Abstract
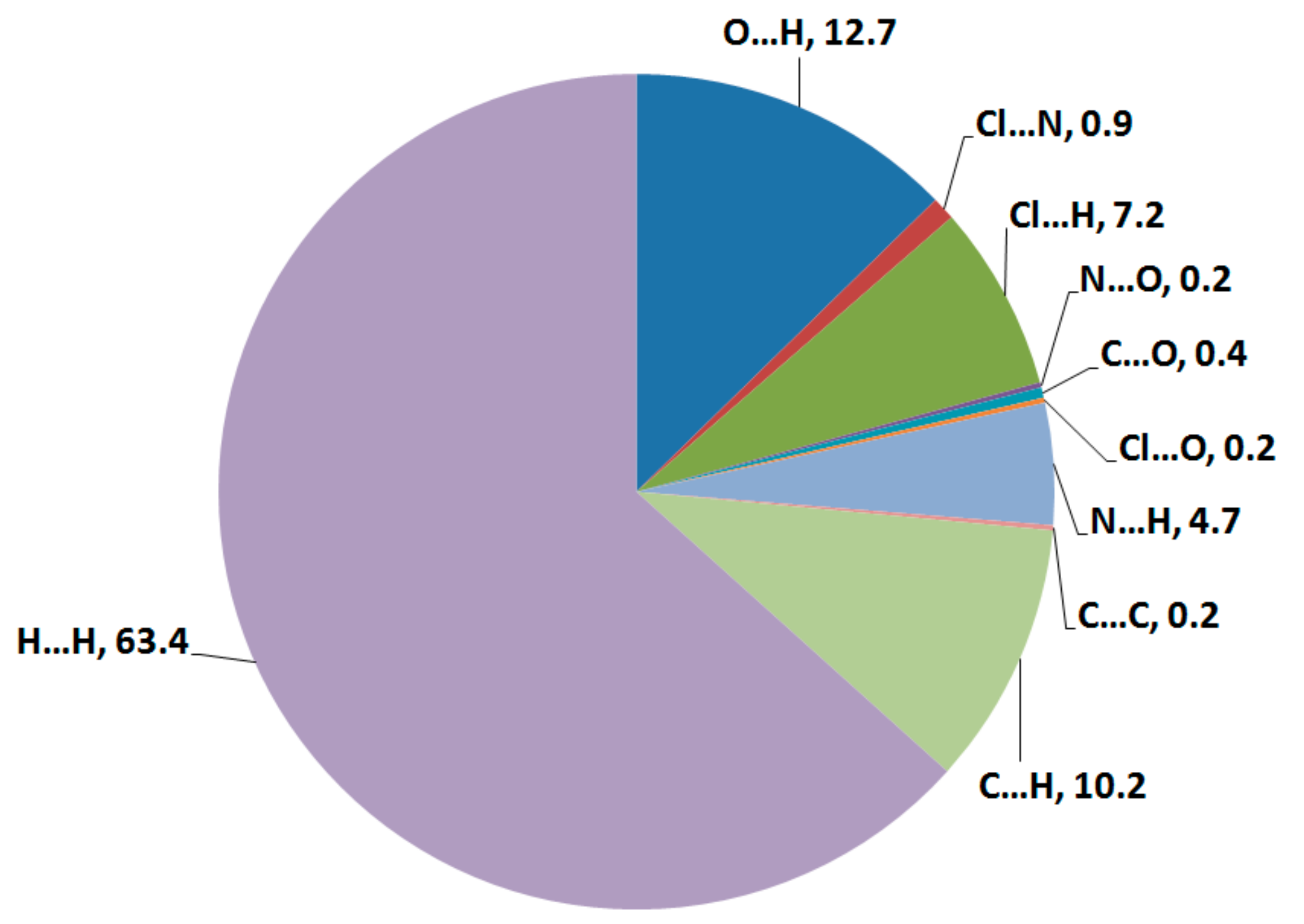
The one-pot fashion of three multi-component reaction provides the desired hydroacridinone-based hydrazino-s-triazine scaffold 4. Compound 4 was crystallized in an orthorhombic crystal system and Pbca space group with a = 11.6271(2) Å, b = 18.2018(4) Å, c = 32.4721(6) Å, and α = β = γ = 90° with one formula unit per asymmetric unit and eight molecules per unit cell. Additionally, structural features, Hirshfeld surfaces, and DFT studies were also investigated. Its packing in the crystal is controlled by H…H (63.4%), O…H (12.7%), Cl…H (7.2%), N…H (4.7%), and C…H (10.2%) contacts, where the O…H and Cl…H contacts were found the strongest. In vitro urease inhibition evaluation showed that the hydroacridinone-based hydrazino-s-triazine is more active (IC50 = 17.9 ± 0.47 µM) than the standard acetohydroxamic acid (IC50 = 20.3 ± 0.43 µM).
1. Introduction
Urease is a metalloenzyme-containing nickel that frequently exists in various plants, bacteria, fungi, and algae and leads to the growing of different bacteria pathogen (e.g., Helicobacter pylori) in acidic condition inside the stomach. Several health complications including hepatic coma, urinary tract infections, gastric lymphoma, gastric ulcers, gastric carcinoma, pyelonephritis, and kidney stones proved to be related to these bacterial infections [1,2]. In spite of numerous compounds being well-known to have potential urease inhibition, few of them are in the market for urease treatment. Thus, the discovery of effective and safe urease inhibitors is a very important issue in pharmaceutical research due to the contribution of ureases in different pathological conditions.
On the other hand, acridine-based compounds have been proven to display different pharmaceutical targets including anti-protozoal drugs [3], anti-bacterial drugs [4], anti-malarial agents [5], anti-HIV drugs [6], anti-inflammatory drugs [7], and antiviral drugs [8], and are known as cytotoxic agents and fluorescent probes [9,10]. Several acridine-based heterocyclic compounds have previously been reported to have a wide range of biological and therapeutic activities [11,12,13,14,15].
Similarly, s-triazine is one of the constituent molecular scaffolds found in natural products and synthetic drugs [16,17]. The s-triazine ring-based compounds have been proven to display different pharmaceutical targets for example MAO inhibitors [18], anticancer [19], antiviral [20], drug delivery systems [21], antimalarial [22], antiprotozoal [23], antimicrobial [24], antifungal [25], and antileishmanial activity [26]. The s-triazine moiety linked with other groups is of a lipophilic character and is already reported to have remarkable antifungal and antibacterial activities [27,28,29,30].
According to our knowledge, the search of potent acridine-based s-triazine compounds as urease inhibitors is not reported so far. Accordingly, we synthesized a hydroacridinone-based hydrazino-s-triazine derivative via a one-pot multi-component reaction. The in vitro anti-urease inhibition of the hydroacridinone-based hydrazino-s-triazine was evaluated. In addition, its structural features, Hirshfeld surfaces, and DFT were demonstrated.
2. Materials and Methods
2.1. General Methods
All melting points were determined using Mel-Temp electrothermal apparatus (Electrothermal, Staffordshire, ST15, UK) and were uncorrected. Thin-layer chromatography (TLC) was conducted on silica gel (Kiesel gel G, Merck) and spots were detected under UV light at 254 nm. IR spectra were recorded in a KBr matrix with a Perkin Elmer, Spectrum 100 FT-IR spectrophotometer (FT-IR, Perkin Elmer, Waltham, MA, USA). Furthermore, 1H and 13C NMR spectra were recorded in CDCl3 as solvent using JEOL 400 MHz (JEOL, Ltd, Tokyo, Japan), and the chemical shifts (δ) values were given in ppm. X-ray crystallographic analysis was collected by using Bruker SMART APEX II D8 Venture diffractometer at Karachi University.
2.2. Synthesis of Hydroacridinone Compound 4
9-(4-Chlorophenyl)-3,3,6,6-tetramethyl-10-((4-morpholino-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)amino)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4).
A mixture of 5,5-dimethylcyclohexane-1,3-dione (dimedone, 1) (2 mmol, 280 mg), p-chlorobenzaldehyde 2 (1 mmol, 141 mg), 3 (1 mmol, 279 mg), and glacial acetic acid (5 mL) was heated under reflux overnight. The solid product was isolated by simple filtration. Compound 4 was obtained in 85% yield as yellow powder, m.p: 275–286 °C. IR (KBr, cm−1): 3292, 2959, 2934, 2856, 1640, 1581, 1520, 1498, 1444, 1365; 1H-NMR (CDCl3): δ 7.37–7.15 (m, 4H, Ph), 4.7 (s, 1H, PhCH), 3.85–3.71 (m, 12H, morpholino & piperidinyl), 2.44 (s, 4H, CH2 hydroacridinone), 2.20 (s, 2H, CH2 hydroacridinone), 2.15 (s, 2H, CH2 hydroacridinone), 1.52 (m, 7H, piperidinyl and amino), 1.23 (s, 12H, 4CH3); 13C-NMR (CDCl3): δ 196.3, 195.5, 195.4, 162.4, 150.4, 142.7, 131.7, 130.0, 129.9, 129.8, 129.6, 128.2, 127.9, 127.8, 115.3, 114.7, 66.5, 50.7, 50.2, 50.0, 44.7, 44.5, 44.0, 41.3, 40.9, 38.8, 38.5, 38.1, 33.3, 32.7, 32.4, 32.0, 31.5, 30.0, 29.7; GC/MS (EI+): 645.3; Anal. for C35H44ClN7O3; Calcd: C, 65.05; H, 6.86; N, 15.17; Found: C, 65.25; H, 6.99; N, 15.35.
Single crystals were obtained from ethanol-diethyl ether (1:2) by slow evaporation at room temperature.
2.3. Single-Crystal X-ray Diffraction Analysis
Single-crystal X-ray diffraction analysis of compound 4 was carried out by mounting an appropriate crystal with dimensions 0.15 × 0.09 × 0.07 mm3 on a Bruker D8 Venture equipped with CCD Photon II detector and graphite monochromator having Cu Kα radiation (λ = 1.54178 Å) at T = 173 K for data collection. For the integration and reduction of data, SAINT (Bruker 1998) program was used [31]. The structure solution was done by direct method and Fourier transformation techniques, and further refined by full-matrix least-squares techniques on F2 using SHELXL-2018 program. PLATON (Spek 2008) [32] and SHELXL (Sheldrick 2015) [33] programs were employed for the final refinement of the solved structure (Table 1). CCDC 1967280 contains supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12, Union 26 Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033; email: deposit@ccdc.cam.uk or at http://www.ccdc.cam.ac.uk.

Table 1.
Summary of data collection and structure refinements of crystal of 4.
2.4. Hirshfeld Surface Analysis
The topology analyses based on CIF data were performed using Crystal Explorer 17.5 program [34] in order to determine the percentages of the different intermolecular interactions in the crystal structure of the studied compound.
2.5. Computational Methods
All DFT calculations were performed using Gaussian 09 software package [35,36]. The optimized geometry in gas phase was calculated using B3LYP/6-31G(d,p) method. The starting input was the X-ray structure coordinates. No imaginary frequencies were obtained indicating a real energy minimum.
2.6. Urease Inhibition
The urease inhibition assay was performed spectrophotometrically. The final volume of the reaction mixture was 200 µL, comprising 25 µL of urease enzyme solution (1 U/well is defined as the release of 1 µM of substrate per unit time under the specified conditions). This mixture was incubated with 5 µL of test compound (500 µM) for 15 min at 30 °C (urease enzyme was prepared in phosphate buffer pH 6.8, concentration 4 mM). Thereafter, 55 µL of urea (substrate) at a concentration of 100 mM was added, and the plate was again incubated for 15 min at 30 °C. After incubation, 45 µL of phenol reagents (1% w/v phenol and 0.005% w/v sodium nitroprusside), and 70 µL of alkali reagents (0.5% w/v sodium hydroxide and 0.1% sodium hypochlorite) were added to each well. The plate was re-incubated for 50 min at 30 °C. The continuous production of ammonia by urease was monitored following the Weatherburn method, and absorbance was recorded at 630 nm on an ELISA plate reader (Spectra Max M2, Molecular Devices, CA, USA). Acetohydroxamic acid was used as a reference compound (Standard Drug, available under the brand name Lithostate) [37,38].
3. Results and Discussion
3.1. Chemistry
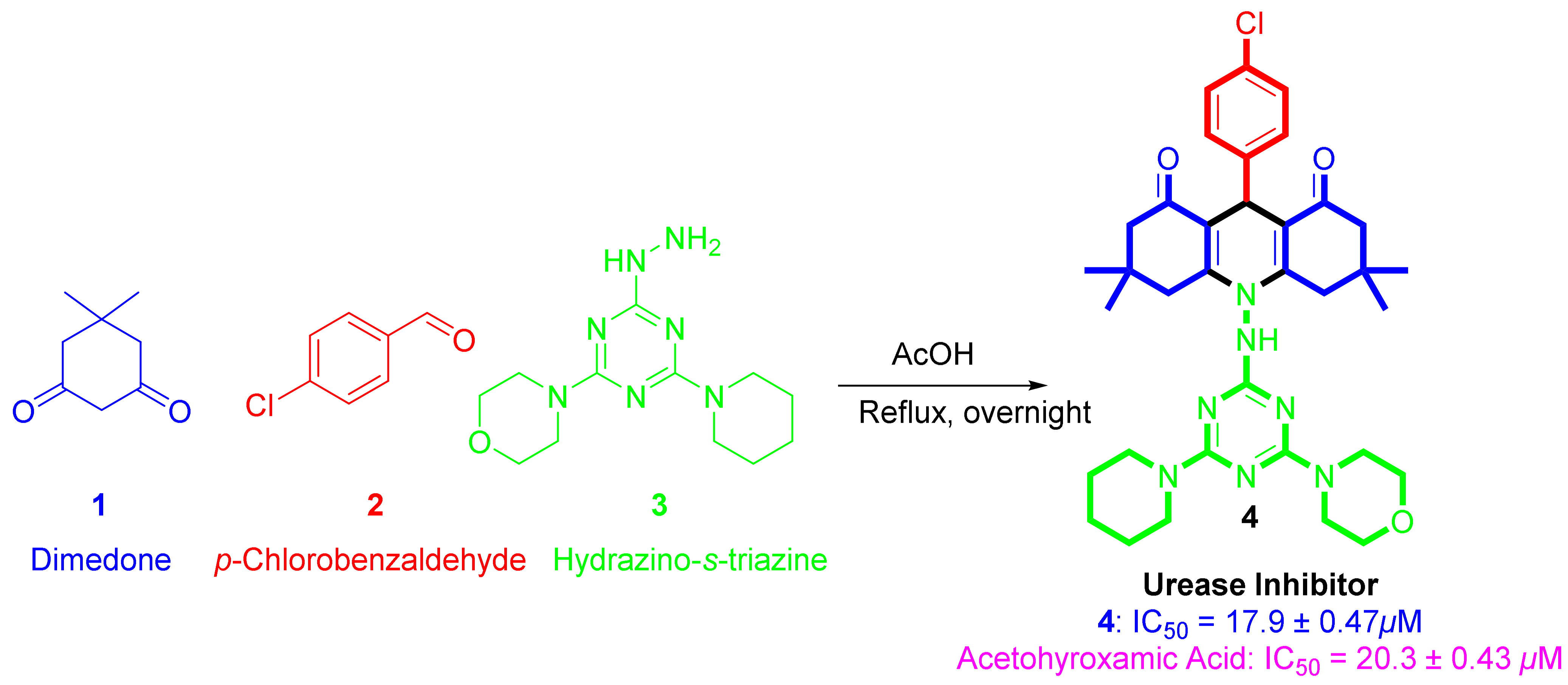
The synthesis of 4 was outlined in Scheme 1, where the starting material including dimedone 1, p-chlorobenzaldehyde 2, and 4-(4-hydrazinyl-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)morpholine 4 in acetic acid under reflux applying the one-pot fashion multi-component approach [39]. The structural feature of the desired hydroacridinone-based hydrazino-s-triazine was assigned based on sets of spectroscopic tools including FT-IR, mass spectroscopy (MS), nuclear magnetic resonance (NMR), and single-crystal X-ray diffraction technique.

Scheme 1.
Synthetic route for the hydroacridinone-based hydrazino-s-triazine, 4.
The 1H-NMR spectra are consistent with the assigned structure of 4. A multiplet signal was shown at δ 7.37–7.15 ppm and the singlet at δ 4.7 ppm could be assigned for the aromatic protons and the benzylic proton, respectively. In the aliphatic chemical shift region, a multiplet at δ 3.85–3.71 ppm for the morpholino and piperidinyl moieties protons was observed. The hydroacridinone proton was detected at δ 2.44–2.15 ppm. Additionally, the signal δ 1.52 ppm could be assigned to 7H of the piperidinyl ring (3CH2), which overlays with the NH proton. Finally, the singlet signal assigned to the four methyl groups with 12 protons was detected at δ 1.23 ppm. 13C-NMR spectrum and MS are matching with the expected product.
3.2. Crystal Structural Description
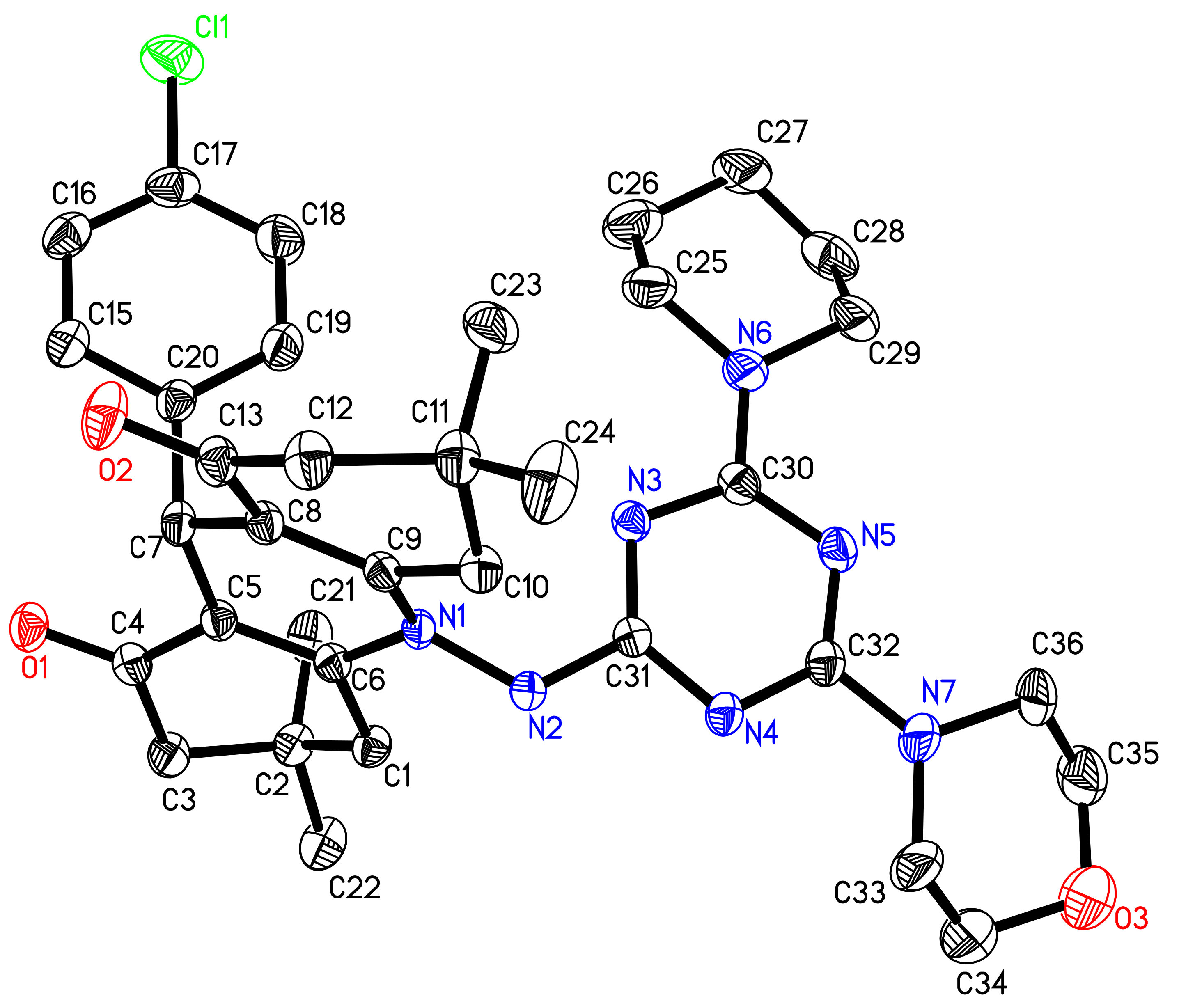
The 3D structure of compound 4 was represented by the ORTEP diagram (Figure 1). Structurally, compound 4 was found to be comprised of 1,8-dioxo-decahydroacridine (N1/O1-O2/C1-C13) moiety substituted with planar p-chlorophenyl ring (Cl1/C15-C20) at C7 and dimethyl groups (C21-C22/C23-C24) at C2 and C11 atom, linked via N1-N2 linkage to planar s-triazine ring (N3-N5/C30-C32). The piperidinyl ring (N6/C25-C29) was found in twist-boat conformation at C30 and morpholino moiety (N7/O3/C33-C36) in a chair conformation at C32 atom, respectively. The torsion angle between N1/N2/C6/C31 was found to be 112.8(2)°. A list of dihedral angles is given in Table S1 (Supplementary Data).
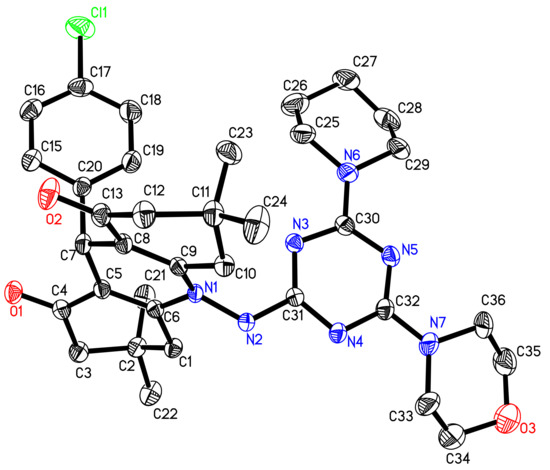
Figure 1.
The ORTEP view of compound 4 showing atom numbering drawn at 50% probability level. H atoms have been omitted for clarity.
The puckering parameters and the distortion of the two non-planer rings, morpholino, and piperidinyl moieties were calculated using PLATON [32,40]. The six-membered morpholino and piperidinyl rings were in the target molecule 4 with puckering parameters: Q, θ, and φ = 0.541(4) Å, 4.2(4)° and 6(5)°, respectively (in case of morpholine moiety); Q, θ, and φ = 0.560(4) Å, 177.1(4)° and 30 (7)°, respectively (in case of piperidino moiety), which indicated the slightly distorted conformation.
3.3. Crystal Packing
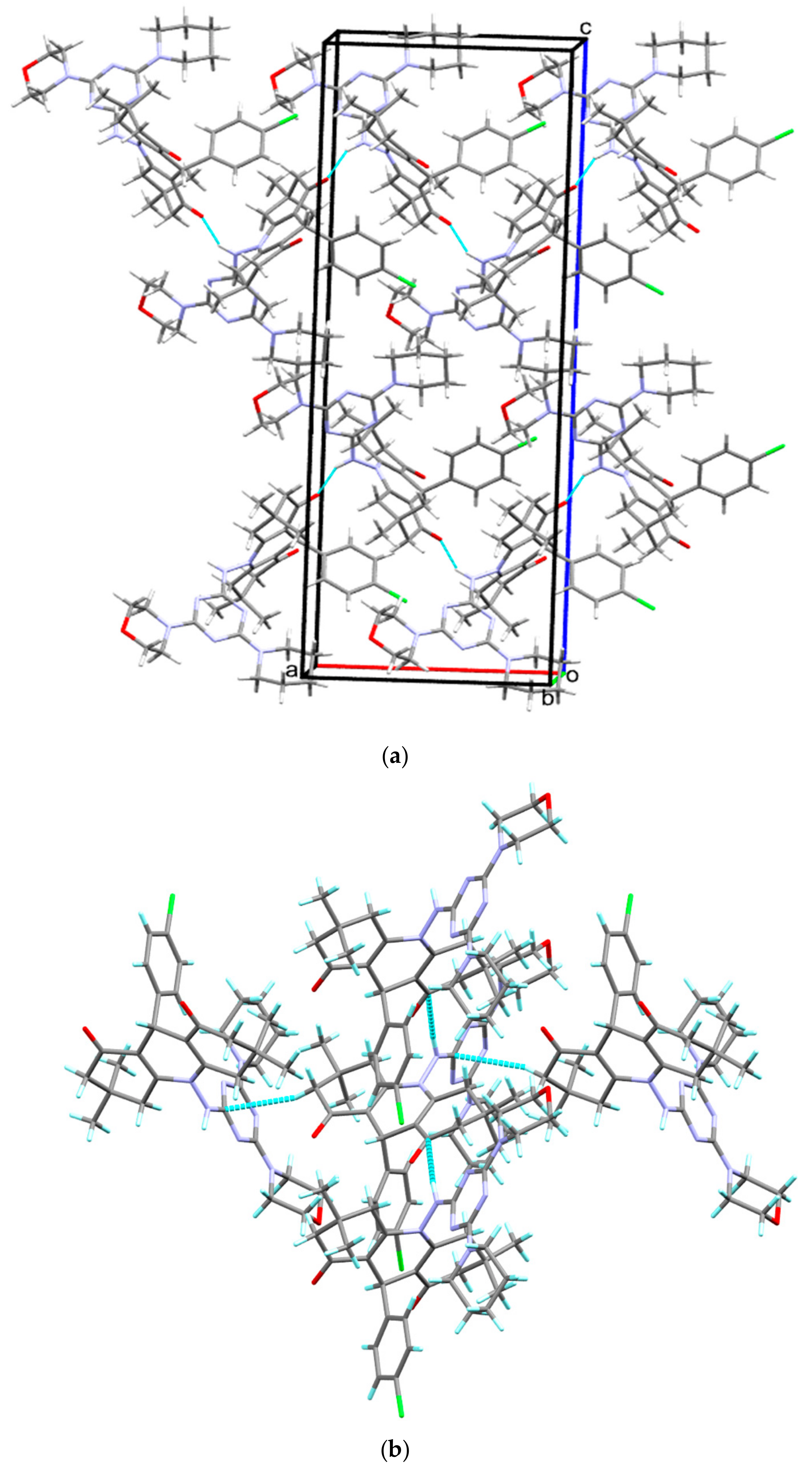
In crystal lattice of compound 4, molecules are found to be interlinked via H1…O1 interactions with donor-acceptor distance of 2.727(3) Å (Table 2), arranged to form a three-dimensional network. The C-H…π interactions further strengthen the crystal structure with H12B…Cg3 (N3-N5/C30-C32) and a distance of 2.71 Å (Table 2). These inter-molecular interactions existing in crystal packing of 4 are shown Figure 2.

Table 2.
The hydrogen bond distances (Å) and angles (°) in 4.

Figure 2.
Crystal packing diagram of compound 4. (a) The most important hydrogen bonding interaction (N-H…O) is presented as dotted turquoise line. (b) The weak C-H…π interactions are also presented in the same color in the lower part of this illustration.
3.4. Hirshfeld Analysis of Molecular Packing
In Hirshfeld surface analysis, the dnorm is a normalized contact distance as defined by Equation (1). The di is normalized by the van der Waals radius of the atoms involved; de is similarly normalized, and the sum of these two quantities is the dnorm property. Where atoms make intermolecular contacts closer than the sum of their van der Waals radii, these contacts will be highlighted in red on the dnorm surface. Longer contacts are blue and contacts around the sum of van der Waals radii are white [41].
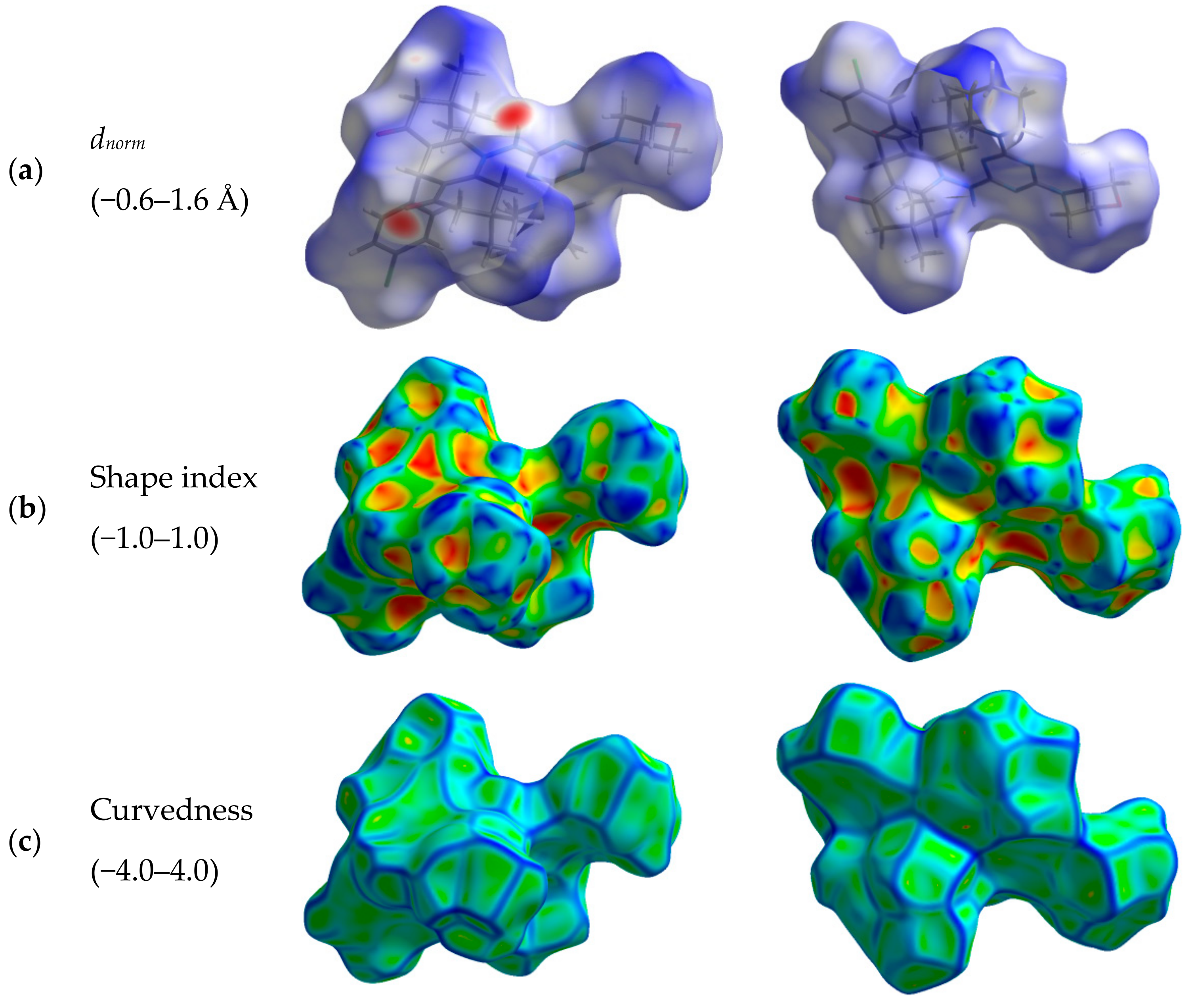
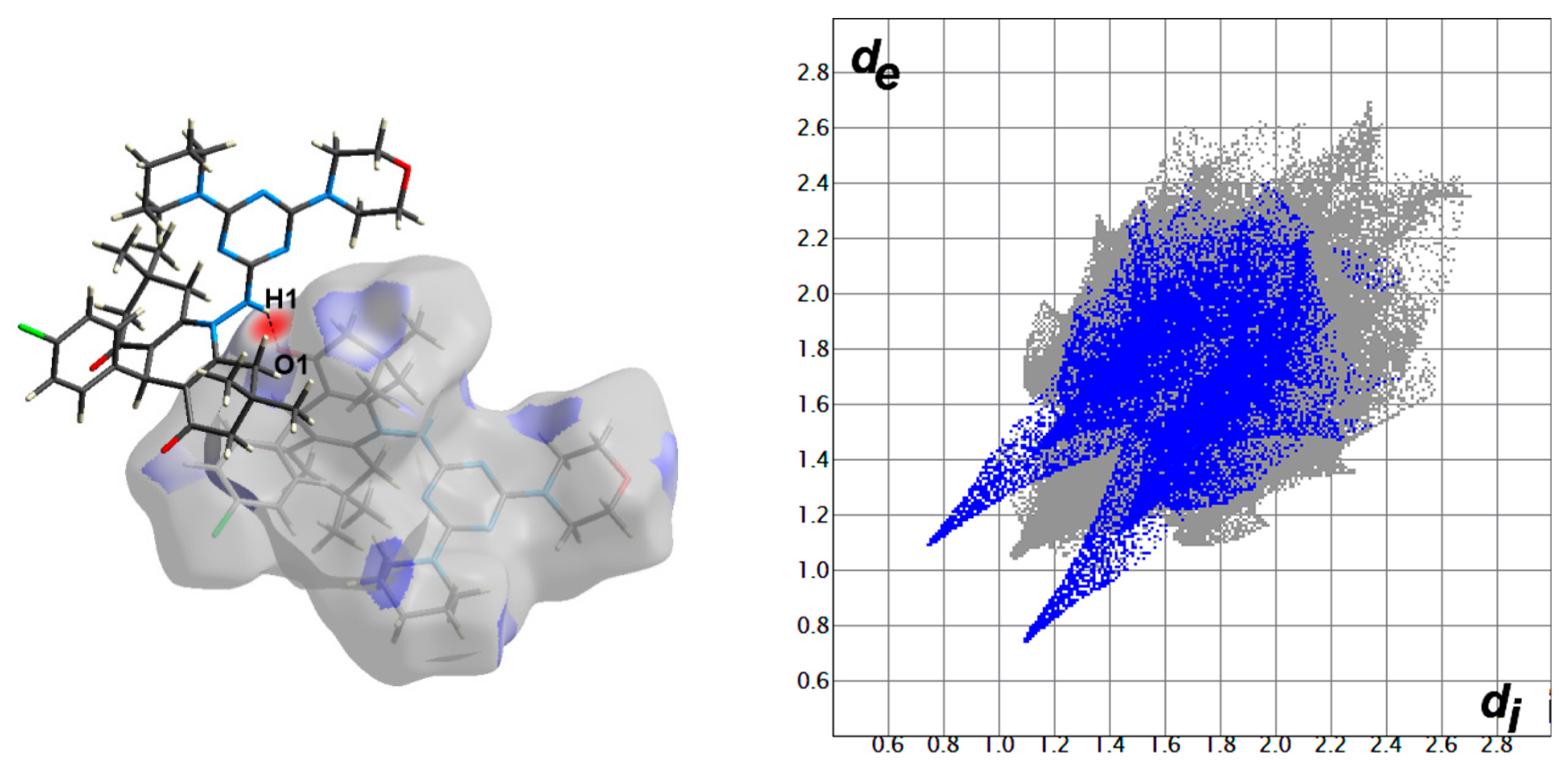
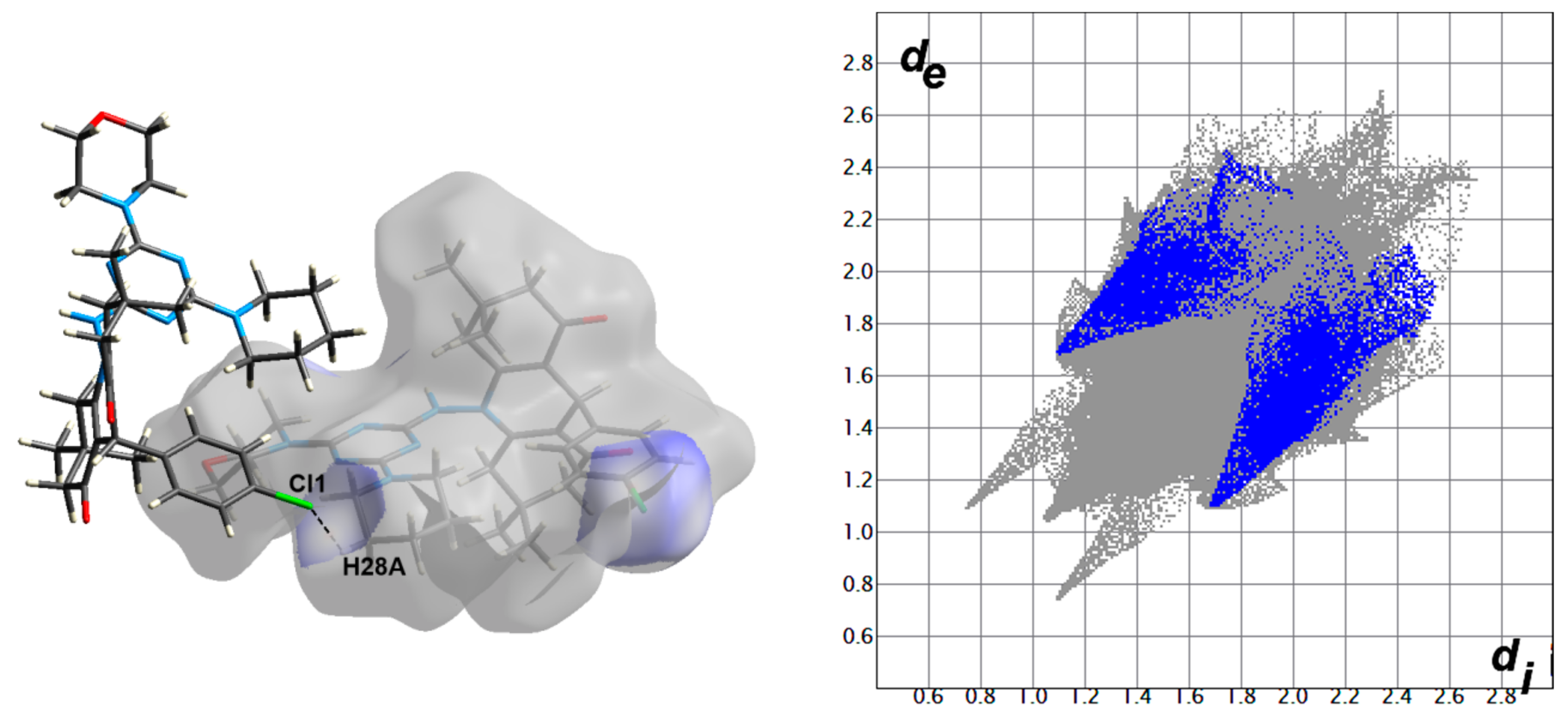
Hirshfeld surfaces for compound 4 are shown in Figure 3. The decomposed fingerprint plots indicated that the most common contacts are H…H (63.4%), O…H (12.7%), Cl…H (7.2%), N…H (4.7%), and C…H (10.2%) as shown in Figure 4. Among them, the O…H and Cl…H contacts appeared as intense and faded red spots in the dnorm maps, respectively, while the rest of other contacts appeared as blue regions. From this point of view, the O…H and Cl…H contacts are considered the strongest among the others. These interactions appeared as relatively sharp spikes in the fingerprint (FP) plots (Figure 5). The intermolecular distances are 1.831 Å and 2.791 Å for the O1…H1 and Cl1…H28A, respectively.

Figure 3.
Hirshfeld (a) dnorm, (b) shape index, and (c) curvedness maps for compound 4.

Figure 4.
The intermolecular contacts in 4 and their percentages.
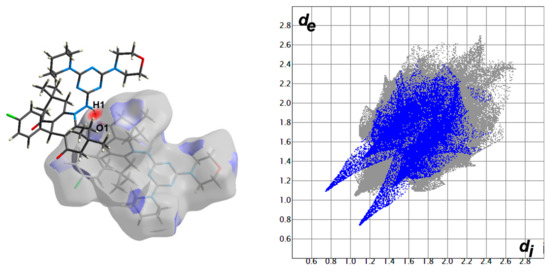
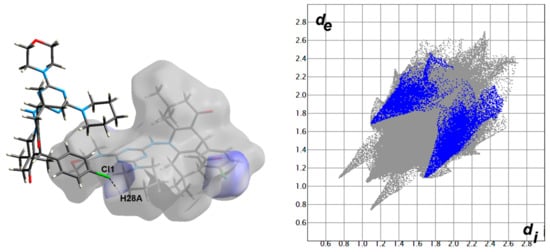
Figure 5.
The dnorm maps (left) and FP plots (right) of the O1…H1 (1.831 Å) and Cl1…H28A (2.791 Å) contacts in the studied molecule.
3.5. Geometric Parameters
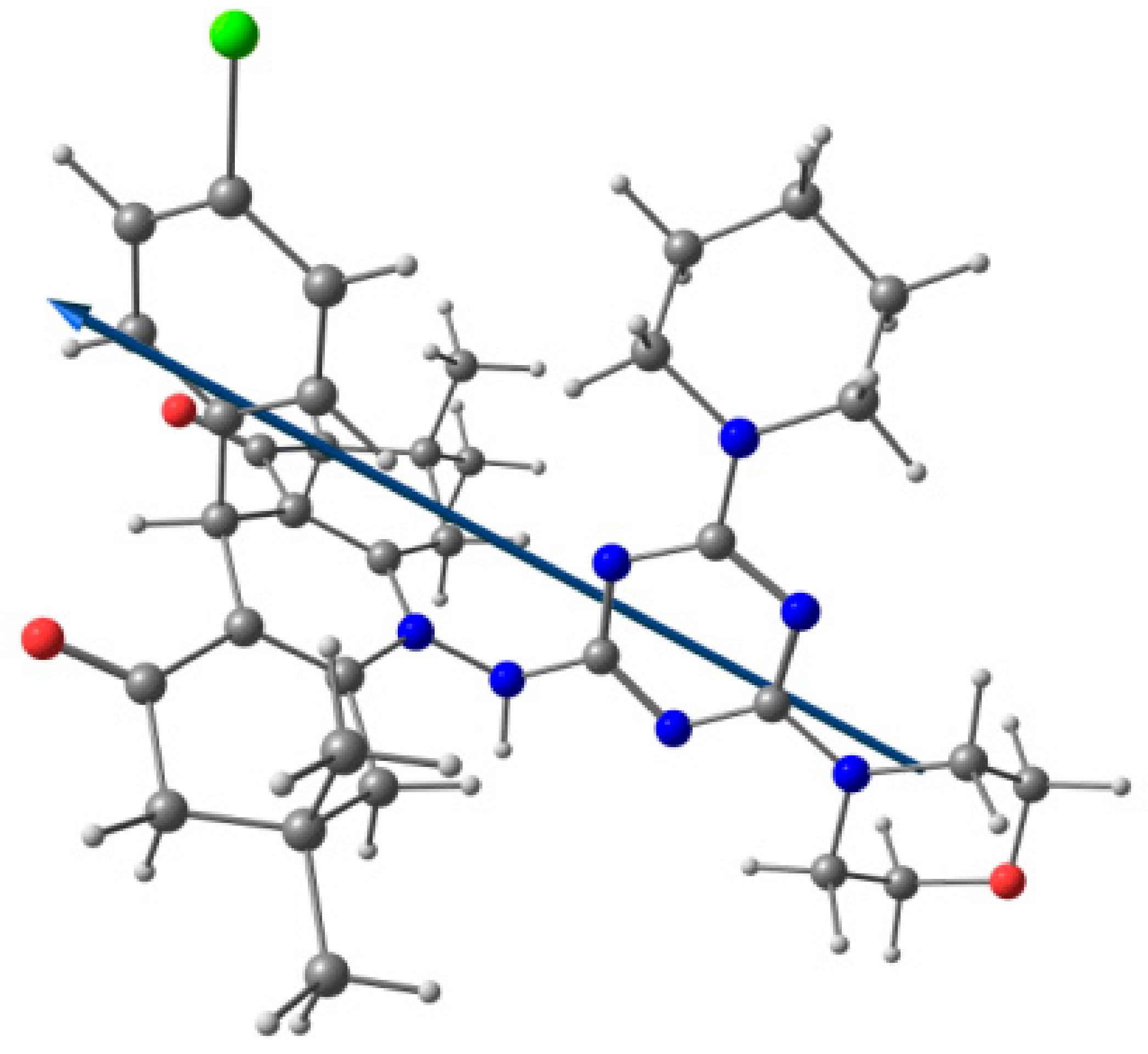
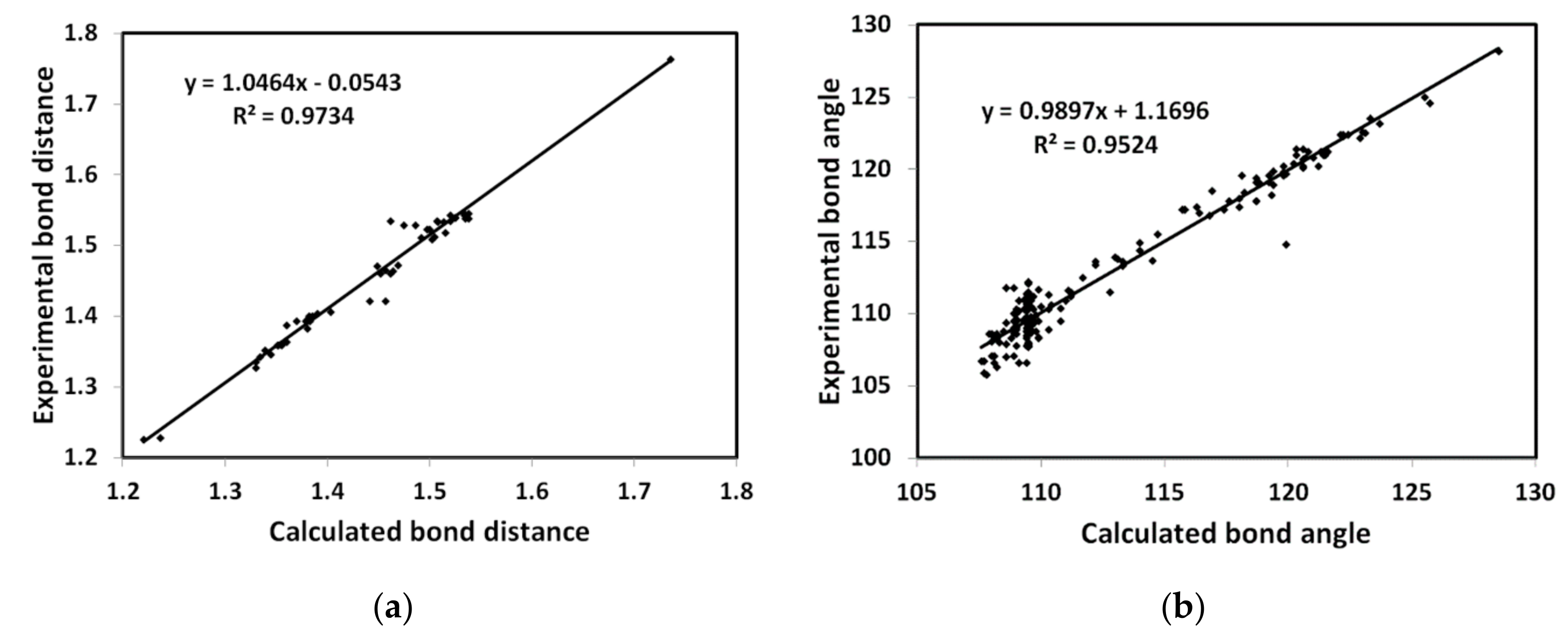
The crystal structure was left to relax using DFT B3LYP/6-31G(d,p) method and the resulting optimized structure is presented in Figure 6. Bond distances and angles are given in Table S2 (Supplementary Materials). Figure 7 showed the good correlations between the calculated and experimental bond distances (R2 = 0.973), and angles (R2 = 0.952). The presence of many polar atoms related to the highly electronegative atoms such as oxygen, nitrogen, and chlorine leads to a polar molecule with a high dipole moment of 9.268 Debye. The natural atomic charges and the direction of the dipole moment vector are given in Table S3 (Supplementary Materials) and Figure 6, respectively. The dipole moment vector is oriented toward the most polar region in the molecule, which is the carbonyl oxygen atoms.

Figure 6.
Optimized molecular structure showing the dipole moment vector of 4; C: Grey, N: blue, O: red and H: white.

Figure 7.
The calculated versus experimental (a) bond distances and (b) angles.
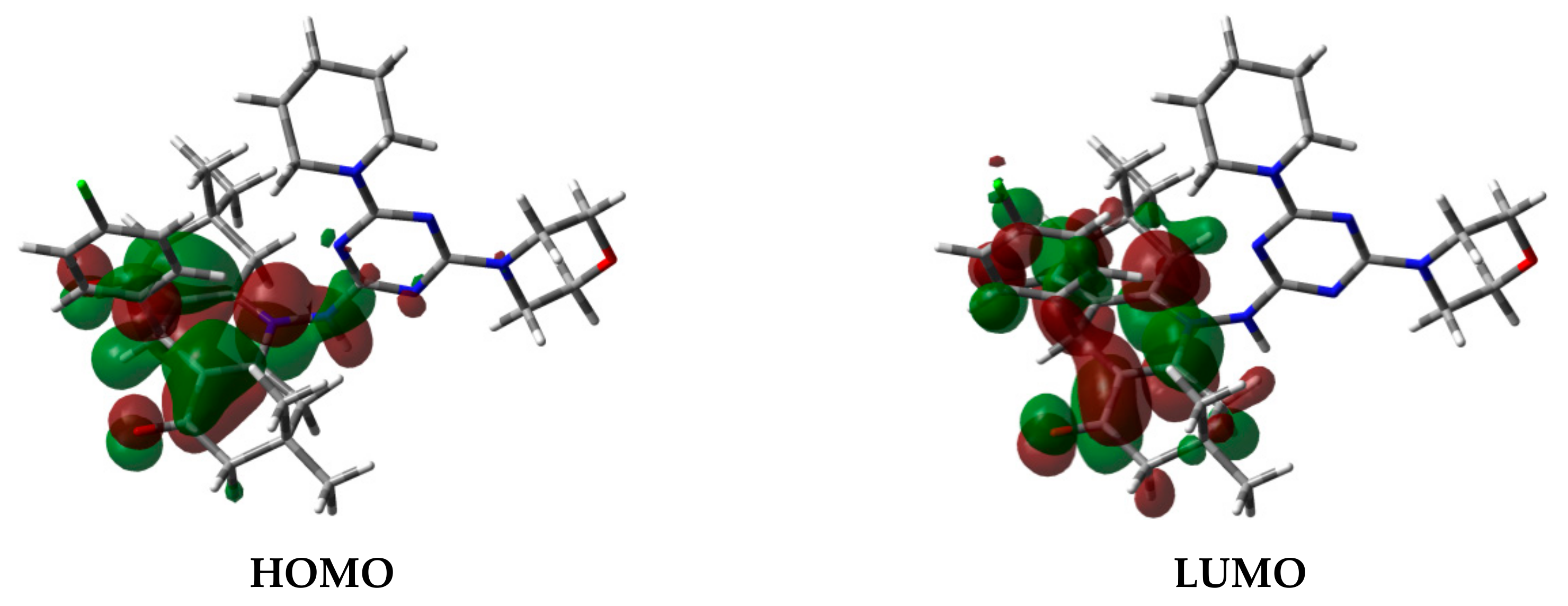
On the other hand, the HOMO (−5.722 eV) and LUMO (−1.516 eV) orbitals are distributed over the π-system of the fused ring (Figure 8). The energy of the HOMO–LUMO intramolecular charge transfer is calculated to be 4.206 eV. The reactivity indices [42,43,44,45,46,47,48,49,50] of the studied compound such as ionization potential (I), electron affinity (A), chemical potential (μ), hardness (η), softness (S), and electrophilicity (ω) were calculated based on the HOMO and LUMO energies to be 5.722, 1.516, −3.619, 4.206, 0.238, and 1.557, respectively. These descriptors are important for the biological reactivity of the compounds.

Figure 8.
HOMO and LUMO of 4.
3.6. Biological Activity
In a recent publication [51], we reported the synthesis of several hydrazone derivatives based on s-triazine and their corresponding anti-proliferative activities. Of these derivatives, only three inhibited the growth of lung carcinoma A549 and hepatocyte carcinoma HepG2 cells. Our results showed the combination of morpholino and piperidinyl moieties conferred greater selectivity for A549 cells and had a reasonable inhibitory effect on HepG2 cells [52].
Based on the previous results [52,53], 4-(4-hydrazinyl-6-(piperidin-1-yl)-1,3,5-triazin-2-yl) morpholine 3 was used as an example for the synthesis of hydroacridinone-based hydrazino-s-triazine 4. Compound 4 was evaluated in vitro against-urease inhibition and the results are shown in Table 3. The desired compound exhibited high efficacy compared to acetohyroxamic acid (IC50 = 20.3 ± 0.43 µM), where the IC50 is equal to 17.9 ± 0.47 µM (Table 3). The structure of the compound consists of hydroacridinone-based hydrazino-s-triazine having lipophilic moieties like morpholine and piperidine provided high potency, which can be considered as a lead compound to discover new drugs as urease inhibitor.

Table 3.
Result of in vitro urease enzyme inhibition potential.
4. Conclusions
The present report describes the design and synthesis of hydroacridinone-based ydrazine-s-triazine derivative as a promising anti-urease agent. The synthesized hydroacridinone based hydrazino-s-triazine derivative (4) showed more potency than acetohyroxamic acid as reference. Thus, the hydroacridinone-based s-triazine could be considered as a compound of potential lead for further optimization and drug discovery in future work. Hirshfeld analysis indicated the importance of the H…H (63.4%), O…H (12.7%), Cl…H (7.2%), N…H (4.7%), and C…H (10.2%) in the molecular packing of 4. DFT calculations indicated that 4 is polar compound with a total dipole moment of 9.268 Debye. Its reactivity indices were computed using the frontier molecular orbital energies. The computed bond distances and angles were found in good correlations with the experimental results.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/10/1/14/s1: Figure S1, The atom numbering of the calculated molecular structure of the studied compound; Table S1, The calculated bond distances and angles of the studied organic compound compared to the experimental X-ray structure data; Table S2, The natural atomic charge analysis of the studied compound.
Author Contributions
Conceptualization, A.B. and A.E-F.; data curation, S.M.S. and S.Y.; formal analysis, S.Y.; funding acquisition, A.B.; investigation, M.A.; methodology, M.A.; software, S.M.S. and S.Y.; validation, A.M.A.; visualization, A.M.A. and M.I.C.; writing—original and final draft was carried by A.B., S.M.S., and A.E-F.; final revision and editing was carried out by M.I.C. All authors have read and agreed to the published version of the manuscript.
Funding
Researchers Supporting Project, King Saud University.
Acknowledgments
The authors would like to extend their sincere appreciation to Researchers Supporting Project Number (RSP-2019/64), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Qazi, S.U.; Naz, S.; Ishtiaq, M.; Khan, K.M. A patent update on therapeutic applications of urease inhibitors (2012–2018). Expert Opin. Ther. Pat. 2019, 29, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Rego, Y.F.; Queiroz, M.P.; Brito, T.O.; Carvalho, P.G.; de Queiroz, V.T.; de Fatima, A.; Macedo, F., Jr. A review on the development of urease inhibitors as antimicrobial agents against pathogenic bacteria. J. Adv. Res. 2018, 13, 69–100. [Google Scholar] [CrossRef] [PubMed]
- Sebestik, J.; Hlavacek, J.; Stibor, I. A role of the 9-aminoacridines and their conjugates in a life science. Curr. Protein Pept. Sci. 2007, 8, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Huo, L.; Jaiswal, Y.; Huang, J.; Zhong, Z.; Zhong, J.; Williams, L.; Xia, X.; Liang, Y.; Yan, Z. Design, Synthesis, Antimicrobial, and Anticancer Activities of Acridine Thiosemicarbazides Derivatives. Molecules 2019, 24, 2065. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.O.; Sherrill, J.; Madrid, P.B.; Liou, A.P.; Weisman, J.L.; DeRisi, J.L.; Guy, R.K. Parallel synthesis of 9-aminoacridines and their evaluation against chloroquine-resistant Plasmodium falciparum. Bioorganic Med. Chem. 2006, 14, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Luedtke, N.W.; Liu, Q.; Tor, Y. RNA—Ligand interactions: Affinity and specificity of aminoglycoside dimers and acridine conjugates to the HIV-1 Rev response element. Biochemistry 2003, 42, 11391–11403. [Google Scholar] [CrossRef]
- Sondhi, S.M.; Singh, J.; Rani, R.; Gupta, P.P.; Agrawal, S.K.; Saxena, A.K. Synthesis, anti-inflammatory and anticancer activity evaluation of some novel acridine derivatives. Eur. J. Med. Chem. 2010, 45, 555–563. [Google Scholar] [CrossRef]
- Bondinell, W.; Reader, V.; Ku, T. SmithKline Beecham Corp, Substituted Bis-Acridines and Related Compounds as CCR5 Receptor Ligands, Anti-Inflammatory Agents and Anti-Viral Agents. U.S. Patent Application 09/833,044, 18 October 2001. [Google Scholar]
- Belmont, P.; Bosson, J.; Godet, T.; Tiano, M. Acridine and acridone derivatives, anticancer properties and synthetic methods: Where are we now? Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2007, 7, 139–169. [Google Scholar] [CrossRef]
- Denny, W.A.; Pereira, R.C.; Pontinha, A.D.R.; Pineiro, M.; de Melo, J.S.S. A comprehensive spectral, photophysical and electrochemical study of synthetic water-soluble acridones. A new class of pH and polarity sensitive fluorescent probes. Dye. Pigment. 2019, 166, 203–210. [Google Scholar]
- Denny, W.A. Acridine derivatives as chemotherapeutic agents. Curr. Med. Chem. 2002, 9, 1655–1665. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.; Li, B.; Gao, C.; Jiang, Y. Acridine and its derivatives: A patent review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Belmont, P.; Dorange, I. Acridine/acridone: A simple scaffold with a wide range of application in oncology. Expert Opin. Ther. Pat. 2008, 18, 1211–1224. [Google Scholar] [CrossRef]
- Galdino-Pitta, M.R.; Pitta, M.G.R.; Lima, M.C.A.; Galdino, L.S.; Pitta, R.I. Niche for acridine derivatives in anticancer therapy. Mini Rev. Med. Chem. 2013, 13, 1256–1271. [Google Scholar] [PubMed]
- Kaur, J.; Singh, P. Acridine derivatives: A patent review (2009–2010). Expert Opin. Ther. Pat. 2011, 21, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Ballell, L.; Field, R.A.; Duncan, K.; Young, R.J. New small-molecule synthetic antimycobacterials. Antimicrob. Agents Chemother. 2005, 49, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.P.; Chang, Y.T. Chemical genetics. Chem. Rev. 2006, 106, 2476–2530. [Google Scholar] [CrossRef]
- Khattab, S.; Khalil, H.; Bekhit, A.; El-Rahman, M.; El-Faham, A.; Albericio, F. Synthesis and preliminary biological evaluation of 1,3,5-triazine amino acid derivatives to study their MAO inhibitors. Molecules 2015, 20, 15976–15988. [Google Scholar] [CrossRef]
- Xiong, Y.Z.; Chen, F.E.; Balzarini, J.; De Clercq, E.; Pannecouque, C. Non-nucleoside HIV-1 reverse transcriptase inhibitors. Part 11: Structural modulations of diaryltriazines with potent anti-HIV activity. Eur. J. Med. Chem. 2008, 43, 1230–1236. [Google Scholar] [CrossRef]
- Saleh, M.; Abbott, S.; Perron, V.; Lauzon, C.; Penney, C.; Zacharie, B. Synthesis and antimicrobial activity of 2-fluorophenyl-4, 6-disubstituted [1,3,5] triazines. Bioorganic Med. Chem. Lett. 2010, 20, 945–949. [Google Scholar] [CrossRef]
- Khattab, S.N.; Naim, S.E.A.; El-Sayed, M.; El Bardan, A.A.; Elzoghby, A.O.; Bekhit, A.A.; El-Faham, A. Design and synthesis of new s-triazine polymers and their application as nanoparticulate drug delivery systems. New J. Chem. 2016, 40, 9565–9578. [Google Scholar] [CrossRef]
- Zhou, C.; Min, J.; Liu, Z.; Young, A.; Deshazer, H.; Gao, T.; Chang, Y.T.; Kallenbach, N.R. Synthesis and biological evaluation of novel 1,3,5-triazine derivatives as antimicrobial agents. Bioorganic Med. Chem. Lett. 2008, 18, 1308–1311. [Google Scholar] [CrossRef] [PubMed]
- Melato, S.; Prosperi, D.; Coghi, P.; Basilico, N.; Monti, D. A combinatorial approach to 2, 4, 6-trisubstituted triazines with potent antimalarial activity: Combining conventional synthesis and microwave-assistance. ChemMedChem Chem. Enabling Drug Discov. 2008, 3, 873–876. [Google Scholar]
- Nowakowska, Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 2007, 42, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Haiba, N.S.; Khalil, H.H.; Moniem, M.A.; El-Wakil, M.H.; Bekhit, A.A.; Khattab, S.N. Design, synthesis and molecular modeling studies of new series of s-triazine derivatives as antimicrobial agents against multi-drug resistant clinical isolates. Bioorganic Chem. 2019, 89, 103013. [Google Scholar] [CrossRef]
- Khattab, S.N.; Khalil, H.H.; Bekhit, A.A.; Abd El-Rahman, M.M.; de la Torre, B.G.; El-Faham, A.; Albericio, F. 1,3,5-Triazino peptide derivatives: Synthesis, characterization, and preliminary antileishmanial activity. ChemMedChem 2018, 13, 725–735. [Google Scholar] [CrossRef]
- Gavade, S.N.; Markad, V.L.; Kodam, K.M.; Shingare, M.S.; Mane, D.V. Synthesis and biological evaluation of novel 2, 4, 6-triazine derivatives as antimicrobial agents. Bioorganic Med. Chem. Lett. 2012, 22, 5075–5077. [Google Scholar] [CrossRef]
- Courme, C.; Gresh, N.; Vidal, M.; Lenoir, C.; Garbay, C.; Florent, J.C.; Bertounesque, E. Synthesis of aryl phosphates based on pyrimidine and triazine scaffolds. Eur. J. Med. Chem. 2010, 45, 244–255. [Google Scholar] [CrossRef]
- Gahtori, P.; Ghosh, S.K.; Singh, B.; Singh, U.P.; Bhat, H.R.; Uppal, A. Synthesis, SAR and antibacterial activity of hybrid chloro, dichloro-phenylthiazolyl-s-triazines. Saudi Pharm. J. 2012, 20, 35–43. [Google Scholar] [CrossRef]
- Desai, N.C.; Makwana, A.H.; Rajpara, K.M. Synthesis and study of 1, 3, 5-triazine based thiazole derivatives as antimicrobial agents. J. Saudi Chem. Soc. 2016, 20, S334–S341. [Google Scholar] [CrossRef]
- Bruker, A. SAINT Software Reference Manual; Technical Publications Department: Madison, WI, USA, 1998; p. 5465. [Google Scholar]
- Spek, A.L.J. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17; University of Western Australia: Perth, Australia, 2017; Available online: http://hirshfeldsurface.net (accessed on 30 December 2019).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09. Revision A0; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Keith, T.; Millam, J. GaussView, Version 4.1; Dennington, R., Ed.; Semichem Inc.: Shawnee Mission, KS, USA, 2007. [Google Scholar]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Benini, S.; Rypniewski, W.R.; Wilson, K.S.; Miletti, S.; Ciurli, S.; Mangani, S. The complex of Bacillus pasteurii urease with acetohydroxamate anion from X-ray data at 1.55 Å resolution. JBIC J. Biol. Inorg. Chem. 2000, 5, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, C.S.; Sivaguru, P.; Grzegorz Malecki, J.; Lalitha, A. Glacial acetic acid-assisted one-pot synthesis of diverse octahydroacridin-4-methylbenzenesulfonamides via Tandem Cascade reactions. Polycycl. Aromat. Compd. 2018, 1–14. [Google Scholar] [CrossRef]
- Cremer, D.T.; Pople, J.A. General definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Chang, R. Chemistry, 7th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Kosar, B.; Albayrak, C. Spectroscopic investigations and quantum chemical computational study of (E)-4-methoxy-2-[(p-tolylimino)methyl]phenol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 160–167. [Google Scholar] [CrossRef]
- Koopmans, T.A. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den einzelnen Elektronen eines Atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A Theoretical study. J. Org. Chem. 2008, 73, 4615. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Soliman, S.M.; Ghabbour, H.A.; Elnakady, Y.A.; Mohaya, T.A.; Siddiqui, M.R.; Albericio, F. Ultrasonic promoted synthesis of novel s-triazine-Schiff base derivatives; molecular structure, spectroscopic studies and their preliminary anti-proliferative activities. J. Mol. Struct. 2016, 1125, 121–135. [Google Scholar] [CrossRef]
- Farooq, M.; Sharma, A.; Almarhoon, Z.; Al-Dhfyan, A.; El-Faham, A.; Taha, N.A.; Wadaan, M.A.; Beatriz, G.; Albericio, F. Design and synthesis of mono-and di-pyrazolyl-s-triazine derivatives, their anticancer profile in human cancer cell lines, and in vivo toxicity in zebrafish embryos. Bioorganic Chem. 2019, 87, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.R.; Modh, R.P.; Chikhalia, K.H. Privileged s-triazines: Structure and pharmacological applications. Future Med. Chem. 2014, 6, 463–477. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).