Abstract
This study assessed mycotoxin contamination in roasted peanut snacks and kluiklui (fried pressed peanut cake), and consumer exposure in southern Benin. Roasted peanut snacks and kluiklui were sampled from markets across six municipalities, and their production follow-up was conducted on two sites using different processing methods. Mycotoxins were quantified using UPLC-MS/MS, while fungal species were identified via culture-based methods. Exposure to aflatoxin B1, total aflatoxins and ochratoxin A was estimated. Aflatoxin B1 predominated, reaching 169 µg/kg in roasted peanut snacks and 2144.64 µg/kg in marketed kluiklui. In contrast, just-produced kluiklui contained much lower levels (11.73–37.78 µg/kg). Aspergillus flavus and Aspergillus niger predominated in kluiklui from the first processing site, while Aspergillus chevalieri dominated in kluiklui from the second processing site. The grinding step (using public grinder) was identified as the main contamination point. The significative higher mycotoxin levels in kluiklui sampled on markets compared to just-produced kluiklui are probably due to poor storage conditions. Dietary exposure estimates revealed that margins of exposure for aflatoxins were far below the safety threshold of 10,000, and liver cancer risk estimates were particularly high for kluiklui consumers. Kluiklui consumption poses a significant health risk in Benin. Improved hygiene in public grinders and better storage practices are urgently needed to reduce contamination and protect consumers’ health.
Keywords:
Arachis hypogaea; AFB1; total aflatoxins; OTA; MOE; risk assessment; process contribution; kluiklui Key Contribution:
The main contribution of our manuscript lies in determining the extent to which the processing practices of kluiklui and roasted peanuts contribute to contamination by fungi and mycotoxins. In addition, consumer exposure to mycotoxins through the consumption of commercialised and freshly processed products was assessed. These results highlight the urgent need to improve the quality of peanut-based foods and optimise storage conditions in order to mitigate health risks to consumers.
1. Introduction
Mycotoxin contamination of food crops represents one of the most significant threats to global food safety and human health [1]. Mycotoxins are toxic secondary metabolites mainly produced by fungal species of the genera Aspergillus spp., Fusarium spp., and Penicillium spp., and to a lesser extent by Alternaria spp., Claviceps spp., Trichoderma spp. and Trichothecium spp. [2,3,4,5]. A single fungal species can produce multiple mycotoxins, potentially leading to the simultaneous contamination of food commodities with several toxins. In addition, successive colonisation by different fungal species can further contribute to multi-mycotoxin contamination [2]. To date, over 400 mycotoxins have been identified, but only a limited number are considered of major concern for food safety due to their documented occurrence in food products. These include aflatoxins B1 (AFB1), B2 (AFB2), G1 (AFG1), G2 (AFG2); ochratoxins, mainly ochratoxin A (OTA) and fumonisins, notably B1 (FB1) and B2 (FB2) [6,7].
Aflatoxins are mainly produced by Aspergillus flavus and Aspergillus parasiticus. Aflatoxins are classified by the International Agency for Research on Cancer (IARC) as carcinogenic to humans (Group 1) and present hepatotoxic and immunosuppressive properties [8]. Aflatoxin B1 is the most frequently present in contaminated foods and is recognised as the most toxic and carcinogenic. Its metabolite (AFB1 8,9-epoxide) displays several biological activities, including acute toxicity, teratogenicity, mutagenicity and carcinogenicity [development of hepatocellular carcinoma (HCC)] in humans and animals [9]. AFB1 has also been associated with growth suppression and weight loss in children [10] and immune system modulation [11]. The risk of development of HCC due to aflatoxin B1 exposure is higher for people infected with hepatitis B virus (HBV) who present a higher risk of developing liver cancer [12]. Ochratoxins (OTs) are produced by Aspergillus ochraceus and some Penicillium species, with OTA being the most extensively studied. OTA primarily affects the kidneys and is classified as a possible human carcinogen by the IARC (group 2B) [13]. Fumonisins, primarily produced by Fusarium verticillioides and Fusarium proliferatum, are also classified in the group 2B of possible human carcinogens [14]. Among them, fumonisin B1 (FB1) is the most prevalent and has been associated with both nephrotoxicity and hepatotoxicity in various animal species [15,16].
The main route of mycotoxin exposure for humans is through ingestion of contaminated food [17]. Fungal contamination and mycotoxin production can occur at any stage of the food production chain, including pre-harvest, harvest, drying, and storage phases [18]. Crops such as maize, millet, wheat, sorghum, soybean, peanut, and their derived products are among the most susceptible to mycotoxin contamination [19].
Peanuts (Arachis hypogaea) are one of the most widely grown oilseed crops in the world, cultivated across the intertropical zone [20]. Peanuts are important in terms of nutritional and economic aspects, particularly in developing countries [21] where they are processed into a wide range of popular food products [22,23]. Peanuts are consumed fresh, boiled, roasted, or processed into paste, oil or fried pressed-peanut cakes [22,23,24]. Besides peanut economic and nutritional benefits, peanut and peanut-based foods consumption has potential drawbacks due to their high susceptibility to fungal contamination, particularly by A. flavus, which produces aflatoxins [25,26].
Weather conditions are favourable to mycotoxin development in peanuts in Africa [27]. Studies across West Africa (Senegal, Ghana, Mali, Nigeria, Togo, and Benin) have reported mycotoxin contamination in peanuts and peanut-based foods with mycotoxins [28,29,30,31,32,33,34,35]. The reported concentrations widely exceeded the maximum limits established by the Commission Regulation (EU) 2023/915, which are set at 8 µg/kg for aflatoxin B1 and 15 µg/kg for total aflatoxins in raw peanuts intended for direct human consumption, and 2 µg/kg and 4 µg/kg, respectively, in peanut-based processed products [36]. Regular consumption of peanut-based foods may lead to a public health risk.
Also, in West Africa, particularly in Benin, peanut-based foods are primarily obtained through traditional, small-scale processing methods [37]. Mycotoxin levels in peanut-based foods may therefore stem from several factors, including the poor quality of peanut seeds used as raw material, inadequate technological processing practices, and/or inappropriate storage conditions of peanut-based foods.
Although a few studies in Benin have reported the presence of mycotoxins in peanut-based foods such as kluiklui (fried pressed peanut cake) and peanut oil [28,32], there is a significant lack of research assessing the contribution of processing practices to the contamination of these derived products. Moreover, data remain scarce regarding the dietary exposure of Beninese consumers to mycotoxins through peanut-based food consumption. This study, therefore, aims to address these gaps by evaluating the occurrence of mycotoxins and fungal contamination in peanuts and their derived products, and by assessing consumer exposure resulting from their consumption.
2. Results and Discussion
2.1. Occurrence of Mycotoxins in Marketed Roasted Peanut Snack and Kluiklui
Figure 1 illustrates the concentration ranges of main mycotoxins quantified in market samples of roasted peanut snacks and kluiklui in Benin, i.e., AFB1, AFB2, AFG1, AFG2, and OTA and OTB. The detailed results for all analysed mycotoxins are presented in Supplementary Materials (Tables S1 and S2).
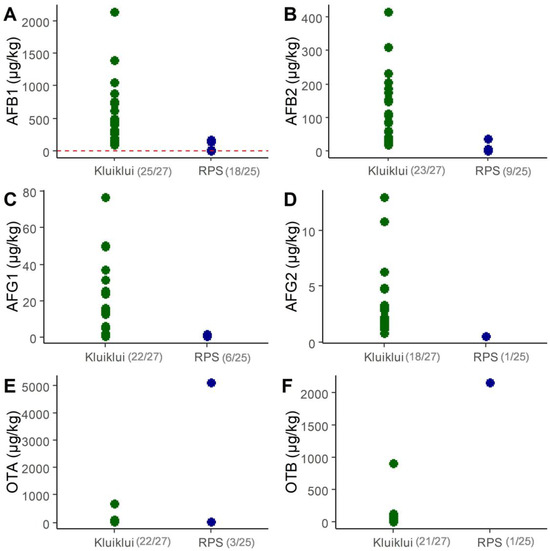
Figure 1.
Concentrations of AFB1 (A), AFB2 (B), AFG1 (C), AFG2 (D), OTA (E) and OTB (F) in samples of marketed kluiklui and roasted peanut snacks (RPS). Only samples above LOQ are shown. LOQ values are 0.2 µg/kg for AFB1, AFB2, AFG1, AFG2, and 0.5 µg/kg for OTA and OTB. The total number of samples was 27 for kluiklui and 25 for roasted peanut snacks. The number of samples above the LOQ among the total number of samples is mentioned in brackets. In Graph A, the red dotted line represents the AFB1 limit of 2 µg/kg for peanut-based foods, as established by the European Commission (EC) [36].
AFB1, AFB2, and AFG1 were the most frequently detected mycotoxins in roasted peanut snacks, with respective concentrations ranging from 0.02 to 169.0 µg/kg, 0.07 to 35.3 µg/kg, and 0.16 to 1.7 µg/kg, respectively, and corresponding median concentrations of 1.0, 0.07, and 0.16 µg/kg, respectively. AOH, FUM B1 and B2 were never detected in roasted peanut (Figure 1; Tables S1 and S2). One particular sample (ID: 20230.0S264, from Aplahoué) displayed inexplicably very high concentrations of OTA and OTB (5085 and 2143 µg/kg, respectively) (Figure 1; Tables S1 and S2).
In kluiklui samples, AFB1, AFB2, AFG1, AFG2, OTA and OTB were detected in almost all samples (Tables S1 and S2). Their concentration ranges were 89.1–2144.6 µg/kg for AFB1, 0.07–416.4 µg/kg for AFB2, 0.16–76.8 µg/kg for AFG1, 0.13–13 µg/kg for AFG2, 0.4–668 µg/kg for OTA, and 0.4–909 µg/kg for OTB (Tables S1 and S2). The corresponding median upper bound (UB) concentrations were 394.8, 83.9, 12.8, 1.7, 23.3, and 16 µg/kg, respectively. The minimum, median, and maximum total aflatoxin UB concentrations were 0.38, 1.36 and 169.36 µg/kg for roasted peanuts, while they were 112.1, 478.4 and 2644.1 µg/kg for kluiklui (Tables S1 and S2).
Several studies have identified AFB1, AFB2, AFG1, and AFG2 as the main mycotoxins frequently occurring in kluiklui, often in conjunction with ochratoxin A (OTA) [28,38]. According to Adjou et al. [28], the concentrations of AFB1, AFB2, AFG1, AFG2, and OTA in kluiklui marketed in Benin in 2011 reached 455, 491, 100, 88 and 2 µg/kg, respectively. Njumbe Ediage et al. [38] reported maximum concentrations of 282 µg/kg for AFB1, 31 µg/kg for AFB2, 79 µg/kg for AFG1, 96 µg/kg for AFG2, and 2 µg/kg for OTA in kluiklui samples collected in Benin in 2010. In Nigeria, a neighbouring country of Benin, in 2011, AFB1 and AFG2 concentrations of 2820 and 477 µg/kg, respectively, were reported in kluiklui [31].
AFB1, AFB2, AFG1 and AFG2 were quantified in roasted peanuts sold in Nigeria, with concentrations reaching up to 165, 26, 20 and 10 µg/kg, respectively [30]. In Iran, concentrations of 244, 6.40, 69.25 and 3.45 µg/kg of AFB1, AFB2, AFG1 and AFG2, respectively, have been reported in marketed roasted peanuts in 2015/2016 [39]. Sombie, et al. [40] also reported the presence of AFB1, AFB2, AFG1 and AFG2 in marketed roasted peanut samples, with concentrations reaching up to 1387, 271, 3328 and 742 µg/kg, respectively, in Serra Leone in 2017. None of these studies reported the presence of ochratoxins in roasted peanut snacks, in contrast to the findings of this study. Benin, Nigeria, and Sierra Leone are West African countries with a tropical climate, under the influence of the Atlantic Ocean, and showing regional variations [41]. These countries are very vulnerable to climate change, which favours mycotoxin contamination in peanuts and peanut-based foods, causing a possible increase in cancer incidence [42,43].
In Europe, no maximal limit exists for ochratoxins in peanut-based food, while for aflatoxins, the European Commission (EC) set a maximum concentration of 2 µg/kg for AFB1 and 4 µg/kg for total aflatoxins (i.e., the sum of AFB1, B2, G1 and G2) in peanut-based food for the final consumer [36]. Among samples above LOQ, all kluiklui samples and 41% of roasted peanut snacks samples had AFB1 concentrations exceeding the established regulatory limits (Figure 1A), with maximum levels of AFB1 being 80 and 1000 times higher than the limit, respectively.
2.2. Contribution of Processing Practices to Mycotoxin Contamination
2.2.1. Occurrence of Mycotoxins in Raw Peanuts Used for Kluiklui and Roasted Peanut Snacks Production
Table 1 presents mycotoxin concentrations of raw peanuts used for kluiklui and roasted peanut snacks production. Since preliminary analyses revealed mycotoxin contamination of pools 11 and 12, individual samples forming these pools were analysed. Thus, pools 1 to 10, the three individual samples forming pool 11, and the three samples forming pool 12 were analysed, for a total of 16 raw material samples (10 pools and 6 individual samples). Table S3 presents the individual data for the raw material samples analysed. Concentrations of AFB1 and total aflatoxins (AFtot) ranged from 0.02 (LOD) to 2.63 µg/kg, and from 0.09 to 2.97 µg/kg, respectively. In case of raw peanuts (to be sorted before human consumption), the EC has established maximum levels of 8 µg/kg for aflatoxin B1 and 15 µg/kg for total aflatoxins [36]. Similarly, in Benin, Decree 2007 No. 0362 MAEP/D-CAB/SGM/DRH/DP/SA, which sets the maximum levels for contaminants in foodstuffs, has also established the same limits for aflatoxin B1 (8 µg/kg) and total aflatoxins (15 µg/kg) [44]. Aflatoxin concentrations in raw peanuts used to produce kluiklui and roasted peanut snacks in this study are below these EC and Benin Government limits. The concentrations of AOH, FUM B1, FUM B2, OTA, and OTB were below the limits of quantification for all analysed raw material samples.

Table 1.
Mycotoxin concentrations of raw peanuts used for kluiklui and roasted peanut snacks (n = 18) (µg/kg, UB).
2.2.2. Kluiklui Processing Practices and Mycotoxins Contamination
The mycotoxin contamination of kluiklui samples collected during processing practices is presented in Table 2 and Table S4. All roasted peanut samples (roasting being the first step of the process, see Figure 4) were below the LOD for all the mycotoxins analysed, showing that roasting did not contribute to mycotoxin contamination during kluiklui production process.

Table 2.
Mycotoxin contamination (µg/kg, UB) in samples collected during kluiklui processing.
On the contrary, aflatoxins were quantified in all peanut paste samples obtained after dehulling and grinding, which are the next steps of the process (Figure 4). Ochratoxins were found as well, but not in all samples, while fumonisins (B1 and B2) and AOH were below the LOD in all peanut paste samples (Table 2 and Table S4). The mean concentrations of AFB1, total aflatoxins, and OTA were 50.56, 60.91 and 3.70 µg/kg, respectively, for peanut paste from the processing without maize flour; and 14.65, 21.60 and 6.13 µg/kg, respectively, for peanut paste from the processing with maize flour. These findings suggest a contamination occurring during the grinding of roasted peanut seeds, as reported by Ndung’u, et al. [45]. Indeed, the grinding is carried out using a public grinder, originally intended for maize or spice grinding. The use of such shared equipment may lead to cross-contamination, as various raw materials of differing hygienic quality are processed in the same grinder. Additionally, as these are public facilities, processors have little control over the sanitary conditions and maintenance of the grinding equipment.
Aflatoxin B1 and total aflatoxin concentrations were significantly higher in peanut paste samples collected from the process without maize flour; with respective p-values of 0.04718 and 0.0479 for AFB1 and total aflatoxins. During peanut processing without maize flour, conducted in the municipality of Covè, the public grinders used were exclusively dedicated to kluiklui production. In contrast, in Aplahoué, the process involving maize flour relied on mixed-use grinders, which were not reserved exclusively for peanuts but were also used for maize grinding. As these grinders in the municipality of Aplahoué are shared with maize processing, a cleaning step is systematically performed prior to peanut grinding, to remove residual maize flour. However, in the municipality of Covè, since the grinders are used exclusively for peanut processing, thorough cleaning is not routinely performed prior to grinding. This could explain the higher level of contamination observed in peanut paste from the process without maize flour. Moreover, the warm and humid climate in southern Benin favours the increase in contamination with fungi and mycotoxins in peanut and ground maize residues from public grinders.
Regarding the maize flour used in kluiklui production, all collected samples were contaminated with aflatoxins and fumonisins, and showed important variation among samples (Table 2 and Table S4). The mean concentrations were 24.11 µg/kg for AFB1, 27.26 µg/kg for AF tot, 991.57 µg/kg for FUM B1, and 333.84 µg/kg for FUM B2. Contamination of maize by fumonisins has also been reported in other studies conducted in Benin, with concentrations of fumonisin B1 and B2 reaching up to 836 and 221 µg/kg, respectively [38], and as high as 14,200 mg/kg and 3750 mg/kg, respectively, in earlier findings [46].
Regarding the final product of both processing methods, all kluiklui samples were contaminated with AFB1 and AFB2. OTA and OTB were found in a total of 12 samples out of 18, FUMB1 and B2 in 3 and 2 samples out of the 9 processed with the addition of maize flour, while AOH was not found in any sample. Kluiklui produced without maize flour and with maize flour had mean AFB1 concentrations of 37.78 and 11.73 µg/kg, respectively; and mean total aflatoxin concentrations of 46.99 and 16.24 µg/kg, respectively. AFB1 (37.78 vs. 11.73 µg/kg) and total aflatoxins concentration (46.99 vs 16.24 µg/kg) were significantly higher in kluiklui samples produced without the addition of maize flour, compared to those produced with maize flour (Table 2). The corresponding p-values were 0.04787 and 0.04584, respectively, for AFB1 and AFtot. These results are in line with those reported by Kayode, et al. [47], who observed higher average concentrations of total aflatoxins in peanut-based snacks (620.9 µg/kg) than in peanut/maize-based snacks (12 µg/kg).
The low levels of FUM B1 and FUM B2 found in some samples of kluiklui produced using maize flour could be explained by the low amount of maize flour added to the peanut paste (about 10 g of maize flour per kg of peanut paste).
AFB1, AFtot and OTA concentrations were significantly lower in just-produced kluiklui (from production follow-up) than marketed kluiklui with p-values of 2.158 × 10−6, 1.924 × 10−6 and 0.001096 for AFB1, AFtot and OTA, respectively (Table S6). These findings suggest that inadequate post-production storage conditions may play a significant role in the contamination of kluiklui with mycotoxins.
2.2.3. Roasted Peanut Processing Practices and Mycotoxin Contamination
Table 3 and Table S5 present the contribution of roasting practices (with or without heat transfer material) to mycotoxin contamination in roasted peanut snacks.

Table 3.
Mycotoxin concentration in roasted peanut snacks according to the process (µg/kg, UB).
AFB1 and AFtot concentrations were 0.50 and 1.56 µg/kg, respectively, in roasted peanut snack samples processed without a heat transfer material; whereas concentrations of 0.12 and 0.43 µg/kg were recorded, respectively, in samples processed using a heat transfer material. Roasting is generally expected to reduce mycotoxin levels in peanut seeds, as reported in several studies conducted in Brazil [48], Togo [49], Senegal [50] and Nigeria [51]. In the present study, the low contamination of peanut seeds by mycotoxins did not allow for an assessment of the effect of roasting processes on the mycotoxin content of roasted peanuts.
There was no AFB1 contamination in the raw seeds used in the process without heat transfer material (<LOQ) and low contamination in those used in the process with heat transfer material (0.35 µg/kg). Despite the absence of mycotoxin contamination in raw peanut seeds used in the roasting process without heat transfer material, AFB1 were detected in the corresponding roasted peanut samples (0.50 µg/kg). Heterogeneity in seed contamination by mycotoxins, as reported by Teixido-Orries, et al. [52], could explain the variability observed in contamination levels between the raw materials used in the two roasting processes. Further studies would be valuable to assess the impact of peanut storage practices, as commonly performed in Benin, on seed contamination by mycotoxins, as well as on their distribution within a batch of seeds.
No significant difference was observed in the concentrations of AFB1 (p-value = 0.1825) and total aflatoxins (p-value = 0.1997) between roasted peanut snacks produced using roasting processes with and without heat transfer material. The use of a heat transfer material did not contribute to the mycotoxin contamination of the roasted peanuts.
It is also important to note that just-produced roasted peanut snacks do not show significantly different concentrations of mycotoxins compared to those available on the market (p > 0.05) (Table S6).
2.3. Dietary Exposure to Aflatoxins and Risk Characterisation
2.3.1. Consumer Exposure to Aflatoxins Through Marketed Peanut-Based Food Consumption
The estimated daily intakes (EDI) of AFB1 for consumers of roasted peanut snacks only, kluiklui only, and both products are summarised in Table 4. According to scenario 1, which was based on the median concentrations of AFB1, the median and 95th percentile daily intakes were 0.0003 and 0.001 µg/kg body weight (bw) per day, respectively, for consumers of roasted peanut snacks; 0.12 and 0.45 µg/kg bw/day for kluiklui consumers; and 0.09 and 0.52 µg/kg bw/day for consumers of both products. In scenario 2 (maximum AFB1 concentrations), the estimated median and 95th percentile daily intakes reached 0.04 and 0.18 µg/kg bw/day, respectively, for roasted peanut snack consumers; 0.63 and 2.45 µg/kg bw/day for kluiklui consumers; and 0.44 and 2.28 µg/kg bw/day for consumers of both peanut-based foods.

Table 4.
Dietary exposure of marketed peanut-based foods consumers to aflatoxin B1 and total aflatoxins.
The risk characterisation, based on median (scenario 1) and maximum (scenario 2) concentrations of AFB1, revealed median MOE values of 1577 and 9 for roasted peanut snack consumers, 3 and 1 for kluiklui consumers, and 4 and 0.9 for consumers of both peanut-based foods, respectively. According to Scenario 1, 94% of roasted peanut snack consumers presented MOE values below the threshold of 10,000, while under Scenario 2, 100% of them fell below this benchmark. For all consumers of kluiklui and both peanut-based foods, MOE values were below 10,000 in both scenarios. Such low MOE values indicate a health concern (development of HCC) related to exposure to AFB1 among the assessed consumer groups.
In Nigeria, Oyedele, et al. [54] reported mean MOE values of 1665 for AFB1 in adult consumers of peanuts from local markets indicate a high risk of liver cancer. Kortei, et al. [55] carried out a risk assessment on peanuts and peanut-based foods collected from local markets in Ghana. The estimated daily intakes ranged from 0.068 to 0.300 µg/kg bw/day, while the corresponding MOE values varied between 1333 and 5882.
For quantitative cancer risk assessment, the estimated median liver cancer risks associated with dietary exposure to AFB1 were 1 and 166 cases per 100,000 persons per year for consumers of roasted peanut snacks, under Scenario 1 and 2, respectively (Table 4). For consumers of kluiklui, the estimated liver cancer risks reached 450 and 2447 cases/100,000 persons/year, and 359 and 1712 cases/100,000 persons/year for consumers of both peanut-based foods. When expressed as a proportion of the overall annual liver cancer incidence, the median risk attributed to AFB1 exposure corresponded to 0.0001% and 0.012% (Scenario 1 and 2, respectively) for roasted peanut snack consumers, 0.03% and 0.18% for kluiklui consumers, and 0.03% and 0.12% for consumers of both peanut-based foods.
Aydemir Atasever, et al. [56] estimated the exposure of the Turkish population to aflatoxin B1 through a daily consumption of 0.8 g of peanut paste, containing an average AFB1 concentration of 3 µg/kg. The resulting EDI was 0.03 ng/kg bw/day, corresponding to an MOE of 12,304 and an estimated annual incidence of hepatocellular carcinoma of 0.000715 cases per 100,000 persons. In Thailand, Kooprasertying, et al. [57] reported an estimated liver cancer risk potency of 0.01 cases per 100,000 individuals per year from a mean consumption of roasted peanuts of 1.3 g/day. Ezekiel, et al. [58] reported a high risk of liver cancer, estimating up to 41 cases per 100,000 population per year in Nigeria due to consumption of peanuts contaminated with AFB1.
The assessment of dietary exposure to total aflatoxins through consumption of marketed kluiklui and roasted peanut snacks is presented in Table 4. Based on scenarios 1 and 2, median total aflatoxins EDIs were 0.0003 and 0.04 µg/kg bw/day for roasted peanut snack consumers; 0.1 and 0.8 µg/kg bw/day for kluiklui consumers, and 0.1 and 0.7 µg/kg bw/day for consumers of both products, respectively.
MOE values were 1160 and 9 for roasted peanut snack consumers; 3 and 1 for kluiklui consumers; and 4 and 1 for consumers of both peanut-based foods, respectively, under scenarios 1 and 2. These MOE values are below the critical threshold of 10,000, indicating a real health concern. A mean MOE of 908 for AFtot was reported for Nigerian consumers of peanuts purchased from local markets [54]. Kortei et al. [55] reported EDI values ranging from 0.087 to 0.380 µg/kg bw/day and corresponding MOE values from 1053 to 4598, based on the consumption of peanuts and peanut-based foods collected from local markets in Ghana.
These estimates highlight the significant public health concern posed by aflatoxin contamination in peanut-based foods, particularly kluiklui marketed in Benin.
2.3.2. Consumer Exposure to Aflatoxins Through “Just-Produced” Peanut-Based Foods Consumption
Since no significant difference was observed in aflatoxin concentrations between roasted peanut samples collected from markets and those just-produced, the risk assessment was re-evaluated using just-produced kluiklui samples from both processing methods.
The EDI of AFB1 among consumers of just-produced kluiklui, obtained from both processing methods, is presented in Table 5. According to scenario 1 (median concentration), the estimated median and 95th percentile of daily AFB1 intakes were 0.01 and 0.03 µg/kg bw/day, respectively, for consumers of kluiklui produced without maize flour; and 0.001 and 0.004 µg/kg bw/day for those consuming kluiklui produced with maize flour. Under scenario 2 (maximum concentration), the estimated median and 95th percentile intakes were 0.03 and 0.1 µg/kg bw/day, respectively, for consumers of kluiklui without maize flour; and 0.01 and 0.04 µg/kg bw/day for consumers of kluiklui with maize flour. The estimated median and 95th percentile intakes of AFB1 were higher through the consumption of kluiklui produced without maize flour.

Table 5.
Dietary exposure of “just produced” kluiklui consumers to aflatoxin B1 and total aflatoxins.
Based on MOE approach, the median MOE values were 46 and 13 for consumers of kluiklui made with maize flour, and 415 and 39 for those consuming kluiklui without maize flour, respectively, under scenarios 1 and 2. As mentioned above, values below 10,000 are indicative of a health concern related to exposure to AFB1. The estimated liver cancer risks associated with median dietary exposure to AFB1 under scenarios 1 and 2 were 34 and 122 cases/100,000 persons/year, respectively, for consumers of kluiklui produced with maize flour, and 4 and 40 cases/100,000 persons/year for those consuming kluiklui produced without maize flour. Consumption of just-produced kluiklui, whether or not produced with maize flour, resulted in lower consumer exposure to AFB1 compared to kluiklui purchased from local markets.
Dietary exposure to AFtot through consumption of just-produced kluiklui is also presented in Table 5.
Based on scenarios 1 and 2, median estimated daily intakes of AFtot were 0.01 and 0.04 µg/kg bw/day for consumers of kluiklui made without maize flour; and 0.001 and 0.01 µg/kg bw/day for kluiklui made with maize flour consumers. The corresponding MOE values were 35 and 10 for kluiklui produced without maize flour consumers; 280 and 32 for kluiklui produced with maize flour consumers. MOE values below the critical threshold of 10,000 suggest a potential health concern related to dietary exposure to AFtot.
It is important to note that MOE values for AFB1 and AFtot were significantly higher for consumers of just-produced kluiklui compared to those of marketed kluiklui (p < 0.05), indicating a lower level of exposure to AFB1 and AFtot for consumers of just-produced kluiklui.
2.4. Dietary Exposure to OTA and Risk Characterisation
2.4.1. Consumer Exposure to OTA Through Marketed Peanut-Based Foods Consumption
The daily OTA intakes of consumers of kluiklui, roasted peanut snacks, and both products are presented in Table 6.

Table 6.
Dietary exposure to OTA through marketed peanut-based food consumption and MOE.
Based on the median (scenario 1) and maximum (scenario 2) concentrations of OTA, the median daily intakes were 0.0001 and 1.29 µg/kg bw per day, respectively, for consumers of roasted peanut snacks; 0.01 and 0.20 µg/kg bw per day for consumers of kluiklui; and 0.01 and 1.52 µg/kg bw per day for consumers of both products.
Considering the risk of neoplastic effects (kidney tumours), the MOE values at the 50th percentile were 142,958 and 11 for roasted peanut snack consumers, 2115 and 74 for kluiklui consumers, and 2654 and 15 for consumers of both products, under scenario 1 and scenario 2, respectively. MOE values below the critical threshold of 10,000 highlight a potential health concern. Regarding non-neoplastic effects (kidney lesions), median MOE values were 46,634 and 4 for roasted peanut snack consumers, 690 and 24 for kluiklui consumers, and 866 and 5 for those consuming both products, under scenario 1 and scenario 2, respectively. MOE values below the threshold of 200 indicate a potential health concern.
Kouadio [60] reported an EDI of OTA ranging from 0.2 to 58 ng/kg bw/day among the Ivorian population, with corresponding MOE values for neoplastic effects varying between 72,500 and 250, based on a daily intake of 22 g of peanuts and peanut paste. Nuhu, et al. [61] assessed consumer exposure to OTA resulting from the consumption of peanut-based foods in Ghana. The EDI ranged from 0.05 to 0.25 ng/kg bw/day, with corresponding MOE values varying between 71 and 326. These low MOE values reflect a concerning public health situation in West Africa regarding chronic OTA exposure from peanut-based foods.
It is important to note, however, that only three roasted peanut samples out of 25 showed OTA concentrations above the limit of quantification. This may have led to an overestimation of consumer exposure to roasted peanuts in the two scenarios considered.
2.4.2. Consumer Exposure to OTA Through “Just-Produced” Peanut-Based Foods Consumption
The median EDI of OTA for consumers of kluiklui without maize flour were 0.002 and 0.007 µg/kg bw/day under scenarios 1 and 2, respectively (Table 7). For consumers of kluiklui with maize flour, the corresponding values were 0.002 and 0.006 µg/kg bw/day. Regarding neoplastic effects (kidney tumours), MOE values derived from the median daily intake were 8832 and 2022 for consumers of kluiklui without maize flour, and 6639 and 2410 for those consuming kluiklui with maize flour, under scenario 1 and scenario 2, respectively. For non-neoplastic effects (kidney lesions), MOE values were 2881 and 660 for consumers of kluiklui without maize flour, and 2166 and 786 for those consuming kluiklui with maize flour.

Table 7.
Dietary exposure to OTA through “just-produced” peanut-based food consumption and MOE.
Considering that 44% of kluiklui samples produced with maize flour (4/9) were below the limit of quantification, the exposure values estimated under both scenarios could potentially have been overestimated.
2.5. Microorganisms Isolated from Peanut-Based Foods
2.5.1. Distribution of Moulds in Samples from Kluiklui Processing
The results of the fungal analysis conducted on samples collected during kluiklui production follow-up are presented in Table 8.

Table 8.
Mould and prevalence of Aspergillus spp. in samples from kluiklui processing.
The mean fungal counts were 1.95, 1.42 and 1.10 log10 CFU/g for raw peanuts, peanut paste, and kluiklui produced without maize flour, respectively. For the production process involving maize flour, the corresponding fungal counts were 2.22, 1.44 and 0.83 log10 CFU/g for raw peanuts, peanut paste, and kluiklui, respectively. The maize flour used in this process showed a fungal count of 2.12 log10 CFU/g.
Fungal contamination was found to be more pronounced in raw peanuts than in kluiklui, regardless of the processing method. A similar observation was made by Norlia, et al. [62], who reported higher levels of fungal contamination in raw peanuts (0.3–3.6 log CFU/g) compared to peanut-based products (0.6–2.7 log CFU/g). In contrast, Hussain, et al. [63] reported higher fungal counts in peanut-based products than in raw peanuts in Pakistan, with mean fungal loads of 4.41 log10 CFU/g (2.6 × 104 CFU/g) in raw peanuts and 4.57 log10 CFU/g (3.7 × 104 CFU/g) in peanut cake.
In a previous study conducted in Benin, Adjou et al. [28] reported mean fungal counts ranging from 1.0 to 8.1 × 102 CFU/g corresponding to 2.0 to 2.9 log10 CFU/g, in kluiklui samples. In the present study, the kluiklui samples produced using both processing methods exhibited lower total fungal counts than those reported by Adjou et al. [28].
Samples collected from the production process incorporating maize flour showed higher total mould counts and a greater fungal diversity, particularly in raw peanuts and maize flour, with 73 and 140 fungal isolates identified, respectively. The number of Aspergillus isolates was also higher in the peanut paste from this processing method (49 isolates), compared to the corresponding product from the process without maize flour (Table 8).
No link is observed between the prevalence of Aspergillus spp. in samples from kluiklui processing and the detected and quantified mycotoxins in this study. In raw peanuts used in the process without maize flour, no mycotoxins were detected and quantified, despite the total number of Aspergillus spp. isolates (32 isolates). On the contrary, in kluiklui samples from the process with maize flour, although only one Aspergillus spp. was isolated, AFB1, AFB2, AFG1, FUM B1, FUM B2, OTA, and OTB were detected and quantified.
2.5.2. Fungal Species Isolated on Samples from Kluiklui Processing
Several fungal species were isolated from samples from kluiklui processing (Figure 2). These included Aspergillus chevalieri, A. flavus, Aspergillus niger, Aspergillus spp., Botryodiplodia theobromae, Circinella muscae, Cladosporium spp., Rhizopus spp., and Talaromyces tumuli. In raw peanut samples, A. chevalieri was the most dominant species, followed by A. niger. For samples from the process without maize flour, A. flavus predominated in both peanut paste and kluiklui; T. tumuli was the second most common species in peanut paste, whereas A. niger was the second most prevalent in kluiklui. In contrast, peanut paste and kluiklui obtained from the process incorporating maize flour were more heavily contaminated with A. chevalieri, while the maize flour itself was predominantly contaminated with A. chevalieri and Aspergillus spp.
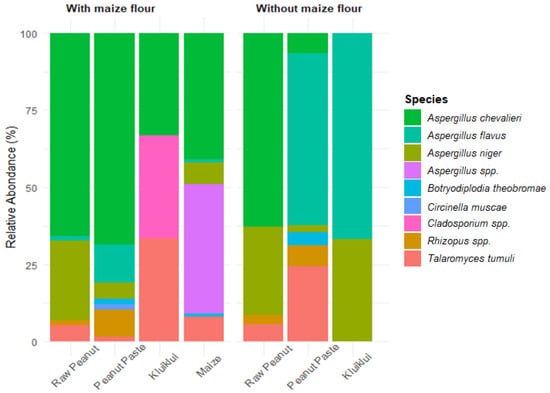
Figure 2.
Fungal diversity associated with the samples from kluiklui processing.
In a previous study conducted in Benin, Adjou et al. [28] identified A. flavus, A. parasiticus and various Aspergillus spp., Fusarium spp., and Penicillium spp. as the dominant fungal species isolated from kluiklui. Similarly, Hussain et al. [63] reported that A. flavus was the most frequently isolated fungal species in peanut-based products, accounting for 29.2% of isolates, followed by A. niger (21.1%), Aspergillus. fumigatus (16.5%), and Penicillium notatum (1.6%).
Although the presence of A. chevalieri is not frequently reported in studies related to fungal contamination of peanuts and their derived products, some investigations have nevertheless documented its occurrence and mechanisms of action in these products. Kamarudin and Zakaria [64], after isolating and identifying fungi from marketed peanuts in Malaysia, reported that 50% of the isolates were similar to A. chevalieri. The authors concluded that the presence of A. chevalieri on peanuts creates favourable conditions for the growth of less xerophilic Aspergillus species, as well as other spoilage-related fungal genera, particularly mycotoxin-producing species, thereby potentially increasing the risk of mycotoxin contamination. Indeed, xerophilic fungi produce metabolic water, which favours an increase in water activity, thereby promoting the growth of other species [65]. Aspergillus chevalieri is a ubiquitous soilborne fungus, considered as one of the most xerophilic and xerotolerant fungal species, and is frequently associated with spoilage of nuts, dried peanuts, dried beans, spices, and stored cereals and grains [66,67,68]. A. chevalieri has been reported to produce gliotoxin and sterigmatocystin [69]. This may account for its high prevalence in peanuts and peanut-derived products observed in the present study. However, despite its widespread occurrence in the analysed samples, only very low concentrations of sterigmatocystin were detected.
Results of the ammonia vapour test carried out on samples from kluiklui processing, regardless of the process method (Table S7), revealed a high proportion of toxigenic A. flavus isolates, particularly in peanut paste (31 isolates) and kluiklui (9 isolates). This high prevalence of toxigenic strains may explain the significant contamination observed in peanut paste and kluiklui from both processing methods.
2.5.3. Distribution of Moulds and Fungal Isolates on Samples from Roasted Peanut Snack Processing
Table 9 shows the concentration of moulds in the roasted peanut samples. In raw peanuts, fungal counts were 2.09 and 1.85 log10 CFU/g for samples used in the roasting process without and with heat transfer material, respectively, with T. tumuli, A. niger, and Rhizopus spp. identified as the predominant fungi.

Table 9.
Mould count and concentration of AFtot in samples from roasted peanut snack processing.
Roasted peanut snacks produced without heat transfer material showed a fungal count of 0.80 log10 CFU/g, with no fungal genera or isolates detected. In contrast, snacks roasted with heat transfer material had a fungal count of 1.30 log10 CFU/g, with four fungal genera identified (T. tumuli, A. niger, B. theobromae, and Penicillium citrinum), and a total of four fungal isolates recovered.
Although roasting without heat transfer material appears to be more effective in eliminating fungal contaminants (Table 9).
When comparing mycotoxin levels in samples from roasted peanut snack processing, it seems that there is no link between mould and the prevalence of Aspergillus spp. and quantified mycotoxins. Although T. tumuli, A. niger, and Rhizopus spp. were isolated in the raw seeds used in the process without heat transfer material, no mycotoxins were detected in these samples. And, in the raw seeds used in the process with heat transfer material, despite the presence of Fusarium spp., only AFB1 was detected.
3. Conclusions
The present study revealed a high level of mycotoxin contamination in peanut-based foods marketed in Southern Benin. AFB1, AFB2, AFG1, AFG2, as well as OTA and OTB, were the predominant mycotoxins detected in roasted peanut snacks and kluiklui. Mycotoxin contamination was found to be higher in kluiklui samples than in roasted peanut snacks, in marketed and just-produced samples. kluiklui collected from local markets were found to be the most contaminated, compared to those collected immediately after production. The storage conditions of kluiklui appear to be a critical factor contributing to its contamination with mycotoxins. During the production of kluiklui, the grinding of roasted peanuts in public grinders significantly contributed to its contamination with mycotoxins, especially when the grinding was carried out in grinders exclusively dedicated to peanut processing. Similarly, fungal contamination was more pronounced in kluiklui, with significantly higher populations of aflatoxigenic fungi, predominantly A. flavus, found in kluiklui samples produced without maize flour; whereas A. chevalieri, Cladosporium spp., and Talaromyces tumuli were the main species identified in kluiklui obtained from the processing method incorporating maize flour. The results of this study also indicate a high level of exposure and significant health concern related to aflatoxins and OTA intake through the consumption of kluiklui, particularly those marketed. In contrast, exposure levels among consumers of just-produced kluiklui and roasted peanuts were significantly lower, meaning that mycotoxin production occurs during storage after processing. Estimated liver cancer risks are considered as high, especially for frequent consumers and individuals with chronic hepatitis B infection. In the current context of climate change and global warming, favourable to fungi proliferation, it is essential to improve the storage conditions of kluiklui as well as the hygienic quality of the grinding equipment used during its production, in order to limit fungal and mycotoxin contamination. Such improvements are crucial to reduce consumer health risks, particularly the development of HCC, as well as kidney tumours and lesions.
4. Methods
4.1. Study Area
This study was conducted in southern Benin. Benin is located at 6°28′ N and 2°36′ E in West Africa. Benin has a tropical monsoon climate in the south and a tropical wet and dry climate in the north, according to the Koppen-Geiger climate type [41]. Benin is one of the most vulnerable countries in global warming scenarios of +2.7 °C by 2070 [42]. Climate change remains the main driving factor in the production of mycotoxins [70,71,72].
4.2. Sampling of Peanut-Based Foods from Beninese Markets
In order to determine mycotoxin concentrations in peanut-based foods marketed in Southern Benin, a total of 52 samples of peanut-based foods were purchased randomly in municipalities representative of peanut production and/or consumption in southern Benin (Abomey, Aplahoué, Covè, Cotonou, Glazoué, and Ouèssè). Figure 3 shows the sampling areas. The collected samples represent the main peanut-based foods produced and consumed in Southern Benin, and include roasted peanut (n = 27) and kluiklui (pressed fried peanut cake) (n = 25). The sampling was performed at production and retail sites (market and supermarket). The samples were ground, packed in sealed containers and stored under dry conditions for further analysis.
Figure 3.
Map of Benin showing the 6 communes of the sampling areas (adapted from a map from the IGN “Institut Géographique National” of Benin using the open source QGIS software, version 3.2).
4.3. Follow-Up of Kluiklui and Roasted Peanut Snacks Production and Sampling
4.3.1. Experimental Design
Figure 4 and Figure 5 illustrate the process for kluiklui and roasted peanut snack production, respectively. Kluiklui can be produced by adding or not adding maize flour during the kneading of the peanut paste. About 10 to 20 g of maize flour per kg of peanut paste is used. Regarding roasted peanuts, roasting can be done using a heat transfer material or not.
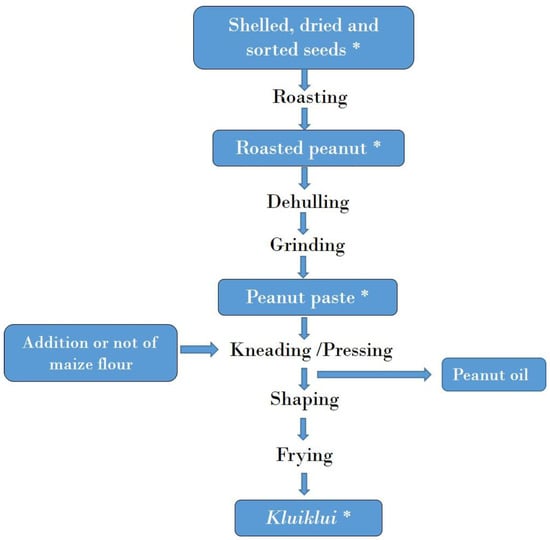
Figure 4.
Flow chart of kluiklui production. *: Samples collected for mycotoxin analysis.
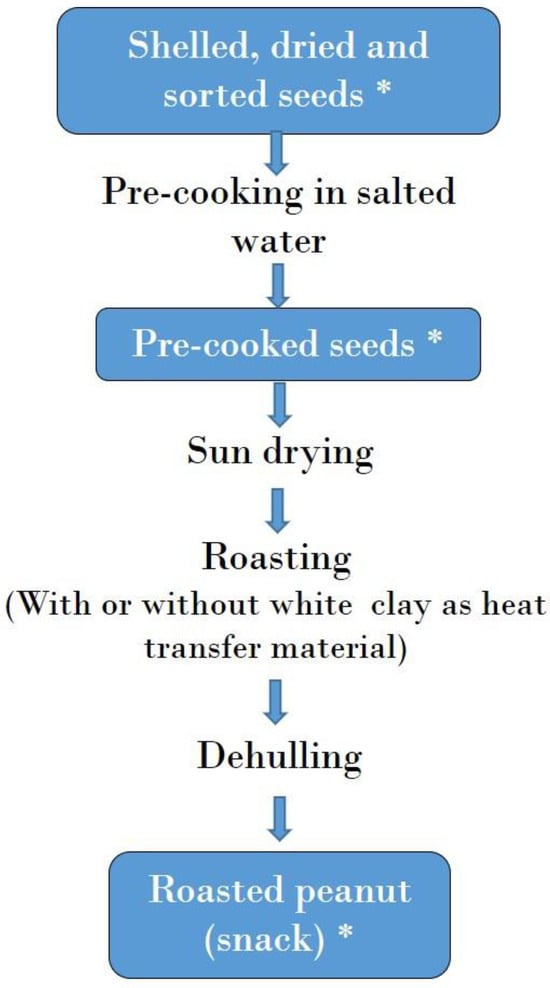
Figure 5.
Flow chart of roasted peanut production. *: Samples collected for mycotoxin analysis.
The comparison between both processes for kluiklui production is particularly relevant, not so much because of the potential role of maize flour as a source of mycotoxins, but rather due to the type of grinder employed. In Covè, the production process without maize flour relies on a grinder dedicated exclusively to peanut grinding, whereas in Aplahoué, the process incorporating maize flour involves the use of a mixed-use grinder. To assess the contribution of peanut processing practices to mould and mycotoxin contamination, kluiklui and roasted peanut snacks production follow-up was conducted on 2 producing sites (Covè and Aplahoué). The study considered both processing methods for kluiklui (with or without the addition of maize flour) and for roasted peanuts (with or without the use of a heat transfer material). Three independent productions, involving three different processors, were carried out for each product and processing method, resulting in a total of 36 trials (Table 10). The 12 processors were selected based on their experience in processing peanuts into kluiklui or roasted peanut snacks.

Table 10.
Experimental design applied to the production of kluiklui and roasted peanuts.
4.3.2. Samples Collected During Follow-Up Experiments
The raw peanuts (unshelled dried seeds) were purchased from a single peanut producer based in Covè. The crop was harvested in November 2023 and underwent two post-harvest drying phases: a first sun-drying in the field for 7 days, followed by a second drying at the producer’s residence for 14 days. After drying, the seeds were stored unshelled in polypropylene bags.
As mentioned before, kluiklui and roasted peanut snack production were performed 3 times by each processor, at intervals of two months between the first and second repetition, and one month between the second and third repetition.
Figure 6 illustrates the distribution of raw material between the 12 selected processors, for one trial. Before each trial, shelling was carried out by the peanut producer using a mechanical sheller. Shelling was stopped once 300 kg of shelled peanuts had been obtained. The shelled seeds were then packaged in 25 kg bags before being distributed to the processors. The 25 kg of shelled seeds were sun-dried and roughly sorted (by visual examination to remove impurities and bad seeds) by each processor before being used for kluiklui or roasted peanut snack production.
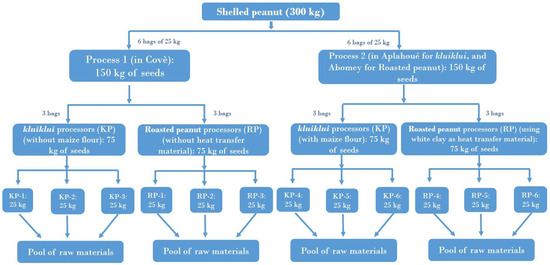
Figure 6.
Distribution plan of raw material (shelled peanut) between the 12 processors, for each trial. This trial was performed 3 times.
For mycotoxin analysis, raw peanuts (shelled, dried and sorted seeds) as well as roasted peanuts, peanut paste, maize flour, and kluiklui were sampled in the follow-up of kluiklui production, while raw peanuts and samples of the final product were collected in the follow-up of roasted peanut snack production.
As the raw material was of the same origin, samples of raw peanuts (shelled, dried and sorted seeds) were pooled. For laboratory analyses, 12 pooled samples of this raw material were created by mixing the 3 samples taken from each trial. Pooling was performed in the laboratory. After freeze-drying and grinding of each individual sample, pools were formed by combining three 50 g subsamples using a blender to obtain a homogeneous mixture. Table S8 presents details of the pooling of raw materials. When pooled raw material samples tested positive for mycotoxins, the corresponding individual samples were analysed, and the resulting data were considered in the present study (Table S8).
During the production of kluiklui, roasted peanut samples were collected immediately after partial roasting and cooling, and just before the dehulling and grinding of seeds. Peanut paste was obtained by grinding the roasted seeds using public grinding services. Samples were collected immediately after grinding. The maize flour used in the process was sourced from household stocks and was produced by grinding maize grains purchased from local markets. It was sampled as well. Kluiklui samples, produced by frying the shaped and pressed peanut paste, were collected immediately after frying and cooling.
Roasted peanut snacks were obtained by precooking and roasting peanut seeds either with or without a heat transfer material (white clay). Samples of roasted peanut snacks were collected immediately after roasting and cooling of seeds.
4.4. Determination of Mycotoxins in Peanut-Based Foods Samples
4.4.1. Standards and Chemicals
Analytical grade standards and solvents were used in this study and sourced from various suppliers. The solvents and reagents included: acetonitrile AR (Biosolve, Dieuze, France), mass spectrometry grade acetonitrile (Biosolve, Dieuze, France), acetic acid AR (Fisher Chemical, Merelbeke, Belgium), MS/MS grade methanol (Biosolve, Dieuze, France), formic acid (Avantor, VWR, Leuven, Belgium), ammonium acetate (Fluka, Mint Street, NC, USA), magnesium sulphate (MgSO4) and sodium chloride (NaCl), both from Roth (Karlsruhe, Germany). Ultrapure water was obtained using a Milli-Q® IQ 7010 purification system (MilliporeSigma, Burlington, MA, USA). Certified analytical standards of mycotoxins were obtained from Romer-Labs (Getzersdorf, Austria) for the following compounds: 15-acetyldeoxynivalenol (15Ac-DON), 3-acetyldeoxynivalenol (3Ac-DON), deoxynivalenol (DON), HT-2 toxin, alternariol (AOH), alternariol monomethyl ether (AME), T-2 toxin, zearalenone (ZEA), aflatoxins B1, B2, G1 and G2, ochratoxin A (OTA), ochratoxin B (OTB), fumonisins B1 (FUM B1), B2 (FUM B2) and B3 (FUM B3), as well as sterigmatocystin (STE). Standards for enniatins A, A1, B, and B1 were purchased from Merck (Merck KGaA, Darmstadt, Germany).
4.4.2. Determination of Mycotoxins
Mycotoxin concentration in marketed kluiklui and roasted peanut snacks and in samples from production follow-up were determined using liquid chromatography tandem mass spectrometry (LC-MS/MS), according to the method developed by Jonard, et al. [73]. A QuEChERS extraction procedure was used for extraction by adding 10 mL of ultrapure water to 5 g of ground sample, accurately weighed into 50 mL polypropylene centrifuge tubes. Then, 10 mL of acetonitrile containing 1% (v/v) acetic acid was added, and samples were homogenised using a Mixer Mill MM400 (Retsch GmbH, Aartselaar, Belgium) at 10 Hz for 10 min. Subsequently, 4 g of anhydrous magnesium sulphate (MgSO4) and 1 g of sodium chloride (NaCl) were added. The tubes were manually shaken and agitated using the Mixer Mill MM400 for 2 min at 10 Hz. The mixtures were then centrifuged at 3000× g for 5 min using a refrigerated centrifuge (Centrifuge 5810 R, Eppendorf, Leipzig, Germany). A 100 μL aliquot of the resulting supernatant was collected and diluted with 900 μL of a 50:50 (v/v) methanol–water solution. The final extracts were transferred into chromatographic vials for analysis. Liquid chromatography–tandem mass spectrometry (LC-MS/MS) analyses were carried out using a Waters ACQUITY I-Class UPLC system (Waters, Milford, MA, USA) equipped with a BEH C18 analytical column (2.1 × 50 mm, 1.7 μm particle size; Waters), coupled to a Xevo TQ-xS mass spectrometer (Waters, Milford, MA, USA). Column temperature was maintained at 50 °C. The injection volume was 2 µL, and the flow rate was set at 0.3 mL/min. An elution gradient was applied using mobile phase A consisting of an ammonium acetate-water solution containing 0.1% of formic acid, and mobile phase B composed of methanol with 0.1% of formic acid. The gradient started at 10% B, held for 3 min and increased linearly to 70% over 7 min. Then, the gradient increased again from 10% in 10 s and remained constant for 2 min. Finally, B decreased to 10% in 10 s followed by an equilibration period of 3 min.
Multiple reaction monitoring mode under positive ionisation mode was used on the mass spectrometer. The capillary voltage was set to 3.4 kV, with a desolvation temperature of 400 °C. The desolvation gas flow and cone gas flow were maintained at 800 L/H and 150 L/H, respectively, and the nebuliser pressure was 7 bar.
The software TargetLynx V4.2 (Waters Corporation, Milford, MA, USA) was used to perform chromatographic peak integration and quantification.
4.4.3. Method Validation
Average recoveries for each mycotoxin were evaluated for peanut, peanut paste and maize flour by spiking “blank” samples containing no mycotoxin above the limit of detection (LOD). A total of 6 “blank” samples were extracted and analysed on three different days. Reproducibility and repeatability were thus also evaluated. LOD and recovery in the different matrices are presented in Table 11. Intra-day and inter-day variability are presented in Table 12.

Table 11.
Limit of detection (LOD) and recovery of mycotoxin of interest in the different matrices.

Table 12.
Average intra-day repeatability and inter-day reproducibility of mycotoxin of interest in roasted peanuts.
4.5. Fungal Analysis
4.5.1. Fungal Enumeration and Isolation
Fungi were enumerated and isolated from samples collected during the follow-up of kluiklui and roasted peanut snack production. For each sample, 10 g were suspended in 90 mL of Buffered Peptone Water (BPW, Bio-Rad, Hemel, Belgium) and homogenised at 230 rpm for 2 min using a stomacher to obtain a 1:10 dilution. Serial dilutions (10−2 and 10−3) were then prepared by transferring 1 mL from the previous dilution into 9 mL of sterile BPW, following the protocol described in ISO 6887-1:2017 [74]. Moulds were enumerated on Yeast Extract Glucose Chloramphenicol (YGC) agar (Bio-Rad), incubated at 25 °C for 5 days, according to ISO 21527-2:2008 [75].
The samples were isolated directly on Water Agar (Micro Agar, Duchefa Biochemie, Haarlem, The Netherlands). Samples were also directly plated on Water Agar (Micro Agar, Duchefa Biochemie, The Netherlands). The fungal colonies observed were transferred to Potato Dextrose Agar (PDA, Scharlau, Barcelona, Spain) and sub-cultured repeatedly until pure isolates were obtained. The plates were incubated at 22 °C for 7 days. The colonies were examined under a microscope for a preliminary identification based on the observation of the macroscopic and microscopic characteristics of the isolates prior to molecular analyses.
4.5.2. Molecular Identification
Genomic DNA was extracted from fungal mycelium using a modified cetyltrimethylammonium bromide (CTAB) protocol as described by Karthikeyan, et al. [76], and amplified by PCR using primers ITS1 and ITS4. The PCR mix was then sequenced for complete identification of the isolated strain. The obtained sequence was compared to those available in the NCBI GenBank database using BLAST algorithm.
4.5.3. Ammonia Vapour Test
A qualitative method was used to identify aflatoxigenic strains of A. flavus. The isolates were cultivated in darkness on coconut agar medium supplemented with 0.3% methyl-β-cyclodextrin and incubated at 28 °C for 7 days. Following incubation, the cultures were exposed to ammonia vapour for 5 to 10 min. A colour change from pink to red was indicative of the strain’s ability to produce aflatoxins [77,78].
4.6. Estimation of Mycotoxins Daily Intake and Risk Characterisation
Exposure assessment and risk characterisation were conducted for aflatoxins B1 and total aflatoxins (B1 + B2 + G1 + G2) and for ochratoxin A, the main mycotoxins found in analysed samples.
4.6.1. Estimation of Mycotoxins Daily Intake
A food consumption survey was performed to estimate the daily intake of kluiklui and roasted peanut snacks. The survey was conducted among 400 adult consumers in municipalities of Abomey, Aplahoué, Covè, Cotonou, Glazoué, and Ouèssè. Individual body weights of consumers and daily intake levels of kluiklui and roasted peanut snacks were recorded. Data collection was carried out through face-to-face interviews conducted in French and local languages, including Fon, Mahi, Adja, Nago, and Idatcha. Prior to each interview, participants were provided with a concise explanation of the study’s objectives to ensure informed participation. Daily consumption of kluiklui and roasted peanut snack data used in this study ranged respectively from 0.4 to 346 g, and from 0.6 to 284 g, with respective medians of 14.4 g and 9.5 g.
The estimated daily intake (EDI) of each mycotoxin was calculated based on the contamination levels determined in kluiklui and roasted peanut snacks, and on these consumption data collected from a previous food consumption survey [79]. For contamination levels, two scenarios were considered: scenario 1 was based on median concentrations (calculated as upper bound values) and scenario 2, considered as a worst-case scenario, was based on the maximum concentration of the mycotoxin of interest.
Estimated daily intake: Estimated amount of mycotoxin ingested daily (µg/kg bw/day); Contamination level: median or maximum mycotoxin content (µg/kg); Consumption rate: amount peanut-based product ingested daily (g/day).
4.6.2. Risk Characterisation Related to Mycotoxin Ingestion Through Kluiklui and Roasted Peanut Snack Consumption
Risk Characterisation Related to the Exposure to Aflatoxin B1 and Total Aflatoxins
Two different approaches previously established by international regulatory agencies were used to assess the potential health risks associated with the consumption of kluiklui and roasted peanut snacks: the Margin of Exposure (MOE) approach recommended by the European Food Safety Authority [12] and a quantitative liver cancer risk approach developed by the FAO and the WHO [53].
The MOE was calculated for AFB1 and total aflatoxins (sum of AFB1, AFB2, AFG1 and AFG2) using a Benchmark Dose (BMDL10) of 0.4 µg/kg body weight (bw)/day derived from the incidence of hepatocellular carcinomas (HCC) in male rats following exposure to AFB1 and total aflatoxins applying equal potency factors for AFB1, AFB2, AFG1 and AFG2, as recommended by EFSA [12].
The following equation was used:
An MOE above 10,000 is considered indicative of a low public health concern related to AFB1 exposure.
The quantitative estimation of liver cancer risk resulting from AFB1 exposure was performed by multiplying the carcinogenic potency (Pcancer) (number of cancers per year per 100,000 individuals per ng AFB1 per kg bw per day) by the estimated daily intake of AFB1 (ng AFB1 per kg bw per day). For estimating the risk, in the present study, it was assumed that 9.9% of the Beninese population is chronically affected by hepatitis B [80], which is a known cofactor enhancing the carcinogenic effect of AFB1. The following equations were used:
With:
PHBsAg+ = 0.3 cancers/year/100,000 individuals/ng AFB1/kg bw/day [42]; PHBsAg- = 0.01 cancers/year/100,000 individuals/ng AFB1/kg bw/day [42]; pop.HBsAg+ = fraction of the Beninese population with Hepatitis B (0.099) [43]; pop.HBsAg- = fraction of the Beninese population without Hepatitis B (0.901) [43]
Risk Characterization Related to Ochratoxin a Exposure Through Peanut-Based Food Consumption
For ochratoxin A, a BMDL10 of 14.5 µg/kg bw/day was used to characterise neoplastic effects (kidney tumours in rats), whereas a BMDL10 of 4.73 µg/kg bw/day was applied to assess neurotoxic effects (kidney lesions in pigs) [59]. Margins of exposure (MOEs) below 10,000 for neoplastic effects and below 200 for neurotoxic effects are considered indicative of a health concern.
4.7. Data Analysis
Microsoft Excel 2019 was used to perform descriptive statistical analyses. Statistical analyses were done using R version 4.4.2 software. Mycotoxin concentration data were expressed on a wet weight basis. Upper-bound (UB) contamination data was used to carry out all calculations. Data were initially tested for normality using the Shapiro–Wilk test and for homogeneity of variances using Levene’s test, prior to conducting further statistical analyses. Differences between the two groups were analysed using an independent sample t-test or a Wilcoxon nonparametric test. All differences were considered statistically significant at p < 0.05.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins17110532/s1, Table S1: Mycotoxins concentrations of marketed roasted peanut snacks and kluiklui (µg/kg); Table S2: Statistics of main mycotoxins concentrations of marketed peanut-based foods (µg/kg, UB); Table S3: Mycotoxins concentration in raw peanut (µg/kg); Table S4: Mycotoxins contamination (µg/kg) in samples collected during kluiklui production; Table S5: Mycotoxins concentration in roasted peanut snacks (µg/kg); Table S6: Mean mycotoxin concentrations in marketed and just-produced peanut-based foods (µg/kg UB); Table S7: Detection of aflatoxigenic and non-aflatoxigenic A. flavus isolates in samples collected during kluiklui production; Table S8: Pooling of raw material (peanut seeds) used for kluiklui and roasted peanut snack processing.
Author Contributions
Conceptualization, C.S.G., M.J.M.A., O.H.I.A., Y.E.M., C.D., M.S. and M.-L.S.; Methodology, C.S.G., M.J.M.A., O.H.I.A., Y.E.M., C.D., M.S. and M.-L.S.; Software, C.S.G., C.J. and O.A.M.; Validation, S.G., C.D. and M.-L.S.; Formal analysis, C.S.G., C.J., O.A.M., S.G. and S.B.; Investigation, C.S.G., and M.J.M.A.; Resources, C.D., Y.E.M., J.M., C.B., M.S., P.A. and M.-L.S.; Data curation, C.S.G., C.J. and O.A.M.; Writing—original draft preparation, C.S.G.; Writing—review and editing, C.S.G., C.J., O.A.M., C.B., J.M., P.A., O.H.I.A., Y.E.M., C.D., M.S. and M.-L.S.; Visualization, C.S.G., C.J. and O.A.M.; Supervision, C.B., J.M., P.A., Y.E.M., C.D., M.S. and M.-L.S.; Project administration, P.A., M.S. and M.-L.S.; Funding acquisition, O.H.I.A., D.G.A., C.B., J.M., P.A., Y.E.M., C.D., M.S. and M.-L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by ARES-CCD (Académie de Recherche et d’Enseignement Supérieur–Coopération au Développement, Belgium) through the funded project “Peanut Quality”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Battilani, P.; Palumbo, R.; Giorni, P.; Dall’Asta, C.; Dellafiora, L.; Gkrillas, A.; Toscano, P.; Crisci, A.; Brera, C.; De Santis, B.; et al. Mycotoxin mixtures in food and feed: Holistic, innovative, flexible risk assessment modelling approach. EFSA Support. Publ. 2020, 17, 1757E. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing Fungi and Mechanisms of Phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef]
- IARC. Improving Public Health Through Mycotoxin Control. IARC Scientific Publication No. 158; International Agency for Research on Cancer: Lyon, France, 2012; Volume 158, p. 168. [Google Scholar]
- Garello, M.; Piombo, E.; Buonsenso, F.; Prencipe, S.; Valente, S.; Meloni, G.R.; Marcet-Houben, M.; Gabaldón, T.; Spadaro, D. Several secondary metabolite gene clusters in the genomes of ten Penicillium spp. raise the risk of multiple mycotoxin occurrence in chestnuts. Food Microbiol. 2024, 122, 104532. [Google Scholar] [CrossRef] [PubMed]
- Hellany, H.; Assaf, J.C.; El-Badan, D.; Khalil, M. Quantification, Prevalence, and Pretreatment Methods of Mycotoxins in Groundnuts and Tree Nuts: An Update. Processes 2023, 11, 3428. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- IARC. Chemical Agents and Related Occupations; IARC: Lyon, France, 2012; Volume 100F, pp. 1–599. [Google Scholar]
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Manyes, L. Toxicity of mycotoxins in vivo on vertebrate organisms: A review. Food Chem. Toxicol. 2020, 137, 111161. [Google Scholar] [CrossRef]
- Nejad, B.G.; Mostafaei, Z.; Rezaabad, A.B.; Mehravar, F.; Zarei, M.; Dehghani, A.; Estabragh, M.A.R.; Karami-Mohajeri, S.; Alizadeh, H. A systematic review with meta-analysis of the relation of aflatoxin B1 to growth impairment in infants/children. BMC Pediatr. 2023, 23, 614. [Google Scholar] [CrossRef]
- Bondy, G. Immunological toxicity of mycotoxins. Stewart Postharvest Rev. 2008, 4, 1–6. [Google Scholar] [CrossRef]
- EFSA. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, 112. [Google Scholar] [CrossRef]
- IARC. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1993; Volume 56, pp. 1–599. [Google Scholar]
- IARC. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2002; Volume 82, pp. 1–557. [Google Scholar]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef] [PubMed]
- Stockmann-Juvala, H.; Savolainen, K. A review of the toxic effects and mechanisms of action of fumonisin B1. Hum. Exp. Toxicol. 2008, 27, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Cai, H.; Luo, B.; Duan, S.; Yang, J.; Zhang, N.; He, Y.; Wu, A.; Liu, H. Recent Progress of Mycotoxin in Various Food Products—Human Exposure and Health Risk Assessment. Foods 2025, 14, 865. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.; Pereg, L. A review on the relation between soil and mycotoxins: Effect of aflatoxin on field, food and finance. Eur. J. Soil Sci. 2019, 70, 882–897. [Google Scholar] [CrossRef]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- USDA. Oilseeds: World Markets and Trade; USDA: Washington, DC, USA, 2024; Volume 39. [Google Scholar]
- Valentine, H. The Role of Peanuts in Global Food Security. In Peanuts; Academic Press: Champaign, IL, USA; AOCS Press: Urbana, IL, USA, 2016; pp. 447–461. [Google Scholar]
- Chang, A.S.; Sreedharan, A.; Schneider, K.R. Peanut and peanut products: A food safety perspective. Food Control 2013, 32, 296–303. [Google Scholar] [CrossRef]
- Variath, M.; Pasupuleti, J. Economic and Academic Importance of Peanut. In The Peanut Genome, Compendium of Plant Genomes; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2017; pp. 7–26. [Google Scholar]
- Adjile, A.; Mongbo, R.; Floquet, A. Les exploitations agricoles familiales arachidières de la commune de Ouessè au centre Bénin: État des lieux, typologies et dynamiques des systèmes de cultures. Ahoho Rev. Géogr. Lomé 2015, 15, 55–67. [Google Scholar]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as functional food: A review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef]
- Bediako, K.A.; Ofori, K.; Offei, S.K.; Dzidzienyo, D.; Asibuo, J.Y.; Amoah, R.A. Aflatoxin contamination of groundnut (Arachis hypogaea L.): Predisposing factors and management interventions. Food Control 2019, 98, 61–67. [Google Scholar] [CrossRef]
- Darko, C.B. Effect of Storage Conditions on Aspergillus Growth and Aflatoxin Production in Peanuts: A Study in Ghana; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2016. [Google Scholar]
- Adjou, E.S.; Yehouenou, B.; Sossou, C.M.; Soumanou, M.M.; De Souza, C.A. Occurrence of mycotoxins and associated mycoflora in peanut cake product (kulikuli) marketed in Benin. Afr. J. Biotechnol. 2012, 11, 14354–14360. [Google Scholar] [CrossRef]
- Awuah, R.T.; Fialor, S.C.; Binns, A.D.; Kagochi, J.; Jolly, C.M. Factors Influencing Market Participants Decision to Sort Groundnuts along the Marketing Chain in Ghana. Peanut Sci. 2009, 36, 68–76. [Google Scholar] [CrossRef]
- Bankole, S.A.; Ogunsanwo, B.M.; Eseigbe, D.A. Aflatoxins in Nigerian dry-roasted groundnuts. Food Chem. 2005, 89, 503–506. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Sulyok, M.; Warth, B.; Odebode, A.C.; Krska, R. Natural occurrence of mycotoxins in peanut cake from Nigeria. Food Control 2012, 27, 338–342. [Google Scholar] [CrossRef]
- Ingenbleek, L.; Sulyok, M.; Adegboye, A.; Hossou, E.S.; Koné, A.Z.; Oyedele, D.A.; Kisito, C.S.K.J.; Dembélé, K.Y.; Leblanc, J.-C.; Le Bizec, B.; et al. Regional Sub-Saharan Africa Total Diet Study in Benin, Cameroon, Mali and Nigeria Reveals the Presence of 164 Mycotoxins and Other Secondary Metabolites in Foods. Toxins 2019, 11, 54. [Google Scholar] [CrossRef]
- Tedihou, E.; Hell, K.; Aziato, K.; Nyaku, A. Évaluation de la Prévalence des Aflatoxines Dans les Produits D’arachide au Togo; Bulletin de la Recherche Agronomique du Gronomique du Bénin (BRAB); Numéro Spécial Productions Végétales, Animales et Halieutiques, Économie Rurale, Sociologie Rurale, Agronomie, Environnement, Développement Durable & Sécurité Alimentaire de l’Institut Togolais de Recherche Agronomique (ITRA); ITRA: Lausanne, Switzerland, 2019; pp. 129–136. [Google Scholar]
- Waliyar, F.; Umeh, V.C.; Traore, A.; Osiru, M.; Ntare, B.R.; Diarra, B.; Sudini, H. Prevalence and distribution of aflatoxin contamination in groundnut (Arachis hypogaea L.) in Mali, West Africa. Crop Prot. 2015, 70, 1–7. [Google Scholar] [CrossRef]
- Watson, S.; Diedhiou, P.M.; Atehnkeng, J.; Dem, A.; Bandyopadhyay, R.; Srey, C.; Gong, Y.Y. Seasonal and geographical differences in aflatoxin exposures in Senegal. World Mycotoxin J. 2015, 8, 525–531. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance); C/2023/35; European Commission: Luxembourg, 2023; pp. 103–157. [Google Scholar]
- Videgla, E.G.; Floquet, A.; Mongbo, R.; Garba, K.; Tossou, H.S.; Toukourou, F. Liens à l’origine et qualité spécifique d’un produit de l’artisanat agroalimentaire du Bénin—Le kluiklui d’Agonlin. Cah. Agric. 2016, 25, 35003. [Google Scholar] [CrossRef][Green Version]
- Njumbe Ediage, E.; Diana Di Mavungu, J.; Monbaliu, S.; Van Peteghem, C.; De Saeger, S. A Validated Multianalyte LC–MS/MS Method for Quantification of 25 Mycotoxins in Cassava Flour, Peanut Cake and Maize Samples. J. Agric. Food Chem. 2011, 59, 5173–5180. [Google Scholar] [CrossRef]
- Bakherad, Z.; Feizy, J. Preliminary Survey of Aflatoxins in Mashhad’s Roasted Red Skin Peanut Kernels during February to May 2016. J. Community Health Res. 2018, 7, 112–118. [Google Scholar]
- Sombie, J.I.N.; Ezekiel, C.N.; Sulyok, M.; Ayeni, K.I.; Jonsyn-Ellis, F.; Krska, R. Survey of roasted street-vended nuts in Sierra Leone for toxic metabolites of fungal origin. Food Addit. Contam. Part A 2018, 35, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, H.W. Using the Köppen classification to quantify climate variation and change: An example for 1901–2010. Environ. Dev. 2013, 6, 69–79. [Google Scholar] [CrossRef]
- World Bank. Benin Country Climate and Development Report. Available online: https://www.worldbank.org/en/country/benin/publication/benin-country-climate-and-development-report (accessed on 16 October 2025).
- Yu, P.; Xu, R.; Yang, Z.; Ye, T.; Liu, Y.; Li, S.; Abramson, M.J.; Kimlin, M.; Guo, Y. Cancer and Ongoing Climate Change: Who Are the Most Affected? ACS Environ. Au 2023, 3, 5–11. [Google Scholar] [CrossRef] [PubMed]
- MAEP. Arrêté 2007 N°0362 MAEP/D-CAB/SGM/DRH/DP/SA Portant Fixation des Teneurs Maximales Pour Certains Contaminants Dans les Denrées Alimentaires en République du Bénin; MAEP: Cotonou, Benin, 2007; p. 17. [Google Scholar]
- Ndung’u, J.W.; Makokha, A.; Onyango, C.A.; Mutegi, C.; Wagacha, M.; Christie, M.; Wanjoya, A. Prevalence and potential for aflatoxin contamination in groundnuts and peanut butter from farmers and traders in Nairobi and Nyanza provinces of Kenya. J. Appl. Biosci. 2013, 65, 4922–4934. [Google Scholar] [CrossRef][Green Version]
- Fandohan, P.; Gnonlonfin, B.; Hell, K.; Marasas, W.F.O.; Wingfield, M.J. Natural occurrence of Fusarium and subsequent fumonisin contamination in preharvest and stored maize in Benin, West Africa. Int. J. Food Microbiol. 2005, 99, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Kayode, O.F.; Sulyok, M.; Fapohunda, S.O.; Ezekiel, C.N.; Krska, R.; Oguntona, C.R. Mycotoxins and fungal metabolites in groundnut- and maize-based snacks from Nigeria. Food Addit. Contam. Part B Surveill. 2013, 6, 294–300. [Google Scholar] [CrossRef]
- Martins, L.M.; Sant’Ana, A.S.; Iamanaka, B.T.; Berto, M.I.; Pitt, J.I.; Taniwaki, M.H. Kinetics of aflatoxin degradation during peanut roasting. Food Res. Int. 2017, 97, 178–183. [Google Scholar] [CrossRef]
- N’pagyendou, L.; Rassimwaï, P.; Tchaou, B.; Banfitebiyi, G.; Essohouna, A.; Simplice, K.D. Impact of traditional transformation processes on the level of aflatoxin B1 in groundnut seeds sold in northern Togo. Int. J. Nov. Res. Life Sci. 2022, 9, 22–30. [Google Scholar]
- Diedhiou, P.M.; Ba, F.; Kane, A.; Mbaye, N. Effect of different cooking methods on aflatoxin fate in peanut products. Afr. J. Food Sci. Technol. 2012, 3, 53–58. [Google Scholar]
- Adeniran, H.A.; Ikujenlola, A.V.; Nd’janton, G.N. Effect of Processing Conditions on the Aflatoxin Content of Kulikuli-A Groundnut-Based Fried Snack. Ann. Clin. Nutr. 2022, 5, 1023. [Google Scholar]
- Teixido-Orries, I.; Molino, F.; Castro-Criado, B.; Jodkowska, M.; Medina, A.; Marín, S.; Verheecke-Vaessen, C. Mapping Variability of Mycotoxins in Individual Oat Kernels from Batch Samples: Implications for Sampling and Food Safety. Toxins 2025, 17, 34. [Google Scholar] [CrossRef]
- JECFA. Safety Evaluation of Certain Contaminants in Food; World Health Organization: Geneva, Switzerland, 2018; Volume 74, p. 992. [Google Scholar]
- Oyedele, O.A.; Ezekiel, C.N.; Sulyok, M.; Adetunji, M.C.; Warth, B.; Atanda, O.O.; Krska, R. Mycotoxin risk assessment for consumers of groundnut in domestic markets in Nigeria. Int. J. Food Microbiol. 2017, 251, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kortei, N.K.; Annan, T.; Akonor, P.T.; Richard, S.A.; Annan, H.A.; Kwagyan, M.W.; Ayim-Akonor, M.; Akpaloo, P.G. Aflatoxins in randomly selected groundnuts (Arachis hypogaea) and its products from some local markets across Ghana: Human risk assessment and monitoring. Toxicol. Rep. 2021, 8, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Aydemir Atasever, M.; Güler İnce, M.B.; Alkan Polat, B.; Özlü, H.; Atasever, M. Aflatoxin B1 levels, dietary exposure and cancer risk assessment in sesame and nut-based foods in Türkiye. Mycotoxin Res. 2025, 41, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Kooprasertying, P.; Maneeboon, T.; Hongprayoon, R.; Mahakarnchanakul, W. Exposure assessment of aflatoxins in Thai peanut consumption. Cogent Food Agric. 2016, 2, 1204683. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Ayeni, K.I.; Akinyemi, M.O.; Sulyok, M.; Oyedele, O.A.; Babalola, D.A.; Ogara, I.M.; Krska, R. Dietary Risk Assessment and Consumer Awareness of Mycotoxins among Household Consumers of Cereals, Nuts and Legumes in North-Central Nigeria. Toxins 2021, 13, 635. [Google Scholar] [CrossRef]
- EFSA. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, e06113. [Google Scholar] [CrossRef]
- Kouadio, J. Risk Assessment of Mycotoxins Intake through the Consumption of Maize, Peanuts, Rice and Cassava in Côte d’Ivoire. Food Nutr. Sci. 2022, 13, 41–54. [Google Scholar] [CrossRef]
- Nuhu, A.H.; Dorleku, W.-P.; Blay, B.; Derban, E.; McArthur, C.O.; Alobuia, S.E.; Incoom, A.; Dontoh, D.; Ofosu, I.W.; Oduro-Mensah, D. Exposure to aflatoxins and ochratoxin A from the consumption of selected staples and fresh cow milk in the wet and dry seasons in Ghana. Food Control 2025, 168, 110968. [Google Scholar] [CrossRef]
- Norlia, M.; Nor-Khaizura, M.A.R.; Selamat, J.; Abu Bakar, F.; Radu, S.; Chin, C.K. Evaluation of aflatoxin and Aspergillus sp. contamination in raw peanuts and peanut-based products along this supply chain in Malaysia. Food Addit. Contam. Part A 2018, 35, 1787–1802. [Google Scholar] [CrossRef]
- Hussain, A.; Rahman, Z.; Amin, M.; Khan, M.; Bangash, J. Diversity of fungal contamination in peanut products locally available in Peshawar Region, Pakistan. Agric. Sci. Res. J. 2021, 11, 169–172. [Google Scholar]
- Kamarudin, N.; Zakaria, L. Characterization of two xerophilic Aspergillus spp. from peanuts (Arachis hypogaea). Malays. J. Microbiol. 2018, 14, 41–48. [Google Scholar] [CrossRef]
- Xing, F.; Ding, N.; Liu, X.; Selvaraj, J.N.; Wang, L.; Zhou, L.; Zhao, Y.; Wang, Y.; Liu, Y. Variation in fungal microbiome (mycobiome) and aflatoxins during simulated storage of in-shell peanuts and peanut kernels. Sci. Rep. 2016, 6, 25930. [Google Scholar] [CrossRef] [PubMed]
- Hubka, V.; Kolařík, M.; Kubátová, A.; Peterson, S.W. Taxonomic revision of Eurotium and transfer of species to Aspergillus. Mycologia 2013, 105, 912–937. [Google Scholar] [CrossRef] [PubMed]
- Molina-Hernandez, J.B.; Landi, L.; De Flaviis, R.; Laika, J.; Romanazzi, G.; Chaves-Lopez, C. Understanding the mechanisms of action of atmospheric cold plasma towards the mitigation of the stress induced in molds: The case of Aspergillus chevalieri. Innov. Food Sci. Emerg. Technol. 2023, 90, 103492. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: Berlin/Heidelberg, Germany, 2009; p. 520. [Google Scholar] [CrossRef]
- Escrivá, L.; Font, G.; Manyes, L.; Berrada, H. Studies on the Presence of Mycotoxins in Biological Samples: An Overview. Toxins 2017, 9, 251. [Google Scholar] [CrossRef]
- Magan, N.; Medina, A.; Aldred, D. Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathol. 2011, 60, 150–163. [Google Scholar] [CrossRef]
- Nji, Q.N.; Babalola, O.O.; Ekwomadu, T.I.; Nleya, N.; Mwanza, M. Six Main Contributing Factors to High Levels of Mycotoxin Contamination in African Foods. Toxins 2022, 14, 318. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. How will climate change affect mycotoxins in food? Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef]
- Jonard, C.; Chandelier, A.; Eylenbosch, D.; Pannecoucque, J.; Godin, B.; Douny, C.; Scippo, M.-L.; Gofflot, S. Multi-Mycotoxin Analyses by UPLC-MS/MS in Wheat: The Situation in Belgium in 2023 and 2024. Foods 2025, 14, 2300. [Google Scholar] [CrossRef]
- ISO 6887-1:2017; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Molds Part 2: Colony Count Technique in Products with Water Activity Less Than or Equal to 0,95. International Organization for Standardization: Geneva, Switzerland, 2008.
- Karthikeyan, V.; Patharajan, S.; Palani, P.; Spadaro, D. Modified simple protocol for efficient fungal DNA extraction highly. Glob. J. Mol. Sci. 2010, 5, 37–42. [Google Scholar]
- Saito, M.; Machida, S. A rapid identification method for aflatoxin-producing strains of Aspergillus flavus and A. parasiticus by ammonia vapor. Mycoscience 1999, 40, 205–208. [Google Scholar] [CrossRef]
- Abd El-Aziz, A.R.M.; Shehata, S.M.; Hisham, S.M.; Alobathani, A.A. Molecular profile of aflatoxigenic and non-aflatoxigenic isolates of Aspergillus flavus isolated from stored maize. Saudi J. Biol. Sci. 2021, 28, 1383–1391. [Google Scholar] [CrossRef]
- AFSCA. Agence Fédérale Pour la Sécurité de la Chaîne Alimentaire: Terminologie en Matière D’analyse des Dangers et des Risques Selon le Codex Alimentarius; AFSCA: Brussels, Belgium, 2005; 46p. [Google Scholar]
- Kpossou, A.R.; Paraiso, M.N.; Sokpon, C.N.; Alassan, K.S.; Vignon, R.K.; Keke, R.K.; Bigot, C.; Domonhédo, C.; Sossa Gbédo, E.; Séhonou, J.; et al. Seroprevalence of viral hepatitis B and its associated factors determined based on data from a screening campaign targeting the general population in Benin. Pan Afr. Med. J. 2020, 37, 247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).