Abstract
The in-situ catalytic fast pyrolysis of pinecone over HY catalysts, HY(30; SiO2/Al2O3), HY(60), and 1% Ni/HY(30), was studied by TGA and Py-GC/MS. Thermal and catalytic TGA indicated that the main decomposition temperature region of pinecone, from 200 to 400 °C, was not changed using HY catalysts. On the other hand, the DTG peak heights were differentiated by the additional use of HY catalysts. Py-GC/MS analysis showed that the efficient conversion of phenols and other oxygenates formed from the pyrolysis of pinecone to aromatic hydrocarbons could be achieved using HY catalysts. Of the HY catalysts assessed, HY(30), showed higher efficiency in the production of aromatic hydrocarbons than HY(60) because of its higher acidity. The aromatic hydrocarbon production was increased further by increasing the pyrolysis temperature from 500 to 600 °C and increasing the amount of catalyst due to the enhanced cracking ability and overall acidity. The use of 1% Ni/HY(30) also increased the amount of monoaromatic hydrocarbons compared to the use of HY(30) due to the additional role of Ni in enhancing the deoxygenation and aromatization of reaction intermediates.
1. Introduction
Recently, the frequency of abnormal weather due to global warming has been increasing, and its intensity has also become stronger than before. For example, the temperature in France in June 2019 approached 45 °C. In this regard, the use of renewable energy is essential to reduce global warming, the main cause of climate change, and biofuels are a significant source of renewable energy [1,2,3,4,5]. Among the biofuels, the importance of bio-oil is increasing because it can be used both as a biofuel and as chemical feedstock. Bio-oil is produced from the pyrolysis of biomass, and the characteristics of bio-oil obtained from biomass pyrolysis are differentiated according to the biomass species [6]. In particular, many studies have been conducted to produce bio-oils by applying non-edible biomass as the pyrolysis source instead of edible biomass [7,8,9]. Among various types of non-edible biomass, lignocellulosic biomass pyrolysis, including pine trees, has been studied intensively [10].
Of the range of woody biomass in Korea, pinewood is the one of the most widely distributed wood species in Korea from ancient times. Pinewood has been used widely to construct Korean traditional houses, and a large amount of pinewood waste is emitted as a byproduct. Therefore, pyrolysis research related to pine waste such as pinecones and needles has been carried out worldwide, including Korea. On the other hand, as more pine trees are cultivated, a larger amount of cones will be generated annually. Recently, pinecones have been regarded as a biomass resource that can be used as biofuel, and the pyrolysis of pinecones has been reported [11]. However, most studies on pinecone pyrolysis have focused on the production and application of biochar, and very few studies have reported on the production and quality analysis of bio-oil from pinecones via thermo-chemical conversion technologies [12,13]. Therefore, the pyrolysis processes that produce bio-oil from pinecones require more research. In addition, the bio-oil obtained from the non-catalytic fast pyrolysis (NCFP) of pinecones can be used as a low-grade fuel only owing to its high oxygen content. Therefore, it is necessary to convert it to high-grade fuels with low oxygen contents or valuable biochemicals with high economic value by applying the appropriate catalytic upgrading [14,15,16]. On the other hand, there are no reports on the production of bio-oils with a low oxygen content or valuable chemical feedstock via the catalytic fast pyrolysis (CFP) of pinecones.
This study examined the CFP of pinecones to produce high-quality biofuel with low oxygen contents by thermogravimetric (TG) analysis and pyrolyzer-gas chromatography/mass spectrometry (Py-GC/MS). For this purpose, HY—a typical microporous catalyst used widely in petrochemical cracking reactions—was selected as a reaction catalyst. The SiO2/Al2O3 ratios of the catalysts were varied to investigate the effects of the acid characteristics of HY on the CFP. To investigate the effects of the process conditions, the reaction temperature and catalyst amount were also differentiated. The effects of Ni impregnation into HY(30) on the CFP of pinecones were also assessed.
2. Results and Discussion
2.1. TG Analysis
Figure 1 shows the thermal and catalytic TG and DTG curves of pinecone over HY catalysts. The non-catalytic TG curve of pinecone suggests that it decomposed rapidly at between 200 and 400 °C because of the main decomposition of the lignocellulosic components of biomass (lignin, hemicellulose, and cellulose) [17] and that this continued up to a higher temperature than 600 °C due to char stabilization [18,19]. The catalytic TG and DTG curves of pinecone also revealed the same decomposition temperature region as those obtained from the non-catalytic TGA. On the other hand, the catalytic DTG peak heights of pinecone over both HY(30) and HY(60) were lower at temperatures less than 400 °C and higher at the temperature greater than 400 °C compared to the non-catalytic DTG curve. This can be explained by the CFP behavior of biomass. The lignocellulosic components of biomass converted to pyrolyzates consisted mainly of oxygen-containing pyrolyzates such as acids, aldehydes, furans, levoglucosan, and phenols during the CFP of biomass. These pyrolyzates are converted to light olefins and aromatic hydrocarbons over the catalysts [20]. Although their formation amounts can be differentiated according to the CFP condition and the catalyst species, coke can form at the surface of the catalyst and inside of the catalyst pores, thereby poisoning the catalyst. Therefore, some CFP intermediates cannot be vaporized and fixed to the catalyst as thermal coke and catalyst coke, as shown in the DTG peak height of pinecones at temperatures lower than 400 °C. If a considerable amount of coke is deposited on the catalyst, the coke is also stabilized at higher temperatures with the elimination of oxygen and the formation of more thermally stable polyaromatic coke [21]. The CFP of pinecone over HY catalysts can increase the amount of volatile emission at temperatures higher than 400 °C. Compared to the catalytic DTG curve of pinecones over HY(60), that over HY(30) showed a lower DTG peak height at temperatures less than 400 °C and higher at temperatures greater than 400 °C. This suggests that HY(30) produces a larger amount of coke at temperatures lower than 400 °C and emits a larger amount of volatiles during the coke stabilization at temperatures higher than 400 °C [22].
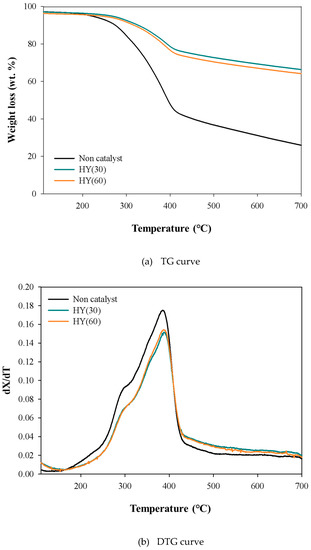
Figure 1.
TG and DTG curves obtained from the thermal and catalytic TG analysis of pinecone at 20 °C/min over HY catalysts.
2.2. Py-GC/MS Analysis
Table 1 lists the absolute MS peak areas for the products obtained from the NCFP of pinecone at 600 °C. The NCFP of pinecones mainly produced large amounts of oxygen-containing pyrolyzates, classified into furans, acids, phenols, aldehydes, ketones, and levoglucosan, along with minor amounts of hydrocarbons and N-containing compounds. Acetic acid and furans are the typical pyrolyzates of woody biomass [23]. Levoglucosan, the primary decomposition product of cellulose [24], comprised the largest portion of oxygen-containing pyrolyzates of pinecone. Many phenolic products, such as phenol, methyl phenol, methoxy phenol, methyl methoxy phenol, ethyl methoxy phenol, vinyl methoxy phenol, eugenol, and propenyl methoxy phenol, are also produced by the decomposition of lignin in pinecones [25,26].

Table 1.
Absolute MS peak area of the products obtained from the non-catalytic fast pyrolysis (NCFP) of pinecone at 600 °C. BTEX: benzene, toluene, ethyl benzene, and xylene.
By applying the CFP of pinecone over HY catalysts, the amounts of oxygen-containing pyrolyzates decreased significantly with the formation of aromatic hydrocarbons, as shown in Figure 2. The contents of phenolics and other oxygenates also decreased after applying both HY catalysts to the pyrolysis of pinecone. This suggests that both HYs are efficient catalysts for the conversion of oxygen-containing pyrolyzates of pinecone to aromatic hydrocarbons. Compared to the CFP of pinecone over HY(60), that over HY(30) produced a larger amount of aromatic hydrocarbons because of its higher acidity. During the CFP of biomass, the lignocellulosic components of biomass (e.g., hemicellulose and cellulose) are converted to oxygen-containing pyrolyzates and light hydrocarbons via catalytic cracking, dehydration, decarboxylation, decarbonylation, dealkylation, and dealkoxylation [27]. These hydrocarbons form a hydrocarbon pool inside the pores of the acid catalysts and produce aromatic hydrocarbons [28]. In the case of the CFP of lignin, its main pyrolyzates (e.g., alkyl phenols and alkoxy phenols) are converted to aromatic hydrocarbons via additional dealkylation and dealkoxylation reactions of phenolic pyrolyzates over acid catalysts [29].
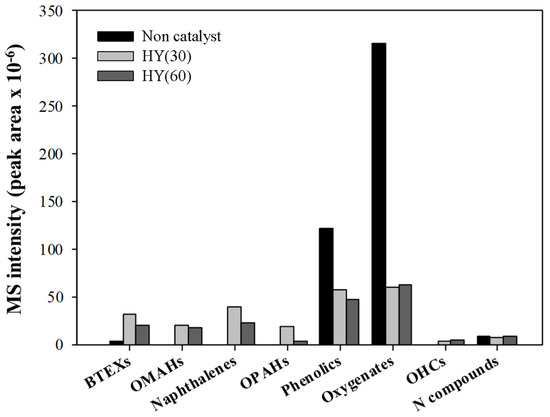
Figure 2.
The absolute MS peak areas for each chemical group obtained from the thermal and catalytic fast pyrolysis of pine cone at 600 °C over HY(30) and HY(60). OMAHs: other monoaromatic hydrocarbons; OPAHs: other polyaromatic hydrocarbons.
Figure 3 shows the effects of the CFP reaction temperature on the formation of aromatic hydrocarbons via the CFP of pinecone over HY(30). Larger amounts of benzene, toluene, ethyl benzene, and xylenes (BTEXs), other monoaromatic hydrocarbons (OMAHs), naphthalenes, and other polyaromatic hydrocarbons (OPAHs) were formed by increasing the reaction temperature from 500 to 600 °C. The amounts of phenolics and other oxygenates decreased with increasing reaction temperature. This also confirms that the formation of aromatic hydrocarbons during the CFP of pinecone is related to the effective conversion of phenolics and other oxygenates to aromatic hydrocarbons via the aromatization of deoxygenated light olefins and the dealkylation and dealkoxylation of large molecular weight phenols. The higher production efficiency of aromatic hydrocarbons by increasing the reaction temperature on the CFP of pinecone can be explained by the increased catalytic cracking efficiency at higher temperatures [22] and the increased diffusion efficiency of large molecular weight reaction intermediates to the expanded catalysts’ pores [22,30]. When the reaction temperature was increased to 600 °C, the yield of naphthalenes (one class of PAHs) was increased. Naphthalene is an important material in the organic chemical industry and is used widely as a resin [31], solvent [32], or dye [33].
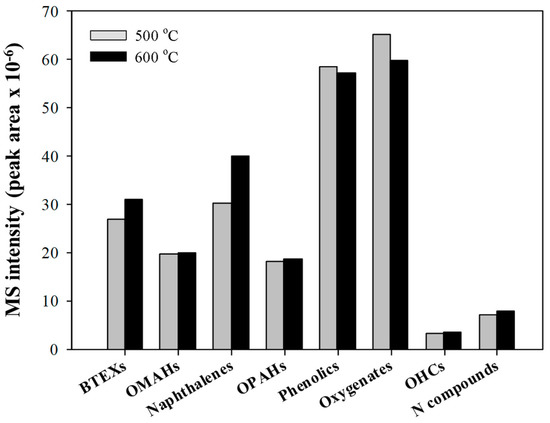
Figure 3.
The absolute MS peak areas for each chemical group obtained from the thermal and catalytic pyrolysis of pine cone at different temperatures over HY(30).
Figure 4 shows the effect of the catalyst amount on the CFP of pinecone over HY catalysts. The level of aromatic hydrocarbon production was also increased by decreasing the sample-to-catalyst ratio from 4:1 to 1:4 on the CFP of the same amount of pinecone (1 mg). This means that an increase in the overall number of acid sites by increasing the catalyst amount can increase the formation efficiency of aromatic hydrocarbons during the CFP of pinecone. In addition, the nitrogen compounds were converted completely when the amount of the catalyst was increased. In particular, most of the product formed from the CFP of pinecone consisted of aromatic hydrocarbons together with a small amount of phenolics when a sample-to-catalyst ratio of 1:4 was applied to the reaction.
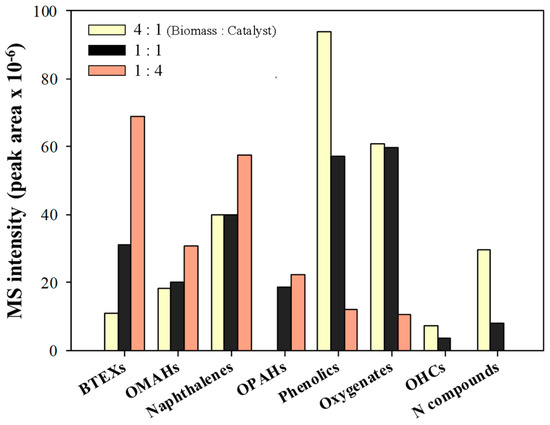
Figure 4.
The absolute MS peak areas for each chemical group obtained from the catalytic fast pyrolysis of pinecone at different sample-to-catalyst ratios at 600 °C over HY(30).
Figure 5 shows the effects of 1% Ni/HY(30) on the production of aromatics during the CFP of pinecone at 600 °C. Compared to HY(30), 1% Ni/HY(30) produced larger amounts of BTEXs. On the other hand, the amounts of naphthalenes and OPAHs were decreased using Ni/HY(30) instead of HY(30). This indicates that the selectivity toward to MAHs can be increased using a Ni-impregnated HY catalyst, although the overall acidity of HY(30) was decreased slightly by the Ni impregnation (Figure 6). In addition, the amount of oxygenates were decreased for Ni/HY(30). This may imply that the catalytic pyrolysis intermediates could be more efficiently converted to MAHs over Ni by enhancing deoxygenation. The effective role of Ni in the formation of MAHs has also been reported by other researchers. Ben and Ragauskas [34] commented that the deoxygenation reaction of biomass pyrolysis intermediates can be increased by the additional use of NiCl2 with HZSM-5. Dai et al. [35] suggested that Ni strongly promoted direct deoxygenation reactions such as the decarboxylation, decarbonylation, and dehydration of oxygenates.
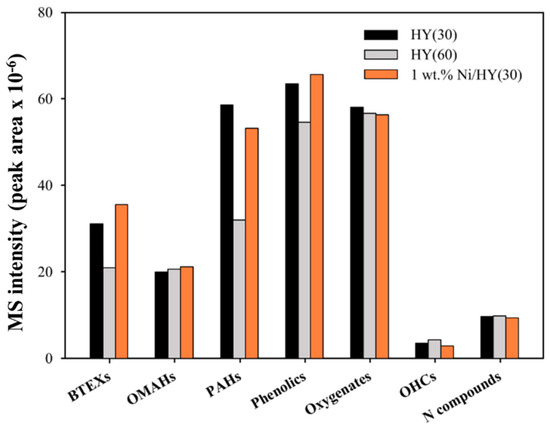
Figure 5.
The absolute MS peak areas for each chemical group obtained from the catalytic fast pyrolysis of pinecone over Ni/HY at 600 °C.
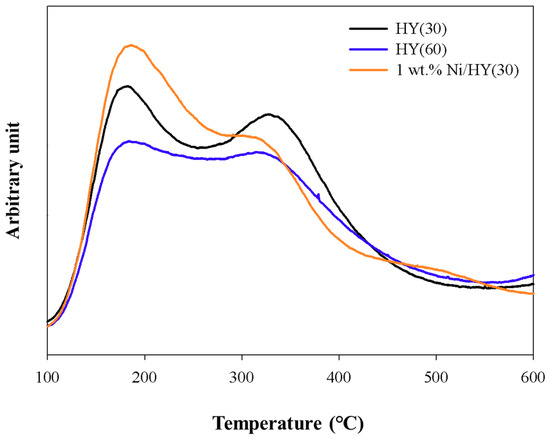
Figure 6.
NH3 TPD of catalysts.
3. Materials and Methods
3.1. Pinecone
Pinecones, which were emitted as waste biomass separated from pinewood by natural free falling, were pulverized to the same size. Ultimate and proximate analyses of pinecone (Table 2) were performed to determine its physico-chemical properties according to the procedure reported elsewhere [36]. Proximate analysis indicated that the pinecone had a high content of volatiles (83.0%). The moisture, fixed carbon, and ash contents of the pinecone were 2.1%, 13.4%, and 1.5%, respectively. Ultimate analysis of pinecone showed that carbon and oxygen accounted for more than 93.0% of the total organic elements together with low hydrogen (5.9%) and nitrogen (0.3%) contents. This suggests that a high-efficiency deoxygenation reaction is required to obtain high-quality oil with high carbon and hydrogen contents.

Table 2.
The ultimate and proximate analysis results of pinecone used in this study.
3.2. Catalysts
Two types of HY—HY(30) and HY(60), having SiO2/Al2O3 ratios of 30 and 60—respectively, were obtained from Zeolyst (Conshohocken, PA, USA). The BET surface areas of HY(30) and HY(60) were given as 780 and 720 m2/g from Zeolyst, respectively. Ni (1 wt.%) was impregnated on HY(30) using nickel nitrate. NH3-temperature programmed desorption (NH3-TPD) was performed to study the acidity of HY zeolites. Additionally, pyridine FT-IR measurements were performed to study different kinds of acid sites (Brönsted and Lewis acid sites). The detailed procedures are described in the Supplementary Information.
As shown in the NH3-TPD curve of the HYs (Figure 6), both HY catalysts had two ammonia desorption temperature regions at approximately 180 °C and 330 °C, which were assigned to weak Brönsted acid sites or Lewis acid sites and medium Brönsted acid sites, respectively [37]. Between the two catalysts, HY(30) showed a larger amount of acid sites than HY(60) (Table 3). The substitution of proton with Ni2+ by the addition of Ni to HY(30) can lead to a reduction in the number of medium Brönsted acid sites and an increase in the number of Lewis acid sites. As shown in Figure 7, Ni addition increased Lewis acid sites density (peak at 1455 cm−1) [38] and decreased Brönsted acid sites (peak at 1545 cm−1) [38].

Table 3.
Amount of acid sites determined by NH3-TPD.
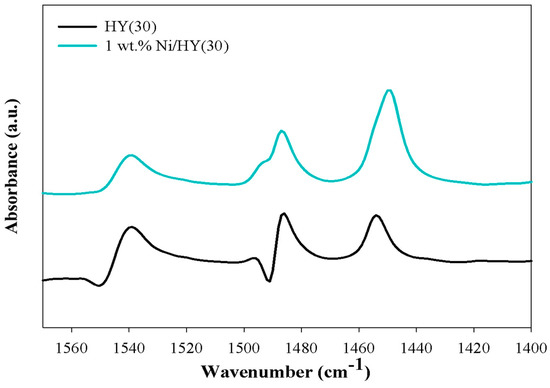
Figure 7.
Pyridine FT-IR of HY(30) and Ni/HY(30) catalysts.
3.3. TGA
TGA (Pyris Diamond, Perkin Elmer) of pinecone was performed. For this, 1 mg of pinecone was heated from ambient temperature to 800 °C under a nitrogen atmosphere with a flow rate of 50 mL/min. In the case of catalytic TGA, an additional amount of catalyst (1 mg) was added to the TGA sample containing 1 mg of pinecone.
3.4. Py-GC/MS Analysis
NCFP and CFP of pinecone over HY catalysts and their product analysis were performed at the same time using a micro-furnace-type pyrolyzer (Py-3030D, Frontier Laboratories, Fukushima, Japan)-GC/MS(7890A/5975C, Agilent Technology, Santa Clara, CA, USA). For the NCFP of pinecone, 1 mg of pinecone in the pyrolyzer sample cup was decomposed at 500 or 600 °C and the product vapor was analyzed by GC/MS. In case of the CFP of pinecone, different amounts of catalyst (0.25, 1.00, or 4.00 mg) were added to the same amount of pinecone sample (1 mg) and mixed in the pyrolysis sample cup. The prepared catalyst/sample mixture was free-fallen into the pyrolyzer heater for the CFP reaction, and the sample pyrolysis temperature and GC analysis conditions used in NCFP were also applied to the CFP. The detailed procedure is shown in the Supplementary Information.
4. Conclusions
The effectiveness of the CFP of biomass over HY catalysts for the production of aromatic hydrocarbons was examined by TGA and Py-GC/MS analysis. Although the main decomposition temperature of pinecone was not changed using the HY catalysts, the amount of aromatic hydrocarbons from the pyrolysis of pinecone was increased significantly using the HY catalysts. Between the two catalysts, HY(30) produced a larger amount of aromatic hydrocarbons than HY(60) because of its high acidity. By increasing the reaction temperature from 500 to 600 °C and decreasing sample-to-catalyst ratio from 4:1 to 1:4, the production of monoaromatic hydrocarbons during the catalytic pyrolysis of pinecone was also increased. The use of 1% Ni/HY(30) on the catalytic pyrolysis of pinecone further increased production efficiency of monoaromatic hydrocarbons, with a decrease in the formation of polyaromatics compared to HY(30) owing to enhancement of deoxygenation and aromatization.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/12/1034/s1.
Author Contributions
Conceptualization, Supervision, Y.-K.P.; Data curation, Formal analysis, Investigation, Methodology, Visualization, S.R., J.J.; H.W.L., R.-s.P., S.-C.J., J.-K.J.; Validation, Writing—original draft, J.J., H.W.L., S.H.J.; Writing—review and editing, Y.-M.K., J.-K.J., Y.-K.P.
Funding
This work was supported by the New and Renewable Energy Core Technology Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resources from the Ministry of Trade, Industry & Energy, Republic of Korea (No. 20173010092430).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, K.; Kim, K.H.; Brown, R.C. Catalytic pyrolysis of individual components of lignocellulosic biomass. Green Chem. 2014, 16, 727–735. [Google Scholar] [CrossRef]
- Mamaeva, A.; Tahmasebi, A.; Yu, J. The effects of mineral salt catalysts on selectivity of phenolic compounds in bio-oil during microwave pyrolysis of peanut shell. Korean J. Chem. Eng. 2017, 34, 672–680. [Google Scholar] [CrossRef]
- Devarajan, Y.; Nagappan, B.K.; Munuswamy, D.B. Performance and emissions analysis on diesel engine fuelled with cashew nut shell biodiesel and pentanol blends. Korean J. Chem. Eng. 2017, 34, 1021–1026. [Google Scholar] [CrossRef]
- Yadav, A.K.; Khan, M.E.; Pal, A. Biodiesel production from oleander (Thevetia Peruviana) oil and its performance testing on a diesel engine. Korean J. Chem. Eng. 2017, 34, 340–345. [Google Scholar] [CrossRef]
- Cherikkallinmel, S.K.; Sugunan, S.; Narayanan, B.N.; Faisal, P.A. Statistical optimization for lithium silicate catalysed production of biodiesel from waste cooking oil. Korean J. Chem. Eng. 2017, 34, 2840–2851. [Google Scholar] [CrossRef]
- Xie, H.; Yu, Q.; Duan, W.; Wang, K.; Li, X.; Shi, X. Pyrolysis characteristics and kinetics of lignin derived from three agricultural wastes. J. Renew. Sustain. Energy 2013, 5, 063119. [Google Scholar] [CrossRef]
- Yang, J.-H.; Shin, H.-Y.; Ryu, Y.-J.; Lee, C.-G. Hydrothermal liquefaction of Chlorella vulgaris: Effect of reaction temperature and time on energy recovery and nutrient recovery. J. Ind. Eng. Chem. 2018, 68, 267–273. [Google Scholar] [CrossRef]
- Phusunti, N.; Phetwarotai, W.; Tekasakul, S. Effects of torrefaction on physical properties, chemical composition and reactivity of microalgae. Korean J. Chem. Eng. 2018, 35, 503–510. [Google Scholar] [CrossRef]
- Kim, I.; Dwiatmoko, A.A.; Choi, J.W.; Suh, D.J.; Jae, J.; Ha, J.M.; Kim, J.K. Upgrading of sawdust pyrolysis oil to hydrocarbon fuels using tungstate-zirconia-supported Ru catalysts with less formation of cokes. J. Ind. Eng. Chem. 2017, 56, 74–81. [Google Scholar] [CrossRef]
- Pang, S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Boutaieb, M.; Guiza, M.; Roman, S.; Nogales, S.; Ledesma, B.; Ouederni, A. Pine cone pyrolysis: Optimization of temperature for energy recovery. Environ. Prog. Sustain. Energy 2019, e13272. [Google Scholar] [CrossRef]
- Park, J.-H.; Wang, J.J.; Kim, S.-H.; Jeong, C.Y.; Jeon, J.-R.; Park, K.H.; Cho, J.-S.; Delaune, R.D.; Sep, D.-C. Cadmiun adsorption characteristics of biochars derived using various pine tree residues and pyrolysis. J. Colloid Interface Sci. 2019, 553, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ng, W.; Wong, B.S.E.; Baeg, G.H.; Wang, C.-H.; Ok, Y.S. Characterization and ecotoxicological investigation of biochar produced via slow pyrolysis: Effect of feedstock composition and pyrolysis conditions. J. Hazard. Mater. 2019, 365, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Shafahat, H.; Kim, J.; Kang, B.S.; Jeon, J.K.; Jung, S.C.; Lee, I.G.; Park, Y.K. Stabilization of bio-oil over a low cost dolomite catalyst. Korean J. Chem. Eng. 2018, 35, 922–925. [Google Scholar] [CrossRef]
- Xue, Y.; Yan, C.; Zhao, X.; Huang, S.; Guo, C. Ni/La2O3-ZrO2 catalyst for hydrogen production from steam reforming of acetic acid as a model compounds of bio-oil. Korean J. Chem. Eng. 2017, 34, 305–313. [Google Scholar] [CrossRef]
- Lee, Y.; Shafaghat, H.; Kim, J.; Jeon, J.K.; Jung, S.C.; Lee, I.G.; Park, Y.K. Upgrading of pyrolysis bio-oil using WO3/ZrO2 and Amberlyst catalysts: Evaluation of acid number and viscosity. Korean J. Chem. Eng. 2017, 34, 2180–2187. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, M.; Zhao, L.; Lu, J. Effects of organic and inorganic metal salts on thermogravimetric pyrolysis of biomass components. Korean J. Chem. Eng. 2017, 34, 3077–3084. [Google Scholar] [CrossRef]
- Zhou, H.; Long, Y.; Meng, A.; Chen, S.; Li, Q.; Zhang, Y. A novel method for kinetics analysis of pyrolysis of hemicellulose, cellulose, and lignin in TGA and macro-TGA. RSC Adv. 2015, 5, 26509–26516. [Google Scholar] [CrossRef]
- Yao, C.; Tian, H.; Hu, Z.; Yin, Y.; Chen, D.; Yan, X. Characteristics and analyses of different genus biomass pyrolysis. Korean J. Chem. Eng. 2018, 35, 511–517. [Google Scholar] [CrossRef]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Lazaridis, P.A.; Fotopoulos, A.A.P.; Karakoulia, S.A.; Triantafyllidis, K.S. Catalytic Fast Pyrolysis of Kraft Lignin with Conventional, Mesoporous and Nanosized ZSM-5 Zeolite for the Production of Alkyl-Phenols and Aromatics. Front. Chem. 2018, 6, 295. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, Y.-M.; Lee, H.W.; Jae, J.; Kim, D.H.; Jung, S.C.; Watanabe, C.; Park, Y.-K. Catalytic Copyrolysis of Cellulose and Thermoplastics over HZSM-5 and HY. ACS Sustain. Chem. Eng. 2016, 4, 1354–1363. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S.-S.; Suh, D.J.; Jang, E.-J.; Min, K.-I.; Woo, H.C. Characterization of the bio-oil and bio-char produced by fixed bed pyrolysis of the brown alga Saccharina japonica. Korean J. Chem. Eng. 2016, 33, 2691–2698. [Google Scholar] [CrossRef]
- Shen, D.; Jin, W.; Hu, J.; Xiao, R.; Luo, K. An overview on fast pyrolysis of the main constituents in lignocellulosic biomass to valued-added chemicals: Structures, pathways and interactions. Renew. Sustain. Energy Rev. 2015, 51, 761–774. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, Q.; Ye, J.; Yao, Q.; Zhao, C. Study on the thermal degradation behaviors and kinetics of alkali lignin for production of phenolic-rich bio-oil using TGA-FTIR and Py-GC/MS. J. Anal. Appl. Pyrolysis 2016, 117, 116–124. [Google Scholar] [CrossRef]
- Bai, X.; Kim, K.H.; Brown, R.C.; Dalluge, E.; Hutchinson, C.; Lee, Y.J.; Dalluge, D. Formation of phenolic oligomers during fast pytolysis of lignin. Fuel 2014, 128, 170–179. [Google Scholar] [CrossRef]
- Rezaei, P.S.; Shafaghat, H.; Daud, W.M.A.W. Production of green aromatics and olefins by catalytic cracking of oxygenate compounds derived from biomass pyrolysis: A review. Appl. Catal. A-Gen. 2014, 469, 490–511. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Huber, G.W. Production of targeted aromatics by using Diels-Alder classes of reactions with Furans and olefins overZSM-5. Green Chem. 2012, 14, 3114–3125. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. Influence of Si/Al ratio of ZSM-5 Zeolite on the Properties of Lignin pyrolysis Products. ACS Sustain. Chem. Eng. 2013, 1, 316–324. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.H.; Park, J.; Kim, J.K.; An, D.; Song, I.K.; Choi, J.W. Catalytic pyrolysis of lignin over HZSM-5 catalysts: Effect of various parameters on the production of aromatic hydrocarbon. J. Anal. Appl. Pyrolysis 2015, 114, 273–280. [Google Scholar] [CrossRef]
- Chung, K.H.; Jeong, S.; Kim, H.; Kim, S.J.; Park, Y.-K.; Jung, S.-C. Highly Selective Catalytic Properties of HZSM-5 Zeolite in the Synthesis of Acetyl Triethyl Citrate by the Acetylation of Triethyl Citrate with Acetic Anhydride. Cataylsts 2017, 7, 321. [Google Scholar] [CrossRef]
- Usman, M.; Li, D.; Razzaq, R.; Yaseen, M.; Li, C.; Zhang, S. Novel MoP/HY catalyst for the selective conversion of naphthalene to tetralin. J. Ind. Eng. Chem. 2015, 23, 21–26. [Google Scholar] [CrossRef]
- Malik, G.M.; Patel, P.C.; Tailor, J.H.; Patel, S.S. Synthesis, Characterization, and Dyeing Performance of Thiadiazole Derivatives. Fiber Polym. 2018, 19, 1670–1677. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. Pyrolysis of Kraft lignin with additives. Energy Fuels 2011, 25, 4662–4668. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; Duan, D.; Zhao, Y.; Yu, Z.; Jiang, L. Catalytic fast pyrolysis of torrefied corn cob to aromatic hydrocarbons over Ni-modified hierarchical ZSM-5 catalyst. Bioresour. Technol. 2019, 272, 407–414. [Google Scholar] [CrossRef]
- Almendros, A.O.I.; Martin-Lara, M.A.; Ronda, A.; Perez, A.; Blazquez, G.; Calero, M. Physico-chemical characterization of pine cone shell and its use as biosorbent and fuel. Bioresour. Technol. 2015, 196, 406–412. [Google Scholar] [CrossRef]
- Vijaykumar, S.M.; Halgeri, A.B. Metal ion-exchanged zeolites as highly active solid acid catalysts for the green synthesis of glycerol carbonate from glycerol. RSC Adv. 2015, 5, 14286–14293. [Google Scholar]
- Busca, G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3–16. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).