Release of Exosomal PD-L1 in Bone and Soft Tissue Sarcomas and Its Relationship to Radiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Exosome Isolation
2.3. Measurement of Interferon Gamma (IFN-γ) Production
2.4. Flow Cytometry Analysis
2.5. Immunofluorescence Staining
2.6. Western Blot Analysis
2.7. PD-L1 Knockout HT1080 Cells/CRISPR-Cas9
2.8. Experiments with Mice
2.9. Statistical Analysis
3. Results
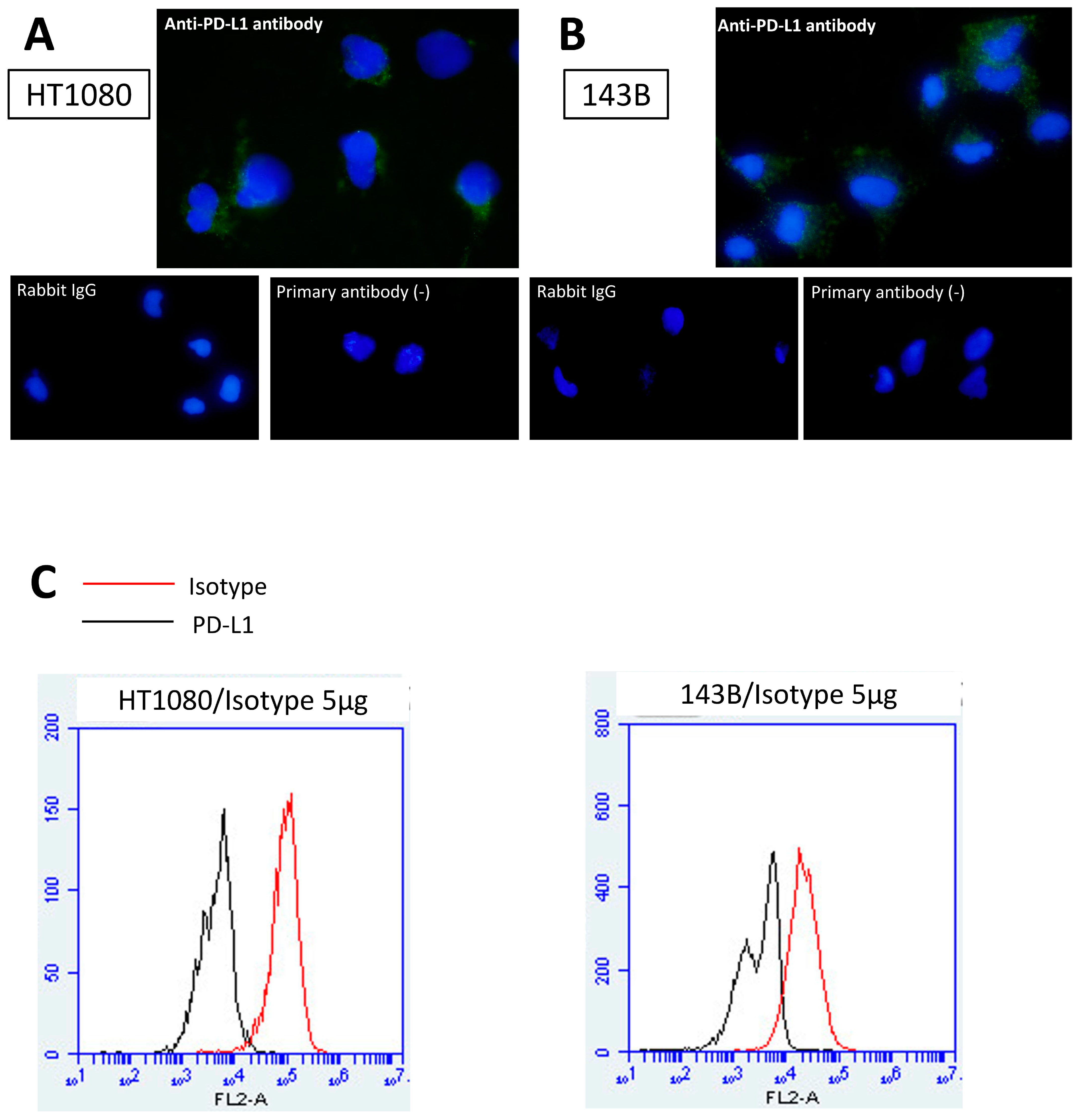
3.1. PD-L1 Expression in Bone and Soft Tissue Sarcoma Cell Lines
3.2. Exosomal PD-L1 Expression in Culture Medium and Serum
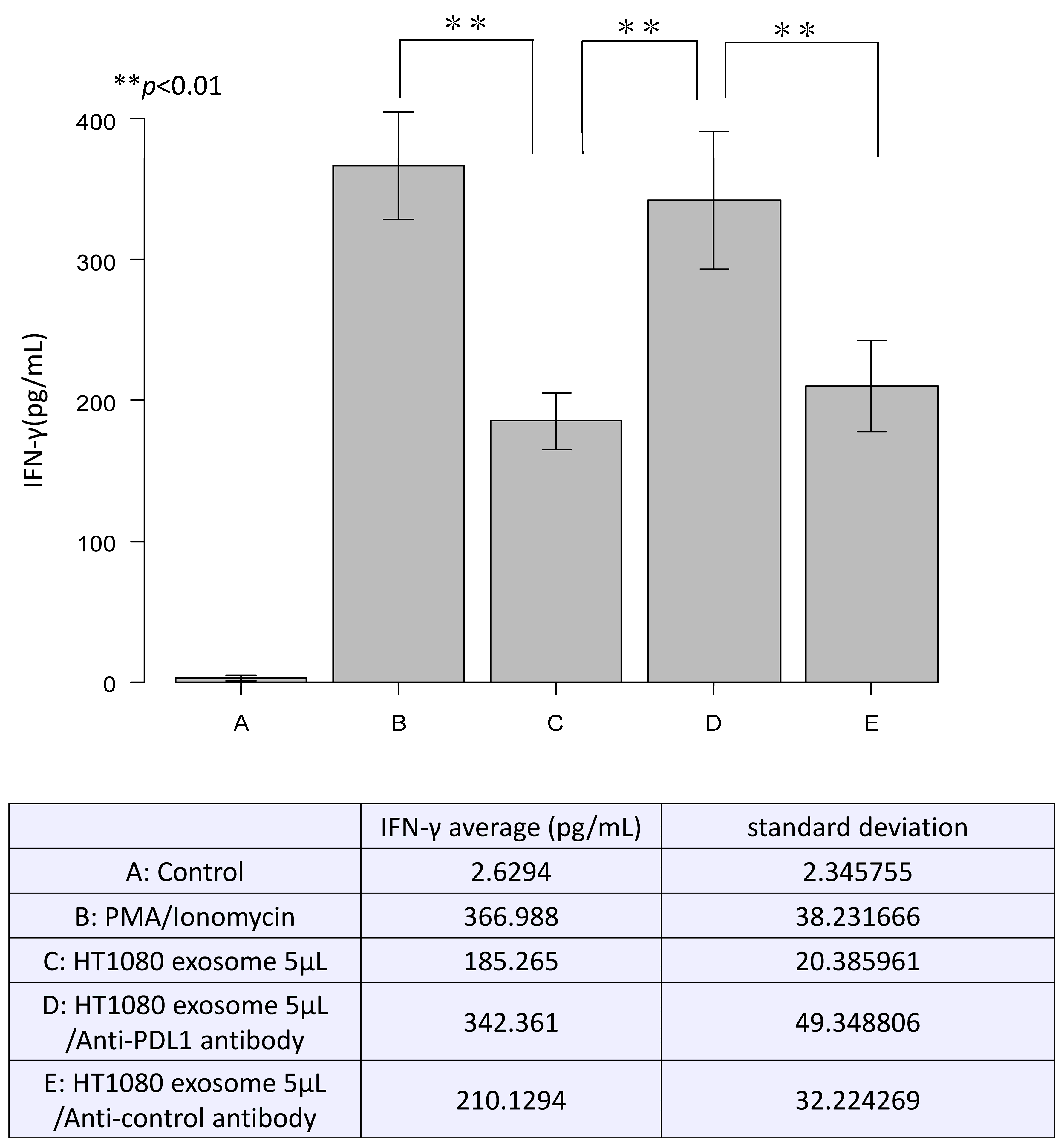
3.3. Inhibitory Effect of Exosomal PD-L1 on T Cell Activation In Vitro
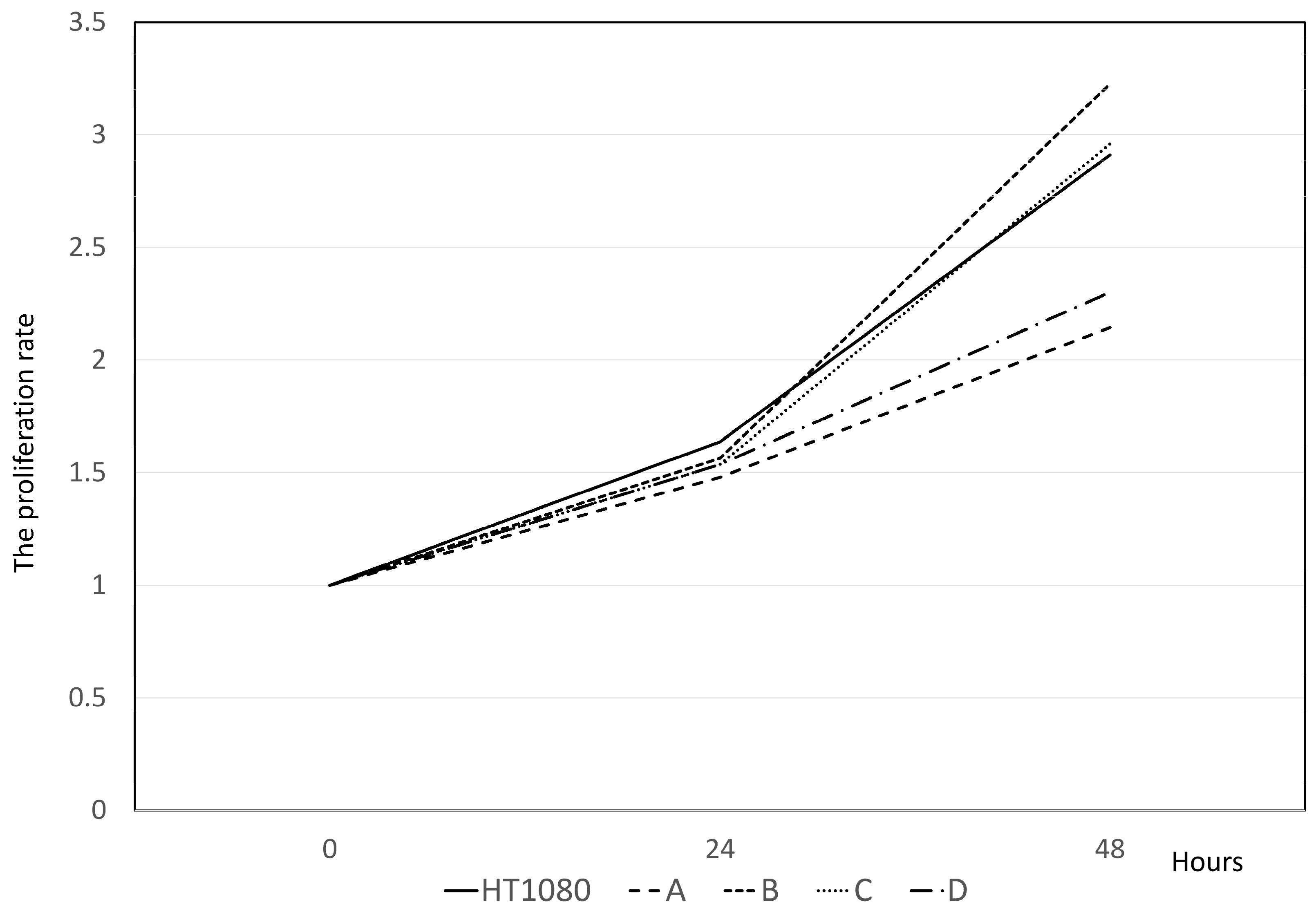
3.4. PD-L1 Knockout (KO) HT1080 Cells/CRISPR-Cas9
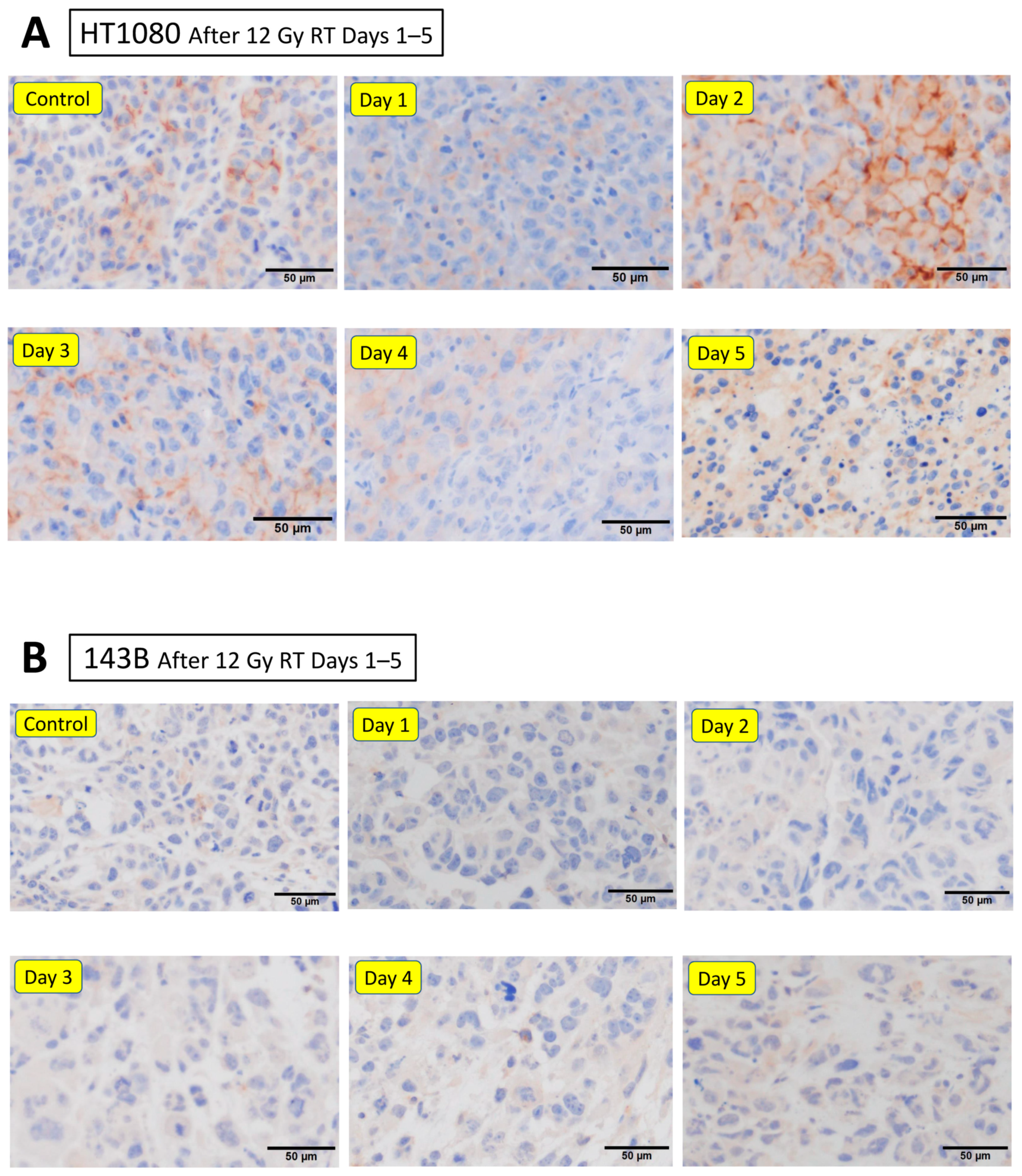
3.5. Effect of Irradiation on Exosomal PD-L1 Protein Levels In Vitro
3.6. Effect of Irradiation on Exosomal PD-L1 Protein Levels In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a nobel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. Immunology beats cancer: A blueprint for successful translation. Nat. Immunol. 2012, 13, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Malas, S.; Harrasser, M.; Lacy, K.E.; Karagiannis, S.N. Antibody therapies for melanoma new and emerging opportunities to active immunity. Oncol. Rep. 2014, 32, 875–876. [Google Scholar] [CrossRef] [PubMed]
- Burgress, M.A.; Crowley, J.; Reinke, D.K.; Riedel, R.F.; George, S.; Movva, S.; Van Tine, B.A.; Davis, L.E.; Schuetze, S.; Hu, J.; et al. SARC 028: A phase II study of the anti-PD1 antibody pembrolizumab (P) in patients (Pts) with advanced sarcomas. J. Clin. Oncol. 2015, 33 (Suppl. 15), TPS10578. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Shoushtari, N.A.; Agaram, N.P.; Kuk, D.; Qin, L.; Carvajal, R.D.; Dickson, A.M.; Gounder, M.; Keohan, M.L.; Schwartz, G.K.; et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum. Pathol. 2015, 46, 357–365. [Google Scholar] [CrossRef]
- Torabi, A.; Amaya, C.N.; Wians, F.H.; Bryan, B.A. PD-1 and PD-L1 expression in bone and soft tissue sarcomas. Pathology 2017, 49, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Xiao, W.; Guan, X.Y.; Liang, Y.; Yan, S.M.; Chen, H.Y.; Li, Q.Q.; Xu, B.S.; Zhou, Z.W.; Zhang, X. PD-L1 Expression Is Associated with FOXP3+ Regulatory T-Cell Infiltration of Soft Tissue Sarcoma and Poor Patient Prognosis. J. Cancer 2017, 8, 2018–2025. [Google Scholar] [CrossRef]
- Kim, J.R.; Moon, J.Y.; Kwon, S.K.; Bae, S.J.; Wagle, S.; Kim, K.M.; Park, S.H.; Lee, H.; Moon, S.W.; Chung, M.J.; et al. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS ONE 2013, 8, e82870. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgress, M.; Bolejack, V.; Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancel Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Poggio, M.; Hu, T.; Pai, C.C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, E.U.; Fu, Q.; Fong, L.; et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell 2019, 177, 414–427.e13. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Shibaki, R.; Matsumoto, Y.; Yoshida, T.; Goto, Y.; Kanda, S.; Horinouchi, H.; Fujiwara, Y.; Yamamoto, N.; Ohe, Y. Association Between Serum Level Soluble Programmed Cell Death Ligand 1 and Prognosis in Patients with Non-Small Cell Lung Cancer Treated with Anti-PD-1 Antibody. Thorac. Cancer 2020, 11, 3585–3595. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, T.; Toyozumi, T.; Sakata, H.; Murakami, K.; Kano, M.; Matsumoto, Y.; Yokoyama, M.; Okada, K.; Kamata, T.; Ryuzaki, T.; et al. Soluble PD-L1 Concentration Is Proportional to the Expression of PD-L1 in Tissue and is Associated with a Poor Prognosis in Esophageal Squamous Cell Carcinoma. Oncology 2022, 100, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, A.; Kwiatkowska, A.; Suszczyk, D.; Chudzik, A.; Tarkowski, R.; Barczynski, B.; Kotarski, J.; Wertel, I. Clinical and Prognostic Value of Antigen-Presenting Cells with PD-L1/PD-L2 Expression in Ovarian Cancer Patients. Int. J. Mol. Sci. 2021, 22, 11563. [Google Scholar] [CrossRef]
- Wu, C.T.; Chen, W.C.; Chang, Y.H.; Lin, Y.W.; Chen, M.F. The role of PD-L1 in the radiation response and clinical outcome for bladder cancer. Sci. Rep. 2016, 6, 19740. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, K.; Nakamura, T.; Hayashi, A.; Okamoto, T.; Iino, T.; Asanuma, Y.; Hagi, T.; Kita, K.; Nakamura, K.; Sudo, A. Soluble programmed death-ligand 1 rather than PD-L1 on tumor cells effectively predicts metastasis and prognosis in soft tissue sarcomas. Sci. Rep. 2020, 10, 9077. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzas, E.I.; Bemis, L.T.; Bora, A.; Lasser, C.; Lotvall, J.; Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Daassi, D.; Mahoney, K.M.; Freeman, G.J. The Importance of Exosomal PD-L1 in Tumour Immune Evasion. Nat. Rev. Immunol. 2020, 20, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Ao, X.; Yang, Y.; Chen, Z.; Xu, X. Soluble Immune Checkpoints in Cancer: Production, Function and Biological Significance. J. Immunother Cancer 2018, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Jing, C.Q.; Li, X.R.; Tan, Z.F.; Li, H.J. Prognostic Significance of Serum PD-L1 Level in Patients with Locally Advanced or Metastatic Esophageal Squamous Cell Carcinoma Treated with Combination Cytotoxic Chemotherapy. Cancer Manag. Res. 2021, 13, 4935–4946. [Google Scholar] [CrossRef] [PubMed]
- Larrinaga, G.; Solano-Iturri, J.D.; Errarte, P.; Unda, M.; Iriarte, A.L.; Fernandez, A.P.; Echevarria, E.; Asumendi, A.; Manini, C.; Angulo, J.C.; et al. Soluble PD-L1 is an Independent Prognostic Factor in Clear Cell Renal Cell Carcinoma. Cancers 2021, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Vikerfors, A.; Davidsson, S.; Frey, J.; Jerlstrom, T.; Carlsson, J. Soluble PD-L1 in Serum and Urine in Urinary Bladder Cancer Patients. Cancers 2021, 13, 5841. [Google Scholar] [CrossRef] [PubMed]
- Mocan, T.; Ilies, M.; Nenu, I.; Craciun, R.; Horhat, A.; Susa, R.; Minciuna, J.; Rusu, I.; Mocan, L.P.; Seicean, A.; et al. Serum Levels of Soluble Programmed Death-Ligand 1 (sPD-L1): A Possible Biomarker in Predicting Post-Treatment Outcomes in Patients with Early Hepatocellular Carcinoma. Int. Immunopharmacol. 2021, 94, 107467. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Mahoney, K.M.; Giobbie-Hurder, A.; Zhao, F.; Lee, S.; Liao, X.; Rodig, S.; Li, J.; Wu, X.; Butterfield, L.H.; et al. Soluble PD-L1 as a Biomarker in Malignant Melanoma Treated with Checkpoint Blockade. Cancer Immunol. Res. 2017, 5, 480–492. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, C.; Wang, Y.; Dai, L. Soluble PD-L1 as a Predictive Biomarker in Lung Cancer: A Systematic Review and Meta-Analysis. Future Oncol. 2022, 18, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, D. Exosomes in Cancer Development, Metastasis, and Immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 455–468. [Google Scholar] [CrossRef]
- Lyu, T.; Wang, Y.; Li, D.; Yang, H.; Qin, B.; Zhang, W.; Li, Z.; Cheng, C.; Zhang, B.; Guo, R.; et al. Exosomes From BM-MSCs Promote Acute Myeloid Leukemia Cell Proliferation, Invasion and Chemoresistance via Upregulation of S100A4. Exp. Hematol. Oncol. 2021, 10, 24. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.; Choi, Y.J.; Kim, Y.S.; Lee, E.J.; Sung, K.J.; Sun, H.Y.; Pack, C.G.; Jung, M.K.; Han, B.; et al. Exosomal PD-L1 Promotes Tumor Growth through Immune Escape in non-Small Cell Lung Cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, C.; Zhi, C.; Liang, W.; Wang, X.; Chen, X.; Lv, T.; Shen, Q.; Song, Y.; Lin, D.; et al. Clinical Significance of PD-L1 Expression in Serum-Derived Exosomes in NSCLC Patients. J. Transl. Med. 2019, 17, 355. [Google Scholar] [CrossRef] [PubMed]
- Lux, A.; Kahlert, C.; Grützmann, R.; Pilarsky, C. C-Met and PD-L1 on Circulating Exosomes as Diagnostic and Prognostic Markers for Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 3305. [Google Scholar] [CrossRef]
- Golden, E.B.; Frances, D.; Pellicciotta, I.; Demaria, S.; Barcellos-Hoff, M.H.; Formenti, S.C. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014, 3, 28518. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-dependent cytosolic DNA Sensing promotes radiation-induced Type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Victor, C.T.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and Dual Checkpoint Blockade Activate Non-Redundant Immune Mechanisms in Cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.; Sato, H.; Multhoff, G.; Shibata, A. Novel approaches to improve the efficacy of immuno-radiotherapy. Front. Oncol. 2019, 9, 156. [Google Scholar] [CrossRef]
- Kosinsky, Y.; Dovedi, S.J.; Peskov, K.; Voronova, V.; Chu, L.; Tomkinson, H.; Al-Huniti, N.; Stanski, D.R.; Helmlinger, G. Radiation and PD-(L)1 treatment combinations: Immune response and dose optimization via a predictive systems model. J. Immunother Cancer 2018, 6, 17. [Google Scholar] [CrossRef]
- Alinezhad, M.; Bakhshandeh, M.; Rostami, E.; Alimohamadi, R.; Mosaffa, N.; Jalali, S.A. Synergistic effects of anti-PDL-1 with ablative radiation comparing to other regimens with same biological effect dose based on different immunogenic response. PLoS ONE 2020, 15, e0231507. [Google Scholar] [CrossRef]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y.; et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef]
- Sato, H.; Niimi, A.; Yasuhara, T.; Permata, B.T.; Hagiwara, Y.; Isono, M.; Nuryadi, E.; Sekine, R.; Oike, T.; Kakoti, S.; et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 2017, 8, 1751. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Dong, H.D.; Liu, X.; Harrington, S.M.; Krco, C.J.; Grams, M.P.; Mansfield, A.S.; Furutani, K.M.; Olivier, K.R.; Kwon, E.D. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol. Res. 2015, 3, 610–619. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; Wit, M.; et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Asanuma, K.; Matsuyama, Y.; Okamoto, T.; Hagi, T.; Nakamura, T.; Sudo, A. Release of Exosomal PD-L1 in Bone and Soft Tissue Sarcomas and Its Relationship to Radiotherapy. Cancers 2024, 16, 2489. https://doi.org/10.3390/cancers16132489
Yoshida K, Asanuma K, Matsuyama Y, Okamoto T, Hagi T, Nakamura T, Sudo A. Release of Exosomal PD-L1 in Bone and Soft Tissue Sarcomas and Its Relationship to Radiotherapy. Cancers. 2024; 16(13):2489. https://doi.org/10.3390/cancers16132489
Chicago/Turabian StyleYoshida, Keisuke, Kunihiro Asanuma, Yumi Matsuyama, Takayuki Okamoto, Tomohito Hagi, Tomoki Nakamura, and Akihiro Sudo. 2024. "Release of Exosomal PD-L1 in Bone and Soft Tissue Sarcomas and Its Relationship to Radiotherapy" Cancers 16, no. 13: 2489. https://doi.org/10.3390/cancers16132489
APA StyleYoshida, K., Asanuma, K., Matsuyama, Y., Okamoto, T., Hagi, T., Nakamura, T., & Sudo, A. (2024). Release of Exosomal PD-L1 in Bone and Soft Tissue Sarcomas and Its Relationship to Radiotherapy. Cancers, 16(13), 2489. https://doi.org/10.3390/cancers16132489






