Abstract
Currently, commercially available pain medications can cause adverse effects. Within this framework, researchers have been exploring new drug candidates derived from animal venoms and toxins. The objective of this study was to investigate the number of molecules with potential for pain relief derived from animal venoms and toxins, which could potentially contribute to the development of new biopharmaceuticals. We conducted a literature search in January 2025, covering the period from 1960 to 2025, in two Latin American and nine international scientific databases. The results consisted of 212 articles selected for review. From these articles, 152 toxins and venoms with analgesic potential were identified and classified into 14 different types of pharmacological targets. The peptides investigated, with masses between 500 Da and 5000 Da, are strong candidates for alternative biopharmaceuticals. Most of the toxins found interact with ion channels, representing an alternative to commercially available drugs.
Key Contribution:
This review provides a comprehensive landscape of 152 venom-derived molecules with analgesic potential, categorizing them into 14 distinct pharmacological targets and highlighting their role as essential blueprints for next-generation non-opioid pain therapies.
1. Introduction
The somatosensory system is part of the physiological process of pain, which is defined as the sensation associated with actual or potential tissue damage. It is always considered a subjective feeling, which encompasses not only physical stimuli but also cognitive and psychological ones, varying from individual to individual and from species to species (Figure 1) [1].
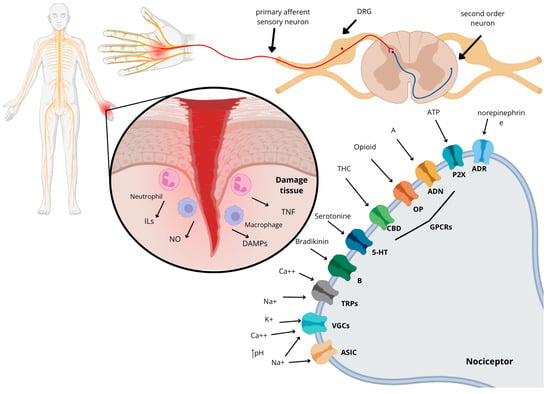
Figure 1.
Mechanism of action of pain from a tissue injury that leads to the release of inflammatory factors such as ILs, nitric oxide (NO), TNF, and DAMPs (damage-associated molecular patterns). This process involves the receptors and ion channels that are associated with the nociceptor of the primary afferent sensory neuron, which respond to the nociceptive stimulus. The receptors mentioned are the same as those found in this review: ASICs (acid-sensitive ion channels), VGCs (voltage-gated channels), TRPs (transient receptor potential), B (bradykinin receptor), 5-HT (serotonin receptor), CBD (cannabinoid receptor), OP (opioid receptor), ADN (adenosine receptor), P2X (purinergic receptor), and ADR (adrenergic receptor).
Pain is one of the most frequently encountered symptoms in medical care, especially when it comes to muscle pain [2]. However, it can also be associated with cancer, metabolic and autoimmune diseases, nutritional disorders, traumatic and post-surgical injuries, age, sex, chemical dependencies, sedentary lifestyle, emotional and psychological disorders, social factors, and demographic variations. These are some of the factors that contribute to the development and exacerbation of the clinical picture of pain [3,4].
There are many different types of pain; one of the main methods of classifying pain is according to its duration. Thus, pain can be divided primarily into acute pain and chronic pain [5]. Acute pain is short-term pain that is triggered after a process of injury or tissue trauma, and its prevalence time is between three and six months [6]. Chronic pain is considered a disease in addition to being a symptom of conditions like cancer, arthritis, or autoimmune diseases; it is defined as long-lasting or intermittent pain that persists for at least 3 months [5,7]. Studies on the prevalence of chronic pain in different countries indicate a variation between 9% and 50%. This range is affected by the social and socioeconomic factors of each population and is more noticeable in developing countries, where the patients are mostly women from low-income families, who have a worsening of this disease compared to people from higher social classes [8,9,10,11].
The most commonly used approach to treat pain includes the administration of analgesic medications [12]. However, the majority of medications marketed for pain treatment come with a series of adverse effects, including dependence, tolerance, and constipation. The latter condition often requires an increased dosage of the drug over time due to reduced efficacy and the return of clinical symptoms [13,14,15,16]. Another growing problem in recent years, which has been occurring in several countries, such as the United States, is the presence of dependence caused by the inappropriate use of opioid drugs, especially fentanyl, resulting from a lack of understanding about the adverse effects. The United States has already experienced several opioid crises in which thousands of people died due to dependence on some type of drug. This has led to the need to develop public and social policies to treat this epidemic [17,18,19,20].
One of the alternatives for the development of new drugs is investment in the study of toxinology with animal venom. Venoms are a complex mixture of several molecules [21]. Researchers have come up with better ways to separate, clean, and study molecules derived from animals. This has made it possible to study how the molecules of venomous animals act in the envenomation process and to determine what pharmaceutical and pharmacological properties they might have [22]. These new technologies have enabled the production of new analgesic biopharmaceuticals, such as Prialt®, which is derived from the cone snail of the species Conus magus and has been the subject of several clinical studies mainly focusing on the treatment of chronic pain [23,24]. Another example is Halneuron®, a biopharmaceutical that is composed of tetrodotoxin, a peptide isolated from puffer fish, which is currently in phase II clinical studies [25,26,27]. In addition, there are many other compounds that are undergoing clinical trials or are already on the market [22].
Therefore, the main objective of this study was to present the antinociceptive molecules found in different animal groups, as well as their different mechanisms of action, and to identify those with the potential to be studied as future biopharmaceuticals.
2. Results
A total of 21,119 articles were found via data collection in databases. After the removal of 4460 duplicates, there were 16,659 articles remaining. During the review of titles, abstracts, and conclusions, it was found that 16,122 articles did not align with the scope of the study and were categorized according to Figure 2 into the following categories: venom characterization, clinical manifestations, envenoming, antimicrobial/parasite, plants, other therapeutic properties, theses, books, congress, and evolution/genetics. Consequently, 537 articles were chosen for full-text reading and included in the qualitative literature study. Following a full-text assessment, 212 articles were selected for the completion of this review. Once the text was read, 152 toxins and venoms with antinociceptive potential were found, separated into 14 different types of ion channel/receptor targets (Figure 2).
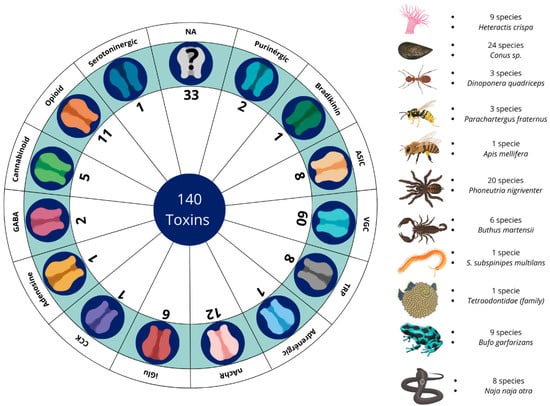
Figure 2.
Summary of the quantities of venoms and toxins with antinociceptive potential across their respective receptors and channels, together with the number of species identified by each animal group and which species were the most prominent in the studies. VGC (voltage-gated channel); TRPs (transient receptor potential); ASIC (acid-sensing ion channel); iGluRs (ionotropic glutamate receptors); nAChRs (nicotinic receptors); GABA (γ-aminobutyric acid); CCK (cholecystokinin); NA (not available).
2.1. Voltage-Gated Channels (VGCs)
Currently, voltage-gated ion channels (VGCs) have been the subject of extensive studies for the purpose of therapeutic innovations aimed at the treatment of chronic and neuropathic pain, tumors, and neurological diseases (Table S1) [28,29,30,31]. These channels are cationic in nature and are subdivided into families, with the sodium ion family having 10 members (1.1–1.9 Nav), potassium channels (1 to 12 Kv members), and calcium ion channels (1.1–1.4, 2.1–2.3, and 3.1–3.3 Cav). Each member of this family is associated with a specific type of nociception and a distinct region of the body with wide distribution in the central and peripheral nervous system. They are present in the membrane of sensory neurons and are activated by changes in the electrical potential near the channels, as well as by the presence of salts in the extracellular medium. This means that the transmission of the sensation of nociception is directly related to the excitability and inhibition of these channels [32,33,34,35,36]. Conus species are widely studied for producing toxins that interact with VGCs; among them, ω-conotoxin MVIIA stands out. Popularly known as ziconotide, this peptide is the main component of the drug Prialt® [23]. It interacts with Cav2.2, leading to its inhibition, which causes an analgesic effect on the body. In models of neuropathic pain caused by nerve injury, chronic pain, postoperative pain, and visceral pain, this molecule showed antinociceptive effects when administered intracerebroventricularly, intranasally, intrathecally, and subcutaneously [37,38,39]. Despite these promising results, scientists have observed that this toxin can cause motor dysfunction.
The transition from parenteral administration to non-invasive routes faces significant challenges, including proteolytic and acid degradation in the gastrointestinal tract, low epithelial permeability due to the hydrophilic nature of peptides, and the restriction imposed by the blood–brain barrier. To mitigate these limitations, the use of nanocarriers, such as liposomes and micelles, has emerged as a promising strategy to increase the metabolic stability and bioavailability of these molecules. Recently, researchers associated this peptide with micelles, and the results demonstrated that the toxin maintained its antinociceptive action without causing adverse effects [39,40]. When compared to opioid drugs, ω-conotoxin MVIIA showed antinociceptive potency about 800 times higher than morphine without inducing pharmacological tolerance. Furthermore, this molecule also potentiates the effect of morphine [41,42].
Other toxins from Conus sp. also exhibit important antinociceptive properties. The toxin Bu8, C. bullatus, inhibits Ba2+ currents in Cav channels, exhibiting an antinociceptive effect twice as high as that of ω-conotoxin MVIIA in models of acute and inflammatory pain induced by acetic acid, presenting low motor dysfunction and toxicity in fish [43]. On the other hand, AM336 (C. catus) showed a dose-dependent antinociceptive effect intrathecally in models of inflammatory pain caused by complete Freund’s adjuvant (CFA) without the manifestation of side effects [44]. The toxin KIIIA derived from C. kinoshitai blocks Nav channels, producing an antinociceptive effect in formalin models when administered intraperitoneally; the tests were performed together with some synthetic analogs, which demonstrated promise for the development of new analgesic drugs [45,46]. The toxin MrVIB, derived from C. marmoreus, inhibits persistent pain behavior by blocking the propagation of action potentials in Nav1.8 channels intrathecally in models of chronic constriction injury. However, in high doses, it can lead to motor problems [47,48,49].
Also, the toxins MoVIA (C. moncuri), RsXXIVA (C. regularis), and SO-3 (C. striatus) block Cav 2.2, thereby reversing pain behavior in neuropathic pain models resulting from nerve damage and inflammatory pain induced by formalin and acetic acid. The toxin TsIIIA from C. tesselatus also blocks Nav 1.8, which has a long-lasting effect on lowering pain and raising the nociceptive threshold [50,51,52,53,54].
In toxin-producing spiders, a total of 20 species that interact with these receptors have been identified, of which Phoneutria nigriventer stands out, presenting the largest quantity of toxins, namely Phα1β, Pntx3-3, and PhTx3-4, which act by inhibiting Cav channels. Phα1β is one of the most extensively studied toxins. In inflammatory pain experiments using the molecule and morphine, the standard drug demonstrated adverse effects such as increased hyperalgesia, while Phα1β reversed these effects [55]. Phα1β also reduced nociception to heat and decreased gastrointestinal transit; experiments with the recombinant molecule presented effects similar to those of the native molecule. It was also demonstrated that the co-administration of Phα1β with morphine potentiated the antinociceptive effect of the drug’s analgesia. However, Phα1β presented motor impairment in mobility tests such as the rotarod [56,57,58,59]. In models of cancer pain induced by melanoma cells in the hind paw of mice, Phα1β caused the reversal of pain hypersensitivity and, compared with ω-conotoxin MVIIA, did not demonstrate adverse effects [60,61,62]. Administration of the Phα1β peptide resulted in the reversal of inflammatory pathology in peripheral glial cells caused by CFA through inhibition of Cav channels, generating an antinociceptive response to inflammatory pain [63].
Pntx3-3, on the other hand, did not reduce inflammatory pain, manifesting action only in models of nociceptive and neuropathic pain (diabetic neuropathy and partial injury to the sciatic nerve), and demonstrated a pain-reducing effect in models of fibromyalgia induced in mice [64,65]. Pntx3-4 may be related to capsaicin-induced glutamate inhibition in a calcium-dependent and independent manner, which may suggest a possible action on NMDA (N-methyl-D-aspartate) receptors. It also did not cause changes in locomotor activity. In tests with formalin, it did not demonstrate an acute nociceptive effect, only showing efficacy in the late phase of the test. The toxin caused an improvement in mechanical hypersensitivity and, in high doses, led to paralysis and weakness [66,67].
Within the genus Cyriopagopus sp., two species are responsible for the production of toxins Ca1a, Ca2a, and Cyriotoxin-1a. These toxins strongly block Nav 1.7 and 1.6, which stops pain responses to formalin and acetic acid and lowers abdominal writhing and thermal nociception [68,69,70]. The toxins Hntx-III and -IV, derived from the species Ornithoctonus hainana, exhibit inhibitory action in Nav 1.7, 1.2, and 1.3, causing a reversal of inflammatory and neuropathic pain; a reduction in abdominal writhing, acute pain, and hyperalgesia; and inhibition of allodynia without motor impairment. However, in high doses, they caused paralysis in mice [71,72,73,74]. Studies involving Agelenopsis aperta have identified a Cav channel-blocking toxin that demonstrated antinociceptive potential in rat models of carrageenan-induced inflammatory knee pain when administered topically [75,76].
The spider, Ceratogyrus darlini, produces a toxin called Cd1a that interacts with Cav2.2 and Nav channels in electrophysiological tests and in models of pain caused by the scorpion toxin OD1 [77]. The species Chilobranchys jingzhao produces the toxin jingzhaotoxin-34, which acts selectively on Nav 1.7 channels and has also demonstrated a more potent and long-lasting effect than morphine in tests of inflammatory pain caused by formalin, especially in the late phase [78]. Phlotoxin-1, derived from Phlogiellus sp., reduces the nociceptive response to formalin in both phase 1 and phase 2, as well as to the scorpion toxin OD1 [79,80]. Protx II/III toxins from Thrixopelma pruriens inhibit Nav1.7 channels, reversing pain induced by OD1 and dorsal root ganglion (DRG) injury [81,82]. Davus fasciatus produces the toxin Drtx-Df, which inhibits Nav1.7 and Cav3 channels, thereby reversing pain behavior induced by OD1 [83].
Grammostola rosea produces the toxins Gptx1 and Gptx1-71 with excellent selectivity for blocking Nav1.7. Both have antihyperalgesic effects on inflammatory pain via intrathecal and intraplantar routes in a dose-dependent manner, manifested by the action of CFA and OD1. The effects were attenuated by opioid antagonists, indicating the possible participation of the toxin in the endogenous opioid system. Furthermore, it also induced spinal analgesia without losing potency over 8 days in models of neuropathic pain and inflammation [84,85,86].
The species Haplopelma lividum secretes the toxin H1a, which acts by inhibiting Nav1.8 channels. It was able to dose-dependently reduce inflammatory and neuropathic pain in formalin, acetic acid, and hot-plate tests, showing a better effect than morphine [87]. The neurotoxin HpTx3, derived from the venom of the huntsman spider, Heteropoda venatoria, has a potent inhibitory effect on Nav 1.7 channels; this activity is dose-dependent and was observed in the following pain models: those induced by formalin, acetic acid, CFA, the hot plate, and nerve injury [88].
Within the spider group, it has been demonstrated that huwentoxins I, IV, and XVI, extracted from Ornithoctonus huwena, block both Cav and Nav channels, resulting in an antinociceptive effect comparable to that of ω-conotoxin MVIIA and morphine. Tests involving inflammatory pain, formalin-induced allodynia, OD1, and incisional injury demonstrated this effect without presenting motor dysfunction [89,90,91]. SNX-482, a toxin from Hysterocrates gigas that acts on Cav channels (specifically Cav2.3), was administered intravenously and shown to reduce pain in models of neuropathic pain caused by spinal nerve ligation injury and mechanical allodynia [92].
The scorpion genus Buthus sp. is the group with the second-highest quantity of molecules with action on VGCs, totaling 15 toxins. These toxins induce pain reduction in models of visceral pain, inflammatory pain, and chronic sciatic nerve constriction [93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]. The scorpion species Hottentotta franzwerneri produces the toxin ω-Buthitoxin-Hf1a, capable of inhibiting calcium influx via Cav3.3 and Cav3.2 channels, producing an antinociceptive effect in mechanical and thermal allodynia caused by the post-surgical pain model [113]. The venom of the scorpion Heterometrus laoticus has a high affinity for Kv1.3 channels. Researchers of one study administered a solution containing the crude venom into animals under study, subjecting them to acetic acid writhing and carrageenan-induced paw edema tests. The crude venom induced an important antinociceptive and anti-inflammatory action [114].
Gln49-PLA2 is a phospholipase A2 that is found in the venom of the snake Gloydius ussuriensis. It raised the threshold of thermal pain in mice in a dose-dependent way, even though it was not lethal. This action was antagonized by naloxone, indicating the possible mediation of opioids in the action of the toxin. However, electrophysiological studies have shown that the main action of this toxin is in the depolarization of Kv channels [115,116].
Tetrodotoxin was first described in the Tetraodontidae fish family [117]. It is a toxin that interacts with Nav channels and has played a significant role as a subcutaneous or topical antinociceptive and local anesthetic in models of inflammatory, neuropathic, and oncological pain. It was able to reduce allodynia and hyperalgesia manifested by carrageenan, formalin, paclitaxel, and DRG injury [118,119,120,121,122,123,124]. The toxin is undergoing clinical studies as part of the drug Halneuron [26].
Other groups of animals, such as amphibians represented by Trachycephalus typhonius and Bufo gargarizans, produce the toxins Tt7 and bufalin, respectively. These toxins inhibit Nav, while bufalin causes the suppression of the inflammatory pain response caused by carrageenan [125]. Tt7 lowered pain caused by heat in the hot-plate test and stopped calcium from working on DRG neurons, which suggests it may be working on ASIC receptors [126]. The skin of the tree frog, Hyla annectans, releases a peptide called anntoxin. This peptide blocks Nav channels and has been shown to be very effective at relieving pain in a number of models, including the tail-flick test, the hot-plate test, the acetic acid-induced writhing test, the formalin-induced paw licking test, and the carrageenan-induced paw edema test [127].
The venom of the snake Naja atra was found to block Nav1.8 channels in models of inflammatory and neuropathic pain without demonstrating toxicity and with an effect more potent than that of morphine [128]. Additionally, the centipede species Scolopendra subspinipes mutilans produces the toxin SSm6a, which blocks Nav1.7 and works better than morphine in models of pain caused by acetic acid and formalin [129].
2.2. Opioid and Cannabinoid Receptors
Opioid receptors are responsible for the pharmacological effects of opioid antinociceptives, the class considered the gold standard for antinociceptive studies. They are located in central and peripheral neurons, as well as in certain cell types, such as endocrine, immune, and dermal cells. In mammals, they are divided into three types: mu (µ), delta (δ), and kappa (κ) [130,131,132]. In addition to the receptors, there is also the endogenous opioid system, which is distributed throughout both the central and peripheral nervous systems and is composed of three classes of peptides called endorphins, enkephalins, and dynorphins. This system performs several functions in the body, including analgesia and mood modulation (Table S2) [133,134,135].
Adolapine is a peptide isolated from bee venom. This molecule consists of 103 amino acids and has a molecular weight of 11 kDa. It demonstrates a potent antinociceptive effect by inhibiting the activity of prostaglandins. Studies indicate that adolapine induces antinociception, a process modulated by opioid receptors, as evidenced by the inhibition of its effects upon administration of opioid receptor antagonists [136].
Some of the toxins discovered in amphibians are known as “Kambô,” a term referring to the venom of frogs that Indigenous Amazonian peoples have historically used as a form of medicine for treating infections, preventing diseases, and as an antinociceptive. Initially researched in the frog species Phyllomedusa bicolor, these medical studies have expanded to encompass various other amphibian species [137]. Among the molecules with antinociceptive properties found in amphibians, three main groups stand out: caeruleins, dermorphins, and deltorphins [138,139].
Dermorphins are heptapeptides with an affinity for µ-type opioid receptors. In preclinical tests, they have demonstrated longer-lasting and more potent effects than morphine, with up to 1000 times greater efficiency when administered intrathecally. Dermorphins are found in the skin of amphibians of the genus Phyllomedusa sp. [140,141]. Deltorphins are also heptapeptides that bind to β-opioid receptors. However, they work a little differently than dermorphins because they depend on glial cells [142,143].
Hannalgesin from the venom of the king cobra (Ophiophagus hannah) is a 72-amino acid neurotoxin exhibiting opioid characteristics. It exhibited an antinociceptive effect even when administered orally, and studies have indicated that the antinociceptive effect of the molecule is related to the presence of nitric oxide. However, depending on the dose administered, it can cause neurological deficits, shrinkage of muscle membranes, and darkening of mitochondria in skeletal muscles [144].
Another snake species known to possess molecules with antinociceptive properties in its venom is the rattlesnake (Crotalus durissus terrificus). The first studies with this species’s crude venom showed that it could relieve pain through the mouth, the bloodstream, the peritoneum, and the skin. These effects were caused by opioid receptors.
Additionally, the active component was identified as a low-mass molecule [145,146]. In 1998, research with a neurotoxin that was about 4.8 kDa showed that it could reduce pain through opioid receptors. Remarkably, this toxin exhibited potency up to 500 times greater than morphine at molar doses while causing no apparent tissue damage in mice. Consequently, it was distinguished as the unique low-molecular-weight antinociceptive molecule identified at that time [146]. Subsequently, investigations were conducted to elucidate the venom’s interaction with opioid receptors and to determine the involvement of nitric oxide and potassium channels in its antinociceptive properties. These experiments were performed using models of hyperalgesia induced by prostaglandin E2 and carrageenan [147,148,149].
In 2003, studies on C. d. terrificus crotamine revealed that, despite behaving similarly to defensins and sharing similar structures, the molecule could also interact with Nav channels [150]. Subsequently, in 2021, a study using recombinant crotamine molecules showed biological activity, pointing to a possible pain-relieving effect through opioid receptors [151,152].
In relation to rattlesnake venom, other bioactive molecules have also been discovered and continue to be studied to this day. In 2008, a low-mass molecule consisting of around 15 amino acids was identified, known as crotalphin, which is a γ chain of crotapotin. Crotalphin exhibited antinociceptive effects via various routes of administration, including oral, intravascular, subcutaneous, intraplantar, intrathecal, and peritoneal, with an efficacy approximately 10 times greater than morphine, particularly in peripheral analgesia [153].
Furthermore, crotalphin showed promising results in models of neuropathic pain induced in the sciatic nerves of mice, demonstrating sustained antinociceptive effects. It exhibited interactions with both opioid and cannabinoid receptors, making it a potential candidate for chronic pain treatment. Crotalphin also worked well in tests that measure inflammatory pain, like the formalin and acetic acid tests, and it was also effective in treating cancer pain [153,154,155].
In the snake Naja n. atra, a molecule called najanalgesin has been discovered that acts on opioid receptors, demonstrating antinociceptive effects with greater efficacy when administered intrathecally and intraperitoneally. Furthermore, there is a significant association with astrocytes, showing positive results in studies involving induced neuropathic pain [156,157]. In studies with the species Micrurus lemniscatus, antinociceptive properties targeting opioid receptors have been identified. However, little is currently known about the specific toxin responsible for this effect [158].
Cannabinoid receptors have been discovered through studies on the medicinal properties of Cannabis sp., which play an important role in immunomodulation. To date, two types of receptors have been discovered—CB1 and CB2—both associated with G proteins [159]. CB1 receptors can be found mainly in the central and peripheral nervous systems, skin, and liver, while CB2 receptors act mainly in the peripheral nervous system and in cells of the immune system [159,160,161]. Both are arranged in vesicles of nerve cells in mammals, mainly in glial cells, divided between the central and peripheral nervous systems, respectively. They have a broad affinity with lipid molecules and can inhibit mechanical, thermal, and chemical nociceptive effects, in addition to being related to the treatment of neuropathic and chronic pain [162,163].
In a study by Jergova et al., the effects of Conus venom on cannabinoid receptors were investigated. For this purpose, HEK293 cells expressing CB1 and CB2 cannabinoid receptors were used to select the fractions with the greatest antinociceptive potential. These selected fractions underwent additional fractionation steps and were tested in experimental pain models. Among the species evaluated, C. textile demonstrated significant antinociceptive effects in the formalin test, in addition to attenuation of thermal and mechanical allodynia in a nerve injury model [164].
PnPP-19 and ε-Ctenitoxin-Pn1a, two toxins found in the spider P. nigriventer, were purified and used to study biological processes connected with pain. Experiments using mechanical, inflammatory, neuropathic, and acute pain models revealed that these toxins exhibit effects on opioid and cannabinoid receptors [165,166,167].
In the case of anuran toxins, it is noteworthy that not all identified toxins are of protein origin; some molecules are organic compounds. For example, bufalin, which is synthesized from the toxins of B. gargarizans, reduces inflammation by blocking anti-inflammatory substances. Furthermore, it interacts with opioid and cannabinoid receptors, thereby alleviating inflammation, inflammatory pain, and cancer-induced pain [168,169].
2.3. TRP (Transient Receptor Potential) Channels
TRP channels were initially characterized in studies with the species Drosophila sp. [170,171]. TRPs are cation channels expressed by sensory neurons and play important roles in the generation of pain when exposed to physical and chemical stimuli, especially temperature variations, such as the TRPV1 channel, which is sensitive to the extreme heat generated by capsaicin [172,173,174,175]. In some cases, certain subunits of these channels present additional sensitivity to protons, contributing to the peripheral perception of inflammatory pain [172,176]. This group of receptors has seven subfamilies: TRPA, TRPV, TRPP, TRPC, TRPM, TRPML, and TRPN. Each of these subfamilies has six different transmembrane domains based on their amino acid sequences and subunit variations [176,177,178]. Calcium and magnesium ions activate these channels, which are non-selective and preferably thermosensitive, but other stimuli can also activate them (Table S3) [179,180].
TRP modulation has been implicated in the antinociceptive properties of toxins. The sea anemone Heteractis crispa exhibited two molecules with action on TRPV1, APHC-1, and APHC-3, both of which consist of 56 amino acid residues and weigh 6 kDa. Electrophysiological experiments have shown that these toxins partially inhibit these receptors. In tests using models of pain and inflammation, an increase in the latency of response to noxious stimuli was observed upon administration of APHC-1 and APHC-3 [181,182].
Another toxin that interacts with this receptor is Ms9a-1, derived from the sea anemone, Metridium senile. Using models of pain and inflammation, the authors investigated its action through in vitro and in vivo tests. The data suggests that, although it cannot activate the receptor directly, the molecule acts as a positive modulator by enhancing receptor activation through different agonists. The results were very positive, since the molecule was able to produce antinociceptive effects and inhibit the neurogenic inflammatory response at low doses and without causing side effects [183]. In a recent study, the molecule showed pain-relieving and anti-inflammatory effects in models of monosodium iodoacetate (MIA)-induced osteoarthritis, with results equivalent to or superior to those observed for the reference drugs, meloxicam and ibuprofen [184]. Another species of anemone, Urticina eques, produces the toxin Ueq 12-1, which acts on TRPA1 receptors similar to Ms9a-1. It also exhibits antibacterial activity in addition to its antinociceptive properties [185].
The spider P. nigriventer has been found to contain two antinociceptive toxins in its venom. One of these is PnTx3-5, which acts on TRPV1 receptors, causing an inhibitory effect. It has demonstrated antinociceptive effects in models of postoperative pain, neuropathic pain, and cancer-induced pain. It has been particularly effective in animals with opioid tolerance [186,187].
Another toxin from P. nigriventer called Phα1β has been shown to effectively block TRPV1 receptors in capsaicin models, leading to pain-relieving and anti-allergic effects. In order to assess its efficacy in relation to ω-conotoxin MVIIA, the molecule was tested in peripheral pain models via intradermal and intracerebral injection [188]. Additionally, Phα1β has been shown to relieve pain without any signs of toxicity when compared to ω-conotoxin MVIIA in models of neuropathic pain and cancer, with effects that last for up to 14 days when injected into the spinal cord [189,190]. It has also been shown that this molecule can lower mechanical hyperalgesia, which happens when TRPA1 channels are activated along with Cav channels [191]. In studies that evaluated pain relief after surgery, low doses of Phα1β toxin increased the pain-relieving effects of morphine while also lowering tolerance, withdrawal syndrome, and pain that was caused by opioids. This result suggests that it could be an effective adjuvant when used in combination with opioids for the treatment of postoperative and cancer pain [55,189].
Experiments with the spider toxin GsMTx-4 from G. rosea in models of neuropathic and inflammatory pain demonstrated a reduction in mechanical hyperalgesia through TRPV4 receptors, in cooperation with two other members of the TRP channel subfamily, TRPC1 and TRPC6, which are expressed in neurons of the DRG [192]. GsMTx-4 was also produced recombinantly in vitro and retained its antinociceptive activity, alleviating mechanical hyperalgesia and allodynia caused by inflammation and nerve damage [193].
The BmK AGAP toxin, derived from the venom of the scorpion B. martensii, has been shown to inhibit TRP receptors. When combined with lidocaine, this molecule can induce long-lasting analgesia, making it suitable for postoperative treatments with local anesthetics administered intrathecally [101,194].
Another toxin that interacts significantly with TRP channels is crotalphine. Experiments on HEK293t kidney cells and dorsal root ganglion cells, both expressing these channels, have demonstrated that crotalphine from C. d. terrificus activates the TRPA1 channel, leading to long-lasting desensitization. This mechanism is crucial for the antinociceptive and antihyperalgesic effects observed in C. d. t. venom [195].
2.4. ASICs (Acid-Sensing Ion Channels)
ASICs are a class of receptors located on neurons in both the central nervous system (CNS) and the peripheral nervous system (PNS). They are also associated with mechanoreceptors and play a crucial role in stimulus mechanical transduction [196]. These channels are activated by the presence of protons in the synaptic space, leading to a mild acidification of the environment, which causes the channels to open or close [197,198]. There are six different subunits in the ASIC channel family. They are called ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4 and are encoded by a different set of four genes. These receptors have a notable sensitivity, being able to respond to pH differences as small as 0.2 units while still being able to recognize very minor pH variations (Table S4) [196,197,198,199,200,201].
Protons released into the extracellular environment during the inflammatory process can activate ASICs, contributing to inflammatory pain [202]. This activity may be associated with pain clusters resulting from a variety of conditions, including ischemia, hematoma, fracture, tumor development, and muscle and skin incisions after surgical procedures, and has been linked to diseases such as arthritis, urogenital tract obstructions, and digestive disorders [200]. Some toxins found in cnidarians have also been found to possess antinociceptive properties by selectively targeting ASIC receptors. In particular, these include toxins from the anemones H. crispa (Hcr1b-1, -2, -3, and -4) [203,204] and Urticina grebenyi (UGR9a-1) [205].
Mambalgins I, II, and III are three neurotoxins with antinociceptive effects found in the venom of the black mamba snake, Dendroaspis polylepis. These molecules, each composed of 57 amino acids, differ by only one amino acid from each other [206]. These toxins have demonstrated a strong affinity for ASIC1a, ASIC2a, and ASIC3, acting quickly and reversibly and causing few side effects. Diochot et al. demonstrated that mambalgins are capable of inhibiting ASICs expressed either in central or peripheral neurons. The authors also found that mambalgins and their isoforms showed promising results in treating neuropathic and inflammatory pain, which shows how useful they could be in the field of pain treatment [206,207].
2.5. Nicotinic Acetylcholine Receptors (nAChRs)
Nicotinic acetylcholine receptors form ion channels through the junction of their five transmembrane domains, and the main subtypes are α6β4, α7, and α9α10. They are distributed throughout the CNS and PNS. These receptors are involved in different physiological functions, such as cognition, memory, learning, and mood modulation (Table S4) [208,209,210]. When activated, nAChRs play roles in pain modulation by inhibiting the release of inflammatory cytokines, thus producing, in addition to an anti-inflammatory action, an analgesic response in the body, mainly in models of neuropathic pain [211,212].
Epibatidine is an alkaloid toxin found in the amphibian species Epipedobates tricolor, which has a similar structure to nicotine and acts as an agonist in nAChRs, with an antinociceptive effect in models of inflammatory and neuropathic pain [213,214,215]. In tests with mice’s spinal cords, epibatidine proved to be a fully selective and potent agonist at these receptors; it also showed tolerance with continuous use [216,217,218].
In the mollusks group, seven toxins (α-conotoxin Lv1d, α-conotoxin BuIA, α-conotoxin RgIA, α-conotoxin Vc1.1, α-conotoxin Mr1.1, lt14a, and αO-conotoxin GeXIVA) from species of the genus Conus sp. (C. lividus, C. bullatus, C. regius, C. victoriae, C. marmoreus, C. literatus, and C. generalis) were found to interact with nAChRs and have shown antinociceptive effects in models of neuropathic and inflammatory pain [219,220,221,222,223,224,225,226,227]. Specifically, RgIA was able to reduce hypersensitivity in a model of neuropathic pain induced by oxaliplatin [224]. In experiments with synthetic α-conotoxin BuIA, the authors demonstrated positive results, as the molecules were able to attenuate pain in the hot-plate model and the paclitaxel-induced peripheral neuropathy model without causing adverse effects [219].
Various species within the Elapidae snake family possess toxins that specifically target nAChRs. Cobrotoxin, from Naja n. atra, is a substance that may induce analgesia in the CNS [228]. Cobratoxin (CTX) isolated from Naja n. kaouthia venom has antinociceptive potential in a dose-dependent cancer-induced bone pain model through nicotinic acetylcholine receptors [229]. CTX was also able to inhibit inflammatory pain caused by formalin, pain caused by CFA-induced arthritis, and neuropathic pain caused by disruption of nerve connections and was shown to be opioid-independent [230,231,232,233].
In models of inflammatory pain, the organic compound cinobufagin, obtained from the amphibian Bufo b. gargarizans, showed an antinociceptive effect by activating nAChRs [234]. The armored spider P. nigriventer also has a toxin in its venom that is capable of modulating pain by activating cholinergic receptors of the muscarinic and nicotinic systems in models of neuropathic and inflammatory pain [235].
2.6. γ-Aminobutyric Acid (GABA) Receptors
γ-Aminobutyric acid (GABA) is an inhibitory neurotransmitter responsible for regulating mammalian behavior, such as sensations, consciousness, sleep, learning, and memory, but it is also related to chronic pain conditions. These molecules target GABA receptors, which are widely distributed throughout the CNS in neurons and glial cells. These receptors can be divided into ionotropic (GABAA and GABAC) and metabotropic (GABAB) types, which are coupled to G proteins [12,236,237]. Ionotropic GABA receptors modulate pain when presynaptic receptors, stimulated by the presence of the neurotransmitter, promote the efflux of Cl− from the afferent neuron to the synaptic cleft, leading to influx through the GABA receptor located in the postsynaptic neuron, generating hyperpolarization, and thus generating the sensation of pain [238,239]. In comparative studies, the antinociceptive function of GABA receptors has shown satisfactory results in models of neuropathic, chronic, and inflammatory pain induced by CFA and formalin (Table S4) [240,241,242].
Several in vitro and in vivo experiments have demonstrated that conotoxins modulate the action of Cav channels while also causing the activation of GABAB receptors [243,244,245].
The conotoxins RgIA, GeXIVA, Vc1.1, and AuIB, along with their respective synthetic analogs, have previously shown antinociceptive potential in models of neuropathic pain, mechanical allodynia, and nerve injury by modulating GABAB receptors [224,225,237,246].
2.7. Ionotropic Glutamate Receptors (iGluRs)
Ionic glutamate receptors are fundamental elements in the regulation of synaptic transmissions in neurons involved in sensory transmission and cerebral stimulus processing. There are four functional groups of receptors: AMPA (amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), kainate, NMDA, and GluD (also called delta receptors) (Table S4) [247,248,249].
iGluRs are responsible for triggering long-lasting responses in synapses; they are associated with processes such as neuropathic pain, learning, and memory. In the treatment of neuropathic and chronic pain, when antagonized, glutamate receptors play a significant role in pain relief. These receptors can also change how endogenous opioids are released in the body and how opioid tolerance is built up by exogenous molecules. This may be important for treating chronic pain and controlling pain in general [250,251,252].
In studies with various toxins with anti-inflammatory and antinociceptive potential from Conus cone snails, some compounds showed affinity for glutamate receptors [253]. An electrophysiological study conducted with conotoxin-AC1 and its synthetic variants demonstrated that the toxin originating from C. achatinus was capable of inhibiting NMDA receptors in HEK293 cells. In murine pain models, the administration of the molecule resulted in dose-dependent analgesia [254]. The species C. geographus has two conantokins, G and T, and their synthetic variants have an affinity for subtypes of NMDA receptors, which exhibit antinociception in an injury-induced pain model [255,256].
Joro spider toxin (JST), obtained from the venom of Trichonephila clavata, can interact with glutamate receptors, specifically the AMPA type. In experiments with pain models, the molecule was able to inhibit the receptor, causing analgesia in models of thermal hyperalgesia and mechanical allodynia pain, but it did not produce results in the inflammatory pain model induced by formalin [257,258].
The P. nigriventer peptide Pntx(5-5) showed an antinociceptive effect in the inflammatory phase of the formalin test by inhibiting NMDAR receptors. This peptide is capable of mediating the antinociceptive effect in the spinal cord, not only on postsynaptic receptors but also through possible interactions with autoreceptors [259].
The Brazilian scorpion Tityus serrulatus produces a toxin called TsNTxP. This toxin may be able to block NMDA receptors by decreasing the amount of glutamate in the body during fluorescence experiments with these receptors. Furthermore, the molecule demonstrated antinociceptive effects in models of allodynia and neuropathic pain induced by CCI and paclitaxel [260].
2.8. Purinergic Receptors
Purinergic receptors are a family of receptors that can be divided into two main types: P1 and P2. P2Y receptors are ATP receptors coupled to G proteins, while the P2X class is also mediated by ATP but is permeable to Na+, K+, and Ca2+ cations (Table S4) [261,262,263].
Activation of these receptors is primarily related to controlled ATP release into the environment, allowing the influx of hypertension and calcium into the cell, and has been associated with various types of pain, including neuropathic pain, cancer pain, allodynia, and acute inflammatory pain [262,264,265]. Geolycosa sp., a genus of spider, was found to produce two toxins, purotoxin-1 and purotoxin-2, that interact with purinergic receptors. Both block P2X3, which lowers the affinity between the agonist and the receptor. This effectively reduces pain in models of inflammatory pain [266,267].
The B. gargarizans bufalin molecule also showed interaction with P2X7-type receptors, inhibiting the channel’s activity and regulating the release of inflammatory cytokines, such as TNF-α, IL-18, IL-1β, and IL-6 in models of neuropathic pain [268].
2.9. Adrenergic Receptors
Adrenergic receptors located in the spinal cord are involved in inflammatory and neuropathic pain [269,270]. These receptors are activated by drugs such as clonidine and dexmedetomidine and are associated with the control of chronic pain (Table S4) [269,271,272].
The bee Apis mellifera venom has been extensively studied as an adjuvant for pain treatment. In several studies, melittin, one of the venom’s toxins, produced an antinociceptive effect via adrenergic receptors in models of paclitaxel-induced neuropathic pain, osteoarthritis, and visceral pain induced by acetic acid [273,274,275,276]. The use of diluted bee venom (DVB) at acupuncture points, a technique known as apipuncture, is an established practice in Eastern medicine for the therapeutic management of various pathological conditions. In an experimental study, the antinociceptive effects of this approach were evaluated in models of neuropathic pain. The results indicated that DVB exerts its effect through the activation of alpha2-adrenoceptors and that the administration of the compound twice daily resulted in a significant reduction in thermal hyperalgesia and mechanical allodynia in the animals tested [276,277].
2.10. Bradykinin Receptors
Bradykinin receptors are a family of G protein-coupled receptors characterized by having seven transmembrane domains. These receptors can be classified into two main groups, known as B1 and B2, and are distributed in tissues such as endothelial, cardiovascular, respiratory, and immune cells, exhibiting different physiological functions [278,279,280]. Bradykinin interacts with receptors, causing excitability in afferent pain receptors and hyperalgesia, in addition to causing the production of inflammatory mediators. Studies with antagonists of the bradykinin B2 receptor showed that inhibiting the receptors generated positive responses for antinociception in models of inflammatory (CFA) and neuropathic (CCI) hyperalgesia [281]. In wasps, the species Polybia occidentalis presents a peptide analogous to Thr6-bradykinin, which shows affinity for the B2 receptor subtype, causing a dose-dependent thermal antinociceptive effect and demonstrating that it is more potent than morphine and bradykinin (Table S4) [282].
2.11. Adenosine Receptors
Adenosine interacts with a family of G-protein-associated receptors, which are distributed in different tissues according to their subtypes (A1R, A2AR, A2BR, and A3R) [283,284]. These receptors play multiple physiological roles, including the regulation of cardiovascular, respiratory, renal, inflammatory, and immunological processes [285]. In pain, adenosine receptors, when activated, promote the inhibition of the action of cAMP, protein kinase A, and calcium channels, leading to reduced pain perception in models of chronic and inflammatory pain induced by formalin and carrageenan [286,287,288]. It has been shown that the venom of the Naja n. atra snake can reduce pain by activating adenosine receptors, especially those in subtypes A1 and A2a. In moderate doses, these effects result in antinociception, while high doses can trigger hyperalgesia. Activation of subtype A1 is associated with antinociception, whereas activation of subtype A2a, at higher concentrations of the neurotoxin, can cause hyperalgesia (Table S4) [289].
2.12. Cholecystokinin Receptors (CCKr)
Cholecystokinin receptors are a class of receptors composed of two main subclasses (CCK-A and CCK-B) distributed mainly throughout the CNS and enteric nervous system (ENS). They are associated with sensitivity, anxiety, and motility of the gastrointestinal system [290,291]. The DRG in the peripheral system and the spinal cord in the CNS both contain this class of receptors, which stimulate the release of neurotransmitters associated with pain, such as glutamate and GABA. The activation of CCKr leads to hyperalgesia and inflammatory pain [292,293,294,295]. CCKr may act to reduce tolerance triggered by opioid molecules (Table S4) [295].
Caeruleins are composed of 10 to 16 peptides and are known for their antinociceptive properties. They were first isolated in the frog Hyla caerulea but have subsequently been found in several other genres [296,297]. They are known as myotropic peptides with contractile effects on the intestines and smooth muscles. These endogenous peptides are significant in the skin of amphibians and have a structure similar to gastrin in mammals and cholecystokinin (CCK). Caeruleins can act through CCKr in the digestive tract, causing constriction and slowing down gastric emptying in response to protein and fat intake [138,294]. Caeruleins are not opioid peptides and have been shown to be 15 times more effective than morphine. Their antinociceptive properties have been evaluated in various indications in oncological, gastrointestinal, and migraine domains [298,299]. Recent years have revived studies with caeruleins, primarily due to their potential in oncological treatment through intrathecal administration into the spinal cord [138].
2.13. Serotonin Receptors
Serotonin (5-HT) is a neurotransmitter that acts on G-protein-coupled receptors that, in addition to being related to mood modulation, play a fundamental role in pain modulation [239]. Serotonin receptors are divided into six families, of which only 5-HT1A, 5-HT3, and 5-HT7 play a role in pain reduction when activated [300,301,302,303]. Only the peptide named bunodosine 391, an acid acylamine from the anemone Bunodosoma cangicum, showed an antinociceptive effect in carrageenan-induced hyperalgesia. Bunodosine does not demonstrate an antinociceptive effect blocked by naloxone; however, its effect was reversed by methysergide, indicating the participation of serotonin receptors in the antinociceptive effect of this toxin (Table S4) [304].
2.14. Not Known Targets
This topic focuses on toxins and crude venoms that exhibit antinociceptive action in animal models, but their pharmacological targets remain unidentified (Table S5). A compound named mygalin was identified from the spider Acanthoscurria gomesiana and presented an antinociceptive effect in an acetic acid-induced pain model. The authors suggest that, as it is an acyl-polyamine, it is possible that this molecule targets NMDA receptors [305]. In another study using the same pain model, the peptide named brachyin from Brachypelma albopilosum presented a similar effect [306].
Antinociceptive activities were also found in bees and wasps. Antinociceptive studies using the CFA-induced chronic arthritis and the capsaicin pain model demonstrated that the crude venom from A. mellifera has antinociceptive action. This effect was not inhibited by naloxone, a non-selective blocker of opioid receptors, indicating that the antinociceptive effect of the venom is not mediated by the opioid system [307,308,309].
In another study carried out with this species, the results obtained demonstrated that bee venom significantly increased the antinociceptive effect of ketoprofen and tramadol in the hot-plate model in mice, and the administration of intraperitoneal crude venom did not cause changes in motor activity [310]. In the formalin-induced inflammatory pain test, AmsBV, which comes from Apis mellifera syriaca, showed a decrease in nociceptive stimulation. The hot plate was used to measure the stimulus caused by the inflammatory agent. AmsBV reduced inflammatory cytokines, demonstrating an immunomodulatory effect [311].
The mastoparan Agelaia-MPI, originating from the wasp Parachartergus fraternus, demonstrated an important antinociceptive action. This effect was more pronounced in the hot-plate test than in the tail-flick test. These results suggest an interaction with non-opioid receptors by partial and reversible blockade of action potential amplitude, possibly in Nav channels. Another possibility would be an interaction with sodium channels, as the peptide was able to partially block the action potential in electrophysiological experiments conducted on the sciatic nerve. Further studies are necessary to establish a relationship between the compound and potential targets. Similar results were found in fractions of Pseudopolybia vespiceps venom [312,313].
The ant toxin named myrmexin found in Pseudomyrmex triplarinus and the crude venom of Dinoponera quadriceps present antinociceptive properties. Pain models using mechanical, thermal, and chemical stimuli have shown their effects in reducing nociception [314,315,316]. Poneratoxin (PoTX), which was isolated from Paraponera clavata, presented similar effects. Its exact pharmacological target has not been found, but it is thought to work on non-opioid receptors [317].
Among the scorpions, the species B. martensii stands out, presenting six different toxins, known as BmK IT-AP, BmK IT AP3, BmK AGAP-SYPU1, BmK AGAP-SYPU2, BmK AngP1, and BmK dITAP3, which induce antinociceptive effects. These toxins did not show toxic effects in blow-fly larvae or mammals; however, these compounds were found to induce dependence when compared to morphine, heroin, and aspirin [318,319,320,321,322,323]. The toxin leptucin, derived from the venom of Hemiscorpius lepturus, exhibited acute toxic effects and possibly interacted with opioid receptors, despite showing antinociceptive potential in thermal stimulus [324].
The centipede S. s. mutilans produces the SsmTX-I toxin, whose antinociceptive potential was evidenced using the formalin-induced pain test; pharmacological target tests were not conducted [325]. Toxins from cnidarians like Stichodactyla mertensii, Stichodactyla gigantea, Pseudopterogorgia elisabethae, and Pelagia noctiluca helped reduce pain in models of arthritis and inflammation caused by acetic acid without presenting toxic effects [326,327,328].
Toxins of seven species of Conus sp., including C. striatus, C. coronatus, C. virgo, and C. frigidus, showed antinociceptive effects in a formalin-induced pain model. C. imperialis toxin induced an antinociceptive effect in the nerve ligation-induced neuropathic pain model. The venom of C. parvatus presented a potent antinociceptive effect in mechanical stimulus, mediated by action in both the CNS and PNS, surpassing the effect of pentazocine [329,330,331]. On the other hand, this venom reduced the motor activity, which compromises the reliability of the results of nociception tests, which are based on motor behavioral responses. The crude venom of C. virgo and C. striatus induced an antinociceptive effect in inflammatory pain caused by formalin, carrageenan, and acetic acid, mainly in the acute phase with lower toxicity and tolerance [332,333].
Several bioactive compounds have been identified in the amphibian group. Bufotenin, an indolealkylamine extracted from toad venom, has demonstrated anti-inflammatory and antinociceptive effects caused by formalin. However, the compound showed toxicity in biological models. To alleviate the toxicity of the compound in the organism, the authors encapsulated it in liposomes, generating satisfactory results, since the desired effects were preserved [334,335,336,337].
The compound analgesin-HJ identified in Hyla japonica showed the ability to attenuate nociception associated with inflammation by modulating factors like TNF-α and IL-1β [338]. Peptide CI5, derived from B. gargarizans, manifested a reduction in abdominal contortion caused by acetic acid and a reduction in edema and inflammatory pain caused by carrageenan and formalin [339].
Neuropeptide B, derived from B. gargarizans, exhibited a transdermal antinociceptive effect in hot-plate and acetic acid pain models when associated with the antennapedia protein, which facilitates transmembrane permeability [340].
A comparative study using specimens of the snake Bungarus fasciatus from different regions of Vietnam demonstrated antinociceptive potential in the acetic acid test [341]. Studies using the C. d. terrificus venom fraction, crotoxin, demonstrated an antinociceptive effect mediated in the central nervous system, without reversal by blocking opioid and acetylcholine receptors [342,343].
Environmental factors, such as diet and geographic variations, significantly influence the potency and composition of venoms, as observed in comparative studies. This variability highlights the importance of identifying isolated molecules to ensure the reproducibility of pharmacological effects.
3. Trends and Challenges
The development of novel antinociceptive drugs encounters significant translational challenges hindering the progression of promising candidates from preclinical research to clinical application. Efforts to mitigate these challenges include a focus on new pharmaceutical agents that interact with receptors distinct from those currently available on the market, such as VGCs, TRPs, and ASICs, as well as consideration of the discrepancies observed between biological models utilizing rodents and human physiology. This highlights the need to develop new techniques to investigate specific pathways of action for pain treatment [344,345,346]. Alternatives have been explored to solve this problem, such as the use of biomarkers and 3D models of nociceptors to facilitate the understanding of the trajectory and effectiveness of new compounds [347,348].
The clinical success of venom-derived analgesics is underscored by the high affinity and specificity of these toxins for ion channels, exemplified by Ziconotide (Prialt®. TerSera Therapeutics LLC, Deerfield, IL, USA)—a synthetic ω-conotoxin MVIIA that targets N-type voltage-gated calcium channels (Cav2.2) to provide relief for refractory pain without opioid-related risks—and Tetrodotoxin (TTX/Halneuron®. Dogwood Therapeutics Inc., Alpharetta, GA, USA), which is utilized in palliative care. These molecules, shaped by millions of years of evolutionary refinement, achieve a level of molecular precision that traditional small-molecule drugs rarely attain, establishing venoms as essential “biological blueprints” for developing biopharmaceuticals that effectively bypass conventional opioid pathways [23,28].
There are still several challenges facing clinical candidates. A major challenge is the narrow therapeutic index; for instance, many Nav1.7 or Nav1.8 blockers derived from spider and scorpion toxins show remarkable efficacy in rodent models but fail in humans due to off-target effects on the cardiovascular or neuromuscular systems. Furthermore, the delivery remains a bottleneck. While Ziconotide requires invasive intrathecal administration to reach its CNS targets, many newer candidates (such as modified ω-conotoxins) have failed Phase II trials because they could not achieve stable therapeutic concentrations in human plasma due to rapid proteolytic degradation. The “translational gap”—where molecules perform well in reflex-based animal pain models but fail to address the complex, multidimensional nature of human chronic pain—remains the primary obstacle for candidates currently in the pipeline.
Furthermore, most antinociceptive toxins, in addition to the drugs mentioned above, have a molecular mass of less than 5 kilodaltons (kDa) and greater than 500 daltons (Da). According to Muttenthaler et al., approximately 75% of drugs marketed in 2019 were based on low-molecular-weight molecules, 20% were biopharmaceuticals, and 5% were peptides [349]. The work of Wang et al. and Craik et al. identified that the future of research on molecules with pharmaceutical potential will focus on peptides located between sizes of 500 Da and 5000 Da, since there is a gap in the development of peptide-based drugs with intermediate sizes, which may yield different results from those found in small and large molecules, such as greater specificity and precision with their pharmacological targets [350,351].
The area of study, called venomics, has achieved numerous advances in the study of animal toxins. By conducting an integrated analysis of the proteomes of the total venom, along with the transcriptomes of venom glands/ducts, it is possible to determine the fundamental molecules found and relate them to potential therapeutics [352].
Despite advances in “venomics”, technical limitations persist, especially in the detection of low-abundance toxins and in the characterization of complex post-translational modifications. The integration of transcriptomic and proteomic data is essential to overcome the gap between genetic identification and the biological functionality of toxins.
Intraspecific genetic variations, resulting from polymorphisms and differential gene regulation, can lead to the expression of isoforms with distinct pharmacological efficacies. Such genetic variability should be considered in the selection of lead molecules, as small structural alterations can significantly impact the interaction with molecular targets and the reproducibility of the observed analgesic effects. Although natural venom extraction faces scalability challenges and high operating costs, the economic viability of these drug candidates lies in the transition to recombinant production and chemical synthesis. These technologies allow for the biotechnological standardization necessary for the pharmaceutical market, mitigating biological variability and reducing mass production costs in the long term.
Furthermore, ethical considerations in the supply of venoms include compliance with animal welfare protocols and biodiversity access legislation. The transition to toxin production via recombinant technology or chemical synthesis, discussed in this review, is fundamental to reducing dependence on animal specimens and ensuring the sustainability of drug discovery.
4. Conclusions
To fully realize the clinical potential of toxins as biopharmaceuticals, research is increasingly focused on pharmacological optimization and innovative delivery systems. Strategies such as the development of synthetic analogs with enhanced stability and selectivity, as demonstrated with KIIIA and AC1 conotoxins, and the use of recombinant technology for scalable production of molecules like Phα1β and crotamine are critical steps toward therapeutic viability. Furthermore, to overcome the limitations of invasive administration—typically associated with intrathecal delivery—new routes are being explored, including intranasal and topical applications that have shown promising antinociceptive effects in preclinical models. The integration of nanotechnology also plays a pivotal role; for instance, the association of toxins with micelles or liposomes has proven effective in protecting these peptides from degradation, facilitating tissue permeation, and significantly reducing motor or systemic side effects. These multifaceted efforts to refine “biological blueprints” through bioengineering and nanotechnology are essential to bridging the translational gap and establishing toxins as the next generation of safe, non-opioid analgesics.
In summary, this review highlighted a wide range of animal venoms and toxins with potential for pain relief, most of which are intermediate-mass peptides and interact with voltage-gated ion channels. Nonetheless, the advantages and limitations in the development of new drugs, such as translatability between animal models and humans, advances in venom study technology, and synthetic peptide production, present promising solutions to bridge the gaps in the production of new drugs.
5. Materials and Methods
To begin this review, we asked the following questions: How many venom molecules and toxins with antinociceptive effects have been identified to date? And which receptors do they act on? Once these questions were posed, we began the process of searching for and selecting relevant articles.
The bibliographic search was conducted in January 2025, covering the period from 1960 to 2025. We used descriptors obtained from the MeSH and DECS platforms and performed searches in both Latin American databases (SciELO and LILACS) and international databases (Scopus, Web of Science, CABI, Cochrane, CINAHL, Embase, and Medline/PubMed) (Figure 3). The descriptor lists are in the Supplementary Material.
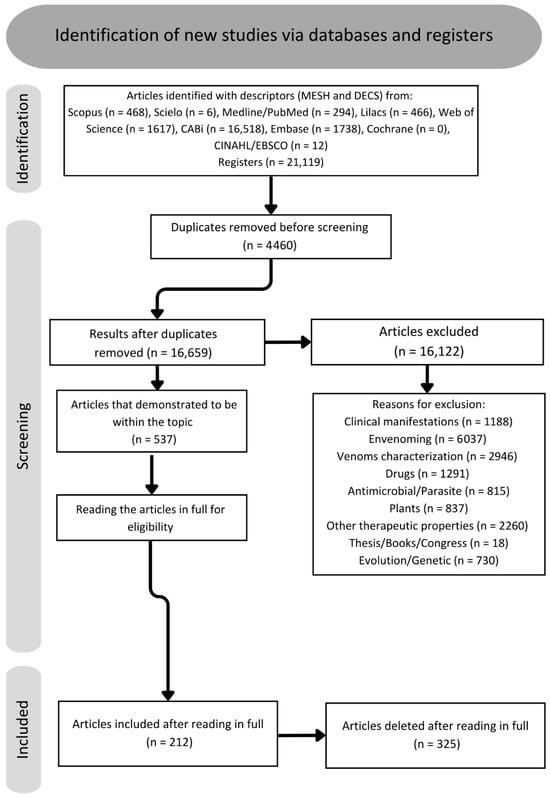
Figure 3.
Flowchart about screening of articles.
We then selected eligible articles based on the inclusion and exclusion criteria specified for this study, which included:
- Elimination of duplicates.
- Elimination of articles that did not address the target topic.
- Inclusion of original research articles and reviews that added new insights.
- Inclusion of articles on molecular characterization.
- Inclusion of experiments with positive and consolidated data.
When the title and abstract were aligned with the theme, the full text was read using the data extraction method. The flowchart below represents the criteria used to exclude and include articles (Figure 3). Furthermore, for each article that met the inclusion and data extraction criteria, we performed additional reading and selection of references within the article itself to delve into the subject and find new references on the study topic, including previous review articles on analgesia.
The information considered in each study included potentially active and antinociceptive molecules present in each animal group, their molecular size, their mechanism of action on related nociceptors, and properties of pharmacokinetic and pharmacodynamic importance.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/toxins18020069/s1, Table S1: Toxins that interact with voltage-gated channels to induce antinociception; Table S2: Toxins found to interact with opioid and cannabinoid receptors that cause antinociception; Table S3: Toxins found to interact with TRP receptors that cause antinociception; Table S4: Toxins found to interact with other receptors that cause antinociception; Table S5: Toxins with no known targets.
Author Contributions
Conceptualization, D.G.A. and R.S.F.J.; writing—review and editing, D.G.A., B.C.J., A.F.M.P., J.d.S.C., C.F.V., D.C.P., and R.S.F.J. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declared that financial support was received for the research, authorship, and/or publication of this article. The APC was funded by FAPESP Proc 2021/11936-3 (RSFJr); 2023/16514-5 (RSFJr); 2024/14131-4 (JSC); 2022/16060-1; 2023/09921-3 (AFMP); 2022/11535-1 (DCP).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to express their gratitude to São Paulo State University (UNESP) and the Center for the Study of Venoms and Venomous Animals (CEVAP) for their institutional support, especially to Professor Benedito Barraviera, director of the Center for Translational Science and Biopharmaceutical Development at FAPESP. The authors are also grateful to the Coordination for the Improvement of Higher Education Personnel (CAPES) and São Paulo Research Foundation (FAPESP) for the scholarship provided, which was essential for the development of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| VGCs | Voltage-gated channels |
| TRPs | Transient receptor potential |
| TNF | Tumor necrosis factor |
| DAMPs | Damage-associated molecular patterns |
| CB | Cannabinoid receptor |
| B | Bradykinin receptor |
| ASIC | Acid-sensitive ion channels |
| OP | Opioid receptor |
| P2X | Purinergic receptor |
| ADR | Adrenergic receptor |
| ADN | Adenosine receptor |
| 5-TH | Serotonin receptor |
| Cav | Voltage-gated calcium channel |
| Nav | Voltage-gated sodium channel |
| Kv | Voltage-gated potassium channel |
| CCK | Cholecystokinin receptors |
| IGluRs | Ionotropic glutamate receptors |
| GABA | γ-Aminobutyric acid |
| kDa | Kilo daltons |
| CFA | Complete Freund’s adjuvant |
| CNS | Central nervous system |
| PNS | Peripheral nervous system |
| CCI | Chronic constriction injury |
| PBS | Phosphate-buffered saline |
| DDPCPX | Dipropylcyclopentylxanthine |
| ZZM241385 | Potent selective adenosine A2A antagonist |
| PMA | Phorbol 12-myristate 13-acetate |
| NO | Nitric oxide |
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Vasconcelos, F.H.; De Araújo, G.C. Prevalence of chronic pain in Brazil: A descriptive study. Braz. J. Pain 2018, 1, 176–179. [Google Scholar] [CrossRef]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- Chen, T.; Taniguchi, W.; Chen, Q.Y.; Tozaki-Saitoh, H.; Song, Q.; Liu, R.H.; Koga, K.; Matsuda, T.; Kaito-Sugimura, Y.; Wang, J.; et al. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat. Commun. 2018, 9, 1886. [Google Scholar] [CrossRef] [PubMed]
- Orr, P.M.; Shank, B.C.; Black, A.C. The role of pain classification systems in pain management. Crit. Care Nurs. Clin. N. Am. 2017, 29, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.B.; Goudas, L.C. Acute pain. Lancet 1999, 353, 2051–2058. [Google Scholar] [CrossRef]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2018, 160, 19–27. [Google Scholar] [CrossRef]
- Raffaeli, W.; Arnaudo, E. Pain as a disease: An overview. J. Pain Res. 2017, 10, 2003–2008. [Google Scholar] [CrossRef] [PubMed]
- Thurston, K.L.; Zhang, S.J.; Wilbanks, B.A.; Billings, R.; Aroke, E.N. A systematic review of race, sex, and socioeconomic status differences in postoperative pain and pain management. J. Perianesth. Nurs. 2022, 38, 504–515. [Google Scholar] [CrossRef]
- Prego-Domínguez, J.; Khazaeipour, Z.; Mallah, N.; Takkouche, B. Socioeconomic status and occurrence of chronic pain: A meta-analysis. Rheumatology 2020, 60, 1091–1105. [Google Scholar] [CrossRef]
- Zimmer, Z.; Fraser, K.; Grol-Prokopczyk, H.; Zajacova, A. A global study of pain prevalence across 52 countries: Examining the role of country-level contextual factors. Pain 2021, 163, 1740–1750. [Google Scholar] [CrossRef]
- Golan, D.E.; Tashjian, A.H., Jr.; Armstrong, E.J.; Armstrong, A.W. Fundamental Principles of Neuropharmacology. In Principles of Pharmacology, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 1–71. [Google Scholar]
- Kim, J.; Ham, S.; Hong, H.; Moon, C.; Im, H.I. Brain reward circuits in morphine addiction. Mol. Cells 2016, 39, 645–653. [Google Scholar] [CrossRef]
- Listos, J.; Łupina, M.; Talarek, S.; Mazur, A.; Orzelska-Górka, J.; Kotlińska, J. The mechanisms involved in morphine Addiction: An overview. Int. J. Mol. Sci. 2019, 20, 4302. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.S.; Hennessy, G.; Borrow, J.A.; Greenfield, S.F.; Weiss, R.D. Substance use histories in patients seeking treatment for controlled-release oxycodone dependence. Drug Alcohol Depend. 2004, 76, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Gallego, A.O.; Barón, M.G.; Arranz, E.E. Oxycodone: A pharmacological and clinical review. Clin. Transl. Oncol. 2007, 9, 298–307. [Google Scholar] [CrossRef]
- Vadivelu, N.; Kai, A.M.; Kodumudi, V.; Sramcik, J.; Kaye, A.D. The Opioid Crisis: A Comprehensive Overview. Curr. Pain Headache Rep. 2018, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Blanco, C. The changing opioid crisis: Development, challenges and opportunities. Mol. Psychiatry 2020, 26, 218–233. [Google Scholar] [CrossRef]
- Zoorob, M. Fentanyl shock: The changing geography of overdose in the United States. Int. J. Drug Policy 2019, 70, 40–46. [Google Scholar] [CrossRef]
- Han, Y.; Yan, W.; Zheng, Y.; Khan, M.Z.; Yuan, K.; Lu, L. The rising crisis of illicit fentanyl use, overdose, and potential therapeutic strategies. Transl. Psychiatry 2019, 9, 282. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef]
- De Castro Figueiredo Bordon, K.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Wiezel, G.A.; Cardoso, I.A.; Ferreira, I.G.; et al. From animal poisons and venoms to medicines: Achievements, challenges and perspectives in drug discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Day, M.; Heavner, J.E. Ziconotide: An update and review. Expert Opin. Pharmacother. 2008, 9, 1575–1583. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Study of the Safety and Efficacy of a Single Dose of CNV1014802 in Patients with Trigeminal Neuralgia. Available online: https://clinicaltrials.gov/study/NCT00047749 (accessed on 10 March 2025).
- ClinicalTrials.gov. A Trial to Evaluate the Safety, Tolerability, and Pharmacokinetics of Halneuron in Healthy Adult Participants. Available online: https://clinicaltrials.gov/study/NCT06541184 (accessed on 10 March 2025).
- WEX Pharmaceuticals Inc. Halneuron® for Pain Management-WEX Pharmaceuticals Inc-Halneuron for Pain Management. Available online: https://wexpharma.com/technology/about-halneuron/ (accessed on 10 March 2025).
- ClinicalTrials.gov. A Study to Evaluate the Safety, Tolerability, and Efficacy of XPF-001 in Patients with Postherpetic Neuralgia (PHN). Available online: https://clinicaltrials.gov/study/NCT05359133 (accessed on 10 March 2025).
- Bourinet, N.E.; Zamponi, N.G.W. Voltage gated calcium channels as targets for analgesics. Curr. Top. Med. Chem. 2005, 5, 539–546. [Google Scholar] [CrossRef]
- Abd-Elsayed, A.; Jackson, M.; Gu, S.L.; Fiala, K.; Gu, J. Neuropathic pain and Kv7 voltage-gated potassium channels: The potential role of Kv7 activators in the treatment of neuropathic pain. Mol. Pain 2019, 15, 1744806919864256. [Google Scholar] [CrossRef]
- Goodwin, G.; McMahon, S.B. The physiological function of different voltage-gated sodium channels in pain. Nat. Rev. Neurosci. 2021, 22, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ou, S.W.; Wang, Y.J. Distribution and function of voltage-gated sodium channels in the nervous system. Channels 2017, 11, 534–554. [Google Scholar] [CrossRef]
- Armstrong, C.M.; Hille, B. Voltage-Gated ion channels and electrical excitability. Neuron 1998, 20, 371–380. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Conti, F.; Franciolini, F. Fifty years of gating currents and channel gating. J. Gen. Physiol. 2023, 155, e202313380. [Google Scholar] [CrossRef]
- Terlau, H.; Stühmer, W. Structure and function of Voltage-Gated Ion Channels. Naturwissenschaften 1998, 85, 437–444. [Google Scholar] [CrossRef]
- Sands, Z.; Grottesi, A.; Sansom, M.S.P. Voltage-gated ion channels. Curr. Biol. 2005, 15, R44–R47. [Google Scholar] [CrossRef] [PubMed]
- Van Hook, M.J.; Nawy, S.; Thoreson, W.B. Voltage- and calcium-gated ion channels of neurons in the vertebrate retina. Prog. Retin. Eye Res. 2019, 72, 100760. [Google Scholar] [CrossRef]
- Wang, Y.X.; Gao, D.; Pettus, M.; Phillips, C.; Bowersox, S.S. Interactions of intrathecally administered ziconotide, a selective blocker of neuronal N-type voltage-sensitive calcium channels, with morphine on nociception in rats. Pain 2000, 84, 271–281. [Google Scholar] [CrossRef] [PubMed]
- White, D.M.; Cousins, M.J. Effect of subcutaneous administration of calcium channel blockers on nerve injury-induced hyperalgesia. Brain Res. 1998, 801, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, Y.; Zhang, S.; Zhang, R.; Chen, D.; Chen, L.; Qi, C.; Li, Y.; Huang, J.; Wang, X. Self-Assembly nanostructure of myristoylated Ω-Conotoxin MVIIA increases the duration of efficacy and reduces side effects. Mar. Drugs 2023, 21, 229. [Google Scholar] [CrossRef]
- Yu, S.; Li, Y.; Chen, J.; Zhang, Y.; Tao, X.; Dai, Q.; Liu, D.; Wu, Y.; Xia, M.; Zhangsun, D.; et al. TAT-Modified Ω-Conotoxin MVIIA for crossing the Blood-Brain barrier. Mar. Drugs 2019, 17, 286. [Google Scholar] [CrossRef]
- Xia, Z.; Chen, Y.; Zhu, Y.; Wang, F.; Xu, X.; Zhan, J. Recombinant Ω-Conotoxin MVIIA Possesses Strong Analgesic Activity. BioDrugs 2006, 20, 275–281. [Google Scholar] [CrossRef]
- Wang, Y.X.; Pettus, M.; Gao, D.; Phillips, C.; Bowersox, S.S. Effects of intrathecal administration of ziconotide, a selective neuronal N-type calcium channel blocker, on mechanical allodynia and heat hyperalgesia in a rat model of postoperative pain. Pain 2000, 84, 151–158. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Yu, S.; Liu, J.; Chen, R.; Zhang, Y.; Luo, S.; Zhangsun, D. A novel ω-conotoxin Bu8 inhibiting N-type voltage-gated calcium channels displays potent analgesic activity. Acta Pharm. Sin. B 2021, 11, 2685–2693. [Google Scholar] [CrossRef]
- Smith, M.T.; Cabot, P.J.; Ross, F.B.; Robertson, A.D.; Lewis, R.J. The novel N-type calcium channel blocker, AM336, produces potent dose-dependent antinociception after intrathecal dosing in rats and inhibits substance P release in rat spinal cord slices. Pain 2002, 96, 119–127. [Google Scholar] [CrossRef]
- Han, T.S.; Zhang, M.; Walewska, A.; Gruszczynski, P.; Robertson, C.R.; Cheatham, T.E.; Yoshikami, D.; Olivera, B.M.; Bulaj, G. Structurally minimized Μ-Conotoxin analogues as sodium channel blockers: Implications for designing Conopeptide-Based therapeutics. ChemMedChem 2009, 4, 406–414. [Google Scholar] [CrossRef]
- Zhao, Z.; Pan, T.; Chen, S.; Harvey, P.J.; Zhang, J.; Li, X.; Wang, X.; Li, D.; Zhangsun, D.; Luo, S.; et al. Design, synthesis, and mechanism of action of novel μ-conotoxin KIIIA analogues for inhibition of the voltage-gated sodium channel Nav1.7. J. Biol. Chem. 2023, 299, 103068. [Google Scholar] [CrossRef]
- Ekberg, J.; Jayamanne, A.; Vaughan, C.W.; Aslan, S.; Thomas, L.; Mould, J.; Drinkwater, R.; Baker, M.D.; Abrahamsen, B.; Wood, J.N.; et al. μO-conotoxin MrVIB selectively blocks Na v 1.8 sensory neuron specific sodium channels and chronic pain behavior without motor deficits. Proc. Natl. Acad. Sci. USA 2006, 103, 17030–17035. [Google Scholar] [CrossRef]
- Bulaj, G.; Zhang, M.M.; Green, B.R.; Fiedler, B.; Wei, S.; Nielsen, J.S.; Low, S.J.; Klein, B.D.; Wagstaff, J.D.; Chicoine, L.; et al. Synthetic μO-Conotoxin MrVIB Blocks TTX-Resistant Sodium Channel NaV1.8 and Has a Long-Lasting Analgesic Activity. Biochemistry 2006, 45, 7404–7414. [Google Scholar] [CrossRef]
- Gao, B.; Zhangsun, D.; Hu, Y.; Wu, Y.; Sheng, L.; Fang, L.; Wu, X.; Zhu, X.; Luo, S. Expression and secretion of functional recombinant μO-conotoxin MrVIB-His-tag in Escherichia coli. Toxicon 2013, 72, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.R.; McArthur, J.R.; Brust, A.; Bhola, R.F.; Rosengren, K.J.; Ragnarsson, L.; Deuis, J.R.; Dutertre, S.; Vetter, I.; Alewood, P.F.; et al. Novel analgesic ω-conotoxins from the vermivorous cone snail Conus moncuri provide new insights into the evolution of conopeptides. Sci. Rep. 2018, 8, 13397. [Google Scholar] [CrossRef] [PubMed]
- Bernáldez, J.; Román-González, S.A.; Martínez, O.; Jiménez, S.; Vivas, O.; Arenas, I.; Corzo, G.; Arreguín, R.; García, D.E.; Hernández-López, R.A.; et al. A Conus regularis Conotoxin with a Novel Eight-Cysteine Framework Inhibits CaV2.2 Channels and Displays an Anti-Nociceptive Activity. Mar. Drugs 2013, 11, 1188–1202. [Google Scholar] [CrossRef]
- Dai, Q.; Liu, F.; Zhou, Y.; Lu, B.; Yu, F.; Huang, P. The Synthesis of SO-3, a Conopeptide with High Analgesic Activity Derived from Conus striatus. J. Nat. Prod. 2003, 66, 1276–1279. [Google Scholar] [CrossRef]
- Yan, L.D.; Liu, Y.L.; Zhang, L.; Dong, H.J.; Zhou, P.L.; Su, R.B.; Gong, Z.H. Spinal antinociception of synthetic omega-conotoxin SO-3, a selective N-type neuronal voltage-sensitive calcium channel blocker, and its effects on morphine analgesia in chemical stimulus tests in rodent. Eur. J. Pharmacol. 2010, 636, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhao, S.; Min, X.; Shao, M.; Chen, Y.; Chen, Z.; Liu, Y.; Li, Z.; Xu, X.; Liang, S. A novel μ-conotoxin from worm-hunting Conus tessulatus that selectively inhibit rat TTX-resistant sodium currents. Toxicon 2017, 130, 11–18. [Google Scholar] [CrossRef]
- Tonello, R.; Trevisan, G.; Luckemeyer, D.; Castro-Junior, C.J.; Gomez, M.V.; Ferreira, J. Phα1β, a dual blocker of TRPA1 and Cav2.2, as an adjuvant drug in opioid therapy for postoperative pain. Toxicon 2020, 188, 80–88. [Google Scholar] [CrossRef]
- Tonello, R.; Rigo, F.; Gewehr, C.; Trevisan, G.; Pereira, E.M.R.; Gomez, M.V.; Ferreira, J. Action of Phα1β, a Peptide from the Venom of the Spider Phoneutria nigriventer, on the Analgesic and Adverse Effects Caused by Morphine in Mice. J. Pain 2014, 15, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Rigo, F.K.; Rossato, M.F.; Borges, V.; Da Silva, J.F.; Pereira, E.M.R.; De Ávila, R.A.M.; Silva, M.A.; De Castro Junior, C.J.; Trevisan, G.; Ferreira, J.; et al. Analgesic and side effects of intravenous recombinant Phα1β. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190070. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.H.; Ferreira, J.; Cordeiro, M.D.N.; Vieira, L.B.; De Castro, C.J.; Trevisan, G.; Reis, H.; Souza, I.A.; Richardson, M.; Prado, M.A.M.; et al. Analgesic effect in rodents of native and recombinant Phα1β toxin, a high-voltage-activated calcium channel blocker isolated from armed spider venom. Pain 2008, 140, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, J.; De Assis, P.M.; Gonçalves, E.C.D.; Bobermin, L.D.; Quincozes-Santos, A.; Raposo, N.R.B.; Gomez, M.V.; Souza, A.H. Systemic, intrathecal, and intracerebroventricular antihyperalgesic effects of the calcium channel blocker CTK 01512–2 toxin in persistent pain models. Mol. Neurobiol. 2022, 59, 4436–4452. [Google Scholar] [CrossRef]
- Rigo, F.K.; Trevisan, G.; Rosa, F.; Dalmolin, G.D.; Otuki, M.F.; Cueto, A.P.; de Castro Junior, C.J.; Sebben, V.; Sousa, S.R.V.; Cordeiro, M.D.N.; et al. Spider peptide Phα1β induces analgesic effect in a model of cancer pain. Cancer Sci. 2013, 104, 1226–1230. [Google Scholar] [CrossRef]
- Aoki, C.T.; Moura, R.A.; Ferreira, L.A.; Mendes, M.G.; Santos, D.C.; Rezende, M.J.; De Lima, M.E.; Picolo, G.; Gomez, M.V.; Ferreira, J. Isobolographic analysis reveals antinociceptive synergism between Phα1β recombinant toxin and morphine in a model of cancer pain in C57BL/6J mice. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20210022. [Google Scholar] [CrossRef]
- De Melo Cardoso, M.; Scussel, R.; Da Silva Abel, J.; Pereira, F.O.; Cruz, L.A.; Da Costa Constante, F.; Milioli, A.M.; Rigo, F.K.; Gomez, M.V.; Ferreira, J. Intravenous administration of recombinant Phα1β: Antinociceptive properties and morphine tolerance reversal in a cancer-associated pain model. Toxicon 2024, 243, 107717. [Google Scholar] [CrossRef]
- Tenza-Ferrer, H.; Magno, L.A.V.; Romano-Silva, M.A.; Da Silva, J.F.; Gomez, M.V. PHA1B spider toxin reverses glial structural plasticity upon peripheral inflammation. Front. Cell. Neurosci. 2019, 13, 306. [Google Scholar] [CrossRef]
- Dalmolin, G.D.; Silva, C.R.; Rigo, F.K.; Gomes, G.M.; Cordeiro, M.D.N.; Richardson, M.; Silva, M.A.; Prado, M.A.M.; Gomez, M.V.; Ferreira, J. Antinociceptive effect of Brazilian armed spider venom toxin Tx3–3 in animal models of neuropathic pain. Pain 2011, 152, 2224–2232. [Google Scholar] [CrossRef]
- Pedron, C.; Antunes, F.T.T.; Rebelo, I.N.; Campos, M.M.; Correa, Á.P.; Klein, C.P.; de Souza, A.H.; Gomez, M.V.; Ferreira, J. Phoneutria nigriventer Tx3-3 peptide toxin reduces fibromyalgia symptoms in mice. Neuropeptides 2020, 85, 102094. [Google Scholar] [CrossRef]
- Da Silva, J.F.; Castro-Junior, C.J.; Oliveira, S.M.; Dalmolin, G.D.; Silva, C.R.; Vieira, L.B.; Ribeiro, F.M.; Cordeiro, M.D.N.; Gomez, M.V.; Ferreira, J. Characterization of the antinociceptive effect of PhTx3-4, a toxin from Phoneutria nigriventer, in models of thermal, chemical and incisional pain in mice. Toxicon 2015, 108, 53–61. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Ferreira, J.; Prado, M.A.M.; Cordeiro, M.N.; Richardson, M.; Pinheiro, A.C.D.N.; Gomez, M.V. The Effect of Spider Toxin PhTx3-4, ω-Conotoxins MVIIA and MVIIC on Glutamate Uptake and on Capsaicin-Induced Glutamate Release and [Ca2+]i in Spinal cord Synaptosomes. Cell. Mol. Neurobiol. 2011, 31, 277–283. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Peng, D.Z.; Zhang, Q.F.; Huang, B.; Yang, Q.C.; Tang, D.F.; Li, J.Y.; Liu, L.L.; Li, D.S.; Xiong, X.Y.; et al. µ-TRTX-Ca1a: A novel neurotoxin from Cyriopagopus albostriatus with analgesic effects. Acta Pharmacol. Sin. 2019, 40, 859–866. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, D.; Huang, B.; Yang, Q.; Zhang, Q.; Chen, M.; Wu, C.; Xiong, X.; Li, J.; Liu, L.; et al. Discovery of a Novel Nav1.7 Inhibitor From Cyriopagopus albostriatus Venom With Potent Analgesic Efficacy. Front. Pharmacol. 2018, 9, 1158. [Google Scholar] [CrossRef]
- Gonçalves, T.C.; Benoit, E.; Kurz, M.; Lucarain, L.; Fouconnier, S.; Combemale, S.; Goudet, C.; Belghazi, M.; Leprince, J.; Dutertre, S.; et al. From identification to functional characterization of cyriotoxin-1a, an antinociceptive toxin from the spider Cyriopagopus schioedtei. Br. J. Pharmacol. 2019, 176, 1298–1314. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, J.; Zhang, Y.; Xun, X.; Tang, D.; Peng, D.; Zhang, Y.; Xiong, X.; Li, D.; Chen, M.; et al. Synthesis and analgesic effects of Μ-TRTX-HHN1B on models of inflammatory and neuropathic pain. Toxins 2014, 6, 2363–2378. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Wang, Q.; Wang, Z.; Zhang, Y. HNTX-III alleviates inflammatory and neuropathic pain in animal models. Int. J. Pept. Res. Ther. 2019, 25, 799–806. [Google Scholar] [CrossRef]
- Xiao, Y.; Liang, S. Inhibition of neuronal tetrodotoxin-sensitive Na+ channels by two spider toxins: Hainantoxin-III and hainantoxin-IV. Eur. J. Pharmacol. 2003, 477, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Peng, D.; Zhang, Q.; Yang, Q.; Li, J.; Liu, L.; Li, D.; Xiong, X.; Chen, M.; et al. Engineering of highly potent and selective HNTX-III mutant against hNav1.7 sodium channel for treatment of pain. J. Biol. Chem. 2021, 296, 100326. [Google Scholar] [CrossRef] [PubMed]
- Nebe, J.; Vanegas, H.; Neugebauer, V.; Schaible, H.-G. ω-Agatoxin IVA, a P-Type Calcium Channel Antagonist, Reduces Nociceptive Processing in Spinal Cord Neurons with Input from the Inflamed But Not from the Normal Knee Joint—An Electrophysiological Study in the Rat In Vivo. Eur. J. Neurosci. 1997, 9, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Mintz, I.M.; Venema, V.J.; Swiderek, K.M.; Lee, T.D.; Bean, B.P.; Adams, M.E. P-type calcium channels blocked by the spider toxin ω-Aga-IVA. Nature 1992, 355, 827–829. [Google Scholar] [CrossRef]
- Sousa, S.R.; Wingerd, J.S.; Brust, A.; Bladen, C.; Ragnarsson, L.; Herzig, V.; Deuis, J.R.; Dutertre, S.; Vetter, I.; Zamponi, G.W.; et al. Discovery and mode of action of a novel analgesic β-toxin from the African spider Ceratogyrus darlingi. PLoS ONE 2017, 12, e0182848. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, P.; Chen, B.; Huang, J.; Lai, R.; Liu, J.; Yang, F. Selective Closed-State Nav1.7 Blocker JZTX-34 Exhibits Analgesic Effects against Pain. Toxins 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, S.; Zoukimian, C.; Bosmans, F.; Montnach, J.; Diochot, S.; Cuypers, E.; Canul-Tec, J.C.; le Gall, F.; Legros, C.; Alami, M.; et al. Chemical synthesis, proper folding, NAV channel selectivity profile and analgesic properties of the spider peptide phlotoxin 1. Toxins 2019, 11, 367. [Google Scholar] [CrossRef]
- Gonçalves, T.C.; Lesport, P.; Kuylle, S.; Stura, E.; Ciolek, J.; Mourier, G.; Servent, D.; Benoit, E. Evaluation of the Spider (Phlogiellus genus) Phlotoxin 1 and Synthetic Variants as Antinociceptive Drug Candidates. Toxins 2019, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.C.; Dekan, Z.; Rosengren, K.J.; Erickson, A.; Vetter, I.; Deuis, J.R.; Herzig, V.; Alewood, P.F.; King, G.F.; Lewis, R.J. Identification and Characterization of ProTx-III [μ-TRTX-Tp1a], a New Voltage-Gated Sodium Channel Inhibitor from Venom of the Tarantula Thrixopelma pruriens. Mol. Pharmacol. 2015, 88, 291–303. [Google Scholar] [CrossRef]
- Torres-Pérez, J.V.; Adamek, P.; Palecek, J.; Vizcaychipi, M.; Nagy, I.; Varga, A. The NAv1.7 blocker protoxin II reduces burn injury-induced spinal nociceptive processing. J. Mol. Med. 2018, 96, 75–84. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Dekan, Z.; Smith, J.J.; Deuis, J.R.; Vetter, I.; Herzig, V.; Alewood, P.F.; King, G.F.; Lewis, R.J. Modulatory features of the novel spider toxin μ-TRTX-Df1a isolated from the venom of the spider Davus fasciatus. Br. J. Pharmacol. 2017, 174, 2528–2544. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, R.; Zhang, M.; Chen, D.; Zhang, Q.; Zhang, N.; Liu, Z.; Yang, S.; Liang, S. NAV1.7 Channel Blocker [ALA5, PHE6, LEU26, ARG28]GPTX-1 attenuates CFA-induced inflammatory hypersensitivity in rats via endogenous enkephalin mechanism. J. Pain 2023, 24, 840–859. [Google Scholar] [CrossRef]
- Chen, C.; Xu, B.; Shi, X.; Zhang, M.; Zhang, Q.; Zhang, T.; Liu, Z.; Yang, S.; Liang, S. GpTx-1 and [Ala5, Phe6, Leu26, Arg28]GpTx-1, two peptide NaV1.7 inhibitors: Analgesic and tolerance properties at the spinal level. Br. J. Pharmacol. 2018, 175, 3911–3927. [Google Scholar] [CrossRef]
- Deuis, J.R.; Wingerd, J.S.; Winter, Z.; Durek, T.; Dekan, Z.; Sousa, S.R.; Zimmermann, K.; Hoffmann, T.; Weidner, C.; Nassar, M.A.; et al. Analgesic effects of GPTX-1, PF-04856264 and CNV1014802 in a mouse model of NAV1.7-Mediated pain. Toxins 2016, 8, 78. [Google Scholar] [CrossRef]
- Meng, P.; Huang, H.; Wang, G.; Yang, S.; Lu, Q.; Liu, J.; Liu, R.; Liu, Z.; Lai, R. A Novel Toxin from Haplopelma lividum Selectively Inhibits the NaV1.8 Channel and Possesses Potent Analgesic Efficacy. Toxins 2017, 9, 7. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Chen, Y.; Xu, D.; Zhang, P.; Wang, X. Newly Discovered Action of HpTx3 from Venom of Heteropoda venatoria on Nav1.7 and Its Pharmacological Implications in Analgesia. Toxins 2019, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Zhang, Y.Q.; Dai, J.; Luo, Z.M.; Liang, S.P. Antinociceptive effects of intrathecally administered huwentoxin-I, a selective N-type calcium channel blocker, in the formalin test in conscious rats. Toxicon 2005, 45, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Rahnama, S.; Deuis, J.R.; Cardoso, F.C.; Ramanujam, V.; Lewis, R.J.; Rash, L.D.; King, G.F.; Vetter, I.; Mobil, M.; Robinson, S.D. The structure, dynamics and selectivity profile of a NaV1.7 potency-optimised huwentoxin-IV variant. PLoS ONE 2017, 12, e0173551. [Google Scholar] [CrossRef]
- Deng, M.; Luo, X.; Xiao, Y.; Sun, Z.; Jiang, L.; Liu, Z.; Zeng, X.; Chen, Y.; Li, W.; Liang, S. Huwentoxin-XVI, an analgesic, highly reversible mammalian N-type calcium channel antagonist from Chinese tarantula Ornithoctonus huwena. Neuropharmacology 2014, 79, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.A.; Bee, L.A.; Stephens, G.J.; Dickenson, A.H. The Cav2.3 calcium channel antagonist SNX-482 reduces dorsal horn neuronal responses in a rat model of chronic neuropathic pain. Eur. J. Neurosci. 2007, 25, 3561–3569. [Google Scholar] [CrossRef]
- Xu, Y.; Meng, X.; Hou, X.; Sun, J.; Kong, X.; Sun, Y.; Li, Z.; Wu, Y.; Yu, L.; Li, H.; et al. A mutant of the Buthus martensii Karsch antitumor-analgesic peptide exhibits reduced inhibition to hNav1.4 and hNav1.5 channels while retaining analgesic activity. J. Biol. Chem. 2017, 292, 18270–18280. [Google Scholar] [CrossRef]
- Cao, P.; Yu, J.; Lu, W.; Cai, X.; Wang, Z.; Gu, Z.; Li, W.; Li, J.; Yan, H.; Yang, H.; et al. Expression and purification of an antitumor-analgesic peptide from the venom of Mesobuthus martensii Karsch by small ubiquitin–related modifier fusion in Escherichia coli. Biotechnol. Prog. 2010, 26, 1240–1244. [Google Scholar] [CrossRef]
- Tan, Z.Y.; Xiao, H.; Mao, X.; Wang, C.Y.; Zhao, Z.Q.; Ji, Y.H. The inhibitory effects of BmK IT2, a scorpion neurotoxin on rat nociceptive flexion reflex and a possible mechanism for modulating voltage-gated Na+ channels. Neuropharmacology 2001, 40, 352–357. [Google Scholar] [CrossRef]
- Wang, C.Y.; Tan, Z.Y.; Chen, B.; Zhao, Z.Q.; Ji, Y.H. Antihyperalgesia effect of BmK IT2, a depressant insect-selective scorpion toxin in rat by peripheral administration. Brain Res. Bull. 2000, 53, 335–338. [Google Scholar] [CrossRef]
- Bai, Z.T.; Liu, T.; Pang, X.Y.; Chai, Z.F.; Ji, Y.H. Suppression by intrathecal BmK IT2 on rat spontaneous pain behaviors and spinal c-Fos expression induced by formalin. Brain Res. Bull. 2007, 73, 248–253. [Google Scholar] [CrossRef]
- Chen, J.; Feng, X.H.; Shi, J.; Tan, Z.Y.; Bai, Z.T.; Liu, T.; Ji, Y.H. The anti-nociceptive effect of BmK AS, a scorpion active polypeptide, and the possible mechanism on specifically modulating voltage-gated Na+ currents in primary afferent neurons. Peptides 2006, 27, 2182–2192. [Google Scholar] [CrossRef]
- Liu, T.; Pang, X.Y.; Jiang, F.; Bai, Z.T.; Ji, Y.H. Anti-nociceptive effects induced by intrathecal injection of BmK AS, a polypeptide from the venom of Chinese-scorpion Buthus martensi Karsch, in rat formalin test. J. Ethnopharmacol. 2008, 117, 332–338. [Google Scholar] [CrossRef]
- Ji, Y.H.; Li, Y.J.; Zhang, J.W.; Song, B.L.; Yamaki, T.; Mochizuki, T.; Hoshino, M.; Yanaihara, N. Covalent structures of BmK AS and BmK AS-1, two novel bioactive polypeptides purified from Chinese scorpion Buthus martensi Karsch. Toxicon 1999, 37, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Cui, Y.; Zhou, Y.; Bao, Y.M.; Yang, W.Y.; Liu, Y.F.; Zhang, J.H. Location of the analgesic domain in Scorpion toxin BmK AGAP by mutagenesis of disulfide bridges. Biochem. Biophys. Res. Commun. 2010, 394, 330–334. [Google Scholar] [CrossRef]
- Liu, Y.F.; Ma, R.L.; Wang, S.L.; Duan, Z.Y.; Zhang, J.H.; Wu, L.J.; Wu, C.F.; Zhang, J.W. Expression of an antitumor–analgesic peptide from the venom of Chinese scorpion Buthus martensii karsch in Escherichia coli. Protein Expr. Purif. 2003, 27, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Liu, X.F.; Li, G.X.; Ban, M.Q.; Chen, J.Z.; Cui, Y.; Zhang, J.H.; Liu, Y.F.; Wu, C.F. Antinociceptive effects of AGAP, a recombinant neurotoxic polypeptide: Possible involvement of the Tetrodotoxin-Resistant sodium channels in small dorsal root ganglia neurons. Front. Pharmacol. 2016, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.P.; Mao, Q.H.; Lu, W.G.; Cai, X.T.; Chen, J.; Li, Q.; Cao, J.L. Inhibition of spinal MAPKs by scorpion venom peptide BmK AGAP produces a sensory-specific analgesic effect. Mol. Pain 2018, 14, 1744806918761238. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Cui, Y.; Song, Y.; Liu, Y.; Zhang, R.; Wu, C.; Zhang, J. Purification, characterization and functional expression of a new peptide with an analgesic effect from Chinese scorpion Buthus martensii Karsch (BmK AGP-SYPU1). Biomed. Chromatogr. 2011, 25, 801–807. [Google Scholar] [CrossRef]
- Yang, R.; Song, Y.; Wang, H.; Chen, C.; Bai, F.; Li, C. BMK DKK13, a Scorpion toxin, alleviates pain behavior in a RAT model of trigeminal neuralgia by modulating Voltage-Gated sodium channels and MAPKS/CREB pathway. Mol. Neurobiol. 2022, 59, 4535–4549. [Google Scholar] [CrossRef]
- Cao, Z.-Y.; Mi, Z.-M.; Cheng, G.-F.; Shen, W.-Q.; Xiao, X.; Liu, X.-M.; Yu, D.-Q.; Zhang, J.-W.; Ji, Y.-H. Purification and characterization of a new peptide with analgesic effect from the scorpion Buthus martensi Karch. J. Pept. Res. 2004, 64, 33–41. [Google Scholar] [CrossRef]
- Bai, F.; Song, Y.; Cao, Y.; Ban, M.; Zhang, Z.; Sun, Y.; Li, C. Scorpion Neurotoxin Syb-prII-1 Exerts Analgesic Effect through Nav1.8 Channel and MAPKs Pathway. Int. J. Mol. Sci. 2022, 23, 7065. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Z.; Zhang, Q.; Li, C.; Jin, W.; Liu, L.; Zhang, J.; Wu, Y.; Ji, Y. Investigation of binding modes and functional surface of scorpion toxins ANEP to sodium channels 1.7. Toxins 2017, 9, 387. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zhu, Y.; Zhang, L.; Ji, J.; Gui, M.; Wu, C.; Zhang, J.; Ji, Y. Study of Anti-Inflammatory and Analgesic Activity of Scorpion Toxins DKK-SP1/2 from Scorpion Buthus martensii Karsch (BmK). Toxins 2021, 13, 498. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, E.; Sang, M.; Wang, Z.; Zhang, Y.; Ye, J.; Wu, Y.; Ji, Y. Makatoxin-3, a thermostable Nav1.7 agonist from Buthus martensii Karsch (BmK) scorpion elicits non-narcotic analgesia in inflammatory pain models. J. Ethnopharmacol. 2022, 288, 114998. [Google Scholar] [CrossRef] [PubMed]
- Maatoug, R.; Jebali, J.; Guieu, R.; De Waard, M.; Kharrat, R. BotAF, a new Buthus occitanus tunetanus scorpion toxin, produces potent analgesia in rodents. Toxicon 2018, 149, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Herzig, V.; Dekan, Z.; Rosengren, K.J.; Payne, C.D.; Hasan, M.M.; Hardy, M.C.; King, G.F.; Vetter, I.; Lewis, R.J. Novel scorpion toxin Ω-Buthitoxin-HF1A selectively inhibits calcium influx via CAV3.3 and CAV3.2 and alleviates allodynia in a mouse model of acute postsurgical pain. Int. J. Mol. Sci. 2024, 25, 4745. [Google Scholar] [CrossRef]
- Hoang, A.N.; Vo, H.D.M.; Vo, N.P.; Kudryashova, K.S.; Nekrasova, O.V.; Feofanov, A.V.; Kasheverov, I.E.; Tsetlin, V.I.; Utkin, Y.N. Vietnamese Heterometrus laoticus scorpion venom: Evidence for analgesic and anti-inflammatory activity and isolation of new polypeptide toxin acting on Kv1.3 potassium channel. Toxicon 2014, 77, 40–48. [Google Scholar] [CrossRef]
- Chang, Y.; Li, Y.; Bao, Y.; An, L. Neurotoxic activity of Gln49 phospholipase A2 from Gloydius ussuriensis snake venom. J. Appl. Toxicol. 2007, 27, 447–452. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, B.; Li, W.; Zhou, C.; Ji, F.; Xie, Q.; Xu, X.; An, L. Mechanisms of Analgesic Action of Gln49-PLA2 from Gloydius ussurensis Snake Venom. Appl. Biochem. Biotechnol. 2010, 160, 773–779. [Google Scholar] [CrossRef]
- Miyazawa, K.; Noguchi, T. Distribution and Origin of Tetrodotoxin. J. Toxicol. Toxin Rev. 2001, 20, 11–33. [Google Scholar] [CrossRef]
- Nieto, F.R.; Cobos, E.J.; Tejada, M.Á.; Sánchez-Fernández, C.; González-Cano, R.; Cendán, C.M. Tetrodotoxin (TTX) as a therapeutic agent for pain. Mar. Drugs 2012, 10, 281–305. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Fields, H.L.; Duncan, K.G.; Duncan, J.L.; Jones, M.R. Experimental study of tetrodotoxin, a long-acting topical anesthetic. Am. J. Ophthalmol. 1998, 125, 481–487. [Google Scholar] [CrossRef]
- Beloeil, H.; Ababneh, Z.; Chung, R.; Zurakowski, D.; Mulkern, R.V.; Berde, C.B. Effects of bupivacaine and tetrodotoxin on carrageenan-induced hind paw inflammation in rats (Part 1). Anesthesiology 2006, 105, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Marcil, J.; Walczak, J.-S.; Guindon, J.; Ngoc, A.H.; Lu, S.; Beaulieu, P. Antinociceptive effects of tetrodotoxin (TTX) in rodents. Br. J. Anaesth. 2006, 96, 761–768. [Google Scholar] [CrossRef]
- Lyu, Y.S.; Park, S.K.; Chung, K.; Chung, J.M. Low dose of tetrodotoxin reduces neuropathic pain behaviors in an animal model. Brain Res. 2000, 871, 98–103. [Google Scholar] [CrossRef]
- Nieto, F.R.; Entrena, J.M.; Cendán, C.M.; Del Pozo, E.; Vela, J.M.; Baeyens, J.M. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain 2008, 137, 520–531. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Duncan, K.G.; Fields, H.L.; Jones, M.R. Tetrodotoxin: Anesthetic activity in the de-epithelialized cornea. Graefes Arch. Clin. Exp. Ophthalmol. 1999, 237, 790–794. [Google Scholar] [CrossRef]
- Tao, J.; Jiang, F.; Liu, C.; Liu, Z.; Zhu, Y.; Xu, J.; Chen, X.; Chen, Y.; Chen, Y.; Chen, Z.; et al. Modulatory effects of bufalin, an active ingredient from toad venom on voltage-gated sodium channels. Mol. Biol. Rep. 2018, 45, 721–740. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, F.; Corrales-Garcia, L.L.; Olamendi-Portugal, T.; Restano-Cassulini, R.; Vega, R.; Vega, M.; Zamudio, F.; Batista, C.V.F.; Possani, L.D. Identification, chemical synthesis and heterologous expression of an antinociceptive peptide from the veined tree frog Trachycephalus typhonius. Process Biochem. 2017, 62, 205–214. [Google Scholar] [CrossRef]
- Wei, L.; Dong, L.; Zhao, T.; You, D.; Liu, R.; Liu, H.; Li, Z.; Lai, R. Analgesic and anti-inflammatory effects of the amphibian neurotoxin, anntoxin. Biochimie 2011, 93, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, C.; Xu, X.; Zhang, Y.; Gong, X.; Yang, Z.; Xu, L.; Chen, Z.; Wu, Y.; Liang, S. Naja atra venom peptide reduces pain by selectively blocking the voltage-gated sodium channel Nav1.8. J. Biol. Chem. 2019, 294, 7324–7334. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xiao, Y.; Kang, D.; Liu, J.; Li, Y.; Undheim, E.A.B.; Klint, J.K.; Rong, M.; Lai, R.; King, G.F. Discovery of a selective Na V 1.7 inhibitor from centipede venom with analgesic efficacy exceeding morphine in rodent pain models. Proc. Natl. Acad. Sci. USA 2013, 110, 17534–17539. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.S. The evolution of vertebrate opioid receptors. Front. Biosci. 2009, 14, 1247–1269. [Google Scholar] [CrossRef]
- Pasternak, G.W. Molecular Biology of Opioid Analgesia. J. Pain Symptom Manag. 2005, 29, S2–S9. [Google Scholar] [CrossRef]
- Gavériaux-Ruff, C.; Kieffer, B.L. Delta opioid receptor analgesia. Behav. Pharmacol. 2011, 22, 405–414. [Google Scholar] [CrossRef]
- Benarroch, E.E. Endogenous opioid systems. Neurology 2012, 79, 807–814. [Google Scholar] [CrossRef]
- Holden, J.E.; Jeong, Y.; Forrest, J.M. The Endogenous Opioid System and Clinical Pain Management. AACN Clin. Issues 2005, 16, 291–301. [Google Scholar] [CrossRef]
- Nandhu, M.S.; Naijil, G.; Smijin, S.; Jayanarayanan, S.; Paulose, C.S. Opioid system functional regulation in neurological disease management. J. Neurosci. Res. 2010, 88, 3215–3221. [Google Scholar] [CrossRef]
- Shkenderov, S.; Koburova, K. Adolapin–A newly isolated analgetic and anti-inflammatory polypeptide from bee venom. Toxicon 1982, 20, 317–321. [Google Scholar] [CrossRef]
- Junior, W.S.; Martins, P.R. KAMBÔ: An Amazonian enigma. J. Venom Res. 2020, 10, 13–17. [Google Scholar]
- Hesselink, J.M.K. Rediscovery of Ceruletide, a CCK Agonist, as an Analgesic Drug. J. Pain Res. 2020, 13, 123–130. [Google Scholar] [CrossRef]
- Anastasi, A.; Erspamer, V.; Endean, R. Isolation and structure of caerulein, an active decapeptide from the skin ofHyla caerulea. Experientia 1967, 23, 699–700. [Google Scholar] [CrossRef]
- Ando, H.; Ukena, K.; Nagata, S. Handbook of Hormones: Comparative Endocrinology for Basic and Clinical Research, 2nd ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 1, pp. 107–109. [Google Scholar]
- Melchiorri, P.; Negri, L. The dermorphin peptide family. Gen. Pharmacol. 1996, 27, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Erspamer, V.; Melchiorri, P.; Falconieri-Erspamer, G.; Negri, L.; Corsi, R.; Severini, C.; Barra, D.; Simmaco, M.; Kreil, G. Deltorphins: A family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites. Proc. Natl. Acad. Sci. USA 1989, 86, 5188–5192. [Google Scholar] [CrossRef]
- Kreil, G.; Barra, D.; Simmaco, M.; Erspamer, V.; Erspamer, G.F.; Negri, L.; Severini, C.; Corsi, R.; Melchiorri, P. Deltorphin, a novel amphibian skin peptide with high selectivity and affinity for δ opioid receptors. Eur. J. Pharmacol. 1989, 162, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.C.; Wong, P.T.H.; Gopalakrishnakone, P. A novel analgesic toxin (hannalgesin) from the venom of king cobra (Ophiophagus hannah). Toxicon 1995, 33, 1425–1431. [Google Scholar] [CrossRef]
- Giorgi, R.; Bernardi, M.M.; Cury, Y. Analgesic effect evoked by low molecular weight substances extracted from Crotalus durissus terrificus venom. Toxicon 1993, 31, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Picolo, G.; Giorgi, R.; Bernardi, M.M.; Cury, Y. The antinociceptive effect of Crotalus durissus terrificus snake venom is mainly due to a supraspinally integrated response. Toxicon 1998, 36, 223–227. [Google Scholar] [CrossRef]
- Picolo, G.; Giorgi, R.; Cury, Y. δ-Opioid receptors and nitric oxide mediate the analgesic effect of Crotalus durissus terrificus snake venom. Eur. J. Pharmacol. 2000, 391, 55–62. [Google Scholar] [CrossRef]
- Picolo, G.; Cassola, A.C.; Cury, Y. Activation of peripheral ATP-sensitive K+ channels mediates the antinociceptive effect of Crotalus durissus terrificus snake venom. Eur. J. Pharmacol. 2003, 469, 57–64. [Google Scholar] [CrossRef]
- Picolo, G. Peripheral neuronal nitric oxide synthase activity mediates the antinociceptive effect of Crotalus durissus terrificus snake venom, a delta and kappaopioid receptor agonist. Life Sci. 2004, 75, 559–573. [Google Scholar] [CrossRef]
- Mancin, A.C.; Soares, A.M.; Andrião-Escarso, S.H.; Faça, V.M.; Greene, L.J.; Zuccolotto, S.; Giglio, J.R. The analgesic activity of crotamine, a neurotoxin from Crotalus durissus terrificus (South American rattlesnake) venom: A biochemical and pharmacological study. Toxicon 1998, 36, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Hang, B.D.O.; Lee, J.S.; Yang, H.C.; Nguyen, A.N.; Krupa, M.; Kini, R.M.; Doley, R. Antinociceptive and Anti-Inflammatory effects of recombinant crotamine in mouse models of pain. Toxins 2021, 13, 707. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, G.; Franzoni, L.; De Chiara, C.; Mancin, A.C.; Giglio, J.R.; Spisni, A. Solution structure of crotamine, a Na+ channel affecting toxin from Crotalus durissus terrificus venom. Eur. J. Biochem. 2003, 270, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Picolo, G.; Gutierrez, V.P.; Brigatte, P.; Zambelli, V.O.; Camargo, A.C.M.; Cury, Y. Crotalphine, a novel potent analgesic peptide from the venom of the South American rattlesnake Crotalus durissus terrificus. Peptides 2008, 29, 1293–1304. [Google Scholar] [CrossRef]
- De Freitas, B.G.; Hösch, N.G.; Pereira, L.M.; Barbosa, T.C.; Picolo, G.; Cury, Y.; Camargo, A.C.M. PKCΖ-Mitogen-Activated protein kinase signaling mediates Crotalphine-Induced Antinociception. Toxins 2021, 13, 912. [Google Scholar] [CrossRef]
- Remuzgo, C.; Sampaio, S.; Picolo, G.; Machini, M.; Cury, Y. Crotalphine: Fluorescent analogues, structural analysis and its effect on co-cultures of peritoneal macrophages and DRG neurons (LB615). FASEB J. 2014, 28, lb615. [Google Scholar] [CrossRef]
- Liang, Y.X.; Jiang, W.J.; Han, L.P.; Zhao, S.J. Peripheral and spinal antihyperalgesic activity of najanalgesin isolated from Naja naja atra in a rat experimental model of neuropathic pain. Neurosci. Lett. 2009, 460, 191–195. [Google Scholar] [CrossRef]
- Jiang, W.J.; Liang, Y.X.; Han, L.P.; Qiu, P.X.; Yuan, J.; Zhao, S.J. Purification and characterization of a novel antinociceptive toxin from Cobra venom (Naja naja atra). Toxicon 2008, 52, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.G.L.D.; Silva, L.L.C.E.; Soares, M.B.P.; Villarreal, C.F. Antinociceptive properties of Micrurus lemniscatus venom. Toxicon 2012, 60, 1005–1012. [Google Scholar] [CrossRef]
- Fine, P.G.; Rosenfeld, M.J. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med. J. 2013, 4, e0022. [Google Scholar] [CrossRef]
- Walker, J.M.; Huang, S.M. Cannabinoid analgesia. Pharmacol. Ther. 2002, 95, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Vučković, S.; Srebro, D.; Vujović, K.S.; Vučetić, Č.; Prostran, M. Cannabinoids and pain: New insights from old molecules. Front. Pharmacol. 2018, 9, 1259. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Tang, Y. The central cannabinoid receptor type-2 (CB2) and chronic pain. Int. J. Neurosci. 2017, 127, 812–823. [Google Scholar] [CrossRef]
- Scott, D.A.; Wright, C.E.; Angus, J.A. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain 2004, 109, 124–131. [Google Scholar] [CrossRef]
- Jergova, S.; Perez, C.; Imperial, J.S.; Gajavelli, S.; Jain, A.; Abin, A.; Spitzer, N.; Pena, N.; Gajavelli, S.; Olivera, B.M.; et al. Cannabinoid receptor agonists from Conus venoms alleviate pain-related behavior in rats. Pharmacol. Biochem. Behav. 2021, 205, 173182. [Google Scholar] [CrossRef]
- Da Fonseca Pacheco, D.; Freitas, A.C.N.; Pimenta, A.M.C.; Duarte, I.D.G.; De Lima, M.E. A spider derived peptide, PnPP-19, induces central antinociception mediated by opioid and cannabinoid systems. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 34. [Google Scholar] [CrossRef]
- Freitas, A.C.N.; Pacheco, D.F.; Machado, M.F.M.; Carmona, A.K.; Duarte, I.D.G.; De Lima, M.E. PnPP-19, a spider toxin peptide, induces peripheral antinociception through opioid and cannabinoid receptors and inhibition of neutral endopeptidase. Br. J. Pharmacol. 2016, 173, 1491–1501. [Google Scholar] [CrossRef]
- Emerich, B.L.; Ferreira, R.C.M.; Cordeiro, M.N.; Borges, M.H.; Pimenta, A.M.C.; Figueiredo, S.G.; Duarte, I.D.G.; De Lima, M.E. δ-Ctenitoxin-Pn1a, a Peptide from Phoneutria nigriventer Spider Venom, Shows Antinociceptive Effect Involving Opioid and Cannabinoid Systems, in Rats. Toxins 2016, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Huang, Y.; Xie, X.; Huang, W.; Yin, J.; Lin, W.; Jia, Q.; Zeng, W. Anti-Inflammatory and antinociceptive activities of bufalin in rodents. Mediat. Inflamm. 2014, 2014, 171839. [Google Scholar] [CrossRef]
- Ji, D.; Liang, Z.; Liu, G.; Zhao, G.; Fang, J. Bufalin attenuates cancer-induced pain and bone destruction in a model of bone cancer. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 1211–1219. [Google Scholar] [CrossRef]
- Cosens, D.J.; Manning, A. Abnormal Electroretinogram from a Drosophila Mutant. Nature 1969, 224, 285–287. [Google Scholar] [CrossRef]
- Montell, C.; Rubin, G.M. Molecular characterization of the drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323. [Google Scholar] [CrossRef]
- White, J.P.M.; Cibelli, M.; Fidalgo, A.R.; Paule, C.C.; Noormohamed, F.; Urban, L.; Nilius, B.; McNaughton, P.A.; Nagy, I. Role of transient receptor potential and acid-sensing ion channels in peripheral inflammatory pain. Anesthesiology 2010, 112, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Pingle, S.C.; Matta, J.A.; Ahern, G.P. Capsaicin receptor: TRPV1 a promiscuous TRP channel. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 155–171. [Google Scholar] [CrossRef]
- Corey, D.P. New TRP channels in hearing and mechanosensation. Neuron 2003, 39, 585–588. [Google Scholar] [CrossRef]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef]
- Montell, C.; Birnbaumer, L.; Flockerzi, V. The TRP Channels, a remarkably functional family. Cell 2002, 108, 595–598. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Owsianik, G.; Nilius, B. TRP channels: An overview. Cell Calcium 2005, 38, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 261. [Google Scholar] [CrossRef]
- Zheng, J. Molecular mechanism of TRP channels. Compr. Physiol. 2013, 3, 221–242. [Google Scholar] [CrossRef]
- Andreev, Y.A.; Kozlov, S.A.; Korolkova, Y.V.; Dyachenko, I.A.; Bondarenko, D.A.; Skobtsov, D.I.; Murashev, A.N.; Kotova, P.D.; Rogachevskaja, O.A.; Kabanova, N.V.; et al. Polypeptide Modulators of TRPV1 Produce Analgesia without Hyperthermia. Mar. Drugs 2013, 11, 5100–5115. [Google Scholar] [CrossRef]
- Andreev, Y.A.; Kozlov, S.A.; Koshelev, S.G.; Ivanova, E.A.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Grishin, E.V. Analgesic Compound from Sea Anemone Heteractis crispa Is the First Polypeptide Inhibitor of Vanilloid Receptor 1 (TRPV1). J. Biol. Chem. 2008, 283, 23914–23921. [Google Scholar] [CrossRef]
- Logashina, Y.A.; Mosharova, I.V.; Korolkova, Y.V.; Shelukhina, I.V.; Dyachenko, I.A.; Palikov, V.A.; Palikova, Y.A.; Murashev, A.N.; Kozlov, S.A.; Andreev, Y.A. Peptide from Sea Anemone Metridium senile Affects Transient Receptor Potential Ankyrin-repeat 1 (TRPA1) Function and Produces Analgesic Effect. J. Biol. Chem. 2017, 292, 2992–3004. [Google Scholar] [CrossRef]
- Maleeva, E.E.; Palikova, Y.A.; Palikov, V.A.; Kazakov, V.A.; Simonova, M.A.; Logashina, Y.A.; Korolkova, Y.V.; Andreev, Y.A. Potentiating TRPA1 by sea anemone peptide MS 9a-1 reduces pain and inflammation in a model of osteoarthritis. Mar. Drugs 2023, 21, 617. [Google Scholar] [CrossRef]
- Logashina, Y.A.; Solstad, R.G.; Mineev, K.S.; Korolkova, Y.V.; Mosharova, I.V.; Dyachenko, I.A.; Palikov, V.A.; Palikova, Y.A.; Arseniev, A.S.; Stensvåg, K.; et al. New Disulfide-Stabilized fold provides sea anemone peptide to exhibit both antimicrobial and TRPA1 potentiating properties. Toxins 2017, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.M.R.; Souza, J.M.; Carobin, N.V.; Silva, J.F.; Santos, D.C.; Júnior, C.A.S.; Castro, I.J.; Reis, H.J.; Gomez, M.V. Phoneutria toxin PnTx3-5 inhibits TRPV1 channel with antinociceptive action in an orofacial pain model. Neuropharmacology 2020, 162, 107826. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Silva, C.R.; Trevisan, G.; Villarinho, J.G.; Cordeiro, M.N.; Richardson, M.; Borges, M.H.; Gomez, M.V.; Ferreira, J. Antinociceptive effect of a novel armed spider peptide Tx3-5 in pathological pain models in mice. Pflugers Arch. 2016, 468, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Castro-Junior, C.J.; Milano, J.; Souza, A.H.; Silva, J.F.; Rigo, F.K.; Dalmolin, G.; Silva, C.R.; Gomes, G.M.; Cordeiro, M.N.; Richardson, M.; et al. Phα1β toxin prevents capsaicin-induced nociceptive behavior and mechanical hypersensitivity without acting on TRPV1 channels. Neuropharmacology 2013, 71, 237–246. [Google Scholar] [CrossRef]
- Ferreira, L.A.; Aoki, C.T.; Santos, D.C.; Rezende, M.J.; Mendes, M.P.; Moura, R.A.; Gomez, M.V.; Ferreira, J. Antinociceptive synergism upon the joint use of methadone and Phα1β in a model of cancer-related pain in C57BL/6J mice. Life Sci. 2021, 278, 119582. [Google Scholar] [CrossRef]
- Rosa, F.; Trevisan, G.; Rigo, F.K.; Tonello, R.; Andrade, E.L.; Cordeiro, M.D.N.; Gomez, M.V.; Ferreira, J. Phα1β, a Peptide from the Venom of the Spider Phoneutria nigriventer Shows Antinociceptive Effects after Continuous Infusion in a Neuropathic Pain Model in Rats. Anesth. Analg. 2014, 119, 196–202. [Google Scholar] [CrossRef]
- Tonello, R.; Fusi, C.; Materazzi, S.; Marone, I.M.; De Logu, F.; Benemei, S.; Gonçalves, M.C.; Coppi, E.; Castro-Junior, C.J.; Gomez, M.V.; et al. The peptide Phα1β, from spider venom, acts as a TRPA1 channel antagonist with antinociceptive effects in mice. Br. J. Pharmacol. 2017, 174, 57–69. [Google Scholar] [CrossRef]
- Alessandri-Haber, N.; Dina, O.A.; Chen, X.; Levine, J.D. TRPC1 and TRPC6 Channels Cooperate with TRPV4 to Mediate Mechanical Hyperalgesia and Nociceptor Sensitization. J. Neurosci. 2009, 29, 6217–6228. [Google Scholar] [CrossRef]
- Park, S.P.; Kim, B.M.; Koo, J.Y.; Cho, H.; Lee, C.H.; Kim, M.; Na, H.S.; Oh, U. A tarantula spider toxin, GsMTx4, reduces mechanical and neuropathic pain. Pain 2008, 137, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Kampo, S.; Cui, Y.; Yu, J.; Anabah, T.W.; Falagán, A.A.; Bayor, M.T.; Wang, X.; Huang, W.; An, L.; Li, Z. Scorpion Venom peptide, AGAP inhibits TRPV1 and potentiates the analgesic effect of lidocaine. Heliyon 2021, 7, e08560. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Touska, F.; Vetter, I.; Kistner, K.; Kichko, T.I.; Teixeira, N.B.; Picolo, G.; Cury, Y.; Lewis, R.J.; Fischer, M.J.M.; et al. Crotalphine desensitizes TRPA1 ion channels to alleviate inflammatory hyperalgesia. Pain 2016, 157, 2504–2516. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, R.; Champigny, G.; Bassilana, F.; Heurteaux, C.; Lazdunski, M. A proton-gated cation channel involved in acid-sensing. Nature 1997, 386, 173–177. [Google Scholar] [CrossRef]
- Bartoi, T.; Augustinowski, K.; Polleichtner, G.; Gründer, S.; Ulbrich, M.H. Acid-sensing ion channel (ASIC) 1a/2a heteromers have a flexible 2:1/1:2 stoichiometry. Proc. Natl. Acad. Sci. USA 2014, 111, 8281–8286. [Google Scholar] [CrossRef]
- Deval, E.; Gasull, X.; Noël, J.; Salinas, M.; Baron, A.; Diochot, S.; Lingueglia, E. Acid-Sensing Ion Channels (ASICs): Pharmacology and implication in pain. Pharmacol. Ther. 2010, 128, 549–558. [Google Scholar] [CrossRef]
- Gründer, S.; Pusch, M. Biophysical properties of acid-sensing ion channels (ASICs). Neuropharmacology 2015, 94, 9–18. [Google Scholar] [CrossRef] [PubMed]
- García-Añoveros, J.; Derfler, B.; Neville-Golden, J.; Hyman, B.T.; Corey, D.P. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc. Natl. Acad. Sci. USA 1997, 94, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.; Lingueglia, E. Pharmacology of acid-sensing ion channels—Physiological and therapeutical perspectives. Neuropharmacology 2015, 94, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Wemmie, J.A.; Taugher, R.J.; Kreple, C.J. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 2013, 14, 461–471. [Google Scholar] [CrossRef]
- Kalina, R.S.; Gladkikh, I.N.; Dmitrenok, P.S.; Chernikov, O.V.; Koshelev, S.G.; Kvetkina, A.N.; Monastyrnaya, M.M.; Isaeva, M.P.; Kozlov, S.A. New APETx-like peptides from sea anemone Heteractis crispa modulate ASIC1a channels. Peptides 2018, 104, 41–49. [Google Scholar] [CrossRef]
- Kozlov, S.A.; Osmakov, D.I.; Andreev, Y.A.; Koshelev, S.G.; Gladkikh, I.N.; Monastyrnaya, M.M.; Loginova, N.A.; Grishin, E.V. A sea anemone polypeptide toxin inhibiting the ASIC3 acid-sensitive channel. Russ. J. Bioorg. Chem. 2012, 38, 578–583. [Google Scholar] [CrossRef]
- Osmakov, D.I.; Kozlov, S.A.; Andreev, Y.A.; Koshelev, S.G.; Sanamyan, N.P.; Sanamyan, K.E.; Dyachenko, I.A.; Bondarenko, D.A.; Murashev, A.N.; Mineev, K.S.; et al. Sea Anemone Peptide with Uncommon β-Hairpin Structure Inhibits Acid-sensing Ion Channel 3 (ASIC3) and Reveals Analgesic Activity. J. Biol. Chem. 2013, 288, 23116–23127. [Google Scholar] [CrossRef]
- Diochot, S.; Alloui, A.; Rodrigues, P.; Dauvois, M.; Friend, V.; Aissouni, Y.; Eschalier, A.; Lingueglia, E.; Baron, A. Analgesic effects of mambalgin peptide inhibitors of acid-sensing ion channels in inflammatory and neuropathic pain. Pain 2016, 157, 552–559. [Google Scholar] [CrossRef]
- Diochot, S.; Baron, A.; Salinas, M.; Douguet, D.; Scarzello, S.; Dabert-Gay, A.S.; Debayle, D.; Friend, V.; Alloui, A.; Lazdunski, M.; et al. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature 2012, 490, 552–555. [Google Scholar] [CrossRef]
- Dani, J.A.; Mayer, M.L. Structure and function of glutamate and nicotinic acetylcholine receptors. Curr. Opin. Neurobiol. 1995, 5, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, J.; Anand, R.; Gerzanich, V.; Peng, X.; Wang, F.; Wells, G. Chapter 10 Structure and function of neuronal nicotinic acetylcholine receptors. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 1996; Volume 109, pp. 125–137. [Google Scholar] [CrossRef]
- Dani, J.A. Overview of nicotinic receptors and their roles in the central nervous system. Biol. Psychiatry 2001, 49, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Hone, A.J.; McIntosh, J.M. Nicotinic acetylcholine receptors in neuropathic and inflammatory pain. FEBS Lett. 2018, 592, 1045–1062. [Google Scholar] [CrossRef]
- Hone, A.J.; McIntosh, J.M. Nicotinic acetylcholine receptors: Therapeutic targets for novel ligands to treat pain and inflammation. Pharmacol. Res. 2023, 190, 106715. [Google Scholar] [CrossRef] [PubMed]
- Cucchiaro, G.; Chaijale, N.; Commons, K.G. The locus coeruleus nucleus as a site of action of the antinociceptive and behavioral effects of the nicotinic receptor agonist, epibatidine. Neuropharmacology 2006, 50, 769–776. [Google Scholar] [CrossRef]
- Kesingland, A.C.; Gentry, C.T.; Panesar, M.S.; Bowes, M.A.; Vernier, J.-M.; Cube, R.; Chapman, K.; Lotarski, S.; El Kouhen, R.; Cortes-Burgos, L.A.; et al. Analgesic profile of the nicotinic acetylcholine receptor agonists, (+)-epibatidine and ABT-594 in models of persistent inflammatory and neuropathic pain. Pain 2000, 86, 113–118. [Google Scholar] [CrossRef]
- Rupniak, N.M.J.; Patel, S.; Marwood, R.; Webb, J.; Traynor, J.R.; Elliott, J.; Freedman, S.B.; Fletcher, S.R.; Hill, R.G. Antinociceptive and toxic effects of (+)-epibatidine oxalate attributable to nicotinic agonist activity. Br. J. Pharmacol. 1994, 113, 1487–1493. [Google Scholar] [CrossRef]
- Damaj, M.I.; Martin, B.R. Tolerance to the antinociceptive effect of epibatidine after acute and chronic administration in mice. Eur. J. Pharmacol. 1996, 300, 51–57. [Google Scholar] [CrossRef]
- Qian, N.C.; Li, N.T.; Shen, T.Y.; Libertine-Garahan, L.; Eckman, J.; Biftu, T.; Ip, S. Epibatidine is a nicotinic analgesic. Eur. J. Pharmacol. 1993, 250, R13–R14. [Google Scholar] [CrossRef]
- Khan, I.M.; Yaksh, T.L.; Taylor, P. Epibatidine binding sites and activity in the spinal cord. Brain Res. 1997, 753, 269–282. [Google Scholar] [CrossRef]
- Liu, C.; Wu, P.; Zhu, H.; Grieco, P.; Yu, R.; Gao, X.; Li, W.; McIntosh, J.M.; Lin, D. Rationally designed A-Conotoxin analogues maintained analgesia activity and weakened side effects. Molecules 2019, 24, 337. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhangsun, D.; Yu, G.; Su, R.; Luo, S. The α9α10 Nicotinic Acetylcholine Receptor Antagonist αO-Conotoxin GeXIVA[1,2] Alleviates and Reverses Chemotherapy-Induced Neuropathic Pain. Mar. Drugs 2019, 17, 265. [Google Scholar] [CrossRef]
- Peng, C.; Tang, S.; Pi, C.; Liu, J.; Wang, F.; Wang, L.; Zhou, M.; Chen, X.; Xu, A. Discovery of a novel class of conotoxin from Conus litteratus, lt14a, with a unique cysteine pattern. Peptides 2006, 27, 2174–2181. [Google Scholar] [CrossRef]
- Qiang, Y.; Wu, Y.; Zhao, D.; Zhao, B.; Wang, F.; Ren, S.; Zhang, L.; Zhangsun, D.; Luo, S. Discovery and characterization of the novel conotoxin Lv1d from Conus lividus that presents analgesic activity. Toxicon 2021, 194, 70–78. [Google Scholar] [CrossRef]
- Peng, C.; Chen, W.; Sanders, T.; Chew, G.; Liu, J.; Hawrot, E.; Chi, C.; Wang, C. Chemical synthesis and characterization of two α4/7-conotoxins. Acta Biochim. Biophys Sin 2010, 42, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Pacini, A.; Micheli, L.; Maresca, M.; Branca, J.J.V.; McIntosh, J.M.; Ghelardini, C.; Di Cesare Mannelli, L. The α9α10 nicotinic receptor antagonist α-conotoxin RgIA prevents neuropathic pain induced by oxaliplatin treatment. Exp. Neurol. 2016, 282, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Gajewiak, J.; Christensen, S.B.; Dowell, C.; Hararah, F.; Fisher, F.; Huynh, P.N.; Vetter, D.E.; Wack, M.F.; McIntosh, J.M. Selective Penicillamine Substitution Enables Development of a Potent Analgesic Peptide that Acts through a Non-Opioid-Based Mechanism. J. Med. Chem. 2021, 64, 9271–9278. [Google Scholar] [CrossRef] [PubMed]
- Sandall, D.W.; Satkunanathan, N.; Keays, D.A.; Polidano, M.A.; Liping, X.; Pham, V.; Down, J.G.; Khalil, Z.; Livett, B.G.; Gayler, K.R. A novel A-Conotoxin identified by gene sequencing is active in suppressing the vascular response to selective stimulation of sensory nerves in vivo. Biochemistry 2003, 42, 6904–6911. [Google Scholar] [CrossRef]
- Belgi, A.; Burnley, J.V.; MacRaild, C.A.; Chhabra, S.; Elnahriry, K.A.; Robinson, S.D.; Livett, B.G.; Robinson, A.J.; Norton, R.S. Alkyne-Bridged A-Conotoxin VC1.1 potently reverses mechanical allodynia in neuropathic pain models. J. Med. Chem. 2021, 64, 3222–3233. [Google Scholar] [CrossRef]
- Chen, R.; Robinson, S.E. The effect of cholinergic manipulations on the analgesic response to cobrotoxin in mice. Life Sci. 1990, 47, 1949–1954. [Google Scholar] [CrossRef]
- Xu, L.; Wang, S.; Zhang, L.; Liu, B.; Zheng, S.; Yao, M. Cobratoxin Alleviates Cancer-Induced Bone Pain in Rats via Inhibiting CaMKII Signaling Pathway after Acting on M4 Muscarinic Cholinergic Receptors. ACS Chem. Neurosci. 2022, 13, 1422–1432. [Google Scholar] [CrossRef]
- Shi, G.N.; Liu, Y.L.; Lin, H.M.; Yang, S.L.; Feng, Y.L.; Reid, P.F.; Qin, Z.H. Involvement of cholinergic system in suppression of formalin-induced inflammatory pain by cobratoxin. Acta Pharmacol. Sin. 2011, 32, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Lin, H.M.; Zou, R.; Wu, J.C.; Han, R.; Raymond, L.N.; Reid, P.F.; Qin, Z.H. Suppression of complete Freund’s adjuvant-induced adjuvant arthritis by cobratoxin. Acta Pharmacol. Sin. 2009, 30, 219–227. [Google Scholar] [CrossRef]
- Cheng, B.C.; Zhou, X.P.; Zhu, Q.; Gong, S.; Qin, Z.H.; Reid, P.F.; Raymond, L.N.; Zhang, J.W.; Jiang, Z.H. Cobratoxin inhibits pain-evoked discharge of neurons in thalamic parafascicular nucleus in rats: Involvement of cholinergic and serotonergic systems. Toxicon 2009, 54, 224–232. [Google Scholar] [CrossRef]
- Gong, S.; Liang, Q.; Zhu, Q.; Ding, D.; Yin, Q.; Tao, J.; Jiang, Z.H.; Zhang, J.W.; Qin, Z.H. Nicotinic acetylcholine receptor α7 subunit is involved in the cobratoxin-induced antinociception in an animal model of neuropathic pain. Toxicon 2015, 93, 31–36. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Feng, Q.; Zheng, Y.; Ni, H.; Shen, H.; Zhang, L.; Yao, M. Alpha-7 Nicotinic Receptor-Targeted cinobufagin induces antinociception and inhibits NF-ΚB signaling pathway in DRG neurons. ACS Chem. Neurosci. 2019, 10, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Rigo, F.K.; Rossato, M.F.; Trevisan, G.; De Prá, S.D.T.; Ineu, R.P.; Duarte, M.B.; de Castro Junior, C.J.; Ferreira, J.; Gomez, M.V. PhKv a toxin isolated from the spider venom induces antinociception by inhibition of cholinesterase activating cholinergic system. Scand. J. Pain 2017, 17, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Maemura, K.; Kanbara, K.; Tamayama, T.; Hayasaki, H. GABA and GABA receptors in the central nervous system and other organs. Int. Rev. Cytol. 2002, 213, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Chebib, M.; Johnston, G.A.R. The ‘ABC’ of Gaba Receptors: A Brief Review. Clin. Exp. Pharmacol. Physiol. 1999, 26, 937–940. [Google Scholar] [CrossRef]
- Munro, G.; Hansen, R.R.; Mirza, N.R. GABAA receptor modulation: Potential to deliver novel pain medicines? Eur. J. Pharmacol. 2013, 716, 17–23. [Google Scholar] [CrossRef]
- Qian, X.; Zhao, X.; Yu, L.; Yin, Y.; Zhang, X.D.; Wang, L.; Li, H.; Zhang, Y. Current status of GABA receptor subtypes in analgesia. Biomed. Pharmacother. 2023, 168, 115800. [Google Scholar] [CrossRef]
- Patel, S.; Naeem, S.; Kesingland, A.; Froestl, W.; Capogna, M.; Urban, L.; Fox, A. The effects of GABAB agonists and gabapentin on mechanical hyperalgesia in models of neuropathic and inflammatory pain in the rat. Pain 2001, 90, 217–226. [Google Scholar] [CrossRef]
- Dirig, D.M.; Yaksh, T.L. A novel alpha-conotoxin identified by gene sequencing is active in suppressing the vascular response to selective stimulation of sensory nerves in vivo. J. Pharmacol. Exp. Ther. 1995, 274, 635–640. [Google Scholar]
- Bravo-Hernández, M.; Corleto, J.A.; Barragán-Iglesias, P.; González-Ramírez, R.; Pineda-Farias, J.B.; Felix, R.; Calcutt, N.A.; Delgado-Lezama, R.; Marsala, M.; Yaksh, T.L. The α5 subunit containing GABAA receptors contribute to chronic pain. Pain 2016, 157, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.; Haythornthwaite, A.; Berecki, G.; Clark, R.J.; Craik, D.J.; Adams, D.J. Analgesic A-Conotoxins VC1.1 and RG1A inhibit N-Type calcium channels in rat sensory neurons via GABAB Receptor activation. J. Neurosci. 2008, 28, 10943–10951. [Google Scholar] [CrossRef]
- Huynh, T.G.; Cuny, H.; Slesinger, P.A.; Adams, D.J. Novel Mechanism of Voltage-Gated N-type (Cav2.2) Calcium Channel Inhibition Revealed through α-Conotoxin Vc1.1 Activation of the GABAB Receptor. Mol. Pharmacol. 2015, 87, 240–250. [Google Scholar] [CrossRef]
- Cuny, H.; De Faoite, A.; Huynh, T.G.; Yasuda, T.; Berecki, G.; Adams, D.J. Γ-Aminobutyric acid type B (GABAB) receptor expression is needed for inhibition of N-type (CAV2.2) calcium channels by analgesic A-Conotoxins. J. Biol. Chem. 2012, 287, 23948–23957. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, M.; Yu, S.; Huang, Q.; Chen, R.; Xu, S.; Zhangsun, D.; Luo, S. A Single Amino Acid Replacement Boosts the Analgesic Activity of α-Conotoxin AuIB through the Inhibition of the GABABR-Coupled N-Type Calcium Channel. Mar. Drugs 2022, 20, 750. [Google Scholar] [CrossRef]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, function, and pharmacology of glutamate receptor ion channels. Pharmacol. Rev. 2021, 73, 1469–1658. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.C.; Jane, D.E. The glutamate story. Br. J. Pharmacol. 2006, 147, S100–S108. [Google Scholar] [CrossRef]
- Gintzler, A.R.; Liu, N.J. Arbiters of endogenous opioid analgesia: Role of CNS estrogenic and glutamatergic systems. Transl. Res. 2021, 234, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Budai, D.; Larson, A.A. GYKI 52466 inhibits AMPA/kainate and peripheral mechanical sensory activity. NeuroReport 1994, 5, 881–884. [Google Scholar] [CrossRef]
- Cumberbatch, M.J.; Chizh, B.A.; Headley, P.M. AMPA receptors have an equal role in spinal nociceptive and non-nociceptive transmission. NeuroReport 1994, 5, 877–880. [Google Scholar] [CrossRef]
- Bleakman, D.; Alt, A.; Nisenbaum, E.S. Glutamate receptors and pain. Semin. Cell Dev. Biol. 2006, 17, 592–604. [Google Scholar] [CrossRef]
- Brassai, A.; Suvanjeiev, R.-G.; Bán, E.G.; Lakatos, M. Role of synaptic and nonsynaptic glutamate receptors in ischaemia induced neurotoxicity. Brain Res. Bull. 2015, 112, 1–6. [Google Scholar] [CrossRef]
- Liu, X.; Yao, G.; Wang, K.; Liu, Y.; Wan, X.; Jiang, H. Structural and Functional Characterization of Conotoxins from Conus achatinus Targeting NMDAR. Mar. Drugs 2020, 18, 135. [Google Scholar] [CrossRef]
- Xiao, C.; Huang, Y.; Dong, M.; Hu, J.; Hou, S.; Castellino, F.J.; Prorok, M.; Dai, Q. NR2B-selective conantokin peptide inhibitors of the NMDA receptor display enhanced antinociceptive properties compared to non-selective conantokins. Neuropeptides 2009, 43, 127–133. [Google Scholar] [CrossRef]
- Malmberg, A.B.; Gilbert, H.; McCabe, R.T.; Basbaum, A.I. Powerful antinociceptive effects of the cone snail venom-derived subtype-selective NMDA receptor antagonists conantokins G and T. Pain 2003, 101, 109–116. [Google Scholar] [CrossRef]
- Sorkin, L.S.; Yaksh, T.L.; Doom, C.M. Pain models display differential sensitivity to CA2+-Permeable Non-NMDA glutamate receptor antagonists. Anesthesiology 2001, 95, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Savidge, J.R.; Bristow, D.R. Ca2+ permeability and Joro spider toxin sensitivity of AMPA and kainate receptors on cerebellar granule cells. Eur. J. Pharmacol. 1998, 351, 131–138. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, M.M.; Binda, N.S.; Reis, M.P.F.C.A.; Diniz, D.M.; Cordeiro, M.N.; Borges, M.H.; Gomez, M.V. Spinal antinociceptive effect of the PnTx4(5-5) peptide is possibly mediated by the NMDA autoreceptors. J. Venom. Anim. Toxins Incl. Trop. Dis. 2024, 30, e20230103. [Google Scholar] [CrossRef]
- Rigo, F.K.; Bochi, G.V.; Pereira, A.L.; Adamante, G.; Ferro, P.R.; De Prá, S.D.T.; Milioli, A.M.; de Souza, A.H.; da Silveira, V.P.; de Oliveira, S.M.; et al. TsNTxP, a non-toxic protein from Tityus serrulatus scorpion venom, induces antinociceptive effects by suppressing glutamate release in mice. Eur. J. Pharmacol. 2019, 855, 65–74. [Google Scholar] [CrossRef]
- Burnstock, G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 2007, 87, 659–797. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic receptors and pain. Curr. Pharm. Des. 2009, 15, 1717–1735. [Google Scholar] [CrossRef] [PubMed]
- Donnelly-Roberts, D.; McGaraughty, S.; Shieh, C.C.; Honore, P.; Jarvis, M.F. Painful purinergic receptors. J. Pharmacol. Exp. Ther. 2008, 324, 409–415. [Google Scholar] [CrossRef]
- Spinaci, A.; Buccioni, M.; Ben, D.D.; Marucci, G.; Volpini, R.; Lambertucci, C. P2X3 receptor ligands: Structural features and potential therapeutic applications. Front. Pharmacol. 2021, 12, 653561. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic mechanisms and pain. Adv. Pharmacol. 2016, 75, 91–137. [Google Scholar] [CrossRef]
- Grishin, E.V.; Savchenko, G.A.; Vassilevski, A.A.; Korolkova, Y.V.; Boychuk, Y.A.; Viatchenko-Karpinski, V.Y.; Nadezhdin, K.D.; Arseniev, A.S.; Pluzhnikov, K.A.; Kulyk, V.B.; et al. Novel peptide from spider venom inhibits P2X3 receptors and inflammatory pain. Ann. Neurol. 2010, 67, 680–683. [Google Scholar] [CrossRef]
- Kabanova, N.V.; Vassilevski, A.A.; Rogachevskaja, O.A.; Bystrova, M.F.; Korolkova, Y.V.; Pluzhnikov, K.A.; Romanov, R.A.; Khokhlov, A.A.; Kazin, E.M.; Grishin, E.V. Modulation of P2X3 receptors by spider toxins. Biochim. Biophys. Acta 2012, 1818, 2868–2875. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zhong, W.; Guo, L.; Ji, J.; Nie, H. Effect of Bufalin-PLGA microspheres in the alleviation of neuropathic pain via the CCI model. Front. Pharmacol. 2022, 13, 910885. [Google Scholar] [CrossRef] [PubMed]
- Bantel, C.; Eisenach, J.C.; Duflo, F.; Tobin, J.R.; Childers, S.R. Spinal nerve ligation increases α2-adrenergic receptor G-protein coupling in the spinal cord. Brain Res. 2005, 1038, 76–82. [Google Scholar] [CrossRef]
- Smith, M.S.; Schambra, U.B.; Wilson, K.H.; Page, S.O.; Hulette, C.; Light, A.R.; Schwinn, D.A. α2-Adrenergic receptors in human spinal cord: Specific localized expression of mRNA encoding α2-adrenergic receptor subtypes at four distinct levels. Mol. Brain Res. 1995, 34, 109–117. [Google Scholar] [CrossRef]
- Kalso, E.A.; Pöyhiä, R.; Rosenberg, P.H. Spinal antinociception by dexmedetomidine, a highly selective A2-Adrenergic agonist. Pharmacol. Toxicol. 1991, 68, 140–143. [Google Scholar] [CrossRef]
- Gao, J.; Sun, Z.; Xiao, Z.; Du, Q.; Niu, X.; Wang, G.; Zhang, S.; Wang, M.Q.; Sun, T. Dexmedetomidine modulates neuroinflammation and improves outcome via alpha2-adrenergic receptor signaling after rat spinal cord injury. Br. J. Anaesth. 2019, 123, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.B.; Kang, M.S.; Han, H.J.; Beitz, A.J.; Lee, J.H. Visceral antinociception produced by bee venom stimulation of the Zhongwan acupuncture point in mice: Role of α2 adrenoceptors. Neurosci. Lett. 2001, 308, 133–137. [Google Scholar] [CrossRef]
- Baek, Y.H.; Huh, J.E.; Lee, J.D.; Choi, D.Y.; Park, D.S. Antinociceptive effect and the mechanism of bee venom acupuncture (Apipuncture) on inflammatory pain in the rat model of collagen-induced arthritis: Mediation by α2-Adrenoceptors. Brain Res. 2006, 1073, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jeon, C.; Lee, J.; Jang, J.; Quan, F.; Lee, K.; Kim, W.; Park, D.; Lee, S.; Kim, S.K. Suppressive effects of BEE venom acupuncture on Paclitaxel-Induced neuropathic pain in rats: Mediation by spinal A2-Adrenergic receptor. Toxins 2017, 9, 351. [Google Scholar] [CrossRef]
- Roh, D.H.; Kwon, Y.B.; Kim, H.W.; Ham, T.W.; Yoon, S.Y.; Kang, S.Y.; Han, H.J.; Lee, H.J.; Choi, S.M.; Ryu, Y.H.; et al. Acupoint stimulation with diluted bee venom (apipuncture) alleviates thermal hyperalgesia in a rodent neuropathic pain model: Involvement of spinal alpha2-adrenoceptors. J. Pain 2004, 5, 297–303. [Google Scholar] [CrossRef]
- Kang, S.Y.; Roh, D.H.; Yoon, S.Y.; Moon, J.Y.; Kim, H.W.; Lee, H.J.; Beitz, A.J.; Lee, J.H. Repetitive treatment with diluted Bee venom reduces neuropathic pain via potentiation of locus coeruleus noradrenergic neuronal activity and modulation of spinal NR1 phosphorylation in rats. J. Pain 2012, 13, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.M. Bradykinin receptors. Gen. Pharmacol. 1997, 28, 1–6. [Google Scholar] [CrossRef]
- Hall, J.M. Bradykinin receptors: Pharmacological properties and biological roles. Pharmacol. Ther. 1992, 56, 131–190. [Google Scholar] [CrossRef]
- Steranka, L.R.; Manning, D.C.; DeHaas, C.J.; Ferkany, J.W.; Borosky, S.A.; Connor, J.R.; Vavrek, R.J.; Stewart, J.M.; Snyder, S.H. Bradykinin as a pain mediator: Receptors are localized to sensory neurons, and antagonists have analgesic actions. Proc. Natl. Acad. Sci. USA 1988, 85, 3245–3249. [Google Scholar] [CrossRef]
- Burgess, G.M.; Perkins, M.N.; Rang, H.P.; Campbell, E.A.; Brown, M.C.; McIntyre, P.; Urban, L.; Dziadulewicz, E.K.; Ritchie, T.J.; Hallett, A.; et al. Bradyzide, a potent non-peptide B2 bradykinin receptor antagonist with long-lasting oral activity in animal models of inflammatory hyperalgesia. Br. J. Pharmacol. 2000, 129, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Mortari, M.R.; Cunha, A.O.S.; Carolino, R.O.G.; Coutinho-Netto, J.; Tomaz, J.C.; Lopes, N.P.; dos Santos, W.F. Inhibition of acute nociceptive responses in rats after i.c.v. injection of Thr6-bradykinin, isolated from the venom of the social wasp, Polybia occidentalis. Br. J. Pharmacol. 2007, 151, 860–869. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Arslan, G.; Halldner, L.; Kull, B.; Schulte, G.; Wasserman, W. Structure and function of adenosine receptors and their genes. Naunyn-Schmiedebergs Arch. Pharmacol. 2000, 362, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of adenosine receptors: The state of the art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Palmer, T.M.; Stiles, G.L. Adenosine receptors. Neuropharmacology 1995, 34, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Varani, K. Adenosine as a Multi-Signalling Guardian Angel in Human Diseases: When, Where and How Does it Exert its Protective Effects? Trends Pharmacol. Sci. 2016, 37, 419–434. [Google Scholar] [CrossRef]
- Lima, F.O.; Souza, G.R.; Verri, W.A.; Parada, C.A.; Ferreira, S.H.; Cunha, F.Q.; Cunha, T.M. Direct blockade of inflammatory hypernociception by peripheral A1 adenosine receptors: Involvement of the NO/cGMP/PKG/KATP signaling pathway. Pain 2010, 151, 506–515. [Google Scholar] [CrossRef]
- Maione, S.; De Novellis, V.; Cappellacci, L.; Palazzo, E.; Vita, D.; Luongo, L.; Stella, L.; Franchetti, P.; Marabese, I.; de Chiaro, M.; et al. The antinociceptive effect of 2-chloro-2′-C-methyl-N6-cyclopentyladenosine (2′-Me-CCPA), a highly selective adenosine A1 receptor agonist, in the rat. Pain 2007, 131, 281–292. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, J.; Yang, Q.; Ye, Y. Cobra neurotoxin produces central analgesic and hyperalgesic actions via adenosine A1 and A2A receptors. Mol. Pain 2017, 13, 1744806917720336. [Google Scholar] [CrossRef]
- Wank, S.A. Cholecystokinin receptors. Am. J. Physiol. 1995, 269, G628–G646. [Google Scholar] [CrossRef]
- Crawley, J.N.; Corwin, R.L. Biological actions of cholecystokinin. Peptides 1994, 15, 731–755. [Google Scholar] [CrossRef]
- Roca-Lapirot, O.; Fossat, P.; Ma, S.; Egron, K.; Trigilio, G.; López-González, M.J.; Salio, C.; Darbon, P.; Landry, M.; Radwani, H. Acquisition of analgesic properties by the cholecystokinin (CCK)/CCK2 receptor system within the amygdala in a persistent inflammatory pain condition. Pain 2019, 160, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Danigo, A.; Bourthoumieu, S.; Mroué, M.; Desmoulière, A.; Sturtz, F.; Funalot, B.; Demiot, C. The cholecystokinin type 2 receptor, a pharmacological target for pain management. Pharmaceuticals 2021, 14, 1185. [Google Scholar] [CrossRef]
- Baber, N.S.; Dourish, C.T.; Hill, D.R. The role of CCK, caerulein, and CCK antagonists in nociception. Pain 1989, 39, 307–328. [Google Scholar] [CrossRef]
- DeSantana, J.M.; Da Silva, L.F.S.; Sluka, K.A. Cholecystokinin receptors mediate tolerance to the analgesic effect of TENS in arthritic rats. Pain 2009, 148, 84–93. [Google Scholar] [CrossRef]
- Jackway, R.J.; Maselli, V.M.; Musgrave, I.F.; Maclean, M.J.; Tyler, M.J.; Bowie, J.H. Skin peptides from anurans of the Litoria rubella Group: Sequence determination using electrospray mass spectrometry. Opioid activity of two major peptides. Rapid Commun. Mass Spectrom. 2009, 23, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Ellis-Steinborner, S.T.; Scanlon, D.; Musgrave, I.F.; Tran, T.T.N.; Hack, S.; Wang, T.; Bowie, J.H.; Tyler, M.J. An unusual kynurenine-containing opioid tetrapeptide from the skin gland secretion of the Australian red tree frog Litoria rubella. Sequence determination by electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Basso, N.; Bagarani, M.; Materia, A.; Gizzonio, D.; De Paolis, C.; Praga, C.; Speranza, V. Effect of caerulein in patients with biliary colic pain. Gastroenterology 1985, 89, 605–609. [Google Scholar] [CrossRef]
- Zetler, G. Analgesia and ptosis caused by caerulein and cholecystokinin octapeptide (CCK-8). Neuropharmacology 1980, 19, 415–422. [Google Scholar] [CrossRef]
- Godínez-Chaparro, B.; Barragán-Iglesias, P.; Castañeda-Corral, G.; Rocha-González, H.I.; Granados-Soto, V. Role of peripheral 5-HT4, 5-HT6, and 5-HT7 receptors in development and maintenance of secondary mechanical allodynia and hyperalgesia. Pain 2011, 152, 687–697. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chang, W.J.; Lin, C.S.; Huang, C.Y.; Wang, H.F.; Sun, W.H. Serotonin receptor 5-HT2BMediates Serotonin-Induced Mechanical hyperalgesia. J. Neurosci. 2011, 31, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Obata, H.; Kawahara, K.; Saito, S.; Goto, F. Peripheral 5-HT2A receptor antagonism attenuates primary thermal hyperalgesia and secondary mechanical allodynia after thermal injury in rats. Pain 2006, 122, 130–136. [Google Scholar] [CrossRef]
- Sommer, C. Serotonin in pain and analgesia: Actions in the periphery. Mol. Neurobiol. 2004, 30, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Zaharenko, A.J.; Picolo, G.; Ferreira, W.A.; Murakami, T.; Kazuma, K.; Hashimoto, M.; Cury, Y.; Satake, M.; Konno, K. Bunodosine 391: An Analgesic Acylamino Acid from the Venom of the Sea Anemone Bunodosoma cangicum. J. Nat. Prod. 2011, 74, 378–382. [Google Scholar] [CrossRef]
- Medeiros, A.C.; Medeiros, P.; De Freitas, R.L.; Da Silva Júnior, P.I.; Coimbra, N.C.; Santos, W.F.D. Acanthoscurria gomesiana spider-derived synthetic mygalin in the dorsal raphe nucleus modulates acute and chronic pain. J. Biochem. Mol. Toxicol. 2021, 35, e22877. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Song, B.; Mo, G.; Yuan, M.; Li, H.; Wang, P.; Li, D.; Lai, R. A Novel Neurotoxin from Venom of the Spider, Brachypelma albopilosum. PLoS ONE 2014, 9, e110221. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Kim, Y.; Choi, J.; Jeon, S.; Kim, D.; Kim, H.; Lee, H.; Kang, K. Antiallodynic effects of Bee venom in an animal model of complex regional pain syndrome Type 1 (CRPS-I). Toxins 2017, 9, 285. [Google Scholar] [CrossRef]
- Kwon, Y.B.; Lee, J.D.; Lee, H.J.; Han, H.J.; Mar, W.C.; Kang, S.K.; Beitz, A.J.; Lee, J.H. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain 2001, 90, 271–280. [Google Scholar] [CrossRef]
- Chen, H.S.; Qu, F.; He, X.; Liao, D.; Kang, S.M.; Lu, S.J. The anti-nociceptive effect and the possible mechanism of acupoint stimulation caused by chemical irritants in the bee venom pain model. Brain Res. 2010, 1355, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zagaja, M.; Zagaja, A.; Szala-Rycaj, J.; Szewczyk, A.; Maruszewska, A.; Łuszczki, J.; Andres-Mach, M. Influence of bee venom on antinociceptive activity of selected analgesic drugs in hot plate test in mice. Ann. Agric. Environ. Med. 2024, 31, 506–512. [Google Scholar] [CrossRef]
- Ayoub, M.; Fayjaloun, S.; Roufayel, R.; Obeid, D.E.; Fajloun, Z.; Rima, M. Influence of Apis mellifera syriaca Bee Venom on Nociception and Inflammatory Cytokine Profiles in Experimental Hyperalgesia. Toxins 2025, 17, 18. [Google Scholar] [CrossRef]
- Mortari, M.R.; Anjos, L.D.; Gonçalves, J.C.; De Araujo, B.P. Antinociceptive activity of the peptide fraction from the venom of social wasp Pseudopolybia vespiceps testacea. Pharmacogn. Mag. 2019, 15, 322–327. [Google Scholar] [CrossRef]
- Gonçalves, J.; Rangel, M.; Biolchi, A.; Alves, E.; Moreira, K.; Silva, L.; Mortari, M. Antinociceptive properties of the mastoparan peptide Agelaia-MPI isolated from social wasps. Toxicon 2016, 120, 15–21. [Google Scholar] [CrossRef]
- Pan, J.; Hink, W.F. Isolation and characterization of myrmexins, six isoforms of venom proteins with anti-inflammatory activity from the tropical ant, Pseudomyrmex triplarinus. Toxicon 2000, 38, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Sousa, P.L.; Quinet, Y.P.; Ponte, E.L.; Vale, J.F.D.; Torres, A.F.C.; Pereira, M.G.; Assreuy, A.M.S. Venom’s antinociceptive property in the primitive ant Dinoponera quadriceps. J. Ethnopharmacol. 2012, 144, 213–216. [Google Scholar] [CrossRef]
- Assreuy, A.M.S.; Adjafre, B.L.; Sousa, É.R.O.; Alves, A.P.N.N.; De Freitas Pires, A.; Quinet, Y.P.; Martins, A.M.C.; Mota, M.R.L.; Nascimento, N.R.F. Venom of the giant ant Dinoponera quadriceps attenuates inflammatory pain in mouse cutaneous wound healing model. Acta Sci. Biol. Sci. 2020, 42, e47680. [Google Scholar] [CrossRef]
- Rykaczewska-Czerwińska, M.; Radosz, A.; Konopińska, D.; Wróbel, M.; Plenć, A. Antinociceptive effect of poneratoxin (PoTX) in rats. Pestycydy 2008, 1-2, 135–141. [Google Scholar]
- Xiong, Y.M.; Lan, Z.D.; Wang, M.; Liu, B.; Liu, X.Q.; Fei, H.; Xu, L.; Yang, X.; Wang, C.; Wu, Y.; et al. Molecular characterization of a new excitatory insect neurotoxin with an analgesic effect on mice from the scorpion Buthus martensi Karsch. Toxicon 1999, 37, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.J.; Wang, C.G.; Wang, M.; Wang, D.C. A depressant insect toxin with a novel analgesic effect from scorpion Buthus martensii Karsch. Biochim. Biophys. Acta 2001, 1549, 9–18. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Wang, X.; Zhang, Z.; Zhang, J.; Zhao, Y. The role of the arginine residue in site RC for the analgesic activity of the recombinant Chinese scorpion Buthus martensii Karsch, BmK AGP-SYPU1. Comput. Biol. Chem. 2018, 74, 247–252. [Google Scholar] [CrossRef]
- Shao, J.H.; Cui, Y.; Zhao, M.Y.; Wu, C.F.; Liu, Y.F.; Zhang, J.H. Purification, characterization, and bioactivity of a new analgesic-antitumor peptide from Chinese scorpion Buthus martensii Karsch. Peptides 2014, 53, 89–96. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, F.; Liu, Y.; Cui, Y.; Zhang, J. Purification, characterization and cDNA cloning of an analgesic peptide from the Chinese scorpion Buthus martensii Karsch (BmK AGP-SYPU2). Mol. Biol. 2011, 45, 956–962. [Google Scholar] [CrossRef]
- Guan, R.-J.; Wang, M.; Wang, D.; Wang, D.-C. A new insect neurotoxin AngP1 with analgesic effect from the scorpion Buthus martensii Karsch: Purification and characterization. J. Pept. Res. 2001, 58, 27–35. [Google Scholar] [CrossRef]
- Bagheri-Ziari, S.; Shahbazzadeh, D.; Sardari, S.; Sabatier, J.M.; Bagheri, K.P. Discovery of a New Analgesic Peptide, Leptucin, from the Iranian Scorpion, Hemiscorpius lepturus. Molecules 2021, 26, 2580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Yang, M.; Wu, C.; Zou, Z.; Tang, J.; Wu, Y.; Lai, R. Centipede venom peptide SsmTX-I with two intramolecular disulfide bonds shows analgesic activities in animal models. J. Pept. Sci. 2017, 23, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, S.; Bragadeeswaran, S. Assessment of biomedical and pharmacological activities of sea anemones Stichodactyla mertensii and Stichodactyla gigantea from Gulf of Mannar Biosphere Reserve, southeast coast of India. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 53–61. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Jacobson, P.B.; Fenical, W.; Jacobs, R.S.; Glaser, K.B. Pharmacological characterization of the pseudopterosins: Novel anti-inflammatory natural products isolated from the Caribbean soft coral, Pseudopterogorgia elisabethae. Life Sci. 1998, 62, PL401–PL407. [Google Scholar] [CrossRef]
- Ayed, Y.; Dellai, A.; Mansour, H.B.; Bacha, H.; Abid, S. Analgesic and antibutyrylcholinestrasic activities of the venom prepared from the Mediterranean jellyfish Pelagia noctiluca (Forsskal, 1775). Ann. Clin. Microbiol. Antimicrob. 2012, 11, 15. [Google Scholar] [CrossRef]
- Rajabi, H.; Zolgharnein, H.; Ronagh, M.T.; Amiri Moghaddam, J.; Crüsemann, M. Conus coronatus and Conus frigidus Venom: A New Source of Conopeptides with Analgesic Activity. Avicenna J. Med. Biotechnol. 2020, 12, 179–185. [Google Scholar]
- Yu, S.; Du, T.; Liu, Z.; Wu, Q.; Feng, G.; Dong, M.; Luo, S. Im10A, a short conopeptide isolated from Conus imperialis and possesses two highly concentrated disulfide bridges and analgesic activity. Peptides 2016, 81, 15–20. [Google Scholar] [CrossRef]
- Balamurgan, K.; Akalanka Dey, A.; Sivakumar, G.; Diwakar Bobbila, D.; Shah Jignashu, S. Some Neuropharamacological Effects of the Crude Extract of Conus parvatus in Mice. Pak. J. Biol. Sci. 2007, 10, 4136–4139. [Google Scholar] [CrossRef]
- Qiang, Y.; Niu, J.; Wu, Y.; Di, Z.; Wang, F.; Zhang, L.; Zhangsun, D.; Luo, S. Discovery of a Novel Cysteine Framework XXIV Conotoxin from Conus striatus, S24a, with Potential Analgesic Activity. Int. J. Pept. Res. Ther. 2021, 27, 615–625. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Nabil, Z.I.; El-Naggar, M.S. Prospecting for candidate molecules from Conus virgo toxins to develop new biopharmaceuticals. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20220028. [Google Scholar] [CrossRef]
- Shen, L.; Lv, X.; Yang, X.; Deng, S.; Liu, L.; Zhou, J.; Ma, H.; Wang, J.; Xu, D. Bufotenines-loaded liposome exerts anti-inflammatory, analgesic effects and reduce gastrointestinal toxicity through altering lipid and bufotenines metabolism. Biomed. Pharmacother. 2022, 153, 113492. [Google Scholar] [CrossRef]
- Wang, J.; Xu, D.; Shen, L.; Zhou, J.; Lv, X.; Ma, H.; Xu, D.; Yang, X.; Deng, S. Anti-inflammatory and analgesic actions of bufotenine through inhibiting lipid metabolism pathway. Biomed. Pharmacother. 2021, 140, 111749. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, J.; Chen, W.; Yang, X.; Zhou, J.; Ma, H.; Xu, D.; Shen, L.; Deng, S. Evaluation of analgesic and anti-inflammatory actions of indolealkylamines from toad venom in mice using lipidomics and molecular docking. J. Ethnopharmacol. 2021, 269, 113677. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, G.I.M.C.; Carvalho, I.F.; Coelho, E.B.S.; Monteiro, M.R.B.; Medeiros, R.L.; Carvalho, E.D.F.; Silva, P.T.A.; Carvalho, D.M.F.; Uchoa, D.E.A.; Silveira, E.R.; et al. Potent nonopioid antinociceptive activity of telocinobufagin in models of acute pain in mice. Pain Rep. 2019, 4, e791. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Liu, H.; He, X.; Zhang, Y.; Jin, J.; Che, J.; Li, C.; Chen, W.; Lai, R.; et al. Novel analgesic peptides from the tree frog of Hyla japonica. Biochimie 2014, 99, 38–43. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, S.; Zhang, C.; Ren, S.; Zhou, Z.; Wang, F.; Wang, Y.; Chen, Q.; Wang, Y.; Lee, W.-H.; et al. Novel analgesic peptide derived from Cinobufacini injection suppressing inflammation and pain via ERK1/2/COX-2 pathway. Int. Immunopharmacol. 2024, 141, 112918. [Google Scholar] [CrossRef]
- Peng, X.; Tao, H.; Xia, F.; Zhu, M.; Yang, M.; Liu, K.; Hou, B.; Li, X.; Li, S.; He, Y.; et al. Molecular design, construction and analgesic mechanism insights into the novel transdermal fusion peptide ANTP-BgNPB. Bioorganic Chem. 2024, 148, 107482. [Google Scholar] [CrossRef]
- Tran, T.V.; Nguyen, T.T.T.; Hoang, A.N.; Utkin, Y.N. Comparative study of the analgesic effects of Bungarus fasciatus snake venom from Vinh Phuc and Tien Giang Provinces of Vietnam. Trop. J. Pharm. Res. 2022, 21, 1915–1921. [Google Scholar] [CrossRef]
- Wolz-Richter, S.; Esser, K.H.; Hess, A. Antinociceptive activity of crotoxin in the central nervous system: A functional Magnetic Resonance Imaging study. Toxicon 2013, 74, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Han, R.; Chen, Z.X.; Chen, B.W.; Gu, Z.L.; Reid, P.F.; Raymond, L.N.; Qi, Z.-H. Opiate and acetylcholine-independent analgesic actions of crotoxin isolated from Crotalus durissus terrificus venom. Toxicon 2006, 48, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, M.; Kummer, K.K.; Kress, M. Towards bridging the translational gap by improved modeling of human nociception in health and disease. Pflug. Arch. 2022, 474, 965–978. [Google Scholar] [CrossRef]
- Yezierski, R.P.; Hansson, P. Inflammatory and neuropathic pain from bench to bedside: What went wrong? J. Pain 2018, 19, 571–588. [Google Scholar] [CrossRef]
- Botz, B.; Bölcskei, K.; Helyes, Z. Challenges to develop novel anti-inflammatory and analgesic drugs. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1427. [Google Scholar] [CrossRef]
- Malheiro, A.; Harichandan, A.; Bernardi, J.; Seijas-Gamardo, A.; Konings, G.F.; Volders, P.G.A.; Romano, A.; Mota, C.; Wieringa, P.; Moroni, L. 3D culture platform of human iPSCs-derived nociceptors for peripheral nerve modeling and tissue innervation. Biofabrication 2022, 14, 014105. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.D.; Aghaeepour, N.; Ahn, A.H.; Angst, M.S.; Borsook, D.; Brenton, A.; Burczynski, M.E.; Crean, C.; Edwards, R.; Gaudilliere, B.; et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: Challenges and opportunities. Nat. Rev. Neurol. 2020, 16, 381–400. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef] [PubMed]
- El-Aziz, T.M.A.; Soares, A.G.; Stockand, J.D. Advances in venomics: Modern separation techniques and mass spectrometry. J. Chromatogr. B 2020, 1160, 122352. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.