The Impact of Soil Water Content on Yield, Composition, Energy, and Water Indicators of the Bioenergy Grass Saccharum spontaneum ssp. aegyptiacum under Three-Growing Seasons
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Trial
2.2. Measurements and Determinations
2.3. Statistical Analysis
3. Results
3.1. Meteorological Trend
3.2. Biomass Yield and Composition
3.3. Energy and Water Indicators
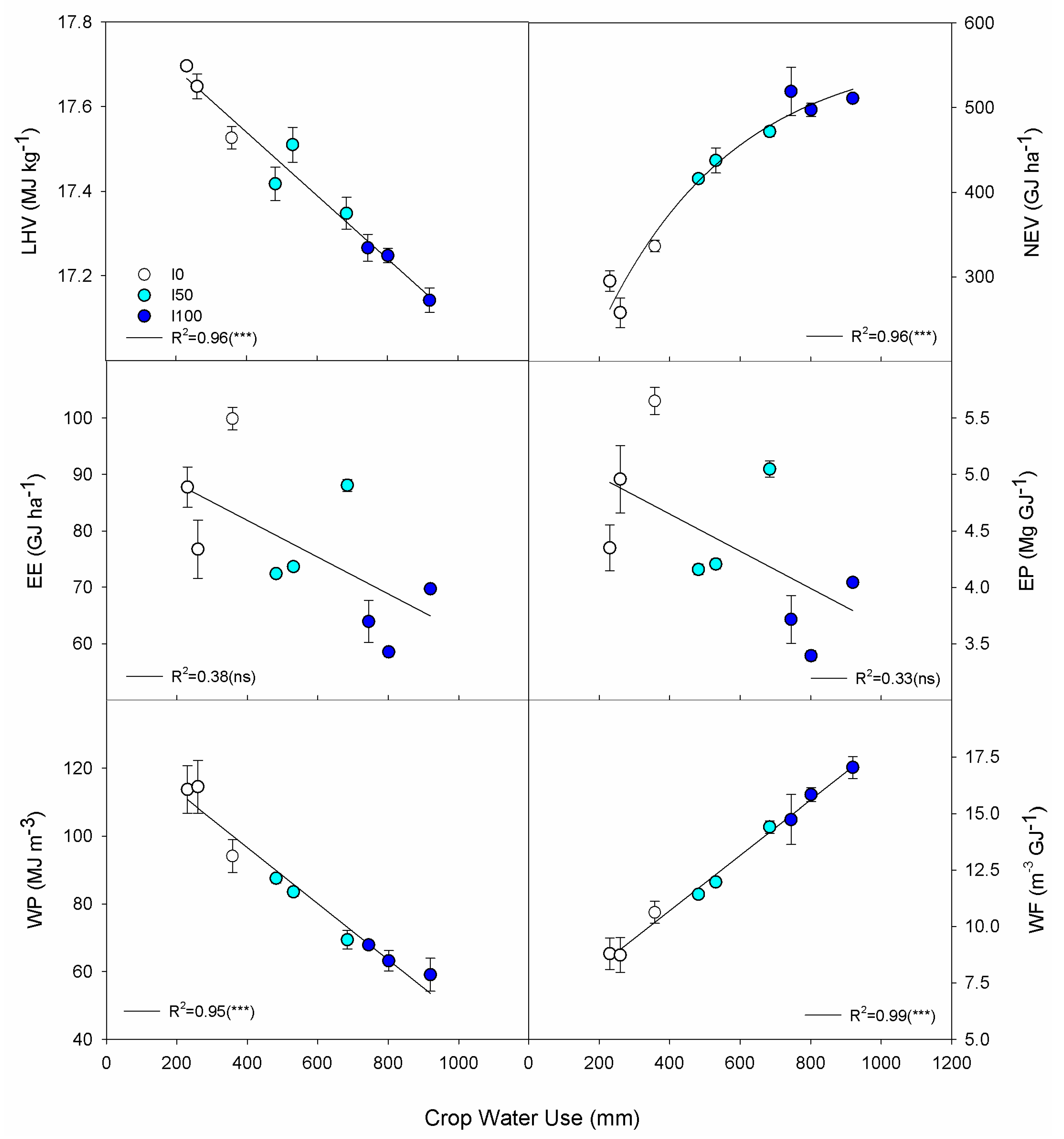
3.4. Relationships among Crop Water Use, Yield, Composition, Energy and Water
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Energy Outlook 2014; International Energy Agency: Paris, France, 2014.
- Fader, M.; Shi, S.; Von Bloh, W.; Bondeau, A.; Cramer, W. Mediterranean irrigation under climate change: More efficient irrigation needed to compensate increases in irrigation water requirements. Hydrol. Earth Syst. Sci. 2016, 20, 953–973. [Google Scholar] [CrossRef]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.P.; Iglesias, A.; Lange, M.A.; Llasat, M.C.; Paz, S.; Penuelas, J.; et al. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Chang. 2018, 8, 972–980. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.F.; Monforti-Ferrario, F.; Nita, V. The role of biomass and bioenergy in a future bioeconomy: Policies and facts. Env. Dev. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L. Perennial Energy Grasses: Resilient Crops in a Changing European Agriculture. Agriculture 2019, 9, 169. [Google Scholar] [CrossRef]
- Von Cossel, M.; Lewandowski, I.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; Iqbal, Y.; Mantel, S.; Scordia, D.; Testa, G.; Cosentino, S.L.; et al. Marginal agricultural land low-input systems for biomass production. Energies 2019, 12, 3123. [Google Scholar] [CrossRef]
- Perpiña Castillo, C.; Kavalov, B.; Diogo, V.; Jacobs-Crisioni, C.; Batista e Silva, F.; Lavalle, C. Agricultural land abandonment in the EU within 2015–2030. JRC Policy Insight. JRC113718; European Commission, 2018. Available online: https://ec.europa.eu/jrc/en/luisa (accessed on 10 July 2020).
- Panoutsou, C.; Chiaramonti, D. Socio-Economic Opportunities from Miscanthus Cultivation in Marginal Land for Bioenergy. Energies 2020, 13, 2741. [Google Scholar] [CrossRef]
- Soldatos, P.; Lychnaras, V.; Panoutsou, C.; Cosentino, S.L. Economic viability of energy crops in the EU: The farmer’s point of view. Biofuels Bioprod. Bioref. 2010, 4, 637–657. [Google Scholar] [CrossRef]
- Von Cossel, M.; Wagner, M.; Lask, J.; Magenau, E.; Bauerle, A.; Cossel, V.V.; Warrach-Sagi, K.; Elbersen, B.; Staritsky, I.; van Eupen, M.; et al. Prospects of bioenergy cropping systems for a more social-ecologically sound bioeconomy. Agronomy 2019, 9, 605. [Google Scholar] [CrossRef]
- Fernando, A.L.; Duarte, M.P.; Almeida, J.; Boléo, S.; Mendes, B. Environmental impact assessment of energy crops cultivation in Europe. Biofuel Bioprod. Bioref. 2010, 4, 594–604. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, Y.A. Water footprint benchmarks for crop production: A first global assessment. Ecol. Indic. 2014, 46, 214–223. [Google Scholar] [CrossRef]
- Schmidt, T.; Fernando, A.L.; Monti, A.; Rettenmaier, N. Life Cycle Assessment of Bioenergy and Bio-Based Products from Perennial Grasses Cultivated on Marginal Land in the Mediterranean Region. Bioenergy Res. 2015, 8, 1548–1561. [Google Scholar] [CrossRef]
- Lewandowski, I.; Scurlock, J.M.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Alexopoulou, E.; Zanetti, F.; Scordia, D.; Zegada-Lizarazu, W.; Christou, M.; Testa, G.; Cosentino, S.L.; Monti, A. Long-Term Yields of Switchgrass, Giant Reed, and Miscanthus in the Mediterranean Basin. Bioenergy Res. 2015, 8, 1492–1499. [Google Scholar] [CrossRef]
- Fernando, A.L.; Boléo, S.; Barbosa, B.; Costa, J.; Duarte, M.P.; Monti, A. Perennial Grass Production Opportunities on Marginal Mediterranean Land. Bioenergy Res. 2015, 8, 1523–1537. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Copani, V.; Scalici, G.; Scordia, D.; Testa, G. Soil erosion mitigation by perennial species under Mediterranean environment. Bioenergy Res. 2015, 8, 1538–1547. [Google Scholar] [CrossRef]
- Triana, F.; Nassi o Di Nasso, N.; Ragaglini, G.; Roncucci, N.; Bonari, E. Evapotranspiration, crop coefficient and water use efficiency of giant reed (Arundo donax L.) and miscanthus (Miscanthus × giganteus Greef et Deu.) in a Mediterranean environment. GCB Bioenergy 2015, 7, 811–819. [Google Scholar] [CrossRef]
- Jones, M.B.; Finnan, J.; Hodkinson, T.R. Morphological and physiological traits for higher biomass production in perennial rhizomatous grasses grown on marginal land. GCB Bioenergy 2015, 7, 375–385. [Google Scholar] [CrossRef]
- Sulas, L.; Franca, A.; Sanna, F.; Re, G.A.; Melis, R.; Porqueddu, C. Biomass characteristics in Mediterranean populations of Piptatherum miliaceum—A native perennial grass species for bioenergy. Ind. Crop Prod. 2015, 75, 76–84. [Google Scholar] [CrossRef]
- Scordia, D.; Testa, G.; Copani, V.; Patanè, C.; Cosentino, S.L. Lignocellulosic biomass production of Mediterranean wild accessions (Oryzopsis miliacea, Cymbopogon hirtus, Sorghum halepense and Saccharum spontaneum) in a semi-arid environment. Field Crops Res. 2017, 214, 56–65. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Copani, V.; Testa, G.; Scordia, D. Saccharum spontaneum L. ssp. aegyptiacum (Willd.) Hack. a potential perennial grass for biomass production in marginal land in semi-arid Mediterranean environment. Ind. Crop. Prod. 2015, 75, 93–102. [Google Scholar] [CrossRef]
- Scordia, D.; Testa, G.; Csentino, S.L.; Copani, V.; Patanè, C. Soil water effect on crop growth, leaf gas exchange, water and radiation use efficiency of Saccharum spontaneum L. ssp. aegyptiacum (Willd.) Hackel in semi-arid Mediterranean environment. Ital. J. Agron. 2015, 10, 185–191. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L.; Jeffries, T.W. Second generation bioethanol production from Saccharum spontaneum L. spp. aegyptiacum (Willd.) Hack. Bioresour. Technol. 2010, 101, 5358–5365. [Google Scholar] [CrossRef] [PubMed]
- Scordia, D.; Testa, G.; Cosentino, S.L. Perennial grasses as lignocellulosic feedstock for second-generation bioethanol production in Mediterranean environment. Ital. J. Agron. 2014, 9, 84–92. [Google Scholar] [CrossRef]
- Scordia, D.; van den Berg, D.; van Sleen, P.; Alexopoulou, E.; Cosentino, S.L. Are herbaceous perennial grasses suitable feedstock for thermochemical conversion pathways? Ind Crop Prod. 2016, 91, 350–357. [Google Scholar] [CrossRef]
- Copani, V.; Cosentino, S.L.; Testa, G.; Scordia, D. Agamic propagation of giant reed (Arundo donax L.) in semi-arid Mediterranean environment. Ital. J. Agron. 2013, 8, 18–24. [Google Scholar]
- Scordia, D.; Zanetti, F.; Varga, S.S.; Alexopoulou, E.; Cavallaro, V.; Monti, A.; Copani, V.; Cosentino, S.L. New Insights into the Propagation Methods of Switchgrass, Miscanthus and Giant Reed. Bioenergy Res. 2015, 8, 1480–1491. [Google Scholar] [CrossRef]
- Cavallaro, V.; Scordia, D.; Cosentino, S.L.; Copani, V. Up-scaling agamic propagation of giant reed (Arundo donax L.) by means of single-node stem cuttings. Ind. Crop. Prod. 2019, 128, 534–544. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Scordia, D.; Sanzone, E.; Testa, G.; Copani, V. Response of giant reed (Arundo donax L.) to nitrogen fertilization and soil water availability in semi-arid Mediterranean environment. Eur. J. Agron. 2014, 60, 22–32. [Google Scholar] [CrossRef]
- Doorenbos, J.; Pruitt, W.O. Guidelines for predicting crop water requirements. In Irrigation and Drainage Paper No. 24; FAO: Rome, Italy, 1977; Volume 179. [Google Scholar]
- Odum, E.P. Basi di Ecologia; Piccin: Padova, Italy, 1988; p. 584. [Google Scholar]
- Zanetti, F.; Scordia, D.; Calcagno, S.; Acciai, M.; Grasso, A.; Cosentino, S.L.; Monti, A. Trade-off between harvest date and lignocellulosic crop choice for advanced biofuel production in the Mediterranean area. Ind. Crop. Prod. 2019, 138, 111439. [Google Scholar] [CrossRef]
- Pari, L.; Curt, M.D.; Sànchez, J.; Santangelo, E. Economic and energy analysis of different systems for giant reed (Arundo donax L.) harvesting in Italy and Spain. Ind. Crops Prod. 2016, 84, 176–188. [Google Scholar] [CrossRef]
- Borsato, E.; Martello, M.; Marinello, F.; Bortolini, L. Environmental and Economic Sustainability Assessment for Two Di_erent Sprinkler and A Drip Irrigation Systems: A Case Study on Maize Cropping. Agriculture 2019, 9, 187. [Google Scholar] [CrossRef]
- Climate Change, Water and Food Security; Water Reports No. 36; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011.
- Vicente-Serrano, S.M.; Lopez-Moreno, J.I.; Beguería, S.; Lorenzo-Lacruz, J.; Sanchez-Lorenzo, A.; García-Ruiz, J.M.; Azorin-Molina, C.; Morán-Tejeda, E.; Revuelto, J.; Trigo, R.; et al. Evidence of increasing drought severity caused by temperature rise in southern. Eur. Environ. Res. Lett. 2014, 9, 044001IEA. [Google Scholar] [CrossRef]
- The Water-Energy-Food Nexus. A New approach in Support of Food Security and Sustainable Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; Available online: http://www.fao.org/3/a-bl496e.pdf (accessed on 27 July 2020).
- Haworth, M.; Marino, G.; Riggi, E.; Avola, G.; Brunetti, C.; Scordia, D.; Testa, G.; Gomes, M.T.G.; Loreto, F.; Cosentino, S.L.; et al. The effect of summer drought on the yield of Arundo donax is reduced by the retention of photosynthetic capacity and leaf growth later in the growing season. Ann. Bot. 2019, 124, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Clifton-Brown, J.; Harfouche, A.; Casler, M.D.; Jones, H.D.; Macalpine, W.J.; Murphy-Bokern, D.; Smart, L.B.; Adler, A.; Ashman, C.; Awty-Carroll, D.; et al. Breeding progress and preparedness for mass-scale deployment of perennial lignocellulosic biomass crops switchgrass, miscanthus, willow and poplar. Glob. Chang. Biol. Bioenergy 2019, 11, 118–151. [Google Scholar] [CrossRef] [PubMed]
- Stavridou, E.; Webster, R.J.; Robson, P.R.H. Novel Miscanthus genotypes selected for different drought tolerance phenotypes show enhanced tolerance across combinations of salinity and drought treatments. Ann. Bot. 2019, 124, 653–674. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Schwarz, K.U.; Awty-Carroll, D.; Iurato, A.; Meyer, H.; Greef, J.; Gwyn, J.; Mos, M.; Ashman, C.; Hayes, C.; et al. Breeding strategies to improve Miscanthus as a sustainable source of biomass for bioenergy and biorenewable products. Agronomy 2019, 9, 673. [Google Scholar] [CrossRef]
- Scordia, D.; Scalici, G.; Clifton-Brown, J.; Robson, P.; Patanè, C.; Cosentino, S.L. Wild miscanthus germplasm in a drought-affected area: Physiology and agronomy appraisals. Agronomy 2020, 10, 679. [Google Scholar] [CrossRef]
- Tillman, D.; Socolow, R.; Foley, J.A.; Hill, J.; Larson, E.; Lynd, L.; Pacala, S.; Reilly, J.; Searchinger, T.; Somerville, C.; et al. Beneficial biofuels—The food, energy, and environment trilemma. Science 2009, 325, 270–271. [Google Scholar] [CrossRef]
- Confalonieri, R.; Jones, B.; Van Diepen, K.; Van Orshoven, J. Scientific contribution on combining biophysical criteria underpinning the delineation of agricultural areas affected by specific constraints. In Methodology, Factsheets for Plausible Criteria Combinations; Terres, J.M., Hagyo, A., Wania, A., Eds.; Scientific and Technical Research Series; European Commission, Joint Research Centre, Institute for Environment and Sustainability: Luxembourg, 2014; ISSN 1831-9424. ISBN 978-92-79-44340-4. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Pasqui, M.; Menduni, G.; Messeri, G.; Gozzini, B.; Grifoni, D.; Rossi, M.; Maracchi, G. Sensitivity of meteorological high-resolution numerical simulations of the biggest floods occurred over the Arno river basin, Italy, in the 20th century. J. Hydrol. 2004, 288, 37–56. [Google Scholar] [CrossRef]
- Caloiero, T.; Veltri, S.; Caloiero, P.; Frustaci, F. Drought Analysis in Europe and in the Mediterranean Basin Using the Standardized Precipitation Index. Water 2018, 10, 1043. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Patanè, C.; Sanzone, E.; Testa, G.; Scordia, D. Leaf gas exchange, water status and radiation use efficiency of giant reed (Arundo donax L.) in a changing soil nitrogen fertilization and soil water availability in a semi-arid Mediterranean area. Eur. J. Agron. 2016, 72, 56–69. [Google Scholar] [CrossRef]
- Haworth, M.; Cosentino, S.L.; Marino, G.; Brunetti, C.; Scordia, D.; Testa, G.; Riggi, E.; Avola, G.; Loreto, F.; Centritto, M. Physiological responses of Arundo donax ecotypes to drought: A common garden study. GCB Bioenergy 2017, 9, 132–143. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L.; Jeffries, T.W. Enzymatic hydrolysis, simultaneous saccharification and ethanolfermentation of oxalic acid pretreated giant reed (Arundo donax L.). Ind. Crop. Prod. 2013, 49, 392–399. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Breuer, J.; Jones, M.B. Carbon mitigation by the energy crop, Miscanthus. Glob. Chang. Biol. Bioenergy 2007, 13, 2296–2307. [Google Scholar] [CrossRef]
- Angelini, L.G.; Ceccarini, L.; Nassi o Di Nasso, N.; Bonari, E. Comparison of Arundo donax L. and Miscanthus x giganteus in a long-term field experiment in Central Italy: Analysis of productive characteristics and energy balance. Biomass Bioenergy 2009, 33, 635–643. [Google Scholar] [CrossRef]
- Larsen, S.U.; Jørgensen, U.; Kjeldsen, J.B.; Lærke, P.E. Long-term Miscanthus yields influenced by location, genotype, row distance, fertilization and harvest season. Bioenergy Res. 2014, 7, 620–635. [Google Scholar] [CrossRef]
- Mantineo, M.; D’Agosta, G.M.; Copani, V.; Patanè, C.; Cosentino, S.L. Biomass yield and energy balance of three perennial crops for energy use in the semi-arid Mediterranean environment. Field Crop. Res. 2009, 114, 204–213. [Google Scholar] [CrossRef]
- Monti, A.; Di Virgilio, N.; Venturi, G. Mineral composition and ash content of six major energy crops. Biomass Bioenergy 2008, 32, 216–223. [Google Scholar] [CrossRef]
- Monti, A.; Zanetti, F.; Scordia, D.; Testa, G.; Cosentino, S.L. What to harvest when? Autumn, winter, annual and biennial harvesting of giant reed, miscanthus and switchgrass in northern and southern Mediterranean area. Ind. Crop Prod. 2015, 75, 129–134. [Google Scholar] [CrossRef]
- Gulías, J.; Melis, R.; Scordia, D.; Cifre, J.; Testa, G.; Cosentino, S.L.; Porqueddu, C. Exploring the potential of wild perennial grasses as a biomass source in semi-arid Mediterranean environments. Ital. J. Agron. 2018, 13, 937. [Google Scholar] [CrossRef]
- Parikh, J.; Channiwala, S.A.; Ghosal, G.K. A correlation for calculating HHV from proximate analysis of solid fuels. Fuel 2005, 84, 487–494. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Patane, C.; Sanzone, E.; Copani, V.; Foti, S. Effect of soil water content and nitrogen supply on the productivity of Miscanthus × giganteus Greef and Deu. in Mediterranean environment. Ind. Crop. Prod. 2007, 25, 75–88. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. The green, blue and grey water footprint of crops and derived crop products. Hydrol. Earth Syst. Sci. 2011, 15, 1577–1600. [Google Scholar] [CrossRef]
- Gerbens-Leenes, W.; Hoekstra, A.Y.; van der Meer, T.H. The water footprint of bioenergy. Proc. Natl. Acad. Sci. USA 2009, 106, 10219–10223. [Google Scholar] [CrossRef] [PubMed]


| Source | DF | DMY | NDS | HC | CL | ADL | ASH |
|---|---|---|---|---|---|---|---|
| Adj MS | |||||||
| Water (W) | 2 | 374.68 *** | 32.22 *** | 3.534 *** | 12.426 *** | 2.959 *** | 2.651 *** |
| Season (S) | 2 | 13.91 *** | 0.443 ** | 0.655 ** | 1.030 ** | 0.146 ** | 0.454 ** |
| W × S | 4 | 4.12 ** | 0.199 ** | 0.120 *** | 0.0196 ** | 0.066 * | 0.0607 ** |
| Error(T) | 12 | 0.095 | 0.009 | 0.001 | 0.001 | 0.003 | 0.003 |
| Error | 6 | 0.433 | 0.109 | 0.005 | 0.004 | 0.006 | 0.016 |
| W | S | Mg ha−1 | % w/w | % w/w | % w/w | % w/w | % w/w |
| I0 | 2017 | 14.8 ± 0.69 | 18.1 ± 0.37 | 30.4 ± 0.07 | 37.0 ± 0.04 | 8.3 ± 0.03 | 6.1 ± 0.14 |
| I50 | 2017 | 25.3 ± 0.28 | 20.8 ± 0.52 | 29.4 ± 0.02 | 35.3 ± 0.03 | 7.7 ± 0.11 | 6.6 ± 0.11 |
| I100 | 2017 | 29.3 ± 0.42 | 22.5 ± 0.12 | 28.8 ± 0.05 | 34.2 ± 0.02 | 6.9 ± 0.04 | 7.4 ± 0.06 |
| I0 | 2018 | 19.2 ± 0.40 | 19.0 ± 0.39 | 29.5 ± 0.07 | 35.8 ± 0.08 | 7.9 ± 0.06 | 6.7 ± 0.11 |
| I50 | 2018 | 27.3 ± 0.41 | 21.0 ± 0.14 | 29.0 ± 0.12 | 34.7 ± 0.09 | 7.3 ± 0.06 | 7.2 ± 0.18 |
| I100 | 2018 | 30.1 ± 0.25 | 22.7 ± 0.09 | 28.5 ± 0.07 | 34.1 ± 0.07 | 7.0 ± 0.11 | 7.6 ± 0.5 |
| I0 | 2019 | 16.8 ± 1.01 | 18.5 ± 0.71 | 29.8 ± 0.01 | 36.7 ± 0.06 | 8.2 ± 0.04 | 6.3 ± 0.10 |
| I50 | 2019 | 24.2 ± 0.30 | 20.7 ± 0.15 | 28.9 ± 0.14 | 35.3 ± 0.02 | 7.6 ± 0.07 | 6.9 ± 0.20 |
| I100 | 2019 | 29.3 ± 1.83 | 22.5 ± 0.11 | 28.6 ± 0.06 | 34.2 ± 0.10 | 6.9 ± 0.12 | 7.3 ± 0.07 |
| LSD(W × S) | ≤0.05 | 1.044 | 0.524 | 0.112 | 0.100 | 0.123 | 0.634 |
| Source | DF | LHV | NEV | EE | EP | WP | WF |
|---|---|---|---|---|---|---|---|
| Adj MS | |||||||
| Water (W) | 2 | 0.3742 *** | 106412 *** | 1377.15 *** | 3.857 *** | 4644.3 *** | 102.62 *** |
| Season (S) | 2 | 0.0050 * | 3633 *** | 539.70 *** | 1.975 *** | 715.9 *** | 17.66 *** |
| W × S | 4 | 0.0025 * | 1822 ** | 75.52 ** | 0.161 * | 31.85 *** | 0.405 * |
| Error(T) | 12 | 0.0001 | 21.0 | 0.62 | 0.002 | 2.13 | 0.068 |
| Error | 6 | 0.0007 | 119.0 | 5.48 | 0.018 | 54.98 | 0.856 |
| W | S | MJ kg−1 | GJ ha−1 | GJ ha−1 | Mg GJ−1 | MJ m−3 | m3 GJ−1 |
| I0 | 2017 | 17.7 ± 0.01 | 294.9 ± 12.2 | 87.8 ± 3.59 | 4.35 ± 0.20 | 113.7 ± 6.97 | 8.79 ± 0.70 |
| I50 | 2017 | 17.5 ± 0.04 | 437.5 ± 3.93 | 73.6 ± 0.61 | 4.20 ± 0.04 | 83.5 ± 1.35 | 12.0 ± 0.14 |
| I100 | 2017 | 17.2 ± 0.02 | 497.4 ± 7.87 | 58.5 ± 0.83 | 3.39 ± 0.05 | 63.2 ± 3.01 | 15.8 ± 0.31 |
| I0 | 2018 | 17.5 ± 0.02 | 336.2 ± 6.81 | 99.8 ± 2.01 | 5.65 ± 0.12 | 94.1 ± 4.86 | 10.6 ± 0.49 |
| I50 | 2018 | 17.3 ± 0.03 | 471.7 ± 6.02 | 88.1 ± 1.05 | 5.04 ± 0.07 | 69.4 ± 2.75 | 14.4 ± 0.27 |
| I100 | 2018 | 17.1 ± 0.01 | 511.0 ± 4.95 | 69.7 ± 0.69 | 4.04 ± 0.03 | 56.1 ± 4.86 | 17.8 ± 0.05 |
| I0 | 2019 | 17.6 ± 0.03 | 257.6 ± 17.5 | 76.8 ± 5.15 | 4.96 ± 0.29 | 114.5 ± 7.76 | 8.73 ± 0.77 |
| I50 | 2019 | 17.4 ± 0.04 | 416.0 ± 3.88 | 72.4 ± 0.64 | 4.15 ± 0.05 | 87.5 ± 1.45 | 11.4 ± 0.15 |
| I100 | 2019 | 17.2 ± 0.01 | 519.1 ± 32.2 | 63.9 ± 3.72 | 3.71 ± 0.21 | 67.9 ± 1.10 | 14.7 ± 1.10 |
| LSD(W × S) | ≤0.05 | 0.043 | 17.30 | 3.714 | 0.215 | 11.75 | 1.468 |
| Relationship | Equation | Coefficient | Value | SE | t-Value | p-Value |
|---|---|---|---|---|---|---|
| CWU vs. DMY | y = a × (1 − exp(−bx)) | a | 33.635 | 0.774 | 43.430 | <0.001 |
| b | 0.0024 | 0.0001 | 18.759 | <0.001 | ||
| CWU vs. NDS | y = y0 + ax | y0 | 16.772 | 0.3814 | 43.976 | <0.001 |
| a | 0.007 | 0.0006 | 11.038 | <0.001 | ||
| CWU vs. HC | y = y0 + () | y0 | 28.153 | 0.1657 | 169.888 | <0.001 |
| a | 485.588 | 66.400 | 7.313 | 0.002 | ||
| CWU vs. CL | y = y0 + () | y0 | 33.261 | 0.1662 | 200.106 | <0.001 |
| a | 892.410 | 66.601 | 13.399 | <0.001 | ||
| CWU vs. ADL | y = y0 + () | y0 | 6.622 | 0.1381 | 47.939 | <0.001 |
| a | 419.799 | 55.351 | 7.584 | 0.001 | ||
| CWU vs. ASH | y = y0 + ax | y0 | 5.7670 | 0.1183 | 48.7447 | <0.001 |
| a | 0.0021 | 0.0002 | 10.5519 | <0.001 |
| Relationship | Equation | Coefficient | Value | SE | t-Value | p-Value |
|---|---|---|---|---|---|---|
| CWU vs. LHV | y = y0 + ax | y0 | 17.8392 | 0.0360 | 495.4335 | <0.001 |
| a | −0.0007 | 0.00005 | −12.5399 | <0.001 | ||
| CWU vs. NEV | y = a × (1 − exp(−bx)) | a | 570.603 | 26.850 | 21.251 | <0.001 |
| b | 0.0027 | 0.0003 | 8.905 | <0.001 | ||
| CWU vs. EE | y = y0 + ax | y0 | 94.9085 | 9.5250 | 9.9642 | <0.001 |
| a | −0.0326 | 0.0158 | −2.0648 | 0.078 | ||
| CWU vs. EP | y = y0 + ax | y0 | 5.3091 | 0.5375 | 9.8771 | <0.001 |
| a | −0.0016 | 0.0009 | −1.8482 | 0.107 | ||
| CWU vs. WP | y = y0 + ax | y0 | 129.8059 | 3.8662 | 33.5744 | <0.001 |
| a | −0.0829 | 0.0064 | −12.9270 | <0.001 | ||
| CWU vs. WF | y = y0 + ax | y0 | 5.8007 | 0.2569 | 22.5805 | <0.001 |
| a | 0.0122 | 0.0004 | 28.7397 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scordia, D.; Calcagno, S.; Piccitto, A.; Patanè, C.; Cosentino, S.L. The Impact of Soil Water Content on Yield, Composition, Energy, and Water Indicators of the Bioenergy Grass Saccharum spontaneum ssp. aegyptiacum under Three-Growing Seasons. Agronomy 2020, 10, 1105. https://doi.org/10.3390/agronomy10081105
Scordia D, Calcagno S, Piccitto A, Patanè C, Cosentino SL. The Impact of Soil Water Content on Yield, Composition, Energy, and Water Indicators of the Bioenergy Grass Saccharum spontaneum ssp. aegyptiacum under Three-Growing Seasons. Agronomy. 2020; 10(8):1105. https://doi.org/10.3390/agronomy10081105
Chicago/Turabian StyleScordia, Danilo, Silvio Calcagno, Alessandra Piccitto, Cristina Patanè, and Salvatore Luciano Cosentino. 2020. "The Impact of Soil Water Content on Yield, Composition, Energy, and Water Indicators of the Bioenergy Grass Saccharum spontaneum ssp. aegyptiacum under Three-Growing Seasons" Agronomy 10, no. 8: 1105. https://doi.org/10.3390/agronomy10081105
APA StyleScordia, D., Calcagno, S., Piccitto, A., Patanè, C., & Cosentino, S. L. (2020). The Impact of Soil Water Content on Yield, Composition, Energy, and Water Indicators of the Bioenergy Grass Saccharum spontaneum ssp. aegyptiacum under Three-Growing Seasons. Agronomy, 10(8), 1105. https://doi.org/10.3390/agronomy10081105







