1. Introduction
Teff [
Eragrostis tef (
Zucc.)
Trotter], commonly referred to as teff, is an annual self-pollinated, allotetraploid (2
n = 4x = 40) warm season crop belonging to the Poaceae (grass) family [
1,
2]. It is a major food crop native to Ethiopia and Eritrea for the production of a range of traditional foods and beverages including Injera (flatbread).
Teff is a C
4 plant that has high chlorophyll a/b ratios and utilizes CO
2 very efficiently during photosynthesis. Teff is adapted to a range of growing environmental conditions [
3]. Teff grain also presents excellent storage properties. Therefore, it plays an important role in food security in Eastern Africa and in combating global climate change [
4].
In recent years, teff is becoming popular in the health-food markets of developed countries due to its attractive nutritional properties and gluten-free nature. The inability to separate the bran from the seed makes teff flour rich in fiber and thus has health benefits as an anti-oxidative and improves the hemoglobin level in the human body [
4,
5].
Despite teff’s versatility in adapting to extreme environmental conditions, teff is susceptible to lodging, which can drastically reduce yield and grain quality, and complicates harvesting [
5]. Lodging can limit productivity directly by reducing the photosynthetic capacity due to changes in sun/shade architecture. Lodging also limits the use of high input Nitrogen fertilizer to boost yield.
Lodging is a process through which the shoot cereals are displaced from vertical orientation (upright position) and settle in a permanent horizontal position [
6]. It is a complex phenomenon that is influenced by many factors, including wind, rain, geography, landscaping, soil type, crop history, agricultural system, and disease [
5].
Stem lodging results from bending or breaking of the lower culm internodes, and root lodging results from a failure in root soil integrity [
7]. Lodging is worsened by the use of fertilizers, reducing the yield potential of teff. The problems of lodging can be reduced by decreasing plant height, however, yield might be reduced when plants are shortened too much with dwarfing genes or plant growth regulators [
5]. Hence, it was suggested to target traits other than height for further improvement in lodging resistance in teff.
Teff has weak stems that easily succumb to lodging caused by wind or rain [
1]. Various attempts were made to develop lodging-resistant teff cultivars but presently no cultivar with reasonable lodging resistance has been obtained [
1]. Despite lodging being the greatest cause for yield loss in teff, its genetic and physiological control is understudied in terms of molecular breeding techniques and biotechnology [
5].
Ethiopia is teff’s origin and the center of its biodiversity, harboring landraces with a wide array of phenotypic diversity, wild progenitors, and related wild species. The genetic diversity of teff is represented in a very large collection of accessions [
8] from across its cultivation range at the Ethiopian Institute of Biodiversity. There has been a major increase in the collection size over the last decades, which demonstrates the presence of both a wide diversity of germplasm in Ethiopia, as well as the commitment of institutes and individuals to collect and preserve these germplasms for future use [
1].
The genetic diversity in teff was also discovered by using a range of molecular markers [
4] and its genome has been sequenced [
9]. Great genetic diversity in yield, lodging index, and stem strength related traits was recorded in teff [
10,
11].
Phenotypic variability in teff was recorded in—grain yield, grain color and size, days to panicle emergence, days to maturity (21 to 81 and 50 to 140, respectively), number of grains/plant (9000–90,000), plant height (20–156 cm), number of tillers/plant (5–35), and culm diameter (1.2–5 mm) [
10,
11].
Teff breeding should target the improvement in the following traits—grain yield, shoot biomass, lodging resistance, grain size and color, grain coat properties, nitrogen-use efficiency, osmotic adjustment root depth, tolerance to drought, salinity, and acidity, nutritional values, physicochemical, and palatability [
1]. Variability for the culm internode diameter is a key factor for improved lodging resistance [
4].
Overall, there is a limited amount of research on the genetic basis for the processing and nutritional quality of teff and its components as food [
11]. Therefore, it is necessary to assess the genetic diversity of this crop for potential improvement of agronomic as well as food-processing traits [
4].
The preparation of Injera, the Ethiopian sourdough type flat bread, involves fermentation processes of the teff flour [
12]. The fermentation preparation consists of two stages of natural fermentation, which last for about 24 to 72 h, depending on ambient temperatures [
13]. Good quality Injera will have uniformly-spaced honeycomb-like “eyes” or holes, and no blind spot (flat area with no holes) on its surface. The major factor that decrease Injera quality result from inadequate fermentation. Good quality Injera becomes soft and pliable in texture, which enables the consumer to wrap and pick up sauce in the Injera with fingers [
5]. In Ethiopia, people prefer their Injera to be white [
5]. Texture is determined by touch and refers to the degree of fluffiness, roughness, smoothness, hardness, or softness.
Four phenotypes of seed coat color (grain color) were documented in teff—dark brown, medium brown, yellowish-white, and grayish-white. However, the dark and medium brown are difficult to differentiate so they are both included as brown. A duplicate gene pair is known to be involved in seed color inheritance, with simple dominance and additive gene effects. Tests of independence showed that lemma and seed color are inherited independently [
14].
There is scarce documentation on seed size and seed coat in teff [
1]. Depending on the varieties, the color of teff grain can be ivory, light tan to deep brown or dark reddish-brown to purple [
1,
5]. Based on people’s preference for their consumption, white teff is the most expensive, while in terms of benefit, red teff is more nutritious and gains acceptance by the health-oriented consumers in Ethiopia and worldwide [
5].
Different teff varieties have different mineral concentrations. Red teff has a higher content of iron and calcium than mixed or white teff varieties, and in contrast, white teff has a higher copper content than the red and mixed teff varieties [
5].
Thirteen teff plants were found among commercial teff plots in Israel (cultivar name White) and were selfed. These plants were randomly distributed in the plots and looked different from the general population in terms of lodging (
Figure S1). A single panicle was collected from these thirteen Teff plants for further proliferation and study. These plants were propagated off-season in a greenhouse (
Figure S2), and from these thirteen F1, eleven F2 populations were selected for further detailed analyses under field conditions.
In a teff plot, we found plants that looked different from the surrounding population. First, and most striking, was the lodging resistance presented by these single plants under the prevailing environmental conditions, compared to the surrounding plants. Secondly, these plants, randomly distributed within the plot, were different from the common white cultivar in stem diameter, leaf size, phenology, and inflorescence coloration. From each of these plants a single panicle was collected for further characterization.
The objective of this study was to characterize the relations between lodging and other agronomic and sensory traits in the newly discovered populations.
3. Results
3.1. Off-Season Phenotyping and Line Segregation
The subjected teff populations were propagated in 10 L pots in the greenhouse off-season. Each line originated from a single panicle of a single plant. The initial collected seed set AGW (0.23–0.60 mg) as well as AGW scored from the greenhouse (0.22–0.50 mg) are presented in
Table 1. MPH ranged from 147 to 200 cm. The white cultivar and the pure brown cultivar 44A-163-B presented similar heights. The white cultivar had more stems per pot and panicle emergence was about a week earlier than the rest of the populations under this condition.
Three populations that had mixed color seeds were separated as detailed in
Table 1. The proportion between the brown to white seeds was tripled from 0.021 to 0.064, within a generation of prorogation. Whereas in 53-2 and 53-3, no dark brown seeds were detected upon collection. However, after a second round of propagation in the field, dark brown seeds were found in those populations (0.105 and 0.015 for 53-2 and 53-3, respectively).
3.2. Phenotyping
Analysis of variation for most traits showed a significant line effect (ANOVA in
Table 2). Within the agronomic and plant physiological parameters tested, a very wide range of values was found for EGC and Chlb/gFW (higher than 3 folds,
Table 3 and
Table 4). A 2–3 fold range was recorded for—TDM, GY, HI, LSD, Chla/gFW, Chl/gFW, Chl a/b ratio, and AGW. A low range of values (under 2 folds) was found for—USD, DSP, DPM, DSM, SL, and MPH. ANOVA analyses of all sensory traits (except for ‘Odor intensity’) reveled a significant effect of variations between the populations (
Table 2).
3.3. Biomass Production
A range of GY was observed in the current study. The white commercial cultivar and pure brown 44A-163-B were ranked relatively high in GY and HI, and low in TDM, with the former showing a significantly higher GY compared to the latter (
Table 3). Interestingly, the brown populations (53-1-B, 53-2-B, and 53-3-B) exhibited a similar pattern and were also ranked higher in GY and HI and lower in TDM, as compared to most of their white counterparts (
Table 3). The best performance in terms of GY was the white cultivar. Second was 44B-163-W, which was not significantly different from the pure brown 44A-163-B, as well as from the other brown populations.
3.4. Early Growth Cover and Chlorophyll Measurements
Early growth cover (
Table 3 and
Supplementary Figure S4) of the white commercial cultivar and 53-3-B was low as compared to other populations (such as 53-1-W) at 33 DAS. 53-1-B and 53-2-B exhibited the highest values of EGC.
Leaf chlorophyll content was measured at 42 DAS (a, b, and total Chl). Both the commercial white cultivar and pure brown 44A-163-B line had a very similar midrange value (
Table 4). Both 53-3-B and 53-1-B contained the highest Chl (b, and total Chl) among the evaluated populations. Whereas 53-2-B exhibited the opposite patterns in terms of Chl levels. Interestingly, all three brown populations presented a low Chla/Chlb ratio.
3.5. Phenology
The difference in panicle emergence time ranged between 53 to 64 DAS, the white commercial cultivar being the earliest to enter the reproductive stage at 53 DAS (
Table 5), along with 53-1-B. In general, the brown populations exhibited earlier heading, compared to their white-counterparts. 53-3-W was the latest to head and had the highest DSP, DPM, and DSM within the collection. While the white commercial cultivar was significantly earlier than brown 44A-163-B, their grain filling period (indicated by DPM) was not statistically different. Both the pure brown 44A-163-B and the white cultivar exhibited the lowest DSM in the collection.
3.6. Stem Phenotyping
Stem width was measured at 70 DAS during stem elongation and the lower and upper basal stem widths were measured (
Table 3). The brown populations (as well as the white commercial cultivar) tend to group as having a narrower stem (low USD and LSD) than the white populations (
Table 3). The single plant selected 53-2-2-W exhibited the highest LSD in the collection.
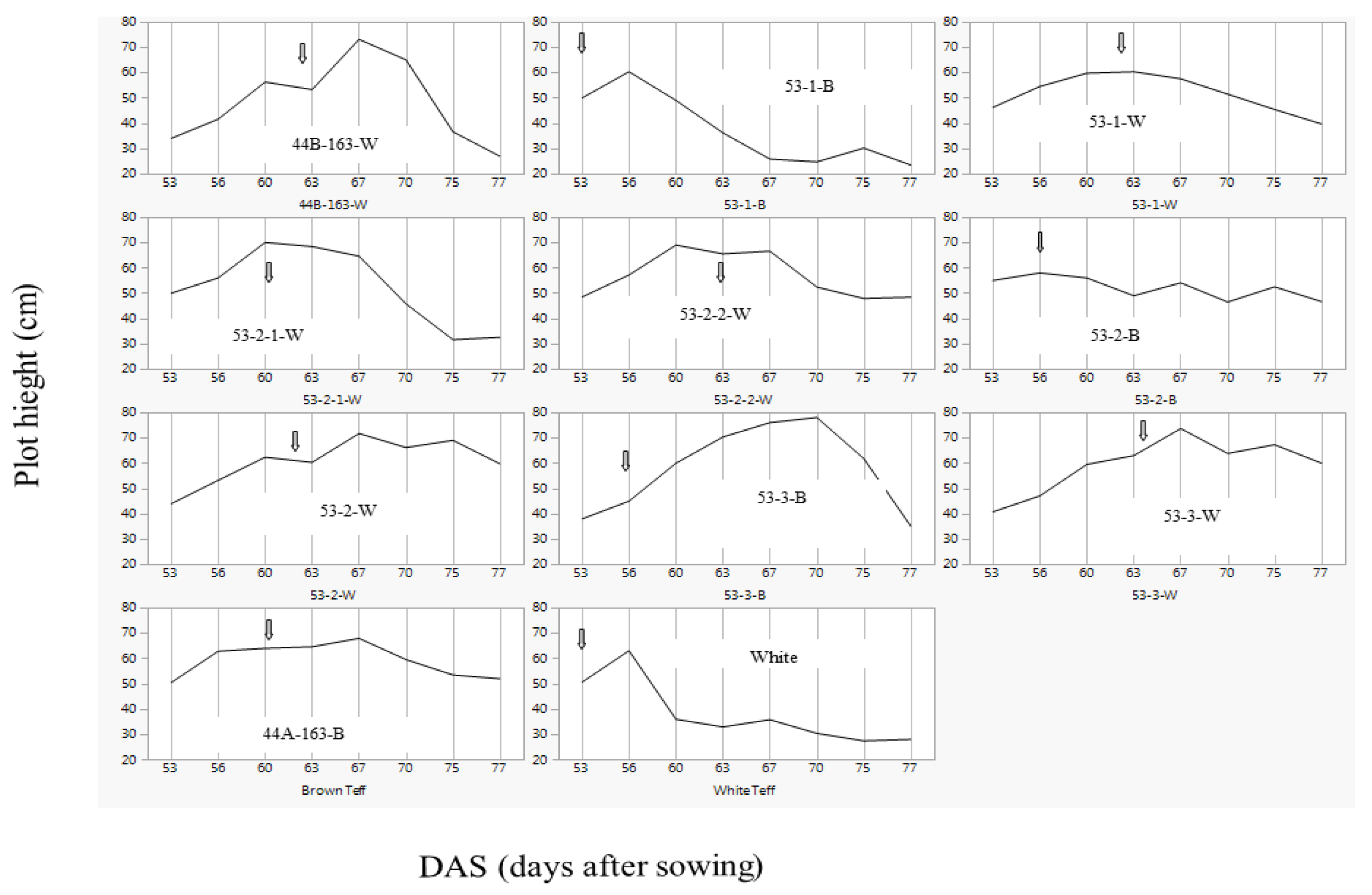
3.7. Plot Plant Height Dynamics and Lodging
Maximal plot plant height of the white commercial cultivar and two of the brown populations, 53-1-B and 53-2-B, was lower compared to the other white populations (
Table 5). The dynamics of plant height, which was documented in detail from 53 DAS to 87 DAS (
Figure 1,
Table 5), revealed distinct patterns among the studied populations.
White, the earliest to head, started exhibiting lodging three days after panicle emergence. However, this lodging was later revealed to be essentially different from the lodging observed in other populations. The main differences were that while the white commercial cultivar was relatively uniform in its lodging across the plot (
Figure S7b), as well as relatively static/stable in terms of the plot’s height throughout the grain filling period (at around 35 cm above ground), the other populations did not lodge so soon after panicle emergence and their lodging was not uniform across the plot. For example line 44A-163-B started lodging between 70–77 DAS (
Figure 1 and
Figure S7b), and the plot’s height reached around 50 cm above ground. Other populations, like 44-B-163-W exhibited strong lodging between 70 and 77 going from a plot height of 77 cm to 27 cm in seven days (
Figure S7b). This line was among the tallest populations and its heavy panicles filling seemed to bend the entire plant downwards. Some of the populations, such as 53-2-2-W, exhibited a relatively prolonged period of erect posture during grain filling, before lodging (
Figure S7b), which was not severe.
3.8. Seed and Spikelet Phenotyping
AGW of the brown segregated populations 53-1-B and 53-3-B was significantly higher, as compared to their white counterparts (
Table 6). The commercial cultivar exhibited midrange values of AGW. A similar pattern was obtained for SL, where this time all three brown segregated populations exhibited significantly higher averaged values, as compared to their white counterparts (
Table 6).
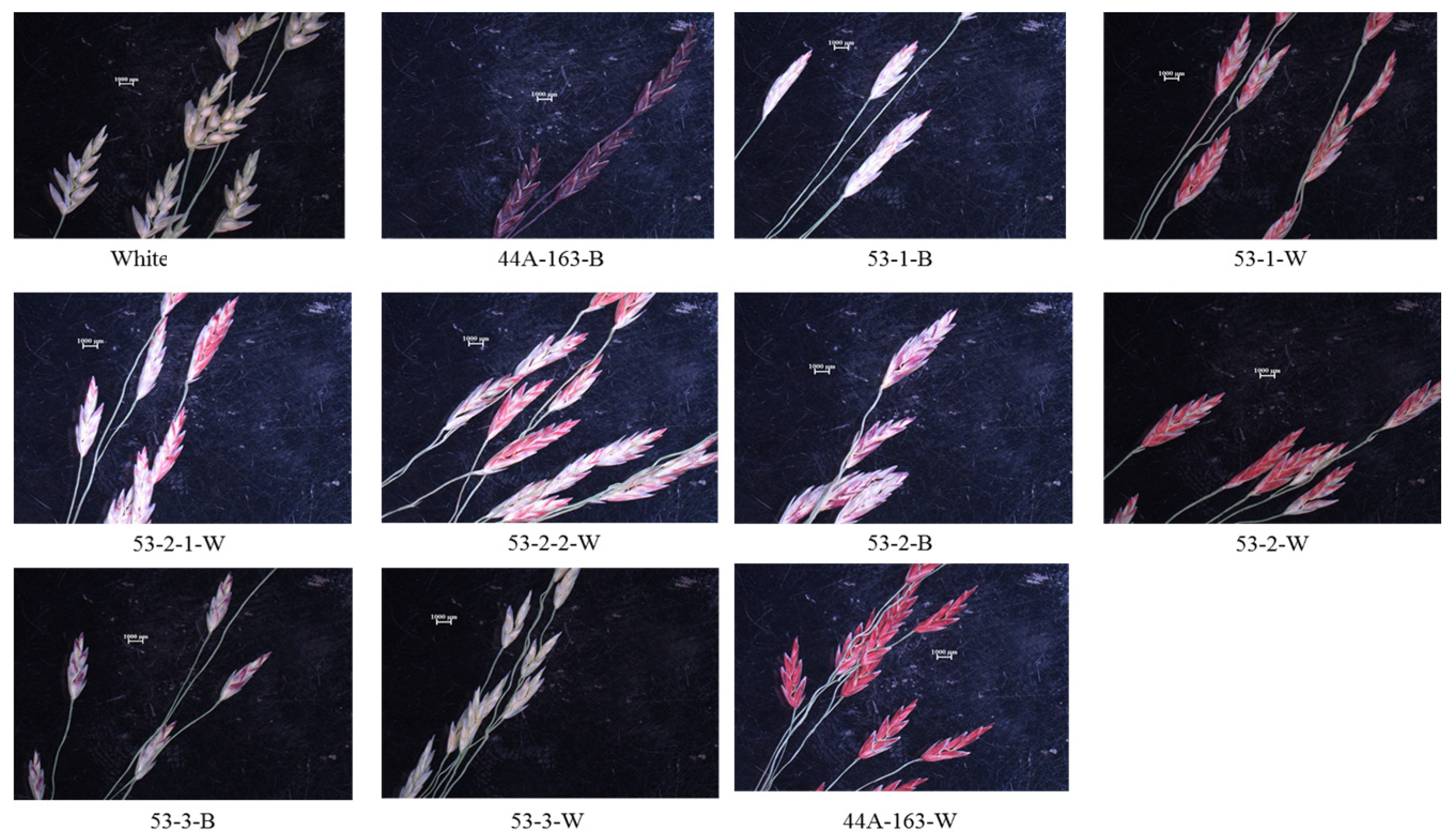
Coloration of the Spikelet lemma was also documented (
Figure 2). Whit’s lemma were gray-purplish and highly transparent. 44A-163-B exhibited a dark-purple coloration and was transparent as well. 44B-163-W exhibited bright pink lemma with whitish-gray outer borders and veins. The brown seeded line of 53-1 exhibited much less pink coloration (mainly at the outer borders), compared to their white counterparts, which had bright pink lemma with white gray outer borders and veins. The second brown line of 53-2 exhibited purple coloration at the outer border of the lemma, as compared to their white counterparts which were pink—not purple. The third brown line of 53-3 also exhibited purple coloration at the outer borders of the lemma, as compared to the white counterparts, which had whitish-gray lemma (similar to white in terms of coloration).
3.9. Injera Sensory Evaluation
The sensory evaluation acceptability trials of Injera made from 14 flours samples of the 11 populations grown in the ARO field experiment with additional three commercial samples bought in local markets are presented in
Table 7 and
Figure S9.
The sensory evaluation scored values for all sensory attributes were sampled 8 h after baking the Injera by a panel of 14 Ethiopian judges, to assess the degree of consumer acceptance of Injera prepared from different populations.
44B-163-W and 53-3-B were significantly preferable in terms of Injera appearance, color, and odor, which significantly differed from some of the populations, but not from the commercial ones. In terms of Injera appearance, color, and odor, 53-2-1-W was significantly the least preferable. 53-2-1-W also presented the highest (unpleasant) odor intensity, compared to 44A-163-B, which had the lowest odor intensity.
Both 44B-163-W and 53-3-B exhibited a relativity low odor intensity (which is apparently preferable). The texture of all market samples as well as that of the White was generally ranked higher than the rest of the populations. The acidity level of 53-2-2-W was the highest among the collection while the market samples were generally less acidic in taste The highest ranked in terms of flavor were 53-3-B, 44A-163-B (around 3.2), and the lowest were 53-2-B and 53-2-1-W (around 1.6).
3.10. Principal Component Analysis
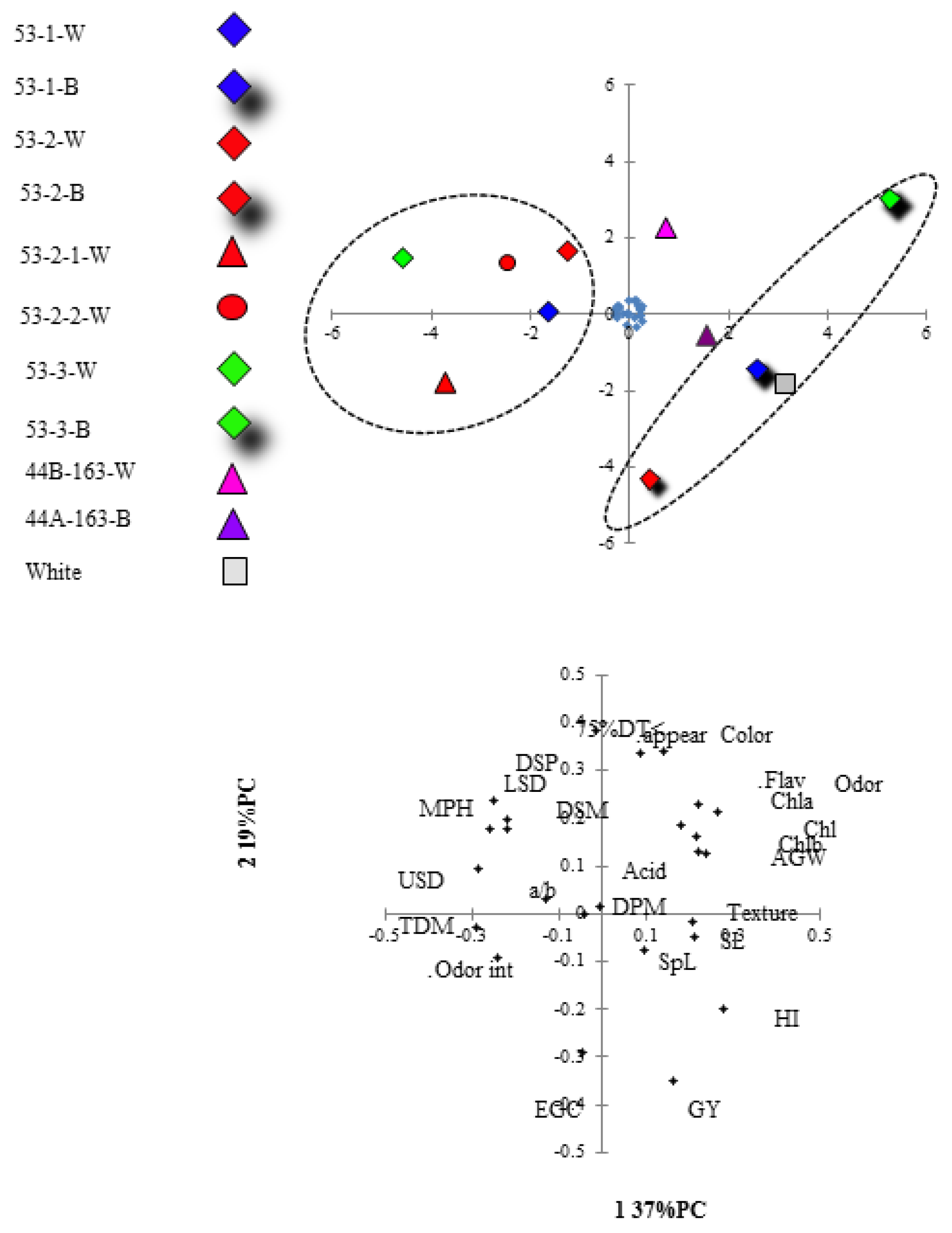
Principal component analysis (PCA) was conducted for all studied traits (
Figure 3). Two components were extracted using eigenvalues > 1 to ensure meaningful implementation of the data by each factor. The PCA of the 11 lines extracted two major principal components (eigenvalues > 1) that accounted collectively for 56% of the variance between the populations. Principal component 1 (PC1,
X-axis) explained 37% of the data set variation, and PC2 (
Y-axis) explained 19% of the data set variation.
Both the correlations and the PCA showed a negative association between the two components representing reproductive variables (GY and HI) and MPH, DSP, DSM, DT < 75%, LSD (r = −0.8 **, −0.69 *, −0.62 *, −0.64 * with GY, respectively). Along the axis of association, white as well as the brown segregants were the highest yielding and had the lowest MPH, DSP, DSM, DT < 75%, LSD.
Injera color and appearance were grouped and were negatively associated (r = −0.61 *) with EGC. Another group which was obtained was negatively associated with TDM and odor intensity, which included the traits—Chl Tot, Chlb, flavor, and AGW (r = −0.71 *, −0.8 **, −0.6 *, −0.64 *, with TDM respectively).
3.11. Correlations
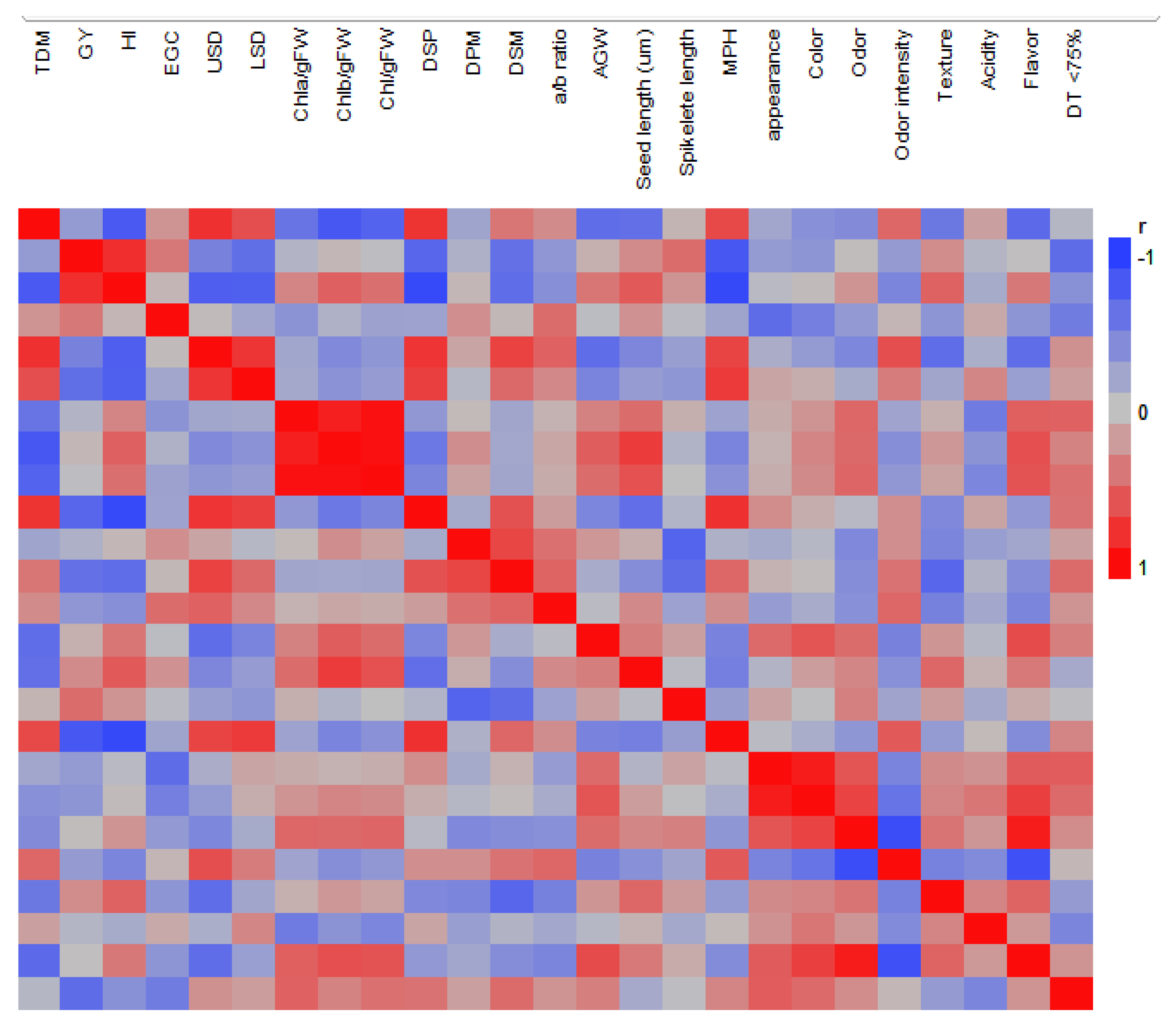
Figure 4 shows the heat-map correlation matrix obtained for the studied traits. TDM was found to be significantly negatively correlated with Chlb/gFW (
r = −0.8 **) and Chl/gFW (−0.71 **), and positively correlated with USD (0.79 **) and LSD (0.63 *). GY was negatively correlated with MPH (−0.8 **) and with LSD (0.63 *). The correlation between Chla/gFW and Chlb/gFW was found strongly significant (0.88 ***). MPH was correlated with TDM, USD, and LSD (0.66 *, 0.68 *, and 0.74 **).
Low DSM or DSP were correlated with increased GY (r = −0.62 * and −0.69 * respectively) and SL (−0.65 * and −0.63 *).
In the sensory parameters evaluated, flavor was found to be positively correlated with odor (0.9 ***) and color (0.7 *), and negatively with odor intensity (−0.86 ***). Odor and odor intensity were negatively correlated (−0.86 ***), and color and appearance were positively correlated (0.90 ***). Odor and color were positively correlated (0.7 *).
Flavor was positively correlated to Chlb/gFW (0.6 *) and negatively with TDM (−0.61 *) and USD (−0.64 *). AGW was found to be positively correlated with odor intensity and negatively with acidity (−0.73 ***). Texture was found to be negatively correlated (−0.64 *) with USD, and USD was positively correlated with odor intensity.
4. Discussion
The teff populations evaluated in this study exhibited a wide phenotypic variation (
Supplementary Figure S7a) was comparable to previous literature reports [
8,
18,
19,
20]. For example, the GY range in the current field experiment was equivalent to 1.3–2.7 t/ha, where the national average farmer’s yield was around 1 t/ha, and 2.5 t/ha under experimental conditions in Ethiopia [
4,
18,
19,
21]. Teff has a potential for yielding 4.6–5 t/ha if lodging can be resolved [
22]. The harvest index values previously reported [
23] were of a similar range to our field experiment (
Table 3). In addition, the phenological values (
Table 6) were in accordance with the previously reported ranges for teff cultivation [
24,
25].
Within the genetic material tested, no correlation was found between TDM and GY, whereas in others, a positive [
5] or negative correlation was reported [
26]. However, when analyzing the white and brown separately (and excluding white which appeared to be much different) there appeared to be some degree of correlation (
r = 0.6 and 0.7 for the white and black, respectively), yet not statistically significant. We found correlation between GY and HI (0.8 ***) that was in agreement with previous reports [
27].
Plant height was previously reported to range between 74 and 116 cm [
18]. Under our field conditions, within the growing season, MPH was 58–78 cm (
Table 5), and in the greenhouse off-season, MPH was 180–200 cm (
Table 1), which was more than double the field growth but with a narrower range across populations. Differences in day length and other environmental factors might account for these differences. Some of the populations were ranked similarly in both experiments (
Table 1 and
Table 6) in terms of MPH.
Since there are two duplicate genes for grain color in teff, which are known to be dominant [
14], the small fraction of the brown seeds found within the seeds propagated from the initial collected panicle (0.064, 0.105 and 0.015 for 53-1, 53-2, 53-3, respectively,
Table 1,
Figure 5) could only be explained by an external foreign pollination. The increase in brown seed ratio in 53-1 from the first collected generation to the greenhouse next-generation, was from 0.021 to 0.064—as would be expected from the segregation of heterozygosity of the grain color loci (A/a). The small fraction of A/a in the collected panicle would be expected to triple (a total of 1 A/A and 2 A/a) over the course of a single generation. The data also support the hypothesis that the brown populations are half-siblings (hybrids) to their white counterparts; these half-siblings share the maternal side but differ in the paternal one. It is very likely that these hybridizations were most probably wind-driven. As opposed to 53-1, no dark brown seeds could be detected within the grain of the collected panicles of these two populations, so there must have been undetectable light brown seeds that were in a heterozygous state A/a and were later segregated.
The brown populations in this study (pure and segregating) exhibited an overall advantage over the white populations in terms of directing their biomass towards grain production (
Table 3, PCA
Figure 3, excluding the case of the ‘White’). White, the pure brown 44A-163-B, and some of the segregating brown populations were the highest ranked for GY (
Table 3, PCA
Figure 3).
A clear pattern emerges that all brown hybrids were earlier to flower (along with the white,
Table 6) as compared to their white half-siblings. Therefore, it is possible that the pollen donor/s was/were a relatively early flowering type. Additionally, the significant differences in plant height, observed between 53-2-W and 53-2-B (76 cm vs. 58 cm,
Table 6) might indicate that the pollen donor in this case had a shorter stature than the maternal line.
53-3-B, which originated from the segregation of the mixed color line 53-3 into brown and white seeds (
Table 1), was especially interesting. This line exhibited relatively low TDM and high HI (
Table 3), low EGC (
Table 3), and high Chl levels (
Table 4). In contrast, this line was also relatively tall, as indicated by its high MPH (
Table 5) and thin stems (
Table 3). In terms of sensory evaluation, 53-3-B was the most promising line with its preferable taste, smell, and appearance (
Table 7).
Our hypothesis was that each of the three half-siblings originated from a different pollen donor and was strengthened by the large variations in Chl levels between the three half-siblings. 53-2-B had the lowest Chl levels, and 53-1-B and 53-3-B exhibited the highest levels among the collection (
Table 4 and
Figure 3). Interestingly, 53-2 which presented the lowest Chl levels had the highest GY among the three. Another result was the grouping of the traits—Chl Tot, Chlb, flavor, and AGW that was negatively correlated with TDM (
Figure 3). This was in agreement with the literature that suggest that a smaller plant might contain denser leaves and more chloroplast and Chl per g FW of leaf tissue [
28]. The positive correlation obtained between flavor and Chl might be indirect, however, these correlations might have importance for future breeding programs as initial phenotypes for selection.
Results from the six crosses of parental populations derived from the Ethiopian gene bank collected from diverse climate and elevation, differing in lemma color (purple, red grey, and yellowish-white), showed that at least four pairs of genes control the inheritance of lemma color in teff [
14], with dominance complementary and epistatic gene actions. Berhe [
14] suggested the following model. C is a gene for basic anthocyanin color; P1 and P2 are duplicate genes responsible for development of purple lemma color in the presence of dominant C (either P1 or P2 alone); p1 and p2 are genes responsible for the red lemma color in the presence of dominant C; G is gene for the gray lemma color, visible only when the dominant C is absent; g is gene for yellowish-white lemma color in the absence of C and G [
14].
Following the genetic model of lemma color [
14], it appeared that both the maternal line of 53-2 and 53-3 had the basic p1 or p2 genes in the background of the dominant C gene, thus, resulting in red lemma color, and that the hybridization with an unknown brown donor introduced a P1 or P2, thus, resulting in purple lemma color. There seems to be differences that might have come from maternal differences between 53-2 and 53-3 in lemma color, because 53-3-W was not red like 53-2-W, but rather was gray (
Figure 2).
In this work we characterized in detail the lodging phenomena in the studied teff populations. The simplified scoring system for lodging [
29], which is commonly used, does not take into account at which growth stage lodging starts, nor the uniformity of lodging within a plot. Therefore, we chose to document plot-height over the course of the reproductive period (
Figure 1), as well as to calculate the days to 75% lodging in a plot. This detailed inspection and documentation explained mechanisms related to teff lodging (
Figure 1,
Figure S7a,b), which were very much context-dependent in terms of environmental conditions. With respect to lodging, the lodging resistance—the reason for collecting these populations to begin with (
Supplementary Figure S1)—it appeared that since the collected plants grew randomly as single plants within a homogeneous lodging with inclined genetic population, and occurred at very low-density planted areas, they showed a lodging-resistant phenotype. In well-irrigated, well-fertilized and low-stress conditions of our experiments, a complete lodging-resistant line was not found, and lodging seemed to be a flash-mob phenomenon where several plants start a lodging movement that sweeps the reset of the field. However, we showed that our in-depth documentation and interpretation of lodging here might be useful for future breeding of lodging resistance characteristics in teff.
High yielding populations tend to lodge at harvest time [
30]. The lack of variation in lodging resistance might be a result of unfavorable associations of lodging resistance with productivity promoting traits like plant height, panicle length, grain, and shoot biomass [
20,
29]. Improvement of lodging-related traits—like culm length, overall-height, and diameter of the culm internodes—through breeding is expected to be a demanding task due to their relatively low heritability and lack of reliable genetic advance-estimates [
31]. Therefore, increasing our understanding of the lodging phenomena and its phenotyping can improve our ability to breed for high yielding lodging-resistant cultivars. This study showed that the nature of lodging was variable in terms of timing and strength (
Figure S7a). In terms of timing, we observed an early type of lodging that was most likely triggered by the fast inflorescence weight increase exhibited by the white (
Figure S7b) and 53-1-B (
Figure 1). Other populations were ‘strong’ enough to carry the inflorescence during most of the grain-filling period, like 44A-163-B and 44B-163-W (
Figure S7b). Therefore, the rate at which panicle increased in weight, prior and throughout grain filling, appeared to vary and might be important from a breeding perspective.
Surprisingly, white, which was the first to lodge, was mostly stable in plot-height once lodged (around 35 cm above ground surface) during the entire grain filling and was the best yielding line. In white, despite stem weakness and the plant being bent towards the ground, the panicles were mostly above ground level. This pattern created a medium level of lodging that appeared to be different from the strong lodging where the plant was heavier and was totally bent to the ground (
Figure S7b: 44B-163-W and 53-2-2-W).
Despite the large number of studies screening teff populations [
11,
19,
20,
32] there are only few that include in-depth characterization of different populations. In addition, there are hardly any studies that present agronomic as well as sensory traits, side by side, to analyze possible links between them. The processing, eating, and nutritional quality of food products might be greatly influenced by teff variety. Therefore, it is necessary to assess the genetic diversity of this crop for potential improvements of agronomic, as well as edible traits. New breed varieties must be subjected to sensory analysis, for consumer acceptance, to make the research efforts commercially meaningful [
4]. We report a significant genotypic effect on most of the sensory traits evaluated (
Table 7). The effectiveness of flour grinding was found to be crucial for texture, across all market samples. The white cultivars were ranked higher than the rest of the populations. The size of the grain might also effect Injera acidity and odor, as AGW was found to be positively correlated with odor intensity and negatively with acidity. There is a need to explore the variability of flower grinding parameters as well.
The current study growing conditions (pots and field experiments) were not favorable to observe the lodging resistance that was observed during the original seed collection. The experiment did not replicate the specific environmental context of a single seed developing in a low-density planted area and was not surrounded by a homogenous population of the plots from which it was collected. A wide genotypic variance was found in the current study for stem width and plant height. However, under an abundance of water and nutrient and, at a high plant density, a thick stem did not ensure lodging resistance. It appeared, however, that under low density or some sort of environmental stress might lead to increased stem lignification and hardening, allowing the plant to carry the grain load at an erect posture. Future experimentation to test this hypothesis might include combinations of agro-technics implementations like seed-coverage to enlarge seeds, using a mixture of teff populations, reducing sowing density to reduce plant density, and the introduction of controlled stresses like water deficiency and salinity. It is clear that late maturing, thick stem, and tall teff varieties possess deeper root systems than early maturing populations of shorter height [
25]. Therefore, a combination that includes populations that are characterized by thick stems with stress can improve lodging resistance. Integration between a wide range of parameters and the correlations obtained between agronomic and sensory traits might improve our ability to breed towards a “real world” better end-product.