Genipin as An Emergent Tool in the Design of Biocatalysts: Mechanism of Reaction and Applications
Abstract
1. Introduction
1.1. Biocatalysis and Biocatalysts Design
1.2. Genipin Uses
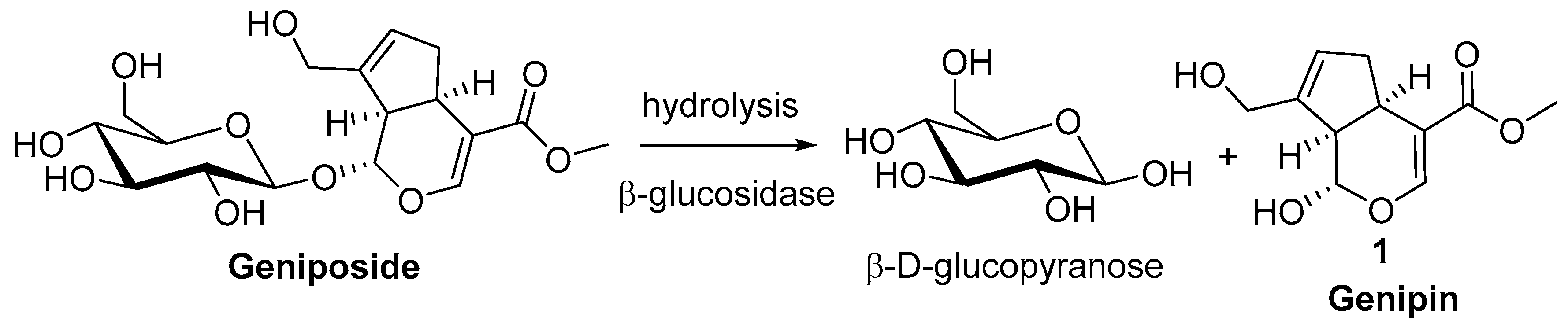
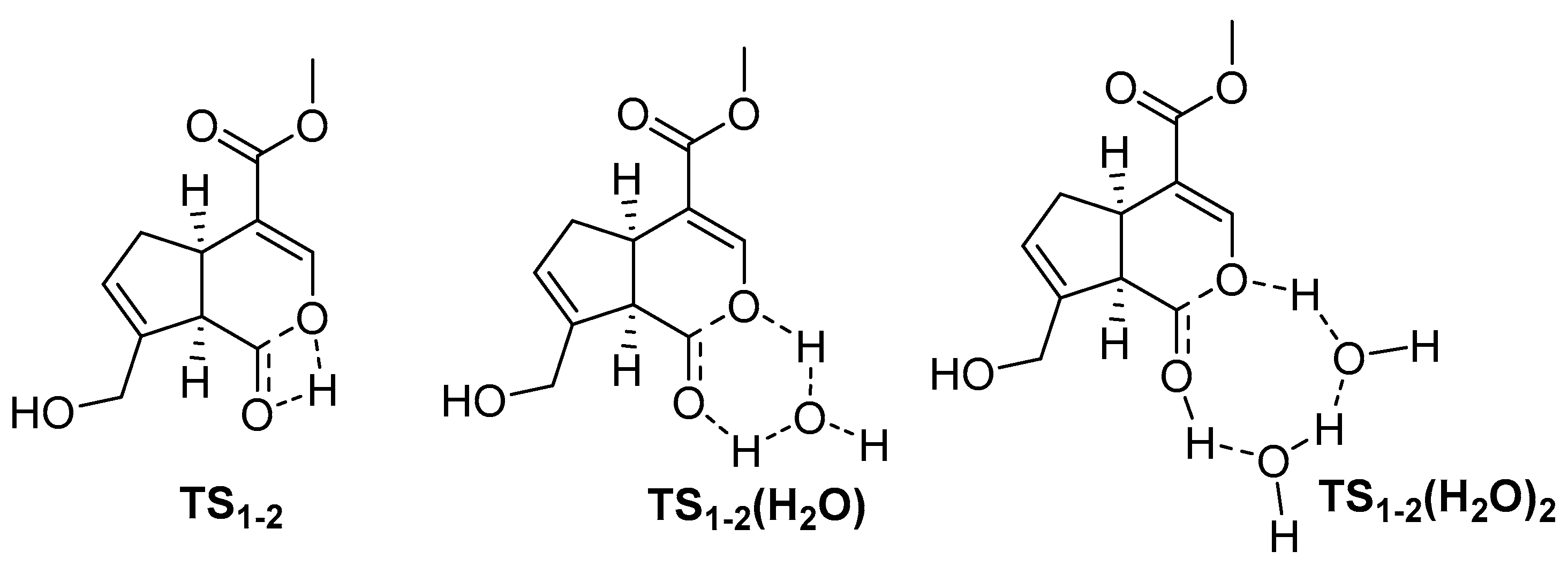
2. Chemisty of Genipin
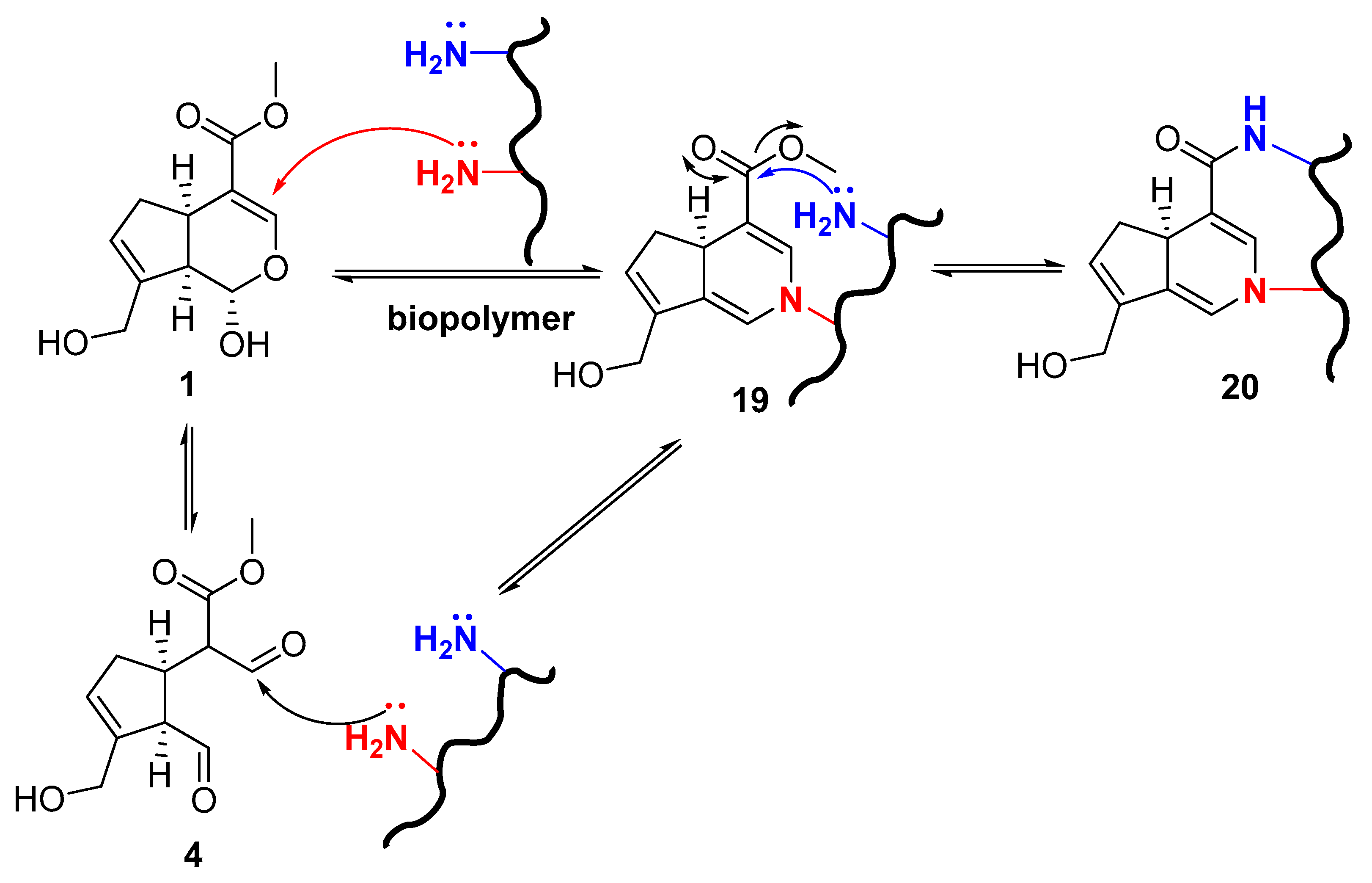
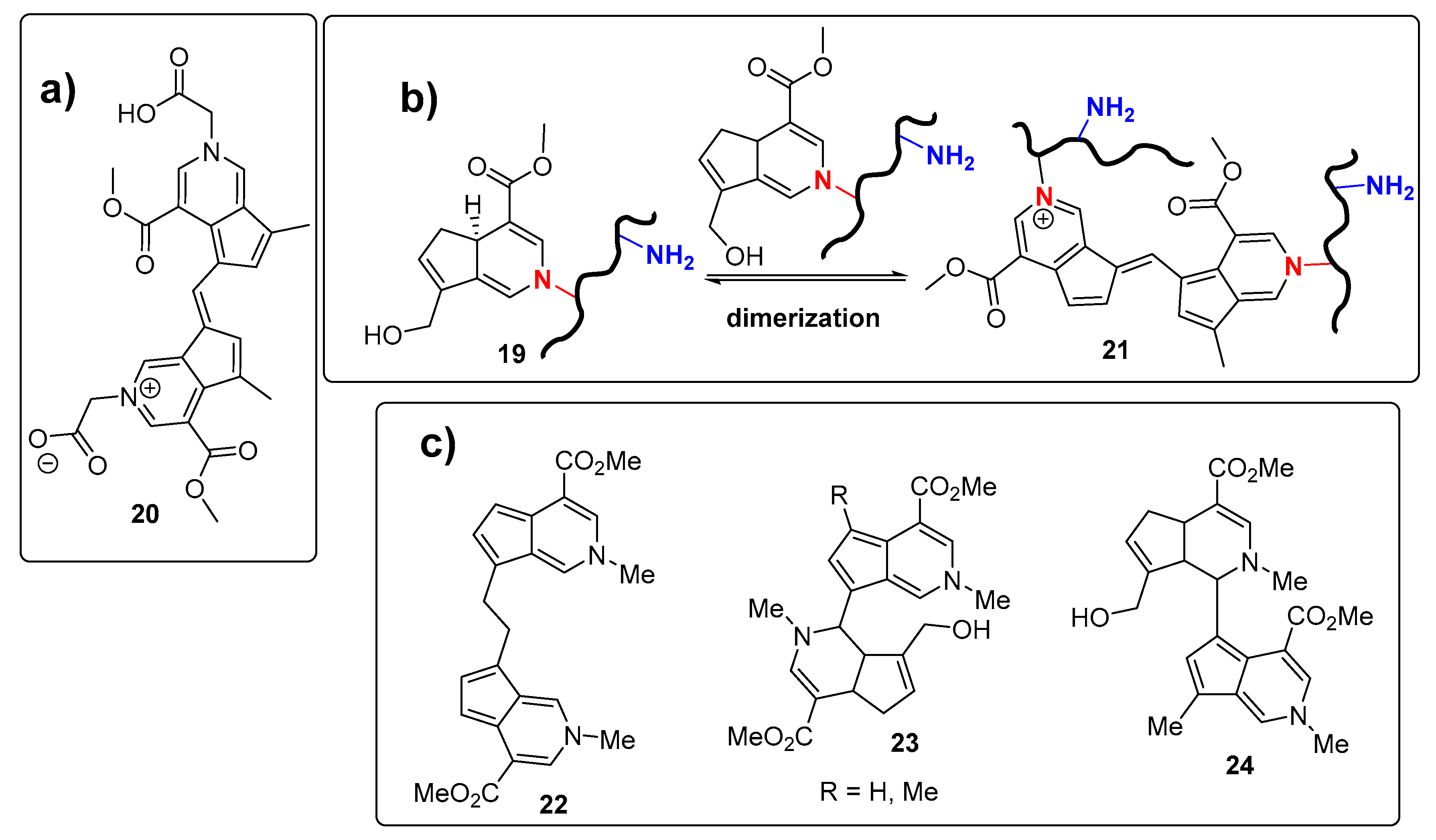
Reactivity of Genipin as Cross-Linker Agent
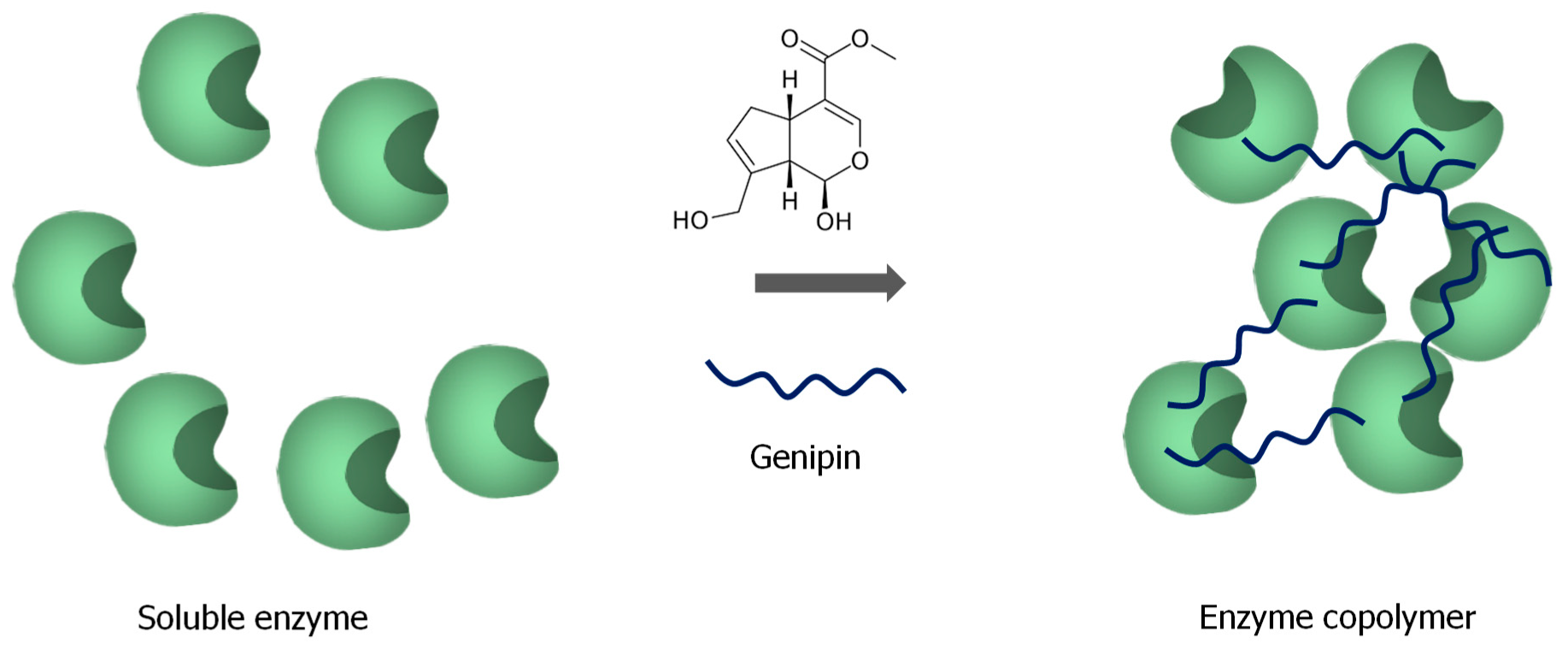
3. Use of Genipin in Biocatalysts Design
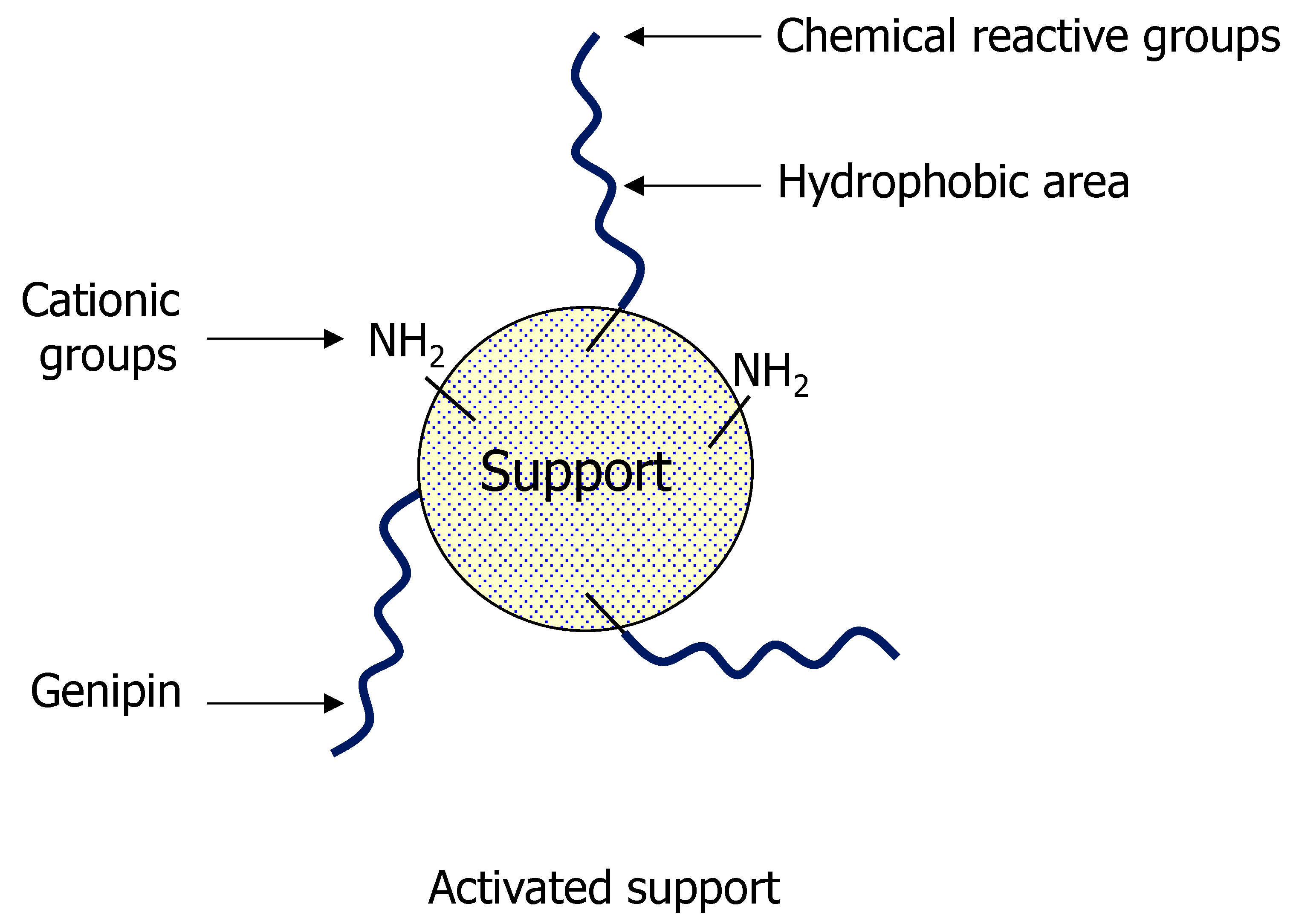
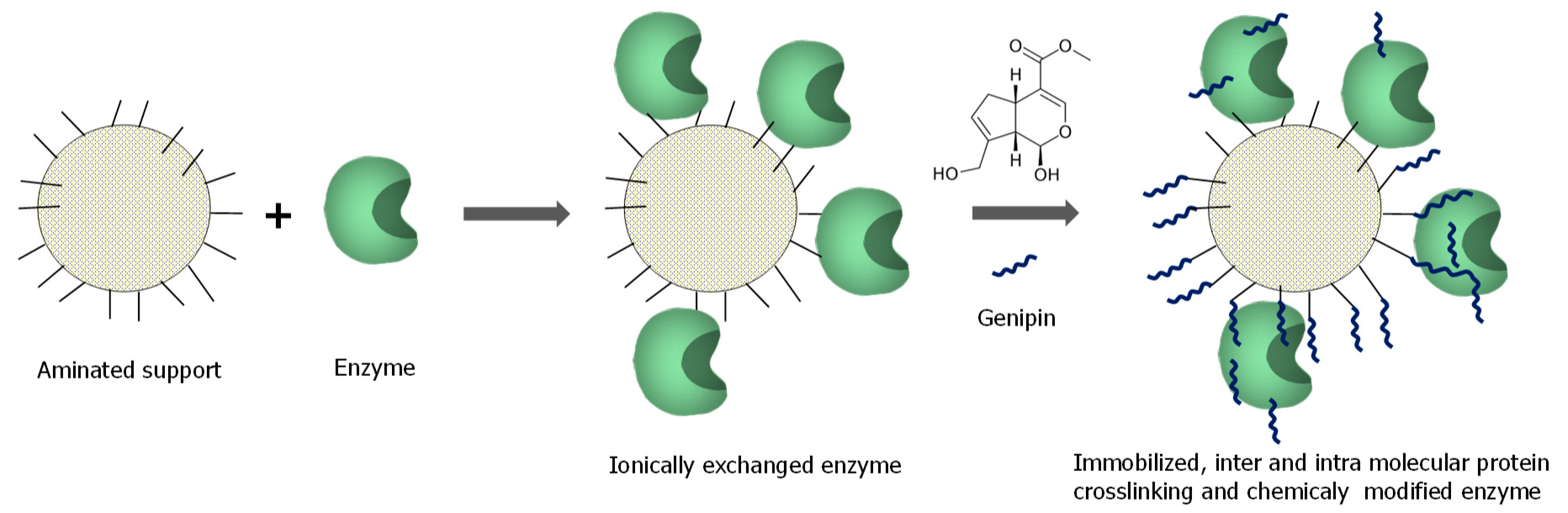
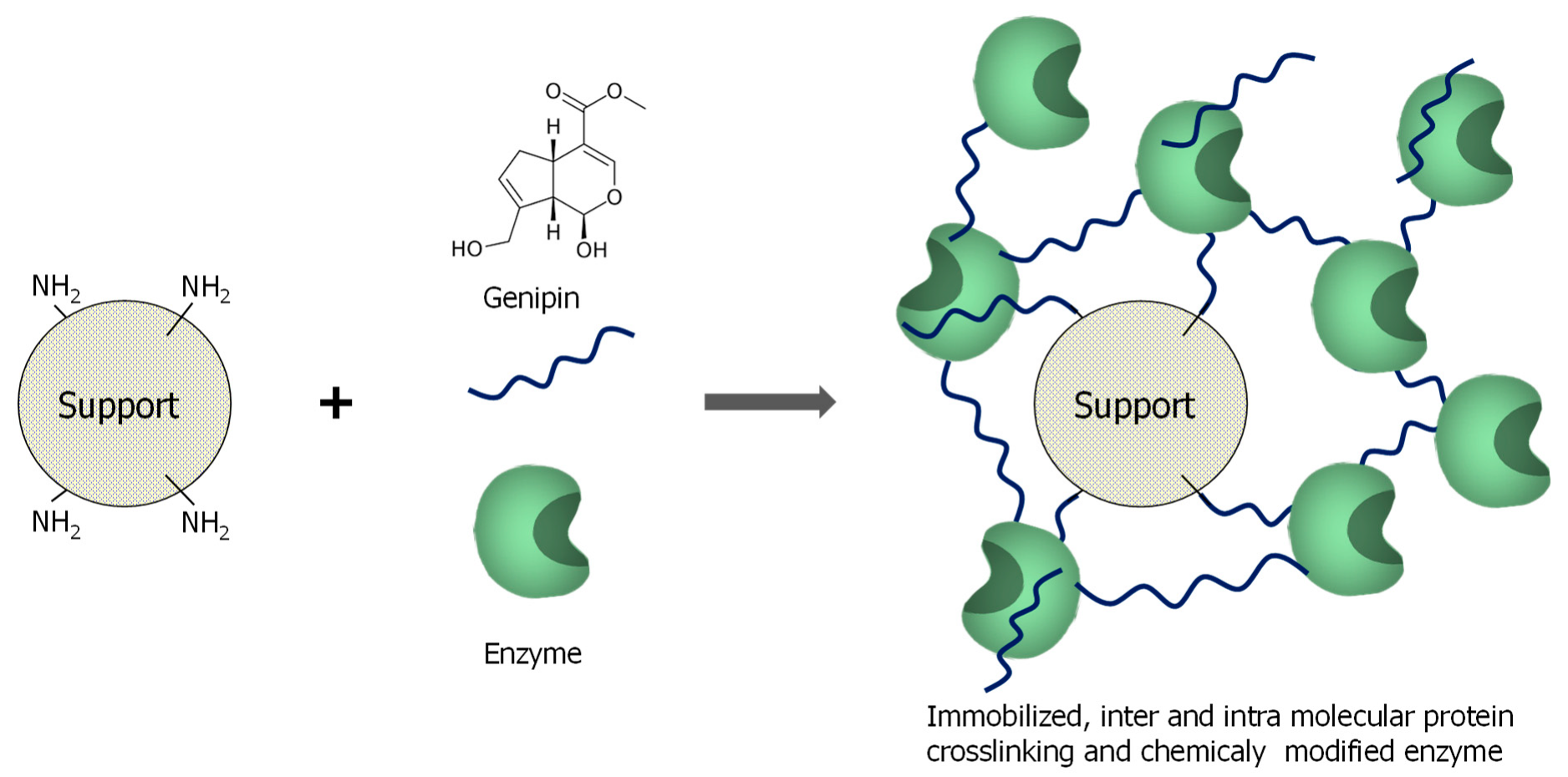
3.1. General Aspects of Use of Genipin in Enzyme Immobilization
3.2. Enzyme Immobilization on Preexisting Supports Using Genipin
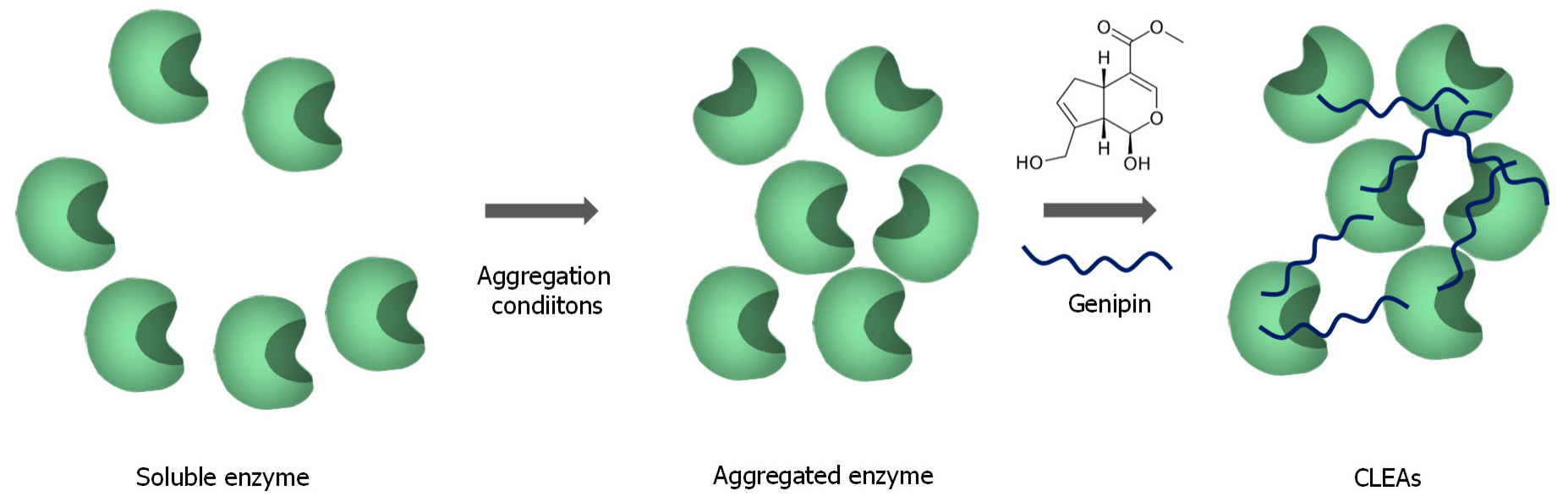
3.3. Enzyme Immobilization without Support Using Genipin to Stabilize the Solid
3.4. Immobilization of Cells Using Genipin
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schoemaker, H.E.; Mink, D.; Wubbolts, M.G. Dispelling the myths-biocatalysis in industrial synthesis. Science 2003, 299, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Dordick, J.S.; Hauer, B.; Kiener, A.; Wubbolts, M.; Witholt, B. Industrial biocatalysis today and tomorrow. Nature 2001, 409, 258. [Google Scholar] [CrossRef] [PubMed]
- Pollard, D.J.; Woodley, J.M. Biocatalysis for pharmaceutical intermediates: The future is now. Trends Biotechnol. 2007, 25, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T. Biocatalysis in organic chemistry and biotechnology: Past, present, and future. J. Am. Chem. Soc. 2013, 135, 12480–12496. [Google Scholar] [CrossRef] [PubMed]
- Woodley, J.M. New opportunities for biocatalysis: Making pharmaceutical processes greener. Trends Biotechnol. 2008, 26, 321–327. [Google Scholar] [CrossRef]
- Robles-Medina, A.; González-Moreno, P.A.; Esteban-Cerdán, L.; Molina-Grima, E. Biocatalysis: Towards ever greener biodiesel production. Biotechnol. Adv. 2009, 27, 398–408. [Google Scholar] [CrossRef]
- Patel, R.N. Synthesis of chiral pharmaceutical intermediates by biocatalysis. Coordin. Chem. Rev. 2008, 252, 659–701. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Eijsink, V.G.H.; Gåseidnes, S.; Borchert, T.V.; van den Burg, B. Directed evolution of enzyme stability. Biomol. Eng. 2005, 22, 21–30. [Google Scholar] [CrossRef]
- Polizzi, K.M.; Bommarius, A.S.; Broering, J.M.; Chaparro-Riggers, J.F. Stability of biocatalysts. Curr. Opin. Chem. Biol. 2007, 11, 220–225. [Google Scholar] [CrossRef]
- Castilla, I.A.; Woods, D.F.; Reen, F.J.; O’Gara, F. Harnessing marine biocatalytic reservoirs for green chemistry applications through metagenomic technologies. Mar. Drugs 2018, 16, 227. [Google Scholar] [CrossRef] [PubMed]
- Peña-García, C.; Martínez-Martínez, M.; Reyes-Duarte, D.; Ferrer, M. High throughput screening of esterases, lipases and phospholipases in mutant and metagenomic libraries: A review. Comb. Chem. High Throughput Screen. 2016, 19, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.A.; Ramond, J.-B.; Makhalanyane, T.P.; De Maayer, P. Metagenomics of extreme environments. Curr. Opin. Microbiol. 2015, 25, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arrojo, L.; Guazzaroni, M.-E.; López-Cortés, N.; Beloqui, A.; Ferrer, M. Metagenomic era for biocatalyst identification. Curr. Opin. Biotechnol. 2010, 21, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J. Directed evolution drives the next generation of biocatalysts. Nat. Chem. Biol. 2009, 5, 567. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.R.; Fidantsef, A.L. Directed evolution of industrial enzymes: An update. Curr. Opin. Biotechnol. 2003, 14, 438–443. [Google Scholar] [CrossRef]
- Romero, P.A.; Arnold, F.H. Exploring protein fitness landscapes by directed evolution. Nat. Rev. Mol. Cell Biol. 2009, 10, 866. [Google Scholar] [CrossRef]
- Arnold, F.H.; Volkov, A.A. Directed evolution of biocatalysts. Curr. Opin. Chem. Biol. 1999, 3, 54–59. [Google Scholar] [CrossRef]
- Boutureira, O.; Bernardes, G.J. Advances in chemical protein modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef]
- Cowan, D.A.; Fernandez-Lafuente, R. Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzym. Microb. Technol. 2011, 49, 326–346. [Google Scholar] [CrossRef]
- Sakamoto, S.; Hamachi, I. Recent progress in chemical modification of proteins. Anal. Sci. 2018, 18R003. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Adv. Synth. Catal. 2011, 353, 2216–2238. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Ortiz, C.; Torres, R.; Barbosa, O.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Chemical modification in the design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Chem. Rec. 2016, 16, 1436–1455. [Google Scholar] [CrossRef] [PubMed]
- Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7490. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Virgen-OrtÍz, J.J.; Pedrero, S.G.; Lopez-Carrobles, N.; Gorines, B.C.; Otero, C.; Fernandez-Lafuente, R. Optimization of the coating of octyl-CALB with ionic polymers to improve stability and decrease enzyme leakage. Biocatal. Biotransformation 2018, 36, 47–56. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Virgen-Ortíz, J.J.; Gorines, B.C.; Otero, C.; Fernandez-Lafuente, R. Physical crosslinking of lipase from Rhizomucor miehei immobilized on octyl agarose via coating with ionic polymers: Avoiding enzyme release from the support. Process Biochem. 2017, 54, 81–88. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzym. Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Rueda, N.; Barbosa, O.; Fernández-Sánchez, J.F.; Medina-Castillo, A.L.; Ramón-Márquez, T.; Arias-Martos, M.C.; Millán-Linares, M.C.; Pedroche, J.; del Mar Yust, M. Characterization of supports activated with divinyl sulfone as a tool to immobilize and stabilize enzymes via multipoint covalent attachment. Application to chymotrypsin. RSC Adv. 2015, 5, 20639–20649. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.-K. From protein engineering to immobilization: Promising strategies for the upgrade of industrial enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernandez-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
- Brady, D.; Jordaan, J. Advances in enzyme immobilisation. Biotechnol. Lett. 2009, 31, 1639. [Google Scholar] [CrossRef]
- Tran, D.N.; Balkus, K.J., Jr. Perspective of recent progress in immobilization of enzymes. ACS Catal. 2011, 1, 956–968. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Eq. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Ortiz, C.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Lecitase ultra: A phospholipase with great potential in biocatalysis. Mol. Catal. 2019, 473, 110405. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernandez-Lafuente, R. Amination of enzymes to improve biocatalyst performance: Coupling genetic modification and physicochemical tools. RSC Adv. 2014, 4, 38350–38374. [Google Scholar] [CrossRef]
- López-Gallego, F.; Montes, T.; Fuentes, M.; Alonso, N.; Grazu, V.; Betancor, L.; Guisán, J.M.; Fernández-Lafuente, R. Improved stabilization of chemically aminated enzymes via multipoint covalent attachment on glyoxyl supports. J. Biotechnol. 2005, 116, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef]
- Rios, N.S.; Mendez-Sanchez, C.; Arana-Peña, S.; Rueda, N.; Ortiz, C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Immobilization of lipase from Pseudomonas fluorescens on glyoxyl-octyl-agarose beads: Improved stability and reusability. Biochim. Biophys. Acta BBA Proteins Proteom. 2019, 1867, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Gk, M.S.; Rounaghi, G.H.; Chamsaz, M. An optical sensor for determination of low pH values based on covalent immobilization of Congo red on triacetyl cellulose films via epichlorohydrin. Sensors Actuat. B Chem. 2018, 254, 177–181. [Google Scholar]
- Santos, J.C.; Paula, A.V.; Nunes, G.F.M.; De Castro, H.F. Pseudomonas fluorescens lipase immobilization on polysiloxane–polyvinyl alcohol composite chemically modified with epichlorohydrin. J. Mol. Catal. B Enzym. 2008, 52, 49–57. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Peng, L.-J.; Wang, Y.; Li, Y.-Q. Lipase immobilization on epoxy-activated poly (vinyl acetate-acrylamide) microspheres. Colloid Surf. B 2015, 129, 206–210. [Google Scholar] [CrossRef]
- Rios, N.S.; Neto, D.M.A.; dos Santos, J.C.S.; Fechine, P.B.A.; Fernández-Lafuente, R.; Gonçalves, L.R.B. Comparison of the immobilization of lipase from Pseudomonas fluorescens on divinylsulfone or p-benzoquinone activated support. Int. J. Biol. Macromol. 2019, 134, 936–945. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Rueda, N.; Sanchez, A.; Villalonga, R.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Versatility of divinylsulfone supports permits the tuning of CALB properties during its immobilization. RSC Adv. 2015, 5, 35801–35810. [Google Scholar] [CrossRef]
- Pinheiro, B.B.; Rios, N.S.; Aguado, E.R.; Fernandez-Lafuente, R.; Freire, T.M.; Fechine, P.B.A.; dos Santos, J.C.S.; Gonçalves, L.R.B. Chitosan activated with divinyl sulfone: A new heterofunctional support for enzyme immobilization. Application in the immobilization of lipase B from Candida antarctica. Int. J. Biol. Macromol. 2019, 130, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Rosell, C.M.; Rodriguez, V.; Guisan, J.M. Strategies for enzyme stabilization by intramolecular crosslinking with bifunctional reagents. Enzym. Microb. Technol. 1995, 17, 517–523. [Google Scholar] [CrossRef]
- Siar, E.-H.; Arana-Peña, S.; Barbosa, O.; Zidoune, M.N.; Fernandez-Lafuente, R. Solid phase chemical modification of agarose glyoxyl-ficin: Improving activity and stability properties by amination and modification with glutaraldehyde. Process. Biochem. 2018, 73, 109–116. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Kowal, R.; Parsons, R.G. Stabilization of proteins immobilized on Sepharose from leakage by glutaraldehyde crosslinking. Anal. Biochem. 1980, 102, 72–76. [Google Scholar] [CrossRef]
- Zaak, H.; Fernandez-Lopez, L.; Otero, C.; Sassi, M.; Fernandez-Lafuente, R. Improved stability of immobilized lipases via modification with polyethylenimine and glutaraldehyde. Enzym. Microb. Technol. 2017, 106, 67–74. [Google Scholar] [CrossRef]
- López-Gallego, F.; Betancor, L.; Mateo, C.; Hidalgo, A.; Alonso-Morales, N.; Dellamora-Ortiz, G.; Guisán, J.M.; Fernández-Lafuente, R. Enzyme stabilization by glutaraldehyde crosslinking of adsorbed proteins on aminated supports. J. Biotechnol. 2005, 119, 70–75. [Google Scholar] [CrossRef]
- Liu, X.; Lou, H. Synthesis of monoterpene alkaloid derivatives from the iridoid glucoside geniposide. Nat. Prod. Res. 2007, 21, 1157–1164. [Google Scholar] [CrossRef]
- Isoe, S.; Katsumura, S.; Okada, T.; Yamamoto, K.; Takemoto, T.; Inaba, H.; Han, Q.; Nakatani, K. Novel synthesis of (−)-Secologanin aglucon-O-silyl ether from (+)-genipin via oxidative fragmentation of γ-hydroxyalkylstannane. Tetrahedron Lett. 1987, 28, 5865–5868. [Google Scholar] [CrossRef]
- Samprasit, W.; Akkaramongkolporn, P.; Jaewjira, S.; Opanasopit, P. Design of alpha mangostin-loaded chitosan/alginate controlled-release nanoparticles using genipin as crosslinker. J. Drug Deliv. Sci. Technol. 2018, 46, 312–321. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Daniele, M.; Perez, V.; Gentili, A.; Gasperi, T.; Lupi, S.; Palocci, C. A physico-chemical approach to the study of genipin crosslinking of biofabricated peptide hydrogels. Process. Biochem. 2018, 70, 110–116. [Google Scholar] [CrossRef]
- Somers, P.; De Somer, F.; Cornelissen, M.; Bouchez, S.; Gasthuys, F.; Narine, K.; Cox, E.; Van Nooten, G. Genipin blues: An alternative non-toxic crosslinker for heart valves? J. Heart Valve Dis. 2008, 17, 682. [Google Scholar] [PubMed]
- Tsai, C.C.; Huang, R.N.; Sung, H.W.; Liang, H.C. In vitro evaluation of the genotoxicity of a naturally occurring crosslinking agent (genipin) for biologic tissue fixation. J. Biomed. Mater. Res. 2000, 52, 58–65. [Google Scholar] [CrossRef]
- Roether, J.; Oelschlaeger, C.; Willenbacher, N. Hyaluronic acid cryogels with non-cytotoxic crosslinker genipin. Mater. Lett. 2019, 4, 100027. [Google Scholar] [CrossRef]
- Manickam, B.; Sreedharan, R.; Elumalai, M. ‘Genipin’—The natural water soluble cross-linking agent and its importance in the modified drug delivery systems: An overview. Curr. Drug Deliv. 2014, 11, 139–145. [Google Scholar] [CrossRef]
- Di Tommaso, S.; David, P.; Picolet, K.; Gabant, M.; David, H.; Morançais, J.-L.; Gomar, J.; Leroy, F.; Adamo, C. Structure of genipin in solution: A combined experimental and theoretical study. RSC Adv. 2013, 3, 13764–13771. [Google Scholar] [CrossRef]
- Di Tommaso, S.; David, H.; Gomar, J.; Leroy, F.; Adamo, C. From iridoids to dyes: A theoretical study on genipin reactivity. RSC Adv. 2014, 4, 11029–11038. [Google Scholar] [CrossRef]
- Trevor, S.L.; Butler, M.F.; Adams, S.; Laity, P.R.; Burley, J.C.; Cameron, R.E. Structure and phase transitions of genipin, an herbal medicine and naturally occurring cross-linker. Cryst. Growth Des. 2008, 8, 1748–1753. [Google Scholar] [CrossRef]
- Fujikawa, S.; Nakamura, S.; Koga, K. Genipin, a new type of protein crosslinking reagent from gardenia fruits. Agric. Biol. Chem. 1988, 52, 869–870. [Google Scholar]
- Neri-Numa, I.A.; Pessoa, M.G.; Paulino, B.N.; Pastore, G.M. Genipin: A natural blue pigment for food and health purposes. Trends Food Sci. Technol. 2017, 67, 271–279. [Google Scholar] [CrossRef]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Pol. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Touyama, R.; Inoue, K.; Takeda, Y.; Yatsuzuka, M.; Ikumoto, T.; Moritome, N.; Shingu, T.; Yokoi, T.; Inouye, H. Studies on the blue pigments produced from genipin and methylamine. II. On the formation mechanisms of brownish-red intermediates leading to the blue pigment formation. Chem. Pharm. Bull. 1994, 42, 1571–1578. [Google Scholar] [CrossRef]
- Dimida, S.; Demitri, C.; De Benedictis, V.M.; Scalera, F.; Gervaso, F.; Sannino, A. Genipin-cross-linked chitosan-based hydrogels: Reaction kinetics and structure-related characteristics. J. Appl. Polym. Sci. 2015, 132, 42256. [Google Scholar] [CrossRef]
- Pujana, M.A.; Pérez-Álvarez, L.; Iturbe, L.C.C.; Katime, I. Biodegradable chitosan nanogels crosslinked with genipin. Carbohyd. Polym. 2013, 94, 836–842. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-crosslinked chitosan gels and scaffolds for tissue engineering and regeneration of cartilage and bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef]
- Fujikawa, S.; Fukui, Y.; Koga, K.; Iwashita, T.; Komura, H.; Nomoto, K. Structure of genipocyanin G1, a spontaneous reaction product between genipin and glycine. Tetrahedron Lett. 1987, 28, 4699–4700. [Google Scholar] [CrossRef]
- Yoo, J.S.; Kim, Y.J.; Kim, S.H.; Choi, S.H. Study on genipin: A new alternative natural crosslinking agent for fixing heterograft tissue. Korean J. Thorac. Cardiovasc. Surg. 2011, 44, 197–207. [Google Scholar] [CrossRef]
- Sung, H.W.; Chang, Y.; Liang, I.L.; Chang, W.H.; Chen, Y.C. Fixation of biological tissues with a naturally occurring crosslinking agent: Fixation rate and effects of pH, temperature, and initial fixative concentration. J. Biomed. Mater. Res. 2000, 52, 77–87. [Google Scholar] [CrossRef]
- Chang, Y.; Tsai, C.-C.; Liang, H.-C.; Sung, H.-W. Reconstruction of the right ventricular outflow tract with a bovine jugular vein graft fixed with a naturally occurring crosslinking agent (genipin) in a canine model. J. Thorac. Cardiovasc. 2001, 122, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, I.; Fathima, N.N.; Jonnalagadda, R.R.; Nair, B.U. Elucidation of hydration dynamics of locust bean gum-collagen composites by impedance and thermoporometry. Carbohydr. Polym. 2014, 103, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Touyama, R.; Takeda, Y.; Inoue, K.; Kawamura, I.; Yatsuzuka, M.; Ikumoto, T.; Shingu, T.; Yokoi, T.; Inouye, H. Studies on the blue pigments produced from genipin and methylamine. I. Structures of the brownish-red pigments, intermediates leading to the blue pigments. Chem. Pharm. Bull. 1994, 42, 668–673. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Brown, E.M.; Keyong, T. Characterization and thermal properties of polygenipin-crosslinked hide powders. J. Am. Leather Chem. Assoc. 2018, 113, 96–104. [Google Scholar]
- Dai, Y.; Zhang, X. Stable and biocompatible genipin-inducing interlayer-crosslinked micelles for sustained drug release. J. Nanopart. Res. 2017, 19, 164. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Ma, H.-F.; Meng, G.; Cui, B.-K.; Si, J.; Dai, Y.-C. Chitosan crosslinked with genipin as supporting matrix for biodegradation of synthetic dyes: Laccase immobilization and characterization. Chem. Eng. Res. Des. 2018, 132, 664–676. [Google Scholar] [CrossRef]
- Flores, E.E.E.; Cardoso, F.D.; Siqueira, L.B.; Ricardi, N.C.; Costa, T.H.; Rodrigues, R.C.; Klein, M.P.; Hertz, P.F. Influence of reaction parameters in the polymerization between genipin and chitosan for enzyme immobilization. Process. Biochem. 2019, 84, 73–80. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional supports in enzyme immobilization: From traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef]
- Fujikawa, S.; Yokota, T.; Koga, K. Immobilization of β-glucosidase in calcium alginate gel using genipin as a new type of cross-linking reagent of natural origin. Appl. Microbiol. Biotechnol. 1988, 28, 440–441. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, Y.; Zhou, L.; Gao, J. Comparison of the properties of lipase immobilized onto mesoporous resins by different methods. Appl. Biochem. Biotechnol. 2011, 164, 561–572. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin-and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Chiou, S.H.; Hung, T.C.; Giridhar, R.; Wu, W.T. Immobilization of lipase to chitosan beads using a natural cross-linker. Prep. Biochem Biotechnol. 2007, 37, 265–275. [Google Scholar] [CrossRef]
- Gracida, J.; Arredondo-Ochoa, T.; García-Almendárez, B.E.; Escamilla-García, M.; Shirai, K.; Regalado, C.; Amaro-Reyes, A. Improved Thermal and Reusability Properties of Xylanase by Genipin Cross-Linking to Magnetic Chitosan Particles. Appl. Biochem. Biotechnol. 2019, 188, 395–409. [Google Scholar] [CrossRef]
- Fernandes, S.C.; de Oliveira Santos, D.M.P.; Vieira, I.C. Genipin-cross-linked chitosan as a support for laccase biosensor. Electroanalysis 2013, 25, 557–566. [Google Scholar] [CrossRef]
- Rangel-Rodríguez, A.M.; Conxita, S.; Susana, V.; Flores-Gallardo, S.G.; Contreras-Esquivel, J.C.; Licea-Jiménez, L. Immobilization of pectinesterase in genipin-crosslinked chitosan membrane for low methoxyl pectin production. Appl. Biochem. Biotechnol. 2014, 174, 2941–2950. [Google Scholar] [CrossRef]
- Klein, M.P.; Hackenhaar, C.R.; Lorenzoni, A.S.G.; Rodrigues, R.C.; Costa, T.M.H.; Ninow, J.L.; Hertz, P.F. Chitosan crosslinked with genipin as support matrix for application in food process: Support characterization and β-d-galactosidase immobilization. Carbohydr. Polym. 2016, 137, 184–190. [Google Scholar] [CrossRef]
- Cavello, I.A.; Contreras-Esquivel, J.C.; Cavalitto, S.F. Immobilization of a keratinolytic protease from Purpureocillium lilacinum on genipin activated-chitosan beads. Process. Biochem. 2014, 49, 1332–1336. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Wang, L.; Wang, S. Stability and catalytic properties of lipase immobilized on chitosan encapsulated magnetic nanoparticles cross-linked with genipin and glutaraldehyde. J. Chem. Technol. Biotechnol. 2016, 91, 1359–1367. [Google Scholar] [CrossRef]
- Phadungcharoen, N.; Winotapun, W.; Khomniyawanit, A.; Krataichan, F.; Rojanarata, T. Facile and green fabrication of biocatalytic chitosan beads by one-step genipin-mediated β-glucosidase immobilization for production of bioactive genistein. Sustain. Chem. Pharm. 2019, 14, 100187. [Google Scholar] [CrossRef]
- Salazar-Leyva, J.A.; Lizardi-Mendoza, J.; Ramirez-Suarez, J.C.; Valenzuela-Soto, E.M.; Ezquerra-Brauer, J.M.; Castillo-Yañez, F.J.; Pacheco-Aguilar, R. Acidic proteases from Monterey sardine (Sardinops sagax caerulea) immobilized on shrimp waste chitin and chitosan supports: Searching for a by-product catalytic system. Appl. Biochem. Biotechnol. 2013, 171, 795–805. [Google Scholar] [CrossRef]
- Metin, A.Ü. Immobilization of laccase onto polyethyleneimine grafted chitosan films: Effect of system parameters. Macromol. Res. 2013, 21, 1145–1152. [Google Scholar] [CrossRef]
- Dai, Q.; Ma, Y.; Wang, S.; Kankala, R.K.; Liu, Y. Investigation of various cross-linking methods for the immobilization of cytosine arabinoside on bacterial magnetosomes. J. Nanomater. 2017, 2017, 6738484. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Versatility of glutaraldehyde to immobilize lipases: Effect of the immobilization protocol on the properties of lipase B from Candida antarctica. Process. Biochem. 2012, 47, 1220–1227. [Google Scholar] [CrossRef]
- Vazquez-Ortega, P.G.; Alcaraz-Fructuoso, M.T.; Rojas-Contreras, J.A.; López-Miranda, J.; Fernandez-Lafuente, R. Stabilization of dimeric β-glucosidase from Aspergillus niger via glutaraldehyde immobilization under different conditions. Enzym. Microb. Technol. 2018, 110, 38–45. [Google Scholar] [CrossRef]
- Zaak, H.; Peirce, S.; de Albuquerque, T.; Sassi, M.; Fernandez-Lafuente, R. Exploiting the versatility of aminated supports activated with glutaraldehyde to immobilize β-galactosidase from Aspergillus oryzae. Catalysts 2017, 7, 250. [Google Scholar] [CrossRef]
- Siar, E.-H.; Arana-Peña, S.; Barbosa, O.; Zidoune, M.; Fernandez-Lafuente, R. Immobilization/stabilization of ficin extract on glutaraldehyde-activated agarose beads. Variables that control the final stability and activity in protein hydrolyses. Catalysts 2018, 8, 149. [Google Scholar] [CrossRef]
- De Andrades, D.; Graebin, N.G.; Kadowaki, M.K.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Immobilization and stabilization of different β-glucosidases using the glutaraldehyde chemistry: Optimal protocol depends on the enzyme. Int. J. Biol. Macromol. 2019, 129, 672–678. [Google Scholar] [CrossRef]
- Yang, Q.; Lan, F.; Liu, Z.; Ma, S.; Li, W.; Wu, Y.; Gu, Z. Uniform Superparamagnetic Fe3O4/CMCS Composite Nanospheres for Lysozyme Adsorption. J. Nanosci. Nanotechnol. 2016, 16, 2233–2238. [Google Scholar] [CrossRef]
- Ma, W.; Tang, C.-H.; Yin, S.-W.; Yang, X.-Q.; Qi, J.-R. Genipin-crosslinked gelatin films as controlled releasing carriers of lysozyme. Food Res. Int. 2013, 51, 321–324. [Google Scholar] [CrossRef]
- Beldengruün, Y.; Aragon, J.; Prazeres, S.F.; Montalvo, G.; Miras, J.; Esquena, J. Gelatin/Maltodextrin Water-in-Water (W/W) emulsions for the preparation of Cross-Linked Enzyme-Loaded microgels. Langmuir 2018, 34, 9731–9743. [Google Scholar] [CrossRef]
- Cao, L.; van Langen, L.; Sheldon, R.A. Immobilised enzymes: Carrier-bound or carrier-free? Curr. Opin. Biotechnol. 2003, 14, 387–394. [Google Scholar] [CrossRef]
- Wilson, L.; Betancor, L.; Fernández-Lorente, G.; Fuentes, M.; Hidalgo, A.; Guisán, J.M.; Pessela, B.C.C.; Fernández-Lafuente, R. Cross-linked aggregates of multimeric enzymes: A simple and efficient methodology to stabilize their quaternary structure. Biomacromolecules 2004, 5, 814–817. [Google Scholar] [CrossRef]
- Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates: A simple and effective method for the immobilization of penicillin acylase. Org. Lett. 2000, 2, 1361–1364. [Google Scholar] [CrossRef]
- Cao, L.; Van Langen, L.M.; Van Rantwijk, F.; Sheldon, R.A. Cross-linked aggregates of penicillin acylase: Robust catalysts for the synthesis of β-lactam antibiotics. J. Mol. Catal. B Enzym. 2001, 11, 665–670. [Google Scholar] [CrossRef]
- Yanjun, J.; Qi, W.; Wenqin, W.; Liya, Z.; Jing, G. Preparation of immobilized lipase through combination of cross-linked enzyme aggregates and biomimetic silicification. Chin. J. Catal. 2012, 33, 857–862. [Google Scholar]
- Zhang, Q.; Zha, X.; Zhou, N.; Tian, Y. Preparation of crosslinked enzyme aggregates (CLEAs) of acid urease with urethanase activity and their application. J. Basic Microb. 2016, 56, 422–431. [Google Scholar] [CrossRef]
- Müller, F.; Torger, B.; Allertz, P.J.; Jähnichen, K.; Keßler, S.; Müller, M.; Simon, F.; Salchert, K.; Mäurer, H.; Pospiech, D. Multifunctional crosslinkable itaconic acid copolymers for enzyme immobilization. Eur. Polym. J. 2018, 102, 47–55. [Google Scholar] [CrossRef]
- Pollak, A.; Blumenfeld, H.; Wax, M.; Baughn, R.L.; Whitesides, G.M. Enzyme immobilization by condensation copolymerization into crosslinked polyacrylamide gels. J. Am. Chem. Soc. 1980, 102, 6324–6336. [Google Scholar] [CrossRef]
- Cui, C.; Chen, H.; Chen, B.; Tan, T. Genipin cross-linked glucose oxidase and catalase multi-enzyme for gluconic acid synthesis. Appl. Biochem. Biotechnol. 2017, 181, 526–535. [Google Scholar] [CrossRef]
- Hernandez, K.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Hydrogen peroxide in biocatalysis. A dangerous liaison. Curr. Org. Chem. 2012, 16, 2652–2672. [Google Scholar] [CrossRef]
- Ko, C.-S.; Chu, I.M. Immobilized cells biocatalyst for the production of S-acetylthio-2-methyl propionic acid. Enzyme Microb. Technol. 2004, 35, 619–623. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tacias-Pascacio, V.G.; García-Parra, E.; Vela-Gutiérrez, G.; Virgen-Ortiz, J.J.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Genipin as An Emergent Tool in the Design of Biocatalysts: Mechanism of Reaction and Applications. Catalysts 2019, 9, 1035. https://doi.org/10.3390/catal9121035
Tacias-Pascacio VG, García-Parra E, Vela-Gutiérrez G, Virgen-Ortiz JJ, Berenguer-Murcia Á, Alcántara AR, Fernandez-Lafuente R. Genipin as An Emergent Tool in the Design of Biocatalysts: Mechanism of Reaction and Applications. Catalysts. 2019; 9(12):1035. https://doi.org/10.3390/catal9121035
Chicago/Turabian StyleTacias-Pascacio, Veymar G., Esmeralda García-Parra, Gilber Vela-Gutiérrez, Jose J. Virgen-Ortiz, Ángel Berenguer-Murcia, Andrés R. Alcántara, and Roberto Fernandez-Lafuente. 2019. "Genipin as An Emergent Tool in the Design of Biocatalysts: Mechanism of Reaction and Applications" Catalysts 9, no. 12: 1035. https://doi.org/10.3390/catal9121035
APA StyleTacias-Pascacio, V. G., García-Parra, E., Vela-Gutiérrez, G., Virgen-Ortiz, J. J., Berenguer-Murcia, Á., Alcántara, A. R., & Fernandez-Lafuente, R. (2019). Genipin as An Emergent Tool in the Design of Biocatalysts: Mechanism of Reaction and Applications. Catalysts, 9(12), 1035. https://doi.org/10.3390/catal9121035








