Abstract
Orobanche cumana is an obligate holoparasitic plant with noxious effects in sunflower crops. Bellardia trixago is a facultative hemiparasitic plant that infects ruderal plants without noxious significance in agriculture and is known to produce a wide spectrum of bioactive metabolites. The objective of this study was to evaluate the allelopathic effects of B. trixago on the growth of O. cumana seedlings. Three different extracts using solvents of increasing polarity (n-hexane, dichloromethane and ethyl acetate) were prepared from the flowers, aerial green organs and roots of two populations, a white-flowered and a yellow-flowered population of B. trixago, both collected in southern Spain. Each extract was studied using allelopathic screenings on O. cumana which resulted in the identification of allelopathic activity of the ethyl acetate extracts against Orobanche radicles. Five iridoid glycosides were isolated together with benzoic acid from the ethyl acetate extract of aerial green organs by bio-guided purification. These compounds were identified as bartsioside, melampyroside, mussaenoside, gardoside methyl ester and aucubin. Among them, melampyroside was found to be the most abundant constituent in the extract (44.3% w/w), as well as the most phytotoxic iridoid on O. cumana radicle, showing a 72.6% inhibition of radicle growth. This activity of melampyroside was significantly high when compared with the inhibitory activity of benzoic acid (25.9%), a phenolic acid with known allelopathic activity against weeds. The ecotoxicological profile of melampyroside was evaluated using organisms representing different trophic levels of the aquatic and terrestrial ecosystems, namely producers (green freshwater algae Raphidocelis subcapitata and macrophyte Lepidium sativum), consumers (water flea Daphnia magna and nematode Caenorhabditis elegans) and decomposers (bacterium Aliivibrio fischeri). The ecotoxicity of melampyroside differed significantly depending on the test organism showing the highest toxicity to daphnia, nematodes and bacteria, and a lower toxicity to algae and macrophytes. The findings of the present study may provide useful information for the generation of green alternatives to synthetic herbicides for the control of O. cumana.
Key Contribution:
Five iridoid glycosides and benzoic acid were isolated from the ethyl acetate extract of aerial vegetative organs from plants of a white-flowered Bellardia trixago population. Bartsioside, melampyroside and mussaenoside were identified for the first time as inhibitors of Orobanche cumana radicle growth. Melampyroside was found to be the most phytotoxic compound and could be considered as a promising biocontrol agent for O. cumana considering the absence of relevant toxic effects observed in a preliminary ecotoxicological study.
1. Introduction
Approximately 1% of all angiosperms are parasites with the ability to infect other plants. Some parasitic plants are facultative parasites, capable of living autotrophically but shifting to a parasitic life form when a host is available, while others are obligated parasites requiring the infection of another plant shortly after germination. Some parasitic plants are hemiparasites with the ability to photosynthesize, while others are holoparasitic plants without photosynthetic competence, relying on their host for photoassimilates. Parasitic plants are distributed among 28 dicotyledonous families, and among them, the Orobanchaceae contains examples of parasitic species from all cases of host dependency [1,2]. Orobanchaceae contains facultative hemiparasitic plants, such as the non-weedy Bellardia trixago L. (syn. Bartsia trixago L.) with a Mediterranean origin that parasitizes the roots of ruderal species [3,4]. Orobanchaceae also contains obligate holoparasitic weeds from Orobanche genus from which control is limited or non-existent [5].
Among Orobanche species, Orobanche cumana Wallr. is one of the most noxious biotic stresses for sunflower crops [6]. Sunflower infection by O. cumana occurs in southern and eastern Europe, in the Mediterranean basin and in Asia [7]. Both types of sunflower, the oilseed type and the confectionary type, are severely affected by O. cumana [8]. The most feasible crop protection measures against the infection of Orobanche species are the cultivation of resistant varieties and chemical control [1,9]. However, for the specific sunflower problem caused by O. cumana, the resistant varieties are not durable, since their bred resistance is overcome by new races of O. cumana [10]. On the other hand, the chemical solution to control O. cumana is the use of imidazolinone herbicides that inhibit the enzyme acetohydroxy acid synthase [8,11]. However, the capacity to evolve imidazolinone-resistance in weeds, and the lack of alternative chemical methods for O. cumana threatens the sustainability of chemical control of O. cumana in sunflower [12]. Characterization of novel modes of allelopathic action against O. cumana in previously known natural compounds is an alternative solution to provide efficacy and sustainability in strategies for parasitic weed management [13].
B. trixago is a source of several bioactive compounds [14,15,16,17] but the screening of B. trixago as source of herbicidal compounds has not been performed before. Different B. trixago extracts have been screened for insecticidal activity [18,19]. Formisano et al. [19] demonstrated variation in insecticidal activity among different parts of B. trixago plants, with root extracts being significantly more active than extracts from aerial parts. In addition, Barrero et al. [16] demonstrated that qualitative and quantitative differences exist in the plant chemical composition among different populations of B. trixago. Here, we report the isolation and identification of iridoid glycosides—bartsioside, melampyroside and mussaenoside—with inhibitory activity on O. cumana radicle growth. Furthermore, the ecotoxicity of melampyroside (the most phytotoxic compound isolated) was assessed considering aquatic and terrestrial ecosystems as well as different trophic levels to reveal its toxicity and safety to environment and human health in order to be used as potent biocontrol agent.
2. Results and Discussion
Three different organs (flowers, aerial vegetative green organs and roots) of two B. trixago populations (a white-flowered and yellow-flowered population) were extracted by maceration with a hydroalcoholic solution and then employing three different solvents of increasing polarity (n-hexane, dichloromethane and ethyl acetate) in sequential order as described in the Materials and Methods section. The allelopathic activity of the resulting B. trixago extracts was analysed at 100 μg/mL using radicle growth bioassays on O. cumana (Figure 1). The inhibition of radicle growth was significantly affected by the B. trixago population and by the solvent used for the extraction, but not by the plant organ (ANOVA, p = 0.022, p < 0.001 and p = 0.347 respectively). Significant effects on radicle growth inhibition were observed by the interaction of B. trixago population × plant organ (ANOVA, p = 0.002), by the interaction B. trixago population × solvent used for the extraction (ANOVA, p < 0.007) and also by the interaction plant organ × solvent used for the extraction (ANOVA, p < 0.009). Previously, quantitative and qualitative variations in the chemical profiles have been reported among different populations of B. trixago [16] and among different plant organs of B. trixago plants [19]. Formisano et al. [19] located the insecticidal activity in the roots of one B. trixago population collected in Italy, whereas aerial parts of plants of the same population were less active.
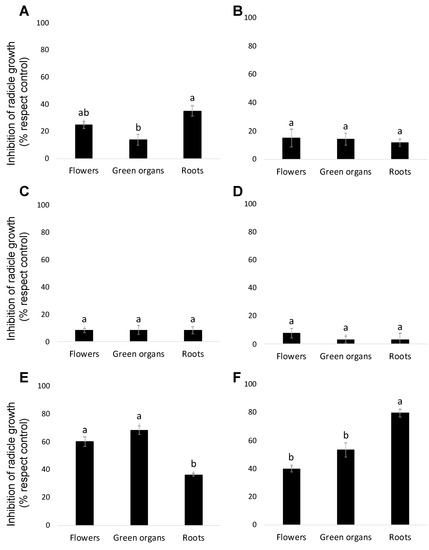
Figure 1.
Allelopathic effects on Orobanche cumana radicle growth induced by extracts prepared from sequential extractions with n-hexane (A,B), dichloromethane (C,D), and ethyl acetate (E,F) of three types of Bellardia trixago organs: flowers, aerial green organs and roots of two Bellardia trixago populations—a white-flowered population (A,C,E) and yellow-flowered population (B,D,F). In each figure, bars with different letters are significantly different according to the Tukey test (p = 0.05). Error bars represent the standard error of the mean.
In both populations studied, the main inhibitory activity was obtained with the ethyl acetate (EtOAc) extraction (Figure 1E,F). In the white-flowered population, the strongest inhibitory activity was found in the EtOAc extract of the aerial parts, mainly in the green vegetative organs followed by the EtOAc extract of the flowers (68.31 ± 2.9% and 60.1 ± 3.4% inhibition, respectively, in comparison with control). In the yellow-flowered B. trixago population, the strongest Orobanche inhibition activity was found in the EtOAc extract of roots (inhibition average of 79.5 ± 2.9%), followed by the EtOAc extracts of aerial parts of plants, both green organs and flowers (53.5 ± 5.1% and 40.1 ± 2.5% inhibition, respectively, in comparison with control). As a result of the allelopathic screening, a preliminary qualitative evaluation of the chromatographic profiles of all EtOAc extracts was performed. This evaluation revealed the common presence of a main compound and a common pattern of secondary metabolites among the different populations and plant organs (data not showed). This fact and also the larger amount of vegetative green tissues available in the laboratory allowed the selection of EtOAc extract of green organs of the white-flowered population as the source for the isolation and characterization of inhibitors of O. cumana radicle growth.
Thus, an amount of 189.0 g of lyophilized green organs of the white population were extracted following the procedure described in the Materials and Methods section. The sample yielded 1.45 g (0.77%) of EtOAc organic extract which was fractionated by different steps of purification by column chromatography and preparative TLCs, as reported in Scheme S1, obtaining six pure compounds which were identified as benzoic acid (1, 10.8 mg), bartsioside (2, 13.9 mg), aucubin (3, 12.4 mg), melampyroside (4, 642.3 mg), gardoside methyl ester (5, 2.0 mg), and mussaenoside (6, 6.1 mg) (Figure 2). The structures of these compounds were confirmed by NMR spectroscopy and MS, and by comparison with the data reported in the literature. Optical rotation allowed us to unequivocally identify the stereochemistry of the compounds by comparing with the values of the natural iridoids, which is a well-established family of natural products among which absolute stereochemistry was previously reported by chiroptical methods and X Ray [20,21]. Compounds 1 [16] and 2–6 were previously isolated from B. trixago [17,22] and other iridoid-containing plants [23,24,25,26,27,28,29,30].
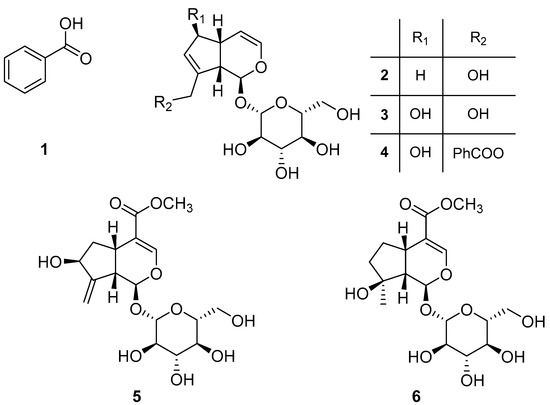
Figure 2.
Chemical structures of benzoic acid (1), bartsioside (2), aucubin (3), melampyroside (4), gardoside methyl ester (5), and mussaenoside (6).
The allelopathic effects of compounds 1–6 were assayed at 100 μg/mL on O. cumana radicles (Figure 3). Compounds 3 and 5 showed no significant inhibitory activity in the growth of O. cumana radicles in comparison with radicles treated with the control. On the other hand, compound 4 showed the strongest inhibition of radicle growth (72.6 ± 0.9%), followed by the inhibition activity induced by compounds 2 and 6 (61.1 ± 1.5% and 65.9 ± 2.9%, respectively). This is the first time that the inhibitory activity of Orobanche radicle growth has been reported in compounds 2, 4 and 6. Their activity was significantly higher than the activity of compound 1, a phenolic acid with recognized weedicide activity [31]. Compound 1 showed low but significant inhibitory activity on O. cumana radicle (25.9 ± 0.3%), which agrees with the moderate inhibitory activity observed by a previously study on the radicles of the legume-specific parasitic plant Orobanche crenata [32]. Compound 1 has been previously described as a growth-regulating agent, affecting plant growth in a dose-dependent manner on different plants [33,34,35]. Previous reports describe formulations including 1 in a combination with other components as an herbicide or growth-regulating agent [36,37,38].
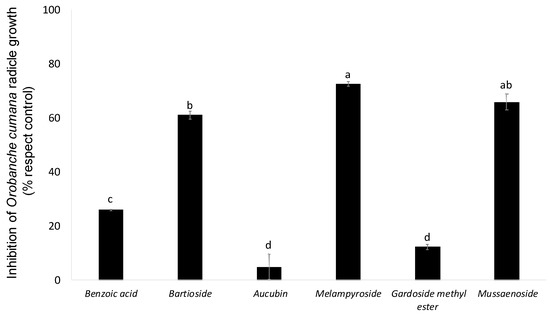
Figure 3.
Inhibition of Orobanche cumana radicle growth induced by benzoic acid (1), bartioside (2), aucubin (3), melampyroside (4), gardoside methyl ester (5) and mussaenoside (6) at 100 µg/mL. Bars with different letters are significantly different according to the Tukey test (p = 0.05). Error bars represent the standard error of the mean.
Compound 4 was firstly reported as an iridoid in Melampyrum silvaticum L. [39] while compounds 2, 3, 5 and 6 were first isolated from B. trixago [15], Aucuba japonica Thunb. [40], Melampyrum arvense L. [28] and Mussaenda parviflora Miq. [41], respectively. In the recent literature, it has been reported that compound 4 has anti-inflammatory activity [42], along with other iridoids not reported herein, which linked to a study on the potential activity of Odontites vulgaris against rheumatoid arthritis. In an even earlier study [43] including other iridoids and compound 4, the latter was also found to be cardioactive in Wistar rats.
Despite the similar chemical structures of 3 and 4, differences in their biological activities have been reported before. Compounds 4, 1 and 3, isolated from M. arvense, were reported to display antiprotozoal effects on different species, showing a certain degree of species-specificity [24]. Among the most active compounds 2, 4 and 6 on O. cumana growth, compound 4 induced some degree of phytotoxicity observed as darkening in the O. cumana radicles (Figure 4). Compounds 3, 4 and 6 were tested for antioxidant activity in previous reports [23,44]. Although no DPPH scavenging activity was found for these compounds, interestingly, through a β-carotene bleaching assay [23], compound 6 was found to be an antioxidant, 4 a pro-oxidant (by inducing a faster than spontaneous oxidation of β-carotene) and 3 was inactive. The pro-oxidation effect of 4 could damage the tissue of the plant explaining phytotoxicity; however, whether and how the pro-oxidant activity and the lack of antioxidant activity of 3 might be related with the observed (or not) inhibition of growth is unclear.

Figure 4.
Growth of Orobanche cumana radicles treated with melampyroside (4) at 100 µg/mL (A) and control (B).
CLogP was calculated for the isolated compounds in an effort to correlate the observed inhibitory activity of compounds 1–6 (Table 1). Negative values were obtained for all the compounds except 1, indicating a preference for the aqueous media instead of the organic. A good solubility in water is needed in order to favor the transport phenomena for the compound to reach the active site; however, a very low lipophilicity might jeopardize the ability of such to traverse through the cell membrane [45,46]. Thus, the compounds with the highest absolute CLogP values (over |2|) have the least bioactivity (3 and 5), while the most active ones 4 (−1.153), 6 (−1.849) and 2 (−1.941) have lower CLogP values. In the case of compound 4, the less affinity to water is joined to the presence of the benzoyl group which may have a positive effect on the bioactivity by release of this group by enzymatic transformation to benzoic acid inside the cell. As mentioned above, it has been reported that standalone benzoic acid has phytotoxic properties. The much lower activity of compound 1 when compared with 4 might be caused by two factors: their lipophilicity (CLogP: +1.885) and a possible synergistic effect with 4 after metabolization.

Table 1.
Calculated LogP for compounds (1–6).
The ecotoxicological tests are considered a valuable tool for preliminary toxicity screening of compounds, especially plant extracts [47]. The ecotoxicity of compound 4 was determined in three aquatic and two terrestrial organisms with different concentrations starting from 100 µg/mL, namely the effective concentration used for O. cumana. The influence of compound 4 on the observed effect in R. subcapitata, L. sativum, D. magna, C. elegans and A. fischeri, was shown in Figure 5.
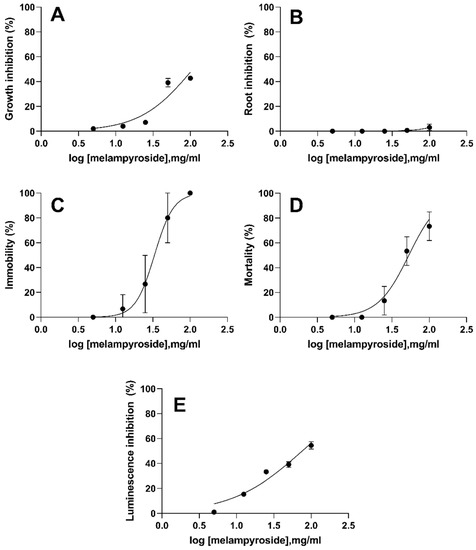
Figure 5.
Concentration–response curves of melampyroside (4) for R. subcapitata (A), L. sativum (B), D. magna (C), C. elegans (D) and A. fischeri (E). Error bars correspond to 95% confidence intervals. Dotted lines represent the fitting to the effect equation.
The results of ecotoxicity showed significant differences in the sensitivity of tested organisms. The differences were reflected in the sensitivity of the plant species and the other organisms to compound 4. While melampyroside showed the highest toxicity to daphnia (24 h EC50 = 33.26 µg/mL), nematodes (24 h EC50 = 57.23 µg/mL) and bacteria (EC50 30′ = 76.05 µg/mL), it was less toxic for plant species, such as algae and macrophytes with 72 h EC50 ≥ 100 µg/mL. It is noteworthy that, when compared the effect in L. sativum by concentration, the difference was not statistically significant from 5 µg/mL to 100 µg/mL, and the growth observed with these treatments was not significantly different from the growth of control group (Figure 5B). One explanation for this different species-specific sensitivity to melampyroside may be related to the non-absorption of this compound by some plants suggesting a selectivity of melampyroside to inhibit radicle growth of O. cumana [48].
Considering the EU-Directive 93/67/ECC (EC, 1996) [49] (whereby EC50 values < 1.0 µg/mL were considered highly toxic; 1.0–10 µg/mL are considered toxic, 10–100 µg/mL were classified as slightly toxic and above 100 µg/mL were non-toxic), the response of the investigated organisms revealed that compound 4 had little or no toxicity. In contrast to other compounds that are exceedingly toxic, melampyroside could be considered as potential antiparasitic weed agent with an optimal toxicity/selectivity ratio [50,51].
3. Conclusions
Ethyl acetate extracts from different organs of B. trixago exhibited significant levels of growth inhibition on radicles of O. cumana at extract concentration as low as 100 µg/mL. Subsequently, we isolated and identified the active compounds contained in the ethyl acetate extract of aerial vegetative organs. We found three iridoid glycosides bartsioside (2), melampyroside (4) and mussaenoside (6) with growth inhibition activity in the radicles of O. cumana. The radicle of Orobanche species is a parasitic organ that grows towards the host and upon host contact, it allows infection. The use of allelochemicals to inhibit the normal growth of Orobanche radicles inhibits crop infection and, as a consequence, the death of this parasitic weed. Ecotoxicological tests carried out on compound 4 (the most abundant and phytotoxic iridoid isolated from ethyl acetate extract) showed little or no toxicity according to the EU Directive 93/67/ECC [49]. Plant species-specific phytotoxicity is a desirable trait in the development of novel molecules with herbicidal action to satisfy the principle of pesticide selectivity recommended for integrated pest management [52]. Future studies should determine, at the molecular level, the mode of action of the active iridoid glycosides on the radicles of O. cumana.
4. Materials and Methods
4.1. General Experimental Procedures
A JASCO P-1010 digital polarimeter (Tokyo, Japan) was used to measure the optical rotations. 1H NMR spectra were recorded at 400/100 MHz on a Bruker 400 Anova Advance (Karlsruhe, Germany) spectrometer or at 500/125 MHz on a Varian Inova 500 (Palo Alto, CA, USA). The spectra were recorded using CDCl3 or CD3OD and the same solvents were used as internal standards. Column chromatography (CC) was performed using silica gel (Kieselgel 60, 0.063–0.200 mm, Merck, Darmstadt, Germany). Thin-layer chromatography (TLC) was performed on analytical and preparative silica gel plates (Kieselgel 60, F254, 0.25 and 0.5 mm, respectively, Merck, Darmstadt, Germany). The spots were visualized via exposure to UV light (254 nm) and/or iodine vapours and/or by spraying first with 10% H2SO4 in MeOH and then with 5% phosphomolybdic acid in EtOH, followed by heating at 110 °C for 10 min. Electrospray ionization mass spectra (ESIMS) were performed using the LC/MS TOF system AGILENT 6230B (Agilent Technologies, Milan, Italy), HPLC 1260 Infinity. Sigma-Aldrich Co. (St. Louis, MO, USA) supplied all the reagents and the solvents.
4.2. Plant Material
Plants of two populations of Bellardia trixago—a white-flowered population and yellow-flowered population—were harvested at the phenological stage of flowering in spring of 2021 in Cordoba, southern Spain (coordinates 37.856 N, 4.806 W, datum WGS84). Bellardia trixago plants were immediately carried to the laboratory, and the plants were separated into three compartments: flowers, aerial green organs (stems and leaves) and roots. Each compartment was immediately frozen with liquid nitrogen, stored at −80 °C, subsequently lyophilized and the dry material stored in the dark at 4 °C until use. Orobanche seeds were collected from mature plants of O. cumana infecting sunflowers in southern Spain. Dry parasitic seeds were separated from capsules using winnowing combined with a sieve of 0.6 mm mesh size and then stored dry in the dark at room temperature until use for this work.
4.3. Extractions of Bellardia trixago Organs
A total of 3.0 g of each B. trixago organs were extracted, following a previously reported protocol often used for the extraction of plant material [53,54], in order to perform a preliminary activity screening against parasitic plants. In particular, the flowers, green organs and roots of each population were extracted separately by H2O/MeOH (1/1, v/v), under stirred conditions at room temperature for 24 h. The hydroalcoholic suspensions were centrifuged at 7000 rpm and extracted with n-hexane (3 × 50 mL), CH2Cl2 (3 × 50 mL), and after removing methanol under reduced pressure, with EtOAc (3 × 50 mL). The yield of each extract is reported in SI (Table S1).
4.4. Isolation and Identification of Metabolites from Bellardia trixago Green Organs of the White-Flowered Population
White green organs (189.0 g) were extracted (1 × 500 mL) by H2O/MeOH (1/1, v/v), under stirred conditions at room temperature for 24 h, the suspension centrifuged, and the supernatant extracted by n-hexane (3 × 300 mL) and successively with CH2Cl2 (3 × 300 mL) and, after removing methanol under reduced pressure, with EtOAc (3 × 200 mL). The residue (1.45 g) of EtOAc organic extract was purified by CC eluted with CH2Cl2/MeOH (8.5/1.5, v/v) yielding nine homogeneous fractions (F1-9), as reported in Scheme S1. The residue (54.6 mg) of F3 was purified by TLC eluted with EtOAc/MeOH/H2O (9/0.75/0.25, v/v/v), yielding six groups of homogeneous fractions (F3.1-F3.6). F3.1 was identified as benzoic acid (1, 10.8 mg) and F3.5 as melampyroside (4, 8.4 mg). The residue (584.7 mg) of fraction F4 yielded pure melampyroside (4). The residue (77.9 mg) of F5 was purified by TLC eluted by CH2Cl2/EtOAc/MeOH (2/2/1, v/v/v), yielding two homogeneous fractions. The first fraction of the latter purification yielded a further amount of melampyroside (4, 20.4 mg, for a total of 613.5 mg). The residue (498.4 mg) of F6 was purified by CC eluted with CH2Cl2/EtOAc/MeOH (2/2/1, v/v/v), yielding seven fractions (F6.1-F6.7). The residue (36.5 mg) of F6.4 was further purified by reverse-phase TLC eluted with MeCN/H2O (4/6, v/v), yielding gardoside methyl ester (5, 2.0 mg), bartsioside (2, 13.9 mg), and mussaenoside (6, 6.1 mg). The residue (64.7 mg) of F7 was purified by TLC eluted with EtOAc/MeOH/H2O (8.5/1/0.5, v/v/v) giving further amount of mussaenoside (6, 2.3 mg, for a total of 8.4 mg) and aucubin (3, 12.4 mg).
- Benzoic acid (1): 1H NMR spectrum (Figure S1) was in agreement with data previously reported [55]. ESI MS (-) m/z: 121 [M − H]−.
- Bartsioside (2): [α]D22-71.9 (c 0.64, MeOH) [lit. [56]: [α]D25-86.4 (c 0.5 MeOH)]. 1H NMR spectrum (Figure S2) was in agreement with data previously reported [57]. ESI-MS (+), m/z: 330 [M + H]+.
- Aucubin (3): [α]D22-89.8 (c 1.0, MeOH) [lit. [27]: [α]D26-92.8 (c 0.27, MeOH)]. 1H NMR spectrum (Figure S3) was in agreement with data previously reported [17,58,59]. ESI-MS (+), m/z: 347 [M + H]+.
- Melampyroside (4): [α]D22-69.6 (c 0.79, MeOH) [lit. [27]: [α]D26-52.9 (c 0.31, MeOH)]. 1H and 13C NMR spectra (Figures S4 and S5) were in agreement with data previously reported [11,49], while its NOESY spectrum is reported in Figure S6. ESI MS (+) m/z: 451 [M + H]+.
- Gardoside methyl ester (5): [α]D22-49.8 (c 0.20, MeOH) [lit. [28]: [α]D20-46 (c 0.3, MeOH)]. 1H NMR spectrum (Figure S7) was in agreement with data previously reported [11,22]. ESI-MS (+), m/z: 389 [M + H]+.
- Mussaenoside (6): [α]D22-81.3 (c 0.30, MeOH) [lit. [27]: [α]D26-77.9 (c 0.32, MeOH)]. 1H NMR spectrum (Figure S8) was in agreement with data previously reported [17,59,60,61]. ESI-MS (+), m/z: 391 [M + H]+.
4.5. Bioactivity on Parasitic Weed Seeds
Allelopathic effects of each B. trixago extracts and isolated compounds were tested on Orobanche radicle growth according to previous protocols [62]. Seeds of Orobanche cumana, were surface-sterilized by immersion in 0.5% (w/v) NaOCl and 0.02% (v/v) Tween 20, for 5 min, rinsed thoroughly with sterile distilled water, and dried in a laminar airflow cabinet. Approximately 100 seeds of Orobanche seeds were placed separately on 9 mm-diameter glass fiber filter paper disks (GFFP) (Whatman International Ltd., Maidstone, UK), moistened with 50 μL of sterile distilled water, and placed in incubators at 23 °C for 10 days inside Parafilm-sealed Petri dishes, to allow seed conditioning. Then, GFFP disks containing conditioned Orobanche seeds were transferred onto a sterile sheet of filter paper and transferred to new 9 cm sterile Petri dishes. Stock solutions of each B. trixago extract and isolated metabolite were respectively dissolved in dimethyl sulfoxide and subsequently individually diluted to 100 μg/mL using an aqueous solution of GR24 (10−6 M). The final concentration of dimethyl sulfoxide was 2% in all test treatments. For each assay, 50 μL aliquots of each sample were applied to GFFP discs containing conditioned Orobanche seeds. Triplicate aliquots of a treatment only containing GR24 and 2% dimethyl sulfoxide was used as a control. Treated seeds were incubated in the dark at 23 °C for 7 days and radicle growth was determined for each GFFP disc, using a stereoscopic microscope (Leica S9i, Leica Microsystems GmbH, Wetzlar, Germany). For the characteristic of radicle growth, the value used was the average of 10 randomly selected radicles per GFFP disc [63]. The percentage of radicle growth inhibition of each treatment was then calculated relative to the average radicle growth of control treatment.
4.6. Molecular Modelling
CLogP were calculated using ChemOffice v20.1 (PerkinElmer, Waltham, MA, USA) by means of the appropriate tool in ChemDraw Professional [64].
4.7. Ecotoxicity Analysis on Melampyroside
The ecotoxicological tests were carried out on green freshwater algae Raphidocelis subcapitata, macrophyte Lepidium sativum, water flea Daphnia magna, nematode Caenorhabditis elegans and bacterium Aliivibrio fischeri to expand the range of endpoints due to differences in species sensitivity and exposure. Testing on R. subcapitata was performed using as endpoint the algal growth inhibition after 72 h of exposure and was based on ISO 8692:2012 [65]. The algal density was determined by spectrophotometric analysis (DR5000, Hach Lange GbH, Weinheim, Germany). Ecotoxicity tests were carried out in triplicate, at 25 ± 1 °C with constant illumination of 6700 lux. Testing on L. sativum was performed according to ISO 11269-1:2012 [66] considering germination and root elongation as endpoint after 72 h. Seeds (n = 10) were exposed in triplicate in Petri dishes and incubated at 25 ± 1 °C in darkness. Daphnia magna test was conducted according to UNI EN ISO 6341:2013 [67] and the endpoint evaluated was the immobilization after 24 h. Daphnids (less than 24 h old) were exposed to the samples at 20 ± 2 °C, in darkness without feeding. Testing on C. elegans was carried out, with a few modifications, according to the ASTM E2172-01 Standard Method (2014) using the 24 h mortality endpoint. The test was performed using age-synchronous adult nematodes exposed at 20 °C to compound 4, without feeding. Testing on A. fischeri was based on ISO 11348-3:2007 [68] and the inhibition of the bioluminescence of the bacterium after 30 min of exposure was measured as endpoint. The test was performed using Microtox® Model 500 (M500) analyzer with osmotic adjustment solution (OAS) at 15 ± 1 °C.
4.8. Data Analyses
All bioassays were performed using a completely randomized design. Percentage data in Orobanche assays were approximated to normal frequency distribution by means of angular transformation and subjected to analysis of variance (ANOVA) using SPSS software for Windows (SPSS Inc., Chicago, IL, USA). The significance of mean differences among treatments was evaluated by the Tukey test. The null hypothesis was rejected at the level of 0.05. Results of ecotoxicological tests were given as the mean of effect ± standard error. Median effect concentrations EC50, EC20 and EC5 were calculated as mean values and relative 95% confidence limit values for compound 4. Statistical analysis was carried out via XLSTAT and GraphPad Prism 2.5. (Systat Software, San Jose, CA, USA).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/toxins14080559/s1, Table S1: Extract weights of organic extracts obtained from different Bartsia trixago organs; Scheme S1: Purification scheme of EtOAc extract of B. trixago white green organs; Figure S1: 1H NMR spectrum of benzoic acid, 1 (CDCl3, 400 MHz); Figure S2: 1H NMR spectrum of bartsioside, 2 (MeOD, 500 MHz); Figure S3: 1H NMR spectrum of aucubin, 3 (MeOD, 400 MHz); Figure S4: 1H NMR spectrum of melampyroside, 4 (MeOD, 400 MHz); Figure S5: 13C NMR spectrum of melampyroside, 4 (MeOD, 100 MHz); Figure S6: NOESY spectrum of melampyroside, 4 (MeOD, 400 MHz); Figure S7: 1H NMR spectrum of gardoside methyl ester, 5 (MeOD, 400 MHz). Figure S8: 1H NMR spectrum of mussaenoside, 6 (MeOD, 500 MHz).
Author Contributions
Conceptualisation, M.F.-A., M.M., and A.C.; formal analysis, A.S., M.F.-A., and A.C.P.; writing—original draft preparation, G.S., A.S., M.F.-A., A.C.P., and M.M.; data curation, G.S., A.S., M.F.-A., A.C.P., A.M.-R.; writing—review and editing, G.S., A.S., M.F.-A., A.C.P., M.M.; M.G., and A.C.; Supervision, A.C.; Funding acquisition, M.F.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Agencia Estatal de Investigación (projects PID2020-114668RB-I00 and RYC-2015-18961). Authors wish to express gratitude for the Ph.D. grant to Gabriele Soriano funded by INPS (Istituto Nazionale Previdenza Sociale), and for the Galileo grant from Córdoba University-Diputación to Antonio Moreno-Robles.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the “CSIC Interdisciplinary Thematic Platform (PTI) Optimization of Agricultural and Forestry Systems (PTI-AGROFOR)”, the “Consejería de Transformación Económica, Industria, Conocimiento y Universidades de la Junta de Andalucía, project ID: QUAL21 023 IAS” and “Máster Universitario en Agroalimentación, Córdoba University, Spain”. A.C.P. expresses his sincere gratitude to the “Plan Propio—UCA 2022-2023” (REF. EST2022-087), the “Consejería de Economía, Conocimiento, Empresas y Universidad de la Junta de Andalucía” and the “Programa Operativo Fondo Social Europeo de Andalucía 2014–2020” for their financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parker, C.; Riches, C.R. Parasitic Weeds of the World: Biology and Control; CAB International: Wallingford, UK, 1993; ISBN 9780851988733. [Google Scholar]
- Westwood, J.H.; Yoder, J.I.; Timko, M.P.; dePamphilis, C.W. The evolution of parasitism in plants. Trends Plant Sci. 2010, 15, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Press, M.C.; Parsons, A.N.; Mackay, A.W.; Vincent, C.A.; Cochrane, V.; Seel, W.E. Gas exchange characteristics and nitrogen relations of two Mediterranean root hemiparasites: Bartsia trixago and Parentucellia viscosa. Oecologia 1993, 95, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Convers, S.; Tank, D.C. Phylogenetic revision of the genus Bartsia (Orobanchaceae): Disjunct distributions correlate to independent lineages. Syst. Bot. 2016, 41, 672–684. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Sillero, J.C.; Rubiales, D. Resistance to broomrape species (Orobanche spp.) in common vetch (Vicia sativa L.). Crop. Prot. 2009, 28, 7–12. [Google Scholar] [CrossRef]
- Molinero-Ruiz, L.; Delavault, P.; Pérez-Vich, B.; Pacureanu-Joita, M.; Bulos, M.; Altieri, E.; Domínguez, J. History of the race structure of Orobanche cumana and the breeding of sunflower for resistance to this parasitic weed: A review. Span. J. Agric. Res. 2015, 13, e10R01. [Google Scholar] [CrossRef]
- Parker, C. Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag. Sci. 2009, 65, 453–459. [Google Scholar] [CrossRef]
- Eizenberg, H.; Hershenhorn, J.; Ephrath, J.E. Factors affecting the efficacy of Orobanche cumana chemical control in sunflower. Weed Res. 2008, 49, 308–315. [Google Scholar] [CrossRef]
- Joel, D.M.; Hershenhorn, J.; Eizenberg, H.; Aly, R.; Ejeta, G.; Rich, P.J.; Ransom, J.K.; Sauerborn, J.; Rubiales, D. Biology and management of weedy root parasites. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons: New York, NY, USA, 2007; Volume 33, pp. 267–350. [Google Scholar]
- Labrousse, P.; Arnaud, M.C.; Griveau, Y.; Fer, A.; Thalouarn, P. Analysis of resistance criteria of sunflower recombined inbred lines against Orobanche cumana Wallr. Crop Prot. 2004, 23, 407–413. [Google Scholar] [CrossRef]
- Aly, R.; Goldwasser, Y.; Eizenber, H.; Hershenhorn, J.; Golan, S.; Kleifeld, Y. Broomrape (Orobanche cumana) control in sunflower (Helianthus annuus) with imazapic. Weed Technol. 2009, 15, 306–3009. [Google Scholar] [CrossRef]
- Lerner, F.; Pfenning, M.; Picard, L.; Lerchl, J.; Hollenbach, E. Prohesadione calcium is herbicidal to the sunflower root parasite Orobanche cumana. Pest Manag. Sci. 2021, 77, 1893–1902. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Delavault, P.; Timko, M.P. Management of infection by parasitic weeds: A Review. Plants 2020, 9, 1184. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Cole, M.D.; Garcia-Viguera, C.; Tomas-Lorente, F.; Guirado, A. Epicuticular flavonoids from Bellardia trixago and their antifungal fully methylated derivatives. Int. J. Crude Drug Res. 1990, 28, 57–60. [Google Scholar] [CrossRef]
- Bianco, A.; Guiso, M.; Iavarone, C.; Trogolo, C. Iridoids. XX. Bartsioside, structure and configuration. Gazz. Chim. Ital. 1976, 106, 725–732. [Google Scholar]
- Barrero, A.F.; Sánchez, J.F.; Cuenca, F.G. Dramatic variation in diterpenoids of different populations of Bellardia trixago. Phytochemistry 1988, 27, 3676–3678. [Google Scholar] [CrossRef]
- Ersöz, T.; Yalçin, F.N.; Taşdemir, D.; Sticher, O.; Çaliş, İ. Iridoid and lignan glucosides from Bellardia trixago (L.) All. Turk. J. Med. Sci. 1998, 28, 397–400. [Google Scholar]
- Pascual-Villalobos, M.J.; Robledo, A. Screening for anti-insect activity in Mediterranean plants. Ind. Crops Prod. 1998, 8, 183–194. [Google Scholar] [CrossRef]
- Formisano, C.; Rigano, D.; Senatore, F.; Simmonds, M.S.J.; Bisio, A.; Bruno, M.; Rosselli, S. Essential oil composition and antifeedant properties of Bellardia trixago (L.) All. (sin. Bartsia trixago L.) (Scrophulariaceae). Biochem. Syst. Ecol. 2008, 36, 454–457. [Google Scholar] [CrossRef]
- Weinges, K.; Ziegler, H.J. Chemie und Stereochemie der Iridoide, XIV. Aucubin und Scandosid aus Catalpol. Liebigs Ann. Chem. 1990, 1990, 715–717. [Google Scholar] [CrossRef]
- Tietze, L.F.; Niemeyer, U.; Marx, P.; Glüsenkamp, K.H.; Schwenen, L. Iridoide—XIII: Bestimmung der absoluten konfiguration und konformation isomerer iridoidglycoside mit hilfe chiroptischer methoden. Tetrahedron 1980, 36, 735–739. [Google Scholar] [CrossRef]
- Venditti, A.; Ballero, M.; Serafini, M.; Bianco, A. Polar compounds from Parentucellia viscosa (L.) Caruel from Sardinia. Nat. Prod. Res. 2015, 29, 602–606. [Google Scholar] [CrossRef]
- Cuendet, M.; Potterat, O.; Hostettmann, K. Iridoid glucosides, phenylpropanoid derivatives and flavanoids from Bartsia alpina. Pharm. Biol. 1999, 37, 318–320. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Atay, I.; Kaiser, M.; Brun, R.; Cartagena, M.M.; Carballeira, N.M.; Yesilada, E.; Tasdemir, D. Antiprotozoal activity of Melampyrum arvense and its metabolites. Phytother. Res. 2011, 25, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Tamura, K.; Matsumoto, T.; Terao, H.; Tabata, M.; Fujita, T.; Honda, G.; Sezik, E.; Yesiladat, E. 6′-O-benzoylshanzhiside methyl ester from Rhinanthus angustifolius subsp. grandiflorus. Phytochemistry 1993, 33, 623–625. [Google Scholar] [CrossRef]
- Bianco, A.; Bolli, D.; Passacantilli, P. 6-O-β-Glucopyranosylaucubin, a new irodoid from Odontites verna. Planta Med. 1982, 44, 97–99. [Google Scholar] [CrossRef]
- Takeda, Y.; Fujita, T. Iridoid glucosides of Melampyrum laxum. Planta Med. 1981, 41, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Damtoft, S.; Hansen, S.B.; Jacobsen, B.; Jensen, S.R.; Nielsen, B.J. Iridoid glucosides from Melampyrum. Phytochemistry 1984, 23, 2387–2389. [Google Scholar] [CrossRef]
- Bianco, A.; Passacantilli, P.; Righi, G.; Nicoletti, M. Iridoid glucosides from Parentucellia viscosa. Phytochemistry 1985, 24, 1843–1845. [Google Scholar] [CrossRef]
- Petitto, V.; Serafini, M.; Ballero, M.; Foddai, S.; Stanzione, A.; Nicoletti, M. Iridoids from Euphrasia genargentea, a rare Sardinian endemism. Nat. Prod. Res. 2009, 23, 431–435. [Google Scholar] [CrossRef]
- Khanh, T.D.; Anh, L.H.; Nghia, L.T.; Trung, K.H.; Hien, P.B.; Trung, D.M.; Xuan, T.D. Allelopathic responses of rice seedlings under some different stresses. Plants 2018, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, M.; Cimmino, A.; Evidente, A.; Rubiales, D. Inhibition of Orobanche crenata seed germination and radicle growth by allelochemicals identified in cereals. J. Agric. Food Chem. 2013, 61, 9797–9803. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, L.-Y.; Hu, L.-Y.; Cao, W.; Sun, K.; Sun, Q.-B.; Siddikee, A.; Shi, R.-H.; Dai, C.-C. Evidence for the involvement of auxin, ethylene and ROS signaling during primary root inhibition of Arabidopsis by the allelochemical benzoic acid. Plant Cell Physiol. 2018, 59, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Chen, Y.; Chen, J.; Zhang, Y.; Zhang, T.; He, H. Comparison of allelopathic effects of two typical invasive plants: Mikania micrantha and Ipomoea cairica in Hainan island. Sci. Rep. 2020, 10, 11332. [Google Scholar] [CrossRef] [PubMed]
- Baziramakenga, R.; Leroux, G.D.; Simard, R.R. Effects of benzoic and cinnamic acids on membrane permeability of soybean roots. J. Chem. Ecol. 1995, 21, 1271–1285. [Google Scholar] [CrossRef]
- Earl, D.F. Method of Treating Plants. U.S. Patent US2723909A, 21 August 1952. [Google Scholar]
- Pang, J. One Kind Greening Momordica Grosvenori Herbicide and Preparation Method Thereof. Chinese Patent CN107372626A, 14 August 2017. [Google Scholar]
- Jin, X.; Lin, Y.; Li, Y.; Lin, D.; Lin, J.; Lin, Z. Organic Crop Herbicide Containing Organic Acid and Mineral Oil and Preparation Method Thereof. Chinese Patent CN113925063A, 14 January 2022. [Google Scholar]
- Ahn, B.Z.; Pachaly, P. Melampyrosid, ein neus iridoid aus Melampyrum silvaticum L. Tetrahedron 1974, 30, 4049–4054. [Google Scholar] [CrossRef]
- Karrer, P.; Schmid, H. Über die Konstitution des Aucubins. Helv. Chim. Acta 1946, 29, 525–552. [Google Scholar] [CrossRef]
- Takeda, Y.; Nishimura, H.; Inouye, H. Two new iridoid glucosides from Mussaenda parviflora and Mussaenda shikokiana. Phytochemistry 1977, 16, 1401–1404. [Google Scholar] [CrossRef]
- Ji, M.; Wang, C.; Yang, T.; Meng, X.; Wang, X.; Li, M. Integrated phytochemical analysis based on UPLC–MS/MS and network pharmacology approaches to explore the effect of Odontites vulgaris Moench on rheumatoid arthritis. Front. Pharmacol. 2021, 12, 707687. [Google Scholar] [CrossRef]
- Pennacchio, M.; Syah, Y.M.; Ghisalberti, E.L.; Alexander, E. Cardioactive iridoid glycosides from Eremophila species. Phytomedicine 1997, 4, 325–330. [Google Scholar] [CrossRef]
- Çaliş, I.; Kirmizibekmez, H.; Taşdemir, D.; Ireland, C.M. Iridoid glycosides from Globularia davisiana. Chem. Pharm. Bull. 2002, 50, 678–680. [Google Scholar] [CrossRef]
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and its relationship with passive drug permeation. Pharm. Res. 2011, 28, 962–977. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Afolayan, A.J.; Ohikhena, F.U.; Wintola, O.A. Toxicity assessment of different solvent extracts of the medicinal plant, Phragmanthera capitata (Sprengel) Balle on brine shrimp (Artemia salina). Int. J. Pharmacol. 2016, 12, 701–710. [Google Scholar]
- Page, S.W. Antiparasitic drugs. In Small Animal Clinical Pharmacology; Maddison, J.E., Page, S.W., Church, D.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 198–260. [Google Scholar]
- European Commission (EC). Technical Guidance Document in Support of the Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances and Commission Regulation (EC) No 1488/94 on Risk Assessment for Existing Substances; Parts 1–4; Office for Official Publications of the EC: Luxembourg, 1996. [Google Scholar]
- Koko, W.S.; Jentzsch, J.; Kalie, H.; Schobert, R.; Ersfeld, K.; Al Nasr, I.S.; Biersack, B. Evaluation of the antiparasitic activities of imidazol-2-ylidene-gold (I) complexes. Arch. Pharm. 2020, 353, e1900363. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.W.; Phillipson, J.D. Natural products and the development of selective antiprotozoal drugs. Phytother. Res. 1990, 4, 127–139. [Google Scholar] [CrossRef]
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Sattin, M. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Cimmino, A.; Roscetto, E.; Masi, M.; Tuzi, A.; Radjai, I.; Gahdab, C.; Paolillo, R.; Guarino, A.; Catania, M.R.; Evidente, A. Sesquiterpene lactones from Cotula cinerea with antibiotic activity against clinical isolates of Enterococcus faecalis. Antibiotics 2021, 10, 819. [Google Scholar] [CrossRef]
- Soriano, G.; Petrillo, C.; Masi, M.; Bouafiane, M.; Khelil, A.; Tuzi, A.; Isticato, R.; Fernández-Aparicio, M.; Cimmino, A. Specialized metabolites from the allelopathic plant Retama raetam as potential biopesticides. Toxins 2022, 14, 311. [Google Scholar] [CrossRef]
- Cui, L.-Q.; Liu, K.; Zhang, C. Effective oxidation of benzylic and alkane C–H bonds catalyzed by sodium o-iodobenzenesulfonate with Oxone as a terminal oxidant under phase-transfer conditions. Org. Biomol. Chem. 2011, 9, 2258–2265. [Google Scholar] [CrossRef]
- Delicato, A.; Masi, M.; de Lara, F.; Rubiales, D.; Paolillo, I.; Lucci, V.; Falco, G.; Calabro, V.; Evidente, A. In vitro characterization of iridoid and phenylethanoid glycosides from Cistanche phelypaea for nutraceutical and pharmacological applications. Phytother. Res. 2022, 1–12. [Google Scholar] [CrossRef]
- Andrzejewska-Golec, E.; Ofterdinger-Daegel, S.; Calis, I.; Światek, L. Chemotaxonomic aspects of iridoids occurring in Plantago subg. Psyllium (Plantaginaceae). Plant Syst. Evol. 1993, 185, 85–89. [Google Scholar] [CrossRef]
- Chaudhuri, R.K.; Afifi-Yazar, F.Ü.; Sticher, O.; Winkler, T. 13C NMR spectroscopy of naturally occurring iridoid glucosides and their acylated derivatives. Tetrahedron 1980, 36, 2317–2326. [Google Scholar] [CrossRef]
- Chu, H.-B.; Tan, N.-H.; Zhang, Y.-M. Chemical constituents from Pedicularis rex C. B. Clarke. Z. Naturforsch. B 2007, 62, 1465–1470. [Google Scholar] [CrossRef]
- Otsuka, H.; Watanabe, E.; Yuasa, K.; Ogimi, C.; Takushi, A.; Takeda, Y. A verbascoside iridoid glucoside conjugate from Premna corymbosa var. abtusifolia. Phytochemistry 1993, 32, 983–986. [Google Scholar] [CrossRef]
- Gardner, D.R.; Narum, J.; Zook, D.; Stermitz, F.R. New iridoid glucosides from Castilleja and Besseya: 6-Hydroxyadoxoside and 6-isovanillylcatapol. J. Nat. Prod. 1987, 50, 485–489. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Moral, A.; Kharrat, M.; Rubiales, D. Resistance against broomrapes (Orobanche and Phelipanche spp.) in faba bean (Vicia faba) based in low induction of broomrape seed germination. Euphytica 2012, 186, 897–905. [Google Scholar] [CrossRef]
- Westwood, J.H.; Foy, C.L. Influence of nitrogen on germination and early development of broomrape (Orobanche spp.). Weed Sci. 1999, 47, 2–7. [Google Scholar] [CrossRef]
- Cala, A.; Zorrilla, J.G.; Rial, C.; Molinillo, J.M.G.; Varela, R.M.; Macías, F.A. Easy access to alkoxy, amino, carbamoyl, hydroxy, and thiol derivatives of sesquiterpene lactones and evaluation of their bioactivity on parasitic weeds. J. Agric. Food Chem. 2019, 67, 10764–10773. [Google Scholar] [CrossRef]
- ISO 8692:2012; Water Quality—Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae. ISO: Geneva, Switzerland, 2012.
- ISO 11269-1:2012; Soil Quality—Determination of the Effects of Pollutants on Soil Flora—Part 1: Method for the Measurement of Inhibition of Root Growth. ISO: Geneva, Switzerland, 2012.
- UNI EN ISO 6341:2013; Water Quality—Determination of the Inhibition of the Mobility of Daphnia magna Straus (Cladoc-Era, Crustacea)—Acute Toxicity Test. Ente Nazionale Italiano di Unificazione (UNI): Milan, Italy, 2013.
- ISO 11348-3:2007; Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent Bacteria Test)—Part 3: Method Using Freeze-Dried Bacteria. ISO: Geneva, Switzerland, 2007.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).