Aflatoxins in Mexican Maize Systems: From Genetic Resources to Agroecological Resilience and Co-Occurrence with Fumonisins
Abstract
1. Introduction
1.1. Maize in Mexico and the World
1.2. Climate Change and Mycotoxins of Major Relevance in Maize
1.3. Regulation of Aflatoxins and Fumonisins
2. Types of Germplasm and the Aflatoxin Production
2.1. Advances in Resistance Development
2.2. Resilience and Susceptibility to Aflatoxins in Maize Landrace
2.3. Hybrid Maize and Its Relationship with the Presence of Aflatoxins
| Maize Code | Name | Characteristics | Current Distribution (State Level) | Reference |
|---|---|---|---|---|
| Landrace maize | ||||
| MLR 2006-23 | Tabloncillo | Elongated cobs with jagged or semicrystalline grains varying from white to orange | Michoacán, Jalisco, Nayarit, Sinaloa, and Sonora | [48,49,50] |
| MLR 2007–06 | Vandeño | Cylindrical cobs with a thick ear and white jagged grains | Chiapas, Oaxaca and Guerrero | [48,50] |
| Tropical white maize hybrid | ||||
| NB-722 | Novasem | Excellent stability, adaptability, and Fusarium tolerance | Tamaulipas | [53] |
| AG-2525 | Anzu | Cob health and high yields | Tamaulipas | [53] |
| P-3057 | Pioneer | Early maturity, strong stalks, and high yields | Tamaulipas | [53] |
| CORONEL | Iyadilpro | Excellent plant health, good ear coverage, and tolerance to stalk lodging | Campeche | [53] |
| P-4028 | Pioneer | Good foliar and grain health | Campeche | [53] |
| P-4279 | Pioneer | Good foliar and grain health | Campeche | [53] |
| TORNADO | Ceres | Excellent plant health and tolerance to stalk lodging | Campeche | [53] |
3. Agricultural Production Systems and Aflatoxin Incidences
3.1. Impact of Agronomic Practices on Aflatoxin Contamination in Grain
3.2. Environmental and Edaphic Factors Associated with Aflatoxin Incidence
3.3. Prevalence of Aflatoxins in Maize-Producing Regions in Mexico
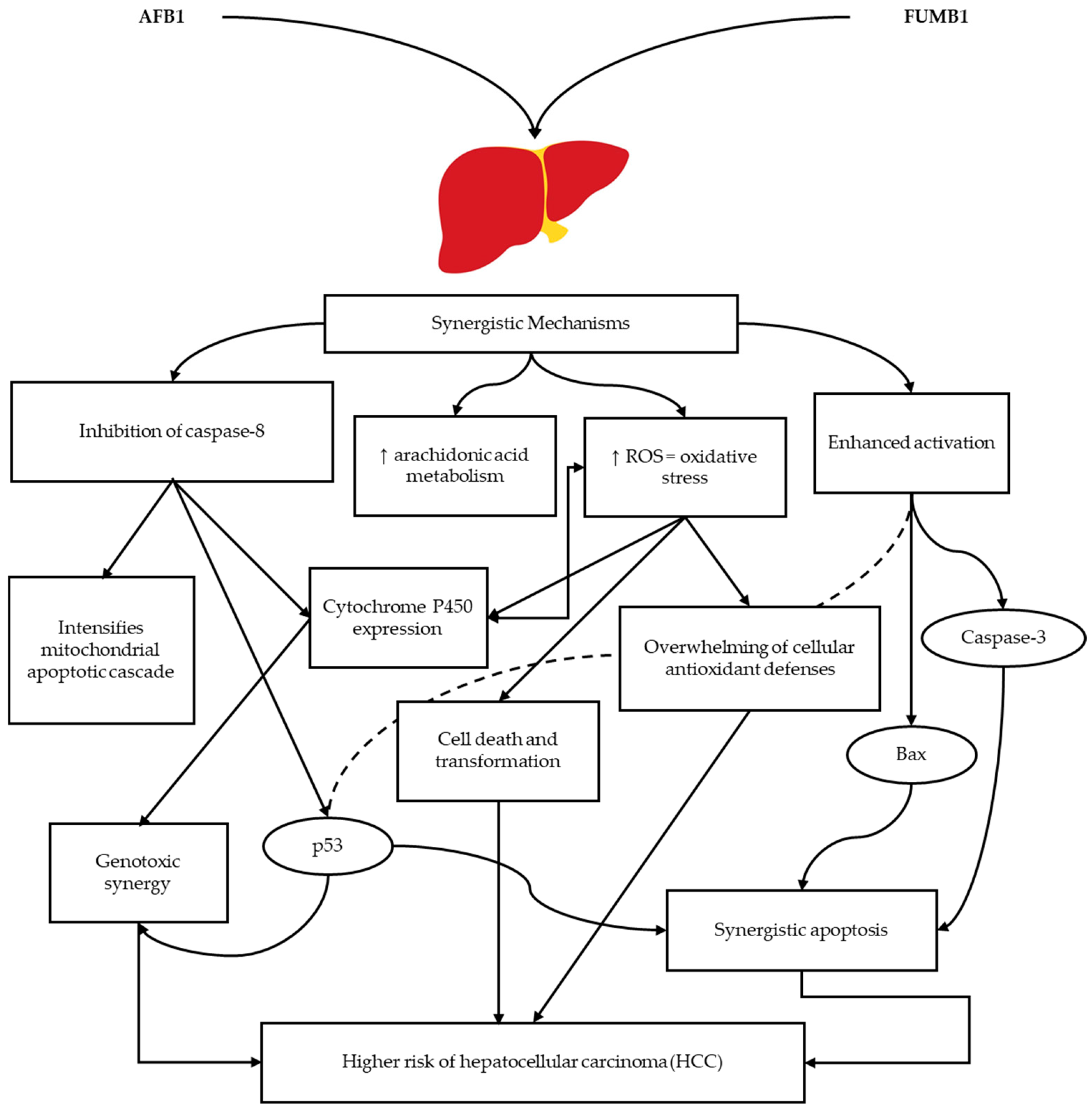
4. Co-Occurrences of Aflatoxins and Fumonisins
4.1. Factors Associated with Co-Occurrence
4.2. Health, Food Safety, and Trade Implications
4.3. Use of Biomarkers to Assess Exposure to Mycotoxins
5. Strategies to Mitigate Aflatoxin and Fumonisin Contamination
5.1. Sustainable Agricultural Practices
5.2. Genetic Resistance in the Maize Plant
| Inbred Line 1 | Germplasm Source 2 | Inbred Line 1 | Germplasm Source 2 |
|---|---|---|---|
| Drought-tolerant, resistant to ear rot and major foliar diseases, tropical white for Latin America | |||
| CML515 | CML247/IR | CML576 | CLFAWW11/CML494 |
| CML549 | CML498/CLRCW36 | CML596 | CL04325/CML401 |
| CML550 | P25HSRRS | CML600 | CLRCW88/CLRCW96 |
| CML552 | CML495/CML401 | CML601 | CLRCW79/CLRCW98 |
| CML553 | CML264/CLRCW41 | CML636B | CML269/CL02221 |
| CML554 | CML491/CLQRCWQ13 | CML638A | CLG2305/CML401 |
| CML555 | H132 | CML639B | CML555/CLQRCWQ121 |
| CML556 | CML502/CLQRCWQ26 | CML640B | CL02221/CLRCW72//CML556 |
| CML557 | CML176/CML264 | ||
| Drought-tolerant, resistant to ear rot and major foliar diseases, yellow for Latin America | |||
| CML551 | P27FRRS | CML598 | CML413/CML287 |
| CML575 | CML451/CLRCW29 | CML599 | P390AM |
| CML577 | CML454/CML451 | CML602 | CLRCY040/CML451 |
| CML597 | CML285/CL00356 | CML637B | CML451/CML551 |
| Drought-tolerant, resistant to ear rot and major foliar diseases, white for Eastern and Southern Africa | |||
| CML569 | LAPOSTASEQ/CML395 | CML609A | CML495/PHG39 |
| CML570 | LAPOSTASEQ/CML444 | CML610A | CKL05017/LAPOSTASEQ |
| CML607B | LAPOSTASEQ/CML395 | CML618B | CML384/(MBR/MDR |
| CML608B | ZM521B/LAPOSTASEQ | CML620B | CML543/(CML444//CML395///DTPW |
| Drought-tolerant and provitamin A-enhanced tropical mid-altitude, yellow for Southern Africa | |||
| CML628B | KUICAROTENOIDSYN/CML297///KUI3/SC55 | ||
| CML629B | CML488/(BETASYN)BC1//G9BTSR///ATZT-VC82 | ||
| CML630B | CLQRCWQ97/KUICAROTENOIDSYN///KU1409 | ||
5.3. Biological Control
5.3.1. Atoxigenic Strains of A. flavus
| Commercial Product | Strain Name | Isolation Source | Place of Application | Use in Crops |
|---|---|---|---|---|
| AF36 Prevail® 1 | AF36 | Cottonseed | United States | Cotton, maize, fig, almond, pistachio |
| Afla-Guard® 2 | NRRL21882 | Peanut | United States | Maize, peanut, almond, pistachio |
| Aflasafe™ 3 | Ka16127, La3279, La3304, Og0222 | Maize soils | Nigeria | Maize, peanut |
| Aflasafe KE01™ | C6-E, C8-F, E63-I, R7-H | Maize soils | Kenya | Maize |
| Aflasafe SN01 | M2-7, M21-11, Ms14-19, Ss19-14 | Maize and peanut soils | Senegal, Gambia | Maize, peanut |
| Aflasafe BF01 | M011-8, G018-2, M109-2, M110-7 | Maize and peanut soils | Burkina Faso | Maize, peanut |
| Aflasafe GH01 | GHG079-4, GHG083-4, GHG321-2, GHM174-1 | Maize and peanut soils | Ghana | Maize, peanut, sorghum |
| Aflasafe GH02 | GHM511-3, GHM109-4, GHM001-5, GHM287-10 | Maize and peanut soils | Ghana | Maize, peanut, sorghum |
| Aflasafe TZ01 | TMS199-3, TMH104-9, TGS364-2, TMH 30-8 | Maize and peanut soils | Tanzania | Maize, peanut |
| Aflasafe TZ02 | TMS64-1, TGS55-6, TMS205-5, TMS137-3 | Maize and peanut soils | Tanzania | Maize, peanut |
| Aflasafe MWMZ01 | GP5G-8, GP1H-12, MZM594-1, MZM029-7 | Maize and peanut soils | Mozambique | Maize, peanut |
| Aflasafe MWMZ01 | MW199-1, MW097-8, MW246-2, MW238-2 | Maize and peanut soils | Malawi | Maize, peanut |
| Aflasafe MZ02 | GP5G-8, MZG071-6, MZM028-5, MZM250-8 | Maize and peanut soils | Mozambique | Maize, peanut |
| Aflasafe MW02 | MW258-6, MW332-10, MW248-11, MW204-7 | Maize and peanut soils | Malawi | Maize, peanut |
| Aflasafe ZM01 | 110MS-05, 38MS-03, 46MS-02, 03MS-10 | Maize and peanut soils | Zambia | Maize, peanut |
| Aflasafe ZM02 | 31MS-12, 12MS-10, 47MS-12, 64MS-03 | Maize and peanut soils | Zambia | Maize, peanut |
| AF-X1® 4 | MUCL54911 | Maize cob | Italy | Maize |
| FourSure™ 5 | TC16F, TC35C, TC38B, TC46G–FFDCA | Maize fields | Texas | Maize |
5.3.2. Soil Microbiome
6. Conclusions
7. Future Directions
7.1. Other Significant Mycotoxins Found in Maize
7.2. Modern Strategies to Optimize Maize Breeding
7.3. Improving Microbial Understanding in Biocontrol Development
7.4. Aflatoxins’ Predictive Risk Models in Maize
7.5. Clinical and Epidemiological Studies on Mycotoxins
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasanna, B.M. Diversity in Global Maize Germplasm: Characterization and Utilization. J. Biosci. 2012, 37, 843–855. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 15 June 2025).
- SIAP Servicio de Información Agroalimentaria y Pesquera. Intención de Siembra y de Cosecha en Mexico, Ciclo 2025. Available online: https://nube.agricultura.gob.mx/agroprograma/ (accessed on 19 June 2025).
- Atlin, G.N.; Palacios, N.; Babu, R.; Das, B.; Twumasi-Afriyie, S.; Friesen, D.K.; De Groote, H.; Vivek, B.; Pixley, K.V. Quality Protein Maize: Progress and Prospects. In Plant Breeding Reviews; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2010; pp. 83–130. ISBN 978-0-470-88057-9. [Google Scholar]
- García-Salazar, J.A.; Ramírez-Jaspeado, R. El Mercado de la Semilla Mejorada de Maíz (Zea mays L.) en México: Análisis del saldo comercial por entidad federativa. Rev. Fitotec. Mex. 2014, 37, 69–77. [Google Scholar] [CrossRef]
- Santillán-Fernández, A.; Salinas-Moreno, Y.; Valdez-Lazalde, J.R.; Bautista-Ortega, J.; Pereira-Lorenzo, S. Spatial Delimitation of Genetic Diversity of Native Maize and Its Relationship with Ethnic Groups in Mexico. Agronomy 2021, 11, 672. [Google Scholar] [CrossRef]
- Plasencia, J. Aflatoxins in Maize: A Mexican Perspective. J. Toxicol. Toxin Rev. 2004, 23, 155–177. [Google Scholar] [CrossRef]
- Camardo Leggieri, M.; Giorni, P.; Pietri, A.; Battilani, P. Aspergillus flavus and Fusarium verticillioides Interaction: Modeling the Impact on Mycotoxin Production. Front. Microbiol. 2019, 10, 2653. [Google Scholar] [CrossRef]
- Cai, Y. How Does Climate Change Affect Regional Sustainable Development? Empirical Evidence from 186 Countries around the World. Int. Rev. Econ. Financ. 2025, 99, 104047. [Google Scholar] [CrossRef]
- Zhao, Z.-W.; Leng, P.; Han, X.-J.; Shang, G.-F. Global Maize Yield Responses to Essential Climate Variables: Assessment Using Atmospheric Reanalysis and Future Climate Scenarios. Comput. Electron. Agric. 2025, 232, 110140. [Google Scholar] [CrossRef]
- Ureta, C.; González, E.J.; Espinosa, A.; Trueba, A.; Piñeyro-Nelson, A.; Álvarez-Buylla, E.R. Maize Yield in Mexico under Climate Change. Agric. Syst. 2020, 177, 102697. [Google Scholar] [CrossRef]
- Conde, C.; Estrada, F.; Martínez, B.; Sánchez, O.; Gay, C. Regional Climate Change Scenarios for México. Atmósfera 2011, 24, 125–140. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Miller, J.D. Chapter 4—Mycotoxins: Still with Us after All These Years. In Present Knowledge in Food Safety; Knowles, M.E., Anelich, L.E., Boobis, A.R., Popping, B., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 62–78. ISBN 978-0-12-819470-6. [Google Scholar]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited Review: Remediation Strategies for Mycotoxin Control in Feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef]
- Habschied, K.; Krstanović, V.; Zdunić, Z.; Babić, J.; Mastanjević, K.; Šarić, G.K. Mycotoxins Biocontrol Methods for Healthier Crops and Stored Products. J. Fungi 2021, 7, 348. [Google Scholar] [CrossRef]
- McCormick, S.P. Microbial Detoxification of Mycotoxins. J. Chem. Ecol. 2013, 39, 907–918. [Google Scholar] [CrossRef]
- Andrade, P.D. Dietary Risk Assessment for Fumonisins: Challenges and Prospects. Curr. Opin. Food Sci. 2023, 54, 101080. [Google Scholar] [CrossRef]
- Santini, A.; Ritieni, A.; Santini, A.; Ritieni, A. Aflatoxins: Risk, Exposure and Remediation. In Aflatoxins—Recent Advances and Future Prospects; IntechOpen: London, UK, 2013; ISBN 978-953-51-0904-4. [Google Scholar]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as Human Carcinogens—The IARC Monographs Classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Elabed, S.; Khaled, R.; Farhat, N.; Madkour, M.; Mohammad Zadeh, S.A.; Shousha, T.; Taneera, J.; Semerjian, L.; Abass, K. Assessing Aflatoxin Exposure in the United Arab Emirates (UAE): Biomonitoring AFM1 Levels in Urine Samples and Their Association with Dietary Habits. Environ. Toxicol. Pharmacol. 2025, 114, 104644. [Google Scholar] [CrossRef] [PubMed]
- NOM. NORMA Oficial Mexicana NOM. Available online: https://salud.gob.mx/unidades/cdi/nom/188ssa12.html (accessed on 15 April 2025).
- FDA. Guidance for Industry: Fumonisin Levels in Human Foods and Animal Feeds. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-fumonisin-levels-human-foods-and-animal-feeds (accessed on 16 June 2025).
- Soni, P.; Gangurde, S.S.; Ortega-Beltran, A.; Kumar, R.; Parmar, S.; Sudini, H.K.; Lei, Y.; Ni, X.; Huai, D.; Fountain, J.C.; et al. Functional Biology and Molecular Mechanisms of Host-Pathogen Interactions for Aflatoxin Contamination in Groundnut (Arachis hypogaea L.) and Maize (Zea mays L.). Front. Microbiol. 2020, 11, 227. [Google Scholar] [CrossRef]
- Fountain, J.; Scully, B.; Ni, X.; Kemerait, R.; Lee, D.; Chen, Z.-Y.; Guo, B. Environmental Influences on Maize-Aspergillus flavus Interactions and Aflatoxin Production. Front. Microbiol. 2014, 5, 40. [Google Scholar] [CrossRef]
- Womack, E.D.; Williams, W.P.; Windham, G.L.; Xu, W. Mapping Quantitative Trait Loci Associated with Resistance to Aflatoxin Accumulation in Maize Inbred Mp719. Front. Microbiol. 2020, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Bazan, V.; Mahuku, G.; Bibbins-Martinez, M.; Arroyo-Bacerra, A.; Villalobos-López, M.Á. Dissection of Mechanisms of Resistance to Aspergillus flavus and Aflatoxin Using Tropical Maize Germplasm. World Mycotoxin J. 2018, 25, 215–224. [Google Scholar] [CrossRef]
- Ogunola, O.; Smith, J.S.; Xu, W.; Bhattramakki, D.; Jeffers, D.; Williams, W.P.; Warburton, M.L. Characterization of a Source of Resistance to Aflatoxin Accumulation in Maize. Agrosyst. Geosci. Environ. 2021, 4, e20203. [Google Scholar] [CrossRef]
- Baisakh, N.; Da Silva, E.A.; Pradhan, A.K.; Rajasekaran, K. Comprehensive Meta-Analysis of QTL and Gene Expression Studies Identify Candidate Genes Associated with Aspergillus flavus Resistance in Maize. Front. Plant Sci. 2023, 14, 1214907. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Guo, X.; Zhang, J.; Wang, S.; Sun, X.; Duan, H.; Xie, H.; Ding, D.; Tang, J.; et al. Identification of Novel QTL Contributing to Resistance Against Aspergillus flavus in Maize (Zea mays L.) Using an Enlarged Genotype Panel. J. Integr. Agric. 2025, in press. [Google Scholar] [CrossRef]
- Scott, G.E.; Zummo, N. Registration of Mp313E Parental Line of Maize. Crop Sci. 1990, 30, 1378. [Google Scholar] [CrossRef]
- Scott, G.E.; Zummo, N. Registration of Mp420 Germplasm Line of Maize. Crop Sci. 1992, 32, 1296. [Google Scholar] [CrossRef]
- Williams, W.P.; Windham, G.L. Registration of Maize Germplasm Line Mp715. Crop Sci. 2001, 41, 1374. [Google Scholar] [CrossRef]
- Williams, W.P.; Windham, G.L. Registration of Maize Germplasm Line Mp717. Crop Sci. 2006, 46, 1407. [Google Scholar] [CrossRef]
- Williams, W.P.; Windham, G.L. Registration of Mp718 and Mp719 Germplasm Lines of Maize. J. Plant Regist. 2012, 6, 200–202. [Google Scholar] [CrossRef]
- Betrán, F.J.; Isakeit, T.; Odvody, G. Aflatoxin Accumulation of White and Yellow Maize Inbreds in Diallel Crosses. Crop Sci. 2002, 42, 1894–1901. [Google Scholar] [CrossRef]
- Guo, B.Z.; Widstrom, N.W.; Lee, R.D.; Coy, A.E.; Lynch, R.E. Registration of Maize Germplasm GT601 (AM-1) and GT602 (AM-2). J. Plant Reg. 2007, 1, 153–154. [Google Scholar] [CrossRef]
- Guo, B.Z.; Krakowsky, M.D.; Ni, X.; Scully, B.T.; Lee, R.D.; Coy, A.E.; Widstrom, N.W. Registration of Maize Inbred Line GT603. J. Plant Regist. 2011, 5, 211–214. [Google Scholar] [CrossRef]
- Williams, W.; Krakowsky, M.D.; Windham, G.L.; Balint-Kurti, P.; Hawkins, L.K.; Henry, W. Identifying Maize Germplasm with Resistance to Aflatoxin Accumulation. Toxin Rev. 2008, 27, 319–345. [Google Scholar] [CrossRef]
- Fountain, J.C.; Abbas, H.K.; Scully, B.T.; Li, H.; Lee, R.D.; Kemerait, R.C.; Guo, B. Evaluation of Maize Inbred Lines and Topcross Progeny for Resistance to Pre-Harvest Aflatoxin Contamination. Crop J. 2019, 7, 118–125. [Google Scholar] [CrossRef]
- Mayfield, K.; Betrán, F.J.; Isakeit, T.; Odvody, G.; Murray, S.C.; Rooney, W.L.; Landivar, J.C. Registration of Maize Germplasm Lines Tx736, Tx739, and Tx740 for Reducing Preharvest Aflatoxin Accumulation. J. Plant Regist. 2012, 6, 88–94. [Google Scholar] [CrossRef]
- Murray, S.C.; Mayfield, K.; Pekar, J.; Brown, P.; Lorenz, A.; Isakeit, T.; Odvody, G.; Xu, W.; Betran, J. Tx741, Tx777, Tx779, Tx780, and Tx782 Inbred Maize Lines for Yield and Southern United States Stress Adaptation. J. Plant Regist. 2019, 13, 258–269. [Google Scholar] [CrossRef]
- Brown, R.L.; Williams, W.P.; Windham, G.L.; Menkir, A.; Chen, Z.-Y. Evaluation of African-Bred Maize Germplasm Lines for Resistance to Aflatoxin Accumulation. Agronomy 2016, 6, 24. [Google Scholar] [CrossRef]
- Okoth, S.; Rose, L.J.; Ouko, A.; Beukes, I.; Sila, H.; Mouton, M.; Flett, B.C.; Makumbi, D.; Viljoen, A. Field Evaluation of Resistance to Aflatoxin Accumulation in Maize Inbred Lines in Kenya and South Africa. J. Crop Improv. 2017, 31, 862–878. [Google Scholar] [CrossRef]
- Brown, R.L.; Chen, Z.-Y.; Menkir, A.; Cleveland, T.E.; Cardwell, K.; Kling, J.; White, D.G. Resistance to Aflatoxin Accumulation in Kernels of Maize Inbreds Selected for Ear Rot Resistance in West and Central Africa. J. Food Prot. 2001, 64, 396–400. [Google Scholar] [CrossRef]
- Mboup, M.; Aduramigba-Modupe, A.O.; Maazou, A.-R.S.; Olasanmi, B.; Mengesha, W.; Meseka, S.; Dieng, I.; Bandyopadhyay, R.; Menkir, A.; Ortega-Beltran, A. Performance of Testers with Contrasting Provitamin A Content to Evaluate Provitamin A Maize for Resistance to Aspergillus flavus Infection and Aflatoxin Production. Front. Plant Sci. 2023, 14, 1167628. [Google Scholar] [CrossRef]
- Suwarno, W.B.; Hannok, P.; Palacios-Rojas, N.; Windham, G.; Crossa, J.; Pixley, K.V. Provitamin A Carotenoids in Grain Reduce Aflatoxin Contamination of Maize While Combating Vitamin A Deficiency. Front. Plant Sci. 2019, 10, 30. [Google Scholar] [CrossRef]
- Ortega-Beltran, A.; Guerrero-Herrera, M.D.J.; Ortega-Corona, A.; Vidal-Martinez, V.A.; Cotty, P.J. Susceptibility to Aflatoxin Contamination among Maize Landraces from Mexico. J. Food Prot. 2014, 77, 1554–1562. [Google Scholar] [CrossRef]
- Ortega-Beltran, A.; Cotty, P.J. Influence of Wounding and Temperature on Resistance of Maize Landraces From Mexico to Aflatoxin Contamination. Front. Plant Sci. 2020, 11, 572264. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Beltran, A.; Jaime, R.; Cotty, P.J. Resistance of Maize Landraces from Mexico to Aflatoxin Contamination: Influence of Aflatoxin-Producing Fungi Genotype and Length of Incubation. Eur. J. Plant Pathol. 2022, 162, 237–246. [Google Scholar] [CrossRef]
- Warburton, M.L.; Williams, W.P.; Windham, G.L.; Murray, S.C.; Xu, W.; Hawkins, L.K.; Duran, J.F. Phenotypic and Genetic Characterization of a Maize Association Mapping Panel Developed for the Identification of New Sources of Resistance to Aspergillus flavus and Aflatoxin Accumulation. Crop Sci. 2013, 53, 2374–2383. [Google Scholar] [CrossRef]
- Xu, F.; Baker, R.C.; Whitaker, T.B.; Luo, H.; Zhao, Y.; Stevenson, A.; Boesch, C.J.; Zhang, G. Review of Good Agricultural Practices for Smallholder Maize Farmers to Minimise Aflatoxin Contamination. World Mycotoxin J. 2022, 15, 171–186. [Google Scholar] [CrossRef]
- Muñoz-Zavala, C.; Molina-Macedo, A.; Toledo, F.H.; Telles-Mejía, E.; Cabrera-Soto, L.; Palacios-Rojas, N. Combating Aflatoxin Contamination by Combining Biocontrol Application and Adapted Maize Germplasm in Northeastern and Southeastern Mexico. Biol. Control 2025, 204, 105727. [Google Scholar] [CrossRef]
- Peña Betancourt, S.D.; Carranza, B.V.; Manzano, E.P. Estimation of Mycotoxin Multiple Contamination in Mexican Hybrid Seed Maize by HPLC-MS/MS. Agric. Sci. 2015, 06, 1089–1097. [Google Scholar] [CrossRef]
- USDA United States Department of Agriculture. World Agricultural Production. Mexico Corn: Production Cut on Lower Expected Area. Available online: https://ipad.fas.usda.gov/cropexplorer/pecad_stories.aspx?regionid=ca&ftype=prodbriefs (accessed on 5 June 2025).
- Saldivia-Tejeda, A.; Uribe-Guerrero, M.Á.; Rojas-Cruz, J.M.; Guera, O.G.M.; Verhulst, N.; Fonteyne, S. Conservation Agriculture Enhances Maize Yields and Profitability in Mexico’s Semi-Arid Highlands. Sci. Rep. 2024, 14, 29638. [Google Scholar] [CrossRef]
- Bowles, T.M.; Mooshammer, M.; Socolar, Y.; Calderón, F.; Cavigelli, M.A.; Culman, S.W.; Deen, W.; Drury, C.F.; Garcia, A.G.y; Gaudin, A.C.M.; et al. Long-Term Evidence Shows That Crop-Rotation Diversification Increases Agricultural Resilience to Adverse Growing Conditions in North America. One Earth 2020, 2, 284–293. [Google Scholar] [CrossRef]
- Renwick, L.L.R.; Deen, W.; Silva, L.; Gilbert, M.E.; Maxwell, T.; Bowles, T.M.; Gaudin, A.C.M. Long-Term Crop Rotation Diversification Enhances Maize Drought Resistance through Soil Organic Matter. Environ. Res. Lett. 2021, 16, 084067. [Google Scholar] [CrossRef]
- Yamini, V.; Singh, K.; Antar, M.; El Sabagh, A. Sustainable Cereal Production through Integrated Crop Management: A Global Review of Current Practices and Future Prospects. Front. Sustain. Food Syst. 2025, 9, 1428687. [Google Scholar] [CrossRef]
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human Pathogen, Allergen and Mycotoxin Producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [CrossRef]
- Horn, B.W.; Pitt, J.I. Yellow Mold and Aflatoxin. Compend. Peanut Dis. 1997, 2, 40–42. [Google Scholar]
- Prasanna, B.M.; Huesing, J.E.; Eddy, R.; Peschke, V.M. Fall Armyworm in Africa: A Guide for Integrated Pest Management; USAID: Washington, DC, USA, 2018.
- Olmstead, D.L.; Nault, B.A.; Shelton, A.M. Biology, Ecology, and Evolving Management of Helicoverpa zea (Lepidoptera: Noctuidae) in Sweet Corn in the United States. J. Econ. Entomol. 2016, 109, 1667–1676. [Google Scholar] [CrossRef]
- García-Lara, S.; Espinosa Carrillo, C.; Bergvinson, D.J. Manual de Plagas en Granos Almacenado y Tecnologías Alternas para su Manejo y Control; CIMMyT: Texcoco, Mexico, 2007; ISBN 970-648-154-0. [Google Scholar]
- Blacutt, A.A.; Gold, S.E.; Voss, K.A.; Gao, M.; Glenn, A.E. Fusarium verticillioides: Advancements in Understanding the Toxicity, Virulence, and Niche Adaptations of a Model Mycotoxigenic Pathogen of Maize. Phytopathology 2018, 108, 312–326. [Google Scholar] [CrossRef]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin Contamination, Its Impact and Management Strategies: An Updated Review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Wang, D.; Chu, Q.; Zhang, Q.; Yue, X.; Zhu, M.; Dong, J.; Li, L.; Jiang, X.; et al. Discovery of the Relationship between Distribution and Aflatoxin Production Capacity of Aspergillus species and Soil Types in Peanut Planting Areas. Toxins 2022, 14, 425. [Google Scholar] [CrossRef]
- Caceres, I.; Al Khoury, A.; El Khoury, R.; Lorber, S.; Oswald, I.P.; El Khoury, A.; Atoui, A.; Puel, O.; Bailly, J.-D. Aflatoxin Biosynthesis and Genetic Regulation: A Review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.; More, C.; Korz, S.; Muñoz, K. Soil Microbial Responses to Aflatoxin Exposure: Consequences for Biomass, Activity and Catabolic Functionality. Soil. Syst. 2023, 7, 23. [Google Scholar] [CrossRef]
- Odjo, S.; Alakonya, A.E.; Rosales-Nolasco, A.; Molina, A.L.; Muñoz, C.; Palacios-Rojas, N. Occurrence and Postharvest Strategies to Help Mitigate Aflatoxins and Fumonisins in Maize and Their Co-Exposure to Consumers in Mexico and Central America. Food Control 2022, 138, 108968. [Google Scholar] [CrossRef]
- Palacios-Cabrera, H.; Fracari, J.; Copetti, M.V.; Mallmann, C.A.; Almeida, M.; Meléndez-Jácome, M.R.; Vásquez-Castillo, W. Toxigenic Fungi and Co-Occurring Mycotoxins in Maize (Zea mayz L.) Samples from the Highlands and Coast of Ecuador. Foods 2025, 14, 2630. [Google Scholar] [CrossRef]
- Wall-Martínez, H.A.; Ramírez-Martínez, A.; Wesolek, N.; Brabet, C.; Durand, N.; Rodríguez-Jimenes, G.C.; García-Alvarado, M.A.; Salgado-Cervantes, M.A.; Robles-Olvera, V.J.; Roudot, A.C. Risk Assessment of Exposure to Mycotoxins (Aflatoxins and Fumonisins) through Corn Tortilla Intake in Veracruz City (Mexico). Food Addit. Contam. Part A 2019, 36, 929–939. [Google Scholar] [CrossRef]
- Monge, A.; Romero, M.; Groopman, J.D.; McGlynn, K.A.; Santiago-Ruiz, L.; Villalpando-Hernández, S.; Mannan, R.; Burke, S.M.; Remes-Troche, J.M.; Lajous, M. Aflatoxin Exposure in Adults in Southern and Eastern Mexico in 2018: A Descriptive Study. Int. J. Hyg. Environ. Health 2023, 253, 114249. [Google Scholar] [CrossRef]
- Monge, A.; McGlynn, K.; Santiago-Ruiz, L.; Remes-Troche, J.M.; Hernandez-Flores, K.; Villapando-Hernandez, S.; Romero, M.; Groopman, J.; Lajous, M. Abstract 4221: Aflatoxin Exposure in a Population-Based Representative Sample of Adults in Mexico in 2018. Cancer Res. 2023, 83, 4221. [Google Scholar] [CrossRef]
- Jiménez-Pérez, C.; Alatorre-Santamaría, S.; Tello-Solís, S.R.; Gómez-Ruiz, L.; Rodríguez-Serrano, G.; García-Garibay, M.; Cruz-Guerrero, A. Analysis of Aflatoxin M1 Contamination in Milk and Cheese Produced in Mexico: A Review. World Mycotoxin J. 2021, 14, 269–286. [Google Scholar] [CrossRef]
- Lino-Silva, L.S.; Lajous, M.; Brochier, M.; Santiago-Ruiz, L.; Melchor-Ruan, J.; Xie, Y.; Wang, M.; Wu, D.; Higson, H.; Jones, K.; et al. Aflatoxin Levels and Prevalence of TP53 Aflatoxin-Mutations in Hepatocellular Carcinomas in Mexico. Salud Pública México 2022, 64, 35–40. [Google Scholar] [CrossRef]
- Monter-Arciniega, A.; Cruz-Cansino, N.d.S.; Castañeda-Ovando, A.; Jiménez-Osorio, A.S.; Tello-Solís, S.R.; Jiménez-Pérez, C.; Rodríguez-Serrano, G.M. Aflatoxin M1 Levels in Commercial Cows’ Milk in Mexico: Contamination and Carcinogenic Risk Assessment. Appl. Sci. 2025, 15, 6106. [Google Scholar] [CrossRef]
- Anguiano-Ruvalcaba, G.L.; Vargas-Cortina, A.V.; Guzmán-De Peña, D. Inactivación de Aflatoxina B1 y Aflatoxicol Por Nixtamalización Tradicional Del Maíz y Su Regeneración Por Acidificación de La Masa. Salud Pública México 2005, 47, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Molina-Pintor, I.B.; Ruíz-Arias, M.A.; Guerrero-Flores, M.C.; Rojas-García, A.E.; Barrón-Vivanco, B.S.; Medina-Díaz, I.M.; Bernal-Hernández, Y.Y.; Ortega-Cervantes, L.; Rodríguez-Cervantes, C.H.; Ramos, A.J.; et al. Preliminary Survey of the Occurrence of Mycotoxins in Cereals and Estimated Exposure in a Northwestern Region of Mexico. Int. J. Environ. Health Res. 2021, 32, 2271–2285. [Google Scholar] [CrossRef] [PubMed]
- Da Luna-López, M.C.; Valdivia-Flores, A.G.; Jaramillo-Juárez, F.; Reyes, J.L.; Ortiz-Martínez, R.; Quezada-Tristán, T. Association between Aspergillus flavus Colonization and Aflatoxins Production in Immature Grains of Maize Genotypes. J. Food Sci. Eng. 2013, 3, 688. [Google Scholar] [CrossRef]
- Saez-Gomez, K.; Avila-Sosa, R.; Huerta-Lara, M.; Avelino-Flores, F.; Munguia-Pérez, R. Determination of Mycotoxigenic Fungi and Total Aflatoxins in Stored Corn from Sites of Puebla and Tlaxcala, Mexico. Nat. Environ. Pollut. Technol. 2024, 23, 583–589. [Google Scholar] [CrossRef]
- Zuki-Orozco, B.A.; Batres-Esquivel, L.E.; Ortiz-Pérez, M.D.; Juárez-Flores, B.I.; Díaz-Barriga, F. Aflatoxins Contamination in Maize Products from Rural Communities in San Luis Potosi, Mexico. Ann. Glob. Health 2018, 84, 300–305. [Google Scholar] [CrossRef]
- Gilbert Sandoval, I.; Wesseling, S.; Rietjens, I.M.C.M. Aflatoxin B1 in Nixtamalized Maize in Mexico; Occurrence and Accompanying Risk Assessment. Toxicol. Rep. 2019, 6, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Urueta, P.; Carvajal, M.; Méndez, I.; Meza, F.; Gálvez, A. Survey of Aflatoxins in Maize Tortillas from Mexico City. Food Addit. Contam. Part B 2011, 4, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Morales-Moo, T.; Hernández-Camarillo, E.; Carvajal-Moreno, M.; Vargas-Ortiz, M.; Robles-Olvera, V.; Salgado-Cervantes, M.A. Human Health Risk Associated with the Consumption of Aflatoxins in Popcorn. Risk Manag. Healthc. Policy 2020, 13, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Albores, J.A.; Arámbula-Villa, G.; Preciado-Ortíz, R.E.; Moreno-Martínez, E. Aflatoxins in Pozol, a Nixtamalized, Maize-Based Food. Int. J. Food Microbiol. 2004, 94, 211–215. [Google Scholar] [CrossRef]
- Sharma, M.; Márquez, C. Determination of Aflatoxins in Domestic Pet Foods (Dog and Cat) Using Immunoaffinity Column and HPLC. Anim. Feed Sci. Technol. 2001, 93, 109–114. [Google Scholar] [CrossRef]
- Morrison, D.M.; Shrestha, R.K.; Bianchini, A.; Morrison, D.M.; Shrestha, R.K.; Bianchini, A. Aflatoxins in Staple Foods in Latin America and the Caribbean Countries and Decontamination Strategies; IntechOpen: London, UK, 2025; ISBN 978-1-83634-451-3. [Google Scholar]
- Hernandez-Rauda, R.; Peña-Rodas, O.; Arbaiza-Rodríguez, G.; Cuadra-Escobar, M.; Guzman-Rodríguez, M. Aflatoxins and Fumonisins in Nixtamalized Corn Dough from El Salvador: A Two-Year Survey. Toxicol. Rep. 2025, 15, 102087. [Google Scholar] [CrossRef]
- Foerster, C.; Müller-Sepúlveda, A.; Copetti, M.V.; Arrúa, A.A.; Monsalve, L.; Ramirez, M.L.; Torres, A.M. A Mini Review of Mycotoxin’s Occurrence in Food in South America in the Last 5 Years: Research Gaps and Challenges in a Climate Change Era. Front. Chem. Biol. 2024, 3, 1400481. [Google Scholar] [CrossRef]
- Demonte, L.D.; Cendoya, E.; Nichea, M.J.; Romero Donato, C.J.; Ramirez, M.L.; Repetti, M.R. Occurrence of Modified Mycotoxins in Latin America: An up-to-Date Review. Mycotoxin Res. 2024, 40, 467–481. [Google Scholar] [CrossRef]
- Satterlee, T.R.; Hawkins, J.A.; Mitchell, T.R.; Wei, Q.; Lohmar, J.M.; Glenn, A.E.; Gold, S.E. Fungal Chemical Warfare: The Role of Aflatoxin and Fumonisin in Governing the Interaction between the Maize Pathogens, Aspergillus flavus and Fusarium verticillioides. Front. Cell. Infect. Microbiol. 2025, 14, 1513134. [Google Scholar] [CrossRef]
- Zorzete, P.; Castro, R.S.; Pozzi, C.R.; Israel, A.L.M.; Fonseca, H.; Yanaguibashi, G.; Corrêa, B. Relative Populations and Toxin Production by Aspergillus flavus and Fusarium verticillioides in Artificially Inoculated Corn at Various Stages of Development under Field Conditions. J. Sci. Food Agric. 2008, 88, 48–55. [Google Scholar] [CrossRef]
- Brown, D.W.; Butchko, R.A.E.; Busman, M.; Proctor, R.H. The Fusarium verticillioides FUM Gene Cluster Encodes a Zn(II)2Cys6 Protein That Affects FUM Gene Expression and Fumonisin Production. Eukaryot. Cell 2007, 6, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-Occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef]
- Casu, A.; Camardo Leggieri, M.; Toscano, P.; Battilani, P. Changing Climate, Shifting Mycotoxins: A Comprehensive Review of Climate Change Impact on Mycotoxin Contamination. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13323. [Google Scholar] [CrossRef]
- Perrone, G.; Ferrara, M.; Medina, A.; Pascale, M.; Magan, N. Toxigenic Fungi and Mycotoxins in a Climate Change Scenario: Ecology, Genomics, Distribution, Prediction and Prevention of the Risk. Microorganisms 2020, 8, 1496. [Google Scholar] [CrossRef]
- Ortega-Beltran, A.; Jaime, R.; Cotty, P.J. Aflatoxin-Producing Fungi in Maize Field Soils from Sea Level to over 2000 Masl: A Three Year Study in Sonora, Mexico. Fungal Biol. 2015, 119, 191–200. [Google Scholar] [CrossRef]
- Alvarado-Carrillo, M.; Díaz-Franco, A.; Delgado-Aguirre, E.; Montes-García, N. Impact of Corn Agronomic Management on Aflatoxin (Aspergillus flavus) Contamination and Charcoal Stalk Rot (Macrophomina phaseolina) Incidence. Trop. Subtrop. Agroecosyst. 2010, 12, 575–582. [Google Scholar]
- García, S.; Heredia, N. Mycotoxins in Mexico: Epidemiology, Management, and Control Strategies. Mycopathologia 2006, 162, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Dombrink-Kurtzman, M.A.; Dvorak, T.J. Fumonisin Content in Masa and Tortillas from Mexico. J. Agric. Food Chem. 1999, 47, 622–627. [Google Scholar] [CrossRef]
- Torres, O.; Matute, J.; Waes, J.G.; Maddox, J.R.; Gregory, S.G.; Ashley-Koch, A.E.; Showker, J.L.; Voss, K.A.; Riley, R.T. Human Health Implications from Co-Exposure to Aflatoxins and Fumonisins in Maize-Based Foods in Latin America: Guatemala as a Case Study. World Mycotoxin J. 2015, 8, 143–160. [Google Scholar] [CrossRef]
- Solís-Martínez, O.; Monge, A.; Groopman, J.D.; McGlynn, K.A.; Romero-Martínez, M.; Palacios-Rojas, N.; Batis, C.; Lamadrid-Figueroa, H.; Riojas-Rodríguez, H.; Lajous, M. Maize Consumption and Circulating Aflatoxin Levels in Mexican Middle- and Older-Aged Adults: A Cross-Sectional Analysis. Am. J. Clin. Nutr. 2025, 121, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.Y.; Torres-Sanchez, L.; Lopez-Carrillo, L.; Peng, J.H.; Sutcliffe, A.E.; White, K.L.; Humpf, H.-U.; Turner, P.C.; Wild, C.P. Association between Tortilla Consumption and Human Urinary Fumonisin B1 Levels in a Mexican Population. Cancer Epidemiol. Biomark. Prev. 2008, 17, 688–694. [Google Scholar] [CrossRef]
- Elmore, S.E.; Treviño-Espinosa, R.S.; Garcia-Mazcorro, J.F.; González-Barranco, P.; Sánchez-Casas, R.M.; Phillips, T.D.; Marroquín-Cardona, A.G. Evaluation of Aflatoxin and Fumonisin Co-Exposure in Urine Samples from Healthy Volunteers in Northern Mexico. Toxicol. Rep. 2021, 8, 1734–1741. [Google Scholar] [CrossRef]
- Du, M.; Liu, Y.; Zhang, G. Interaction of Aflatoxin B1 and Fumonisin B1 in HepG2 Cell Apoptosis. Food Biosci. 2017, 20, 131–140. [Google Scholar] [CrossRef]
- Mary, V.S.; Arias, S.L.; Otaiza, S.N.; Velez, P.A.; Rubinstein, H.R.; Theumer, M.G. The Aflatoxin B1-Fumonisin B1 Toxicity in BRL-3A Hepatocytes Is Associated to Induction of Cytochrome P450 Activity and Arachidonic Acid Metabolism. Environ. Toxicol. 2017, 32, 1711–1724. [Google Scholar] [CrossRef]
- Chen, X.; Abdallah, M.F.; Chen, X.; Rajkovic, A. Current Knowledge of Individual and Combined Toxicities of Aflatoxin B1 and Fumonisin B1 In Vitro. Toxins 2023, 15, 653. [Google Scholar] [CrossRef]
- Turner, P.C.; Snyder, J.A. Development and Limitations of Exposure Biomarkers to Dietary Contaminants Mycotoxins. Toxins 2021, 13, 314. [Google Scholar] [CrossRef] [PubMed]
- Chhaya, R.S.; Nag, R.; Cummins, E. Human Health Risk from Co-Occurring Mycotoxins in Dairy: A Feed-to-Fork Approach. Food Control 2025, 168, 110954. [Google Scholar] [CrossRef]
- von Mérey, G.E.; Veyrat, N.; de Lange, E.; Degen, T.; Mahuku, G.; Valdez, R.L.; Turlings, T.C.J.; D’Alessandro, M. Minor Effects of Two Elicitors of Insect and Pathogen Resistance on Volatile Emissions and Parasitism of Spodoptera frugiperda in Mexican Maize Fields. Biol. Control 2012, 60, 7–15. [Google Scholar] [CrossRef]
- Cruz-Esteban, S.; Hernández-Ledesma, P.; Malo, E.A.; Rojas, J.C. Cebos Feromonales Para La Captura de Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) En Cultivos de Maíz Adyacentes a Cultivos de Fresas. Acta Zoológica Mex. 2020, 36, e3612255. [Google Scholar]
- Ávila-Rodríguez, V.; Nava-Camberos, U.; García-Hernández, J.L.; Martínez-Carrillo, J.L.; Blanco, C.A. Insect Diversity in Conventional and Bt Cottons in the Comarca Lagunera, Mexico. Southwest. Entomol. 2019, 44, 383–392. [Google Scholar] [CrossRef]
- Zaman, S.; Ahmad, F.; Javed, N. Pathogenicity of Entomopathogenic Fungi against Sitophilus granarius (L.) (Coleoptera: Curculionidae) under Abiotic Factors. Pak. J. Agric. Sci. 2020, 57, 79–86. [Google Scholar]
- Pellan, L.; Durand, N.; Martinez, V.; Fontana, A.; Schorr-Galindo, S.; Strub, C. Commercial Biocontrol Agents Reveal Contrasting Comportments Against Two Mycotoxigenic Fungi in Cereals: Fusarium graminearum and Fusarium verticillioides. Toxins 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, D.; Qi, G.; Mao, Z.; Hu, X.; Du, B.; Liu, K.; Ding, Y. Effects of Bacillus velezensis FKM10 for Promoting the Growth of Malus hupehensis Rehd. and Inhibiting Fusarium verticillioides. Front. Microbiol. 2020, 10, 2889. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, J.R.; You, M.P.; Laudinot, V.; Barbetti, M.J.; Aubertot, J.-N. Revisiting Sustainability of Fungicide Seed Treatments for Field Crops. Plant Dis. 2020, 104, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, B.M.; Cairns, J.E.; Zaidi, P.H.; Beyene, Y.; Makumbi, D.; Gowda, M.; Magorokosho, C.; Zaman-Allah, M.; Olsen, M.; Das, A.; et al. Beat the Stress: Breeding for Climate Resilience in Maize for the Tropical Rainfed Environments. Theor. Appl. Genet. 2021, 134, 1729–1752. [Google Scholar] [CrossRef] [PubMed]
- Braun, H.J.; Atlin, G.; Payne, T. Multi-Location Testing as a Tool to Identify Plant Response to Global Climate Change. In Climate Change and Crop Production; CABI Climate Change Series; CABI: Wallingford, UK, 2010; pp. 115–138. ISBN 978-1-84593-633-4. [Google Scholar]
- Atlin, G.N.; Cairns, J.E.; Das, B. Rapid Breeding and Varietal Replacement Are Critical to Adaptation of Cropping Systems in the Developing World to Climate Change. Glob. Food Secur. 2017, 12, 31–37. [Google Scholar] [CrossRef]
- Werner, C.R.; Zaman-Allah, M.; Assefa, T.; Cairns, J.E.; Atlin, G.N. Accelerating Genetic Gain through Early-Stage on-Farm Sparse Testing. Trends Plant Sci. 2025, 30, 17–20. [Google Scholar] [CrossRef]
- Cotty, P.J. Virulence and Cultural Characteristics of Two Aspergillus flavus Strains Pathogenic on Cotton. Phytopathology 1989, 79, 808–814. [Google Scholar] [CrossRef]
- Collinge, D.B.; Jensen, D.F.; Rabiey, M.; Sarrocco, S.; Shaw, M.W.; Shaw, R.H. Biological Control of Plant Diseases—What Has Been Achieved and What Is the Direction? Plant Pathol. 2022, 71, 1024–1047. [Google Scholar] [CrossRef]
- Maxwell, L.A.; Callicott, K.A.; Bandyopadhyay, R.; Mehl, H.L.; Orbach, M.J.; Cotty, P.J. Degradation of Aflatoxins B1 by Atoxigenic Aspergillus flavus Biocontrol Agents. Plant Dis. 2021, 105, 2343–2350. [Google Scholar] [CrossRef]
- Huang, C.; Jha, A.; Sweany, R.; DeRobertis, C.; Kenneth, E.; Damann, J. Intraspecific Aflatoxin Inhibition in Aspergillus flavus Is Thigmoregulated, Independent of Vegetative Compatibility Group and Is Strain Dependent. PLoS ONE 2011, 6, e23470. [Google Scholar] [CrossRef]
- Moore, G.G.; Lebar, M.D.; Carter-Wientjes, C.H. Cumulative Effects of Non-Aflatoxigenic Aspergillus flavus Volatile Organic Compounds to Abate Toxin Production by Mycotoxigenic Aspergilli. Toxins 2022, 14, 340. [Google Scholar] [CrossRef]
- Mehl, H.L.; Jaime, R.; Callicott, K.A.; Probst, C.; Garber, N.P.; Ortega-Beltran, A.; Grubisha, L.C.; Cotty, P.J. Aspergillus flavus Diversity on Crops and in the Environment Can Be Exploited to Reduce Aflatoxin Exposure and Improve Health. Ann. N. Y. Acad. Sci. 2012, 1273, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Moral, J.; Garcia-Lopez, M.T.; Camiletti, B.X.; Jaime, R.; Michailides, T.J.; Bandyopadhyay, R.; Ortega-Beltran, A. Present Status and Perspective on the Future Use of Aflatoxin Biocontrol Products. Agronomy 2020, 10, 491. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Vetukuri, R.R.; Kelbessa, B.G.; Gepts, P.; Heslop-Harrison, P.; Araujo, A.S.F.; Sharma, S.; Ortiz, R. Exploitation of Rhizosphere Microbiome Biodiversity in Plant Breeding. Trends Plant Sci. 2025, 30, P1033–P1045. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Hernández, G.; Tijerina-Castro, G.D.; Cortés-Pérez, S.; Ferrera-Cerrato, R.; Alarcón, A. Evaluation of Functional Plant Growth-Promoting Activities of Culturable Rhizobacteria Associated to Tunicate Maize (Zea mays Var. Tunicata A. St. Hil), a Mexican Exotic Landrace Grown in Traditional Agroecosystems. Front. Microbiol. 2024, 15, 1478807. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Ma, Y.; Li, Y.; Jin, J.; Lian, T. The Rhizospheric Microbiome Becomes More Diverse with Maize Domestication and Genetic Improvement. J. Integr. Agric. 2022, 21, 1188–1202. [Google Scholar] [CrossRef]
- Schamann, A.; Soukup, S.T.; Geisen, R.; Kulling, S.; Schmidt-Heydt, M. Comparative Analysis of the Genomes and Aflatoxin Production Patterns of Three Species within the Aspergillus Section flavi Reveals an Undescribed Chemotype and Habitat-Specific Genetic Traits. Commun. Biol. 2024, 7, 1134. [Google Scholar] [CrossRef] [PubMed]
- Medina, Á.; Rodríguez, A.; Magan, N. Climate Change and Mycotoxigenic Fungi: Impacts on Mycotoxin Production. Curr. Opin. Food Sci. 2015, 5, 99–104. [Google Scholar] [CrossRef]
- García-Reyes, V.; Solano-Báez, A.R.; Leyva-Mir, S.G.; De León-García de Alba, C.; Rodríguez-Mendoza, J.; Quezada-Salinas, A.; Márquez-Licona, G. Molecular Confirmation of Stenocarpella maydis Causing Ear Rot of Maize in Mexico. J. Plant Pathol. 2022, 104, 775–779. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- He, B.; Pan, S.; Zhao, J.; Zou, X.; Liu, X.; Wu, S. Maize Improvement Based on Modern Breeding Strategies: Progress and Perspective. ACS Agric. Sci. Technol. 2024, 4, 274–282. [Google Scholar] [CrossRef]
- Deressa, T.; Adugna, G.; Suresh, L.M.; Bekeko, Z. Resistance of Maize (Zea mays L.) Genotypes against Ear Rot Causing Pathogens in Southern and Western Ethiopia. Phytoparasitica 2025, 53, 67. [Google Scholar] [CrossRef]
- Hua, S.S.T.; Palumbo, J.D.; Parfitt, D.E.; Sarreal, S.B.L.; O’Keeffe, T.L. Development of a Droplet Digital PCR Assay for Population Analysis of Aflatoxigenic and Atoxigenic Aspergillus flavus Mixtures in Soil. Mycotoxin Res. 2018, 34, 187–194. [Google Scholar] [CrossRef]
- Moore, G.G.; Olarte, R.A.; Horn, B.W.; Elliott, J.L.; Singh, R.; O’Neal, C.J.; Carbone, I. Global Population Structure and Adaptive Evolution of Aflatoxin-Producing Fungi. Ecol. Evol. 2017, 7, 9179–9191. [Google Scholar] [CrossRef]
- Sweany, R.R.; Breunig, M.; Opoku, J.; Clay, K.; Spatafora, J.W.; Drott, M.T.; Baldwin, T.T.; Fountain, J.C. Why Do Plant-Pathogenic Fungi Produce Mycotoxins? Potential Roles for Mycotoxins in the Plant Ecosystem. Phytopathology 2022, 112, 2044–2051. [Google Scholar] [CrossRef]
- Weaver, M.A.; Abbas, H.K. Field Displacement of Aflatoxigenic Aspergillus flavus Strains Through Repeated Biological Control Applications. Front. Microbiol. 2019, 10, 1788. [Google Scholar] [CrossRef]
- Baloch, N. Microbial Contributions to Maize Crop Production: A Comprehensive Review of Challenges and Future Perspectives. Discov. Agric. 2025, 3, 10. [Google Scholar] [CrossRef]
- Patel, R.; Mehta, K.; Prajapati, J.; Shukla, A.; Parmar, P.; Goswami, D.; Saraf, M. An Anecdote of Mechanics for Fusarium Biocontrol by Plant Growth Promoting Microbes. Biol. Control 2022, 174, 105012. [Google Scholar] [CrossRef]
- Camardo Leggieri, M.; Mazzoni, M.; Battilani, P. Machine Learning for Predicting Mycotoxin Occurrence in Maize. Front. Microbiol. 2021, 12, 661132. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Liu, C.; Dudaš, T.N.; Loc, M.Č.; Bagi, F.F.; van der Fels-Klerx, H.J. Improved Aflatoxins and Fumonisins Forecasting Models for Maize (PREMA and PREFUM), Using Combined Mechanistic and Bayesian Network Modeling—Serbia as a Case Study. Front. Microbiol. 2021, 12, 643604. [Google Scholar] [CrossRef]
- Li, F.; Gates, D.J.; Buckler, E.S.; Hufford, M.B.; Janzen, G.M.; Rellán-Álvarez, R.; Rodríguez-Zapata, F.; Navarro, J.A.R.; Sawers, R.J.H.; Snodgrass, S.J.; et al. Environmental data provide marginal benefit for predicting climate adaptation. PLoS Genet. 2025, 21, e1011714. [Google Scholar] [CrossRef] [PubMed]
- Costa-Neto, G.; Crossa, J.; Fritsche-Neto, R. Enviromic Assembly Increases Accuracy and Reduces Costs of the Genomic Prediction for Yield Plasticity in Maize. Front. Plant Sci. 2021, 12, 717552. [Google Scholar] [CrossRef]
- Cairns, J.E.; Sonder, K.; Zaidi, P.H.; Verhulst, N.; Mahuku, G.; Babu, R.; Nair, S.K.; Das, B.; Govaerts, B.; Vinayan, M.T.; et al. Chapter One— Maize Production in a Changing Climate: Impacts, Adaptation, and Mitigation Strategies. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 114, pp. 1–58. [Google Scholar]
- Castano-Duque, L.; Avila, A.; Mack, B.M.; Winzeler, H.E.; Blackstock, J.M.; Lebar, M.D.; Moore, G.G.; Owens, P.R.; Mehl, H.L.; Su, J.; et al. Prediction of Aflatoxin Contamination Outbreaks in Texas Corn Using Mechanistic and Machine Learning Models. Front. Microbiol. 2025, 16, 1528997. [Google Scholar] [CrossRef] [PubMed]
- Branstad-Spates, E.; Castano-Duque, L.; Mosher, G.; Hurburgh, C., Jr.; Rajasekaran, K.; Owens, P.; Edwin Winzeler, H.; Bowers, E. Predicting Fumonisins in Iowa Corn: Gradient Boosting Machine Learning. Cereal Chem. 2024, 101, 1261–1272. [Google Scholar] [CrossRef]
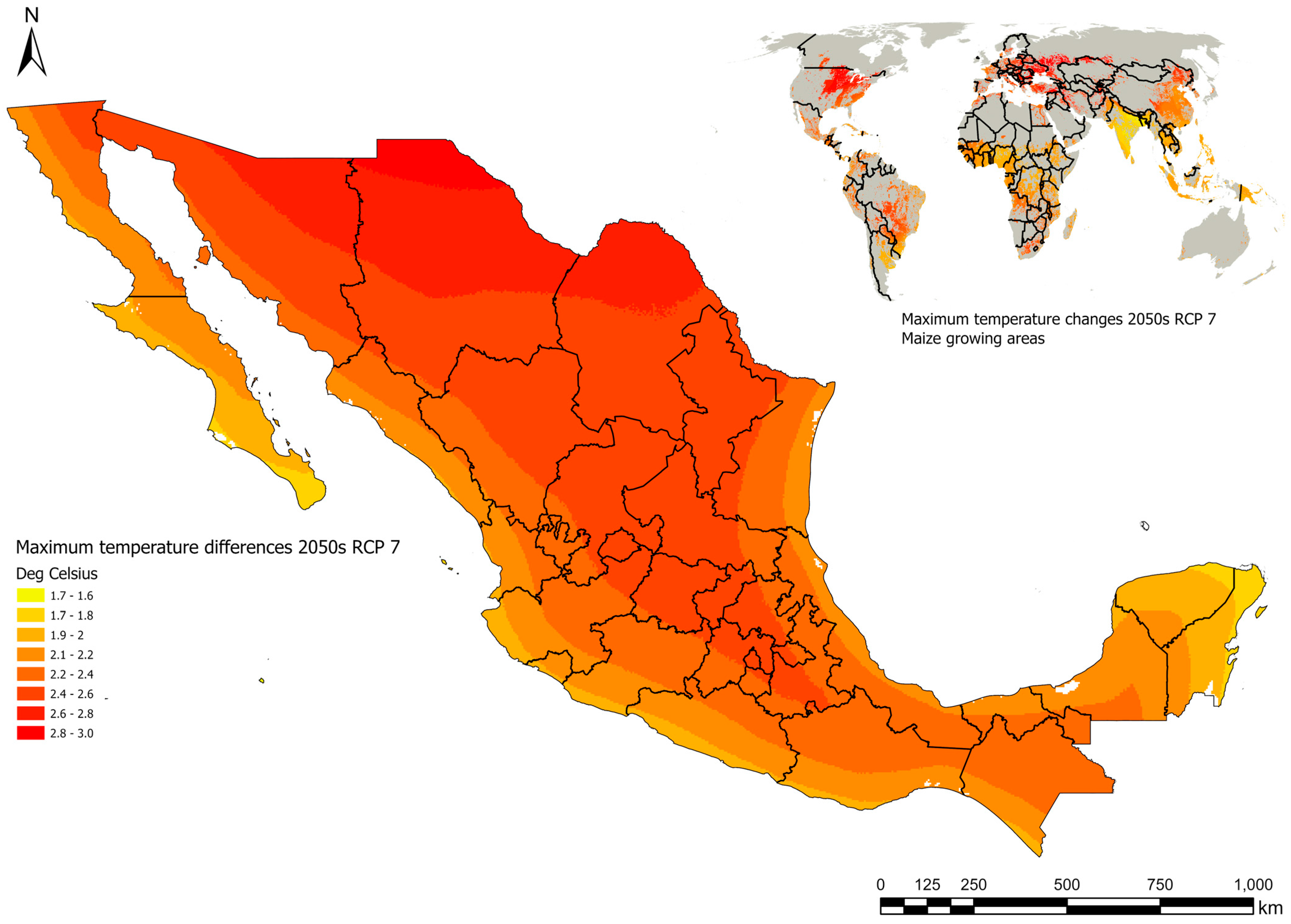


| Commodity | Upper Limit µg/kg | |
|---|---|---|
| AF B1 | FUM B1 + B2 | |
| All products destined for humans | 20 1 | 4000 4 |
| Nixtamalized maize flour and masa for tortillas | 12 2 | 2000 4 |
| Milk | 0.5 3 | N/A 5 |
| All products destined for poultry | 100 1 | N/A |
| Swine | 200 1 | N/A |
| Cattle | 300 1 | N/A |
| Inbred Line Code | Sourced Institution | Ref. |
|---|---|---|
| Mp313E, Mp 420, Mp 715, Mp717, Mp 718 and Mp719 | Mississippi State University, USA | [31,32,33,34,35] |
| CML176, CML269 and CML322 | CIMMYT 1 and Texas A&M University, USA | [36] |
| GT-601, GT-602 and GT-603 | University of Georgia Coastal Plain, USA | [37,38] |
| CML348, NC388, NC400, NC408 and NC458 | CIMMYT and North Carolina State University, USA | [39] |
| CML52, CML69, GEMS-0005, Hi63, Hp301 and M37 W | CIMMYT and University of Georgia, USA | [40] |
| Tx736, Tx739, Tx740, Tx741, Tx777, Tx779, Tx780 and Tx782 | Texas A&M and Texas AgriLife Research Maize, USA | [41,42] |
| TZAR101, TZAR102, TZAR103, TZAR104, TZAR105 and TZAR106 | IITA 2, West and Central Africa | [43] |
| CML247, CML444 and CML495 | CIMMYT and University of Nairobi, Kenya, and South Africa | [44,45] |
| CML247 and CML495 | CIMMYT, Southern Mexico | [27] |
| Parameter | Intensive Practices | Agroecological Practices | Relationship with Mycotoxin Contamination |
|---|---|---|---|
| Soil fertility | Heavy reliance on chemical fertilizers, leading to nutrient imbalances and soil degradation. | Combines organic and inorganic inputs, promoting balanced nutrition and improved soil structure and fertility. | Favorable, balanced nutritional conditions improve plant defenses; e.g., optimal nitrogen application reduces mycotoxin contamination. |
| Nutrient management | Generalized fertilizer application without soil testing, often resulting in inefficiencies. | Site-specific nutrient management based on scientific assessments, such as soil health cards, for optimal nutrient use. | Periodic soil testing helps determine the specific nutritional needs of the crop and allows for targeted fertilizer application. |
| Water management | Inefficient irrigation methods, leading to water wastage and salinization. | Promotes efficient techniques like micro-irrigation, drip systems, rainwater harvesting, and scheduling based on crop needs. | Maintaining optimal soil moisture levels creates unfavorable conditions for mycotoxin-producing fungi and improves the resilience of drought-tolerant maize. |
| Crop diversification | Monocropping dominates, increasing vulnerability to pests, diseases, and market risks. | Encourages diverse cropping systems, including rotations and intercropping with cereals, pulses, and horticultural crops. | Crop rotation disrupts the life cycle of mycotoxigenic fungi and improves microbial diversity. |
| Resource use efficiency | Overuse of inputs like water, fertilizers, and pesticides, reducing long-term productivity. | Focuses on precise and judicious use of inputs to enhance efficiency and reduce costs and environmental impact. | Pesticides reduce pest populations associated with mycotoxin contamination. However, excessive use reduces the number of natural enemies and can lead to pesticide resistance. |
| Pest and disease management | Sole reliance on chemical pesticides, leading to resistance and ecological imbalance. | Advocates integrated pest management (IPM) and agroecological pest management (APM), combining biological, cultural, and chemical controls to manage pests sustainably. | IPM or APM approaches can significantly reduce mycotoxin contamination and improve crop quality. |
| Conservation agriculture (CA) | Rarely adopted, leading to soil erosion and loss of organic matter. | Incorporates practices like minimum tillage, residue retention, and crop rotations to conserve soil and water resources. | CA promotes soil health and creates a less favorable environment for Aspergillus and Fusarium. |
| Yield and productivity | Short-term yield gains but declining productivity over time due to resource degradation. | Maintains or improves yields sustainably through holistic management of inputs, pests, and environmental factors. | High-yield practices help reduce plant stress and the risk of fungal infection. |
| Economic viability | High input costs and diminishing returns in the long run. | Reduces input costs through efficient practices, improving profit margins for farmers. | Cost-effectiveness and economic incentives are crucial for adopting control methods across different agricultural sectors. |
| Environmental impact | Contributes to environmental issues like water pollution, greenhouse gas emissions, and loss of biodiversity. | Minimizes environmental footprint by reducing reliance on synthetic inputs and adopting eco-friendly practices. | The different agroecological practices help prevent and reduce the conditions that favor the growth of mycotoxin-producing fungi in the field and during postharvest. |
| Insect/Pathogen | Morphological Description | Habits and Pest Structures | Critical Period | Ref. |
|---|---|---|---|---|
| Budworm: Spodoptera frugiperda (Lepidoptera: Noctuidae) | The adult is a dark gray moth with a white spot on the wings and lays its eggs on the underside of leaves. After six larval stages, the grayish-brown maggot measures 3 cm. | The cannibalistic larva is a bud and leaf chewer. Before pupating, it falls to the ground and may feed on tender stalks. | Vegetative | [62] |
| Corn earworm: Helicoverpa zea (Lepidoptera: Noctuidae) | The adult is a brown moth, laying eggs at the R1 stage. The first instar larva is gray with a black head, and in the last instar (sixth) it is pink. | The larva feeds on stigmas, silk, and cob. Noctuid moths tend to fly hundreds of miles in search of food. | Reproductive | [63] |
| Maize weevil: Sitophilus zeamais (Coleoptera: Curculionidae) | The adult is black, 3.5 mm, with a long proboscis, and lays its eggs inside the grain. The larvae are creamy white. Between 6 and 7 generations are produced per year. | The flying adult and larva feed on the grain, affecting seed germination during feeding and facilitating the introduction of Aspergillus. | Maturity and postharvest | [64] |
| Ear rot: Fusarium verticillioides (Telemorph: Gibberella moniliformis) | The fungus produces ovoid microconidia in chains and macroconidia in purplish pink aerial mycelium. | With the first rains or irrigations, the conidia germinate and are spread by the wind to infect several points distributed in the ear and/or asymptomatic grains. | Reproductive and Maturity | [65] |
| Ear rot: Aspergillus flavus (Teleomorph: Petromyces flavus) | The fungus produces purplish-brown-green conidiophores in aerial mycelium. | Sclerotia survive in the soil under warm weather and drought conditions. Airborne and insect dispersal of conidia are associated with infection. | Harvest and storage | [66] |
| States | Maize Product | Number of Samples (n) | AFB1 µg/kg (Maximum Level Found) | Year 4 | Ref. |
|---|---|---|---|---|---|
| Tamaulipas and Campeche | Grain | 1479 | 4405 | 2025 | [53] |
| Tamaulipas | Grain | 35 | 955 | 2005 | [79] |
| Nayarit | Grain | 49 | 21 | 2021 | [80] |
| Aguascalientes | Grain | 11 | 26 | 2013 | [81] |
| Puebla and Tlaxcala | Grain | 80 | 12 | 2024 | [82] |
| San Luis Potosí | Nixtamalized grain | 327 | 287 | 2018 | [83] |
| México city | Nixtamalized grain | 88 | 16 | 2019 | [84] |
| Veracruz | Tortilla local market | 120 | 22 | 2019 | [73] |
| Mexico City | Tortilla local market | 396 | 20 | 2011 | [85] |
| Veracruz | Popcorn | 30 | 26 | 2020 | [86] |
| Chiapas | Pozol 2 | 111 | 21 | 2004 | [87] |
| Mexico | Domestic pet foods (dog and cat) 3 | 35 | 72.4 | 2001 | [88] |
| Mycotoxin | Temperature (°C) | Rainfall/Drought | Grain Moisture (%) | Reporting States | Reference |
|---|---|---|---|---|---|
| Aflatoxins | 30–36 | Drought | ≥14 | Sonora, Tamaulipas, Campeche Veracruz, Chiapas, Yucatán, and Guerrero. | [53,100,,101] |
| Fumonisins | 28–34 | Rainfall | ≥18 | Puebla, Guanajuato, Jalisco, Nayarit, Sinaloa, Coahuila, Chihuahua, Veracruz, and Chiapas. | [102,103] |
| Aflatoxins and Fumonisins | 30–34 | Drought and rainfall intervals | 18–25 | Veracruz and Chiapas | [73,,102,104] |
| Practice | Spodoptera frugiperda | Helicoverpa zea | Sitophilus zeamais | Fusarium verticillioides | Aspergillus flavus |
|---|---|---|---|---|---|
| Genetic | Conduct pilot tests with several commercial hybrids and/or landraces with good adaptation to have genetic variation and to serve as a protective barrier to prevent the spread of pests. | Maize with excellent ear coverage and tolerance to drought, high temperatures, and insects significantly reduces fungal infestation and aflatoxin production (Table 9). | |||
| Agronomical | Soil removal before planting to expose larvae and pupae to the sun. | Weed control and densities ≤75 thousand plants/ha. | Dry and store grain at humidity ≤16%. | Sow pathogen-free seed. | Harvest when grain moisture is ≤25%. Adjust the threshing machine to avoid grain breakage. Dry and store grain at moisture ≤13% [71]. |
| Biological | Campoletis sonorensis and Cotesia marginiventris [113]. Phero-SF pheromones [114]. | Trichogramma spp., Hippodamia convergens and Bacillus thuringiensis [115]. | Metarhizium anisopliae and Beauveria bassiana [116]. | Trichoderma asperellum [117]. Bacillus spp. [118]. | Use of atoxigenic strains of A. flavus (Table 10). |
| Chemical | Spinetoram (Palgus-Dow). Dosage: 75–100 mL/ha. Flubendiamide (Belt-Bayer): Dosage: 100–125 mL/ha. Application: Direct spraying to leaves and buds when 30–40% of plants with perforated leaves, larvae, or droppings are observed. | Chlorantraniliprole (Coragen-FMC). Dosage: 200–250 mL/ha. Avermectin (Denim-Syngenta). Dosage: 100–200 mL/ha. Application: Make three applications to the foliage at 10-day intervals. Start the first one at flowering. | Phosphine (Phostoxin-Degesch) Dosage: 3 tablets/t. Application: Must gas for 72 h. Then ventilate the area for 24 h. Repeat after 3 months. | Seed treatment: Fludioxonil + metalaxyl (Maxim-Syngenta). Dosage: 100 mL/kg seed. It is recommended to mix with systemic insecticides such as azoxystrobin and trifloxystrobin [119]. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Zavala, C.; Solís-Martínez, O.; Valencia-Luna, J.B.; Sonder, K.; Hernández-Anguiano, A.M.; Palacios-Rojas, N. Aflatoxins in Mexican Maize Systems: From Genetic Resources to Agroecological Resilience and Co-Occurrence with Fumonisins. Toxins 2025, 17, 531. https://doi.org/10.3390/toxins17110531
Muñoz-Zavala C, Solís-Martínez O, Valencia-Luna JB, Sonder K, Hernández-Anguiano AM, Palacios-Rojas N. Aflatoxins in Mexican Maize Systems: From Genetic Resources to Agroecological Resilience and Co-Occurrence with Fumonisins. Toxins. 2025; 17(11):531. https://doi.org/10.3390/toxins17110531
Chicago/Turabian StyleMuñoz-Zavala, Carlos, Obed Solís-Martínez, Jessica Berenice Valencia-Luna, Kai Sonder, Ana María Hernández-Anguiano, and Natalia Palacios-Rojas. 2025. "Aflatoxins in Mexican Maize Systems: From Genetic Resources to Agroecological Resilience and Co-Occurrence with Fumonisins" Toxins 17, no. 11: 531. https://doi.org/10.3390/toxins17110531
APA StyleMuñoz-Zavala, C., Solís-Martínez, O., Valencia-Luna, J. B., Sonder, K., Hernández-Anguiano, A. M., & Palacios-Rojas, N. (2025). Aflatoxins in Mexican Maize Systems: From Genetic Resources to Agroecological Resilience and Co-Occurrence with Fumonisins. Toxins, 17(11), 531. https://doi.org/10.3390/toxins17110531





