Abstract
Hybrid materials, i.e., the organically modified silicates (ORMOSIL) based on zincphthalocyanine (ZnPc) and silica glass matrix were synthesized by the sol-gel method using protic solvents (methanol, ethanol, isopropanol, butanol) and aprotic solvent (N,N-dimethylformamide; DMF). The effect of an alkaline environment with NaOH addition (a single-stage process) and acid–alkaline environment with CH3COOH-NH4OH and HCl-NaOH (a two-stage process) was analyzed. UV-Vis spectroscopy was used to study the stability of ZnPc in the sol. The highest stability of zinc phthalocyanine in the glass was obtained for synthesis with isopropanol in the presence of the alkaline catalyst. The lowest stability of ZnPc was observed when the aprotic solvent was used. The structure and optical properties of the gels were studied by SEM, FTIR, and XRD techniques and optically stimulated luminescence (OSL) and thermoluminescence (TL), respectively. The thermal stability of the materials was analyzed by TG-DSC methods.
1. Introduction
Phthalocyanines are one of the most popular heterocyclic compounds, which have found applications in optoelectronics [1,2]. They generate free charge carriers during exposition on the light, which induces luminescence. The structure of phthalocyanine is based on four benzopyrrole units, linked by nitrogen atoms. Its macrocycle forms the characteristic chromophore system, which is similar to porphyrins which are naturally occurring analogues of phthalocyanines. Photochemistry of the compounds (metal-free phthalocyanine and their metal complexes) is determined by 18 delocalized electrons present in the macrocycle ring [3,4,5]. The electronic properties of phthalocyanines are strongly related to the type of the coordinated metal or metals cation/s (e.g., electron configuration), and character and position of substituents [6,7]. The chromophore system of phthalocyanine is very stable and strongly absorbs photons in the near-UV (300−400 nm) and visible (600−800 nm) regions [4,5], and can act as photosensitizer [8,9]. Additionally, phthalocyanines reveal other useful properties such as magnetic, redox, and primally semiconducting and photoconducting properties [10,11,12].
Organic–inorganic hybrid glasses (OI hybrids) are one of the commonly used matrixes for immobilization of organic molecules and metalloorganic compounds, dyes, and enzymes (biomolecules). These kinds of materials have found applications in nonlinear optics (NLO) and as biosensors [13,14,15,16]. Due to the low thermal stability of organic compounds, the OI hybrids should be obtained by low thermal processes, i.e., a sol-gel method [17,18,19]. In the organically modified silicate matrix (ORMOSIL), the alkoxides are modified by organic groups (e.g., epoxy, amine, phenyl groups). It results in significant improvement of the hydrophobic/hydrophilic character and diminishing porosity of the material. Conditions for conducting the sol-gel synthesis, i.e., mainly the kind of catalysts and pH values of sols, type of solvents, and molar ratio of precursors affect the rate of hydrolysis and condensation of precursors and gelation, and consequently change the structure, and finally, the optical properties of material [17,18,19,20,21,22].
In this work, we analyzed the influence of protic and aprotic solvents and a two-stage sol-gel process (acid-base conditions) on the stability of zinc phthalocyanine and the structure and luminescence of organic–inorganic hybrid materials.
2. Experiments
2.1. Materials
Commercial zinc phthalocyanine without further purification (purity 96%, Acros) was used. The following starting reagents: tetraethyl orthosilicate (TEOS) (purity 98%), (3-Glycidyloxypropyl)trimethoxysilane (GPTMS) (purity ≥ 98%), triethoxyphenylsilane (PTES) (purity 98%) and methanol (purity 99.5%), ethanol (anhydrous), 2-propanol (purity 99.5%), butanol (purity 99.5%), N,N-dimethylformamide (purity 99.5%) hydrochloric acid (1 M), sodium hydroxide (1 M), acetic acid (0.5%), and ammonia solution (~20%) were supplied by Sigma Aldrich (Saint Louis, MO, USA) and POCH S.A. (Gliwice, Poland).
The compounds used for sol-gel synthesis play different roles, i.e., TEOS delivers network former (SiO2); GPTMS and PTES supply network modification due to the presence of non-hydrolyzed phenyl groups and aliphatic chains with the epoxide ring at the end. Organoalkoxides affect hydrophobicity of the matrix which allows immobilization of the organic compounds with luminescence properties. Zinc phthalocyanine, which is what we used, has no other substituents. Thus, a hybrid matrix seems to be the best way for immobilization.
2.2. Preparation of Samples
TEOS, GPTMS, and PTES were mixed together in the molar ratio (7:49:3) with alcohol or DMF added to achieve a volume of 20 mL. Then, 10 μL (1 M HCl) or 100 μL (0.5% CH3COOH) or 10 μL NaOH (1 M) were added to the mixture and stirred for 24 h. After two days, the mixtures were neutralized by adding sodium hydroxide or ammonia solution (Table 1). Then, 5 mL of 1 × 10−4 M of ZnPc solution in DMF was added to each sample and stirred for 15 min. Syntheses were performed in glass bottles wrapped in aluminum foil. After two months, 5 mL of sols was poured into a beaker and dried in the laboratory dryer at 37 °C up to gelation. The synthesis was conducted in ambient conditions. The sol samples were stored in laboratory glass bottles in ambient conditions without access to light. The obtained gels were ground to powder in a mortar after 3 weeks and tested by structural methods (XRD, FTIR, TG-DTG-DSC) and thermoluminescence and optically stimulated luminescence.

Table 1.
Samples preparation regarding the reagents.
2.3. Methods of Experiments
SEM/EDS investigations were performed using JEOL JSM 5400 LV X-ray analyzer 300 Series LINK ISIS. The samples were coated by carbon to maintain their electrical conductivity. The X-ray diffraction pattern (Philips X’Pert Diffractometer, Amsterdam, The Netherlands) with CuKα radiation) was used to confirm the amorphous nature of the samples using step size 0.05° of 2θ in the range of 10–70° and counting time 1 s for each step.
All sol samples were measured for two months in order to confirm the immobilization/presence and stability of zinc phthalocyanine in a hybrid matrix obtained via sol-gel synthesis. A JASCO V-630 UV-Vis-NIR Spectrophotometer was used in the range of 200−1100 nm. All absorption spectra were measured for sol using the 10 mm cuvette.
Thermal stability analysis was performed with DSC method, using NETZSCH STA 449 F3 equipment and a platinum crucible in air atmosphere. Approximately 60 mg of ground glasses were used for DSC measurement from 50 °C to 900 °C at the constant heating rate of 10 K/s. Peak temperature of decomposition (Td) and mass loss were calculated using NETZSCH software.
FTIR spectra were recorded with a Bruker Company Vertex 70 v spectrometer. Spectra were collected in the range of 4000–400 cm−1, typically using 128 scans at 4 cm−1 resolution. The 6−8 mg of each powder sample was prepared by the standard KBr pellet method.
Samples for TL and OSL measurements were ground to the form of pellets of around 5 mm diameter and thickness of 1 mm. Before the irradiations, the samples were heated to the temperature of 270 °C at the constant heating rate of 5 K/s to erase the residual signal coming from the previous exposures. TL signal was recorded with the heating rate of 2 K/s. The samples were irradiated with beta radiation (90 Sr/90 Y source) and with alpha particle (241 Am source) doses of 100 Gy, respectively. The sample response to alpha and beta radiation was studied in consecutive measurements with an additional erasing readout between them. The automatic Risø TL/OSL-DA−20 reader with BG39 and U-340 optical filters were used for measurements. All curves were analyzed using GlowVIEW software.
3. Results and Discussion
3.1. SEM and XRD Study
The hybrid gels were flat, smooth, and had a color ranging from yellow to green. All the samples showed similar appearance under SEM microscope. Figure 1 illustrates an example of the gel cross-section. The material was homogeneous. This allowed the conclusion that the selected proportion of precursors used for the synthesis was suitable for optoelectronic purposes, demanding uniformity and the lowest possible porosity. The other samples were also uniform (see Supplementary Information). The EDX results confirmed chemical homogeneity of the sample (Figure 2). As we can see, three detected elements: C, O, and Si demonstrated high concentrations in the sample, which are derived from alkoxide precursors used for the sol-gel synthesis. It must be noted that all samples are sputtered by carbon, which explains the large concentration of the element. However, nitrogen is also detected, which may confirm presence of the phthalocyanine, because benzopyrrole units build up macrocycle ring of this dye. Zn and N concentrations in the samples are too low to confirm a reliable presence of the phthalocyanine in the matrix by EDX. Furthermore, the molar concentration of zinc phthalocyanine, based on the UV-Vis spectra (Section 3.2), is relatively limited and ranges from 10−5 to 10−8 M.
Figure 1.
Surface morphology of the sample no. 1 (based on isopropanol-NaOH synthesis). Magnification 100,000×. Numbers indicate areas of EDX analysis.
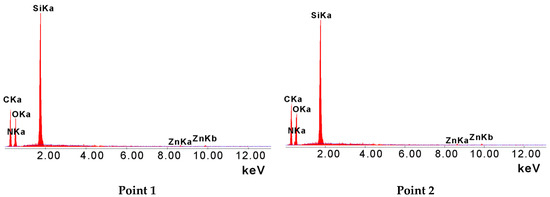
Figure 2.
EDX spectrum of the hybrid gel sample referring to two points 1 and 2 depicted in Figure 1.
XRD study reveals that all samples are amorphous. However, Figure 3 shows XRD patterns with two broad peaks at the range 15−25 and 25−35 of 2θ, respectively, which may indicate that structure of gels consists of two sub-networks, i.e., inorganic and organic. Such effects are observed for glasses based on two glass formers, e.g., borosilicate glass [23].
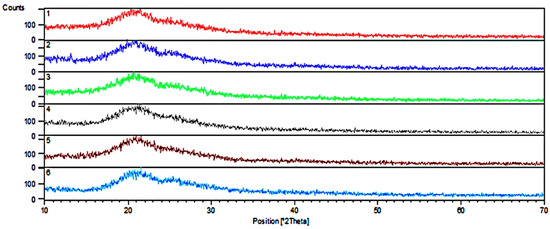
Figure 3.
XRD patterns of the gels. Samples identification in accordance with Table 1.
3.2. UV-Vis Analyzes
All tested samples were intensive blue after addition of zinc phthalocyanine solution to DMF. Only a sample, derived from synthesis in ethanol and in the presence of the base catalyst NH4OH, was intense green. The green color is due to the addition of a blue dye solution to a slightly yellow-colored sol, resulting from addition of the ammonia solution. The spectra recorded after addition of dye solution to sols confirmed the presence of zinc phthalocyanine in the analyzed system [24,25,26]. The series of protic solvents were used for sol-gel synthesis to develop influence of the acidic character of alcohols. Stability of zinc phthalocyanine in the matrix of the finally hybrid gel is changing in the order: methanol > ethanol > isopropanol > butanol on. Based on the presented spectra (Figure 4), isopropanol, a solvent, is the most suitable environment for zinc phthalocyanine incorporation to the glass matrix as is. The lowest condition shows the aprotic solvent DMF for sol-gel synthesis. Photodegradation of zinc phthalocyanine occurs very slowly in the sample with ethanol and isopropanol compared to the samples with methanol or butanol. Moreover, the samples based on the basic condition (with addition of NaOH solution) show better results than a two-step sol-gel synthesis (CH3COOH-NH4OH). As we can see on the spectra, every sample shows decrease of absorption in due course, which is related to the degradation (photobleaching) of zinc phthalocyanine. There are several factors which induce photobleaching, such as type of used solvent, acid condition, type of the central metal, character and number of substituents, ambient condition, etc. Firstly, in our experiments all used solvents are very weakly coordinated to the central metal phthalocyanine, thus they do not protect the central metal well. Aprotic solvent DMF has the lowest affinity for coordination of the zinc, which explains the fast degradation of phthalocyanine in sample no. 6. Secondly, sol-gel synthesis and UV-Vis measurement of the sols were carried out in the ambient condition. A complex of ZnPc produces singlet oxygen upon photolysis with visible light. The singlet oxygen is responsible for photobleaching of phthalocyanine, because it can oxidize the unsaturated bond of the macrocycle ring of this dye [27]. The spectrum of the sol samples changes with the time, and the background increases. This effect is derived from sol to gel transition of the matrix which was also mentioned in the works [28,29].
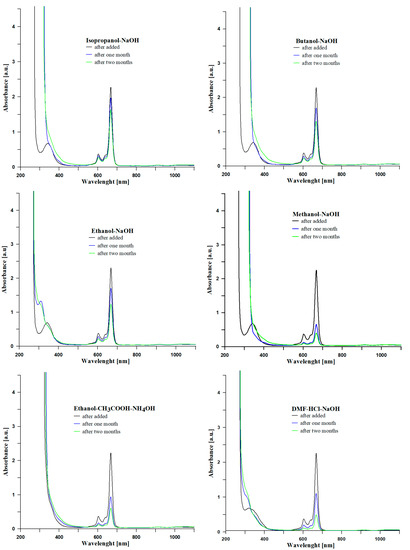
Figure 4.
Evaluation of UV-Vis absorbance according to lifetimes.
3.3. FTIR Study
No significant differences can be observed between the particular spectra (Figure 5). Bands in the range 480−486 cm−1 are derived from ring vibration of the phenyl group and deformation vibration of O-Si-O units [30]. Phenyl group bonded to silicate network shows a band at 700 cm−1 [31]. Bands located at around 762 cm−1, 854 cm−1, 1050 cm−1, and 1200 cm−1 are derived from Si-O-Si linkages of the glass network [30,32,33]. The band at ~1137 cm−1, which is located in the Si-O-Si vibration region and visible as a shoulder on the main band, indicates Si-C bond vibrations [34] and/or symmetric bending vibration of -CH2 units derived from GPTMS [35]. The C-H deformation vibrations and stretching vibration occur between 1456−1458 cm−1 and 2850−2970 cm−1, respectively [30,32,33,35]. The low-intensity band at 1437 cm−1 indicates C=C stretching vibration in the aromatic ring [34]. Vibrational bands observed at around 1200 cm−1 and 3001 cm−1 can be assigned to the epoxide ring of GPTMS [35]. Moreover, C-H and C-O vibrations of the epoxy ring are visible as the bands located at 1350−1380 cm−1 and 1730−1750 cm−1, respectively [32]. These bands are higher intensities for samples no. 5 and no. 6 on the FTIR spectra. Symmetric and asymmetric stretching vibrations of CH3 and CH2 are revealed in the range 2800–3000 cm−1 [30,32,33,36]. The band recorded between 3000−3100 cm−1 corresponds to vibrations of C-H bonds in the phenyl ring. Vibrations between 3460−3495 cm−1 are derived from overlapping O-H stretching bonds from hydrogen bonds between water molecules and SiO-H stretching of surface silanols hydrogen bonded to molecular water [33]. Observed differences in the intensity of the bands on the FTIR spectra can result from different types of catalysts and solvents that affect the process of the hydrolysis and precursor condensation, as the proportion of precursors was constant for all samples.
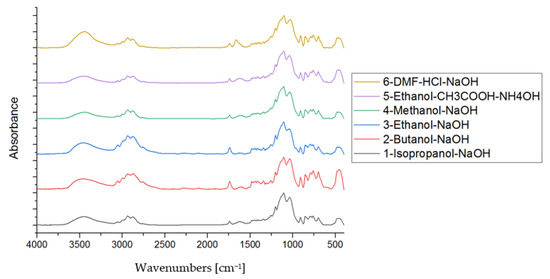
Figure 5.
Normalized FTIR spectra of hybrid gels.
3.4. Thermal Analysis
Thermal analysis reveals a few exothermal peaks accompanied by mass loss during the samples heating to 900 °C (Figure 6). Low loss of mass was observed at the temperature range from 25 °C to 60 °C for materials based on alcohol synthesis. The mass loss varies from 1.31−1.54% and 2.75−4.54% for single-stage and two-stage processes, respectively. This effect is connected with progressive water evaporation absorbance on the samples. The highest mass loss for samples fabricated by the two-stage process can result from aqueous solution of the second catalyst. The loss of physiosorbed water is accompanied by an exothermal peak visible at approximately 30 °C on the DSC curves. Absorbed in the water chemicals together with the rest of solvents and catalysts retained in the gels evaporate in the temperature range from 60 °C to 220 °C. The third exothermal peak at temperature of 237−257 °C, visible on all curves (see Supplementary Information), can be related to polymerization of the silica matrix. It can be correlated with degeneration of the Si-OH vibration band at 912 cm−1 as well as the release of alcohol molecules from the glass network, which is manifested in lowering intensity of the band corresponding to the –CH2 vibration in the FTIR spectra [32,36]. The highest mass loss (>30%), accompanied by two exothermal peaks, is revealed at temperature 260−420 °C (Table 2) on TG curves, which is due to degradation of organic groups such as glycidyloxypropyl units derived from GPTMS, n-propyl units, and initial pyrolysis of phenyl groups (PTES) [31]. The pyrolysis of the organic groups continues at the temperature range 400−900 °C [31,36,37,38,39]. Simultaneously, the silica matrix is thermally stable and does not degrade.
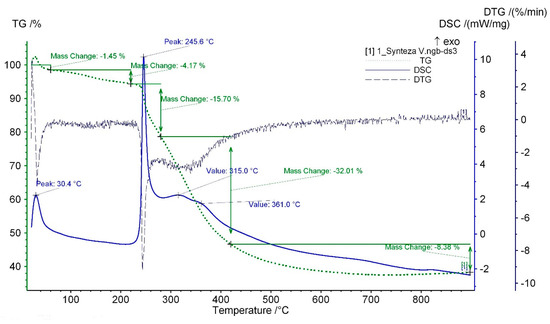
Figure 6.
Thermal analysis TG-DTG-DSC of isopropanol-NaOH sample.

Table 2.
Recorded effects based on thermal analysis.
3.5. Luminescence
3.5.1. Thermoluminescence (TL)
The process of synthesis reflects on optical properties of the gels (Figure 7). The lowest TL shows materials obtained in the two-stage process (samples no. 5 and 6). The amount of dye is lower due to significant degradation of metallophthalocyanine, which is manifested in the UV-Vis spectra. The environment of anhydrous ethanol and alkaline catalyst inhibits ZnPc degradation, which contributes to higher thermoluminescence. A similar result was obtained for the material with isopropanol processing (sample no. 1). The sample based on isopropanol had the best chemical stability (Figure 4). However, the presented results of the luminescence show that this sample is average compared to the other samples. A sample synthesized in the presence of butanol showed a slightly lower stability than the gel based on isopropanol. However, in this case, a lower intensity of luminescence is visible, due to a lower content of dye in the material.
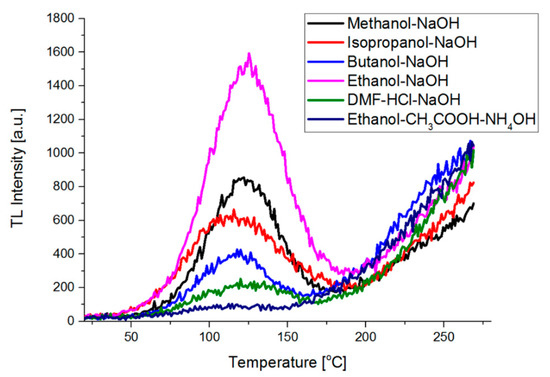
Figure 7.
Emission spectra based on thermoluminescence measurements of the gels.
The high TL of the material synthetized with the presence of butanol is also a big surprise as degradation of ZnPc was similar to a sample fabricated with the presence of DMF.
3.5.2. Optically Stimulated Luminescence (OSL)
OSL of samples obtained by a two-stage process characterizes the lowest luminescence intensity that is similar to TL results (Figure 8). OSL signals for butanol and isopropanol solvents are not significantly different. It may result from the similar rate of degradation and thus the similar concentration of ZnPc in both cases. However, the highest intensity of OSL shows a sample with methanol solvent, where the final concentration of ZnPc is low (see Figure 4). It is the reverse effect compared to TL results.
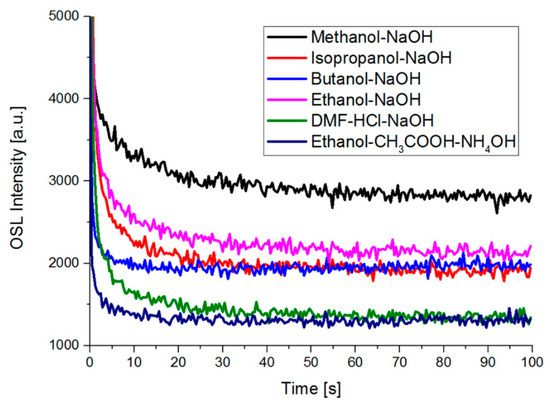
Figure 8.
OSL spectra of gel samples.
4. Conclusions
Phthalocyanine is thermally stable only to a temperature of c.a. 300 °C. Thus, the low thermal synthesis is a big advantage of the sol-gel method. The method enables production of the low porosity layers appropriate for optoelectronics.
Zinc phthalocyanine (ZnPc) was introduced successfully to a silicate amorphous matrix using the sol-gel method. In this form, the phthalocyanine shows luminescence properties that make the material valuable for optoelectronics, e.g., NLO. The results indicate that stability of phthalocyanine is sensitive to process of the synthesis. The highest stability of zinc phthalocyanine was obtained for the synthesis with isopropanol in the presence of the alkaline catalyst and the lowest when the aprotic solvent was used. The different ways of fabrication, i.e., the kind of solvent and catalysts that affect the optical properties of ZnPc in the glass matrix. The results show that optical active layers of ORMOSIL with phthalocyanine could be fabricated by the sol-gel method on the glass substrate, i.e., by dip coating. This gives us the ability to develop dye sensitizer solar cells and organic light emitting diodes.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst11060592/s1, Figure S1: SEM image of hybrid butanol-NaOH gel (magnification: 100,000× left and 30,000× right); raw data; Figure S2: SEM image of hybrid ethanol-NaOH gel (magnification 100,000×); raw data; Figure S3: SEM image of methanol-NaOH gel (magnification 100,000×); raw data; Figure S4: SEM image of hybrid ethanol-CH3COOH gel (magnification 100,000×); raw data; Figure S5: SEM image of hybrid DMF-HCl-NaOH gel (magnification 100,000×); raw data; Figure S6: TG-DSC analysis of butanol-NaOH gel; Figure S7: TG-DSC analysis of ethanol-NaOH gel; Figure S8: TG-DSC analysis of methanol-NaOH gel; Figure S9: TG-DSC analysis of ethanol-CH3COOH-NH4OH gel; Figure S10: TG-DSC analysis of DMF-HCl-NaOH gel.
Author Contributions
Conceptualization: B.P.; Methodology: B.P.; Validation: B.P. and M.Ś.; Investigation: B.P. and M.Ś.; Resources: M.Ś. and K.C.-K.; Writing–Original Draft Preparation: B.P. and M.Ś.; Writing–Review & Editing: B.P. and M.Ś.; Visualization: B.P. and M.Ś.; Supervision: M.Ś.; Project Administration: B.P. and M.Ś.; Funding Acquisition: M.Ś. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported from the subsidy of the Ministry of Education and Science for the AGH University of Science and Technology in Kraków (Project No 16.16.160.557).
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
Authors are very thankful to Magdalena Szumera, (AGH University of Science, Kraków, Poland), Barbara Trybalska (AGH University of Science, Kraków, Poland), and Wojciech Gieszczyk (Institute of Nuclear Physics, Polish Academy of Sciences, Kraków, Poland) for assistance in thermal analysis, SEM-EDS, and OSL/TL measurements, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shirota, Y.; Kageyama, H. Handbook of Organic Materials for Optical Devices; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kalyani, N.T.; Swart, H.; Dhoble, S. Principles and Applications of Organic Light Emitting Diodes (OLEDs); Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Arslan, S. Phthalocyanines: Structure, synthesis, purification and applications. J. Life Sci. 2016, 6, 188–197. [Google Scholar]
- Trytek, M.; Makarska, M.; Polska, K.; Radzki, S.; Fiedurek, J. Porfiryny i ftalocyjaniny Cz, I. Właściwości i niektóre zastosowania. Biotechnologia 2005, 71, 109–127. [Google Scholar]
- Isago, H. Optical Spectra of Phthalocyanines and Related Compounds; Springer: Tokyo, Japan, 2015. [Google Scholar]
- Słota, R.; Dyrda, G. UV Photostability of Metal phthalocyanines in organic solvents. Inorg. Chem. 2003, 42, 5743–5750. [Google Scholar] [CrossRef] [PubMed]
- Kadish, K.M.; Smith, K.M.; Guilard, R. Handbook of Porphyrin Science: With Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine; World Scientific: Singapore, 2010. [Google Scholar]
- Belogorokhov, I.A.; Ryabchikov, Y.V.; Tikhonov, E.V. Photoluminescence in semiconductor structures based on butyl-substituted erbium phthalocyanine complexes. Semiconductors 2008, 42, 321–324. [Google Scholar] [CrossRef]
- Eastwood, D.; Edwards, L.; Gourterman, M. Spectra of porphyrins part VII: Vapor absorption and emission of phthalocyanines. J. Mol. Spectrosc. 1966, 20, 381–390. [Google Scholar] [CrossRef]
- Dyrda, G.; Zakrzyk, M.; Broda, M.A. Hydrogen bond-mediated conjugates involving lanthanide diphthalocyanines and trifluoroacetic acid (LnPc2@TFA): Structure, photoactivity and stability. Molecules 2020, 25, 3638. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Andre, J.J. Molecular Semiconductors: Photoelectrical Properties and Solar Cells; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Hunger, K. Industrial Dyes: Chemistry, Properties, Application; Wiley VCH: Weinheim, Germany, 2003. [Google Scholar]
- Xia, H.; Nogami, M. Copper phthalocyanine bonding with gel and their optical properties. Opt. Mater. 2000, 15, 93–98. [Google Scholar] [CrossRef]
- Guglielmi, M.; Brusatin, G.; Della Giustina, G. Hybrid glass-like films through sol–gel techniques. J. Non Cryst. Solids 2007, 353, 1681–1687. [Google Scholar] [CrossRef]
- Cheng-Shane, C. Optical fiber oxygen sensor based on Pd(II) complex embedded in sol–gel matrix. J. Lumin. 2013, 135, 5–9. [Google Scholar]
- Dash, S.; Mishra, S.; Patel, S.; Mishra, B.K. Organically modified silica: Synthesis and applications due to its surface interaction with organic molecules. Adv. Colloid Interface Sci. 2008, 140, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, Y.; Kurumada, K. Percolation transition of siloxane domain in partially phenylated organic/inorganic hybrid glass. J. Non Cryst. Solids 2007, 353, 2521–2527. [Google Scholar] [CrossRef]
- Kuniyoshi, M.; Takahashi, M.; Tokuda, Y.; Yoko, T. Thermosoftening phenyl siloxane glasses prepared via sol concentration. J. Non Cryst. Solids 2007, 353, 4162–4169. [Google Scholar] [CrossRef]
- Kamimura, Y.; Kurumada, K. Evaluation of molecular volume of siloxane bonding phenyl group in partially phenylated organic/inorganic hybrid glass. J. Non Cryst. Solids 2008, 354, 3414–3417. [Google Scholar] [CrossRef]
- Ou, D.L.; Seddon, A.B. Near-and mid-infrared spectroscopy of sol–gel derived ormosils: Vinyl phenyl silicates. J. Non Cryst. Solids 1997, 210, 187–203. [Google Scholar] [CrossRef]
- Wu, Z.; You, L.; Xiang, H.; Jiang, Y. Comparison of dye adsorption by mesoporous hybrid gels: Understanding the interactions between dyes gel surfaces. J. Colloid Interface Sci. 2006, 303, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Ulatowska, A.; Kudrawiec, K.; Podbielska, H.; Bryja, L.; Misiewicz, J. Transmittance examination in sol-gel derived matrices for optoelectronic application. Opt. Mater. 2001, 17, 247–250. [Google Scholar] [CrossRef]
- Wright, A.C.; Feller, S.A.; Hannon, A.C. (Eds.) Borate Glasses, Crystals and Melts. The Society of Glass Technology: Sheffield, UK, 1997. [Google Scholar]
- Ghani, F.; Kristen, J.; Riegler, H. Solubility properties of unsubstituted metal phthalocyanines in different types of solvents. J. Chem. Eng. Data 2012, 57, 439–449. [Google Scholar] [CrossRef]
- Ogunsipe, A.O.; Idowu, M.A.; Ogunbayo, T.B.; Akinbulu, I.A. Protonation of some non-transition metal phthalocyanines—Spectral photophysicochemical consequences. J. Porphyr. Phthalocyanines 2012, 16, 885–894. [Google Scholar] [CrossRef]
- Ogunsipe, A.; Maree, D.; Nyokong, T. Solvent effects on the photochemical fluorescence properties of zinc phthalocyanine derivatives. J. Mol. Struct. 2003, 650, 131–140. [Google Scholar] [CrossRef]
- Kuznetsova, N.A.; Kaliya, O.L. Oxidative photobleaching of phthalocyanines in solution. J. Porphyr. Phthalocyanines 2012, 16, 705–712. [Google Scholar] [CrossRef]
- Baldovi, H.G.; Blas-Ferrando, V.M.; Ortiz, J.; Garcia, H.; Fernández-Lázaro, F.; Sastre-Santos, Á. Phthalocyanine–Gold Nanoparticle Hybrids: Modulating Quenching with a Silica Matrix Shell. Chem. Phys. Chem. 2016, 17, 1579–1585. [Google Scholar] [CrossRef]
- Gerasymchuk, Y.S.; Chernii, V.Y.; Tomachynski, L.A.; Legendziewicz, L.; Radzki, S. Spectroscopic characterization of zirconium(IV) and hafniumf(IV) gallate phthalocyanines in monolithic silica gels obtained by sol–gel method. Opt. Mater. 2005, 27, 1484–1494. [Google Scholar] [CrossRef]
- Purcar, V.; Stamatin, I.; Cinteza, O.; Petcu, C.; Raditoiu, V.; Ghiurea, M.; Miclaus, T.; Andronie, A. Fabrication of hydrophobic and antireflective coatings based on hybrid silica films by sol–gel proces. Surf. Coat. Technol. 2012, 206, 4449–4454. [Google Scholar] [CrossRef]
- Moriones, P.; Ríos, X.; Echeverría, J.C.; Garrido, J.J.; Pires, J.; Pinto, M. Hybrid organic–inorganic phenyl stationary phases for the gas separation of organic binary mixtures Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 69–75. [Google Scholar] [CrossRef]
- Hernández-Escolano, M.; Ramis, X.; Jiménez-Morales, A.; Juan-Díaz, M.; Suay, J. Study of the thermal degradation of bioactive sol–gel coatings for the optimization of its curing process. J. Therm. Anal. Calorim. 2012, 107, 499–508. [Google Scholar] [CrossRef][Green Version]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R”Si(OR0)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Yong-Ho, K.; Byunghwan, L.; Kwang-Ho, C.; Sang-June, C. Selective adsorption of bisphenol A by organic–inorganic hybrid mesoporous silicas. Microporous Mesoporous Mater. 2011, 138, 184–190. [Google Scholar]
- Juan-Díaz, M.J.; Martínez-Ibáñez, M.; Lara-Sáez, I.; da Silva, S.; Izquierdo, R.; Gurruchaga, M.; Goñi, I.; Suay, J. Development of hybrid sol–gel coatings for the improvement of metallic biomaterials performance. Prog. Org. Coat. 2016, 96, 42–51. [Google Scholar] [CrossRef]
- Ying-Sing, L.; Yu, W.; Sorrie, C. Vibrational spectra of phenyltriethoxysilane, phenyltrimethoxysilane and their sol–gels. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 71, 1819–1824. [Google Scholar]
- Jana, R.N.; Bhunia, H. Thermal stability and proton conductivity of silane based nanostructured composite membranes. Solid State Ion. 2008, 178, 1872–1878. [Google Scholar] [CrossRef]
- Wu, K.H.; Chao, C.M.; Yang, C.J.; Chang, T.C. Synthesis and characterization of polydimethylsiloxane-cured organically modified silicate hybrid coatings. Polym. Degrad. Stab. 2006, 91, 2917–2923. [Google Scholar] [CrossRef]
- Li, G.; Kanezashi, M.; Tsuru, T. Preparation of organic–inorganic hybrid silica membranes using organoalkoxysilanes: The effect of pendant groups. J. Membr. Sci. 2011, 379, 287–295. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).