Abstract
The stability of homogeneous catalytic systems is an industrially crucial topic, which, however, receives comparatively little attention from academic research. Phosphites are among the most frequently used ligands in industrial, rhodium-catalyzed n-regioselective hydroformylation. However, they are particularly vulnerable to hydrolysis. Since the decomposition of ligands should be dependent on the substitution patterns, phenyl, tert-butyl and condensed ring systems of benzopinacolphosphites were evaluated concerning their activity, regioselectivity and hydrolysis stability. A series of twelve strongly related phosphites were synthesized, tested in the hydroformylation of isomeric n-octenes, and studied in hydrolysis experiments using in situ NMR spectroscopy. Our results show that substituents in the ortho-position, especially tert-butyl substituents, enhance hydrolysis stability while maintaining compelling activity and regioselectivity. In contrast, substituents in the para-position may destabilize the phosphite.
1. Introduction
The hydroformylation of olefins is one of the most relevant, industrially applied chemical transformations worldwide [1,2,3,4,5,6]. The annual production of over 10 million tons of aldehydes reflects its importance [1,3]. Principally, hydroformylation is the reaction of syngas—a mixture of hydrogen and carbon monoxide—with olefins under the formation of aldehydes (Scheme 1). Formally, this reaction can be described as the addition of hydrogen and a formyl group to the double bond of the alkene in an atom-economical manner. Aldehydes themselves are not only valuable final products in bulk and fine-chemistry, but also important intermediates for further reactions [1,3,7]. They can be, for example, transformed into alcohols by reduction, to carboxylic acids by oxidation, or to amines by reductive amination.
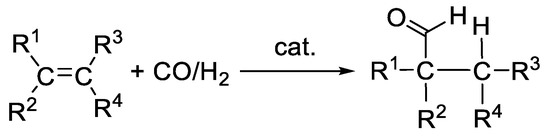
Scheme 1.
Hydroformylation.
A catalyst is a chemical compound that alters the rate of a reaction without being consumed. Thus, in theory, it is assumed that the catalyst is fully regenerated after each catalytic cycle. However, in practice, the catalyst is always subjected to modifications, for example, due to degradation reactions with components present in the catalytic system (e.g., starting products, (side)products, solvents and impurities), which result in a decrease in the concentration of the catalyst. This may consequently affect activity and/or selectivity [8]. Especially prone to alterations are organic ligands, which form in homogeneous metal catalysis, together with a metal of the catalytically active species.
When employed in hydroformylation, ligands fulfil several crucial tasks. They improve general catalytic properties, such as activity, and regio- and chemo-selectivity. Moreover, they can significantly contribute to the stability of the organometallic complex against the attack of water, aldehydes or acids. For several decades, mainly trivalent phosphorus compounds—like phosphines, phosphinites, phosphonites and phosphites—have been used in rhodium-catalyzed hydroformylation. A perusal of patent literature indicates that phosphites are the most common. Phosphorus ligands offer significant advantages, by employing moderate syngas pressure (1.8–6.0 MPa) and temperatures (85–130 °C) in comparison to related ligand-free processes.
Whereas phosphines are prone to oxidation and P–C bond activation, phosphites and similar P–O bond-containing compounds are mostly instable towards hydrolysis [1,8,9]. Due to the reaction with water, pentavalent species are formed which cannot coordinate to the metal center because they are missing a free electron pair. As a result, unmodified metal carbonyl complexes emerge, which are less active, selective, and, in the worst case, cause a loss of the precious metal by the formation of catalytically inactive clusters, followed by metal deposition [9]. Since, however, the pentavalent form of phosphoric acid diester is in tautomeric equilibrium with a trivalent species, the coordination of the degradation products to the metal center is possible (Scheme 2). When all the aryloxy groups are gradually substituted, phosphoric acid remains as the final product. The acid will catalyze together with acidic organic phosphorous intermediates (phosphoric acid esters) during the hydrolysis and lead to the autocatalyzed decomposition of the ligand [10].
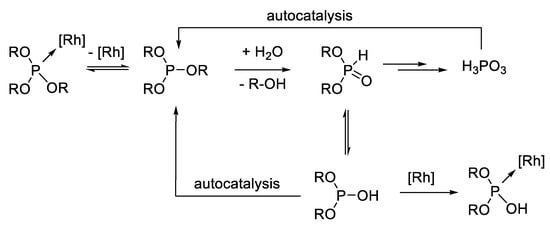
Scheme 2.
Degradation of phosphites by (autocatalytic) hydrolysis.
The hydrolytic stability of phosphites is an important issue in industrial processes, because water will inevitably be formed during hydroformylation as a by-product of the aldol condensation of the product aldehydes. Therefore, the study of their behavior towards water is of crucial importance for the design of new ligands, as well as adjusting proper reaction conditions. Of course, measures against hydrolysis should not affect the catalytic properties of the ligands. In the literature, some phosphites are described which combine both desired properties. Prominent examples are the monodentate ligand Alkanox® (also named Alkanox 240) [11,12,13,14,15] and the bidentate ligand Biphephos [16,17,18] (Figure 1). Both are decorated with several tert-butyl groups.
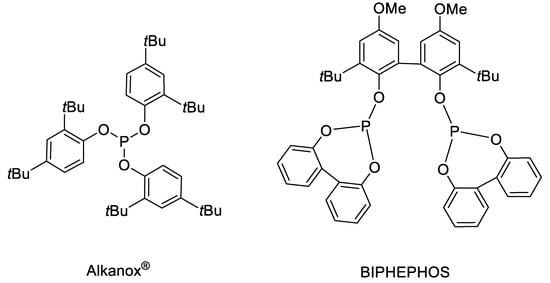
Figure 1.
Structures of Alkanox® and Biphephos.
Due to the large number of ligands with such varying properties, the unique effect of a single substituent on stability and catalytic properties does not become clear; therefore, especially dedicated studies are highly requested.
To date, only a few investigations concerning the hydrolysis of phosphites have been reported in the literature. One remarkable study was reported by van Leeuwen and co-workers several years ago, where they utilized a simple heteroatom-substituted phosphine oxide (HP(O)Ph2), often referred to as a HASPO, in platinum-catalyzed hydroformylation, suggesting that the catalytic impact of such degradation products should not be underestimated [19].
We also found that the degradation products of industrially used mono- and bi-dentate ligands can coordinate to rhodium (I) [9,20,21]. Several ligand-modified mono- and di-nuclear rhodium complexes were formed which exhibited different activity and n-regioselectivity in the hydroformylation of 1-octene and n-octenes compared to the parent catalyst or to unmodified (“naked”) rhodium.
Studies addressing the stabilizing and destabilizing effects of substituents on the stability of phosphites are even rarer. Since highly varied analytic methods and reaction conditions have been applied, the comparison of results reported in the literature is almost impossible. For example, the hydrolysis stability of several bulky phosphites bearing tert-butyl groups (e.g., Alkanox P-24, Ultranox U641, Alkanox 240) used in polymer chemistry as antioxidants was investigated by the research group, by the research group of Edge [22]. Recently, Oberhauser and Manca reported on the catalytic hydrolysis of aromatic and aliphatic tertiary phosphites by cationic phosphametallocene-based platinum (II) aqua complexes under neutral reaction conditions [23]. Evidence was given that the selective cleavage of a P–O bond occurs in the coordination sphere of the platinum (II) due to the transfer of a water molecule to the phosphite. The results of the experiments also show a clear difference in the reaction rate of the hydrolysis between aromatic and aliphatic phosphites, with the aromatic structures exhibiting a significantly slower decomposition rate.
In 2016, we studied the hydrolysis of a non-symmetrical bidentate phosphite and both of its monophosphite constituents, respectively [24]. The concerned non-symmetrical bidentate ligand was characterized by a biphenolphosphite and an acylphosphite moiety. In this study, the biphenolphosphite unit was proved to be of a magnitude more stable toward hydrolysis by water than the acylphosphite unit, which decomposed rapidly to salicylic acid and phosphoric acid. In addition, the influence of different substituents on the hydrolytic stability of the acylphosphite moiety was investigated. In principle, organic substituents with electron-donating or -accepting properties alter the Lewis basicity of phosphorus and oxygen. The rate of hydrolysis can also be controlled by the introduction of bulky substituents which cause kinetic inhibition. Noteworthy, bulky and electron-donating groups (e.g., tert-butyl or MeO) improve the stability, whereas electron-withdrawing substituents, such as chlorine, have a destabilizing effect.
In the aforementioned paper, decomposition was studied using in situ NMR spectroscopic methods, which were also applied in the actual report to gain comparable results and to obtain detailed insights into stabilizing effects. Several monodentate benzopinacolphosphites with varying substitution patterns were synthesized. The ligand class of benzopinacolphosphites was selected because they exhibited superior catalysis properties in previous investigations [25,26,27,28]. In this way, the influence of various substituents on the hydroformylation performance and hydrolysis stability can be investigated independently from other structural alterations. Having positioned electron-donating and electron-withdrawing aryl substituents in ortho-, meta- and para-positions to the phosphite group, the precise effect of phenyl-substituents, condensed ring systems, and tert-butyl substituents, was then studied.
2. Results and Discussion
2.1. Synthesis of Benzopinacolphosphites
For the sake of this study, 12 benzopinacolphosphites with varying substituents were prepared (Scheme 3, Table 1). Some of these compounds were already described previously [25,26,27,28,29]. First, the required phosphorchloridite 1 was synthesized using a reaction of phosphorus trichloride and benzopinacol, in the presence of triethylamine as a HCl scavenger [30].
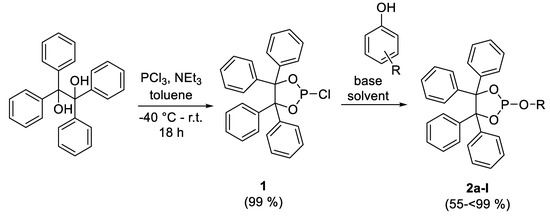
Scheme 3.
General synthesis of phosphites.

Table 1.
Reaction conditions and yields of the investigated benzopinacolphosphites.
Afterwards, the product was reacted with the relevant substituted phenol in the presence of triethylamine as supporting base. Alternatively, the phenol was first deprotonated with n-BuLi to form the lithium salt, which was subsequently reacted with the phosphorchloridite 1. The low reaction temperatures served to slow down the formation of HCl, so that it was only present in low concentrations in the reaction solution before it reacted with triethylamine to give the corresponding ammonium salt. Higher concentrations of HCl could lead to the degradation of the phosphite. Moreover, the formation of the ammonium salt, generated from evolving HCl, shifts the reaction equilibrium towards the phosphite. It was observed that the phenyl-substituted phenols only needed the relatively weak base, triethylamine, for deprotonation. Phenols with more than one tert-butyl group, or condensed aromatic ring systems, usually required the application of the stronger base, n-butyllithium. Otherwise, the reaction was much more unselective, and the work-up was massively hampered.
Crystals suitable for X-ray crystal structure analysis could be obtained, e.g., using phosphites 2a and 2d (Figure 2). The comparison of both structures reveals that a phenyl substituent in the meta position does not significantly influence the length of the central P–O bonds.
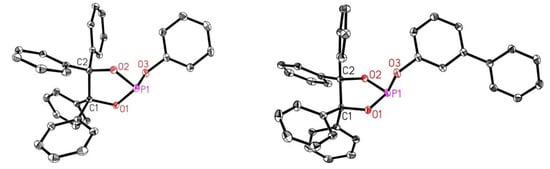
Figure 2.
The molecular structure of phosphites 2a (left), and 2d (right). The displacement ellipsoids correspond to 30% probability. Hydrogen atoms are omitted for clarity. Selected bond lengths [Ǻ] and angles [°]: 2a: P1–O1 1.6275(9), P1–O2 1.6223(9), P1–O3 1.6403(9); O2–P1–O1 94.20(4), O2–P1–O3 106.71(5), O1–P1–O3 94.53(5); 2d: P1–O1 1.6292(9), P1–O2 1.6189(9), P1–O3 1.6397(9); O2–P1–O1 94.10(4), O2–P1–O3 105.61(5), O1–P1–O3 95.53(5).
2.2. Hydroformylation Experiments
To investigate the activity and n-selectivity, n-octenes—a technical relevant mixture of isomeric octenes—were used as a model substrate in the hydroformylation. The rate constant kobs. of the conversion was determined whenever possible by the exponential curve-fitting of the measured gas consumption during the reaction. In this case, pseudo-first-order kinetics could be applied because of the vast excess of synthesis gas used. The kinetic can be described using the following equation:
with [A] representing the concentration of the starting product, and k the reaction rate. The results are collected in Table 2.

Table 2.
Hydroformylation a of n-octenes b with benzopinacolphosphites.
The highest yields after 4 h of reaction time were found using phosphites 2b, 2e, 2f and 2h—each at over 97% (Table 2, entries 2, 5, 7 and 11). Taking the rate constant kobs. into account, ligands 2b, 2e, 2h and 2j induced the highest activity (entries 2, 5, 11 and 15). Generally, substituents in an ortho-position, especially electron-accepting phenyl-substituents and condensed aromatic systems, allowed for the achievement of high activities. However, tert-butyl groups (2j, entry 15) may also contribute beneficially to the activity. The following general tendency can be derived (Scheme 4).
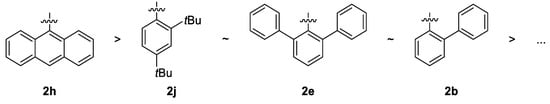
Scheme 4.
The order of activity in hydroformylation in dependence on position and nature of substituents.
All n-selectivities were within a range of 10% and 41%. However, the highest n-selectivities were associated with relatively low yields and activities. The highest n-selectivity (29.7%) paired with reasonable activity (93%, 0.067 min−1) was obtained using ligand 2k (entry 16). Furthermore, ligand 2f exhibited comparable properties with an n-selectivity of 26.5%, a yield of 93% and a rate constant kobs of 0.067 min−1. Phosphites 2g and 2h, with condensed aromatic structures, gave slightly better n-selectivities than the parent phenyl ligand. Phenyl-substituents in ortho-position, and tert-butyl substituents in para-position, lowered the regioselectivity.
It can be noted that the observed tendencies for yield and n-selectivity seen with a change of the reaction temperature are not the same for each ligand. In the cases of 2f (Table 2, entries 6 and 7) and 2g (entries 8 and 9), the decrease in temperature led to an increase in the yield of aldehyde and in n-selectivity. In contrast, the same transformation with 2h only gave an increase in yield in the beginning, but a further decrease in the temperature to 100 °C did not affect the yield. Moreover, lower reaction temperatures led to a lowered n-selectivity in this particular case.
2.3. Hydrolysis Experiments
To test the phosphites’ stability against water, a definite amount of phosphite was dissolved in 1,4-dioxane in order to gain a 0.049 M solution. Afterwards, 100 equivalents of deoxygenized water were added. The use of a high excess of 100 equivalents of water opened up the possibility of applying pseudo-first-order kinetics to determine the reaction constant and half-life time of the reaction. As previously mentioned, pseudo-first-order kinetics can be applied when one reagent is present in large excess, and therefore stays approximately constant during the reaction, as shown in Equation (1).
When the concentrations of starting material A are plotted against time t, an exponential curve results. Logarithm, on the other hand, provides a linear correlation for the decrease in concentration of the starting product. The exemplary plots of 2a are shown in Figure 3.
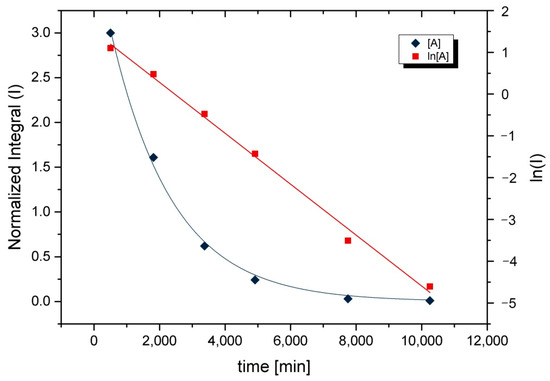
Figure 3.
Direct and logarithmized plots of the concentration profile of 2a during hydrolysis. Conditions: [L] = 0.049 mol·L−1; H2O/L = 100; T = room temperature; solvent: 1,4-dioxane.
To obtain the half-life of the phosphites, the rate constant kobs. is needed, which can be derived from the equations of the plotted concentration profiles. The half-life of a pseudo-first-order reaction can be calculated using Equation (2):
The half-life could not be obtained for all the decomposition experiments, as some phosphites decomposed too quickly, whereby not enough sufficient data points existed to determine the rate constant. Moreover, some other phosphites did not decompose at all, and some experimental data did not show the behavior of first-order reactions. Therefore, besides the half-life time, the complete decomposition time of the phosphites was also determined.
The hydrolysis of the phosphites was studied at room temperature to obtain sufficient differentiation (Table 3), and at 90 °C to simulate common conditions for hydroformylation reactions (Table 4). At both temperatures, similar trends could be observed. The two structures, 2k and 2l, which possess tert-butyl substituents in both ortho-positions, did not show any sign of decomposition, either at room temperature or at 90 °C. Also, the other phosphites with aryl substituents in their ortho-positions showed significantly higher stabilities compared to the unsubstituted structures (e.g., 2e, 2f and 2h). Surprisingly, 2b did not follow the same tendency. The reason for this unique behavior is not clear.

Table 3.
The hydrolysis of benzopinacolphosphites at room temperature a.

Table 4.
Hydrolysis of benzopinacolphosphites at 90 °C a.
While 2k and 2l were astonishingly stable, 2d and 2i decomposed rapidly. Therefore, no rate constants for these phosphites could be derived. Furthermore, not all benzopinacolphosphites exhibited an exponential decomposition behavior throughout the whole reaction time. The decomposition rates of 2e, 2f and 2g increased to a certain point, which may be rationalized by the autocatalysis induced by the formation of acidic degradation products. Their hydrolysis behavior at 90 °C is shown in Figure 4.
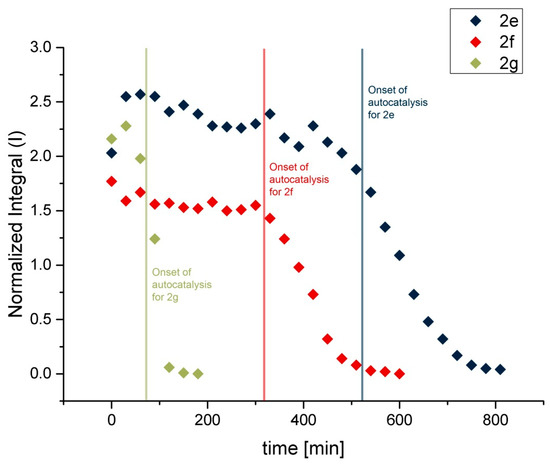
Figure 4.
Hydrolysis of 2e, 2f and 2g at 90 °C (conditions see Table 3).
It is significant that sterically demanding substituents in ortho-position to the phosphorous moiety increase the hydrolysis stability to an exceptional level. For example, 2e, 2f, 2h, 2k, and 2l are the most robust phosphites at room temperature (Figure 5).
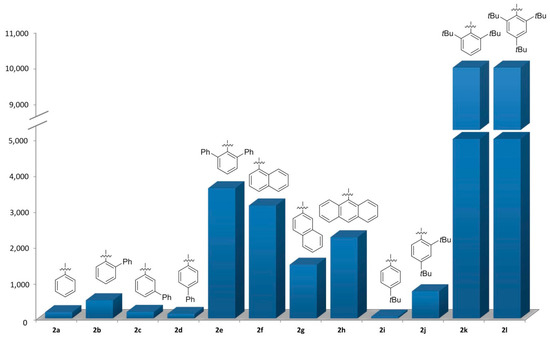
Figure 5.
Complete decomposition times of benzopinacolphosphites at room temperature.
The extremely high stability of phosphites 2k and 2l, which both have tert-butyl groups in the ortho-position, should be particularly emphasized. Phosphites with substituents in the para-position can be considered slightly more labile towards hydrolysis than the unsubstituted structure (2a). The latter exhibits a decomposition time over 60 times shorter at room temperature, and over 225 times shorter at 90 °C, than the most stable phosphites (2k and 2l).
3. Materials and Methods
3.1. Synthesis of Phosphites
Phosphorchloridite 2-chloro-4,4,5,5-tetraphenyl-1,3,2-dioxaphospholane (1), the unsubstituted benzopinacolphosphite 2-phenoxy-4,4′,5,5′-tetraphenyl-1,3,2-dioxaphospholane (2a), the four phenyl-substituted benzopinacolphosphites 2-([1,1′-biphenyl]-2-yloxy)-4,4,5,5-tetraphenyl-1,3,2- dioxaphospholane (2b), 2-([1,1′-biphenyl]-3-yloxy)-4,4,5,5-tetraphenyl-1,3,2-dioxaphospholane (2c), 2-([1,1′-biphenyl]-4-yloxy)-4,4,5,5-tetraphenyl-1,3,2-dioxaphospholane (2d), 2-([1,1′:3′,1″-terphenyl]- 2′-yloxy)-4,4,5,5-tetraphenyl-1,3,2-dioxaphospholane (2e), the three benzopinacolphosphites with condensed ring systems 2-(naphthalen-1-yloxy)-4,4,5,5-tetraphenyl-1,3,2-dioxaphospholane (2f), 2-(naphthalen-2-yloxy)-4,4,5,5-tetraphenyl-1,3,2-dioxaphospholane (2g), 2-(anthracen-9-yloxy)- 4,4,5,5-tetraphenyl-1,3,2-dioxaphospholane (2h) and the four benzopinacolphosphites with tert-butyl-substituents 2-(4-(tert-butyl)phenoxy)-4,4,5,5-tetraphenyl-1,3,2-dioxaphospholane (2i), 2-(2,4-di-tert-butylphenoxy)-4,4,5,5-tetraphenyl-1,3,2-dioxaphospholane (2j), 2-(2,6-di-tert- butylphenoxy)-4,4,5,5-tetraphenyl-1,3,2-dioxaphospholane (2k) and 4,4,5,5-tetraphenyl-2-(2,4,6-tri- tert-butylphenoxy)-1,3,2-dioxaphospholane (2l) were synthesized. Detailed synthesis procedures and experimental data are given in the supporting information. The structures and yields are shown in Figure 6.
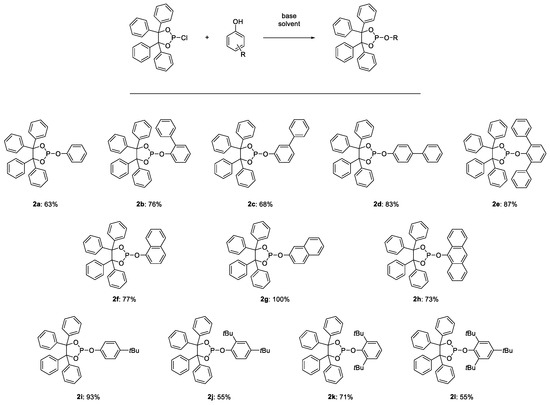
Figure 6.
Structures and yields of the synthesized benzopinacolphosphites.
3.2. X-ray Crystal Structual Analyses of 2a and 2d
Data were collected on a Bruker Kappa APEX II Duo diffractometer. The structures were solved by direct methods (SHELXS-97: Sheldrick, G. M. Acta Cryst. 2008, A64, 112.) and refined using full-matrix least-squares procedures on F2 (SHELXL-2014: Sheldrick, G. M. Acta Cryst. 2015, C71, 3.). XP (Bruker AXS) was used for graphical representations.
Crystal data for 2a: C32H25O3P, M = 488.49, monoclinic, space group P21/n, a = 14.9694(4), b = 9.5530(2), c = 18.7199(5) Å, β = 112.7503(6)°, V = 2468.72(11) Å3, T = 150(2) K, Z = 4, 50,826 reflections measured, 5970 independent reflections (Rint = 0.026), final R values (I > 2σ(I)): R1 = 0.0355, wR2 = 0.0898, final R values (all data): R1 = 0.0425, wR2 = 0.0963, 325 parameters.
Crystal data for 2d: C38H29O3P, M = 564.58, monoclinic, space group P21/n, a = 14.9935(3), b = 9.8857(2), c = 20.5571(5) Å, β = 104.0748(6)°, V = 2955.52(11) Å3, T = 150(2) K, Z = 4, 60,717 reflections measured, 7483 independent reflections (Rint = 0.031), final R values (I > 2σ(I)): R1 = 0.0368, wR2 = 0.0894, final R values (all data): R1 = 0.0488, wR2 = 0.0984, 379 parameters.
CCDC 1964104-1964105 contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.
3.3. Hydroformylation Experiments
The hydroformylation experiments were performed in a 150 mL autoclave (Premex Reactor AG, Lengnau, Switzerland) equipped with a gas inlet stirrer, a thermocouple, a storage vessel allowing the addition of substrate under pressure applied, a Bronkhorst hitec® pressure controller, and a Bronkhorst hitec® gas flow meter. The autoclave periphery allowed us to fill in and to remove any media and reagents through the exclusion of air. A high-grade pure syngas (99.997%; CO/H2 = 1:1) was purchased from Linde AG. The composition of the technical octene mixture used as a substrate for the hydroformylation reaction was: 3.3% 1-octene, 48.4% Z/E-2-octene, 29.2% Z/E-3-octene, 16.4% Z/E-4-octene, 2.1% skeletal C8-olefinic isomers, 0.6% n-octane. Olefins were refluxed in an argon atmosphere over sodium metal and freshly distilled prior to use.
For the hydroformylation experiments, the reaction solution was prepared under an atmosphere of argon. The mass of toluene was defined for GC analysis. The stirred reaction solution was pressurized with 32 bar of syngas and heated to the assigned temperature. After reaching the reaction temperature, the pressure was elevated to 46 bar and the n-octene was added with a pressure of 54 bar. The reaction was kept at constant pressure of 50 bar for 4 h. The current gas flow was determined in a time cycle of 3 s simultaneously with the reaction process. The gas consumption curve thus obtained was used to determine the observed first-order velocity constant kobs. After the reaction time, the autoclave was cooled down to room temperature, the pressure released and purged with argon. An amount of 0.1 mL of the reaction solution was diluted with 1 mL pentane and analyzed with gas chromatography (HP 5890 Series II plus, PONA, 50 m × 0.2 mm × 0.5 µm). The quantitative amount of remaining olefin and newly formed aldehyde were determined using toluene as an internal standard.
3.4. Hydrolysis Experiments
NMR samples were filled in an argon atmosphere using standard Schlenk techniques. 1,4-Dioxane was purchased from Acros Organics and, being extremely dry, was stored under argon protection. 1,4-Dioxane-d8 was purchased from Deutero and distilled in an argon atmosphere prior to use. Tri-n-octylphosphine oxide and p-xylene-d10 were purchased from Sigma–Aldrich (St. Louis, MO, USA). The in situ 31P NMR experiments were carried out in a sealed 9 inch, 5 mm NMR tube (Deutero) equipped with a sealed 12.5 cm capillary (Sigma–Aldrich) of an inner diameter of 1 mm, which was filled with a 0.259 M solution of tri-n-octylphosphine oxide (TOPO) in p-xylene-d10, along with the sample solution as an external reference, and the lock solvent. The samples were dissolved in 1,4-dioxane at a concentration of 0.049 M. Water was distilled in an argon atmosphere and added into the specimen before measurement. In situ NMR experiments were carried out on a Bruker AVANCE 300 III spectrometer at a Lamor frequency of 300 MHz for 1H, 121 MHz for 31P, and 75 MHz for 13C NMR or a Bruker AVANCE 250 spectrometer at a Lamor frequency of 250 MHz for 1H, 101 MHz for 31P and 63 MHz for 13C NMR. Quantitative 31P NMR spectra were acquired with a relaxation delay of 5 s and a scan number of 256, referenced by the chemical shift of TOPO set at 41 ppm, whose integral was set as 1.
4. Conclusions
A series of monophosphites with substituted benzopinacol backbones were synthesized and tested as ligands in the rhodium-catalyzed hydroformylation of n-octenes. Among these ligands, phosphites with a single substituent in the ortho-position of the phosphorous moiety exhibited especially high activities and yields of the desired aldehyde. This holds for phenyl as well as for tert-butyl groups. Surprisingly, phosphites 2c and 2d, with a single phenyl group in either the meta or para position, can be considered as ineligible for the hydroformylation of n-octenes—at least under our reaction conditions. The same holds for 2l, where the phenyl ring is decorated with three tert-butyl groups. For 2c and 2d, this failure can be rationalized by their low stability, which leads to catalytically unreactive species. The catalyst derived from phosphite 2l might be inactive because of the large steric hindrance exerted by the three tert-butyl substituents. Probably, this substitution pattern inhibits the coordination of the phosphorus to rhodium. Generally, tert-butyl groups in the ortho-position stabilize the ligand toward hydrolysis. This is in full agreement with former results, where bulky ortho-alkyl substituents were incorporated, e.g., in the prominent monophosphite Alkanox® or the diphosphite Biphephos (vide supra).
In terms of regioselectivity, the catalysts of those ligands that induced low activities usually exhibited high n-selectivities. Among the ligands favoring an n-aldehyde yield over 20%, the highest regioselectivities were produced by 2f, 2h and 2k. All of them are characterized by sterically demanding structures. Another very sterically demanding phosphite (2e) displayed a very high activity, but a mediocre n-selectivity. This result must arise from the fast reaction with the non-isomerized internal olefins. In general, our study gives evidence that a compromise must be found, where the catalytic and stabilizing properties of ligands have to be matched.
5. Patents
A. Börner, K. M. Dyballa, R. Franke, D. Fridag, F. Geilen, D. Hess, D. Selent (to Evonik Industries), Monophosphites with structural unit 4,4,5,5-tetraphenyl-1,3,2-dioxaphospholan as ligands for hydroformylation catalysts, EP000003029013 (2014).
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/12/1036/s1, General Preparation, Synthesis procedures for compounds 1 and 2a–2l, Figures S1–S6: Hydrolysis diagrams, Figures S7–S29: NMR spectra of hydrolysis reactions, Figures S30–S69: NMR spectra of compounds 1 and 2a–2l.
Author Contributions
Conceptualization, A.B. and R.F.; investigation, S.K., D.S., A.S. and M.S.; writing—original draft preparation, S.K.; writing—review and editing, A.B.; visualization, S.K.; supervision, A.B.; project administration, A.B.; funding acquisition, A.B.
Funding
The research was supported by Evonik Industries and the Leibniz-Institut für Katalyse e.V.
Acknowledgments
We thank Evonik Industries for financial support. We acknowledge highly skilled technical and analytic support by K. Romeike, H. Borgwaldt, M. Lange and D. Michalik. We are grateful to Marcus Uhlemann for the preparation of phosphites 2a, 2c and 2d.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Börner, A.; Franke, R. Hydroformylation. Fundamentals, Processes, and Applications in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- van der Slot, S.C.; Duran, D.; Luten, J.; Kamer, P.C.J.; van Leeuwen, P.N.M. Rhodium Catalyzed Hydroformylation; van Leeuwen, P.W.N.M., Claver, C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 4 December 2014. [Google Scholar]
- Franke, R.; Selent, D.; Börner, A. Applied hydroformylation. Chem. Rev. 2012, 112, 5675–5732. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, H.-W.; Cornils, B. Hydroformylation of alkenes: An industrial view of the status and importance. Adv. Catal. 2002, 47, 1–64. [Google Scholar] [CrossRef]
- Cornils, B.; Herrmann, W.A.; Beller, M.; Paciello, R. Applied Homogeneous Catalysis with Organometallic Compounds. A Comprehensive Handbook in Four Volumes, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Wiese, K.-D.; Obst, D. Hydroformylation. In Catalytic Carbonylation Reactions; Beller, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–33. [Google Scholar]
- Gusevskaya, E.V.; Jiménez-Pinto, J.; Börner, A. Hydroformylation in the Realm of Scents. ChemCatChem 2014, 6, 382–411. [Google Scholar] [CrossRef]
- van Leeuwen, P.W.N.M.; Chadwick, J.C. Homogeneous Catalysts. Activity—Stability—Deactivation; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Christiansen, A.; Selent, D.; Spannenberg, A.; Köckerling, M.; Reinke, H.; Baumann, W.; Jiao, H.; Franke, R.; Börner, A. Heteroatom-substituted secondary phosphine oxides (HASPOs) as decomposition products and preligands in rhodium-catalysed hydroformylation. Chem. Eur. J. 2011, 17, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, S.K.; Alam, T.M. 17O NMR investigation of phosphite hydrolysis mechanisms. Magn. Reson. Chem. 2007, 45, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Roobeek, C.F.; van Leeuwen, P.W.N.M. A Process for the Hydroformylation of Olefins. EP000000054986, 22 December 1980. [Google Scholar]
- Abatjoglou, A.G.; Bryant, D.R.; Maher, J.M. Stabilization of Phosphite Ligands in Hydroformylation Process. EP000000697391, 18 August 1995. [Google Scholar]
- van Leeuwen, P.W.N.M.; Roobeek, C.F. Hydroformylation of less reactive olefins with modified rhodium catalysts. J. Organomet. Chem. 1983, 258, 343–350. [Google Scholar] [CrossRef]
- van Rooy, A.; Orij, E.N.; Kamer, P.C.J.; van den Aardweg, F.; van Leeuwen, P.W.N.M. Hydroformylation of oct-1-ene with extremely high rates using rhodium catalysts containing bulky phosphites. J. Chem. Soc. Chem. Commun. 1991, 1096. [Google Scholar] [CrossRef]
- Jongsma, T.; Challa, G.; van Leeuwen, P.W.N.M. A mechanistic study of rhodium tri(o-t-butylphenyl)phosphite complexes as hydroformylation catalysts. J. Organomet. Chem. 1991, 421, 121–128. [Google Scholar] [CrossRef]
- Abatjoglou, A.G.; Billig, E.; Bryant, D.R. Transition Metal Complex Catalyzed Processes. US000004769498, 6 September 1988. [Google Scholar]
- Cuny, G.D.; Buchwald, S.L. Practical, high-yield, regioselective, rhodium-catalyzed hydroformylation of functionalized α.-olefins. J. Am. Chem. Soc. 1993, 115, 2066–2068. [Google Scholar] [CrossRef]
- Abatjoglou, A.G.; Billig, E.; Bryant, D.R. Bis-Phosphite Compounds. EP000000213639, 4 September 1986. [Google Scholar]
- van Leeuwen, P.W.N.M.; Roobeek, C.F.; Frijns, J.H.G.; Orpen, A.G. Characterization of the intermediates in the hydroformylation reaction catalyzed by platinum diphenylphosphinous acid complexes. Organometallics 1990, 9, 1211–1222. [Google Scholar] [CrossRef]
- Christiansen, A.; Selent, D.; Spannenberg, A.; Baumann, W.; Franke, R.; Börner, A. Reaction of Secondary Phosphine Oxides with Rhodium(I). Organometallics 2010, 29, 3139–3145. [Google Scholar] [CrossRef]
- Christiansen, A.; Li, C.; Garland, M.; Selent, D.; Ludwig, R.; Franke, R.; Börner, A. Secondary Phosphane Oxides as Preligands in Rhodium-Catalyzed Hydroformylation. ChemCatChem 2010, 2, 1278–1285. [Google Scholar] [CrossRef]
- Johnson, B.; Keck-Antoine, K.; Dejolier, B.; Allen, N.; Ortuoste, N.; Edge, M. Impact of improved phosphite hydrolytic stability on the processing stabilization of polypropylene. J. Vinyl Addit. Techn. 2005, 11, 136–142. [Google Scholar] [CrossRef]
- Oberhauser, W.; Manca, G. Catalytic Phosphite Hydrolysis under Neutral Reaction Conditions. Inorg. Chem. 2018, 57, 4824–4827. [Google Scholar] [CrossRef]
- Zhang, B.; Jiao, H.; Michalik, D.; Kloß, S.; Deter, L.M.; Selent, D.; Spannenberg, A.; Franke, R.; Börner, A. Hydrolysis Stability of Bidentate Phosphites Utilized as Modifying Ligands in the Rh-Catalyzed n-Regioselective Hydroformylation of Olefins. ACS Catal. 2016, 6, 7554–7565. [Google Scholar] [CrossRef][Green Version]
- Börner, A.; Dyballa, K.M.; Franke, R.; Selent, D. Phosphites with a Dihydroxyterphenyl with a Tetraphenyl Dioxaphospholane. EP000003293192, 7 September 2016. [Google Scholar]
- Börner, A.; Dyballa, K.M.; Franke, R.; Fridag, D.; Selent, D. Bis-Phosphites with an Asymmetric Biaryl Central Component. EP3029048, 4 December 2014. [Google Scholar]
- Börner, A.; Hess, D.; Kreidler, B.; Selent, D.; Wiese, K.-D. Bisphosphite Ligands for Hydroformylation Catalyzed by Transition Metals. WO002008071508, 13 December 2006. [Google Scholar]
- Börner, A.; Dyballa, K.M.; Franke, R.; Selent, D. Bis-Phosphites with 2,3-Biphenol Unit as Central Component. EP000003029045, 4 December 2014. [Google Scholar]
- Dyballa, K.M.; Franke, R. Novel Monophosphite Compound with an Ester Group. EP000003088407, 14 April 2016. [Google Scholar]
- Selent, D.; Franke, R.; Kubis, C.; Spannenberg, A.; Baumann, W.; Kreidler, B.; Börner, A. A New Diphosphite Promoting Highly Regioselective Rhodium-Catalyzed Hydroformylation. Organometallics 2011, 30, 4509–4514. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).