (100:100:1, v/v/v) at 70 °C for 2 h. Fatty acid methyl esters were analyzed using a GC-MS QP2010 PLUS system (Shimadzu, Kyoto, Japan) equipped with a BPX70 capillary column (25 m × 0.22 mm × 0.25 µm; SGE Analytical Science, Victoria, Australia). Helium was used as the carrier gas with a flow rate of 0.9 mL/min. The temperatures were as follows: the injector was −230 °C; the column was programmed in the range from 150 °C to 250 °C; the interface of GC-MS was −240 °C; the ion source was −240 °C; and the electron energy was 70 eV. Individual fatty acid methyl esters were identified on the basis of their retention times and mass spectra, and results were expressed as the percentage (w/w) of each fatty acid in total fatty acids based on their peak areas.
2.4. Quality and Composition of Bioactive Compounds in Pumpkin Seed Lipids
The content of tocopherols was determined by HPLC, according to the method described by Czaplicki et al. [30]. Samples were diluted in hexane and centrifuged (11,500× g for 10 min) in a 5417R-type Eppendorf centrifuge (Eppendorf AG, Hamburg, Germany). The analysis was carried out using an Agilent Technologies 1200 RP-HPLC apparatus with a fluorescence detector (Santa Clara, CA, USA). Separation was performed on a LiChrospher Si60 column (Merck, Darmstadt, Germany). The mobile phase was 0.7% iso-propanol in a hexane solution and the flow rate was 1 mL/min. The fluorescence detector was set at excitation and emission wavelengths of 296 and 330 nm, respectively. The content of tocopherols was calculated based on the external calibration curves. The repeatability for α-, γ-, and δ-tocopherol determination (expressed as coefficient of variation) was 2.5%. Limit of quantification was respectively 0.45, 0.4 and 0.2 µg/g of sample, while linearity of calibration curve was confirmed in range of 0.02–16 µg/mL.
The content of squalene and sterols was determined according to the procedure described in the work of Roszkowska et al. [31]. The oil samples were dissolved in hexane, and a 5α-cholestane solution was added as an internal standard. The oil sample with the 5α-cholestane (internal standard) hexane solution was saponified in 2 M KOH in a methanolic solution at 70 °C for 30 min. Sterols were extracted three times with diethyl ether. The collected extracts were evaporated to dryness in the R-210 type rotary evaporator (Büchi Labortechnik AG, Essen, Germany) under an N2 stream. Then, the residue was re-dissolved in pyridine and N,O-bis-(trimethylsilyl)trifluoroacetamide (BSTFA) with 1% (w/w) trimethylchlorosilane (TMCS), and derivatized at 60 °C for 60 min. The mixture was re-dissolved in heptane and analyzed using a GC-MS QP2010 PLUS system (Shimadzu, Kyoto, Japan) equipped with a ZB-5ms capillary column (30 m × 0.25 mm × 0.25 mm; Phenomenex, Torrance, CA, USA). Helium was used as the carrier gas with a flow rate of 0.9 mL/min. The temperatures were as follows: the injector was −230 °C; the column was programmed in the range from 70 °C to 310 °C; the interface of GC-MS was −240 °C; the ion source was −220 °C; and the electron energy was 70 eV. The sterols were identified on the basis of their retention times and mass spectra. Quantitative analysis of individual compounds was made based on the internal standard method with the use of 5α-cholestane. The repeatability for 5α-cholestane determination (expressed as coefficient of variation) was 2.5%. Limit of quantification was 0.05 μg/g of sample.
2.5. Oxidative Stability of Pumpkin Seed Lipids
A 743 Rancimat (Metrohm, Herisau, Switzerland) eight-channel oxidative stability instrument was used to evaluate the oxidative stability of the native and encapsulated oils. Briefly, 2.5 g of the oil sample was weighed into a reaction vessel. The capped vessel was placed in a thermostated electric heating block. The temperature was set at 110 °C and an air flow rate of 20 L/h was applied. The oxidative stability index (OSI) was expressed as hours.
2.6. Statistical Analysis
Results of determined compounds are presented as an average value with standard deviation quantified for both years of cultivation. The differences between the hybrids/variety were determined using the analysis of variance (ANOVA) with the Duncan test. Additionally, two-way analysis of variance was employed to show the impact of genotype, cultivation year, and interaction, and linear Pearson’s correlation coefficients were used to show the impact of determined lipid class content (free and bound), fatty acid composition, and phytochemicals with antioxidant properties (tocopherols and squalene) on the oxidative stability of obtained oils (in Rancimat assay). Results were analyzed using Statistica 13.1 PL software (StatSoft, Kraków, Poland) at the p ≤ 0.05 significance level.
3. Results and Discussion
3.1. Seed Lipid Content and Fatty Acid Composition
Results of an average content of total, free, and bound lipids in pumpkin seeds from two years of cultivation are presented in Table 1. The total lipid content (determined according to the Folch procedure) varied from 45.0% of seed dry matter (DM) (hybrid 78) to 49.3% of DM (hybrid 75). Only hybrid 78 significantly differed from the variety Junona used in this study (48.8% of DM). The determined lipid content in the Junona variety was higher than that of the same variety cultivated in the warmer south-western zone of Poland [9]. The degree of pumpkin fruit ripeness can have an additional impact on the total lipid content in seeds. To date, there is no data on the variation in lipid content of C. pepo var. styriaca seeds during fruit ripening, but Petkova and Antonova [32] found the lipid content in seeds of C. moschata depended on the stage of seed development, and was 41.1 and 47.1% in seeds at 60 and 90 days after flowering, respectively. This points to a successive increase in the lipid content of seeds along with their maturation. This effect could also be expected for other Cucurbita species, such as C. pepo var. styriaca. A tendency for an increase in the total lipid content in seeds of pumpkins cultivated in the relatively warmer year 2015 (46.9% of DM) vs. year 2014 (45.5% of DM) was also observed. In 2015, the average temperature in the final stages of pumpkin fruit ripening was higher by ca. 3 °C in August and by ca. 1 °C in September than in the respective months of 2014 (Table S1). However, it was accompanied by slightly lower fruit and seed yields (Table S2). Among the determined total lipid classes, easily extractable (so-called free) lipids ranged from 94.1% (hybrid 75) to 99.3% (hybrid 74). In general, bound lipids are mainly represented by various phospholipids and glycolipids, which are structural parts of cell membranes and organelles [33]. The higher content of bound lipids can indicate lower maturity of these seeds because the share of polar lipids significantly diminishes during ripening [32]. Given this statement, it appears that in the present study, the ripest were the seeds of the 74 and 78 hybrids. Another possible explanation for these differences could be the different size of storage oil bodies between hybrids. Membranes of these organelles are built by phospholipids [34], so larger oil bodies deposit relatively lower content of these polar lipids. An indicator of the validity of this assumption is the highest effect of genotype on bound lipid content (Table 2). In contrast, the most decisive interaction for free lipid content was that of genotype × year of cultivation.

Table 1.
Lipid content and fatty acid composition of total fat from seeds of studied Styrian oil pumpkins.

Table 2.
Effect of year and Styrian oil pumpkin genotype (% of explained variance) on analyzed chemical compounds of seed lipids and oxidative stability index (OSI).
The fatty acid composition of the examined pumpkin genotypes is presented in Table 1. The content of predominant linoleic acid (C18:2; n-6) varied from 50.9% (hybrid 78) to 62.9% (hybrid 75) and was accompanied by the highest fluctuation in the content of the second major fatty acid in these hybrids, oleic acid (C18:1), i.e., from 20.3% to 32.6%, respectively. Detailed analysis showed additionally that hybrid 78 was the most distinctive genotype in this regard. In summary, oleic and linoleic acids represented from 81.2% to 84.1% of total fatty acids, and their contents were negatively correlated (calculations not shown). In the case of other quantified fatty acids, the variations in their contents were small and reached 9.9–11.9%, 4.1–5.8%, and 0.6–0.9% for palmitic, stearic, and linolenic acids, respectively. Although both years of pumpkin cultivation differed slightly in temperature during seed maturation, this did not cause significant changes in the composition of fatty acids (Table 2). Only the content of minor linolenic acid was less dependent on genotype (23.6%) than on the impact of both years and genotype × year interaction (19.3% and 15.9%, respectively) (Table 2).
Seed development is a critical stage in the life cycle of a plant [35]. Although the fatty acid profile is a phenotypic feature of each plant seed, the changes in the fatty acid composition can also be a natural consequence of the different stages of fatty acid biosynthesis or climate impact. According to Petkova and Antonova [32], in the first stage of ripening, the saturated fatty acids are accumulated; then, the rate of polyunsaturated fatty acids (PUFAs) biosynthesis increases. In plants, seed-specific delta-12 fatty acid desaturase 2 (FAD2) is responsible for the high content of linoleic acid by inserting a double bond at the delta-12 (omega-6) position of oleic acid [36]. Another factor that affects the fatty acid composition of mature seeds is the climate. Under cold stress, membrane flexibility through increased lipid unsaturation allows for maintaining a homeostatic environment that is integral to the cell’s functioning [37]. Drastic changes in temperature result in plant adaptation with modified PUFA concentrations in their membranes and storage lipids [35]. This phenomenon can explain differences in the fatty acid composition of Junona seeds analyzed in the present study and in the research previously published by Nawirska-Olszańska et al. [9]. We determined ca. 14% higher content of linoleic acid accompanied by ca. 10% lower content of oleic acid; however, the field where the pumpkin was cultivated in the current study is located in a relatively cold north-eastern region of Poland in Cibory Gałeckie village. The field experiment of Nawirska-Olszańska et al. [9] was conducted near Wroclaw in a statistically warmer south-western region of Poland (unfortunately, climate conditions are not presented in the cited study) and pumpkin fruits were harvested in mid-September.
3.2. Tocopherol Content and Composition
Tocopherol content and composition of the examined pumpkin seed oils are presented in Table 3. The total content of these compounds varied from 25.5 mg/100 g (line 77) to 48.9 mg/100 g (line 73), indicating almost two-fold differences between the most distinctive genotypes. Among these, γ-tocopherol prevailed, with its content ranging from 89.8% (line 73) to 99.1% (line 79). Except for this homolog, small amounts of α- and δ-tocopherols were determined, which did not exceed 2.93 mg/100 g and 2.72 mg/100 g, respectively. Although temperature fluctuation between 2014 and 2015 was not high (ca. 3 and 1 °C in August and September, respectively), we found a significant impact of year and genotype × year interaction on tocopherol content (Table 2), especially in the case of γ-tocopherol. This could be explained by drought stress in August of 2015, since the sum of precipitation in this month was ca. 12-fold lower than that in 2014 (5.2 mm vs. 59.6 mm; Table S1). It was previously found that many plants accumulate significantly higher amounts of tocopherols under water deficit; for example, in Arabidopsis plants the level of α- and γ-tocopherols increased by 3.6 and 13.5-fold, respectively [38].

Table 3.
Tocopherol content in the total content of lipids from seeds of studied Styrian oil pumpkins.
The content and composition of tocopherols determined in our study were similar to those found in the majority of previous studies [9,11,39,40]. However, some authors have reported significantly higher total contents, such as ca. 83–100 mg/100 g [41,42], or very low contents, such as ca. 4 mg/100 g presented by Lelley et al. [14]. The explanation of differences in tocopherol accumulation in pumpkin seeds appears to be still open. Additionally, identification of tocopherol homologs should be improved since Butinar et al. [41] determined a high share of unique homologs, such as α- and γ-tocomonoenols, with only one double bond in the side-chain.
Unfortunately, the tocopherol composition determined in pumpkin oils is not ideal from the nutritional point of view. Although all of these homologs are still summarized as “vitamin E”, this name should only be used to define molecules that prevent the human deficiency disease “Ataxia with vitamin E deficiency” [43]. The content of α-tocopherol, the only molecule that can be named “vitamin E″, did not exceed 2.28 mg/100 g in the studied pumpkin oils. The Recommended Dietary Allowance (RDA) for α-tocopherol varies with human age and sex. Adults and teenagers need about 15 mg daily, while children between 9 and 13 years of age require 11 mg. Younger children, between 1 and 8 years old, need 6 to 7 mg per day, while newborns and infants should consume 4 or 5 mg daily [44]. This indicates that pumpkin seeds or oil are rather poor sources of vitamin E in a human diet. However, the high concentration of γ-tocopherol in pumpkin oil, with the highest antioxidant properties [9], can protect labile PUFAs and other phytochemicals during oil storage and/or can protect the human body against reactive oxygen species after being absorbed from the gastrointestinal tract [45]. Additionally, γ-tocopherol, but not α-tocopherol, inhibits cyclooxygenase activity and, thus, possesses anti-inflammatory properties [46]. Some human and animal studies also indicate that plasma concentrations of γ-tocopherol are inversely associated with the incidence of cardiovascular disease [46,47].
3.3. Sterols Content and Composition
The sterol content and composition of the pumpkin genotypes studied are presented in Table 4. Their content varied from 276 mg/100 g (line 77) to 352 mg/100 g (line 79). The average content was determined at 318 mg/100 g, with a relatively low coefficient of variation between the genotypes (CV = 5.2%). Among the quantified homologs, the sum of spinasterol + β-sitosterol prevailed (47.1–56.0% of total sterols), followed by Δ7,25-stigmastadienol (17.4–23.5%) and Δ7,22,25-stigmastatrienol (18.5–22.2%). In addition, minor amounts of Δ7-stigmastenol and campesterol were found in the oils examined. Although we quantified spinasterol + β-sitosterol together, the β-sitosterol content in pumpkin oil is at least ca. 4-fold lower than that of spinasterol, which is representative of Δ7-sterols [48].

Table 4.
Sterol content in total content of lipids from seeds of studied Styrian oil pumpkins.
The sterol content was affected mostly by genotype impact (42.9–83.8% of the determined variation), followed by the significant impact of the year (only one exception for Δ7,22,25-stigmastatrienol) and interaction of these traits (Table 2). The content and composition of sterols determined in the current study were in range of 225–343 mg/100 g reported in the previous studies [49,50,51]. Significantly more abundant in sterols are, for example, rapeseed oil (ca. 890 mg/100 g), corn oil (ca. 990 mg/100 g), and rice bran oil (ca. 1890 mg/100 g) [52]. In contrast, sterol content in pumpkin oils is close to that of soybean, peanut, sunflower, olive, walnut, and grapeseed oils [52].
Although sterol content in pumpkin seed oil is not distinctive, this oil is unique considering its sterol composition. β-sitosterol prevails in the majority of typical plant oils [53], while Δ7-sterols dominate in pumpkin oil, accounting for at least 91% [14,50]. A lower level of these Δ7-sterols detected in commercial pumpkin oil can indicate its adulteration [54]. It appears that these unique sterols have a beneficial impact on the human body. For example, Ramak et al. [55] reported that esterified Δ7-sterols were effective agents in the treatment of prostatic hyperplasia. Additionally, Tsai et al. [56] concluded that pumpkin seed oil alone or combined with Phytosterol-F could block the testosterone/prazosin-induced increases in prostate size ratio in the animal rat model. This was also confirmed in the Heim et al. [57] study, in which Uromedic® pumpkin (variety of Cucurbita pepo L. convar. citrullinina Greb. var. styriaca Greb.) seed soft extract (active ingredients of GRANUFINK® Prosta forte 500 mg), seed oil, and isolated Δ7-sterols inhibited 5α-reductases and competitively bound to the androgen receptor in vitro. It appears likely that Δ7-sterols exert clinically positive effects on the prostate, as well as exhibit bladder-strengthening effects.
3.4. Squalene Content
Squalene content in the examined pumpkin seeds varied from 210 mg/100 g (hybrid 73) to 305 mg/100 g (hybrid 71), reaching an average value of 241 mg/100 g, with a coefficient of variation between the tested genotypes of 8.8% (Table 5). Its content determined in our study was consistent with the range of 164–258 mg/100 g reported by Naziri et al. [42] for cold-pressed pumpkin oils from plants cultivated in Serbia. However, significantly higher contents of squalene were determined by Nyam et al. [58] in seeds from a local market in Malaysia and by Gorjanović et al. [39] in seeds of plants grown in Serbia. These authors found from 549 to 788 mg of this compound in 100 g of pumpkin seed oil. In general, squalene is a precursor of various sterols in plant tissues [59]. In the present study, its mass share to total sterols varied from 0.64 (line 73) to 0.98 (line 71). Its content was affected in 80.8% by genotype impact (Table 2).

Table 5.
Squalene content in total content of lipids from seeds of studied Styrian oil pumpkins.
Currently, squalene is recognized as a health-promoting food component [60,61]. From a nutritional point of view, squalene is a molecule with pharmacological, cosmetic, and nutritional potential. Scientific research has shown it reduces skin damage by UV radiation, reduces LDL and cholesterol levels in the blood, prevents cardiovascular diseases, and has antitumor and anticancer effects against ovarian, breast, lung, and colon cancers. However, squalene intake is low due to the lack of its abundant natural sources [61]. Among the plant sources reporting squalene content, the richest are amaranth (5942 mg/100 g) and olive (564 mg/100 g) oils, while soybean, rice, wheat germ, grape seed, and peanut oils contain from 9.9 to 27.4 mg/100 g of this compound [61]. It appears that pumpkin oil can be a good source of squalene in the human diet.
3.5. Oxidative Stability Index (OSI)
OSI values of the examined pumpkin oils are presented in Figure 2. They ranged from 7.3 h (hybrid 75) to 12.3 h (hybrid 78), with a coefficient of variation reaching 14.3%. It is worth mentioning that line 75 was characterized by the highest (62.9%), while hybrid 78 by the lowest (50.9%) level of double-unsaturated linoleic acid. Data presented in Table 6 show that the OSI value was positively correlated with γ-tocopherol content (r = 0.75) in the oil and share of stearic acid (r = 0.82).
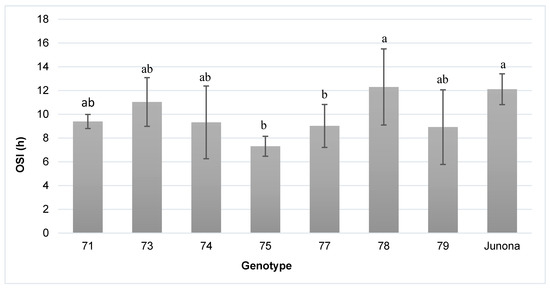
Figure 2.
Oxidative stability index (OSI) determined for total lipids of studied Styrian oil pumpkin seeds (average value (calculated for two years) and standard deviation). a,b—average values with the same letter are not significantly different at p ≤ 0.05.

Table 6.
Pearson’s correlation coefficients between OSI values and selected pumpkin oil chemical compounds.
The OSI values obtained for pumpkin seed oils were consistent with the range of 9.7–16.6 h reported for tests conducted at 110 °C [62]. For experiments conducted at 120 °C [40,42,63], these values ranged from 3.4 to 11.1 h, while for tests conducted at 100 °C [11,40], from 17 to 31 h. Considering that every 10 °C increase doubles the results [64], the values determined in our study fit within all of the cited ranges. Pumpkin seed oil is relatively oxidatively stable because it contains minor or trace amounts of linolenic acid. The positive effect of γ-tocopherol was expected because of the highest antioxidative properties of this tocopherol homolog [65].
4. Conclusions
The hybrids of Styrian type pumpkins newly developed in Poland, and cultivated in the north-eastern part of the country, produce seeds rich in lipids (ca. 45–49%), with a relatively higher share of linoleic acid (up to 63%) compared to plants cultivated in warmer regions. The content and composition of major homologs of determined phytochemicals are similar to those of other pumpkin oils studied previously. The majority of the quantified compounds were affected primarily by genotype impact (at a low variation in climate conditions in the years 2014 and 2015). However, the year of pumpkin cultivation was significant for the contents of selected sterols, γ-tocopherol, and free lipids. The oxidative stability of the examined oils was mostly positively correlated with the content of γ-tocopherol (r = 0.75) and with the share of stearic acid (r = 0.82). There was also a visible tendency to diminish OSI value with the increased share of linoleic acid (r = −0.63).
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/10/8/1104/s1, Table S1: Mean temperature and precipitation during conducted field experiments (Białystok–44.7 km from Cibory Gałęckie, Poland), Table S2: General characteristics of the genotypes of Styrian oil pumpkins (Cucurbita pepo L. subsp. pepo var. styriaca Greb.) used in the study.
Author Contributions
Supervised the research project, carried out the lab studies, edited and revised the manuscript, M.T.; carried out the lab studies, edited and revised the manuscript, D.O.; hybrids field-performance characterization, drafted and revised the manuscript, G.B.; developed the hybrids, participated in hybrids field-performance characterization, A.K.; drafted and revised the manuscript, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Authors thank “Szarłat” M. and W. Lenkiewicz General Partnership company for providing research material and Katarzyna Niemirowicz-Szczytt (Warsaw University of Life Sciences, Poland) for her support for this research study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caili, F.U.; Huan, S.; Quanhong, L.I. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum. Nutr. 2006, 61, 70–77. [Google Scholar] [CrossRef]
- Yadav, M.; Jain, S.; Tomar, R.; Prasad, G.B.K.S.; Yadav, H. Medicinal and biological potential of pumpkin: An updated review. Nutr. Res. Rev. 2010, 23, 184–190. [Google Scholar] [CrossRef]
- Kaur, S.; Panghal, A.; Garg, M.; Mann, S.; Khatkar, S.; Sharma, P.; Chhikara, N. Functional and nutraceutical properties of pumpkin—A review. Nutr. Food Sci. 2019, 50. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, S.; Kumar, V.; Thakur, A. Comprehensive review on nutraceutical significance of phytochemicals as functional food ingredients for human health management. J. Pharmacogn. Phytochem. 2019, 8, 385–395. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, G.; Kehinde, B.A.; Chhikara, N.; Panghal, A.; Kaur, H. Pharmacological and biomedical uses of extracts of pumpkin and its relatives and applications in the food industry: A review. Int. J. Veg. Sci. 2020, 26, 79–95. [Google Scholar] [CrossRef]
- Alfawaz, M.A. Chemical composition and oil characteristics of pumpkin (Cucurbita maxima) seed kernels. Food Sci. Agric. 2004, 2, 5–18. [Google Scholar]
- Fruhwirth, G.O.; Hermetter, A. Production technology and characteristics of Styrian pumpkin seed oil. Eur. J. Lipid Sci. Technol. 2008, 110, 637–644. [Google Scholar] [CrossRef]
- Gohari, A.A.; Farhoosh, R.; Haddad, K.M. Chemical composition and physicochemical properties of pumpkin seeds (Cucurbita pepo subsp. pepo var. Styriaka) grown in Iran. J. Agric. Sci. Technol. 2011, 13, 1053–1063. [Google Scholar]
- Nawirska-Olszańska, A.; Kita, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z. . Characteristics of antioxidant activity and composition of pumpkin seed oils in 12 cultivars. Food Chem. 2013, 139, 155–161. [Google Scholar] [CrossRef]
- Procida, G.; Stancher, B.; Cateni, F.; Zacchigna, M. Chemical composition and functional characterization of commercial pumpkin seed oil. In Proceedings of the ChimAlSi 2012, IX Italian Congress of Food,” Food, Functional Foods and Nutraceuticals”, Faculty of Food and Agriculture, UAE University, Ischia, Italy, 03–07 June 2012; p. 63. [Google Scholar]
- Neđeral, S.; Petrović, M.; Vincek, D.; Pukec, D.; Škevin, D.; Kraljić, K.; Obranović, M. Variance of quality parameters and fatty acid composition in pumpkin seed oil during three crop seasons. Ind. Crops Prod. 2014, 60, 15–21. [Google Scholar] [CrossRef]
- Potočnik, T.; Ogrinc, N.; Potočnik, D.; Košir, I.J. Fatty acid composition and δ13C isotopic ratio characterisation of pumpkin seed oil. J. Food Compos. Anal. 2016, 53, 85–90. [Google Scholar] [CrossRef]
- Kachel, M.; Matwijczuk, A.; Przywara, A.; Kraszkiewicz, A.; Koszel, M. Profile of fatty acids and spectroscopic characteristics of selected vegetable oils extracted by cold maceration. Agric. Eng. 2018, 22, 61–71. [Google Scholar] [CrossRef]
- Lelley, T.; Loy, B.; Murkovic, M. Hull-less oil seed pumpkin. In Oil Crops; Springer: New York, NY, USA, 2009; pp. 469–492. [Google Scholar]
- Paris, H.S. Overview of the origins and history of the five major cucurbit crops: Issues for ancient DNA analysis of archaeological specimens. Veg. Hist. Archaeobotany 2016, 25, 405–414. [Google Scholar] [CrossRef]
- Chomicki, G.; Schäfer, H.; Renner, S.S. Origin and domestication of Cucurbitaceae crops: Insights from phylogenies, genomics and archaeology. New Phytol. 2020, 226, 1240–1255. [Google Scholar] [CrossRef] [PubMed]
- Ferriol, M.; Picó, B. Pumpkin and Winter Squash; Springer Science and Business Media LLC: Berlin, Germany, 2008; Volume 1, pp. 317–349. [Google Scholar]
- Korzeniewska, A.; Witek, M.; Gałecka, T.; Niemirowicz-Szczytt, K. Evaluation of selected traits in hull-less seed squash (Cucurbita pepo subsp. pepo var. styriaca Greb.). Pol. J. Agron. 2013, 12, 32–37. (In Polish) [Google Scholar]
- Winkler, J. The origin and breeding of the hull-less seeded Styrian oil-pumpkin varieties in Austria. Rep. Cucurbit Genet. Coop. 2000, 23, 101–104. [Google Scholar]
- Cui, H.; Loy, J.B. Heterosis for seed yield exhibited in hull-less seeded pumpkin. In Cucurbitaceae; ASHS Press: Alexandria, VA, USA, 2002; pp. 323–329. [Google Scholar]
- Darrudi, R. , Nazeri, V.; Soltani, F.; Shokrpour, M.; Ercolano, M.R. Evaluation of combining ability in Cucurbita pepo L. and Cucurbita moschata Duchesne accessions for fruit and seed quantitative traits. J. Appl. Res. Med. Aromat. Plants 2018, 9, 70–77. [Google Scholar]
- Council Regulation (EEC). No. 2081/92. Application for Registration: Art. 17, PGI (National application No: 1215-GR/95). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31992R2081 (accessed on 7 June 2020).
- Adam, E.; Bernhart, M.; Müller, H.; Winkler, J.; Berg, G. The Cucurbita pepo seed microbiome: Genotype-specific composition and implications for breeding. Plant Soil 2018, 422, 35–49. [Google Scholar] [CrossRef]
- Baxter, G.G.; Murphy, A.; Paech, A. The potential to produce pumpkin seed for processing in North East Victoria. Rural Ind. Dev. Corp. 2012, 11/145, 5–36. [Google Scholar]
- Polish National List of Vegetable Plant Varieties. Available online: http://www.coboru.pl/English/index_eng.aspx (accessed on 10 May 2020).
- Korzeniewska, A.; Niemirowicz-Szczytt, K. Possibilities of using presently underrated oil yielding plants exemplified by oil seed pumpkin. In Biological Diversity of Agricultural Ecosystems and Possibilities of Its Protection in Ecological Farms; Tyburski, J., Kostrzewska, M.K., Eds.; Uniwersytet Warmińsko-Mazurski, Olsztyn: Olsztyn, Poland, 2013; pp. 43–51. (In Polish) [Google Scholar]
- Polish Standard PN-73/R-66164. Determination of Fat Content in Seeds, Oil Fruits and Post-Extraction Meal. Polish Committee for Standardization. (In Polish). Available online: https://www.pkn.pl/en/standardization (accessed on 7 June 2020).
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Zadernowski, R.; Sosulski, F. Composition of total lipids in rapeseed. J. Am. Oil Chem. Soc. 1978, 55, 870–872. [Google Scholar] [CrossRef]
- Czaplicki, S.; Ogrodowska, D.; Derewiaka, D.; Tańska, M.; Zadernowski, R. Bioactive compounds in unsaponifiable fraction of oils from unconventional sources. Eur. J. Lipid Sci. Technol. 2011, 113, 1456–1464. [Google Scholar] [CrossRef]
- Roszkowska, B.; Tańska, M.; Czaplicki, S.; Konopka, I. Variation in the composition and oxidative stability of commercial rapeseed oils during their shelf life. Eur. J. Lipid Sci. Technol. 2015, 117, 673–683. [Google Scholar] [CrossRef]
- Petkova, Z.Y.; Antova, G.A. Changes in the composition of pumpkin seeds (Cucurbita moschata) during development and maturation. Grasas Aceites 2015, 66, 058. [Google Scholar] [CrossRef]
- van der Schoot, C.; Paul, L.K.; Paul, S.B.; Rinne, P.L. Plant lipid bodies and cell-cell signaling: A new role for an old organelle? Plant Signal. Behav. 2011, 6, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Nikiforidis, C.V. Structure and functions of oleosomes (oil bodies). Adv. Colloid Interface Sci. 2019, 274, 102039. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yan, T.; Chen, X.; Li, Z.; Wu, D.; Hua, S.; Jiang, L. Effect of high night temperature on storage lipids and transcriptome changes in developing seeds of oilseed rape. J. Exp. Bot. 2018, 69, 1721–1733. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, F.D.; Yarizade, K. Bioinformatics study of delta-12 fatty acid desaturase 2 (FAD2) gene in oilseeds. Mol. Biol. Rep. 2014, 41, 5077–5087. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Semchuk, N.M. Tocopherol biosynthesis: Chemistry, regulation and effects on environmental factors. Acta Physiol. Plant. 2012, 34, 1607–1628. [Google Scholar] [CrossRef]
- Gorjanović, S.Ž.; Rabrenović, B.B.; Novaković, M.M.; Dimić, E.B.; Basić, Z.N.; Sužnjević, D.Ž. Cold-pressed pumpkin seed oil antioxidant activity as determined by a DC polarographic assay based on hydrogen peroxide scavenge. J. Am. Oil Chem. Soc. 2011, 88, 1875–1882. [Google Scholar] [CrossRef]
- Vujasinovic, V.; Djilas, S.; Dimic, E.; Basic, Z.; Radocaj, O. The effect of roasting on the chemical composition and oxidative stability of pumpkin oil. Eur. J. Lipid Sci. Technol. 2012, 114, 568–574. [Google Scholar] [CrossRef]
- Butinar, B.; Bučar-Miklavčič, M.; Mariani, C.; Raspor, P. New vitamin E isomers (gamma-tocomonoenol and alpha-tocomonoenol) in seeds, roasted seeds and roasted seed oil from the Slovenian pumpkin variety ‘Slovenska golica’. Food Chem. 2011, 128, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Naziri, E.; Mitić, M.N.; Tsimidou, M.Z. Contribution of tocopherols and squalene to the oxidative stability of cold-pressed pumpkin seed oil (Cucurbita pepo L.). Eur. J. Lipid Sci. Technol. 2016, 118, 898–905. [Google Scholar] [CrossRef]
- Azzi, A. Tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox Biol. 2019, 101259. [Google Scholar] [CrossRef]
- Foods High in Alpha-Tocopherol. Available online: https://healthyeating.sfgate.com/foods-high-alphatocopherol-3483.html (accessed on 7 June 2020).
- Ali, M.A.; Nargis, A.; Othman, N.H.; Noor, A.F.; Sadik, G.; Hossen, J. Oxidation stability and compositional characteristics of oils from microwave roasted pumpkin seeds during thermal oxidation. Int. J. Food Prop. 2017, 20, 2569–2580. [Google Scholar] [CrossRef]
- Jiang, Q.; Christen, S.; Shigenaga, M.K.; Ames, B.N. γ-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 2001, 74, 714–722. [Google Scholar] [CrossRef]
- Burbank, A.J.; Duran, C.G.; Pan, Y.; Burns, P.; Jones, S.; Jiang, Q.; Yang, C.; Jenkins, S.; Wells, H.; Alexis, N.; et al. Gamma tocopherol-enriched supplement reduces sputum eosinophilia and endotoxin-induced sputum neutrophilia in volunteers with asthma. J. Allergy Clin. Immunol. 2018, 141, 1231–1238. [Google Scholar] [CrossRef]
- Dulf, F.V.; Bele, C.; Unguresan, M.; Parlog, R.; Socaciu, C. Phytosterols as markers in identification of the adulterated pumpkin seed oil with sunflower oil. Bull. Uasvm Agric. 2009, 66, 301–307. [Google Scholar]
- Ogrodowska, D.; Laaksonen, O.; Tańska, M.; Konopka, I.; Linderborg, K.M. Pumpkin oil addition and encapsulation process as methods to improve oxidative stability of fish oil. Lwt—Food Sci. Technol. 2020, 124, 109142. [Google Scholar] [CrossRef]
- Konopka, I.; Roszkowska, B.; Czaplicki, S.; Tańska, M. Optimization of pumpkin oil recovery by using aqueous enzymatic extraction and comparison of the quality of the obtained oil with the quality of cold-pressed oil. Food Technol. Biotechnol. 2016, 54, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Hrabovski, N.; Sinadinović-Fišer, S.; Nikolovski, B.; Sovilj, M.; Borota, O. Phytosterols in pumpkin seed oil extracted by organic solvents and supercritical CO2. Eur. J. Lipid Sci. Technol. 2012, 114, 1204–1211. [Google Scholar] [CrossRef]
- Yang, R.; Xue, L.; Zhang, L.; Wang, X.; Qi, X.; Jiang, J.; Yu, L.; Wang, X.; Zhang, W.; Zhang, Q.; et al. Phytosterol contents of edible oils and their contributions to estimated phytosterol intake in the Chinese diet. Foods 2019, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, O.N.; Przybylski, R.; Rudzińska, M. Lipid components of flax, perilla, and chia seeds. Eur. J. Lipid Sci. Technol. 2012, 114, 794–800. [Google Scholar] [CrossRef]
- Mandl, A.; Reich, G.; Lindner, W. Detection of adulteration of pumpkin seed oil by analysis of content and composition of specific Δ7-phytosterols. Eur. Food Res. Technol. 1999, 209, 400–406. [Google Scholar] [CrossRef]
- Ramak, P.; Mahboubi, M. The beneficial effects of pumpkin (Cucurbita pepo L.) seed oil for health condition of men. Food Rev. Int. 2019; 35, 166–176. [Google Scholar]
- Tsai, Y.S.; Tong, Y.C.; Cheng, J.T.; Lee, C.H.; Yang, F.S.; Lee, H.Y. Pumpkin seed oil and phytosterol-F can block testosterone/prazosin-induced prostate growth in rats. Urol. Int. 2006, 77, 269–274. [Google Scholar] [CrossRef]
- Heim, S.; Seibt, S.; Stier, H.; Moré, M.I. Uromedic® pumpkin seed derived Δ7-sterols, extract and oil inhibit 5α-reductases and bind to androgen receptor in vitro. Pharm. Pharm. 2018, 9, 193. [Google Scholar]
- Nyam, K.L.; Tan, C.P.; Lai, O.M.; Long, K.; Man, Y.C. Physicochemical properties and bioactive compounds of selected seed oils. Lwt-Food Sci. Technol. 2009, 42, 1396–1403. [Google Scholar] [CrossRef]
- Busquets, A.; Keim, V.; Closa, M.; Del Arco, A.; Boronat, A.; Arró, M.; Ferrer, A. Arabidopsis thaliana contains a single gene encoding squalene synthase. Plant Mol. Biol. 2008, 67, 25–36. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Fairus, S.; Zulfarina, M.S.; Naina Mohamed, I. The efficacy of squalene in cardiovascular disease risk—A systematic review. Nutrients 2020, 12, 414. [Google Scholar] [CrossRef]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant sources, extraction methods, and uses of squalene. Int. J. Agron. 2018. [Google Scholar] [CrossRef]
- Jafari, M.; Goli, S.A.H.; Rahimmalek, M. The chemical composition of the seeds of Iranian pumpkin cultivars and physicochemical characteristics of the oil extract. Eur. J. Lipid Sci. Technol. 2012, 114, 161–167. [Google Scholar] [CrossRef]
- Bardaa, S.; Halima, N.B.; Aloui, F.; Mansour, R.B.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.N.; Breyen, M.D. (Eds.) Joining and Assembly of Medical Materials and Devices; Woodhead Publishing: Sawston, Cambridge, UK, 2013; pp. 386–388. [Google Scholar]
- van Hoed, V.; Sampaio, K.A.; Felkner, B.; Bavec, F.; Scippo, M.L.; Brose, F.; Bavec, M.; Verhé, R. Tocopherols and polyphenols in pumpkin seed oil are moderately affected by industrially relevant roasting conditions. Eur. J. Lipid Sci. Technol. 2017, 119, 1700110. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).