DFT Investigation into Adsorption–Desorption Properties of Mg/Ni-Doped Calcium-Based Materials
Abstract
1. Introduction
2. Simulation Details
3. Results and Discussion
4. Conclusions
- (a)
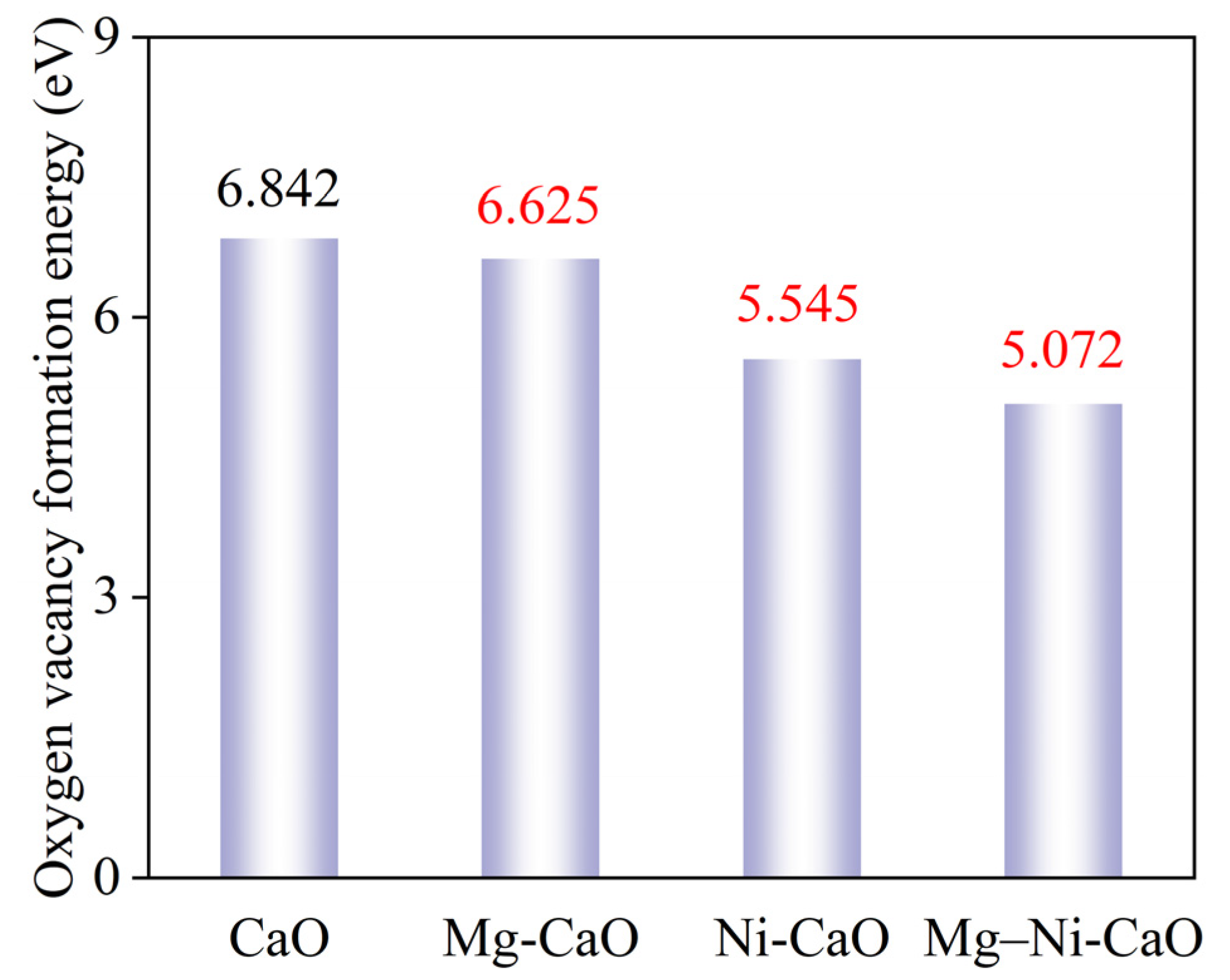
- Both single doping and co-doping of Mg and Ni can significantly regulate the energy state of the CaO system. The oxygen vacancy formation energy for pure CaO is 6.842 eV, while for Mg-CaO, Ni-CaO, and Mg–Ni-CaO, it drops to 6.625 eV, 5.545 eV, and 5.072 eV, respectively. Mg–Ni co-doping exhibits stronger synergistic effects, further reducing the formation energy of oxygen vacancies and making them easier to form. During O2− diffusion, the energy barrier on a pure CaO surface is 4.606 eV. On the surfaces of the Mg-CaO, Ni-CaO, and Mg–Ni-CaO doped systems, the O2− diffusion energy barriers show significant differences, with values of 4.519, 3.927, and 2.692 eV, respectively. The synergistic doping of Mg and Ni can significantly reduce the energy threshold for ion migration, significantly enhancing O2− diffusion efficiency and providing a more favorable ion transport pathway for CO2 adsorption.
- (b)
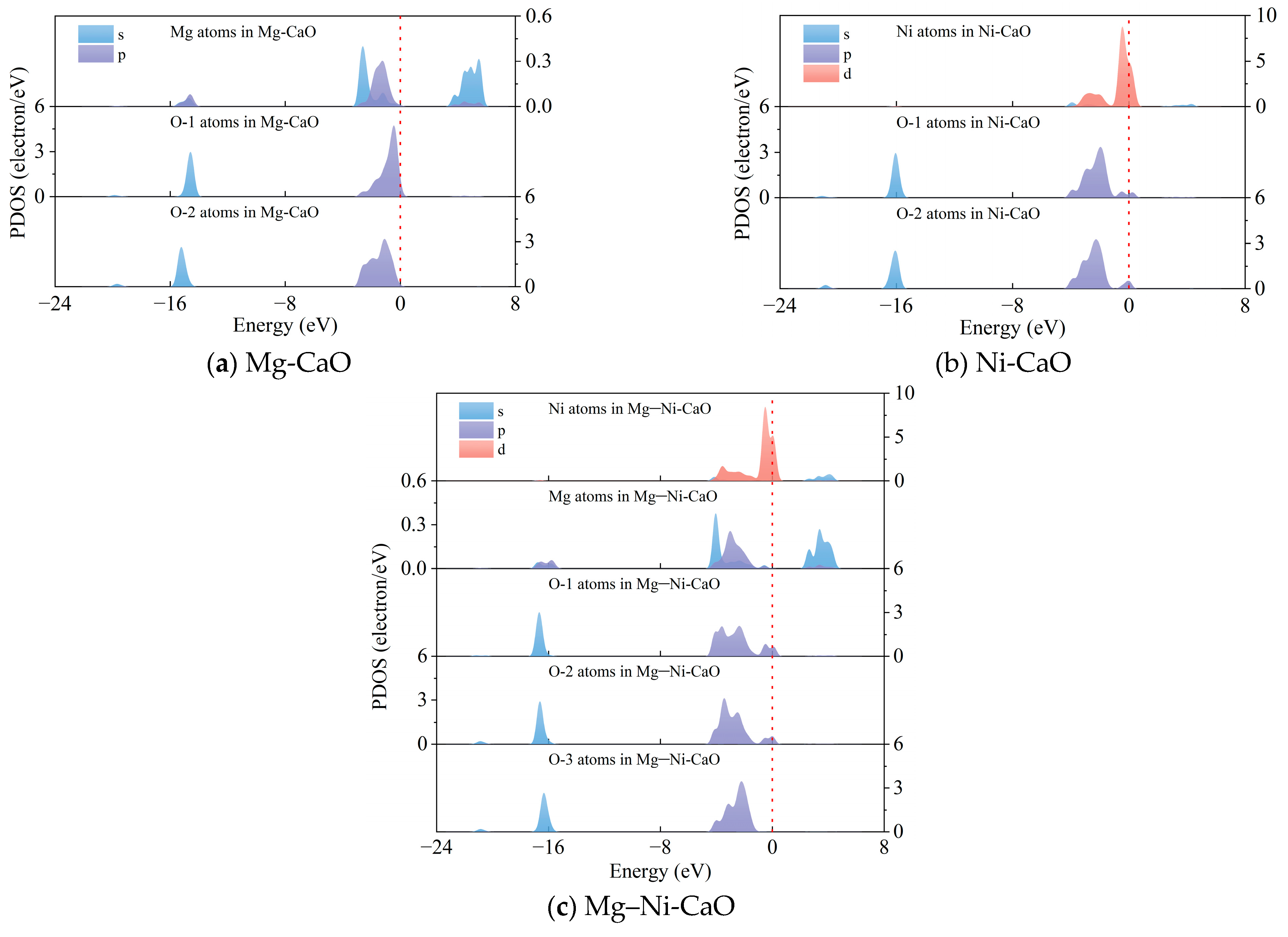
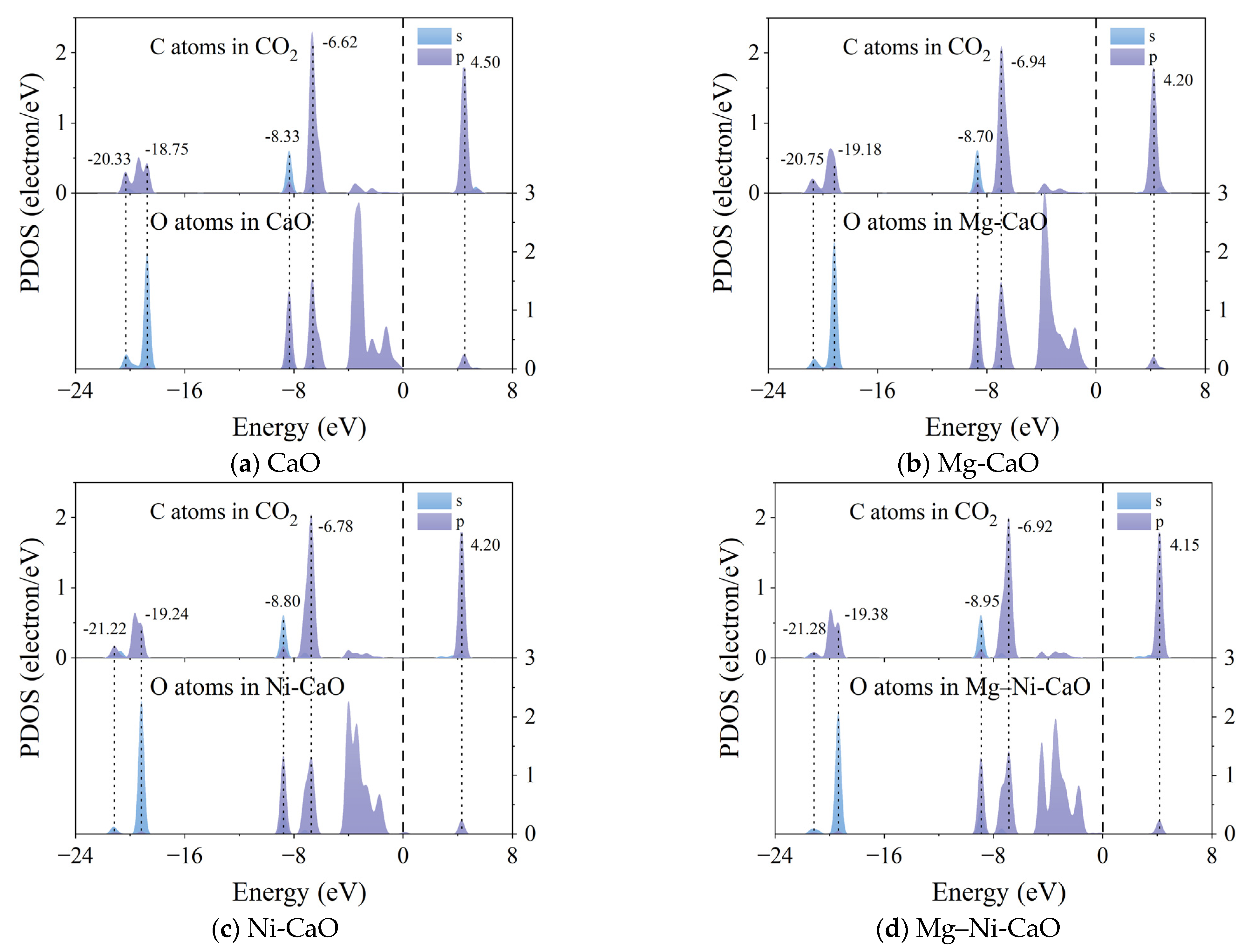
- Doping with Mg and Ni improved the adsorption capacity of CaO for CO2. On the surfaces of CaO, Mg-CaO, Ni-CaO, and Mg–Ni-CaO, CO2 forms stable CO32− structures with surface O atoms, with adsorption energies of −1.484 eV, −1.522 eV, −1.627 eV, and −1.703 eV, respectively. Mg–Ni-CaO exhibits the highest CO2 adsorption energy, indicating that it has the strongest adsorption capacity for CO2. The PDOS analysis further revealed the microscopic mechanism of Mg and Ni doping enhancing CO2 adsorption capacity. Namely, Mg and Ni doping shifted the energy of the resonance peak downward, and the energy shift was more obvious with co-doping, significantly enhancing the interaction between C and O atoms.
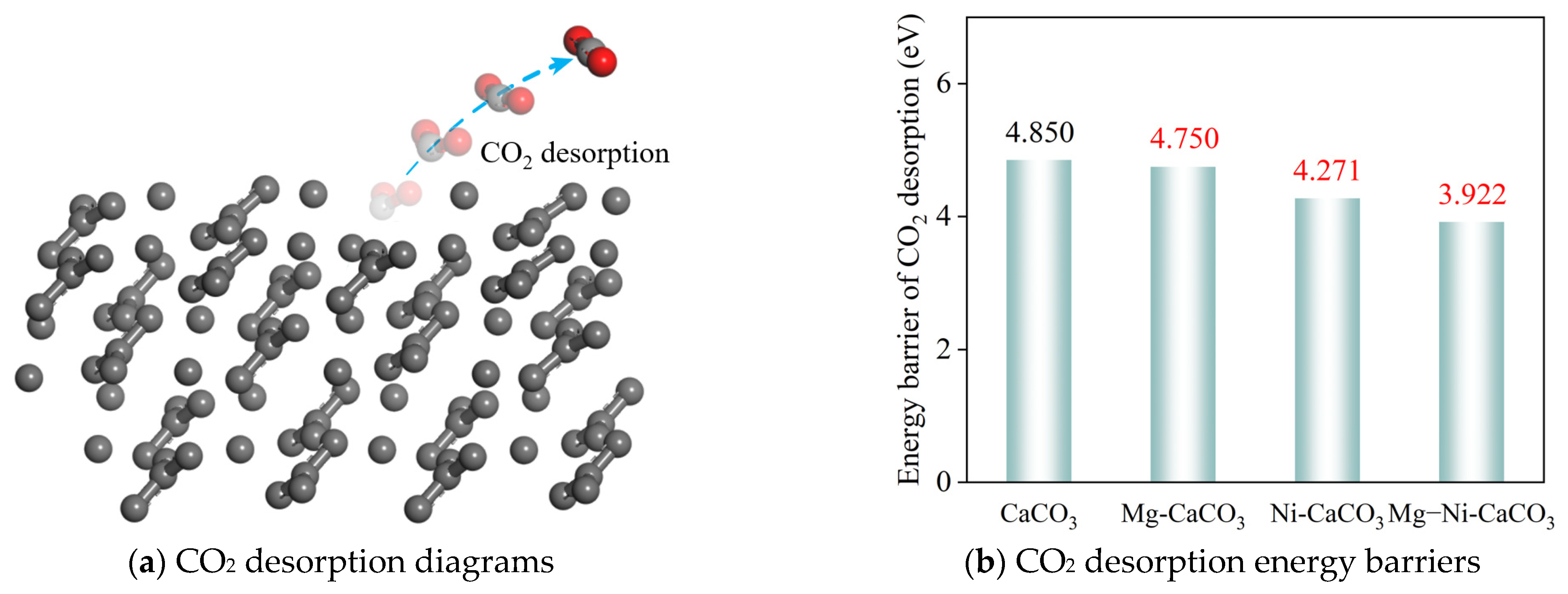
- (c)
- The doping of Mg and Ni promotes the desorption process of CO2. On the surfaces of CaCO3, Mg-CaCO3, Ni-CaCO3, and Mg–Ni-CaCO3, the energy barriers for CO2 desorption are 4.850 eV, 4.750 eV, 4.271 eV, and 3.922 eV, respectively. The doping of Mg and Ni and their synergistic effect significantly reduced the CO2 desorption energy barrier, greatly promoting the CO2 desorption process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, C.; Ju, S.; Xue, Y.; Ren, T.; Ji, Y.; Chen, X. China’s energy transitions for carbon neutrality: Challenges and opportunities. Carbon Neutrality 2022, 1, 7. [Google Scholar] [CrossRef]
- Tian, X.; Guo, S.; Lv, X.; Lin, S.; Zhao, C.-Y. Progress in multiscale research on calcium-looping for thermochemical energy storage: From materials to systems. Prog. Energy Combust. Sci. 2025, 106, 101194. [Google Scholar] [CrossRef]
- Wu, H.; Luo, C.; Luo, T.; Zhang, L.; Zhang, X.; Wu, F. Review of Solar Thermochemical Heat Storage Equipment and Systems Based on Calcium-Looping. J. Energy Storage 2024, 103, 114146. [Google Scholar] [CrossRef]
- Lu, Y.; Xuan, Y.; Teng, L.; Liu, J.; Wang, B. A cascaded thermochemical energy storage system enabling performance enhancement of concentrated solar power plants. Energy 2024, 288, 129749. [Google Scholar] [CrossRef]
- Jose, M.V. The Ca-looping process for CO2 capture and energy storage: Role of nanoparticle technology. J. Nanopart. Res. 2018, 20, 39. [Google Scholar]
- Ortiz, C.; Romano, M.C.; Valverde, J.M.; Binotti, M.; Chacartegui, R. Process integration of Calcium-Looping thermochemical energy storage system in concentrating solar power plants. Energy 2018, 155, 535–551. [Google Scholar] [CrossRef]
- Alami, A.H.; Hawili, A.A.; Hassan, R.; Al-Hemyari, M.; Aokal, K. Experimental study of carbon dioxide as working fluid in a closed-loop compressed gas energy storage system. Renew. Energy 2019, 134, 603–611. [Google Scholar] [CrossRef]
- Ramos, J.P.; Fernandes, C.M.; Stora, T.; Senos, A.M.R. Sintering kinetics of nanometric calcium oxide in vacuum atmosphere. Ceram. Int. 2015, 41, 8093–8099. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. Multicycle activity of natural CaCO3 minerals for thermochemical energy storage in Concentrated Solar Power plants. Sol. Energy 2017, 153, 188–199. [Google Scholar] [CrossRef]
- Tian, X.K.; Lin, S.C.; Yan, J.; Zhao, C.Y. Sintering mechanism of calcium oxide/calcium carbonate during thermochemical heat storage process. Chem. Eng. J. 2022, 428, 131229. [Google Scholar] [CrossRef]
- Raganati, F.; Chirone, R.; Ammendola, P. Calcium-looping for thermochemical energy storage in concentrating solar power applications: Evaluation of the effect of acoustic perturbation on the fluidized bed carbonation. Chem. Eng. J. 2020, 392, 123658. [Google Scholar] [CrossRef]
- Sun, J.; Liu, W.; Li, M.; Yang, X.; Wang, W.; Hu, Y.; Chen, H.; Li, X.; Xu, M. Mechanical Modification of Naturally Occurring Limestone for High-Temperature CO2 Capture. Energy Fuels 2016, 30, 6597–6605. [Google Scholar] [CrossRef]
- Sanchez-Jimenez, P.E.; Valverde, J.M.; Perejón, A.; De La Calle, A.; Medina, S.; Pérez-Maqueda, L.A. Influence of ball milling on CaO crystal growth during limestone and dolomite calcination: Effect on CO2 capture at Calcium Looping conditions. Cryst. Growth Des. 2016, 16, 7025–7036. [Google Scholar] [CrossRef]
- Feng, J.; Guo, H.; Wang, S.; Zhao, Y.; Ma, X. Fabrication of multi-shelled hollow Mg-modified CaCO3 microspheres and their improved CO2 adsorption performance. Chem. Eng. J. 2017, 321, 401–411. [Google Scholar] [CrossRef]
- Wang, A.; Deshpande, N.; Fan, L.S. Steam Hydration of Calcium Oxide for Solid Sorbent Based CO2 Capture: Effects of Sintering and Fluidized Bed Reactor Behavior. Energy Fuels 2014, 29, 321–330. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, Y.; Nie, B.; An, N.; Zhu, Z.; Chen, Q. CO2 adsorption and desorption across the Fe-doped Ca-based composites in the presence of H2O: A DFT approach. Phys. B Condens. Matter 2024, 673, 415483. [Google Scholar] [CrossRef]
- Valverde, J.M.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A. Role of precalcination and regeneration conditions on postcombustion CO2 capture in the Ca-looping technology. Appl. Energy 2014, 136, 347–356. [Google Scholar] [CrossRef]
- Valverde, J.M.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A. Effect of Heat Pretreatment/Recarbonation in the Ca-Looping Process at Realistic Calcination Conditions. Energy Fuels 2014, 28, 4062–4067. [Google Scholar] [CrossRef]
- Manovic, V.; Anthony, E.J. Thermal activation of CaO-based sorbent and self-reactivation during CO2 capture looping cycles. Environ. Sci. Technol. 2008, 42, 4170–4174. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Bian, Z.; Yan, X.; Wang, Z.; Liu, W. Thermochemical energy storage performances of Ca-based natural and waste materials under high pressure during CaO/CaCO3 cycles. Energy Convers. Manag. 2019, 197, 111885. [Google Scholar] [CrossRef]
- Sarrión, B.; Perejón, A.; Sánchez-Jiménez, P.E.; Amghar, N.; Chacartegui, R.; Valverde, J.M.; Pérez-Maqueda, L.A. Calcination under low CO2 pressure enhances the calcium Looping performance of limestone for thermochemical energy storage. Chem. Eng. J. 2021, 417, 127922. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Y.; Gao, W.; Harada, T.; Qin, Q.; Zheng, Q.; Hatton, T.A.; Wang, Q. Alkali Carbonate Molten Salt Coated Calcium Oxide with Highly Improved Carbon Dioxide Capture Capacity. Energy Technol. 2017, 5, 1328–1336. [Google Scholar] [CrossRef]
- Xu, Y.; Donat, F.; Luo, C.; Chen, J.; Kierzkowska, A.; Naeem, M.A.; Zhang, L.; Müller, C.R. Investigation of K2CO3-modified CaO sorbents for CO2 capture using in-situ X-ray diffraction. Chem. Eng. J. 2023, 453, 139913. [Google Scholar] [CrossRef]
- Choi, D.; Park, A.; Park, Y. Effects of eutectic alkali chloride salts on the carbonation reaction of CaO-based composites for potential application to a thermochemical energy storage system. Chem. Eng. J. 2022, 437, 135481. [Google Scholar] [CrossRef]
- González, B.; Blamey, J.; McBride-Wright, M.; Carter, N.; Dugwell, D.; Fennell, P.; Abanades, J.C. Calcium looping for CO2 capture: Sorbent enhancement through doping. Energy Procedia 2011, 4, 402–409. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, X.; Wang, X.; Song, C.; Zheng, H.; Tian, C.; Gao, K.; Sun, N.; Jiang, Z.; Xuan, Y.; et al. Rapid and stable calcium-looping solar thermochemical energy storage via co-doping binary sulfate and Al-Mn-Fe oxides. Green Energy Environ. 2023, 9, 1290–1305. [Google Scholar] [CrossRef]
- Kong, D.; Nie, B.; Zhang, Y.; Chen, Q.; An, N.; He, N.; Yao, L.; Zhai, Y. A comprehensive understanding of the role of sodium sulfate in calcium looping via in-situ experiments and DFT studies: Performance and mechanism. Fuel 2024, 371, 131968. [Google Scholar] [CrossRef]
- Kong, D.; Nie, B.; Zhang, Y.; Chen, Q.; An, N.; He, N.; Yao, L.; Wang, Z. Influence of Na2SO4-NaCl-ZnO co-doping on the thermochemical energy storage in CaO looping. Fuel 2024, 375, 132650. [Google Scholar] [CrossRef]
- Kong, D.; He, N.; Chen, Q.; Nie, B.; Zhang, Y.; An, N.; Yao, L.; Wang, Z. Enhancement of Thermochemical Energy Storage by Alkali Metal Chloride Salts-Doped Ca-Based Sorbents: A Combined DFT and Experimental Study. Molecules 2024, 29, 6058. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP: Zeitschrift für Kristallographie-Crystalline Materials. Z. Für Krist. 2005, 220, 567–570. [Google Scholar]
- Perdew, J.P.; Yue, W. Accurate and simple density functional for the electronic exchange energy: Generalized gradient approximation. Phys. Rev. B Condens. Matter 1986, 33, 8800–8802. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B Condens. Matter 1992, 46, 6671–6687. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1998, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Halgren, T.A.; Lipscomb, W.N. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem. Phys. Lett. 1977, 49, 225–232. [Google Scholar] [CrossRef]
- Morris, A.J.; Nicholls, R.J.; Pickard, C.J.; Yates, J.R. OptaDOS: A tool for obtaining density of states, core-level and optical spectra from electronic structure codes. Comput. Phys. Commun. 2014, 185, 1477–1485. [Google Scholar] [CrossRef]
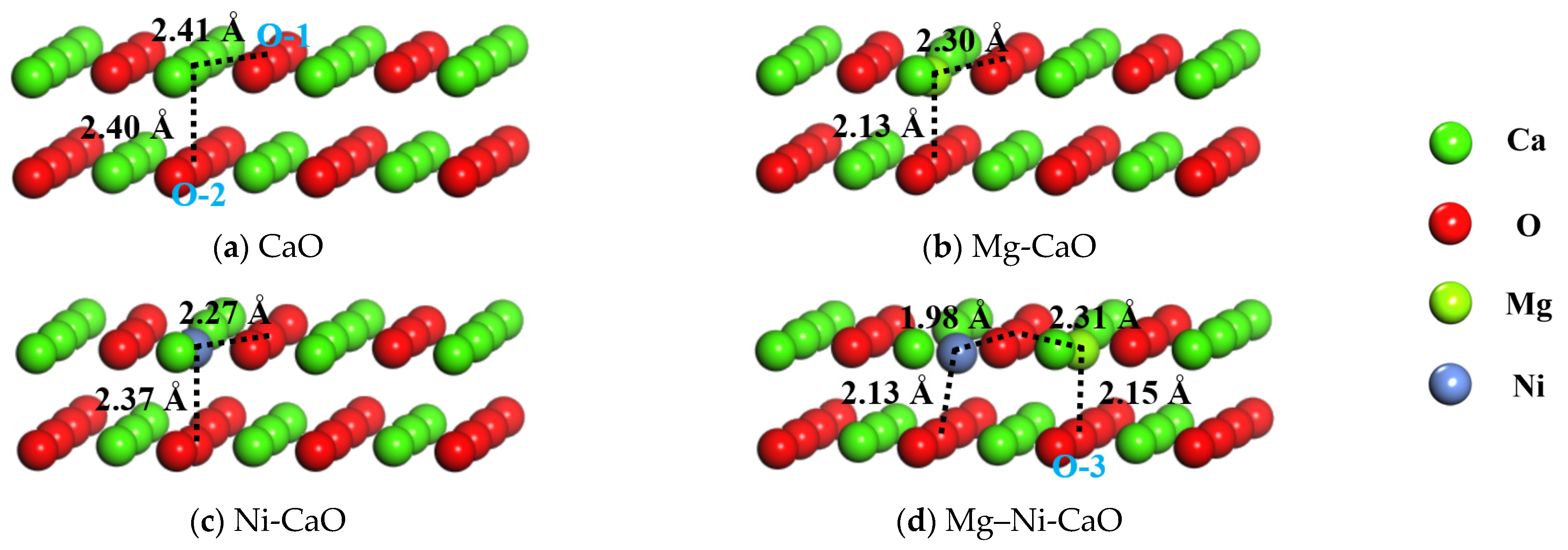




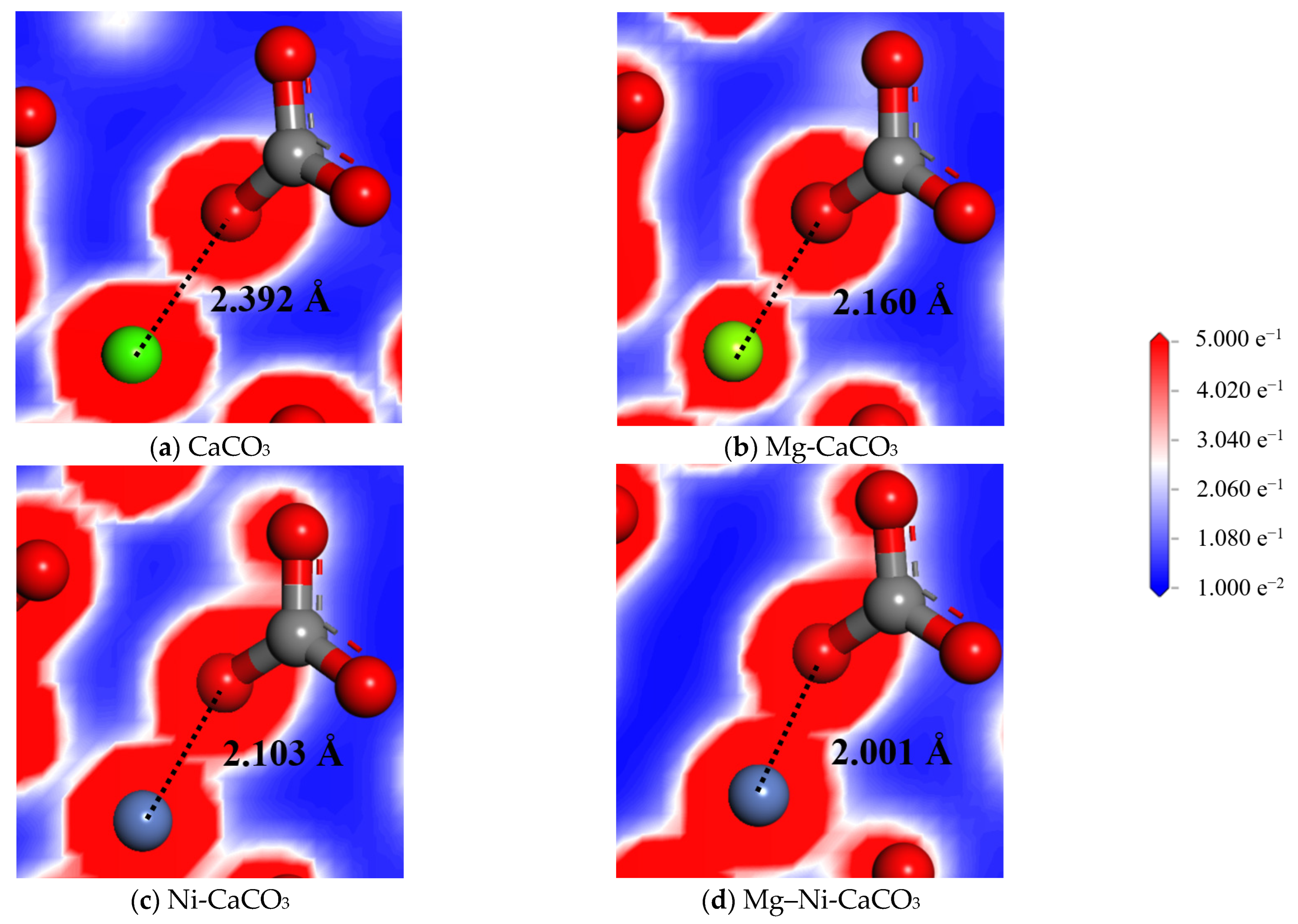
| Structure | Atomic Pair | Distance (Å) | Atomic Pair | Distance (Å) |
|---|---|---|---|---|
| CaO | Ca-O1 | 2.41 | Ca-O2 | 2.40 |
| Mg-CaO | Mg-O1 | 2.30 | Mg-O2 | 2.13 |
| Ni-CaO | Ni-O1 | 2.27 | Ni-O2 | 2.37 |
| Mg–Ni-CaO | Ni-O1 | 1.98 | Ni-O2 | 2.13 |
| Mg-O1 | 2.31 | Mg-O3 | 2.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Li, R.; Bao, X.; Yang, H.; Kong, D. DFT Investigation into Adsorption–Desorption Properties of Mg/Ni-Doped Calcium-Based Materials. Crystals 2025, 15, 711. https://doi.org/10.3390/cryst15080711
Shi W, Li R, Bao X, Yang H, Kong D. DFT Investigation into Adsorption–Desorption Properties of Mg/Ni-Doped Calcium-Based Materials. Crystals. 2025; 15(8):711. https://doi.org/10.3390/cryst15080711
Chicago/Turabian StyleShi, Wei, Renwei Li, Xin Bao, Haifeng Yang, and Dehao Kong. 2025. "DFT Investigation into Adsorption–Desorption Properties of Mg/Ni-Doped Calcium-Based Materials" Crystals 15, no. 8: 711. https://doi.org/10.3390/cryst15080711
APA StyleShi, W., Li, R., Bao, X., Yang, H., & Kong, D. (2025). DFT Investigation into Adsorption–Desorption Properties of Mg/Ni-Doped Calcium-Based Materials. Crystals, 15(8), 711. https://doi.org/10.3390/cryst15080711





