Preparation of Gradient Materials with Molten Salts Electrodeposition
Abstract
1. Introduction
2. Materials and Methods
2.1. The Preparation of Cu–Ni Gradient Materials
2.2. The Ni-W Gradient Layer Material Preparation
2.3. Preparation of Cu–Ni–W Gradient Layer Materials
3. Results
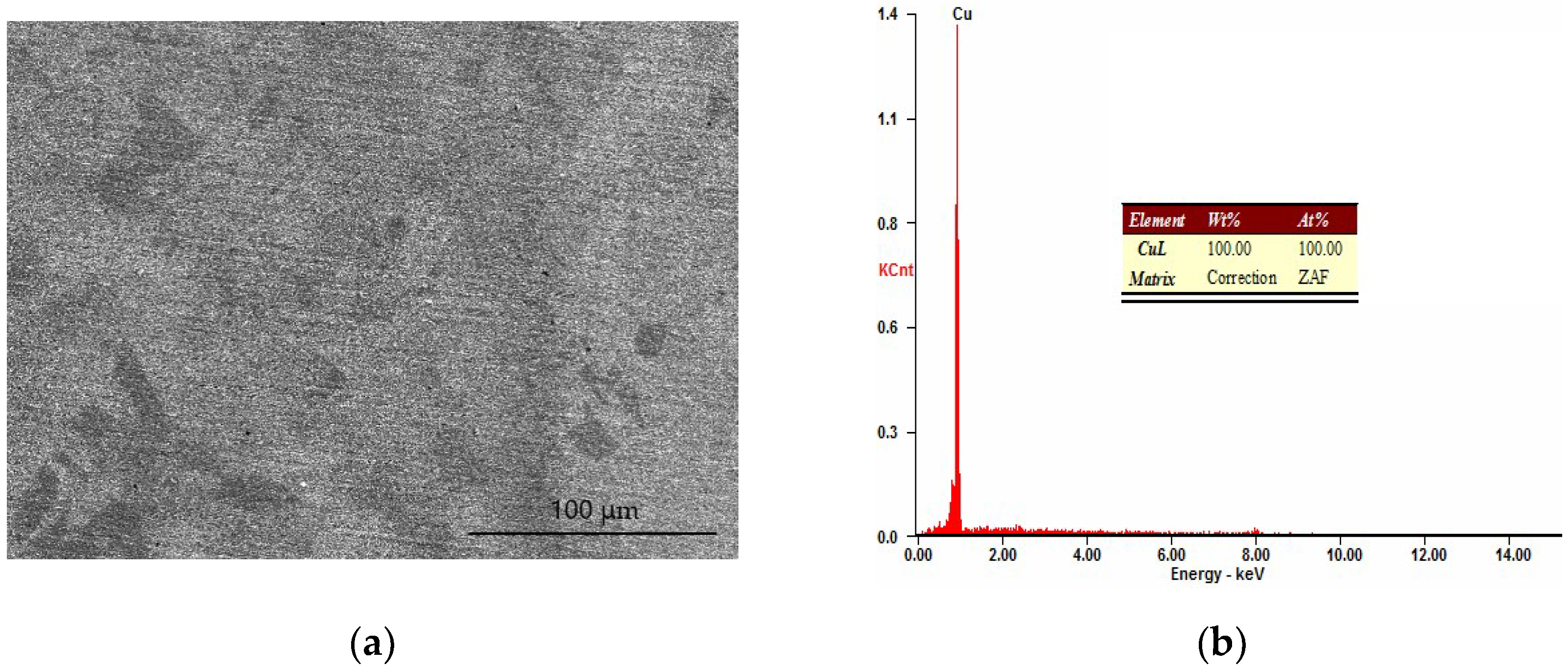
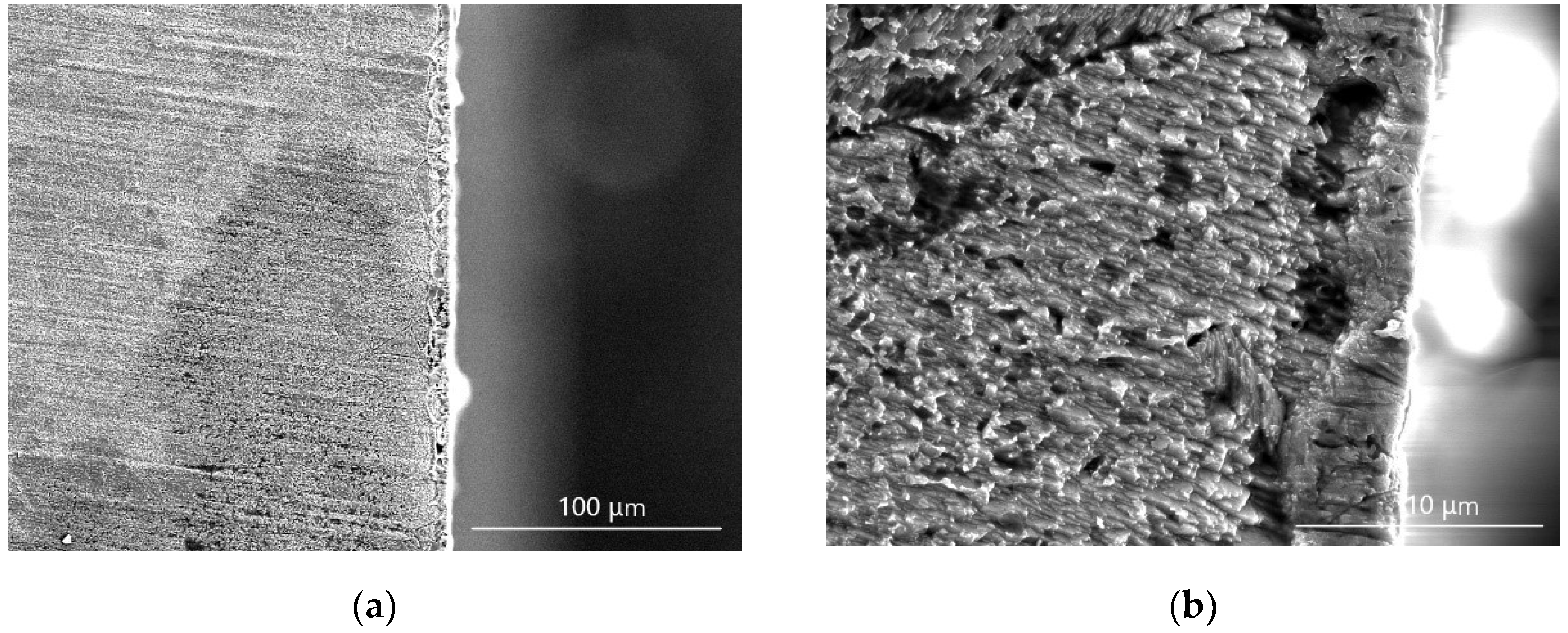
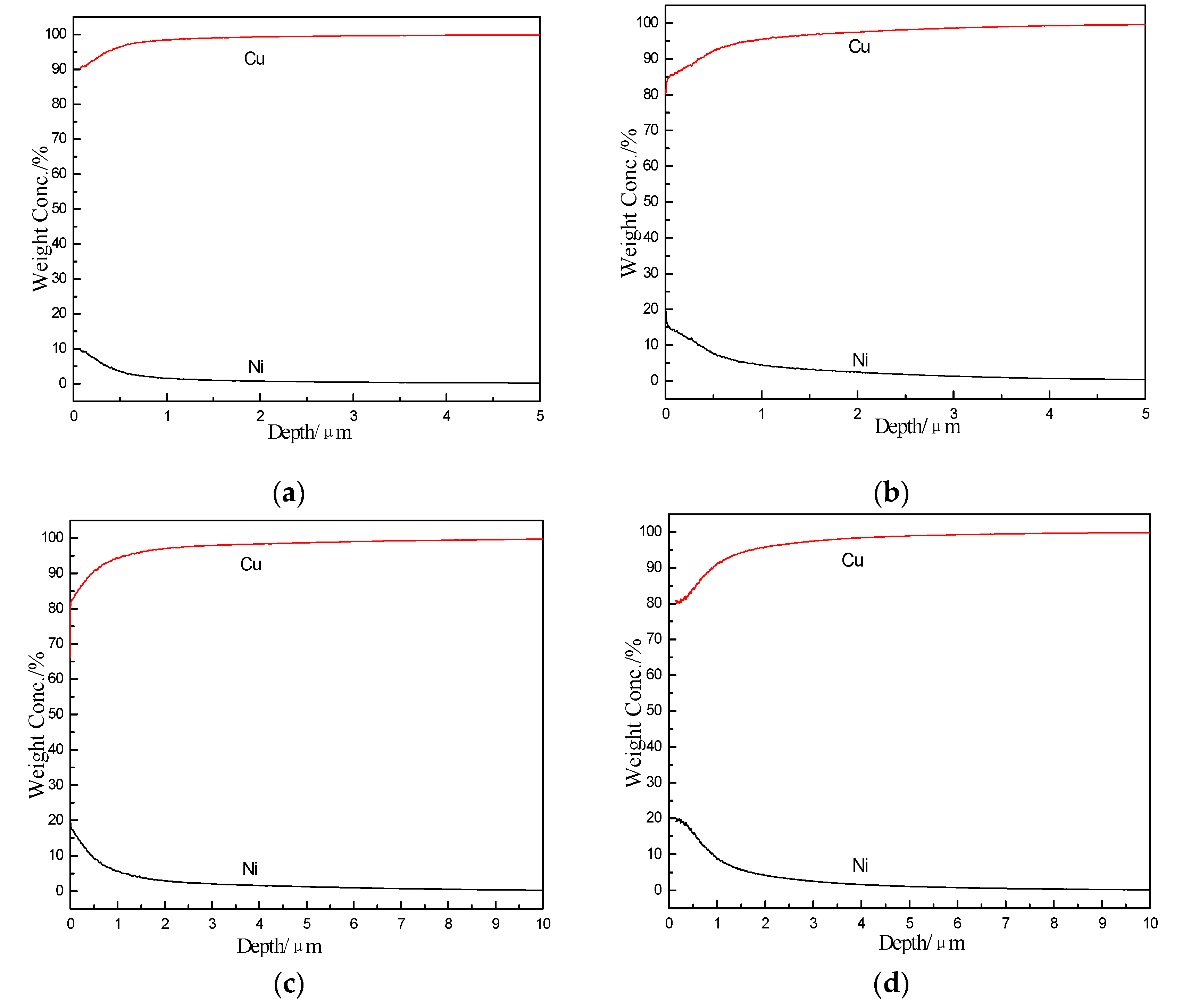
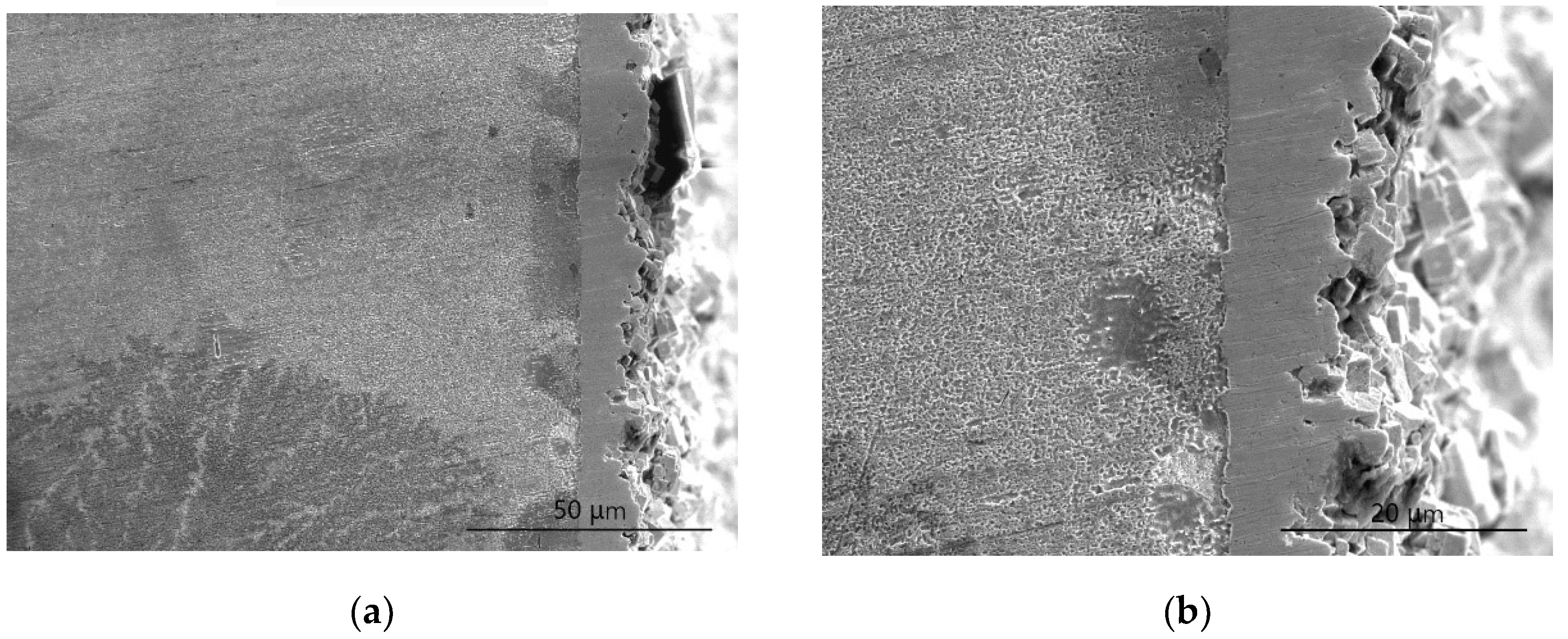
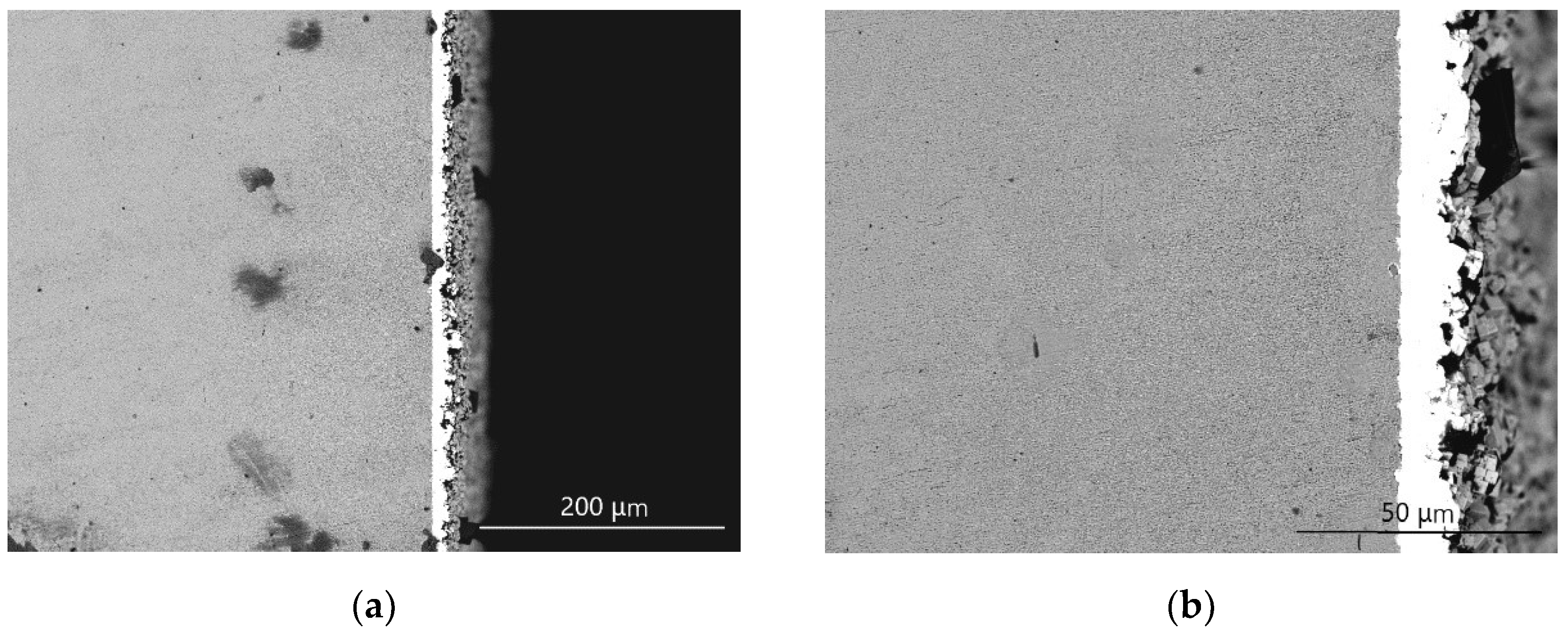
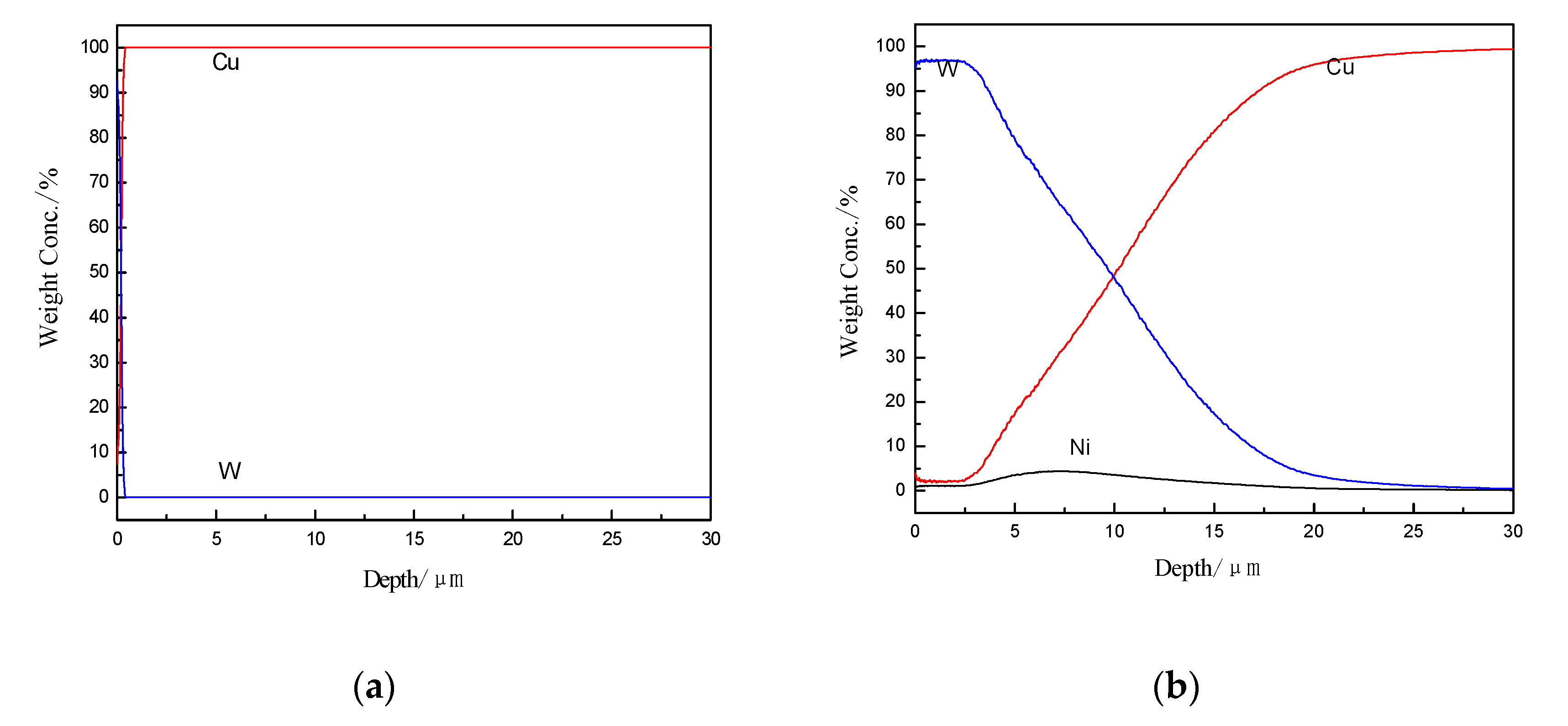
3.1. Analysis of Cu–Ni Gradient Layer Formation Mechanism
3.2. The Feature of Ni-W Gradient Layer
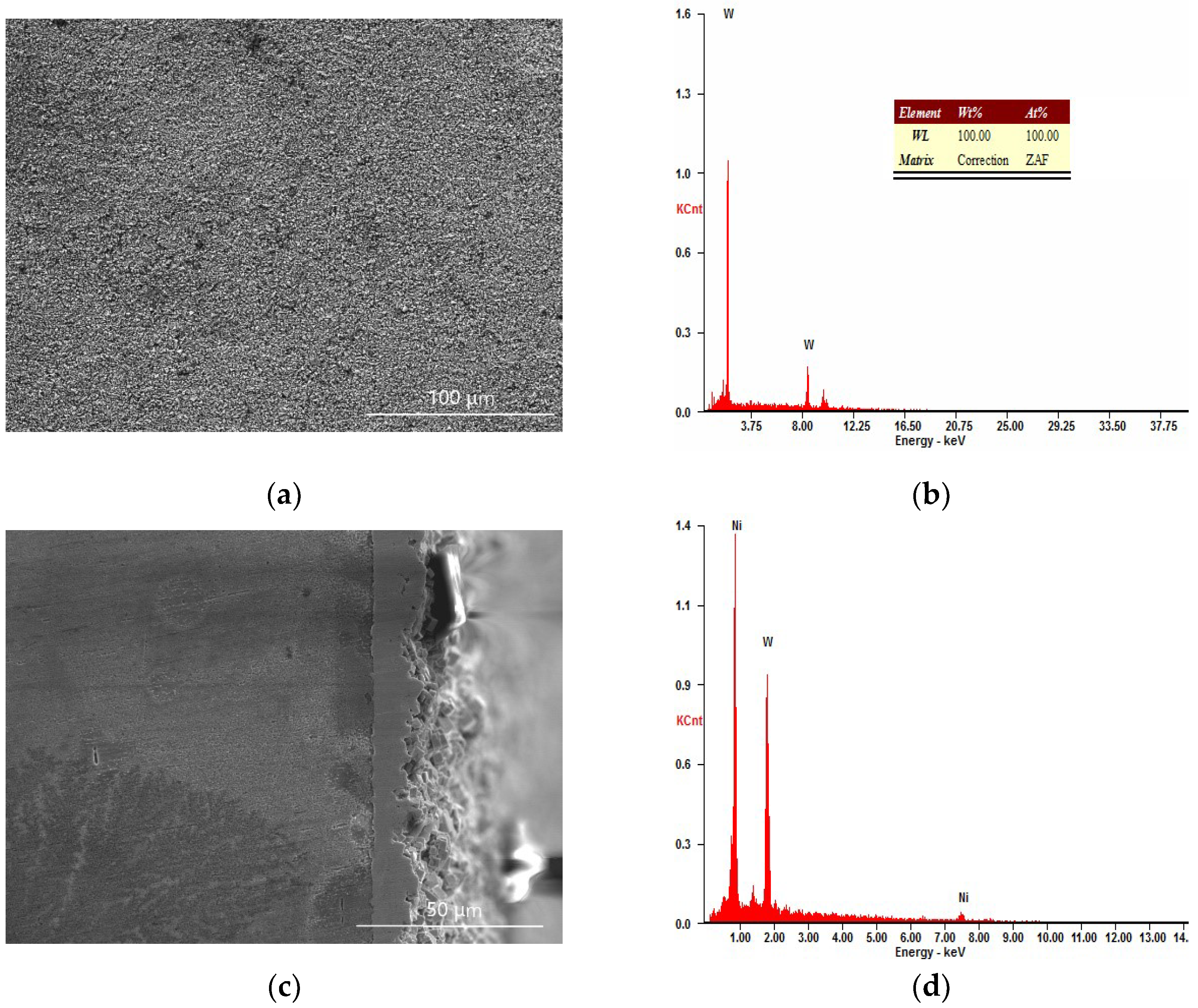
3.2.1. The Analysis of the Constituents
3.2.2. The Analysis of Sample Cross-section
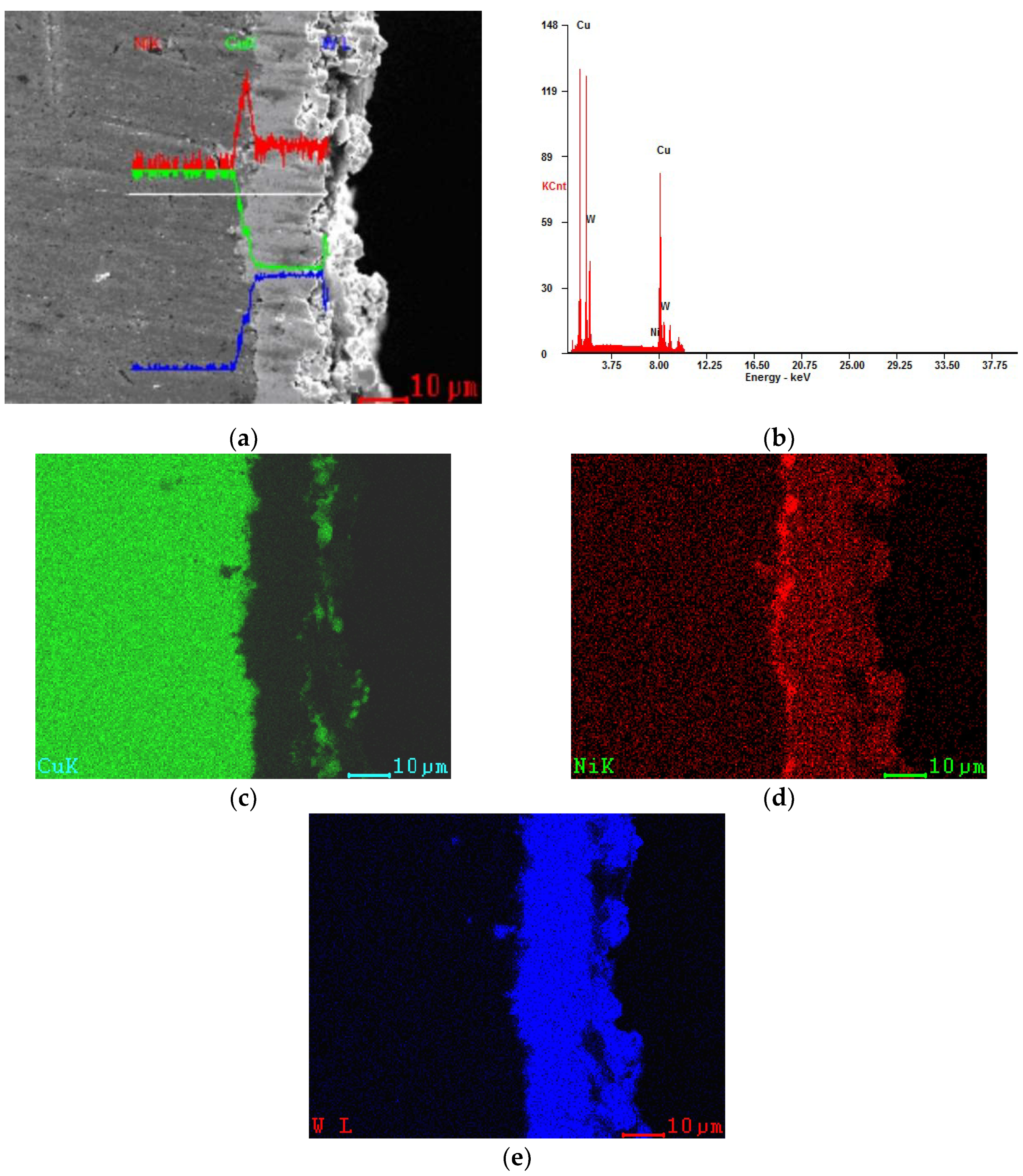
3.3. The Characteristics of Cu–Ni–W Gradient Layer
3.4. The Role of Ni to Cu–W Gradient Layer’s Formation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, C.C.; Zhou, Z.; Ling, Y.H. New progress of metal-based functionally graded plasma-facing materials in China. Mater. Sci. Forum 2003, 423, 11–16. [Google Scholar] [CrossRef]
- Han, J.C.; Xu, L.; Wang, B.L.; Zhang, X.H. Progress and prospects of functional gradient materials. J. Solid Rocket Technol. 2004, 27, 207–215. [Google Scholar]
- Ling, Y.; Bai, X.; Zhou, Z.; Li, J.; Ge, C. Thermal shock behavior of W/Cu functionally gradient material. Rare Met. Mater. Eng. 2004, 33, 819–822. [Google Scholar]
- You, J.H.; Bolt, H. Analytical method for thermal stress analysis of plasma facing materials. J. Nucl. Mater. 2001, 299, 9–19. [Google Scholar] [CrossRef]
- Chapa, J.; Reimanis, I. Modeling of thermal stresses of a graded Cu/W joint. J. Nucl. Mater. 2002, 303, 131–136. [Google Scholar] [CrossRef]
- Senderoff, S.; Mellors, G. Coherent coatings of refractory metal. Science 1966, 153, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Takenishi, H.; Katagiri, A. Electrodeposition of tungsten and related voltammetric study in a basic ZnCl2-NaCl (40–60 mol %) melt. J. Electrochem. Soc. 2001, 148, C59. [Google Scholar] [CrossRef]
- Pavlovskii, V.A. Tungsten galvanic coatings deposited from salt melts. Prot. Met. 2006, 42, 170–173. [Google Scholar] [CrossRef]
- Pavlovskii, V.A. Density and electrical conductivity of NaF–NaCl–WO3 melts. Inorg. Mater. 2003, 39, 1208–1211. [Google Scholar] [CrossRef]
- Koyama, K.; Morishita, M.; Umezu, T. Design of molten salt bath on the basis of acid-base cooperative reaction mechanism. Smooth electrodeposition of tungsten from KF-B2O3-WO3 molten salt. Electrochemistry 1999, 67, 677–683. [Google Scholar] [CrossRef]
- Nitta, K.; Nohira, T.; Hagiwara, R.; Majima, M.; Inazawa, S. Characteristics of a tungsten film electrodeposited in a KF–B2O3–WO3 melt and preparation of W–Cu–W three-layered films for heat sink application. J. Appl. Electrochem. 2010, 40, 1443–1448. [Google Scholar] [CrossRef]
- Boire-Lavigne, S.; Moreau, C.; Saint-Jacques, R. Relationship between the microstructure and thermal diffusivity of plasma-sprayed tungsten coatings. J. Therm. Spray Technol. 1995, 4, 261–267. [Google Scholar] [CrossRef]
- Dhote, A.; Ogale, S. Deposition of tungsten films by pulsed excimer laser ablation technique. Appl. Phys. Lett. 1994, 64, 2809–2811. [Google Scholar] [CrossRef]
- Varacalle, D.J., Jr.; Lundberg, L.; Jacox, M.; Hartenstine, J.; Ii, W.; Herman, H.; Bancke, G. Fabrication of tungsten coatings and monoliths using the vacuum plasma spray process. Surf. Coat. Technol. 1993, 61, 79–85. [Google Scholar]
- Philipps, V. Tungsten as material for plasma-facing components in fusion devices. J. Nucl. Mater. 2011, 415, 2–9. [Google Scholar] [CrossRef]
- Maier, H.; Kötterl, S.; Krieger, K.; Neu, R.; Balden, M. Performance of tungsten coatings as plasma facing components used in ASDEX Upgrade. J. Nucl. Mater. 1998, 258, 921–926. [Google Scholar] [CrossRef]
- Fan, J.L.; Yan, D.J.; Huang, B.Y. The current research status of W–Cu composite material at home and abroad. Powder Metal. Ind. 2003, 13, 9–14. [Google Scholar]
- Riccardi, B.; Montanari, R.; Casadei, M.; Costanza, G.; Chioni, G. Optimisation and characterisation of tungsten thick coatings on copper based alloy substrates. J. Nucl. Mater. 2006, 352, 29–35. [Google Scholar] [CrossRef]
- Liu, X.; Yang, L.; Tamura, S.; Tokunaga, K.; Yoshida, N.; Noda, N.; Xu, Z. Thermal response of plasma sprayed tungsten coating to high heat flux. Fusion Eng. Des. 2004, 70, 341–349. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Liu, Y.; Xu, L.; Jiang, F. Research development of electrodeposited tungsten and tungsten alloys coating. Int. Mater. Rev. 2012, 26, 142–146. [Google Scholar]
- Wang, X.Y.; Yang, H.L.; Feng, C.; Wang, Y.L.; Li, Y.G. Relationship between primary crystallization temperature of NaCl-KCl-NaF molten salt system and composition. Nonferr. Met. 2015, 5, 62–65. [Google Scholar]
- Zhao, P.; Xie, F.Z.; Sun, W.S. Materials Science; Harbin Institute of Technology Press: Harbin, China, 1999; pp. 163–164. [Google Scholar]
- Cui, M.; Zhao, P. The Effect of cold plastic deformation on nitrogen in the deffusion activation energy of 38CrMoAlA steel. J. Met. Heat Treat. 1994, 15, 48–54. [Google Scholar]


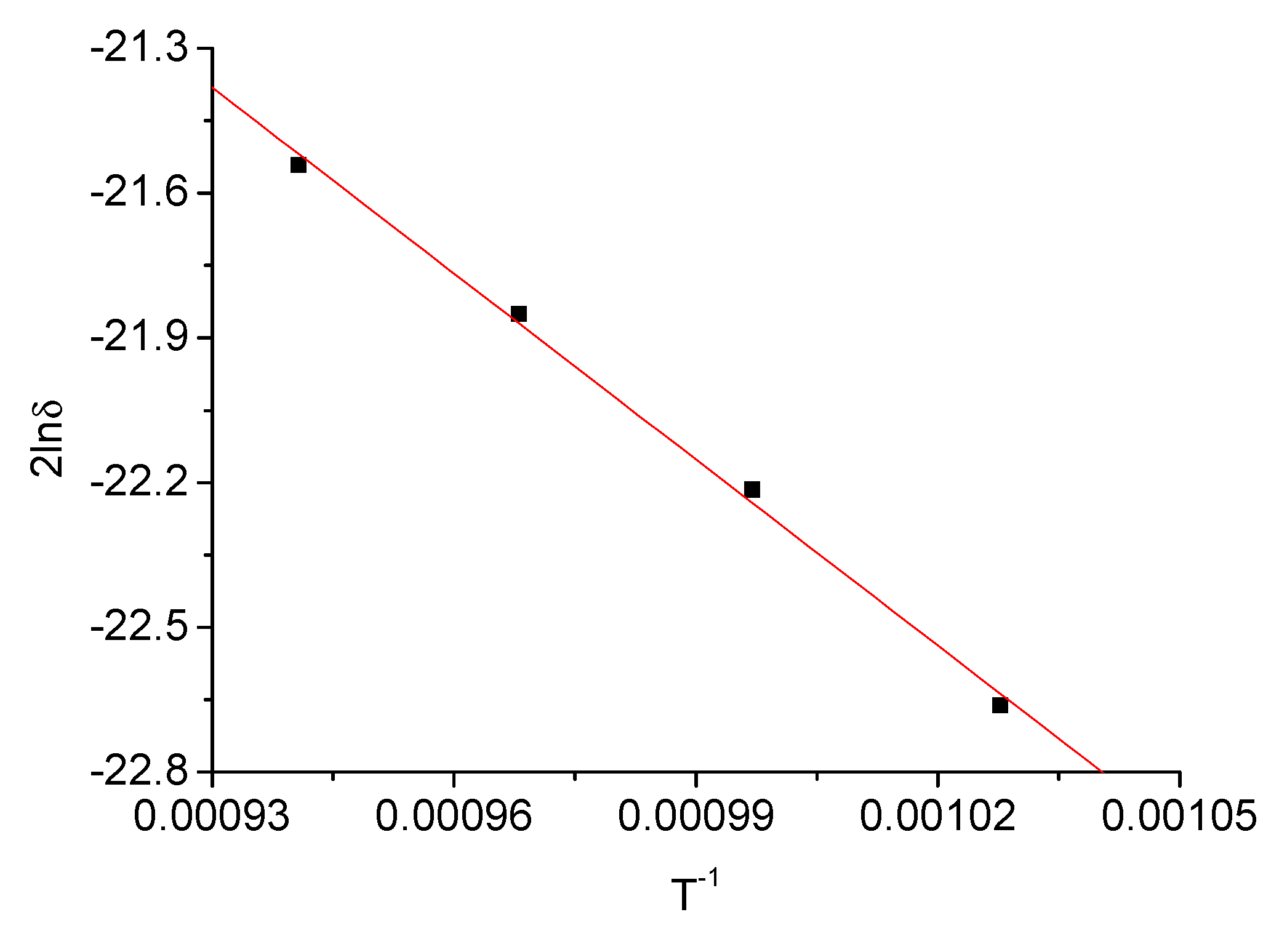
| Deposition Temperature T/K | 973 | 1003 | 1033 | 1063 |
| Thickness of Cu–Ni–W layer δ/μm | 12 | 15 | 18 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liang, J.; Yan, H.; Li, Y.; Wang, L. Preparation of Gradient Materials with Molten Salts Electrodeposition. Crystals 2021, 11, 590. https://doi.org/10.3390/cryst11060590
Li H, Liang J, Yan H, Li Y, Wang L. Preparation of Gradient Materials with Molten Salts Electrodeposition. Crystals. 2021; 11(6):590. https://doi.org/10.3390/cryst11060590
Chicago/Turabian StyleLi, Hui, Jinglong Liang, Hongyan Yan, Yungang Li, and Le Wang. 2021. "Preparation of Gradient Materials with Molten Salts Electrodeposition" Crystals 11, no. 6: 590. https://doi.org/10.3390/cryst11060590
APA StyleLi, H., Liang, J., Yan, H., Li, Y., & Wang, L. (2021). Preparation of Gradient Materials with Molten Salts Electrodeposition. Crystals, 11(6), 590. https://doi.org/10.3390/cryst11060590





