Abstract
A photomicroscopic growth apparatus was used to study the growth rates of calcium oxalate crystals in a new synthetic urine without inhibitors and with various inhibitors, including magnesium ions, citrate ions, chondroitin sulfate ions, and phytate ions. The dependence of growth rates on supersaturation at different temperatures without inhibitors was investigated using a power law model in terms of the Arrhenius form. The effects of various inhibitors on the growth rates of calcium oxalate indicated that the inhibition of growth rates increases in the order magnesium ions < citrate ions < chondroitin sulfate ions < phytate ions. The polymorphic forms of calcium oxalate crystals without inhibitors and with various inhibitors were examined by scanning electron microscopy.
1. Introduction
Renal stone disease is a common pathological disorder which affects about 10% of the global population [1]. Among all types of renal stones, calcium oxalate (CaOx) is the most common composition found in clinical stone formation [2]. Calcium oxalate crystallization yields different hydrates, including the thermodynamically most stable calcium oxalate monohydrate (COM) [3], the metastable calcium oxalate dihydrate (COD) [4] and the unstable calcium oxalate trihydrate (COT) [5]. As COM has the strongest affinity for renal tubule cell membranes among the three hydrates, COM easily forms urinary stones; consequently, it is more difficult to eject out in urine than COT or COD [6].
The ease of calcium oxalate stone formation in urine depends on the degree of supersaturation, crystallization promoters, and crystallization inhibitors. The degree of supersaturation is generally dependent on the total concentrations of calcium ion and oxalate ion in the urinary solutions, respectively. The presence of preformed particles can act as heterogeneous nucleants and promote the stone formation. Many inorganic and organic substances, such as magnesium, citrate, hydroxycitrate, chondroitin sulfate and phytate, have been reported to inhibit renal stone formation. Deficiency of inhibitors in the urine can facilitate the stone formation [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. Some studies indicated that different amino acids can both inhibit and promote the nucleation and crystallization of calcium oxalate depending on the acid structure [27,28].
As it is difficult to perform urolithiasis research using real human urine, a number of protocols have been adopted for preparing various synthetic urines for the study of the renal stone formation [16,22,29]. Due to the wide variety of synthetic urines adopted in the test systems, understanding the relative importance of various inhibitors in the renal stone formation is a challenging task. Hsu et al. [30] have recently studied the effects of various inhibitors on the nucleation of calcium oxalate crystals using a new synthetic urine to more simulate the human urine. The objective of this work is to study the growth kinetics of calcium oxalate crystals in the synthetic urine adopted by Hsu et al. [30] using a photomicroscopic growth apparatus. The growth rates of calcium oxalate crystals were compared between without inhibitors and with inhibitors, including magnesium ions, citrate ions, chondroitin sulfate ions, and phytate ions. The polymorphic forms of calcium oxalate crystals without inhibitors and with inhibitors are examined by scanning electron microscopy (SEM).
2. Experiments
The photomicroscopic experiments were performed to investigate the growth rates of calcium oxalate using the synthetic urine. A schematic diagram of this photomicroscopic growth cell with a description of features is shown in Figure 1 [31]. The growth cell has a solution chamber of in the upper part and a chamber for temperature-controlled water in the lower part. The flowing water in the lower chamber was used to control the temperature of stagnant solution in the upper chamber. The experiments were conducted isothermally. The photomicroscopic growth cell was placed on the stage of an optical microscope for observing the growth of nuclei in the supersaturated solution. Chemicals of analytical reagent grade purity were dissolved in the deionized water to prepare the desired synthetic urine solutions. The solutions were filtered through 0.45 µm pore filters before use.

Figure 1.
The photomicroscopic growth apparatus. (a) The real picture of growth cell; (b) schematic diagram of growth cell with the features: (1) solution chamber; (2) thermistor; (3) solution inlet and outlet; (4) constant-temperature water chamber; (5) water inlet and outlet.
As listed in Table 1, the synthetic urine proposed for the nucleation study by Hsu et al. [30] was adopted in this work. The unique feature of this synthetic urine is that urea, uric acid and creatinine within the normal physiological ranges suggested by Chutipongtanate and Thongboonkerd [29] were added to the commonly used synthetic urine proposed by Robertson and Scurr [16] and Grases et al. [22] to simulate human urine more closely. At the beginning of the experiments, equal volumes of solution 1 and solution 2 were mixed into the growth cell for the current study. The mixed synthetic urine solution was kept at pH = 6.5 during the growth measurements, which is close to the pH value of human urine and within the physiological range for human urine. The urine pH of normal men is , while for women [32]. Fresh solutions were prepared for each experiment.

Table 1.
The initial concentrations of all the components in solution 1 and solution 2, respectively, before mixing for the synthetic urine adopted in this study.
Finlayson [33] proposed the following relation to calculate the supersaturation with respect to calcium oxalate in the urinary solutions based on the urinary ion equilibrium:
where and represent the total concentration of calcium ions and oxalate ions in the urinary solutions, respectively. As listed in Table 1, as was kept the same at 2.50 mM in the mixed synthetic urine solution, varied with . For example, leads to , while leads to . For the synthetic urine in Table 1, sodium phosphate monobasic dihydrate and sodium phosphate dibasic dodecahydrate were added to control the urine solution at pH = 6.5. Consequently, as the concentration of phosphate ions was very low at pH = 6.5, the formation of calcium phosphate was eliminated. Robertson and Scurr [16] confirmed the formation of calcium oxalate crystals by X-ray diffraction analysis using the similar synthetic urine.
In preparation of the synthetic urine, various inhibitors were added to solution 2 before mixing. For the final solutions formed after mixing, the inhibitor concentration, , for magnesium sulfate heptahydrate ranged from 100 ppm to 800 ppm, for trisodium citrate dihydrate ranged from 100 ppm to 600 ppm, for sodium chondroitin sulfate ranged from 10 ppm to 30 ppm, and for potassium phytate ranged from 0.5 ppm to 1.5 ppm. The ranges of for various inhibitors were chosen based on the related inhibition studies in the literature [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. Note that 1 ppm = 1 mg/L = 10−3 kg/m3.
As equal volumes of solution 1 and solution 2 were mixed into the growth cell, the supersaturated mixed solution started to nucleate. The growth of nuclei settled on the bottom of the upper chamber in Figure 1 was monitored photographically through the optical microscope and analyzed by the image analyzer to determine the area of each crystal. As some crystals exhibited irregular shapes, the characteristic size of the small crystal was taken for simplicity as the equivalent circular diameter, i.e., , leading to . The sizes were then plotted against time for each crystal with the slope equal to the growth rate and the intercept equal to the initial size. Since different faces of a crystal may grow at different rates, the obtained growth rate is considered as the characteristic growth rate of crystal.
To examine the polymorphic forms of calcium oxalate crystals, crystals were prepared by mixing 100 mL of solution 1 and 100 mL of solution 2 into a 500 mL crystallizer immersed in a constant temperature water bath. The crystallizer was equipped with a magnetic stirrer with a constant stirring rate of 350 rpm [30]. The crystals were removed and filtered after 120 min. The final dried crystals were examined using SEM (Hitachi, SU8220, Tokyo, Japan).
Sodium sulfate decahydrate (Na2SO4·10H2O, purity 99%), calcium chloride anhydrous (CaCl2, purity 96%), urea (CH4N2O, purity 99%), uric acid (C5H4N4O3, purity > 99%), creatinine (C4H7N3O, purity > 99%), sodium oxalate (Na2C2O4, purity > 95%) and sodium chondroitin sulfate (C14H22NNaO15S, purity > 95%) were purchased from Acros. Ammonium chloride (NH4Cl, purity 99%), potassium chloride (KCl, purity 99.5%), sodium chloride (NaCl, purity 99.5%), trisodium citrate dihydrate (Na3C6H5O7·2H2O, purity 99%) and magnesium sulfate heptahydrate (MgSO4·7H2O, purity 100%) were purchased from Showa. Sodium phosphate monobasic dihydrate (NaH2PO4·2H2O, purity 100%) and sodium phosphate dibasic dodecahydrate (Na2HPO4·12H2O, purity > 98%) were purchased from Aencore. Potassium phytate (C6H16O24P6K2, purity > 95%) was purchased from Sigma (St. Louis, MO, USA).
3. Results and Discussion
Figure 2 shows sizes plotted against time for eight individual calcium oxalate crystals obtained from the photomicroscopic growth cell for at without inhibitors. Figure 3 shows sizes plotted against time for eight individual calcium oxalate crystals for at with 800 ppm magnesium ions. Similar plots are obtained for other experimental conditions. It is evident in these figures that a linear relationship exists between the size and time for each crystal. Note that the slope of each line is equal to the growth rate for each individual crystal. As an individual crystal has an inherent, constant growth rate, but different crystals have different inherent growth rates at each condition, the phenomenon of growth rate dispersion is observed [31,34,35]. In each run 8–10 crystals, which would not agglomerate with neighboring nuclei in the growth period of the experiment, were randomly selected to calculate the mean growth rate among these crystals. As different crystals might nucleate at different times in each run, the small variations of initial sizes are observed. Each experiment was generally finished within 30 min. The measured growth rate data of calcium oxalate crystals without inhibitors for various from to are listed in Tables S1–S3 (see Supplementary Materials). The measured growth rate data of calcium oxalate crystals with various inhibitors for at are listed in Tables S4–S7 (see Supplementary Materials).
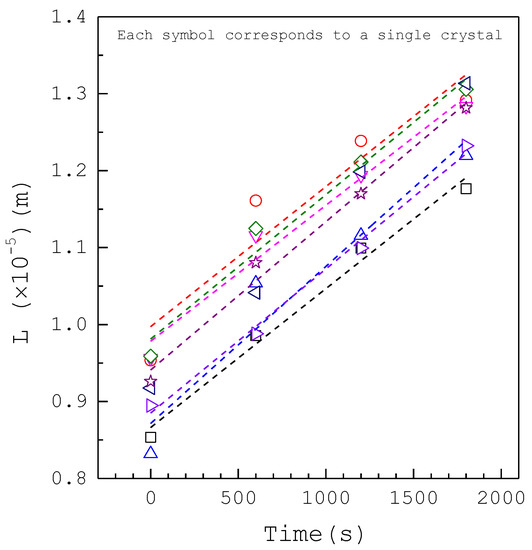
Figure 2.
Sizes versus time plots for eight individual crystals for at without inhibitors. L represents circular diameter of a crystal. Each line represents the linear regression for a single crystal.
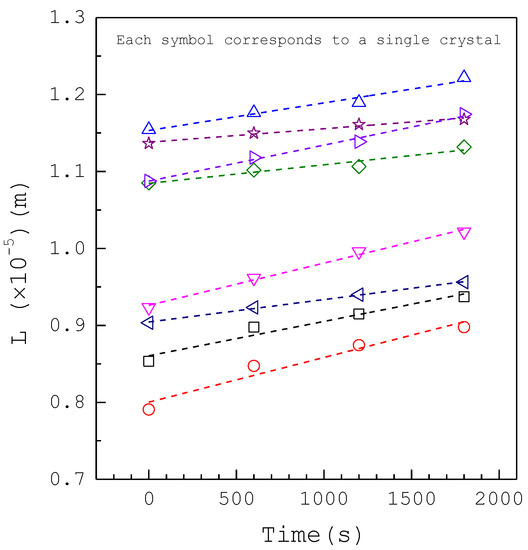
Figure 3.
Sizes versus time plots for eight individual crystals for at with 800 ppm magnesium. L represents circular diameter of a crystal. Each line represents the linear regression for a single crystal.
Figure 4 shows the mean growth rate data of calcium oxalate crystals without inhibitors for various from to , where the error bar represents the 95% confidence interval for the growth rate. For example, the mean growth rate increases from to as increases from 10.09 to 18.01 at . As the growth rate is often expressed in terms of supersaturation by a power law model and the temperature dependence of the kinetics model can be correlated in the Arrhenius form [36], the growth rate can be written as:
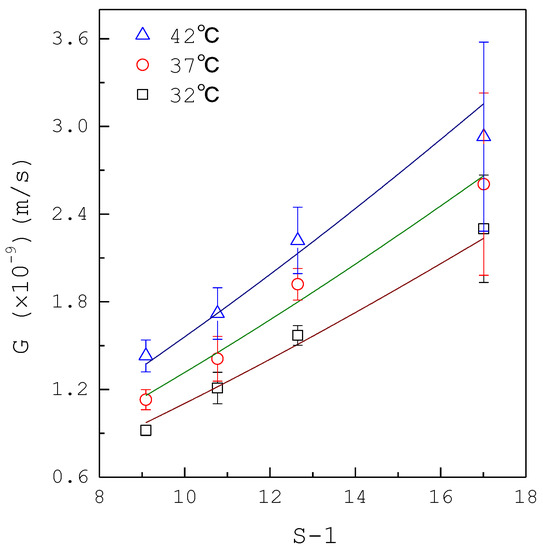
Figure 4.
The growth rate data of calcium oxalate crystals without inhibitors. Each point represents the mean growth rate for eight crystals and the error bar represents the 95% confidence interval for the growth rate. Lines correspond to the best-fit regression of Equation (2).
The growth rate data in Figure 4 were fitted to Equation (2), leading to , and with correlation coefficients . The growth rate order falls in the range 1 to 2 for many inorganic salts crystallized from aqueous solutions [36]. As the activation energy is usually for diffusion and for surface integration [36], it is speculated that the growth rates of calcium oxalate crystals in the synthetic urine without inhibitors are influenced by both diffusion and surface integration.
Figure 5 shows that the mean growth rate decreases with increasing inhibitor concentration for magnesium ions in the range or for citrate ions in the range for at , where the error bar represents the 95% confidence interval for the growth rate. The presence of magnesium ions or citrate ions leads to a gradual decrease of growth rate while the inhibition for citrate ions is slightly stronger than that for magnesium ions. Figure 6 shows that the mean growth rate decreases with increasing inhibitor concentration for chondroitin sulfate ions in the range or phytate ions in the range for at , where the error bar represents the 95% confidence interval for the growth rate. The presence of chondroitin sulfate ions or phytate ions leads to a rapid decrease of growth rate, while the inhibition for phytate ions is much stronger than that for chondroitin sulfate ions.
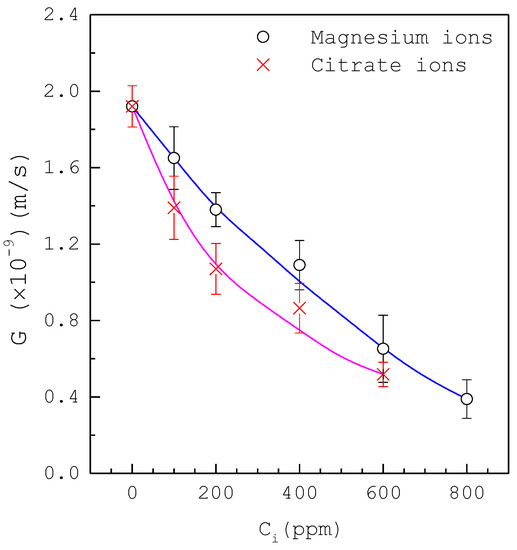
Figure 5.
The decrease of the growth rate with increasing inhibitor concentration of magnesium ions or citrate ions for at . Each point represents the mean growth rate for eight crystals and the error bar represents the 95% confidence interval for the growth rate. Lines are visual guides.
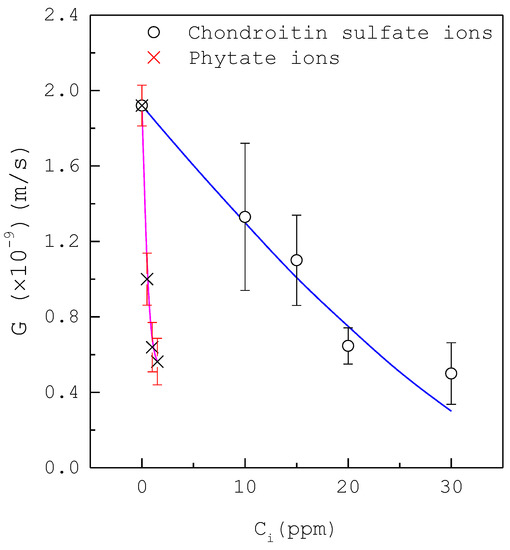
Figure 6.
The decrease of the growth rate with increasing inhibitor concentration of chondroitin sulfate ions or phytate ions for at . Each point represents the mean growth rate for eight crystals and the error bar represents the 95% confidence interval for the growth rate. Lines are visual guides.
As compared in Figure 5 and Figure 6, the inhibition on the mean growth rate increases in the order magnesium ions < citrate ions < chondroitin sulfate ions < phytate ions. For example, the mean growth rate without inhibitors corresponds to for at ; the presence of 400 ppm magnesium ions or 200 ppm citrate ions can reduce the mean growth rate by half compared to the presence of 15 ppm chondroitin sulfate ions or 0.5 ppm phytate ions.
Figure 7 displays the SEM images of calcium oxalate crystals obtained from the 500 mL crystallizer for at . As compared to the morphologies of the different hydrates for calcium oxalate crystals [37], prismatic monohydrate COM crystals are formed without inhibitors (Figure 7a) and in the presence of 400 ppm magnesium ions (Figure 7b) or 200 ppm citrate ions (Figure 7c). The presence of 15 ppm chondroitin sulfate ions (Figure 7d) or 0.5 ppm phytate ions (Figure 7e) can induce the formation of tetragonal dipyramidal dihydrate COD crystals. The SEM results from this study are consistent with those obtained by Grases et al. [22] for at , where COM crystals formed without inhibitors and in the presence of 100 ppm magnesium ions or 600 ppm citrate ions while COD crystals formed in the presence of 1 ppm phytate ions. As COM more easily forms urinary stones than COD, phytate ions and chondroitin sulfate ions can be used to inhibit the formation of COM crystals.
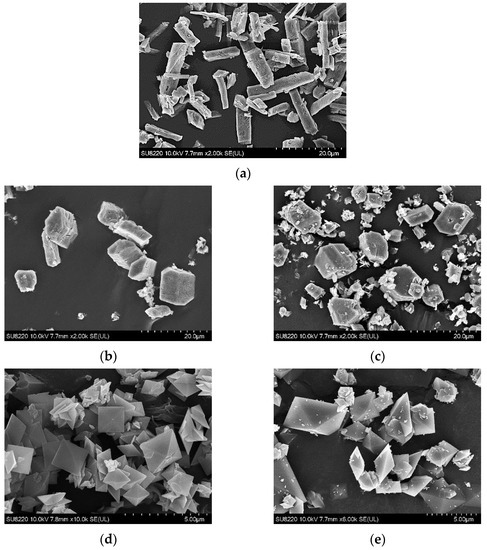
Figure 7.
The images of calcium oxalate crystals obtained after 2-h incubation in a crystallizer under various conditions using SEM: (a) COM without inhibitors; (b) COM in the presence of 400 ppm magnesium ions; (c) COM in the presence of 200 ppm citrate ions; (d) COD in the presence of 15 ppm chondroitin sulfate ions; (e) COD in the presence of 0.5 ppm phytate ions (COM = calcium oxalate monohydrate; COD = calcium oxalate dihydrate; SEM = scanning electron microscopy).
As shown in Figure 7, the crystal morphologies of calcium oxalate crystals are varied due to the presence of different inhibitors. However, SEM is not sufficient for an unambiguous particular phase determination between COM and COD. A phase analysis is needed for the determination of different hydrates in each case. Furthermore, in acid urine, most phosphate is present as the divalent ion hydrogen diphosphate, which is soluble, and phosphate does not precipitate. As urine becomes more alkaline and the pH rises, an increasing proportion of phosphate is present as the monovalent ion hydrogen phosphate, which is much less soluble. As the mixed synthetic urine solution was kept at pH = 6.5 during the growth measurements, the calcium oxalate crystals might contain a small component of calcium hydrogen phosphate and calcium dihydrogen phosphate.
Magnesium ions have been known to act as a competitor of calcium ions in binding with oxalate ions [12,14]. Similarly, citrate ions [19,21] or chondroitin sulfate ions [18,24] have been reported to complex with calcium ions, leading to a reduction of calcium ions in binding with oxalate ions. Small amounts of phytate ions (e.g., 1 ppm) have been indicted to greatly inhibit nucleation and crystal growth of calcium oxalate [20,22,26]. It is speculated in the current study that chondroitin sulfate ions or phytate ions are adsorbed into the calcium oxalate crystal lattice and, subsequently, alter the crystal morphology of calcium oxalate from COM to COD. Thus, small amounts of phytate ions or chondroitin sulfate ions can greatly inhibit the growth rate of calcium oxalate. On the other hand, it is speculated that magnesium ions or citrate ions are not adsorbed into the calcium oxalate crystal lattice, and subsequently, the crystal morphology of COM is not altered. As magnesium ions complex with oxalate ions and citrate ions complex with calcium ions, the presence of magnesium ions or citrate ions leads to a slower growth rate due to a lower supersaturation.
4. Conclusions
The growth rates of calcium oxalate crystals in the new synthetic urine without inhibitors for the supersaturation of 10.09 to 18.01 in the range to were determined from the photomicroscopic growth experiments. The results were fitted to a power law model in terms of the Arrhenius relation, leading to a growth rate of 1.33 and a growth activation energy of . The effects of various inhibitors on the mean growth rates of calcium oxalate crystals were compared for the supersaturation of 13.65 at . The results indicated that the mean growth rate without inhibitors corresponds to for at . The presence of 400 ppm magnesium ions or 200 ppm citrate ions can reduce the mean growth rate by half compared to the presence of 15 ppm chondroitin sulfate ions or 0.5 ppm phytate ions. Thus, the inhibition on growth rates increases in the order magnesium ions < citrate ions < chondroitin sulfate ions < phytate ions. The SEM images of calcium oxalate crystals for the supersaturation of 13.65 at showed that prismatic monohydrate COM crystals are formed in the new synthetic urine without inhibitors and in the presence of 400 ppm magnesium ions or 200 ppm citrate ions. The presence of 15 ppm chondroitin sulfate ions or 0.5 ppm phytate ions can induce the formation of tetragonal dipyramidal dihydrate COD crystals. Consequently, as COM more easily forms urinary stones than COD, the presence of phytate ions or chondroitin sulfate ions can induce the formation of COD and significantly reduce the growth rate of calcium oxalate crystals.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/11/3/223/s1, Table S1: The measured growth rate data of calcium oxalate crystals without inhibitors for various at , Table S2: The measured growth rate data of calcium oxalate crystals without inhibitors for various at , Table S3. The measured growth rate data of calcium oxalate crystals without inhibitors for various at , Table S4. The measured growth rate data of calcium oxalate crystals with magnesium ions for at , Table S5. The measured growth rate data of calcium oxalate crystals with citrate ions for at , Table S6. The measured growth rate data of calcium oxalate crystals with chondroitin sulfate ions for at , Table S7. The measured growth rate data of calcium oxalate crystals with phytate ions for at .
Author Contributions
Conceptualization, L.-D.S.; Data curation, Y.-C.H. and L.-C.P.; Formal analysis, Y.-C.H. and L.-C.P.; Investigation, Y.-C.H. and L.-C.P.; Methodology, L.-D.S.; Validation, Y.-C.H.; Writing—original draft, L.-D.S.; Writing—review & editing, L.-D.S. All authors have read and agreed to the published version of the manuscript.
Funding
Research was funded by Chang Gung Memorial Hospital (CMRPD2G0242) and the Ministry of Science and Technology of Taiwan (MOST108-2221-E-182-034).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Chang Gung Memorial Hospital (CMRPD2G0242) and the Ministry of Science and Technology of Taiwan (MOST108-2221-E-182-034) for financial support of this research.
Conflicts of Interest
The authors declare no conflict of interest.
Notation
| A | area |
| AG | growth kinetic parameter |
| Ci | impurity concentration |
| EG | growth activation energy |
| G | growth rate |
| g | growth rate order |
| L | size |
| R | ideal gas constant |
| S | supersaturation |
| T | temperature |
References
- Scales, C.D., Jr.; Smith, A.C.; Hanley, J.M.; Saigal, C.S. Prevalence of kidney stones in the United States. Eur. Urol. 2012, 62, 160–165. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Abram, V.; Coe, F.L. Isolation of calcium oxalate crystal growth inhibitor from rat kidney and urine. Am. J. Physiol. 1984, 247, 765–772. [Google Scholar] [CrossRef]
- Ogbuji, L.U.; Batich, C.D. Ultrastructure of whewellite kidney stones: Electron-analytical investigation. J. Ultrastruct Res. 1985, 90, 1–8. [Google Scholar] [CrossRef]
- Kaloustian, J.; El-Moselhy, T.F.; Portugal, T.F. Determination of calcium oxalate (mono-and dihydrate) in mixtures with magnesium ammonium phosphate or uric acid: The use of simultaneous thermal analysis in urinary calculi. Clin. Chim. Acta 2003, 334, 117–129. [Google Scholar] [CrossRef]
- Opalko, F.J.; Adair, J.H.; Khan, S.R. Heterogeneous nucleation of calcium oxalate trihydrate in artificial urine by constant composition. J. Cryst. Growth 1997, 181, 410–417. [Google Scholar] [CrossRef]
- Rabinovich, Y.I.; Esayanur, M.; Daosukho, S.; Byer, K.J.; El-Shall, H.E.; Khan, S.R. Adhesion force between calcium oxalate monohydrate crystal and kidney epithelial cells and possible relevance for kidney stone formation. J. Colloid Interface Sci. 2006, 300, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Coe, F.L. Acidic peptide and polyribonucleotide crystal growth inhibitors in human urine. Am. J. Physiol. 1977, 233, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Drach, G.W.; Randolph, A.D.; Miller, J.D. Inhibition of calcium oxalate dihydrate crystallization by chemical modifiers. I. Pyrophosphate and methylene blue. J. Urol. 1978, 119, 99–103. [Google Scholar] [CrossRef]
- Hallson, P.C.; Rose, G.A. Uromucoids and urinary stone formation. Lancet 1979, 1, 1000–1002. [Google Scholar] [CrossRef]
- Randolph, A.D.; Drach, G.W. Some measurements of calcium oxalate nucleation and growth rates in urine-like liquors. J. Cryst. Growth 1981, 53, 195–201. [Google Scholar] [CrossRef]
- Robertson, W.G.; Scurr, D.S. Factors influencing the crystallization of calcium oxalate-a critique. J. Cryst. Growth 1981, 53, 182–194. [Google Scholar] [CrossRef]
- Ryall, R.L.; Harnett, R.M.; Marshall, V.R. The effect of urine, pyrophosphate, citrate, magnesium and glycosaminoglycans on the growth and aggregation of calcium oxalate crystals in vitro. Clin. Chim. Acta 1981, 112, 349–356. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Abram, V.; Kezdy, F.J.; Kaiser, E.T.; Coe, F.L. Purification and characterization of the principal inhibitor of calcium oxalate crystal growth in human urine. J. Biol. Chem. 1983, 258, 12594–12600. [Google Scholar] [CrossRef]
- Li, M.K.; Blacklock, N.J.; Garside, J. Effects of magnesium on calcium oxalate crystallization. J. Urol. 1985, 133, 123–125. [Google Scholar] [CrossRef]
- Robertson, W.G.; Scurr, D.S.; Sergeant, V.J. Ionic and macromolecular modifiers of crystallization of calcium salts in urine. Fortschr. Urol. Nephrol. 1985, 23, 1–11. [Google Scholar]
- Robertson, W.G.; Scurr, D.S. Modifiers of calcium oxalate crystallization found in urine. I. Studies with a continuous crystallizer using an artificial urine. J. Urol. 1986, 86, 1322–1326. [Google Scholar] [CrossRef]
- Pak, C.Y. Citrate and renal calculi: New insights and future directions. Am. J. Kidney Dis. 1991, 17, 420–425. [Google Scholar] [CrossRef]
- Ryall, R.L. Urinary inhibitors of calcium oxalate crystallization and their potential role in stone formation. World J. Urol. 1997, 15, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Marangella, M.; Bagnis, C.; Bruno, M.; Vitale, C.; Petrarulo, M.; Ramello, A. Crystallization inhibitors in the pathophysiology and treatment of nephrolithiasis. Urol. Int. 2004, 72, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Isern, B.; Sanchis, P.; Perello, J.; Torres, J.J.; Costa-Bauza, A. Phytate acts as an inhibitor in formation of renal calculi. Front. Biosci. 2007, 12, 2580–2587. [Google Scholar] [CrossRef]
- Farmanesh, S.; Ramamoorthy, S.; Chung, J.; Asplin, J.R.; Karande, P.; Rimer, J.D. Specificity of Growth Inhibitors and their Cooperative Effects in Calcium Oxalate Monohydrate Crystallization. J. Am. Chem. Soc. 2013, 136, 367–376. [Google Scholar] [CrossRef]
- Grases, F.; Rodriguez, A.; Costa-Bauza, A. Efficacy of mixtures of magnesium, citrate and phytate as calcium oxalate crystallization inhibitors in urine. J. Urol. 2015, 194, 812–819. [Google Scholar] [CrossRef]
- Chung, J.; Granja, I.; Taylor, M.G.; Mpourmpakis, G.; Asplin, J.R.; Rimer, J.D. Molecular modifiers reveal a mechanism of pathological crystal growth inhibition. Nature 2016, 536, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.L.; Jackson, G.E. Determination of thermodynamic parameters for complexation of calcium and magnesium with chondroitin sulfate isomers using isothermal titration calorimetry: Implications for calcium kidney-stone research. J. Cryst. Growth 2017, 463, 14–18. [Google Scholar] [CrossRef]
- Kim, D.; Rimer, J.D.; Asplin, J.R. Hydroxycitrate: A potential new therapy for calcium urolithiasis. Urolithiasis 2019, 47, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Costa-Bauza, A. Key Aspects of Myo-Inositol Hexaphosphate (Phytate) and Pathological Calcifications. Molecules 2019, 24, 4434. [Google Scholar] [CrossRef] [PubMed]
- Izatulina, A.R.; Golovanova, O.A.; Punin, Y.O. Effect of Amino Acids, Magnesium Ions and Hydroxyapatite on the Formation of Oxalate Nephroliths. Chem. Sustain. Dev. 2008, 2, 163–167. [Google Scholar]
- Golovanova, O.A.; Punin, Y.O.; Izatulina, A.R.; Korol’kov, V.V. Crystallization of calcium oxalate monohydrate in the presence of amino acids: Features and regularities. J. Struct. Chem. 2014, 55, 1356–1370. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Thongboonkerd, V. Systematic comparisons of artificial urine formulas for in vitro cellular study. Anal. Biochem. 2010, 402, 110–112. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Lin, Y.H.; Shiau, L.D. Effects of various inhibitors on the nucleation of calcium oxalate in synthetic urine. Crystals 2020, 10, 333. [Google Scholar] [CrossRef]
- Shiau, L.D. The distribution of dislocation activities among crystals in sucrose crystallization. Chem. Eng. Sci. 2003, 58, 5299–53042. [Google Scholar] [CrossRef]
- Coe, F.; Parks, J.H. Defenses of an unstable compromise: Crystallization inhibitors and the kidney’s role in mineral regulation. Kidney Int. 1990, 38, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, B. Calcium stones: Some physical and clinical aspects, Chapter 10. In Calcium Metabolism in Renal Failure and Nephrolithiasis; David, D.S., Ed.; John Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Shiau, L.D.; Berglund, K.A. Growth kinetic of fructose crystals formed by contact nucleation. AIChE J. 1987, 33, 1028–1033. [Google Scholar] [CrossRef]
- Shiau, L.D.; Berglund, K.A. Growth rate dispersion in batch crystallization. AIChE J. 1990, 36, 1669–1678. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystallization; Butterworth-Heinemann: Oxford, UK, 1993. [Google Scholar]
- Yu, H.; Sheikholeslami, R.; Doherty, W.O.S. The effects of silica and sugar on the crystallographic and morphological properties of calcium oxalate. J. Cryst. Growth 2004, 265, 592–603. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).