Evaluation of Viscosity Changes and Rheological Properties of Diutan Gum, Xanthan Gum, and Scleroglucan in Extreme Reservoirs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Solution Preparation
2.2.1. Preparation Method for Diutan Gum and Scleroglucan
2.2.2. Preparation Method for Xanthan Gum
2.3. Experimental Instruments
2.4. Experimental Test Methods
2.4.1. Viscosity Increase Performance Test
2.4.2. Rheological Properties Tests
- Steady-state rheological shear performance test.
- 2.
- Linear viscoelastic zone LVR and oscillation frequency scanning tests.
2.4.3. Long-Term Stability Performance Test
3. Results
3.1. Comparison of the Viscosity-Increasing Properties of Three Biopolymers
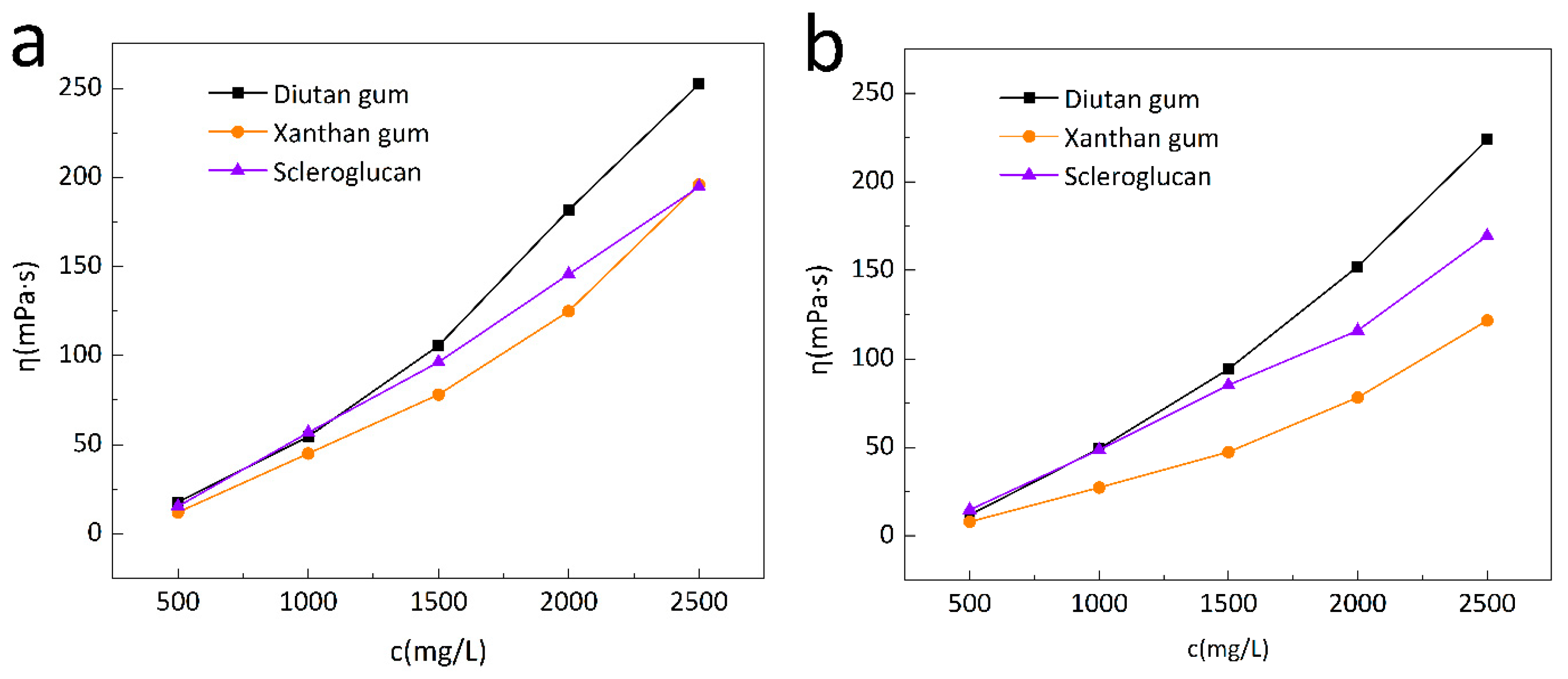
3.1.1. Viscosity Concentration Relationships of Three Biopolymers
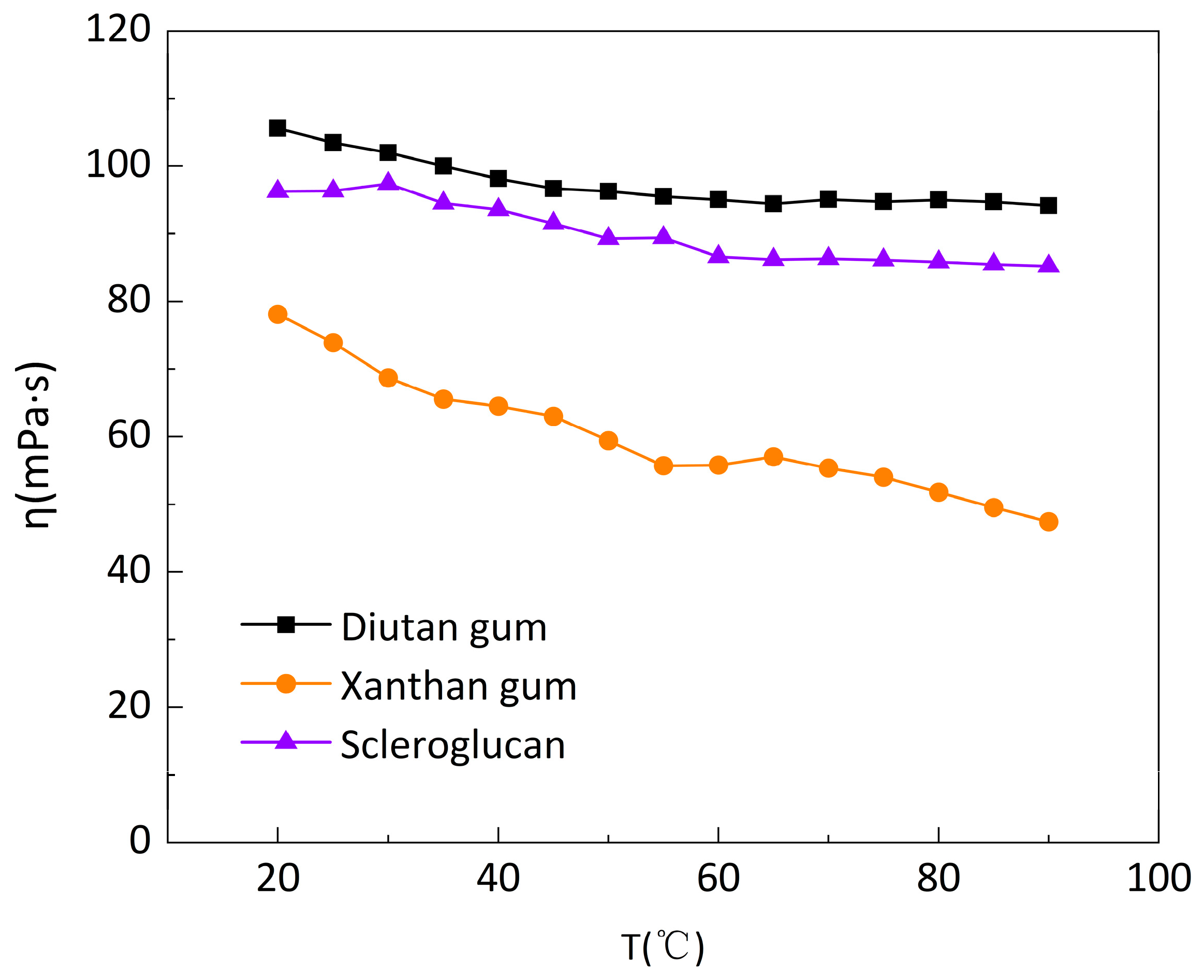
3.1.2. Temperature Resistance Performance Test
3.1.3. Comparison of Salt Resistance of Three Biopolymers
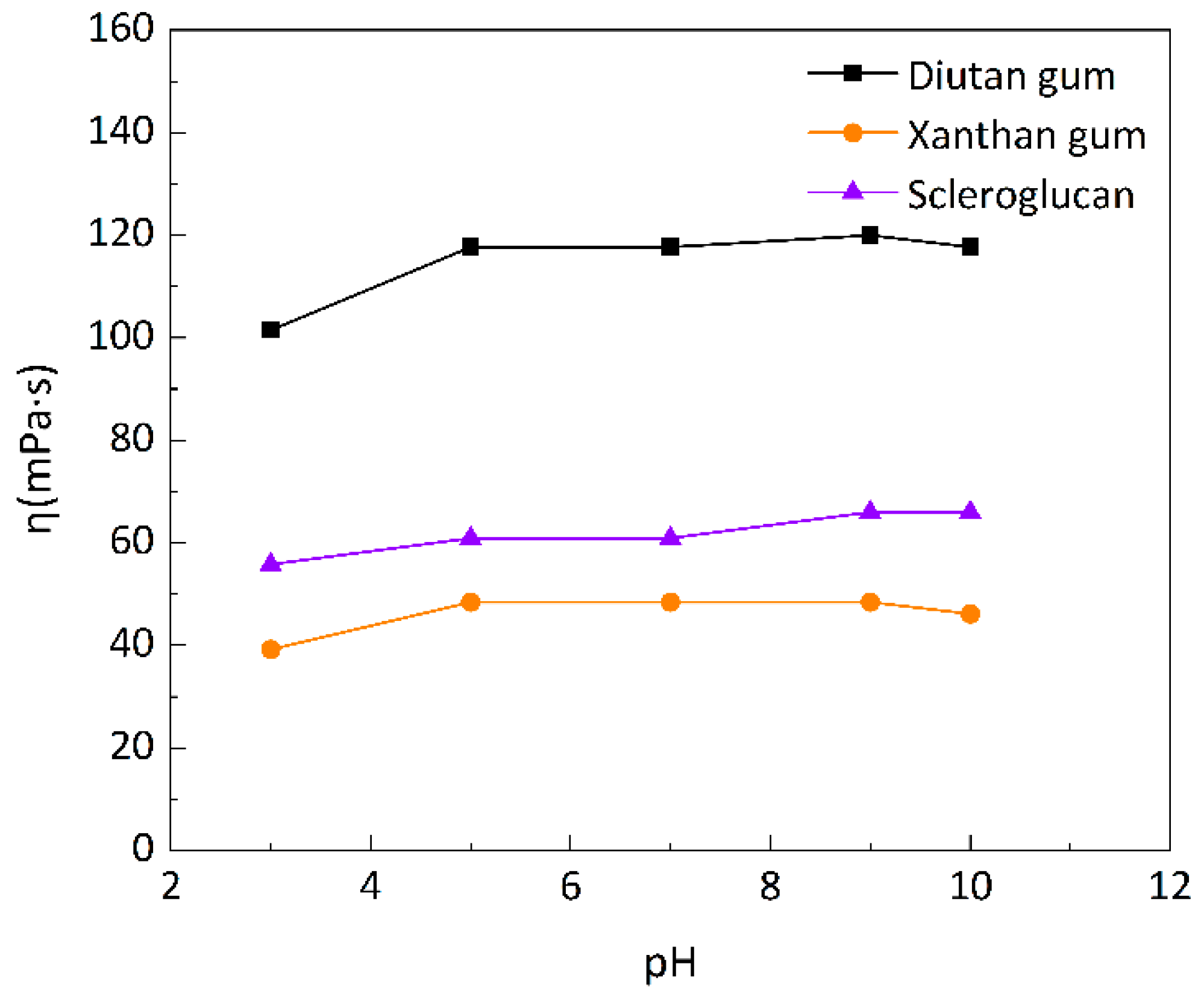
3.1.4. Comparisons of the Acid and Alkali Resistance of Three Biopolymers
3.2. Rheological Properties of Three Biopolymers
3.2.1. Steady-State Rheological Shear Test
3.2.2. Linear Viscoelastic Zone LVR Test
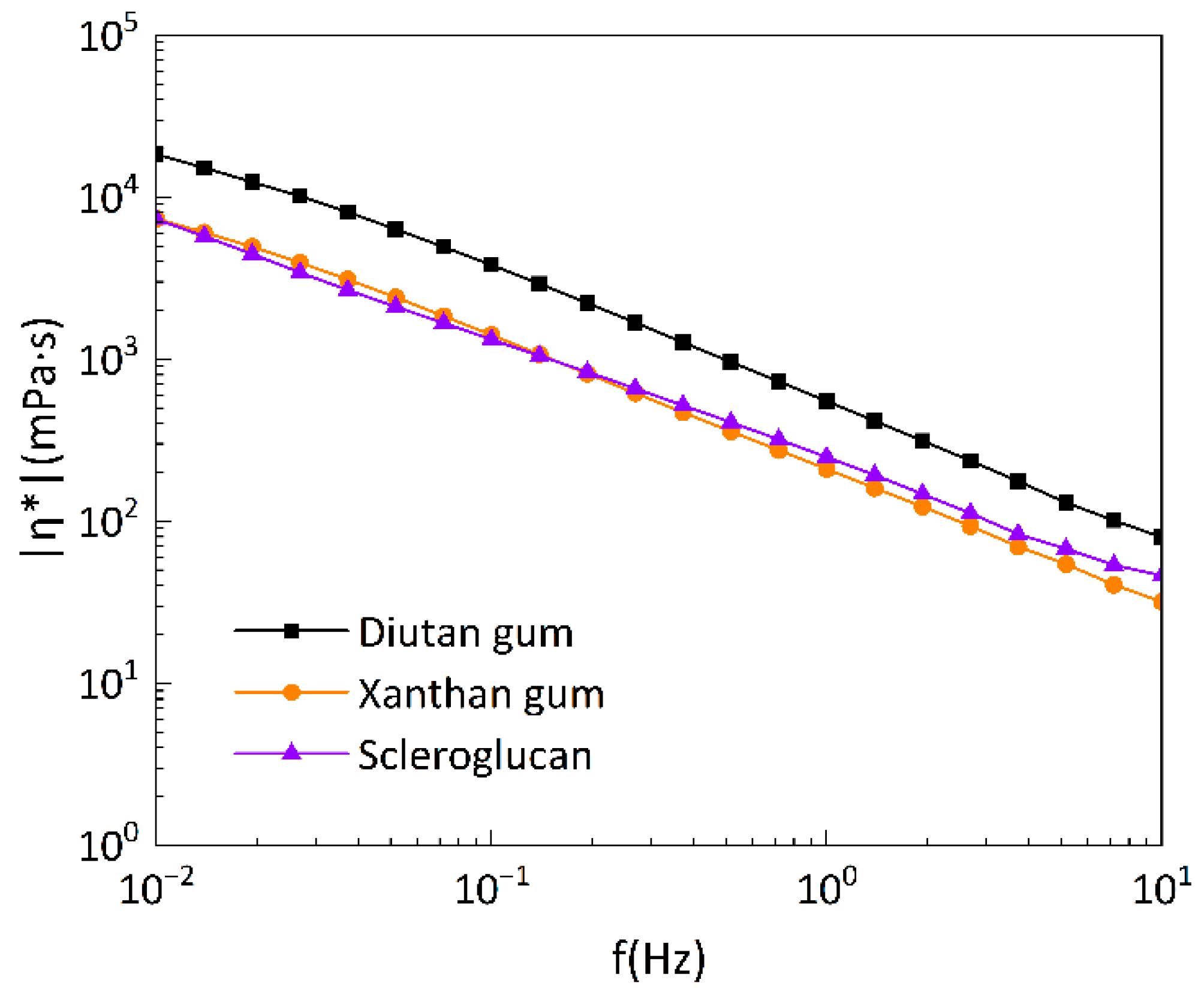
3.2.3. Oscillation Frequency Sweep Test
3.3. Long-Term Stability of Diutan Gum and Scleroglucan
4. Conclusions
- (1)
- At the same temperature and mineralization degree, the thickening and viscosity-increasing performance of diutan gum were better than those of xanthan gum and scleroglucan, and the amount of diutan gum required to achieve the same apparent viscosity was lower than that of xanthan gum and scleroglucan.
- (2)
- Diutan gum and scleroglucan showed better temperature resistance than xanthan gum. Diutan gum could withstand a maximum temperature of 100 °C, with an apparent viscosity retention rate of 89%; scleroglucan’s viscosity was stable up to 150 °C, with an apparent viscosity retention rate of 118.27%.
- (3)
- Diutan gum and scleroglucan showed better salt resistance than xanthan gum, and both could tolerate up to 220 g/L of mineralized simulated brine, with a 90.12% apparent viscosity retention of diutan gum and 70.04 % of scleroglucan, with minor apparent viscosity loss. The acid and alkali resistance of the three biopolymer solutions was relatively high, with a slight decrease in apparent viscosity under heavily acidic conditions and an approximately constant apparent viscosity under alkaline conditions.
- (4)
- In the rheological test, the solutions of diutan gum, xanthan gum, and scleroglucan all distinctly showed pseudoplastic fluid behavior, and the diutan gum had strong water retention and viscoelastic properties due to its peculiar double-helix structure.
- (5)
- Both diutan gum and scleroglucan have certain long-term stability in terms of temperature and salt resistance. Diutan gum can remain stable for about 10 days at 100 °C and 220 g/L mineralization, and the apparent viscosity can be maintained at 90 mPa·s. Scleroglucan can remain stable for approximately 40 days in the same reservoir environment, with an apparent viscosity retention rate of 89.54%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, W.M.; Yu, L.; Xiu, J.L.; Yi, L.N.; Huang, L.X.; Peng, B.F. Research progress on application of biopolymer in petroleum industry. Appl. Chem. Ind. 2023, in press. [Google Scholar]
- Xu, L.; Gong, H.J.; Dong, M.Z.; Li, Y.J. Rheological properties and thickening mechanism of aqueous diutan gum solution: Effects of temperature and salts. Carbohydr. Polym. 2015, 132, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.C.; Carmona, J.A.; Santos, J.; Alfaro, M.C.; Munoz, J. Effect of temperature and shear on the microstructure of a microbial polysaccharide secreted by Sphingomonas species in aqueous solution. Int. J. Biol. Macromol. 2018, 118, 2071–2075. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Jan, B.M. Herschel-Bulkley rheological parameters of a novel environmentally friendly lightweight biopolymer drilling fluid from xanthan gum and starch. J. Appl. Polym. Sci. 2012, 124, 595–606. [Google Scholar] [CrossRef]
- Sulaimon, A.A.; Akintola, S.A.; Johari, M.A.B.M.; Isehunwa, S.O. Evaluation of drilling muds enhanced with modified starch for HPHT well applications. J. Pet. Explor. Prod. Technol. 2021, 11, 203–218. [Google Scholar] [CrossRef]
- Hamed, S.B.; Belhadri, M. Rheological properties of biopolymers drilling fluids. J. Pet. Sci. Eng. 2009, 67, 84–90. [Google Scholar] [CrossRef]
- Gao, C. Potential of Welan Gum as mud thickener. J. Pet. Explor. Prod. Technol. 2015, 5, 109–112. [Google Scholar] [CrossRef]
- Luz, R.C.S.D.; Fagundes, F.P.; Balaban, R.D.C. Water-based drilling fluids: The contribution of xanthan gum and carboxymethylcellulose on filtration control. Chem. Pap. Slovak Acad. Sci. 2017, 71, 2365–2373. [Google Scholar]
- Xia, S.X.; Zhang, L.B.; Davletshin, A.; Li, Z.R.; You, J.H.; Tan, S.Y. Application of Polysaccharide Biopolymer in Petroleum Recovery. Polymers 2020, 12. [Google Scholar] [CrossRef]
- Li, Y.; Guo, J.C.; Wang, S.B.; Wang, C. Research status and development trend of high temperature resistant fracturing fluid system. Mod. Chem. Ind. 2019, 4. [Google Scholar]
- Quraishi, M.; Bhatia, S.K.; Pandit, S.; Gupta, P.K.; Rangarajan, V.; Lahiri, D.; Varjani, S.; Mehariya, S.; Yang, Y.H. Exploiting Microbes in the Petroleum Field: Analyzing the Credibility of Microbial Enhanced Oil Recovery (MEOR). Energies 2021, 14, 4684. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Hu, S.Y.; Jin, Y. China achieving carbon neutral in 2060, fossil energy to fossil resource era. Mod. Chem. Ind. 2021, 41, 5. [Google Scholar]
- Zhou, H.T. Properties of novel microbial polysaccharides suitable for the polymer flooding of harsh oil reservoirs. Oil Drill. Prod. Technol. 2019, 41, 6. [Google Scholar]
- Mao, H.; Qiu, Z.; Shen, Z.; Huang, W.; Zhong, H.; Dai, W. Novel hydrophobic associated polymer based nano-silica composite with core–shell structure for intelligent drilling fluid under ultra-high temperature and ultra-high pressure. Prog. Nat. Sci. Mater. Int. 2015, 25, 90–93. [Google Scholar] [CrossRef]
- Cao, J.; Tan, Y.; Che, Y.; Ma, Q. Synthesis of copolymer of acrylamide with sodium vinylsulfonate and its thermal stability in solution. J. Polym. Res. 2011, 18, 171–178. [Google Scholar] [CrossRef]
- Mccormick, C.L.; Nonaka, T.; Johnson, C.B. Water-soluble copolymers: 27. Synthesis and aqueous solution behaviour of associative acrylamide N-alkylacrylamide copolymers. Polymer 1988, 29, 731–739. [Google Scholar] [CrossRef]
- Mei-Long, F.U.; Jie, L. Characterization of the structure of comb-like polymer comb-1. J. Xi’an Shiyou Univ. (Nat. Sci. Ed.) 2009. [Google Scholar]
- Zheng, C.H.; Zhi, Y. Self-assembly and regulation of hydrophobic associating polyacrylamide with excellent solubility prepared by aqueous two-phase polymerization. Colloids and Surfaces, A. Physicochem. Eng. Asp. 2018, 555, 621–629. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, R.; Cui, Q.; Han, S.; Dong, H.; Zhang, Y. Biosurfactant production under diverse conditions by two kinds of biosurfactant-producing bacteria for microbial enhanced oil recovery. J. Pet. Sci. Eng. 2017, 157, 124–130. [Google Scholar] [CrossRef]
- Geetha, S.J.; Banat, I.M.; Joshi, S.J. Biosurfactants: Production and potential applications in microbial enhanced oil recovery (MEOR). Biocatal. Agric. Biotechnol. 2018, 14, 23–32. [Google Scholar] [CrossRef]
- Du, C.; Cai, Y.; Yan, Y.; Gao, M. Research progress on novel chemical oil displacement technology. Mod. Chem. Ind. 2022, 42, 35–39. [Google Scholar] [CrossRef]
- Wang, B.B. Measures to Improve Tertiary Oil Recovery. Chem. Eng. Equip. 2022, 110–111. [Google Scholar] [CrossRef]
- Peng, Y.S. Basis and Progress of Microbial Oil Recovery; Petroleum Industry Press: Beijing, China, 2005; pp. 255–257. ISBN 9787502148195. [Google Scholar]
- Zhang, X.F.; Gao, Y.W.; Wang, B.L.; Qin, C.W. A preliminary study on the feasibility of biopolymer modulation drive technology for Weyland gums. Drill. Prod. Technol. 2019, 42, 98–101+7. [Google Scholar]
- Clinckspoor, K.J.; Ferreira, V.; Moreno, R. Bulk rheology characterization of biopolymer solutions and discussions of their potential for enhanced oil recovery applications. CT F–Cienc. Tecnol. Futuro 2021, 11, 123–135. [Google Scholar] [CrossRef]
- Lai, N.J.; Wen, Y.P.; Yang, Z.D.; Chen, J.L.; Zhao, X.B.; Wang, D.D.; He, W.; Chen, Y.M. Polymer flooding in high-temperature and high-salinity heterogeneous reservoir by using diutan gum. J. Pet. Sci. Eng. 2020, 188, 20. [Google Scholar] [CrossRef]
- Margaritis, A.; Zajic, J.E. Mixing, mass transfer, and scale-up of polysaccharide fermentations. Biotechnol. Bioeng. 1978, 20, 939–1001. [Google Scholar] [CrossRef]
- Mota, G.P.; Pereira, R.G. A comparison of the rheological behavior of xanthan gum and diutan gum aqueous solutions. J. Braz. Soc. Mech. Sci. Eng. 2022, 44, 11. [Google Scholar] [CrossRef]
- Liang, K.; Han, P.; Chen, Q.; Su, X.; Feng, Y. Comparative Study on Enhancing Oil Recovery under High Temperature and High Salinity: Polysaccharides Versus Synthetic Polymer. ACS Omega 2019, 4, 10620–10628. [Google Scholar] [CrossRef]
- Annable, T.; Buscall, R.; Ettelaie, R.; Whittlestone, D. The rheology of solutions of associating polymers: Comparison of experimental behavior with transient network theory. J. Rheol. 1993, 37, 695–726. [Google Scholar] [CrossRef]
- Modig, K.; Pfrommer, B.G.; Halle, B. Temperature-Dependent Hydrogen-Bond Geometry in Liquid Water. Phys. Rev. Lett. 2003, 90, 75502. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Xu, X.J. Recent Progress in Chain Conformation and Function of Triple Helical Polysaccharides. J. Funct. Polym. 2016, 29, 134–152. [Google Scholar] [CrossRef]
- Han, M. Structures and properties of scleroglucan. Oilfield Chem. 1993, 375–379. [Google Scholar] [CrossRef]
- Lange, P.; Keilhofer, G. Xanthan Gum and Scleroglucan–How both differ at elevated temperatures. Oil Gas Eur. Mag. 2004, 30, 174–178. [Google Scholar]
- Nok, C.; Lecourtier, J. Studies on scleroglucan conformation by rheological measurements versus temperature up to 150 °C. Polymer 1993, 34, 150–157. [Google Scholar] [CrossRef]
- Kalpakci, B.; Jeans, Y.T.; Magri, N.F.; Padolewski, J.P. Thermal stability of scleroglucan at realistic reservoir conditions. In Proceedings of the SPE/DOE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 19–22 April 1990. [Google Scholar]
- Sun, L.; Wei, P.; Fu, Q.; Zhang, J.; Zeng, D. Research advance of xanthan system with temperature resistance and salt resistant in the oilfield development. Appl. Chem. Ind. 2014, 43, 2279. [Google Scholar]
- Banerjee, P.; Mukherjee, I.; Bhattacharya, S.; Datta, S.; Moulik, S.P.; Sarkar, D. Sorption of water vapor, hydration, and viscosity of carboxymethylhydroxypropyl guar, diutan, and xanthan gums, and their molecular association with and without salts (NaCl, CaCl2, HCOOK, CH3COONa, (NH4)2SO4 and MgSO4) in aqueous solution. Langmuir 2009, 25, 11647–11656. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, T.; Chen, Y.; Gong], H. The comparison of rheological properties of aqueous welan gum and xanthan gum solutions. Carbohydr. Polym. 2013, 92, 516–522. [Google Scholar] [CrossRef]
- Farina, J.I.; Sineriz, F.; Molina, O.E.; Perotti, N.I. Isolation and physicochemical characterization of soluble scleroglucan from Sclerotium rolfsii. Rheological properties, molecular weight and conformational characteristics. Carbohydr. Polym. 2001, 44, 41–50. [Google Scholar] [CrossRef]
- Jiran, L. Application of Biopolymer to Improve Oil Recovery in High Temperature High Salinity Carbonate Reservoirs. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 9–12 November 2015. [Google Scholar]
- Zhu, H.; Wu, W.D.; Wang, Y.Q.; Li, Y.J.; Shi, W.R. Study on the effect of pH on the rheological properties of xanthan gum solutions. Farm. Machinery. 2012, 9, 122–125. [Google Scholar] [CrossRef]
- Chagas, B.S.; Machado, D.L.P., Jr.; Haag, R.B.; Souza, C.R.D.; Lucas, E.F. Evaluation of hydrophobically associated polyacrylamide-containing aqueous fluids and their potential use in petroleum recovery. J. Appl. Polym. Sci. 2004, 91, 3686–3692. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Gong, H.; Ding, B.; Dong, M.; Li, Y. A Microbial Exopolysaccharide Produced by Sphingomonas Species for Enhanced Heavy Oil Recovery at High Temperature and High Salinity. Energy Fuels 2017, 31, 3960–3969. [Google Scholar] [CrossRef]
- Marcotte, M.; Hoshahili, A.R.T.; Ramaswamy, H.S. Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Res. Int. 2001, 34, 695–703. [Google Scholar] [CrossRef]
- Wang, B.; Wang, L.J.; Li, D.; Özkan, N.; Li, S.J.; Mao, Z.H. Rheological properties of waxy maize starch and xanthan gum mixtures in the presence of sucrose. Carbohydr. Polym. 2009, 77, 472–481. [Google Scholar] [CrossRef]
- Hu, J.G.; Zhao, L.; Dai, G.C. Rheological Properties of Xanthan Gum Solutions. J. East China Univ. Sci. (Technol. Nat. Sci. Ed.) 2011, 16–19. [Google Scholar] [CrossRef]












| Composition (g/L) | Total Mineralization (g/L) | ||
|---|---|---|---|
| NaCl | CaCl2 | MgCl2·6H2O | |
| 192.5 | 16.5 | 11 | 220 |
| 148.75 | 12.75 | 8.5 | 170 |
| 70 | 6 | 4 | 80 |
| 21.875 | 1.875 | 1.25 | 25 |
| 13.125 | 1.125 | 0.75 | 15 |
| 5.3 | 0.45 | 0.3 | 6 |
| Biopolymer Type | K (mPa·sn) | n | R2 | Shear Rate Range (s−1) |
|---|---|---|---|---|
| Diutan gum | 444.06 | 0.250 | 0.9908 | 0.01002 <  < 1000 < 1000 |
| Scleroglucan | 334.93 | 0.248 | 0.9985 | 0.01001 <  < 1000 < 1000 |
| Xanthan gum | 186.10 | 0.305 | 0.9956 | 0.01003 <  < 1000 < 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Huang, L.; Xiu, J.; Yi, L.; Zhao, Y. Evaluation of Viscosity Changes and Rheological Properties of Diutan Gum, Xanthan Gum, and Scleroglucan in Extreme Reservoirs. Polymers 2023, 15, 4338. https://doi.org/10.3390/polym15214338
Gao X, Huang L, Xiu J, Yi L, Zhao Y. Evaluation of Viscosity Changes and Rheological Properties of Diutan Gum, Xanthan Gum, and Scleroglucan in Extreme Reservoirs. Polymers. 2023; 15(21):4338. https://doi.org/10.3390/polym15214338
Chicago/Turabian StyleGao, Xin, Lixin Huang, Jianlong Xiu, Lina Yi, and Yongheng Zhao. 2023. "Evaluation of Viscosity Changes and Rheological Properties of Diutan Gum, Xanthan Gum, and Scleroglucan in Extreme Reservoirs" Polymers 15, no. 21: 4338. https://doi.org/10.3390/polym15214338
APA StyleGao, X., Huang, L., Xiu, J., Yi, L., & Zhao, Y. (2023). Evaluation of Viscosity Changes and Rheological Properties of Diutan Gum, Xanthan Gum, and Scleroglucan in Extreme Reservoirs. Polymers, 15(21), 4338. https://doi.org/10.3390/polym15214338





