Abstract
The possibility of using Lecitase® Ultra as a novel alternative biocatalyst for the kinetic resolution of model racemic allyl esters of (E)-4-phenylbut-3-en-3-ol: Acetate (4a) and propionate (4b) through their enantioselective hydrolysis was investigated. Reaction afforded (+)-(R)-alcohol (3) and unreacted (−)-(S)-ester (4a or 4b). Hydrolysis of propionate 4b proceeded with higher enantioselectivity than acetate 4a. (R)-Alcohol (3) with highest enantiomeric excess (93–99%) was obtained at 20–30 °C by hydrolysis of propionate 4b, while the highest optical purity of unreacted substrate was observed for (S)-acetate 4a (ee = 34–56%). The highest enantioselectivity was found for the hydrolysis of propionate 4b catalyzed at 30 °C (E = 38). Reaction carried out at 40 °C significantly lowered enantiomeric excess of produced alcohol 3 and enantioselectivity in resolution. Lecitase® Ultra catalyzed the enantioselective hydrolysis of allyl esters 4a,b according to Kazlauskas’ rule to produce (R)-alcohol 3 and can find application as a novel biocatalyst in the processes of kinetic resolution of racemic allyl esters.
1. Introduction
Enantiomers of alcohols are widely used as building blocks in the synthesis of many biologically active compounds or directly as medicines [1], antifeedants [2], odorants [3], or pheromones [4]. The well-established method for the production of enantiomerically enriched alcohols is their enzyme-catalyzed transesterification or hydrolysis of their racemic esters. For this purpose, commercially available lipases are commonly used to afford high enantioselectivity and enantiomeric purity of the products [5,6,7,8], but new biocatalysts are also developed, among others phospholipases.
Development of new biocatalysts useful in the kinetic resolution of racemic mixtures is still a challenge in biotransformation. Enzymes belonging to the class of phospholipases seem to be good candidates as substitutes of lipases because of the similarity in the mechanism of their action and interfacial activation and ability to the hydrolysis of ester bonds in a wide range of structurally different substrates. In nature, phospholipases catalyze the hydrolysis of phospholipids to their lyso form [9]. One of the enzymatic preparations with phospholipase A1 activity is cheap and easily accessible Lecitase® Ultra, which is the product of genes fusion of lipase from Thermomyces lanuginosus and lipase from Fusarium oxysporum. It presents the stability of the lipase and the activity of the phospholipase. Designed mainly for industrial degumming of vegetable oils [10], Lecitase® Ultra was also applied to the production of structured phospholipids, due to the ability to catalyze acidolysis or interesterification [11,12]. Nevertheless, little effort has been made in exploitation of its lipase activity in asymmetric synthesis. Up to now, due to its hydrolytic activity, Lecitase® Ultra has found application for the resolution of racemic esters, that is, esters of 2-hydroxy carboxylic acids [13,14,15], glycidate esters [15,16], N-acetyl-α-amino acid methyl esters [15], as well as for asymmetric hydrolysis of dimethyl 3-phenylglutarate [17]. This enzyme was also used to the regioselective hydrolysis of peracetylated mono- and disaccharides [18].
Herein, we present the preliminary results of Lecitase® Ultra-mediated hydrolysis of (E)-4-phenylbut-3-en-2-yl esters as the model substrates. Compounds with a 4-arylbut-3-en-2-ol system are valuable chiral synthons in the synthesis of different biologically active compounds, i.a. Verapamil [19], Baclofen [20], or β-aryl substituted lactones with antiproliferative activity [21]. Many studies concerning their kinetic resolution by transesterification [22,23,24,25] or hydrolysis [25,26] have been developed recently. To the best of our knowledge, Lecitase® Ultra has not been reported yet as the biocatalyst in these processes.
2. Results and Discussion
Substrates of enzymatic reactions: Racemic (E)-4-phenylbut-3-en-2-yl acetate (4a) and propionate (4b) were obtained through a three-step synthesis. In the first step, benzaldehyde 1 was subjected to Claisen–Schmidt condensation to obtain α,β-unsaturated ketone 2, which was reduced with sodium borohydride to racemic allyl alcohol 3. The alcohol was treated with acetyl or propionyl chloride to afford the corresponding esters 4a,b (Scheme 1).
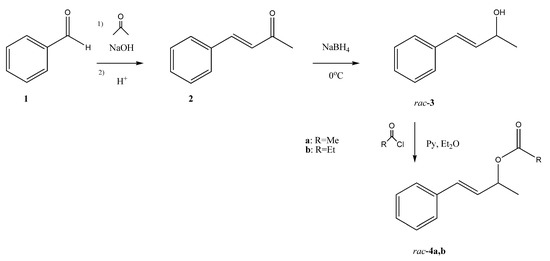
Scheme 1.
Three-step synthesis of racemic acetate 4a and propionate 4b.
Because of the inseparability of enantiomers of alcohol 3 during chiral gas chromatography (CGC), before analysis, samples taken from the enzymatic reaction were directly treated with corresponding acyl chloride. In the case of hydrolysis of acetate 4a, produced alcohol 3 was derivatized into propionate and unreacted acetate was unchanged. A similar procedure was applied in the case of hydrolysis of propionate 4b, where alcohol 3 was transformed into acetate. This method allowed us to determine the enantiomeric composition of unreacted ester and produced alcohol (as ester derivative) in one Chiral Gas Chromatography (CGC) analysis. The described procedure was succesfully applied in our earlier studies on the transesterification of allyl alcohols [21,27].
The first experiments of the hydrolysis of esters 4a and 4b with Lecitase® Ultra as a biocatalyst (Scheme 2) were carried out at room temperature (20 °C). In the case of resolution of acetate 4a, after 24 h the conversion was 14% and successively increased in time to reach 40% after 168 h (Figure 1A). The enantiomeric excess (ee) of the resulting alcohol 3 after 24 h was 92% and it decreased in time to reach 85% after 168 h (Figure 2A). Significantly lower enantiomeric purity was observed for unreacted acetate 4a—in the first 24 h of the process its enantiomeric excess was only 15%, while a significant increase (up to 56%) was noticed after 168 h. The enantioselectivity of the resolution was moderate (E = 22, Table 1, Entry 1).
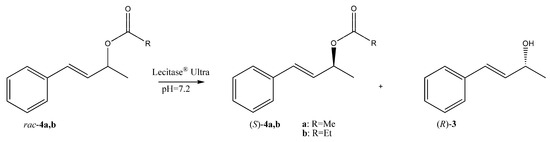
Scheme 2.
Resolution of racemic esters 4a,b through Lecitase® Ultra-catalyzed hydrolysis.
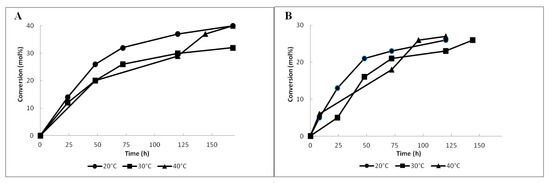
Figure 1.
Effect of temperature on the conversion of acetate 4a (A) and propionate 4b (B) during Lecitase® Ultra-catalyzed hydrolysis.

Figure 2.
Effect of temperature on the enantiomeric excess of the products during Lecitase® Ultra-catalyzed hydrolysis of acetate 4a (A) and propionate 4b (B).

Table 1.
Kinetic resolution of allyl esters 4a,b catalyzed by Lecitase® Ultra-catalyzed hydrolysis at different temperatures.
Under the same conditions, propionate 4b was hydrolyzed with a lower conversion degree which did not exceed 30% after 120 h (Figure 1B), but a higher ee of alcohol 3 was determined. During the first 8 h, enantiomerically pure alcohol was produced, and after 120 h its optical purity was reduced to 90%. A significantly lower ee of unreacted propionate 4b (28%) (Figure 2B) compared to that of acetate 4a (Figure 2A) was determined after 72 h. In comparison to the hydrolysis of acetate 4a, a slightly higher enantioselectivity of the process was observed (E = 26, Table 1, Entry 4).
The results of Lecitase® Ultra-catalyzed hydrolysis of both racemic esters 4a,b were compared with the results obtained in our working group under the same conditions, using commercially available lipase B from Candida antarctica. In the case of the resolution of racemic acetate 4a, after 6 h of reaction, approximately 50% of the ester conversion was achieved, with 80% ee of alcohol 3 and 97% ee of unreacted acetate 4a (E = 37). In the case of the hydrolysis of racemic propionate 4b, a good resolution was achieved after 2 h: ee of alcohol 3 92%, ee of unreacted propionate 4b 85%, conversion degree 57%, and high enantioselectivity (E = 65). Comparable results of the resolution of the enantiomers of acetate 4a with lipase B from C. antarctica were previously obtained by Ghanem and Schurig [26] during the hydrolysis carried out in phosphate buffer (pH 6) and toluene as the solvent for substrate. After 24 h, at 45% conversion, the ee of alcohol 3 and unreacted acetate 4a were 99% and 80%, respectively, with excellent enantioselectivity (E > 200).
Based on the previous studies conducted by Mishra and et al. [15], confirming the thermal stability of Lecitase® Ultra in the range of 30–50 °C, the effect of temperature on the hydrolytic activity towards esters 4a and 4b was determined. For this purpose, the reactions were carried out at 30 °C and 40 °C. At 30 °C, the hydrolysis of acetate 4a proceeded in a similar way as that described for the process at 20 °C, but a lower conversion degree (32%) and lower enantioselectivity (E = 13, Table 1, Entry 2) were observed after 168 h (Figure 1A). A significant decrease of ee was observed for unreacted acetate 4a, which, after 168 h, reached only 38%. Optical purity of alcohol 3 was slightly lower than that observed at 20 °C, and decreased from 88% after 24 h to 80% after 168 h (Figure 2A). The prolongation of the reaction time did not affect the enantiomeric excesses of both alcohol 3 and acetate 4a.
A significantly negative effect on the reaction rate and decreased enantioselectivity (E = 3, Table 1, Entry 3) was noticed when hydrolysis of acetate 4a was carried out at 40 °C. In this case, similarly to the process catalyzed at 20 °C, the highest conversion (40%) was achieved only after 168 h (Figure 1A). The highest ee of alcohol 3 (67%) was observed after 48 h at 36% conversion, while the ee of acetate 4a was only 17%. From this moment, a significant drop of enantiomeric excess of alcohol 3 to 41% was observed after 168 h, with a simultaneous slight increase of ee for acetate 4a to 27% (Figure 2A).
For propionate 4b, raising the temperature of hydrolysis to 30 °C had a positive effect on the enantioselectivity of resolution of propionate 4b (E = 38, Table 1, Entry 5). After 24 h of reaction, the enantiomeric excess of the resulting alcohol and unreacted substrate was 97% and 5%, respectively, but the conversion of propionate 4b was only 5%. Continuation of the reaction caused the gradual decrease of ee of produced alcohol and, after 144 h, it amounted to 93%. Simultaneously, the ee of unreacted propionate reached the level of 32%. The reaction carried out at 40 °C was characterized by a significantly lower enantioselectivity (E = 12, Table 1, Entry 6) and clearly impacted the optical purity of produced alcohol, whose ee decreased from 88% after 8 h to 80% after 120 h of the process. Enantiomeric excesses of the recovered propionate 3 at higher (30 °C and 40°C) temperatures (32% and 29%, respectively) were comparable to those observed during the reaction carried out at 20 °C (Figure 2B), whereas the conversions were comparable to reactions catalyzed in all temperatures studied.
The R configuration of the enantiomerically enriched, dextrorotatory alcohol 3 was confirmed by comparison of its rotation sign with the literature data [28]. Determined configuration indicates that, similar to most lipases, Lecitase® Ultra catalyzes the hydrolysis of esters 4a,b according to the Kazlauskas rule [29], according to which the configuration of more rapidly hydrolyzed enantiomers of these compounds can be predicted as R.
In summary, the obtained results show that Lecitase® Ultra can be applied as a biocatalyst to obtain enantiomerically enriched (R,E)-4-phenylbut-3-en-2-ol (3) by enantioselective hydrolysis of its esters. For both acetate 4a and propionate 4b, 50% conversion was not achieved, but higher enantiomeric purities of alcohol 3 were observed in the case of hydrolysis of propionate 4b. From a practical point of view, the best results were obtained during hydrolysis of 4b at 30 °C, where enantiomerically enriched alcohol (ee = 93–96%) was obtained at 16–26% conversion degree and the highest enantioselectivity of resolution was observed (E = 38, Table 1). Conducting the process at 40 °C decreased the enantioselectivity of the reaction, particularly in the case of hydrolysis of acetate 4a.
Our pioneering, preliminary results showed the ability of Lecitase® Ultra to achieve hydrolysis of allyl esters with moderate to good enantioselectivity. However, they indicated the necessity of further studies to develop Lecitase® Ultra preparation as a biocatalyst in kinetic resolution of racemates. We are going to focus on the effect of various parameters of process (the type of organic solvent added to dissolve the substrate, pH of the reaction medium) as well as immobilization techniques on the increase of enantioselectivity and improvement of kinetic resolution. Substrate scope must be also investigated for future applications, not only to hydrolysis of esters, but also to transesterification of alcohols. Considering the low price of Lecitase® Ultra in comparison to most lipases, we believe that these studies will finally allow the expansion of the practical applications of this enzyme.
3. Materials and Methods
3.1. Materials and Chemicals
Benzaldehyde (1) (99%), acetyl chloride (≥99%), propionyl chloride (98%), and sodium borhydride (96%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Lecitase® Ultra (10,000 U g−1) was purchased from Novozymes A/S (Bagsvaerd, Denmark). Lipase B from Candida antarctica (>5000 U g−1) was purchased from Sigma-Aldrich. Other chemicals, including solvents, were of analytical grade.
3.2. Analysis
The composition of the reaction products was analyzed through gas chromatography (GC) on an Agilent Technologies 6890N instrument (Santa Clara, CA, USA), using a DB-5HT column (Agilent, Santa Clara, CA, USA), polyimide-coated fused silica tubing, 30 m × 0.25 mm × 0.10 μm) and hydrogen as a gas carrier. The following temperature program was applied: Injector 280 °C, detector (FID) 280 °C, initial temperature column 100 °C, 20–200 °C (rate 20 °C·min−1), 200–300 °C (rate 30 °C·min−1), final temperature column 300 °C (hold 1 min). The enantiomeric composition of the products of enzymatic hydrolysis was analyzed through chiral gas chromatography (CGC) on an Agilent Technologies 6890 N instrument A Varian CP Chirasil-DEX CB column (Santa Clara, CA, USA, cyclodextrin bonded to dimethylpolysiloxane, 25 m × 0.25 mm × 0.25 µm) was used with the following temperature program: Injector 200 °C, detector (FID) 250 °C, column 80–200 °C (5 °C min−1), 200 °C (1 min). Samples taken from the reaction mixture were analyzed after treatment with propionyl or acetyl chloride to derivatize inseparable enantiomers of alcohol 3 into corresponding esters (paragraph 3.4). The retention times of analyzed esters were as follow: (R)-acetate 4a tR= 11.73 min, (S)-acetate 4a tR = 11.99 min, (R)-propionate 4b tR = 13.46 min, (S)-propionate 4b tR = 13.55 min.
For the determination of the specific rotation of produced isomer of alcohol 3, hydrolysis of 200 mg of propionate 4b was carried out and (+)-(R)-alcohol 3 was isolated through column chromatography. The specific rotation was measured in CH2Cl2 on a Jasco P-2000-Na digital polarimeter (Easton, MD, USA) with an intelligent Remote Module controller. ([α] = +19.3 (c 2.0, CH2Cl2, ee = 90%), lit. [28] [α] = + 22.9 (c 1.6, CH2Cl2, ee = 99%).
Synthesized compounds were purified through silica gel column chromatography (Kieselgel 60, 230−400 mesh, Merck, Darmstadt, Germany). Analytical Thin Layer Chromatography (TLC) was carried out on silica gel coated aluminium plates (DC-Alufolien Kieselgel 60 F254, Merck, Darmstadt, Germany) using hexane/acetone 4:1 system. Compounds were visualized by spraying the plates with a solution of 1% Ce(SO4)2 and 2% H3[P(Mo3O10)4] in 10% H2SO4.
3.3. Synthesis of Esters 4a,b
3.3.1. (E)-4-Phenylbut-3-en-2-one (2)
Benzaldehyde (1) (47 mmol) was dissolved in acetone (100 mL) and a 10% solution of NaOH (2 mL) was added dropwise to the reaction mixture, stirred in a water bath. After 24 h, the mixture was acidified with 1M HCl and the crude product was extracted with methylene chloride (3 × 40 mL). The organic extract was washed with brine, dried over anhydrous MgSO4, and filtered. The solvent was removed by evaporation in vacuo and pure ketone (2) was obtained in 97% yield (6.7 g) with physical and spectral data as reported previously [30].
3.3.2. Rac-(E)-4-Phenylbut-3-en-2-ol (3)
A solution of ketone (2) in methanol (150 mL) was placed in an ice bath and NaBH4 (2.7 g) dissolved in water (6 mL) was added dropwise. When the ketone reacted completely (GC, TLC, 24 h), the mixture was diluted with hot water and the product was extracted with methylene chloride (3 × 40 mL). Pooled extracts were washed with brine and dried. The solvent was evaporated in vacuo and pure racemic alcohol 3 was obtained in 98% yield (6.6 g). Its physical and spectroscopic data are consistent with those reported earlier [28].
3.3.3. (E)-4-Phenylbut-3-en-3-yl Acetate (4a) and (E)-4-Phenylbut-3-en-3-yl Propionate (4b)
Alcohol 3 (2.2 g) was dissolved in 100 mL of dry diethyl ether and 10 mL of pyridine. The mixture was stirred in an ice bath and 5 ml of acetyl or propionyl chloride was added dropwise. The reaction was continued at room temperature until the alcohol reacted completely (24 h, TLC). The reaction mixture was acidified with 1M HCl, the product was extracted with diethyl ether (3 × 40 mL) and separated through column chromatography (hexane/acetone, 10:1) to afford known esters: acetate 4a (2.7 g, 96% yield) [26] and propionate 4b [31] (2.9 g, 96% yield). Their spectroscopic data are consistent with those reported in the literature.
3.4. General Procedure for Enzymatic Hydrolysis of Allyl Esters (4a,b)
Two milliliters of Lecitase® Ultra (or 50 mg lipase B from Candida antarctica CALB) and 0.2 g of substrate (4a or 4b) dissolved in 0.5 mL of acetone were placed into 10 mL screw cap glass vials containing 3.5 mL of phosphate buffer (pH 7.2). Vials were shaken at 750 rpm. Samples (0.6 mL) from the reaction with Lecitase® Ultra were taken at different time intervals and extracted with diethyl ether. The extracts were dried and solvent was removed by evaporation in vacuo. Before analysis, samples were treated with 0.3 mL of corresponding acyl chloride (propionyl chloride, in the case of hydrolysis of 4a, or acetyl chloride, in the case of hydrolysis of 4b) in 1mL of dry diethyl ether and 0.5 mL of pyridine. The mixtures were stirred for 0.5 h at room temperature, diluted with 1 mL of 1 M HCl and 1 mL of diethyl ether. Ethereal layer was separated, washed with saturated NaHCO3, brine, and dried over anhydrous MgSO4. Extracts were filtrated and solvent was completely evaporated in vacuo. The residues were dissolved in 1 mL of hexane, transferred to the vials, and analyzed by CGC. A similar procedure was followed for the reaction with CALB, but the enzyme was removed from the reaction mixture through filtration.
Author Contributions
A.L., W.G. conceived and designed the experiments; A.L. carried out the experiments, gas chromatography analysis; A.L., A.C. and W.G. analyzed the data; A.L. and W.G. wrote the paper.
Acknowledgments
Publication supported by Wroclaw Centre of Biotechnology, program The Leading National Research Centre (KNOW) for years 2014–2018 (http://know.wroc.pl).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hayes, P.Y.; Chow, S.; Rahm, F.; Bernhardt, P.V.; De Voss, J.J.; Kitching, W. Synthesis of the sponge-derived plakortone series of bioactive compounds. J. Org. Chem. 2010, 75, 6489–6501. [Google Scholar] [CrossRef] [PubMed]
- Grudniewska, A.; Dancewicz, K.; Białońska, A.; Ciunik, Z.; Gabryś, B.; Wawrzeńczyk, C. Synthesis of piperitone-derived halogenated lactones and their effect on aphid probing, feeding, and settling behavior. RSC Adv. 2011, 1, 498–510. [Google Scholar] [CrossRef]
- Mehl, F.; Bombarda, I.; Vanthuyne, N.; Faure, R.; Gaydou, E.M. Hemisynthesis and odour properties of δ-hydroxy-γ-lactones and precursors derived derived from linalool. Food Chem. 2010, 121, 98–104. [Google Scholar] [CrossRef]
- Tashiro, T.; Mori, K. Enzyme-assisted synthesis of (S)-1,3-dihydroxy-3,7-dimethyl-6-octen-2-one, the male-produced aggregation pheromone of the Colorado potato beetle, and its (R)-enantiomer. Tetrahedron Asymmetry 2005, 16, 1801–1806. [Google Scholar] [CrossRef]
- Kamezawa, M.; Raku, T.; Tachibana, H.; Ohtani, T.; Naoshima, Y. Enzymatic hydrolysis of alken- and alkyn-3-ol acetates in an acetone—Water solvent system: Effect of unsaturation on the enantioselectivity of pseudomonas cepacia lipase-catalyzed hydrolysis. Biosci. Biotechnol. Biochem. 1995, 59, 549–551. [Google Scholar] [CrossRef]
- Ohtani, T.; Nakatsukasa, H.; Kamezawa, M.; Tachibana, H.; Naoshima, Y. Enantioselectivity of Candida antarctica lipase for some synthetic substrates including aliphatic secondary alcohols. J. Mol. Catal. B Enzym. 1998, 4, 53–60. [Google Scholar] [CrossRef]
- Singh, A.; Goel, Y.; Rai, A.K.; Banerjee, U.C. Lipase catalyzed kinetic resolution for the production of (S)-3-[5-(4-fluoro-phenyl)-5-hydroxy-pentanoyl]-4-phenyl-oxazolidin-2-one: An intermediate for the synthesis of ezetimibe. J. Mol. Catal. B Enzym. 2013, 85–86, 99–104. [Google Scholar] [CrossRef]
- Angajala, G.; Pavan, P.; Subashini, R. Lipases: An overview of its current challenges and prospectives in the revolution of biocatalysis. Biocatal. Agric. Biotechnol. 2016, 7, 257–270. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Trono, D. Recombinant lipases and phospholipases and their use as biocatalysts for industrial applications. Int. J. Mol. Sci. 2015, 16, 20774–20840. [Google Scholar] [CrossRef] [PubMed]
- De Maria, L.; Vind, J.; Oxenbøll, K.M.; Svendsen, A.; Patkar, S. Phospholipases and their industrial applications. Appl. Microbiol. Biotechnol. 2007, 74, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Vijeeta, T.; Reddy, J.R.C.; Rao, B.V.S.K.; Karuna, M.S.L.; Prasad, R.B.N. Phospholipase-mediated preparation of 1-ricinoleoyl-2-acyl-sn-glycero-3-phosphocholine from soya and egg phosphatidylcholine. Biotechnol. Lett. 2004, 26, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.J.; Wang, X.Y.; Yang, D.; Zhang, H.; Shin, J.A.; Hong, S.T.; Park, S.H.; Lee, K.T. Emulsifying properties of lecithin containing different fatty acids obtained by immobilized Lecitase Ultra-catalyzed reaction. J. Am. Oil Chem. Soc. 2014, 91, 579–590. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R. Effect of the immobilization protocol in the activity, stability, and enantioselectivity of Lecitase® Ultra. J. Mol. Catal. B Enzym. 2007, 47, 99–104. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Cabrera, Z.; Godoy, C.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties. Process Biochem. 2008, 43, 1061–1067. [Google Scholar] [CrossRef]
- Mishra, M.K.; Kumaraguru, T.; Sheelu, G.; Fadnavis, N.W. Lipase activity of Lecitase® Ultra: Characterization and applications in enantioselective reactions. Tetrahedron Asymmetry 2009, 20, 2854–2860. [Google Scholar] [CrossRef]
- Mishra, M.K.; Harini, M.; Kumaraguru, T.; Lakshmi Prasanna, T.; Fadnavis, N.W. A porous vessel bioreactor for gel entrapped biocatalysts: Kinetic resolution of trans-methyl (4-methoxyphenyl)glycidate by Lecitase® Ultra in gelatin organogel (Gelozyme). J. Mol. Catal. B Enzym. 2011, 71, 56–62. [Google Scholar] [CrossRef]
- Cabrera, Z.; Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R. Asymmetric hydrolysis of dimethyl 3-phenylglutarate catalyzed by Lecitase Ultra®. Effect of the immobilization protocol on its catalytic properties. Enzym. Microb. Technol. 2008, 43, 531–536. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Filice, M.; Terreni, M.; Guisan, J.M.; Fernandez-Lafuente, R.; Palomo, J.M. Lecitase® Ultra as regioselective biocatalyst in the hydrolysis of fully protected carbohydrates. Strong modulation by using different immobilization protocols. J. Mol. Catal. B Enzym. 2008, 51, 110–117. [Google Scholar] [CrossRef]
- Brenna, E.; Fuganti, C.; Grasselli, P.; Serra, S. Enzyme-mediated synthesis of (S) and (R)-verapamil. Eur. J. Org. Chem. 2001, 2001, 1349–1357. [Google Scholar] [CrossRef]
- Brenna, E.; Caraccia, N.; Fuganti, C.; Fuganti, D.; Grasselli, P. Enantioselective synthesis of β-substituted butyric acid derivatives via orthoester Claisen rearrangement of enzymatically resolved allylic alcohols: Application to the synthesis of (R)-(−)-baclofen. Tetrahedron Asymmetry 1997, 8, 3801–3805. [Google Scholar] [CrossRef]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Gliszczyńska, A.; Czarnecka, M.; Pawlak, A.; Obmińska-Mrukowicz, B.; Maciejewska, G.; Białońska, A. Chiral δ-iodo-γ-lactones derived from cuminaldehyde, 2,5-dimethylbenzaldehyde and piperonal: Chemoenzymatic synthesis and antiproliferative activity. Tetrahedron Asymmetry 2016, 27, 227–237. [Google Scholar] [CrossRef]
- Morgan, B.; Oehlschlager, A.C.; Stokest, T.M. Enzyme Reactions in Apolar Solvent. 5. The effect of adjacent unsaturation on the PPL-catalyzed kinetic resolution of secondary alcohols. J. Org. Chem. 1992, 57, 3231–3236. [Google Scholar] [CrossRef]
- Brenna, E.; Fuganti, C.; Gatti, F.G.; Passoni, M.; Serra, S. Enantioselective synthesis of benzylic stereocentres via Claisen rearrangement of enantiomerically pure allylic alcohols: Preparation of (R) and (S)-3-methyl-2-phenylbutylamine. Tetrahedron Asymmetry 2003, 14, 2401–2406. [Google Scholar] [CrossRef]
- Lindner, E.; Ghanem, A.; Warad, I.; Eichele, K.; Mayer, A. Asymmetric hydrogenation of an α,β-unsaturated ketone by diamine (ether–phosphine) ruthenium (II) complexes and lipase-catalyzed kinetic resolution: A consecutive approach. Tetrahedron Asymmetry 2003, 14, 1045–1053. [Google Scholar] [CrossRef]
- Zhu, L.; Kedenburg, J.P.; Xian, M.; Wang, P.G. A systematic strategy for preparation of uncommon sugars through enzymatic resolution and ring-closing metathesis. Tetrahedron Lett. 2005, 46, 811–813. [Google Scholar] [CrossRef]
- Ghanem, A.; Schurig, V. Lipase-catalyzed access to enantiomerically pure (R) and (S)-trans-4-phenyl-3-butene-2-ol. Tetrahedron Asymmetry 2003, 14, 57–62. [Google Scholar] [CrossRef]
- Gładkowski, W.; Gliszczyńska, A.; Siepka, M.; Czarnecka, M.; Maciejewska, G. Kinetic resolution of (E)-4-(2′,5′-dimethylphenyl)-but-3-en-2-ol and (E)-4-(benzo[d][1′,3′]dioxol-5′-yl)-but-3-en-2-ol through lipase-catalyzed transesterification. Tetrahedron Asymmetry 2015, 26, 702–709. [Google Scholar] [CrossRef]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Siepka, M.; Białońska, A. Convenient chemoenzymatic route to optically active β-aryl-δ-iodo-γ-lactones and β-aryl-γ-iodo-δ-lactones with the defined configurations of stereogenic centers. Eur. J. Org. Chem. 2015, 2015, 605–615. [Google Scholar] [CrossRef]
- Kazlauskas, R.J.; Weissfloch, A.N.E.; Rappaport, A.T.; Cuccia, L.A. A rule to predict which enantiomer of a secondary alcohol reacts faster in reactions catalyzed by cholesterol esterase, lipase from Pseudomonas cepacia, and lipase from Candida rugosa. J. Org. Chem. 1991, 56, 2656–2665. [Google Scholar] [CrossRef]
- Rahman, A.F.M.M.; Ali, R.; Jahng, Y.; Kadi, A.A. A facile solvent free claisen-schmidt reaction: Synthesis of α,α’-bis-(Substituted-benzylidene)cycloalkanones and α,α’-bis-(Substituted-alkylidene)cycloalkanones. Molecules 2012, 17, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Birman, V.B.; Jiang, H. Kinetic resolution of alcohols using a 1,2-dihydroimidazo[1,2-a]quinoline enantioselective acylation catalyst. Org. Lett. 2005, 7, 3445–3447. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).