Abstract
Chondroitin sulfates are linear anionic sulfated polysaccharides found in biological tissues, mainly within the extracellular matrix, which are degraded and altered by specific lyases depending on specific time points. These polysaccharides have recently acquired relevance in the pharmaceutical industry due to their interesting therapeutic applications. As a consequence, chondroitin sulfate (CS) lyases have been widely investigated as tools for the development of new pharmaceuticals based on these polysaccharides. This review focuses on the major breakthrough represented by chondroitin sulfate-degrading enzymes and their structures and mechanisms of function in addition to their major applications.
1. Introduction
Proteoglycans consist of a central protein core with O-linked glycosaminoglycan (GAG) side-chains. They can be categorized into four main groups based on differences between the repeating disaccharide units comprising GAGs: heparan sulfate (HS), chondroitin sulfate (CS), dermatan sulfate (DS) and hyaluronic acid (HA) (Figure 1A).
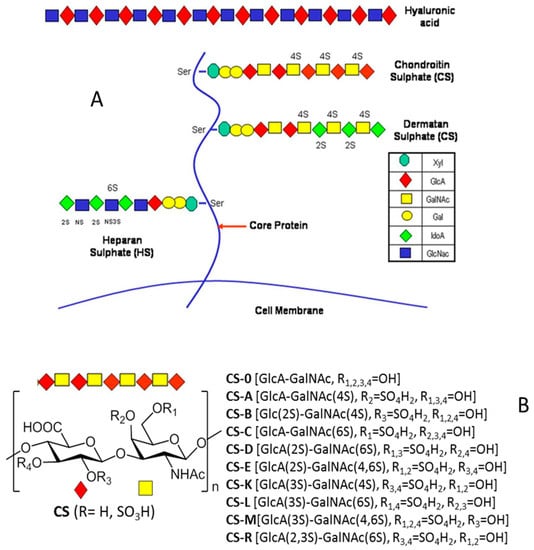
Figure 1.
(A) The general structure of proteoglycans. The chemical structures of glycosaminoglycans (GAGs) are shown. (B) The natural sulfation patterns of the chondroitin sulfates (CSs).
CSs are a family of highly sulfated polysaccharides that have recently acquired relevance in the pharmaceutical industry due to their interesting therapeutic applications [1]. Structurally, these are linear polysaccharides and their basic unit is a disaccharide composed of a D-glucopyranosyluronic acid (◊) or L-idopyranosiduronic acid (◊) glycosidically linked (β (1→3)) to an N-acetyl-D-galactosamine residue (□) in which the hydroxyl groups undergo sulfation at one or more positions. The disaccharide subunits found in natural CSs are shown in Figure 1B.
Their ubiquity in the human body and their essential functions for life have aroused great interest in their medical applications [2]. In addition to their well-known applications in the treatment of osteoarthritis [3] and thrombosis [4], other potential pharmaceutical applications have been proposed [5]. Table 1 summarizes the physiological functions of CSs in animal cells and tissues and their potential medical/pharmacological applications.

Table 1.
Physiological functions in animal cells and tissues and potential medical/pharmacological applications of CSs.
Additionally, CSs are widely used in other pharmacological applications such as coating materials for implants, hydrogels in controlled release applications, components of 3D-constructs such as tissue engineering scaffolds and even as biosensors in diagnostic devices [8,28]. Table 2 summarizes these applications of CS.

Table 2.
Applications of CS in 2D and 3D systems.
However, CSs present not only positive outcomes with regard to their pharmaceutical applications—numerous downsides have also been pointed out. On the one hand, CSs extracted from natural sources have a high structural diversity in terms of their molecular weight (Mw) and degree of sulfation. On the other hand, the preservation of the functionality of CSs while maintaining their biocompatibility is a challenging task and must be addressed depending on the specific application.
To provide solutions to these drawbacks, new approximations have been described during the last decade, with the enzymatic modification of CSs being one of the most widely employed [52,53]. In this context, glycosaminoglycan lyases (GAGLs) can be combined with separation methods for the preparation of CS oligosaccharides for biological evaluations as well as for disaccharide analysis and polysaccharide sequencing [54].
These enzymes have important therapeutic value for the treatment of diseases related to GAGs [55]. Hence, chondroitinase ABC, for example, is being tested in clinical trials for the treatment of spinal cord injury [56]. The same CSase inhibits melanoma invasion, proliferation and angiogenesis [57], and has also been applied as a subretinal injection [58] and for the treatment of intervertebral disc protrusion [59]. On the other hand, HAase has been successfully used as an adjuvant for infiltration anesthesia due to the increased membrane permeability induced by the hydrolysis of HA [60]. Finally, some GAG-degrading enzymes are used as an adjuvant therapy in cancer, in which their administration to reduce the progression of metastatic breast cancer is well tolerated without adverse events [61,62].
Despite the great interest in these direct medical applications, in this review we focused only on the GAG-degrading enzymes, specifically CSases as biocatalytic tools for the development of new pharmaceuticals based on GAGs.
2. Types, Mechanism and Structure of CS Lyases
GAG-degrading enzymes are widely distributed in nature and are structurally diverse depending on whether they are produced by eukaryotic or prokaryotic organisms. These enzymes catalyze the depolymerization of GAGs and are classified according to their enzymatic mechanism in two categories; hydrolases and lyases. They act with an extremely high degree of stereospecificity. Additionally, a classification based on amino acid sequence similarities has been proposed (http:www.cazy.org).
Mammalian enzymes are hydrolases and their mechanism is the same as glucosidases in which the glycosyl–oxygen (C1–O) bond is hydrolyzed by the addition of a water molecule [63], affording saturated oligosaccharide products. In contrast, bacterial enzymes degrade GAGs either through hydrolysis or by a β-elimination reaction (lyases). The latter degrades GAGs by cleaving the oxygen–aglycone (O–C4) linkages on the non-reducing side of uronic acids yielding unsaturated C4–C5 products [64,65,66]. The mechanism of action of these enzymes is shown in Figure 2. In the first step, the negative charge on the C5 carboxylate group is neutralized presumably by interaction with a positively charged arginine or calcium ion, thereby reducing the acidity of the C5 proton. Next, the proton at C5 of the GlcA is abstracted by a His residue, leading to the elimination of the 4-O–glycosidic bond and the formation of a double bond between C4–C5 of the uronic acid. Finally, an acidic residue of the protein (Tyr) donates a proton to the O-leaving group of the glucosamine, reconstituting the hydroxyl functional group at the reducing end of the cleaved bond and releasing the products.
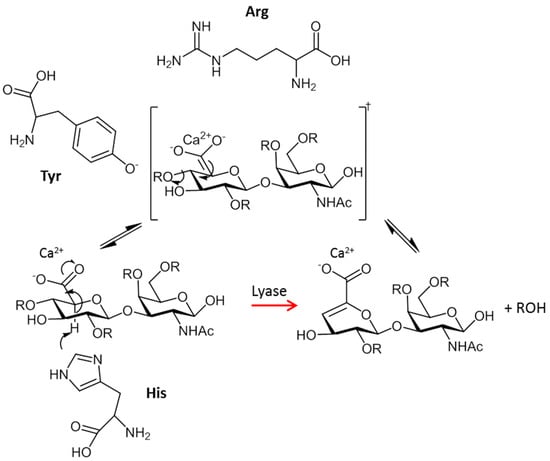
Figure 2.
The catalytic mechanism of the GAG-degrading enzymes by elimination cleavage (lyases).
These enzymes catalyze reactions with an extremely high degree of stereospecificity, with extensive variation in specificity among lyases for different GAG classes [54]. Accordingly, these enzymes can be divided into three groups depending on the composition of the repeating disaccharide unit: GlcA-β(1-4)-GlcNAc for heparinases (Hsases) and hyaluronidases (HAases), and GlcA-β(1-3)-GlcNAc for chondroitinases (CSases) [67]. In this review we focused on those that degrade polysaccharides in which the uronic acids are β(1→3) linked to N-acetyl-D-galactosamine (CS/DS) or to N-acetyl-D-glucosamine (HA) (Figure 3). Some exceptions have been found to this stereospecificity. Hence, an HSase isolated from Erpobdellidae (Nephelopsis obscura and Erpobdella punctata), for example, degrades HA by hydrolysis [68,69] while HAase lyase from Streptococcus pneumoniae degrades HA/CS by β-elimination [66].
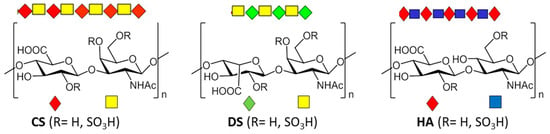
Figure 3.
The chemical structure of GAG disaccharide-repeat building blocks of CS, dermatan sulfate (DS) and hyaluronic acid (HA).
In addition to the GAG-class specificity, the majority of these enzymes degrade glycosidic bonds with absolute uronic acid epimer specificity towards either GlcA or IdoA, as well as being dependent on their sulfation pattern. Thus, CSase AC specifically degrades the glucoronic acid-containing glycosidic bonds present in CS-A (chondroitin 4-sulfate) and CS-C(chondroitin 6-sulfate). In fact, the nomenclature for the CSases has been established based on this sulfation pattern specificity. Hence, CSase AC, for instance, cleaves CS-A and CS-C but not DS (chondroitin 2,4-Disulfate, CS-B).
Finally, the GAG-degrading enzymes can present endolytic or exolytic modes of action. In the former, the cleavage occurs in the middle of the GAG chain, yielding a mixture of disaccharides, tetrasaccharides and longer oligosaccharides. In the latter, the enzyme degrades the chain from the end, releasing only disaccharide products.
Table 3 and the following text summarize the findings regarding the classes, mechanisms and structures of the CS-specific lyases.

Table 3.
CS-degrading enzymes (lyases).
2.1. Chondriotinases ABC
Chondriotinases ABC (EC 4.2.2.4) with endo activity (CSase ABC I) [73] or exo activity (CSase ABC II D) [70] are catalysts with a high degree of stereospecificity. CSases ABC can degrade chondroitin, CS (A and C), DS and HA independent of their sulfation pattern by β-elimination, producing unsaturated disaccharides and tetrasaccharides. They are not active against keratan sulfate, HS and heparin. In rare cases, a single enzyme is able to degrade both uronic acid isomers (IdoA and GlcA) efficiently.
2.1.1. CSase ABC I Endolyase
CSase ABC I endolyase from Proteus vulgaris (EC:4.4.4.20) presents three domains (ID:1HNO); an N-terminal domain with a fold similar to that of carbohydrate-binding domains, a middle domain with an (α/α)5 fold (typical for CSase AC) and a C-terminal domain with β-sheet folding (typical for CSase B) (Figure 4). The substrate-binding site is in the middle domain, a wide-open cleft with two structural folds that evolved to perform these reactions in an epimer-specific fashion [70,71,72,73] (Figure 4). The putative catalytic residues of CSase ABC I from P. vulgaris are His501, Tyr508, Arg560 and Glu653, which were identified by site-directed mutagenesis [74]. The His501 residue plays the critical role of the proton abstraction of C5 from the IdoA/GlcA moiety during catalysis, the Glu653 residue is involved in a hydrogen bonding network in the active site, the Tyr508 residue is essential in the protonation of the leaving group in the GAG and the Arg560 residue near the IdoA/GlcA is able to stabilize the carbanion intermediate formed during catalysis, making the C5 proton more labile (Figure 4). This enzyme presents a shared identity of over 90% in the following species: Shigella sp. FC1655, Proteus mirabilis, Klebsiella pneumoniae, Proteus hauseri and Proteus penneri. This enzyme is able to promote functional recovery in the injured central nervous system via its role in the disruption of the normal organization of the extracellular matrix.
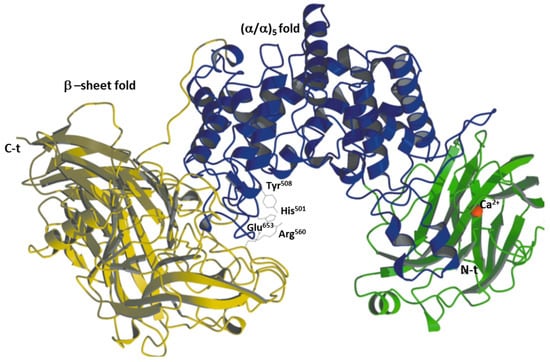
Figure 4.
CSase ABC I from P. vulgaris. The N-terminal domain is colored green, the middle (catalytic) domain is in blue and the C-terminal domain is in yellow. The Ca2+ ion is shown as a red sphere. The catalytic tetrad is shown by the stick (His501, Tyr508 and Arg560).
2.1.2. CSase ABC II Exolyase from Bacteroides thetaiotaomicron
CSase ABC II exolyase from Bacteroides thetaiotaomicron (gene BT_3324) is a broad-specificity lyase which degrades CSs and DS to yield only disaccharide products. This enzyme has a preference for CS-A over CS-C and exhibits low activity against HA. The enzyme presents three structural domains; an N-t domain which adopts a β-jellyroll fold (carbohydrate binding), a central domain which adopts an (α/α)5 incomplete toroid and a C-t domain which contains four antiparallel β-sheets. Glu628, Tyr461, His454 and Arg514 contribute to the catalytic tetrad and one structural Ca2+ ion is located in the N-t domain [70]. Tyr461 in a deprotonated state acts as the catalytic base abstracting the C5-bound proton from glucuronic acid, His454 serves as a catalytic base and Glu628 plays a part in positioning both His454 and Arg514 in the precise orientation necessary for effective CS/DS degradation, but is not directly involved in catalysis. Similar proteins with a shared identity of 90% are found in other species, including: Bacteroides faecis CAG:32, Bacteroides thetaiotaomicron CAG:40, Bacteriodes sp. AR20 and Klebsiella oxytoca.
2.1.3. CSase ABC II Exolyase from Proteus vulgaris
CSase ABC II exolyase from Proteus vulgaris has a broad specificity of GAG activity which preferentially degrades the tetra- and hexasaccharide derivatives of CS and DS produced by the CS ABC endolyase, to yield the respective disaccharides. This enzyme is inhibited by Ni2+. The catalytic residues are His453 (proton acceptor) and Tyr 460 (proton donor).
2.1.4. Other CSases
Recently, CSase ABC from Acinetobacter sp. C26 has been described, with the finding that its activity increases in the presence of a number of different ions (Na+, K+, Mn2+) and is strongly inhibited by other kinds of ions (Cu2+, Hg2+, Al3+) [82].
CSase ABC displays a more open cleft in the central domain (substrate binding), which is different from the other lyases.
2.2. CSases AC
CSases AC with endo activity (from Flavobacterium heparinum) [83] or exo activity (from Arthrobacter aurescens) [83] degrade CS (CS-A or CS-C) and HA, generating unsaturated disaccharides. These enzymes show a low level of homology with several hyaluronate lyases, although they share its fold. CSase AC from Flavobacterium heparinum is composed of two domains; an α-helical domain (N-t) within enzymatic activity residues and a β-sheet domain (C-t). These enzymes are sensitive to the 5-epimerization of the GlcA moiety, so they can only degrade CSs containing a domain with an (α/α)5 toroid fold. The crystal structures of the lyase–GAG complex showed that His225 is the candidate for the catalysis and the Tyr–His–Glu–Arg residues are present in the catalytic center. Four residues—His225, Tyr234, Arg288 and Glu371—are near the catalytic site of chondroitin AC lyase from Flavobacterium heparinium (Figure 5) [78]. The His225 residue is a candidate for the general base and the removes the proton attached to C5 of the glucuronic acid, the Tyr234 residue is able to protonate the leaving group and the Arg288 residue contributes to charge neutralization and stabilization of the enolate anion intermediate. These enzymes could have endolytic or exolytic activity depending on the microorganism (Table 3), while the activity itself is independent of metal ions [78]. The structural alignment of CSases AC and CSase ABC shows that the Tyr–His–Arg–Glu catalytic tetrads of CSases AC have counterparts in CSase ABC [78].
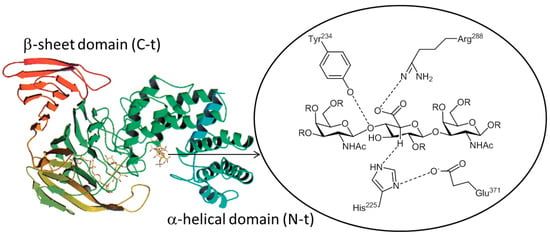
Figure 5.
Ribbon stereo drawing of the chondroitinase AC I from Flavobacterium heparinum showing the N-t domain ((α/α)5 (green), β-sheet sandwich C-t domain (orange)) and active site.
2.3. CSase B
CSase B from Flavobacterium heparinum [83] cleaves endolytically on the GAG DS, generating oligosaccharides, tetrasaccharides and unsaturated 4-sulfate-disaccharides. This enzyme is often used in studies to assess the structural characterization and antithrombin activity of DS by chromatographic techniques. The first crystallographic study of this enzyme with DS facilitated the identification of the subsites in the active site [79,80]. Later, establishing the structure of the lyase–CS complex provided a more complete picture of the active site of the enzyme including the identification of the catalytic residues Lys250, Arg271 and His272 [79,84]. Mutation of the amino acid Lys resulted in the inactivation of the enzyme, which is attributed to the role of the residue in stabilizing the carbanion of C5 formed during catalysis. The 3D X-ray structure revealed the presence of a divalent ion coordinated by conserved acidic residues (one asparagine and two glutamates) and utilized for charge neutralization of the acidic group of iduronic acid [84] (Figure 6). The activity of this enzyme is inhibited by Co2+, Fe2+ and Ba2+ ions. CSase B adopts a β-helical fold, typical of several polysaccharide lyases and hydrolases (Figure 6).
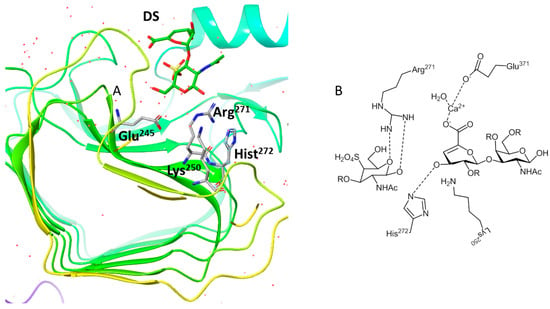
Figure 6.
(A) Ribbon stereo drawing of Chondroitinase B from Flavobacterium heparinum (pdb ID:1egu); (B) the active site of the enzyme CSase B. Figure generated by Maestro and ChemDraw.
2.4. HAases
HAases from Streptococcus pneumoniae degrade hyaluronan and chondroitin/CSs only in four positions of the sulfate group based on the β-elimination mechanism [85]. These lyases show low levels of homology with chondroitinases of type AC. The enzymatic activity residues are within the N-terminal domain. The enzyme molecule is composed of two domains, the catalytic domain having a (α/α)5 barrel fold (N-t) and the C-t domain comprising an antiparallel β-sandwich (Figure 7). The function of the C-t domain is the modulation of the oligosaccharide substrate access to the catalytic cleft present in the N-t domain. Any changes in the binding mode of the protein were detected when used as disaccharides of CS-O, CS-A and CS-C.
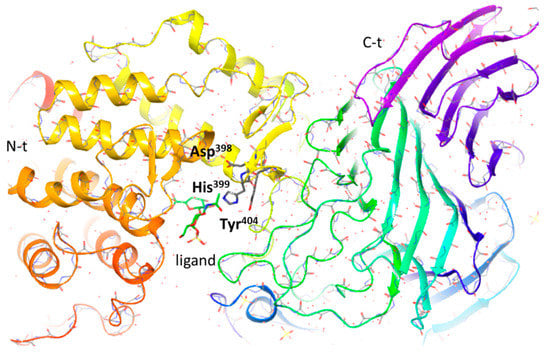
Figure 7.
Ribbon stereo drawing of the hyaluronidase from Streptococcus pneumoniae (pdb ID:1ojo). Figure generated by Maestro.
The HAase from Vibrio sp. FC509 degrades hyaluronan and CS variants except CS-E (GlcA-GalNAc(4,6S)), while the desulfation of the GalNAc unit abrogates its activity on the β(1-4) linkage between the disaccharide units. This protein uses a general acid–base catalysis mechanism as with the other lyases [62].
3. Applications of CSases
3.1. Synthetic Applications: Preparation of Low Molecular Weight Chondroitin Sulphate (LMWCS) as Therapeutic Agents
The preparation of low molecular weight chondroitin sulphate (LMWCS) has mainly been accomplished through acidic, basic or oxidative treatment or by enzymatic depolymerization, allowing oligosaccharides with different molecular weights and degrees of polydispersity to be obtained depending on the employed degradation conditions [86] (Table 4). In the case of the chemical procedures, fairly drastic conditions can cause undesirable reactions and partial or total desulfation of the obtained oligosaccharides, modifying the biological properties of the resulting oligosaccharides [87].

Table 4.
The Mw of natural and depolymerized CS.
On the contrary, enzymatic depolymerizations are more specific and allow better control of the processes, as well as being environmentally friendly. Additionally, the polysaccharide substrates and enzymes are relatively inexpensive, meaning the oligosaccharides can be prepared in large quantities at a low cost. For this reason, several specific (CSase AC I, CSase AC II and CSase ABC) and non-specific enzymes (HAases) have been employed in the preparation of LMWCS.
These oligosaccharides have therapeutic applications, the depolymerization of natural CSs being a strategy that can be used to remove their main limitations in many medical applications. It is well understood that CSs of natural origin have high polydispersity, varying significantly in chain length even when isolated from a single source [88,89]. Furthermore, their high molecular weights preclude their use in many medical uses, impacting not only their biological activities but also their equally important pharmacological properties [90]. For these reasons, their degradation products (LMWCS) have been found to be much more useful than native CSs.
Several studies have demonstrated that changes in the Mw cause different immune responses and that the use of long-chain CSs can even result in the cancellation of the anti-inflammatory activity [91,92]. In a similar way, LMWCS has demonstrated a superior effect on collagen-induced arthritis as compared to that of intact CSs [93]. To this must be added that even though the Mw is similar, the biological effect is further augmented in the case of CSs with a narrow range of molecular weights, i.e., polysaccharides with low polydispersity [94]. An explanation for these results is that only LMWC derivatives reach the bloodstream, as absorption through the gastrointestinal tract is a Mw-dependent process [95,96]. In fact, it has been reported that LMWCS administered orally for osteoarthritis treatment is more readily absorbed and hence arrives at the joint and is distributed into the cartilage more effectively than native CSs [97]. Finally, the Mw also has an important effect on the elimination of exogenously administered CSs. The use of LMWCS prevents its hepatic accumulation and improves its renal filtration [98,99].
Accordingly, the preparation of LMWCS for osteoarthritis treatment has attracted much attention in recent years, especially with the cloning, expression and characterization of new GAG lyases such as chondroitin lyase AC II from Arthrobacter aurescens [100] and the chondroitinase ABC I from Proteus vulgaris with a maltose-binding protein [101] or with glyceraldehyde-3-phosphate dehydrogenase [102] as fusion proteins.
LMWCS 4-sulfated polysaccharides have been prepared by the degradation of CS-A from bovine trachea tissue using a bovine testicular hyaluronidase [103]. These 4-sulfated polysaccharides (CS-A) are known for their antioxidant activity, a capacity that is closely related to the treatment of diseases such as cancer, cardiovascular and cerebrovascular diseases and ischemia, as well as aging processes [104]. In a similar way, the digestion of CS-A from cow cartilage with an extracellular chondroitinase ABC produced by Sphingomonas paucimobilis afforded CS-A oligosaccharides. These promote in vitro cardiocytoprotection, decreasing the well-known damage induced by isoproterenol and accelerating the recovery of myocardial cells [105].
Recently, the preparation of low molecular weight fucosylated chondroitin sulfates (LMWfCSs) has attracted much attention [106] (Figure 8). Fucosylated CSs (fCSs) are CSs with fucose branches invariably extending from the 3-O-position of the GlcA. These have demonstrated anticoagulant and antithrombotic activities as substitutes for heparin [107] and have attracted considerable attention as potential antitumor drugs [108,109] and for their application as a treatment of hyperlipidemia [110,111]. Unfortunately, native fCSs can also cause side effects such as the activation of FXII, platelet aggregation [112], hypertension and spontaneous bleeding in humans [87], limiting their therapeutic applications. However, LMWfCSs have demonstrated that in addition to the retention or even enhancement of biological activities in comparison with native fCS, depolymerized polysaccharides exhibit negligible adverse effects [113].
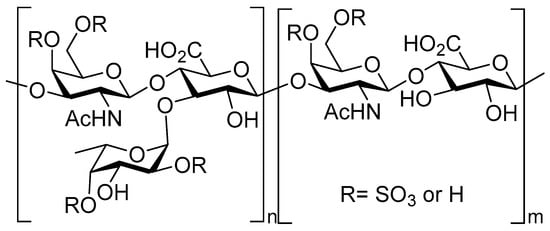
Figure 8.
Chemical structure of fucosylated CS (fCS).
3.2. Analytical Applications
3.2.1. Oligosaccharide Mapping
Knowledge of the oligosaccharide sequences that contain GAGs is an important prerequisite for a better understanding of their biological roles and the development of pharmaceuticals based on them. Oligosaccharide mapping is an approach comparable to the peptide mapping of proteins that has been applied to GAGs in order to provide information on which oligosaccharide sequences are the bioactive domains.
The mapping technique involves specific enzymatic scission of polysaccharide chains, followed by high-resolution separation of the degradation products by chromatographic methods and the final analysis of the obtained oligosaccharides [114]. In this context, the use of enzymes that can specifically isolate certain domains through the selective digestion of other domains has been reported. Hence for example, a lyase from the marine bacterium Vibrio sp. FC509 has been used to isolate several CS-E oligosaccharides and their interaction with herpes simplex virus receptors has been analyzed [115]. As such, the bioactive domain in the binding to the virus has been established [115], opening the door to the development of new antiviral drugs (Figure 9).
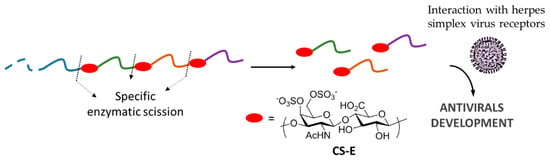
Figure 9.
Oligosaccharide mapping for the discovery of new antivirals.
In a similar way, CS from shark fin cartilage has been digested with chondroitinase AC-I from Flavobacterium heparinum, an enzyme which cannot act on the galactosaminidic linkages bound to GlcA(2-O-sulfate)-GalNAc(6-O-sulfate) disaccharides (CS-D units) (Figure 10A) [116]. This digestion afforded five novel hexasaccharide sequences in addition to three previously reported sequences containing D-D-tetrasaccharide motifs (Figure 10B), which might be useful for the establishment of useful structure–function relationships in neuroglycobiological fields with regard to novel biomarkers.
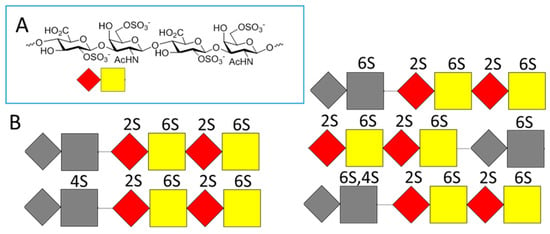
Figure 10.
(A) The chemical structure of D-D-tetrasaccharide motifs. (B) The general structure of novel hexasaccharide sequences.
3.2.2. Compositional Analysis of GAGs
Another important application of GAG lyases is the compositional analysis of polysaccharides, both from natural sources and semi-synthetic products. Normally, a small quantity of the polysaccharide is exhaustively depolymerized using the proper lyase; then, the CS disaccharides obtained from this process are analyzed by High Perfermance Liquid Chromatograpgy (HPLC) and quantified by calculating the total peak areas of the disaccharides derived from a CS calibration curve.
As an example of the analysis of CS from natural sources, very recently a comprehensive disaccharide analysis of polysaccharides from different shellfish was performed to better understand the GAG structures in marine organisms [117]. According to the obtained results, the degree of sulfation in CSs depends on the species and, surprisingly, ∆Di-diSE is present in most shellfish (Figure 11).
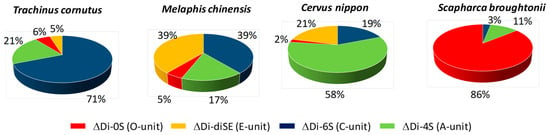
Figure 11.
Unsaturated disaccharide composition of CSs from different types of shellfish.
Finally, enzymatic digestion followed by disaccharide analysis using HPLC is a very useful procedure for evaluation of the composition of semi-synthetic CSs. For example, we employed this method to determine the composition of a library of polysaccharides recently prepared in order to establish novel structure–function relationships [118] (Figure 12). Concretely, we showed that the particular sulfate distribution within the disaccharide repeating-unit plays a key role in the binding of growth factors, modulating the surface charge of the helical structure that, interestingly, has a significant influence on the binding capacity of CSs with several Grow Factors [119]. These findings provide additional strategies in the development of new CSs as growth factor binders for a broad range of therapeutically relevant applications [120].
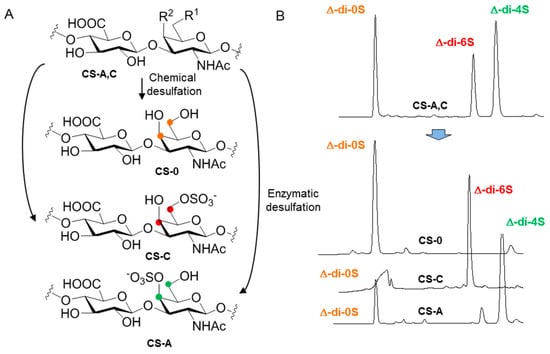
Figure 12.
(A) The semi-synthesis of CS-0, CS-A and CS-C. (B) The analysis of disaccharides by HPLC after CSase ABC digestions.
4. Conclusions and Future Perspectives
The types, structure and activity of CS-degrading enzymes were the focus of this review. These lyases can be employed as tools for the development of new pharmaceuticals based on CS structures. During the last decades, these structures have been demonstrated to be involved not only in structural functions, but also in modulating numerous biological processes, such as angiogenesis, cell differentiation, growth and migration. These findings provide a promising avenue in the development of new pharmaceuticals based on CSs for a broad range of therapeutically relevant applications, including new drugs, drug carriers and medical implants.
However, it should be note that their development still faces challenges, which includes the discovery of more efficient and universal methods to synthesize LMWCSs with precisely controllable structures. Moreover, their interactions with growth factors and cells as well as their biological processes still need to be studied further. Thus, among other things, deep insight into CSs sequences as well as their relation to biological functions is required.
In our opinion, chondroitin sulfate-degrading enzymes will gradually become more present in the pharmaceutical industry in the next years, not only as excellent drug candidates [55,56,57,58,59,60,61,62] but also as important tools for the sustainable development of CS-based pharmaceutical products.
Author Contributions
Conceptualization, A.B. and J.R.; resources, S.Z. and R.B.-A; writing—review and editing, J.R. and A.B.
Funding
The authors thank the Ministerio de Economía y Competitividad (grants MAT2015- 65184-C2-2-R and CTQ2016-79255-P) for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Volpi, N. Therapeutic applications of glycosaminoglycans. Curr. Med. Chem. 2006, 13, 1799–1810. [Google Scholar] [CrossRef] [PubMed]
- Stick, R.V.; Williams, S. Glycoproteins and proteoglycans. In Carbohydrates: The Essential Molecules of Life; Stick, R.V., Williams, S., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2008; pp. 369–412. [Google Scholar]
- Hermann, W.; Lambova, S.; Muller-Ladner, U. Current treatment options for osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Mourão, A.P. Perspective on the use of sulfated polysaccharides from marine organisms as a source of new antithrombotic drugs. Mar. Drugs 2015, 13, 2770–2784. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Sugahara, K. Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr. Drug Discov. Tech. 2008, 5, 289–301. [Google Scholar] [CrossRef]
- Milstone, L.M.; Houghmonroe, L.; Kugelman, L.C.; Bender, J.R.; Haggerty, J.G. Epican, a heparan/chondroitin sulfate proteoglycan form of CD44, mediates cell-cell adhesion. J. Cell Sci. 1994, 107, 3183–3190. [Google Scholar] [PubMed]
- Stabler, T.V.; Huang, Z.; Montell, E.; Verges, J.; Kraus, V.B. Chondroitin sulphate inhibits NF-κB activity induced by interaction of pathogenic and damage associated molecules. Osteoarthritis Cartilage 2017, 25, 166–174. [Google Scholar] [CrossRef]
- Wu, F.F.; Zhou, C.H.; Zhou, D.D.; Ou, S.Y.; Liu, Z.J.; Huang, H.H. Immune-enhancing activities of chondroitin sulfate in murine macrophage RAW 264.7 cells. Carbohydr. Polym. 2018, 198, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, S.J.; Chaiyaroj, S.C.; Ng, K.; Reeder, J.C.; Brown, G.V. Chondroitin sulfate A is a cell-surface receptor for Plamodium-falciparum infected erythrocytes. J. Exp. Med. 1995, 182, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Clausen, T.M.; Pehrson, C.; Mao, Y.; Resende, M.; Daugaard, M.; Kristensen, A.R.; Spliid, C.; Mathiesen, L.; Knudsen, L.E.; et al. Placental Sequestration of Plasmodium falciparum malaria parasites is mediated by the interaction between VAR2CSA and Chondroitin sulfate A on Syndecan-1. PloS Pathog. 2016, 12, e1005831. [Google Scholar] [CrossRef]
- Mourao, P.A.S.; Pereira, M.S.; Pavao, M.S.G.; Mulloy, B.; Tollefsen, D.M.; Mowinckel, M.C.; Abildgaard, U. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm.Sulfated fucose branches on the polysaccharide account for its high anticoagulant action. J. Biol. Chem. 1996, 271, 23973–23984. [Google Scholar] [CrossRef]
- Glauser, B.F.; Pereira, M.S.; Monteiro, R.Q.; Mourao, P.A.S. Serpin-independent anticoagulant activity of a fucosylated chondroitin sulfate. Thromb. Haemos. 2008, 100, 420–428. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Panina, E.G.; Sanamyan, N.P.; Dmitrenok, A.S.; Tsvetkova, E.A.; Ushakova, N.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure and anti-inflammatory activity of a new unusual fucosylated chondroitin sulfate from Cucumaria djakonovi. Mar. Drugs 2018, 16, 389. [Google Scholar] [CrossRef] [PubMed]
- Lovu, M.; Dumais, G.; du Souich, P. Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis Cartilage 2008, 16, S14–S18. [Google Scholar]
- Bergefall, K.; Trybala, E.; Johansson, M.; Uyama, T.; Naito, S.; Yamada, S.; Kitagawa, H.; Sugahara, K.; Bergström, T. Chondroitin sulfate characterized by the E-disaccharide unit is a potent inhibitor of herpes simplex virus infectivity and provides the virus binding sites on gro2C cells. J. Biol. Chem. 2005, 280, 32193–32199. [Google Scholar] [CrossRef] [PubMed]
- Mycroft-West, C.J.; Yates, E.A.; Skidmore, M.A. Marine glycosaminoglycan-like carbohydrates as potential drug candidates for infectious disease. Biochem. Soc. Trans. 2018, 46, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Muthusamy, A.; Miao, J.; Cui, L.W.; Salanti, A.; Winzeler, E.A.; Gowda, D.C. Targeted disruption of a ring-infected erythrocyte surface antigen (RESA)-like export protein gene in Plasmodium falciparum confers stable chondroitin 4-sulfate cytoadherence Capacity. J. Biol.l Chem. 2014, 289, 34408–34421. [Google Scholar] [CrossRef] [PubMed]
- Alkhalil, A.; Achur, R.N.; Valiyaveettil, M.; Ockenhouse, C.F.; Gowda, D.C. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. J. Biol. Chem. 2000, 275, 40357–40364. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulou, A.P.; Theocharis, A.D.; Tzanakakis, G.N.; Karamanos, N.K. The biological role of chondroitin sulfate in cancer and chondroitin-based anticancer agents. In Vivo 2008, 22, 385–389. [Google Scholar] [PubMed]
- Borsig, L.; Wang, L.; Cavalcante, M.C.M.; Cardilo-Reis, L.; Ferreira, P.L.; Mourão, P.A.S.; Esko, J.D.; Pavão, M.S.G. Selectin blocking activity of a fucosylated chondroitin sulfate glycosaminoglycan from sea cucumber: Effect on tumor metastasis and neutrophil recruitment. J. Biol. Chem. 2007, 282, 14984–14991. [Google Scholar] [CrossRef]
- Merida-de-Barros, D.A.; Chaves, S.P.; Belmiro, C.L.R.; Wanderley, J.L.M. Leishmaniasis and glycosaminoglycans: a future therapeutic strategy? Parasit. Vectors 2018, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Vallen, M.J.E.; van Tilborg, A.A.G.; Tesselaar, M.H.; ten Dam, G.B.; Bulten, J.; van Kuppevelt, T.H.; Massuger, L. Novel single-chain antibody GD3A10 defines a chondroitin sulfate biomarker for ovarian cancer. Biomark. Med. 2014, 8, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Tamaki, H.; Fukui, S. Detection of oligosaccharide ligands for Hepatocyte growth factor/Scatter factor (HGF/SF), Keratinocyte growth factor (KGF/FGF-7), RANTES and Heparin cofactor II by neoglycolipid microarrays of glycosaminoglycan-derived oligosaccharide fragments. Glycoconjugate J. 2006, 23, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Djerbal, L.; Lortat-Jacob, H.; Kwok, J. Chondroitin sulfates and their binding molecules in the central nervous system. Glycoconjugate J. 2017, 34, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.M.; Hsieh-Wilson, L.C. Sugar-dependent modulation of neuronal development, regeneration, and plasticity by chondroitin sulfate proteoglycans. Exp. Neurol. 2015, 274, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Gao, J.; Hou, L.; Wang, L.; Zhang, F.; Sun, F.; Zhang, T.; Xu, P.; Shi, Z.; Hu, F.; et al. Neuroprotective effect of chondroitin sulfate on SH-SY5Y cells overexpressing wild-type or A53T mutant α-synuclein. Mol. Med. Rep. 2017, 16, 8721–8728. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.H.; Jiang, Y.Z.; Zhang, G.R.; Jin, H.M.; Hieu, N.T.M.; Ouyang, H.W. Specific interactions between human fibroblasts and particular chondroitin sulfate molecules for wound healing. Acta Biomater. 2009, 5, 1588–1595. [Google Scholar] [CrossRef]
- Kowitsch, A.; Zhou, G.Y.; Groth, T. Medical application of glycosaminoglycans: A review. J. Tissue Eng. Regen. Med. 2018, 12, E23–E41. [Google Scholar] [CrossRef] [PubMed]
- Altgarde, N.; Nileback, E.; de Battice, L.; Pashkuleva, I.; Reis, R.L.; Becher, J.; Moller, S.; Schnabelrauch, M.; Svedhem, S. Probing the biofunctionality of biotinylated hyaluronan and chondroitin sulfate by hyaluronidase degradation and aggrecan interaction. Acta Biomater 2013, 9, 8158–8166. [Google Scholar] [CrossRef] [PubMed]
- Kliemt, S.; Lange, C.; Otto, W.; Hintze, V.; Möller, S.; von Bergen, M.; Hempel, U.; Kalkhof, S. Sulfated Hyaluronan Containing Collagen Matrices Enhance Cell-Matrix-Interaction, Endocytosis, and Osteogenic Differentiation of Human Mesenchymal Stromal Cells. J. Proteome Res. 2013, 12, 378–389. [Google Scholar] [CrossRef]
- Korn, P.; Schulz, M.C.; Hintze, V.; Range, U.; Mai, R.; Eckelt, U.; Schnabelrauch, M.; Moller, S.; Becher, J.; Scharnweber, D.; Stadlinger, B. Chondroitin sulfate and sulfated hyaluronan-containing collagen coatings of titanium implants influence peri-implant bone formation in a minipig model. J. Biomed. Mater. Res. Part A 2014, 102, 2334–2344. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.A.; Melchels, F.P.W.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014, 10, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.S.; Liu, Y.C.; Shu, X.Z.; Prestwich, G.D. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials 2005, 26, 6054–6067. [Google Scholar] [CrossRef] [PubMed]
- Elia, R.; Newhide, D.R.; Pedevillano, P.D.; Reiss, G.R.; Firpo, M.A.; Hsu, E.W.; Kaplan, D.L.; Prestwich, G.D.; Peattie, R.A. Silk-hyaluronan-based composite hydrogels: A novel, securable vehicle for drug delivery. J. Biomater. Appl. 2013, 27, 749–762. [Google Scholar] [CrossRef]
- Shu, X.Z.; Ahmad, S.; Liu, Y.C.; Prestwich, G.D. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J. Biomed. Mater. Res. Part A 2006, 79A, 902–912. [Google Scholar] [CrossRef]
- Jin, R.; Lou, B.; Lin, C. Tyrosinase-mediated in situ forming hydrogels from biodegradable chondroitin sulfate-tyramine conjugates. Polym. Int. 2013, 62, 353–361. [Google Scholar] [CrossRef]
- Ni, Y.L.; Tang, Z.R.; Cao, W.X.; Lin, H.; Fan, Y.J.; Guo, L.K.; Zhang, X.D. Tough and elastic hydrogel of hyaluronic acid and chondroitin sulfate as potential cell scaffold materials. Int. J. Biol. Macromol. 2015, 74, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.Q.; Yeo, Y.; Clifton, R.J.; Jiao, T.; Kohane, D.S.; Kobler, J.B.; Zeitels, S.M.; Langer, R. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules 2006, 7, 3336–3344. [Google Scholar] [CrossRef]
- Kirker, K.R.; Luo, Y.; Nielson, J.H.; Shelby, J.; Prestwich, G.D. Glycosaminoglycan hydrogel films as bio-interactive dressings for wound healing. Biomaterials 2002, 23, 3661–3671. [Google Scholar] [CrossRef]
- Philandrianos, C.; Andrac-Meyer, L.; Mordon, S.; Feuerstein, J.-M.; Sabatier, F.; Veran, J.; Magalon, G.; Casanova, D. Comparison of five dermal substitutes in full-thickness skin wound healing in a porcine model. Burns 2012, 38, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, Q.; Wang, J.; Liu, Y.; Lu, S.; Li, M.; Kaplan, D.L. Silk fibroin/chondroitin sulfate/hyaluronic acid ternary scaffolds for dermal tissue reconstruction. Acta Biomater. 2013, 9, 6771–6782. [Google Scholar] [CrossRef] [PubMed]
- Kesti, M.; Mueller, M.; Becher, J.; Schnabelrauch, M.; D’Este, M.; Eglin, D.; Zenobi-Wong, M. A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater. 2015, 11, 162–172. [Google Scholar] [CrossRef]
- Daamen, W.F.; van Moerkerk, H.T.B.; Hafmans, T.; Buttafoco, L.; Poot, A.A.; Veerkamp, J.H.; van Kuppevelt, T.H. Preparation and evaluation of molecularly-defined collagen-elastin-glycosaminoglycan scaffolds for tissue engineering. Biomaterials 2003, 24, 4001–4009. [Google Scholar] [CrossRef]
- McFadden, T.M.; Duffy, G.P.; Allen, A.B.; Stevens, H.Y.; Schwarzmaier, S.M.; Plesnila, N.; Murphy, J.M.; Barry, F.P.; Guldberg, R.E.; O’Brien, F.J. The delayed addition of human mesenchymal stem cells to pre-formed endothelial cell networks results in functional vascularization of a collagen-glycosaminoglycan scaffold in vivo. Acta Biomater. 2013, 9, 9303–9316. [Google Scholar] [CrossRef]
- Ko, C.-S.; Huang, J.-P.; Huang, C.-W.; Chu, I.M. Type II collagen-chondroitin sulfate-hyaluronan scaffold cross-linked by genipin for cartilage tissue engineering. J. Biosci. Bioeng. 2009, 107, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Lee, H.-P.; Chan, H.-Y.; Sung, L.-Y.; Chen, H.-C.; Hu, Y.-C. Composite chondroitin-6-sulfate/dermatan sulfate/chitosan scaffolds for cartilage tissue engineering. Biomaterials 2007, 28, 2294–2305. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-Y.; Chen, C.-H.; Hsiao, C.-Y.; Chen, J.-P. Incorporation of chitosan in biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel for cartilage tissue engineering. Carbohyd. Polym. 2015, 117, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.L.; McCoy, M.G.; Grant, S.A. Electrospinning collagen and hyaluronic acid nanofiber meshes. J. Mater. Sci. Mater. Med. 2012, 23, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ghosh, K.; Li, B.; Sokolov, J.C.; Clark, R.A.F.; Rafailovich, M.H. Dual-syringe reactive electrospinning of cross-linked hyaluronic acid hydrogel nanofibers for tissue engineering applications. Macromol. Biosci. 2006, 6, 811–817. [Google Scholar] [CrossRef]
- Zhong, S.P.; Teo, W.E.; Zhu, X.; Beuertnan, R.; Ramakrishna, S.; Yung, L.Y.L. Development of a novel collagen-GAG nanofibrous scaffold via electrospinning. Mater. Sci. Eng. C Biomim. Supramol. Syst. 2007, 27, 262–266. [Google Scholar] [CrossRef]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Chitosan-chondroitin sulphate nanoparticles for controlled delivery of platelet lysates in bone regenerative medicine. J. Tissue Eng. Regen. Med. 2012, 6, s47–s59. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Wang, X. Synthetic oligosaccharide libraries and microarray technology: A powerful combination for the success of current glycosaminoglycan interactomics. ChemMedChem 2018, 13, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.Y.; DeAngelis, P.L.; Liu, J.; Linhardt, R.J. Enzymatic synthesis of glycosaminoglycans: improving on nature. In Frontiers in Modern Carbohydrate Chemistry; Demchenko, A.V., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2007; Volume 960, pp. 253–284. [Google Scholar]
- Linhardt, R.J.; Avci, F.Y.; Toida, T.; Kim, Y.S.; Cygler, M. CS lyases: Structure, activity, and applications in analysis and the treatment of diseases. Adv. Pharmacol. 2006, 53, 187–215. [Google Scholar] [PubMed]
- Kasinathan, N.; Volety, S.M.; Josyula, V.R. Chondroitinase: A promising therapeutic enzyme. Crit. Rev. Microbiol. 2016, 42, 474–484. [Google Scholar] [CrossRef]
- Orr, M.B.; Gensel, J.C. Spinal cord injury scarring and inflammation: Therapies targeting glial and inflammatory responses. Neurotherapeutics 2018, 15, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Denholm, E.M.; Lin, Y.Q.; Silver, P.J. Anti-tumor activities of chondroitinase AC and chondroitinase B: inhibition of angiogenesis, proliferation and invasion. Eur. J. Pharmacol. 2001, 416, 213–221. [Google Scholar] [CrossRef]
- Yao, X.Y.; Hageman, G.S.; Marmor, M.F. Recovery of retinal adhesion after enzymatic perturbation of the interphotoreceptor matrix. Invest. Ophthalmol. Vis. Sci. 1992, 33, 498–503. [Google Scholar]
- Kato, F.; Iwata, H.; Mimatsu, K.; Miura, T. Experimental chemonucleolysis with chondroitinase ABC. Clin. Orthop. Relat. Res. 1990, 253, 301–308. [Google Scholar] [CrossRef]
- Buhren, B.A.; Schrumpf, H.; Hoff, N.P.; Bolke, E.; Hilton, S.; Gerber, P.A. Hyaluronidase: From clinical applications to molecular and cellular mechanisms. Eur. J. Med. Res. 2016, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Rzany, B.; Becker-Wegerich, P.; Bachmann, F.; Erdmann, R.; Wollina, U. Hyaluronidase in the correction of hyaluronic acid-based fillers: A review and a recommendation for use. J. Cosmet. Dermatol. 2009, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Niazi, Z.R.; Rehman, F.U.; Akhtar, A.; Khan, M.M.; Khan, S.; Baloch, N.; Khan, S. Hyaluronidases: A Therapeutic Enzyme. Protein Pept. Lett. 2018, 25, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Zechel, D.L.; Withers, S.G. Glycosidase mechanisms: Anatomy of a finely tuned catalyst. Acc. Chem. Res. 2000, 33, 11–18. [Google Scholar] [PubMed]
- Linhardt, R.J.; Galliher, P.M.; Cooney, C.L. Polysaccharide lyases. Appl. Biochem. Biotechnol. 1986, 12, 135–176. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Bernard, T.; Rancurel, C.; Brumer, H.; Coutinho, P.M.; Henrissat, B. A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem. J. 2010, 432, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Garron, M.L.; Cygler, M. Structural and mechanistic classification of uronic acid-containing polysaccharide lyases. Glycobiology 2010, 20, 1547–1573. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Li, F. Hyaluronidase and chondroitinase. Adv. Exp. Med. Biol. 2017, 925, 75–87. [Google Scholar] [PubMed]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their genomics, structures, and mechanisms of action. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef]
- Hovingh, P.; Linker, A. Hyaluronidase activity in leeches (Hirudinea). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1999, 124, 319–326. [Google Scholar] [CrossRef]
- Shaya, D.; Hahn, B.S.; Bjerkan, T.M.; Kim, W.S.; Park, N.Y.; Sim, J.S.; Kim, Y.S.; Cygler, M. Composite active site of chondroitin lyase ABC accepting both epimers of uronic acid. Glycobiology 2008, 18, 270–277. [Google Scholar] [CrossRef]
- Linn, S.; Chan, T.; Lipeski, L.; Salyers, A.A. Isolation and characterization of two chondroitin lyases from Bacteroides thetaiotaomicron. J. Bacteriol. 1983, 156, 859–866. [Google Scholar]
- Yamagata, T.; Saito, H.; Habuchi, O.; Suzuki, S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J. Biol. Chem. 1968, 243, 1523–1535. [Google Scholar]
- Huang, W.; Lunin, V.V.; Li, Y.; Suzuki, S.; Sugiura, N.; Miyazono, H.; Cygler, M. Crystal structure of Proteus vulgaris chondroitin sulfate ABC lyase I at 1.9A resolution. J. Mol. Biol. 2003, 328, 623–634. [Google Scholar] [CrossRef]
- Prabhakar, V.; Raman, R.; Capila, I.; Bosques, C.J.; Pojasek, K.; Sasisekharan, R. Biochemical characterization of the chondroitinase ABC I active site. Biochem. J. 2005, 390, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Lunin, V.V.; Li, Y.; Linhardt, R.J.; Miyazono, H.; Kyogashima, M.; Kaneko, T.; Bell, A.W.; Cygler, M. High-resolution crystal structure of Arthrobacter aurescens chondroitin AC lyase: an enzyme-substrate complex defines the catalytic mechanism. J. Mol. Biol. 2004, 337, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Elmabrouk, Z.H.; Vincent, F.; Zhang, M.; Smith, N.L.; Turkenburg, J.P.; Charnock, S.J.; Black, G.W.; Taylor, E.J. Crystal structures of a family 8 polysaccharide lyase reveal open and highly occluded substrate-binding cleft conformations. Proteins 2011, 79, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Fethiere, J.; Eggimann, B.; Cygler, M. Crystal structure of chondroitin AC lyase, a representative of a family of glycosaminoglycan degrading enzymes. J. Mol. Biol. 1999, 288, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Boju, L.; Tkalec, L.; Su, H.; Yang, H.O.; Gunay, N.S.; Linhardt, R.J.; Kim, Y.S.; Matte, A.; Cygler, M. Active site of chondroitin AC lyase revealed by the structure of enzyme-oligosaccharide complexes and mutagenesis. Biochemistry 2001, 40, 2359–2372. [Google Scholar] [CrossRef] [PubMed]
- Pojasek, K.; Raman, R.; Kiley, P.; Venkataraman, G.; Sasisekharan, R. Biochemical characterization of the chondroitinase B active site. J. Biol. Chem. 2002, 277, 31179–31186. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Matte, A.; Li, Y.; Kim, Y.S.; Linhardt, R.J.; Su, H.; Cygler, M. Crystal structure of chondroitinase B from Flavobacterium heparinum and its complex with a disaccharide product at 1.7 A resolution. J. Mol. Biol. 1999, 294, 1257–1269. [Google Scholar] [CrossRef]
- Rigden, D.J.; Jedrzejas, M.J. Structures of Streptococcus pneumoniae hyaluronate lyase in complex with chondroitin and chondroitin sulfate disaccharides. Insights into specificity and mechanism of action. J. Biol. Chem. 2003, 278, 50596–50606. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, J.; Zhang, J.; Jiang, Y.; Shen, Z.; Guan, H.; Jiang, X. Purification and characterization of chondroitinase ABC from Acinetobacter sp. C26. Int. J. Biol. Macromol. 2017, 95, 80–86. [Google Scholar] [CrossRef]
- Jandik, K.A.; Gu, K.; Linhardt, R.J. Action pattern of polysaccharide lyases on glycosaminoglycans. Glycobiology 1994, 4, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Michel, G.; Pojasek, K.; Li, Y.; Sulea, T.; Linhardt, R.J.; Raman, R.; Prabhakar, V.; Sasisekharan, R.; Cygler, M. The structure of chondroitin B lyase complexed with glycosaminoglycan oligosaccharides unravels a calcium-dependent catalytic machinery. J. Biol. Chem. 2004, 279, 32882–32896. [Google Scholar] [CrossRef] [PubMed]
- Cordula, C.R.; Lima, M.A.; Shinjo, S.K.; Gesteira, T.F.; Pol-Fachin, L.; Coulson-Thomas, V.J.; Verli, H.; Yates, E.A.; Rudd, T.R.; Pinhal, M.A.; Toma, L.; Dietrich, C.P.; Nader, H.B.; Tersariol, I.L. On the catalytic mechanism of polysaccharide lyases: Evidence of His and Tyr involvement in heparin lysis by heparinase I and the role of Ca2+. Mol. BioSyst. 2014, 10, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Feng, D.; Xu, L.; Yin, F.; Zang, H.; Liu, C.; Wang, F. Preparation of low molecular weight chondroitin sulfates, screening of a high anti-complement capacity of low molecular weight chondroitin sulfate and its biological activity studies in attenuating osteoarthritis. Int. J. Mol. Sci. 2016, 17, 1685. [Google Scholar] [CrossRef] [PubMed]
- Buyue, Y.; Sheehan, J.P. Fucosylated chondroitin sulfate inhibits plasma thrombin generation via targeting of the factor IXa heparin-binding exosite. Blood 2009, 114, 3092–3100. [Google Scholar] [CrossRef] [PubMed]
- Tat, S.K.; Pelletier, J.P.; Mineau, F.; Duval, N.; Martel-Pelletier, J. Variable effects of 3 different chondroitin sulfate compounds on human osteoarthritic cartilage/chondrocytes: relevance of purity and Production Process. J. Rheumatol. 2010, 37, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C. Isolation and purification of chondroitin sulfate. Adv. Pharmacol. 2006, 53, 21–31. [Google Scholar] [PubMed]
- Volpi, N. Analytical aspects of pharmaceutical grade chondroitin sulfates. J. Pharm. Sci. 2007, 96, 3168–3180. [Google Scholar] [CrossRef] [PubMed]
- Leeb, B.F.; Schweitzer, H.; Montag, K.; Smolen, J.S. A metaanalysis of chondroitin sulfate in the treatment of osteoarthritis. J. Rheumatol. 2000, 27, 205–211. [Google Scholar]
- Surapaneni, L.; Haley-Zitlin, V.; Bodine, A.; Jiang, X.P.; Brooks, J. Examination of chondroitin sulfate molecular weights on in vitro anti-inflammatory activity. FASEB J. 2013, 27, 1. [Google Scholar]
- Cho, S.Y.; Sim, J.S.; Jeong, C.S.; Chang, S.Y.; Choi, D.W.; Toida, T.; Kim, Y.S. Effects of low molecular weight chondroitin sulfate on type II collagen-induced arthritis in DBA/1J mice. Biol. Pharm. Bull. 2004, 27, 47–51. [Google Scholar] [CrossRef]
- Igarashi, N.; Takeguchi, A.; Sakai, S.; Akiyama, H.; Higashi, K.; Toida, T. Effect of molecular sizes of chondroitin sulfate on interaction with L-Selectin. Int. J. Carbohydr. Chem. 2013, 2013, 856142. [Google Scholar] [CrossRef]
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.S.; Mitra, A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013, 447, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, P.; Cheng, Y.; Zhang, X.; Sheng, J.; Wang, D.; Li, J.; Zhang, Q.; Zhong, C.; Cao, R.; Wang, F. Enhancing the intestinal absorption of low molecular weight chondroitin sulfate by conjugation with alpha-linolenic acid and the transport mechanism of the conjugates. Int. J. Pharm. 2014, 465, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Farran, A.; Montell, E.; Verges, J.; Pelletier, J.P. Discrepancies in composition and biological effects of different formulations of chondroitin sulfate. Molecules 2015, 20, 4277–4289. [Google Scholar] [CrossRef] [PubMed]
- Pecly, I.M.D.; Melo, N.M.; Mourao, P.A.S. Effects of molecular size and chemical structure on renal and hepatic removal of exogenously administered chondroitin sulfate in rats. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N. About oral absorption and human pharmacokinetics of chondroitin sulfate. Osteoarthritis Cartilage 2010, 18, 1104–1105. [Google Scholar] [CrossRef]
- Williams, A.; He, W.Q.; Cress, B.F.; Liu, X.Y.; Alexandria, J.; Yoshizawa, H.; Nishimura, K.; Toida, T.; Koffas, M.; Linhardt, R.J. Cloning and expression of recombinant chondroitinase acii and its comparison to the arthrobacter aurescens enzyme. Biotechnol. J. 2017, 12, 1700239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Y.; Yuan, Q. Expression, purification and thermostability of MBP-chondroitinase ABC I from Proteus vulgaris. Int. J. Biol. Macromol. 2015, 72, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Zhou, Z.; Yuan, Q. Expression, purification and characterization of GAPDH-ChSase ABC I from Proteus vulgaris in Escherichia coli. Protein Expr. Purif. 2016, 128, 36–41. [Google Scholar] [CrossRef]
- Wang, J.P.; Zhang, L.; Jin, Z.Y. Separation and purification of low-molecular-weight chondroitin sulfates and their anti-oxidant properties. Bangladesh J. Pharmacol. 2016, 11, S61–S67. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Jiang, Z.; Chang, J.; Han, B.; Liu, W.; Peng, Y. Purification, characterization of Chondroitinase ABC from Sphingomonas paucimobilis and in vitro cardiocytoprotection of the enzymatically degraded CS-A. Int. J. Biol. Macromol. 2018, 115, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.A.; Sucupira, I.D.; Guedes, A.L.; Queiroz, I.N.; Frattani, F.S.; Fonseca, R.J.; Pomin, V.H. Anticoagulant and antithrombotic properties of three structurally correlated sea urchin sulfated glycans and their low-molecular-weight derivatives. Mar. Drugs 2018, 16, 304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, M.; Xiao, C.; Yang, L.; Zhou, L.; Gaoa, N.; Li, Z.; Chen, J.; Chen, J.; Liu, J.; et al. Discovery of an intrinsic tenase complex inhibitor: Pure nonasaccharide from fucosylated glycosaminoglycan. Proc. Natl. Acad. Sci. USA 2015, 112, 8284–8289. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, J.; Hu, S.; Wang, Y.; Xue, C.; Li, H. The effects of fucosylated chondroitin sulfate isolated from Isostichopus badionotus on antimetastatic activity via down-regulation of Hif-1α and Hpa. Food Technol. Biotechnol. 2014, 23, 1643–1651. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, D.; Wang, S.; Tao, L.; Wang, A.; Chen, W.; Zhu, Z.; Zheng, S.; Gao, X.; Lu, Y. Holothurian glycosaminoglycan inhibits metastasis and thrombosis via targeting of nuclear factor-κB/tissue factor/Factor Xa pathway in melanoma B16F10 cells. PLoS ONE 2013, 8, e56557. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Yan, L.; Ding, T.; Linhardt, R.J.; Yu, Y.; Liu, X.; Liu, D.; Ye, X.; Chen, S. Fucosylated chondroitin sulfate oligosaccharides exert anticoagulant activity by targeting at intrinsic tenase complex with low FXII activation: Importance of sulfation pattern and molecular size. Eur. J. Med. Chem. 2017, 139, 191–200. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.; Ye, X.; Hu, Y.; Ding, T.; Chen, S. Sulfation pattern of fucose branches affects the anti-hyperlipidemic activities of fucosylated chondroitin sulfate. Carbohyd. Polym. 2016, 147, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, G.; Wu, N.; Guo, X.; Liao, N.; Ye, X.; Liu, D.; Xue, C.; Chai, W. Sulfation pattern of the fucose branch is important for the anticoagulant and antithrombotic activities of fucosylated chondroitin sulfates. BBA Gen. Subjects 2013, 1830, 3054–3066. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.P.; Walke, E.N. Depolymerized holothurian glycosaminoglycan and heparin inhibit the intrinsic tenase complex by a common antithrombin-independent mechanism. Blood 2006, 107, 3876–3882. [Google Scholar] [CrossRef] [PubMed]
- Solakyildirim, K.; Zhang, Z.; Linhardt, R.J. Ultraperformance liquid chromatography with electrospray ionization ion trap mass spectrometry for chondroitin disaccharide analysis. Anal. Biochem. 2010, 397, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.N.; Wang, Q.B.; Wang, S.M.; Wang, W.S.; Jiao, R.M.; Han, W.J.; Li, F.C. A chondroitin sulfate and hyaluronic acid lyase with poor activity to glucuronyl 4,6-O-disulfated N-acetylgalactosamine (E-type)-containing structures. J. Biol. Chem. 2018, 293, 4230–4243. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, S.; Murakoshi, S.; Kalayanamitra, K.; Deepa, S.S.; Fukui, S.; Kongtawelert, P.; Yamada, S.; Sugahara, K. Highly sulfated hexasaccharide sequences isolated from chondroitin sulfate of shark fin cartilage: Insights into the sugar sequences with bioactivities. Glycobiology 2013, 23, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Higashi, K.; Linhardt, R.J.; Toida, T. Comprehensive analysis of glycosaminoglycans from the edible shellfish. Carbohyd. Polym. 2018, 184, 269–276. [Google Scholar] [CrossRef]
- Benito-Arenas, R.; Doncel-Pérez, E.; Fernández-Gutiérrez, M.; Garrido, L.; García-Junceda, E.; Revuelta, J.; Bastida, A.; Fernández-Mayoralas, A. A holistic approach to unravelling chondroitin sulfation: Correlations between surface charge, structure and binding to growth factors. Carbohyd. Polym. 2018, 202, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Doncel-Pérez, E.; Aranaz, I.; Bastida, A.; Revuelta, J.; Camacho, C.; Acosta, N.; Garrido, L.; Civera, C.; García-Junceda, E.; Heras, A.; et al. Synthesis, physicochemical characterization and biological evaluation of chitosan sulfate as heparan sulfate mimics. Carbohyd. Polym. 2018, 191, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Pudełko, A.; Wisowski, G.; Olczyk, K.; Koźma, E.M. The dual role of the glycosaminoglycan chondroitin-6-sulfate in the development, progression and metastasis of cancer. FEBS J. 2019. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).