Head and Neck Cancer: A Potential Risk Factor for Parkinson’s Disease?
Abstract
Simple Summary
Abstract
1. Introduction
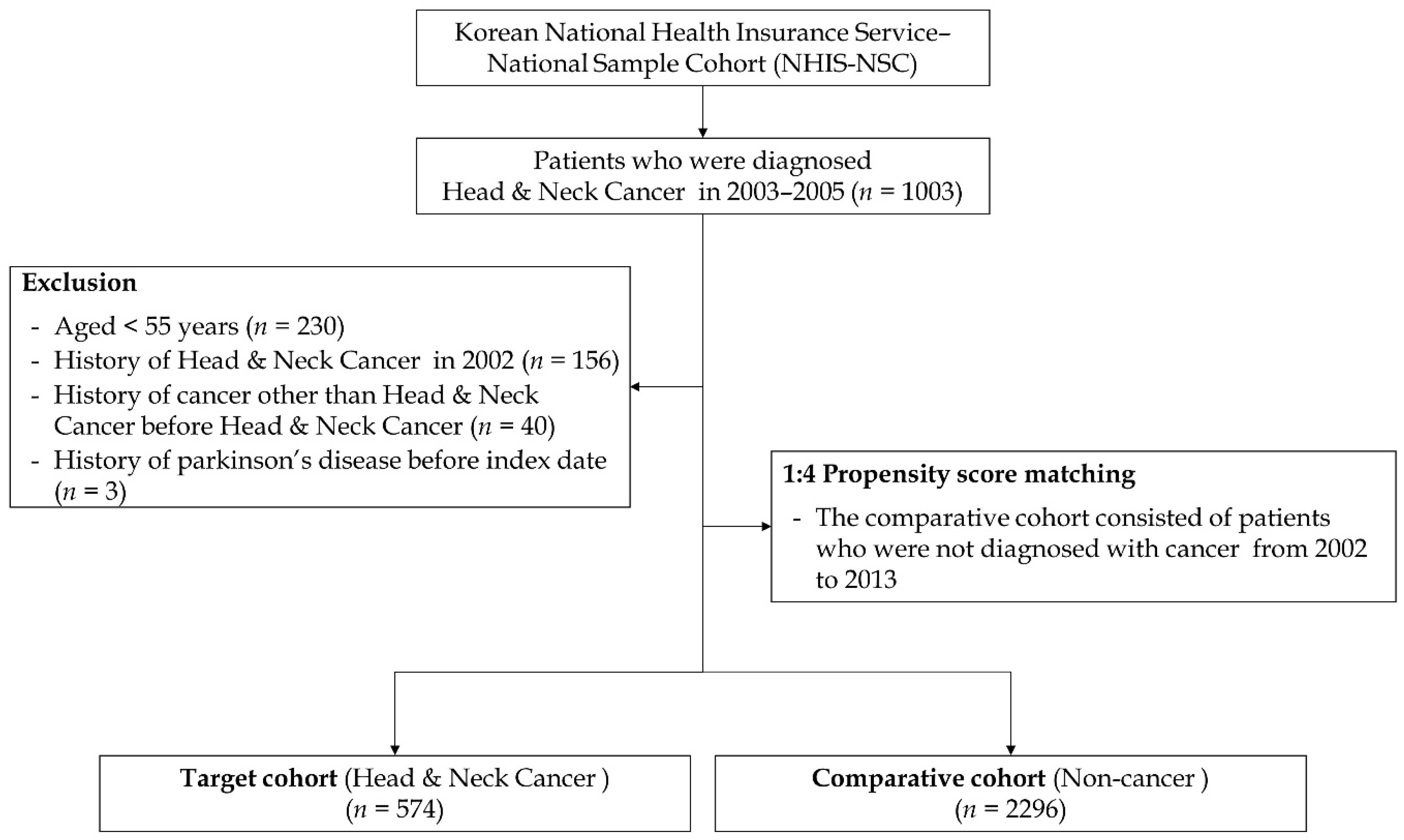
2. Materials and Methods
2.1. Study Design
2.2. Study Population
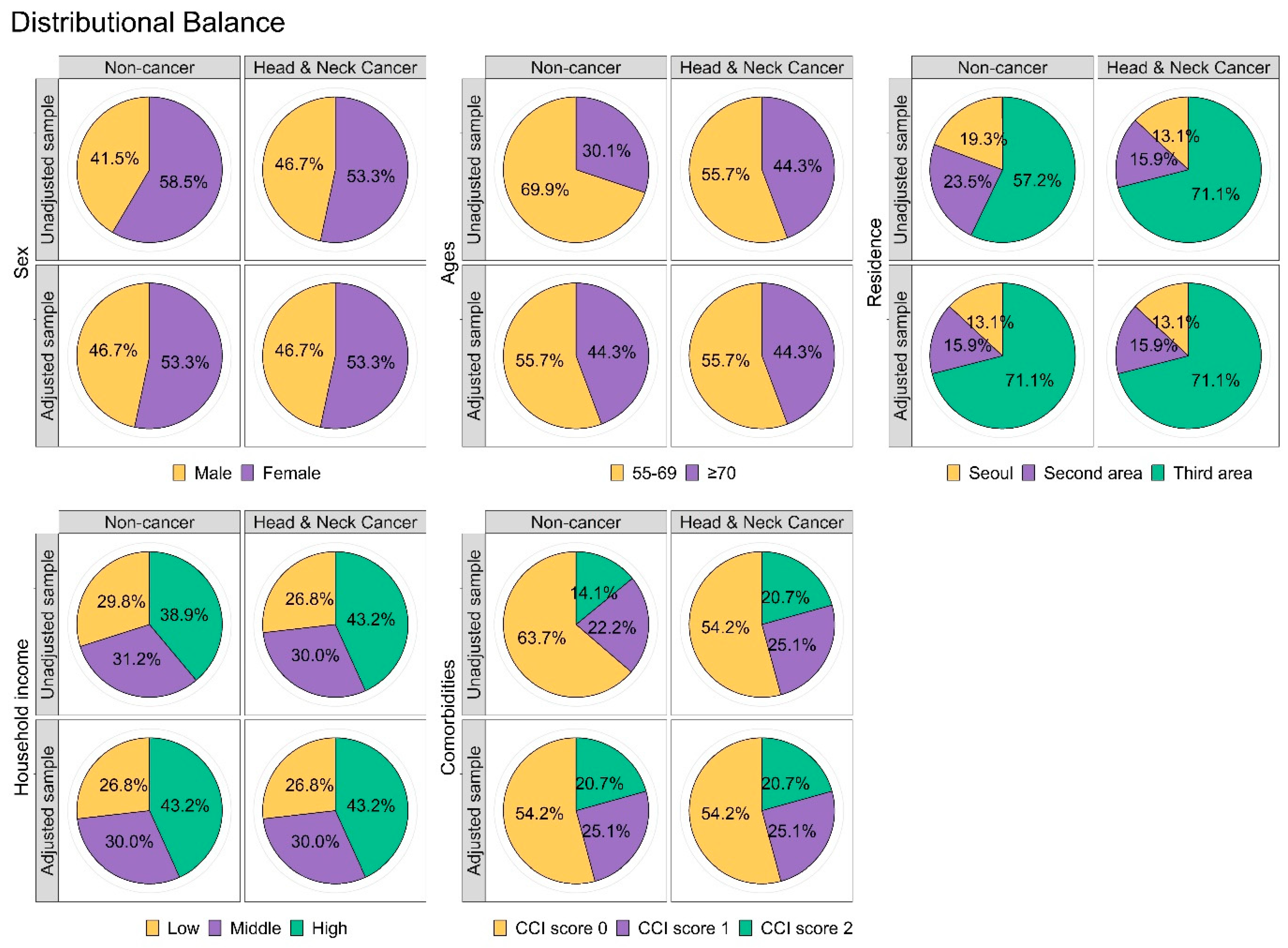
2.3. Statistical Analysis
3. Results
3.1. General Characteristics of the Cohort
3.2. Incidence Rate of PD
3.3. Risk Rate of PD
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simard, E.P.; Torre, L.A.; Jemal, A. International trends in head and neck cancer incidence rates: Differences by country, sex and anatomic site. Oral Oncol. 2014, 50, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Jung, K.W.; Bang, S.H.; Choi, S.H.; Park, E.H.; Yun, E.H.; Kim, H.J.; Kong, H.J.; Im, J.S.; Seo, H.G.; et al. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2020. Cancer Res. Treat. 2023, 55, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.J.; Park, J.H.; Choi, M.; Jung, J.H.; Han, K.; Kwon, D.Y.; Kim, D.H.; Park, Y.G. Parkinson’s disease and skin cancer risk: A nationwide population-based cohort study in Korea. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2775–2780. [Google Scholar] [CrossRef]
- Bajaj, A.; Driver, J.A.; Schernhammer, E.S. Parkinson’s disease and cancer risk: A systematic review and meta-analysis. Cancer Causes Control 2010, 21, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.M.; Wu, J.; Chen, H.; Engels, E.A.; Enewold, L.R.; Freedman, N.D.; Goedert, J.J.; Kuncl, R.W.; Gail, M.H.; Pfeiffer, R.M. Associations between cancer and Parkinson’s disease in U.S. elderly adults. Int. J. Epidemiol. 2016, 45, 741–751. [Google Scholar] [CrossRef]
- Becker, C.; Brobert, G.P.; Johansson, S.; Jick, S.S.; Meier, C.R. Cancer risk in association with Parkinson disease: A population-based study. Park. Relat. Disord. 2010, 16, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guarin, D.; Mohammadzadehhonarvar, N.; Chen, X.; Gao, X. Parkinson’s disease and cancer: A systematic review and meta-analysis of over 17 million participants. BMJ Open 2021, 11, e046329. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.D.; Cai, W.; Chen, X. The associations between Parkinson’s disease and cancer: The plot thickens. Transl. Neurodegener. 2015, 4, 20. [Google Scholar] [CrossRef]
- Cui, X.; Liew, Z.; Hansen, J.; Lee, P.C.; Arah, O.A.; Ritz, B. Cancers preceding Parkinson’s disease after adjustment for bias in a Danish population-based case-control study. Neuroepidemiology 2019, 52, 136–143. [Google Scholar] [CrossRef]
- Boursi, B.; Mamtani, R.; Haynes, K.; Yang, Y.X. Parkinson’s disease and colorectal cancer risk-A nested case control study. Cancer Epidemiol. 2016, 43, 9–14. [Google Scholar] [CrossRef]
- Quinn, P.M.J.; Moreira, P.I.; Ambrósio, A.F.; Alves, C.H. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol. Commun. 2020, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Kitada, T.; Ardah, M.T.; Haque, M.E. History of Parkinson’s disease-associated gene, parkin: Research over a quarter century in quest of finding the physiological substrate. Int. J. Mol. Sci. 2023, 24, 16734. [Google Scholar] [CrossRef]
- Jiang, B.; Ni, H.; Zhou, Z.; Li, Y. Parkin enhances sensitivity of paclitaxel to NPC by arresting cell cycle. Pathol. Res. Pract. 2020, 216, 152755. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Zhou, Z.; Jiang, B.; Yuan, X.; Cao, X.; Huang, G.; Li, Y. Inactivation of parkin by promoter methylation correlated with lymph node metastasis and genomic instability in nasopharyngeal carcinoma. Tumour Biol. 2017, 39, 1010428317695025. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.A.; Khandelwal, A.R.; Wolf, G.T.; Rodrigo, J.P.; Mäkitie, A.A.; Saba, N.F.; Forastiere, A.A.; Bradford, C.R.; Ferlito, A. TP53 mutations in head and neck cancer. Mol. Carcinog. 2022, 61, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Sun, W.; Wang, Y.F.; Li, J.; Li, D.W. Association of p53 with neurodegeneration in Parkinson’s disease. Park. Dis. 2022, 2022, 6600944. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.K.; Anderson, M.D.; Vijayakumar, S.; Vengaloor Thomas, T. Parkinson’s syndrome after cranial radiotherapy: A case report. Cureus 2022, 14, e24411. [Google Scholar] [CrossRef] [PubMed]
- Azizova, T.V.; Bannikova, M.V.; Grigoryeva, E.S.; Rybkina, V.L.; Hamada, N. Occupational exposure to chronic ionizing radiation increases risk of Parkinson’s disease incidence in Russian Mayak workers. Int. J. Epidemiol. 2020, 49, 435–447. [Google Scholar] [CrossRef]
- Robbins, J.H.; Otsuka, F.; Tarone, R.E.; Polinsky, R.J.; Brumback, R.A.; Nee, L.E. Parkinson’s disease and Alzheimer’s disease: Hypersensitivity to X rays in cultured cell lines. J. Neurol. Neurosurg. Psychiatry 1985, 48, 916–923. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Prithivirajsingh, S.; Story, M.D.; Bergh, S.A.; Geara, F.B.; Ang, K.K.; Ismail, S.M.; Stevens, C.W.; Buchholz, T.A.; Brock, W.A. Accumulation of the common mitochondrial DNA deletion induced by ionizing radiation. FEBS Lett. 2004, 571, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Büeler, H. Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson’s disease. Exp. Neurol. 2009, 218, 235–246. [Google Scholar] [CrossRef]
- Götze, H.; Friedrich, M.; Taubenheim, S.; Dietz, A.; Lordick, F.; Mehnert, A. Depression and anxiety in long-term survivors 5 and 10 years after cancer diagnosis. Support. Care Cancer 2020, 28, 211–220. [Google Scholar] [CrossRef]
- Archer, J.; Hutchison, I.; Korszun, A. Mood and malignancy: Head and neck cancer and depression. J. Oral Pathol. Med. 2008, 37, 255–270. [Google Scholar] [CrossRef]
- Shiba, M.; Bower, J.H.; Maraganore, D.M.; McDonnell, S.K.; Peterson, B.J.; Ahlskog, J.E.; Schaid, D.J.; Rocca, W.A. Anxiety disorders and depressive disorders preceding Parkinson’s disease: A case-control study. Mov. Disord. 2000, 15, 669–677. [Google Scholar] [CrossRef]
- Schuurman, A.G.; van den Akker, M.; Ensinck, K.T.; Metsemakers, J.F.; Knottnerus, J.A.; Leentjens, A.F.; Buntinx, F. Increased risk of Parkinson’s disease after depression: A retrospective cohort study. Neurology 2002, 58, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Saint-Hilaire, M.H.; Cupples, L.A.; Thomas, C.A.; Burchard, A.E.; Feldman, R.G.; Myers, R.H. Environmental, medical, and family history risk factors for Parkinson’s disease: A New England-based case control study. Am. J. Med. Genet. 1999, 88, 742–749. [Google Scholar] [CrossRef]
- Leentjens, A.F.G.; Van den Akker, M.; Metsemakers, J.F.M.; Lousberg, R.; Verhey, F.R.J. Higher incidence of depression preceding the onset of Parkinson’s disease: A register study. Mov. Disord. 2003, 18, 414–418. [Google Scholar] [CrossRef]
- Nilsson, F.M.; Kessing, L.V.; Bolwig, T.G. Increased risk of developing Parkinson’s disease for patients with major affective disorder: A register study. Acta Psychiatr. Scand. 2001, 104, 380–386. [Google Scholar] [CrossRef]
- Tran, A.A.; De Smet, M.; Grant, G.D.; Khoo, T.K.; Pountney, D.L. Investigating the convergent mechanisms between major depressive disorder and Parkinson’s disease. Complex Psychiatry 2021, 6, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Palta, P.; Samuel, L.J.; Miller, E.R., 3rd; Szanton, S.L. Depression and oxidative stress: Results from a meta-analysis of observational studies. Psychosom. Med. 2014, 76, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Kotzbauer, P.T.; Uryu, K.; Leight, S.; Trojanowski, J.Q.; Lee, V.M.Y. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J. Neurosci. 2008, 28, 7687–7698. [Google Scholar] [CrossRef] [PubMed]

| Variables | Control (n = 2296) | Head and Neck Cancer (n = 574) | p-Value |
|---|---|---|---|
| Sex | 1.000 | ||
| Male | 1072 (46.7%) | 268 (46.7%) | |
| Female | 1224 (53.3%) | 306 (53.3%) | |
| Ages (years) | 1.000 | ||
| 55–69 | 1280 (55.7%) | 320 (55.7%) | |
| >69 | 1016 (44.3%) | 254 (44.3%) | |
| Residence | 1.000 | ||
| Seoul | 300 (13.1%) | 75 (13.1%) | |
| Second area | 364 (15.9%) | 91 (15.9%) | |
| Third area | 1632 (71.1%) | 408 (71.1%) | |
| Household income | 1.000 | ||
| Low (0–30%) | 616 (26.8%) | 154 (26.8%) | |
| Middle (30–70%) | 688 (30.0%) | 172 (30.0%) | |
| High (70–100%) | 992 (43.2%) | 248 (43.2%) | |
| CCI | 1.000 | ||
| 0 | 1244 (54.2%) | 311 (54.2%) | |
| 1 | 576 (25.1%) | 144 (25.1%) | |
| ≥2 | 476 (20.7%) | 119 (20.7%) |
| Variables | N | Cases | Person-Years | Incidence Rate | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|
| Parkinson’s disease | ||||||
| Comparison | 2296 | 42 | 19,301.1 | 2.18 | 1.00 (ref) | 1.00 (ref) |
| Head and Neck Cancer | 574 | 17 | 4077.7 | 4.17 | 1.90 (1.08–3.34) * | 1.89 (1.08–3.33) * |
| Time (Year) | Number of Events | Adjusted HR (95% CI) | |
|---|---|---|---|
| Comparison | Head and Neck Cancer | ||
| 1 | 2 | 1 | 1.98 (0.18−21.89) |
| 2 | 6 | 3 | 2.03 (0.51−8.13) |
| 3 | 11 | 6 | 2.27 (0.84−6.14) |
| 4 | 17 | 7 | 1.74 (0.72−4.21) |
| 5 | 20 | 8 | 1.71 (0.75−3.89) |
| 6 | 22 | 10 | 1.97 (0.93−4.16) |
| 7 | 25 | 13 | 2.27 (1.16−4.44) * |
| 8 | 34 | 15 | 1.97 (1.07−3.62) * |
| 9 | 40 | 17 | 1.97 (1.11−3.48) * |
| 10 | 41 | 17 | 1.93 (1.10−3.41) * |
| 11 | 42 | 17 | 1.89 (1.08−3.33) * |
| Age | 55−69 | >69 | ||
|---|---|---|---|---|
| Comparison | Head and Neck Cancer | Comparison | Head and Neck Cancer | |
| Parkinson’s disease | ||||
| Unadjusted HR (95% CI) | 1.00 (ref) | 2.70 (1.24−5.84) * | 1.00 (ref) | 1.33 (0.57−3.09) |
| Adjusted HR (95% CI) | 1.00 (ref) | 2.74 (1.26−5.95) * | 1.00 (ref) | 1.33 (0.57−3.10) |
| Variables | N | Cases | Person-Years | Incidence Rate | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|
| Cancer type | ||||||
| Comparison | 2296 | 42 | 19,301.1 | 2.18 | 1.00 (ref) | 1.00 (ref) |
| Oral cavity | 423 | 14 | 3183.3 | 4.40 | 2.00 (1.09−3.66) * | 1.93 (1.05−3.55) * |
| Salivary gland | 8 | 0 | 45.3 | - | N/A | N/A |
| Oropharynx | 21 | 1 | 134.4 | 7.44 | 3.43 (0.47−24.90) | 3.70 (0.51−27.04) |
| Nasopharynx | 23 | 1 | 124.6 | 8.03 | 3.69 (0.51−26.80) | 4.80 (0.65−35.29) |
| Hypopharynx | 8 | 0 | 46.3 | - | N/A | N/A |
| Sinonasal tract | 7 | 0 | 24.1 | - | N/A | N/A |
| Larynx | 84 | 1 | 519.6 | 1.92 | 0.89 (0.12−6.45) | 1.05 (0.14−7.79) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.H.; Kim, D.-K. Head and Neck Cancer: A Potential Risk Factor for Parkinson’s Disease? Cancers 2024, 16, 2486. https://doi.org/10.3390/cancers16132486
Lee IH, Kim D-K. Head and Neck Cancer: A Potential Risk Factor for Parkinson’s Disease? Cancers. 2024; 16(13):2486. https://doi.org/10.3390/cancers16132486
Chicago/Turabian StyleLee, Il Hwan, and Dong-Kyu Kim. 2024. "Head and Neck Cancer: A Potential Risk Factor for Parkinson’s Disease?" Cancers 16, no. 13: 2486. https://doi.org/10.3390/cancers16132486
APA StyleLee, I. H., & Kim, D.-K. (2024). Head and Neck Cancer: A Potential Risk Factor for Parkinson’s Disease? Cancers, 16(13), 2486. https://doi.org/10.3390/cancers16132486






