Abstract
Molecularly imprinted polymers (MIPs) for silver sorption were synthesized using diethylene glycol dimethacrylate (DEGDMA) and divinylbenzene (DVB) as crosslinking agents. Synthesis was carried out using a ratio template: monomer: monomer: cross-linker = 1:2:2:8. The yield of obtained imprinting structures was 63.2% and 67.8% for MIP(DEGDMA) and MIP(DVB), respectively. The MIPs were analyzed by FTIR analysis, which showed the presence of characteristic peaks indicating the presence of monomers and crosslinkers in the MIP structure. According to the results of SEM analysis, the average cavity size for MIP(DEGDMA) is 0.81 ± 0.20 μm and for MIP(DVB) is 0.68 ± 0.23 μm in diameter. MIP(DEGDMA)’s sorption degree is 66.08%, and its sorption capacity is 3.31 g/g; MIP(DVB)’s sorption degree is 78.35%, and its sorption capacity is 3.92 g/g. The desorption degree is 69.85% for MIP(DEGDMA) and 69.52% for MIP(DVB). For analysis of sorption kinetics, the Radushkevich and Elovich kinetic models were applied.
1. Introduction
Noble metals (Au, Ag, Pt, Pd, Rh, etc.) play a key role in high-tech industries, the economy, and everyday life. Their importance is growing with the development of green energy, medicine, and electronics due to their unique properties—chemical resistance, electrical conductivity, and catalytic activity [1,2,3,4]. However, since noble metals are non-renewable, their natural reserves are finite. As a result, extracting noble metals from discarded industrial and electronic waste not only helps mitigate the scarcity of these valuable resources but also contributes to reducing environmental pollution. Nowadays, the trend of growing demand for recycled metals is observed, which requires innovative methods of their extraction and processing [5,6,7,8,9,10,11,12].
The special place from the noble metals is given to silver. The rising demand driven by renewable energy technologies and electronic devices is surpassing current silver production levels, leading to potential shortages. Silver remains a critical metal for electronics and microelectronics [13,14]; medicine and biotechnologies [15,16,17,18,19]; and energy and green technologies [20,21,22].
Sorption is an effective method for concentrating and extracting silver from dilute solutions (ore leaching, industrial waste, electronic waste). The method is based on the physical adsorption or chemical binding of Ag+ ions with the functional groups of the sorbent. Nowadays, the following sorbents for silver extraction can be named: activated coal; ion-exchange resins; inorganic sorbents; and biosorbents [23,24,25,26,27,28,29]. As an alternative to the above-mentioned sorbents, the application of molecularly imprinted polymers (MIPs) can be assumed.
MIPs are synthetic, tailor-made materials designed to selectively recognize and bind target molecules (templates), mimicking natural molecular recognition [30,31]. They offer high selectivity, stability, and reusability, making them superior to conventional sorbents in many applications [32,33]. Key applications of MIPs can be named as follows: metal recovery [34]; environmental remediation [35]; medicine and diagnostics [36]; sensors [37,38]; and food safety and analysis [39,40]. The advantages of MIPs over traditional sorbents are presented in Table 1 [41,42,43,44,45].

Table 1.
Advantages of MIPs over traditional sorbents.
Molecularly imprinted polymers are created by forming a polymer around a target molecule using functional monomers and cross-linkers; once the template is removed, the resulting cavities enable selective rebinding of the target from complex mixtures [46]. There are several studies on developing MIPs based on various monomers and crosslinkers for the extraction of silver [47,48]. For example, a biopolymer chitosan was used as a raw material and glutaraldehyde as a crosslinker for obtaining MIP for selective removal and preconcentration of Ag(I) ions from aqueous solutions [49,50]. As for organic monomers, various acrylic and pyridine compounds were used. The most often used functional monomers among pyridine ones are 2-vinylpyridine (2-VP) and 4-vinylpyridine (4-VP). For example, in [51], ion-imprinted polymer (UA-DSPE-IIP) nanoparticles based on 2-VP were developed for the selective extraction of silver ions using ultrasonic-assisted dispersive solid-phase extraction. The binding properties of silver ion-imprinted polymers based on 4-VP were characterized by equilibrium and kinetic models [52]. 4-VP was used for obtaining magnetic ion imprinted polymers using functionalized magnetite (Fe3O4) nanoparticles [53] and by coating on the surface of magnetic graphene oxide [54] as a magnetic core. Another example of magnetic MIP using Fe3O4-SiO2-TiO2 nanoparticles as a magnetic support of adsorbent and Ag(I)-2,4-diamino-6-phenyl-1,3,5-triazine (DPT) complex as the template molecule was obtained based on the most often used acrylic monomer—methacrylic acid (MAA) [55]. The latter was also used for obtaining an Ag(I)-ion imprinted polymer with silver (Ag) dithizone complex as a template [56] and by the formation of 2-(4-hydroxypent-3-en-2-ylideneamine) phenol complex with silver ions [57]. The peculiarity of all these studies is the use of a single monomer for obtaining MIP. Therefore, our study was devoted to the preparation of MIPs based on two monomers—MAA and 4-VP.
Another peculiarity of these studies is the use of ethylene glycol dimethacrylate (EGDMA) as a crosslinker. The crosslinker is an important component in MIP synthesis, determining the structural stability, binding capacity, and selectivity of the polymer [58]. The common crosslinkers used in molecular imprinting are ethylene glycol dimethacrylate (EGDMA), divinylbenzene (DVB), diethylene glycol di-methacrylate (DEGDMA), N,N′-Methylenebisacrylamide (MBA), and others [59]. Its type and concentration directly affect the mechanical rigidity of the polymer network, the accessibility of imprinted cavities, and the rebinding kinetics of the target molecule [60,61]. Therefore, the aim of the work is to study the influence of the crosslinker type on the sorption properties of MIPs in relation to silver.
2. Materials and Methods
2.1. Materials
Silver nitrate with the composition of the main product ≥ 99.9%, provided by “Laborpharma” (Kazakhstan), was used as a template.
Methacrylic acid (MAA) produced by “Fluka” (Buchs, Switzerland) with a purity of ≥98% was used as a monomer.
4-vinylpyridine (4-VP) produced by “Sigma Aldrich” (Burlington, MA, USA) with a purity of ≥95% was used as a monomer.
Azobisisobutyronitrile (AIBN) in powder form, with the composition of the main product ≥ 95%, produced by “AK Scientific” (Union City, CA, USA), was used as an initiator without pre-treatment.
Di(ethylene glycol) dimethacrylate (DEGDMA) with a purity of ≥95% produced by “Sigma Aldrich” (USA) was used as a cross-linking agent.
Divinylbenzene (DVB) produced by “Sigma Aldrich” (USA) was used as a cross-linking agent (contains > 300 ppm of stabilizer).
2-hydroxyethyl cellulose (HEC), average Mv = 90,000, viscosity 100–180 cP, density 0.6 g/mL at 25 °C, produced by “Sigma Aldrich” (USA), was used as a stabilizer.
Toluene marked as “pure for analysis” and provided by “Skat” (Kazakhstan) was used as a porogen.
Nitric acid (96%) provided by “Laborpharma” (Kazakhstan) was used for the purification of synthesized copolymers.
Ethanol produced by “Talgar Spirtzavod” (Kazakhstan), with the composition of the main product ≥ 96%, was used for the purification of synthesized copolymers.
Dialysis membranes with a pore size of 12–14 kDa produced by “Medicell Membranes Ltd.” (London, England) were used for the purification of synthesized copolymers.
Argon was used to create an inert medium during synthesis.
2.2. MIPs Synthesis
The synthesis of molecularly imprinted polymer was carried out based on the methodology described in [62]. The synthesis of MIPs was carried out via bulk polymerization in the presence of two different cross-linkers: DEGDMA and DVB. A general representation of the MIP synthesis is shown in Figure 1.
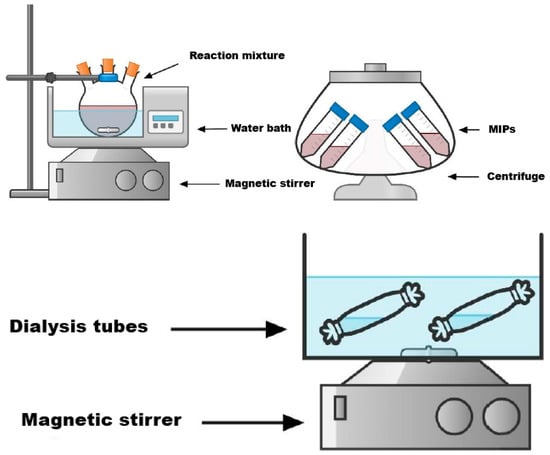
Figure 1.
Synthesis of the MIPs.
Synthesis procedure sequence: template, two monomers, and crosslinker were taken in a ratio of 1:2:2:8. Firstly, 0.8493 g (5 mmol) of AgNO3, 0.848 mL (10 mmol) of MAA, 1.0769 mL (10 mmol) of 4-VP, and 40 mL of distilled water were mixed and stirred until the mixture was dissolved. Then, a 1% solution of HEC was prepared, 20 mL of which was added to the mixture along with 10 mL of toluene, and the mixture was stirred for 15 min. Afterwards, a definite amount of crosslinker (8.956 mL (40 mmol) of DEGDMA or 5.697 mL (40 mmol) of DVB) and 0.019 g of AIBN were added, and the mixture was stirred for 5 min. Inert argon was bubbled through the mixture in order to remove the oxygen. Then, the mixture was placed in a water bath at 60 °C for 6 h. The obtained product was put in a centrifuge to separate the thick part from the liquid part. After centrifugation, the thick part was purified by dialysis for 4 days: the first day in a solution of ethanol to remove unreacted monomers, the crosslinker, and porogen; the second day in distilled water to remove the unreacted monomers and HEC; the third day in a weak solution (0.5 M) of nitric acid to remove template molecules; and the last day again in distilled water to remove the remaining acid solution. Finally, the copolymer was freeze-dried. As a result, two MIP samples were obtained: MIP(DEGDMA) and MIP(DVB).
2.3. Physico-Chemical Analysis
Freeze drying method was used to dry the MIPs from water and obtain a powdered product using a Labconco 7934030 freeze dryer (Labconco Corporation, Kansas City, MO, USA). MIPs were first frozen at approximately −20 °C and then freeze-dried.
The yield of the reaction product is the ratio of the mass of the resulting product to the mass that should theoretically be obtained. The theoretical mass of the substances was taken as the total mass of the monomers used. The yield of products was calculated using the following formula:
Fourier Transform Infrared (FTIR) Analysis was conducted using an FTIR Spectrometer, Nicolet iS10 FT-IR Spectrometer (Thermo Electron Corporation, Waltham, MA, USA), for the determination of the MIP structure. Samples for analysis were prepared using the ATR technique.
Scanning Electron Microscopy (SEM) was conducted using a ZEISS Crossbeam 540 SEM (Carl Zeiss Microscopy GmbH, Jena, Germany) to identify the product’s surface morphology.
Adsorption method was used to study the sorption parameters (sorption degree, sorption capacity) of the imprinted structures. The optimal wavelength for a silver solution with a concentration of 0.5 g/100 mL vs. distilled water was obtained using a “Shimadzu UV-1900i” UV-VIS Spectrophotometer (Shimadzu, Kyoto, Japan). Then, 5 different concentrations of solutions (0.3125, 0.625, 1.25, 2.5, 5 g/L) were made, and a calibration curve for silver nitrate with a wavelength of 425 nm was constructed as shown in Figure 2. The sorption experiment was carried out as follows: each MIP (0.2 g) was placed in a solution of silver nitrate with a concentration of 0.1 g/mL. During sorption, the absorbance was measured on the spectrophotometer every 15 min for 3 h for further concentration calculations (using a calibration curve). After 24 h, control measurements were made. Based on the obtained results, the sorption degree and sorption capacity were calculated.
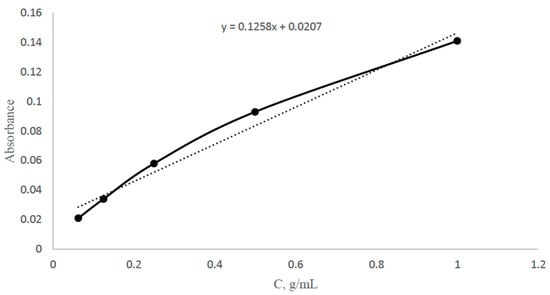
Figure 2.
Calibration curve of silver nitrate at wavelength of 425 nm.
Sorption degree:
where C0 is an initial concentration of silver nitrate in a solution, in g/mL, and Ce is a concentration of silver nitrate in a solution at a certain time interval, in g/mL.
Sorption capacity:
where C0 is an initial concentration of silver nitrate in a solution, in g/mL; Ce is a concentration of silver nitrate in a solution at a certain time interval, in g/mL; Vsolution is a volume of solution of terbium nitrate taken for sorption experiments, in mL; msorbent is the mass of the MIPs taken for sorption experiments, in g.
Desorption experiments were conducted to study the polymer’s surface release properties. The samples of the imprinted structures after sorption were placed into beakers containing a weak solution of nitric acid at a concentration of 0.05 M for this purpose. Similar to the adsorption analysis, the absorbance was determined using a “Shimadzu UV-1900i” UV-VIS Spectrophotometer every 15 min for 3 h. A control absorbance measurement during desorption was performed at 24 h.
3. Results and Discussion
The obtained MIPs are shown in Figure 3: MIP(DEGDMA) (a) and MIP(DVB) (b). Firstly, to analyze the obtained polymers, an organoleptic analysis was carried out (Table 2). The MIPs differ from each other in terms of appearance: the MIP(DVB) is dense while the MIP(DEGDMA) is more liquidized and looser. Due to the fact that 4-VP has a dark brown color, its presence in the MIPs also provides a dark color. The yield of the obtained imprinting structures was 63.2 and 67.8% for MIP(DEGDMA) and MIP(DVB), respectively. Thus, the yield of the synthesis product is around 65%, which is considered to be quite a productive result. The slight difference in values of the MIPs obtained with different crosslinkers can be explained by differences in the structure and reactivity (including steric factors) of the latter.

Figure 3.
Synthesized MIPs: MIP(DEGDMA) (a) and MIP(DVB) (b).

Table 2.
Organoleptic analysis of obtained MIPs.
3.1. FTIR Analysis
The results of the FTIR spectroscopy are shown in Figure 4 for MIP(DEGDMA) and Figure 5 for MIP(DVB). The description of the peaks of the functional groups was analyzed according to the handbook in [63] and is shown in Table 3. As shown in Table 3, both monomers are present in structures on the obtained MIPs. The presence of methacrylic acid is shown by signals characterizing a carboxylic group: intra- and intermolecular hydrogen bonds between -OH groups at 3418.59 cm−1 for MIP(DEGDMA) and 3420 cm−1 for MIP(DVB); a -COOH group at 1629.57 cm−1 for MIP(DEGDMA) and 1628.91 cm−1 for MIP(DVB); an -OH in the carboxylic group at 887.15 cm−1 for MIP(DEGDMA) and 892,49 cm−1 for MIP(DVB). The presence of 4-vynilpyridine is characterized by signals of a pyridine ring at 1069.89 cm−1 for MIP(DEGDMA) and 1030.52 cm−1 for MIP(DVB). Also, both structures are characterized by a peak of -(CH2)x- at 708.02 cm−1 for MIP(DEGDMA) and 706.38 cm−1 for MIP(DVB), which shows that both monomers were polymerized during synthesis. The difference in the obtained MIPs is due to the crosslinkers used during synthesis, which have different structures. This was reflected in the signals observed in the FTIR spectra. The presence of DEGDMA is characterized by the following peaks: a -CH2-CO- group at 2920.06 and 1441.52 cm−1; a carbonyl group -C=O at 1285.25 cm−1; and a -C-O-C- group at 1176.37 cm−1. The structure of DVB in the obtained MIPs is shown by bands of variable intensity of the pyridine group at 1508.65 and 1483.93 cm−1 and by out-of-plane deformation vibrations of the 1,4-substitution at 794.67 cm−1. Thus, the results of the FTIR spectrometry show that the synthesis was successful.
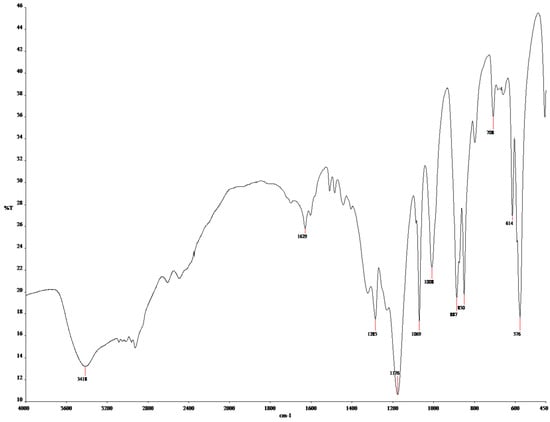
Figure 4.
FTIR spectra of MIP(DEGDMA).
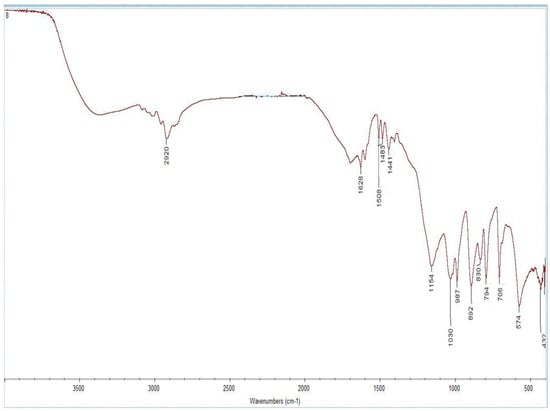
Figure 5.
FTIR spectra of MIP(DVB).

Table 3.
Comparison of FTIR spectra results for MIP(DEGDMA) and for MIP(DVB).
3.2. Reaction Mechanism
The reaction mechanism for obtaining MIPs is proposed based on the literature [45,62] and the results of FTIR spectrometry, and is shown in the example of MIPs with DVB in Figure 6. The suggested mechanism firstly shows the formation of a pre-polymerization complex, in which the monomers and template are involved. Donor–acceptor bonds are formed between the pyridine group of the monomer 4-VP and template ions, and hydrogen bonds are formed between the monomer MAA and template molecules. Furthermore, the pre-polymerization complex is polymerized due to the disclosure of the double bonds of MAA and 4-VP. The polymerization process occurs in the presence of the initiator AIBN, which initiates the polymerization, and then, due to the presence of a crosslinker, the mesh structure is formed. Finally, during the process of dialysis, the template molecule is washed off or removed, and a polymer with molecular imprints is obtained. Molecular imprints are formed as complementary cavities.
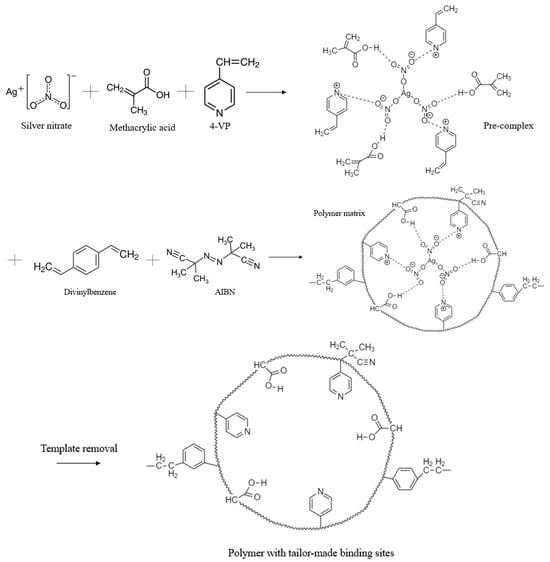
Figure 6.
Proposed reaction mechanism for obtaining MIP(DVB).
3.3. SEM Analysis
To prove the presence of the above-mentioned cavities, SEM analysis was carried out. The results of the SEM analysis are presented in Figure 7 and Figure 8. As can be seen from Figure 7 and Figure 8, the surface of the MIPs is not smooth, and they have some irregularities that look like cavities. Also, it can be seen that the structure of the MIPs is heterogeneous and dense, having in some places cavities due to the harsh formation of the monomeric structure, and these cavities are the molecular imprints, which were obtained in various forms and types. As the structure is heterogeneous, it can be concluded that the bond between the template and the monomer is not stable because the silver is ionizing, and perhaps it is present in an ionic form, which is not a stable bond. Also, the diameter of the cavities was measured using the program ImageJ (version 1.46r). As a result, the average cavity size for MIP(DEGDMA) is 0.81 ± 0.20 μm and for MIP(DVB) is 0.68 ± 0.23 μm in diameter. The difference in sizes of cavities could be explained by the difference in the structures of the crosslinkers: the DEGDMA molecule is more flexible due to its long chain structure, and DVB has an aromatic ring that can affect the steric factor.

Figure 7.
SEM image of MIP(DEGDMA).
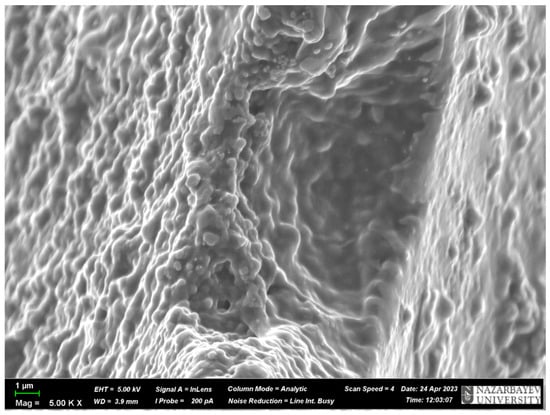
Figure 8.
SEM image of MIP(DVB).
3.4. Sorption Experiments
The obtained MIPs underwent a study of the sorption parameters in the case of the sorption of silver. Figure 9 shows the residual concentration of silver in the solution and the concentration of silver accumulated in the MIPs. According to the results, it is observed that sorption occurs throughout the entire experiment, and the curves lie too close to each other. After one hour, the MIP(DEGDMA) starts to sorb a slightly higher concentration of silver, but at 150 min, the value becomes constant, and its final concentration is less than is shown for MIP(DVB).
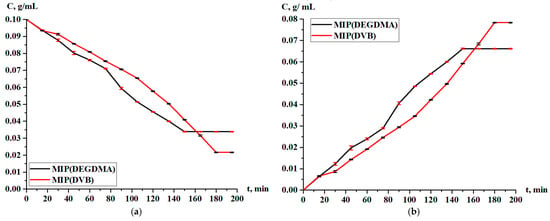
Figure 9.
Concentration of silver nitrate during sorption in solution (a) and in the MIPs (b).
Based on the residual concentration data (Figure 9), the sorption parameters (sorption degree, sorption capacity) of MIP(DEGDMA) and MIP(DVB) were calculated.
The sorption degree and sorption capacity of the MIPs are shown in Figure 10 and Figure 11, respectively. The parameters increase with time, and more intense sorption of silver is observed in the case with MIP(DEGDMA). In the first 15 min, 6.39% of silver is sorbed by MIP(DEGDMA) and 6.62% of silver is sorbed by MIP(DVB). After that time, the characteristics of the sorption change, and MIP(DEGDMA) extracts more metal than MIP(DVB) up to 150 min (the difference is approximately 4–14%). The biggest difference is observed at 105 min: 48.56% vs. 34.59%. After 150 min, MIP(DEGDMA) reaches the equilibrium state, while MIP(DVB) continues the sorption of silver up to 180 min, after which equilibrium is reached. The values of the equilibrium sorption degree for MIP(DEGDMA) and MIP(DVB) are presented in Table 4, from which it is seen that after 180 min, equilibrium is reached (control measurements were obtained at 24 h). From the experimental data, it is seen that the sorption efficiency of MIP(DVB) is higher compared with MIP(DEGDMA). It can be supposed that MIP(DVB) has more stable cavities and higher binding capacity with silver molecules, while it has a looser structure. The same patterns are observed for the sorption capacity of MIPs. Also, it can be concluded that the saturation of MIP(DEGDMA) is faster than for MIP(DVB). Thus, the average sorption degree is approximately 72% and the average sorption capacity is 3.6 g/g, which is considered to be quite a productive result for MIPs.
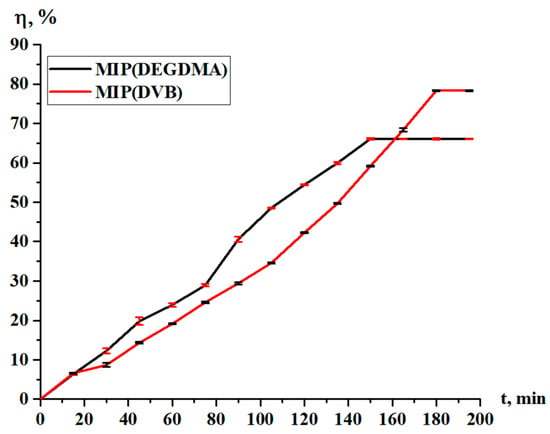
Figure 10.
Sorption degree of the MIPs in relation to silver.
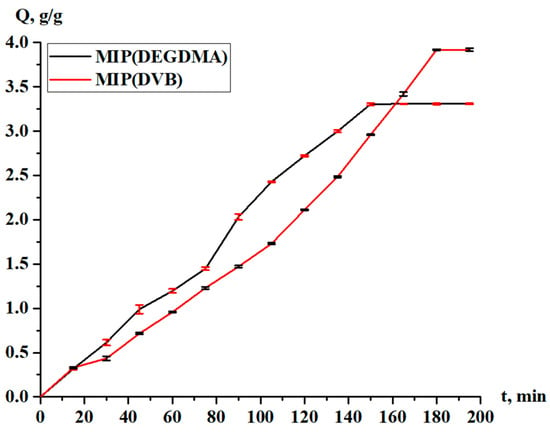
Figure 11.
Sorption capacity of the MIPs in relation to silver.

Table 4.
Sorption parameters of MIP(DEGDMA) and MIP(DVB) after 24 h.
3.5. Analysis of Sorption Kinetics
3.5.1. Radushkevich Kinetic Model
The Radushkevich equation in the non-linear type is:
where qe is the equilibrium sorption capacity (from the experimental data), in g/g; qt is an amount adsorbed at time t, in g/g; kR is a Radushkevich rate constant, in min−1; and t is the time, in min.
Linearization of the equation leads to the form:
Using the sorption data, the curve of ln(1 − qt/qe) = f(t) is plotted (Figure 12 and Figure 13), from which the following parameter is obtained:
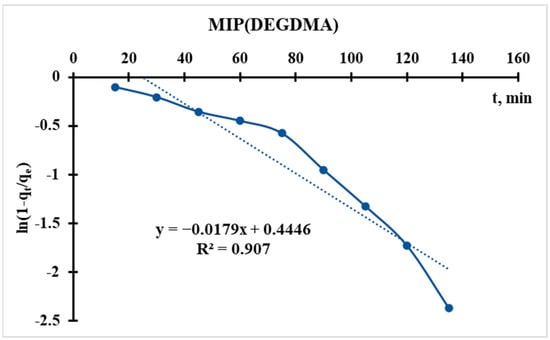
Figure 12.
Radushkevich kinetic model’s curve for MIP(DEGDMA).
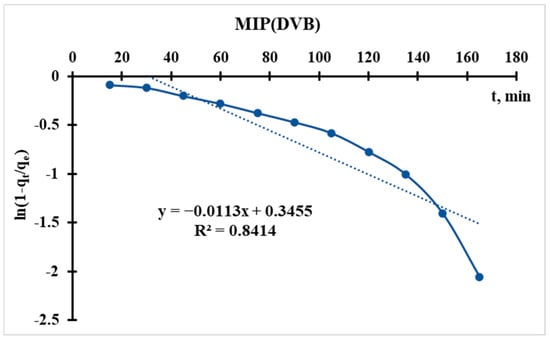
Figure 13.
Radushkevich kinetic model’s curve for MIP(DVB).
In the Radushkevich model, a higher kR indicates a faster sorption rate toward equilibrium. This suggests that MIP(DEGDMA) reaches equilibrium more quickly than MIP(DVB) under the same conditions.
The Radushkevich model is derived based on assumptions about the energy distribution in heterogeneous adsorption systems. A higher kR implies that the adsorption process is more favorable or that the sorbent has a higher affinity for the adsorbate, leading to quicker saturation. MIP(DEGDMA) is likely more efficient in terms of kinetics, as it adsorbs the solute faster than MIP(DVB).
The difference in kR values could arise from variations in:
- The cavity structure and surface area: MIP(DEGDMA) may have a more accessible cavity network or a higher surface area, facilitating faster diffusion and adsorption.
- The chemical affinity: MIP(DEGDMA) may have stronger interactions (e.g., electrostatic, van der Waals, or chemical bonding) with the adsorbate.
- The particle size: Smaller particles (if applicable) could lead to faster kinetics due to shorter diffusion paths.
Thus, according to the Radushkevich model, MIP(DEGDMA) saturates faster during silver sorption in comparison with MIP(DVB), but the latter sorbs a higher concentration of silver. These results correlate with the results of sorption experiments.
3.5.2. Elovich Kinetic Model
The model is expressed as:
where qt is the sorption capacity at time t, in g/g; α is the initial sorption rate, in g·g−1·min−1; and β is the surface coverage parameter, in g/g (related to activation energy).
For further analysis, the initial equation should be linearized (for plotting Figure 14 and Figure 15):
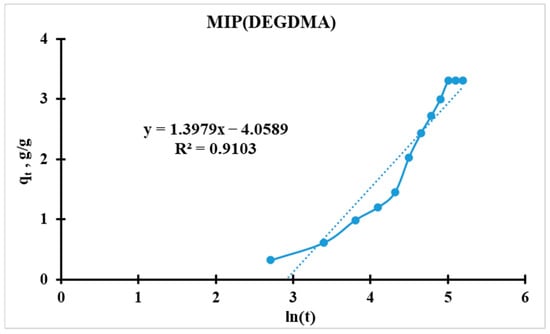
Figure 14.
Elovich kinetic model’s curve for MIP(DEGDMA).
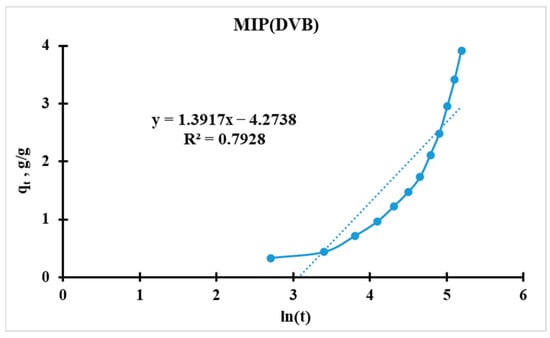
Figure 15.
Elovich kinetic model’s curve for MIP(DVB).
Slope(b) = 1/β;
Intercept(a) = (1/β) ∗ ln(α ∗ β).
For the calculation of α and β, the following equations were used:
Higher α indicates faster initial adsorption. MIP(DEGDMA) has a 24% higher initial rate than MIP(DVB) (0.0172 vs. 0.0139 min−1). MIP(DEGDMA) extracts silver more quickly at the early stages. These values correlate with the results calculated by the Radushkevich model.
Possible reasons for MIP(DEGDMA) higher α values:
- Higher porosity/microporosity: Faster diffusion of adsorbate into pores.
- More active surface sites: Functional groups (e.g., -OH, -COOH) accelerate initial binding.
- Smaller particle size: Larger surface area-to-volume ratio.
Thus, MIP(DEGDMA) saturates faster than MIP(DVB), which correlates with the results of the sorption experiments and the Radushkevich model.
Both sorbents have nearly identical β values (~0.715–0.719 g/g), suggesting similar binding energies and surface heterogeneity. Nearly identical β values imply:
- The same adsorption mechanism (e.g., chemisorption via ion exchange or complexation).
- Comparable surface heterogeneity (energy distribution of binding sites).
The surface coverage parameter of MIP(DVB) is slightly more than for MIP(DEGDMA). A higher β indicates stronger adsorbate–adsorbent interactions (chemisorption). These results correlate with the results of the sorption experiments, where it was proposed that the binding of MIP(DVB) with silver is stronger.
The results of both kinetic models (Radushkevich and Elovich) are similar and correlate with the results of the sorption experiments. The values of the sorption rate in both models are close to each other (Table 5); however, MIP(DEGDMA) has a higher sorption rate. Also, both kinetic models showed that MIP(DVB) sorbs a higher concentration of silver in comparison with MIP(DEGDMA).

Table 5.
Comparison of sorption rates for MIPs using kinetic models.
Thus, there is a similarity in the sorption process of both MIPs, so the sorption is provided mainly by complexation with monomer units, but slight differences are influenced by the differences in the structures of the crosslinkers.
3.6. Desorption Experiments
For effective use of the synthesized MIPs, it is necessary to study their desorption properties. The process of desorption is closely connected with the regeneration procedure of the sorption parameters of the sorbents. Desorption was carried out with 0.05 M nitric acid.
Figure 16 represents the concentration of silver in the solution and in the MIPs during desorption. Figure 17 shows the desorption degree of the MIPs. From the obtained experimental data, the following dependence is observed: in the first 90 min, the desorption rate of MIP(DEGDMA) is 1–5% greater in comparison with MIP(DVB), starting from 9.10% vs. 7.80% (15 min) and ending with 35.17% vs. 35.11% (90 min). After 90 min, the desorption of silver from the MIP(DEGDMA) matrix occurs more slowly, while desorption from MIP(DVB) becomes more rapid for 3–9%. Only at the end of the experiment (180 min), the final values of the desorption degree are slightly higher for MIP(DEGDMA). Control measurements were obtained after 24 h, and the results are presented in Table 6. The equilibrium value of the desorption degree at 24 h reaches almost 80%. Thus, it can be concluded that MIPs have quite high desorption efficiency, and they could be applicable as sorbents for silver recovery.
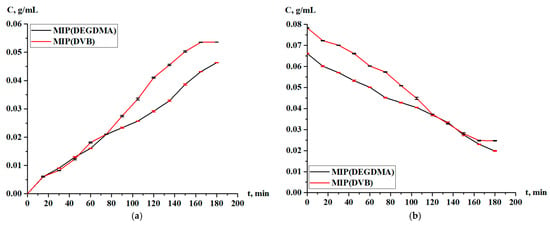
Figure 16.
Concentration of silver nitrate during desorption in solution (a) and in the MIPs (b).
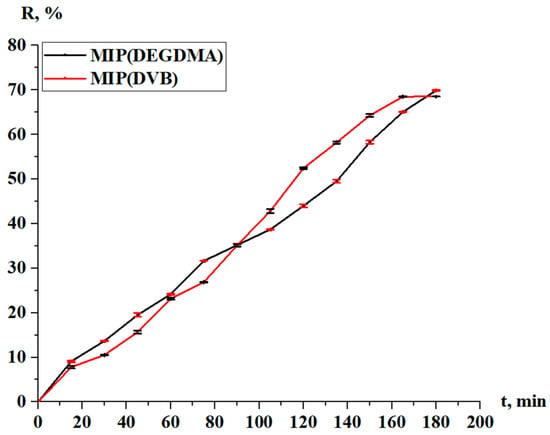
Figure 17.
Desorption degree of the MIPs.

Table 6.
Desorption degree of MIP(DEGDMA) and MIP(DVB) after 24 h.
From the obtained results, it can be observed that the desorption degree of the MIP(DEGDMA) is slightly higher than that of the MIP(DVB). This can be explained by the binding properties of DVB and DEGDMA. The binding in the MIP(DVB) is stronger; thus, the molecules of the silver template are placed tightly in the cavities. The molecular chain of DEGDMA is longer than that of DVB; consequently, the structure of the MIPs will have some voids between the cavities. Because of that, the structure becomes less dense, and the molecules of the silver template will also be less dense, which leads to easier extraction of the silver template molecules from the copolymer matrix.
4. Conclusions
Based on the data obtained, the following conclusions can be made:
- (1)
- The molecularly imprinted structures with silver salt as the template were synthesized with the ratio of template:monomer:monomer:cross-linker = 1:2:2:8. The obtained imprinted structures have a dark brown color due to the presence of the monomer 4-VP. MIP(DVB) has a dense structure, whereas MIP(DEGDMA) has a looser structure.
- (2)
- The structure of the MIPs is dense and heterogeneous, with molecular imprint cavities formed due to unstable bonding between the template and the monomer, likely influenced by the ionized state of silver. The average cavity sizes were 0.81 ± 0.20 μm for MIP(DEGDMA) and 0.68 ± 0.23 μm for MIP(DVB), with the difference attributed to the flexibility of DEGDMA’s long-chain structure compared to DVB’s rigid aromatic ring.
- (3)
- A mechanism of chemical reaction for obtaining a molecularly imprinted system with a silver template was suggested, which includes the formation of a pre-polymerization complex, the polymerization of monomers, and cross-linker around a template molecule. In the formation of a pre-polymerization complex, it is supposed that donor–acceptor bonds are formed between the pyridine group of the monomer 4-vinylpyridine and template ions, and hydrogen bonds are formed between the monomer methacrylic acid and template molecules.
- (4)
- MIP(DEGDMA) saturates faster during silver sorption in comparison with MIP(DVB), but the latter sorbs a higher concentration of silver. The average sorption degree is approximately 72% and the average sorption capacity is 3.6 g/g, which is considered to be quite a productive result for MIPs.
- (5)
- The sorption process was described using kinetic models (Radushkevich and Elovich). Both kinetic models showed identical results that correlated with the results of the sorption experiments: MIP(DEGDMA) saturates faster during silver sorption in comparison with MIP(DVB), but the latter sorbs a higher concentration of silver.
- (6)
- The desorption degree reaches nearly 80% after 24 h, indicating that the MIPs possess high desorption efficiency and may be effectively used as sorbents for silver recovery.
Based on the results mentioned above, it can be concluded that the synthesized MIPs are promising as sorbents for the selective extraction of silver. Both MIPs exhibit similar sorption behavior, primarily driven by complexation with monomer units. However, minor variations arise due to structural differences in the crosslinkers.
Author Contributions
Conceptualization, L.A. and R.K.; data curation, L.A., Y.M., D.K., and R.K.; formal analysis, L.A., Y.M., D.K., and R.K.; funding acquisition, R.K.; investigation, L.A. and R.K.; methodology, L.A. and R.K.; project administration, L.A. and R.K.; resources, R.K.; supervision, L.A. and R.K.; validation, L.A., Y.M., and D.K.; visualization, L.A. and R.K.; writing—original draft, L.A. and R.K.; writing—review and editing, L.A. and R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Ministry of Science and Higher Education of the Republic of Kazakhstan, grant number AP19674527.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Munziya Abutalip (Nazarbayev University, Astana, the Republic of Kazakhstan) for conducting SEM analysis of the imprinted structures.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MIP | Molecularly imprinted polymer |
| MAA | Methacrylic acid |
| 4-VP | 4-vinylpyridine |
| DEGDMA | Diethylene glycol dimethacrylate |
| DVB | Divinylbenzene |
| AIBN | Azobisisobutyronitrile |
| HEC | Hydroxyethyl cellulose |
| FTIR | Fourier transform infrared spectroscopy |
| SEM | Scanning electron microscopy |
References
- Tan, K.B.; Sun, D.H.; Huang, J.L.; Odoom-Wubah, T.; Li, Q.B. State of arts on the bio-synthesis of noble metal nanoparticles and their biological application. Chin. J. Chem. Eng. 2021, 30, 272–290. [Google Scholar] [CrossRef]
- Shou, W.; Yang, S.T.; Wang, Y.L.; Guo, L.H. Preparation of Noble Metal Nanoparticles and Hydrogel Composite Materials and Their Application in Analytical Chemistry. Chin. J. Analyt. Chem. 2021, 49, 676–685. [Google Scholar] [CrossRef]
- Zeng, X.; Zhao, Y.; Hu, X.; Stucky, G.D.; Moskovits, M. Rational Component and Structure Design of Noble-Metal Composites for Optical and Catalytic Applications. Small Struct. 2021, 2, 2000138. [Google Scholar] [CrossRef]
- Liu, X.Q.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.H.; Zhao, S.Q.; Li, Z.; Lin, Z.Q. Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, W.; Gao, J. Trash or treasure? Sustainable noble metal recovery. Green Chem. 2024, 26, 5684–5707. [Google Scholar] [CrossRef]
- Magoda, K.; Mekuto, L. Biohydrometallurgical Recovery of Metals from Waste Electronic Equipment: Current Status and Proposed Process. Recycling 2022, 7, 67. [Google Scholar] [CrossRef]
- Xing, W.D.; Sohn, S.H.; Lee, M.S. A Review on the Recovery of Noble Metals from Anode Slimes. Mineral Proc. Extr. Met. Rev. 2019, 41, 130–143. [Google Scholar] [CrossRef]
- Dinh, T.; Dobo, Z.; Kovacs, H. Phytomining of noble metals—A review. Chemosphere 2022, 286, 131805. [Google Scholar] [CrossRef]
- Padamata, S.K.; Yasinskiy, A.S.; Polyakov, P.V.; Pavlov, E.A.; Varyuhin, D.Y. Recovery of Noble Metals from Spent Catalysts: A Review. Met. Mater. Trans. B Proc. 2020, 51, 2413–2435. [Google Scholar] [CrossRef]
- Islam, A.; Ahmed, T.; Awual, M.R.; Rahman, A.; Sultana, M.; Abd Aziz, A.; Monir, M.U.; Teo, S.H.; Hasan, M. Advances in sustainable approaches to recover metals from e-waste—A review. J. Clean. Prod. 2020, 244, 118815. [Google Scholar] [CrossRef]
- Kaczorowska, M.A. The Use of Polymer Inclusion Membranes for the Removal of Metal Ions from Aqueous Solutions—The Latest Achievements and Potential Industrial Applications: A Review. Membranes 2022, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Keskin, B.; Zeytuncu, B.; Koyuncu, I. Efficient Separation and Recovery of Precious Metal Ions by Polymer Inclusion Membranes (PIMs): A Mini Review. Solv. Extr. Ion Exch. 2024, 42, 193–215. [Google Scholar] [CrossRef]
- Barek, J. Voltammetric and amperometric applications of silver amalgam electrodes for monitoring of reducible organic compounds. TrAC Trends Analyt. Chem. 2024, 170, 117416. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, L.Q. Development of silver oxide electrode in silver-zinc storage batteries. Rare Met. Mat. Eng. 2008, 37, 1124–1128. [Google Scholar]
- Devi, R.S.; Girigoswami, A.; Siddharth, M.; Girigoswami, K. Applications of Gold and Silver Nanoparticles in Theranostics. Appl. Biochem. Biotechnol. 2022, 194, 4187–4219. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef]
- Maillard, J.Y.; Hartemann, P. Silver as an antimicrobial: Facts and gaps in knowledge. Crit. Rev. Microbiol. 2012, 39, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, D. Silver nanoparticles as labels for applications in bioassays. TrAC Trends Analyt. Chem. 2014, 61, 67–73. [Google Scholar] [CrossRef]
- Mathur, P.; Jha, S.; Ramteke, S.; Jain, N.K. Pharmaceutical aspects of silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 115–126. [Google Scholar] [CrossRef]
- Halbes-Letinois, U.; Weibel, J.M.; Pale, P. The organic chemistry of silver acetylides. Chem. Soc. Rev. 2007, 36, 759–769. [Google Scholar] [CrossRef]
- Zhao, Q.X.; Zhao, M.M.; Qiu, J.Q.; Lai, W.Y.; Pang, H.; Huang, W. One Dimensional Silver-based Nanomaterials: Preparations and Electrochemical Applications. Small 2017, 13, 1701091. [Google Scholar] [CrossRef]
- Grancaric, A.M.; Rybicki, E.; Tarbuk, A.; Pavlovic, G.; Botteri, L. Nanoparticles of silver in antimicrobial treatment of textiles. Tekstil 2011, 60, 629–639. [Google Scholar]
- Syed, S. Silver recovery aqueous techniques from diverse sources: Hydrometallurgy in recycling. Waste Manag. 2016, 50, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, E.; Alorro, R.D.; Encila, A.N.; Yoo, K. Magnetic Adsorbents for the Recovery of Precious Metals from Leach Solutions and Wastewater. Metals 2017, 7, 529. [Google Scholar] [CrossRef]
- Guo, J.Q.; Wu, Y.F.; Wang, Z.H.; Yu, J.M.; Li, J.R. Review: Adsorbents for the recovery of precious metals from wastewater. J. Mater. Sci. 2022, 57, 10886–10911. [Google Scholar] [CrossRef]
- Guibal, E.; Vincent, T.; Navarro, R. Metal ion biosorption on chitosan for the synthesis of advanced materials. J. Mater. Sci. 2014, 49, 5505–5518. [Google Scholar] [CrossRef]
- Guo, W.K.; Liu, J.L.; Tao, H.J.; Meng, J.; Yang, J.; Shuai, Q.; Asakura, Y.; Huang, L.J.; Yamauchi, Y. Covalent Organic Framework Nanoarchitectonics: Recent Advances for Precious Metal Recovery. Adv. Mater. 2024, 36, 2405399. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.C.; Cheng, C.L.; Han, Y.L.; Chen, B.Y.; Chang, J.S. Recovery of high-value metals from geothermal sites by biosorption and bioaccumulation. Biores. Technol. 2014, 160, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Foley, S.R.; Wilson, L.D. An Overview of Modified Chitosan Adsorbents for the Removal of Precious Metals Species from Aqueous Media. Molecules 2022, 27, 978. [Google Scholar] [CrossRef]
- Ying, T.L.; Gao, M.J.; Zhang, X.L. Highly selective technique-molecular imprinting. Chin. J. Analyt. Chem. 2001, 29, 99–102. [Google Scholar]
- Komiyama, M.; Takeuchi, T.; Mukawa, T.; Asanuma, H. Molecular Imprinting-from Fundamentals to Applications; Wiley: New York, NY, USA, 2003; 578p. [Google Scholar]
- Chen, L.X.; Wang, X.Y.; Lu, W.H.; Wu, X.Q.; Li, J.H. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Szatkowska, P.; Koba, M.; Koslinski, P.; Szablewski, M. Molecularly Imprinted Polymers’ Applications: A Short Review. Mini-Rev. Org. Chem. 2013, 10, 400–408. [Google Scholar] [CrossRef]
- Wu, H.R.; Lin, G.; Liu, C.C.; Chu, S.Y.; Mo, C.; Liu, X.B. Progress and challenges in molecularly imprinted polymers for adsorption of heavy metal ions from wastewater. Trends Environ. Analyt. Chem. 2022, 36, e00178. [Google Scholar] [CrossRef]
- Sarpong, K.A.; Xu, W.; Huang, W.; Yang, W. The Development of Molecularly Imprinted Polymers in the Clean-Up of Water Pollutants: A Review. Am. J. Analyt. Chem. 2019, 10, 202–226. [Google Scholar] [CrossRef]
- Dmitrienko, E.V.; Pyshnaya, I.A.; Martyanov, O.N.; Pyshny, D.V. Molecularly imprinted polymers for biomedical and biotechnological applications. Russ. Chem. Rev. 2016, 85, 513–536. [Google Scholar] [CrossRef]
- Saylan, Y.; Yilmaz, F.; Ozgur, E.; Derazshamshir, A.; Yavuz, H.; Denizli, A. Molecular Imprinting of Macromolecules for Sensor Applications. Sensors 2017, 17, 898. [Google Scholar] [CrossRef]
- Bhogal, S.; Kaur, K.; Malik, A.K.; Sonne, C.; Lee, S.S.; Kim, K.H. Core-shell structured molecularly imprinted materials for sensing applications. TrAC Trends Anal. Chem. 2020, 133, 16043. [Google Scholar] [CrossRef]
- Irem Kaya, S.; Cetinkaya, A.; Ozkan, S.A. Molecularly imprinted polymers as highly selective sorbents in sample preparation techniques and their applications in environmental water analysis. Trends Environ. Analyt. Chem. 2023, 37, e00193. [Google Scholar] [CrossRef]
- Cheong, W.J.; Yang, S.H.; Ali, F. Molecular imprinted polymers for separation science: A review of reviews. J. Sep. Sci. 2013, 36, 609–628. [Google Scholar] [CrossRef]
- Vijayan, P.P.; Chithra, P.G.; Krishna, S.V.A.; Ansar, E.B.; Parameswaranpillai, J. Development and Current Trends on Ion Exchange Materials. Sep. Purif. Rev. 2024, 53, 40–60. [Google Scholar] [CrossRef]
- Samonin, V.V.; Spiridonova, E.A.; Khokhlachev, S.P.; Podvyaznikov, M.L. Promising Directions for Production and Application of Inorganic Sorbent Materials. Russ. J. Inorg. Chem. 2024, 69, 392–397. [Google Scholar] [CrossRef]
- Melnikov, Y.; Kondaurov, R.; Agibayeva, L. Effect of Lanthanum Sorption on the Behavior of Rarely Crosslinked Acidic and Basic Polymer Hydrogels during Remote Interaction. Polymers 2023, 15, 1420. [Google Scholar] [CrossRef]
- Kondaurov, R.; Melnikov, Y.; Agibayeva, L. Application of Molecular Imprinting for Creation of Highly Selective Sorbents for Extraction and Separation of Rare-Earth Elements. Polymers 2023, 15, 846. [Google Scholar] [CrossRef]
- Agibayeva, L.; Melnikov, Y.; Berdaly, A.; Kondaurov, R. Development of Optimal Conditions for Synthesis of Molecularly Imprinted Polymers for Effective Terbium Sorption. Polymers 2025, 17, 1398. [Google Scholar] [CrossRef] [PubMed]
- Cormack, P.A.G.; Elorza, A.Z. Molecularly imprinted polymers: Synthesis and characterization. J. Chromatogr. B 2004, 804, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Bikiaris, D.N. Molecular Imprinting for High-Added Value Metals: An Overview of Recent Environmental Applications. Adv. Mater. Sci. Eng. 2014, 2014, 932637. [Google Scholar] [CrossRef]
- Şarkaya, K.; Bakhshpour, M.; Denizli, A. Ag+ ions imprinted cryogels for selective removal of silver ions from aqueous solutions. Sep. Sci. Technol. 2018, 54, 2993–3004. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Helleur, R. Selective adsorption of Ag+ by ion-imprinted O-carboxymethyl chitosan beads grafted with thiourea–glutaraldehyde. Chem. Eng. J. 2015, 264, 56–65. [Google Scholar] [CrossRef]
- Shawky, H.A. Synthesis of ion-imprinting chitosan/PVA crosslinked membrane for selective removal of Ag(I). J. Appl. Polym. Sci. 2009, 114, 2608–2615. [Google Scholar] [CrossRef]
- Behbahani, M.; Omidi, F.; Kakavandi, M.G.; Hesam, G. Selective and sensitive determination of silver ions at trace levels based on ultrasonic-assisted dispersive solid-phase extraction using ion-imprinted polymer nanoparticles. Appl. Organometal. Chem. 2017, 31, e3758. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N. Characterization of binding properties of silver ion-imprinted polymers with equilibrium and kinetic models. J. Mol. Liq. 2015, 212, 133–141. [Google Scholar] [CrossRef]
- Kazemi, E.; Haji Shabani, A.M.; Dadfarnia, S. Synthesis and characterization of a nanomagnetic ion imprinted polymer for selective extraction of silver ions from aqueous samples. Microchim. Acta 2015, 182, 1025–1033. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Shafizadeh, F. Fabrication of a novel hydrophilic ion-imprinted polymer based on silica-modified magnetic graphene oxide as a sensitive sorbent for the fast separation of silver ions from aqueous media. J. Disp. Sci. Technol. 2023, 46, 574–590. [Google Scholar] [CrossRef]
- Jalilian, R.; Taheri, A. Synthesis and application of a novel core-shell-shell magnetic ion imprinted polymer as a selective adsorbent of trace amounts of silver ions. e-Polymers 2018, 18, 123–134. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Yahyazadeh, F.; Vardini, M.T. Synthesis of a New Molecularly Imprinted Polymer for Sorption of the Silver Ions from Geological and Antiseptic Samples for Determination by Flame Atomic Absorption Spectrometry. J. AOAC Int. 2014, 97, 1434–1438. [Google Scholar] [CrossRef]
- Mirzaei, M.; Hafezi, M. Selective ion-imprinted polymers for preconcentration and determination of silver in water and hair by electrothermal atomic absorption spectrometry. J. Analyt. Chem. 2017, 72, 70–75. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Nasr-Esfahani, P. Chapter 2. Fundamental aspects of molecular imprinting. In Molecularly Imprinted Polymer Composites: Synthesis, Characterisation and Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 2–15. [Google Scholar] [CrossRef]
- Lisichkin, G.V.; Krutyakov, Y.A. Molecularly imprinted materials: Synthesis, properties, applications. Uspekhi Khimii. 2006, 75, 998–1017. [Google Scholar] [CrossRef]
- Ghiurea, M.; Donescu, D. Influence of crosslinker/porogen ratio upon imprinted polymer parameters. U.P.B. Sci. Bull. Ser. B 2011, 73, 163–172. [Google Scholar]
- Ensafi, A.A.; Kazemifard, N.; Dehkordi, Z.S. Chapter 3. Parameters that affect molecular imprinting polymers. In Molecularly Imprinted Polymer Composites: Synthesis, Characterisation and Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 21–48. [Google Scholar] [CrossRef]
- Jiang, Y.; Kim, D. Effect of solvent/monomer feed ratio on the structure and adsorption properties of Cu2+-imprinted microporous polymer particles. Chem. Eng. J. 2011, 166, 435–444. [Google Scholar] [CrossRef]
- Tarasevich, B.N. IR Spectra of the Main Classes of Organic Compounds; Reference Materials: Moscow, Russia, 2012; 55p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).