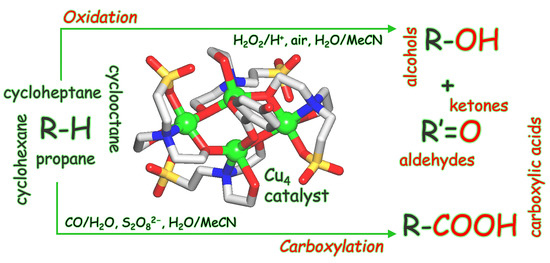
Tetracopper(II) Cores Driven by an Unexplored Trifunctional Aminoalcohol Sulfonic Acid for Mild Catalytic C–H Functionalization of Alkanes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Compounds 1–3
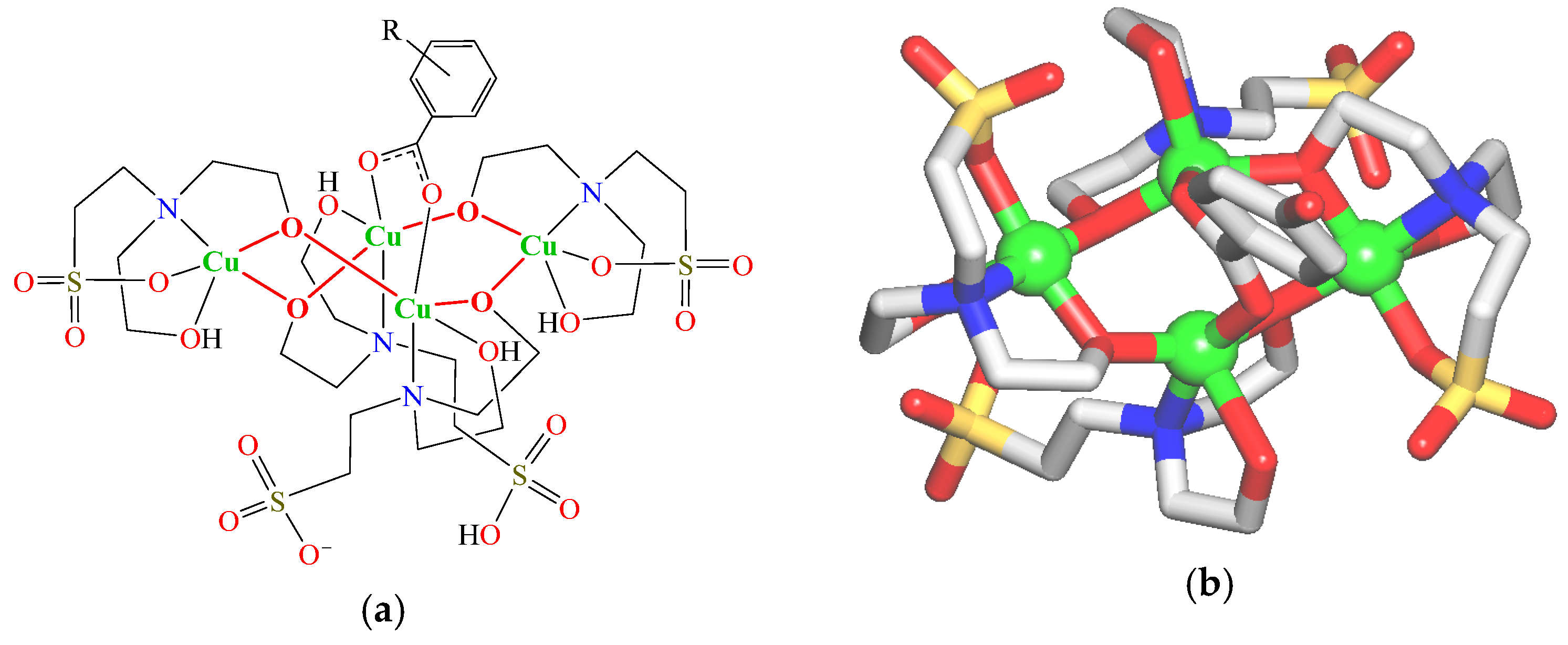
2.2. Structural Description
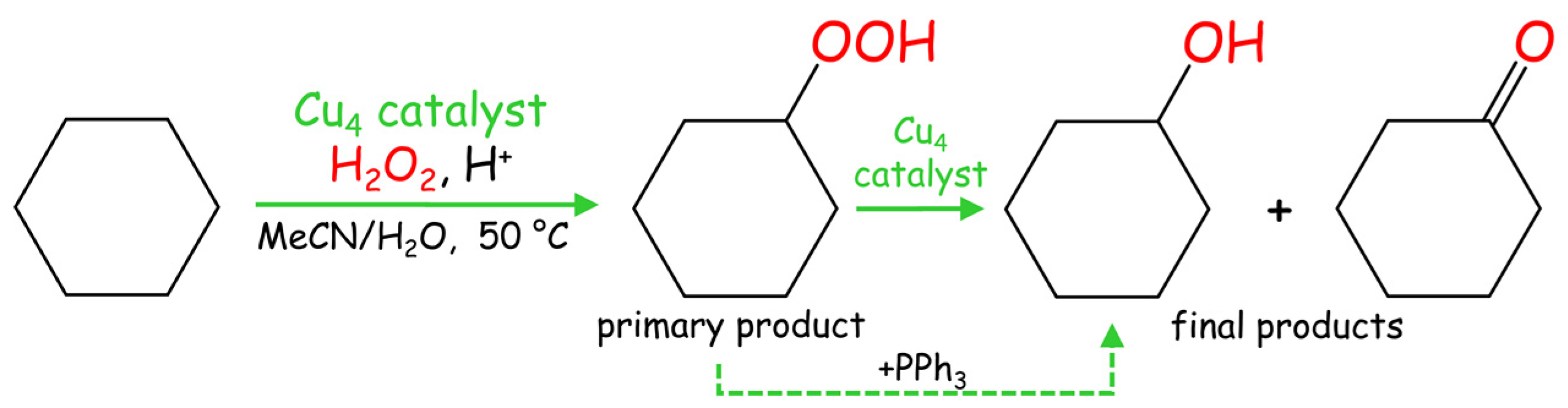
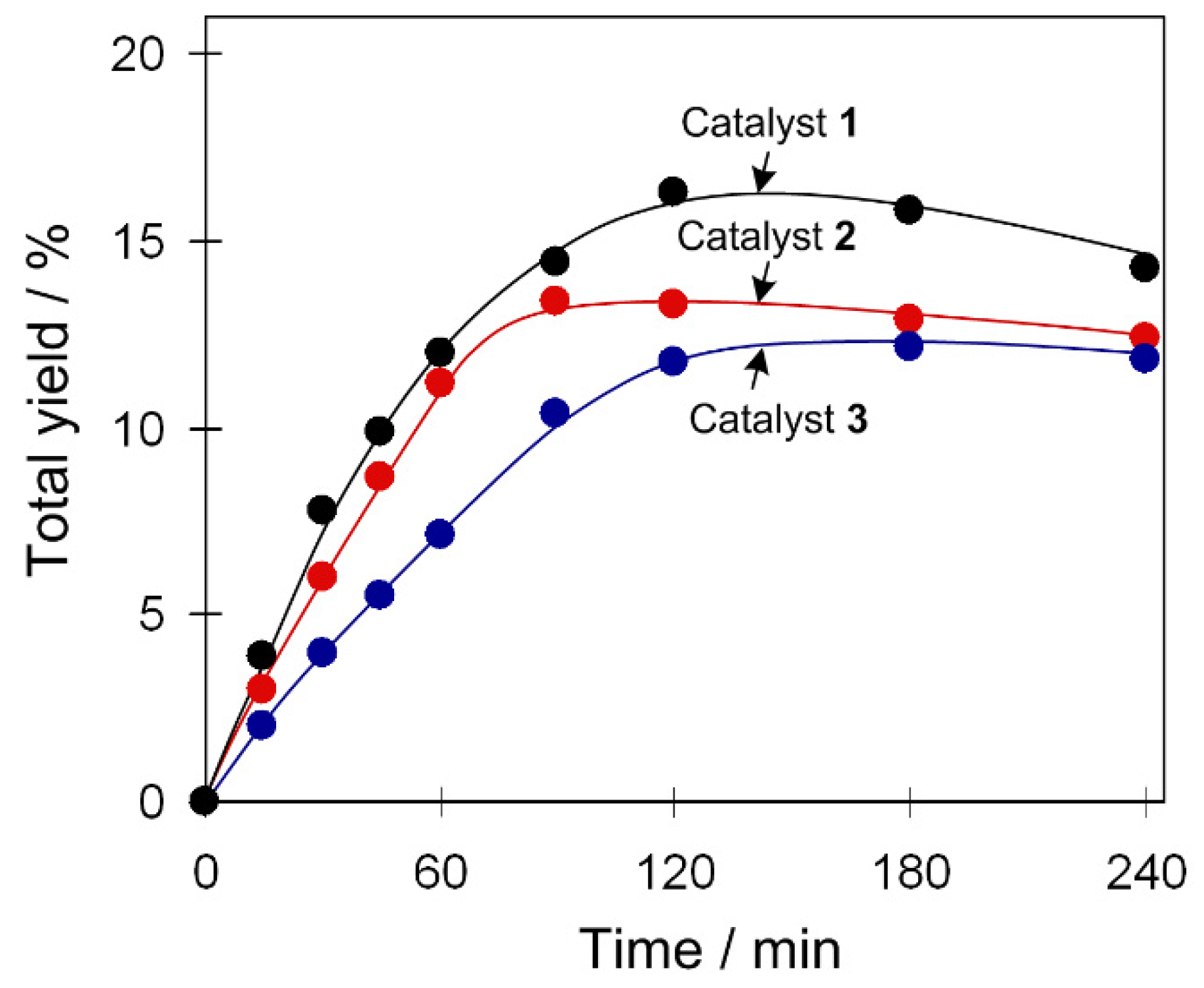
2.3. Mild Catalytic Oxidation of Cycloalkanes
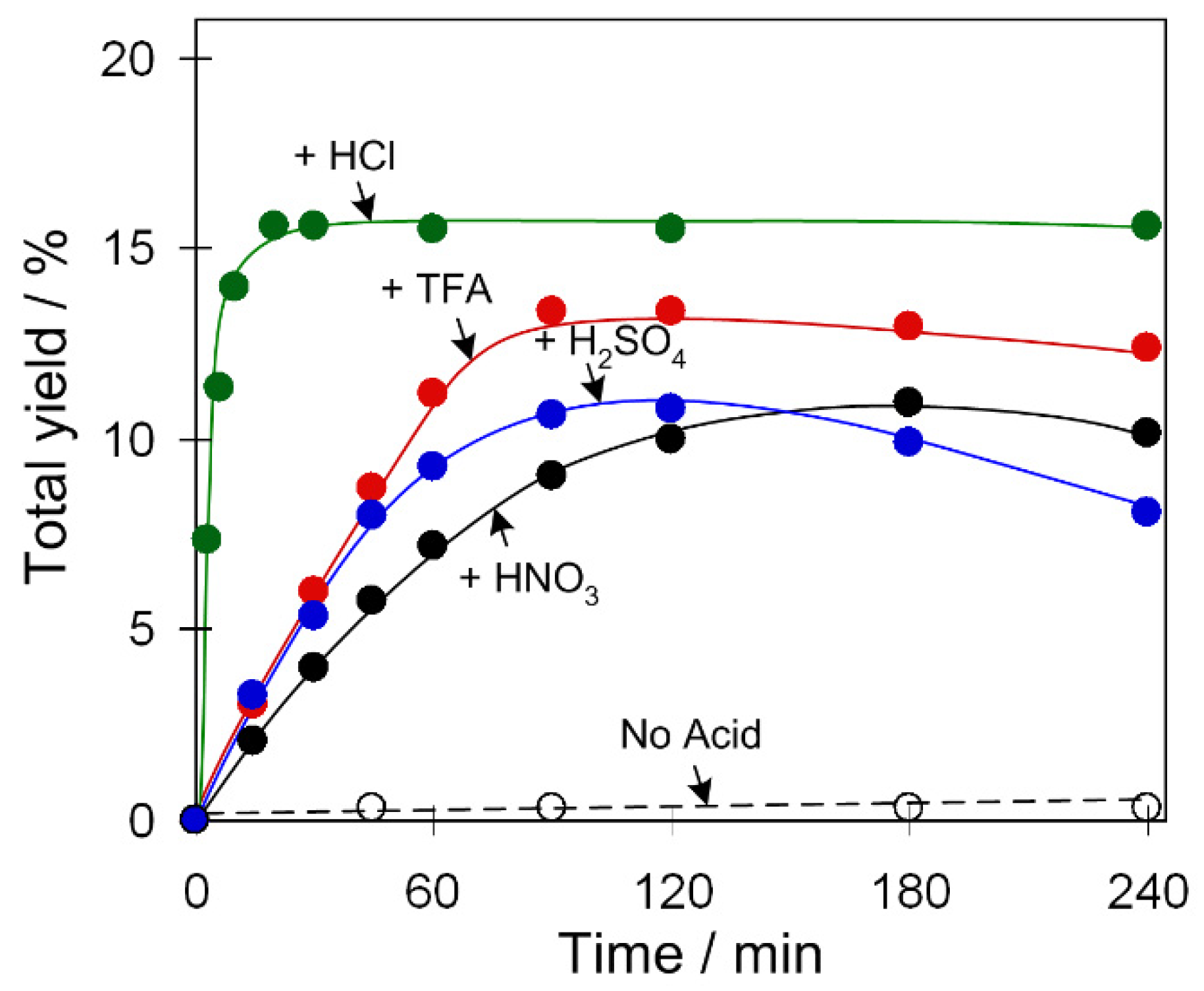
2.3.1. Acid Promoter Effect
2.3.2. Effect of Catalyst Amount
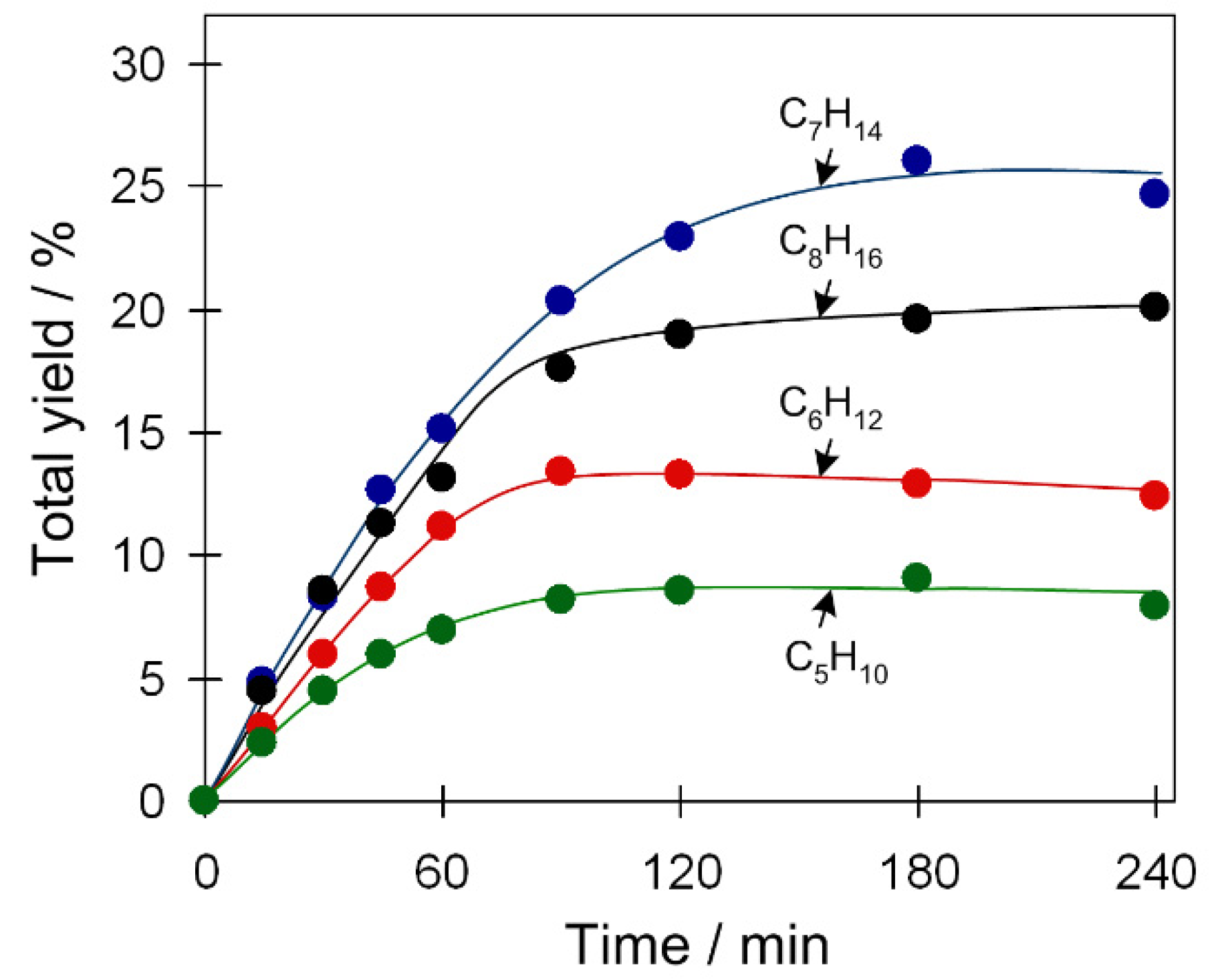
2.3.3. Substrate Scope
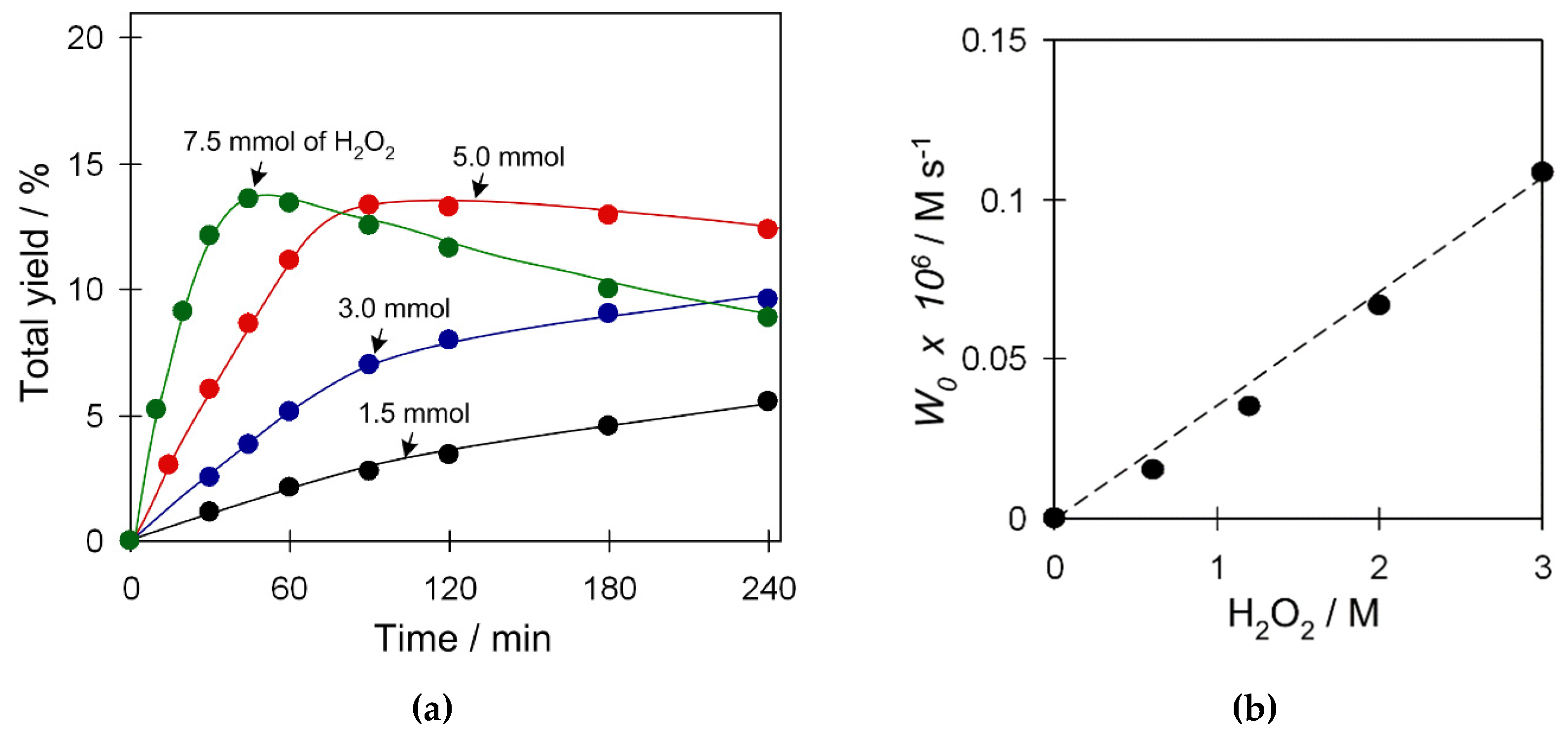
2.3.4. Effect of Substrate and Oxidant Amount
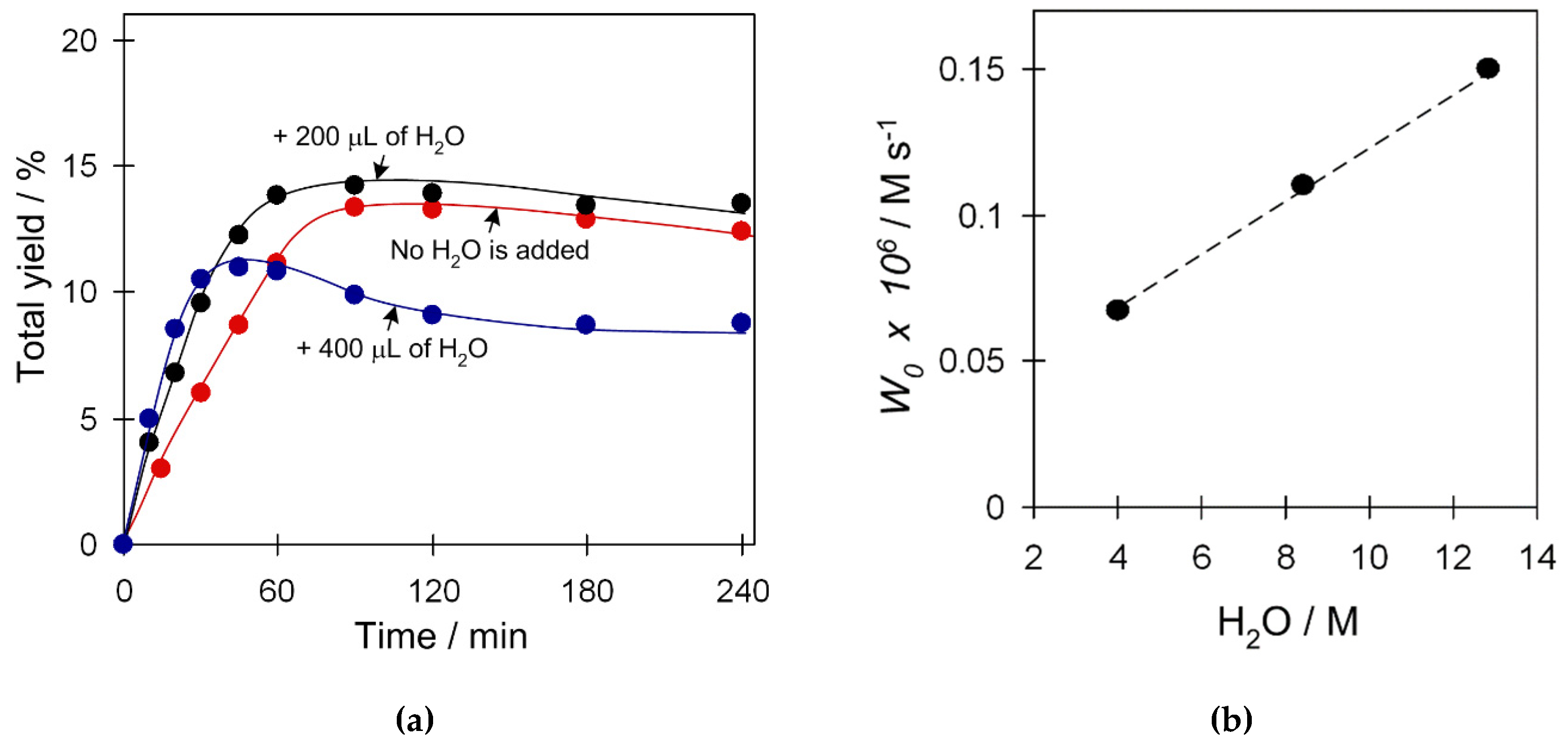
2.3.5. Effect of Water
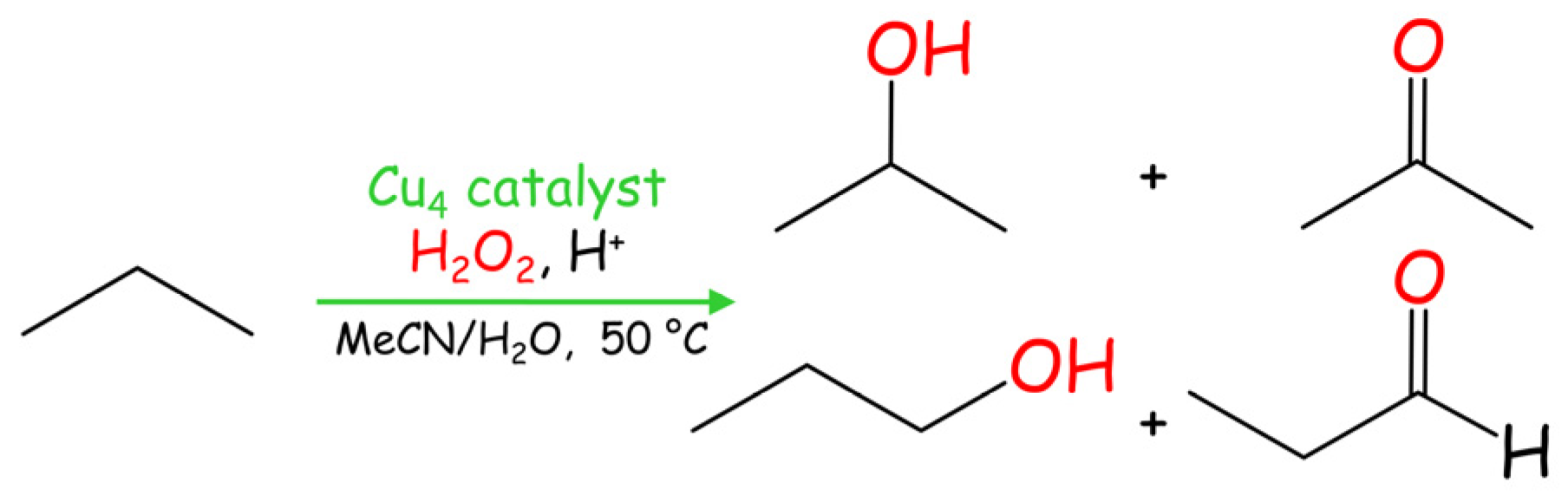
2.4. Mild Cu-Catalyzed Oxidation of Propane
2.5. Selectivity Parameters and Mechanistic Considerations in Alkane Oxidation
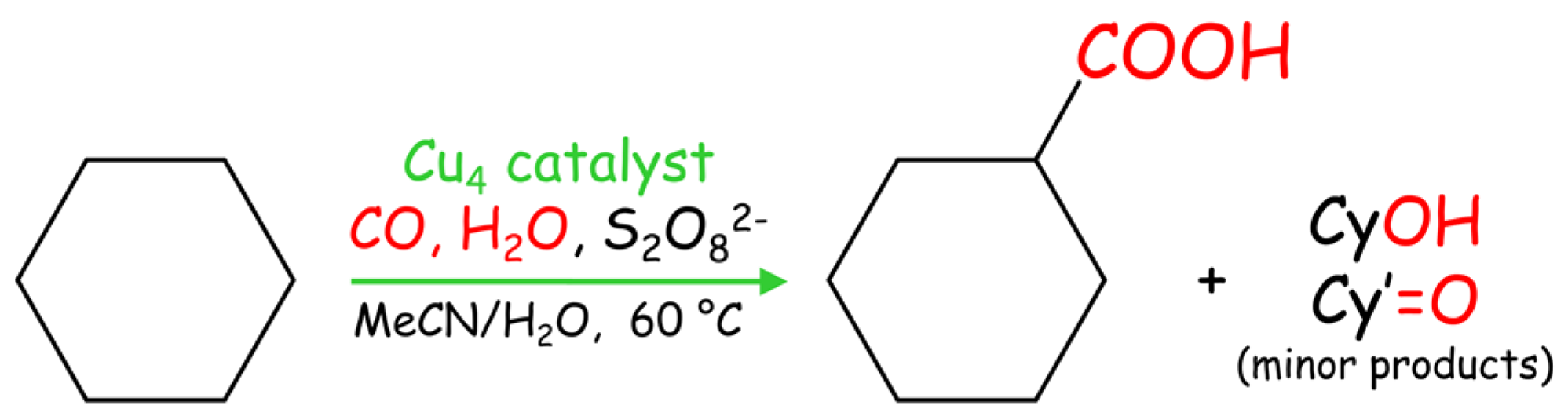
2.6. Mild Cu-Catalyzed Carboxylation of C5–C8 Cycloalkanes
2.7. Mild Cu-Catalyzed Carboxylation of Propane
3. Experimental
3.1. Materials and Methods
3.2. Synthetic Procedure and Analytical Data for 1–3
3.3. X-ray Diffraction
3.4. Catalytic Oxidation of Cycloalkanes
3.5. Catalytic Oxidation of Propane
3.6. Catalytic Carboxylation of Alkanes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shilov, A.E.; Shul’pin, G.B. Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes; Kluwer Acad. Publ.: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Olah, G.A.; Molnár, Á. Hydrocarbon Chemistry; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Alkane, C.-H. Activation by Single-Site Metal Catalysis; Pérez, P.J., Ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Nesterov, D.S.; Nesterova, O.V.; Pombeiro, A.J.L. Homo- and heterometallic polynuclear transition metal catalysts for alkane C-H bonds oxidative functionalization: Recent advances. Coord. Chem. Rev. 2018, 355, 199–222. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Hydrocarbon Oxygenations with Peroxides Catalyzed by Metal Compounds. Mini-Rev. Org. Chem. 2009, 6, 95–104. [Google Scholar] [CrossRef]
- Fokin, A.A.; Schreiner, P.R. Selective Alkane Transformations via Radicals and Radical Cations: Insights into the Activation Step from Experiment and Theory. Chem. Rev. 2002, 102, 1551–1594. [Google Scholar] [CrossRef] [PubMed]
- Labinger, J.A.; Bercaw, J.E. Understanding and exploiting C–H bond activation. Nature 2002, 417, 507–514. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Kirillova, M.V.; Pombeiro, A.J.L. Multicopper complexes and coordination polymers for mild oxidative functionalization of alkanes. Coord. Chem. Rev. 2012, 256, 2741–2759. [Google Scholar] [CrossRef]
- Nesterova, O.V.; Kopylovich, M.N.; Nesterov, D.S. Stereoselective oxidation of alkanes with m-CPBA as an oxidant and cobalt complex with isoindole-based ligands as catalysts. RSC Adv. 2016, 6, 93756–93767. [Google Scholar] [CrossRef]
- Antonangelo, A.R.; Grazia Bezzu, C.; McKeown, N.B.; Nakagaki, S. Highly active manganese porphyrin-based microporous network polymers for selective oxidation reactions. J. Catal. 2019, 369, 133–142. [Google Scholar] [CrossRef]
- Talsi, E.P.; Bryliakov, K.P. Chemo- and stereoselective CH oxidations and epoxidations/cis-dihydroxylations with H2O2, catalyzed by non-heme iron and manganese complexes. Coord. Chem. Rev. 2012, 256, 1418–1434. [Google Scholar] [CrossRef]
- Ottenbacher, R.V.; Talsi, E.P.; Bryliakov, K.P. Direct Selective Oxidative Functionalization of C–H Bonds with H2O2: Mn-Aminopyridine Complexes Challenge the Dominance of Non-Heme Fe Catalysts. Molecules 2016, 21, 1454. [Google Scholar] [CrossRef]
- Shilov, A.E.; Shul’pin, G.B. Activation of C−H Bonds by Metal Complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef]
- Shul’pin, G.B. New Trends in Oxidative Functionalization of Carbon–Hydrogen Bonds: A Review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Nesterova, O.V.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Catalytic behaviour of a novel Fe(III) Schiff base complex in the mild oxidation of cyclohexane. Catal. Sci. Technol. 2015, 5, 1801–1812. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Nesterova, O.V.; Kopylovich, M.N.; Pombeiro, A.J.L. Pronounced retention of stereoconfiguration upon sp3 C-H bonds hydroxylation of dimethylcyclohexanes and decahydronaphthalenes with m-CPBA oxidant and a Co-phthalocyanine catalyst. Mol. Catal. 2018, 459, 8–15. [Google Scholar] [CrossRef]
- Gupta, S.; Kirillova, M.V.; da Silva, M.F.C.G.; Pombeiro, A.J.L.; Kirillov, A.M. Alkali metal directed assembly of heterometallic Vv/M (M = Na, K, Cs) coordination polymers: Structures, topological analysis, and oxidation catalytic properties. Inorg. Chem. 2013, 52, 8601–8611. [Google Scholar] [CrossRef]
- Bäckvall, J.-E. (Ed.) Modern Oxidation Methods, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Karlin, K.D.; Tyeklar, Z. (Eds.) Bioinorganic Chemistry of Copper; Springer: Berlin, Germany, 2012. [Google Scholar]
- Itoh, S.; Rokita, S. (Eds.) Copper-Oxygen Chemistry; Wiley: New York, NY, USA, 2011. [Google Scholar]
- Brissos, R.S.; Garcia, S.; Presa, A.; Gamez, P. Bio-related copper-mediated oxidative processes. Comments Inorg. Chem. 2011, 32, 219–245. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Santos, C.I.M.; André, V.; Kłak, J.; Kirillova, M.V.; Kirillov, A.M. Aqua-Soluble Copper(II) Coordination Polymers Self-assembled from Aminoalcohols and Pyromellitic Acid: Highly Active Pre-catalysts for the Mild Water-promoted Oxidation of Alkanes. Inorg. Chem. 2016, 55, 125–135. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Santos, C.I.M.; André, V.; Dias, S.S.P.; Kirillova, M.V.; Kirillov, A.M. New aqua-soluble dicopper(II) aminoalcoholate cores for mild and water-assisted catalytic oxidation of alkanes. Catal. Sci. Technol. 2016, 6, 4584–4593. [Google Scholar] [CrossRef]
- Dias, S.S.P.; Kirillova, M.V.; André, V.; Kłak, J.; Kirillov, A.M. New tricopper(II) cores self-assembled from aminoalcohol biobuffers and homophthalic acid: Synthesis, structural and topological features, magnetic properties and mild catalytic oxidation of cyclic and linear C5–C8 alkanes. Inorg. Chem. Front. 2015, 2, 525–537. [Google Scholar] [CrossRef]
- Dias, S.S.P.; Kirillova, M.V.; André, V.; Kłak, J.; Kirillov, A.M. New Tetracopper (II) Cubane Cores Driven by a Diamino Alcohol: Self-assembly Synthesis, Structural and Topological Features, and Magnetic and Catalytic Oxidation Properties. Inorg. Chem. 2015, 54, 5204–5212. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Korlyukov, A.A.; Shul’pina, L.S.; Bantreil, X.; Lamaty, F.; Shubina, E.S.; Levitsky, M.M.; Ikonnikova, N.S.; Shul’pin, G.B. A new “bicycle helmet”-like copper(II) sodiumphenylsilsesquioxane. Synthesis, structure and catalytic activity. Dalton Trans. 2018, 47, 15666–15669. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, M.M.; Yalymov, A.I.; Kulakova, A.N.; Petrov, A.A.; Bilyachenko, A.N. Cage-like metallsilsesquioxanes in catalysis: A review. J. Mol. Cat. A Chem. 2017, 426, 297–304. [Google Scholar] [CrossRef]
- Good, N.E.; Winget, G.D.; Winter, W.; Connolly, T.N.; Izawa, S.; Sing, R.M.M. Hydrogen ion buffers for biological research. Biochemistry 1966, 5, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, W.J.; Braunschweiger, K.I.; Braunschweiger, W.R.; Smith, J.R.; McCormick, J.J.; Wasmann, C.C.; Jarvis, N.P.; Bell, D.H.; Good, N.E. Hydrogen ion buffers for biological research. Anal. Biochem. 1980, 104, 300–310. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Coelho, J.A.S.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Nesterov, D.S.; Gruenwald, K.R.; Haukka, M.; Pombeiro, A.J.L. Bringing an “Old” Biological Buffer to Coordination Chemistry: New 1D and 3D Coordination Polymers with [Cu4(Hbes)4] Cores for Mild Hydrocarboxylation of Alkanes. Inorg. Chem. 2010, 49, 6390–6392. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kirillov, A.M.; Martins, A.N.C.; Graiff, C.; Tiripicchio, A.; Pombeiro, A.J.L. Topologically Unique Heterometallic CuII/Li Coordination Polymers Self-Assembled from N,N-bis(2-Hydroxyethyl)-2-aminoethanesulfonic Acid Biobuffer: Versatile Catalyst Precursors for Mild Hydrocarboxylation of Alkanes to Carboxylic Acids. Inorg. Chem. 2012, 51, 5224–5234. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kirillov, A.M.; Pombeiro, A.J.L. Mild, single-pot hydrocarboxylation of gaseous alkanes to carboxylic acids in metal-free and copper-promoted aqueous systems. Chem. Eur. J. 2010, 16, 9485–9493. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, M.V.; Kirillov, A.M.; Kuznetsov, M.L.; Silva, J.A.L.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Alkanes to carboxylic acids in aqueous medium: Metal-free and metal-promoted highly efficient and mild conversions. Chem. Commun. 2009, 2353–2355. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kirillov, A.M.; Pombeiro, A.J.L. Metal-free and copper-promoted single-pot hydrocarboxylation of cycloalkanes to carboxylic acids in aqueous medium. Adv. Synth. Catal. 2009, 351, 2936–2948. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Haukka, M.; da Silva, M.F.C.G.; Pombeiro, A.J.L. Preparation and crystal structures of benzoylhydrazido- and -diazenidorhenium complexes with N,O-ligands and their catalytic activity towards peroxidative oxidation of cycloalkanes. Eur. J. Inorg. Chem. 2005, 2071–2080. [Google Scholar] [CrossRef]
- Schuchardt, U.; Cardoso, D.; Sercheli, R.; Pereira, R.; da Cruz, R.S.; Guerreiro, M.C.; Mandelli, D.; Spinace, E.V.; Pires, E.L. Cyclohexane Oxidation Continues to be a Challenge. Appl. Catal. A Gen. 2001, 211, 1–17. [Google Scholar] [CrossRef]
- Wittcoff, H.; Reuben, B.G.; Plotkin, J.S. Industrial Organic Chemicals, 2nd ed.; Wiley: New York, NY, USA, 2004. [Google Scholar]
- Kirillov, A.M.; Shul’pin, G.B. Pyrazinecarboxylic acid and analogs: Highly efficient co-catalysts in the metal-complex-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2013, 257, 732–754. [Google Scholar] [CrossRef]
- Armakola, E.; Colodrero, R.M.P.; Bazaga-García, M.; Salcedo, I.R.; Choquesillo-Lazarte, D.; Cabeza, A.; Kirillova, M.V.; Kirillov, A.M.; Demadis, K.D. Three-Component Copper-Phosphonate-Auxiliary Ligand Systems: Proton Conductors and Efficient Catalysts in Mild Oxidative Functionalization of Cycloalkanes. Inorg. Chem. 2018, 57, 10656–10666. [Google Scholar] [CrossRef] [PubMed]
- APEX2. Ver. 2014.11-0; Bruker-AXS: Billerica, MA, USA, 2014. [Google Scholar]
- APEX3. Ver. 2017.3-0; Bruker-AXS: Billerica, MA, USA, 2017. [Google Scholar]
- SAINT; Bruker-AXS: Billerica, MA, USA, 2014/2017.
- SADABS; Bruker-AXS: Billerica, MA, USA, 2014/2017.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX—Version 1.80.05. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Ayala, M.; Torres, E. Enzymatic activation of alkanes: Constraints and prospective. Appl. Catal. A 2004, 272, 1–13. [Google Scholar] [CrossRef]




| Substrate | Catalyst | Yield (%) b | ||
|---|---|---|---|---|
| Alcohol | Ketone | Total | ||
| Cyclopentane | 1 | 6.1 | 7.2 | 13.3 |
| 2 | 4.0 | 5.0 | 9.0 | |
| 3 | 2.6 | 4.3 | 6.9 | |
| Cyclohexane | 1 | 8.9 | 6.9 | 15.8 |
| 2 | 7.6 | 5.3 | 12.9 | |
| 3 | 7.8 | 4.3 | 12.1 | |
| Cycloheptane | 1 | 13.1 | 12.8 | 25.9 |
| 2 | 13.4 | 12.6 | 26.0 | |
| 3 | 9.6 | 13.2 | 22.8 | |
| Cyclooctane | 1 | 9.7 | 16.3 | 26.0 |
| 2 | 6.5 | 13.1 | 19.6 | |
| 3 | 6.5 | 13.2 | 19.6 | |
| Catalyst | Product Yield, % b | TON c | ||||
|---|---|---|---|---|---|---|
| i-Propanol | Acetone | n-Propanol | Propanal | Total | ||
| 1 | 1.8 | 3.4 | 1.1 | 0.8 | 7.1 | 60 |
| 2 | 2.7 | 4.2 | 1.4 | 0.9 | 9.2 | 77 |
| 3 | 2.8 | 3.4 | 1.2 | 0.6 | 8.0 | 67 |
| Selectivity Parameter | Catalyst | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Regioselectivity | |||
| C(1):C(2):C(3):C(4) (n-heptane) b | 1:4:5:8 | 1:5:5:7 | 1:5:5:8 |
| Bond selectivity | |||
| 1°:2°:3° (methylcyclohexane) c | 1:5:15 | 1:5:19 | 1:6:13 |
| 2°:3° (adamantane) d | 1:4 | 1:3.6 | 1:3.6 |
| Stereoselectivity | |||
| trans/cis (cis-dimethylcyclohexane) e | 0.9 | 0.8 | 1.1 |
| Substrate | Catalyst | Yield (%) b | |||
|---|---|---|---|---|---|
| Cycloalkane Carboxylic Acid | Cyclic Ketone | Cyclic Alcohol | Total c | ||
| Cyclopentane | 1 | 30.9 | 3.1 | 0.8 | 34.8 |
| 2 | 21.0 | 2.7 | 1.1 | 24.8 | |
| 3 | 27.1 | 3.5 | 0.8 | 31.4 | |
| Cyclohexane | 1 | 41.4 | 1.5 | 0.3 | 43.2 |
| 2 | 42.9 | 2.6 | 0.6 | 46.1 | |
| 3 | 40.0 | 2.4 | 0.5 | 42.9 | |
| Cycloheptane | 1 | 22.5 | 10.9 | 3.6 | 37.0 |
| 2 | 22.4 | 9.1 | 3.5 | 35.0 | |
| 3 | 27.3 | 10.4 | 3.1 | 40.8 | |
| Cyclooctane | 1 | 11.8 | 12.0 | 9.3 | 33.1 |
| 2 | 10.2 | 11.4 | 7.5 | 29.1 | |
| 3 | 14.2 | 10.8 | 11.8 | 36.8 | |
| Catalyst | Yield (%) b,c | ||
|---|---|---|---|
| i-Butyric Acid | n-Butyric Acid | Total d | |
| 1 | 28.2 | 5.7 | 33.9 |
| 2 | 23.1 | 4.8 | 27.9 |
| 3 | 33.3 | 6.7 | 40.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, I.F.M.; Kirillova, M.V.; André, V.; Fernandes, T.A.; Kirillov, A.M. Tetracopper(II) Cores Driven by an Unexplored Trifunctional Aminoalcohol Sulfonic Acid for Mild Catalytic C–H Functionalization of Alkanes. Catalysts 2019, 9, 321. https://doi.org/10.3390/catal9040321
Costa IFM, Kirillova MV, André V, Fernandes TA, Kirillov AM. Tetracopper(II) Cores Driven by an Unexplored Trifunctional Aminoalcohol Sulfonic Acid for Mild Catalytic C–H Functionalization of Alkanes. Catalysts. 2019; 9(4):321. https://doi.org/10.3390/catal9040321
Chicago/Turabian StyleCosta, Inês F. M., Marina V. Kirillova, Vânia André, Tiago A. Fernandes, and Alexander M. Kirillov. 2019. "Tetracopper(II) Cores Driven by an Unexplored Trifunctional Aminoalcohol Sulfonic Acid for Mild Catalytic C–H Functionalization of Alkanes" Catalysts 9, no. 4: 321. https://doi.org/10.3390/catal9040321
APA StyleCosta, I. F. M., Kirillova, M. V., André, V., Fernandes, T. A., & Kirillov, A. M. (2019). Tetracopper(II) Cores Driven by an Unexplored Trifunctional Aminoalcohol Sulfonic Acid for Mild Catalytic C–H Functionalization of Alkanes. Catalysts, 9(4), 321. https://doi.org/10.3390/catal9040321