Simple Summary
This paper addresses the complex diagnostic challenges of pigmented lesions in the oral mucosa, ranging from benign to potentially malignant conditions. Our study provides a narrative review with a clinical case overview to guide clinicians in differentiating these lesions. By classifying pigmented lesions based on clinical and histological features, we emphasize the need for a structured approach to diagnosis. The study incorporates a retrospective analysis of cases from our oral medicine experience, utilizing clinical pictures and relevant histology. Our findings aim to enhance early and precise diagnosis, improving patient management and outcomes in dental and medical practice.
Abstract
This paper examines the clinical differentiation of pigmented lesions in the oral mucosa, which poses significant diagnostic challenges across dental and medical disciplines due to their spectrum from benign to potentially malignant conditions. Through a literature review and analysis of clinical cases, this study clarifies current diagnostic methodologies, with an emphasis on differential diagnosis, to provide a practical guide for clinicians. The classification of pigmented lesions, such as endogenous, focal melanocytic, and multifocal pigmentation, based on clinical and histological features, highlights the necessity for a structured and informed approach. A retrospective examination of cases from our oral medicine and pathology clinic, coupled with analysis of photographic and histological records, aids in classifying these lesions. This fosters a better understanding and promotes informed discussions among clinicians, ultimately aiming to enhance early and precise diagnosis, thus improving patient management and outcomes.
1. Introduction
The human oral mucosa displays a vast spectrum of colors, ranging from pale coral pink to yellow, red, and brown, extending beyond mere physiological variations to encompass an array of pathological states. This diversity in hue and tone is influenced by several factors, notably the degree of keratinization, the density and melanogenic activity of melanocytes within the basal epithelial layer, the extent of vascularization, and the intrinsic properties of the submucosal tissue, which may include muscle, bone, or cartilage. In this context, the inherent coloration of the oral mucosa in individuals with lighter skin tones may vary from shades of white to deep red-purple, while in those with darker complexions, the gums, buccal mucosa, and labial tissue often exhibit a range of brown shades. This chromatic variability not only reflects the genetic factors that regulate melanin synthesis by melanocytes and its subsequent transfer to adjacent keratinocytes but is also influenced by external factors such as trauma, inflammation, hormonal fluctuations, exposure to certain pharmacological agents, external agents that can pigment the mucosa, and radiation, all of which can induce an increase in melanin production.
The clinical landscape of pigmented lesions within the oral mucosa is vast and complex, necessitating a rigorous and nuanced approach to differential diagnosis. These lesions, ranging from benign melanotic macules and physiological pigmentation to melanomas and indicators of systemic diseases, pose formidable diagnostic challenges. Accurate evaluation of these lesions is critical, as it spans a diagnostic continuum that includes benign conditions such as freckles (ephelides), oral melanotic macules, and smoker’s melanosis, to more severe concerns like oral malignant melanoma and pigmentation indicative of systemic conditions such as Addison’s disease and Peutz–Jeghers Syndrome. Moreover, the evaluation of pigmented lesions in the oral cavity is further complicated by multifactorial etiologies, including endogenous factors like nevi and exogenous causes such as amalgam tattoos, making accurate diagnosis and management of these lesions pivotal in clinical practice.
The identification and appropriate management of these conditions underscore the intersection of oral medicine and dermatology. It necessitates a comprehensive understanding of the physiological variations in oral mucosa coloration, alongside an ability to discern pathological states from normal conditions.
2. Melanin-Associated Pigmented Lesions
Melanin-associated pigmented lesions in the oral cavity encompass a wide range of conditions, from benign entities such as ephelides (freckles) and melanotic macules, to melanomas. These lesions, characterized by increased deposition of melanin in the oral mucosa, present a diagnostic challenge due to their varied etiology and clinical presentation. A thorough understanding of these melanin-rich lesions is crucial for clinicians to accurately differentiate between benign and non-benign conditions, ensuring appropriate management and patient care. The ability to distinguish these lesions based on their clinical and histopathological features is essential for effective diagnosis and underscores the importance of a comprehensive examination and, when necessary, biopsy and histological analysis.
2.1. Ephelides (Freckles)
Ephelides, commonly referred to as freckles, are benign, hyperpigmented entities characterized by augmented melanin synthesis and deposition, without a proportional increase in melanocyte density [1]. They predominantly appear in sun-exposed dermal areas in individuals with lighter skin tones and can also manifest on the vermilion border of the lips (Figure 1), presenting unique diagnostic challenges [2].
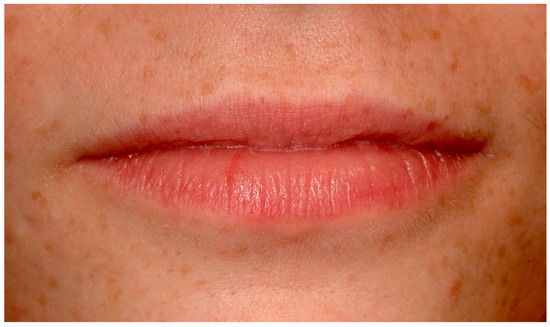
Figure 1.
Ephelides on the perioral skin and labial psudomucosae on a 22-year-old patient. Patient refers to these lesions from birth. (archive S.A., patient signed the consent for clinical pictures).
The genesis of ephelides is intricately linked to genetic predispositions, notably associated with polymorphisms in the melanocortin-1 receptor (MC1R) gene, a key regulator of melanin biosynthesis [3]. These genetic determinants, in conjunction with environmental catalysts such as ultraviolet (UV) radiation exposure, precipitate localized melanin overproduction characteristic of ephelides [4]. Unlike other melanin-rich lesions, ephelides tend to intensify under sun exposure and fade in their absence, illustrating the dynamic modulation of melanin synthesis in response to UV radiation [5].
The management protocol for oral ephelides primarily focuses on vigilant monitoring for changes in dimensions, pigmentation, or morphology indicative of an alternate pathology. Given their benign nature, therapeutic intervention is generally unwarranted [1]. Nonetheless, educating patients about the importance of UV protection is paramount to mitigate the aggravation of existing lesions and the emergence of new ones, particularly in individuals predisposed to skin cancers due to substantial sun exposure or genetic susceptibilities [5].
2.2. Oral/Labial Melanotic Macules
In the field of oral medicine, oral melanotic macules are recognized as a prevalent category of benign pigmented lesions, characterized by a localized increase in melanin concentration [6]. This enhanced melanin deposition occurs without a corresponding proliferation of melanocytes, distinguishing these macules from other pigmented oral pathologies. Clinically, these macules range in hues from light brown to a more pronounced black, primarily dictated by the depth and density of melanin within the epithelial and, occasionally, subepithelial layers (Figure 2) [7].
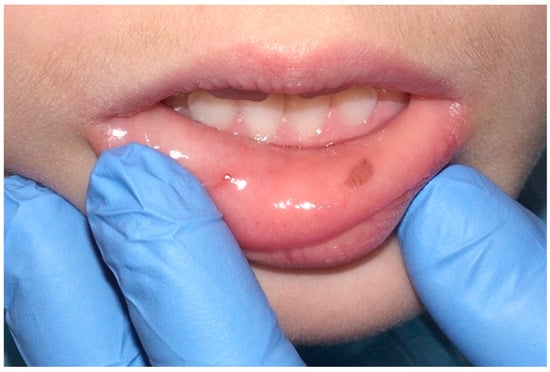
Figure 2.
Oral melanotic macula on the lower labial vestibular mucosa on a 7-year-old patient. Parents referred these lesions as being present for over 6 months. (archive S.A., patient signed the consent for clinical pictures).
The etiology of these macules is multifactorial, combining idiopathic elements with a spectrum of systemic diseases, hormonal fluctuations, and specific incidents of localized trauma. The precise mechanisms leading to the heightened activity of melanocytes in melanin production remain partially understood. It is speculated to involve a complex interplay of both intrinsic genetic factors and extrinsic environmental influences, such as exposure to ultraviolet light, which is known to trigger melanin production as a protective response [8]. Additionally, various medications and systemic illnesses have been associated with the emergence of oral melanotic macules, indicating a possible correlation between systemic health and concentrated oral pigmentation phenomena [6].
Clinically, these macules are commonly situated on the lower lip. However, their presence is not restricted to this region alone; presentations on the buccal mucosa, gingiva, hard and soft palates, and the dorsal surface of the tongue have been documented (Figure 3). These macules, typically isolated in appearance, vary in size, often occupying a few millimeters in diameter. Despite their benign character, the identification of oral melanotic macules warrants a comprehensive clinical assessment [9].
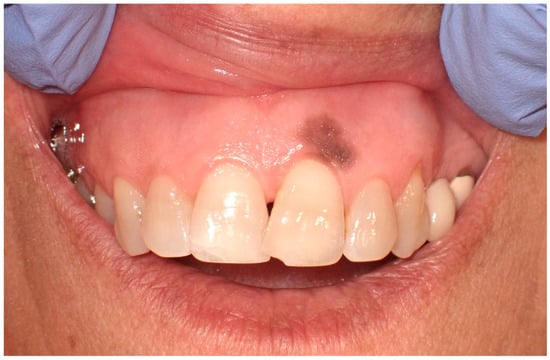
Figure 3.
Oral melanotic macula on the upper gingival mucosa on a 40-year-old patient. Patient refers to these lesions as being present since over 6 months. (archive S.A., patient signed the consent for clinical pictures).
The clinical evaluation of oral melanotic macules typically relies on comprehensive visual inspection and an in-depth review of the patient’s medical history. However, atypical features or documented changes in the lesion’s appearance necessitate additional diagnostic measures [10]. In this case, histological assessment through biopsy becomes essential. Histopathological findings often reveal increased melanin deposition in the epithelial basal layer and may include melanin-laden macrophages within the connective tissue, without a corresponding rise in the number of melanocytes [9].
Management of oral melanotic macules is generally conservative, prioritizing ongoing surveillance to monitor for any alterations indicative of malignant transformation or other clinical concerns [11].
2.3. Racial Pigmentation
Racial or ethnic physiological pigmentation refers to the alteration of the typical coral pink pigmentation of the oral mucosa due to increased cellular activity of melanocytes [12,13], notably an augmented quantity of melanin deposited on the epithelial basal layer [8,14]. This form of pigmentation is the most commonly observed in the oral mucosa, representing 39.9% of all oral pigmentations [15]. It predominantly affects individuals with dark skin tones without gender predisposition and is most frequently diagnosed in the first two decades of life [16,17]. Oral ethnic pigmentation has been documented in 13.5% of children in Israel [18].
Pigmentation manifests as dark brown to black in color, can intensify with age, and is influenced by systemic therapies, smoking, and hormones [19,20]. The most affected site is the gingiva, particularly the attached gingiva, excluding the marginal border [21,22], in 72% of cases of ethnic pigmentation [15] other affected sites include the buccal mucosa, lips, palate, and tongue (Figure 4) [16,19]. Microscopically, there is no observed increase in the number of melanocyte cells or their migration, but rather an increased deposition of melanin on the epithelial basal layer and lamina propria.
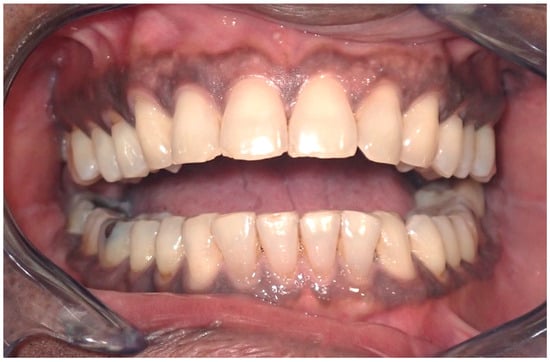
Figure 4.
Ethnic pigmentation of the gingival tissues on a 60-year-old patient. Patient refers to these pigmentation from decades. (archive S.A., patient signed the consent for clinical pictures).
This type of pigmentation is included in the differential diagnosis with some systemic syndromes, including Peutz–Jeghers syndrome, Addison’s disease, and drug-induced pigmentation. The diagnosis is based on oral clinical examination, and since it is asymptomatic and innocuous, ethnic pigmentation does not require any treatment except for aesthetic reasons [13,20].
2.4. Oral Melanoma
Oral melanoma is a rare malignancy originating from the malignant transformation of melanocytes within the oral cavity. Unlike the more common cutaneous melanomas, oral melanomas exhibit distinct features and challenges. Primary oral melanoma accounts for approximately 1% of all melanomas [23,24] and 0.26–0.5% of all malignant neoplasms in the oral cavity [25,26]. Increased incidence rates have been noted in dark-skinned populations, particularly in Uganda [27]. Gender predisposition remains unclear; however, some studies indicate a male-to-female ratio of 2.5–3:1. [25], while others report a female predominance with a ratio of 1:1.7 [28]. Consensus exists regarding the typical onset age in the sixth and seventh decades of life [25,28,29]. It is a subset of mucosal melanomas, a rare but aggressive form of melanoma occurring in the head and neck regions [30]. These diseases often result in diagnostic delays, leading to a low outlook for patients. The 5-year survival rate is approximately 25% [31].
Another form of oral melanoma is oral amelotic melanoma, which lacks pigmentation, with histological analysis showing melanin in less than 5% of tumor cells. Though amelanotic melanomas are uncommon, representing less than 2% of all melanoma cases, they constitute 40% up to 75% of melanomas found in the oral cavity [32]. Oral amelanotic melanoma predominantly affects the maxillary gingiva, followed by the palate, and infrequently involves the mandibular gingiva [33]. The clinical lack of pigmentation poses a diagnostic challenge for this type of melanoma. It appears as asymptomatic, irregular, erythematous, flat, or nodular, lesions and It is more likely to involve regional lymph nodes and metastasize to distant sites, particularly the lungs [34].
The etiology of oral melanoma remains largely unclear: unlike the more common cutaneous melanomas, which are primarily caused by the sun’s ultraviolet (UV) rays in 86% of cases, some researchers suggest that tobacco use and chronic irritation from malocclusion may play a role in its development. [35,36]. In approximately 30% of cases, the onset of the disease is preceded by a benign pigmented lesion [37,38], though it typically develops de novo on seemingly normal oral mucosa. The initial signs and symptoms often include a lesion that is soft to palpation, usually pigmented, initially asymptomatic, with irregular borders and a color spectrum ranging from dark brown to bluish-black, occasionally presenting variations of gray, purple, and red due to ulcerations, or depigmentation (Figure 5) [39,40]. The primary sites of affliction include the mucosa of the hard palate, followed by the maxillary gingiva and alveolar mucosa [29,41].
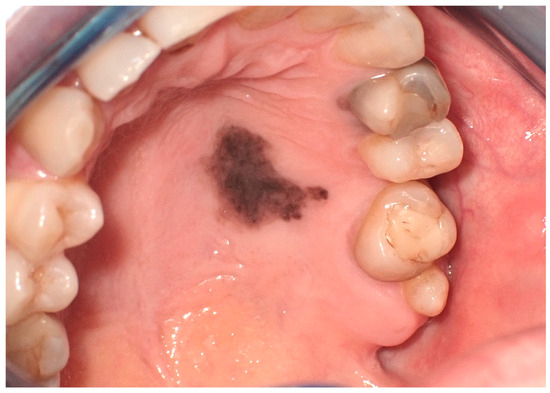
Figure 5.
Oral malignant melanoma, in the hard palatal mucosa on a 67-year-old female patient. Patient refers to these lesions as being present for 3 months. (archive S.A., patient signed the consent for clinical pictures).
Unlike its cutaneous counterpart, oral melanoma lacks histopathological parameters that can reliably predict prognosis, for example, the Clark and Breslow classification and the ABCDEFG diagnostic scale that is normally used to diagnose nevi on the skin is not used for the pigmented lesions of the mouth mucosa. Additionally, the depth of infiltration does not correlate with disease outcome [42].
The prognosis is dire, with a 5-year survival rate between 10 and 20% and a median survival of 25 months. The 10-year survival rate is reported to be 0% [24,27,43], reflecting the challenge of achieving wide surgical margins due to anatomical constraints. Many patients develop widespread metastases affecting the lungs, liver, brain, bone, and lymph nodes. Accurate diagnosis typically requires an incisional or excisional biopsy for histological examination. Immunohistochemical biomarkers, including S-100 (90% positivity), HMB-45 (95.5% positivity), and Melan-A (100% positivity), play a fundamental role in confirming the diagnosis. The World Health Organization (WHO) categorizes melanoma into four primary types: superficial spreading, nodular, acral lentiginous, and lentigo maligna [28,44]. The two most common growth patterns of oral melanoma are (i) a superficial spreading radial growth pattern, featuring pagetoid spread with large melanoma cells in the superficial epithelium, and (ii) a less common but highly aggressive nodular growth pattern that often infiltrates deeply into the skin and presents as a dark, raised bump [45]. The TNM staging system was updated by the American Joint Committee on Cancer in 2017 with the 8th edition of the Cancer Staging Manual, excluding the possibility of having stage I and II disease. Stages are classified as follows: stage III, tumors limited to the mucosa and immediately underlying soft tissue; stage IVa, a moderately advanced local disease involving deep soft tissue, cartilage, bone, or skin; and stage IVb, an advanced local disease involving the brain, dura, skull base, lower cranial nerves, and masticatory space [46,47]
Proper imaging, including MRI and PET/CT scans, is crucial for assessing the anatomical relationships between healthy tissues and the neoplasm. Surgical excision remains the primary treatment for oral melanoma. Achieving wide and clear excision margins, although challenging, is essential for favorable patient outcomes [41,48].
Adjuvant systemic therapies such as immunotherapy and radiotherapy are also employed in their management. Unfortunately, both have shown limited success in treating advanced-stage melanoma [30,49].
The first-line treatments involve surgical removal with clear margins; although data is limited due to the rarity of these cancers, both radiation therapy and systemic treatments are also employed in their management [31,50].
The management of oral amelanotic melanoma generally includes a combination of surgical intervention and radiotherapy or chemotherapy. Given the rarity, there is a scarcity of data, with only a few case reports and series available. As a result, knowledge about this type of melanoma is still developing, highlighting the need for additional research to more accurately define its clinicopathological characteristics, immune profile, and responses to various treatments [51].
Second-line treatment, such as therapy and immunotherapy for advanced melanoma, including the less common acral and mucosal subtypes, has demonstrated promising results. Traditional chemotherapy using dacarbazine has achieved limited success, although regimens combining albumin-bound paclitaxel and carboplatin appear more effective. Immunotherapy involving immune checkpoint inhibitors (ICIs) such as nivolumab and pembrolizumab has shown reduced efficacy in acral melanoma (AM) compared to cutaneous melanoma. However, targeted therapies aimed at KIT and CDK4/6 mutations have shown potential. The combination of targeted therapies with ICIs or other agents could enhance treatment outcomes [52].
2.5. Oral Tobacco Pigmentation/Smoker’s Melanosis
Tobacco-associated oral melanosis is characterized by diffuse, brown to brown-black hyperpigmentation of the oral mucosa, observed in approximately 20–30% of chronic tobacco users (Figure 6). This condition predominantly affects the gingiva, hard palate, and buccal mucosa, with the severity of pigmentation directly proportional to the cumulative exposure to tobacco. It is hypothesized that melanogenesis induced by tobacco consumption acts as a defensive mechanism against the cytotoxic components of tobacco smoke, including polyaromatic hydrocarbons, nicotine, and benzopyrene [53,54]. An investigative study on the Indian population described the prevalence of oral melanin pigmentation among both smoke and smokeless tobacco users, thereby broadening the understanding of tobacco’s impact on oral health beyond conventional smoking practices [55]. Notably, cessation of tobacco use is associated with a gradual reduction in melanotic manifestations, highlighting the reversible nature of smoker’s melanosis upon withdrawal [56].
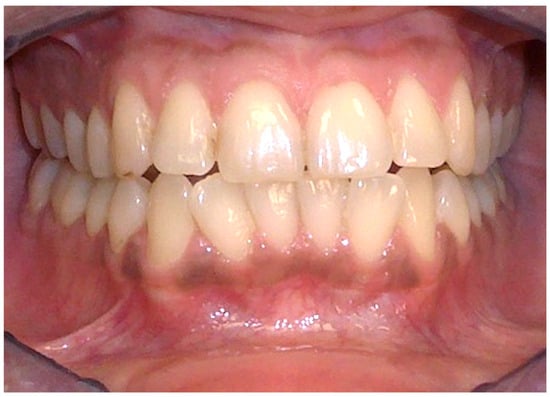
Figure 6.
Smoker’s Melanosis in the lower gingival mucosa of a 38-year-old heavy smoker male patient. Patient refers to these lesions as being present for 6 years. (archive S.A., patient signed the consent for clinical pictures).
Despite its benign pathology, differential diagnosis of smoker’s melanosis is imperative to distinguish it from other melanin-rich oral lesions such as idiopathic melanotic macules, inflammatory melanosis, and medication-induced pigmentation, which share clinical and histopathological features [57]. Importantly, smoker’s melanosis lacks malignant potential, distinguishing it from other pigmented oral pathologies with neoplastic implications. Moreover, the systemic consequences of tobacco use are profound, with epidemiological evidence demonstrating a correlative increase in colorectal cancer risk among tobacco users, thereby highlighting the extensive adverse health outcomes associated with tobacco consumption [58]. Clinically, smoker’s melanosis appears as widespread, uneven darkening, mainly impacting the anterior vestibular mucosa. The degree of this pigmentation is closely linked to how long and how intensely the individual has smoked. [53].
This condition underscores the importance of a comprehensive medical and social history, including tobacco use, in the differential diagnosis of oral pigmentation [57]. The reversibility of smoker’s melanosis upon cessation of smoking further emphasizes its direct link to tobacco exposure [55].
2.6. Melanoacanthoma
Oral melanoacanthoma is recognized as a non-neoplastic, benign lesion characterized predominantly by excessive proliferation of dendritic melanocytes within the epithelial layer of the oral mucosa, presenting a clinical and histopathological profile distinct from other pigmented oral lesions [59]. Typically manifesting as solitary, well-circumscribed macules or plaques, these lesions exhibit brown to black pigmentation and show a marked predilection for the buccal mucosa, although they may also affect the lips, palate, and gingiva [60].
The sudden appearance and rapid expansion of oral melanoacanthoma highlight the necessity of differential diagnosis to distinguish it from other malignant conditions, such as melanoma and various pigmented pathologies. Histopathological examination typically reveals a notable increase in dendritic melanocytes throughout the spinous layer, without evidence of cellular atypia [61]. In such cases, an incisional biopsy is mandatory. Following diagnosis, treatment is generally not indicated [7].
3. Non-Melanin Associated Pigmented Lesions
3.1. Endogenous Pigmentation
Oral pigmented lesions caused by endogenous pigments, particularly those derived from blood, represent a significant clinical concern. These lesions can be classified based on their origin and the nature of the pigments involved. For blood pigments, the coloration is primarily due to the presence of hemoglobin, hemosiderin, and other hemoglobin degradation products, which impart a characteristic red, bluish-purple, brown, or black color to the lesions [2].
Blood extravasation into oral tissues can lead to various forms of pigmented lesions, such as hematomas, petechiae, purpura, and ecchymoses. These manifestations result from the accumulation and subsequent degradation of hemoglobin into bilirubin and biliverdin. Common traumatic events in the oral cavity that may cause blood extravasation and subsequent hyperpigmentation include bites and iatrogenic procedures such as dental interventions [62].
The diagnosis of these lesions is primarily based on clinical examination, which can be implemented with the clinical test of diascopy (vitroscopy). This procedure differentiates pigmented from vascular lesions by removing the camouflaging effect of congested blood to reveal the true color of underlying lesions. It involves pressing a glass or plastic diascopy device, usually a microscope slide, against a cutaneous or mucous lesion. The characteristic blanching effect occurs due to blood dissipating intravascularly under compression, giving the tissue a pale appearance [63].
Patients with hemochromatosis, a condition also known as “bronze diabetes”, often present with a grayish-blue pigmentation of the hard palate, gingiva, and buccal mucosa [64]. This pigmentation results from the accumulation of iron-containing pigments, (ferritin and hemosiderin) within the skin and mucous membranes. This iron accumulation results from a defect in iron metabolism, leading to excessive absorption and deposition in body tissues [65].
In patients with beta-thalassemia, diffuse black-brown pigmentation can be observed. This inherited blood disorder results in abnormal hemoglobin production and commonly presents at the junction between the hard and soft palate (Figure 7). The pigmentation is due to the increased deposition of hemosiderin, a degradation product of hemoglobin. Beta-thalassemia causes accelerated red blood cell destruction, resulting in the release and accumulation of hemosiderin in the tissues [66,67].
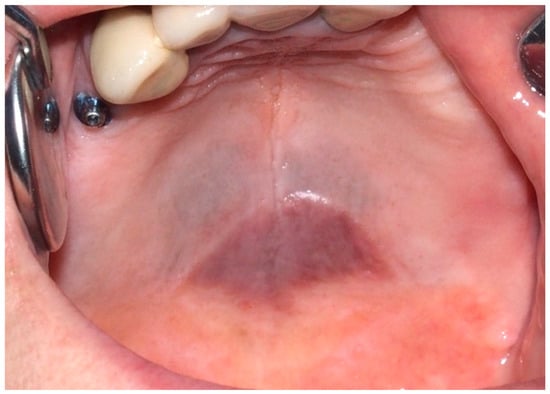
Figure 7.
Grayish-blue dark pigmentation at the border between hard and soft palate due to hemosiderin deposition in a 65-year-old female patient affected by beta-thalassemia. Patient refers to these lesions as being present for 10 years. (archive S.A., patient signed the consent for clinical pictures).
Oral varices (OV) are one of the most frequent changes in the oral mucosa, typically found under the tongue and in the lower lips in elderly patients. They are benign, painless, blue to purple dilatations of veins and/or venules with a nodular pattern. As they become more prominent with age, they can interfere with mastication. The pathogenesis is not clear; regular follow-ups are necessary in order to monitor this condition [68].
3.2. Exogenous Pigmentation and Amalgam Tattoos
Exogenous oral pigmentation is a noteworthy subject due to its frequent occurrence and the diverse sources from which it arises. Typically benign, this pigmentation results from foreign substances introduced into the oral mucosa [69]. A common example is the amalgam tattoo, which occurs when small particles from dental amalgam fillings become accidentally embedded in the soft tissues of the mouth during dental procedures (Figure 8). These tattoos appear as gray, blue, or black spots, usually near the site of dental work [70]. Amalgam tattoos are a prevalent form of exogenous pigmentation, arising typically from dental procedures where amalgam material is inadvertently embedded in the soft tissues. They are characterized by their distinctive blue or black coloration and are identified near areas of dental restorations [71]. Exogenous pigmentations can also occur from old materials used in endodontic fillings, which can traverse the bone and tissues, causing pigmentation of the mucosa at the level of the involved tooth apex. Diagnosis primarily relies on clinical examination, but radiographs or biopsy should be employed when the origin of the pigmentation is unclear, to distinguish them from more serious conditions. Radiographic examination is preferred for the differential diagnosis of amalgam/metal tattoos when suspected. A biopsy is recommended when radiographic examination does not reveal the presence of metal particles, even if clinical suspicion leans towards an amalgam tattoo, particularly in solitary cases, since these lesions enter into differential diagnosis with nevi and melanoma [72]. Management of these pigmented lesions generally does not require intervention unless there is an aesthetic concern or a need to confirm the benign nature of the pigmentation. The prognosis for patients with amalgam tattoos and similar forms of exogenous pigmentation is generally good, underscoring the importance of awareness and understanding of these conditions among dental professionals [73].
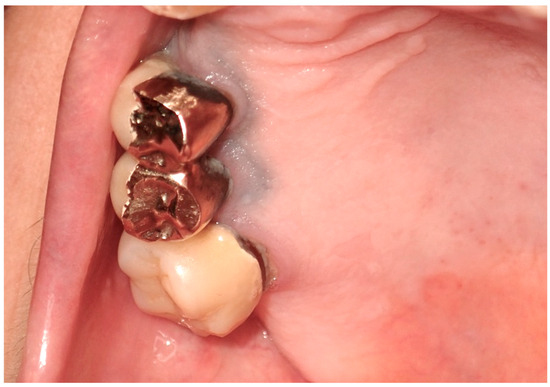
Figure 8.
The so-called “amalgam tatoo”, greyish pigmentation of the marginal gingival from particles of an amalgam of the previous dental restoration of upper premolar teeth sprayed during the preparation of the prosthetic abutments in a 67-year-old patient. Patient refers to these lesions as being present for 20 years. (archive S.A., patient signed the consent for clinical pictures).
4. Drug Induced Oral Melanosis
Drug-Induced Mucosal Pigmentation
Drug-induced oral melanosis results from the chromatic alteration of the oral mucosa, with an estimated 10–20% of acquired oral pigmentations induced by certain medications. Antimalarials such as chloroquine, hydroxychloroquine, and quinacrine are prominently implicated in the development of drug-induced oral pigmentations [74]. Additional medications involved include antipsychotics such as chlorpromazine; chemotherapeutics like bleomycin, imatinib, busulfan, fluorouracil; antifungal drugs including ketoconazole; antimicrobial drugs such as tetracycline and minocycline; and antiretroviral drugs such as zidovudine [7,75,76]. Pigmentation may affect all oral surfaces, be localized to a single site, or spread to multiple sites, with the hard palate, gingiva, and tongue being the most affected (Figure 9).
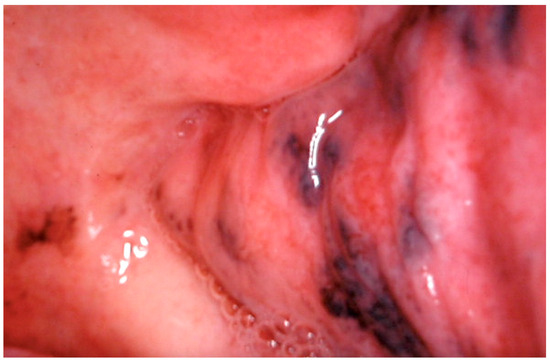
Figure 9.
Extensive oral mucosal pigmentation due to long term therapy with systemic hydroxychloroquine in a 70-year-old patient. Patient refer to these lesions as being present for over 10 years. (archive S.A., patient signed the consent for clinical pictures).
At the pathophysiological level, two mechanisms underlie the formation of these pigmentations: (i) directly or indirectly inducing the synthesis and release of melanin by melanocytes, resulting in true pigmentation; (ii) through the deposition of drug precipitates in the submucosa or direct binding of the drug to melanin thus imparting a blue to black coloration to the oral mucosa [7]. The specific mechanism by which melanin synthesis is increased is not fully understood, though it is possible that metabolites of some drugs may stimulate melanogenesis. Microscopically, hyperpigmentation and melanin leakage are observed with no increase in the melanocyte numbers. Clinical diagnosis is simplified if a temporal association is established between drug intake and the onset of pigmentation; otherwise, a diagnostic biopsy is recommended. In most cases, the discoloration tends to fade within a few months after the drug is discontinued [74]. Malignant transformation of these lesions has not been reported [17].
5. Systemic Disorders
5.1. Peutz–Jeghers Syndrome
Peutz–Jeghers Syndrome (PJS) is a hereditary autosomal dominant disorder characterized by the formation of unique polyps in the gastrointestinal tract and specific mucocutaneous pigmentation. Mutations in the STK11 gene on chromosome 19p13.3 are strongly linked to this syndrome. Those with PJS are at a much higher risk of developing several types of cancer, especially in the breast and gastrointestinal regions [77]. The rarity of PJS poses challenges in establishing evidence-based surveillance and management protocols. However, the primary focus of early endoscopic surveillance is to identify polyps that could lead to intussusception or obstruction rather than for cancer detection, with cancer surveillance becoming a critical component of management as patients age [78].
The diagnosis of Peutz–Jeghers Syndrome (PJS) can be made if a person has two or more histologically confirmed Peutz–Jeghers polyps, any Peutz–Jeghers polyps along with a family history of the syndrome, or the typical mucocutaneous pigmentation in the presence of a family history of PJS [77]. The syndrome has a higher risk of malignancy compared to the general population, including heightened risks for gastrointestinal, breast, lung, and genitourinary cancers [79].
The oral manifestations, particularly the pigmented lesions on the lips and inside the mouth, serve as crucial diagnostic features and visual markers that can alert healthcare providers to the potential presence of PJS [80]. While these lesions are benign and highly indicative of the syndrome, differential diagnosis is essential, as oral pigmentation can also be associated with melanotic macules, nevi, and ephelides on the lips (Figure 10). Accurately identifying the pigmented lesions of PJS and differentiating them from conditions such as melanotic macules, nevi, and ephelides is crucial for guiding appropriate clinical management and surveillance [79].
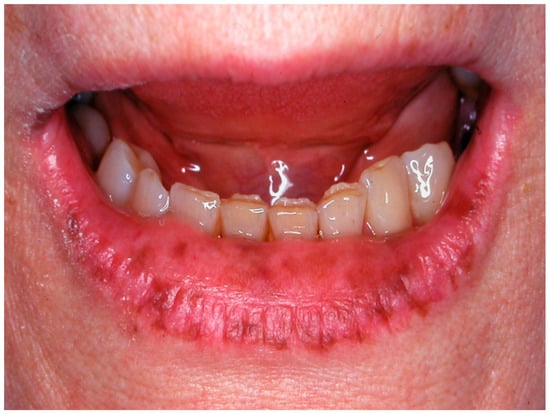
Figure 10.
Labial ephelides in a woman affected by Peutz–Jeghers Syndrome in a 63-year-old patient. Patient refer to these lesions as being present for 45 years. (archive S.A., patient signed the consent for clinical pictures).
5.2. Addison’s Disease
Addison’s disease, also known as adrenal insufficiency or primary hypoadrenalism, is an endocrinopathy characterized by reduced glucocorticoid production due to progressive destruction of the adrenal cortex, typically caused by autoimmune disease, cancer, or infection [81]. The diminished production and subsequent release of adrenocorticoid hormones in the blood lead to dysfunction of the hypothalamus-pituitary-adrenal gland axis, particularly resulting in elevated levels of adrenocorticotropic hormone (ACTH). This increased ACTH production stimulates melanocyte-stimulating hormone (α-, β-, and γ-melanotropins), thereby causing diffuse pigmentation on both cutaneous and oral surfaces (Figure 11).
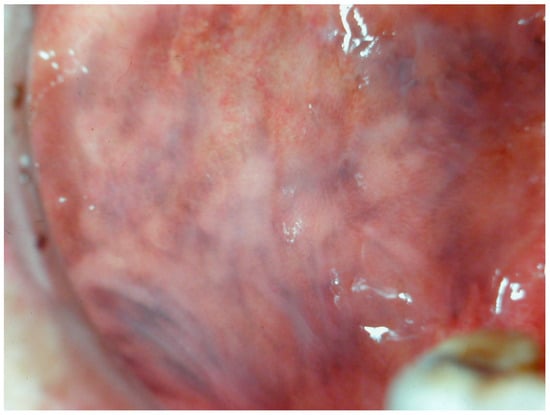
Figure 11.
Pigmentation of the cheek mucosa in a patient with Addison’s disease in a 35-year-old female patient. Patient refer to these lesions as being since years. (archive S.A., patient signed the consent for clinical pictures).
Cutaneo-mucosal hyperpigmentation affects 92% of Addison’s disease patients [82]. These oral pigmented lesions present as diffuse brown macules that can affect all oral surfaces, typically appearing and progressing in adult life [16]. Diagnosis of these oral pigmented lesions is primarily based on the patient’s clinical history. Oral pigmented lesions caused by Addison’s disease do not require any treatment.
6. Post-Inflammatory Pigmentations
Pigmented Oral Lichen Planus
Oral lichen planus (OLP) is a persistent inflammatory disorder that targets the mucous membranes within the mouth. It manifests as white, lace-like patches or red, inflamed tissues, often leading to considerable discomfort, such as pain and a burning feeling. The precise cause of OLP is not fully understood, but it is thought to involve an immune-mediated process, where the immune system erroneously attacks the cells of the mucous membranes. [83].
Among the various forms of lichen planus, there exists a distinct pigmented variant known as lichen planus pigmentosus (LPP). This variant was first identified in India by Bhutani et al. in 1974, who introduced the term to describe its unique presentation. LPP is distinguished by its slow onset and the appearance of small, dark brown or black macules that typically emerge on sun-exposed areas of the skin (Figure 12). Over time, these macules can merge to form larger patches of hyperpigmentation. Although LPP primarily impacts the face, torso, and upper limbs, LPP rarely involves the oral mucosa. Notably, the condition does not affect the palms, soles, or nails [84,85].
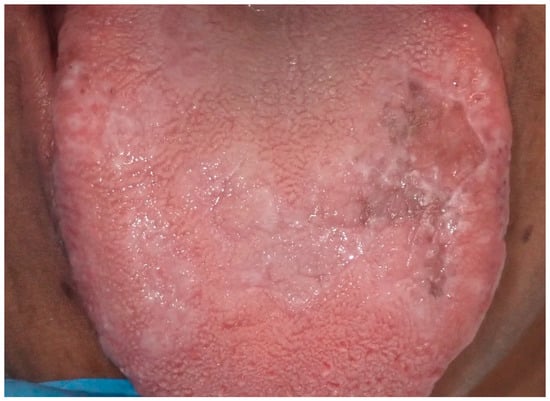
Figure 12.
Area of tongue mucosa pigmentation in a patient with reticular oral lichen planus in a 42-year-old male patient. Patient refers to these lesions as being present for several years. (archive S.A., patient signed the consent for clinical pictures).
Histologically, LPP exhibits notable atrophic alterations in the epidermis, vacuolar degeneration of the basal cell layer, and melanin incontinence within the dermis. This is further accompanied by the presence of dispersed melanophages and a sparse infiltrate around hair follicles or blood vessels. This pigmented variant shows considerable histopathological overlap with erythema dyschromicum persistant, though differences in clinical presentation and immunologic features suggest they may be distinct entities. Despite this, some dermatologists debate whether these conditions should be considered separate diseases [86,87].
Patients with LPP have been found to have associations with several systemic conditions, such as hepatitis C virus infection, frontal fibrosing alopecia, Bazex syndrome, and nephrotic syndrome. An unusual form of LPP, known as LPP inversus, was reported in 2001. Unlike the typical presentation, LPP inversus affects intertriginous areas such as the groins and axillae, predominantly occurring in individuals with lighter skin tones. This variant shares clinical and histopathological features with LPP but presents in areas not exposed to sunlight [88].
7. Conclusions
This narrative review comprehensively addresses the complexities involved in the differential diagnosis of pigmented lesions within the oral mucosa. As evidenced through the detailed examination of various case studies and extensive literature review, the accurate differentiation and timely diagnosis of these lesions are crucial due to their diverse etiologic spectrum ranging from benign discolorations to potentially malignant conditions.
In the following Table 1, the clinical features, the causes, the proposed treatments and differential diagnosis of the lesions and conditions described in this review are summarized and compared.

Table 1.
Clinical features of pigmented lesions affecting the oral mucosa and differential diagnosis.
A cornerstone of effective management of pigmented oral lesions is the early and precise identification through rigorous clinical examination and histopathological evaluation via biopsy. The role of the dental hygienist is pivotal, often serving as the first point of clinical contact. Dental hygienists are uniquely positioned to recognize abnormal pigmentation during routine examinations, thereby initiating a referral pathway that leads to specialized care from oral medicine specialists or otolaryngologists.
The differentiation between benign lesions and those with malignant potential cannot be understated, as it directly influences treatment decisions and patient prognosis. Conditions such as melanotic macules, which are generally harmless, and malignant melanomas, which require aggressive management, exemplify the spectrum of clinical presentations encountered in practice. This delineation is supported by a thorough histopathological examination, underscoring the necessity of biopsy in ambiguous cases.
Moreover, the significance of detailed photographic documentation emerges as a valuable tool in monitoring the evolution of pigmented lesions over time, providing a visual record that can be invaluable in tracking changes and responding appropriately.
Comprehensive patient history collection also remains crucial, with the oral medicine specialist integrating data regarding potential systemic diseases, medication use, and lifestyle factors like smoking. This thorough collection of patient information not only aids in crafting a complete diagnostic picture but also enhances the understanding of the systemic implications of oral pigmentation.
In conclusion, the ability to conduct a detailed and accurate differential diagnosis, timely referral to specialists, and the integration of clinical findings with histopathological insights form the cornerstone of managing pigmented lesions in the oral mucosa. Future enhancements in the training of all oral and dental professionals, including dental hygienists, and advances in diagnostic technologies, promise to improve patient outcomes through earlier detection and tailored intervention strategies.
Author Contributions
Conceptualization, S.A., G.F.S. and L.F.; methodology, S.A., G.F.S. and L.F.; validation, S.A., G.F.S., L.F. and E.P.; formal analysis, S.A., G.F.S. and L.F.; investigation, S.A., G.F.S. and L.F.; resources, S.A., G.F.S. and L.F.; data curation, S.A., G.F.S. and L.F.; writing—original draft preparation, S.A., G.F.S. and L.F.; writing—review and editing, S.A., G.F.S. and L.F.; visualization, S.A., G.F.S. and L.F.; supervision, S.A., G.F.S., L.F. and E.P.; project administration, S.A., G.F.S. and L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to acknowledge the administrative and technical support provided by the staff of IRCCS San Raffaele Hospital and University Vita Salute San Raffaele.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carson Dewitt, R. The Gale Encyclopedia of Dermatology, 1st ed.; Gale: Farmington Hills, MI, USA, 2017; pp. 244–246. [Google Scholar]
- Ko, E.; Panchal, N. Pigmented Lesions. Dermatol. Clin. 2020, 38, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, N.; Healy, E.; Ray, A.; Philips, S.; Todd, C.; Jackson, I.J.; Birch-Machin, M.A.; Rees, J.L. Pleiotropic effects of the melanocortin 1 receptor (MC1R) gene on human pigmentation. Hum. Mol. Genet. 2000, 9, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, M.; ter Huurne, J.; Gruis, N.; Bergman, W.; Westendorp, R.; Vermeer, B.J.; Bouwes Bavinck, J.N. The melanocortin-1-receptor gene is the major freckle gene. Hum. Mol. Genet. 2000, 10, 1701–1708. [Google Scholar] [CrossRef]
- Praetorius, C.; Sturm, R.A.; Steingrimsson, E. Sun-induced freckling: Ephelides and solar lentigines. Pigment Cell Melanoma Res. 2000, 27, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Rosebush, M.S.; Briody, A.N.; Cordell, K.G. Black and Brown: Non-neoplastic Pigmentation of the Oral Mucosa. Head Neck Pathol. 2019, 13, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Alawi, F. Pigmented lesions of the oral cavity: An update. Dent. Clin. N. Am. 2013, 57, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Gondak, R.O.; da Silva-Jorge, R.; Jorge, J.; Lopes, M.A.; Vargas, P.A. Oral pigmented lesions: Clinicopathologic features and review of the literature. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e919–e924. [Google Scholar] [CrossRef]
- Tavares, T.S.; Meirelles, D.P.; de Aguiar, M.C.F.; Caldeira, P.C. Pigmented lesions of the oral mucosa: A cross-sectional study of 458 histopathological specimens. Oral Dis. 2018, 24, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Ison, J.; Clark, A. Pigmented Lesions of the Oral Cavity. Oral Maxillofac. Surg. Clin. N. Am. 2023, 35, 153–158. [Google Scholar] [CrossRef]
- Eisen, D.; Voorhees, J.J. Oral melanoma and other pigmented lesions of the oral cavity. J. Am. Acad. Dermatol. 1991, 24, 527–537. [Google Scholar] [CrossRef]
- Gaeta, G.M.; Satriano, R.A.; Baroni, A. Oral pigmented lesions. Clin. Dermatol. 2002, 20, 286–288. [Google Scholar] [CrossRef]
- Müller, S. Melanin-associated pigmented lesions of the oral mucosa: Presentation, differential diagnosis, and treatment. Dermatol. Ther. 2010, 23, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Fricain, J.C.; Sibaud, V. Pigmentations de la muqueuse buccale. Presse Med. 2017, 46, 303–319. [Google Scholar] [CrossRef]
- Hassona, Y.; Sawair, F.; Al-Karadsheh, O.; Scully, C. Prevalence and clinical features of pigmented oral lesions. Int. J. Dermatol. 2016, 55, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Kauzman, A.; Pavone, M.; Blanas, N.; Bradley, G. Pigmented lesions of the oral cavity: Review, differential diagnosis, and case presentations. J. Can. Dent. Assoc. 2004, 70, 682–683. [Google Scholar]
- Tarakji, B.; Umair, A.; Prasad, D.; Alsakran-Altamimi, M. Diagnosis of oral pigmentations and malignant transformations. Singap. Dent. J. 2014, 35, 39–46. [Google Scholar] [CrossRef]
- Amir, E.; Gorsky, M.; Buchner, A.; Sarnat, H.; Gat, H. Physiologic pigmentation of the oral mucosa in Israeli children. Oral.Surg. Oral Med. Oral Pathol. 1991, 71, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D. Disorders of pigmentation in the oral cavity. Clin. Dermatol. 2000, 18, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Meleti, M.; Vescovi, P.; Mooi, W.J.; van der Waal, I. Pigmented lesions of the oral mucosa and perioral tissues: A flow-chart for the diagnosis and some recommendations for the management. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, 606–616. [Google Scholar] [CrossRef]
- Lenane, P.; Powell, F.C. Oral pigmentation. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 448–465. [Google Scholar] [CrossRef]
- Ciçek, Y.; Ertaş, U. The normal and pathological pigmentation of oral mucous membrane: A review. J. Contemp. Dent. Pract. 2003, 4, 76–86. [Google Scholar]
- McLaughlin, C.C.; Wu, X.C.; Jemal, A.; Martin, H.J.; Roche, L.M.; Chen, V.W. Incidence of noncutaneous melanomas in the U.S. Cancer 2005, 103, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Hashemi-Pour, M.S. Malignant melanoma of the oral cavity: A review of literature. Indian J. Dent. Res. 2008, 19, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Hicks, M.J.; Flaitz, C.M. Oral mucosal melanoma: Epidemiology and pathobiology. Oral Oncol. 2000, 36, 152–169. [Google Scholar] [CrossRef]
- Lourenço, S.V.; Fernandes, J.D.; Hsieh, R.; Coutinho-Camillo, C.M.; Bologna, S.; Sangueza, M.; Nico, M.M. Head and neck mucosal melanoma: A review. Am. J. Dermatopathol. 2014, 36, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E.; Gilde, K.; Remenár, E.; Esik, O. Malignant mucosal melanoma of the head and neck. Pathol. Oncol. Res. 2003, 9, 7–12. [Google Scholar] [CrossRef]
- Smith, M.H.; Bhattacharyya, I.; Cohen, D.M.; Islam, N.M.; Fitzpatrick, S.G.; Montague, L.J.; Damm, D.D.; Fowler, C.B. Melanoma of the Oral Cavity: An Analysis of 46 New Cases with Emphasis on Clinical and Histopathologic Characteristics. Head Neck Pathol. 2016, 10, 298–305. [Google Scholar] [CrossRef]
- Femiano, F.; Lanza, A.; Buonaiuto, C.; Gombos, F.; Di Spirito, F.; Cirillo, N. Oral malignant melanoma: A review of the literature. J. Oral Pathol. Med. 2008, 37, 383–388. [Google Scholar] [CrossRef]
- Yde, S.S.; Sjoegren, P.; Heje, M.; Stolle, L.B. Mucosal Melanoma: A Literature Review. Curr. Oncol. Rep. 2018, 20, 28. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Vlajkovic, S.; Jovanovic, P.; Stefanovic, V. Primary mucosal melanomas: A comprehensive review. Int. J. Clin. Exp. Pathol. 2012, 5, 739–753. [Google Scholar]
- Bansal, S.P.; Dhanawade, S.S.; Arvandekar, A.S.; Mehta, V.; Desai, R.S. Oral Amelanotic Melanoma: A Systematic Review of Case Reports and Case Series. Head Neck Pathol. 2022, 16, 513–524. [Google Scholar] [CrossRef]
- Williams, M.D. Update from the 4th edition of the World Health Organization Classification of head and neck tumours: Mucosal melanomas. Head Neck Pathol. 2017, 11, 110–117. [Google Scholar] [CrossRef] [PubMed]
- de Paulo, L.F.B.; Servato, J.P.S.; Rosa, R.R.; Oliveira, M.T.F.; de Faria, P.R.; da Silva, S.J.; Cardoso, S.V.; Loyola, A.M. Primary amelanotic mucosal melanoma of the oronasal region: Report of two new cases and literature review. Oral Maxillofac. Surg. 2015, 19, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Umeda, M.; Komatsubara, H.; Shibuya, Y.; Yokoo, S.; Komori, T. Premalignant melanocytic dysplasia and malignant melanoma of the oral mucosa. Oral Oncol. 2002, 38, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.M.; Epstein, J.B.; Morton, T.H., Jr. Intraoral melanoma: Long-term follow-up and implication for dental clinicians. A case report and literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2003, 96, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.P.; Hampel, A.; Gorlin, R.J. Primary malignant melanoma of the oral cavity: A review of 105 cases. Cancer 1958, 11, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Chatzistefanou, I.; Kolokythas, A.; Vahtsevanos, K.; Antoniades, K. Primary mucosal melanoma of the oral cavity: Current therapy and future directions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Barker, B.F.; Carpenter, W.M.; Daniels, T.E.; Kahn, M.A.; Leider, A.S.; Lozada-Nur, F.; Lynch, D.P.; Melrose, R.; Merrell, P.; Morton, T.; et al. Oral mucosal melanomas: The WESTOP Banff workshop proceedings. Western Society of Teachers of Oral Pathology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1997, 83, 672–679. [Google Scholar] [CrossRef]
- Mohan, M.; Sukhadia, V.Y.; Pai, D.; Bhat, S. Oral malignant melanoma: Systematic review of literature and report of two cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e247–e254. [Google Scholar] [CrossRef]
- Meleti, M.; Leemans, C.R.; Mooi, W.J.; Vescovi, P.; van der Waal, I. Oral malignant melanoma: A review of the literature. Oral Oncol. 2007, 43, 116–121. [Google Scholar] [CrossRef]
- Prasad, M.L.; Patel, S.G.; Huvos, A.G.; Shah, J.P.; Busam, K.J. Primary mucosal melanoma of the head and neck: A proposal for microstaging localized, Stage I (lymph node-negative) tumors. Cancer 2004, 100, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- McLean, N.; Tighiouart, M.; Muller, S. Primary mucosal melanoma of the head and neck. Comparison of clinical presentation and histopathologic features of oral and sinonasal melanoma. Oral Oncol. 2008, 44, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Prera, J.C. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: The Neck and Lymph Nodes, Metastasis, and Melanocytic Tumors. Head Neck Pathol. 2022, 16, 110–122. [Google Scholar] [CrossRef]
- Bunnell, A.M.; Nedrud, S.M.; Fernandes, R.P. Classification and Staging of Melanoma in the Head and Neck. Oral Maxillofac. Surg. Clin. N. Am. 2022, 34, 221–234. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Bondi, S.; Vinciguerra, A.; Lissoni, A.; Rizzo, N.; Barbieri, D.; Indelicato, P.; Abati, S. Mucosal Melanoma of the Hard Palate: Surgical Treatment and Reconstruction. Int. J. Environ. Res. Public Health 2021, 18, 3341. [Google Scholar] [CrossRef]
- Lim, G.F.; Cusack, C.A.; Kist, J.M. Perioral lesions and dermatoses. Dent. Clin. N. Am. 2014, 58, 401–435. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Accorona, R.; Botti, G.; Farina, D.; Fossati, P.; Gatta, G.; Gogas, H.; Lombardi, D.; Maroldi, R.; Nicolai, P.; et al. Mucosal melanoma of the head and neck. Crit. Rev. Oncol. Hematol. 2017, 112, 136–152. [Google Scholar] [CrossRef]
- Soares, C.D.; Carlos, R.; Andrade, B.A.B.D.; Cunha, J.L.S.; Agostini, M.; Romañach, M.J.; Hernandez-Guerrero, J.C.; Mosqueda-Taylor, A.; de Almeida, O.P.; Jorge, J. Oral amelanotic melanomas: Clinicopathologic features of 8 cases and review of the literature. Int. J. Surg. Pathol. 2021, 29, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N.; Munhoz, R.R.; Kuk, D.; Ott, P.A.; Johnson, D.B.; Tsai, K.K.; Rapisuwon, S.; Eroglu, Z.; Sullivan, R.J.; Luke, J.J.; et al. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer 2016, 122, 3354–3362. [Google Scholar] [CrossRef]
- Hedin, C.A. Smokers’ melanosis. Occurrence and localization in the attached gingiva. Arch. Dermatol. 1977, 113, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Motamayel, F.; Falsafi, P.; Hayati, Z.; Rezaei, F.; Poorolajal, J. Prevalence of oral mucosal lesions in male smokers and nonsmokers. Chonnam Med. J. 2013, 49, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Sarswathi, T.R.; Kumar, S.N.; Kavitha, K.M. Oral melanin pigmentation in smoked and smokeless tobacco users in India. Clinico-pathological study. Indian J. Dent. Res. 2003, 14, 101–106. [Google Scholar] [PubMed]
- Hedin, C.A.; Pindborg, J.J.; Axéll, T. Disappearance of smoker’s melanosis after reducing smoking. J. Oral Pathol. Med. 1993, 22, 228–230. [Google Scholar] [CrossRef]
- Lambertini, M.; Patrizi, A.; Fanti, P.A.; Melotti, B.; Caliceti, U.; Magnoni, C.; Misciali, C.; Baraldi, C.; Ravaioli, G.M.; Dika, E. Oral melanoma and other pigmentations: When to biopsy? J. Eur. Acad. Dermatol. Venereol. 2018, 32, 209–214. [Google Scholar] [CrossRef]
- Giovannucci, E. An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol. Biomark. 2001, 10, 725–731. [Google Scholar]
- Cantudo-Sanagustín, E.; Gutiérrez-Corrales, A.; Vigo-Martínez, M.; Serrera-Figallo, M.Á.; Torres-Lagares, D.; Gutiérrez-Pérez, J.L. Pathogenesis and clinicohistopathological caractheristics of melanoacanthoma: A systematic review. J. Clin. Exp. Dent. 2016, 8, e327–e336. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, V.; Ranganathan, K. Oral melanoacanthoma: A case report and review of the literature. J. Med. Case Rep. 2009, 3, 11. [Google Scholar] [CrossRef]
- Goode, R.K.; Crawford, B.E.; Callihan, M.D.; Neville, B.W. Oral melanoacanthoma. Review of the literature and report of ten cases Oral Surg. Oral Med. Oral Pathol. 1983, 56, 622–628. [Google Scholar] [CrossRef]
- Sreeja, C.; Ramakrishnan, K.; Vijayalakshmi, D.; Devi, M.; Aesha, I.; Vijayabanu, B. Oral pigmentation: A review. J. Pharm. Bioallied Sci. 2015, 7 (Suppl. 2), S403–S408. [Google Scholar] [CrossRef] [PubMed]
- Rudd, M.; Eversole, R.; Carpenter, W. Diascopy: A clinical technique for the diagnosis of vascular lesions. Gen. Dent. 2001, 49, 206–209. [Google Scholar] [PubMed]
- Al Wayli, H.; Rastogi, S.; Verma, N. Hereditary hemochromatosis of tongue. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 111, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Anne Marie, U.; Murererehe, J.; Rehman, M.; Chittilla, M.; Uwambaye, P.; Razzaque, M.S. Oral manifestations of iron imbalance. Front. Nutr. 2023, 10, 1272902. [Google Scholar] [CrossRef]
- Wang, Y.P.; Chang, J.Y.; Wu, Y.C.; Cheng, S.J.; Chen, H.M.; Sun, A. Oral manifestations and blood profile in patients with thalassemia trait. J. Formos. Med. Assoc. 2013, 112, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Nabi, A.T.; Muttu, J.; Chhaparwal, A.; Mukhopadhyay, A.; Pattnaik, S.J.; Choudhary, P. Implications of β-thalassemia on oral health status in patients: A cross-sectional study. J. Family Med. Prim. Care 2022, 11, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Lazos, J.P.; Piemonte, E.D.; Panico, R.L. Oral varix: A review. Gerodontology 2015, 32, 82–89. [Google Scholar] [CrossRef]
- Zahid, E.; Bhatti, O.; Zahid, M.A.; Stubbs, M. Overview of common oral lesions. Malays. Fam. Physician 2022, 17, 9–21. [Google Scholar] [CrossRef]
- Vera-Sirera, B.; Risueño-Mata, P.; Ricart-Vayá, J.M.; de la Hermosa, C.B.R.; Vera-Sempere, F. Clinicopathological and immunohistochemical study of oral amalgam pigmentation. Acta Otorrinolaringol. 2012, 63, 376–381. [Google Scholar] [CrossRef]
- Buchner, A. Amalgam tattoo (amalgam pigmentation) of the oral mucosa: Clinical manifestations, diagnosis and treatment. Refuat Hapeh Vehashinayim 2004, 21, 25–28, 92. [Google Scholar]
- Buchner, A.; Hansen, L.S. Amalgam pigmentation (amalgam tattoo) of the oral mucosa. A clinicopathologic study of 268 cases. Oral Surg. Oral Med. Oral Pathol. 1980, 49, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Owens, B.M.; Johnson, W.W.; Schuman, N.J. Oral amalgam pigmentations (tattoos): A retrospective study. Quintessence Int. 1992, 23, 805–810. [Google Scholar]
- Dereure, O. Drug-induced skin pigmentation. Epidemiology, diagnosis and treatment. Am. J. Clin. Dermatol. 2001, 2, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Moraes, P.C.; Noce, C.W.; Thomaz, L.A.; Cintra, M.L.; Correa, M.E. Pigmented lichenoid drug eruption secondary to chloroquine therapy: An unusual presentation in lower lip. Minerva Stomatol. 2011, 60, 327–332. [Google Scholar]
- Yuan, A.; Woo, S.B. Adverse Drug Events in the Oral Cavity. Dermatol. Clin. 2020, 38, 523–533. [Google Scholar] [CrossRef]
- McGarrity, T.J.; Amos, C.I.; Baker, M.J. Peutz-Jeghers Syndrome GeneReviews®; University of Washington: Seattle, WA, USA, 2001. [Google Scholar]
- Klimkowski, S.; Ibrahim, M.; Ibarra Rovira, J.J.; Elshikh, M.; Javadi, S.; Klekers, A.R.; Abusaif, A.A.; Moawad, A.W.; Ali, K.; Elsayes, K.M. Peutz–Jeghers Syndrome and the Role of Imaging: Pathophysiology, Diagnosis, and Associated Cancers. Cancers 2021, 13, 5121. [Google Scholar] [CrossRef]
- Yamamoto, H.; Sakamoto, H.; Kumagai, H.; Abe, T. Clinical Guidelines for Diagnosis and Management of Peutz-Jeghers Syndrome in Children and Adults. Digestion 2023, 104, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, E.F.; Stănescu, L.; Simionescu, C.; Georgescu, I.; Ionescu, R.; Florescu, G. Peutz-Jeghers syndrome: Case report and literature review. Rom. J. Morphol. Embryol. 2008, 49, 241–245. [Google Scholar]
- Kim, H.W. Generalized oral and cutaneous hyperpigmentation in Addison’s disease. Odontostomatol. Trop. 1988, 11, 87–90. [Google Scholar]
- Bugălă, N.M.; Carsote, M.; Stoica, L.E.; Albulescu, D.M.; Ţuculină, M.J.; Preda, S.A.; Boicea, A.-R.; Alexandru, D.O. New Approach to Addison Disease: Oral Manifestations Due to Endocrine Dysfunction and Comorbidity Burden. Diagnostics 2022, 12, 2080. [Google Scholar] [CrossRef]
- Alrashdan, M.S.; Cirillo, N.; Cullough, M. Oral lichen planus: A literature review and update. Arch. Dermatol. Res. 2016, 308, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Coondoo, A. Lichen Planus Pigmentosus: The Controversial Consensus. Indian J. Dermatol. 2016, 61, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Kartland Vieira, D.L.P.; De Lima, M.H.A.; De Oliveira Lima, A.L.; Peixoto, F.B.; Beder Ribeiro, C.M.; De Melo Franco, A.V.; Ferreira Soares, S.M. Pigmented Oral Lichen Planus: Report of Three Cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, e185. [Google Scholar] [CrossRef]
- Chiang, C.P.; Yu-Fong Chang, J.; Wang, Y.P.; Wu, Y.H.; Lu, S.Y.; Sun, A. Oral lichen planus—Differential diagnoses, serum autoantibodies, hematinic deficiencies, and management. J. Formos. Med. Assoc. 2018, 117, 756–765. [Google Scholar] [CrossRef]
- Kusari, A.; Ahluwalia, J. Lichen Planus. N. Engl. J. Med. 2018, 379, 567. [Google Scholar] [CrossRef]
- Rieder, E.; Kaplan, J.; Kamino, H.; Sanchez, M.; Pomeranz, M.K. Lichen planus pigmentosus. Dermatol. Online J. 2013, 19, 20713. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).