Facile Synthesis of High-Quality Nano-Size 10B-Enriched Fibers of Hexagonal Boron Nitride
Abstract
1. Introduction
2. Materials and Methods
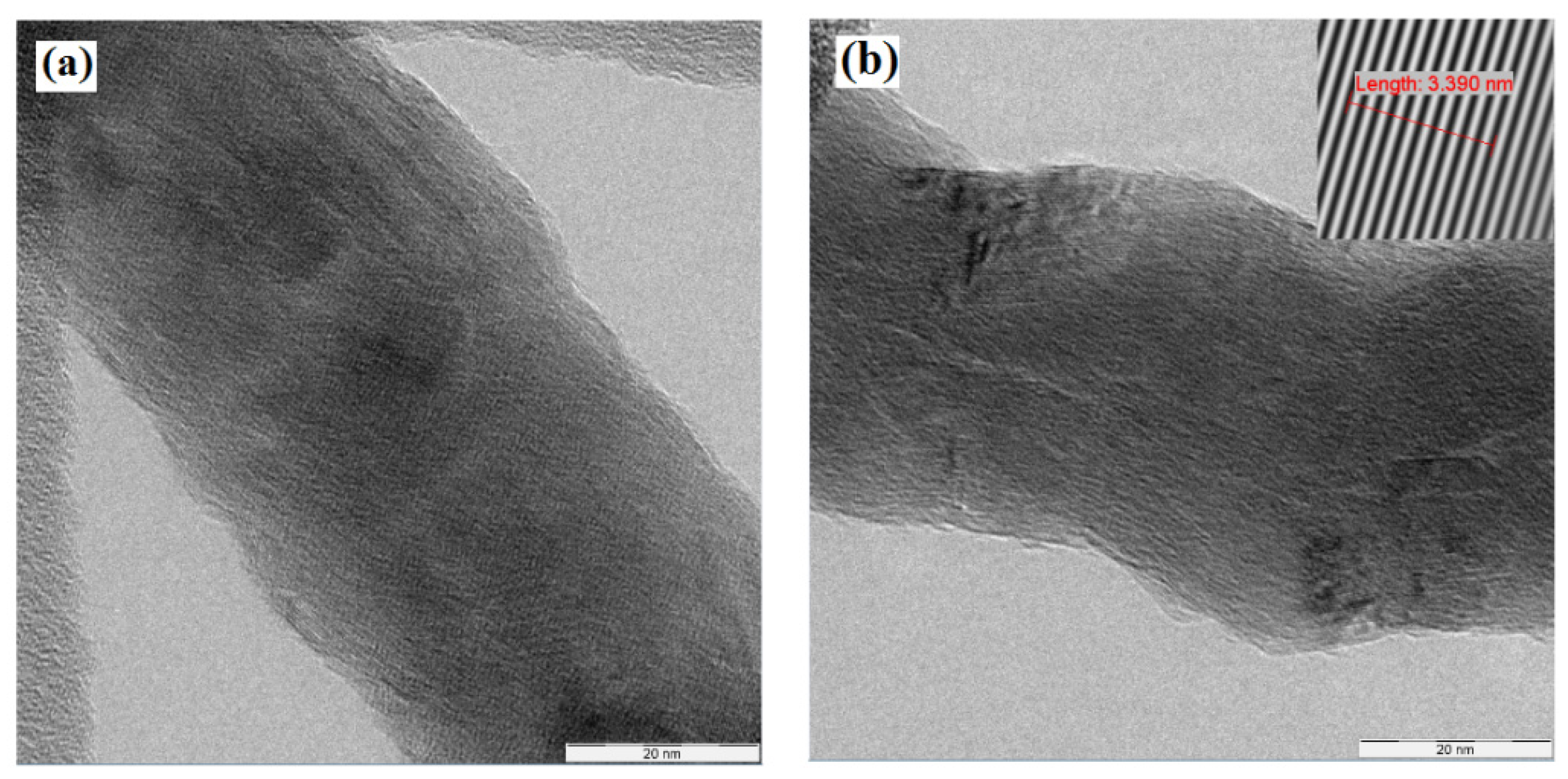
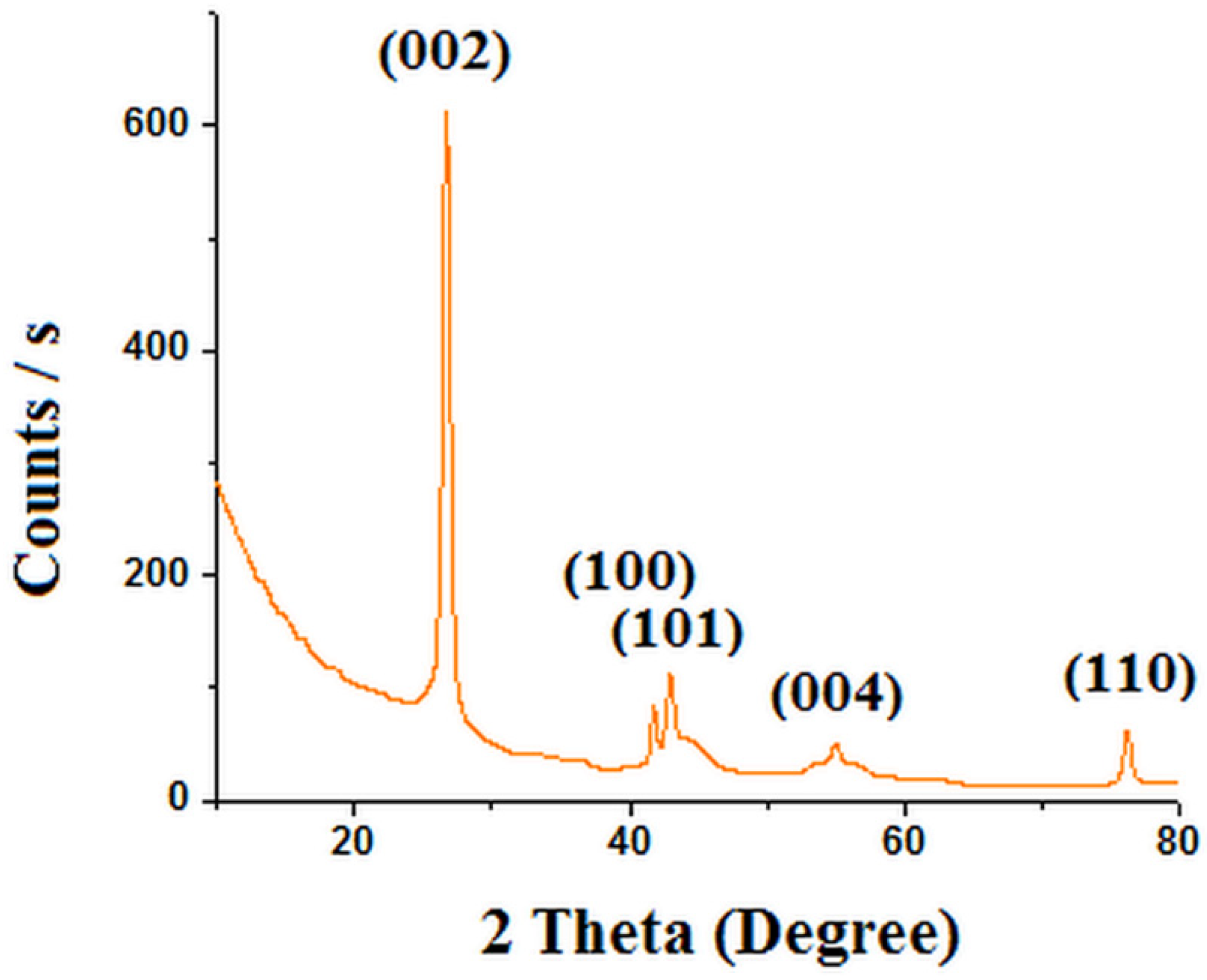
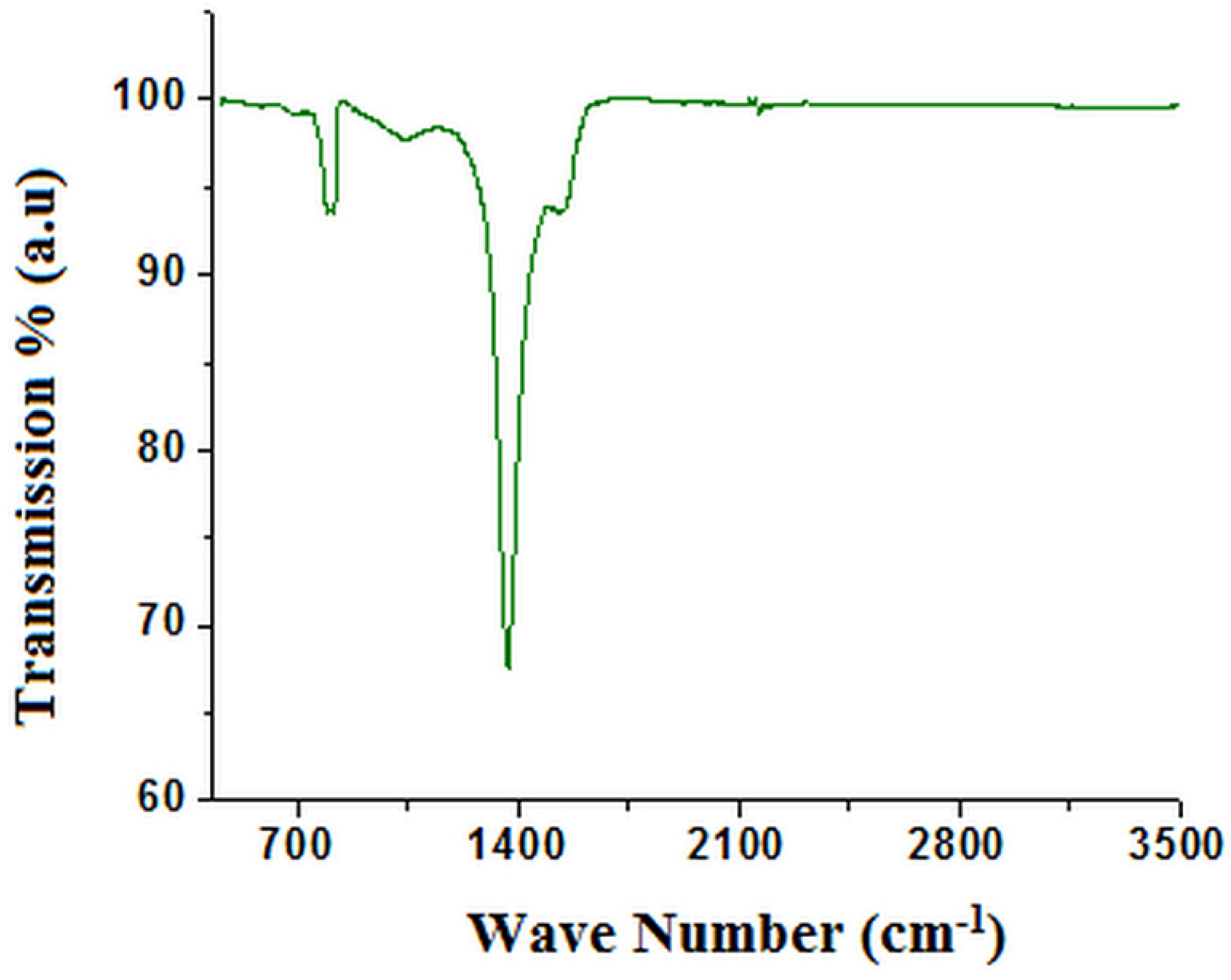
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salles, V.; Bernard, S.; Brioude, A.; Cornu, D.; Miele, P. A new class of boron nitride fibers with tunable properties by combining an electrospinning process and the polymer-derived ceramics route. Nanoscale 2010, 2, 215–217. [Google Scholar] [CrossRef]
- Hou, X.; Yu, Z.; Chou, K.-C. Facile synthesis of hexagonal boron nitride fibers with uniform morphology. Ceram. Int. 2013, 39, 6427–6431. [Google Scholar] [CrossRef]
- Economy, J.; Anderson, R. Boron nitride fibers. J. Polym. Sci. Part C Polym. Symp. 1967, 19, 283–297. [Google Scholar] [CrossRef]
- Paine, R.T.; Narula, C.K. Synthetic routes to boron nitride. Chem. Rev. 1990, 90, 73–91. [Google Scholar] [CrossRef]
- Toury, B.; Bernard, S.; Cornu, D.; Chassagneux, F.; Letoffe, J.-M.; Miele, P. High-performance boron nitride fibers obtained from asymmetric alkylaminoborazine. J. Mater. Chem. 2003, 13, 274–279. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, J.; Rafique, J.; Yin, J.; Bai, X.; Wang, E. Large-scale production of aligned long boron nitride nanofibers by multijet/multicollector electrospinning. J. Phys. Chem. C 2009, 113, 11228–11234. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, J.; Yin, J.; Tan, C.; Zhou, X.; Bai, X.; Wang, E. Synthesis of continuous boron nitride nanofibers by solution coating electrospun template fibers. Nanotechnology 2009, 20, 345603. [Google Scholar] [CrossRef]
- Bernard, S.; Ayadi, K.; Berthet, M.-P.; Chassagneux, F.; Cornu, D.; Letoffe, J.-M.; Miele, P. Evolution of structural features and mechanical properties during the conversion of poly [(methylamino) borazine] fibers into boron nitride fibers. J. Solid State Chem. 2004, 177, 1803–1810. [Google Scholar] [CrossRef]
- Li, J.; Dahal, R.; Majety, S.; Lin, J.; Jiang, H. Hexagonal boron nitride epitaxial layers as neutron detector materials. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2011, 654, 417–420. [Google Scholar] [CrossRef]
- Reed, B.C. The Physics of the Manhattan Project; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Ahmad, P.; Khandaker, M.U.; Amin, Y.M.; Khan, G.; Ramay, S.M.; Mahmood, A.; Amin, M.; Muhammad, N. Catalytic growth of vertically aligned neutron sensitive 10Boron nitride nanotubes. J. Nanopart. Res. 2016, 18, 25. [Google Scholar] [CrossRef]
- Tang, C.; Bando, Y.; Sato, T.; Kurashima, K. A novel precursor for synthesis of pure boron nitride nanotubes. Chem. Commun. 2002, 1290–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhi, C.; Bando, Y.; Tan, C.; Golberg, D. Effective precursor for high yield synthesis of pure BN nanotubes. Solid State Commun. 2005, 135, 67–70. [Google Scholar] [CrossRef]
- Ahmad, P.; Khandaker, M.U.; Khan, Z.R.; Amin, Y.M. Synthesis of boron nitride nanotubes via chemical vapour deposition: A comprehensive review. Rsc Adv. 2015, 5, 35116–35137. [Google Scholar] [CrossRef]
- Ahmad, P.; Khandaker, M.U.; Amin, Y.M. Synthesis of highly crystalline multilayers structures of 10BNNTs as a potential neutron sensing element. Ceram. Int. 2015, 41, 4544–4548. [Google Scholar] [CrossRef]
- Ahmad, P.; Uddin Khandaker, M.; Mohd Amin, Y.; Amin, M.; Imran Irshad, M.; Ud Din, I. Low temperature synthesis of high quality BNNTs via argon supported thermal CVD. Ceram. Int. 2015, 41, 15222–15226. [Google Scholar] [CrossRef]
- Ahmad, P.; Khandaker, M.U.; Amin, Y.M.; Muhammad, N. Synthesis of Highly Crystalline Multilayered Boron Niride Microflakes. Sci. Rep. UK 2016, 6, 21403. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Khandaker, M.U.; Amin, Y.M.; Muhammad, N.; Khan, G.; Khan, A.S.; Numan, A.; Rehman, M.A.; Ahmed, S.M.; Khan, A. Synthesis of hexagonal boron nitride fibers within two hour annealing at 500 °C and two hour growth duration at 1000 °C. Ceram. Int. 2016, 42, 14661–14666. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, J.; Tang, C.; Bando, Y.; Zhi, C.; Zhai, T.; Dierre, B.; Sekiguchi, T.; Golberg, D. Bulk synthesis, growth mechanism and properties of highly pure ultrafine boron nitride nanotubes with diameters of sub-10 nm. Nanotechnology 2011, 22, 145602. [Google Scholar] [CrossRef]
- Cai, P.; Chen, L.; Shi, L.; Yang, Z.; Zhao, A.; Gu, Y.; Huang, T.; Qian, Y. One convenient synthesis route to boron nitride nanotube. Solid State Commun. 2005, 133, 621–623. [Google Scholar] [CrossRef]
- Han, W.Q.; Yu, H.G.; Zhi, C.; Wang, J.; Liu, Z.; Sekiguchi, T.; Bando, Y. Isotope effect on band gap and radiative transitions properties of boron nitride nanotubes. Nano Lett. 2008, 8, 491–494. [Google Scholar] [CrossRef]
- Arenal, R.; Ferrari, A.C.; Reich, S.; Wirtz, L.; Mevellec, J.Y.; Lefrant, S.; Rubio, A.; Loiseau, A. Raman spectroscopy of single-wall boron nitride nanotubes. Nano Lett. 2006, 6, 1812–1816. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, P.; Khandaker, M.U.; Rehman, F.; Muhammad, N.; Faruque, M.R.I.; Ullah, Z.; Khan, M.A.R.; Khan, G.; Khan, M.I.; Ali, H.; et al. Facile Synthesis of High-Quality Nano-Size 10B-Enriched Fibers of Hexagonal Boron Nitride. Crystals 2021, 11, 222. https://doi.org/10.3390/cryst11030222
Ahmad P, Khandaker MU, Rehman F, Muhammad N, Faruque MRI, Ullah Z, Khan MAR, Khan G, Khan MI, Ali H, et al. Facile Synthesis of High-Quality Nano-Size 10B-Enriched Fibers of Hexagonal Boron Nitride. Crystals. 2021; 11(3):222. https://doi.org/10.3390/cryst11030222
Chicago/Turabian StyleAhmad, Pervaiz, Mayeen Uddin Khandaker, Fida Rehman, Nawshad Muhammad, Mohammad Rashed Iqbal Faruque, Zahoor Ullah, Muhammad Abdul Rauf Khan, Ghulamullah Khan, Muhammad Imtiaz Khan, Hazrat Ali, and et al. 2021. "Facile Synthesis of High-Quality Nano-Size 10B-Enriched Fibers of Hexagonal Boron Nitride" Crystals 11, no. 3: 222. https://doi.org/10.3390/cryst11030222
APA StyleAhmad, P., Khandaker, M. U., Rehman, F., Muhammad, N., Faruque, M. R. I., Ullah, Z., Khan, M. A. R., Khan, G., Khan, M. I., Ali, H., Haq, S., Iqbal, Y., Alzimami, K., Jambi, L., & Bradley, D. A. (2021). Facile Synthesis of High-Quality Nano-Size 10B-Enriched Fibers of Hexagonal Boron Nitride. Crystals, 11(3), 222. https://doi.org/10.3390/cryst11030222








